3ad49e01341573a158464b0f633369ae.ppt
- Количество слайдов: 50
 Chemotherapy for Lung Cancer Giorgio V. Scagliotti University of Torino Dept. Clinical & Biological Sciences giorgio. scagliotti@unito. it www. oncologiapolmonare. it
Chemotherapy for Lung Cancer Giorgio V. Scagliotti University of Torino Dept. Clinical & Biological Sciences giorgio. scagliotti@unito. it www. oncologiapolmonare. it
 Therapeutic Research in Lung Cancer Non-Small Cell Lung Cancer
Therapeutic Research in Lung Cancer Non-Small Cell Lung Cancer
 SCLC: Treatment Options Overview • Limited-Stage Disease • • • surgery platinum-based combination chemotherapy thoracic irradiation prophylactic cranial irradiation (PCI) [for responders] New Agents (taxanes, topoisomerase I inhibitors, pemetrexed) • Extensive-Stage Disease • combination chemotherapy • radiotherapy + combination chemotherapy or vice versa • prophylactic cranial irradiation (PCI) [for responders]
SCLC: Treatment Options Overview • Limited-Stage Disease • • • surgery platinum-based combination chemotherapy thoracic irradiation prophylactic cranial irradiation (PCI) [for responders] New Agents (taxanes, topoisomerase I inhibitors, pemetrexed) • Extensive-Stage Disease • combination chemotherapy • radiotherapy + combination chemotherapy or vice versa • prophylactic cranial irradiation (PCI) [for responders]
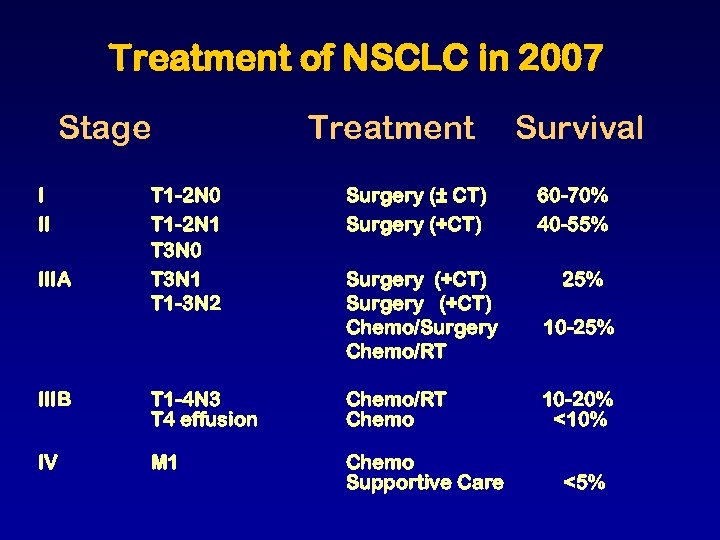 Treatment of NSCLC in 2007 Stage I II IIIA Treatment T 1 -2 N 0 T 1 -2 N 1 T 3 N 0 T 3 N 1 T 1 -3 N 2 Surgery (± CT) Surgery (+CT) Chemo/Surgery Chemo/RT IIIB T 1 -4 N 3 T 4 effusion Chemo/RT Chemo IV M 1 Chemo Supportive Care Survival 60 -70% 40 -55% 25% 10 -20% <10% <5%
Treatment of NSCLC in 2007 Stage I II IIIA Treatment T 1 -2 N 0 T 1 -2 N 1 T 3 N 0 T 3 N 1 T 1 -3 N 2 Surgery (± CT) Surgery (+CT) Chemo/Surgery Chemo/RT IIIB T 1 -4 N 3 T 4 effusion Chemo/RT Chemo IV M 1 Chemo Supportive Care Survival 60 -70% 40 -55% 25% 10 -20% <10% <5%
 Established Key Points in 2007 • Chemotherapy prolongs survival • Platinum-based doublets with 3 rd generation cytotoxics (Gemcitabine, Taxane, Irinotecan and Vinorelbine) are the standard • Elderly patients may benefit from chemotherapy but patients with poor KPS may not • Cisplatin has slight advantage over carboplatin in terms of survival but more disadvantageous in terms of toxicity
Established Key Points in 2007 • Chemotherapy prolongs survival • Platinum-based doublets with 3 rd generation cytotoxics (Gemcitabine, Taxane, Irinotecan and Vinorelbine) are the standard • Elderly patients may benefit from chemotherapy but patients with poor KPS may not • Cisplatin has slight advantage over carboplatin in terms of survival but more disadvantageous in terms of toxicity
 NSCLC Meta-Analysis: Results with First-Line Cisplatin-Based CT Treatments Hazard ratio (95% CI) P value Reduction risk of death (%) Surgery vs Surgery + CT 0. 87 (0. 74– 1. 20) 0. 08 Surgery + RT vs Surgery + RT + CT 0. 94 (0. 79– 1. 11) RT vs RT + CT BSC vs BSC + CT Absolute benefit (%) 2 years 5 years 13 3 5 0. 46 6 2 2 0. 87 (0. 79– 0. 96) <0. 01 13 4 2 0. 73 (0. 63– 0. 96) <0. 001 27 10 (1 year) MST >1. 5 months BSC: best supportive care; CT: chemotherapy; MST: median survival time; RT: radiotherapy. NSCLC Collaborative Group BMJ 1995.
NSCLC Meta-Analysis: Results with First-Line Cisplatin-Based CT Treatments Hazard ratio (95% CI) P value Reduction risk of death (%) Surgery vs Surgery + CT 0. 87 (0. 74– 1. 20) 0. 08 Surgery + RT vs Surgery + RT + CT 0. 94 (0. 79– 1. 11) RT vs RT + CT BSC vs BSC + CT Absolute benefit (%) 2 years 5 years 13 3 5 0. 46 6 2 2 0. 87 (0. 79– 0. 96) <0. 01 13 4 2 0. 73 (0. 63– 0. 96) <0. 001 27 10 (1 year) MST >1. 5 months BSC: best supportive care; CT: chemotherapy; MST: median survival time; RT: radiotherapy. NSCLC Collaborative Group BMJ 1995.
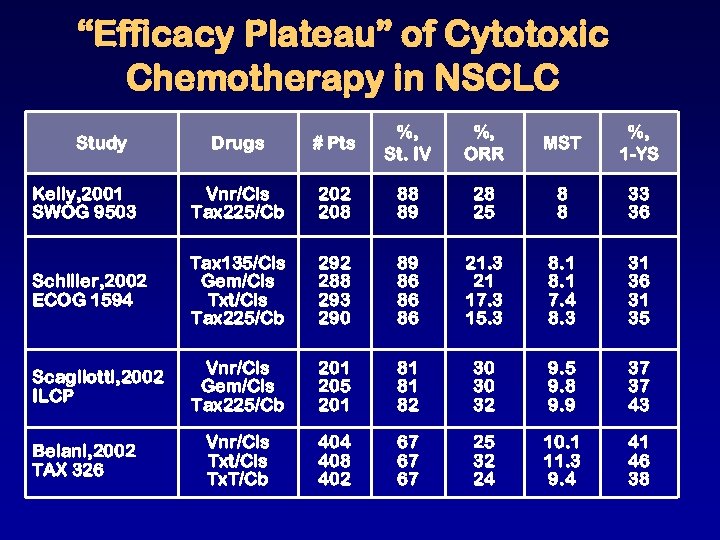 “Efficacy Plateau” of Cytotoxic Chemotherapy in NSCLC Drugs # Pts %, St. IV %, ORR MST %, 1 -YS Kelly, 2001 SWOG 9503 Vnr/Cis Tax 225/Cb 202 208 88 89 28 25 8 8 33 36 Schiller, 2002 ECOG 1594 Tax 135/Cis Gem/Cis Txt/Cis Tax 225/Cb 292 288 293 290 89 86 86 86 21. 3 21 17. 3 15. 3 8. 1 7. 4 8. 3 31 36 31 35 Scagliotti, 2002 ILCP Vnr/Cis Gem/Cis Tax 225/Cb 201 205 201 81 81 82 30 30 32 9. 5 9. 8 9. 9 37 37 43 Vnr/Cis Txt/Cis Tx. T/Cb 404 408 402 67 67 67 25 32 24 10. 1 11. 3 9. 4 41 46 38 Study Belani, 2002 TAX 326
“Efficacy Plateau” of Cytotoxic Chemotherapy in NSCLC Drugs # Pts %, St. IV %, ORR MST %, 1 -YS Kelly, 2001 SWOG 9503 Vnr/Cis Tax 225/Cb 202 208 88 89 28 25 8 8 33 36 Schiller, 2002 ECOG 1594 Tax 135/Cis Gem/Cis Txt/Cis Tax 225/Cb 292 288 293 290 89 86 86 86 21. 3 21 17. 3 15. 3 8. 1 7. 4 8. 3 31 36 31 35 Scagliotti, 2002 ILCP Vnr/Cis Gem/Cis Tax 225/Cb 201 205 201 81 81 82 30 30 32 9. 5 9. 8 9. 9 37 37 43 Vnr/Cis Txt/Cis Tx. T/Cb 404 408 402 67 67 67 25 32 24 10. 1 11. 3 9. 4 41 46 38 Study Belani, 2002 TAX 326
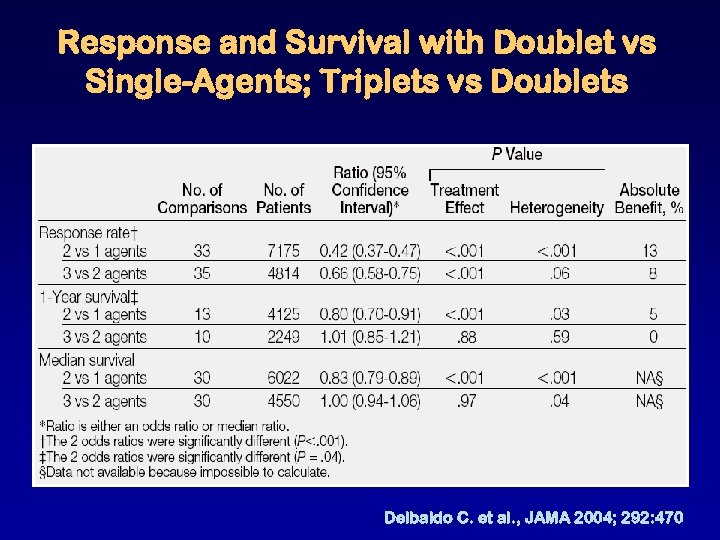 Response and Survival with Doublet vs Single-Agents; Triplets vs Doublets Delbaldo C. et al. , JAMA 2004; 292: 470
