8d125deaf19374ad63b86744f2eaa1c3.ppt
- Количество слайдов: 55
 CHROMATOGRAPHICAL APPLICATIONS Dr. S. TURE UCL, Imperial College, Ph. D. University of London
CHROMATOGRAPHICAL APPLICATIONS Dr. S. TURE UCL, Imperial College, Ph. D. University of London
 Chromatography basically involves the separation of mixtures due to differences in the distribution coefficient of sample components between 2 different phases. One of these phases is a mobile phase and the other is a stationary phase.
Chromatography basically involves the separation of mixtures due to differences in the distribution coefficient of sample components between 2 different phases. One of these phases is a mobile phase and the other is a stationary phase.
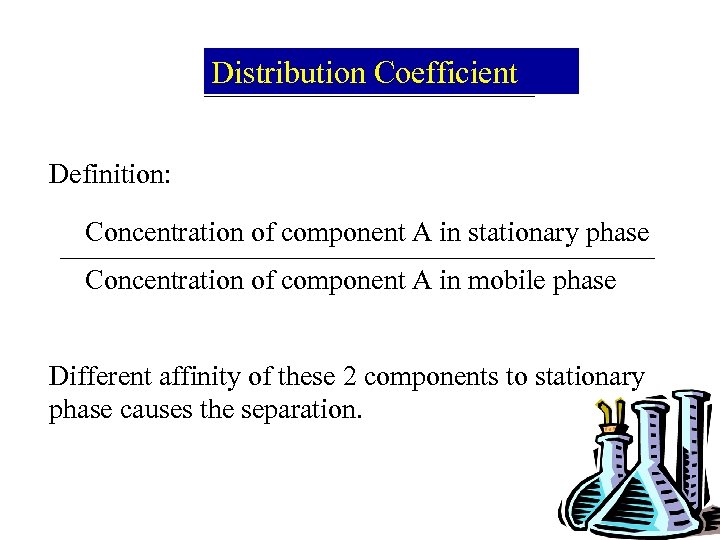 Distribution Coefficient Definition: Concentration of component A in stationary phase Concentration of component A in mobile phase Different affinity of these 2 components to stationary phase causes the separation.
Distribution Coefficient Definition: Concentration of component A in stationary phase Concentration of component A in mobile phase Different affinity of these 2 components to stationary phase causes the separation.
 Definition of Chromatography Simplified Definition: Chromatography separates the components of a mixture by their distinctive attraction to the mobile phase and the stationary phase. Explanation: • • Compound is placed on stationary phase Mobile phase passes through the stationary phase Mobile phase solubilizes the components Mobile phase carries the individual components a certain distance through the stationary phase, depending on their attraction to both of the phases
Definition of Chromatography Simplified Definition: Chromatography separates the components of a mixture by their distinctive attraction to the mobile phase and the stationary phase. Explanation: • • Compound is placed on stationary phase Mobile phase passes through the stationary phase Mobile phase solubilizes the components Mobile phase carries the individual components a certain distance through the stationary phase, depending on their attraction to both of the phases
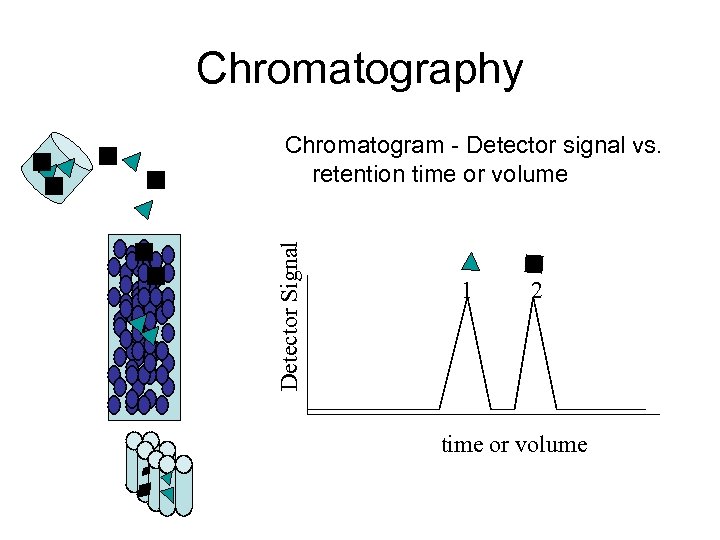 Chromatography Detector Signal Chromatogram - Detector signal vs. retention time or volume 1 2 time or volume
Chromatography Detector Signal Chromatogram - Detector signal vs. retention time or volume 1 2 time or volume
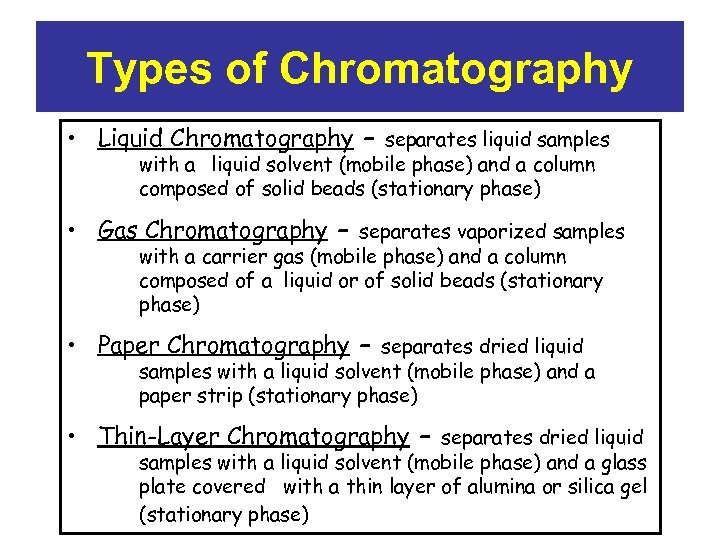 Types of Chromatography • Liquid Chromatography – separates liquid samples with a liquid solvent (mobile phase) and a column composed of solid beads (stationary phase) • Gas Chromatography – separates vaporized samples with a carrier gas (mobile phase) and a column composed of a liquid or of solid beads (stationary phase) • Paper Chromatography – separates dried liquid samples with a liquid solvent (mobile phase) and a paper strip (stationary phase) • Thin-Layer Chromatography – separates dried liquid samples with a liquid solvent (mobile phase) and a glass plate covered with a thin layer of alumina or silica gel (stationary phase)
Types of Chromatography • Liquid Chromatography – separates liquid samples with a liquid solvent (mobile phase) and a column composed of solid beads (stationary phase) • Gas Chromatography – separates vaporized samples with a carrier gas (mobile phase) and a column composed of a liquid or of solid beads (stationary phase) • Paper Chromatography – separates dried liquid samples with a liquid solvent (mobile phase) and a paper strip (stationary phase) • Thin-Layer Chromatography – separates dried liquid samples with a liquid solvent (mobile phase) and a glass plate covered with a thin layer of alumina or silica gel (stationary phase)
 Types of Chromatography LIQUID MOBILE PHASE Liquid-Liquid Chromatography (Partition) FORMAT STATIONARY PHASE Normal Phase Mobile Phase - Nonpolar Stationary phase Polar Liquid-Solid (Adsorption) Chromatography Solid Liquid Reverse Phase Normal Phase Mobile Phase Polar Stationary phase - Nonpolar Reverse Phase
Types of Chromatography LIQUID MOBILE PHASE Liquid-Liquid Chromatography (Partition) FORMAT STATIONARY PHASE Normal Phase Mobile Phase - Nonpolar Stationary phase Polar Liquid-Solid (Adsorption) Chromatography Solid Liquid Reverse Phase Normal Phase Mobile Phase Polar Stationary phase - Nonpolar Reverse Phase

 Sifat Fisika kimia kertas untuk Kromatografi • • Kertas terdiri dari 98 -99 % selulose, 0, 3 -10 % selulose, dan 0, 4 -0, 8 % pentosan. Juga mempunyai gugus karboksilat yang dapat menimbulkan muatan negatif pada kertas. Kertas kromatografi terdapat kontaminan asam amino yang mempunyai kadar Nitrogen 15 mg/kg kertas. Senyawa lipofilik 25 mg/kg. dan senyawa an organik (kadar abu) 0, 04 -0, 07%, Senyawa kontaminan tidak mengganggu dalam pemisahan sampel pada kromatografi. Yang penting kemampuan absorbsi dan kenaikkan kapileritas masing kertas. Whatman no. 1 sebagai kertas standard yang digunakan, no. 3 MM digunakan untuk preparatif. Sedangkan no. 4 untuk elusi yang cepat, dan 33 ET untuk elusi sangat cepat.
