73f3ce01d2bd31d2d4967b91751bd1d8.ppt
- Количество слайдов: 42
 CHHIPS Controlling Hypertension and Hypotension Immediately Post-Stroke Trial
CHHIPS Controlling Hypertension and Hypotension Immediately Post-Stroke Trial
 CHHIPS Background - Hypertension w Hypertension is common following acute stroke. w Management of acute stroke hypertension is uncertain, but: n n Acute stroke hypertension may be detrimental. The benefits and risks of treatment of acute stroke hypertension are not clear.
CHHIPS Background - Hypertension w Hypertension is common following acute stroke. w Management of acute stroke hypertension is uncertain, but: n n Acute stroke hypertension may be detrimental. The benefits and risks of treatment of acute stroke hypertension are not clear.
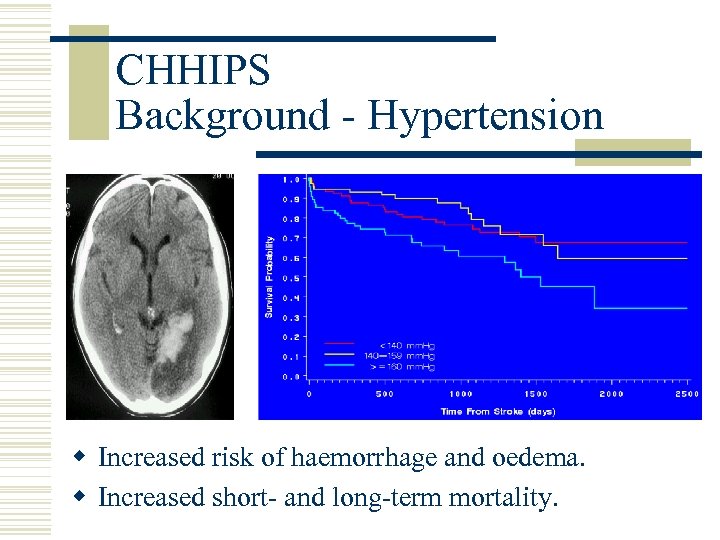 CHHIPS Background - Hypertension w Increased risk of haemorrhage and oedema. w Increased short- and long-term mortality.
CHHIPS Background - Hypertension w Increased risk of haemorrhage and oedema. w Increased short- and long-term mortality.
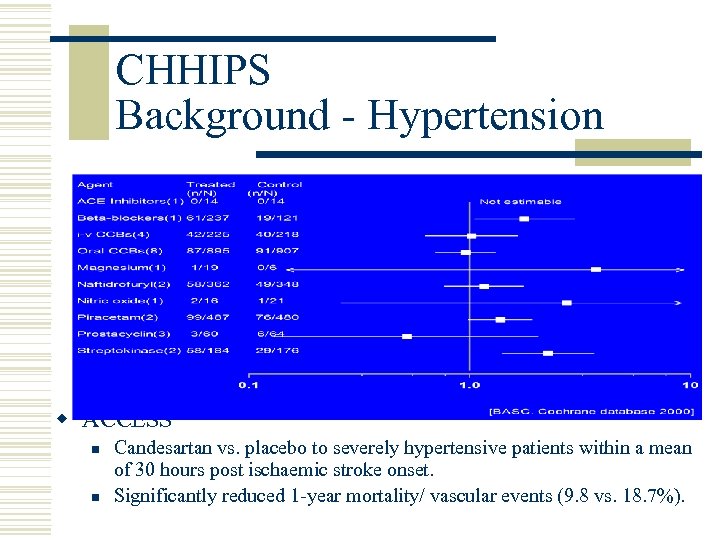 CHHIPS Background - Hypertension w ACCESS n n Candesartan vs. placebo to severely hypertensive patients within a mean of 30 hours post ischaemic stroke onset. Significantly reduced 1 -year mortality/ vascular events (9. 8 vs. 18. 7%).
CHHIPS Background - Hypertension w ACCESS n n Candesartan vs. placebo to severely hypertensive patients within a mean of 30 hours post ischaemic stroke onset. Significantly reduced 1 -year mortality/ vascular events (9. 8 vs. 18. 7%).