Response and Survival with Doublet vs Single-Agents; Triplets vs Doublets Delbaldo C. et al. , JAMA 2004; 292: 470
 New Cytotoxics in Lung Cancer • Platinum Analogs • New Antimetabolites (Pemetrexed, Raltitrexed) • Second Generation Taxanes • Epothilons • Hypoxic Cytotoxins • Oral Agents (Vinorelbine, Taxanes, Camptothecins) • Cell Cycle Inhibitors
New Cytotoxics in Lung Cancer • Platinum Analogs • New Antimetabolites (Pemetrexed, Raltitrexed) • Second Generation Taxanes • Epothilons • Hypoxic Cytotoxins • Oral Agents (Vinorelbine, Taxanes, Camptothecins) • Cell Cycle Inhibitors
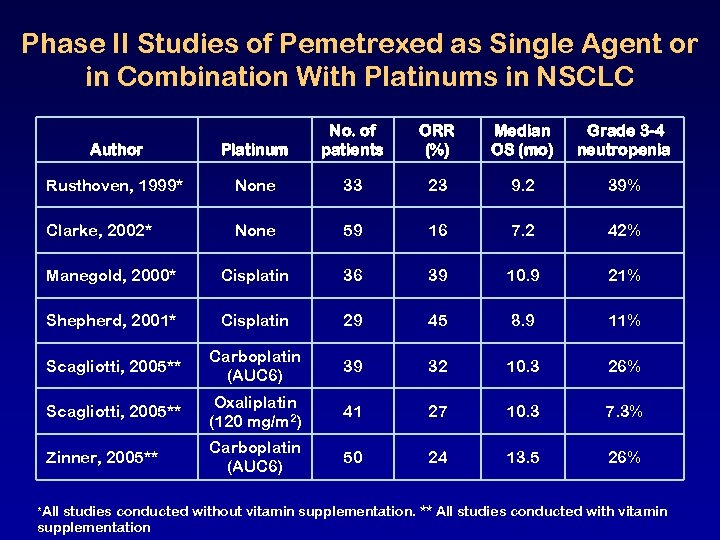 Phase II Studies of Pemetrexed as Single Agent or in Combination With Platinums in NSCLC Author Platinum No. of patients ORR (%) Median OS (mo) Grade 3 -4 neutropenia Rusthoven, 1999* None 33 23 9. 2 39% Clarke, 2002* None 59 16 7. 2 42% Manegold, 2000* Cisplatin 36 39 10. 9 21% Shepherd, 2001* Cisplatin 29 45 8. 9 11% Scagliotti, 2005** Carboplatin (AUC 6) 39 32 10. 3 26% Scagliotti, 2005** Oxaliplatin (120 mg/m 2) 41 27 10. 3 7. 3% Zinner, 2005** Carboplatin (AUC 6) 50 24 13. 5 26% *All studies conducted without vitamin supplementation. ** All studies conducted with vitamin supplementation
Phase II Studies of Pemetrexed as Single Agent or in Combination With Platinums in NSCLC Author Platinum No. of patients ORR (%) Median OS (mo) Grade 3 -4 neutropenia Rusthoven, 1999* None 33 23 9. 2 39% Clarke, 2002* None 59 16 7. 2 42% Manegold, 2000* Cisplatin 36 39 10. 9 21% Shepherd, 2001* Cisplatin 29 45 8. 9 11% Scagliotti, 2005** Carboplatin (AUC 6) 39 32 10. 3 26% Scagliotti, 2005** Oxaliplatin (120 mg/m 2) 41 27 10. 3 7. 3% Zinner, 2005** Carboplatin (AUC 6) 50 24 13. 5 26% *All studies conducted without vitamin supplementation. ** All studies conducted with vitamin supplementation
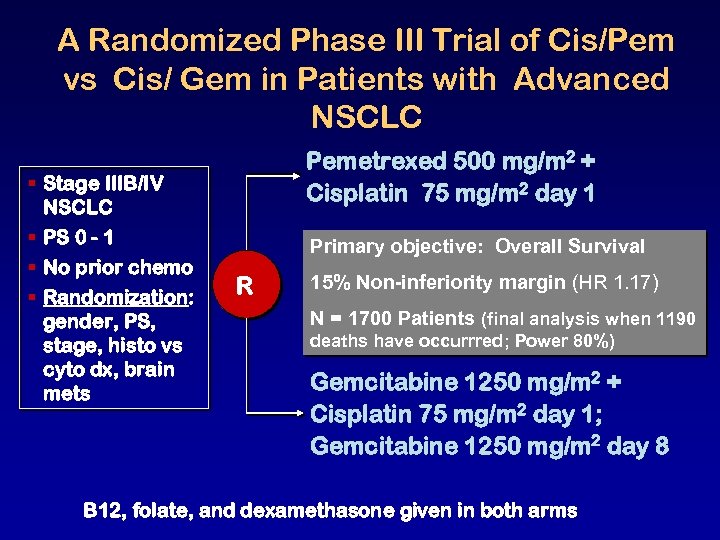 A Randomized Phase III Trial of Cis/Pem vs Cis/ Gem in Patients with Advanced NSCLC § Stage IIIB/IV NSCLC § PS 0 - 1 § No prior chemo § Randomization: gender, PS, stage, histo vs cyto dx, brain mets Pemetrexed 500 mg/m 2 + Cisplatin 75 mg/m 2 day 1 Primary objective: Overall Survival R 15% Non-inferiority margin (HR 1. 17) N = 1700 Patients (final analysis when 1190 deaths have occurrred; Power 80%) Gemcitabine 1250 mg/m 2 + Cisplatin 75 mg/m 2 day 1; Gemcitabine 1250 mg/m 2 day 8 B 12, folate, and dexamethasone given in both arms
A Randomized Phase III Trial of Cis/Pem vs Cis/ Gem in Patients with Advanced NSCLC § Stage IIIB/IV NSCLC § PS 0 - 1 § No prior chemo § Randomization: gender, PS, stage, histo vs cyto dx, brain mets Pemetrexed 500 mg/m 2 + Cisplatin 75 mg/m 2 day 1 Primary objective: Overall Survival R 15% Non-inferiority margin (HR 1. 17) N = 1700 Patients (final analysis when 1190 deaths have occurrred; Power 80%) Gemcitabine 1250 mg/m 2 + Cisplatin 75 mg/m 2 day 1; Gemcitabine 1250 mg/m 2 day 8 B 12, folate, and dexamethasone given in both arms
 Clinical Efficacy and Safety Results § § Overall survival: Cisplatin/Pemetrexed non-inferior to Cisplatin/Gemcitabine – 10. 3 vs 10. 3 mo [HR 0. 94, 95% CI: 0. 84 1. 05] PFS and ORR: Cisplatin/Pemetrexed non-inferior to Cisplatin/Gemcitabine – – PFS: 4. 8 vs 5. 1 mo [HR 1. 04, 95% CI: 0. 94 1. 15] ORR: 31% vs 28% Cisplatin/Pemetrexed appears to have better efficacy in adenocarcinoma and large cell carcinoma than in squamous cell carcinoma Cisplatin/Pemetrexed showed a significantly better safety profile than Cisplatin/Gemcitabine Scagliotti G. et al. Proc. JTO 2007
Clinical Efficacy and Safety Results § § Overall survival: Cisplatin/Pemetrexed non-inferior to Cisplatin/Gemcitabine – 10. 3 vs 10. 3 mo [HR 0. 94, 95% CI: 0. 84 1. 05] PFS and ORR: Cisplatin/Pemetrexed non-inferior to Cisplatin/Gemcitabine – – PFS: 4. 8 vs 5. 1 mo [HR 1. 04, 95% CI: 0. 94 1. 15] ORR: 31% vs 28% Cisplatin/Pemetrexed appears to have better efficacy in adenocarcinoma and large cell carcinoma than in squamous cell carcinoma Cisplatin/Pemetrexed showed a significantly better safety profile than Cisplatin/Gemcitabine Scagliotti G. et al. Proc. JTO 2007
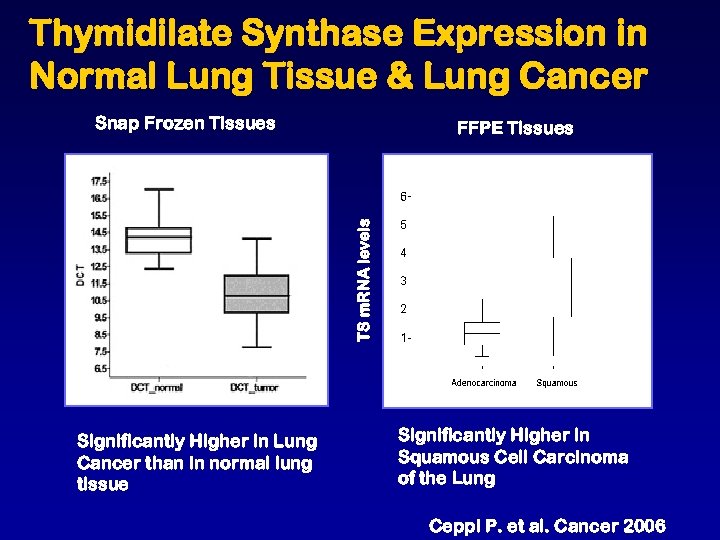 Thymidilate Synthase Expression in Normal Lung Tissue & Lung Cancer Snap Frozen Tissues TS m. RNA levels FFPE Tissues Significantly Higher in Lung Cancer than in normal lung tissue Significantly Higher in Squamous Cell Carcinoma of the Lung Ceppi P. et al. Cancer 2006
Thymidilate Synthase Expression in Normal Lung Tissue & Lung Cancer Snap Frozen Tissues TS m. RNA levels FFPE Tissues Significantly Higher in Lung Cancer than in normal lung tissue Significantly Higher in Squamous Cell Carcinoma of the Lung Ceppi P. et al. Cancer 2006
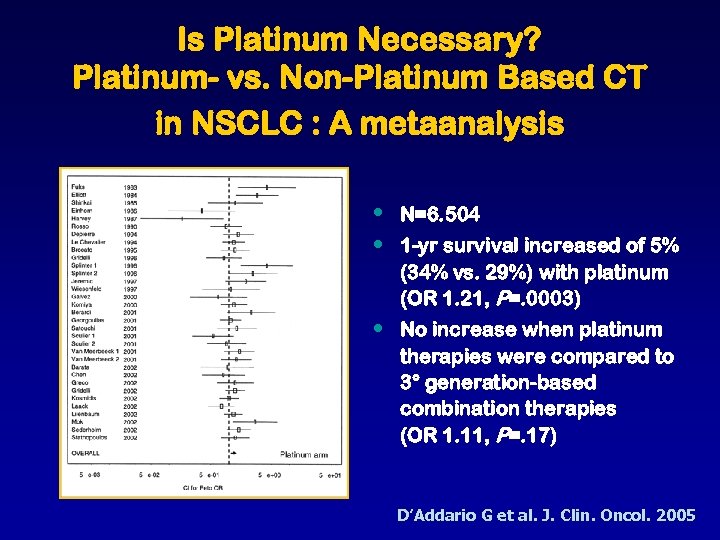 Is Platinum Necessary? Platinum- vs. Non-Platinum Based CT in NSCLC : A metaanalysis • • N=6. 504 • No increase when platinum therapies were compared to 3° generation-based combination therapies (OR 1. 11, P=. 17) 1 -yr survival increased of 5% (34% vs. 29%) with platinum (OR 1. 21, P=. 0003) D’Addario G et al. J. Clin. Oncol. 2005
