c6853a9f0a239a7e56e252808ba6f88e.ppt
- Количество слайдов: 54
 CHM 1045: General Chemistry and Qualitative Analysis Unit # 3 Stoichiometry: Calculations with Chemical Formulas and Equations Dr. Jorge L. Alonso Miami-Dade College – Kendall Campus Miami, FL Textbook Reference: • Module #3 & 4 Stoichiometry
CHM 1045: General Chemistry and Qualitative Analysis Unit # 3 Stoichiometry: Calculations with Chemical Formulas and Equations Dr. Jorge L. Alonso Miami-Dade College – Kendall Campus Miami, FL Textbook Reference: • Module #3 & 4 Stoichiometry
 Chemical Reaction H 2 O(g) The actual phenomenon that occurs when chemical interact with each other. CO 2 (g) flame Methane gas is mixed with air and then it is light-up by a spark. O 2 (g) What is happening here? CH 4 (g) Stoichiometry
Chemical Reaction H 2 O(g) The actual phenomenon that occurs when chemical interact with each other. CO 2 (g) flame Methane gas is mixed with air and then it is light-up by a spark. O 2 (g) What is happening here? CH 4 (g) Stoichiometry
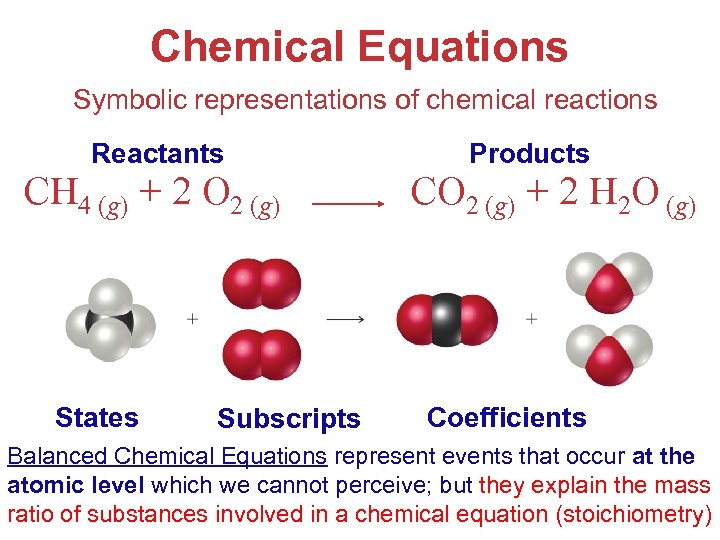 Chemical Equations Symbolic representations of chemical reactions Reactants CH 4 (g) + 2 O 2 (g) States Subscripts Products CO 2 (g) + 2 H 2 O (g) Coefficients Balanced Chemical Equations represent events that occur at the Stoichiometry atomic level which we cannot perceive; but they explain the mass ratio of substances involved in a chemical equation (stoichiometry)
Chemical Equations Symbolic representations of chemical reactions Reactants CH 4 (g) + 2 O 2 (g) States Subscripts Products CO 2 (g) + 2 H 2 O (g) Coefficients Balanced Chemical Equations represent events that occur at the Stoichiometry atomic level which we cannot perceive; but they explain the mass ratio of substances involved in a chemical equation (stoichiometry)
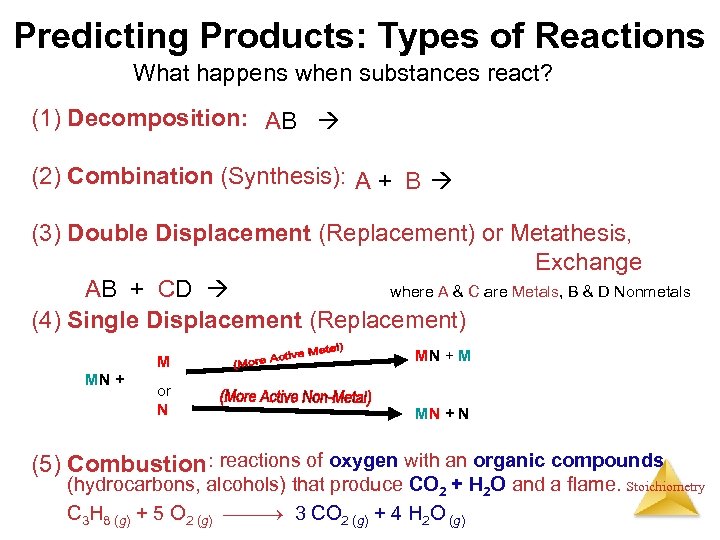 Predicting Products: Types of Reactions What happens when substances react? (1) Decomposition: AB A + B (2) Combination (Synthesis): A + B AB (3) Double Displacement (Replacement) or Metathesis, Exchange AB + CD AD + CB where A & C are Metals, B & D Nonmetals (4) Single Displacement (Replacement) M MN + M or N MN + N (5) Combustion : reactions of oxygen with an organic compounds (hydrocarbons, alcohols) that produce CO 2 + H 2 O and a flame. Stoichiometry C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g)
Predicting Products: Types of Reactions What happens when substances react? (1) Decomposition: AB A + B (2) Combination (Synthesis): A + B AB (3) Double Displacement (Replacement) or Metathesis, Exchange AB + CD AD + CB where A & C are Metals, B & D Nonmetals (4) Single Displacement (Replacement) M MN + M or N MN + N (5) Combustion : reactions of oxygen with an organic compounds (hydrocarbons, alcohols) that produce CO 2 + H 2 O and a flame. Stoichiometry C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g)
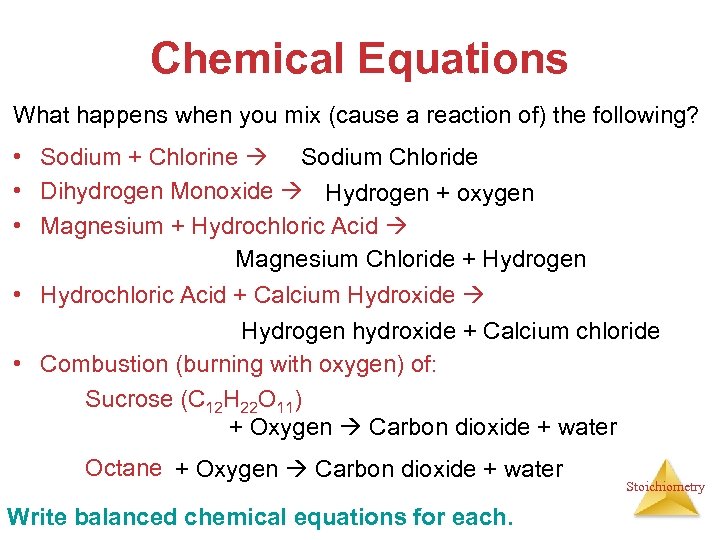 Chemical Equations What happens when you mix (cause a reaction of) the following? • Sodium + Chlorine Sodium Chloride • Dihydrogen Monoxide Hydrogen + oxygen • Magnesium + Hydrochloric Acid Magnesium Chloride + Hydrogen • Hydrochloric Acid + Calcium Hydroxide Hydrogen hydroxide + Calcium chloride • Combustion (burning with oxygen) of: Sucrose (C 12 H 22 O 11) + Oxygen Carbon dioxide + water Octane + Oxygen Carbon dioxide + water Write balanced chemical equations for each. Stoichiometry
Chemical Equations What happens when you mix (cause a reaction of) the following? • Sodium + Chlorine Sodium Chloride • Dihydrogen Monoxide Hydrogen + oxygen • Magnesium + Hydrochloric Acid Magnesium Chloride + Hydrogen • Hydrochloric Acid + Calcium Hydroxide Hydrogen hydroxide + Calcium chloride • Combustion (burning with oxygen) of: Sucrose (C 12 H 22 O 11) + Oxygen Carbon dioxide + water Octane + Oxygen Carbon dioxide + water Write balanced chemical equations for each. Stoichiometry
