d45e3d77c55538295e900bdfeedbf7fe.ppt
- Количество слайдов: 51
 Chemistry Which GV science teacher looks like this guy?
Chemistry Which GV science teacher looks like this guy?
 Slide 2 of 20
Slide 2 of 20
 Slide 3 of 20
Slide 3 of 20
 Slide 4 of 20
Slide 4 of 20
 Slide 5 of 20
Slide 5 of 20
 Slide 6 of 20
Slide 6 of 20
 Slide 7 of 20
Slide 7 of 20
 Slide 8 of 20
Slide 8 of 20
 Slide 9 of 20
Slide 9 of 20
 Slide 10 of 20
Slide 10 of 20
 Slide 11 of 20
Slide 11 of 20
 Slide 12 of 20
Slide 12 of 20
 Slide 13 of 20
Slide 13 of 20
 Slide 14 of 20
Slide 14 of 20
 Slide 15 of 20
Slide 15 of 20
 Slide 16 of 20
Slide 16 of 20
 Slide 17 of 20
Slide 17 of 20
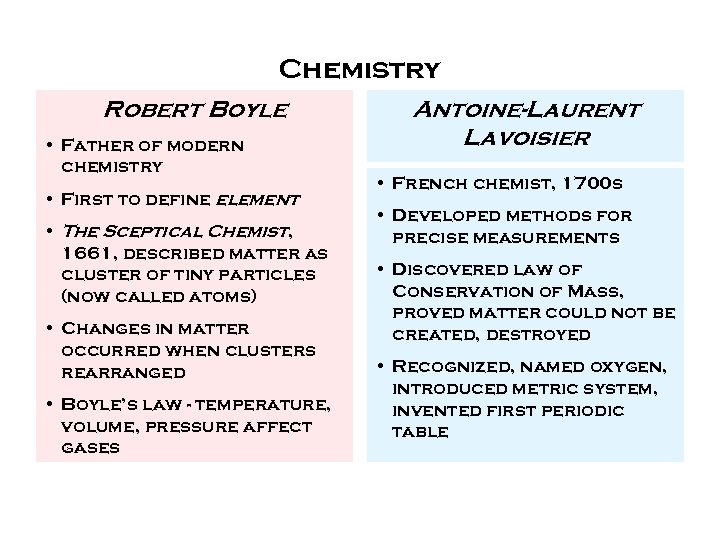 Chemistry Robert Boyle • Father of modern chemistry • First to define element • The Sceptical Chemist, 1661, described matter as cluster of tiny particles (now called atoms) • Changes in matter occurred when clusters rearranged • Boyle’s law - temperature, volume, pressure affect gases Antoine-Laurent Lavoisier • French chemist, 1700 s • Developed methods for precise measurements • Discovered law of Conservation of Mass, proved matter could not be created, destroyed • Recognized, named oxygen, introduced metric system, invented first periodic table
Chemistry Robert Boyle • Father of modern chemistry • First to define element • The Sceptical Chemist, 1661, described matter as cluster of tiny particles (now called atoms) • Changes in matter occurred when clusters rearranged • Boyle’s law - temperature, volume, pressure affect gases Antoine-Laurent Lavoisier • French chemist, 1700 s • Developed methods for precise measurements • Discovered law of Conservation of Mass, proved matter could not be created, destroyed • Recognized, named oxygen, introduced metric system, invented first periodic table
 Slide 18 of 20
Slide 18 of 20
 Slide 19 of 20
Slide 19 of 20
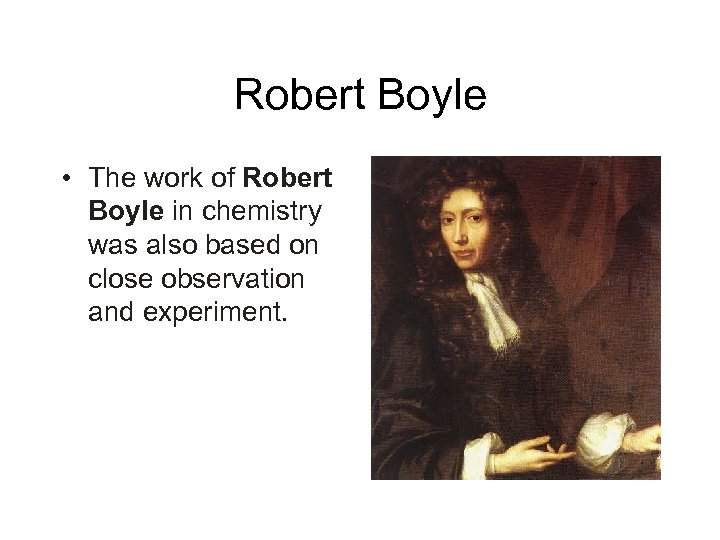 Robert Boyle • The work of Robert Boyle in chemistry was also based on close observation and experiment.