Sifat Fisika kimia kertas untuk Kromatografi • • Kertas terdiri dari 98 -99 % selulose, 0, 3 -10 % selulose, dan 0, 4 -0, 8 % pentosan. Juga mempunyai gugus karboksilat yang dapat menimbulkan muatan negatif pada kertas. Kertas kromatografi terdapat kontaminan asam amino yang mempunyai kadar Nitrogen 15 mg/kg kertas. Senyawa lipofilik 25 mg/kg. dan senyawa an organik (kadar abu) 0, 04 -0, 07%, Senyawa kontaminan tidak mengganggu dalam pemisahan sampel pada kromatografi. Yang penting kemampuan absorbsi dan kenaikkan kapileritas masing kertas. Whatman no. 1 sebagai kertas standard yang digunakan, no. 3 MM digunakan untuk preparatif. Sedangkan no. 4 untuk elusi yang cepat, dan 33 ET untuk elusi sangat cepat.
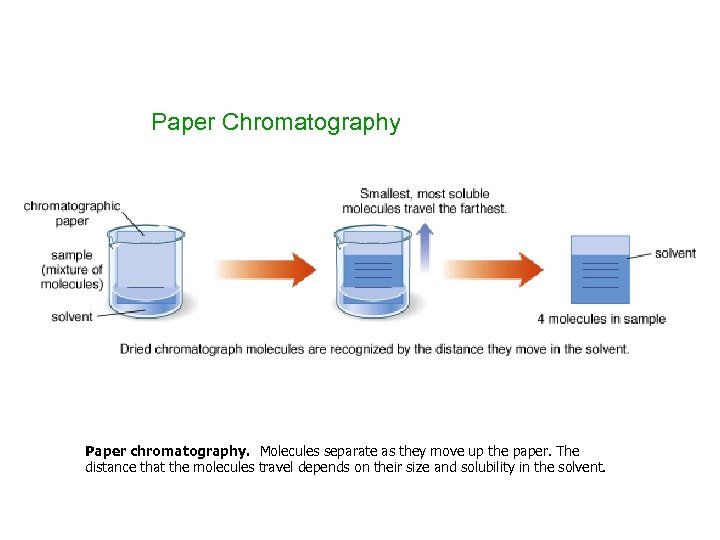 Paper Chromatography Paper chromatography. Molecules separate as they move up the paper. The distance that the molecules travel depends on their size and solubility in the solvent.
Paper Chromatography Paper chromatography. Molecules separate as they move up the paper. The distance that the molecules travel depends on their size and solubility in the solvent.
 Paper Chromatography • Similar to TLC • Stationary phase = H 2 O adsorbed by cellulose • Mobile phase = solvent • Frequently used to polar compounds – Amino acids, carbohydrates, etc.
Paper Chromatography • Similar to TLC • Stationary phase = H 2 O adsorbed by cellulose • Mobile phase = solvent • Frequently used to polar compounds – Amino acids, carbohydrates, etc.
 Stationary phase: Papers (cellulose), mechanism of separation is through partition. Mobile phase: As TLC but more polar mixtures are usually used. Buffers can also be used. Sample application: A line drawn by pencil, spot places are determined as dots. Apply sample as in TLC.
Stationary phase: Papers (cellulose), mechanism of separation is through partition. Mobile phase: As TLC but more polar mixtures are usually used. Buffers can also be used. Sample application: A line drawn by pencil, spot places are determined as dots. Apply sample as in TLC.
 Paper Chromatography
Paper Chromatography
 Types of Paper Chromatography • Radial chromatography • Ascending chromatography • Descending chromatography
Types of Paper Chromatography • Radial chromatography • Ascending chromatography • Descending chromatography
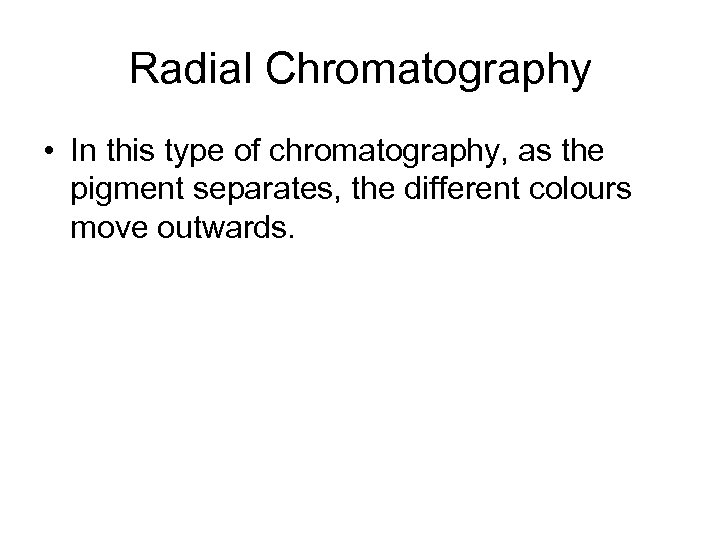 Radial Chromatography • In this type of chromatography, as the pigment separates, the different colours move outwards.
Radial Chromatography • In this type of chromatography, as the pigment separates, the different colours move outwards.
