839e7852d7406c42e9250c777a635ca2.ppt
- Количество слайдов: 25
Chemo-enzymatic peptide synthesis (CEPS): a generally applicable, traceless ligation technology for the synthesis of longer peptides and peptide-to-protein conjugates. Europeptides, 18 th of November 2015 Timo Nuijens timo@enzypep. com www. enzypep. com
Enzy. Pep Spin-off from DSM, founded in July 2012 Labs located at Brightlands Campus in Geleen (NL) Continuation of an enzymatic peptide coupling technology developed at DSM Enzymatic Peptide synthesis DSM transferred all existing IP to Enzypep BV in 2012 Two private investors and 3 investment funds. 5 employees, growing Target: development of scalable processes for pharmaceutical peptides with lower cost -prices (> 30% reduction) and significantly reduced timelines using enzymatic coupling techniques. 2
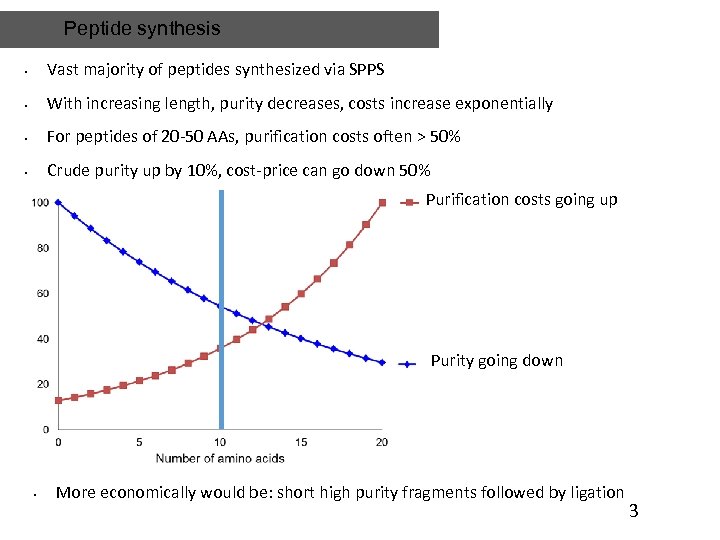
Peptide synthesis • Vast majority of peptides synthesized via SPPS • With increasing length, purity decreases, costs increase exponentially • For peptides of 20 -50 AAs, purification costs often > 50% • Crude purity up by 10%, cost-price can go down 50% Purification costs going up Purity going down • More economically would be: short high purity fragments followed by ligation 3
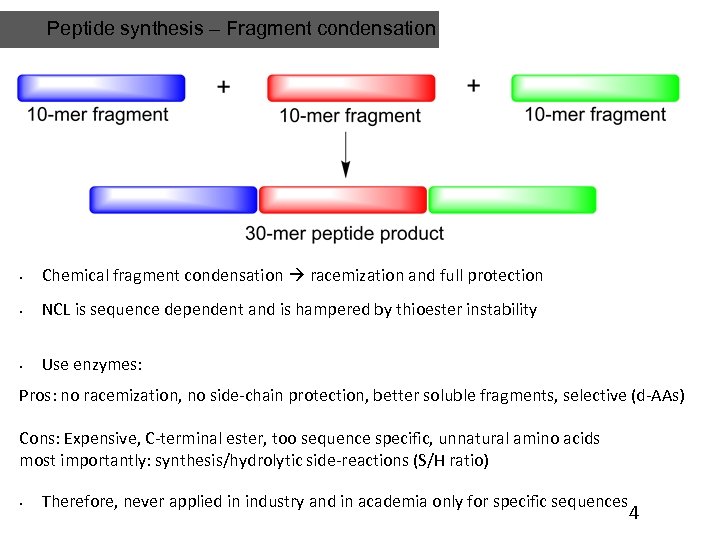