Robert Boyle • The work of Robert Boyle in chemistry was also based on close observation and experiment.
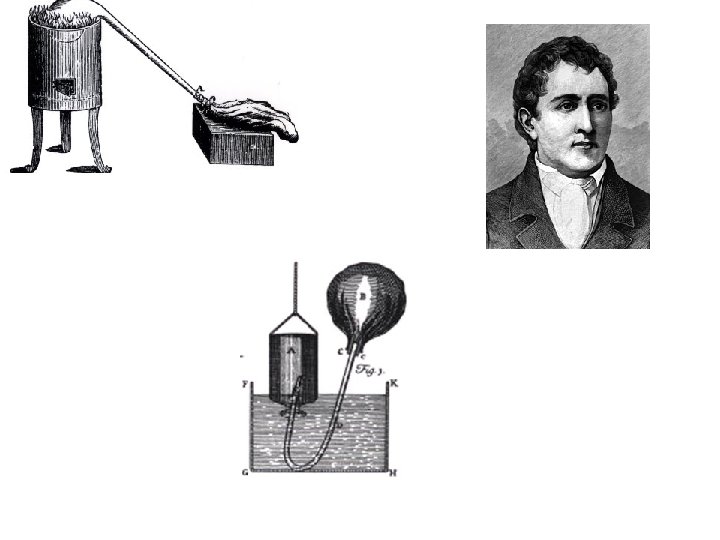
 Robert Boyle: 1627 – 1691 AD • Irish chemist • Inspired by Bacon • First to prove that alchemy did not work • However, he still thought that gold was a compound and could be made if you mixed the right elements. • Wrote a new book suggesting a theory about chemicals but did not have the proof to support it • Boyle suggested that some basic chemicals could not be divided up into any other basic kinds. • These were called elements and each element was a type of atom. • Other more complex chemical mixtures were called compounds.
Robert Boyle: 1627 – 1691 AD • Irish chemist • Inspired by Bacon • First to prove that alchemy did not work • However, he still thought that gold was a compound and could be made if you mixed the right elements. • Wrote a new book suggesting a theory about chemicals but did not have the proof to support it • Boyle suggested that some basic chemicals could not be divided up into any other basic kinds. • These were called elements and each element was a type of atom. • Other more complex chemical mixtures were called compounds.
 Robert Boyle (1627 -1691) • Through careful experimentation, Boyle established chemistry as a pure science • experiments using a vacuum § Discovered “Boyle’s Law” : the idea that the volume of gas varies inversely with pressure § Studied the physical nature of air, through numerous § Supported corpuscularism: a form of atomism which discussed reality and change in terms of particles and their motion § Boyle thought that chemical experiments would be able to show the truth of corpuscularism § Made use of Hooke’s microscope § Used an air pump to prove that sound travels through air § Completed numerous experiments on air by burning a candle in a closed air pump
Robert Boyle (1627 -1691) • Through careful experimentation, Boyle established chemistry as a pure science • experiments using a vacuum § Discovered “Boyle’s Law” : the idea that the volume of gas varies inversely with pressure § Studied the physical nature of air, through numerous § Supported corpuscularism: a form of atomism which discussed reality and change in terms of particles and their motion § Boyle thought that chemical experiments would be able to show the truth of corpuscularism § Made use of Hooke’s microscope § Used an air pump to prove that sound travels through air § Completed numerous experiments on air by burning a candle in a closed air pump
 Boyle Cont. • Spent time at Oxford during the English Civil Wars, where he worked with a group experimenting – The group was committed to the New Philosophy, which emphasized observation and experiment as having equal importance to logical thinking in scientific discovery and comprehension – Foreshadowed the Royal Society, founded in 1660 – Here, Boyle came in contact with numerous philosophical writings including those of Gassendi and Descartes § All of Boyle’s writing were published in English and in Latin translations
Boyle Cont. • Spent time at Oxford during the English Civil Wars, where he worked with a group experimenting – The group was committed to the New Philosophy, which emphasized observation and experiment as having equal importance to logical thinking in scientific discovery and comprehension – Foreshadowed the Royal Society, founded in 1660 – Here, Boyle came in contact with numerous philosophical writings including those of Gassendi and Descartes § All of Boyle’s writing were published in English and in Latin translations
 Boyle • Boyle’s work encouraged scientists to mix chemicals in a more scientific way • He founded a brand new science--chemistry!