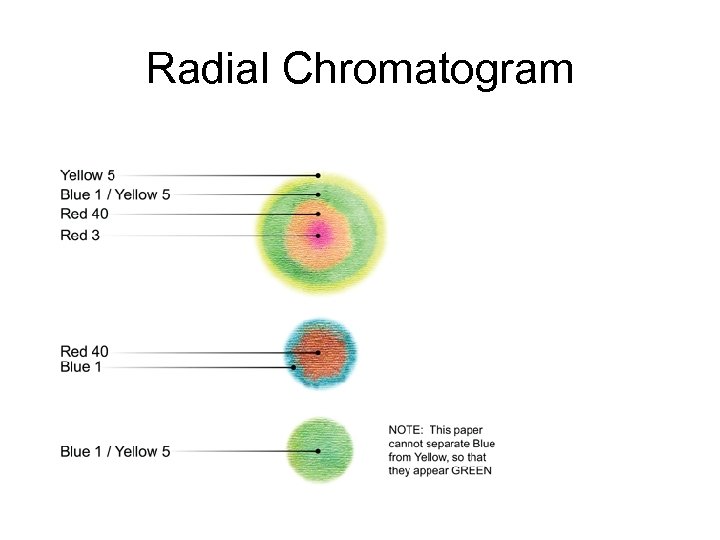 Radial Chromatogram
Radial Chromatogram
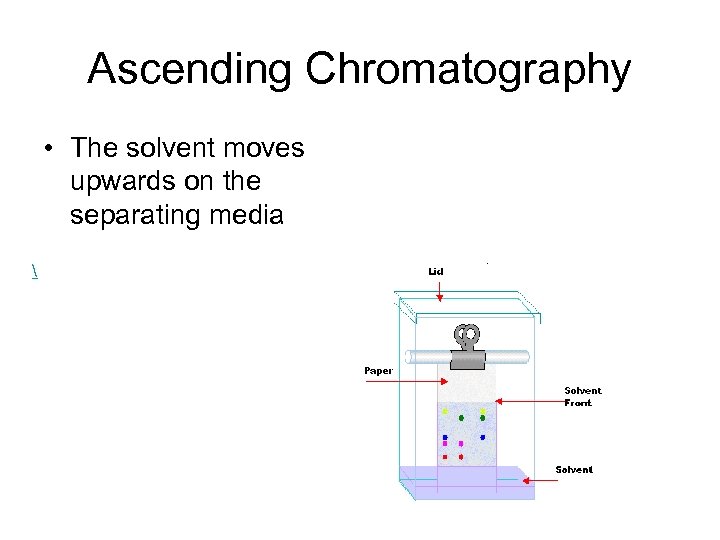 Ascending Chromatography • The solvent moves upwards on the separating media
Ascending Chromatography • The solvent moves upwards on the separating media
 Development Type of Ascending: 1 - Single development: The solvent system is allowed to move through the stationary phase one time only against gravity. 2 - Repeated developments: a- Multiple developments: The plated are developed more than one time using the same solvent system. The plates must be completely dried after each development. b- Stepwise developments: The plated are developed more than one time using different solvent systems.
Development Type of Ascending: 1 - Single development: The solvent system is allowed to move through the stationary phase one time only against gravity. 2 - Repeated developments: a- Multiple developments: The plated are developed more than one time using the same solvent system. The plates must be completely dried after each development. b- Stepwise developments: The plated are developed more than one time using different solvent systems.
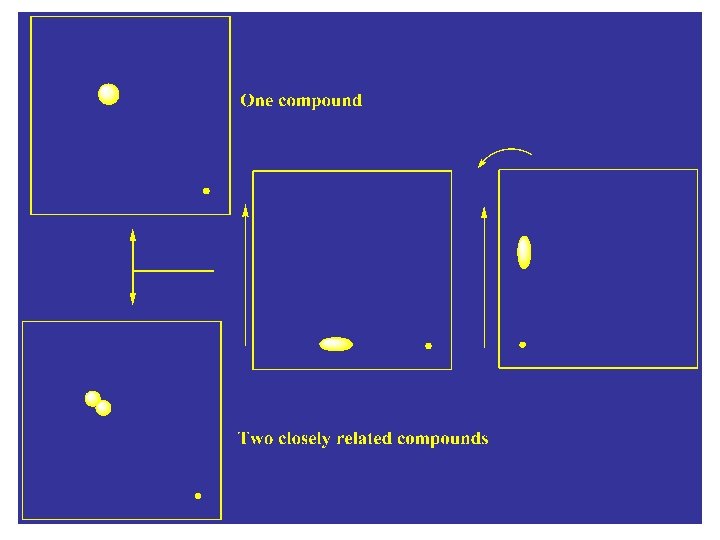
 3 - Two-dimensional development: Is used to verify if a given spot on TLC using the above methods of development (one Dimensional) is one pure compound or mixture of two closely related compounds. The spots are applied to one corner and the plate developed as usual. The plate is then rotated 90 ˚C and then developed again. This method allow better separation of related compounds.
3 - Two-dimensional development: Is used to verify if a given spot on TLC using the above methods of development (one Dimensional) is one pure compound or mixture of two closely related compounds. The spots are applied to one corner and the plate developed as usual. The plate is then rotated 90 ˚C and then developed again. This method allow better separation of related compounds.
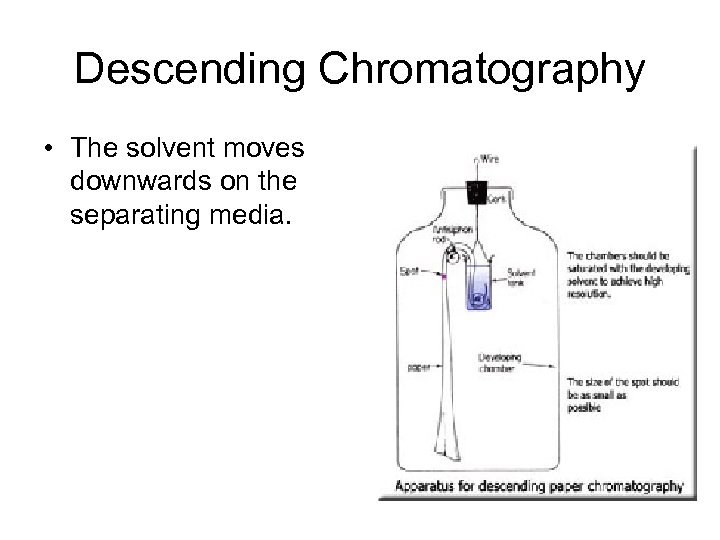 Descending Chromatography • The solvent moves downwards on the separating media.
Descending Chromatography • The solvent moves downwards on the separating media.
 • Pada kromatografi kertas lebih banyak digunakan sistem menurun sehingga lebih cepat perambatan nya. Keuntungan yang lain kromatografi kertas dapat digunakan lembaran kertas yang lebih panjang sehingga dapat dipisahkan campuran yang lebih kompleks. • Pemisahan yang terjadi berdasar atas peristiwa partisi, karena fase gerak yang digunakan adalah pelarut organik yang semi polar. • Dan umumnya pelarut yang digunakan mengandung air sehingga air akan mudah terikat oleh selulosa, dan selulosa dapat mengembang menyerap air, maka air akan berfungsi sebagai fase diam. • Komposisi Fase gerak yang dikenal dengan nama BAW (Butanol, Acetic Acid Water). Banyak digunakan untuk pemisahan flavanoid.
• Pada kromatografi kertas lebih banyak digunakan sistem menurun sehingga lebih cepat perambatan nya. Keuntungan yang lain kromatografi kertas dapat digunakan lembaran kertas yang lebih panjang sehingga dapat dipisahkan campuran yang lebih kompleks. • Pemisahan yang terjadi berdasar atas peristiwa partisi, karena fase gerak yang digunakan adalah pelarut organik yang semi polar. • Dan umumnya pelarut yang digunakan mengandung air sehingga air akan mudah terikat oleh selulosa, dan selulosa dapat mengembang menyerap air, maka air akan berfungsi sebagai fase diam. • Komposisi Fase gerak yang dikenal dengan nama BAW (Butanol, Acetic Acid Water). Banyak digunakan untuk pemisahan flavanoid.
 Fase gerak yang berupa pelarut organik akan berkompetisi melarutkan sampel yang dianalisis Kromatografi kertas dapat diubah polaritasnya dengan cara inpregnasi atau pembaceman, antara lain dengan asetilasi, foforilasi, fomilasi. Atau dengan senyawa yang bersifat lifofilik seperti parafin, vaselin, undekan. Pembaceman sistemnya seperti pada KLT, hanya pada kromatografi kertas dengan arah yang menurun atau desenden. Dengan cara tersebut kromatografi kertaspun dapat digunakan sebagai kromatografi fase terbalik. Arah elusi dari kertas untuk kromatografi biasa nya ditunjukkan oleh panah, kalau tak ada, digunakan arah yang memanjang dari kertas.
