39079106d290846163ffdbc26b0d2f35.ppt
- Количество слайдов: 64
 Chemistry of Coordination Compounds David P. White University of North Carolina, Wilmington Chapter 24 Copyright 1999, PRENTICE HALL Chapter 24 1
Chemistry of Coordination Compounds David P. White University of North Carolina, Wilmington Chapter 24 Copyright 1999, PRENTICE HALL Chapter 24 1
 The Structure of Complexes • We know Lewis acids are electron pair acceptors. • Coordination compounds: metal compounds formed by Lewis acid-base interactions. • Complexes: Have a metal ion (can be zero oxidation state) bonded to a number of ligands. Complex ions are charged. Example, [Ag(NH 3)2]+. • Ligands are Lewis bases. • Square brackets enclose the metal ion and ligands. • Coordination Sphere: The area of space encompassing the ligands and metal ion. Copyright 1999, PRENTICE HALL Chapter 24 2
The Structure of Complexes • We know Lewis acids are electron pair acceptors. • Coordination compounds: metal compounds formed by Lewis acid-base interactions. • Complexes: Have a metal ion (can be zero oxidation state) bonded to a number of ligands. Complex ions are charged. Example, [Ag(NH 3)2]+. • Ligands are Lewis bases. • Square brackets enclose the metal ion and ligands. • Coordination Sphere: The area of space encompassing the ligands and metal ion. Copyright 1999, PRENTICE HALL Chapter 24 2
 The Structure of Complexes • Ligands can alter properties of the metal: Ag+(aq) + e- Ag(s), E = +0. 799 V [Ag(CN)2]-(aq) + e- Ag(s) + 2 CN-(aq), E = -0. 031 V • Most metal ions in water exist as [M(H 2 O)6]n+. Charges, Coordination Numbers and Geometries • Charge on complex ion = charge on metal + charges on ligands. • Donor atom: the atom bonded directly to the metal. • Coordination number: the number of ligands attached to the metal. Copyright 1999, PRENTICE HALL Chapter 24 3
The Structure of Complexes • Ligands can alter properties of the metal: Ag+(aq) + e- Ag(s), E = +0. 799 V [Ag(CN)2]-(aq) + e- Ag(s) + 2 CN-(aq), E = -0. 031 V • Most metal ions in water exist as [M(H 2 O)6]n+. Charges, Coordination Numbers and Geometries • Charge on complex ion = charge on metal + charges on ligands. • Donor atom: the atom bonded directly to the metal. • Coordination number: the number of ligands attached to the metal. Copyright 1999, PRENTICE HALL Chapter 24 3
 The Structure of Complexes Charges, Coordination Numbers and Geometries – Most common coordination numbers are 4 and 6. – Some metal ions have constant coordination number (e. g. Cr 3+ and Co 3+ have coordination numbers of 6). – The size of the ligand affects the coordination number (e. g. [Fe. F 6]3 - forms but only [Fe. Cl 4]- is stable). – The amount of charge transferred from ligand to metal affects coordination number (e. g. [Ni(NH 3)6]2+ is stable but only [Ni(CN)4]2 - is stable). • Four coordinate complexes are either tetrahedral or square planar (commonly seen for d 8 metal ions). • Six coordinate complexes are octahedral. Copyright 1999, PRENTICE HALL Chapter 24 4
The Structure of Complexes Charges, Coordination Numbers and Geometries – Most common coordination numbers are 4 and 6. – Some metal ions have constant coordination number (e. g. Cr 3+ and Co 3+ have coordination numbers of 6). – The size of the ligand affects the coordination number (e. g. [Fe. F 6]3 - forms but only [Fe. Cl 4]- is stable). – The amount of charge transferred from ligand to metal affects coordination number (e. g. [Ni(NH 3)6]2+ is stable but only [Ni(CN)4]2 - is stable). • Four coordinate complexes are either tetrahedral or square planar (commonly seen for d 8 metal ions). • Six coordinate complexes are octahedral. Copyright 1999, PRENTICE HALL Chapter 24 4
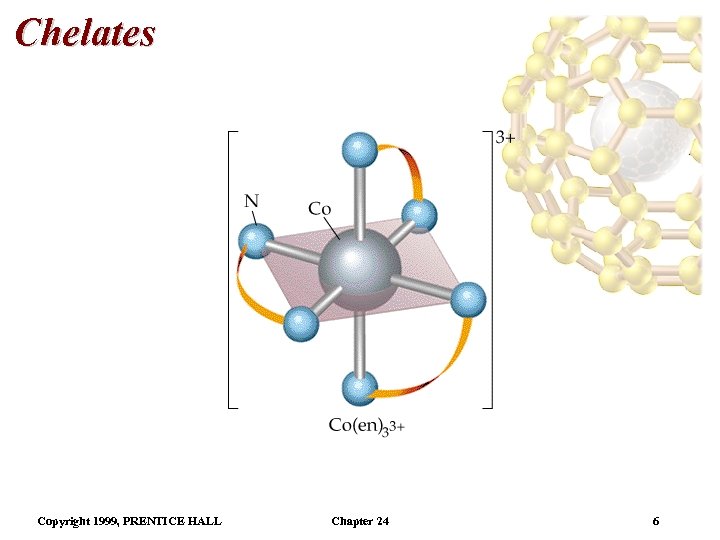
 Chelates • Monodentate ligands bind through one donor atom only. – Therefore they occupy only one coordination site. • Polydentate ligands (or chelating agents) bind through more than one donor atom per ligand. – Example, ethylenediamine (en), H 2 NCH 2 NH 2. • The octahedral [Co(en)3]3+ is a typical en complex. • Chelate effect: More stable complexes are formed with chelating agents than the equivalent number of monodentate ligands. Copyright 1999, PRENTICE HALL Chapter 24 5
