fa7c36b16ccbe9fece2a710e1f1d5a3a.ppt
- Количество слайдов: 11
 CI-1 Certican® (everolimus) Cardiovascular and Renal Drugs Advisory Committee Meeting November 16, 2005
CI-1 Certican® (everolimus) Cardiovascular and Renal Drugs Advisory Committee Meeting November 16, 2005
 CI-2 Certican® (everolimus) Introduction and Regulatory Background Mathias Hukkelhoven, Ph. D Senior Vice President Global Head, Drug Regulatory Affairs Novartis Pharmaceuticals Corporation
CI-2 Certican® (everolimus) Introduction and Regulatory Background Mathias Hukkelhoven, Ph. D Senior Vice President Global Head, Drug Regulatory Affairs Novartis Pharmaceuticals Corporation
 122 -5 Certican® Clinical Development Program u Most comprehensive clinical development program in transplantation – 25 trials (1 heart, 7 kidney, 17 clin-pharm) • Approximately 3000 patients enrolled, 1800 patients treated • 220 investigators worldwide u Phase 3 heart study B 253 – 634 patients – Follow-up at 6, 12, 24, and 48 months 3
122 -5 Certican® Clinical Development Program u Most comprehensive clinical development program in transplantation – 25 trials (1 heart, 7 kidney, 17 clin-pharm) • Approximately 3000 patients enrolled, 1800 patients treated • 220 investigators worldwide u Phase 3 heart study B 253 – 634 patients – Follow-up at 6, 12, 24, and 48 months 3
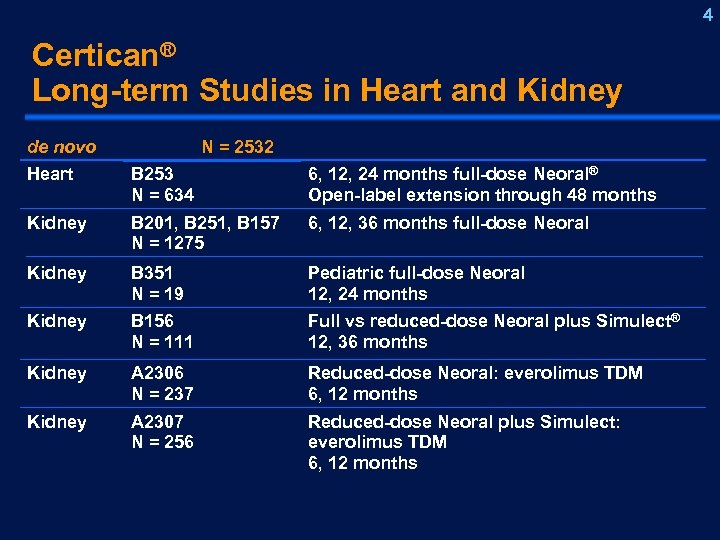 4 Certican® Long-term Studies in Heart and Kidney de novo N = 2532 Heart B 253 N = 634 6, 12, 24 months full-dose Neoral® Open-label extension through 48 months Kidney B 201, B 251, B 157 N = 1275 6, 12, 36 months full-dose Neoral Kidney B 351 N = 19 Pediatric full-dose Neoral 12, 24 months Kidney B 156 N = 111 Full vs reduced-dose Neoral plus Simulect® 12, 36 months Kidney A 2306 N = 237 Reduced-dose Neoral: everolimus TDM 6, 12 months Kidney A 2307 N = 256 Reduced-dose Neoral plus Simulect: everolimus TDM 6, 12 months
4 Certican® Long-term Studies in Heart and Kidney de novo N = 2532 Heart B 253 N = 634 6, 12, 24 months full-dose Neoral® Open-label extension through 48 months Kidney B 201, B 251, B 157 N = 1275 6, 12, 36 months full-dose Neoral Kidney B 351 N = 19 Pediatric full-dose Neoral 12, 24 months Kidney B 156 N = 111 Full vs reduced-dose Neoral plus Simulect® 12, 36 months Kidney A 2306 N = 237 Reduced-dose Neoral: everolimus TDM 6, 12 months Kidney A 2307 N = 256 Reduced-dose Neoral plus Simulect: everolimus TDM 6, 12 months
 5 Certican® Global Registration Total country approvals in kidney and heart 48 1200 patients on commercial drug • 75% are heart transplant patients Sweden reference state—kidney and heart approvals Jul 2003 Recommended approvals by 15 of 17 EU member states Dec 2003 Approval by 10 new EU member states Dec 2004 Australia, South Africa, Switzerland, South America (9), Central America (6), Israel 2003 - 2005 Japan—new drug application submitted—heart Jun 2005 3 countries have not approved Certican— Canada, Ireland, United Kingdom
5 Certican® Global Registration Total country approvals in kidney and heart 48 1200 patients on commercial drug • 75% are heart transplant patients Sweden reference state—kidney and heart approvals Jul 2003 Recommended approvals by 15 of 17 EU member states Dec 2003 Approval by 10 new EU member states Dec 2004 Australia, South Africa, Switzerland, South America (9), Central America (6), Israel 2003 - 2005 Japan—new drug application submitted—heart Jun 2005 3 countries have not approved Certican— Canada, Ireland, United Kingdom
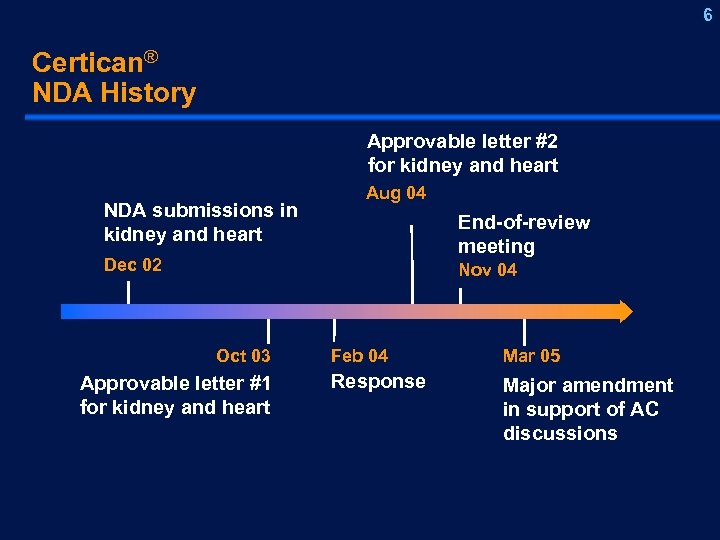 208 -1 6 Certican® NDA History Approvable letter #2 for kidney and heart NDA submissions in kidney and heart Aug 04 End-of-review meeting Dec 02 Nov 04 Oct 03 Approvable letter #1 for kidney and heart Feb 04 Mar 05 Response Major amendment in support of AC discussions