Fase gerak yang berupa pelarut organik akan berkompetisi melarutkan sampel yang dianalisis Kromatografi kertas dapat diubah polaritasnya dengan cara inpregnasi atau pembaceman, antara lain dengan asetilasi, foforilasi, fomilasi. Atau dengan senyawa yang bersifat lifofilik seperti parafin, vaselin, undekan. Pembaceman sistemnya seperti pada KLT, hanya pada kromatografi kertas dengan arah yang menurun atau desenden. Dengan cara tersebut kromatografi kertaspun dapat digunakan sebagai kromatografi fase terbalik. Arah elusi dari kertas untuk kromatografi biasa nya ditunjukkan oleh panah, kalau tak ada, digunakan arah yang memanjang dari kertas.
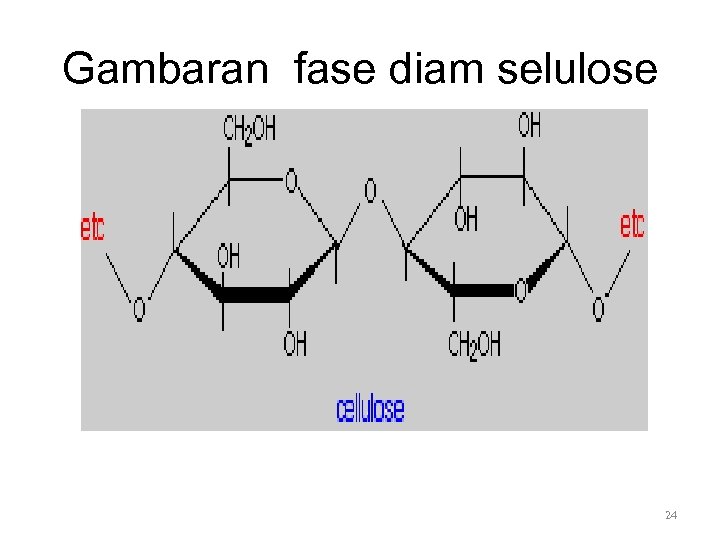 Gambaran fase diam selulose 24
Gambaran fase diam selulose 24
 PENGUBAHAN GUGUS HIDROKSIL Asetilasi (CH 3 COOH) (C 6 H 12 O 6)n + n x 4 (CH 3 COOH) O-CO- CH 3 O OH HH O-CO- CH 3 CH 2 O-CO- CH H 3 HO H O-CO- CH 3 25
PENGUBAHAN GUGUS HIDROKSIL Asetilasi (CH 3 COOH) (C 6 H 12 O 6)n + n x 4 (CH 3 COOH) O-CO- CH 3 O OH HH O-CO- CH 3 CH 2 O-CO- CH H 3 HO H O-CO- CH 3 25
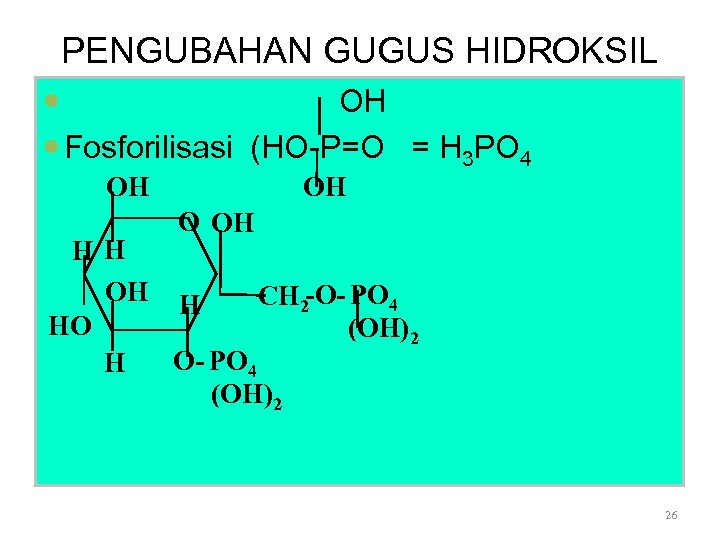 PENGUBAHAN GUGUS HIDROKSIL OH Fosforilisasi (HO-P=O = H 3 PO 4 OH OH O OH HH OH H CH 2 -O- PO 4 HO (OH)2 O- PO 4 H (OH)2 26
PENGUBAHAN GUGUS HIDROKSIL OH Fosforilisasi (HO-P=O = H 3 PO 4 OH OH O OH HH OH H CH 2 -O- PO 4 HO (OH)2 O- PO 4 H (OH)2 26
 Principles of Paper Chromatography • Capillary Action – the movement of liquid within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension. The liquid is able to move up the filter paper because its attraction to itself is stronger than the force of gravity. • Solubility – the degree to which a material (solute) dissolves into a solvent. Solutes dissolve into solvents that have similar properties. (Like dissolves like) This allows different solutes to be separated by different combinations of solvents. Separation of components depends on both their solubility in the mobile phase and their differential affinity to the mobile phase and the stationary phase.
Principles of Paper Chromatography • Capillary Action – the movement of liquid within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension. The liquid is able to move up the filter paper because its attraction to itself is stronger than the force of gravity. • Solubility – the degree to which a material (solute) dissolves into a solvent. Solutes dissolve into solvents that have similar properties. (Like dissolves like) This allows different solutes to be separated by different combinations of solvents. Separation of components depends on both their solubility in the mobile phase and their differential affinity to the mobile phase and the stationary phase.
 Visualization (Detection of spots): A- Universal methods: 1 - Destructive methods: The plated are sprayed with corrosive reagents and then heated in oven where organic compounds will give charred spots. After this treatment the materials can not be recovered. e. g. Anisaldehyde / H 2 SO 4 Vanillin / H 2 SO 4
Visualization (Detection of spots): A- Universal methods: 1 - Destructive methods: The plated are sprayed with corrosive reagents and then heated in oven where organic compounds will give charred spots. After this treatment the materials can not be recovered. e. g. Anisaldehyde / H 2 SO 4 Vanillin / H 2 SO 4
 2 - Non – Destructive methods: In these methods the materials can be recovered. – – Day light for colour compounds. UV light for fluorescent compounds (conjugated double bonds). I 2 vapour for any compounds contain at least one double bond Spray with water where organic compounds appear as white opaque spots.
2 - Non – Destructive methods: In these methods the materials can be recovered. – – Day light for colour compounds. UV light for fluorescent compounds (conjugated double bonds). I 2 vapour for any compounds contain at least one double bond Spray with water where organic compounds appear as white opaque spots.