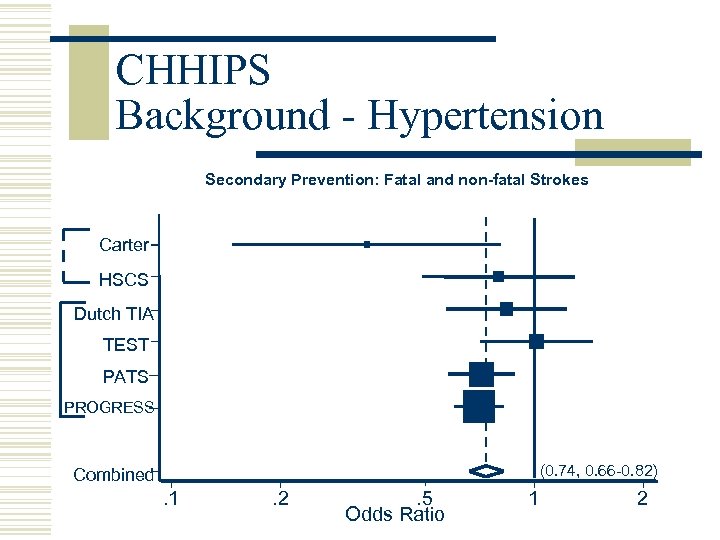 CHHIPS Background - Hypertension Secondary Prevention: Fatal and non-fatal Strokes Carter HSCS Dutch TIA TEST PATS PROGRESS (0. 74, 0. 66 -0. 82) Combined . 1 . 2 . 5 Odds Ratio 1 2
CHHIPS Background - Hypertension Secondary Prevention: Fatal and non-fatal Strokes Carter HSCS Dutch TIA TEST PATS PROGRESS (0. 74, 0. 66 -0. 82) Combined . 1 . 2 . 5 Odds Ratio 1 2
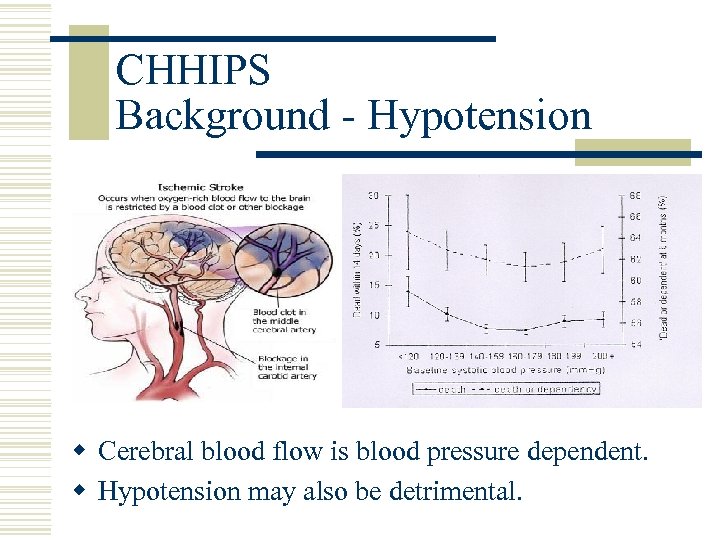 CHHIPS Background - Hypotension w Cerebral blood flow is blood pressure dependent. w Hypotension may also be detrimental.
CHHIPS Background - Hypotension w Cerebral blood flow is blood pressure dependent. w Hypotension may also be detrimental.
 CHHIPS Background - Hypotension w Rordorf et al, Neurology 2001 n n n N=13, <12 hours of acute ischaemic stroke PE (40 to 300 microg/ minute) 20% increase in baseline SBP for >60 minutes 7 responders (infusion maintained for up to 6 days) Improved NIHSS score (>2) Safe and well tolerated
CHHIPS Background - Hypotension w Rordorf et al, Neurology 2001 n n n N=13, <12 hours of acute ischaemic stroke PE (40 to 300 microg/ minute) 20% increase in baseline SBP for >60 minutes 7 responders (infusion maintained for up to 6 days) Improved NIHSS score (>2) Safe and well tolerated
 CHHIPS Study Design and Objectives w Multi-centre, prospective, randomised, double-blind, placebo-controlled, titrateddose trial. w To assess whether hypertension and hypotension should be therapeutically manipulated following acute stroke.
CHHIPS Study Design and Objectives w Multi-centre, prospective, randomised, double-blind, placebo-controlled, titrateddose trial. w To assess whether hypertension and hypotension should be therapeutically manipulated following acute stroke.
 CHHIPS Primary Endpoints w Proportion of patients who are dead or dependent (m. RS >2) at 2 weeks post-stroke.
CHHIPS Primary Endpoints w Proportion of patients who are dead or dependent (m. RS >2) at 2 weeks post-stroke.
 CHHIPS Secondary Endpoints w Early (<72 hours) Neurological Deterioration (NIHSS Increase >4). w Serious Adverse Events. w Treatment Discontinuations. w Trial Withdrawals. w Casual BP Changes (24 hours, 2 weeks). w Fatal and Non-fatal Stroke Recurrence (2 weeks). w Health-related Quality of Life (HUI-3 at 2 weeks, 3 months).
