0c1743c5901f42a4ad78a0beda631e70.ppt
- Количество слайдов: 21
 Chemoprevention for liver Cancer Manufacturing Process of Product By Ms. Davong Oumavong
Chemoprevention for liver Cancer Manufacturing Process of Product By Ms. Davong Oumavong
 1. Why should we prevent cancer ? Prevention is the most successful of treatment 2. What’s new challenge for prevent liver cancer ? “ Taking compound product of non toxic agents as natural product of food supplementary ”
1. Why should we prevent cancer ? Prevention is the most successful of treatment 2. What’s new challenge for prevent liver cancer ? “ Taking compound product of non toxic agents as natural product of food supplementary ”
 New Challenge of Natural products • Including Broccoli sprouted crushed (500 mg ) (Sulforaphane) and Turmaric crushed (450 mg) (Curcumin) combined with Soy Lecithin (50 mg) per box /capsule Within two formulas 1. 250 cc Soy Milk per box, 1 -2 boxes / day 2. 1000 mg Capsules, 1 -2 capsules/ time, 2 time/day (For exactly of their consuming amount will prove on Clinical trial)
New Challenge of Natural products • Including Broccoli sprouted crushed (500 mg ) (Sulforaphane) and Turmaric crushed (450 mg) (Curcumin) combined with Soy Lecithin (50 mg) per box /capsule Within two formulas 1. 250 cc Soy Milk per box, 1 -2 boxes / day 2. 1000 mg Capsules, 1 -2 capsules/ time, 2 time/day (For exactly of their consuming amount will prove on Clinical trial)
 What are our products’ benefits? • Can prevent all of aflatoxin, HBV and also HCV which induce to liver cancer • Prevent not only liver cancer but also other organs and can improve healthy • Clinically proven- it’s a natural product, has no side effect.
What are our products’ benefits? • Can prevent all of aflatoxin, HBV and also HCV which induce to liver cancer • Prevent not only liver cancer but also other organs and can improve healthy • Clinically proven- it’s a natural product, has no side effect.
 products’ benefits cont. • Soy milk product Soy milk • Good taste, easy to buy and take for children or adult • Low cost, safety and give us more than chemoprevention - Including lecithin, mineral agent, vitamin • Capsule product • Easy to safe, take and transport • alternative challenge for people who do not like to drink soy milk
products’ benefits cont. • Soy milk product Soy milk • Good taste, easy to buy and take for children or adult • Low cost, safety and give us more than chemoprevention - Including lecithin, mineral agent, vitamin • Capsule product • Easy to safe, take and transport • alternative challenge for people who do not like to drink soy milk
 I. Set up the Factory • Find out building factory’s location - far from community • Design the building factory and structure and necessary equipments – Follow up WHO Guideline for Good Hygiene Practices, Drug GMP - close system, waste system, enough lighting, water supply system etc • Design storage - for raw materials, finished products and chemicals and analysis laboratory room, testing animal house and cool house