Chelates • Monodentate ligands bind through one donor atom only. – Therefore they occupy only one coordination site. • Polydentate ligands (or chelating agents) bind through more than one donor atom per ligand. – Example, ethylenediamine (en), H 2 NCH 2 NH 2. • The octahedral [Co(en)3]3+ is a typical en complex. • Chelate effect: More stable complexes are formed with chelating agents than the equivalent number of monodentate ligands. Copyright 1999, PRENTICE HALL Chapter 24 5
 Chelates Copyright 1999, PRENTICE HALL Chapter 24 6
Chelates Copyright 1999, PRENTICE HALL Chapter 24 6
![Chelates [Ni(H 2 O)6]2+(aq) + 6 NH 3 [Ni(NH 3)6]2+(aq) + 6 H 2 Chelates [Ni(H 2 O)6]2+(aq) + 6 NH 3 [Ni(NH 3)6]2+(aq) + 6 H 2](https://present5.com/presentation/39079106d290846163ffdbc26b0d2f35/image-7.jpg) Chelates [Ni(H 2 O)6]2+(aq) + 6 NH 3 [Ni(NH 3)6]2+(aq) + 6 H 2 O(l) Kf = 4 108 [Ni(H 2 O)6]2+(aq) + 3 en [Ni(en)3]2+(aq) + 6 H 2 O(l) Kf = 2 1018 • Sequestering agents are chelating agents that are used to remove unwanted metal ions. • In medicine sequestering agents are used to selectively remove toxic metal ions (e. g. Hg 2+ and Pb 2+) while leaving biologically important metals. • One very important chelating agent is ethylenediaminetetraacetate (EDTA 4 -). Copyright 1999, PRENTICE HALL Chapter 24 7
Chelates [Ni(H 2 O)6]2+(aq) + 6 NH 3 [Ni(NH 3)6]2+(aq) + 6 H 2 O(l) Kf = 4 108 [Ni(H 2 O)6]2+(aq) + 3 en [Ni(en)3]2+(aq) + 6 H 2 O(l) Kf = 2 1018 • Sequestering agents are chelating agents that are used to remove unwanted metal ions. • In medicine sequestering agents are used to selectively remove toxic metal ions (e. g. Hg 2+ and Pb 2+) while leaving biologically important metals. • One very important chelating agent is ethylenediaminetetraacetate (EDTA 4 -). Copyright 1999, PRENTICE HALL Chapter 24 7
 Chelates Copyright 1999, PRENTICE HALL Chapter 24 8
Chelates Copyright 1999, PRENTICE HALL Chapter 24 8
![Chelates • EDTA occupies 6 coordination sites, for example [Co. EDTA]- is an octahedral Chelates • EDTA occupies 6 coordination sites, for example [Co. EDTA]- is an octahedral](https://present5.com/presentation/39079106d290846163ffdbc26b0d2f35/image-9.jpg) Chelates • EDTA occupies 6 coordination sites, for example [Co. EDTA]- is an octahedral Co 3+ complex. • Both N atoms (blue) and O atoms (red) coordinate to the metal. • EDTA is used in consumer products to complex the metal ions which catalyze decomposition reactions. Copyright 1999, PRENTICE HALL Chapter 24 9
Chelates • EDTA occupies 6 coordination sites, for example [Co. EDTA]- is an octahedral Co 3+ complex. • Both N atoms (blue) and O atoms (red) coordinate to the metal. • EDTA is used in consumer products to complex the metal ions which catalyze decomposition reactions. Copyright 1999, PRENTICE HALL Chapter 24 9
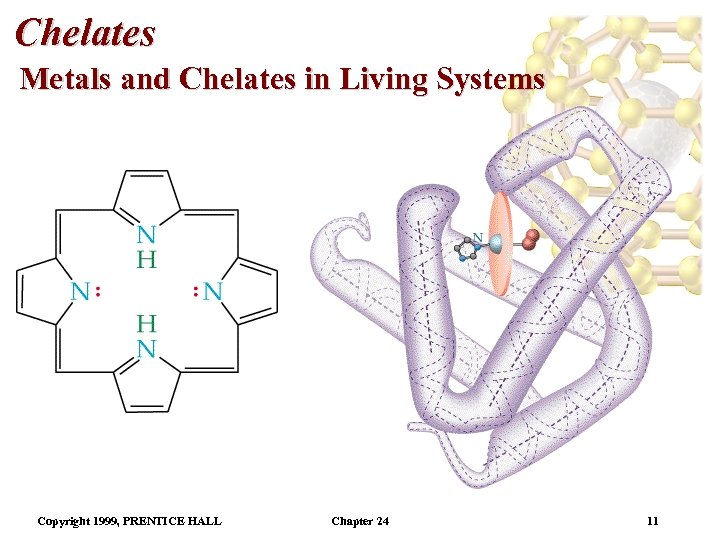
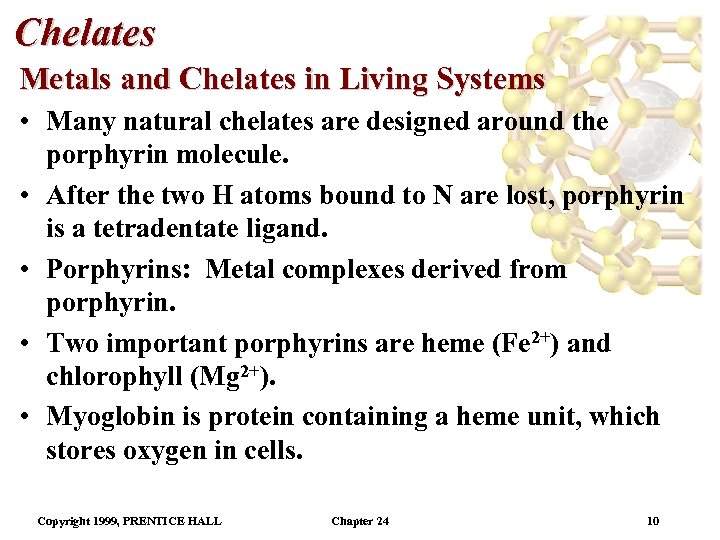 Chelates Metals and Chelates in Living Systems • Many natural chelates are designed around the porphyrin molecule. • After the two H atoms bound to N are lost, porphyrin is a tetradentate ligand. • Porphyrins: Metal complexes derived from porphyrin. • Two important porphyrins are heme (Fe 2+) and chlorophyll (Mg 2+). • Myoglobin is protein containing a heme unit, which stores oxygen in cells. Copyright 1999, PRENTICE HALL Chapter 24 10
Chelates Metals and Chelates in Living Systems • Many natural chelates are designed around the porphyrin molecule. • After the two H atoms bound to N are lost, porphyrin is a tetradentate ligand. • Porphyrins: Metal complexes derived from porphyrin. • Two important porphyrins are heme (Fe 2+) and chlorophyll (Mg 2+). • Myoglobin is protein containing a heme unit, which stores oxygen in cells. Copyright 1999, PRENTICE HALL Chapter 24 10
 Chelates Metals and Chelates in Living Systems Copyright 1999, PRENTICE HALL Chapter 24 11
Chelates Metals and Chelates in Living Systems Copyright 1999, PRENTICE HALL Chapter 24 11
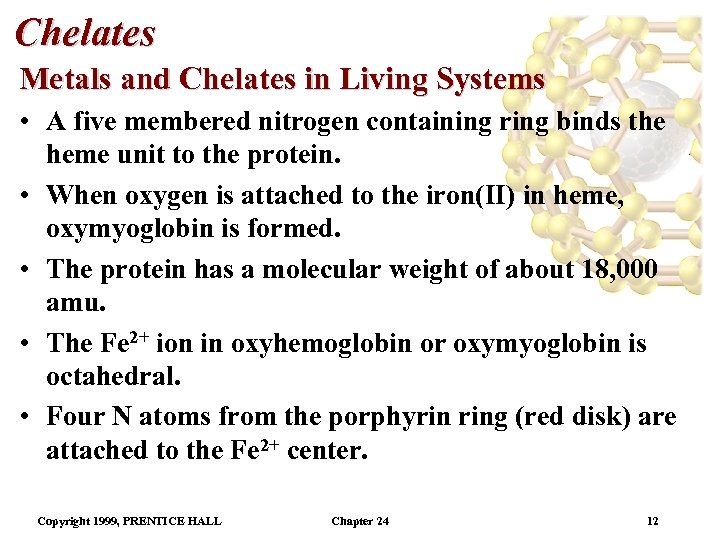 Chelates Metals and Chelates in Living Systems • A five membered nitrogen containing ring binds the heme unit to the protein. • When oxygen is attached to the iron(II) in heme, oxymyoglobin is formed. • The protein has a molecular weight of about 18, 000 amu. • The Fe 2+ ion in oxyhemoglobin or oxymyoglobin is octahedral. • Four N atoms from the porphyrin ring (red disk) are attached to the Fe 2+ center. Copyright 1999, PRENTICE HALL Chapter 24 12