CHHIPS Secondary Endpoints w Early (<72 hours) Neurological Deterioration (NIHSS Increase >4). w Serious Adverse Events. w Treatment Discontinuations. w Trial Withdrawals. w Casual BP Changes (24 hours, 2 weeks). w Fatal and Non-fatal Stroke Recurrence (2 weeks). w Health-related Quality of Life (HUI-3 at 2 weeks, 3 months).
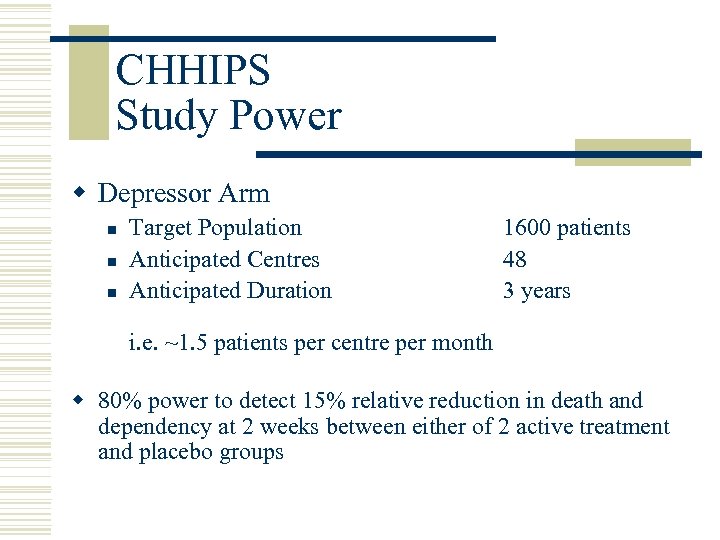 CHHIPS Study Power w Depressor Arm n n n Target Population Anticipated Centres Anticipated Duration 1600 patients 48 3 years i. e. ~1. 5 patients per centre per month w 80% power to detect 15% relative reduction in death and dependency at 2 weeks between either of 2 active treatment and placebo groups
CHHIPS Study Power w Depressor Arm n n n Target Population Anticipated Centres Anticipated Duration 1600 patients 48 3 years i. e. ~1. 5 patients per centre per month w 80% power to detect 15% relative reduction in death and dependency at 2 weeks between either of 2 active treatment and placebo groups
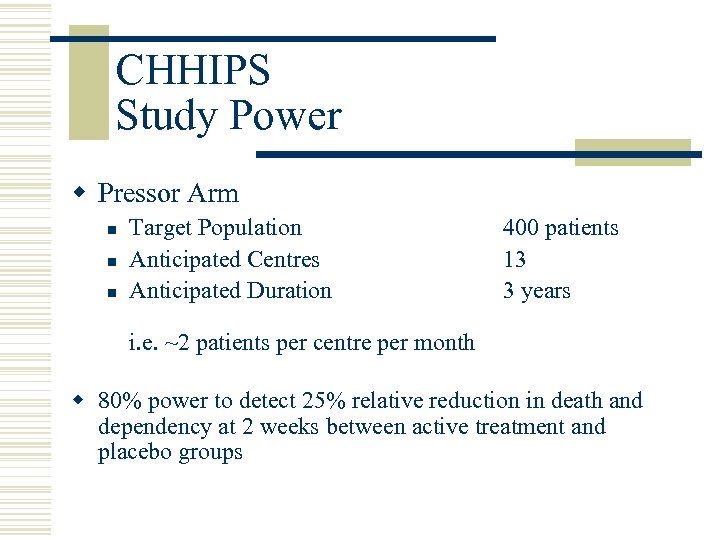 CHHIPS Study Power w Pressor Arm n n n Target Population Anticipated Centres Anticipated Duration 400 patients 13 3 years i. e. ~2 patients per centre per month w 80% power to detect 25% relative reduction in death and dependency at 2 weeks between active treatment and placebo groups
CHHIPS Study Power w Pressor Arm n n n Target Population Anticipated Centres Anticipated Duration 400 patients 13 3 years i. e. ~2 patients per centre per month w 80% power to detect 25% relative reduction in death and dependency at 2 weeks between active treatment and placebo groups
 CHHIPS Study Committees w Data and Safety Monitoring n n Professor Beevers Dr Dewey Professor Jagger Professor Lees w Steering n n n n Professor Bulpitt Dr Drummond Professor Ford Professor Markus Professor Potter Mr Redahan Dr Robinson
