375074e5e7eaab7ba4da2e2a054e2acf.ppt
- Количество слайдов: 12
 CHONDROITIN SULFATE: CLINICAL REVIEW IN OSTEOARTHRITIS José Vergés MD, MSc, Ph. D Clinical Pharmacologist Scientific Director BIOIBERICA S. A. Barcelona, Spain J Chondroitin sulfate: clinical review in osteoarthritis
CHONDROITIN SULFATE: CLINICAL REVIEW IN OSTEOARTHRITIS José Vergés MD, MSc, Ph. D Clinical Pharmacologist Scientific Director BIOIBERICA S. A. Barcelona, Spain J Chondroitin sulfate: clinical review in osteoarthritis
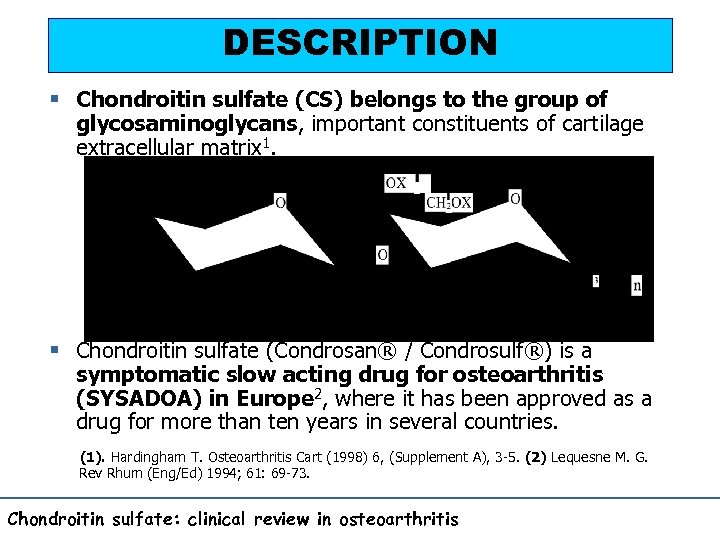 DESCRIPTION § Chondroitin sulfate (CS) belongs to the group of glycosaminoglycans, important constituents of cartilage extracellular matrix 1. § Chondroitin sulfate (Condrosan® / Condrosulf®) is a symptomatic slow acting drug for osteoarthritis (SYSADOA) in Europe 2, where it has been approved as a drug for more than ten years in several countries. (1). Hardingham T. Osteoarthritis Cart (1998) 6, (Supplement A), 3 -5. (2) Lequesne M. G. Rev Rhum (Eng/Ed) 1994; 61: 69 -73. Chondroitin sulfate: clinical review in osteoarthritis
DESCRIPTION § Chondroitin sulfate (CS) belongs to the group of glycosaminoglycans, important constituents of cartilage extracellular matrix 1. § Chondroitin sulfate (Condrosan® / Condrosulf®) is a symptomatic slow acting drug for osteoarthritis (SYSADOA) in Europe 2, where it has been approved as a drug for more than ten years in several countries. (1). Hardingham T. Osteoarthritis Cart (1998) 6, (Supplement A), 3 -5. (2) Lequesne M. G. Rev Rhum (Eng/Ed) 1994; 61: 69 -73. Chondroitin sulfate: clinical review in osteoarthritis
 CHONDROITIN SULFATE ACTION MECHANISMS 3 -4 STIMULATES: proteoglycans HA EFFECT: -anti-inflammatory activity -Membrane stabilising action INHIBITS: cartilage degradative enzymes (collagenase, elastase, proteoglycanase, fosfolipase A 2, N-acetylglucosaminidase, etc. ) cartilage damaging substances (free radicals) apoptosis NO Stromelysin (MMP-3) NF-k. B (3) Ronca F et. al. Osteoarthritis Cart (1998) 6, (Supplement A), 14 -21. (4) Blanco FJ. et. al. Rev. Esp. Reumatol 2001; 28, 1: 12 -17. Chondroitin sulfate: clinical review in osteoarthritis
CHONDROITIN SULFATE ACTION MECHANISMS 3 -4 STIMULATES: proteoglycans HA EFFECT: -anti-inflammatory activity -Membrane stabilising action INHIBITS: cartilage degradative enzymes (collagenase, elastase, proteoglycanase, fosfolipase A 2, N-acetylglucosaminidase, etc. ) cartilage damaging substances (free radicals) apoptosis NO Stromelysin (MMP-3) NF-k. B (3) Ronca F et. al. Osteoarthritis Cart (1998) 6, (Supplement A), 14 -21. (4) Blanco FJ. et. al. Rev. Esp. Reumatol 2001; 28, 1: 12 -17. Chondroitin sulfate: clinical review in osteoarthritis
