aa16d44402af77eb2da2e4bf3bc5cfa6.ppt
- Количество слайдов: 96

Chronic Viral Hepatitis HBV, HCV and HIV Edward Gane NZLTU
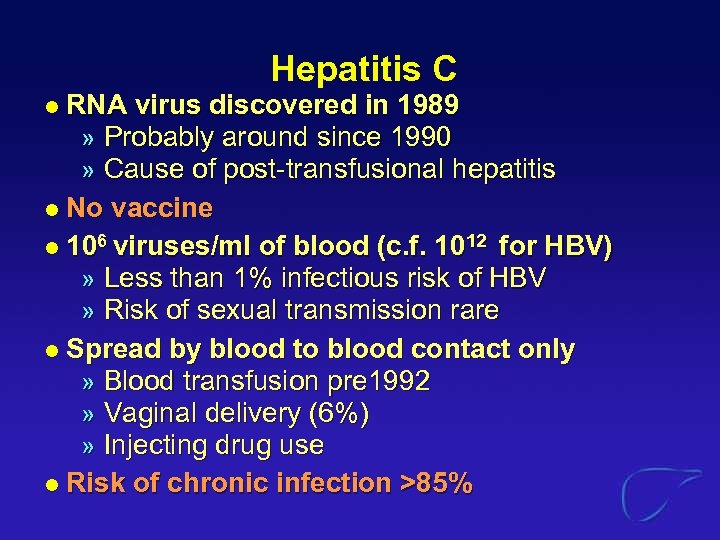
Hepatitis C RNA virus discovered in 1989 » Probably around since 1990 » Cause of post-transfusional hepatitis l No vaccine l 106 viruses/ml of blood (c. f. 1012 for HBV) » Less than 1% infectious risk of HBV » Risk of sexual transmission rare l Spread by blood to blood contact only » Blood transfusion pre 1992 » Vaginal delivery (6%) » Injecting drug use l Risk of chronic infection >85% l
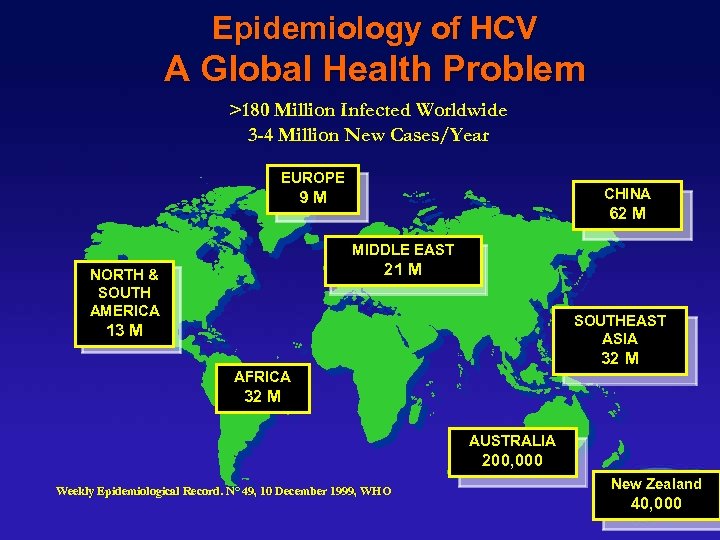
Epidemiology of HCV A Global Health Problem >180 Million Infected Worldwide 3 -4 Million New Cases/Year EUROPE CHINA 9 M 62 M MIDDLE EAST 21 M NORTH & SOUTH AMERICA SOUTHEAST ASIA 13 M 32 M AFRICA 32 M AUSTRALIA 200, 000 Weekly Epidemiological Record. N° 49, 10 December 1999, WHO New Zealand 40, 000
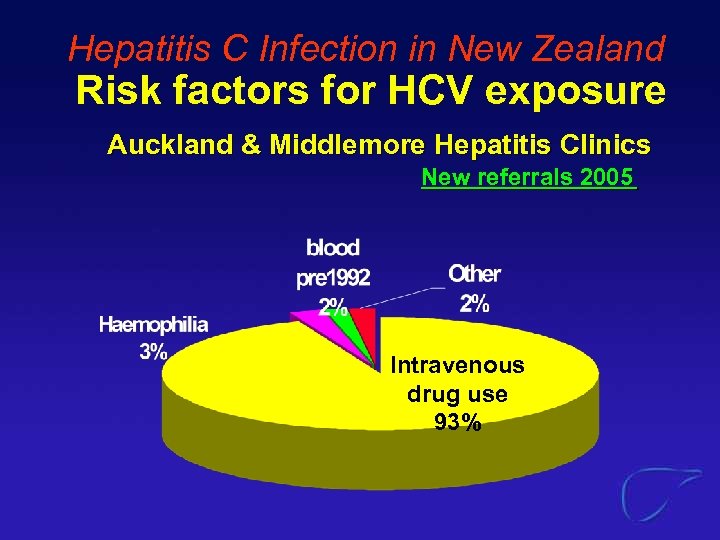
Hepatitis C Infection in New Zealand Risk factors for HCV exposure Auckland & Middlemore Hepatitis Clinics New referrals 2005 Intravenous drug use 93%
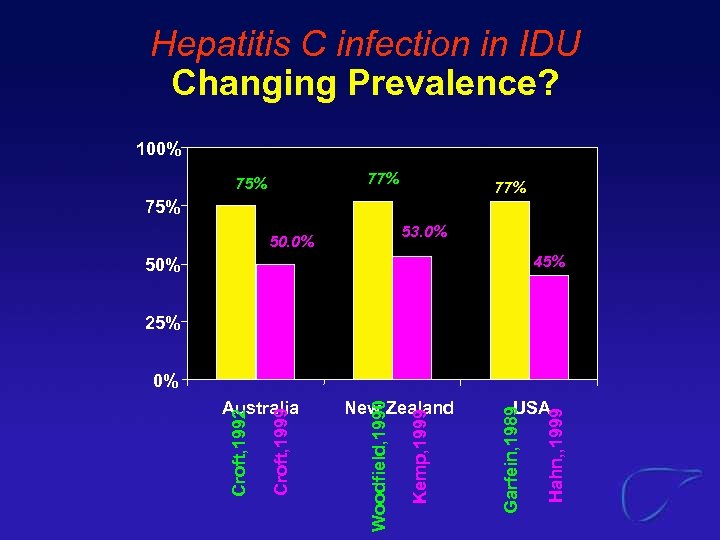
Hepatitis C infection in IDU Changing Prevalence? 100% 77% 75% 53. 0% 50. 0% 45% 50% 25% 0% Hahn, , 1999 USA Garfein, 1989 Kemp, 1999 New Zealand Woodfield, 1990 Croft, 1999 Croft, 1992 Australia
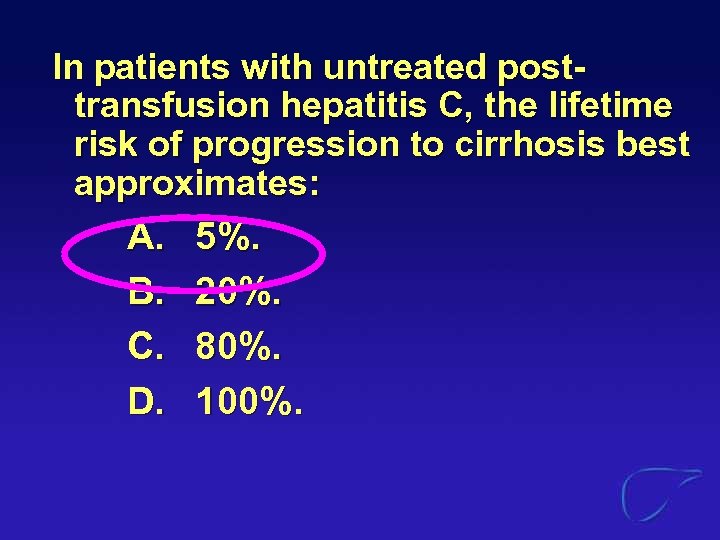
In patients with untreated posttransfusion hepatitis C, the lifetime risk of progression to cirrhosis best approximates: A. B. C. D. 5%. 20%. 80%. 100%.
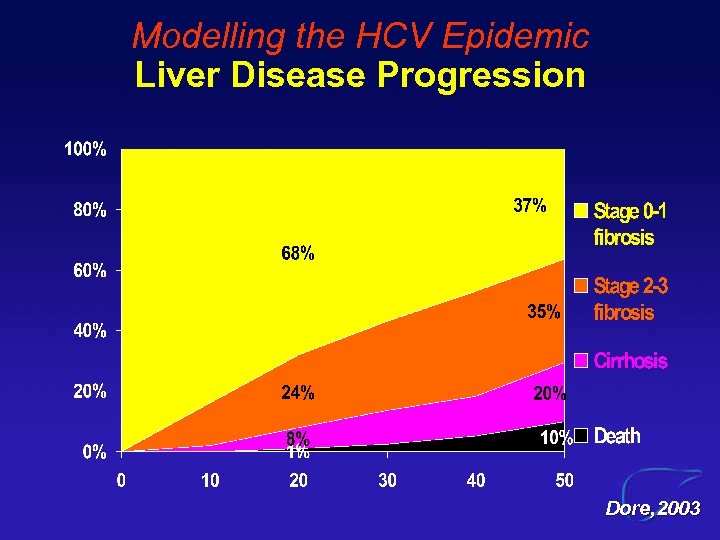
Modelling the HCV Epidemic Liver Disease Progression Dore, 2003
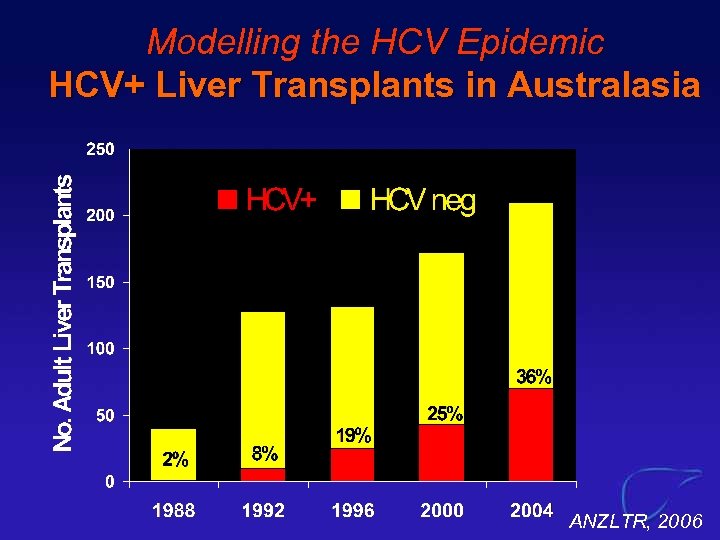
Modelling the HCV Epidemic HCV+ Liver Transplants in Australasia ANZLTR, 2006
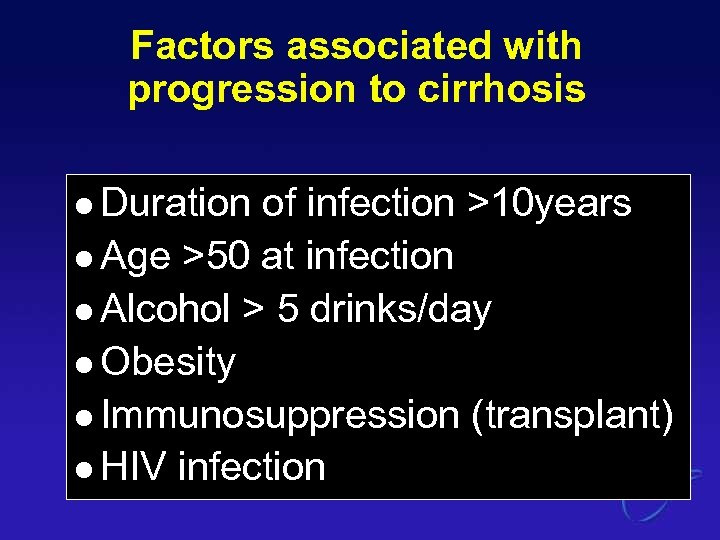
Factors associated with progression to cirrhosis l Duration of infection >10 years l Age >50 at infection l Alcohol > 5 drinks/day l Obesity l Immunosuppression (transplant) l HIV infection
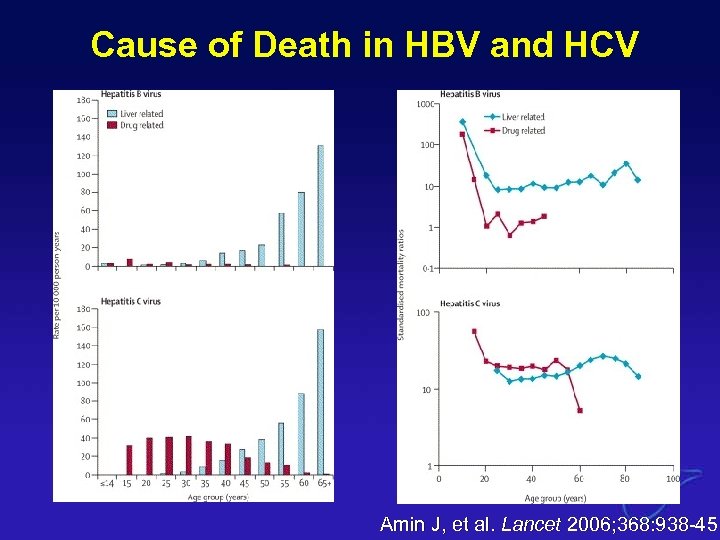
Cause of Death in HBV and HCV Amin J, et al. Lancet 2006; 368: 938 -45
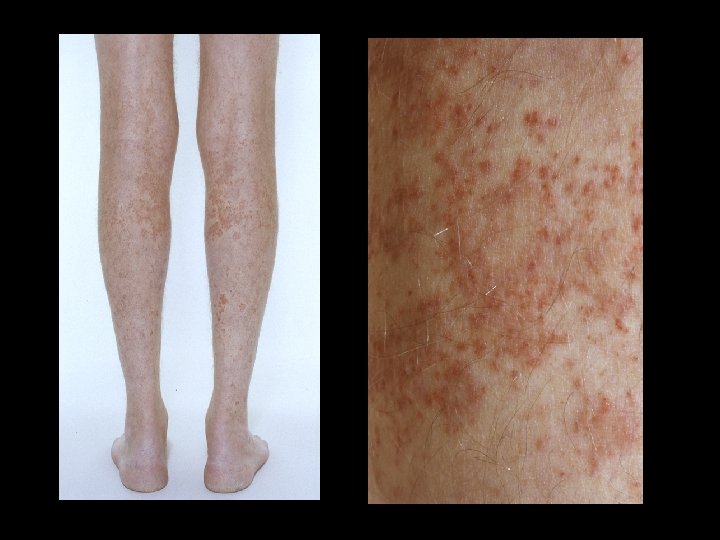

Further tests HBs. Ag neg; anti-HBs+; anti-HBcore+ l Anti-HCV+ l PCR for HCV RNA+ l HCV Genotype 3 a l Liver Biopsy: cirrhosis l What is the rash?
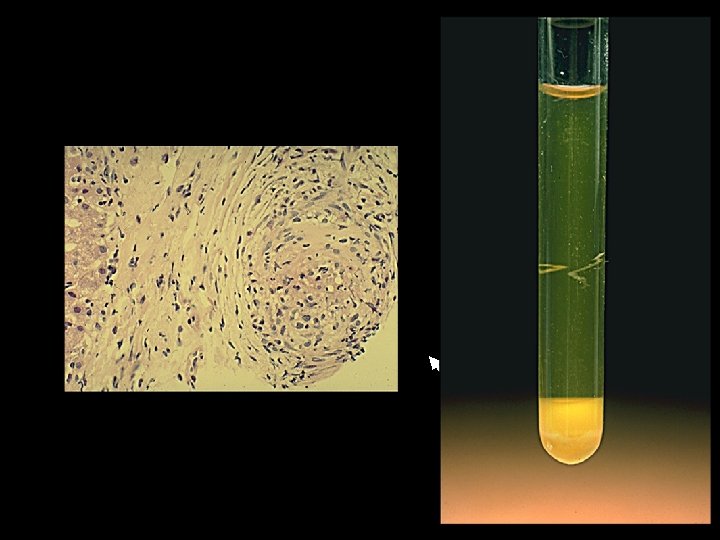
Extrahepatic Manifestations of HCV Cryoglobulins = Ig. M(RF) + Ig. G + Antigen l Ig. M Rheumatoid factor » Polyclonal (Type III) monoclonal (Type II) » Monoclonal RF is highly restricted l Ig. G » Ig. G is heterologous but specific anti-HCV ; structural proteins (anti-core, anti-E 1, E 2) l Antigen » complexed to anti-HCV ; contains E 1, E 2, core, RNA=whole virion ; chronic HCV viremia drives the disease?