CHHIPS Study Committees w Data and Safety Monitoring n n Professor Beevers Dr Dewey Professor Jagger Professor Lees w Steering n n n n Professor Bulpitt Dr Drummond Professor Ford Professor Markus Professor Potter Mr Redahan Dr Robinson
 CHHIPS Co-ordinating Centre Staff w Principal Investigator w Trial Co-ordinator w Nurse Co-ordinator w Statistics Fellow w Database Manager Dr Tom Robinson Dr Penny Eames Ms Janet Hamilton Mr Thomas Hotz Mrs Anne Moore
CHHIPS Co-ordinating Centre Staff w Principal Investigator w Trial Co-ordinator w Nurse Co-ordinator w Statistics Fellow w Database Manager Dr Tom Robinson Dr Penny Eames Ms Janet Hamilton Mr Thomas Hotz Mrs Anne Moore
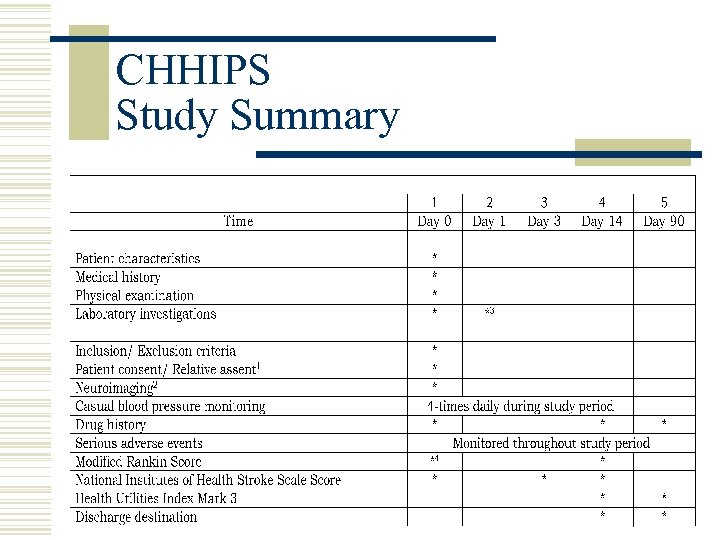 CHHIPS Study Summary
CHHIPS Study Summary
 CHHIPS Treatment Schedule I w Depressor Non-Dysphagic n n T=0 T=4 o 5 mg Lisinopril/ 50 mg Labetalol/ Placebo T=8 o 5 mg Lisinopril/ 50 mg Labetalol/ Placebo (if target SBP NOT achieved: 150 mm. Hg/ 15 mm. Hg Fall) n Days 1 to 14 + 2 l l l Lisinopril 5 to 15 mg mane + Placebo nocte Labetalol 50 to 150 mg BD Placebo BD
CHHIPS Treatment Schedule I w Depressor Non-Dysphagic n n T=0 T=4 o 5 mg Lisinopril/ 50 mg Labetalol/ Placebo T=8 o 5 mg Lisinopril/ 50 mg Labetalol/ Placebo (if target SBP NOT achieved: 150 mm. Hg/ 15 mm. Hg Fall) n Days 1 to 14 + 2 l l l Lisinopril 5 to 15 mg mane + Placebo nocte Labetalol 50 to 150 mg BD Placebo BD
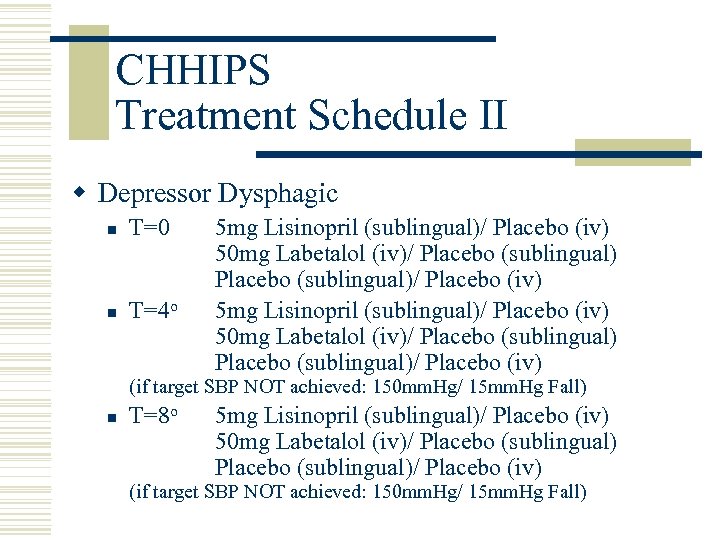 CHHIPS Treatment Schedule II w Depressor Dysphagic n T=0 n T=4 o 5 mg Lisinopril (sublingual)/ Placebo (iv) 50 mg Labetalol (iv)/ Placebo (sublingual)/ Placebo (iv) (if target SBP NOT achieved: 150 mm. Hg/ 15 mm. Hg Fall) n T=8 o 5 mg Lisinopril (sublingual)/ Placebo (iv) 50 mg Labetalol (iv)/ Placebo (sublingual)/ Placebo (iv) (if target SBP NOT achieved: 150 mm. Hg/ 15 mm. Hg Fall)
