23b0668e17c1cd09594096919e726739.ppt
- Количество слайдов: 30

CHIPRA Quality Measures: NAC Subcommittee Discussion November 13, 2009 National Advisory Committee Meeting Kathleen Lohr, Ph. D Timothy Brei, MD

CHIPRA – Title IV
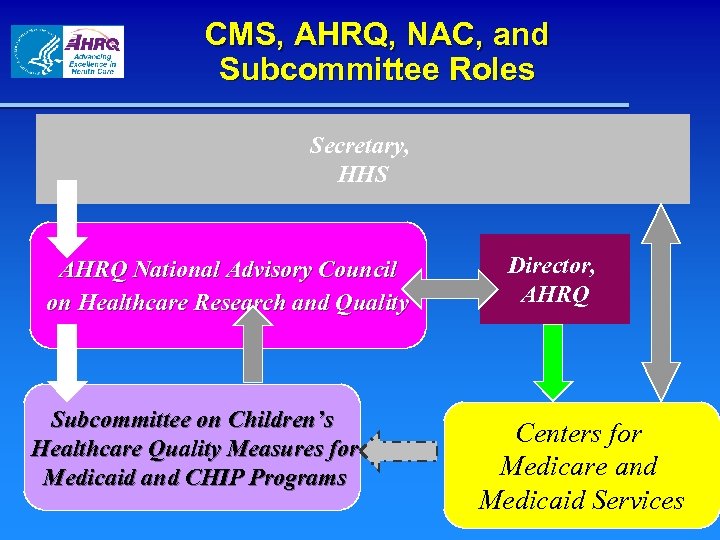
CMS, AHRQ, NAC, and Subcommittee Roles Secretary, HHS AHRQ National Advisory Council on Healthcare Research and Quality Subcommittee on Children’s Healthcare Quality Measures for Medicaid and CHIP Programs Director, AHRQ Centers for Medicare and Medicaid Services

Key Players for SNAC Work SNAC Co-Chairs: n Jeffrey Schiff, MD, MBA Minnesota Department of Human Services n Rita Mangione-Smith, MD, MPH, University of Washington AHRQ Staff: n Denise Dougherty, Ph. D, Senior Advisor, Child Health and Quality Improvement NAC Members: n Timothy Brei, MD, FAAP n Kathleen Lohr, Ph. D Members: n Another ~ 20 experts from clinical, Medicaid, CHIP, quality measurement, research, and policy fields

Subcommittee Charge Identify initial core health care quality measurement set for Medicaid and CHIP programs and in doing this n Provide guidance on criteria for identification of initial core set n Provide guidance on a strategy for identifying additional measures in use for consideration n Review and apply criteria to compilation of measures currently in use by Medicaid/CHIP
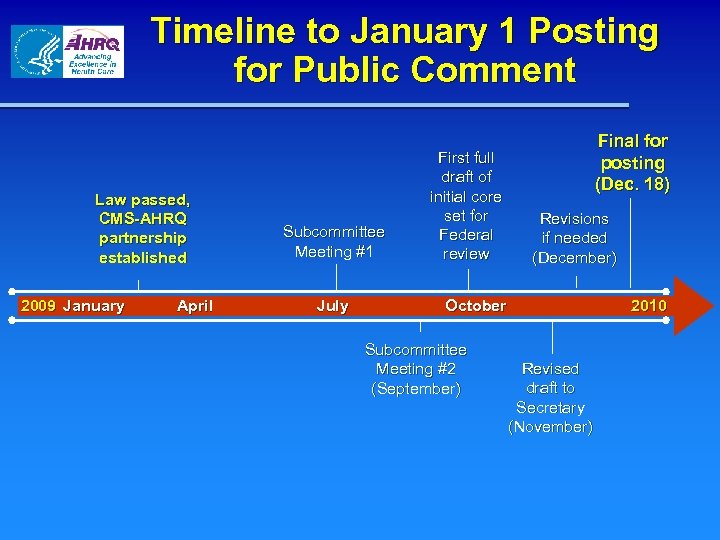
Timeline to January 1 Posting for Public Comment Law passed, CMS-AHRQ partnership established 2009 January April Subcommittee Meeting #1 July First full draft of initial core set for Federal review Final for posting (Dec. 18) Revisions if needed (December) October Subcommittee Meeting #2 (September) 2010 Revised draft to Secretary (November)