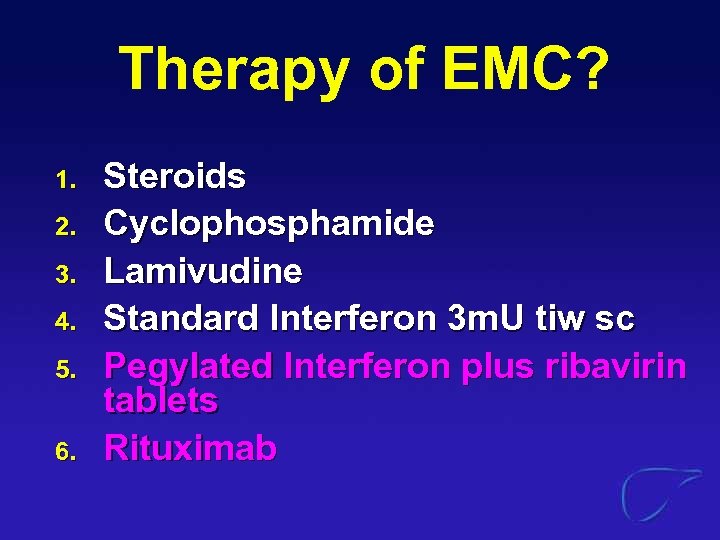
Therapy of EMC? 1. 2. 3. 4. 5. 6. Steroids Cyclophosphamide Lamivudine Standard Interferon 3 m. U tiw sc Pegylated Interferon plus ribavirin tablets Rituximab
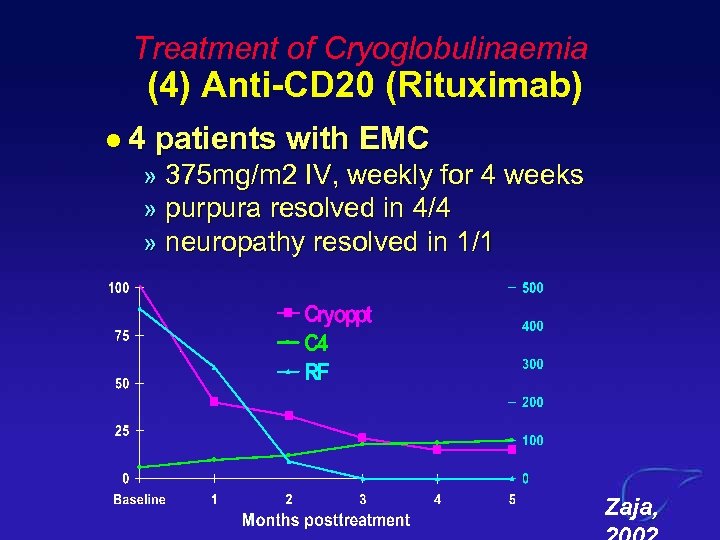
Treatment of Cryoglobulinaemia (4) Anti-CD 20 (Rituximab) l 4 patients with EMC » 375 mg/m 2 IV, weekly for 4 weeks » purpura resolved in 4/4 » neuropathy resolved in 1/1 Zaja,
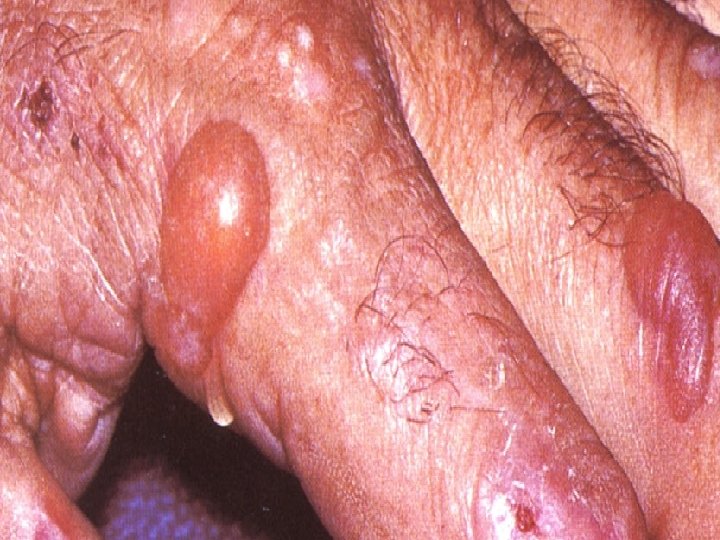
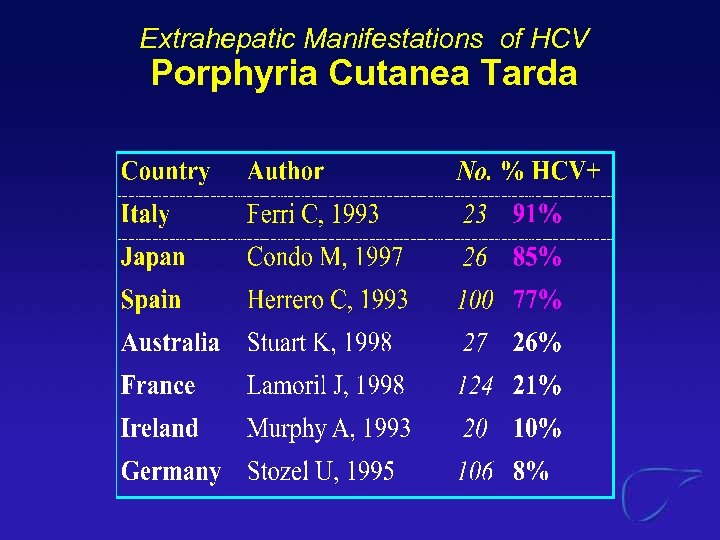
Extrahepatic Manifestations of HCV Porphyria Cutanea Tarda
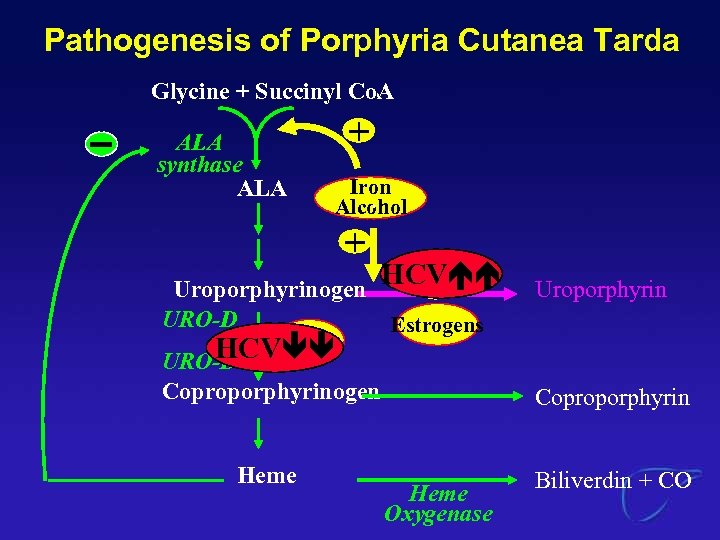
Pathogenesis of Porphyria Cutanea Tarda Glycine + Succinyl Co. A ALA synthase ALA + Iron Alcohol + Uroporphyrinogen URO-D Iron HCV URO-D HCV + Estrogens Coproporphyrinogen Heme Uroporphyrin Coproporphyrin Heme Oxygenase Biliverdin + CO
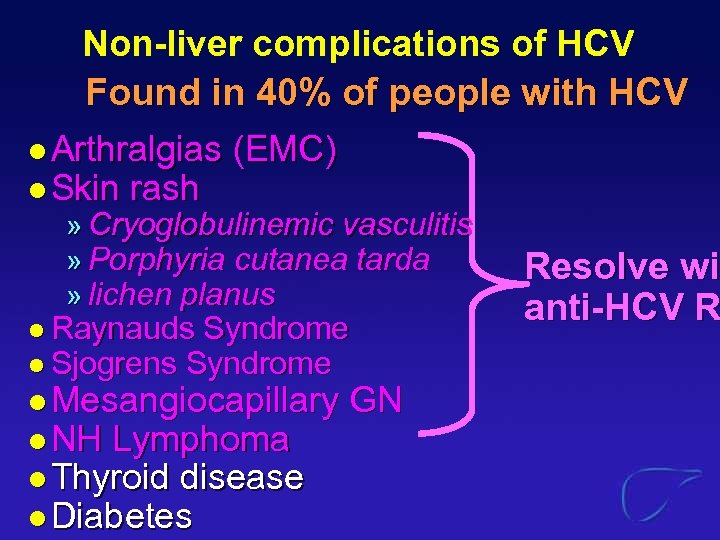
Non-liver complications of HCV Found in 40% of people with HCV l Arthralgias (EMC) l Skin rash » Cryoglobulinemic vasculitis » Porphyria cutanea tarda » lichen planus l Raynauds Syndrome l Sjogrens Syndrome l Mesangiocapillary l NH Lymphoma l Thyroid disease l Diabetes GN Resolve wit wi anti-HCV R
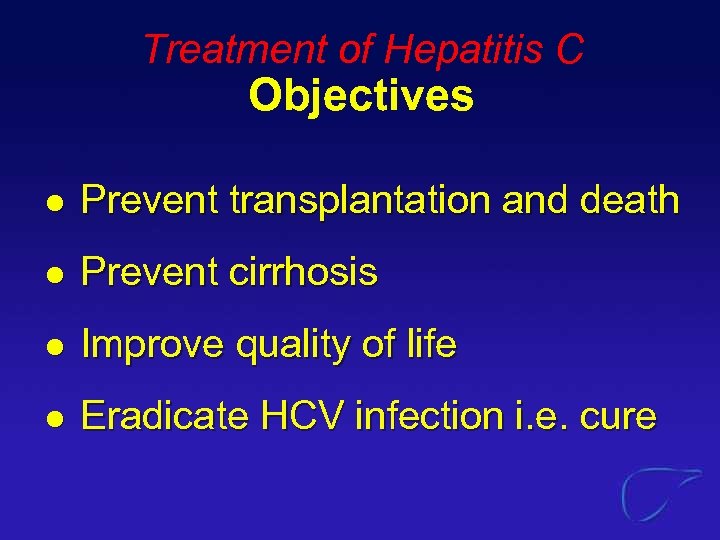
Treatment of Hepatitis C Objectives l Prevent transplantation and death l Prevent cirrhosis l Improve quality of life l Eradicate HCV infection i. e. cure
![IFN- 2 a Pegylated IFN- 2 a [IFN]plasma. (ng/m. L) Mon Tue Wed Thu IFN- 2 a Pegylated IFN- 2 a [IFN]plasma. (ng/m. L) Mon Tue Wed Thu](https://present5.com/presentation/aa16d44402af77eb2da2e4bf3bc5cfa6/image-22.jpg)
IFN- 2 a Pegylated IFN- 2 a [IFN]plasma. (ng/m. L) Mon Tue Wed Thu Fri Sat Sun 30 25 Pegylated IFN- 20 15 10 5 0 IFN- 2 a 0 24 48 72 96 120 Time (hours) 144 168 192
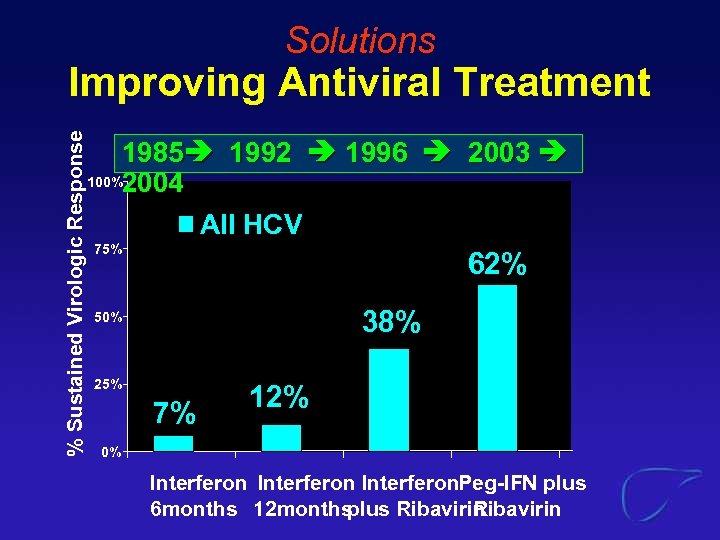
Solutions % Sustained Virologic Response Improving Antiviral Treatment 1985 1992 1996 2003 100%2004 All HCV 75% 62% 38% 50% 25% 7% 12% 0% Interferon. Peg-IFN plus 6 months 12 months plus Ribavirin
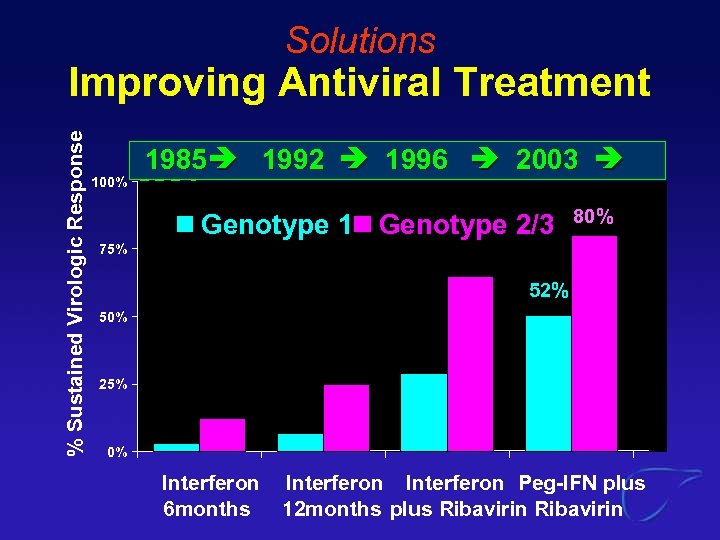
Solutions % Sustained Virologic Response Improving Antiviral Treatment 100% 1985 1992 1996 2003 2004+ Genotype 1 Genotype 2/3 80% 75% 52% 50% 25% 0% Interferon 6 months Interferon Peg-IFN plus 12 months plus Ribavirin
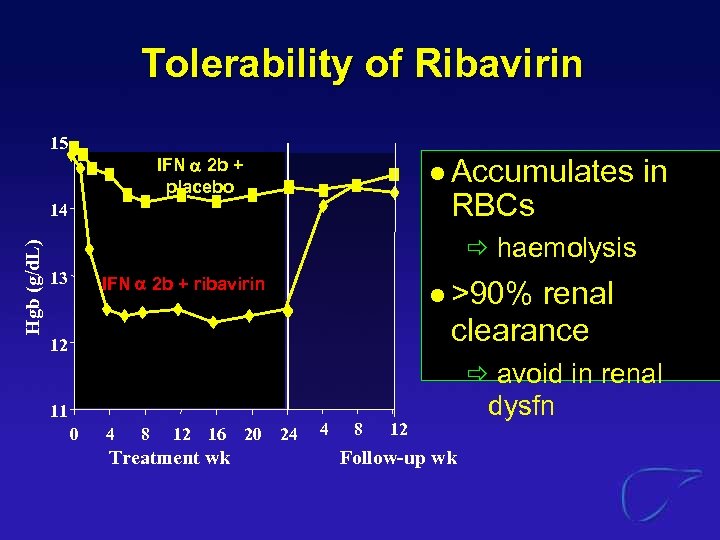
Tolerability of Ribavirin 15 l Accumulates IFN 2 b + placebo RBCs Hgb (g/d. L) 14 in haemolysis 13 IFN 2 b + ribavirin l >90% renal clearance 12 avoid in renal 11 0 4 8 12 16 20 24 Treatment wk 4 8 12 Follow-up wk dysfn
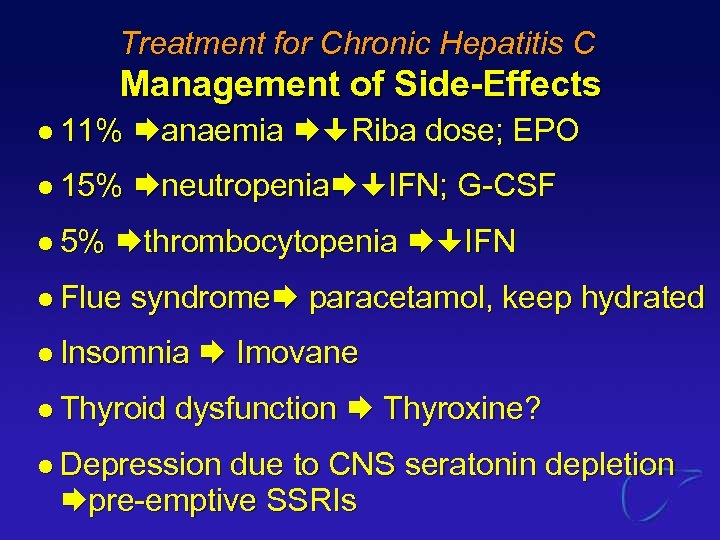
Treatment for Chronic Hepatitis C Management of Side-Effects l 11% anaemia Riba dose; EPO l 15% neutropenia IFN; G-CSF l 5% thrombocytopenia IFN l Flue syndrome paracetamol, keep hydrated l Insomnia l Thyroid Imovane dysfunction Thyroxine? l Depression due to CNS seratonin depletion pre-emptive SSRIs