Boyle • Boyle’s work encouraged scientists to mix chemicals in a more scientific way • He founded a brand new science--chemistry!
 Slide 20 of 20
Slide 20 of 20

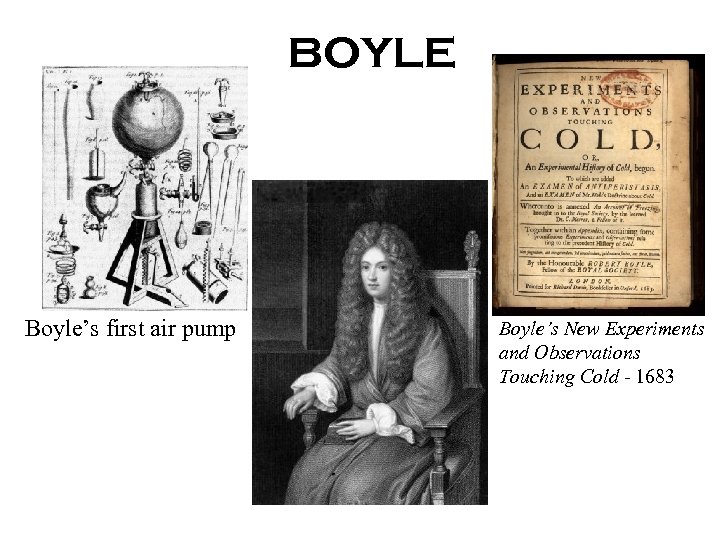 BOYLE Boyle’s first air pump Boyle’s New Experiments and Observations Touching Cold - 1683
BOYLE Boyle’s first air pump Boyle’s New Experiments and Observations Touching Cold - 1683
 Boyle and Lavoisier • He formulated Boyle’s Law about gases— the volume of a gas varies with the pressure exerted on it. • In the eighteenth century Antoine Lavoisier, the founder of modern chemistry, invented a system of naming the chemical elements.
Boyle and Lavoisier • He formulated Boyle’s Law about gases— the volume of a gas varies with the pressure exerted on it. • In the eighteenth century Antoine Lavoisier, the founder of modern chemistry, invented a system of naming the chemical elements.
 Antoine Lavoisier 1743 – 1794 AD • French • Member of the French Academy of Sciences • Worked on a report on the Paris water supply • In 1772, he found that all chemicals could exist as a gas, a liquid, and a solid depending on how hot they were. • In the 1772, Lavoisier found that oxygen is needed for both rusting and burning. He discovered the process called oxidation. • He also showed that water is made up of oxygen and hydrogen.
Antoine Lavoisier 1743 – 1794 AD • French • Member of the French Academy of Sciences • Worked on a report on the Paris water supply • In 1772, he found that all chemicals could exist as a gas, a liquid, and a solid depending on how hot they were. • In the 1772, Lavoisier found that oxygen is needed for both rusting and burning. He discovered the process called oxidation. • He also showed that water is made up of oxygen and hydrogen.
 Lavoisier • Insisted that all the chemicals he used should be carefully weighed and accurate records should be made of all his experiments. • Chemists had not always done this in the past. • This made it possible for the experiments to be duplicated and verified.
Lavoisier • Insisted that all the chemicals he used should be carefully weighed and accurate records should be made of all his experiments. • Chemists had not always done this in the past. • This made it possible for the experiments to be duplicated and verified.