Peptide synthesis – Fragment condensation • Chemical fragment condensation racemization and full protection • NCL is sequence dependent and is hampered by thioester instability • Use enzymes: Pros: no racemization, no side-chain protection, better soluble fragments, selective (d-AAs) Cons: Expensive, C-terminal ester, too sequence specific, unnatural amino acids most importantly: synthesis/hydrolytic side-reactions (S/H ratio) • Therefore, never applied in industry and in academia only for specific sequences 4
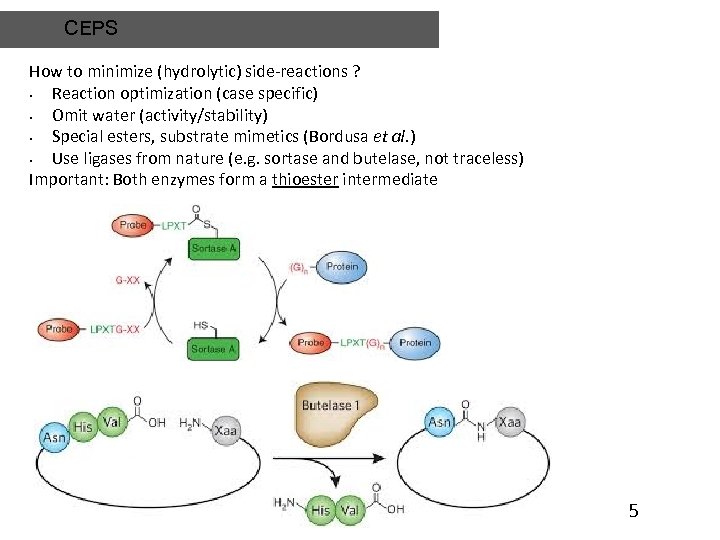
CEPS How to minimize (hydrolytic) side-reactions ? • Reaction optimization (case specific) • Omit water (activity/stability) • Special esters, substrate mimetics (Bordusa et al. ) • Use ligases from nature (e. g. sortase and butelase, not traceless) Important: Both enzymes form a thioester intermediate 5
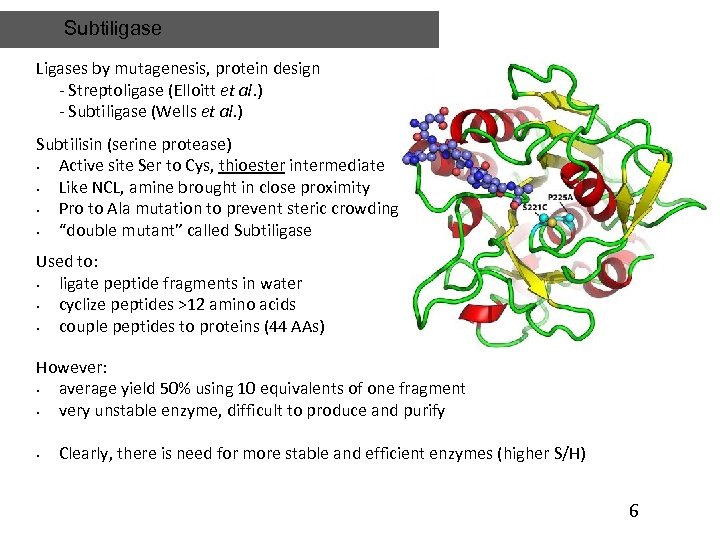
Subtiligase Ligases by mutagenesis, protein design - Streptoligase (Elloitt et al. ) - Subtiligase (Wells et al. ) Subtilisin (serine protease) • Active site Ser to Cys, thioester intermediate • Like NCL, amine brought in close proximity • Pro to Ala mutation to prevent steric crowding • “double mutant” called Subtiligase Used to: • ligate peptide fragments in water • cyclize peptides >12 amino acids • couple peptides to proteins (44 AAs) However: • average yield 50% using 10 equivalents of one fragment • very unstable enzyme, difficult to produce and purify • Clearly, there is need for more stable and efficient enzymes (higher S/H) 6