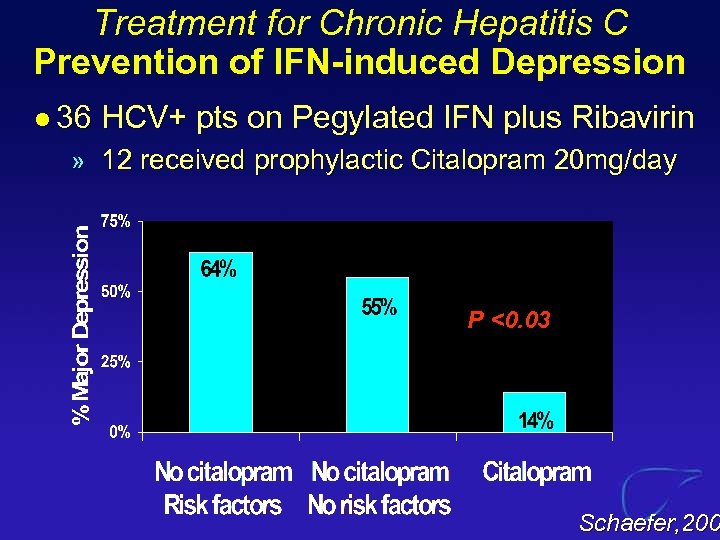
Treatment for Chronic Hepatitis C Prevention of IFN-induced Depression l 36 HCV+ pts on Pegylated IFN plus Ribavirin » 12 received prophylactic Citalopram 20 mg/day P <0. 03 Schaefer, 200

Contraindication to treatment? 1. 2. 3. 4. 5. 6. Thyrotoxicosis Ascites Uncontrolled hypomania Guttate psoriasis Frequent Angina All of above
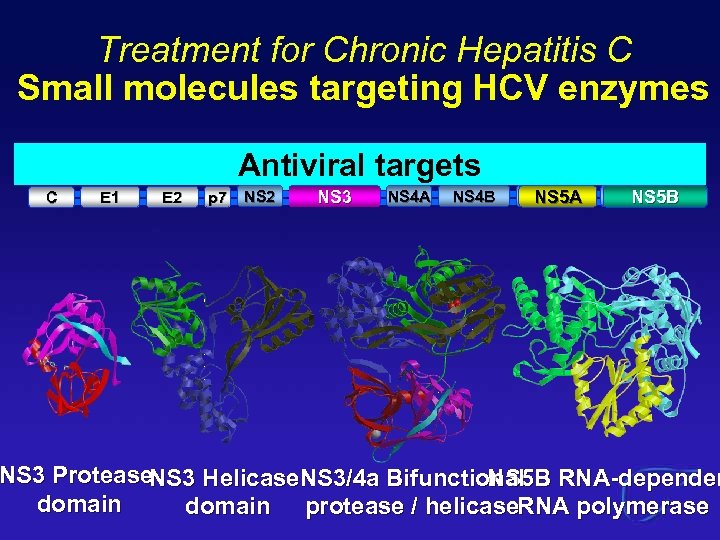
Treatment for Chronic Hepatitis C Small molecules targeting HCV enzymes Therapeutic Immunomodulators Antiviral targets vaccine IFN C E 1 E 2 p 7 NS 2 NS 3 NS 4 A NS 4 B NS 5 A Host target NS 5 B NS 3 Protease. NS 3 Helicase. NS 3/4 a Bifunctional RNA-dependen NS 5 B domain protease / helicase. RNA polymerase
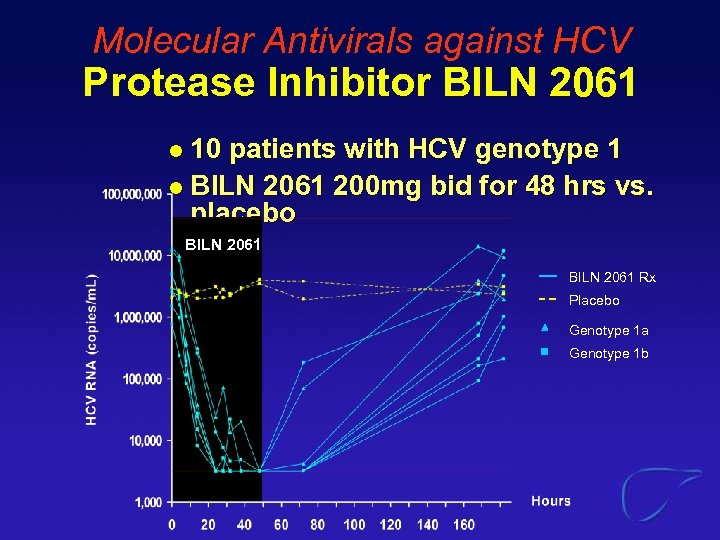
Molecular Antivirals against HCV Protease Inhibitor BILN 2061 10 patients with HCV genotype 1 l BILN 2061 200 mg bid for 48 hrs vs. placebo l BILN 2061 Rx Placebo Genotype 1 a Genotype 1 b
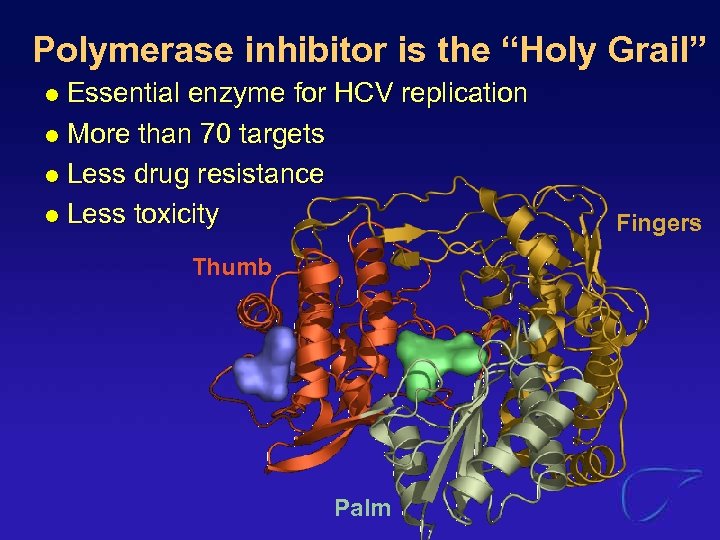
Polymerase inhibitor is the “Holy Grail” Essential enzyme for HCV replication l More than 70 targets l Less drug resistance l Less toxicity l Thumb Palm Fingers
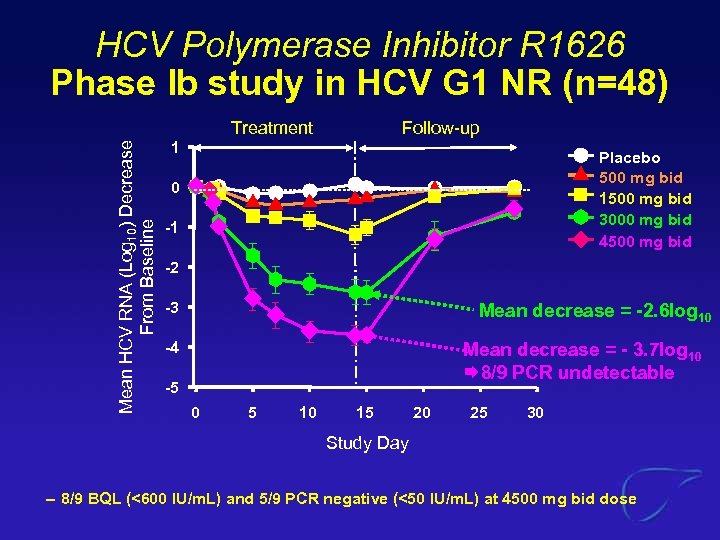
HCV Polymerase Inhibitor R 1626 Phase Ib study in HCV G 1 NR (n=48) Mean HCV RNA (Log 10) Decrease From Baseline Treatment Follow-up 1 Placebo 500 mg bid 1500 mg bid 3000 mg bid 4500 mg bid 0 -1 -2 -3 Mean decrease = -2. 6 log 10 -4 Mean decrease = - 3. 7 log 10 8/9 PCR undetectable -5 0 5 10 15 20 25 30 Study Day – 8/9 BQL (<600 IU/m. L) and 5/9 PCR negative (<50 IU/m. L) at 4500 mg bid dose
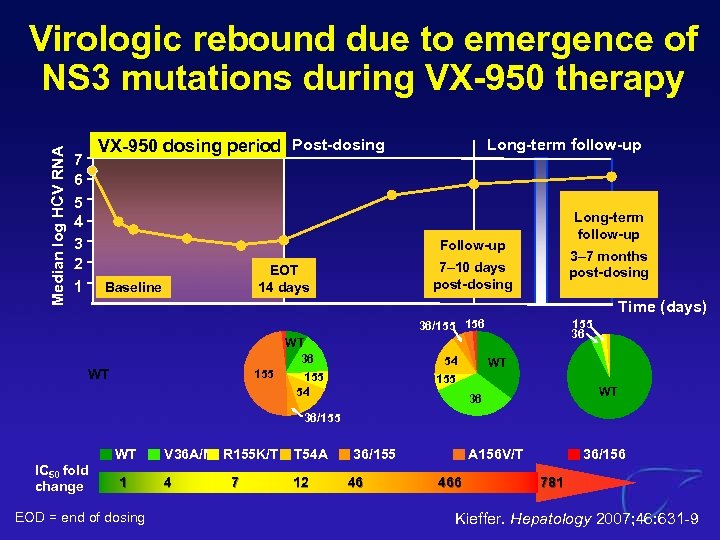
Median log HCV RNA Virologic rebound due to emergence of NS 3 mutations during VX-950 therapy VX-950 dosing period Post-dosing 7 6 5 4 3 2 1 Long-term follow-up Follow-up Baseline 3– 7 months post-dosing 7– 10 days post-dosing EOT 14 days Time (days) 36/155 156 WT 36 155 54 WT 54 155 36 WT 155 WT 36 36/155 IC 50 fold change WT 1 EOD = end of dosing V 36 A/M R 155 K/T T 54 A 4 12 7 36/155 46 A 156 V/T 466 36/156 781 Kieffer. Hepatology 2007; 46: 631 -9

How to overcome drug resistance The Lessons from HIV l Resistance expected for anti-HCV agents » mutation rate higher than HIV » replication rate higher than HIV (1012 per day) ; each new virus contains at least 1 mutation Resistance will prevent cure l Cross-resistance to related compounds Combination therapy is essential l
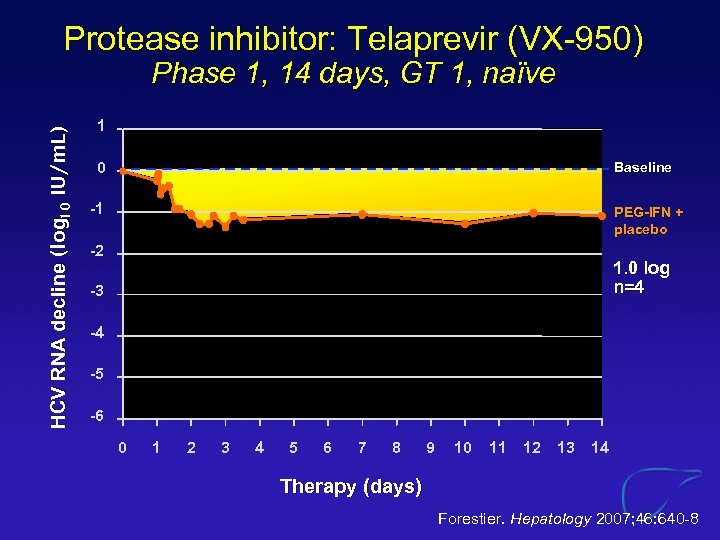
Protease inhibitor: Telaprevir (VX-950) HCV RNA decline (log 10 IU/m. L) Phase 1, 14 days, GT 1, naïve 1 0 Baseline -1 PEG-IFN + placebo -2 1. 0 log n=4 -3 -4 -5 -6 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Therapy (days) Forestier. Hepatology 2007; 46: 640 -8
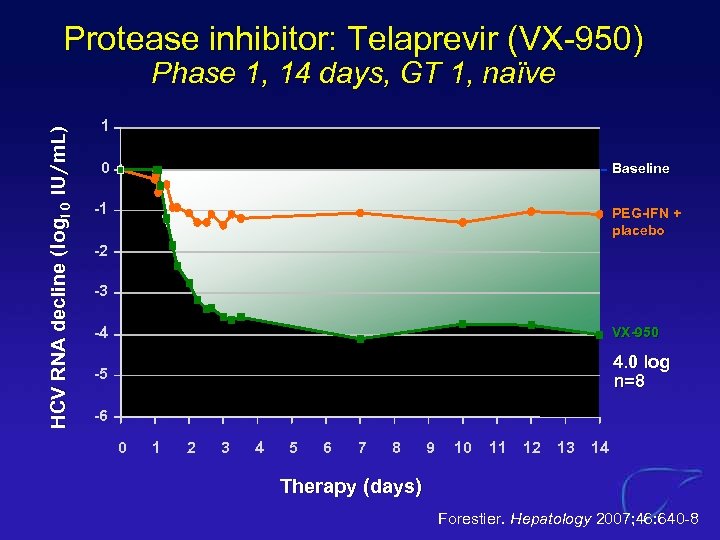