Is Platinum Necessary? Platinum- vs. Non-Platinum Based CT in NSCLC : A metaanalysis • • N=6. 504 • No increase when platinum therapies were compared to 3° generation-based combination therapies (OR 1. 11, P=. 17) 1 -yr survival increased of 5% (34% vs. 29%) with platinum (OR 1. 21, P=. 0003) D’Addario G et al. J. Clin. Oncol. 2005
 A New Platform for Clinical Research in Lung Cancer • Implementation of pharmacogenomic research (study of genome-derived data, including human genetic variation, RNA and protein expression differences, to predict drug response in individual patients or groups of patients) • Integration of molecularly targeted therapies
A New Platform for Clinical Research in Lung Cancer • Implementation of pharmacogenomic research (study of genome-derived data, including human genetic variation, RNA and protein expression differences, to predict drug response in individual patients or groups of patients) • Integration of molecularly targeted therapies
 What’s the Clinical Promise? • Focused treatment by pre-identifying genetic backgrounds likely to respond. • Reduce adverse events by predicting who is at risk • In other words TO INCREASE THERAPEUTIC MARGINS • Way to save drugs in the pipeline that are very effective only in subpopulations. • Better understanding of drug interactions
What’s the Clinical Promise? • Focused treatment by pre-identifying genetic backgrounds likely to respond. • Reduce adverse events by predicting who is at risk • In other words TO INCREASE THERAPEUTIC MARGINS • Way to save drugs in the pipeline that are very effective only in subpopulations. • Better understanding of drug interactions
 Pharmacogenomics Human Genetics • SNPs • Haplotypes • Sequencing Expression Profiling • Specific transcript levels • Total RNA profiling Phenotype • Drug response Proteomics • Specific biochemical markers • Protein profiling • Disease Prediction
Pharmacogenomics Human Genetics • SNPs • Haplotypes • Sequencing Expression Profiling • Specific transcript levels • Total RNA profiling Phenotype • Drug response Proteomics • Specific biochemical markers • Protein profiling • Disease Prediction
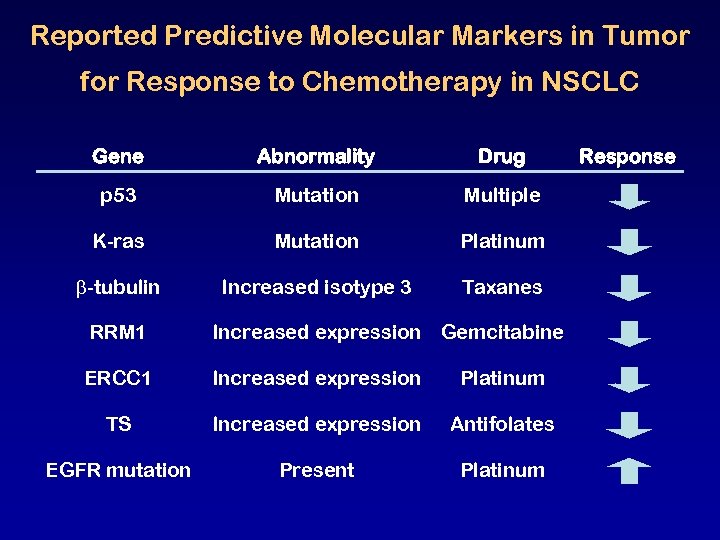 Reported Predictive Molecular Markers in Tumor for Response to Chemotherapy in NSCLC Gene Abnormality Drug p 53 Mutation Multiple K-ras Mutation Platinum -tubulin Increased isotype 3 Taxanes RRM 1 Increased expression Gemcitabine ERCC 1 Increased expression Platinum TS Increased expression Antifolates EGFR mutation Present Platinum Response
Reported Predictive Molecular Markers in Tumor for Response to Chemotherapy in NSCLC Gene Abnormality Drug p 53 Mutation Multiple K-ras Mutation Platinum -tubulin Increased isotype 3 Taxanes RRM 1 Increased expression Gemcitabine ERCC 1 Increased expression Platinum TS Increased expression Antifolates EGFR mutation Present Platinum Response
 Gene Expression and Lung Cancer • ERCC 1, RRM 1, BRCA 1, TS • Mainly retrospective studies, few prospective • Not fully validated techniques, frozen vs. paraffin-embedded tissues, different house-keeping genes, different cut-off values • Predictive vs. prognostic information
Gene Expression and Lung Cancer • ERCC 1, RRM 1, BRCA 1, TS • Mainly retrospective studies, few prospective • Not fully validated techniques, frozen vs. paraffin-embedded tissues, different house-keeping genes, different cut-off values • Predictive vs. prognostic information
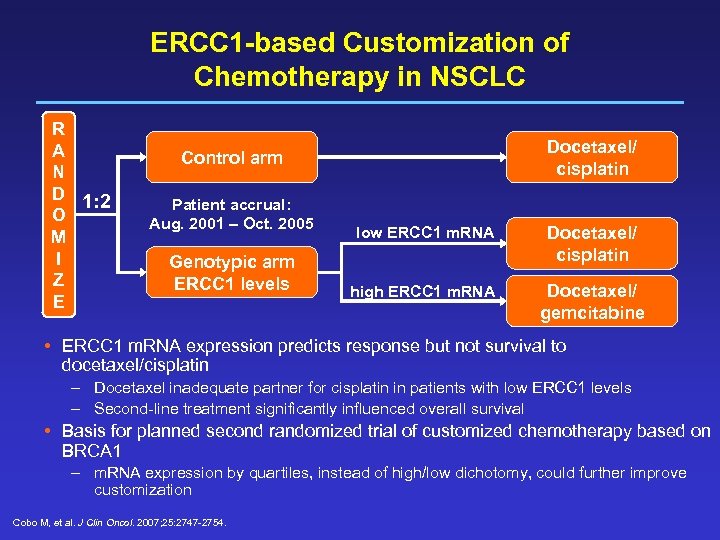 ERCC 1 -based Customization of Chemotherapy in NSCLC R A N D 1: 2 O M I Z E Docetaxel/ cisplatin Control arm Patient accrual: Aug. 2001 – Oct. 2005 Genotypic arm ERCC 1 levels low ERCC 1 m. RNA Docetaxel/ cisplatin high ERCC 1 m. RNA Docetaxel/ gemcitabine • ERCC 1 m. RNA expression predicts response but not survival to docetaxel/cisplatin – Docetaxel inadequate partner for cisplatin in patients with low ERCC 1 levels – Second-line treatment significantly influenced overall survival • Basis for planned second randomized trial of customized chemotherapy based on BRCA 1 – m. RNA expression by quartiles, instead of high/low dichotomy, could further improve customization Cobo M, et al. J Clin Oncol. 2007; 25: 2747 -2754.
ERCC 1 -based Customization of Chemotherapy in NSCLC R A N D 1: 2 O M I Z E Docetaxel/ cisplatin Control arm Patient accrual: Aug. 2001 – Oct. 2005 Genotypic arm ERCC 1 levels low ERCC 1 m. RNA Docetaxel/ cisplatin high ERCC 1 m. RNA Docetaxel/ gemcitabine • ERCC 1 m. RNA expression predicts response but not survival to docetaxel/cisplatin – Docetaxel inadequate partner for cisplatin in patients with low ERCC 1 levels – Second-line treatment significantly influenced overall survival • Basis for planned second randomized trial of customized chemotherapy based on BRCA 1 – m. RNA expression by quartiles, instead of high/low dichotomy, could further improve customization Cobo M, et al. J Clin Oncol. 2007; 25: 2747 -2754.