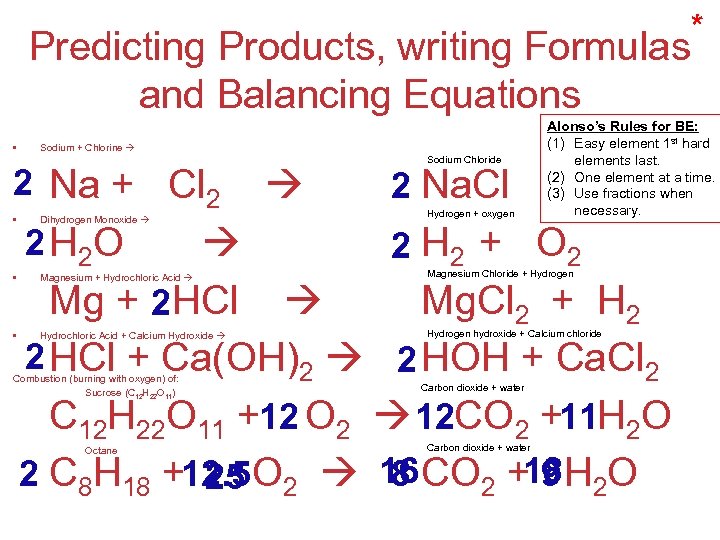 Predicting Products, writing Formulas and Balancing Equations • Sodium + Chlorine 2 Na + Cl 2 • • • Dihydrogen Monoxide 2 H 2 O Sodium Chloride 2 Na. Cl Hydrogen + oxygen * Alonso’s Rules for BE: (1) Easy element 1 st hard elements last. (2) One element at a time. (3) Use fractions when necessary. 2 H 2 + O 2 Mg + 2 HCl Mg. Cl 2 + H 2 2 HCl + Ca(OH)2 2 HOH + Ca. Cl 2 C 12 H 22 O 11 +12 O 2 12 CO 2 +11 H 2 O 2 C 8 H 18 +12. 5 O 2 16 CO 2 +18 H 2 O 8 9 25 Magnesium + Hydrochloric Acid Magnesium Chloride + Hydrogen Hydrochloric Acid + Calcium Hydroxide Hydrogen hydroxide + Calcium chloride Combustion (burning with oxygen) of: Sucrose (C 12 H 22 O 11) Octane Carbon dioxide + water Stoichiometry
Predicting Products, writing Formulas and Balancing Equations • Sodium + Chlorine 2 Na + Cl 2 • • • Dihydrogen Monoxide 2 H 2 O Sodium Chloride 2 Na. Cl Hydrogen + oxygen * Alonso’s Rules for BE: (1) Easy element 1 st hard elements last. (2) One element at a time. (3) Use fractions when necessary. 2 H 2 + O 2 Mg + 2 HCl Mg. Cl 2 + H 2 2 HCl + Ca(OH)2 2 HOH + Ca. Cl 2 C 12 H 22 O 11 +12 O 2 12 CO 2 +11 H 2 O 2 C 8 H 18 +12. 5 O 2 16 CO 2 +18 H 2 O 8 9 25 Magnesium + Hydrochloric Acid Magnesium Chloride + Hydrogen Hydrochloric Acid + Calcium Hydroxide Hydrogen hydroxide + Calcium chloride Combustion (burning with oxygen) of: Sucrose (C 12 H 22 O 11) Octane Carbon dioxide + water Stoichiometry
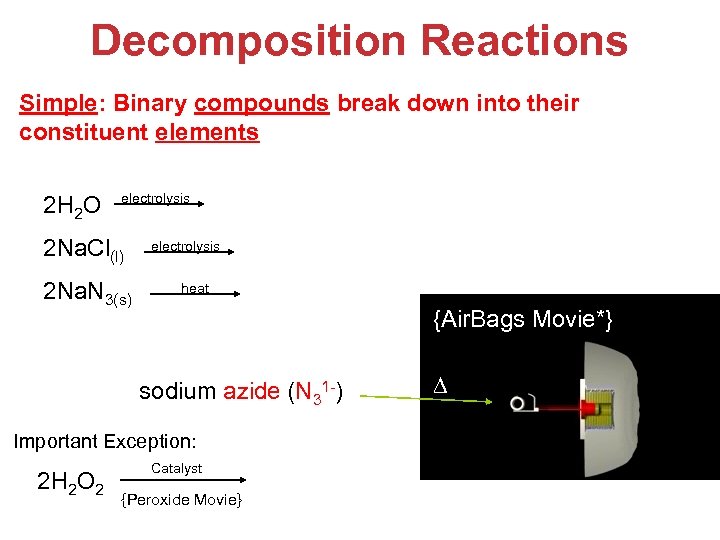 Decomposition Reactions Simple: Binary compounds break down into their constituent elements 2 H 2 O electrolysis 2 Na. Cl(l) 2 Na. N 3(s) 2 H 2 + O 2 electrolysis heat 2 Na (l) + Cl 2(g) 2 Na(s) + 3 N 2(g) sodium azide (N 31 -) {Air. Bags Movie*} ∆ Important Exception: 2 H 2 O 2 Catalyst {Peroxide Movie} 2 H 2 O + O 2 Stoichiometry
Decomposition Reactions Simple: Binary compounds break down into their constituent elements 2 H 2 O electrolysis 2 Na. Cl(l) 2 Na. N 3(s) 2 H 2 + O 2 electrolysis heat 2 Na (l) + Cl 2(g) 2 Na(s) + 3 N 2(g) sodium azide (N 31 -) {Air. Bags Movie*} ∆ Important Exception: 2 H 2 O 2 Catalyst {Peroxide Movie} 2 H 2 O + O 2 Stoichiometry
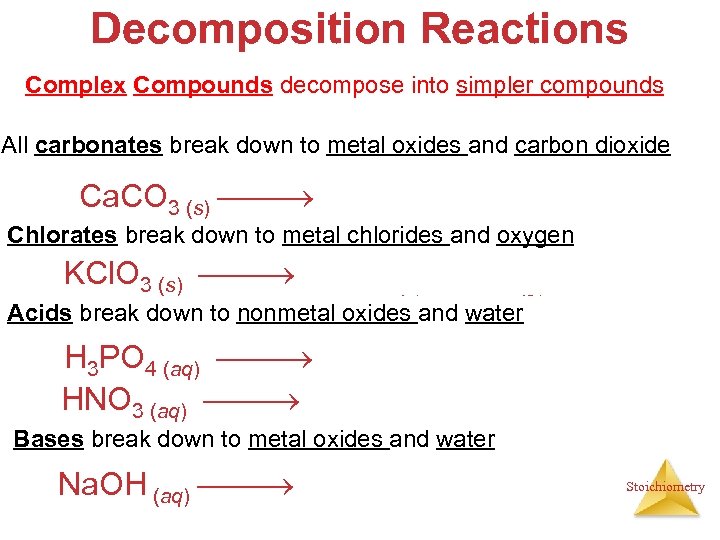 Decomposition Reactions Complex Compounds decompose into simpler compounds All carbonates break down to metal oxides and carbon dioxide Ca. CO 3 (s) Ca. O (s) + CO 2 (g) Chlorates break down to metal chlorides and oxygen 2 KCl. O 3 (s) 2 KCl (s) + 3 O 2 (g) ∆ Acids break down to nonmetal oxides and water 2 H 3 PO 4 (aq) P 2 O 5(g) + 3 H 2 O (l) 2 HNO 3 (aq) N 2 O 5(g) + H 2 O (l) Bases break down to metal oxides and water 2 Na. OH (aq) Na 2 O (s) + H 2 O (l) Stoichiometry
Decomposition Reactions Complex Compounds decompose into simpler compounds All carbonates break down to metal oxides and carbon dioxide Ca. CO 3 (s) Ca. O (s) + CO 2 (g) Chlorates break down to metal chlorides and oxygen 2 KCl. O 3 (s) 2 KCl (s) + 3 O 2 (g) ∆ Acids break down to nonmetal oxides and water 2 H 3 PO 4 (aq) P 2 O 5(g) + 3 H 2 O (l) 2 HNO 3 (aq) N 2 O 5(g) + H 2 O (l) Bases break down to metal oxides and water 2 Na. OH (aq) Na 2 O (s) + H 2 O (l) Stoichiometry
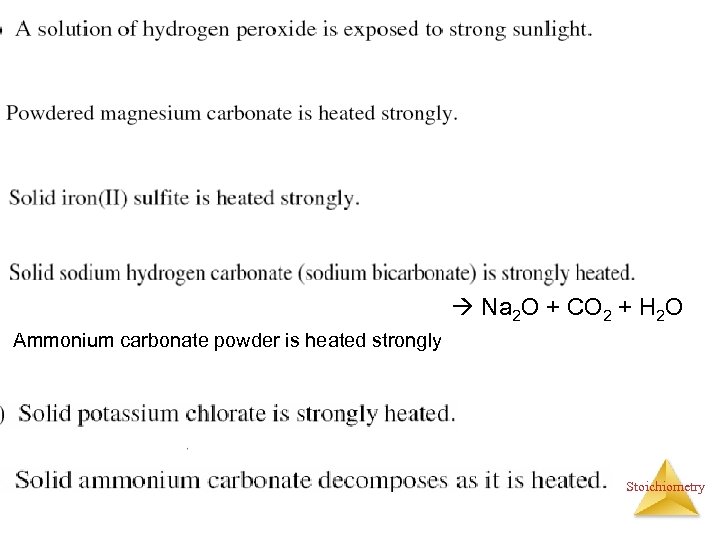 Na 2 O + CO 2 + H 2 O Ammonium carbonate powder is heated strongly Stoichiometry
Na 2 O + CO 2 + H 2 O Ammonium carbonate powder is heated strongly Stoichiometry
 Stoichiometry
Stoichiometry
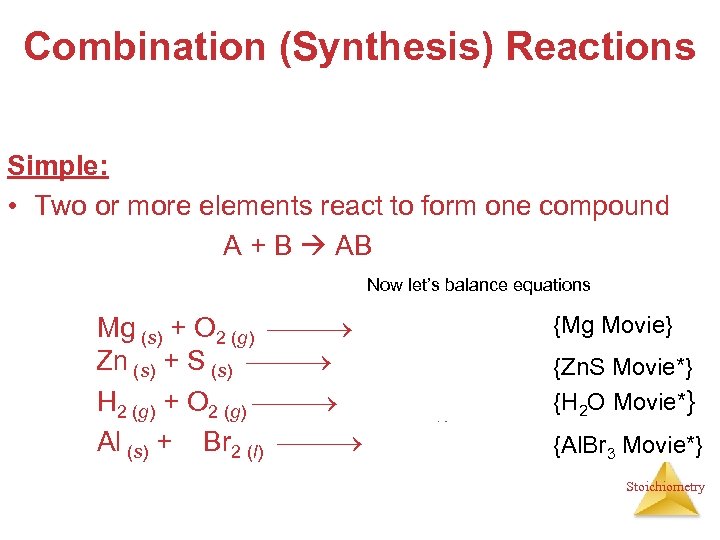 Combination (Synthesis) Reactions Simple: • Two or more elements react to form one compound A + B AB Now let’s balance equations 2 Mg (s) + O 2 (g) 2 Mg. O (s) Zn (s) + S (s) Zn. S (s) 2 H 2 (g) + O 2 (g) 2 H 2 O (l) 2 Al (s) + 3 Br 2 (l) 2 Al. Br 3 (s) {Mg Movie} {Zn. S Movie*} {H 2 O Movie*} {Al. Br 3 Movie*} Stoichiometry
Combination (Synthesis) Reactions Simple: • Two or more elements react to form one compound A + B AB Now let’s balance equations 2 Mg (s) + O 2 (g) 2 Mg. O (s) Zn (s) + S (s) Zn. S (s) 2 H 2 (g) + O 2 (g) 2 H 2 O (l) 2 Al (s) + 3 Br 2 (l) 2 Al. Br 3 (s) {Mg Movie} {Zn. S Movie*} {H 2 O Movie*} {Al. Br 3 Movie*} Stoichiometry
 Bromine liquid is poured over aluminum metal Hydrogen chloride and ammonia gas are mixed together. Sulfur dioxide gas is bubbled into water. Stoichiometry
Bromine liquid is poured over aluminum metal Hydrogen chloride and ammonia gas are mixed together. Sulfur dioxide gas is bubbled into water. Stoichiometry
 Stoichiometry