Protease inhibitor: Telaprevir (VX-950) HCV RNA decline (log 10 IU/m. L) Phase 1, 14 days, GT 1, naïve 1 0 Baseline -1 PEG-IFN + placebo -2 -3 -4 VX-950 -5 4. 0 log n=8 -6 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Therapy (days) Forestier. Hepatology 2007; 46: 640 -8
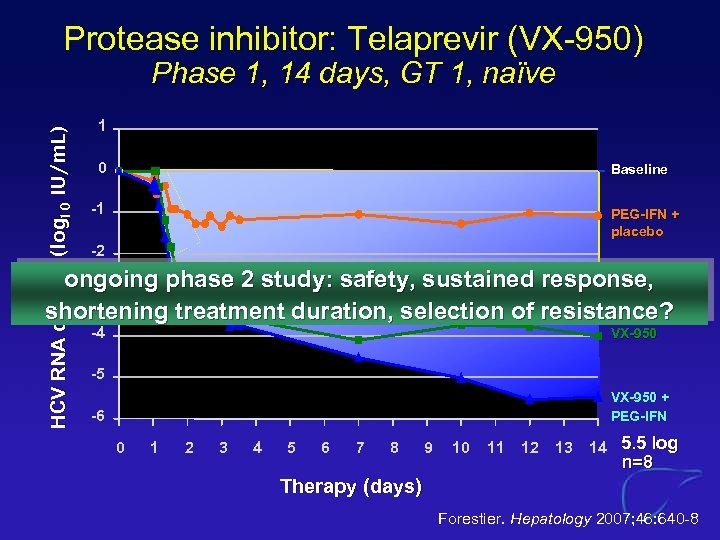
Protease inhibitor: Telaprevir (VX-950) HCV RNA decline (log 10 IU/m. L) Phase 1, 14 days, GT 1, naïve 1 0 Baseline -1 PEG-IFN + placebo -2 ongoing phase 2 study: safety, sustained response, -3 shortening treatment duration, selection of resistance? -4 VX-950 -5 VX-950 + PEG-IFN -6 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 5. 5 log n=8 Therapy (days) Forestier. Hepatology 2007; 46: 640 -8
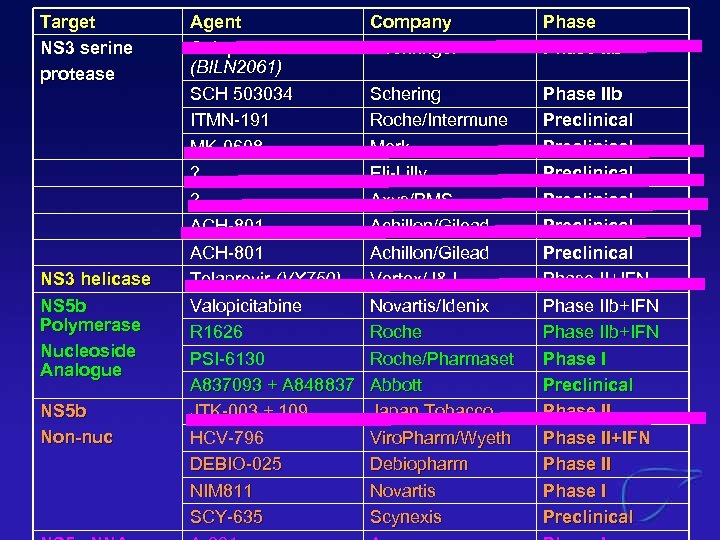
Target NS 3 serine protease NS 3 helicase NS 5 b Polymerase Nucleoside Analogue NS 5 b Non-nuc Agent Celuprivir (BILN 2061) SCH 503034 ITMN-191 MK-0608 ? ? ACH-801 Telaprevir (VX 750) Valopicitabine R 1626 PSI-6130 A 837093 + A 848837 JTK-003 + 109 HCV-796 DEBIO-025 NIM 811 SCY-635 Company Boehringer Phase IIb Schering Roche/Intermune Merk Eli-Lilly Axys/BMS Achillon/Gilead Vertex/J&J Novartis/Idenix Roche/Pharmaset Abbott Japan Tobacco Viro. Pharm/Wyeth Debiopharm Novartis Scynexis Phase IIb Preclinical Preclinical Phase II+IFN Phase IIb+IFN Phase I Preclinical Phase II+IFN Phase II Phase I Preclinical

Risk of Sexual Transmission? 1. 2. 3. 4. 5. <1 % 5% 10% 20 % 50%
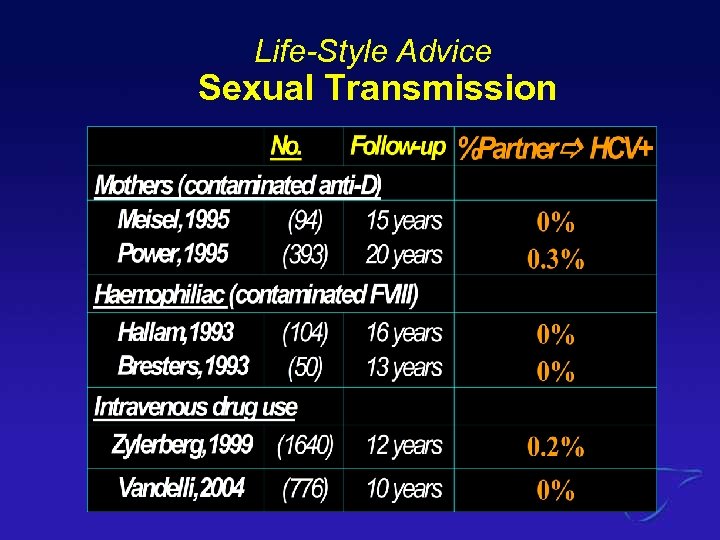
Life-Style Advice Sexual Transmission
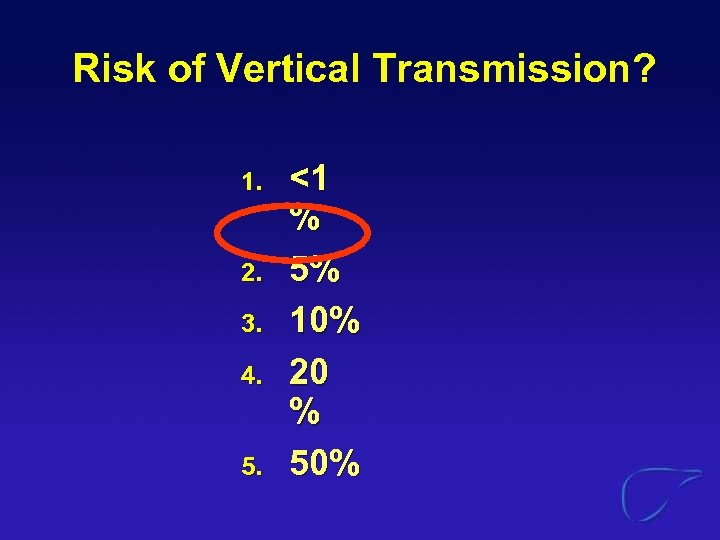
Risk of Vertical Transmission? 1. 2. 3. 4. 5. <1 % 5% 10% 20 % 50%
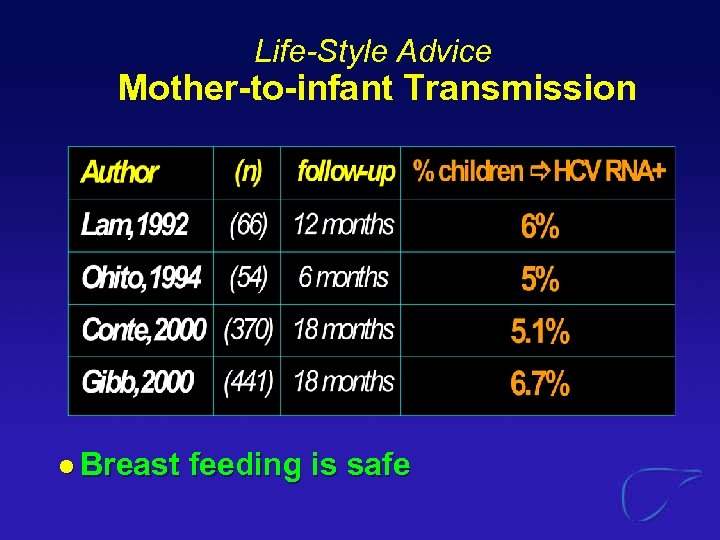
Life-Style Advice Mother-to-infant Transmission l Breast feeding is safe
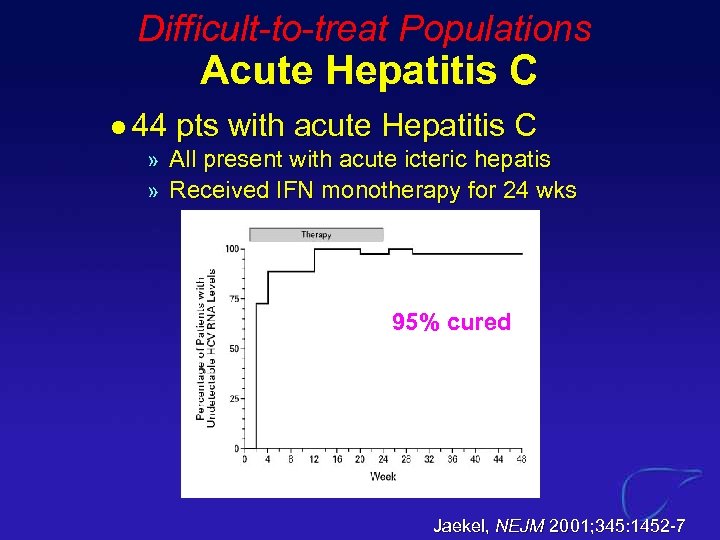
Difficult-to-treat Populations Acute Hepatitis C l 44 pts with acute Hepatitis C » All present with acute icteric hepatis » Received IFN monotherapy for 24 wks 95% cured Jaekel, NEJM 2001; 345: 1452 -7
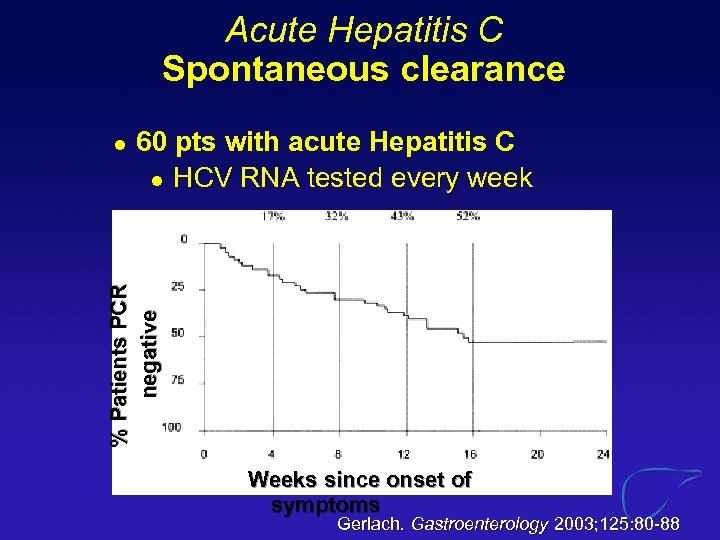
Acute Hepatitis C Spontaneous clearance 60 pts with acute Hepatitis C l HCV RNA tested every week % Patients PCR negative l Weeks since onset of symptoms Gerlach. Gastroenterology 2003; 125: 80 -88
Management of HIV/HCV Co-infection + =?
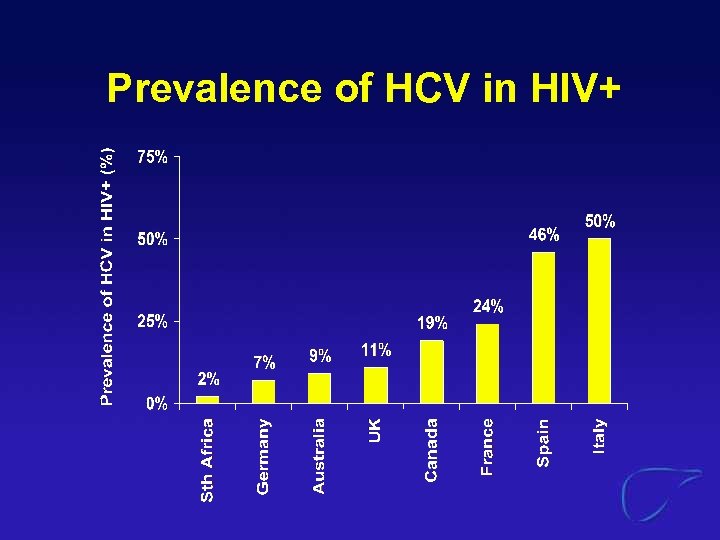
Prevalence of HCV in HIV+
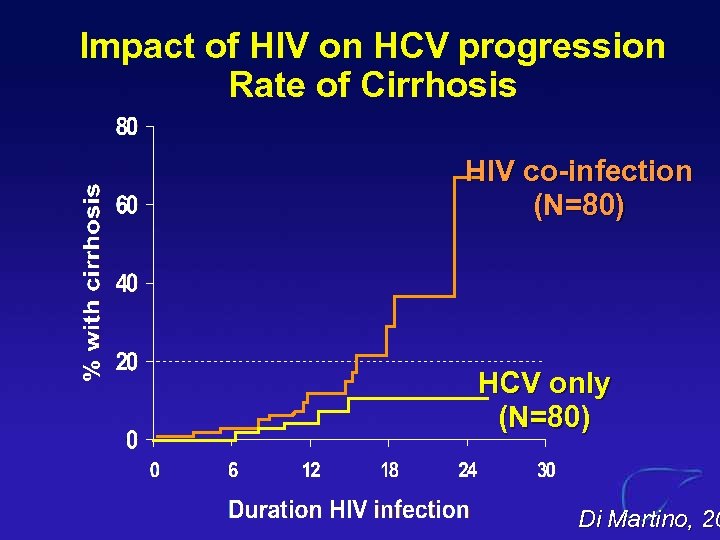
Impact of HIV on HCV progression Rate of Cirrhosis HIV co-infection (N=80) HCV only (N=80) Di Martino, 20
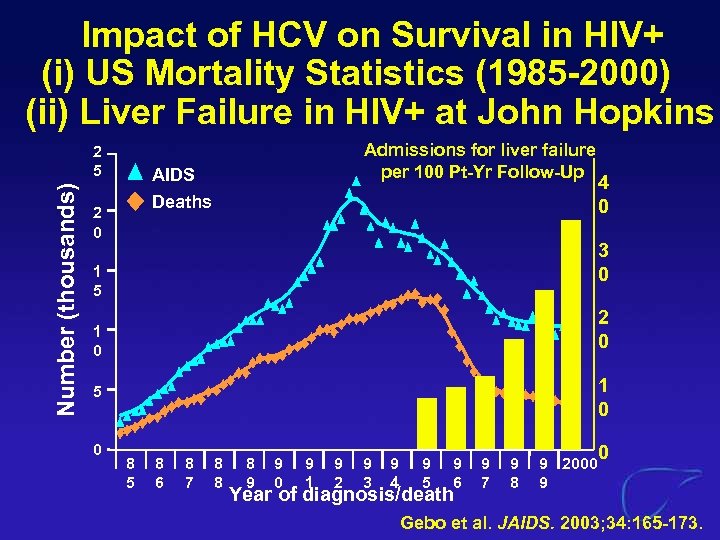
Impact of HCV on Survival in HIV+ (i) US Mortality Statistics (1985 -2000) (ii) Liver Failure in HIV+ at John Hopkins Number (thousands) 2 5 Admissions for liver failure per 100 Pt-Yr Follow-Up AIDS Deaths 2 0 3 0 1 5 2 0 1 0 5 0 4 0 8 5 8 6 8 7 8 8 8 9 9 0 9 1 9 2 9 3 9 4 9 5 9 6 Year of diagnosis/death 9 7 9 8 0 9 2000 9 Gebo et al. JAIDS. 2003; 34: 165 -173.