 ITACA Adjuvant Trial Pharmacogenomic : Yes or No Taxanes High Profile 4 Control TS High Pem Low High ERCC 1 Low Profile 3 Profile 2 Control Cis/Gem Control TS Cis/Pem Low Profile 1 Control = Investigators’ choice; Primary end-point =overall survival; Sample size =700 patients
ITACA Adjuvant Trial Pharmacogenomic : Yes or No Taxanes High Profile 4 Control TS High Pem Low High ERCC 1 Low Profile 3 Profile 2 Control Cis/Gem Control TS Cis/Pem Low Profile 1 Control = Investigators’ choice; Primary end-point =overall survival; Sample size =700 patients
 Challenges for Tomorrow • Inherited component of response to drugs is often polygenic • Two strategies : SNP maps to perform genomewide searches or candidate gene approach • Both with values and limitations • Gene-expression profiling and proteomic studies are evolving fields • Need for well characterized patients who have been uniformly treated and systematically evaluated • Pharmacogenomic relation validated for each therapeutic indication and in different racial and etnic groups.
Challenges for Tomorrow • Inherited component of response to drugs is often polygenic • Two strategies : SNP maps to perform genomewide searches or candidate gene approach • Both with values and limitations • Gene-expression profiling and proteomic studies are evolving fields • Need for well characterized patients who have been uniformly treated and systematically evaluated • Pharmacogenomic relation validated for each therapeutic indication and in different racial and etnic groups.
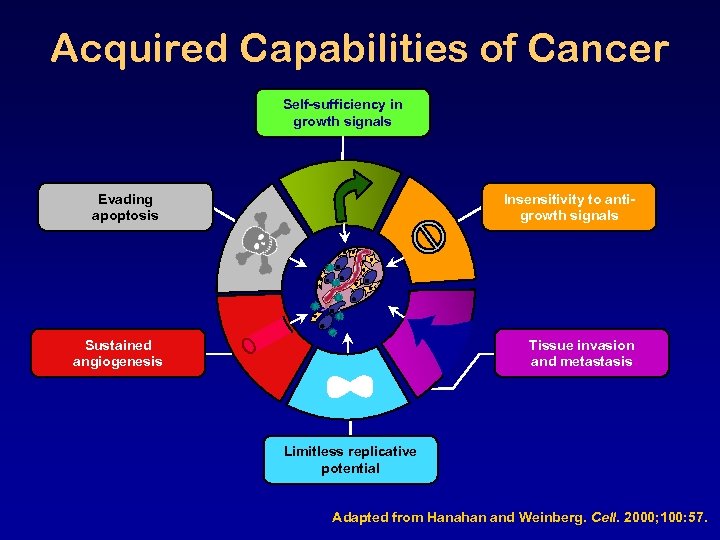 Acquired Capabilities of Cancer Self-sufficiency in growth signals Evading apoptosis Insensitivity to antigrowth signals Sustained angiogenesis Tissue invasion and metastasis Limitless replicative potential Adapted from Hanahan and Weinberg. Cell. 2000; 100: 57.
Acquired Capabilities of Cancer Self-sufficiency in growth signals Evading apoptosis Insensitivity to antigrowth signals Sustained angiogenesis Tissue invasion and metastasis Limitless replicative potential Adapted from Hanahan and Weinberg. Cell. 2000; 100: 57.
 Targeted Therapies are Drugs to Treat : • Biologically homogenous cancer patient population • Tumor specific molecular abnormality • Tumor specific molecular profile • Expression of a specific receptor or antigen • Different cancers likely to require different therapies • Patient population likely to be small
Targeted Therapies are Drugs to Treat : • Biologically homogenous cancer patient population • Tumor specific molecular abnormality • Tumor specific molecular profile • Expression of a specific receptor or antigen • Different cancers likely to require different therapies • Patient population likely to be small
 Most Attractive Targets for Molecular-Targeted Strategies • • • Receptor & non-receptor tyrosine kinases Ras/raf/MAPK pathway PI 3 K/Akt/PTEN pathway Proteosome inhibition Gene expression modification with antisense oligonucleotide and RNAi • Endothelial cell & angiogenesis-associated factors (i. e. , VEGF) • Modulation of Apoptosis
Most Attractive Targets for Molecular-Targeted Strategies • • • Receptor & non-receptor tyrosine kinases Ras/raf/MAPK pathway PI 3 K/Akt/PTEN pathway Proteosome inhibition Gene expression modification with antisense oligonucleotide and RNAi • Endothelial cell & angiogenesis-associated factors (i. e. , VEGF) • Modulation of Apoptosis
 Most Attractive Targets for Molecular-Targeted Strategies • • • Receptor & non-receptor tyrosine kinases Ras/raf/MAPK pathway PI 3 K/Akt/PTEN pathway Proteosome inhibition Gene expression modification with antisense oligonucleotide and RNAi • Endothelial cell & angiogenesis-associated factors (i. e. , VEGF) • Modulation of Apoptosis
Most Attractive Targets for Molecular-Targeted Strategies • • • Receptor & non-receptor tyrosine kinases Ras/raf/MAPK pathway PI 3 K/Akt/PTEN pathway Proteosome inhibition Gene expression modification with antisense oligonucleotide and RNAi • Endothelial cell & angiogenesis-associated factors (i. e. , VEGF) • Modulation of Apoptosis
![Doublets & Targeted Therapies Trial OS [m] 1 -Y [%] RR [%] INTACT 1 Doublets & Targeted Therapies Trial OS [m] 1 -Y [%] RR [%] INTACT 1](https://present5.com/presentation/3ad49e01341573a158464b0f633369ae/image-27.jpg) Doublets & Targeted Therapies Trial OS [m] 1 -Y [%] RR [%] INTACT 1 364 x 3 9. 9 -10. 9 41 -44% 47 -51% INTACT 2 406 x 3 8. 7 -9. 9 37 -42% 29 -30% TALENT 586 x 2 9. 9 -10. 1 TRIBUTE 529 x 2 10. 6 -10. 8 19 -22% Affinitak ? x 2 10. 0 -10. 4 29 -35% Affinitak 300 x 2 9. 7 -10. 0 SPIRIT 1 311 x 2 8. 7 -9. 9 SPIRIT 2 306 x 2 8. 5 -9. 2 E 4599 427 x 2 10. 2 12. 5 41 -42% 36 -37% 44 10 52% 27%
Doublets & Targeted Therapies Trial OS [m] 1 -Y [%] RR [%] INTACT 1 364 x 3 9. 9 -10. 9 41 -44% 47 -51% INTACT 2 406 x 3 8. 7 -9. 9 37 -42% 29 -30% TALENT 586 x 2 9. 9 -10. 1 TRIBUTE 529 x 2 10. 6 -10. 8 19 -22% Affinitak ? x 2 10. 0 -10. 4 29 -35% Affinitak 300 x 2 9. 7 -10. 0 SPIRIT 1 311 x 2 8. 7 -9. 9 SPIRIT 2 306 x 2 8. 5 -9. 2 E 4599 427 x 2 10. 2 12. 5 41 -42% 36 -37% 44 10 52% 27%