Stoichiometry
 Metathesis (Double Displacement) AB + CD AD + CB where A & C are Metals, B & D Nonmetals • Involve two Compounds • Elements (or polyatiomic groups) in the two compounds exchange partners Example: what quantity of Baking Soda will react with 100 m. L of vinegar? Na. HCO 3 (s) + HC 2 H 3 O 2 (l) Na. C 2 H 3 O 2 (aq) + HHCO 3 (aq) H 2 O (l) + CO 2 (g) (2 nd Rx decomposition) Stoichiometry {Movie: Bicarb + Vineg with Stoichio&Limit. Reag *}
Metathesis (Double Displacement) AB + CD AD + CB where A & C are Metals, B & D Nonmetals • Involve two Compounds • Elements (or polyatiomic groups) in the two compounds exchange partners Example: what quantity of Baking Soda will react with 100 m. L of vinegar? Na. HCO 3 (s) + HC 2 H 3 O 2 (l) Na. C 2 H 3 O 2 (aq) + HHCO 3 (aq) H 2 O (l) + CO 2 (g) (2 nd Rx decomposition) Stoichiometry {Movie: Bicarb + Vineg with Stoichio&Limit. Reag *}
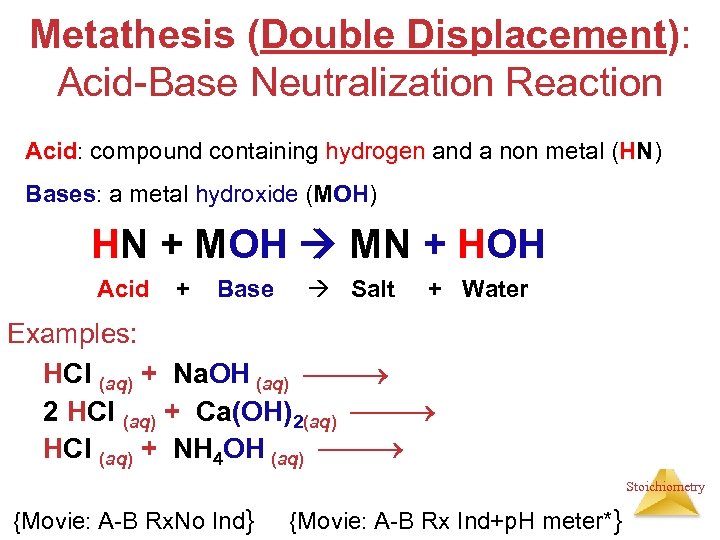 Metathesis (Double Displacement): Acid-Base Neutralization Reaction Acid: compound containing hydrogen and a non metal (HN) Bases: a metal hydroxide (MOH) HN + MOH MN + HOH Acid + Base Salt + Water Examples: HCl (aq) + Na. OH (aq) Na. Cl (aq) + HOH (l) 2 HCl (aq) + Ca(OH)2(aq) Ca. Cl 2 (aq) + 2 HOH (l) HCl (aq) + NH 4 OH (aq) NH 4 Cl (aq) + 2 HOH (l) Stoichiometry {Movie: A-B Rx. No Ind} {Movie: A-B Rx Ind+p. H meter*}
Metathesis (Double Displacement): Acid-Base Neutralization Reaction Acid: compound containing hydrogen and a non metal (HN) Bases: a metal hydroxide (MOH) HN + MOH MN + HOH Acid + Base Salt + Water Examples: HCl (aq) + Na. OH (aq) Na. Cl (aq) + HOH (l) 2 HCl (aq) + Ca(OH)2(aq) Ca. Cl 2 (aq) + 2 HOH (l) HCl (aq) + NH 4 OH (aq) NH 4 Cl (aq) + 2 HOH (l) Stoichiometry {Movie: A-B Rx. No Ind} {Movie: A-B Rx Ind+p. H meter*}
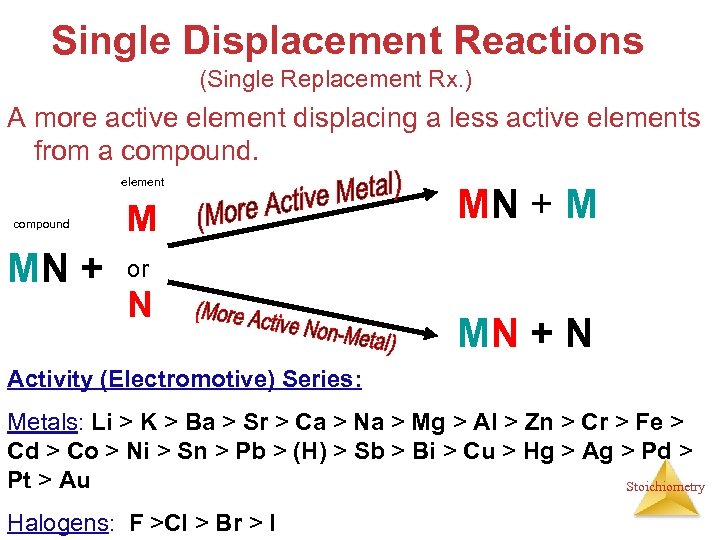 Single Displacement Reactions (Single Replacement Rx. ) A more active element displacing a less active elements from a compound. element compound MN + M or N MN + N Activity (Electromotive) Series: Metals: Li > K > Ba > Sr > Ca > Na > Mg > Al > Zn > Cr > Fe > Cd > Co > Ni > Sn > Pb > (H) > Sb > Bi > Cu > Hg > Ag > Pd > Pt > Au Stoichiometry Halogens: F >Cl > Br > I
Single Displacement Reactions (Single Replacement Rx. ) A more active element displacing a less active elements from a compound. element compound MN + M or N MN + N Activity (Electromotive) Series: Metals: Li > K > Ba > Sr > Ca > Na > Mg > Al > Zn > Cr > Fe > Cd > Co > Ni > Sn > Pb > (H) > Sb > Bi > Cu > Hg > Ag > Pd > Pt > Au Stoichiometry Halogens: F >Cl > Br > I
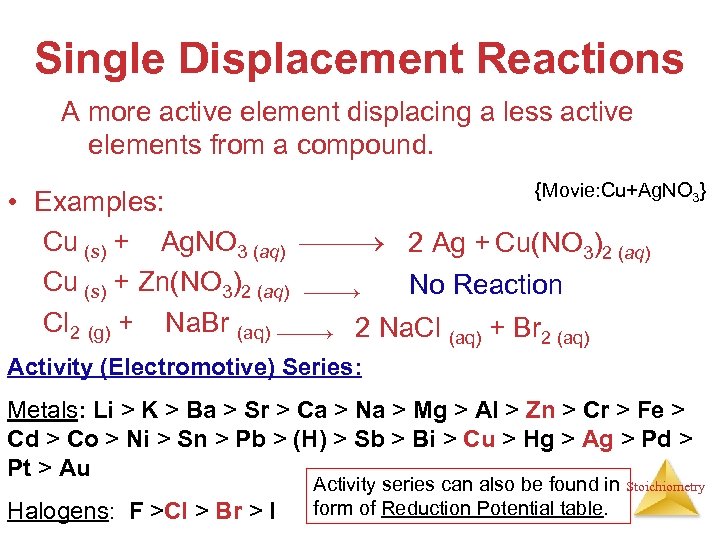 Single Displacement Reactions A more active element displacing a less active elements from a compound. {Movie: Cu+Ag. NO 3} • Examples: Cu (s) + 2 Ag. NO 3 (aq) 2 Ag + Cu(NO 3)2 (aq) Cu (s) + Zn(NO 3)2 (aq) No Reaction Cl 2 (g) + 2 Na. Br (aq) 2 Na. Cl (aq) + Br 2 (aq) Activity (Electromotive) Series: Metals: Li > K > Ba > Sr > Ca > Na > Mg > Al > Zn > Cr > Fe > Cd > Co > Ni > Sn > Pb > (H) > Sb > Bi > Cu > Hg > Ag > Pd > Pt > Au Halogens: F >Cl > Br > I Activity series can also be found in form of Reduction Potential table. Stoichiometry
Single Displacement Reactions A more active element displacing a less active elements from a compound. {Movie: Cu+Ag. NO 3} • Examples: Cu (s) + 2 Ag. NO 3 (aq) 2 Ag + Cu(NO 3)2 (aq) Cu (s) + Zn(NO 3)2 (aq) No Reaction Cl 2 (g) + 2 Na. Br (aq) 2 Na. Cl (aq) + Br 2 (aq) Activity (Electromotive) Series: Metals: Li > K > Ba > Sr > Ca > Na > Mg > Al > Zn > Cr > Fe > Cd > Co > Ni > Sn > Pb > (H) > Sb > Bi > Cu > Hg > Ag > Pd > Pt > Au Halogens: F >Cl > Br > I Activity series can also be found in form of Reduction Potential table. Stoichiometry
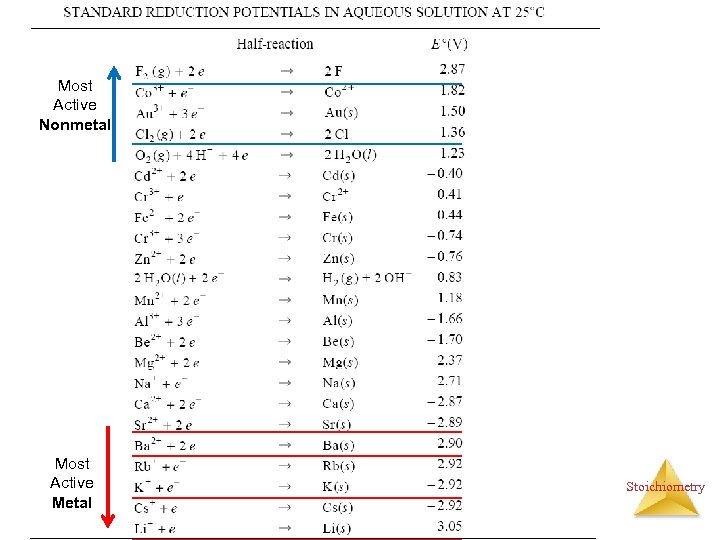 Most Active Nonmetal Most Active Metal Stoichiometry
Most Active Nonmetal Most Active Metal Stoichiometry
 H+OH- Stoichiometry
H+OH- Stoichiometry
 Reactions with Oxygen What is the difference? Oxidation Rx. (1) Oxidation Reactions: are combination reactions involving oxygen. {Movie: Mg, Fe, P, S + conc. O 2 {Metal Oxides*} Combustion Rx (2) Combustion Reaction: Rapid reactions of oxygen with an organic compounds (hydrocarbons, alcohols) that produce CO 2 + H 2 O and a flame. Examples: CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g) Stoichiometry {Movie: CH 3 OH + O 2*}