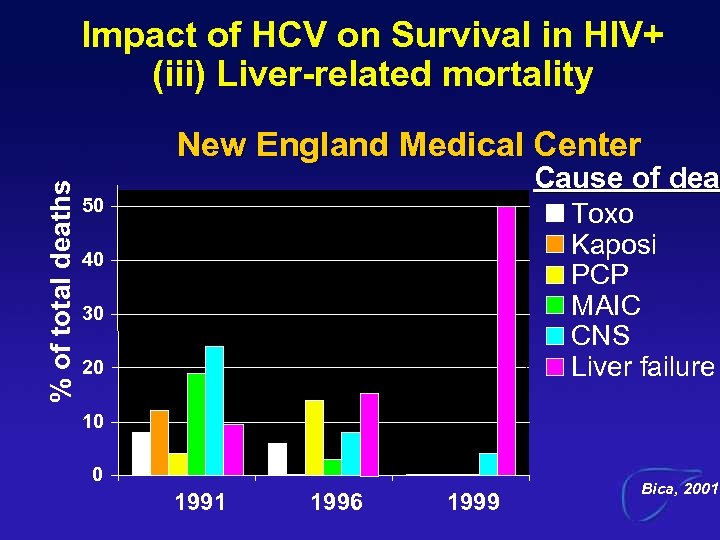
Impact of HCV on Survival in HIV+ (iii) Liver-related mortality % of total deaths New England Medical Center Cause of dea 50 Toxo Kaposi PCP MAIC CNS Liver failure 40 30 20 10 0 1991 1996 1999 Bica, 2001
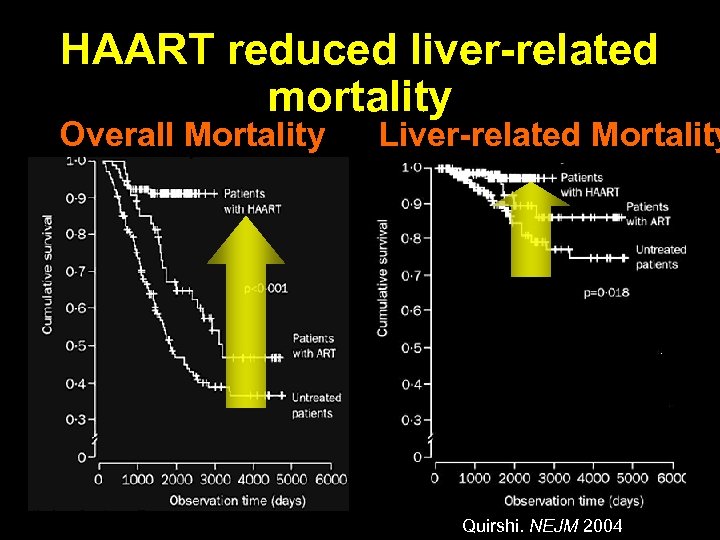
HAART reduced liver-related mortality Overall Mortality Liver-related Mortality Quirshi. NEJM 2004
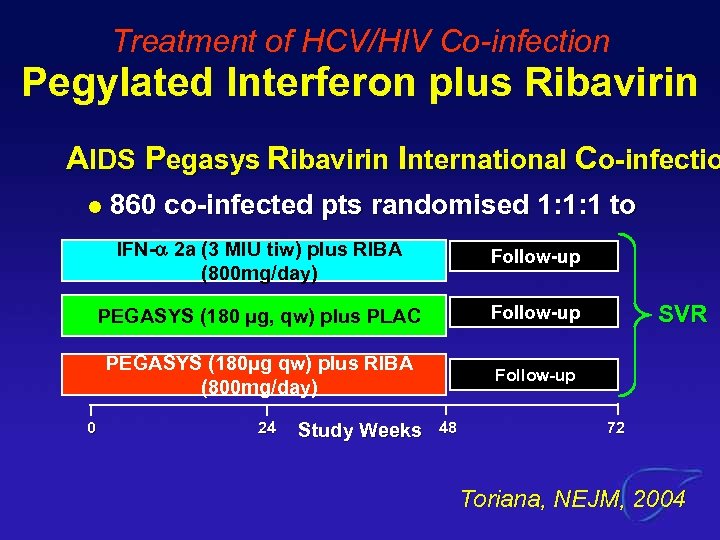
Treatment of HCV/HIV Co-infection Pegylated Interferon plus Ribavirin AIDS Pegasys Ribavirin International Co-infectio l 860 co-infected pts randomised 1: 1: 1 to IFN- 2 a (3 MIU tiw) plus RIBA (800 mg/day) PEGASYS (180 µg, qw) plus PLAC Follow-up PEGASYS (180µg qw) plus RIBA (800 mg/day) 0 Follow-up 24 Study Weeks 48 SVR 72 Toriana, NEJM, 2004
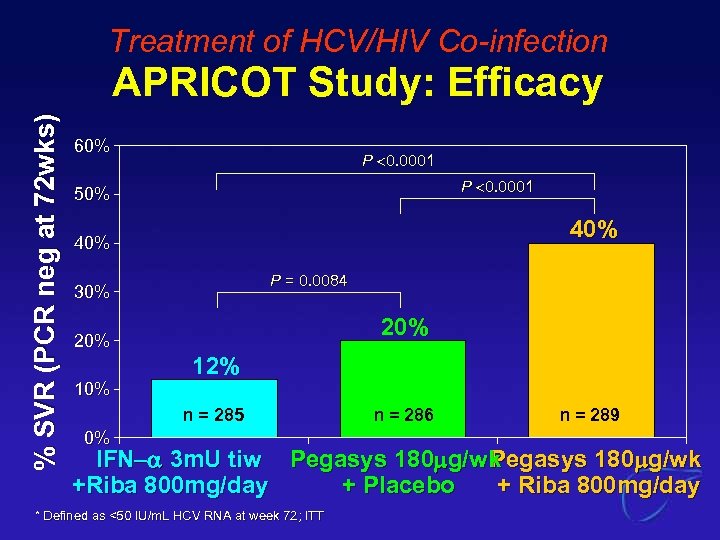
Treatment of HCV/HIV Co-infection % SVR (PCR neg at 72 wks) APRICOT Study: Efficacy 60% P 0. 0001 50% 40% P = 0. 0084 30% 20% 12% n = 285 0% n = 286 n = 289 IFN- 3 m. U tiw Pegasys 180 mg/wk +Riba 800 mg/day + Placebo + Riba 800 mg/day * Defined as <50 IU/m. L HCV RNA at week 72; ITT
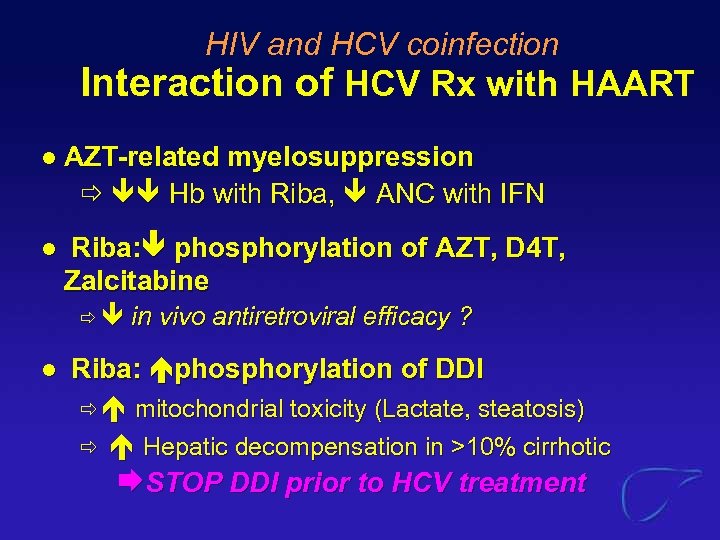
HIV and HCV coinfection Interaction of HCV Rx with HAART l AZT-related myelosuppression Hb with Riba, ANC with IFN l Riba: phosphorylation of AZT, D 4 T, Zalcitabine l in vivo antiretroviral efficacy ? Riba: phosphorylation of DDI mitochondrial toxicity (Lactate, steatosis) Hepatic decompensation in >10% cirrhotic STOP DDI prior to HCV treatment
Chronic Hepatitis B
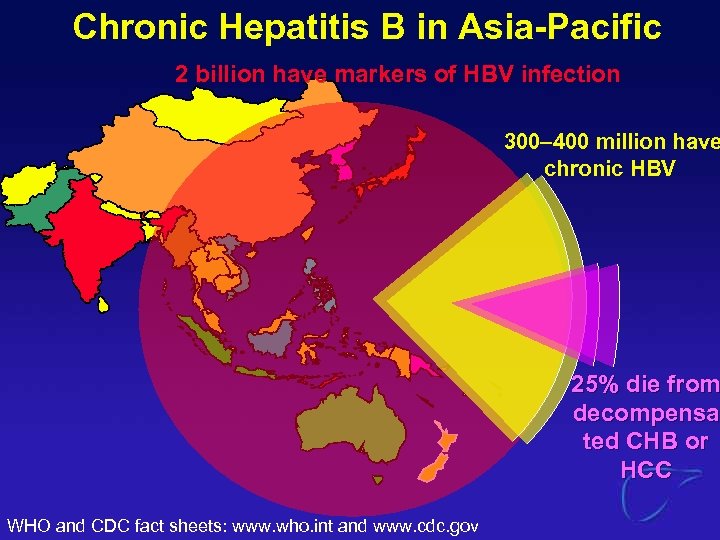
Chronic Hepatitis B in Asia-Pacific 2 billion have markers of HBV infection 300– 400 million have chronic HBV 25% die from decompensa ted CHB or HCC WHO and CDC fact sheets: www. who. int and www. cdc. gov
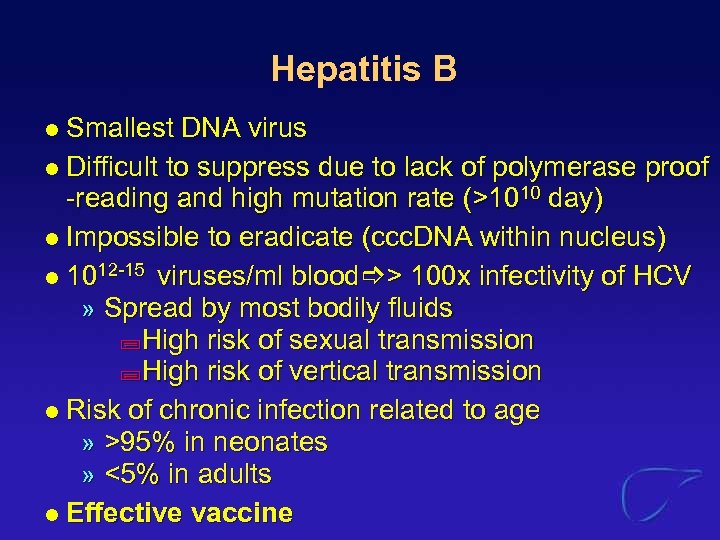
Hepatitis B Smallest DNA virus l Difficult to suppress due to lack of polymerase proof -reading and high mutation rate (>1010 day) l Impossible to eradicate (ccc. DNA within nucleus) l 1012 -15 viruses/ml blood > 100 x infectivity of HCV » Spread by most bodily fluids ; High risk of sexual transmission ; High risk of vertical transmission l Risk of chronic infection related to age » >95% in neonates » <5% in adults l Effective vaccine l
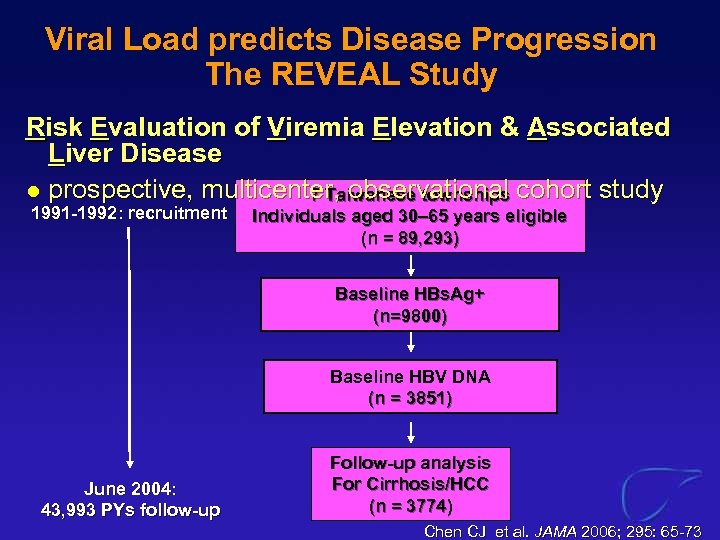
Viral Load predicts Disease Progression The REVEAL Study Risk Evaluation of Viremia Elevation & Associated Liver Disease l prospective, multicenter, observational cohort study 7 Taiwanese townships 1991 -1992: recruitment Individuals aged 30– 65 years eligible (n = 89, 293) Baseline HBs. Ag+ (n=9800) Baseline HBV DNA (n = 3851) June 2004: 43, 993 PYs follow-up Follow-up analysis For Cirrhosis/HCC (n = 3774) Chen CJ et al. JAMA 2006; 295: 65 -73
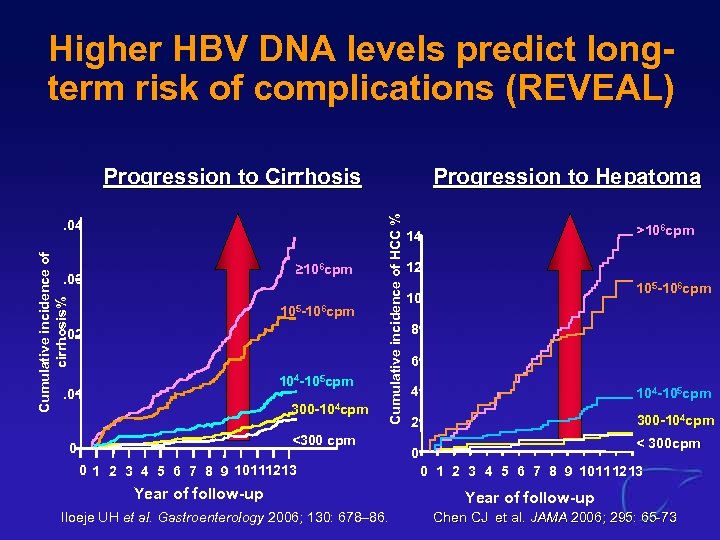
Higher HBV DNA levels predict longterm risk of complications (REVEAL) Cumulative incidence of cirrhosis% . 04 ≥ 106 cpm . 03 105 -106 cpm . 02 104 -105 cpm . 01 300 -104 cpm Progression to Hepatoma Cumulative incidence of HCC % Progression to Cirrhosis <300 cpm 0 0 1 2 3 4 5 6 7 8 9 10111213 Year of follow-up Iloeje UH et al. Gastroenterology 2006; 130: 678– 86. >106 cpm 14 12 105 -106 cpm 10 8 6 4 104 -105 cpm 2 300 -104 cpm < 300 cpm 0 0 1 2 3 4 5 6 7 8 9 10111213 Year of follow-up Chen CJ et al. JAMA 2006; 295: 65 -73
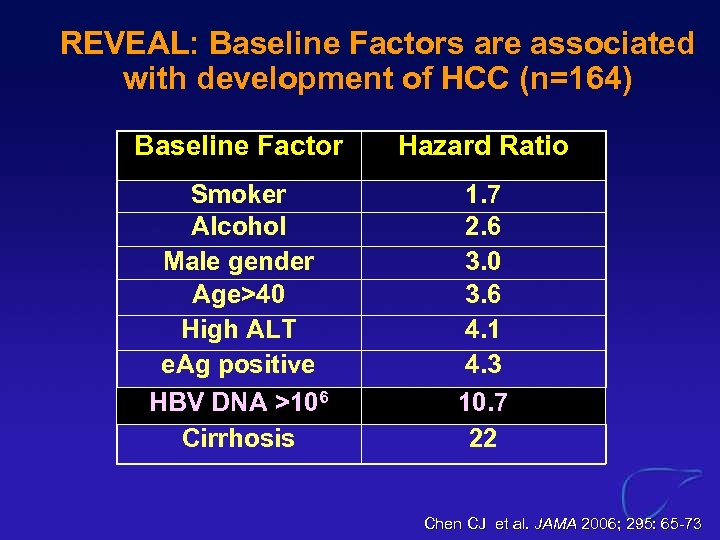