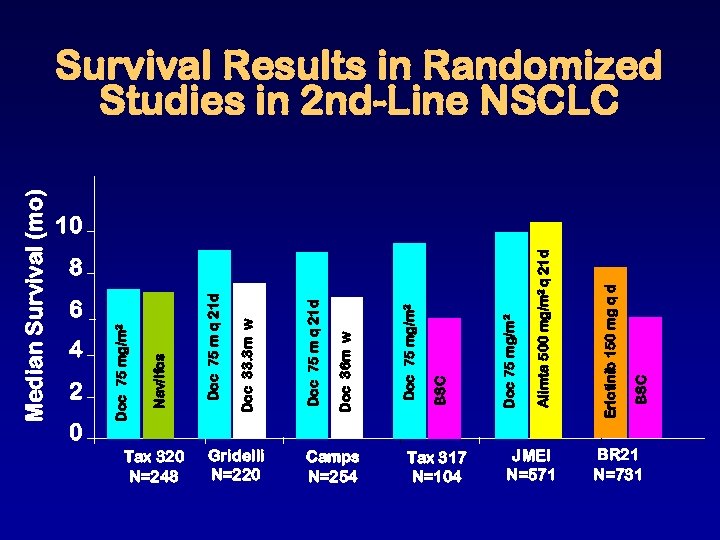 Median Survival (mo) 4 2 0 6 Tax 320 N=248 Gridelli N=220 Camps N=254 Tax 317 N=104 8 JMEI N=571 BSC Erlotinib 150 mg q d Alimta 500 mg/m 2 q 21 d Doc 75 mg/m 2 BSC Doc 75 mg/m 2 Doc 36 m w Doc 75 m q 21 d Doc 33. 3 m w Doc 75 m q 21 d Nav/Ifos Doc 75 mg/m 2 Survival Results in Randomized Studies in 2 nd-Line NSCLC 10 BR 21 N=731
Median Survival (mo) 4 2 0 6 Tax 320 N=248 Gridelli N=220 Camps N=254 Tax 317 N=104 8 JMEI N=571 BSC Erlotinib 150 mg q d Alimta 500 mg/m 2 q 21 d Doc 75 mg/m 2 BSC Doc 75 mg/m 2 Doc 36 m w Doc 75 m q 21 d Doc 33. 3 m w Doc 75 m q 21 d Nav/Ifos Doc 75 mg/m 2 Survival Results in Randomized Studies in 2 nd-Line NSCLC 10 BR 21 N=731
 Determinants of Sensitivity/ Resistance to EGFR-TKIs • Clinical: gender, histology, ethnicity, smoking status • Molecular: EGFR mutations, gene copy number, baseline AKT/MAPK, K-Ras mutations • Post-therapy: skin rash
Determinants of Sensitivity/ Resistance to EGFR-TKIs • Clinical: gender, histology, ethnicity, smoking status • Molecular: EGFR mutations, gene copy number, baseline AKT/MAPK, K-Ras mutations • Post-therapy: skin rash
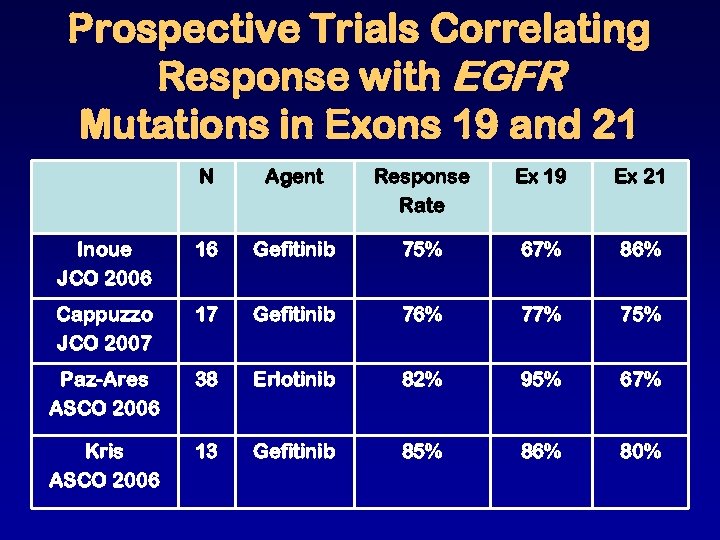 Prospective Trials Correlating Response with EGFR Mutations in Exons 19 and 21 N Agent Response Rate Ex 19 Ex 21 Inoue JCO 2006 16 Gefitinib 75% 67% 86% Cappuzzo JCO 2007 17 Gefitinib 76% 77% 75% Paz-Ares ASCO 2006 38 Erlotinib 82% 95% 67% Kris ASCO 2006 13 Gefitinib 85% 86% 80%
Prospective Trials Correlating Response with EGFR Mutations in Exons 19 and 21 N Agent Response Rate Ex 19 Ex 21 Inoue JCO 2006 16 Gefitinib 75% 67% 86% Cappuzzo JCO 2007 17 Gefitinib 76% 77% 75% Paz-Ares ASCO 2006 38 Erlotinib 82% 95% 67% Kris ASCO 2006 13 Gefitinib 85% 86% 80%
 Phase III Trial of Second-line Gefitinib 250 mg vs Docetaxel in NSCLC (INTEREST): OS Overall 1. 0 High EGFR Gene Copy Number 1. 0 Docetaxel (n = 710) Docetaxel (n = 89) 0. 8 Median OS: 8. 0 vs 7. 6 mo HR (95% CI): 1. 02 (0. 91 -1. 15) 1 -year survival: 34 vs 32% 0. 6 0. 4 0. 2 0. 0 0 8 16 24 32 40 Gefitinib (n = 85) Probability of Survival Gefitinib (n = 723) 0. 8 Median OS: 7. 5 vs 8. 4 mo HR (95% CI): 1. 09 (0. 78 -1. 51) 1 -year survival: 35 vs 32% 0. 6 0. 4 0. 2 0. 0 0 8 16 24 32 40 Months Conclude noninferiority in the overall population Conclude no statistical superiority in EGFR FISH+ patients
Phase III Trial of Second-line Gefitinib 250 mg vs Docetaxel in NSCLC (INTEREST): OS Overall 1. 0 High EGFR Gene Copy Number 1. 0 Docetaxel (n = 710) Docetaxel (n = 89) 0. 8 Median OS: 8. 0 vs 7. 6 mo HR (95% CI): 1. 02 (0. 91 -1. 15) 1 -year survival: 34 vs 32% 0. 6 0. 4 0. 2 0. 0 0 8 16 24 32 40 Gefitinib (n = 85) Probability of Survival Gefitinib (n = 723) 0. 8 Median OS: 7. 5 vs 8. 4 mo HR (95% CI): 1. 09 (0. 78 -1. 51) 1 -year survival: 35 vs 32% 0. 6 0. 4 0. 2 0. 0 0 8 16 24 32 40 Months Conclude noninferiority in the overall population Conclude no statistical superiority in EGFR FISH+ patients
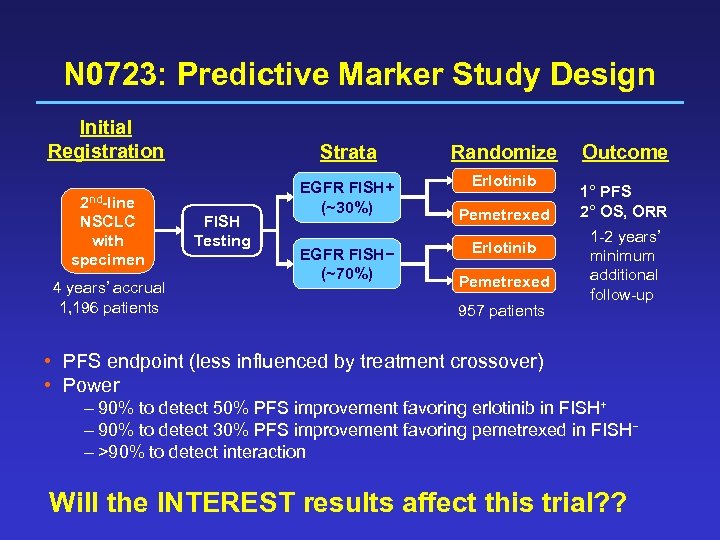 N 0723: Predictive Marker Study Design Initial Registration 2 nd-line NSCLC with specimen 4 years’ accrual 1, 196 patients Strata FISH Testing Randomize EGFR FISH+ (~30%) Erlotinib EGFR FISH− (~70%) Pemetrexed Erlotinib Pemetrexed 957 patients Outcome 1° PFS 2° OS, ORR 1 -2 years’ minimum additional follow-up • PFS endpoint (less influenced by treatment crossover) • Power – 90% to detect 50% PFS improvement favoring erlotinib in FISH+ – 90% to detect 30% PFS improvement favoring pemetrexed in FISH− – >90% to detect interaction Will the INTEREST results affect this trial? ?
N 0723: Predictive Marker Study Design Initial Registration 2 nd-line NSCLC with specimen 4 years’ accrual 1, 196 patients Strata FISH Testing Randomize EGFR FISH+ (~30%) Erlotinib EGFR FISH− (~70%) Pemetrexed Erlotinib Pemetrexed 957 patients Outcome 1° PFS 2° OS, ORR 1 -2 years’ minimum additional follow-up • PFS endpoint (less influenced by treatment crossover) • Power – 90% to detect 50% PFS improvement favoring erlotinib in FISH+ – 90% to detect 30% PFS improvement favoring pemetrexed in FISH− – >90% to detect interaction Will the INTEREST results affect this trial? ?
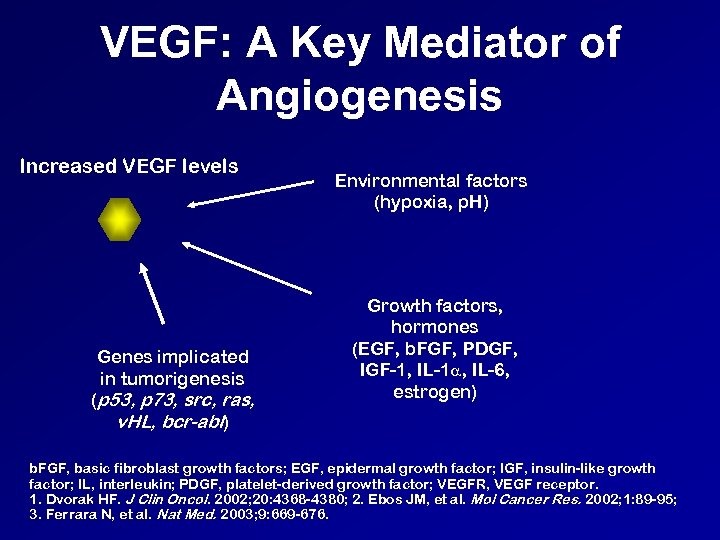 VEGF: A Key Mediator of Angiogenesis Increased VEGF levels Genes implicated in tumorigenesis (p 53, p 73, src, ras, v. HL, bcr-abl) Environmental factors (hypoxia, p. H) Growth factors, hormones (EGF, b. FGF, PDGF, IGF-1, IL-1 , IL-6, estrogen) b. FGF, basic fibroblast growth factors; EGF, epidermal growth factor; IGF, insulin-like growth factor; IL, interleukin; PDGF, platelet-derived growth factor; VEGFR, VEGF receptor. 1. Dvorak HF. J Clin Oncol. 2002; 20: 4368 -4380; 2. Ebos JM, et al. Mol Cancer Res. 2002; 1: 89 -95; 3. Ferrara N, et al. Nat Med. 2003; 9: 669 -676.
VEGF: A Key Mediator of Angiogenesis Increased VEGF levels Genes implicated in tumorigenesis (p 53, p 73, src, ras, v. HL, bcr-abl) Environmental factors (hypoxia, p. H) Growth factors, hormones (EGF, b. FGF, PDGF, IGF-1, IL-1 , IL-6, estrogen) b. FGF, basic fibroblast growth factors; EGF, epidermal growth factor; IGF, insulin-like growth factor; IL, interleukin; PDGF, platelet-derived growth factor; VEGFR, VEGF receptor. 1. Dvorak HF. J Clin Oncol. 2002; 20: 4368 -4380; 2. Ebos JM, et al. Mol Cancer Res. 2002; 1: 89 -95; 3. Ferrara N, et al. Nat Med. 2003; 9: 669 -676.