CHHIPS Treatment Schedule II w Depressor Dysphagic n T=0 n T=4 o 5 mg Lisinopril (sublingual)/ Placebo (iv) 50 mg Labetalol (iv)/ Placebo (sublingual)/ Placebo (iv) (if target SBP NOT achieved: 150 mm. Hg/ 15 mm. Hg Fall) n T=8 o 5 mg Lisinopril (sublingual)/ Placebo (iv) 50 mg Labetalol (iv)/ Placebo (sublingual)/ Placebo (iv) (if target SBP NOT achieved: 150 mm. Hg/ 15 mm. Hg Fall)
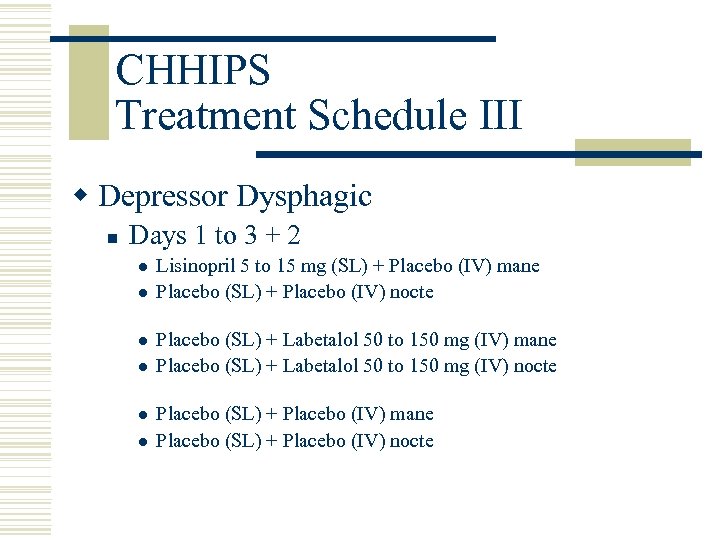 CHHIPS Treatment Schedule III w Depressor Dysphagic n Days 1 to 3 + 2 l l l Lisinopril 5 to 15 mg (SL) + Placebo (IV) mane Placebo (SL) + Placebo (IV) nocte Placebo (SL) + Labetalol 50 to 150 mg (IV) mane Placebo (SL) + Labetalol 50 to 150 mg (IV) nocte Placebo (SL) + Placebo (IV) mane Placebo (SL) + Placebo (IV) nocte
CHHIPS Treatment Schedule III w Depressor Dysphagic n Days 1 to 3 + 2 l l l Lisinopril 5 to 15 mg (SL) + Placebo (IV) mane Placebo (SL) + Placebo (IV) nocte Placebo (SL) + Labetalol 50 to 150 mg (IV) mane Placebo (SL) + Labetalol 50 to 150 mg (IV) nocte Placebo (SL) + Placebo (IV) mane Placebo (SL) + Placebo (IV) nocte
 CHHIPS Treatment Schedule IV w Depressor Dysphagic n Days 5 to 14 + 2 Lisinopril 5 to 15 mg mane + placebo nocte l Labetalol 50 to 150 mg BD l Placebo BD l All as suspensions: l Oral if non-dysphagic l NG/ PEG if dysphagic
CHHIPS Treatment Schedule IV w Depressor Dysphagic n Days 5 to 14 + 2 Lisinopril 5 to 15 mg mane + placebo nocte l Labetalol 50 to 150 mg BD l Placebo BD l All as suspensions: l Oral if non-dysphagic l NG/ PEG if dysphagic
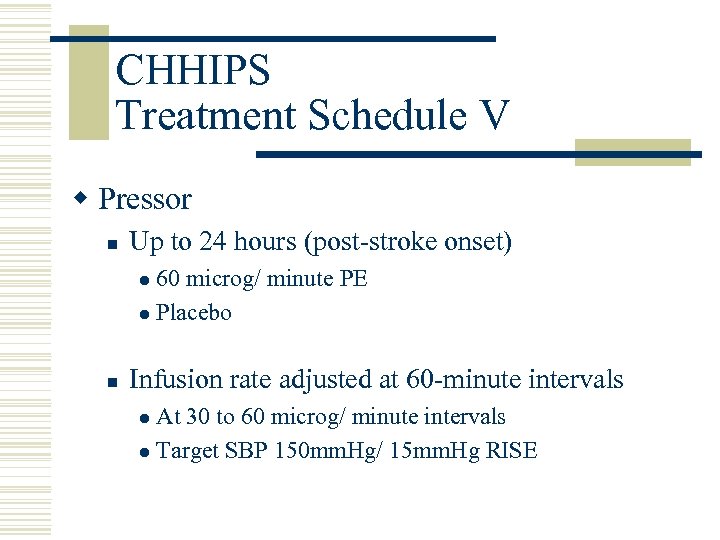 CHHIPS Treatment Schedule V w Pressor n Up to 24 hours (post-stroke onset) 60 microg/ minute PE l Placebo l n Infusion rate adjusted at 60 -minute intervals At 30 to 60 microg/ minute intervals l Target SBP 150 mm. Hg/ 15 mm. Hg RISE l