REVEAL: Baseline Factors are associated with development of HCC (n=164) Baseline Factor Hazard Ratio Smoker Alcohol Male gender Age>40 High ALT e. Ag positive HBV DNA >106 Cirrhosis 1. 7 2. 6 3. 0 3. 6 4. 1 4. 3 10. 7 22 Chen CJ et al. JAMA 2006; 295: 65 -73
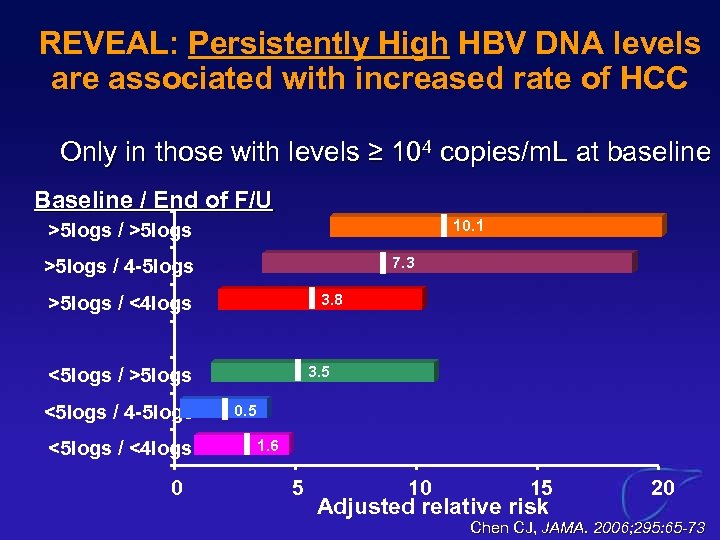
REVEAL: Persistently High HBV DNA levels are associated with increased rate of HCC Only in those with levels ≥ 104 copies/m. L at baseline ( Baseline / End of F/U 10. 1 >5 logs / >5 logs 7. 3 >5 logs / 4 -5 logs 3. 8 >5 logs / <4 logs 3. 5 <5 logs / >5 logs <5 logs / 4 -5 logs <5 logs / <4 logs 0 0. 5 1. 6 5 10 15 Adjusted relative risk 20 Chen CJ, JAMA. 2006; 295: 65 -73

Goals of treatment in CHB: APASL Consensus Statement 2005 “The ultimate long-term goal of therapy is to prevent progression to cirrhosis, decompensation and HCC, and to prolong survival. Sustained viral suppression is the key to the reduction or prevention of hepatic injury and disease progression. Therefore, the primary goal of treatment for chronic hepatitis B is to permanently suppress HBV…. . ” Liaw YF et al. Liver Int 2005
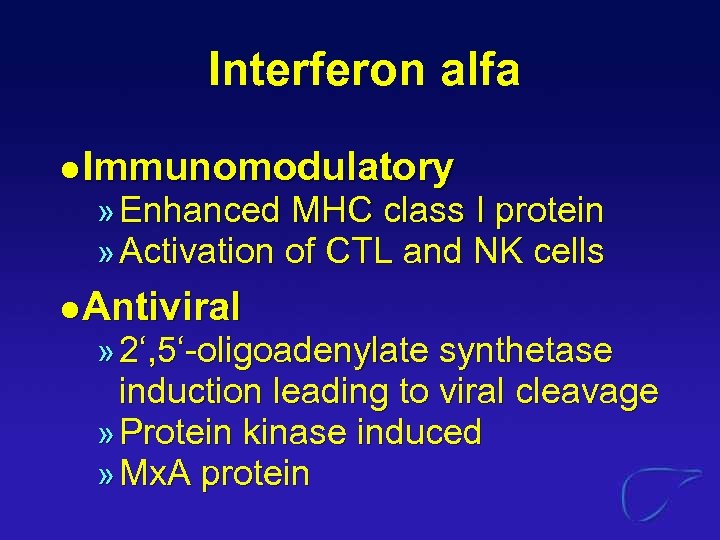
Interferon alfa l Immunomodulatory » Enhanced MHC class I protein » Activation of CTL and NK cells l Antiviral » 2‘, 5‘-oligoadenylate synthetase induction leading to viral cleavage » Protein kinase induced » Mx. A protein
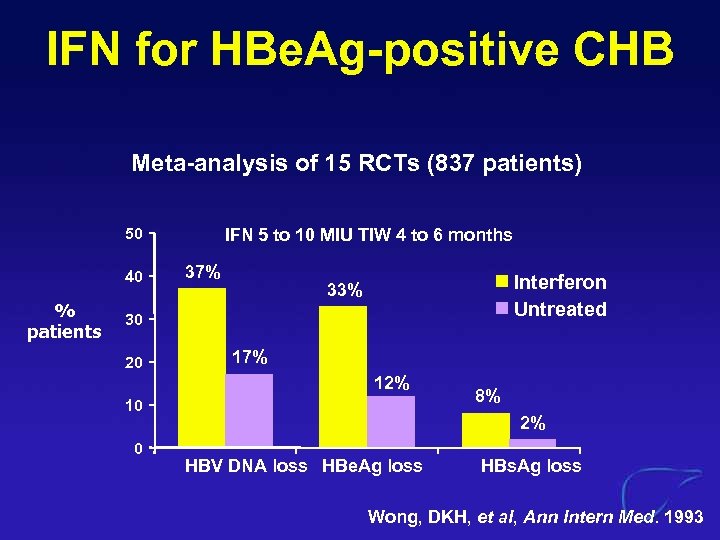
IFN for HBe. Ag-positive CHB Meta-analysis of 15 RCTs (837 patients) 50 40 % patients IFN 5 to 10 MIU TIW 4 to 6 months 37% Interferon Untreated 33% 30 20 17% 12% 10 0 8% 2% HBV DNA loss HBe. Ag loss HBs. Ag loss Wong, DKH, et al, Ann Intern Med. 1993
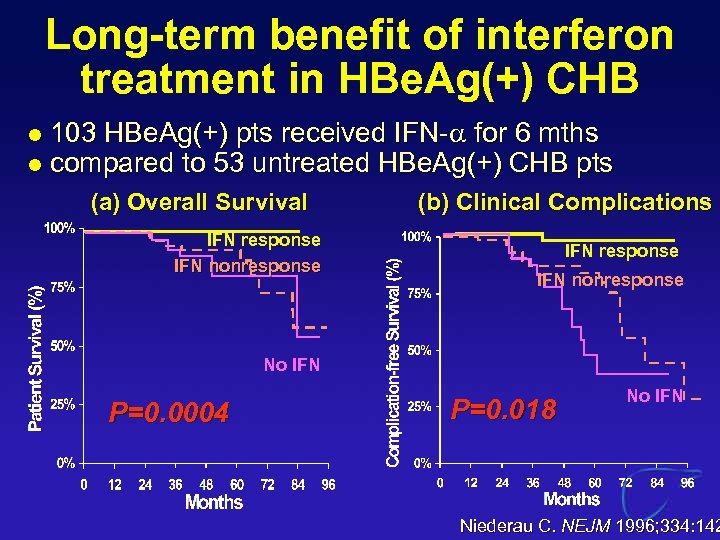
Long-term benefit of interferon treatment in HBe. Ag(+) CHB l l 103 HBe. Ag(+) pts received IFN-a for 6 mths compared to 53 untreated HBe. Ag(+) CHB pts (a) Overall Survival IFN response IFN nonresponse (b) Clinical Complications IFN response IFN nonresponse No IFN P=0. 0004 P=0. 018 No IFN Niederau C. NEJM 1996; 334: 142
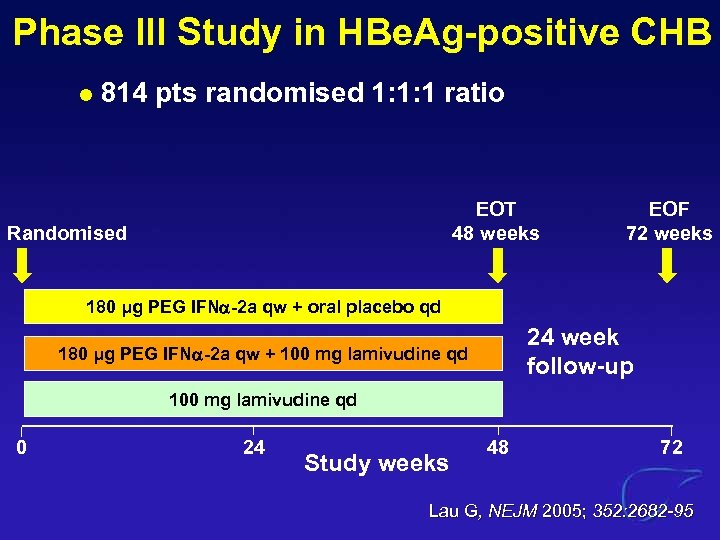
Phase III Study in HBe. Ag-positive CHB l 814 pts randomised 1: 1: 1 ratio EOT 48 weeks Randomised EOF 72 weeks 180 μg PEG IFN -2 a qw + oral placebo qd 24 week follow-up 180 μg PEG IFN -2 a qw + 100 mg lamivudine qd 0 24 Study weeks 48 72 Lau G, NEJM 2005; 352: 2682 -95
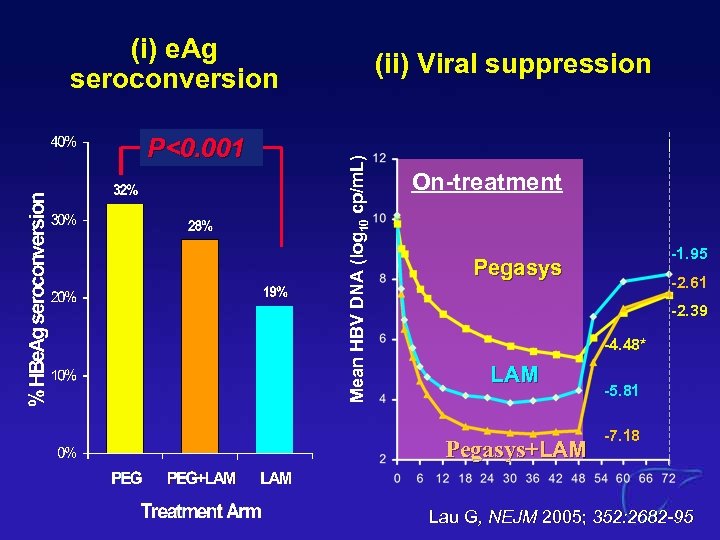
(i) e. Ag seroconversion Mean HBV DNA (log 10 cp/m. L) P<0. 001 (ii) Viral suppression On-treatment -1. 95 Pegasys -2. 61 -2. 39 -4. 48* LAM Pegasys+LAM -5. 81 -7. 18 Lau G, NEJM 2005; 352: 2682 -95
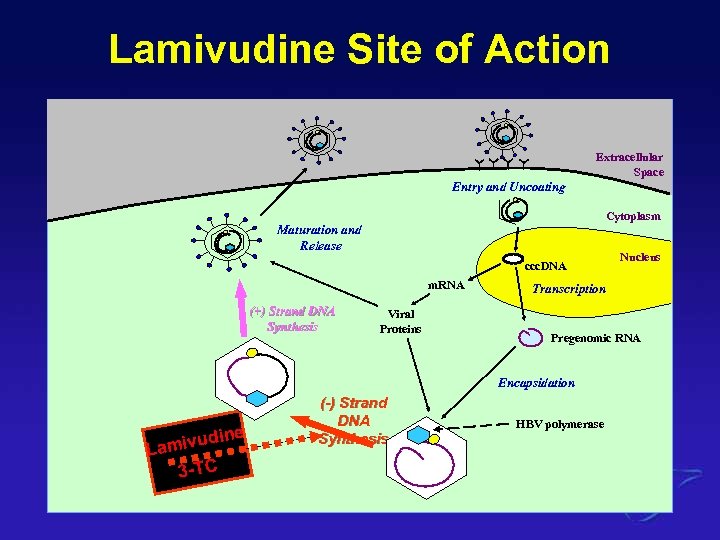
Lamivudine Site of Action Extracellular Space Entry and Uncoating Cytoplasm Maturation and Release ccc. DNA m. RNA (+) Strand DNA Synthesis Viral Proteins Transcription Pregenomic RNA Encapsidation e ivudin Lam 3 -TC (-) Strand DNA Synthesis Nucleus HBV polymerase
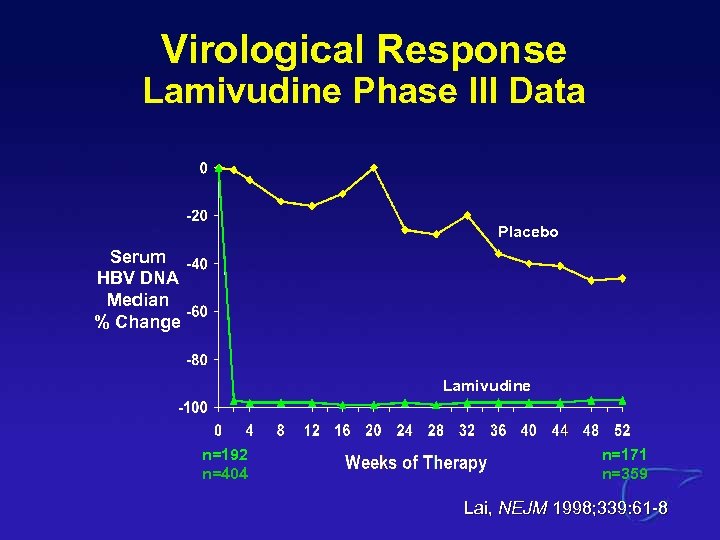
Virological Response Lamivudine Phase III Data Placebo Serum HBV DNA Median % Change Lamivudine n=192 n=404 n=171 n=359 Lai, NEJM 1998; 339: 61 -8
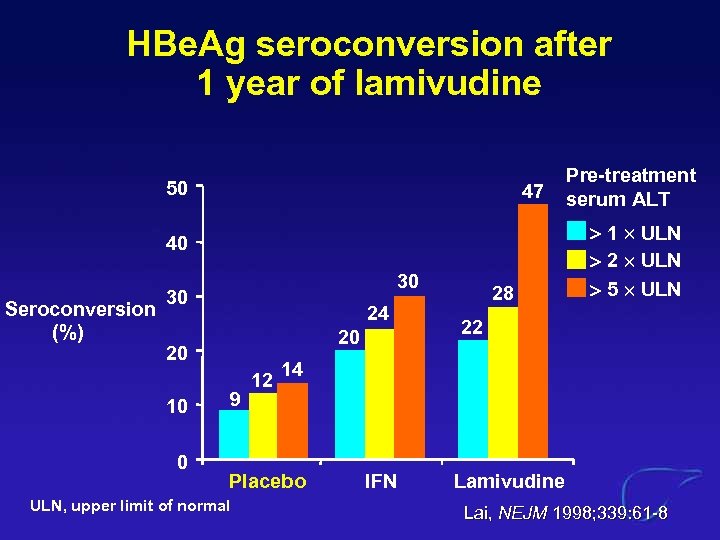
HBe. Ag seroconversion after 1 year of lamivudine 50 47 40 Seroconversion (%) 30 30 24 20 20 10 0 9 12 1 ULN 2 ULN 5 ULN 22 14 Placebo ULN, upper limit of normal 28 Pre-treatment serum ALT IFN Lamivudine Lai, NEJM 1998; 339: 61 -8
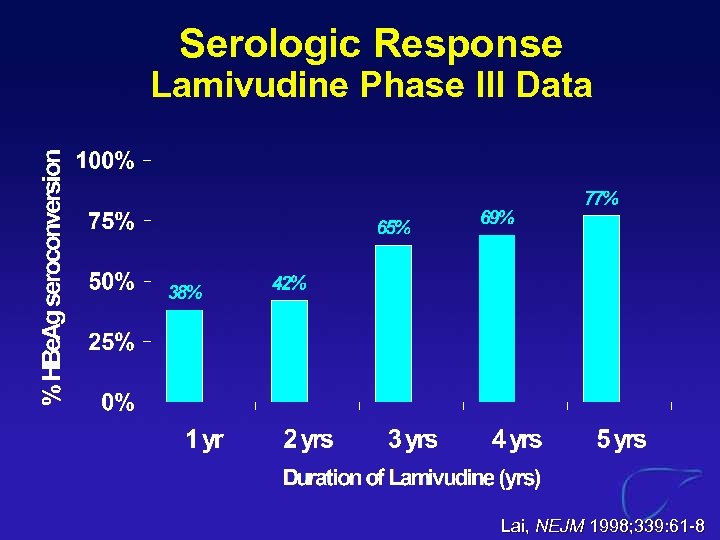
Serologic Response Lamivudine Phase III Data Lai, NEJM 1998; 339: 61 -8