Key Points about Process (I) n Three major criteria for selection of measures – Validity – Feasibility – Importance n Philosophically, preferred to leave an "empty chair" than to recommend quality measures that simply were too weak; n Sought breadth where possible n Sought familiarity with or use of measures on part of Medicaid programs and/or SCHIP programs n Recognized nothing was going to be perfect

Key Points about Process (II) n Short amount of time AHRQ had to do this n Immense effort AHRQ staff and the SNAC Co -Chairs over the summer n Intense involvement, interest, contributions of the people on the subcommittee n Notable collaboration and collegiality with a sister agency (namely CMS)

SNAC Nomination and Scoring Process n n n n n Initial measure identification Delphi I – Validity and Feasibility SNAC definitions of – Validity – Feasibility (with reliability) – Importance Understanding of a “core, grounded, and parsimonious” set Broad measure nomination Delphi II – VFI Ranking process Final vote SNAC key issues

Key Features of the SNAC Public Process n Transparent n Multiple stakeholders per CHIPRA n Public comment including public nominations of measures n Focused on measures in use per CHIPRA n Evidence-informed (per CHIPRA) SNAC Delphi processes June and August
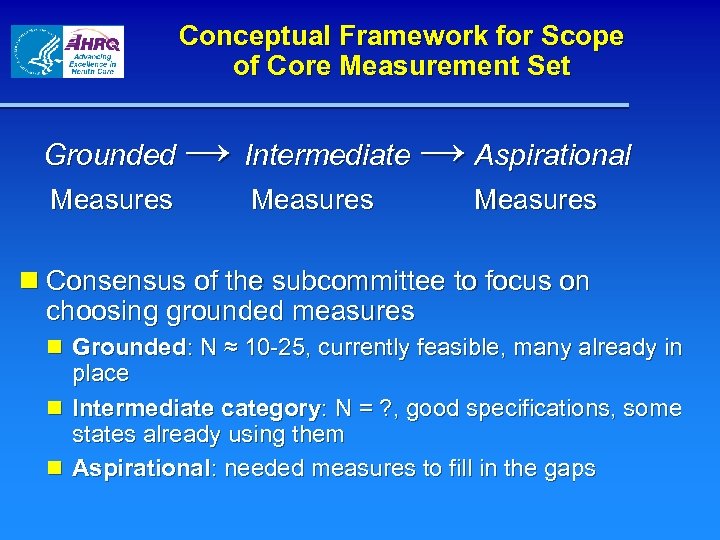
Conceptual Framework for Scope of Core Measurement Set → Intermediate → Aspirational Grounded Measures n Consensus of the subcommittee to focus on choosing grounded measures n Grounded: N ≈ 10 -25, currently feasible, many already in place n Intermediate category: N = ? , good specifications, some states already using them n Aspirational: needed measures to fill in the gaps
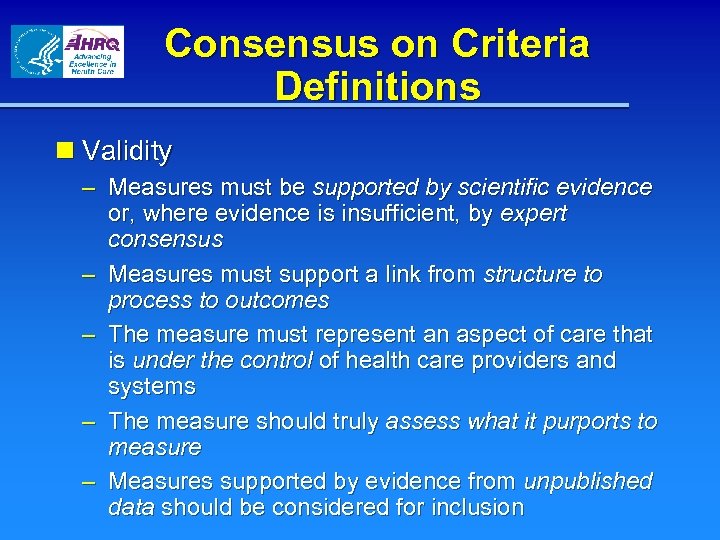
Consensus on Criteria Definitions n Validity – Measures must be supported by scientific evidence or, where evidence is insufficient, by expert consensus – Measures must support a link from structure to process to outcomes – The measure must represent an aspect of care that is under the control of health care providers and systems – The measure should truly assess what it purports to measure – Measures supported by evidence from unpublished data should be considered for inclusion
Consensus on Criteria Definitions n Feasibility – The data necessary to score the measure must be available to state Medicaid and CHIP programs v Administrative data, medical records data, survey data – Detailed specifications must be available for the measure that allow for reliable and unbiased scoring of the measure across states and institutions

Consensus on Criteria Definitions n Importance – The measure should be actionable – Cost of the condition to the nation should be high – Health care systems are clearly accountable for the quality problem assessed by the measure – The extent of the quality problem should be substantial – There should be documented variation in performance on the measure – The measure should be representative of a class of quality problems: “sentinel measure” of QOC provided for preventive care, mental health care, or dental care, etc.