Reactions with Oxygen What is the difference? Oxidation Rx. (1) Oxidation Reactions: are combination reactions involving oxygen. {Movie: Mg, Fe, P, S + conc. O 2 {Metal Oxides*} Combustion Rx (2) Combustion Reaction: Rapid reactions of oxygen with an organic compounds (hydrocarbons, alcohols) that produce CO 2 + H 2 O and a flame. Examples: CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 O (g) C 3 H 8 (g) + 5 O 2 (g) 3 CO 2 (g) + 4 H 2 O (g) Stoichiometry {Movie: CH 3 OH + O 2*}
 When oxygen is scarce…. * Stoichiometry
When oxygen is scarce…. * Stoichiometry
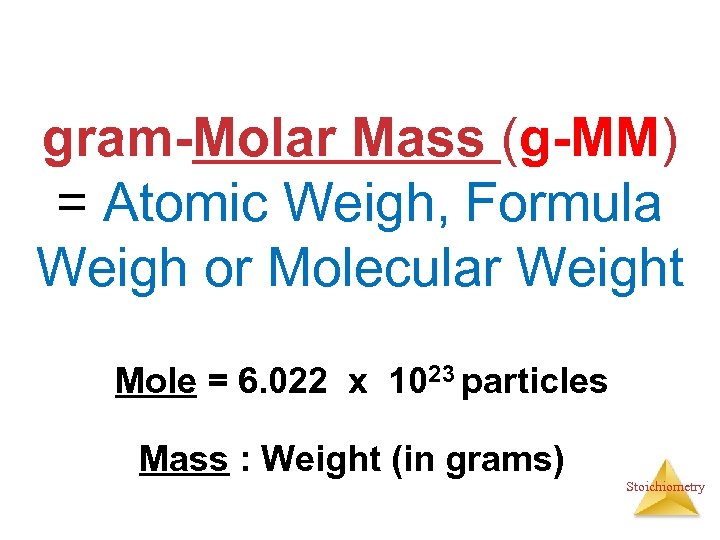 gram-Molar Mass (g-MM) = Atomic Weigh, Formula Weigh or Molecular Weight Mole = 6. 022 x 1023 particles Mass : Weight (in grams) Stoichiometry
gram-Molar Mass (g-MM) = Atomic Weigh, Formula Weigh or Molecular Weight Mole = 6. 022 x 1023 particles Mass : Weight (in grams) Stoichiometry
 gram-Molar Mass (g-MM): AW, FW, MW * the mass in grams of 1 mole of a substance (units= g/mol) For an element we find it on the periodic table. For compounds the same as the formula & molecular weight (but in g/mol) Example: the g-MM of Al 2(SO 4)3, would be 2 Al: 2 x(26. 98 amu) = 53. 96 + 3 S: 3 x(32. 06 amu) = 96. 18 +3 x 4 O: 12 x(16. 00 amu) =192. 00 Stoichiometry 342. 14 amu (g/mol)
gram-Molar Mass (g-MM): AW, FW, MW * the mass in grams of 1 mole of a substance (units= g/mol) For an element we find it on the periodic table. For compounds the same as the formula & molecular weight (but in g/mol) Example: the g-MM of Al 2(SO 4)3, would be 2 Al: 2 x(26. 98 amu) = 53. 96 + 3 S: 3 x(32. 06 amu) = 96. 18 +3 x 4 O: 12 x(16. 00 amu) =192. 00 Stoichiometry 342. 14 amu (g/mol)
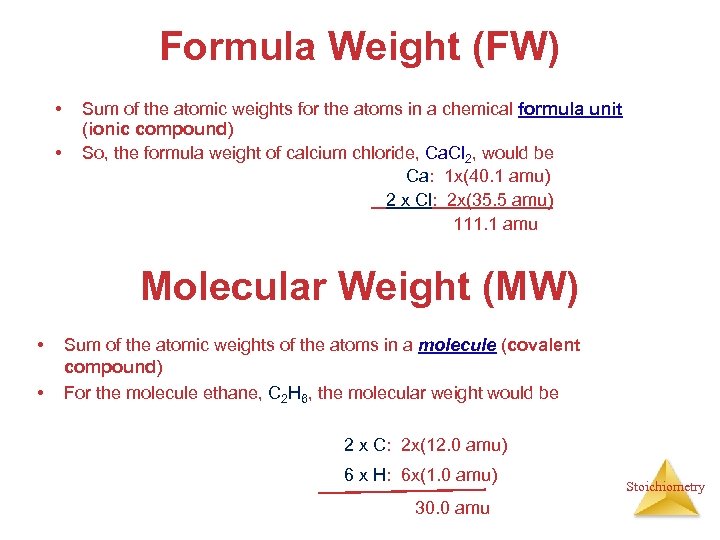 Formula Weight (FW) • • Sum of the atomic weights for the atoms in a chemical formula unit (ionic compound) So, the formula weight of calcium chloride, Ca. Cl 2, would be Ca: 1 x(40. 1 amu) 2 x Cl: 2 x(35. 5 amu) 111. 1 amu Molecular Weight (MW) • • Sum of the atomic weights of the atoms in a molecule (covalent compound) For the molecule ethane, C 2 H 6, the molecular weight would be 2 x C: 2 x(12. 0 amu) 6 x H: 6 x(1. 0 amu) 30. 0 amu Stoichiometry
Formula Weight (FW) • • Sum of the atomic weights for the atoms in a chemical formula unit (ionic compound) So, the formula weight of calcium chloride, Ca. Cl 2, would be Ca: 1 x(40. 1 amu) 2 x Cl: 2 x(35. 5 amu) 111. 1 amu Molecular Weight (MW) • • Sum of the atomic weights of the atoms in a molecule (covalent compound) For the molecule ethane, C 2 H 6, the molecular weight would be 2 x C: 2 x(12. 0 amu) 6 x H: 6 x(1. 0 amu) 30. 0 amu Stoichiometry
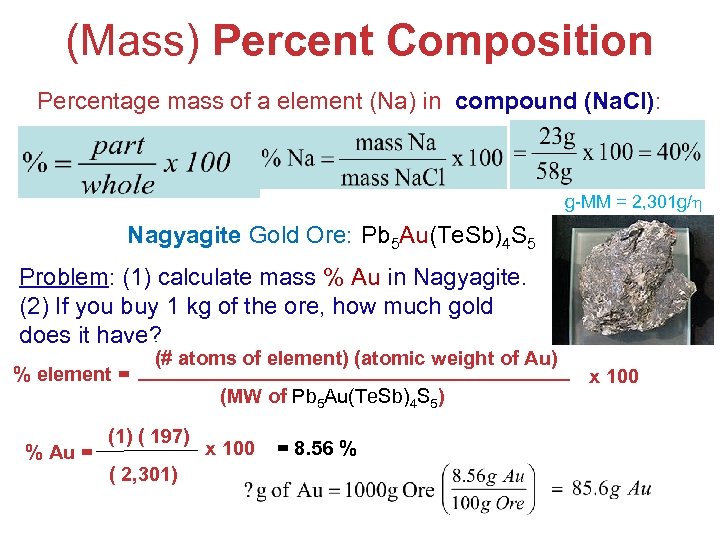 (Mass) Percent Composition Percentage mass of a element (Na) in compound (Na. Cl): g-MM = 2, 301 g/ Nagyagite Gold Ore: Pb 5 Au(Te. Sb)4 S 5 Problem: (1) calculate mass % Au in Nagyagite. (2) If you buy 1 kg of the ore, how much gold does it have? % element = % Au = (# atoms of element) (atomic weight of Au) (1) ( 197) ( 2, 301) (MW of Pb 5 Au(Te. Sb)4 S 5) x 100 = 8. 56 % Stoichiometry
(Mass) Percent Composition Percentage mass of a element (Na) in compound (Na. Cl): g-MM = 2, 301 g/ Nagyagite Gold Ore: Pb 5 Au(Te. Sb)4 S 5 Problem: (1) calculate mass % Au in Nagyagite. (2) If you buy 1 kg of the ore, how much gold does it have? % element = % Au = (# atoms of element) (atomic weight of Au) (1) ( 197) ( 2, 301) (MW of Pb 5 Au(Te. Sb)4 S 5) x 100 = 8. 56 % Stoichiometry
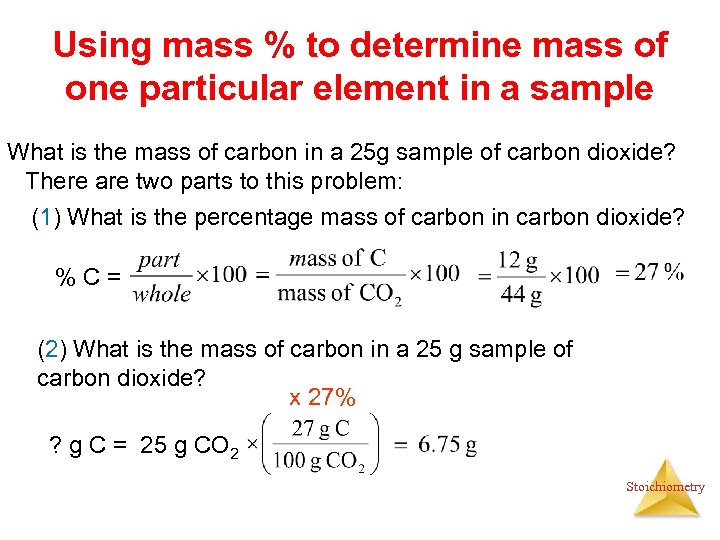 Using mass % to determine mass of one particular element in a sample What is the mass of carbon in a 25 g sample of carbon dioxide? There are two parts to this problem: (1) What is the percentage mass of carbon in carbon dioxide? %C= (2) What is the mass of carbon in a 25 g sample of carbon dioxide? x 27% ? g C = 25 g CO 2 Stoichiometry
