44a57f9c4eb36218165f4eb67c730202.ppt
- Количество слайдов: 16

Chemistry XXI Lab Session 9 How can we use chemical reactions to identify substances? (Two-Week Experiment)

Your Challenge Imagine that you work for a mining company that is about to buy a new mine in Southern Arizona. Chemistry XXI You have been asked to identify the metals present in samples of the ore extracted from the mine. What different metals are present in the ore?
Solubility Chemistry XXI Many ionic compounds dissolve in water, or can be dissolved using acids, and the ions separate in solution. When combined with other substances, the ions may recombine to form new products (chemical reaction) with characteristic properties. We can use some of these chemical reactions as differentiating characteristics to Weak identify the ions. electrolyte
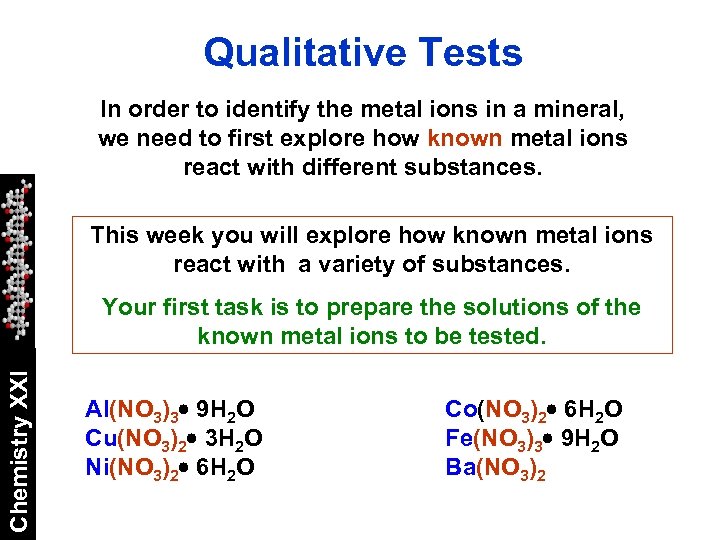
Qualitative Tests In order to identify the metal ions in a mineral, we need to first explore how known metal ions react with different substances. This week you will explore how known metal ions react with a variety of substances. Chemistry XXI Your first task is to prepare the solutions of the known metal ions to be tested. Al(NO 3)3 9 H 2 O Cu(NO 3)2 3 H 2 O Ni(NO 3)2 6 H 2 O Co(NO 3)2 6 H 2 O Fe(NO 3)3 9 H 2 O Ba(NO 3)2

Skill Building Tests Chemistry XXI Your groups needs to prepare 100 m. L of one 0. 1 M of the metallic cations. Save this solution in a labeled bottle (you will need it next week). Discuss how the work will be divided among all of the different groups. Available resources: v Balances; You will have 30 minutes Calculations Preparation v Volumetric glassware; v Ionic salts: Al(NO 3)3 9 H 2 O, Co(NO 3)2 6 H 2 O, Cu(NO 3)2 3 H 2 O, Fe(NO 3)3 9 H 2 O, Ni(NO 3)2 6 H 2 O Ba(NO 3)2

Other Solutions Chemistry XXI Now that we have solutions of the cations we can proceed to react them with solutions of anions. You will use the following anion solutions provided by the prep-room. Na 2 SO 4 – Sodium Sulfate Na 2 C 2 O 4 – Sodium Oxalate Na. OH – Sodium Hydroxide K 3 PO 4 – Potassium Phosphate Na 2 CO 3 – Sodium Carbonate

Your First Challenge Combine all of the cations (6) with all of the anions (5) to determine if there is a characteristic chemical reaction that can be used to identify them. Chemistry XXI Available resources: v Cation and anion solutions; v A well plate v Volumetric glassware. You have 45 minutes
Share Results and Reflect Chemistry XXI Present your results to the class. Decide as a group which data should be shared to facilitate the analysis. v In your groups, discuss how you may identify any given ion. v As a class discuss whether these set of reactions are enough to separate/identify all of the cations.

Additional Tests The reactions that you have observed may not be enough to build a full SEPARATION/IDENTIFICATION Chemistry XXI scheme for all of metallic cations present in a mineral. To enrich our set of analytical tools, let’s explore some other reactions.