Discovery of Peptiligase • Hyperstable variant of subtilisin (Bryan et al. ) - Deletion of the calcium binding domain (9 amino acids) - 18 additional stabilizing mutations including one disulphide bridge - Stable to Urea and Guanidiniumchloride - Stable to organic co-solvents (up to 50 vol%) - Temperature stable (70 degrees) - Very hydrolytically active • Double mutations added to this enzyme scaffold (S/H ratio) • Surprisingly, this new enzyme “Peptiligase” has a 2 fold higher S/H then Subtiligase • Very fast ligation reaction (usually 10 to 60 min) • Stability retained • Easy to produce and purify (g/L range) • Little enzyme needed (1 mg/g of 20 -mer peptide, 1 g/kg) • Robust, perfect scaffold for further protein engineering 7
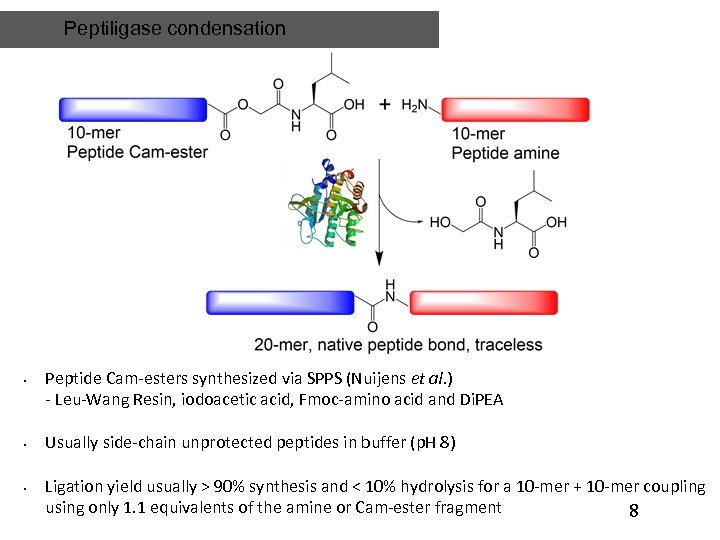
Peptiligase condensation • • • Peptide Cam-esters synthesized via SPPS (Nuijens et al. ) - Leu-Wang Resin, iodoacetic acid, Fmoc-amino acid and Di. PEA Usually side-chain unprotected peptides in buffer (p. H 8) Ligation yield usually > 90% synthesis and < 10% hydrolysis for a 10 -mer + 10 -mer coupling using only 1. 1 equivalents of the amine or Cam-ester fragment 8
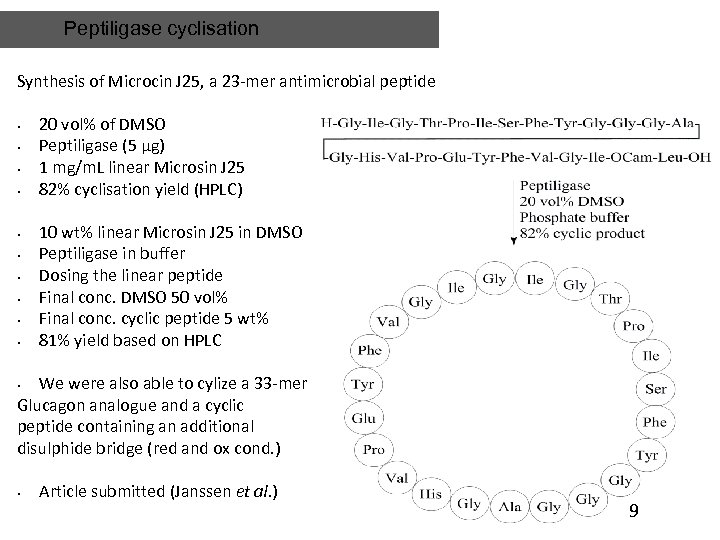
Peptiligase cyclisation Synthesis of Microcin J 25, a 23 -mer antimicrobial peptide • • • 20 vol% of DMSO Peptiligase (5 µg) 1 mg/m. L linear Microsin J 25 82% cyclisation yield (HPLC) 10 wt% linear Microsin J 25 in DMSO Peptiligase in buffer Dosing the linear peptide Final conc. DMSO 50 vol% Final conc. cyclic peptide 5 wt% 81% yield based on HPLC We were also able to cylize a 33 -mer Glucagon analogue and a cyclic peptide containing an additional disulphide bridge (red and ox cond. ) • • Article submitted (Janssen et al. ) 9