 CLINICAL EVIDENCE • 9 randomized, controlled, clinical trials have been conducted in Europe with Condrosan® / Condrosulf®, comparing its effect against placebo (PBO) and sodium diclofenac (SD) (150 mg) in 1163 patients with knee and hand osteoarthritis (OA)5 -13. • The results from these clinical trials conclude that CS is as effective as SD and around 50% more effective than PBO (p < 0. 05) in the reduction of OA symptoms 5, 14, 15. Besides, its efficacy lasts for at least 3 months after treatment suppression (carry-over effect). (5) Morreale, et al. J. Rheumatol. 1996, 23: 1385 -1391. (6) Kissling R. et al. Osteoarthritis Cart 1997, 5 (Supplement A), 9: 70. (7) Bucsi L, et. al. Osteoarthritis Cart 1998, 6 (supplement A): 31 -36. (8). Pavelka K, et al. Litera Rheumatologica 1998, 24: 21 -30. (9). Uebelhart D, et. al. Osteoarthritis Cart 1998, 6, (Supplement A), 39 -46. (10). Uebelhart D, et al. Osteoarthritis Cart 2004, 12: 269 -276. (11) Michel B, et al. . Osteoarthritis Cart 2001, 9 (supplement B), LA 2. (12) Verbruggen G, et al. Osteoarthritis Cart (1998) 6, (Supplement A), 37 -38. (13) Vebruggen G. et al. Clinical Rheumatology 2002, 21: 231 -241. (14) Leeb F, et al. J. Rheumatol. 2000; 27: 1: 205 211. (15) du Souich P, Vergés J. Clin. Pharm. Ther. 2001; 70: 5 -9. Chondroitin sulfate: clinical review in osteoarthritis
CLINICAL EVIDENCE • 9 randomized, controlled, clinical trials have been conducted in Europe with Condrosan® / Condrosulf®, comparing its effect against placebo (PBO) and sodium diclofenac (SD) (150 mg) in 1163 patients with knee and hand osteoarthritis (OA)5 -13. • The results from these clinical trials conclude that CS is as effective as SD and around 50% more effective than PBO (p < 0. 05) in the reduction of OA symptoms 5, 14, 15. Besides, its efficacy lasts for at least 3 months after treatment suppression (carry-over effect). (5) Morreale, et al. J. Rheumatol. 1996, 23: 1385 -1391. (6) Kissling R. et al. Osteoarthritis Cart 1997, 5 (Supplement A), 9: 70. (7) Bucsi L, et. al. Osteoarthritis Cart 1998, 6 (supplement A): 31 -36. (8). Pavelka K, et al. Litera Rheumatologica 1998, 24: 21 -30. (9). Uebelhart D, et. al. Osteoarthritis Cart 1998, 6, (Supplement A), 39 -46. (10). Uebelhart D, et al. Osteoarthritis Cart 2004, 12: 269 -276. (11) Michel B, et al. . Osteoarthritis Cart 2001, 9 (supplement B), LA 2. (12) Verbruggen G, et al. Osteoarthritis Cart (1998) 6, (Supplement A), 37 -38. (13) Vebruggen G. et al. Clinical Rheumatology 2002, 21: 231 -241. (14) Leeb F, et al. J. Rheumatol. 2000; 27: 1: 205 211. (15) du Souich P, Vergés J. Clin. Pharm. Ther. 2001; 70: 5 -9. Chondroitin sulfate: clinical review in osteoarthritis