This Week’s Challenge Perform at least four of the following reaction tests on each of the cations. Create a table of your observations. Available resources: Chemistry XXI v Cation solutions; v Test solutions; Alizarin S Reaction Aluminon Reaction Cinchonine Reaction Dimethylglyoxime Reaction Nitrosonaphthol Reaction Hydrogen Gas Reaction v Well plates; v Glassware. You have 60 minutes

Share Results and Reflect Chemistry XXI Add your results to the chart on the board. As a class, discuss whether you have enough data to build an identification scheme for all of the cations.
Qualitative Analysis Scheme You should now have sufficient tools to create an analysis scheme. Chemistry XXI Your scheme should be able to tell you which of the cations are in a sample regardless of what ever else is there. Your scheme should be constructed as a flow chart.
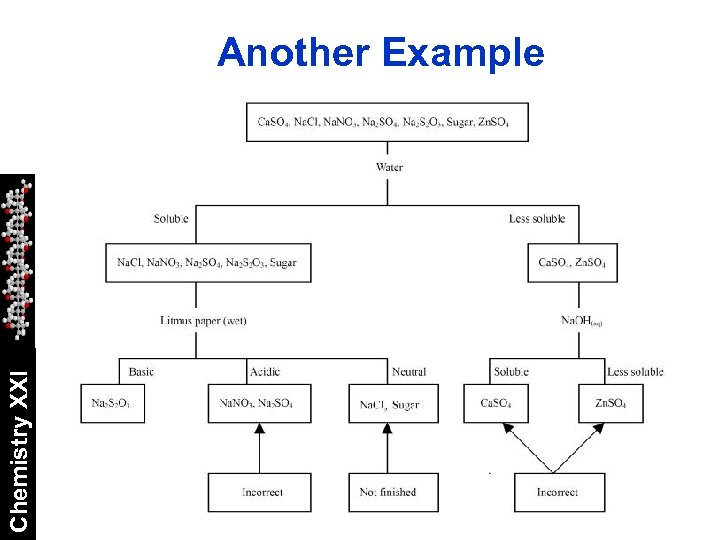
Chemistry XXI Another Example
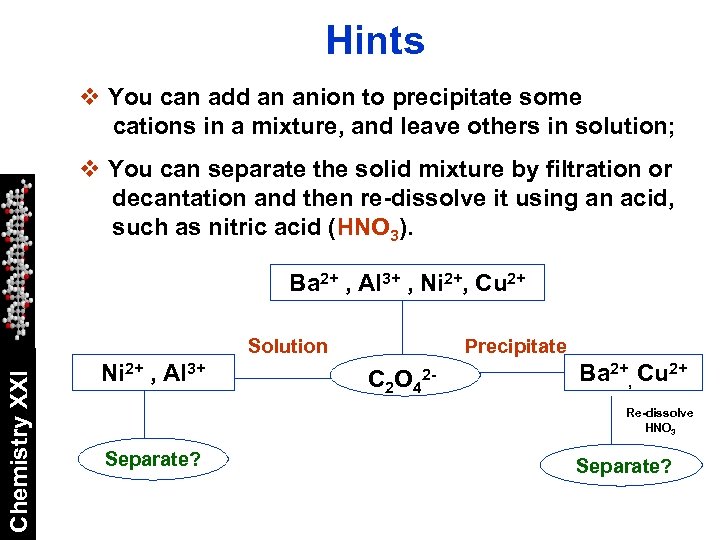
Hints v You can add an anion to precipitate some cations in a mixture, and leave others in solution; v You can separate the solid mixture by filtration or decantation and then re-dissolve it using an acid, such as nitric acid (HNO 3). Ba 2+ , Al 3+ , Ni 2+, Cu 2+ Chemistry XXI Solution Ni 2+ , Al 3+ Precipitate C 2 O 42 - Ba 2+, Cu 2+ Re-dissolve HNO 3 Separate?

Claims and Evidence Chemistry XXI Based on the results of your experiments, present your proposed analysis scheme and the evidence that you have to support your ideas.

No Report of this Week Two Pre-lab Assignment Chemistry XXI v You are to design and construct an appropriate analysis scheme for determining the presence of the selected cations in an ore sample.
44a57f9c4eb36218165f4eb67c730202.ppt