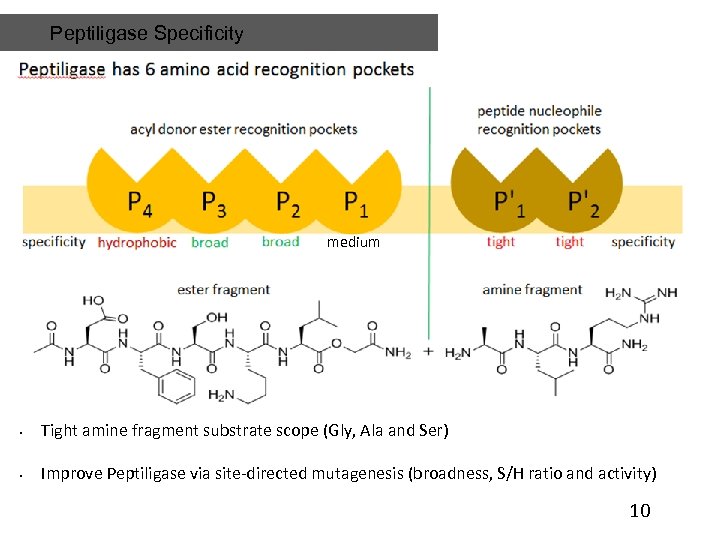
Peptiligase Specificity medium • Tight amine fragment substrate scope (Gly, Ala and Ser) • Improve Peptiligase via site-directed mutagenesis (broadness, S/H ratio and activity) 10
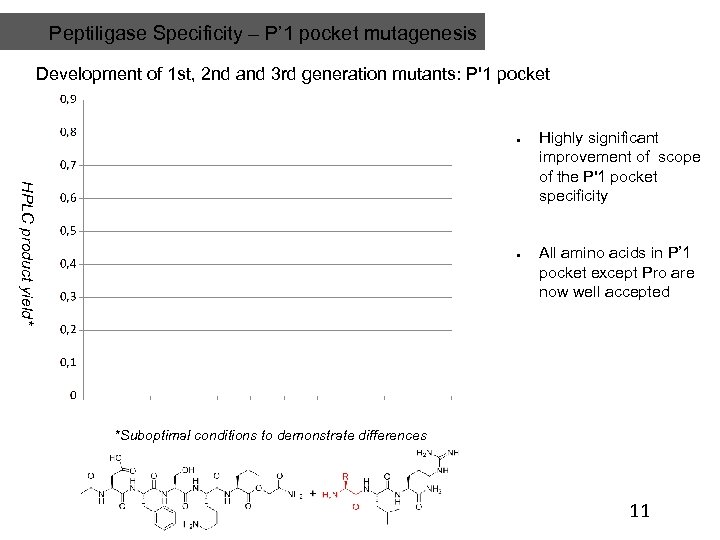
Peptiligase Specificity – P’ 1 pocket mutagenesis Development of 1 st, 2 nd and 3 rd generation mutants: P'1 pocket HPLC product yield* Highly significant improvement of scope of the P'1 pocket specificity All amino acids in P’ 1 pocket except Pro are now well accepted *Suboptimal conditions to demonstrate differences 11
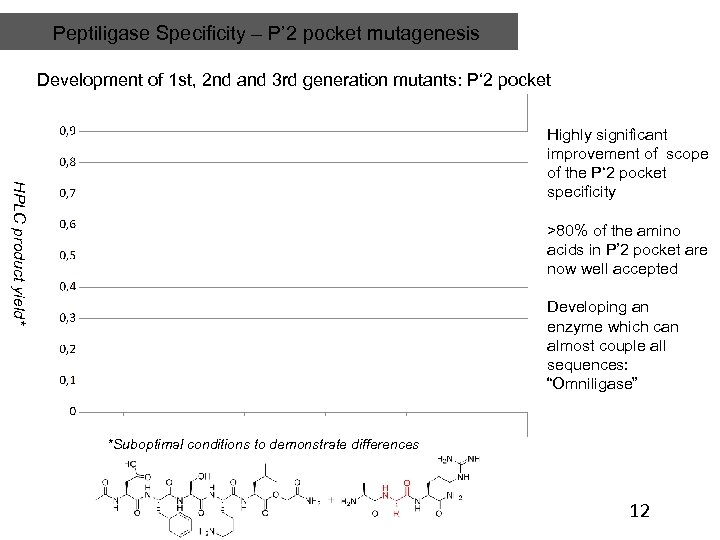
Peptiligase Specificity – P’ 2 pocket mutagenesis Development of 1 st, 2 nd and 3 rd generation mutants: P‘ 2 pocket HPLC product yield* Highly significant improvement of scope of the P‘ 2 pocket specificity >80% of the amino acids in P’ 2 pocket are now well accepted Developing an enzyme which can almost couple all sequences: “Omniligase” *Suboptimal conditions to demonstrate differences 12
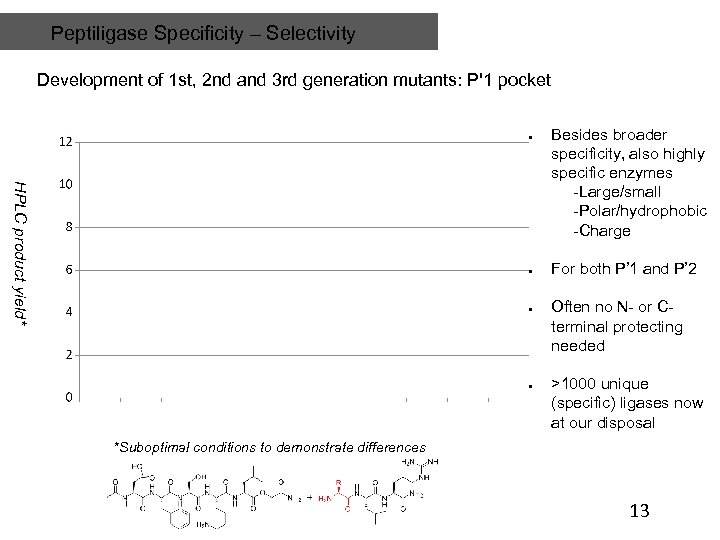
Peptiligase Specificity – Selectivity Development of 1 st, 2 nd and 3 rd generation mutants: P'1 pocket HPLC product yield* Besides broader specificity, also highly specific enzymes -Large/small -Polar/hydrophobic -Charge For both P’ 1 and P’ 2 Often no N- or Cterminal protecting needed >1000 unique (specific) ligases now at our disposal *Suboptimal conditions to demonstrate differences 13