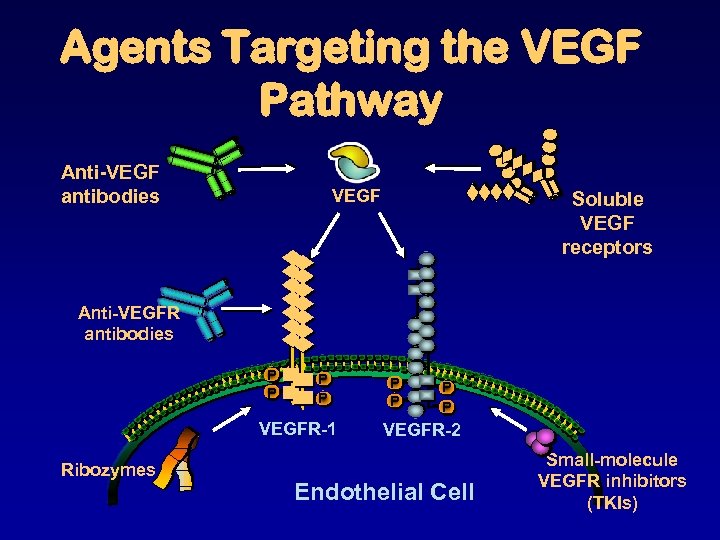 Agents Targeting the VEGF Pathway Anti-VEGF antibodies VEGF Soluble VEGF receptors Anti-VEGFR antibodies P P VEGFR-1 Ribozymes P P VEGFR-2 Endothelial Cell Small-molecule VEGFR inhibitors (TKIs)
Agents Targeting the VEGF Pathway Anti-VEGF antibodies VEGF Soluble VEGF receptors Anti-VEGFR antibodies P P VEGFR-1 Ribozymes P P VEGFR-2 Endothelial Cell Small-molecule VEGFR inhibitors (TKIs)
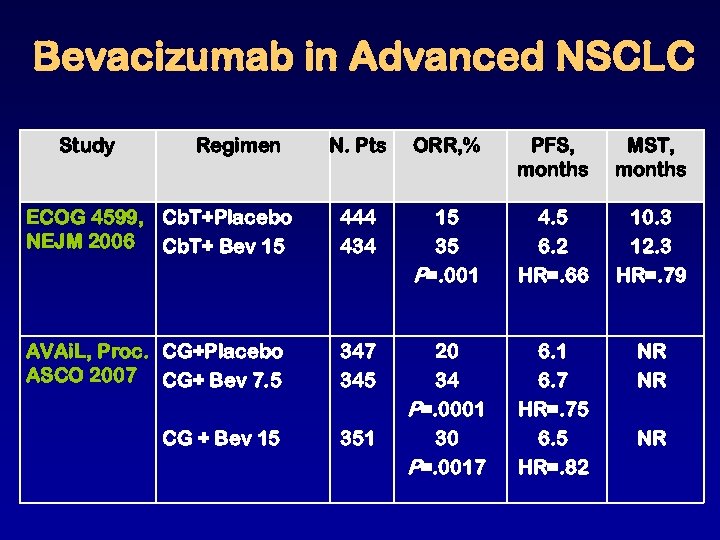 Bevacizumab in Advanced NSCLC Study Regimen N. Pts ORR, % PFS, months MST, months ECOG 4599, Cb. T+Placebo NEJM 2006 Cb. T+ Bev 15 444 434 15 35 P=. 001 4. 5 6. 2 HR=. 66 10. 3 12. 3 HR=. 79 AVAi. L, Proc. CG+Placebo ASCO 2007 CG+ Bev 7. 5 347 345 351 6. 7 HR=. 75 6. 5 HR=. 82 NR NR CG + Bev 15 20 34 P=. 0001 30 P=. 0017 NR
Bevacizumab in Advanced NSCLC Study Regimen N. Pts ORR, % PFS, months MST, months ECOG 4599, Cb. T+Placebo NEJM 2006 Cb. T+ Bev 15 444 434 15 35 P=. 001 4. 5 6. 2 HR=. 66 10. 3 12. 3 HR=. 79 AVAi. L, Proc. CG+Placebo ASCO 2007 CG+ Bev 7. 5 347 345 351 6. 7 HR=. 75 6. 5 HR=. 82 NR NR CG + Bev 15 20 34 P=. 0001 30 P=. 0017 NR
 Anti-VEGF Class Toxicities • • • Hypertension Fatigue Increased clotting events Bleeding (epistaxis, pulmonary hemorrhage, tumor associated) Headache Neurologic events • • Increased LFTs Pain at tumor sites Proteinuria Hypothyroidism?
Anti-VEGF Class Toxicities • • • Hypertension Fatigue Increased clotting events Bleeding (epistaxis, pulmonary hemorrhage, tumor associated) Headache Neurologic events • • Increased LFTs Pain at tumor sites Proteinuria Hypothyroidism?
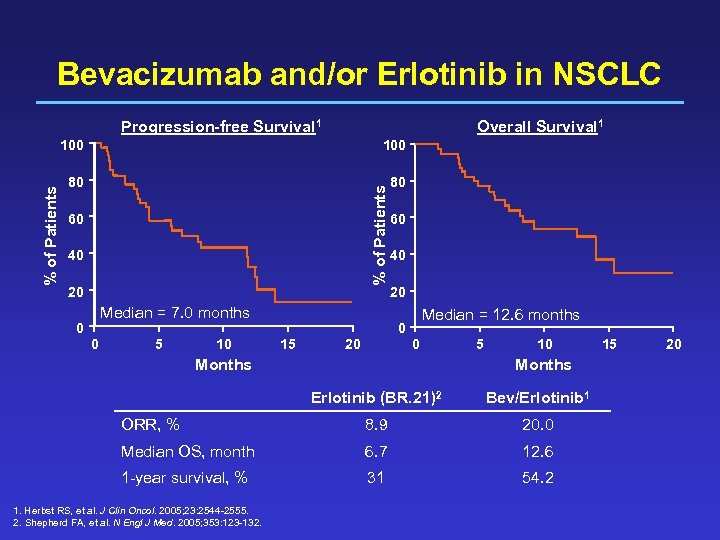 Bevacizumab and/or Erlotinib in NSCLC Progression-free Survival 1 Overall Survival 1 100 80 80 % of Patients 100 60 40 20 Median = 7. 0 months 0 0 5 10 60 40 20 Median = 12. 6 months 0 15 0 20 Months 5 10 Months Erlotinib (BR. 21)2 Bev/Erlotinib 1 ORR, % 8. 9 20. 0 Median OS, month 6. 7 12. 6 1 -year survival, % 31 54. 2 1. Herbst RS, et al. J Clin Oncol. 2005; 23: 2544 -2555. 2. Shepherd FA, et al. N Engl J Med. 2005; 353: 123 -132. 15 20
Bevacizumab and/or Erlotinib in NSCLC Progression-free Survival 1 Overall Survival 1 100 80 80 % of Patients 100 60 40 20 Median = 7. 0 months 0 0 5 10 60 40 20 Median = 12. 6 months 0 15 0 20 Months 5 10 Months Erlotinib (BR. 21)2 Bev/Erlotinib 1 ORR, % 8. 9 20. 0 Median OS, month 6. 7 12. 6 1 -year survival, % 31 54. 2 1. Herbst RS, et al. J Clin Oncol. 2005; 23: 2544 -2555. 2. Shepherd FA, et al. N Engl J Med. 2005; 353: 123 -132. 15 20
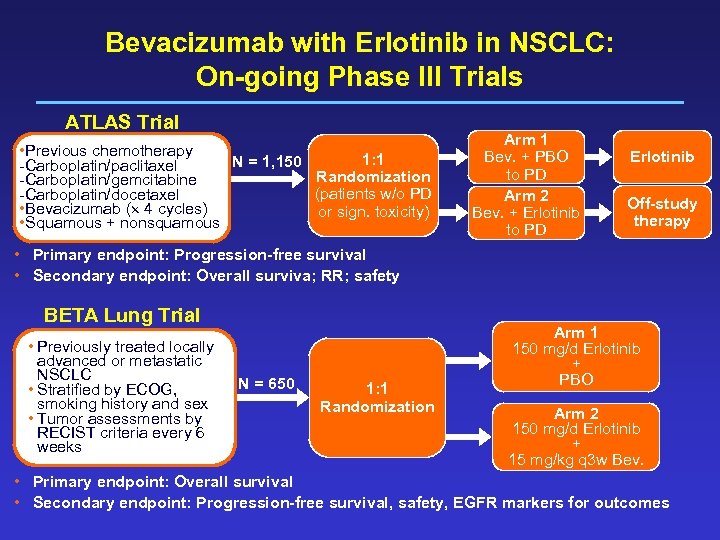 Bevacizumab with Erlotinib in NSCLC: On-going Phase III Trials ATLAS Trial • Previous chemotherapy 1: 1 N = 1, 150 -Carboplatin/paclitaxel Randomization -Carboplatin/gemcitabine (patients w/o PD -Carboplatin/docetaxel • Bevacizumab ( 4 cycles) or sign. toxicity) • Squamous + nonsquamous Arm 1 Bev. + PBO to PD Arm 2 Bev. + Erlotinib to PD Erlotinib Off-study therapy • Primary endpoint: Progression-free survival • Secondary endpoint: Overall surviva; RR; safety BETA Lung Trial • Previously treated locally advanced or metastatic NSCLC • Stratified by ECOG, smoking history and sex • Tumor assessments by RECIST criteria every 6 weeks N = 650 1: 1 Randomization Arm 1 150 mg/d Erlotinib + PBO Arm 2 150 mg/d Erlotinib + 15 mg/kg q 3 w Bev. • Primary endpoint: Overall survival • Secondary endpoint: Progression-free survival, safety, EGFR markers for outcomes
Bevacizumab with Erlotinib in NSCLC: On-going Phase III Trials ATLAS Trial • Previous chemotherapy 1: 1 N = 1, 150 -Carboplatin/paclitaxel Randomization -Carboplatin/gemcitabine (patients w/o PD -Carboplatin/docetaxel • Bevacizumab ( 4 cycles) or sign. toxicity) • Squamous + nonsquamous Arm 1 Bev. + PBO to PD Arm 2 Bev. + Erlotinib to PD Erlotinib Off-study therapy • Primary endpoint: Progression-free survival • Secondary endpoint: Overall surviva; RR; safety BETA Lung Trial • Previously treated locally advanced or metastatic NSCLC • Stratified by ECOG, smoking history and sex • Tumor assessments by RECIST criteria every 6 weeks N = 650 1: 1 Randomization Arm 1 150 mg/d Erlotinib + PBO Arm 2 150 mg/d Erlotinib + 15 mg/kg q 3 w Bev. • Primary endpoint: Overall survival • Secondary endpoint: Progression-free survival, safety, EGFR markers for outcomes
 Tumor Angiogenesis: Bevacizumab and Multitargeted Agents Angiogenic growth factors PDGF Pericyte O 2 Tumor cell Endothelial cell Paracrine factors
Tumor Angiogenesis: Bevacizumab and Multitargeted Agents Angiogenic growth factors PDGF Pericyte O 2 Tumor cell Endothelial cell Paracrine factors
 Rationale for Multitargeted Therapy • Agents acting on single molecular targets may have limited long-term effectiveness – acquired resistance occurs in many tumors 1 • Agents or combinations acting on multiple targets have the potential to overcome this problem • Additive toxicities may limit the potential for combining single-target therapies • An alternative strategy is the use of multitargeted agents such as sunitinib or sorafenib in advanced NSCLC 2– 4 1. Haber DA, et al. Cold Spring Harb Symp Quant Biol 2005; 70: 419– 26. 2. Gatzemeier U, et al. J Clin Oncol 2006; 24(June 20 Suppl. ): 18 s(abstract 7002). 3. Liu B, et al. J Clin Oncol 2006; 24(June 20 Suppl. ): 18 s(abstract 17119). 4. Socinski MA, et al. J Clin Oncol 2006; 24(June 20 Suppl. ): 18 s(abstract 7001).