 S/DMOAD • CS may act as a structure disease modifying OA drug (S/DMOAD), that is, it may slow down disease progression 16. • 3 clinical trials in knee OA have evidenced a stabilization of joint space width with CS treatment as opposed to a narrowing of joint space with PBO 9 -11. • 2 clinical trials in hand OA concluded that disease progression was lower in CStreated patients and less patients from this group developed erosive OA 12 -13. (9). Uebelhart D, et. al. Osteoarthritis Cart 1998, 6, (Supplement A), 39 -46. (10). Uebelhart D, et al. Osteoarthritis Cart 2004, 12: 269 -276. (11) Michel B, et al. . Osteoarthritis Cart 2001, 9 (supplement B), LA 2. (12) Verbruggen G, et al. Osteoarthritis Cart (1998) 6, (Supplement A), 37 -38. (13) Vebruggen G. et al. Clinical Rheumatology 2002, 21: 231 -241. (16). Jordan KM, et al. Ann Rheum Dis 2003; 62: 1145 -1155 Chondroitin sulfate: clinical review in osteoarthritis
S/DMOAD • CS may act as a structure disease modifying OA drug (S/DMOAD), that is, it may slow down disease progression 16. • 3 clinical trials in knee OA have evidenced a stabilization of joint space width with CS treatment as opposed to a narrowing of joint space with PBO 9 -11. • 2 clinical trials in hand OA concluded that disease progression was lower in CStreated patients and less patients from this group developed erosive OA 12 -13. (9). Uebelhart D, et. al. Osteoarthritis Cart 1998, 6, (Supplement A), 39 -46. (10). Uebelhart D, et al. Osteoarthritis Cart 2004, 12: 269 -276. (11) Michel B, et al. . Osteoarthritis Cart 2001, 9 (supplement B), LA 2. (12) Verbruggen G, et al. Osteoarthritis Cart (1998) 6, (Supplement A), 37 -38. (13) Vebruggen G. et al. Clinical Rheumatology 2002, 21: 231 -241. (16). Jordan KM, et al. Ann Rheum Dis 2003; 62: 1145 -1155 Chondroitin sulfate: clinical review in osteoarthritis
 SAFETY PROFILE 1. The tolerance of the product is very well documented; equivalent to PBO and much higher than that of SD 14. 2. It is not metabolized cytochrome P 450. by enzymes from It can not present drug interactions at this level. 1. Pharmacosurveillance data from Europe, where no serious adverse events have been reported for more than 10 years, support the safety of the product. (14) Leeb F, et al. J. Rheumatol. 2000; 27: 1: 205 211. Chondroitin sulfate: clinical review in osteoarthritis
SAFETY PROFILE 1. The tolerance of the product is very well documented; equivalent to PBO and much higher than that of SD 14. 2. It is not metabolized cytochrome P 450. by enzymes from It can not present drug interactions at this level. 1. Pharmacosurveillance data from Europe, where no serious adverse events have been reported for more than 10 years, support the safety of the product. (14) Leeb F, et al. J. Rheumatol. 2000; 27: 1: 205 211. Chondroitin sulfate: clinical review in osteoarthritis
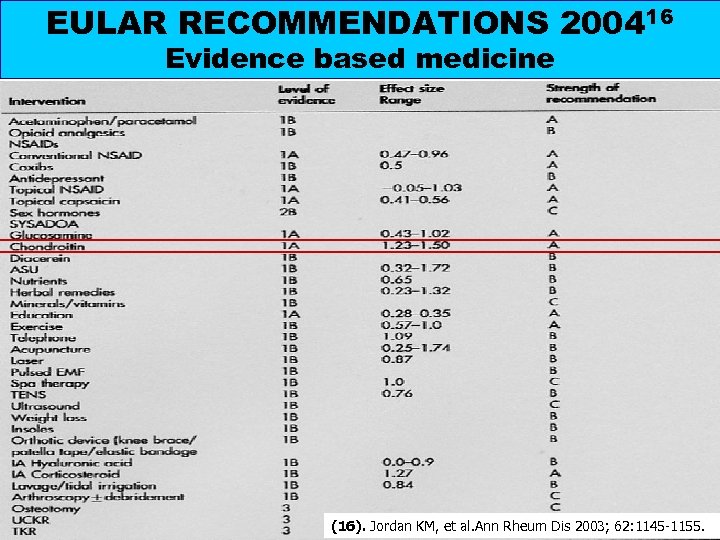 EULAR RECOMMENDATIONS 200416 Evidence based medicine Chondroitin sulfate: clinical review in(16). Jordan KM, et al. Ann Rheum Dis 2003; 62: 1145 -1155. osteoarthritis
EULAR RECOMMENDATIONS 200416 Evidence based medicine Chondroitin sulfate: clinical review in(16). Jordan KM, et al. Ann Rheum Dis 2003; 62: 1145 -1155. osteoarthritis