 Antoine Lavoisier (1743 -1794) • French chemist • Through scientific experiments found that materials do not give off phlogiston when burned and that in fact they consume oxygen – Realized that oxygen is also responsible for acidity, which explains why he called it oxygen (Greek for acid-former) – Showed that organisms disassemble and reassemble air in the same way that something does when it burns • Considered the father of modern chemistry • Lighted the streets of Paris • Through experiment proved that the transmutation of water is impossible • Established the Law of Conservation of Mass which states that matter can never be created or destroyed; it can only change form • Using the experiments of John Priestly, Lavoisier showed that air is made of two parts; However, he tried to take the credit – One part was oxygen and the other he called azote (Greek for no life)
Antoine Lavoisier (1743 -1794) • French chemist • Through scientific experiments found that materials do not give off phlogiston when burned and that in fact they consume oxygen – Realized that oxygen is also responsible for acidity, which explains why he called it oxygen (Greek for acid-former) – Showed that organisms disassemble and reassemble air in the same way that something does when it burns • Considered the father of modern chemistry • Lighted the streets of Paris • Through experiment proved that the transmutation of water is impossible • Established the Law of Conservation of Mass which states that matter can never be created or destroyed; it can only change form • Using the experiments of John Priestly, Lavoisier showed that air is made of two parts; However, he tried to take the credit – One part was oxygen and the other he called azote (Greek for no life)
 Lavoisier Cont. • Discovered Cavendish’s inflammable gas which he called hydrogen, Greek for water-former • Invented a system of chemical nomenclature which he explained in his book Methods of Chemical Nomenclature (1787) • Created the first modern chemical textbook, Elementary Treatise of Chemistry, 1789, which presented new theories, Law of Conservation of Mass, denial of the existence of phlogiston, and a list of elements • Believed that the real existence of atoms was philosophically impossible • Used a calorimeter to estimate the amount of heat created per unit of carbon dioxide created through combustion • Believed the radical theory – that radicals when functioning as one group in a reaction would combine with oxygen • Believed all acids containing oxygen • Lavoisier was beheaded during the French Revolution
Lavoisier Cont. • Discovered Cavendish’s inflammable gas which he called hydrogen, Greek for water-former • Invented a system of chemical nomenclature which he explained in his book Methods of Chemical Nomenclature (1787) • Created the first modern chemical textbook, Elementary Treatise of Chemistry, 1789, which presented new theories, Law of Conservation of Mass, denial of the existence of phlogiston, and a list of elements • Believed that the real existence of atoms was philosophically impossible • Used a calorimeter to estimate the amount of heat created per unit of carbon dioxide created through combustion • Believed the radical theory – that radicals when functioning as one group in a reaction would combine with oxygen • Believed all acids containing oxygen • Lavoisier was beheaded during the French Revolution
 Lavoisier working in his Laboratory Lavoisier • "I have tried. . . to arrive at the truth by linking up facts; to suppress as much as possible the use of reasoning, which is often an unreliable instrument which deceives us, in order to follow as much as possible the torch of observation and of experiment. " - Lavoisier
Lavoisier working in his Laboratory Lavoisier • "I have tried. . . to arrive at the truth by linking up facts; to suppress as much as possible the use of reasoning, which is often an unreliable instrument which deceives us, in order to follow as much as possible the torch of observation and of experiment. " - Lavoisier

 Marie Lavoisier: • Married to Antoine Lavoisier at the age of 13 • Translated English and Latin essays and books for her husband, Antoine Lavoisier • Condensed articles for him • Illustrated, edited, and published his work • Studied chemistry and learned English during her teenage years • Often argued whether she was an aide to Antoine or a large influence on his work as a contributor who has gained no credit • Following her husband’s death, Marie ran a scientific salon