Rationale for Multitargeted Therapy • Agents acting on single molecular targets may have limited long-term effectiveness – acquired resistance occurs in many tumors 1 • Agents or combinations acting on multiple targets have the potential to overcome this problem • Additive toxicities may limit the potential for combining single-target therapies • An alternative strategy is the use of multitargeted agents such as sunitinib or sorafenib in advanced NSCLC 2– 4 1. Haber DA, et al. Cold Spring Harb Symp Quant Biol 2005; 70: 419– 26. 2. Gatzemeier U, et al. J Clin Oncol 2006; 24(June 20 Suppl. ): 18 s(abstract 7002). 3. Liu B, et al. J Clin Oncol 2006; 24(June 20 Suppl. ): 18 s(abstract 17119). 4. Socinski MA, et al. J Clin Oncol 2006; 24(June 20 Suppl. ): 18 s(abstract 7001).
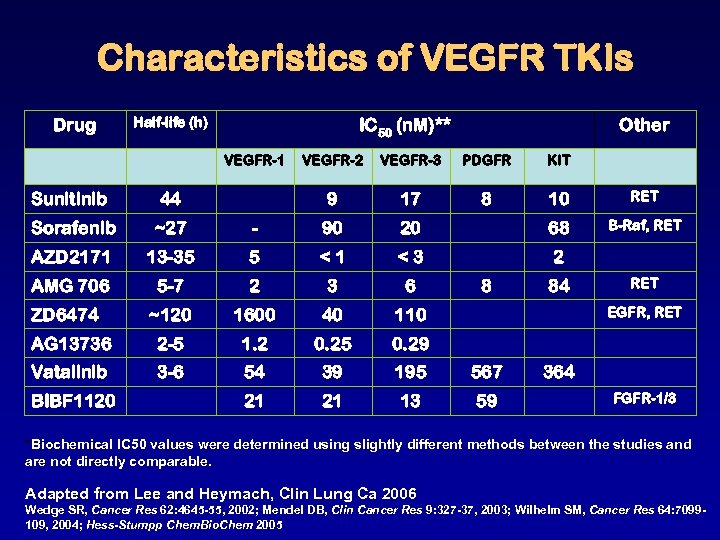 Characteristics of VEGFR TKIs Drug Half-life (h) IC 50 (n. M)** VEGFR-1 Other VEGFR-2 VEGFR-3 PDGFR KIT 9 17 8 10 RET B-Raf, RET Sunitinib 44 Sorafenib ~27 - 90 20 68 AZD 2171 13 -35 5 <1 <3 2 AMG 706 5 -7 2 3 6 ~120 1600 40 110 AG 13736 2 -5 1. 2 0. 25 0. 29 Vatalinib 3 -6 54 39 195 567 21 21 13 59 ZD 6474 BIBF 1120 8 84 RET EGFR, RET 364 FGFR-1/3 *Biochemical IC 50 values were determined using slightly different methods between the studies and are not directly comparable. Adapted from Lee and Heymach, Clin Lung Ca 2006 Wedge SR, Cancer Res 62: 4645 -55, 2002; Mendel DB, Clin Cancer Res 9: 327 -37, 2003; Wilhelm SM, Cancer Res 64: 7099109, 2004; Hess-Stumpp Chem. Bio. Chem 2005
Characteristics of VEGFR TKIs Drug Half-life (h) IC 50 (n. M)** VEGFR-1 Other VEGFR-2 VEGFR-3 PDGFR KIT 9 17 8 10 RET B-Raf, RET Sunitinib 44 Sorafenib ~27 - 90 20 68 AZD 2171 13 -35 5 <1 <3 2 AMG 706 5 -7 2 3 6 ~120 1600 40 110 AG 13736 2 -5 1. 2 0. 25 0. 29 Vatalinib 3 -6 54 39 195 567 21 21 13 59 ZD 6474 BIBF 1120 8 84 RET EGFR, RET 364 FGFR-1/3 *Biochemical IC 50 values were determined using slightly different methods between the studies and are not directly comparable. Adapted from Lee and Heymach, Clin Lung Ca 2006 Wedge SR, Cancer Res 62: 4645 -55, 2002; Mendel DB, Clin Cancer Res 9: 327 -37, 2003; Wilhelm SM, Cancer Res 64: 7099109, 2004; Hess-Stumpp Chem. Bio. Chem 2005
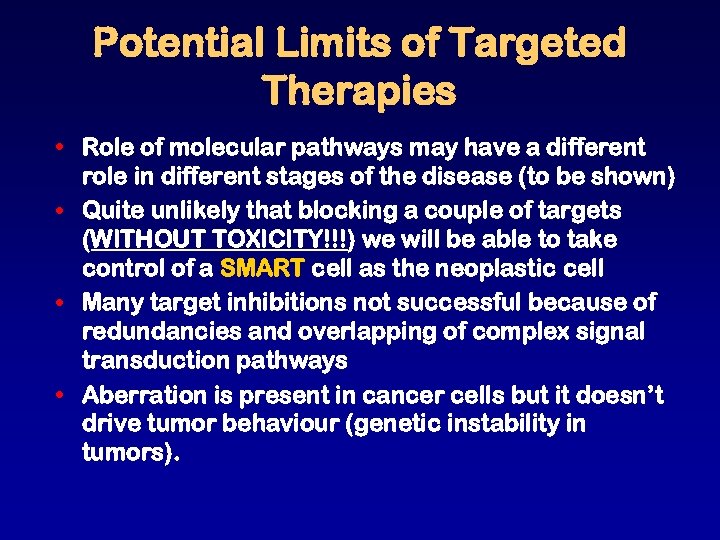 Potential Limits of Targeted Therapies • Role of molecular pathways may have a different role in different stages of the disease (to be shown) • Quite unlikely that blocking a couple of targets (WITHOUT TOXICITY!!!) we will be able to take control of a SMART cell as the neoplastic cell • Many target inhibitions not successful because of redundancies and overlapping of complex signal transduction pathways • Aberration is present in cancer cells but it doesn’t drive tumor behaviour (genetic instability in tumors).
Potential Limits of Targeted Therapies • Role of molecular pathways may have a different role in different stages of the disease (to be shown) • Quite unlikely that blocking a couple of targets (WITHOUT TOXICITY!!!) we will be able to take control of a SMART cell as the neoplastic cell • Many target inhibitions not successful because of redundancies and overlapping of complex signal transduction pathways • Aberration is present in cancer cells but it doesn’t drive tumor behaviour (genetic instability in tumors).