I. Set up the Factory • Find out building factory’s location - far from community • Design the building factory and structure and necessary equipments – Follow up WHO Guideline for Good Hygiene Practices, Drug GMP - close system, waste system, enough lighting, water supply system etc • Design storage - for raw materials, finished products and chemicals and analysis laboratory room, testing animal house and cool house
 I. cont. • Design process of extraction, analysis, packaging and manufacturing • Design useful things for personnel - toilet, changing room, eating room, relaxing room, … • Design responsibility of personnel • Licensing the factory to controlling Agency - Environmental, Industrial, FDA…, and construct the factory
I. cont. • Design process of extraction, analysis, packaging and manufacturing • Design useful things for personnel - toilet, changing room, eating room, relaxing room, … • Design responsibility of personnel • Licensing the factory to controlling Agency - Environmental, Industrial, FDA…, and construct the factory
 II. Finding out sources of medicinal plants or raw materials • Find out location of good soil for cutural control and supply to production - need Good Agricultural Practices ( GAP) and Good Harvesting Practices (GHP)
II. Finding out sources of medicinal plants or raw materials • Find out location of good soil for cutural control and supply to production - need Good Agricultural Practices ( GAP) and Good Harvesting Practices (GHP)
 II. cont. • Selecting factories who produce starting materials / intermediate / bulk /seeds – high quality of goods and has enough for producing supply ( - Broccoli seed and Soy Lecithin / Bean may be buy from companies, Curcuma Longa Rhizome can also plant ( in Thailand or Laos) or buy from Indian Company)
II. cont. • Selecting factories who produce starting materials / intermediate / bulk /seeds – high quality of goods and has enough for producing supply ( - Broccoli seed and Soy Lecithin / Bean may be buy from companies, Curcuma Longa Rhizome can also plant ( in Thailand or Laos) or buy from Indian Company)
 III. Agent Extraction Process • Substance Extraction – freezing dried Rhizome ( Curcuma Longa ) and Broccoli sprouted seeds and crushing • Design for finished product formula and their ingredient quantity - capsules and soy milk with Lecithin
III. Agent Extraction Process • Substance Extraction – freezing dried Rhizome ( Curcuma Longa ) and Broccoli sprouted seeds and crushing • Design for finished product formula and their ingredient quantity - capsules and soy milk with Lecithin
 a. QC for raw/starting materials ) Good laboratory practices in factory laboratory) 1. Purity - Bacteria contamination - Toxin contamination - etc. 2. Quantify for active compound - % of active agent
a. QC for raw/starting materials ) Good laboratory practices in factory laboratory) 1. Purity - Bacteria contamination - Toxin contamination - etc. 2. Quantify for active compound - % of active agent
 IV. Quality Control in Producing process • Quality Management – training personnel, equipments / materials, premises, complaints, internal quality audit, contract manufacture and analysis/ laboratory testing etc • Quality control- sanitation & hygiene, ISO 22000, ISO 17025, Drug GMP ( Safety, effectiveness, Acceptability)