 CS ADVANTAGES ØClinical efficacy on symptom reduction and improvement of functional capacity ØPersistent clinical effect after treatment suppression (evidenced for at least 3 months) ØGreater safety than conventional therapy (analgesics, NSAIDs). It does not cause drug interactions. Chondroitin sulfate: clinical review in osteoarthritis
CS ADVANTAGES ØClinical efficacy on symptom reduction and improvement of functional capacity ØPersistent clinical effect after treatment suppression (evidenced for at least 3 months) ØGreater safety than conventional therapy (analgesics, NSAIDs). It does not cause drug interactions. Chondroitin sulfate: clinical review in osteoarthritis
 CLINICAL BIOEQUIVALENCE I • There is only one CS approved as a drug in several European countries, which is therefore considered as the reference product 17. • This CS is manufactured by BIOIBERICA (CSd. Bio-Active) and marketed in Europe by IBSA and BIOIBERICA; and in the U. S. A. by Nutramax Laboratories, Inc. (under the trademark Cosamin®). • This CS is being used by the NIH for its Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT). [Its IND number is: 59, 181]. (17). Vergés J. , et al. Proceeding of the Western Pharmacology Society 2004 (submitted for publication). Poster Presentation, 47 th Annual Meeting of the Western Pharmacology Society, Hawaii, 25 -29 January (poster N. W-20). Chondroitin sulfate: clinical review in osteoarthritis
CLINICAL BIOEQUIVALENCE I • There is only one CS approved as a drug in several European countries, which is therefore considered as the reference product 17. • This CS is manufactured by BIOIBERICA (CSd. Bio-Active) and marketed in Europe by IBSA and BIOIBERICA; and in the U. S. A. by Nutramax Laboratories, Inc. (under the trademark Cosamin®). • This CS is being used by the NIH for its Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT). [Its IND number is: 59, 181]. (17). Vergés J. , et al. Proceeding of the Western Pharmacology Society 2004 (submitted for publication). Poster Presentation, 47 th Annual Meeting of the Western Pharmacology Society, Hawaii, 25 -29 January (poster N. W-20). Chondroitin sulfate: clinical review in osteoarthritis
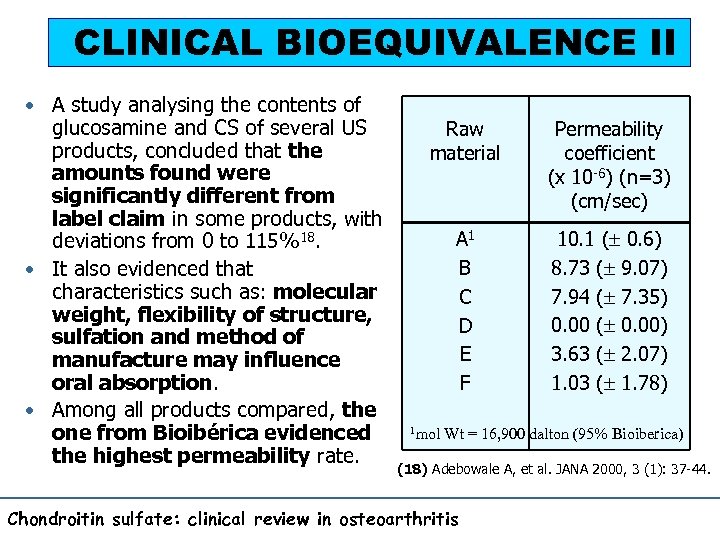 CLINICAL BIOEQUIVALENCE II • A study analysing the contents of glucosamine and CS of several US products, concluded that the amounts found were significantly different from label claim in some products, with deviations from 0 to 115%18. • It also evidenced that characteristics such as: molecular weight, flexibility of structure, sulfation and method of manufacture may influence oral absorption. • Among all products compared, the one from Bioibérica evidenced the highest permeability rate. Raw material Permeability coefficient (x 10 -6) (n=3) (cm/sec) A 1 B C D E F 10. 1 ( 0. 6) 8. 73 ( 9. 07) 7. 94 ( 7. 35) 0. 00 ( 0. 00) 3. 63 ( 2. 07) 1. 03 ( 1. 78) 1 mol Wt = 16, 900 dalton (95% Bioiberica) (18) Adebowale A, et al. JANA 2000, 3 (1): 37 -44. Chondroitin sulfate: clinical review in osteoarthritis