Chelates Metals and Chelates in Living Systems • A five membered nitrogen containing ring binds the heme unit to the protein. • When oxygen is attached to the iron(II) in heme, oxymyoglobin is formed. • The protein has a molecular weight of about 18, 000 amu. • The Fe 2+ ion in oxyhemoglobin or oxymyoglobin is octahedral. • Four N atoms from the porphyrin ring (red disk) are attached to the Fe 2+ center. Copyright 1999, PRENTICE HALL Chapter 24 12
 Chelates Metals and Chelates in Living Systems Copyright 1999, PRENTICE HALL Chapter 24 13
Chelates Metals and Chelates in Living Systems Copyright 1999, PRENTICE HALL Chapter 24 13
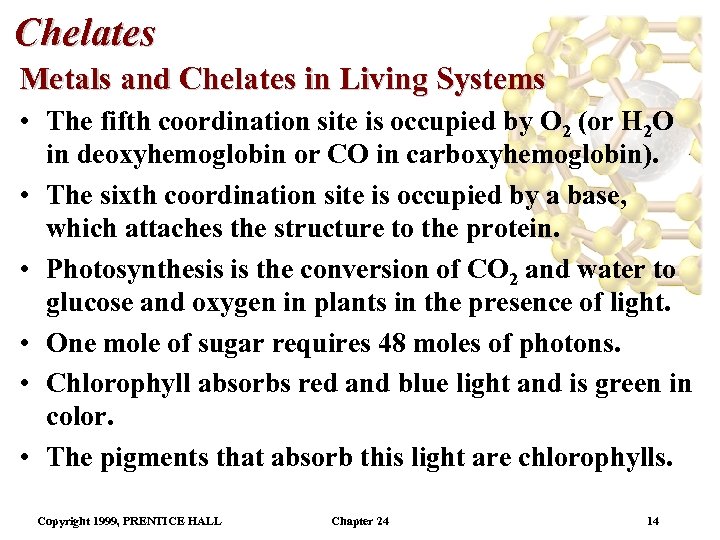 Chelates Metals and Chelates in Living Systems • The fifth coordination site is occupied by O 2 (or H 2 O in deoxyhemoglobin or CO in carboxyhemoglobin). • The sixth coordination site is occupied by a base, which attaches the structure to the protein. • Photosynthesis is the conversion of CO 2 and water to glucose and oxygen in plants in the presence of light. • One mole of sugar requires 48 moles of photons. • Chlorophyll absorbs red and blue light and is green in color. • The pigments that absorb this light are chlorophylls. Copyright 1999, PRENTICE HALL Chapter 24 14
Chelates Metals and Chelates in Living Systems • The fifth coordination site is occupied by O 2 (or H 2 O in deoxyhemoglobin or CO in carboxyhemoglobin). • The sixth coordination site is occupied by a base, which attaches the structure to the protein. • Photosynthesis is the conversion of CO 2 and water to glucose and oxygen in plants in the presence of light. • One mole of sugar requires 48 moles of photons. • Chlorophyll absorbs red and blue light and is green in color. • The pigments that absorb this light are chlorophylls. Copyright 1999, PRENTICE HALL Chapter 24 14
 Chelates Metals and Chelates in Living Systems Copyright 1999, PRENTICE HALL Chapter 24 15
Chelates Metals and Chelates in Living Systems Copyright 1999, PRENTICE HALL Chapter 24 15
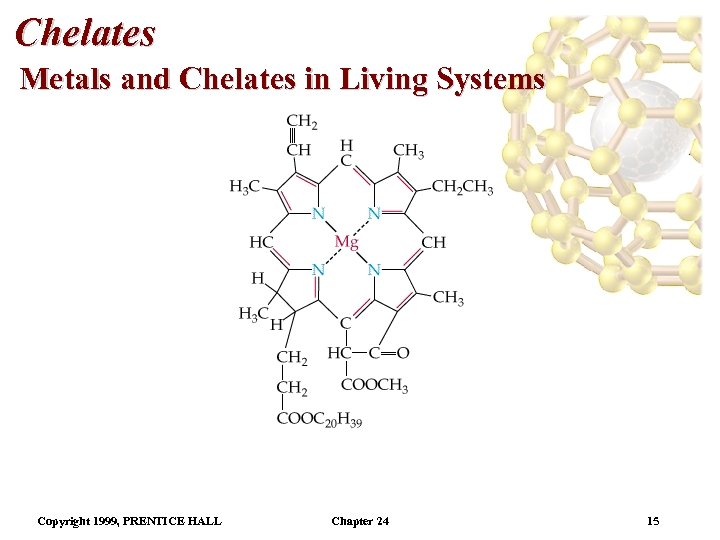
 Chelates Metals and Chelates in Living Systems • Chlorophyll a is the most abundant chlorophyll. • The other chlorophylls differ in the structure of the side chains. • Mg 2+ is in the center of the porphyrin-like ring. • The alternating double bonds give chlorophyll its green color (it absorbs red light). • Chlorophyll absorbs red light (655 nm) and blue light (430 nm). Copyright 1999, PRENTICE HALL Chapter 24 16
Chelates Metals and Chelates in Living Systems • Chlorophyll a is the most abundant chlorophyll. • The other chlorophylls differ in the structure of the side chains. • Mg 2+ is in the center of the porphyrin-like ring. • The alternating double bonds give chlorophyll its green color (it absorbs red light). • Chlorophyll absorbs red light (655 nm) and blue light (430 nm). Copyright 1999, PRENTICE HALL Chapter 24 16
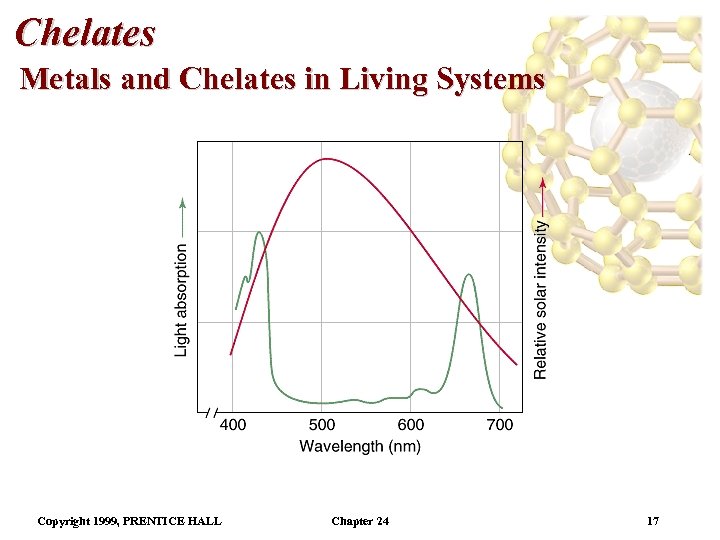 Chelates Metals and Chelates in Living Systems Copyright 1999, PRENTICE HALL Chapter 24 17
Chelates Metals and Chelates in Living Systems Copyright 1999, PRENTICE HALL Chapter 24 17
 Chelates Metals and Chelates in Living Systems • The reaction 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 is highly endothermic. • Plant photosynthesis sustains life on Earth. Copyright 1999, PRENTICE HALL Chapter 24 18
Chelates Metals and Chelates in Living Systems • The reaction 6 CO 2 + 6 H 2 O C 6 H 12 O 6 + 6 O 2 is highly endothermic. • Plant photosynthesis sustains life on Earth. Copyright 1999, PRENTICE HALL Chapter 24 18
 Nomenclature • Rules: – For salts, name the cation before the anion. Example in [Co(NH 3)5 Cl]Cl 2 we name [Co(NH 3)5 Cl]2+ before Cl-. – Within a complex ion, the ligands are named (in alphabetical order) before the metal. Example [Co(NH 3)5 Cl]2+ is tetraamminechlorocobalt(II). Note the tetra portion is an indication of the number of NH 3 groups and is therefore not considered in the alphabetizing of the ligands. – Anionic ligands end in o and neutral ligands are simply the name of the molecule. Exceptions: H 2 O (aqua) and NH 3 (ammine). Copyright 1999, PRENTICE HALL Chapter 24 19