Consensus on Criteria Definitions n Importance – The measure assesses an aspect of health care where there are known disparities – The core set should represent a balanced portfolio of measures and be consistent with the intent of the legislation – Improving on performance for the core set of measures should have the potential to transform care for our nation’s children

SNAC Recommended Quality Measures by Ranking based on SNAC Priority Scores, Legislative Topic, Condition, Age Group, Setting, Source of Data

Recommended Topics by Rank (per SNAC priority score) (I) 1 Immunizations for 2 year-olds (NCQA measure) 2 Frequency of ongoing prenatal care (NCQA measure) 2 Emergency Department Utilization - Average number of emergency room visits per member per reporting period 3 Annual number of asthma patients (> 1 year-old) with > 1 asthma-related ER visit (S/AL Medicaid Program) 4 Body Mass Index documentation 2 -18 year olds (NCQA measure) 5 Well-Child Visits (three NCQA measures): (1) first 15 months of life; (2) third, fourth, fifth and sixth years of life; (3) Adolescent 6 Total eligibles receiving preventive dental services (EPSDT measure) 7 Adolescent immunization (NCQA revised for 2010) 8 HEDIS CAHPS 4. 0 including supplements for children with chronic conditions and Medicaid Plans 9 Timeliness of prenatal care (NCQA measure)

Recommended Topics by Rank (per SNAC priority score) (II) 10 11 % of live births weighing less than 2, 500 grams Rates of screening using standardized screening tools for potential delays in social and emotional development 12 Follow-up care for children prescribed attentiondeficit/hyperactivity disorder (ADHD) (Medication Continuation and Maintenance Phase – NCQA measure) 13 Annual dental visit (NCQA measure) 13 Child and adolescent Major Depressive Disorder (MDD) - suicide risk assessment 13 Annual hemoglobin A 1 C testing (all children and adolescents diagnosed with diabetes)