Marie Lavoisier: • Married to Antoine Lavoisier at the age of 13 • Translated English and Latin essays and books for her husband, Antoine Lavoisier • Condensed articles for him • Illustrated, edited, and published his work • Studied chemistry and learned English during her teenage years • Often argued whether she was an aide to Antoine or a large influence on his work as a contributor who has gained no credit • Following her husband’s death, Marie ran a scientific salon
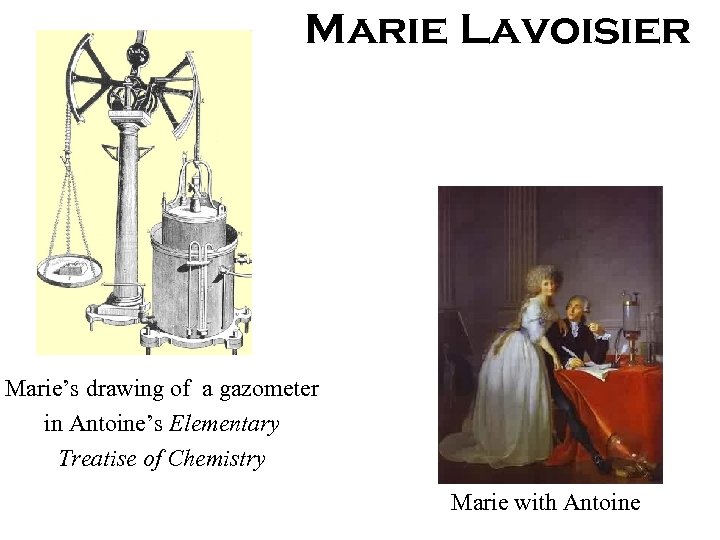 Marie Lavoisier Marie’s drawing of a gazometer in Antoine’s Elementary Treatise of Chemistry Marie with Antoine
Marie Lavoisier Marie’s drawing of a gazometer in Antoine’s Elementary Treatise of Chemistry Marie with Antoine
 Joseph Priestley (1733 -1804) • English chemist and clergyman • Conducted experiments on the properties of air • Studied carbon dioxide; this led to his invention of carbonated beverages • Examined air emitting from numerous substances • Isolated and characterized eight gases, including oxygen • Contributed to the understanding of photosynthesis and respiration • Continually argued with Lavoisier over how to interpret the results of experiments of gases – Priestley used phlogiston – hypothetical principle of flammability that was thought to give metals their characteristics of luster and ductility; widely used to explain combustion, etc. • Completed experiments with candles, plants, and mice in air pumps
Joseph Priestley (1733 -1804) • English chemist and clergyman • Conducted experiments on the properties of air • Studied carbon dioxide; this led to his invention of carbonated beverages • Examined air emitting from numerous substances • Isolated and characterized eight gases, including oxygen • Contributed to the understanding of photosynthesis and respiration • Continually argued with Lavoisier over how to interpret the results of experiments of gases – Priestley used phlogiston – hypothetical principle of flammability that was thought to give metals their characteristics of luster and ductility; widely used to explain combustion, etc. • Completed experiments with candles, plants, and mice in air pumps
 • • The "goodness" of air, a measure of its respirability, interested Priestley. In 1771 he noted the restoration of "injured" or depleted air by green plants. He wrote, The injury which is continually done to the atmosphere by the respiration of such a large number of animals. . . is, in part at least, repaired by the vegetable creation. (5) This balance between animal and plant kingdoms is particularly relevant to our present environmental concerns over global warming and rainforest destruction. Joseph Priestley simplified experimental techniques for the preparation and collection of gases. his pneumatic trough of 1772 was an admirable apparatus. Gases soluble in water, previously difficult to collect, were collected successfully over mercury. in Wiltshire, England, on August 1, 1774 Priestley focused sunlight through a lens in order to heat a sample of mercuric oxide (red calx). The resulting gas supported the burning of a candle with a vigorous flame, was essentially insoluble in water, and accommodated a mouse under glass for some time. In Priestley's own words, I have discovered an air five or six times as good as common air. (5)
• • The "goodness" of air, a measure of its respirability, interested Priestley. In 1771 he noted the restoration of "injured" or depleted air by green plants. He wrote, The injury which is continually done to the atmosphere by the respiration of such a large number of animals. . . is, in part at least, repaired by the vegetable creation. (5) This balance between animal and plant kingdoms is particularly relevant to our present environmental concerns over global warming and rainforest destruction. Joseph Priestley simplified experimental techniques for the preparation and collection of gases. his pneumatic trough of 1772 was an admirable apparatus. Gases soluble in water, previously difficult to collect, were collected successfully over mercury. in Wiltshire, England, on August 1, 1774 Priestley focused sunlight through a lens in order to heat a sample of mercuric oxide (red calx). The resulting gas supported the burning of a candle with a vigorous flame, was essentially insoluble in water, and accommodated a mouse under glass for some time. In Priestley's own words, I have discovered an air five or six times as good as common air. (5)