CHHIPS Treatment Schedule V w Pressor n Up to 24 hours (post-stroke onset) 60 microg/ minute PE l Placebo l n Infusion rate adjusted at 60 -minute intervals At 30 to 60 microg/ minute intervals l Target SBP 150 mm. Hg/ 15 mm. Hg RISE l
 CHHIPS Inclusion Criteria (Depressor) w Age >18 years. w Clinical diagnosis of suspected stroke (neuroimaging not required before entry). w Stroke onset <24 hours (if patient wakes with suspected stroke, document time last normal) w Hypertension (SBP >160 mm. Hg). w Patient consent/ Relative assent.
CHHIPS Inclusion Criteria (Depressor) w Age >18 years. w Clinical diagnosis of suspected stroke (neuroimaging not required before entry). w Stroke onset <24 hours (if patient wakes with suspected stroke, document time last normal) w Hypertension (SBP >160 mm. Hg). w Patient consent/ Relative assent.
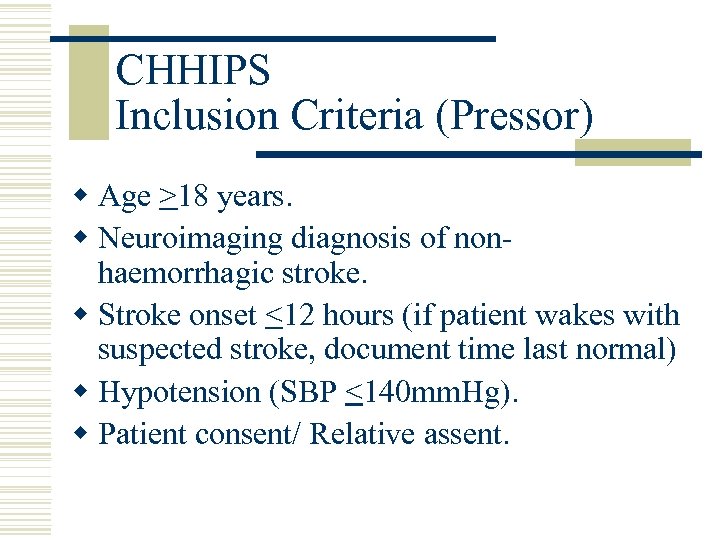 CHHIPS Inclusion Criteria (Pressor) w Age >18 years. w Neuroimaging diagnosis of nonhaemorrhagic stroke. w Stroke onset <12 hours (if patient wakes with suspected stroke, document time last normal) w Hypotension (SBP <140 mm. Hg). w Patient consent/ Relative assent.
CHHIPS Inclusion Criteria (Pressor) w Age >18 years. w Neuroimaging diagnosis of nonhaemorrhagic stroke. w Stroke onset <12 hours (if patient wakes with suspected stroke, document time last normal) w Hypotension (SBP <140 mm. Hg). w Patient consent/ Relative assent.
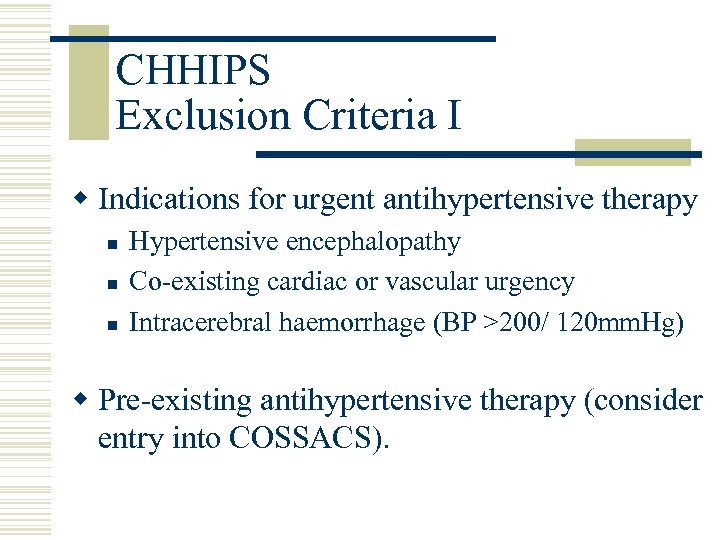 CHHIPS Exclusion Criteria I w Indications for urgent antihypertensive therapy n n n Hypertensive encephalopathy Co-existing cardiac or vascular urgency Intracerebral haemorrhage (BP >200/ 120 mm. Hg) w Pre-existing antihypertensive therapy (consider entry into COSSACS).