Using mass % to determine mass of one particular element in a sample What is the mass of carbon in a 25 g sample of carbon dioxide? There are two parts to this problem: (1) What is the percentage mass of carbon in carbon dioxide? %C= (2) What is the mass of carbon in a 25 g sample of carbon dioxide? x 27% ? g C = 25 g CO 2 Stoichiometry
 The Mole Concept Dermatological Chemical Biological Avogadro's Number: 6. 022, 141, 410, 704, 090, 840, 990, 72 x 1023 602, 214, 141, 070, 409, 084, 099, 072. sextillion pentillion quadrillion trillion billion 602 sextillion million thousand Stoichiometry
The Mole Concept Dermatological Chemical Biological Avogadro's Number: 6. 022, 141, 410, 704, 090, 840, 990, 72 x 1023 602, 214, 141, 070, 409, 084, 099, 072. sextillion pentillion quadrillion trillion billion 602 sextillion million thousand Stoichiometry
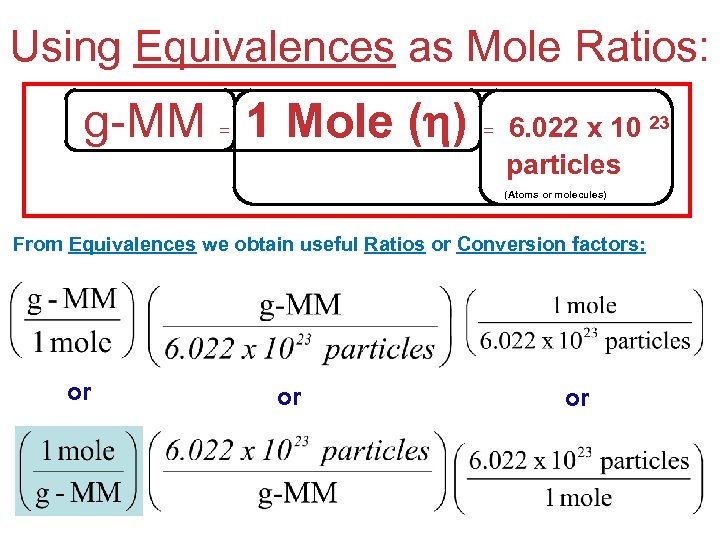 Using Equivalences as Mole Ratios: g-MM 1 Mole ( ) = Na. Cl = 58 g/η = 6. 022 x 10 23 particles (Atoms or molecules) From Equivalences we obtain useful Ratios or Conversion factors: or or or Stoichiometry
Using Equivalences as Mole Ratios: g-MM 1 Mole ( ) = Na. Cl = 58 g/η = 6. 022 x 10 23 particles (Atoms or molecules) From Equivalences we obtain useful Ratios or Conversion factors: or or or Stoichiometry
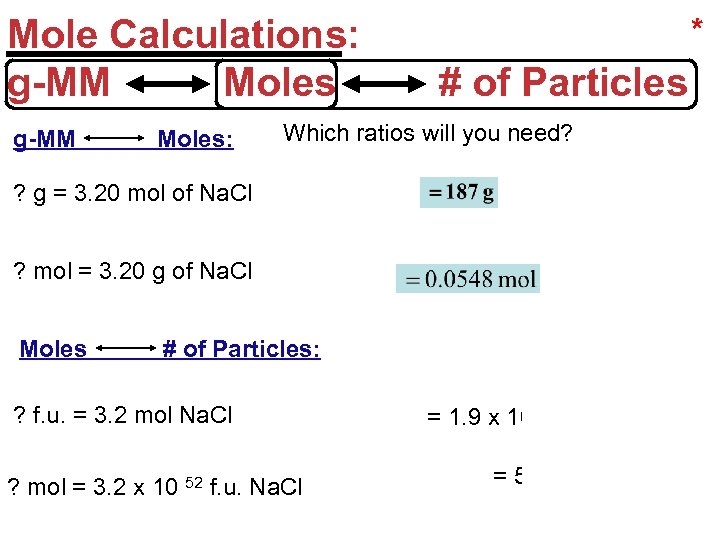 Mole Calculations: g-MM Moles: * # of Particles Which ratios will you need? ? g = 3. 20 mol of Na. Cl or ? mol = 3. 20 g of Na. Cl Moles # of Particles: ? f. u. = 3. 2 mol Na. Cl ? mol = 3. 2 x 10 52 f. u. Na. Cl = 1. 9 x 10 24 f. u. or = 5. 3 x 10 28 Stoichiometry mol
Mole Calculations: g-MM Moles: * # of Particles Which ratios will you need? ? g = 3. 20 mol of Na. Cl or ? mol = 3. 20 g of Na. Cl Moles # of Particles: ? f. u. = 3. 2 mol Na. Cl ? mol = 3. 2 x 10 52 f. u. Na. Cl = 1. 9 x 10 24 f. u. or = 5. 3 x 10 28 Stoichiometry mol
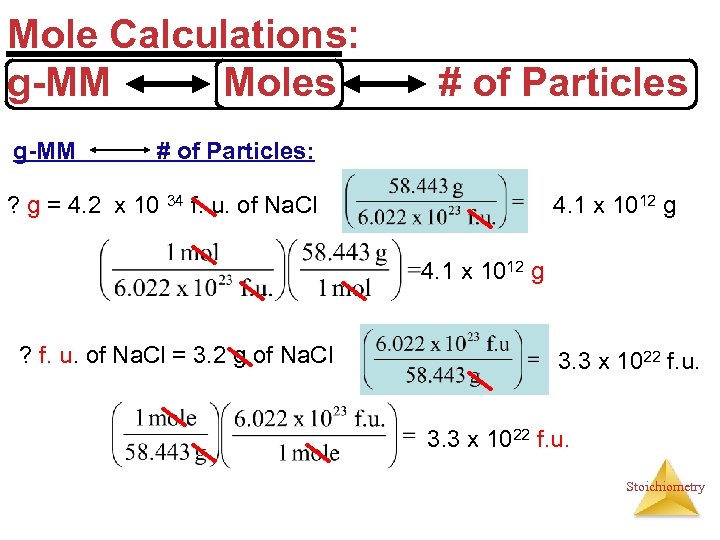 Mole Calculations: g-MM Moles g-MM # of Particles: ? g = 4. 2 x 10 34 f. u. of Na. Cl 4. 1 x 1012 g ? f. u. of Na. Cl = 3. 2 g of Na. Cl 3. 3 x 1022 f. u. Stoichiometry
Mole Calculations: g-MM Moles g-MM # of Particles: ? g = 4. 2 x 10 34 f. u. of Na. Cl 4. 1 x 1012 g ? f. u. of Na. Cl = 3. 2 g of Na. Cl 3. 3 x 1022 f. u. Stoichiometry
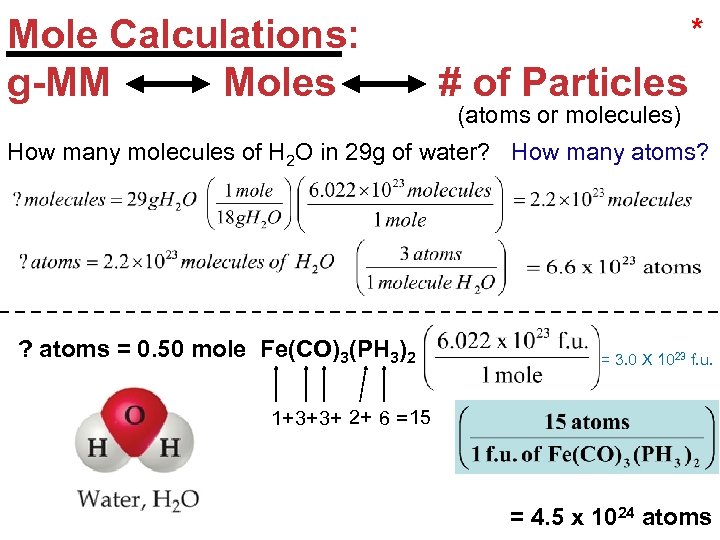 Mole Calculations: g-MM Moles * # of Particles (atoms or molecules) How many molecules of H 2 O in 29 g of water? How many atoms? ? atoms = 0. 50 mole Fe(CO)3(PH 3)2 = 3. 0 X 1023 f. u. 1+3+3+ 2+ 6 = 15 Stoichiometry = 4. 5 x 1024 atoms
Mole Calculations: g-MM Moles * # of Particles (atoms or molecules) How many molecules of H 2 O in 29 g of water? How many atoms? ? atoms = 0. 50 mole Fe(CO)3(PH 3)2 = 3. 0 X 1023 f. u. 1+3+3+ 2+ 6 = 15 Stoichiometry = 4. 5 x 1024 atoms
 The Determination of Empirical Formulas of Compounds by Elemental Analysis C x. H y Combustion furnace Stoichiometry
The Determination of Empirical Formulas of Compounds by Elemental Analysis C x. H y Combustion furnace Stoichiometry
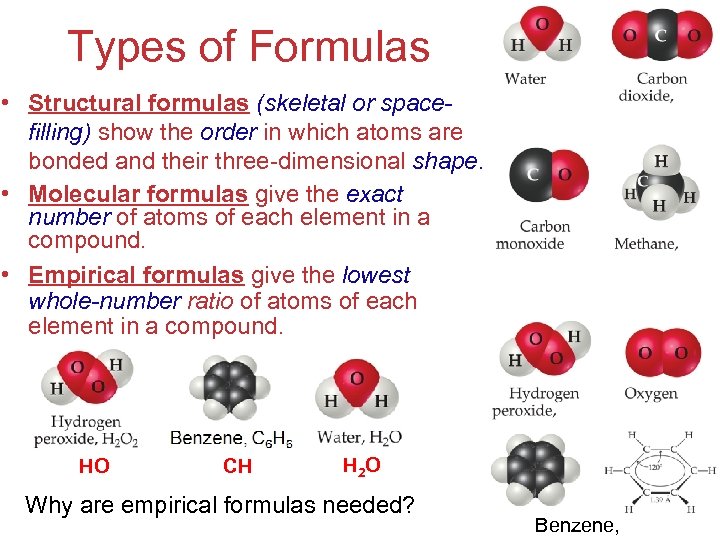 Types of Formulas • Structural formulas (skeletal or spacefilling) show the order in which atoms are bonded and their three-dimensional shape. • Molecular formulas give the exact number of atoms of each element in a compound. • Empirical formulas give the lowest whole-number ratio of atoms of each element in a compound. HO CH H 2 O Why are empirical formulas needed? Stoichiometry Benzene, C 6 H 6
Types of Formulas • Structural formulas (skeletal or spacefilling) show the order in which atoms are bonded and their three-dimensional shape. • Molecular formulas give the exact number of atoms of each element in a compound. • Empirical formulas give the lowest whole-number ratio of atoms of each element in a compound. HO CH H 2 O Why are empirical formulas needed? Stoichiometry Benzene, C 6 H 6
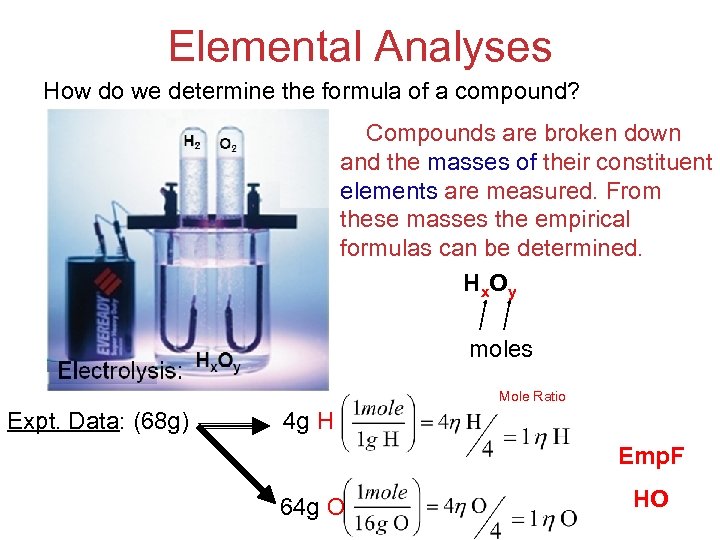 Elemental Analyses How do we determine the formula of a compound? Compounds are broken down and the masses of their constituent elements are measured. From these masses the empirical formulas can be determined. H x. O y moles Mole Ratio Expt. Data: (68 g) 4 g H Emp. F 64 g O Stoichiometry HO