 Priestley Cont. • Religion: – Educated to be a minister in churches opposing the Church of England – Spent much of his life as a preacher or teacher § Supported both the French and American Revolutions § Emigrated to the United States after a mob attacked and destroyed his house and laboratory in 1791 § Planned to create a model community with his sons, but this never materialized § Influenced and encouraged to study by Ben Franklin § Caused Priestley to work with electricity
Priestley Cont. • Religion: – Educated to be a minister in churches opposing the Church of England – Spent much of his life as a preacher or teacher § Supported both the French and American Revolutions § Emigrated to the United States after a mob attacked and destroyed his house and laboratory in 1791 § Planned to create a model community with his sons, but this never materialized § Influenced and encouraged to study by Ben Franklin § Caused Priestley to work with electricity
 • • Among his accomplishments during his years in Birmingham were the publication of the first part of Letters to a Philosophical Unbeliever, an attempt to defend natural religion against the skepticism of David Hume; a History of the Corruptions of Christianity, a direct attack on the central tenets of orthodox religion, particularly the doctrine of the Trinity; and a History of the Early Opinions Concerning Jesus Christ, where he set out to prove that the doctrine of the Trinity was not according to Scripture. These three works catalyzed storms of controversy. Priestley was attacked in pamphlets and periodicals, denounced in pulpits and in the House of Commons, and considered as an agent of the Devil because of his unorthodox views. Under the Test and Corporation Acts in England, the Dissenter was deprived of the rights of citizenship, and , by law, the Unitarian was not even tolerated. When the French Revolution broke out, the sympathies of the Dissenters lay with those who were struggling under the yoke of corruption, tyranny and oppression. Two years later, festivities were planned to celebrate the auspicious event. On the 11 th of July, 1791 a local newspaper printed an advertisement for a dinner to be held at a leading hotel on July 14, to commemorate the auspicious day (Bastille Day) which witnessed the Emancipation of Twenty-six Millions of People from the yoke of Despotism. . . (1)
• • Among his accomplishments during his years in Birmingham were the publication of the first part of Letters to a Philosophical Unbeliever, an attempt to defend natural religion against the skepticism of David Hume; a History of the Corruptions of Christianity, a direct attack on the central tenets of orthodox religion, particularly the doctrine of the Trinity; and a History of the Early Opinions Concerning Jesus Christ, where he set out to prove that the doctrine of the Trinity was not according to Scripture. These three works catalyzed storms of controversy. Priestley was attacked in pamphlets and periodicals, denounced in pulpits and in the House of Commons, and considered as an agent of the Devil because of his unorthodox views. Under the Test and Corporation Acts in England, the Dissenter was deprived of the rights of citizenship, and , by law, the Unitarian was not even tolerated. When the French Revolution broke out, the sympathies of the Dissenters lay with those who were struggling under the yoke of corruption, tyranny and oppression. Two years later, festivities were planned to celebrate the auspicious event. On the 11 th of July, 1791 a local newspaper printed an advertisement for a dinner to be held at a leading hotel on July 14, to commemorate the auspicious day (Bastille Day) which witnessed the Emancipation of Twenty-six Millions of People from the yoke of Despotism. . . (1)
 • The dinner was attended by eighty one men and ended without incident. • Dr. Priestley had declined to attend, to the disappointment of the crowd that had gathered to demonstrate their contrary views to Priestley's revolutionary and heretical writings. • By evening, the crowd reconvened and, fueled by liquor, sacked and burned the New Meetinghouse where Priestley preached. • The Old Meetinghouse was sacked and burned, too. • Warned of the murderous multitude, Priestley and his wife left Fairhill, with nothing more than the clothes we happened to have on. They did not realize the magnitude of the danger they were in and stopped at a friend's house a mile away. • There they received information that the mob was now at their house looking for them. • They moved again a little farther away but not far enough for Priestley to be spared the sight of the holocaust that engulfed his home, his laboratory and especially his library which contained precious manuscripts of works, some of which were as yet unpublished.