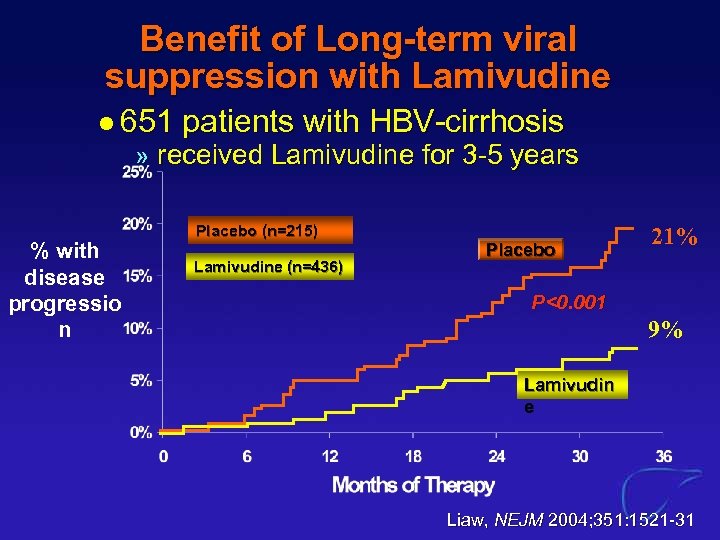
Benefit of Long-term viral suppression with Lamivudine l 651 patients with HBV-cirrhosis » received Lamivudine for 3 -5 years % with disease progressio n Placebo (n=215) Lamivudine (n=436) Placebo 21% P<0. 001 9% Lamivudin e Liaw, NEJM 2004; 351: 1521 -31
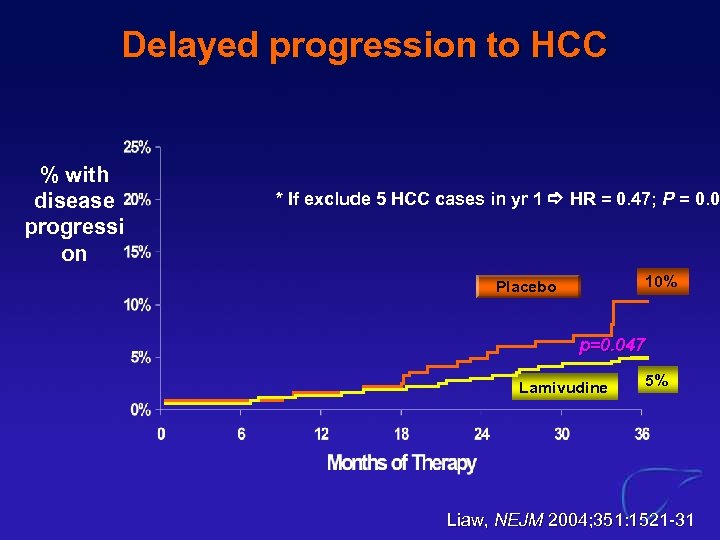
Delayed progression to HCC % with disease progressi on * If exclude 5 HCC cases in yr 1 HR = 0. 47; P = 0. 05 10% Placebo p=0. 047 Lamivudine 5% Liaw, NEJM 2004; 351: 1521 -31
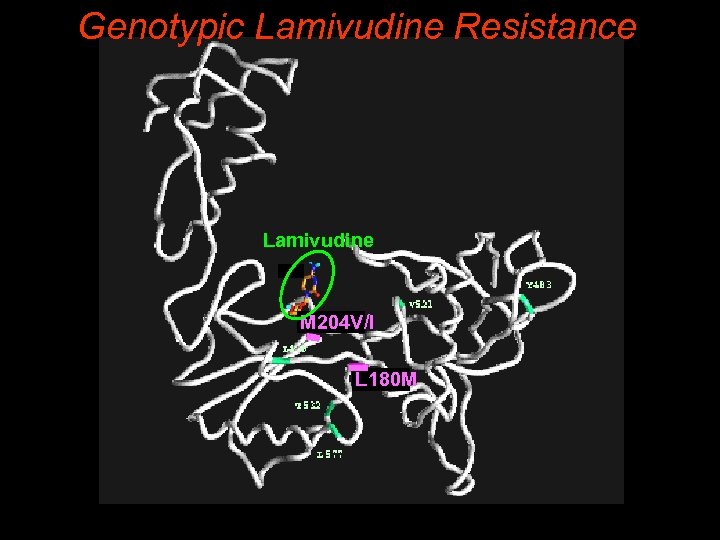
Genotypic Lamivudine Resistance Lamivudine M 204 V/I M 552 L 180 M L 528
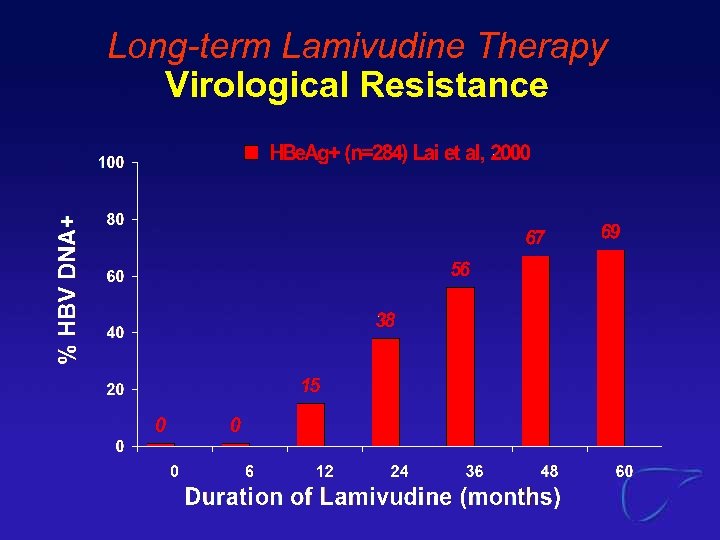
Long-term Lamivudine Therapy Virological Resistance
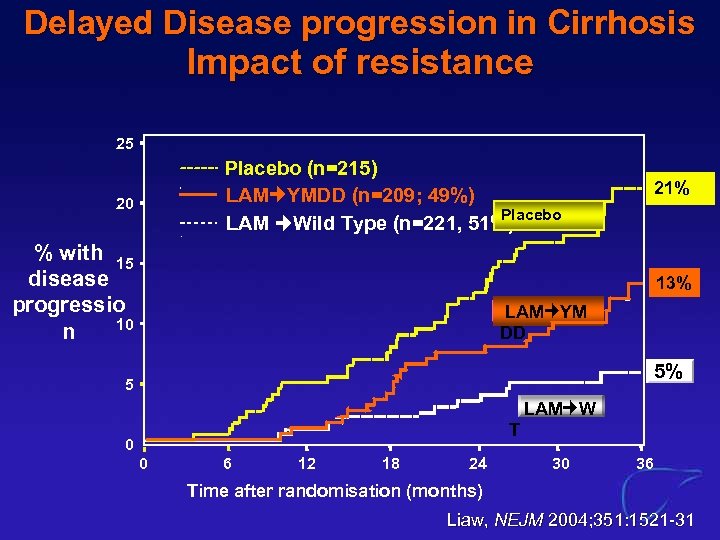
Delayed Disease progression in Cirrhosis Impact of resistance 25 Placebo (n=215) LAM YMDD (n=209; 49%) Placebo LAM Wild Type (n=221, 51%) 20 % with 15 disease progressio 10 n 21% 13% LAM YM DD 5% 5 LAM W T 0 0 6 12 18 24 30 36 Time after randomisation (months) Liaw, NEJM 2004; 351: 1521 -31
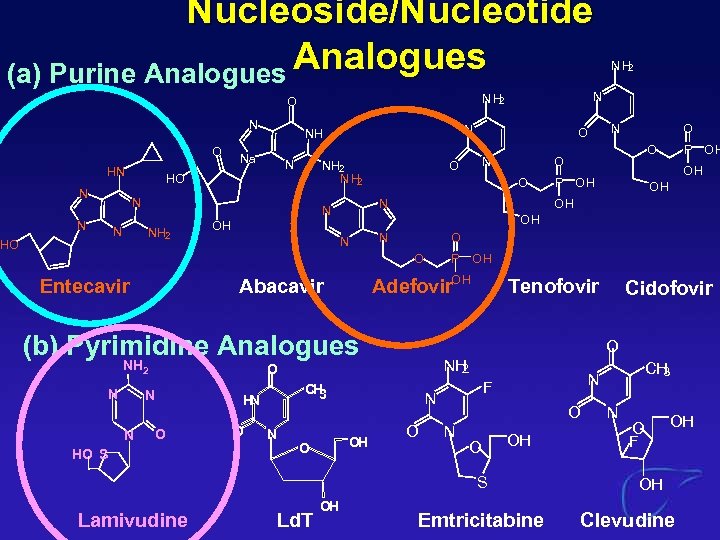
Nucleoside/Nucleotide Analogues (a) Purine Analogues N O HN N NH 2 N N O HO S O Tenofovir NH 2 O O N O OH S Lamivudine Ld. T OH Cidofovir CH 3 N F N OH O CH 3 N OH P OH O H N P OH O Adefovir. OH Abacavir N O OH (b) Pyrimidine Analogues N O OH OH NH 2 N O O O Entecavir N NH 2 HO O O NH 2 HO N N NH Na N NH 2 O NH 2 Emtricitabine N O F OH OH Clevudine
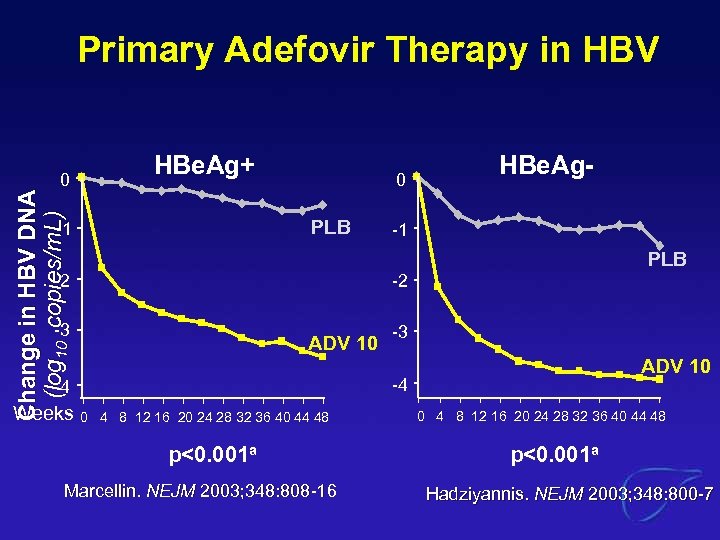
Primary Adefovir Therapy in HBV Change in HBV DNA (log 10 copies/m. L) 0 HBe. Ag+ 0 PLB -1 -1 PLB -2 -2 -3 ADV 10 -4 -4 Weeks HBe. Ag- 0 4 8 12 16 20 24 28 32 36 40 44 48 p<0. 001 a Marcellin. NEJM 2003; 348: 808 -16 0 4 8 12 16 20 24 28 32 36 40 44 48 p<0. 001 a Hadziyannis. NEJM 2003; 348: 800 -7
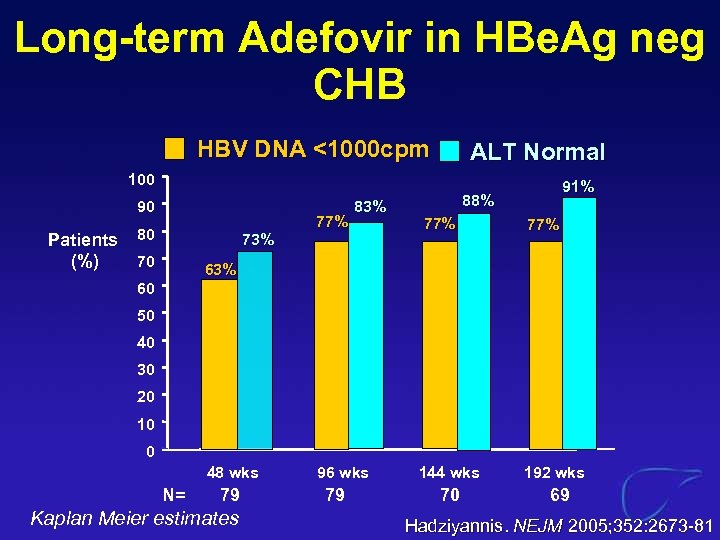
Long-term Adefovir in HBe. Ag neg CHB HBV DNA <1000 cpm ALT Normal 100 90 Patients (%) 80 73% 70 77% 83% 91% 88% 77% 63% 60 50 40 30 20 10 0 48 wks N= 79 Kaplan Meier estimates 96 wks 79 144 wks 70 192 wks 69 Hadziyannis. NEJM 2005; 352: 2673 -81
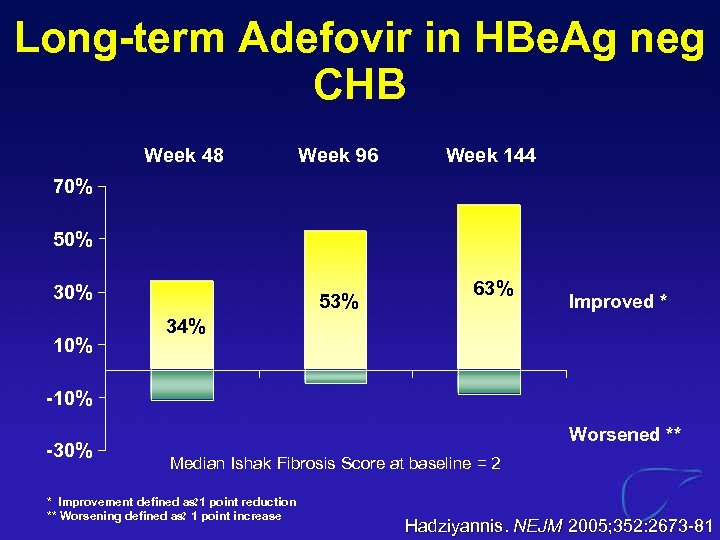
Long-term Adefovir in HBe. Ag neg CHB Week 48 Week 96 Week 144 70% 50% 30% 10% 53% 63% Improved * 34% -10% -30% Worsened ** Median Ishak Fibrosis Score at baseline = 2 * Improvement defined as point reduction ? 1 ** Worsening defined as 1 point increase ? Hadziyannis. NEJM 2005; 352: 2673 -81
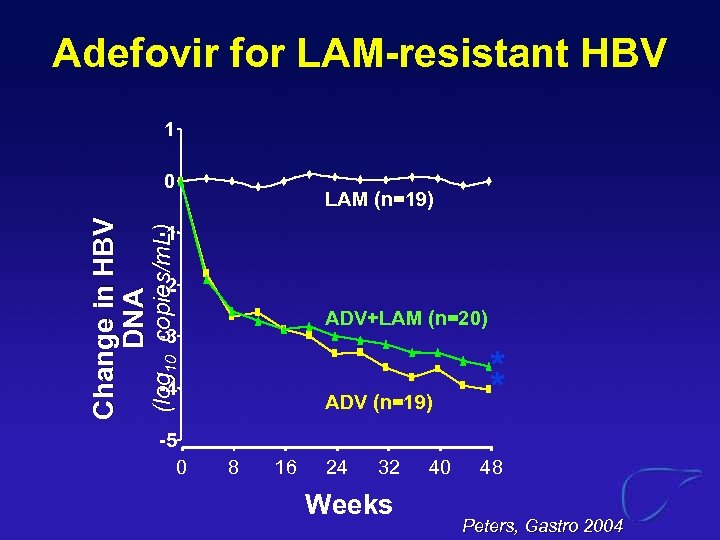
Adefovir for LAM-resistant HBV 1 LAM (n=19) -1 (log 10 copies/m. L) Change in HBV DNA 0 -2 ADV+LAM (n=20) -3 -4 -5 0 ADV (n=19) 8 16 24 32 Weeks 40 * * 48 Peters, Gastro 2004
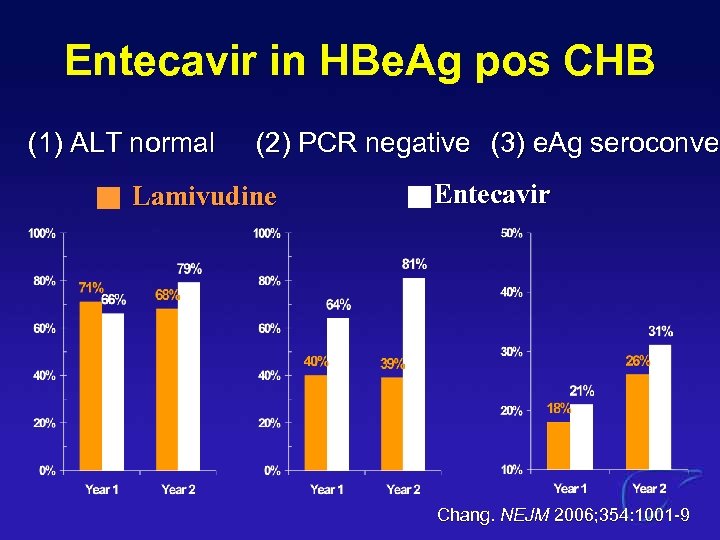
Entecavir in HBe. Ag pos CHB (1) ALT normal (2) PCR negative (3) e. Ag seroconver seroconve Lamivudine Entecavir Chang. NEJM 2006; 354: 1001 -9
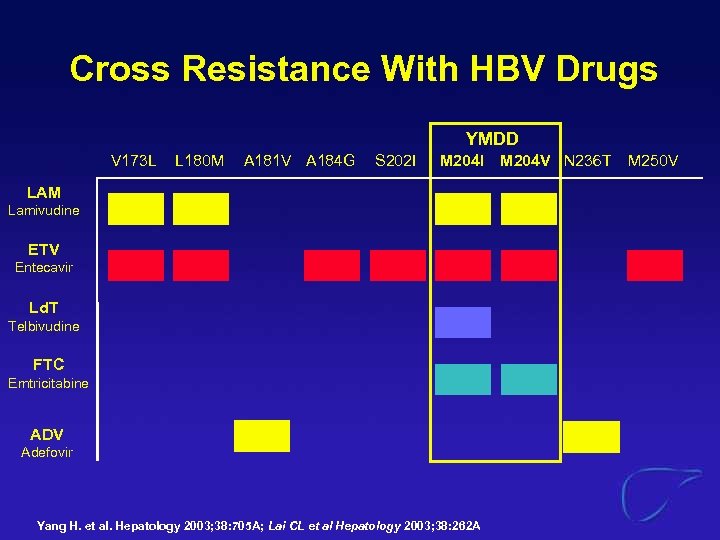
Cross Resistance With HBV Drugs YMDD V 173 L L 180 M A 181 V A 184 G S 202 I M 204 I LAM Lamivudine ETV Entecavir Ld. T Telbivudine FTC Emtricitabine ADV Adefovir Yang H. et al. Hepatology 2003; 38: 705 A; Lai CL et al Hepatology 2003; 38: 262 A M 204 V N 236 T M 250 V
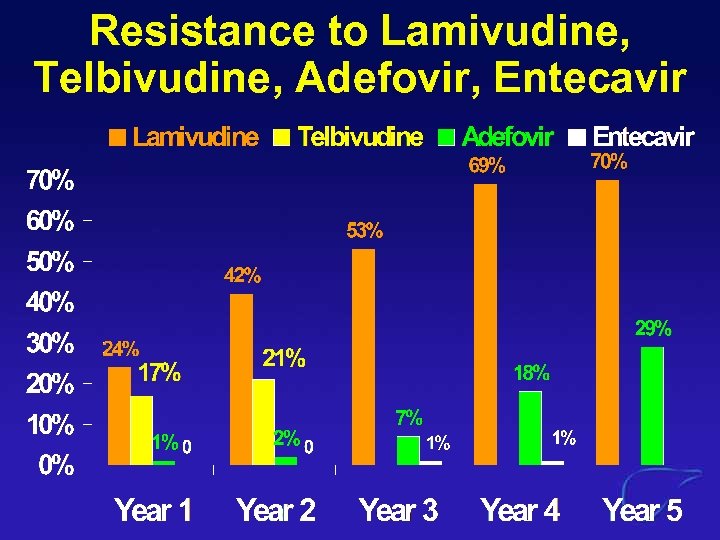
Resistance to Lamivudine, Telbivudine, Adefovir, Entecavir
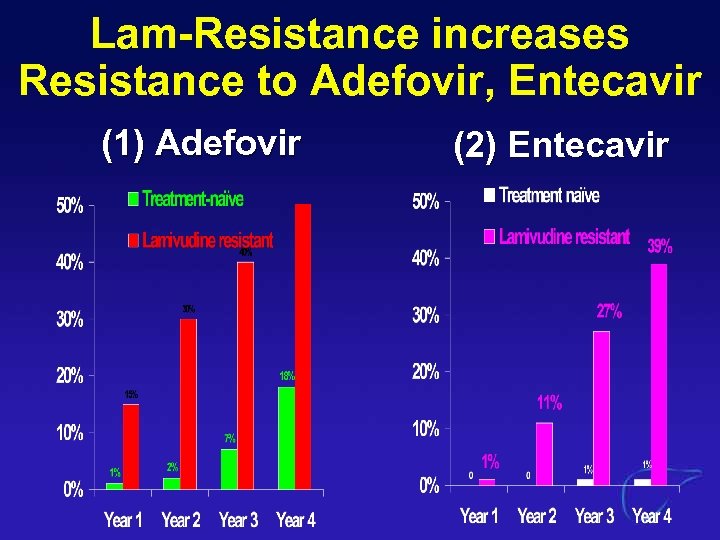
Lam-Resistance increases Resistance to Adefovir, Entecavir (1) Adefovir (2) Entecavir
HBV HIV + =?