Elemental Analyses How do we determine the formula of a compound? Compounds are broken down and the masses of their constituent elements are measured. From these masses the empirical formulas can be determined. H x. O y moles Mole Ratio Expt. Data: (68 g) 4 g H Emp. F 64 g O Stoichiometry HO
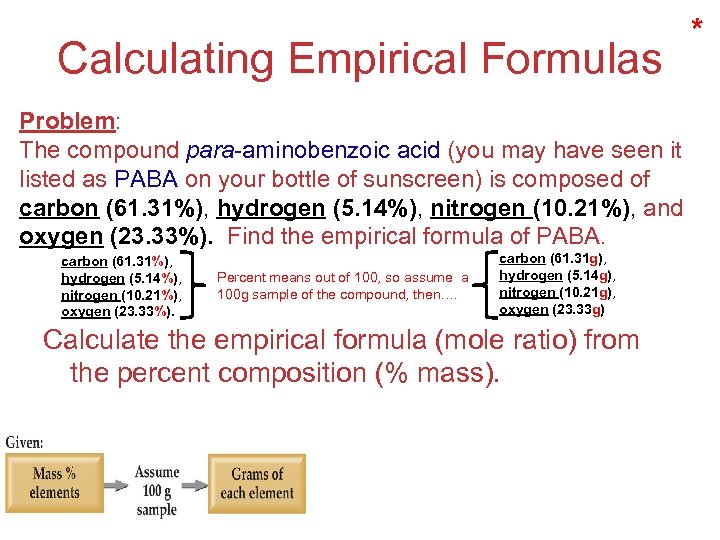 Calculating Empirical Formulas * Problem: The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61. 31%), hydrogen (5. 14%), nitrogen (10. 21%), and oxygen (23. 33%). Find the empirical formula of PABA. carbon (61. 31%), hydrogen (5. 14%), nitrogen (10. 21%), oxygen (23. 33%). Percent means out of 100, so assume a 100 g sample of the compound, then…. carbon (61. 31 g), hydrogen (5. 14 g), nitrogen (10. 21 g), oxygen (23. 33 g) Calculate the empirical formula (mole ratio) from the percent composition (% mass). Stoichiometry
Calculating Empirical Formulas * Problem: The compound para-aminobenzoic acid (you may have seen it listed as PABA on your bottle of sunscreen) is composed of carbon (61. 31%), hydrogen (5. 14%), nitrogen (10. 21%), and oxygen (23. 33%). Find the empirical formula of PABA. carbon (61. 31%), hydrogen (5. 14%), nitrogen (10. 21%), oxygen (23. 33%). Percent means out of 100, so assume a 100 g sample of the compound, then…. carbon (61. 31 g), hydrogen (5. 14 g), nitrogen (10. 21 g), oxygen (23. 33 g) Calculate the empirical formula (mole ratio) from the percent composition (% mass). Stoichiometry
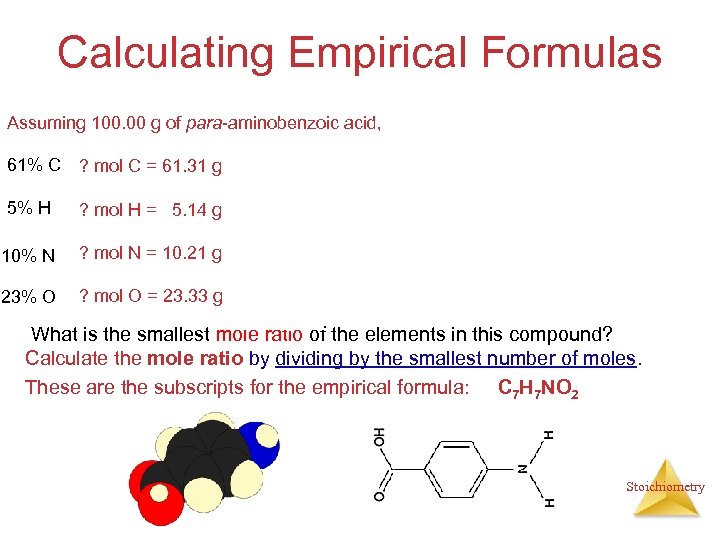 Calculating Empirical Formulas Assuming 100. 00 g of para-aminobenzoic acid, 61% C 5% H 10% N 23% O 1 mol 12. 01 g ? mol H = 5. 14 g x 1 mol 1. 01 g 1 mol ? mol N = 10. 21 g x 14. 01 g ? mol O = 23. 33 g x 1 mol 16. 00 g ? mol C = 61. 31 g x = 5. 105 mol C = 5. 09 mol H = 0. 7288 mol N = 1. 456 mol O 5. 105 mol 0. 7288 mol = 7. 005 7 5. 09 mol = 6. 984 7 0. 7288 mol = 1. 000 0. 7288 mol 1. 458 mol = 2. 001 2 0. 7288 mol What is the smallest mole ratio of the elements in this compound? Calculate the mole ratio by dividing by the smallest number of moles. These are the subscripts for the empirical formula: C 7 H 7 NO 2 Stoichiometry
Calculating Empirical Formulas Assuming 100. 00 g of para-aminobenzoic acid, 61% C 5% H 10% N 23% O 1 mol 12. 01 g ? mol H = 5. 14 g x 1 mol 1. 01 g 1 mol ? mol N = 10. 21 g x 14. 01 g ? mol O = 23. 33 g x 1 mol 16. 00 g ? mol C = 61. 31 g x = 5. 105 mol C = 5. 09 mol H = 0. 7288 mol N = 1. 456 mol O 5. 105 mol 0. 7288 mol = 7. 005 7 5. 09 mol = 6. 984 7 0. 7288 mol = 1. 000 0. 7288 mol 1. 458 mol = 2. 001 2 0. 7288 mol What is the smallest mole ratio of the elements in this compound? Calculate the mole ratio by dividing by the smallest number of moles. These are the subscripts for the empirical formula: C 7 H 7 NO 2 Stoichiometry
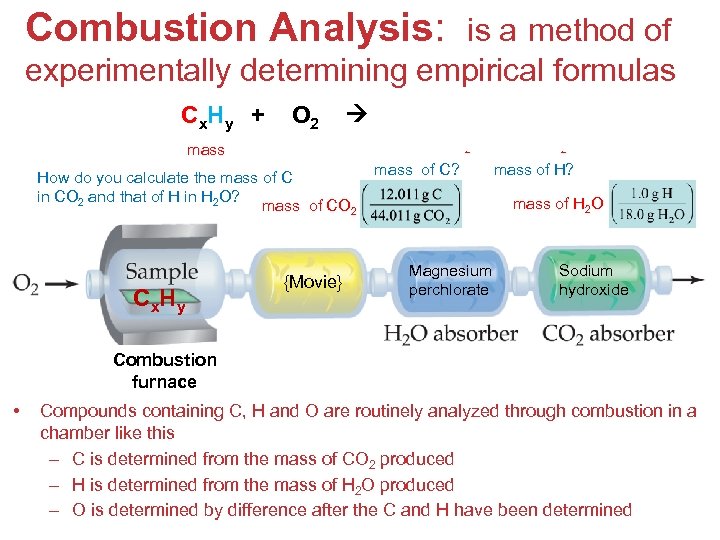 Combustion Analysis: is a method of experimentally determining empirical formulas C x. H y + O 2 + H 2 O mass of CO 2 mass How do you calculate the mass of C in CO 2 and that of H in H 2 O? mass of CO 2 C x. H y CO 2 {Movie} mass of H 2 O mass of C? mass of H? mass of H 2 O Magnesium perchlorate Sodium hydroxide Combustion furnace • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO 2 produced – H is determined from the mass of H 2 O produced Stoichiometry – O is determined by difference after the C and H have been determined
Combustion Analysis: is a method of experimentally determining empirical formulas C x. H y + O 2 + H 2 O mass of CO 2 mass How do you calculate the mass of C in CO 2 and that of H in H 2 O? mass of CO 2 C x. H y CO 2 {Movie} mass of H 2 O mass of C? mass of H? mass of H 2 O Magnesium perchlorate Sodium hydroxide Combustion furnace • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO 2 produced – H is determined from the mass of H 2 O produced Stoichiometry – O is determined by difference after the C and H have been determined
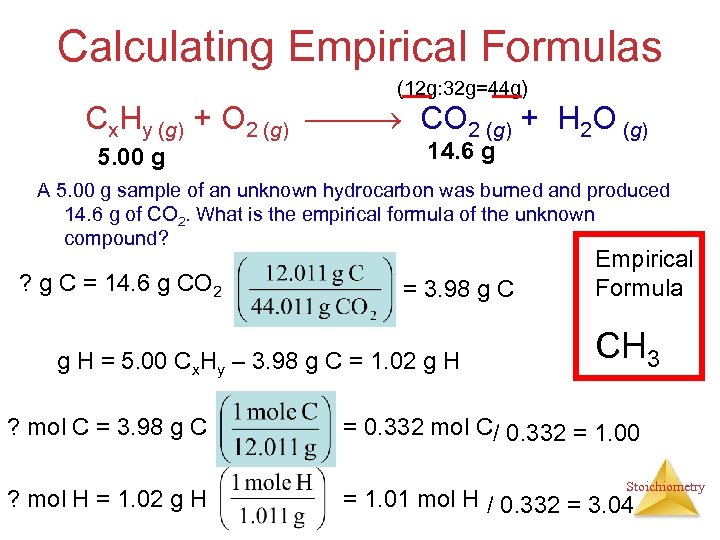 Calculating Empirical Formulas (12 g: 32 g=44 g) Cx. Hy (g) + O 2 (g) CO 2 (g) + H 2 O (g) 5. 00 g 14. 6 g A 5. 00 g sample of an unknown hydrocarbon was burned and produced 14. 6 g of CO 2. What is the empirical formula of the unknown compound? ? g C = 14. 6 g CO 2 = 3. 98 g C g H = 5. 00 Cx. Hy – 3. 98 g C = 1. 02 g H ? mol C = 3. 98 g C ? mol H = 1. 02 g H Empirical Formula CH 3 = 0. 332 mol C/ 0. 332 = 1. 00 Stoichiometry = 1. 01 mol H / 0. 332 = 3. 04