Nomenclature • Rules: – For salts, name the cation before the anion. Example in [Co(NH 3)5 Cl]Cl 2 we name [Co(NH 3)5 Cl]2+ before Cl-. – Within a complex ion, the ligands are named (in alphabetical order) before the metal. Example [Co(NH 3)5 Cl]2+ is tetraamminechlorocobalt(II). Note the tetra portion is an indication of the number of NH 3 groups and is therefore not considered in the alphabetizing of the ligands. – Anionic ligands end in o and neutral ligands are simply the name of the molecule. Exceptions: H 2 O (aqua) and NH 3 (ammine). Copyright 1999, PRENTICE HALL Chapter 24 19
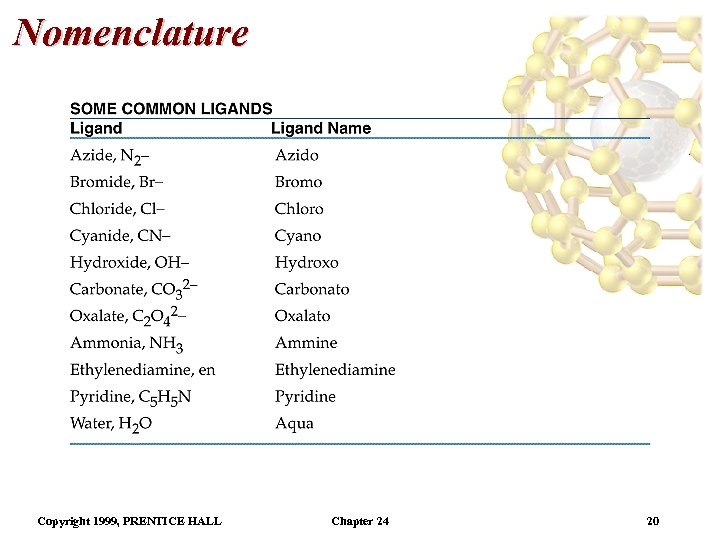 Nomenclature Copyright 1999, PRENTICE HALL Chapter 24 20
Nomenclature Copyright 1999, PRENTICE HALL Chapter 24 20
 Nomenclature • Rules: – Greek prefixes are used to indicate number of ligands (di-, tri-, tetra-, penta-, and hexa-). Exception: if the ligand name has a Greek prefix already. Then enclose the ligand name in parentheses and use bis-, tris-, tetrakis-, pentakis-, and hexakis. • Example [Co(en)3]Cl 3 is tris(ethylenediamine)cobalt(III) chloride. – If the complex is an anion, the name ends in -ate. – Oxidation state of the metal is given in Roman numerals in parenthesis at the end of the complex name. Copyright 1999, PRENTICE HALL Chapter 24 21
Nomenclature • Rules: – Greek prefixes are used to indicate number of ligands (di-, tri-, tetra-, penta-, and hexa-). Exception: if the ligand name has a Greek prefix already. Then enclose the ligand name in parentheses and use bis-, tris-, tetrakis-, pentakis-, and hexakis. • Example [Co(en)3]Cl 3 is tris(ethylenediamine)cobalt(III) chloride. – If the complex is an anion, the name ends in -ate. – Oxidation state of the metal is given in Roman numerals in parenthesis at the end of the complex name. Copyright 1999, PRENTICE HALL Chapter 24 21
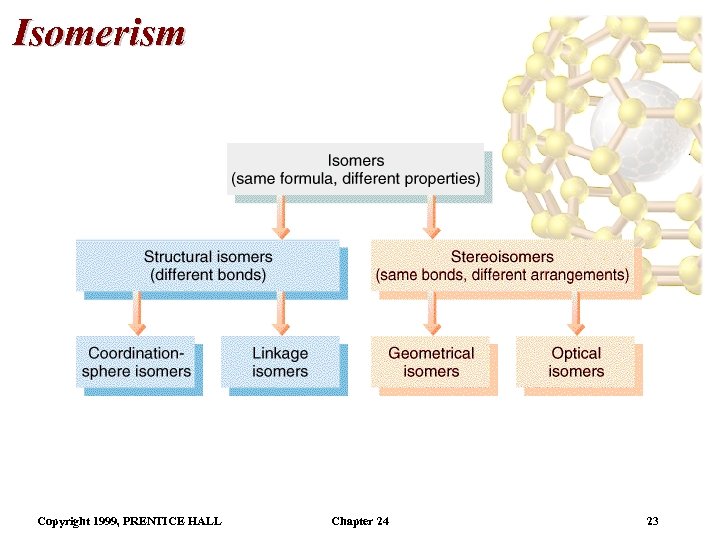
 Isomerism • Isomers: two compounds with the same formulas but different arrangements of atoms. • Coordination-sphere isomers and linkage isomers: have different structures (i. e. different bonds). • Geometrical isomers and optical isomers are stereoisomers (i. e. have the same bonds, but different spatial arrangements of atoms). • Structural isomers have different connectivity of atoms. • Stereoisomers have the same connectivity but different spatial arrangements of atoms. Copyright 1999, PRENTICE HALL Chapter 24 22
Isomerism • Isomers: two compounds with the same formulas but different arrangements of atoms. • Coordination-sphere isomers and linkage isomers: have different structures (i. e. different bonds). • Geometrical isomers and optical isomers are stereoisomers (i. e. have the same bonds, but different spatial arrangements of atoms). • Structural isomers have different connectivity of atoms. • Stereoisomers have the same connectivity but different spatial arrangements of atoms. Copyright 1999, PRENTICE HALL Chapter 24 22
 Isomerism Copyright 1999, PRENTICE HALL Chapter 24 23
Isomerism Copyright 1999, PRENTICE HALL Chapter 24 23
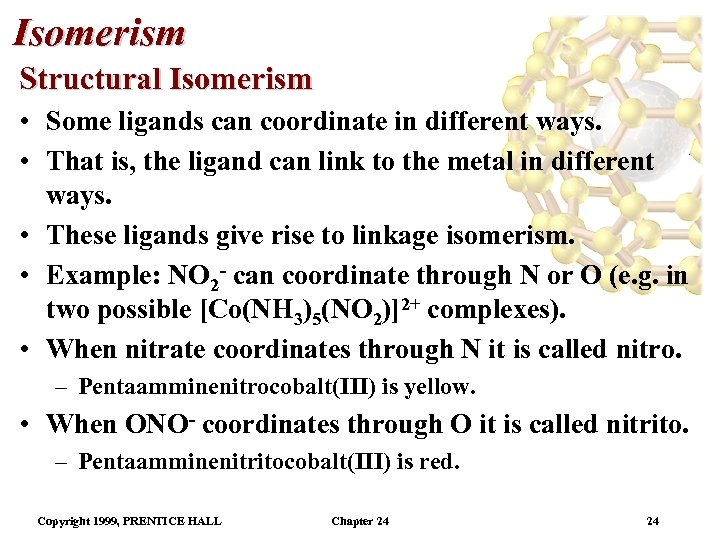 Isomerism Structural Isomerism • Some ligands can coordinate in different ways. • That is, the ligand can link to the metal in different ways. • These ligands give rise to linkage isomerism. • Example: NO 2 - can coordinate through N or O (e. g. in two possible [Co(NH 3)5(NO 2)]2+ complexes). • When nitrate coordinates through N it is called nitro. – Pentaamminenitrocobalt(III) is yellow. • When ONO- coordinates through O it is called nitrito. – Pentaamminenitritocobalt(III) is red. Copyright 1999, PRENTICE HALL Chapter 24 24
Isomerism Structural Isomerism • Some ligands can coordinate in different ways. • That is, the ligand can link to the metal in different ways. • These ligands give rise to linkage isomerism. • Example: NO 2 - can coordinate through N or O (e. g. in two possible [Co(NH 3)5(NO 2)]2+ complexes). • When nitrate coordinates through N it is called nitro. – Pentaamminenitrocobalt(III) is yellow. • When ONO- coordinates through O it is called nitrito. – Pentaamminenitritocobalt(III) is red. Copyright 1999, PRENTICE HALL Chapter 24 24
 Isomerism Structural Isomerism Copyright 1999, PRENTICE HALL Chapter 24 25
Isomerism Structural Isomerism Copyright 1999, PRENTICE HALL Chapter 24 25