208 -1 6 Certican® NDA History Approvable letter #2 for kidney and heart NDA submissions in kidney and heart Aug 04 End-of-review meeting Dec 02 Nov 04 Oct 03 Approvable letter #1 for kidney and heart Feb 04 Mar 05 Response Major amendment in support of AC discussions
 7 Overall Objectives u Review the primary efficacy and safety data from the Certican® (everolimus) pivotal study in heart transplantation (B 253) u Provide dosing recommendations for everolimus in combination with Neoral® u Present data that support a favorable benefit-risk profile for everolimus in heart transplantation
7 Overall Objectives u Review the primary efficacy and safety data from the Certican® (everolimus) pivotal study in heart transplantation (B 253) u Provide dosing recommendations for everolimus in combination with Neoral® u Present data that support a favorable benefit-risk profile for everolimus in heart transplantation
 8 Certican® Proposed Indication u Certican® (everolimus) is indicated for the prophylaxis of organ rejection in adult patients receiving a heart transplant u It is recommended that Certican be used concurrently with Neoral® and corticosteroids
8 Certican® Proposed Indication u Certican® (everolimus) is indicated for the prophylaxis of organ rejection in adult patients receiving a heart transplant u It is recommended that Certican be used concurrently with Neoral® and corticosteroids
 9 Certican® Proposed Dosage and Administration u The pivotal heart study evaluated fixed doses of everolimus at 1. 5 mg per day and 3. 0 mg per day u Initial dose regimen of 1. 5 mg per day in 2 divided doses (BID) adjusted to target trough level of 3 to 8 ng/m. L u Certican should be used with reduced doses of cyclosporine after the first month
9 Certican® Proposed Dosage and Administration u The pivotal heart study evaluated fixed doses of everolimus at 1. 5 mg per day and 3. 0 mg per day u Initial dose regimen of 1. 5 mg per day in 2 divided doses (BID) adjusted to target trough level of 3 to 8 ng/m. L u Certican should be used with reduced doses of cyclosporine after the first month
 10 Today’s Agenda Introduction and Regulatory background Mathias Hukkelhoven, Ph. D Challenges and Opportunities Howard J. Eisen, MD In Cardiac Transplantation Efficacy Results (B 253) Jeffrey Hosenpud, MD IVUS Results (B 253) Jon A. Kobashigawa, MD Safety Kenneth A. Somberg, MD Renal Safety and Dose Recommendations Lawrence Hunsicker, MD Benefit/Risk Assessment Howard J. Eisen, MD
10 Today’s Agenda Introduction and Regulatory background Mathias Hukkelhoven, Ph. D Challenges and Opportunities Howard J. Eisen, MD In Cardiac Transplantation Efficacy Results (B 253) Jeffrey Hosenpud, MD IVUS Results (B 253) Jon A. Kobashigawa, MD Safety Kenneth A. Somberg, MD Renal Safety and Dose Recommendations Lawrence Hunsicker, MD Benefit/Risk Assessment Howard J. Eisen, MD
 11 Novartis Consultants Randall C. Starling, MD, MPh Head, Section of Heart Failure and Cardiac Transplant Medicine Department of Cardiovascular Medicine, Cleveland Clinic Foundation Cleveland, Ohio Lee-Jen Wei, Ph. D Professor of Biostatistics Department of Biostatistics Harvard School of Public Health Boston, MA Hans B. Lehmkuhl, MD Senior Consultant Head of Heart and Lung Failure and Thoracic Transplantation German Heart Center Berlin, Germany
11 Novartis Consultants Randall C. Starling, MD, MPh Head, Section of Heart Failure and Cardiac Transplant Medicine Department of Cardiovascular Medicine, Cleveland Clinic Foundation Cleveland, Ohio Lee-Jen Wei, Ph. D Professor of Biostatistics Department of Biostatistics Harvard School of Public Health Boston, MA Hans B. Lehmkuhl, MD Senior Consultant Head of Heart and Lung Failure and Thoracic Transplantation German Heart Center Berlin, Germany