• The dinner was attended by eighty one men and ended without incident. • Dr. Priestley had declined to attend, to the disappointment of the crowd that had gathered to demonstrate their contrary views to Priestley's revolutionary and heretical writings. • By evening, the crowd reconvened and, fueled by liquor, sacked and burned the New Meetinghouse where Priestley preached. • The Old Meetinghouse was sacked and burned, too. • Warned of the murderous multitude, Priestley and his wife left Fairhill, with nothing more than the clothes we happened to have on. They did not realize the magnitude of the danger they were in and stopped at a friend's house a mile away. • There they received information that the mob was now at their house looking for them. • They moved again a little farther away but not far enough for Priestley to be spared the sight of the holocaust that engulfed his home, his laboratory and especially his library which contained precious manuscripts of works, some of which were as yet unpublished.
 • Priestley fled to London and was never to return to Birmingham. He moved to Tottenham and then to Hackney. • During the following months, Priestley was verbally attacked in the House of Commons, burned in effigy, portrayed in caricatures, denounced in pulpits and subjected to threatening letters. • Priestley had by now become an honorary citizen of France which was at war with England. • He was snubbed by the Royal Society and was forced to resign his membership when several of his colleagues turned against him. • His sons were unable to find work in the area and decided to emigrate to America. • He and his wife decided to join them. They sailed from Gravesend on the Samson on April 7, 1794, two weeks after Priestley's 61 st birthday. • While the Priestleys were on their journey to America, Laviosier met his death at the guillotine in the Place de la Revolution.
• Priestley fled to London and was never to return to Birmingham. He moved to Tottenham and then to Hackney. • During the following months, Priestley was verbally attacked in the House of Commons, burned in effigy, portrayed in caricatures, denounced in pulpits and subjected to threatening letters. • Priestley had by now become an honorary citizen of France which was at war with England. • He was snubbed by the Royal Society and was forced to resign his membership when several of his colleagues turned against him. • His sons were unable to find work in the area and decided to emigrate to America. • He and his wife decided to join them. They sailed from Gravesend on the Samson on April 7, 1794, two weeks after Priestley's 61 st birthday. • While the Priestleys were on their journey to America, Laviosier met his death at the guillotine in the Place de la Revolution.
 • The Priestleys landed in New York and proceeded to the capital, Philadelphia. He refused the offer of a chair in chemistry at the University of Pennsylvania, choosing instead to join his son, Joseph, and friend, Thomas Cooper, who were establishing a colony for English Dissenters in central Pennsylvania. • He moved 130 miles to the north and settled in the small town of Northumberland on the banks of the Susquehanna River. • Within the year, the youngest Priestley son, Harry, died, as did Joseph's wife. • Priestley remained active, writing, preaching and experimenting in his newly established laboratory, but the old fire and cheerfulness were gone.
• The Priestleys landed in New York and proceeded to the capital, Philadelphia. He refused the offer of a chair in chemistry at the University of Pennsylvania, choosing instead to join his son, Joseph, and friend, Thomas Cooper, who were establishing a colony for English Dissenters in central Pennsylvania. • He moved 130 miles to the north and settled in the small town of Northumberland on the banks of the Susquehanna River. • Within the year, the youngest Priestley son, Harry, died, as did Joseph's wife. • Priestley remained active, writing, preaching and experimenting in his newly established laboratory, but the old fire and cheerfulness were gone.
 • • Michael Faraday, Address delivered at the Commemoration of the Centenary of the Birth of Dr Priestley (1833) Dr. Priestley had that freedom of mind, and that independence of dogma and of preconceived notions, by which men are so often bowed down and carried forward from fallacy to fallacy, their eyes not being opened to see what that fallacy is. I am very anxious at this time to exhort you all, - as I trust you all are pursuers of science, - to attend to these things; for DR Priestley made his great discoveries mainly in consequence of his having a mind which could be easily moved from what it had held to the reception of new thoughts and notions; and I will venture to say that all his discoveries followed from the facility with which he could leave a preconceived idea.