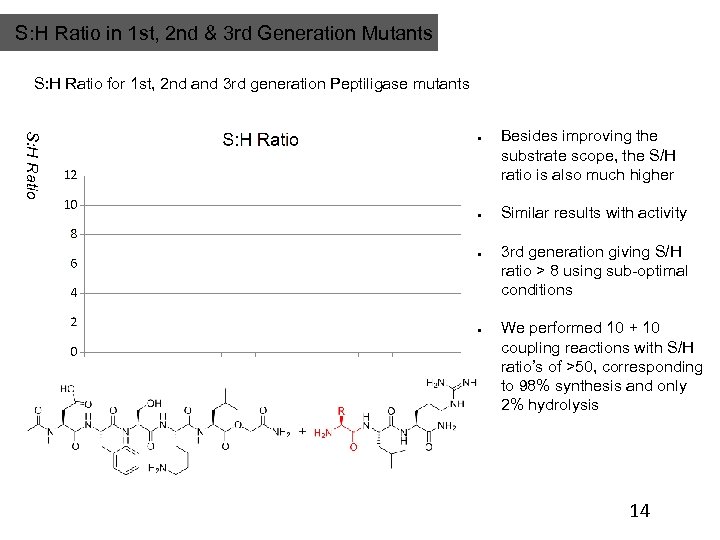
S: H Ratio in 1 st, 2 nd & 3 rd Generation Mutants S: H Ratio for 1 st, 2 nd and 3 rd generation Peptiligase mutants S: H Ratio Besides improving the substrate scope, the S/H ratio is also much higher Similar results with activity 3 rd generation giving S/H ratio > 8 using sub-optimal conditions We performed 10 + 10 coupling reactions with S/H ratio’s of >50, corresponding to 98% synthesis and only 2% hydrolysis 14

Example 1: CEPS of Exenatide (2 -Fragment Strategy) • Exenatide (39 amino acids, type II diabetes, blockbuster) • Exenatide has no Gly or Pro residues at strategic positions • Purified using multiple prep-HPLC runs and overall yields range between 10 -25% • Synthesis of Exenatide from two fragments of similar length • Cam-ester Fragment (21 -mer): H-His 1 -Gly 2 -Glu 3 -Gly 4 -Thr 5 -Phe 6 -Thr 7 -Ser 8 -Asp 9 -Leu 10 -Ser 11 -Lys 12 -Gln 13 Met 14 -Glu 15 -Glu 16 -Glu 17 -Ala 18 -Val 19 -Arg 20 -Leu 21 -OCam-Leu-OH No protection ! • Amine Fragment (18 -mer): H-Phe 22 -Ile 23 -Glu 24 -Trp 25 -Leu 26 -Lys 27 -Asn 28 -Gly 29 -Gly 30 -Pro 31 -Ser 32 -Ser 33 Gly 34 -Ala 35 -Pro 36 -Pro 37 -Pro 38 -Ser 39 -NH 2 Amine Fragment, using Rink resin: Single prep purification: 96. 0% purity, 52% yield • Cam-ester Fragment, using CTC resin Single prep purification: 97. 1% purity, 56% yield • 15
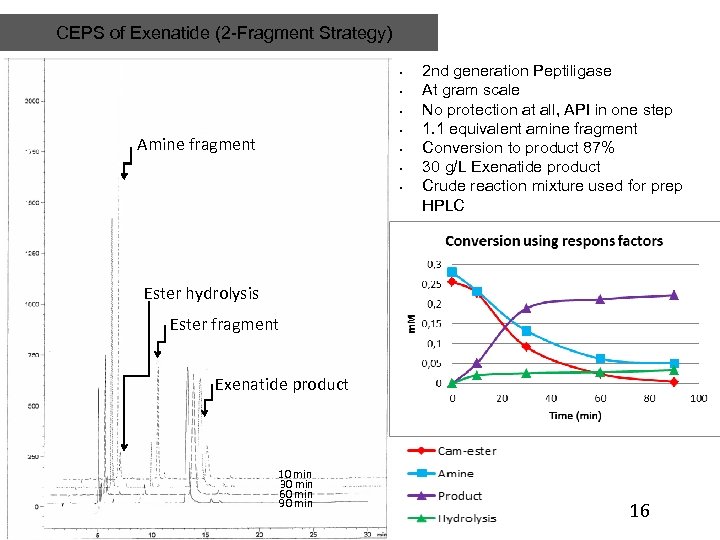