CHHIPS Exclusion Criteria I w Indications for urgent antihypertensive therapy n n n Hypertensive encephalopathy Co-existing cardiac or vascular urgency Intracerebral haemorrhage (BP >200/ 120 mm. Hg) w Pre-existing antihypertensive therapy (consider entry into COSSACS).
 CHHIPS Exclusion Criteria II w Contraindications to study treatments: n Lisinopril l n Labetalol l n Angioneurotic oedema, impaired renal function, renovascular hypertension Asthma, 2 o or 3 o heart block, severe bradycardia, uncontrolled heart failure Phenylephrine l Uncontrolled angina, arrhythmias, occlusive vascular disease, treatment with digoxin quinidine TCA or MAOI
CHHIPS Exclusion Criteria II w Contraindications to study treatments: n Lisinopril l n Labetalol l n Angioneurotic oedema, impaired renal function, renovascular hypertension Asthma, 2 o or 3 o heart block, severe bradycardia, uncontrolled heart failure Phenylephrine l Uncontrolled angina, arrhythmias, occlusive vascular disease, treatment with digoxin quinidine TCA or MAOI
 CHHIPS Exclusion Criteria III w Miscellaneous n n n Impaired conscious level Premorbid dependence (m. RS >3) Life expectancy <6 months (secondary to coexisting morbidity) Females of child-bearing potential Non-stroke diagnoses (on subsequent neuroimaging)
CHHIPS Exclusion Criteria III w Miscellaneous n n n Impaired conscious level Premorbid dependence (m. RS >3) Life expectancy <6 months (secondary to coexisting morbidity) Females of child-bearing potential Non-stroke diagnoses (on subsequent neuroimaging)
 CHHIPS Using the Internet I w This will be an internet-based study. w Website w e-mail www. le. ac. uk/medther chhips@le. ac. uk w Fax 0116 250 2366 w Clinical Help w Technical Help 0116 250 2366 0207 594 3426
CHHIPS Using the Internet I w This will be an internet-based study. w Website w e-mail www. le. ac. uk/medther chhips@le. ac. uk w Fax 0116 250 2366 w Clinical Help w Technical Help 0116 250 2366 0207 594 3426
 CHHIPS Using the Internet II w Secure Website https: //chhips. cvsu. co. uk w What you will need n n n User Name Secret Password (Security fails because passwords are not kept secret) Electronic Signature Certificate and Activation
CHHIPS Using the Internet II w Secure Website https: //chhips. cvsu. co. uk w What you will need n n n User Name Secret Password (Security fails because passwords are not kept secret) Electronic Signature Certificate and Activation
 CHHIPS Using the Internet III w The First Time n n n User Name Password New Password l l n Upper Case Lower Case Number Character Security Question w Thereafter n n n User Name Password will be further required to sign off data forms
CHHIPS Using the Internet III w The First Time n n n User Name Password New Password l l n Upper Case Lower Case Number Character Security Question w Thereafter n n n User Name Password will be further required to sign off data forms
 CHHIPS Randomisation w Select Study Treatment Arm w Patient Name, Hospital Number, Date of birth w Date and Time of Stroke w Review of Inclusion and Exclusion Criteria w Answer specific entry queries w Confirm data entry and intention to randomise w Print randomisation form for medical records
CHHIPS Randomisation w Select Study Treatment Arm w Patient Name, Hospital Number, Date of birth w Date and Time of Stroke w Review of Inclusion and Exclusion Criteria w Answer specific entry queries w Confirm data entry and intention to randomise w Print randomisation form for medical records
 CHHIPS Post-Randomisation Data Form w Contact Details (for 3 -month central f-up) w Investigation Results (Includes Neuroimaging for Pressor Arm) w Baseline BP Data w Baseline Assessment Scale Data NIHSS, Barthel, Modified Rankin Scale
CHHIPS Post-Randomisation Data Form w Contact Details (for 3 -month central f-up) w Investigation Results (Includes Neuroimaging for Pressor Arm) w Baseline BP Data w Baseline Assessment Scale Data NIHSS, Barthel, Modified Rankin Scale
 CHHIPS 24 -hour Data Form w Blood Pressure Data n n Pressor: Hourly Depressor: At 4 and 8 hours w Record of Treatment Doses w SAEs/ AEs
CHHIPS 24 -hour Data Form w Blood Pressure Data n n Pressor: Hourly Depressor: At 4 and 8 hours w Record of Treatment Doses w SAEs/ AEs