Calculating Empirical Formulas (12 g: 32 g=44 g) Cx. Hy (g) + O 2 (g) CO 2 (g) + H 2 O (g) 5. 00 g 14. 6 g A 5. 00 g sample of an unknown hydrocarbon was burned and produced 14. 6 g of CO 2. What is the empirical formula of the unknown compound? ? g C = 14. 6 g CO 2 = 3. 98 g C g H = 5. 00 Cx. Hy – 3. 98 g C = 1. 02 g H ? mol C = 3. 98 g C ? mol H = 1. 02 g H Empirical Formula CH 3 = 0. 332 mol C/ 0. 332 = 1. 00 Stoichiometry = 1. 01 mol H / 0. 332 = 3. 04
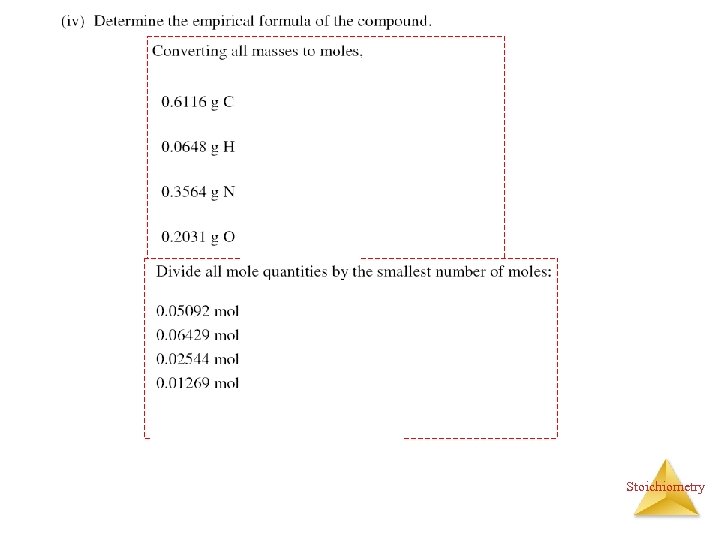
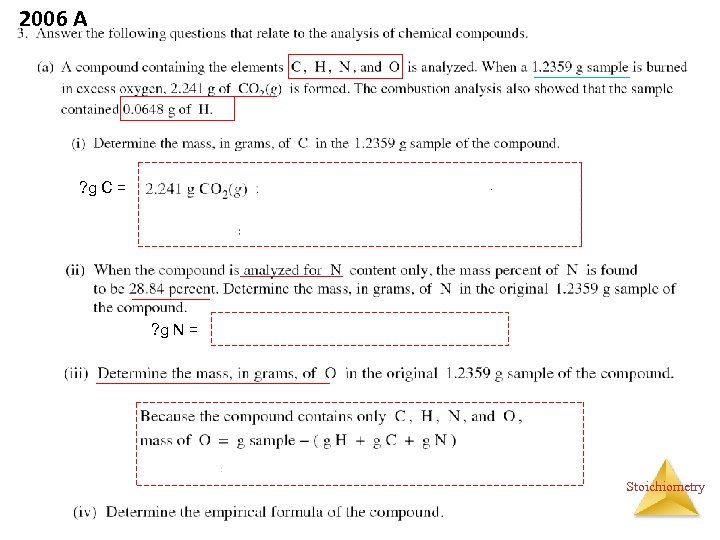 2006 A ? g C = ? g N = Stoichiometry
2006 A ? g C = ? g N = Stoichiometry
 Stoichiometry
Stoichiometry
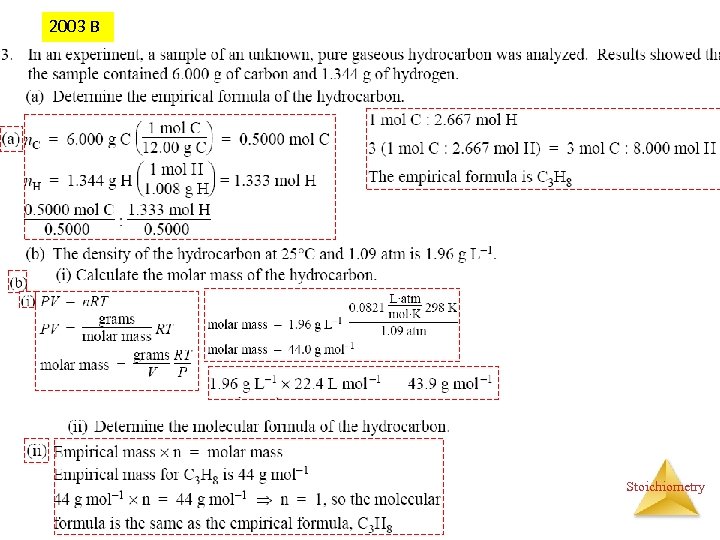 2003 B Stoichiometry
2003 B Stoichiometry
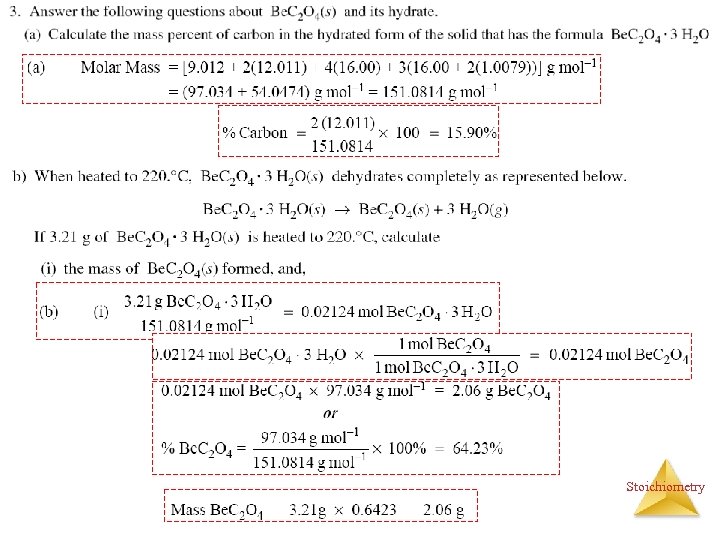 Stoichiometry
Stoichiometry
 Stoichiometry (mass relationships within chemical equations) Stoichiometry
Stoichiometry (mass relationships within chemical equations) Stoichiometry
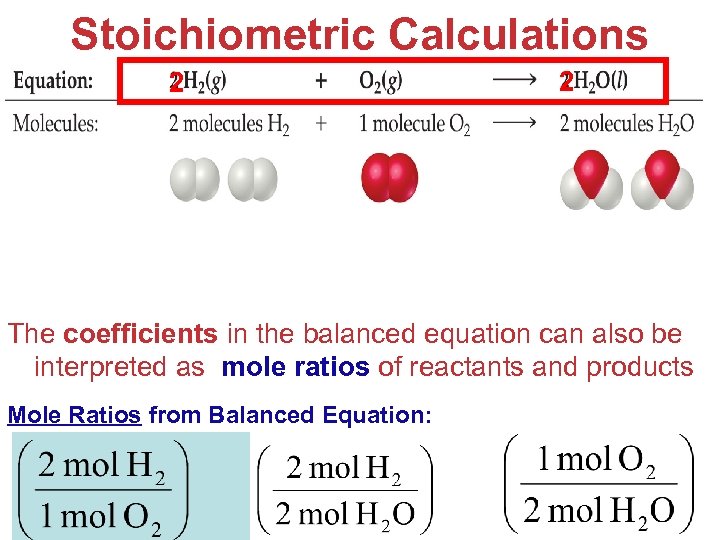 Stoichiometric Calculations 2 2 The coefficients in the balanced equation can also be interpreted as mole ratios of reactants and products Mole Ratios from Balanced Equation: Stoichiometry
Stoichiometric Calculations 2 2 The coefficients in the balanced equation can also be interpreted as mole ratios of reactants and products Mole Ratios from Balanced Equation: Stoichiometry
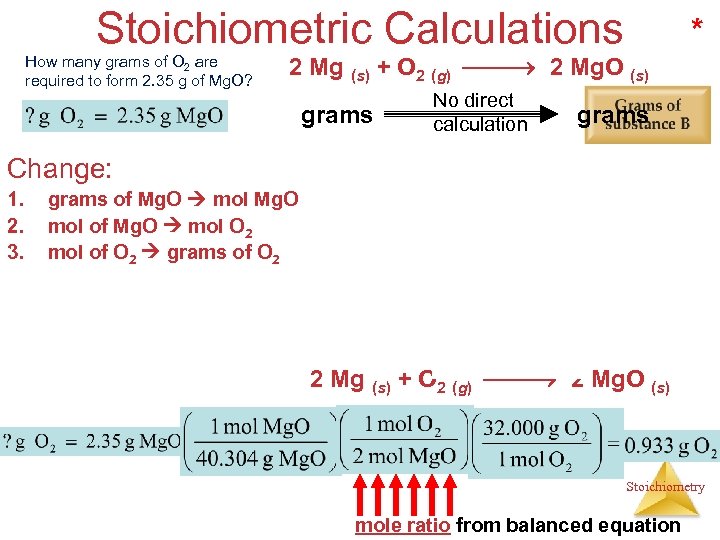 Stoichiometric Calculations How many grams of O 2 are required to form 2. 35 g of Mg. O? * 2 Mg (s) + O 2 (g) 2 Mg. O (s) grams No direct calculation grams Change: 1. 2. 3. grams of Mg. O mol Mg. O mol of Mg. O mol O 2 mol of O 2 grams of O 2 2 Mg (s) + O 2 (g) 2 Mg. O (s) Stoichiometry mole ratio from balanced equation
Stoichiometric Calculations How many grams of O 2 are required to form 2. 35 g of Mg. O? * 2 Mg (s) + O 2 (g) 2 Mg. O (s) grams No direct calculation grams Change: 1. 2. 3. grams of Mg. O mol Mg. O mol of Mg. O mol O 2 mol of O 2 grams of O 2 2 Mg (s) + O 2 (g) 2 Mg. O (s) Stoichiometry mole ratio from balanced equation
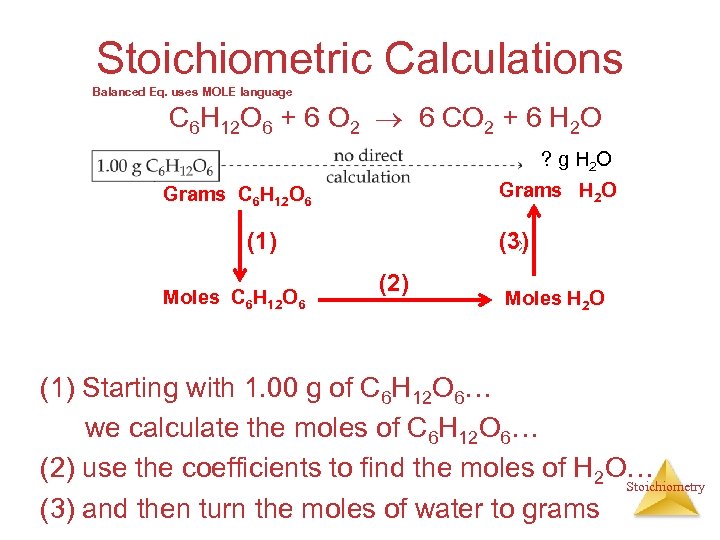 Stoichiometric Calculations Balanced Eq. uses MOLE language C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O ? g H 2 O Grams C 6 H 12 O 6 (3) (1) Moles C 6 H 12 O 6 (2) Moles H 2 O (1) Starting with 1. 00 g of C 6 H 12 O 6… we calculate the moles of C 6 H 12 O 6… (2) use the coefficients to find the moles of H 2 O… Stoichiometry (3) and then turn the moles of water to grams
Stoichiometric Calculations Balanced Eq. uses MOLE language C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O ? g H 2 O Grams C 6 H 12 O 6 (3) (1) Moles C 6 H 12 O 6 (2) Moles H 2 O (1) Starting with 1. 00 g of C 6 H 12 O 6… we calculate the moles of C 6 H 12 O 6… (2) use the coefficients to find the moles of H 2 O… Stoichiometry (3) and then turn the moles of water to grams
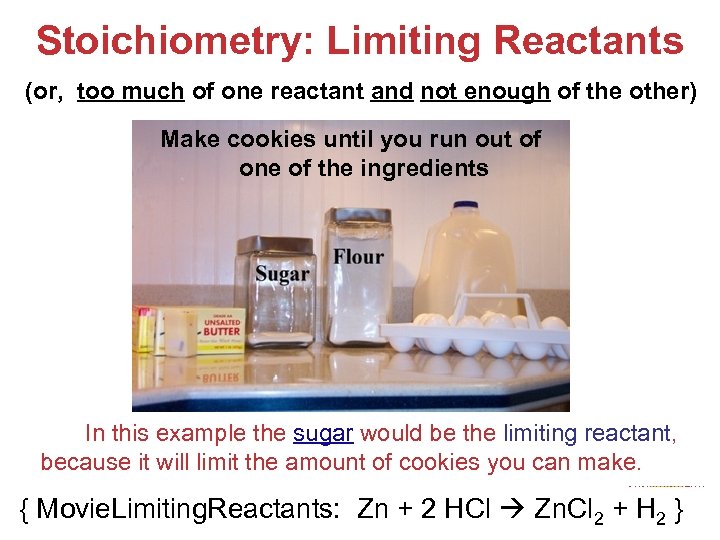 Stoichiometry: Limiting Reactants (or, too much of one reactant and not enough of the other) Make cookies until you run out of one of the ingredients In this example the sugar would be the limiting reactant, because it will limit the amount of cookies you can make. Stoichiometry { Movie. Limiting. Reactants: Zn + 2 HCl Zn. Cl 2 + H 2 }
Stoichiometry: Limiting Reactants (or, too much of one reactant and not enough of the other) Make cookies until you run out of one of the ingredients In this example the sugar would be the limiting reactant, because it will limit the amount of cookies you can make. Stoichiometry { Movie. Limiting. Reactants: Zn + 2 HCl Zn. Cl 2 + H 2 }
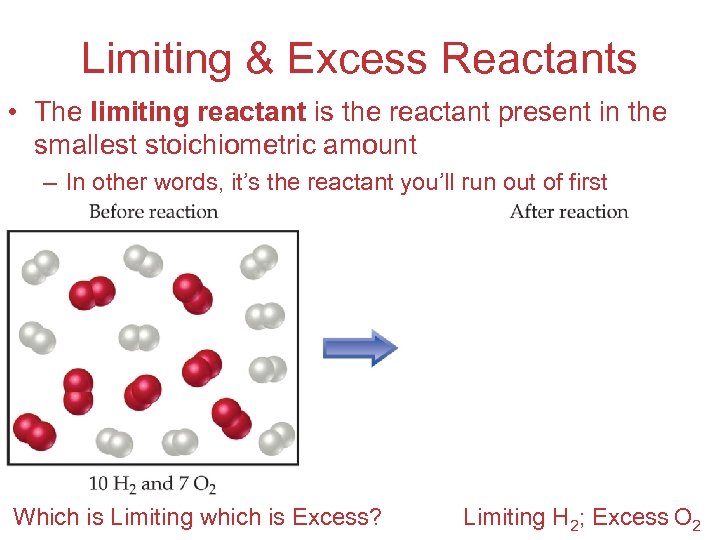 Limiting & Excess Reactants • The limiting reactant is the reactant present in the smallest stoichiometric amount – In other words, it’s the reactant you’ll run out of first Stoichiometry Which is Limiting which is Excess? Limiting H 2; Excess O 2
Limiting & Excess Reactants • The limiting reactant is the reactant present in the smallest stoichiometric amount – In other words, it’s the reactant you’ll run out of first Stoichiometry Which is Limiting which is Excess? Limiting H 2; Excess O 2