CEPS of Exenatide (2 -Fragment Strategy) • • Amine fragment • • • 2 nd generation Peptiligase At gram scale No protection at all, API in one step 1. 1 equivalent amine fragment Conversion to product 87% 30 g/L Exenatide product Crude reaction mixture used for prep HPLC Ester hydrolysis Ester fragment Exenatide product 10 min 30 min 60 min 90 min 16 16
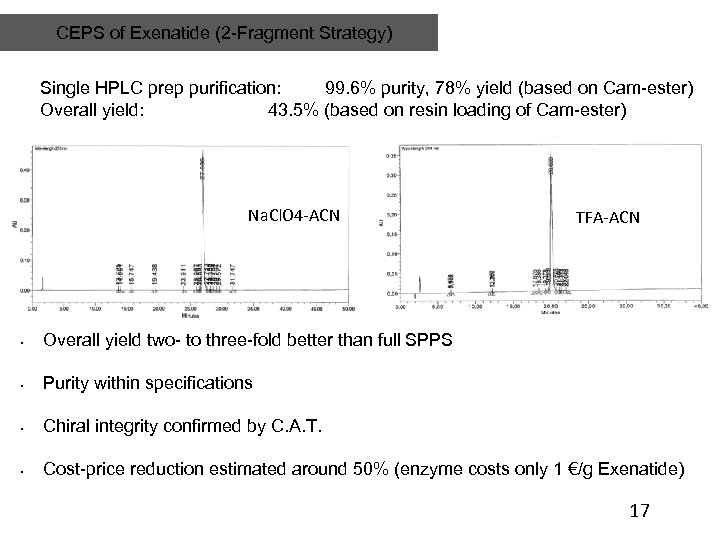
CEPS of Exenatide (2 -Fragment Strategy) Single HPLC prep purification: 99. 6% purity, 78% yield (based on Cam-ester) Overall yield: 43. 5% (based on resin loading of Cam-ester) Na. Cl. O 4 -ACN TFA-ACN • Overall yield two- to three-fold better than full SPPS • Purity within specifications • Chiral integrity confirmed by C. A. T. • Cost-price reduction estimated around 50% (enzyme costs only 1 €/g Exenatide) 17
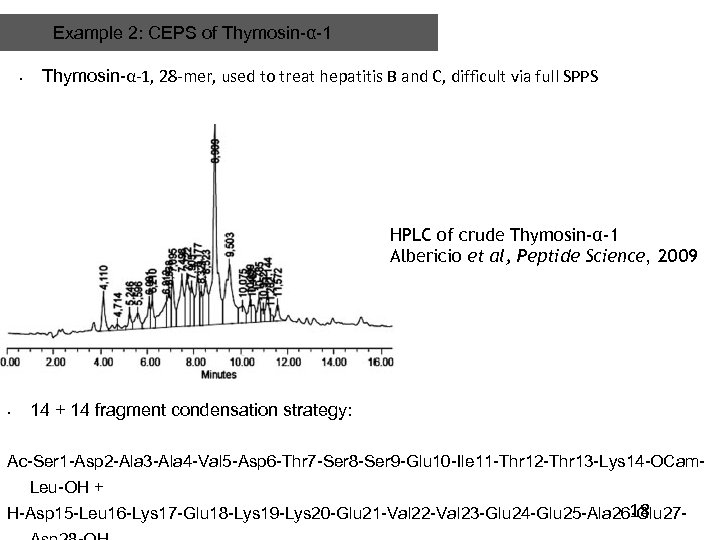
Example 2: CEPS of Thymosin-α-1 • Thymosin-α-1, 28 -mer, used to treat hepatitis B and C, difficult via full SPPS HPLC of crude Thymosin-α-1 Albericio et al, Peptide Science, 2009 • 14 + 14 fragment condensation strategy: Ac-Ser 1 -Asp 2 -Ala 3 -Ala 4 -Val 5 -Asp 6 -Thr 7 -Ser 8 -Ser 9 -Glu 10 -Ile 11 -Thr 12 -Thr 13 -Lys 14 -OCam. Leu-OH + 18 H-Asp 15 -Leu 16 -Lys 17 -Glu 18 -Lys 19 -Lys 20 -Glu 21 -Val 22 -Val 23 -Glu 24 -Glu 25 -Ala 26 -Glu 27 -
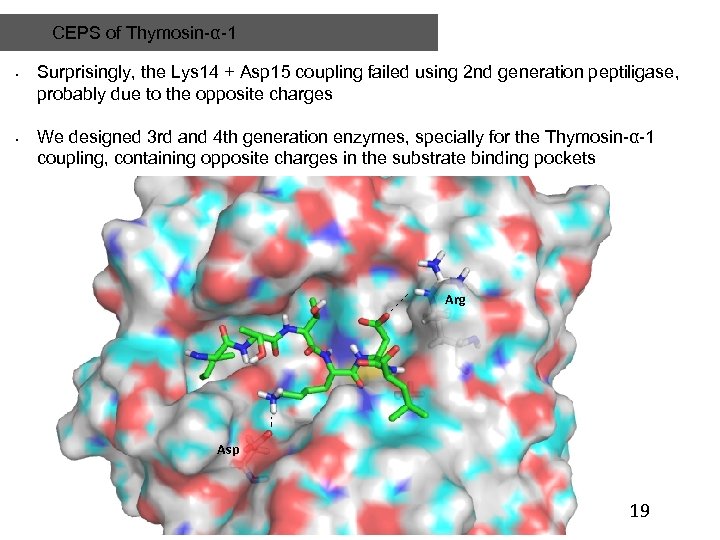
CEPS of Thymosin-α-1 • • Surprisingly, the Lys 14 + Asp 15 coupling failed using 2 nd generation peptiligase, probably due to the opposite charges We designed 3 rd and 4 th generation enzymes, specially for the Thymosin-α-1 coupling, containing opposite charges in the substrate binding pockets Arg Asp 19