 Probable Scenario in the Coming Years • Cytotoxic chemotherapy will remain the backbone of treatment • Its use will be revisited through pharmacogenomic markers. • Development of rationale combinations of targeted agents, based on their mechanism of action and potentiation. • Identification of subsets of patients. • It will be harder to assess targets when combinations will be used. • Control of MRD or prevention of the recurrence will be a therapeutic goal
Probable Scenario in the Coming Years • Cytotoxic chemotherapy will remain the backbone of treatment • Its use will be revisited through pharmacogenomic markers. • Development of rationale combinations of targeted agents, based on their mechanism of action and potentiation. • Identification of subsets of patients. • It will be harder to assess targets when combinations will be used. • Control of MRD or prevention of the recurrence will be a therapeutic goal
 Most Important “Targeted Therapy” for Lung Cancer
Most Important “Targeted Therapy” for Lung Cancer
 Phase III Trial of Bevacizumab in Non-Squamous NSCLC: ECOG 4599 N=855 (eligible) Eligibility: • Non-squamous NSCLC • No Hx of hemoptysis • No CNS metastases Stratification Variables: • RT vs no RT • Stage IIIB or IV vs recurrent • Wt loss <5% vs >5% • Measurable vs non-measurable (PC) Paclitaxel 200 mg/m 2 Carboplatin AUC = 6 (q 3 weeks) x 6 cycles No crossover to Bevacizumab permitted (PCB) PC x 6 cycles + Bevacizumab (15 mg/kg q 3 wks) to PD Sandler, et al. NEJM, Dec 2006
Phase III Trial of Bevacizumab in Non-Squamous NSCLC: ECOG 4599 N=855 (eligible) Eligibility: • Non-squamous NSCLC • No Hx of hemoptysis • No CNS metastases Stratification Variables: • RT vs no RT • Stage IIIB or IV vs recurrent • Wt loss <5% vs >5% • Measurable vs non-measurable (PC) Paclitaxel 200 mg/m 2 Carboplatin AUC = 6 (q 3 weeks) x 6 cycles No crossover to Bevacizumab permitted (PCB) PC x 6 cycles + Bevacizumab (15 mg/kg q 3 wks) to PD Sandler, et al. NEJM, Dec 2006
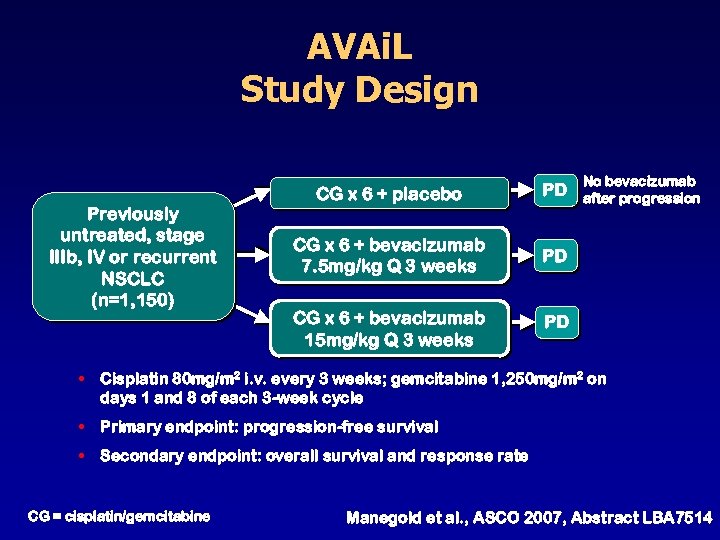 AVAi. L Study Design Previously untreated, stage IIIb, IV or recurrent NSCLC (n=1, 150) CG x 6 + placebo PD CG x 6 + bevacizumab 7. 5 mg/kg Q 3 weeks PD CG x 6 + bevacizumab 15 mg/kg Q 3 weeks No bevacizumab after progression PD • Cisplatin 80 mg/m 2 i. v. every 3 weeks; gemcitabine 1, 250 mg/m 2 on days 1 and 8 of each 3 -week cycle • Primary endpoint: progression-free survival • Secondary endpoint: overall survival and response rate CG = cisplatin/gemcitabine Manegold et al. , ASCO 2007, Abstract LBA 7514
AVAi. L Study Design Previously untreated, stage IIIb, IV or recurrent NSCLC (n=1, 150) CG x 6 + placebo PD CG x 6 + bevacizumab 7. 5 mg/kg Q 3 weeks PD CG x 6 + bevacizumab 15 mg/kg Q 3 weeks No bevacizumab after progression PD • Cisplatin 80 mg/m 2 i. v. every 3 weeks; gemcitabine 1, 250 mg/m 2 on days 1 and 8 of each 3 -week cycle • Primary endpoint: progression-free survival • Secondary endpoint: overall survival and response rate CG = cisplatin/gemcitabine Manegold et al. , ASCO 2007, Abstract LBA 7514
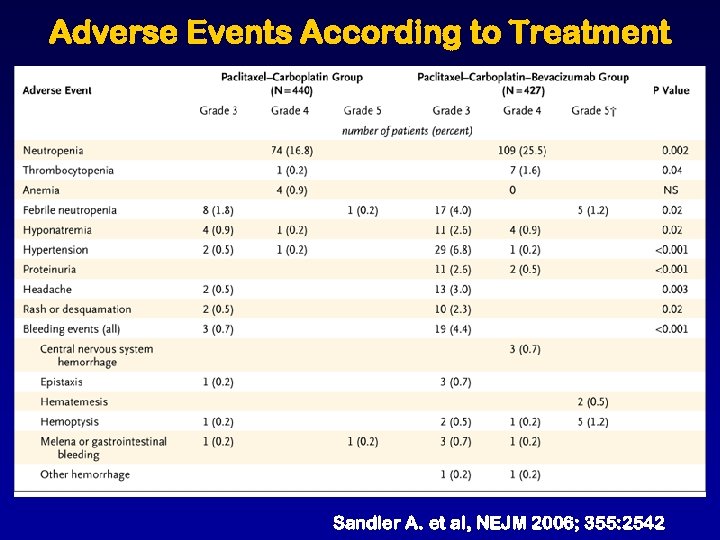 Adverse Events According to Treatment Sandler A. et al, NEJM 2006; 355: 2542
Adverse Events According to Treatment Sandler A. et al, NEJM 2006; 355: 2542
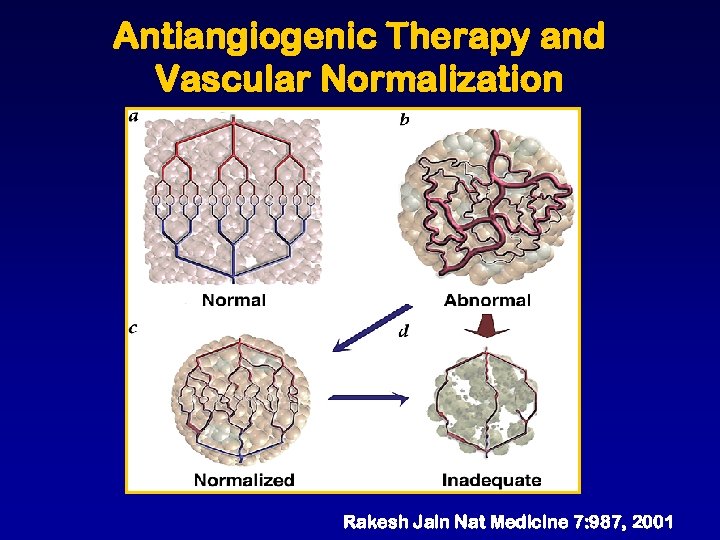 Antiangiogenic Therapy and Vascular Normalization Rakesh Jain Nat Medicine 7: 987, 2001
Antiangiogenic Therapy and Vascular Normalization Rakesh Jain Nat Medicine 7: 987, 2001
 Angiogenesis • Angiogenesis is the formation of new blood vessels from pre-existing vasculature • Angiogenesis is highly dependent on the VEGF signaling pathway • VEGFR-2 is the most important VEGF signaling pathway for angiogenesis • VEGF is frequently overexpressed in cancer and is associated with poor prognosis • Without a blood supply, tumors do not grow larger than 1– 2 mm • As tumors grow they become hypoxic, which leads to the up-regulation of angiogenic factors such as VEGF • Stimulates the production of new vasculature
Angiogenesis • Angiogenesis is the formation of new blood vessels from pre-existing vasculature • Angiogenesis is highly dependent on the VEGF signaling pathway • VEGFR-2 is the most important VEGF signaling pathway for angiogenesis • VEGF is frequently overexpressed in cancer and is associated with poor prognosis • Without a blood supply, tumors do not grow larger than 1– 2 mm • As tumors grow they become hypoxic, which leads to the up-regulation of angiogenic factors such as VEGF • Stimulates the production of new vasculature
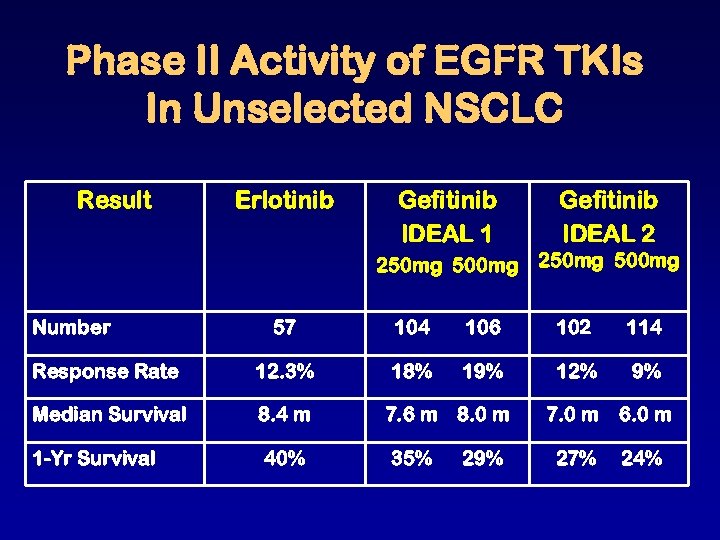 Phase II Activity of EGFR TKIs In Unselected NSCLC Result Erlotinib Gefitinib IDEAL 1 Gefitinib IDEAL 2 250 mg 500 mg Number 57 104 106 102 114 Response Rate 12. 3% 18% 19% 12% 9% Median Survival 8. 4 m 7. 0 m 6. 0 m 1 -Yr Survival 40% 27% 24% 7. 6 m 8. 0 m 35% 29%
Phase II Activity of EGFR TKIs In Unselected NSCLC Result Erlotinib Gefitinib IDEAL 1 Gefitinib IDEAL 2 250 mg 500 mg Number 57 104 106 102 114 Response Rate 12. 3% 18% 19% 12% 9% Median Survival 8. 4 m 7. 0 m 6. 0 m 1 -Yr Survival 40% 27% 24% 7. 6 m 8. 0 m 35% 29%