• • Michael Faraday, Address delivered at the Commemoration of the Centenary of the Birth of Dr Priestley (1833) Dr. Priestley had that freedom of mind, and that independence of dogma and of preconceived notions, by which men are so often bowed down and carried forward from fallacy to fallacy, their eyes not being opened to see what that fallacy is. I am very anxious at this time to exhort you all, - as I trust you all are pursuers of science, - to attend to these things; for DR Priestley made his great discoveries mainly in consequence of his having a mind which could be easily moved from what it had held to the reception of new thoughts and notions; and I will venture to say that all his discoveries followed from the facility with which he could leave a preconceived idea.
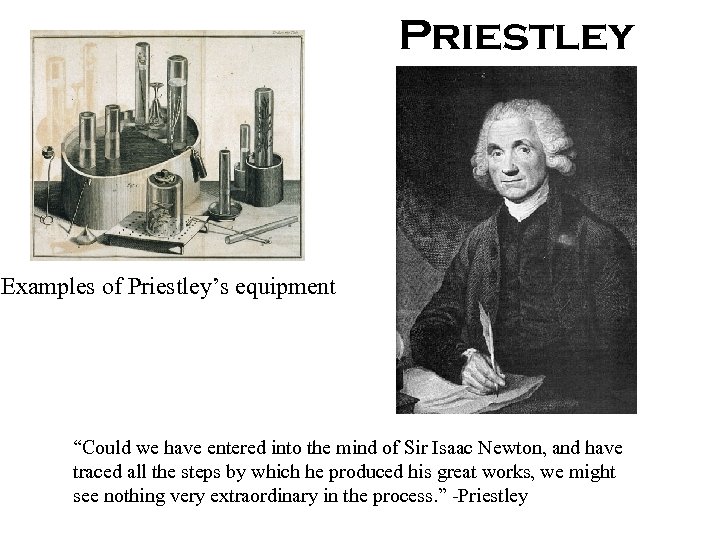 Priestley Examples of Priestley’s equipment “Could we have entered into the mind of Sir Isaac Newton, and have traced all the steps by which he produced his great works, we might see nothing very extraordinary in the process. ” -Priestley
Priestley Examples of Priestley’s equipment “Could we have entered into the mind of Sir Isaac Newton, and have traced all the steps by which he produced his great works, we might see nothing very extraordinary in the process. ” -Priestley
 Henry Cavendish: 1731 – 1810 AD He discovered a whole • • British • Was worth over one hundred million pounds • Son of a Lord and the grandson of the Duke of Devonshire • Spent most of his life doing science experiments at home alone range of new gasses • In 1776 he added acid to marble and the gas that was given off was called fixed air. • He then dripped acid onto iron, and another gas was given off. It seemed that this gas was lighter than air and burnt so easily, so he called it “fire air. ”--What we know as hydrogen.
Henry Cavendish: 1731 – 1810 AD He discovered a whole • • British • Was worth over one hundred million pounds • Son of a Lord and the grandson of the Duke of Devonshire • Spent most of his life doing science experiments at home alone range of new gasses • In 1776 he added acid to marble and the gas that was given off was called fixed air. • He then dripped acid onto iron, and another gas was given off. It seemed that this gas was lighter than air and burnt so easily, so he called it “fire air. ”--What we know as hydrogen.
 Karl Scheele: 1742 – 1786 AD • Swedish • Was a druggist’s assistant • Made a series of discoveries in the 1770 s and 1780 s • He discovered five elements: chlorine, barium, manganese, moylbdenum, and nitrogen. • He also discovered a green dye made up of copper and poisonous arsenic. • He found out how to extract phosphorus from animal bones instead of urine. • He claimed Priestly and Lavoisier stole his discovery of oxygen.
Karl Scheele: 1742 – 1786 AD • Swedish • Was a druggist’s assistant • Made a series of discoveries in the 1770 s and 1780 s • He discovered five elements: chlorine, barium, manganese, moylbdenum, and nitrogen. • He also discovered a green dye made up of copper and poisonous arsenic. • He found out how to extract phosphorus from animal bones instead of urine. • He claimed Priestly and Lavoisier stole his discovery of oxygen.