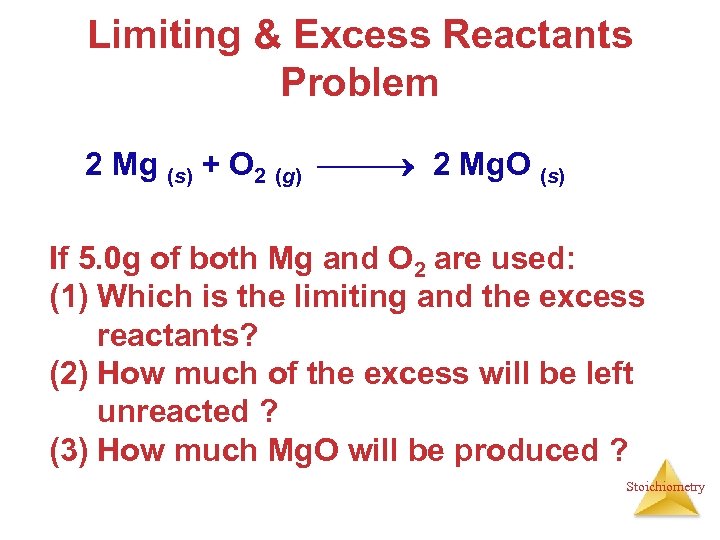 Limiting & Excess Reactants Problem 2 Mg (s) + O 2 (g) 2 Mg. O (s) If 5. 0 g of both Mg and O 2 are used: (1) Which is the limiting and the excess reactants? (2) How much of the excess will be left unreacted ? (3) How much Mg. O will be produced ? Stoichiometry
Limiting & Excess Reactants Problem 2 Mg (s) + O 2 (g) 2 Mg. O (s) If 5. 0 g of both Mg and O 2 are used: (1) Which is the limiting and the excess reactants? (2) How much of the excess will be left unreacted ? (3) How much Mg. O will be produced ? Stoichiometry
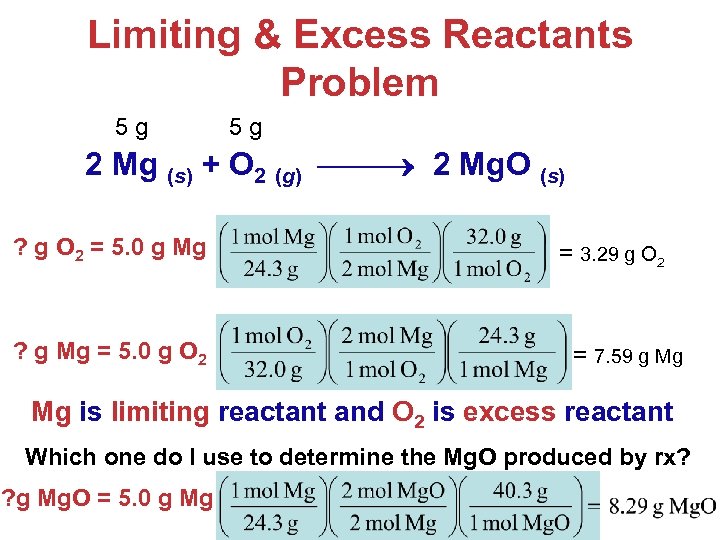 Limiting & Excess Reactants Problem 5 g 5 g 2 Mg (s) + O 2 (g) 2 Mg. O (s) ? g O 2 = 5. 0 g Mg ? g Mg = 5. 0 g O 2 = 3. 29 g O 2 = 7. 59 g Mg Mg is limiting reactant and O 2 is excess reactant Which one do I use to determine the Mg. O produced by rx? ? g Mg. O = 5. 0 g Mg Stoichiometry
Limiting & Excess Reactants Problem 5 g 5 g 2 Mg (s) + O 2 (g) 2 Mg. O (s) ? g O 2 = 5. 0 g Mg ? g Mg = 5. 0 g O 2 = 3. 29 g O 2 = 7. 59 g Mg Mg is limiting reactant and O 2 is excess reactant Which one do I use to determine the Mg. O produced by rx? ? g Mg. O = 5. 0 g Mg Stoichiometry
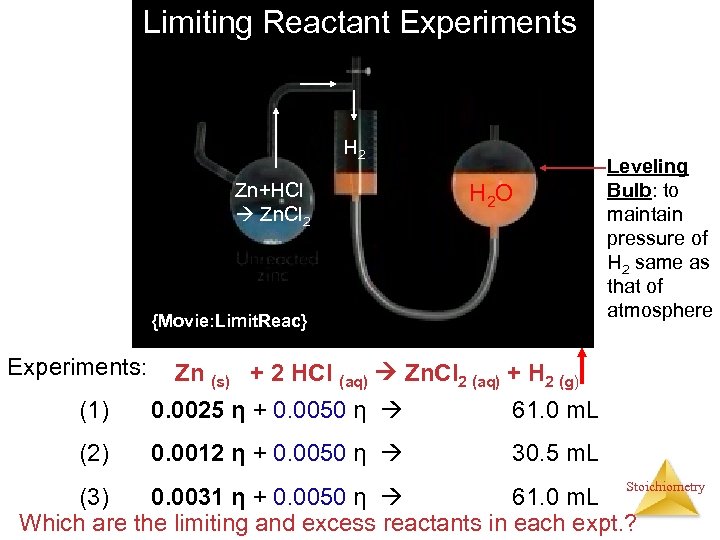 Limiting Reactant Experiments H 2 Zn+HCl Zn. Cl 2 H 2 O {Movie: Limit. Reac} Experiments: Leveling Bulb: to maintain pressure of H 2 same as that of atmosphere Zn (s) + 2 HCl (aq) Zn. Cl 2 (aq) + H 2 (g) (1) 0. 0025 η + 0. 0050 η 61. 0 m. L (2) 0. 0012 η + 0. 0050 η 30. 5 m. L Stoichiometry (3) 0. 0031 η + 0. 0050 η 61. 0 m. L Which are the limiting and excess reactants in each expt. ?
Limiting Reactant Experiments H 2 Zn+HCl Zn. Cl 2 H 2 O {Movie: Limit. Reac} Experiments: Leveling Bulb: to maintain pressure of H 2 same as that of atmosphere Zn (s) + 2 HCl (aq) Zn. Cl 2 (aq) + H 2 (g) (1) 0. 0025 η + 0. 0050 η 61. 0 m. L (2) 0. 0012 η + 0. 0050 η 30. 5 m. L Stoichiometry (3) 0. 0031 η + 0. 0050 η 61. 0 m. L Which are the limiting and excess reactants in each expt. ?
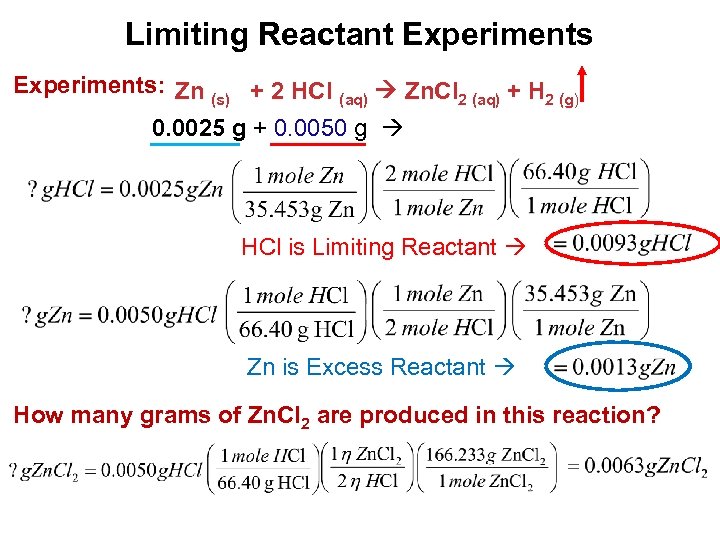 Limiting Reactant Experiments: Zn (s) + 2 HCl (aq) Zn. Cl 2 (aq) + H 2 (g) 0. 0025 g + 0. 0050 g HCl is Limiting Reactant Zn is Excess Reactant How many grams of Zn. Cl 2 are produced in this reaction? Stoichiometry
Limiting Reactant Experiments: Zn (s) + 2 HCl (aq) Zn. Cl 2 (aq) + H 2 (g) 0. 0025 g + 0. 0050 g HCl is Limiting Reactant Zn is Excess Reactant How many grams of Zn. Cl 2 are produced in this reaction? Stoichiometry
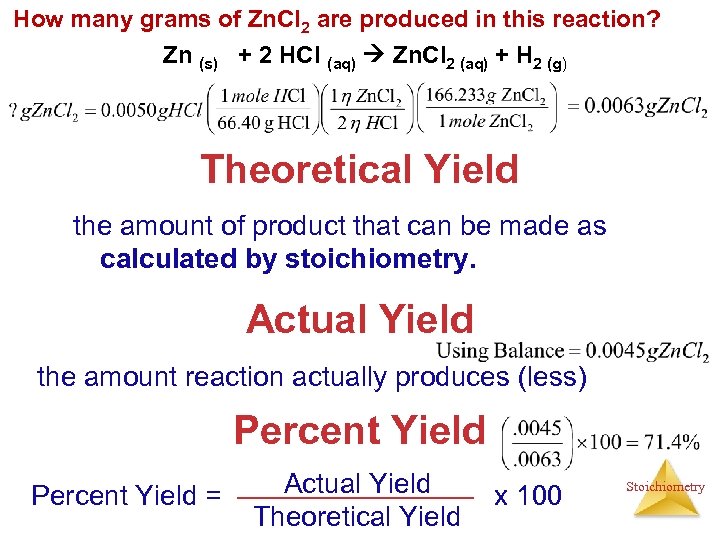 How many grams of Zn. Cl 2 are produced in this reaction? Zn (s) + 2 HCl (aq) Zn. Cl 2 (aq) + H 2 (g) Theoretical Yield the amount of product that can be made as calculated by stoichiometry. Actual Yield the amount reaction actually produces (less) Percent Yield = Actual Yield Theoretical Yield x 100 Stoichiometry
How many grams of Zn. Cl 2 are produced in this reaction? Zn (s) + 2 HCl (aq) Zn. Cl 2 (aq) + H 2 (g) Theoretical Yield the amount of product that can be made as calculated by stoichiometry. Actual Yield the amount reaction actually produces (less) Percent Yield = Actual Yield Theoretical Yield x 100 Stoichiometry
 * The following reaction has a 95% yield: Ge. H 4 + 3 Ge. F 4 4 Ge. F 3 H g-MM: 76. 622 148. 5756 130. 58 Problem: How many grams of the product are formed, when 23. 4 g of Ge. H 4 are reacted with excess Ge. F 4? ? g Ge. F 3 H = 23. 4 g Ge. H 4 Since % yield is only 95%, then actual yield is: What would the yield be if reaction Actually produced 130 g only? Stoichiometry What would be the Theoretical yield if 120 g was a 75% yield ?
* The following reaction has a 95% yield: Ge. H 4 + 3 Ge. F 4 4 Ge. F 3 H g-MM: 76. 622 148. 5756 130. 58 Problem: How many grams of the product are formed, when 23. 4 g of Ge. H 4 are reacted with excess Ge. F 4? ? g Ge. F 3 H = 23. 4 g Ge. H 4 Since % yield is only 95%, then actual yield is: What would the yield be if reaction Actually produced 130 g only? Stoichiometry What would be the Theoretical yield if 120 g was a 75% yield ?