 B- Specific Methods: • These reagents are used for the detection of certain classes of compounds. They are usually destructive. • Dragendorff΄s reagent for Alkaloids. • Ferric Chloride (Fe. Cl 3) for phenolic compounds. • Aniline phthalate for sugars. • Ninhydrine for nitrogenous Amines, Amino acids. compounds as
B- Specific Methods: • These reagents are used for the detection of certain classes of compounds. They are usually destructive. • Dragendorff΄s reagent for Alkaloids. • Ferric Chloride (Fe. Cl 3) for phenolic compounds. • Aniline phthalate for sugars. • Ninhydrine for nitrogenous Amines, Amino acids. compounds as
 Tabel Beberapa penggunaan pelacak bercak pada kromatografi kertas Nama pereaksi l. Sinar UV 254 nm Analit Senyawa flouresen Seny. Amin 2 lodoplatinat ter/kuaterner • 3. Pereaksi furfural Turunan Karbamat Nama pereaksi Analit 9. Pereaksi Marquis Tur. morfin Fenol, aril 10. Peraksi Millon amin Ikatan 11. KMn 04 + tak jenuh as. sulfet 12. Ninhidrin As. amino Heterosiklik amin 13. Nitroso-naftol primer Ergot Karbinol/Sulfonamida 14. Pereaksi alkaloid Turunan Alkaloid/amin kuater, Mandelin ajmalin Ter. Heksa/penta 15. Vanilinas. Barbiturat klorfenol Sulfat fenetoin, Ikatan rangkap/ S senyawa organik Sebyawa fiuoresen Amin ter/kuater. Turunan 4. Pereaksi Simon Heterosik. Uk amin kanabinol, karbamat sulfonamida Alkaloid/Amin kuar 5. DABdlm etanol Ter. Heksa(penta klorfenol) Ikatan rangkap, seny. organik 6. Dragendorff • 7. Uap iodium 8. Uap. NO 2 31 31
Tabel Beberapa penggunaan pelacak bercak pada kromatografi kertas Nama pereaksi l. Sinar UV 254 nm Analit Senyawa flouresen Seny. Amin 2 lodoplatinat ter/kuaterner • 3. Pereaksi furfural Turunan Karbamat Nama pereaksi Analit 9. Pereaksi Marquis Tur. morfin Fenol, aril 10. Peraksi Millon amin Ikatan 11. KMn 04 + tak jenuh as. sulfet 12. Ninhidrin As. amino Heterosiklik amin 13. Nitroso-naftol primer Ergot Karbinol/Sulfonamida 14. Pereaksi alkaloid Turunan Alkaloid/amin kuater, Mandelin ajmalin Ter. Heksa/penta 15. Vanilinas. Barbiturat klorfenol Sulfat fenetoin, Ikatan rangkap/ S senyawa organik Sebyawa fiuoresen Amin ter/kuater. Turunan 4. Pereaksi Simon Heterosik. Uk amin kanabinol, karbamat sulfonamida Alkaloid/Amin kuar 5. DABdlm etanol Ter. Heksa(penta klorfenol) Ikatan rangkap, seny. organik 6. Dragendorff • 7. Uap iodium 8. Uap. NO 2 31 31
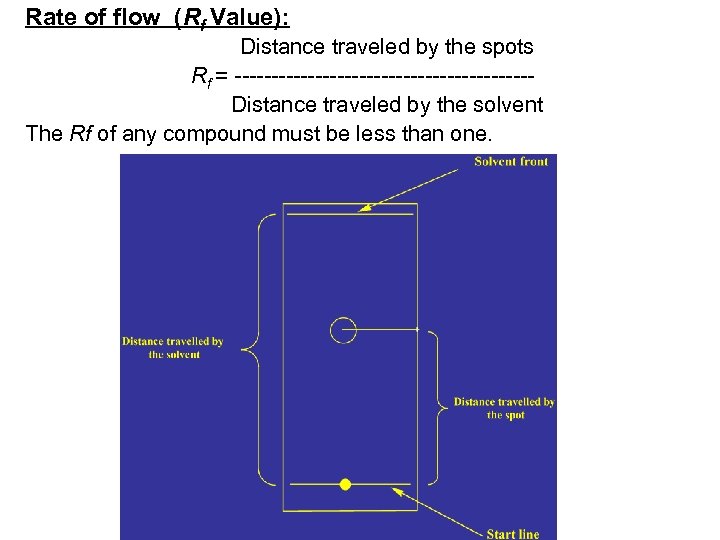 Rate of flow (Rf Value): Distance traveled by the spots Rf = --------------------Distance traveled by the solvent The Rf of any compound must be less than one.
Rate of flow (Rf Value): Distance traveled by the spots Rf = --------------------Distance traveled by the solvent The Rf of any compound must be less than one.
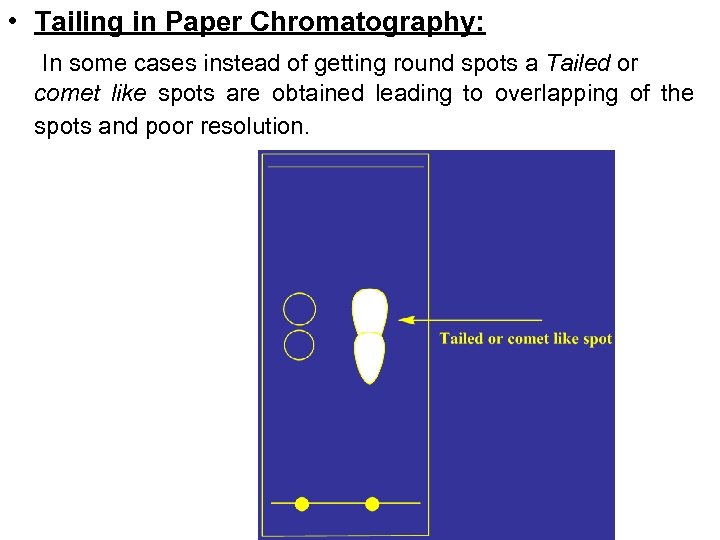 • Tailing in Paper Chromatography: In some cases instead of getting round spots a Tailed or comet like spots are obtained leading to overlapping of the spots and poor resolution.
• Tailing in Paper Chromatography: In some cases instead of getting round spots a Tailed or comet like spots are obtained leading to overlapping of the spots and poor resolution.
 Reasons and solution for tailing problem:
Reasons and solution for tailing problem:
 Application: 1 - Qualitative: Ø Identification through comparison of the Rf value with that of Reference material. Ø Determination of Complexity of mixtures. That will be indicated from number of spots. Ø Determination the purity of materials. Ø Monitoring the progress of Chemical reactions. Ø Monitoring of column chromatography. Ø Development of finger print TLC for extracts, volatile oils or pharmaceutical preparation for future identification and comparison. In this application plates 5× 5, 5× 10 cm with thin film of coating material are usually used.
Application: 1 - Qualitative: Ø Identification through comparison of the Rf value with that of Reference material. Ø Determination of Complexity of mixtures. That will be indicated from number of spots. Ø Determination the purity of materials. Ø Monitoring the progress of Chemical reactions. Ø Monitoring of column chromatography. Ø Development of finger print TLC for extracts, volatile oils or pharmaceutical preparation for future identification and comparison. In this application plates 5× 5, 5× 10 cm with thin film of coating material are usually used.
 • 2 - Quantitative: In this case an accurate volume of samples are applied using syringes. The dimensions of plates range from 5 x 10 to 20 x 20 according to the number pf spots used. The plates are developed as usual in the chromatographic tanks. After development the concentration of material can be determined by: Ø Ø Ø Spot area measurement: Which is directly proportional to the conc. of materials. Photodensitometry: Measure transmittance, reflection or fluorescence of spots. Radioactivity: For radioactive material. These measurements are done using TLC Scanner connected to computer that perform all calculations.
• 2 - Quantitative: In this case an accurate volume of samples are applied using syringes. The dimensions of plates range from 5 x 10 to 20 x 20 according to the number pf spots used. The plates are developed as usual in the chromatographic tanks. After development the concentration of material can be determined by: Ø Ø Ø Spot area measurement: Which is directly proportional to the conc. of materials. Photodensitometry: Measure transmittance, reflection or fluorescence of spots. Radioactivity: For radioactive material. These measurements are done using TLC Scanner connected to computer that perform all calculations.