 Isomerism Structural Isomerism • Similarly, SCN- can coordinate through S or N. – Coordination sphere isomerism occurs when ligands from outside the coordination sphere move inside. – Example: Cr. Cl 3(H 2 O)6 has three coordination sphere isomers: [Cr(H 2 O)6]Cl 3 (violet), [Cr(H 2 O)5 Cl]Cl 2. H 2 O (green), and [Cr(H 2 O)4 Cl 2]Cl. 2 H 2 O (green). Stereoisomerism • Consider square planar [Pt(NH 3)2 Cl 2]. • The two NH 3 ligands can either be 90 apart or 180 apart. • Therefore, the spatial arrangement of the atoms is different. Copyright 1999, PRENTICE HALL Chapter 24 26
Isomerism Structural Isomerism • Similarly, SCN- can coordinate through S or N. – Coordination sphere isomerism occurs when ligands from outside the coordination sphere move inside. – Example: Cr. Cl 3(H 2 O)6 has three coordination sphere isomers: [Cr(H 2 O)6]Cl 3 (violet), [Cr(H 2 O)5 Cl]Cl 2. H 2 O (green), and [Cr(H 2 O)4 Cl 2]Cl. 2 H 2 O (green). Stereoisomerism • Consider square planar [Pt(NH 3)2 Cl 2]. • The two NH 3 ligands can either be 90 apart or 180 apart. • Therefore, the spatial arrangement of the atoms is different. Copyright 1999, PRENTICE HALL Chapter 24 26
 Isomerism Stereoisomerism Copyright 1999, PRENTICE HALL Chapter 24 27
Isomerism Stereoisomerism Copyright 1999, PRENTICE HALL Chapter 24 27
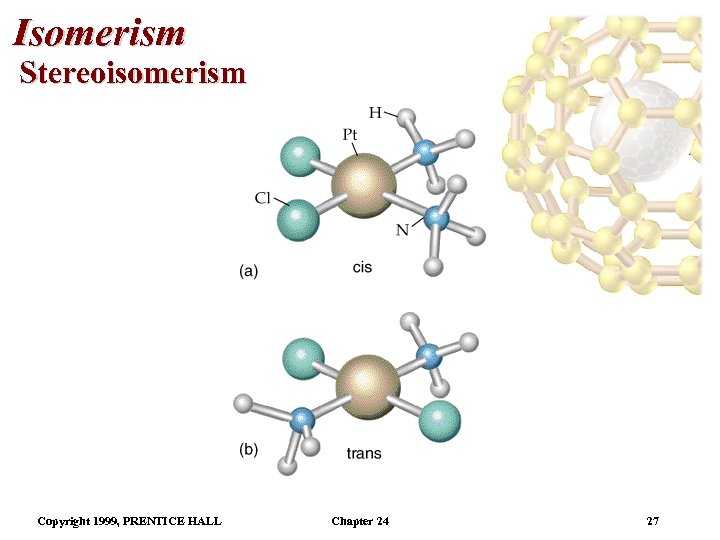
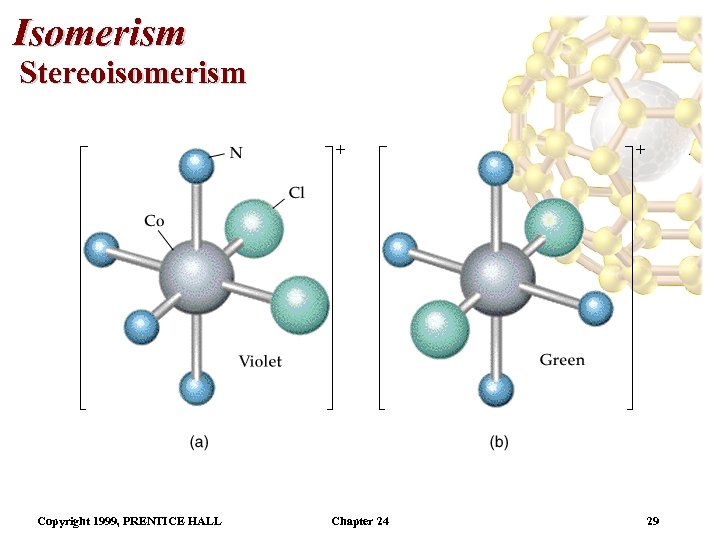
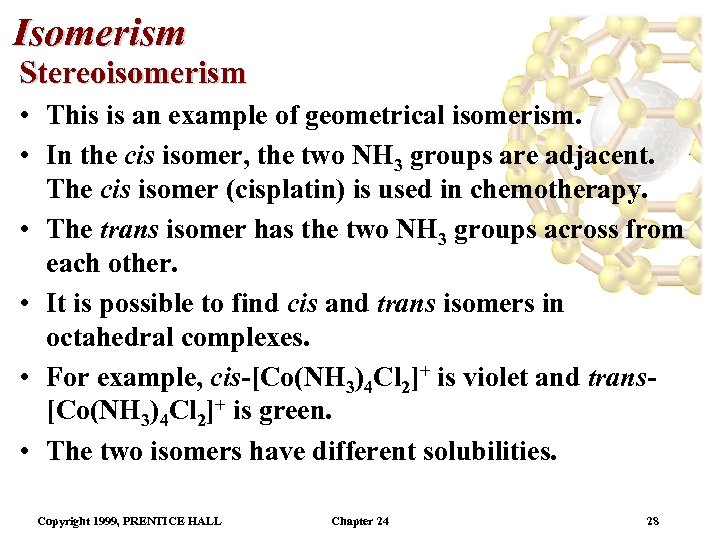 Isomerism Stereoisomerism • This is an example of geometrical isomerism. • In the cis isomer, the two NH 3 groups are adjacent. The cis isomer (cisplatin) is used in chemotherapy. • The trans isomer has the two NH 3 groups across from each other. • It is possible to find cis and trans isomers in octahedral complexes. • For example, cis-[Co(NH 3)4 Cl 2]+ is violet and trans[Co(NH 3)4 Cl 2]+ is green. • The two isomers have different solubilities. Copyright 1999, PRENTICE HALL Chapter 24 28
Isomerism Stereoisomerism • This is an example of geometrical isomerism. • In the cis isomer, the two NH 3 groups are adjacent. The cis isomer (cisplatin) is used in chemotherapy. • The trans isomer has the two NH 3 groups across from each other. • It is possible to find cis and trans isomers in octahedral complexes. • For example, cis-[Co(NH 3)4 Cl 2]+ is violet and trans[Co(NH 3)4 Cl 2]+ is green. • The two isomers have different solubilities. Copyright 1999, PRENTICE HALL Chapter 24 28
 Isomerism Stereoisomerism Copyright 1999, PRENTICE HALL Chapter 24 29
Isomerism Stereoisomerism Copyright 1999, PRENTICE HALL Chapter 24 29
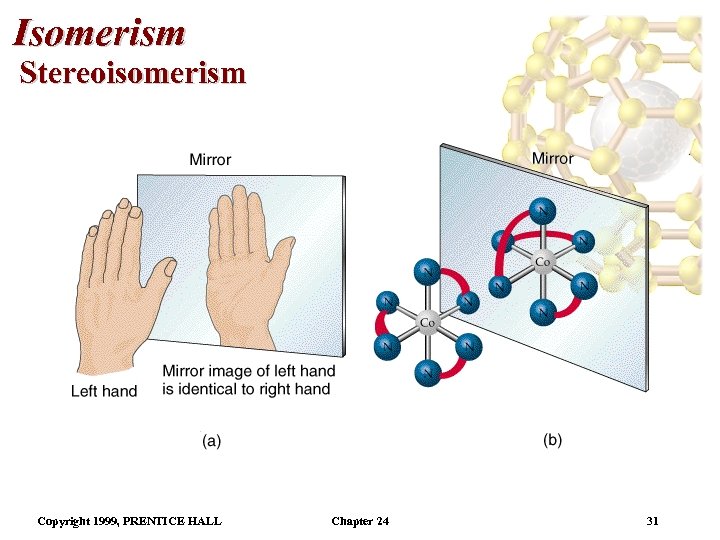
 Isomerism Stereoisomerism • In general, geometrical isomers have different physical and chemical properties. • It is not possible to form geometrical isomers with tetrahedra. (All corners of a tetrahedron are identical. ) • Optical isomers are mirror images which cannot be superimposed on each other. • Optical isomers are called enantiomers. • Complexes which can form enantiomers are chiral. • Most of the human body is chiral (the hands, for example). Copyright 1999, PRENTICE HALL Chapter 24 30
Isomerism Stereoisomerism • In general, geometrical isomers have different physical and chemical properties. • It is not possible to form geometrical isomers with tetrahedra. (All corners of a tetrahedron are identical. ) • Optical isomers are mirror images which cannot be superimposed on each other. • Optical isomers are called enantiomers. • Complexes which can form enantiomers are chiral. • Most of the human body is chiral (the hands, for example). Copyright 1999, PRENTICE HALL Chapter 24 30
 Isomerism Stereoisomerism Copyright 1999, PRENTICE HALL Chapter 24 31
Isomerism Stereoisomerism Copyright 1999, PRENTICE HALL Chapter 24 31
 Isomerism Stereoisomerism • Enzymes are the most highly chiral substances known. • Most physical and chemical properties of enantiomers are identical. • Therefore, enantiomers are very difficult to separate. • Enzymes do a very good job of catalyzing the reaction of only one enantiomer. • Therefore, one enantiomer can produce a specific physiological effect whereas its mirror image produces a different effect. Copyright 1999, PRENTICE HALL Chapter 24 32
Isomerism Stereoisomerism • Enzymes are the most highly chiral substances known. • Most physical and chemical properties of enantiomers are identical. • Therefore, enantiomers are very difficult to separate. • Enzymes do a very good job of catalyzing the reaction of only one enantiomer. • Therefore, one enantiomer can produce a specific physiological effect whereas its mirror image produces a different effect. Copyright 1999, PRENTICE HALL Chapter 24 32