Recommended Topics by Rank (per SNAC priority score) (III) 14 14 16 16 16 17 17 18 18 Chlamydia screening 16 -20 year-old females (NCQA measure) Followup after hospitalization for mental illness (NCQA measure) Cesarean rate for low-risk first birth women Access to primary care practitioners, by age and total Use of clinician & group primary care CAHPS survey for practitioners participating in Medicaid and CHIP (CAHPS family of measures) Total EPSDT eligibles who received dental treatment services (EPSDT) Pediatric catheter-associated blood stream infection rates (PICU/NICU) Pharyngitis: appropriate testing (NCQA measure) Otitis media with effusion: avoidance of inappropriate use of systemic antimicrobials
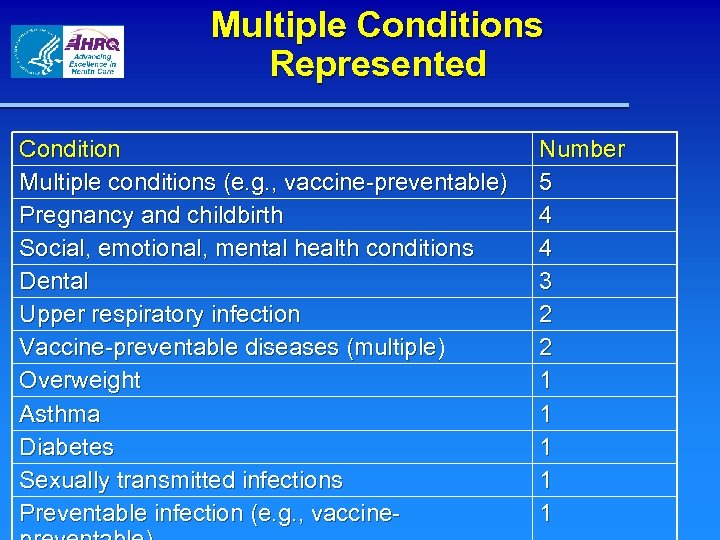
Multiple Conditions Represented Condition Multiple conditions (e. g. , vaccine-preventable) Pregnancy and childbirth Social, emotional, mental health conditions Dental Upper respiratory infection Vaccine-preventable diseases (multiple) Overweight Asthma Diabetes Sexually transmitted infections Preventable infection (e. g. , vaccine- Number 5 4 4 3 2 2 1 1 1
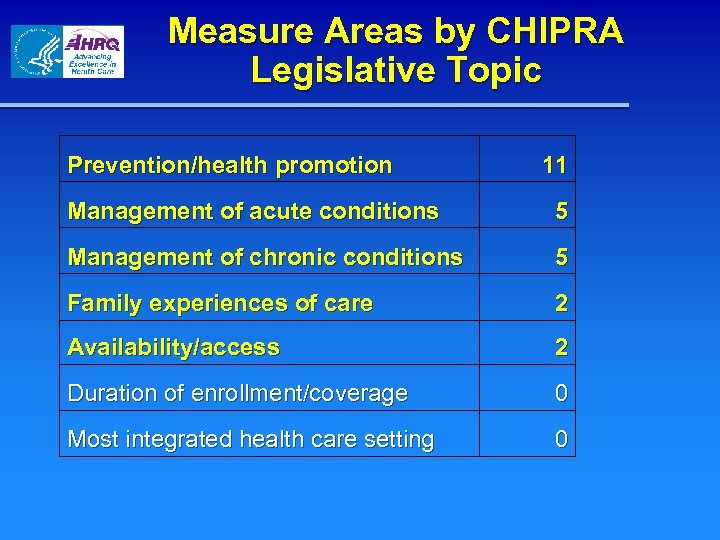
Measure Areas by CHIPRA Legislative Topic Prevention/health promotion 11 Management of acute conditions 5 Management of chronic conditions 5 Family experiences of care 2 Availability/access 2 Duration of enrollment/coverage 0 Most integrated health care setting 0
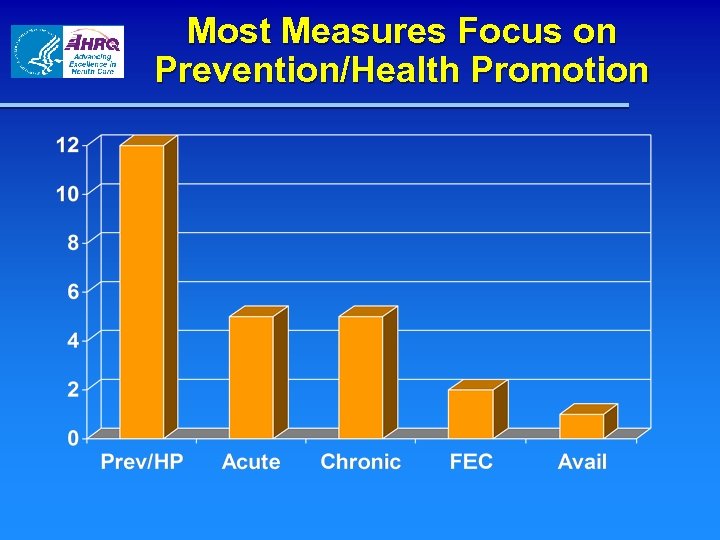
Most Measures Focus on Prevention/Health Promotion
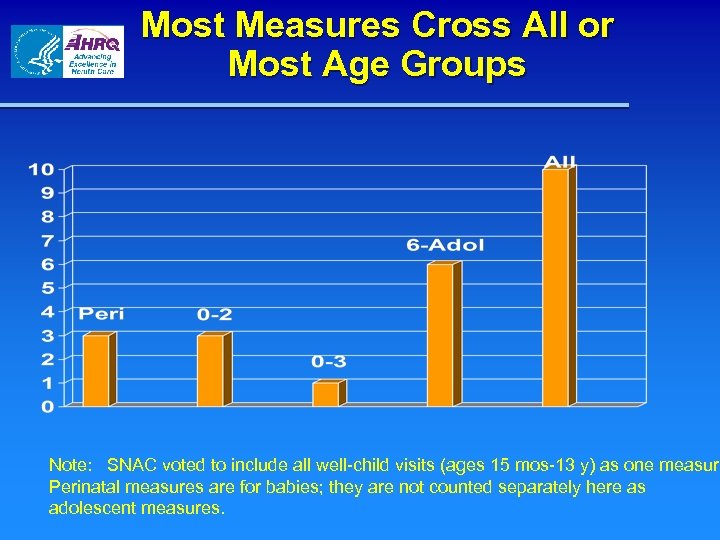
Most Measures Cross All or Most Age Groups Note: SNAC voted to include all well-child visits (ages 15 mos-13 y) as one measure Perinatal measures are for babies; they are not counted separately here as adolescent measures.
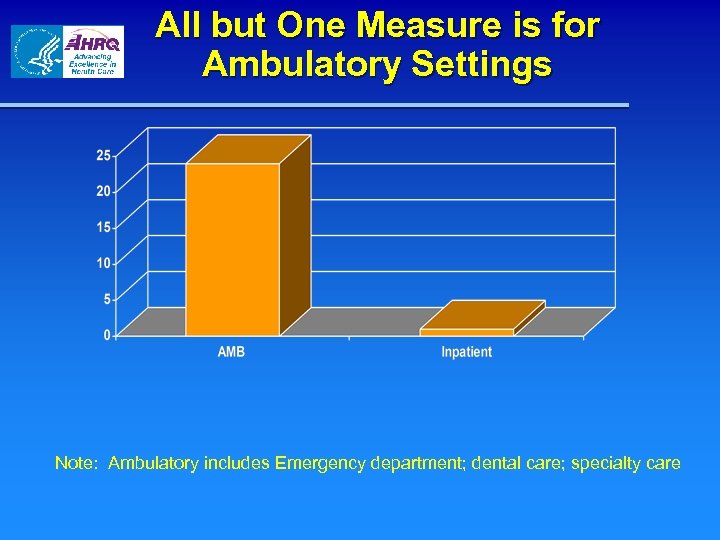
All but One Measure is for Ambulatory Settings Note: Ambulatory includes Emergency department; dental care; specialty care