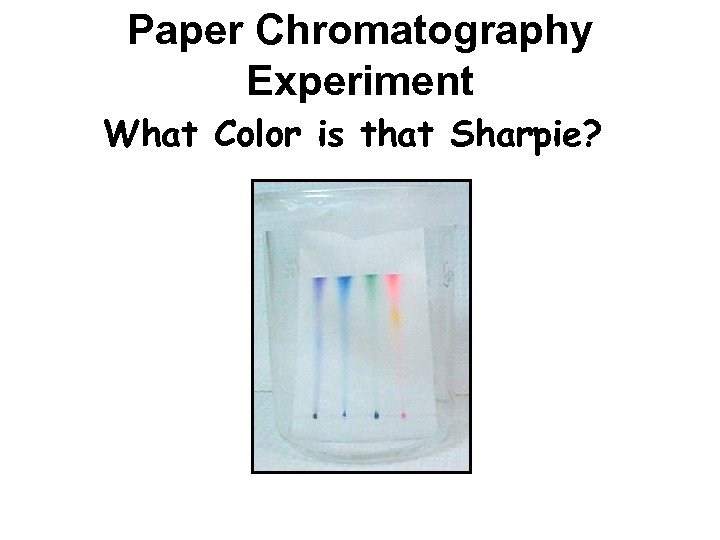 Paper Chromatography Experiment What Color is that Sharpie?
Paper Chromatography Experiment What Color is that Sharpie?
 Overview of the Experiment Purpose: To introduce students to the principles and terminology of chromatography and demonstrate separation of the dyes in Sharpie Pens with paper chromatography. Time Required: Prep. time: 10 minutes Experiment time: 45 minutes
Overview of the Experiment Purpose: To introduce students to the principles and terminology of chromatography and demonstrate separation of the dyes in Sharpie Pens with paper chromatography. Time Required: Prep. time: 10 minutes Experiment time: 45 minutes
 Materials List • • • 6 beakers or jars 6 covers or lids Distilled H 2 O Isopropanol Graduated cylinder 6 strips of filter paper Different colors of Sharpie pens Pencil Ruler Scissors Tape
Materials List • • • 6 beakers or jars 6 covers or lids Distilled H 2 O Isopropanol Graduated cylinder 6 strips of filter paper Different colors of Sharpie pens Pencil Ruler Scissors Tape
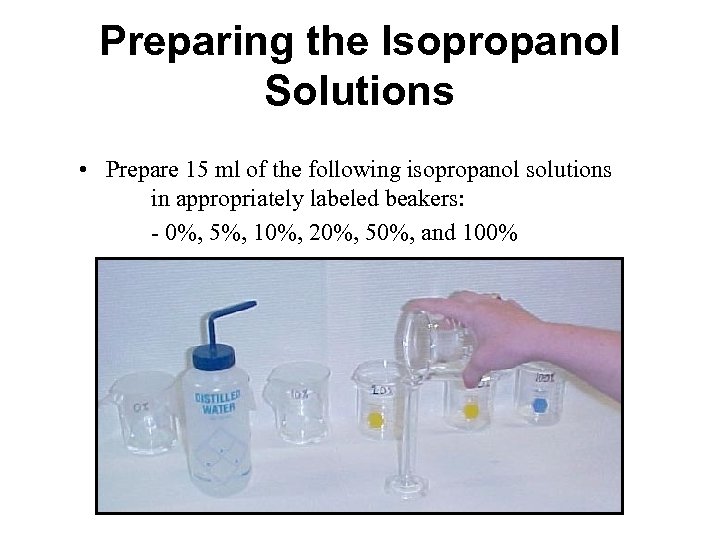 Preparing the Isopropanol Solutions • Prepare 15 ml of the following isopropanol solutions in appropriately labeled beakers: - 0%, 5%, 10%, 20%, 50%, and 100%
Preparing the Isopropanol Solutions • Prepare 15 ml of the following isopropanol solutions in appropriately labeled beakers: - 0%, 5%, 10%, 20%, 50%, and 100%
 Preparing the Chromatography Strips • Cut 6 strips of filter paper • Draw a line 1 cm above the bottom edge of the strip with the pencil • Label each strip with its corresponding solution • Place a spot from each pen on your starting line
Preparing the Chromatography Strips • Cut 6 strips of filter paper • Draw a line 1 cm above the bottom edge of the strip with the pencil • Label each strip with its corresponding solution • Place a spot from each pen on your starting line
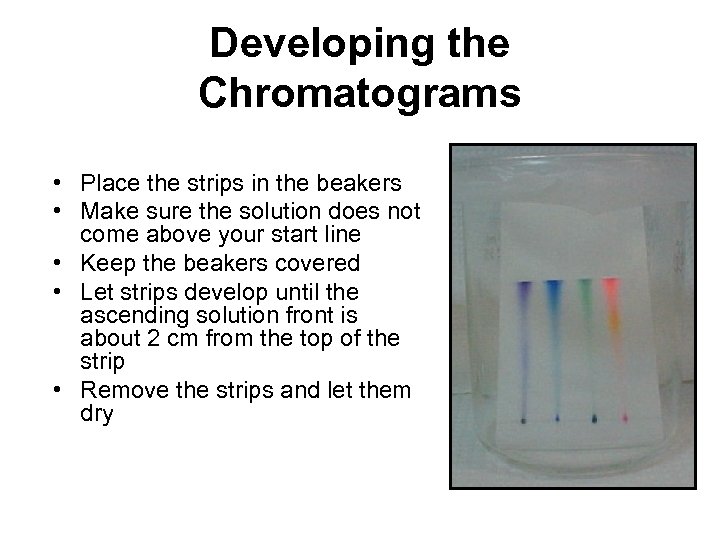 Developing the Chromatograms • Place the strips in the beakers • Make sure the solution does not come above your start line • Keep the beakers covered • Let strips develop until the ascending solution front is about 2 cm from the top of the strip • Remove the strips and let them dry
Developing the Chromatograms • Place the strips in the beakers • Make sure the solution does not come above your start line • Keep the beakers covered • Let strips develop until the ascending solution front is about 2 cm from the top of the strip • Remove the strips and let them dry
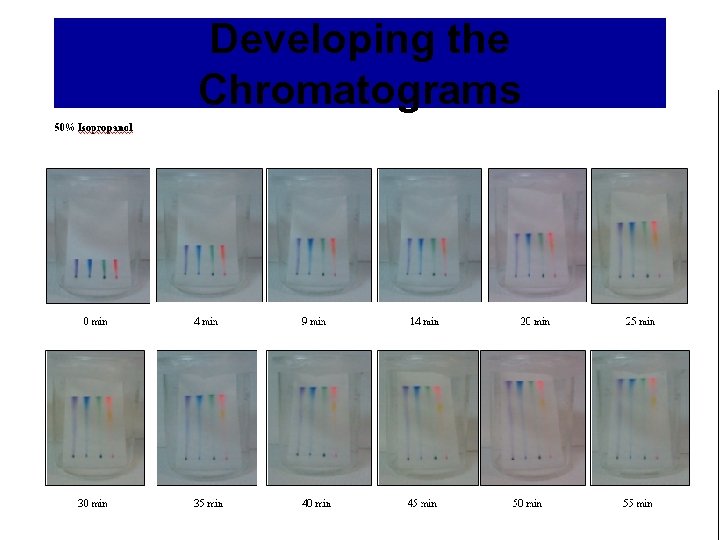 Developing the Chromatograms
Developing the Chromatograms
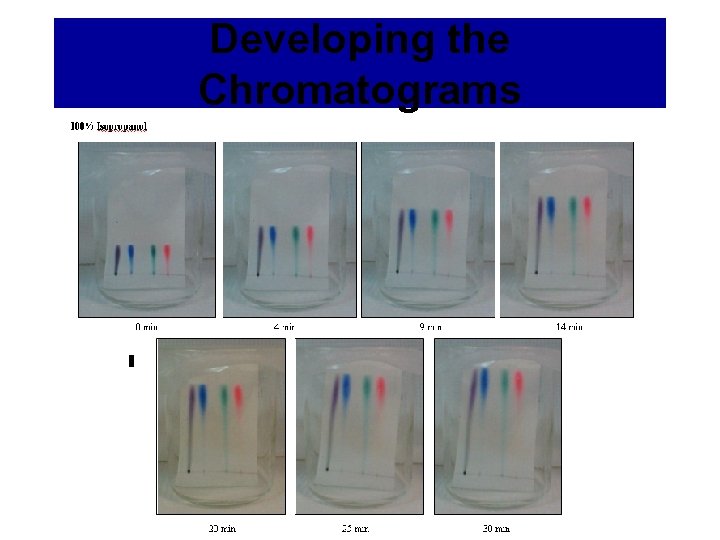 Developing the Chromatograms
Developing the Chromatograms

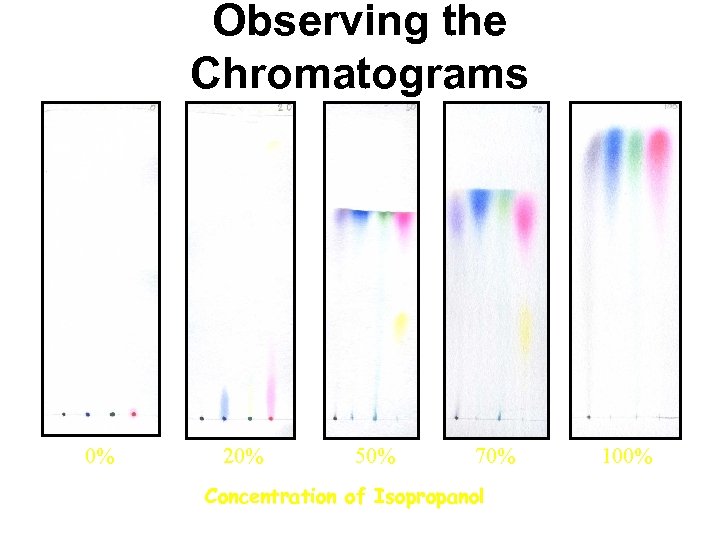 Observing the Chromatograms 0% 20% 50% 70% Concentration of Isopropanol 100%
Observing the Chromatograms 0% 20% 50% 70% Concentration of Isopropanol 100%
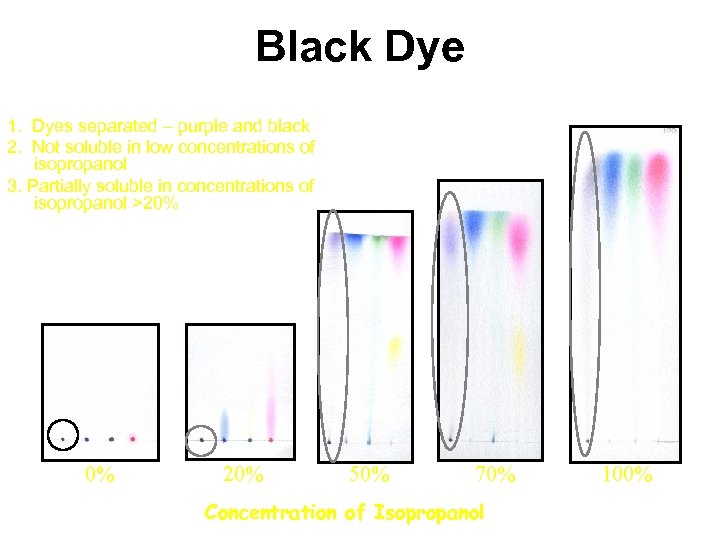 Black Dye 1. Dyes separated – purple and black 2. Not soluble in low concentrations of isopropanol 3. Partially soluble in concentrations of isopropanol >20% 0% 20% 50% 70% Concentration of Isopropanol 100%
Black Dye 1. Dyes separated – purple and black 2. Not soluble in low concentrations of isopropanol 3. Partially soluble in concentrations of isopropanol >20% 0% 20% 50% 70% Concentration of Isopropanol 100%
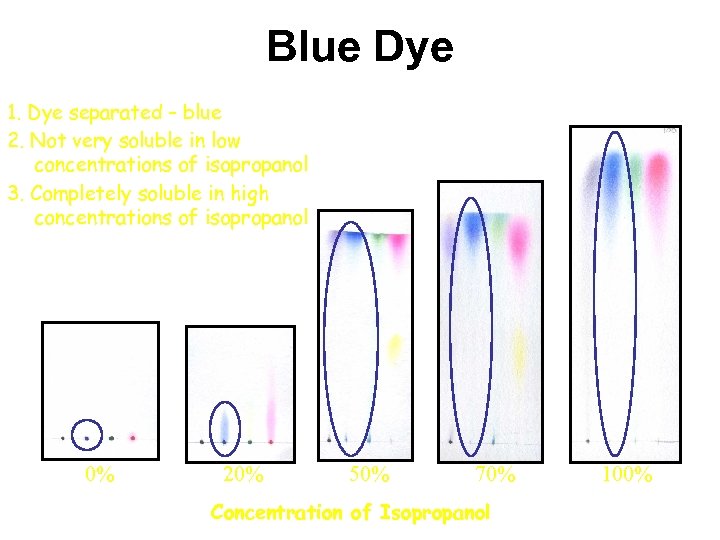 Blue Dye 1. Dye separated – blue 2. Not very soluble in low concentrations of isopropanol 3. Completely soluble in high concentrations of isopropanol 0% 20% 50% 70% Concentration of Isopropanol 100%