 Isomerism Stereoisomerism Copyright 1999, PRENTICE HALL Chapter 24 33
Isomerism Stereoisomerism Copyright 1999, PRENTICE HALL Chapter 24 33
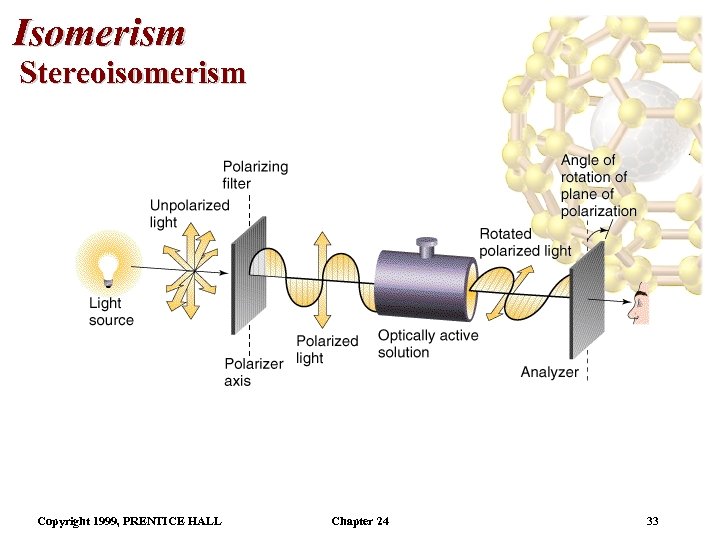
 Isomerism Stereoisomerism • Enantiomers are capable of rotating the plane of polarized light. • Hence, they are called optical isomers. • When horizontally polarized light enters an optically active solution. • As the light emerges from the solution, the plane of polarity has changed. • The mirror image of an enantiomer will rotate the plane of polarized light by the same amount in the opposite direction. Copyright 1999, PRENTICE HALL Chapter 24 34
Isomerism Stereoisomerism • Enantiomers are capable of rotating the plane of polarized light. • Hence, they are called optical isomers. • When horizontally polarized light enters an optically active solution. • As the light emerges from the solution, the plane of polarity has changed. • The mirror image of an enantiomer will rotate the plane of polarized light by the same amount in the opposite direction. Copyright 1999, PRENTICE HALL Chapter 24 34
 Isomerism Stereoisomerism • Dextrorotatory solutions rotate the plane of polarized light to the right. This isomer is called the d-isomer. • Levorotatory solutions rotate the plane of polarized light to the left. This isomer is called the l-isomer. • Chiral molecules are optically active because of their effect on light. • Racemic mixtures contain equal amounts of l- and disomers. They have no overall effect on the plane of polarized light. Copyright 1999, PRENTICE HALL Chapter 24 35
Isomerism Stereoisomerism • Dextrorotatory solutions rotate the plane of polarized light to the right. This isomer is called the d-isomer. • Levorotatory solutions rotate the plane of polarized light to the left. This isomer is called the l-isomer. • Chiral molecules are optically active because of their effect on light. • Racemic mixtures contain equal amounts of l- and disomers. They have no overall effect on the plane of polarized light. Copyright 1999, PRENTICE HALL Chapter 24 35
 Isomerism Stereoisomerism • Pasteur was the first to separate racemic ammonium tartarate (Na. NH 4 C 4 H 9 O 6) by crystallizing the solution and physically picking out the “right-handed” crystals from the mixture using a microscope. • Optically pure tartarate can be used to separate a racemic mixture of [Co(en)3]Cl 3: if d-tartarate is used, d-[Co(en)3]Cl 3 precipitates leaving l-[Co(en)3]Cl 3 in solution. Copyright 1999, PRENTICE HALL Chapter 24 36
Isomerism Stereoisomerism • Pasteur was the first to separate racemic ammonium tartarate (Na. NH 4 C 4 H 9 O 6) by crystallizing the solution and physically picking out the “right-handed” crystals from the mixture using a microscope. • Optically pure tartarate can be used to separate a racemic mixture of [Co(en)3]Cl 3: if d-tartarate is used, d-[Co(en)3]Cl 3 precipitates leaving l-[Co(en)3]Cl 3 in solution. Copyright 1999, PRENTICE HALL Chapter 24 36
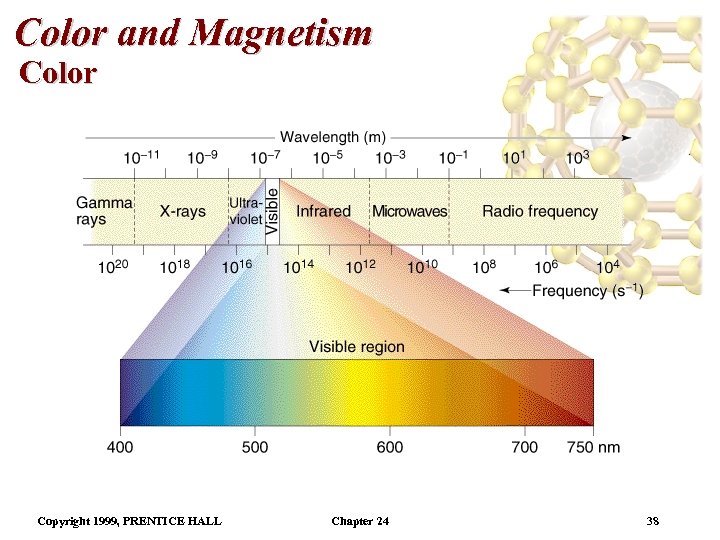
 Color and Magnetism Color • Color of a complex depends on: (i) the metal and (ii) its oxidation state. • Pale blue [Cu(H 2 O)6]2+ can be converted into dark blue [Cu(NH 3)6]2+ by adding NH 3(aq). • A partially filled d orbital is usually required for a complex to be colored. • So, d 0 metal ions are usually colorless. Exceptions: Mn. O 4 - and Cr. O 42 -. • Colored compounds absorb visible light. • The color perceived is the sum of the light not absorbed by the complex. Copyright 1999, PRENTICE HALL Chapter 24 37
Color and Magnetism Color • Color of a complex depends on: (i) the metal and (ii) its oxidation state. • Pale blue [Cu(H 2 O)6]2+ can be converted into dark blue [Cu(NH 3)6]2+ by adding NH 3(aq). • A partially filled d orbital is usually required for a complex to be colored. • So, d 0 metal ions are usually colorless. Exceptions: Mn. O 4 - and Cr. O 42 -. • Colored compounds absorb visible light. • The color perceived is the sum of the light not absorbed by the complex. Copyright 1999, PRENTICE HALL Chapter 24 37
 Color and Magnetism Color Copyright 1999, PRENTICE HALL Chapter 24 38
Color and Magnetism Color Copyright 1999, PRENTICE HALL Chapter 24 38