IV. Quality Control in Producing process • Quality Management – training personnel, equipments / materials, premises, complaints, internal quality audit, contract manufacture and analysis/ laboratory testing etc • Quality control- sanitation & hygiene, ISO 22000, ISO 17025, Drug GMP ( Safety, effectiveness, Acceptability)
 IV. cont. • Producing Control - labeling, clean up, testing lab, toxicology testing, batch product record, documentation, batch control & release • Instruction Product - place / house, cleaning control, good storage practice • Apply to FDA for registration/ licensing
IV. cont. • Producing Control - labeling, clean up, testing lab, toxicology testing, batch product record, documentation, batch control & release • Instruction Product - place / house, cleaning control, good storage practice • Apply to FDA for registration/ licensing
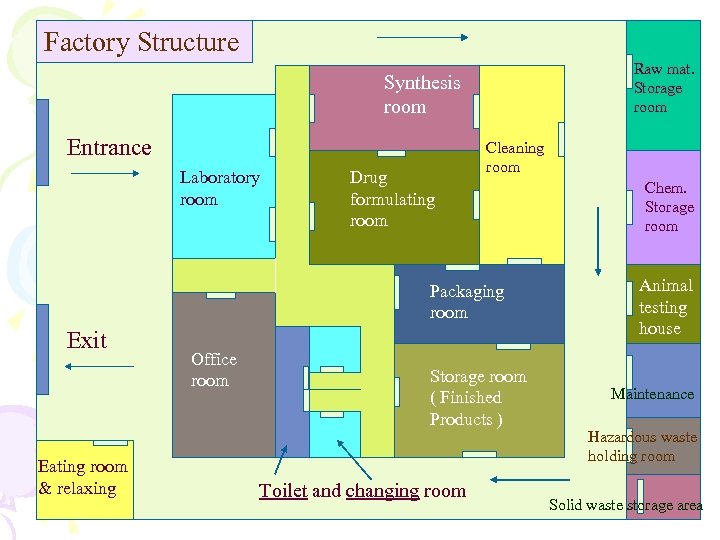 Factory Structure Raw mat. Storage room Synthesis room Entrance Laboratory room Drug formulating room Cleaning room Packaging room Exit Eating room & relaxing Office room Storage room ( Finished Products ) Toilet and changing room Chem. Storage room Animal testing house Maintenance Hazardous waste holding room Solid waste storage area
Factory Structure Raw mat. Storage room Synthesis room Entrance Laboratory room Drug formulating room Cleaning room Packaging room Exit Eating room & relaxing Office room Storage room ( Finished Products ) Toilet and changing room Chem. Storage room Animal testing house Maintenance Hazardous waste holding room Solid waste storage area
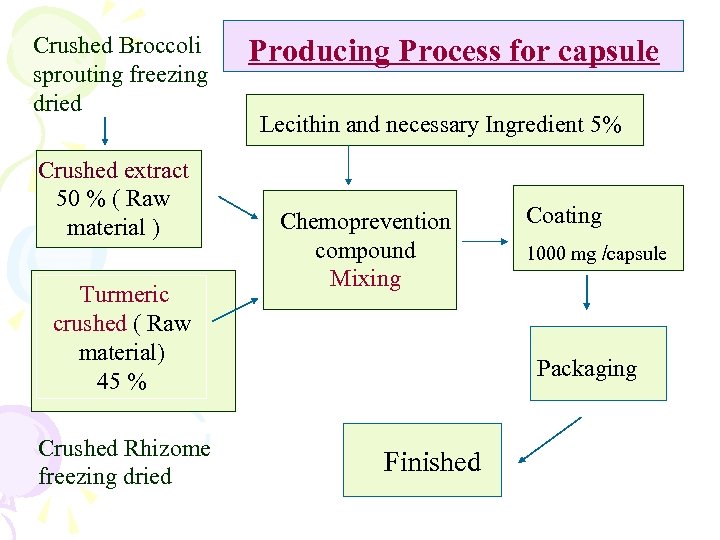 Crushed Broccoli sprouting freezing dried Crushed extract 50 % ( Raw material ) Producing Process for capsule Lecithin and necessary Ingredient 5% Chemoprevention compound Mixing Turmeric crushed ( Raw material) 45 % Crushed Rhizome freezing dried Coating 1000 mg /capsule Packaging Finished
Crushed Broccoli sprouting freezing dried Crushed extract 50 % ( Raw material ) Producing Process for capsule Lecithin and necessary Ingredient 5% Chemoprevention compound Mixing Turmeric crushed ( Raw material) 45 % Crushed Rhizome freezing dried Coating 1000 mg /capsule Packaging Finished
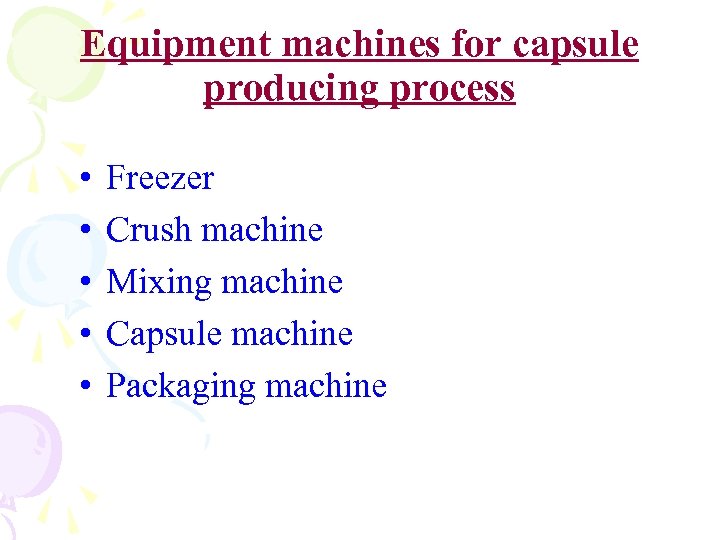 Equipment machines for capsule producing process • • • Freezer Crush machine Mixing machine Capsule machine Packaging machine
Equipment machines for capsule producing process • • • Freezer Crush machine Mixing machine Capsule machine Packaging machine
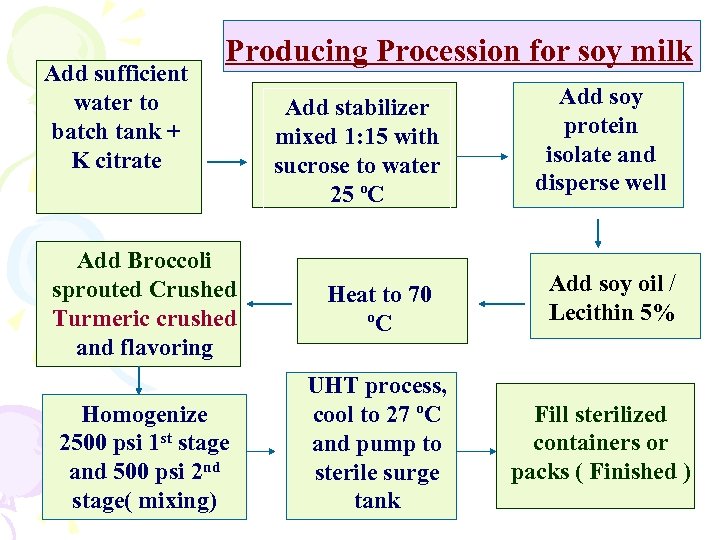 Add sufficient water to batch tank + K citrate Producing Procession for soy milk Add Broccoli sprouted Crushed Turmeric crushed and flavoring Homogenize 2500 psi 1 st stage and 500 psi 2 nd stage( mixing) Add stabilizer mixed 1: 15 with sucrose to water 25 ºC Heat to 70 ºC UHT process, cool to 27 ºC and pump to sterile surge tank Add soy protein isolate and disperse well Add soy oil / Lecithin 5% Fill sterilized containers or packs ( Finished )