Blue Dye 1. Dye separated – blue 2. Not very soluble in low concentrations of isopropanol 3. Completely soluble in high concentrations of isopropanol 0% 20% 50% 70% Concentration of Isopropanol 100%
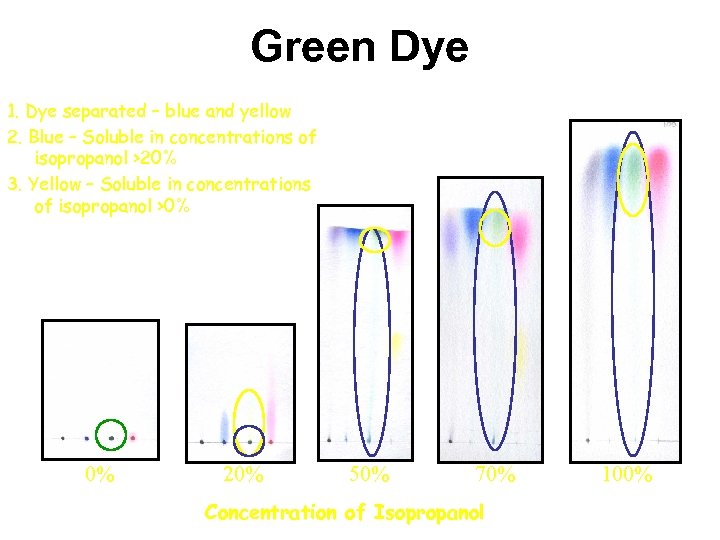 Green Dye 1. Dye separated – blue and yellow 2. Blue – Soluble in concentrations of isopropanol >20% 3. Yellow – Soluble in concentrations of isopropanol >0% 0% 20% 50% 70% Concentration of Isopropanol 100%
Green Dye 1. Dye separated – blue and yellow 2. Blue – Soluble in concentrations of isopropanol >20% 3. Yellow – Soluble in concentrations of isopropanol >0% 0% 20% 50% 70% Concentration of Isopropanol 100%
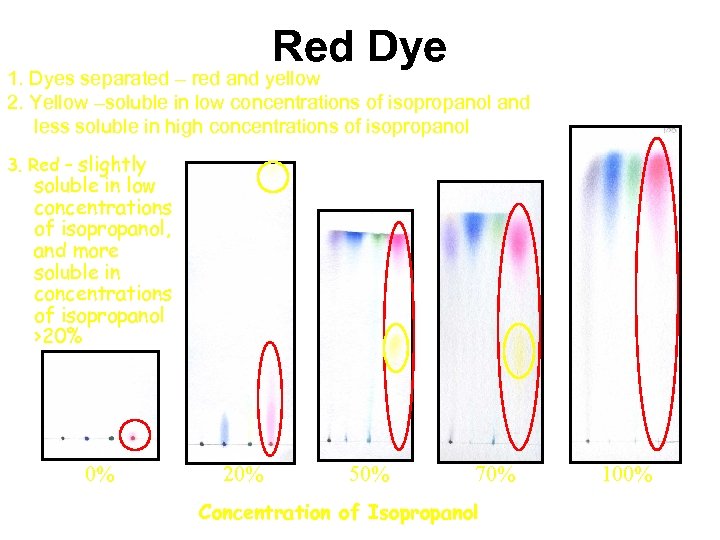 Red Dye 1. Dyes separated – red and yellow 2. Yellow –soluble in low concentrations of isopropanol and less soluble in high concentrations of isopropanol 3. Red – slightly soluble in low concentrations of isopropanol, and more soluble in concentrations of isopropanol >20% 0% 20% 50% 70% Concentration of Isopropanol 100%
Red Dye 1. Dyes separated – red and yellow 2. Yellow –soluble in low concentrations of isopropanol and less soluble in high concentrations of isopropanol 3. Red – slightly soluble in low concentrations of isopropanol, and more soluble in concentrations of isopropanol >20% 0% 20% 50% 70% Concentration of Isopropanol 100%
 Case One “Signed with a Kiss”
Case One “Signed with a Kiss”
 The Discovery… • Before third period, Marie goes to her locker to grab her chemistry book. She and Christopher have shared this locker for the last semester when they started going together • As she pushes Christopher’s geometry books aside, it falls to the floor and a note falls out. – “…can’t wait to see you again, baby. Last nite was so much fun! Call me on my cell after school today. ” • Marie was already bugged out by the words of the note, but on top of that, the girl had the nerve to sign the note with nothing but a kiss!
The Discovery… • Before third period, Marie goes to her locker to grab her chemistry book. She and Christopher have shared this locker for the last semester when they started going together • As she pushes Christopher’s geometry books aside, it falls to the floor and a note falls out. – “…can’t wait to see you again, baby. Last nite was so much fun! Call me on my cell after school today. ” • Marie was already bugged out by the words of the note, but on top of that, the girl had the nerve to sign the note with nothing but a kiss!
 The Plan • After school, Marie calls Mark for support. Between sobs, Marie explains to Mark, “I was so cold to Christopher after Chem class. I’m afraid he thinks I know about the note. ” Mark reassures her, “Naw, he has no idea. • “Okay, ” she says, “let’s continue with our plan. ” • Just make sure you get a sample from each of those girls. Oh yeah, and make sure that you have plenty of nail polish remover. I think that will be the best solvent to separate the mixtures. When I come over tomorrow, I’ll bring the coffee filters. ” • The next day, Marie and Mark meet up at her house to begin the investigation phase of their plan. – Begin lipstick chromatography lab – Paper Chromatography. ppt
The Plan • After school, Marie calls Mark for support. Between sobs, Marie explains to Mark, “I was so cold to Christopher after Chem class. I’m afraid he thinks I know about the note. ” Mark reassures her, “Naw, he has no idea. • “Okay, ” she says, “let’s continue with our plan. ” • Just make sure you get a sample from each of those girls. Oh yeah, and make sure that you have plenty of nail polish remover. I think that will be the best solvent to separate the mixtures. When I come over tomorrow, I’ll bring the coffee filters. ” • The next day, Marie and Mark meet up at her house to begin the investigation phase of their plan. – Begin lipstick chromatography lab – Paper Chromatography. ppt