CLINICAL BIOEQUIVALENCE II • A study analysing the contents of glucosamine and CS of several US products, concluded that the amounts found were significantly different from label claim in some products, with deviations from 0 to 115%18. • It also evidenced that characteristics such as: molecular weight, flexibility of structure, sulfation and method of manufacture may influence oral absorption. • Among all products compared, the one from Bioibérica evidenced the highest permeability rate. Raw material Permeability coefficient (x 10 -6) (n=3) (cm/sec) A 1 B C D E F 10. 1 ( 0. 6) 8. 73 ( 9. 07) 7. 94 ( 7. 35) 0. 00 ( 0. 00) 3. 63 ( 2. 07) 1. 03 ( 1. 78) 1 mol Wt = 16, 900 dalton (95% Bioiberica) (18) Adebowale A, et al. JANA 2000, 3 (1): 37 -44. Chondroitin sulfate: clinical review in osteoarthritis
 CONCLUSION • In order to ensure equivalent clinical results in terms of efficacy and safety, other CS products must show their bioequivalence to the reference formulation 17. • For this purpose, we propose the following method to determine the bioequivalence of two CS formulations: • Only the CS (product b) presenting an equivalent clinical effect to the reference CS (product a) within a variation range of 0. 8 to 1. 2 in the a/b ratio for the Emax, T 50 and values for the 3 following parameters: Lequesne Index, Visual Analogue Scale (VAS) and pain on load, can be considered bioequivalent. (17). Vergés J. , et al. Proceeding of the Western Pharmacology Society 2004 (submitted for publication). Poster Presentation, 47 th Annual Meeting of the Western Pharmacology Society, Hawaii, 25 -29 January (poster N. W-20). Chondroitin sulfate: clinical review in osteoarthritis
CONCLUSION • In order to ensure equivalent clinical results in terms of efficacy and safety, other CS products must show their bioequivalence to the reference formulation 17. • For this purpose, we propose the following method to determine the bioequivalence of two CS formulations: • Only the CS (product b) presenting an equivalent clinical effect to the reference CS (product a) within a variation range of 0. 8 to 1. 2 in the a/b ratio for the Emax, T 50 and values for the 3 following parameters: Lequesne Index, Visual Analogue Scale (VAS) and pain on load, can be considered bioequivalent. (17). Vergés J. , et al. Proceeding of the Western Pharmacology Society 2004 (submitted for publication). Poster Presentation, 47 th Annual Meeting of the Western Pharmacology Society, Hawaii, 25 -29 January (poster N. W-20). Chondroitin sulfate: clinical review in osteoarthritis
 THANK YOU FOR YOUR ATTENTION! Chondroitin sulfate: clinical review in osteoarthritis
THANK YOU FOR YOUR ATTENTION! Chondroitin sulfate: clinical review in osteoarthritis