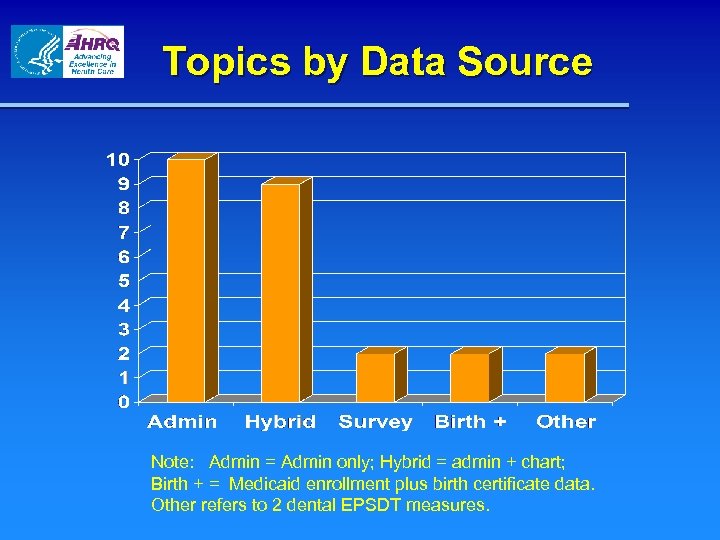
Topics by Data Source Note: Admin = Admin only; Hybrid = admin + chart; Birth + = Medicaid enrollment plus birth certificate data. Other refers to 2 dental EPSDT measures.
Major Issues Identified by the SNAC (I) n Standardization of duration of enrollment measures n Standardization of the denominator across measures n Expansion of use of measures for the whole Medicaid/ CHIP population n Improved disparities reporting (Continued)

Major Issues Identified by the SNAC (II) n Empty chairs” 1. Most integrated health care system (medical home) 2. Specialty care 3. Inpatient care 4. Care for substance abuse 5. Mental health treatment 6. Measures of integration of care with services outside of the health care system 7. Health outcomes “

Other Factors of Interest (I) n n Quality assessment and improvement for federal (insurance) programs is hard, complicated Issues are very broad: e. g. , including dental, mental health, prevention, populations, settings, etc. . . , Medicaid and CHIP programs differ markedly in what they already do and likely can do at the moment Wide range of – probable funding from their states – access to data now and/or what is now reported to them – types of health care systems that deliver care (managed care plans v mostly private practice or whatever) – centralized v. decentralized ways the programs are administered in different states

Other Factors of Interest (II) n The CHIPRA quality measurement activity is voluntary at the moment but n Skepticism that things will stay that way and not become mandatory so n SNAC wanted to be careful about numbers of measures, complexity, and other factors

Discussion Questions n Comments on the recommended core set or the SNAC process? n A model for the Subcommittees of the future? n NAC interest in next phases of AHRQ CHIPRA work? n Other?
23b0668e17c1cd09594096919e726739.ppt