 How to Catch Your Man Cheating! • Lipstick Chromatography Lab Protocol Materials: Chromatography paper or Coffee filters Scissors 3 different Lipsticks similar in shade, but different brands Acetone or Nail Polish remover with Acetone Beaker or Cup large enough for 3 strips of paper (about 500 ml) Tape Procedure: 1. The teacher has smeared samples of the lipstick from the note and each of the suspects onto filter paper. Each group will analyze one suspect or the sample from the note 3 times. 2. Carefully pour 10 ml of solvent into the beaker. 3. Place all three strips of paper into the beaker so that the paper touches the solvent, but that the level of the solvent does NOT reach the lipstick. 4. Secure the top of each paper strip to the beaker with tape if necessary to keep it from slipping. 5. After 15 minutes remove all the samples from the beaker and place the papers flat on the bench top. 6. Measure the distance the acetone traveled up each strip of paper. Also measure the distance each component moved up the paper. 7. Make a data table with average Rf value for each component in your lipstick sample.
How to Catch Your Man Cheating! • Lipstick Chromatography Lab Protocol Materials: Chromatography paper or Coffee filters Scissors 3 different Lipsticks similar in shade, but different brands Acetone or Nail Polish remover with Acetone Beaker or Cup large enough for 3 strips of paper (about 500 ml) Tape Procedure: 1. The teacher has smeared samples of the lipstick from the note and each of the suspects onto filter paper. Each group will analyze one suspect or the sample from the note 3 times. 2. Carefully pour 10 ml of solvent into the beaker. 3. Place all three strips of paper into the beaker so that the paper touches the solvent, but that the level of the solvent does NOT reach the lipstick. 4. Secure the top of each paper strip to the beaker with tape if necessary to keep it from slipping. 5. After 15 minutes remove all the samples from the beaker and place the papers flat on the bench top. 6. Measure the distance the acetone traveled up each strip of paper. Also measure the distance each component moved up the paper. 7. Make a data table with average Rf value for each component in your lipstick sample.
 Epilogue • After discovering that Christopher and her girl Muhsinah were not the friends she thought they were, Marie confronted them with the evidence. Christopher and Muhsinah were shocked at her use of chemistry to catch them. Marie recovered from the nasty breakup with Christopher with the help of Mark's comforting shoulder. . . ; ) • This scene and laboratory protocol were adapted from, "Who's Lipstick? " in Crime Scene Investigations by Pam Walker and Elaine Wood, 1998. The images of the kiss and paper chromatograpy where found at http: //www. ausetute. com. au/ chromato. html and http: //www. consumerreports. com, respectively.
Epilogue • After discovering that Christopher and her girl Muhsinah were not the friends she thought they were, Marie confronted them with the evidence. Christopher and Muhsinah were shocked at her use of chemistry to catch them. Marie recovered from the nasty breakup with Christopher with the help of Mark's comforting shoulder. . . ; ) • This scene and laboratory protocol were adapted from, "Who's Lipstick? " in Crime Scene Investigations by Pam Walker and Elaine Wood, 1998. The images of the kiss and paper chromatograpy where found at http: //www. ausetute. com. au/ chromato. html and http: //www. consumerreports. com, respectively.