 CHHIPS 72 -hour Data Form w SAEs/ AEs w 72 -hour Outcome Data NIHSS w Repeat Dysphagia Screen (Depressor Dysphagic Arm only)
CHHIPS 72 -hour Data Form w SAEs/ AEs w 72 -hour Outcome Data NIHSS w Repeat Dysphagia Screen (Depressor Dysphagic Arm only)
 CHHIPS 2 -week Outcome Form w Is the patient dead? n n Date of death SAE Form completion w Has patient been withdrawn? n n Date of withdrawal Reason for withdrawal
CHHIPS 2 -week Outcome Form w Is the patient dead? n n Date of death SAE Form completion w Has patient been withdrawn? n n Date of withdrawal Reason for withdrawal
 CHHIPS 2 -week outcome w SAEs/ AEs (including recurrent vascular events) w Blood Pressure (daily readings) w Treatment n n Compliance with study treatment Intended Blood Pressure, Cholesterol, Antithrombotic Treatment
CHHIPS 2 -week outcome w SAEs/ AEs (including recurrent vascular events) w Blood Pressure (daily readings) w Treatment n n Compliance with study treatment Intended Blood Pressure, Cholesterol, Antithrombotic Treatment
 CHHIPS 2 -week outcome w Neuroimaging (Result for Depressor Arm only) w 2 -week Outcome Data n n n NIHSS Barthel Index Modified Rankin Score Resource Use Form HUI-3 w Patient Location/ Discharge Destination
CHHIPS 2 -week outcome w Neuroimaging (Result for Depressor Arm only) w 2 -week Outcome Data n n n NIHSS Barthel Index Modified Rankin Score Resource Use Form HUI-3 w Patient Location/ Discharge Destination
 CHHIPS 3 -month outcome w Form completed by Co-ordinating Centre n n GP Information vital Patient/ Carer contact details vital w All efforts are made to ensure that patient is alive w Telephone completion w Carer may be asked to assist in completion
CHHIPS 3 -month outcome w Form completed by Co-ordinating Centre n n GP Information vital Patient/ Carer contact details vital w All efforts are made to ensure that patient is alive w Telephone completion w Carer may be asked to assist in completion
 CHHIPS 3 -month outcome w Death w Trial Withdrawal w Patient Location w Patient/ Carer Diary w HUI-3
CHHIPS 3 -month outcome w Death w Trial Withdrawal w Patient Location w Patient/ Carer Diary w HUI-3
 CHHIPS Serious Adverse Events ‘…an adverse event that results in death, is lifethreatening (i. e. the patient was at immediate risk of death from the adverse event as it occurred), or required inpatient hospitalisation or prolongation of existing hospitalisation…’
CHHIPS Serious Adverse Events ‘…an adverse event that results in death, is lifethreatening (i. e. the patient was at immediate risk of death from the adverse event as it occurred), or required inpatient hospitalisation or prolongation of existing hospitalisation…’
 CHHIPS Serious Adverse Events w Date and Time. w Nature. w Why serious? w Severity. w Relationship to study. w Supporting diagnostic evidence. w Additional information.
CHHIPS Serious Adverse Events w Date and Time. w Nature. w Why serious? w Severity. w Relationship to study. w Supporting diagnostic evidence. w Additional information.
 CHHIPS Monitoring w Responsibility of Co-ordinating Centre. w Random Sample. w Source Data Verification.
CHHIPS Monitoring w Responsibility of Co-ordinating Centre. w Random Sample. w Source Data Verification.
 CHHIPS Summary w Stroke is the most common life-threatening neurological condition. w Hypertension is a common complication. w Hypotension is a less common, but serious, complication. w It is uncertain whether blood pressure should be acutely manipulated following acute stroke. w CHHIPS will help address these questions, and inform the design of definitive trials.
CHHIPS Summary w Stroke is the most common life-threatening neurological condition. w Hypertension is a common complication. w Hypotension is a less common, but serious, complication. w It is uncertain whether blood pressure should be acutely manipulated following acute stroke. w CHHIPS will help address these questions, and inform the design of definitive trials.
 CHHIPS Controlling Hypertension and Hypotension Immediately Post-Stroke Trial
CHHIPS Controlling Hypertension and Hypotension Immediately Post-Stroke Trial