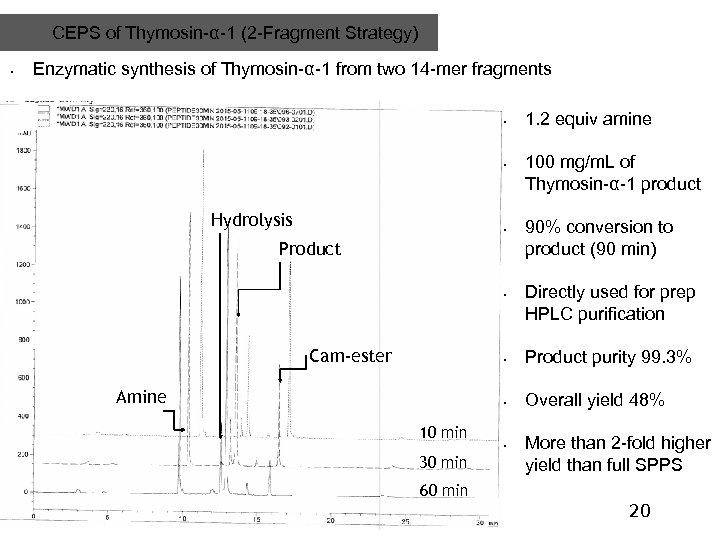
CEPS of Thymosin-α-1 (2 -Fragment Strategy) • Enzymatic synthesis of Thymosin-α-1 from two 14 -mer fragments • • Hydrolysis • Product • Cam-ester 1. 2 equiv amine 100 mg/m. L of Thymosin-α-1 product 90% conversion to product (90 min) Directly used for prep HPLC purification • • Amine 10 min 30 min 60 min 20 Product purity 99. 3% Overall yield 48% • More than 2 -fold higher yield than full SPPS 20
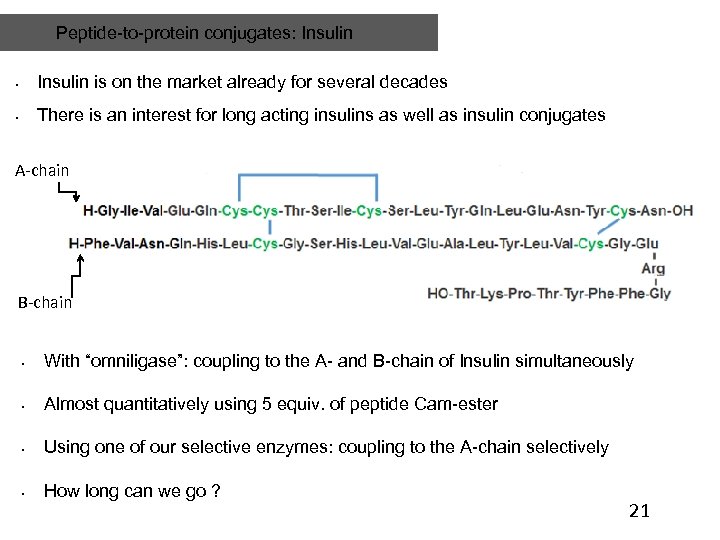
Peptide-to-protein conjugates: Insulin • Insulin is on the market already for several decades • There is an interest for long acting insulins as well as insulin conjugates A-chain B-chain • With “omniligase”: coupling to the A- and B-chain of Insulin simultaneously • Almost quantitatively using 5 equiv. of peptide Cam-ester • Using one of our selective enzymes: coupling to the A-chain selectively • How long can we go ? 21
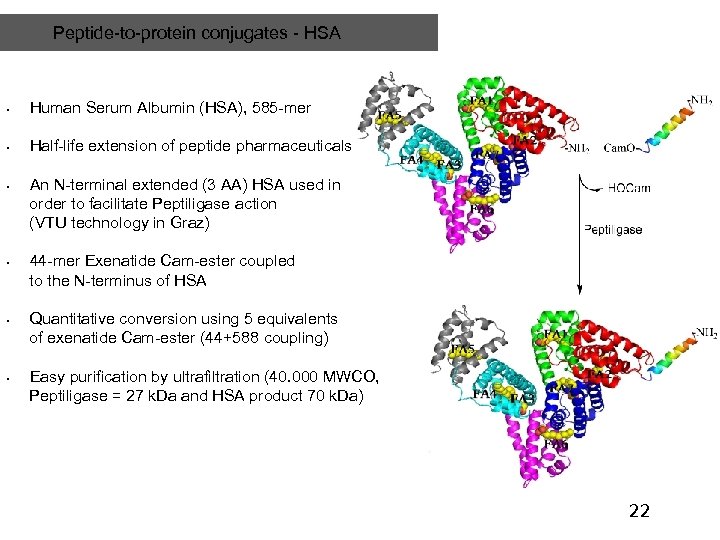
Peptide-to-protein conjugates - HSA • Human Serum Albumin (HSA), 585 -mer • Half-life extension of peptide pharmaceuticals • • An N-terminal extended (3 AA) HSA used in order to facilitate Peptiligase action (VTU technology in Graz) 44 -mer Exenatide Cam-ester coupled to the N-terminus of HSA Quantitative conversion using 5 equivalents of exenatide Cam-ester (44+588 coupling) Easy purification by ultrafiltration (40. 000 MWCO, Peptiligase = 27 k. Da and HSA product 70 k. Da) 22