Add sufficient water to batch tank + K citrate Producing Procession for soy milk Add Broccoli sprouted Crushed Turmeric crushed and flavoring Homogenize 2500 psi 1 st stage and 500 psi 2 nd stage( mixing) Add stabilizer mixed 1: 15 with sucrose to water 25 ºC Heat to 70 ºC UHT process, cool to 27 ºC and pump to sterile surge tank Add soy protein isolate and disperse well Add soy oil / Lecithin 5% Fill sterilized containers or packs ( Finished )
 Equipment machines for soy milk producing process • • Tank, surge tank High shear mixer Heater Mixer machine Freezer Pump Packaging machine
Equipment machines for soy milk producing process • • Tank, surge tank High shear mixer Heater Mixer machine Freezer Pump Packaging machine
 Summary • Every process of manufacturing was done by following Good Development, Marketing and Ethic concern of New Product • Response to finished product quality, might pass International Standard for Quality Control , Safety to health and can reduce liver cancer
Summary • Every process of manufacturing was done by following Good Development, Marketing and Ethic concern of New Product • Response to finished product quality, might pass International Standard for Quality Control , Safety to health and can reduce liver cancer

 Broccoli sprouting process • Seeds not treated with pesticides • Seeds surface-sterilized by a 1 -min rinse in 70% ethanol, a 15 -min exposure to 1. 3% sodium hypochlorite containing 0. 001% Alconox detergent, • and exhaustive rinsing with sterile distilled water. • The broccoli (Brassica oleracea var. italica) cultivar used was SAGA , • unless stated otherwise. • Sprouts were grown without added nutrients either aseptically on 0. 7% agar or in inclined perforated trays (35 × 40 cm ( • watered with four 15 -s gentle spray cycles per h. • All sprouts were grown with a 16 -h light and 8 -h dark photoperiod • and a corresponding 25/20°C cycle for agar-grown sprouts or a constant 25°C for tray-grown sprouts. • Sprouts were rapidly and gently collected from the surface of the agar or spray tray immediately before extraction to minimize hydrolysis of glucosinolates by endogenous myrosinase. • Mature and frozen vegetables were obtained from local supermarkets. Plants not extracted on the day of collection were stored at 80°C.
Broccoli sprouting process • Seeds not treated with pesticides • Seeds surface-sterilized by a 1 -min rinse in 70% ethanol, a 15 -min exposure to 1. 3% sodium hypochlorite containing 0. 001% Alconox detergent, • and exhaustive rinsing with sterile distilled water. • The broccoli (Brassica oleracea var. italica) cultivar used was SAGA , • unless stated otherwise. • Sprouts were grown without added nutrients either aseptically on 0. 7% agar or in inclined perforated trays (35 × 40 cm ( • watered with four 15 -s gentle spray cycles per h. • All sprouts were grown with a 16 -h light and 8 -h dark photoperiod • and a corresponding 25/20°C cycle for agar-grown sprouts or a constant 25°C for tray-grown sprouts. • Sprouts were rapidly and gently collected from the surface of the agar or spray tray immediately before extraction to minimize hydrolysis of glucosinolates by endogenous myrosinase. • Mature and frozen vegetables were obtained from local supermarkets. Plants not extracted on the day of collection were stored at 80°C.