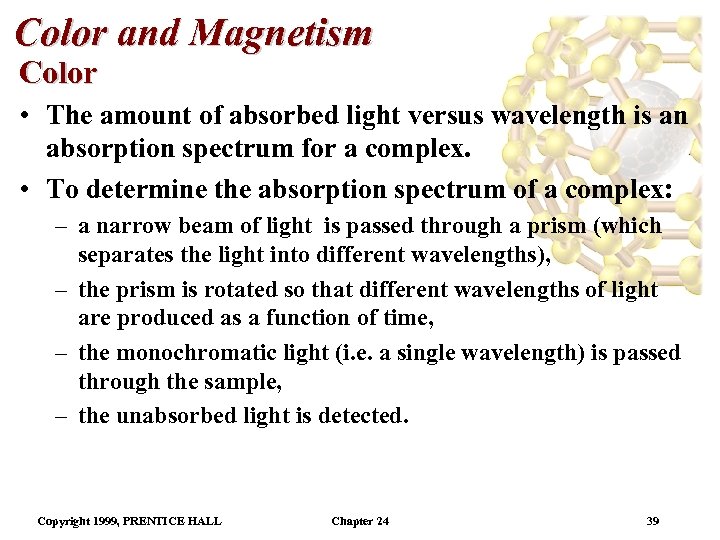 Color and Magnetism Color • The amount of absorbed light versus wavelength is an absorption spectrum for a complex. • To determine the absorption spectrum of a complex: – a narrow beam of light is passed through a prism (which separates the light into different wavelengths), – the prism is rotated so that different wavelengths of light are produced as a function of time, – the monochromatic light (i. e. a single wavelength) is passed through the sample, – the unabsorbed light is detected. Copyright 1999, PRENTICE HALL Chapter 24 39
Color and Magnetism Color • The amount of absorbed light versus wavelength is an absorption spectrum for a complex. • To determine the absorption spectrum of a complex: – a narrow beam of light is passed through a prism (which separates the light into different wavelengths), – the prism is rotated so that different wavelengths of light are produced as a function of time, – the monochromatic light (i. e. a single wavelength) is passed through the sample, – the unabsorbed light is detected. Copyright 1999, PRENTICE HALL Chapter 24 39
 Color and Magnetism Color Copyright 1999, PRENTICE HALL Chapter 24 40
Color and Magnetism Color Copyright 1999, PRENTICE HALL Chapter 24 40
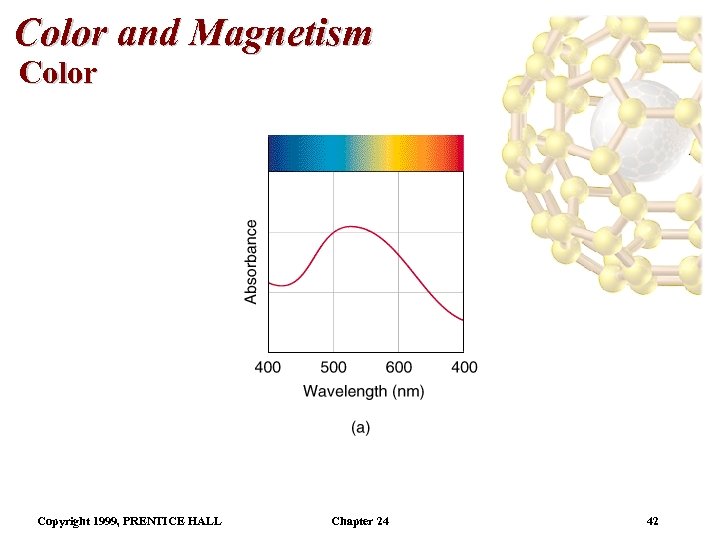
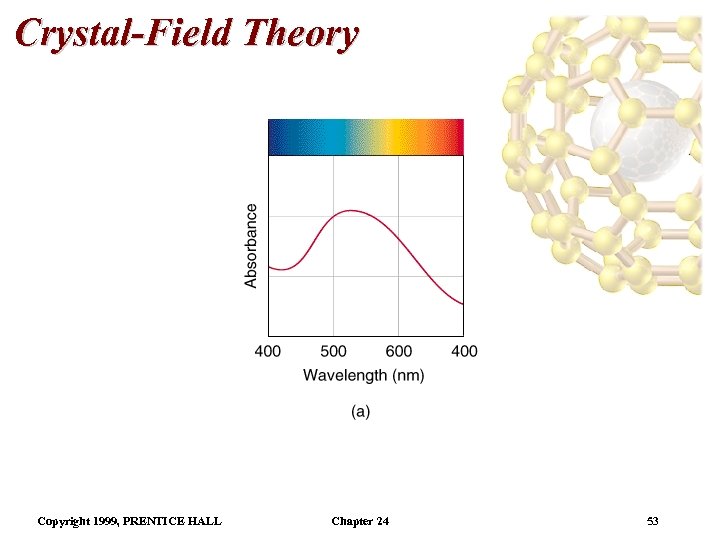
 Color and Magnetism Color • The plot of absorbance versus wavelength is the absorption spectrum. • For example, the absorption spectrum for [Ti(H 2 O)6]3+ has a maximum absorption occurs at 510 nm (green and yellow). • So, the complex transmits all light except green and yellow. • Therefore, the complex is purple. Copyright 1999, PRENTICE HALL Chapter 24 41
Color and Magnetism Color • The plot of absorbance versus wavelength is the absorption spectrum. • For example, the absorption spectrum for [Ti(H 2 O)6]3+ has a maximum absorption occurs at 510 nm (green and yellow). • So, the complex transmits all light except green and yellow. • Therefore, the complex is purple. Copyright 1999, PRENTICE HALL Chapter 24 41
 Color and Magnetism Color Copyright 1999, PRENTICE HALL Chapter 24 42
Color and Magnetism Color Copyright 1999, PRENTICE HALL Chapter 24 42
 Color and Magnetism • Many transition metal complexes are paramagnetic (i. e. they have unpaired electrons). • There are some interesting observations. Consider a d 6 metal ion: – [Co(NH 3)6]3+ has no unpaired electrons, but [Co. F 6]3 - has four unpaired electrons per ion. • We need to develop a bonding theory to account for both color and magnetism in transition metal complexes. Copyright 1999, PRENTICE HALL Chapter 24 43
Color and Magnetism • Many transition metal complexes are paramagnetic (i. e. they have unpaired electrons). • There are some interesting observations. Consider a d 6 metal ion: – [Co(NH 3)6]3+ has no unpaired electrons, but [Co. F 6]3 - has four unpaired electrons per ion. • We need to develop a bonding theory to account for both color and magnetism in transition metal complexes. Copyright 1999, PRENTICE HALL Chapter 24 43
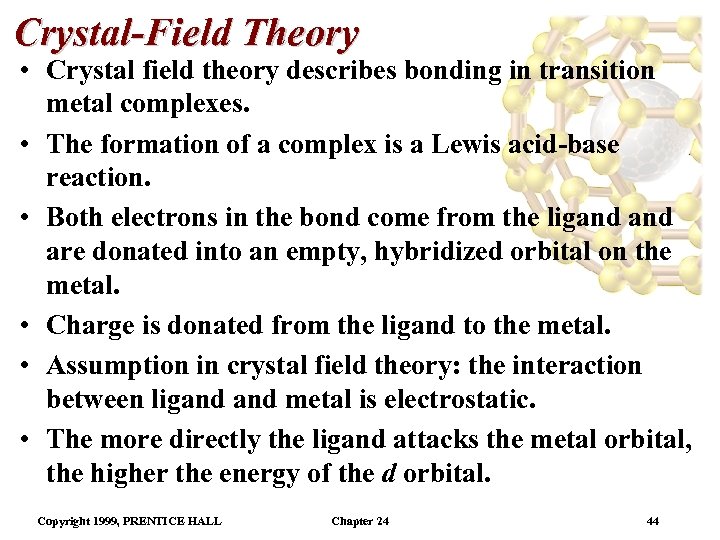 Crystal-Field Theory • Crystal field theory describes bonding in transition metal complexes. • The formation of a complex is a Lewis acid-base reaction. • Both electrons in the bond come from the ligand are donated into an empty, hybridized orbital on the metal. • Charge is donated from the ligand to the metal. • Assumption in crystal field theory: the interaction between ligand metal is electrostatic. • The more directly the ligand attacks the metal orbital, the higher the energy of the d orbital. Copyright 1999, PRENTICE HALL Chapter 24 44
Crystal-Field Theory • Crystal field theory describes bonding in transition metal complexes. • The formation of a complex is a Lewis acid-base reaction. • Both electrons in the bond come from the ligand are donated into an empty, hybridized orbital on the metal. • Charge is donated from the ligand to the metal. • Assumption in crystal field theory: the interaction between ligand metal is electrostatic. • The more directly the ligand attacks the metal orbital, the higher the energy of the d orbital. Copyright 1999, PRENTICE HALL Chapter 24 44
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 45
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 45
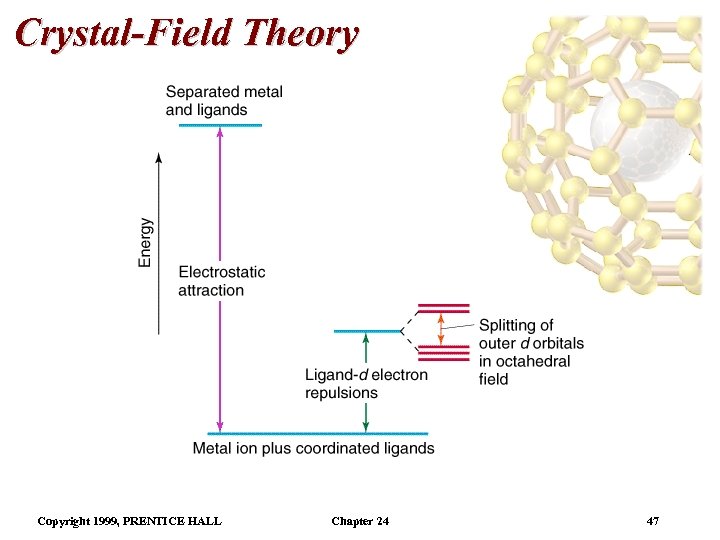