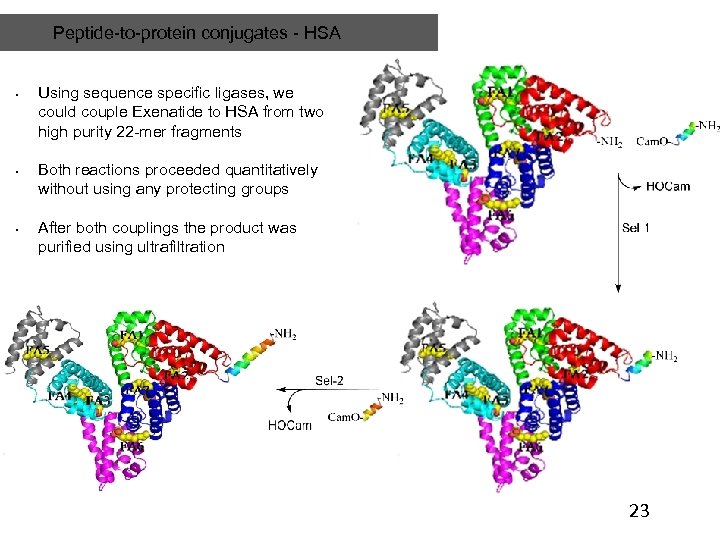
Peptide-to-protein conjugates - HSA • • • Using sequence specific ligases, we could couple Exenatide to HSA from two high purity 22 -mer fragments Both reactions proceeded quantitatively without using any protecting groups After both couplings the product was purified using ultrafiltration 23
Conclusions • Peptiligase, a hyper-stable peptide ligase with surprisingly high S/H ratio in aqueous solution • Condensation of unprotected peptides and head-to-tail macrocyclisation • 1 st, 2 nd and 3 rd generation variants: -”Omniligase”, can couple almost all peptide sequences -”Sequence-specific ligases”, very selective • • • Synthesis of Exenatide and Thymosin-α-1 at gram scale in good overall yield and purity Peptide-to-protein coupling, also possible using peptide fragments (Insulin, Exenatide-HSA) Currently continuing mutagenesis, synthesis of several pharmaceutical peptides, conjugates, macrocycles and polymers (biomaterials) 24

Aknowledgements University of Groningen: Prof. Dick B. Janssen Dr. Ana Toplak Dr. Bian Wu (now at Enzypep) Enzypep: Dr. Peter J. L. M. Quaedflieg B. Sc. Mathijs B. A. C. van de Meulenreek M. Sc. Marcel Schmidt M. Sc. Michel Goldbach M. Sc. Francesco Ventura Scientific advisory board: Prof. Dick B. Janssen (University of Groningen) Dr. Paul ten Kortenaar (MSD, Diosynth) Dr. Rodney Lax (Poly. Peptide Group) Dr. Roland Callans (Corden) Dr. Rinus Broxterman (DSM) 25
839e7852d7406c42e9250c777a635ca2.ppt