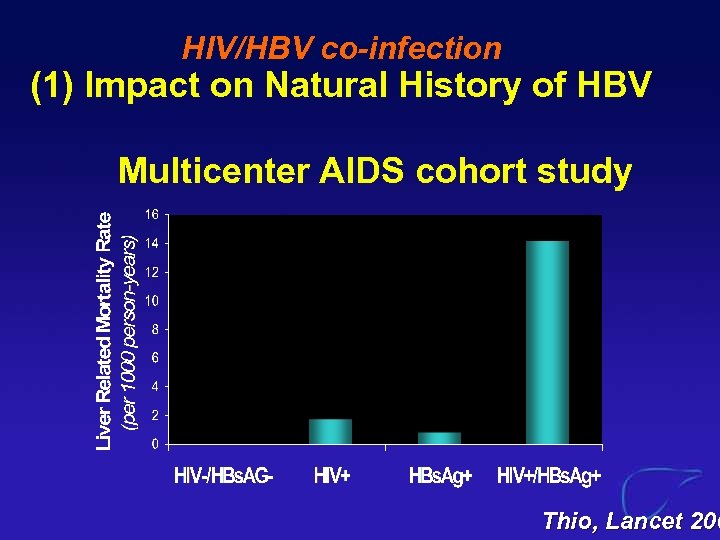
HIV/HBV co-infection (1) Impact on Natural History of HBV Multicenter AIDS cohort study Thio, Lancet 200

HIV/HBV co-infection (1) Impact on Natural History of HBV HIV co-infection associated with: spontaneous clearance of HBe. Ag l spontaneous clearance of HBs. Ag l ALT levels l HBV DNA l progression to cirrhosis l liver-related mortality l
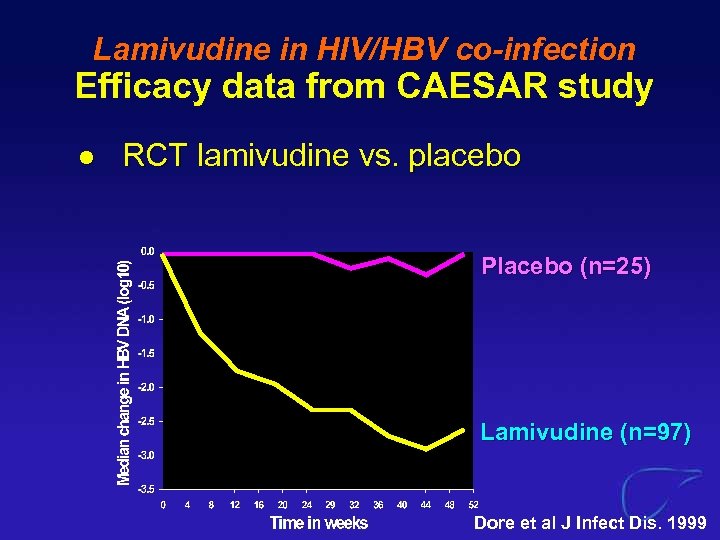
Lamivudine in HIV/HBV co-infection Efficacy data from CAESAR study l RCT lamivudine vs. placebo Placebo (n=25) Lamivudine (n=97) Dore et al J Infect Dis. 1999
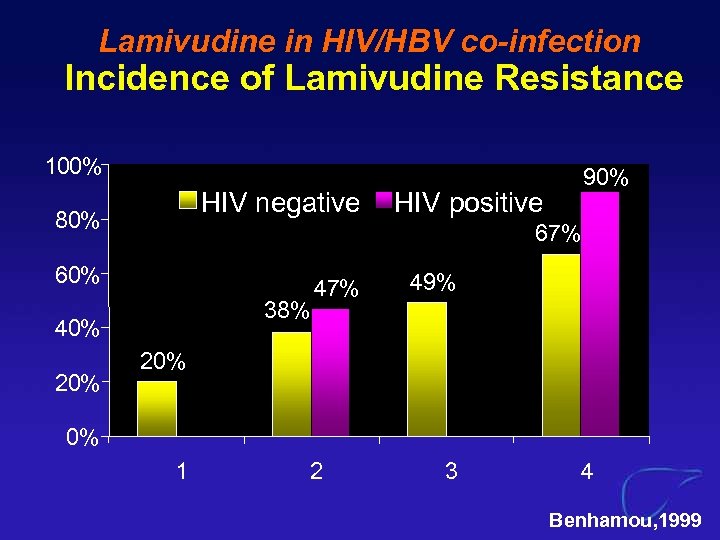
Lamivudine in HIV/HBV co-infection Incidence of Lamivudine Resistance 100% HIV negative 80% HIV positive 67% 60% 38% 40% 20% 90% 47% 49% 20% 0% 1 2 3 4 Benhamou, 1999
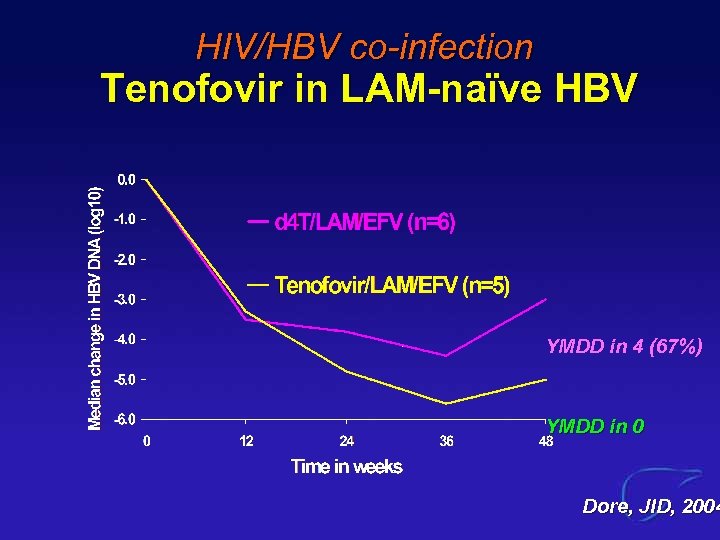
HIV/HBV co-infection Tenofovir in LAM-naïve HBV YMDD in 4 (67%) YMDD in 0 Dore, JID, 2004
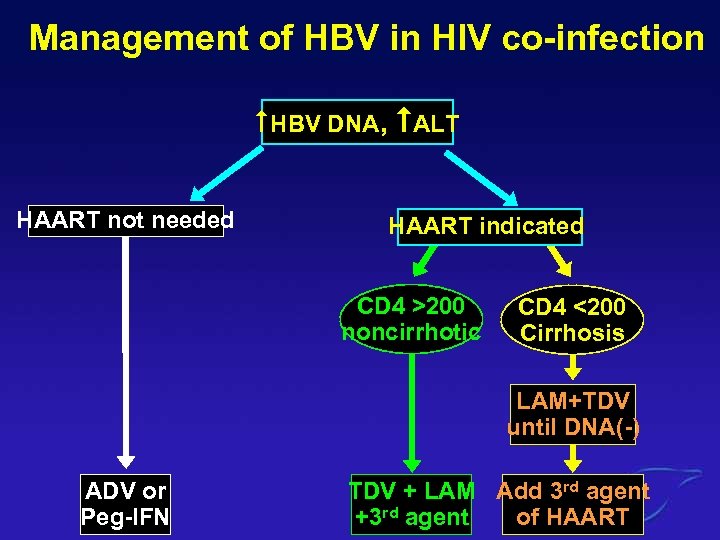
Management of HBV in HIV co-infection HBV DNA, ALT HAART not needed HAART indicated CD 4 >200 noncirrhotic CD 4 <200 Cirrhosis LAM+TDV until DNA(-) ADV or Peg-IFN TDV + LAM Add 3 rd agent of HAART +3 rd agent
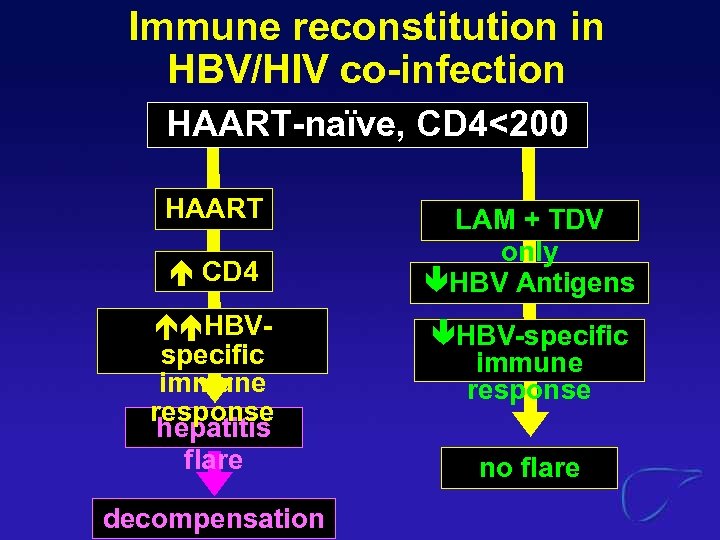
Immune reconstitution in HBV/HIV co-infection HAART-naïve, CD 4<200 HAART CD 4 HBVspecific immune response hepatitis flare decompensation LAM + TDV only äHBV Antigens äHBV-specific immune response no flare
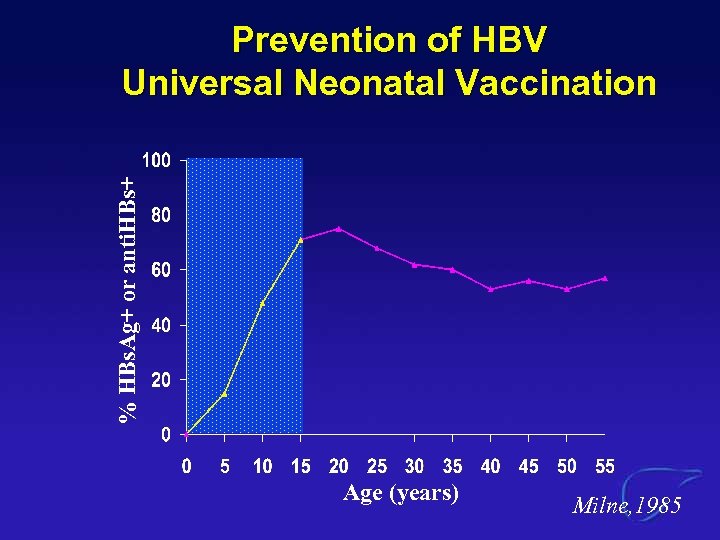
% HBs. Ag+ or anti. HBs+ Prevention of HBV Universal Neonatal Vaccination Age (years) Milne, 1985
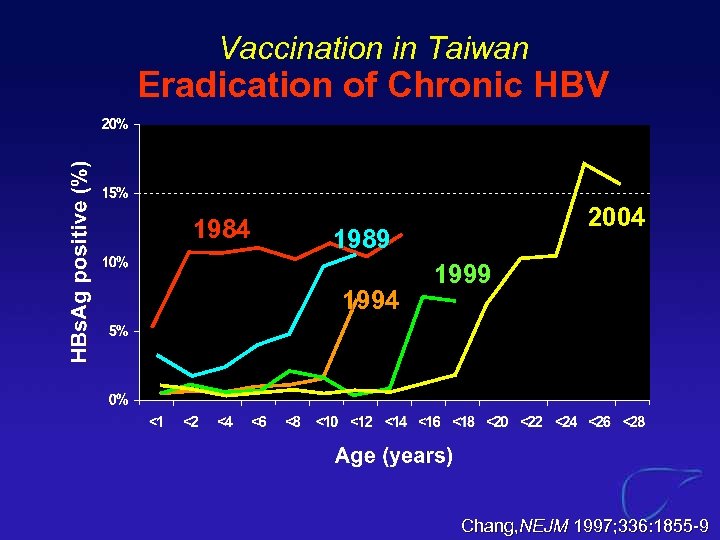
Vaccination in Taiwan Eradication of Chronic HBV 1984 2004 1989 1994 1999 Chang, NEJM 1997; 336: 1855 -9
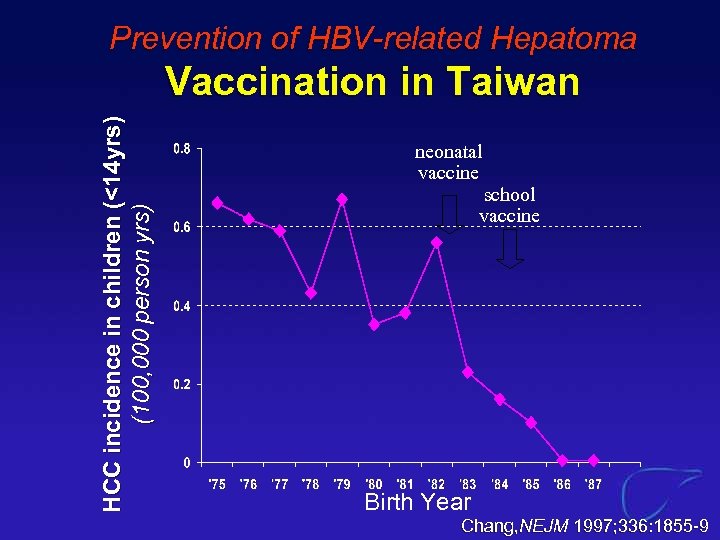
Prevention of HBV-related Hepatoma HCC incidence in children (<14 yrs) (100, 000 person yrs) Vaccination in Taiwan neonatal vaccine school vaccine Birth Year Chang, NEJM 1997; 336: 1855 -9
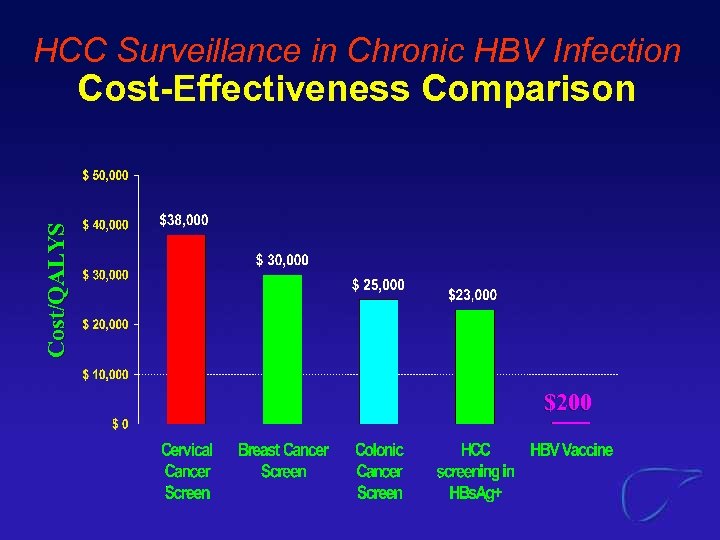
HCC Surveillance in Chronic HBV Infection Cost/QALYS Cost-Effectiveness Comparison $200
aa16d44402af77eb2da2e4bf3bc5cfa6.ppt