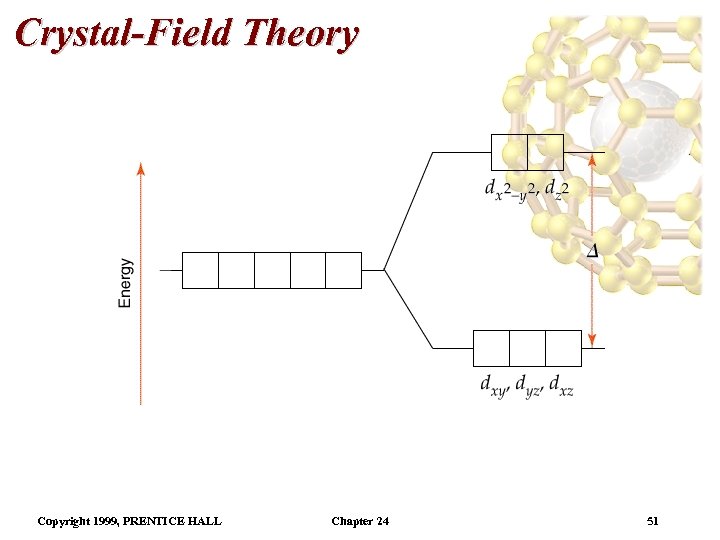
 Crystal-Field Theory • The complex metal ion has a lower energy than the separated metal and ligands. • However, there are some ligand-d-electron repulsions which occur since the metal has partially filled dorbitals. • In an octahedral field, the degeneracy of the five d orbitals is lifted. • In an octahedral field, the five d orbitals do not have the same energy: three degenerate orbitals are higher energy than two degenerate orbitals. • The energy gap between them is called , the crystal field splitting energy. Copyright 1999, PRENTICE HALL Chapter 24 46
Crystal-Field Theory • The complex metal ion has a lower energy than the separated metal and ligands. • However, there are some ligand-d-electron repulsions which occur since the metal has partially filled dorbitals. • In an octahedral field, the degeneracy of the five d orbitals is lifted. • In an octahedral field, the five d orbitals do not have the same energy: three degenerate orbitals are higher energy than two degenerate orbitals. • The energy gap between them is called , the crystal field splitting energy. Copyright 1999, PRENTICE HALL Chapter 24 46
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 47
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 47
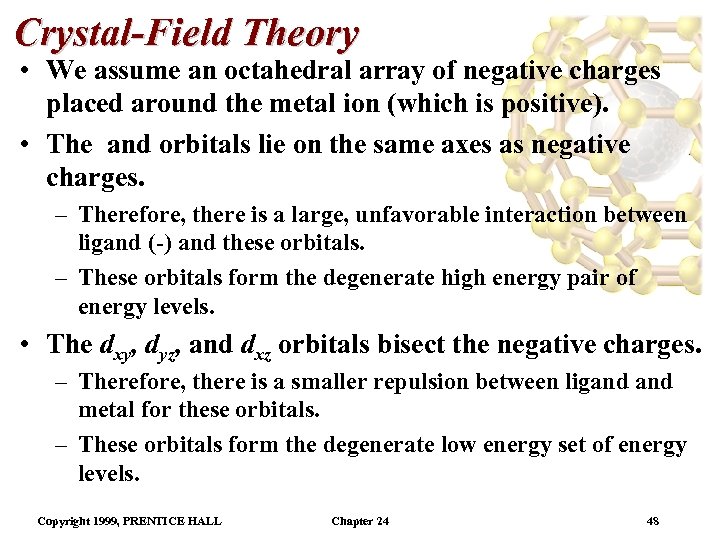 Crystal-Field Theory • We assume an octahedral array of negative charges placed around the metal ion (which is positive). • The and orbitals lie on the same axes as negative charges. – Therefore, there is a large, unfavorable interaction between ligand (-) and these orbitals. – These orbitals form the degenerate high energy pair of energy levels. • The dxy, dyz, and dxz orbitals bisect the negative charges. – Therefore, there is a smaller repulsion between ligand metal for these orbitals. – These orbitals form the degenerate low energy set of energy levels. Copyright 1999, PRENTICE HALL Chapter 24 48
Crystal-Field Theory • We assume an octahedral array of negative charges placed around the metal ion (which is positive). • The and orbitals lie on the same axes as negative charges. – Therefore, there is a large, unfavorable interaction between ligand (-) and these orbitals. – These orbitals form the degenerate high energy pair of energy levels. • The dxy, dyz, and dxz orbitals bisect the negative charges. – Therefore, there is a smaller repulsion between ligand metal for these orbitals. – These orbitals form the degenerate low energy set of energy levels. Copyright 1999, PRENTICE HALL Chapter 24 48
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 49
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 49
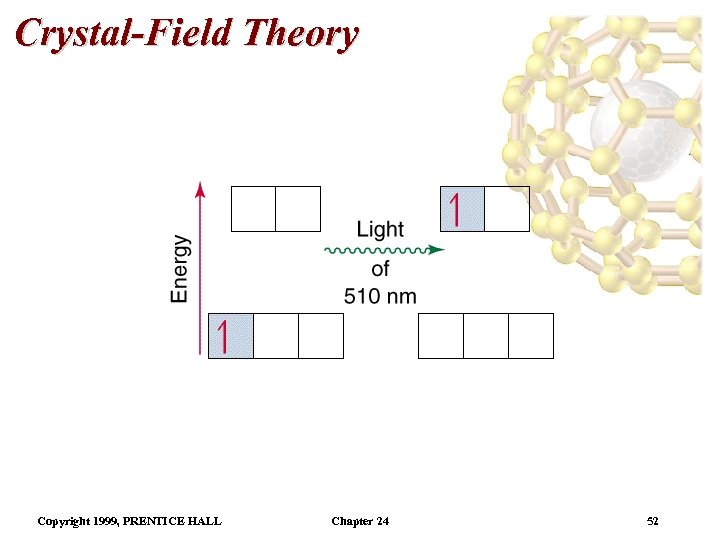
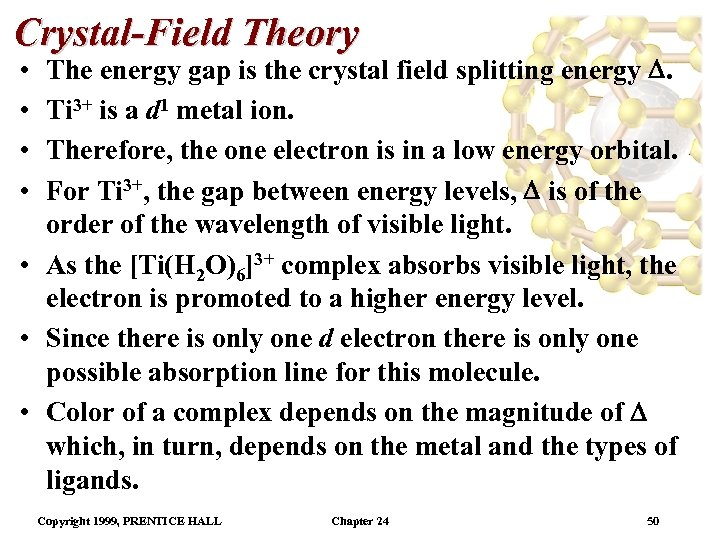 Crystal-Field Theory The energy gap is the crystal field splitting energy . Ti 3+ is a d 1 metal ion. Therefore, the one electron is in a low energy orbital. For Ti 3+, the gap between energy levels, is of the order of the wavelength of visible light. • As the [Ti(H 2 O)6]3+ complex absorbs visible light, the electron is promoted to a higher energy level. • Since there is only one d electron there is only one possible absorption line for this molecule. • Color of a complex depends on the magnitude of which, in turn, depends on the metal and the types of ligands. • • Copyright 1999, PRENTICE HALL Chapter 24 50
Crystal-Field Theory The energy gap is the crystal field splitting energy . Ti 3+ is a d 1 metal ion. Therefore, the one electron is in a low energy orbital. For Ti 3+, the gap between energy levels, is of the order of the wavelength of visible light. • As the [Ti(H 2 O)6]3+ complex absorbs visible light, the electron is promoted to a higher energy level. • Since there is only one d electron there is only one possible absorption line for this molecule. • Color of a complex depends on the magnitude of which, in turn, depends on the metal and the types of ligands. • • Copyright 1999, PRENTICE HALL Chapter 24 50
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 51
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 51
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 52
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 52
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 53
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 53
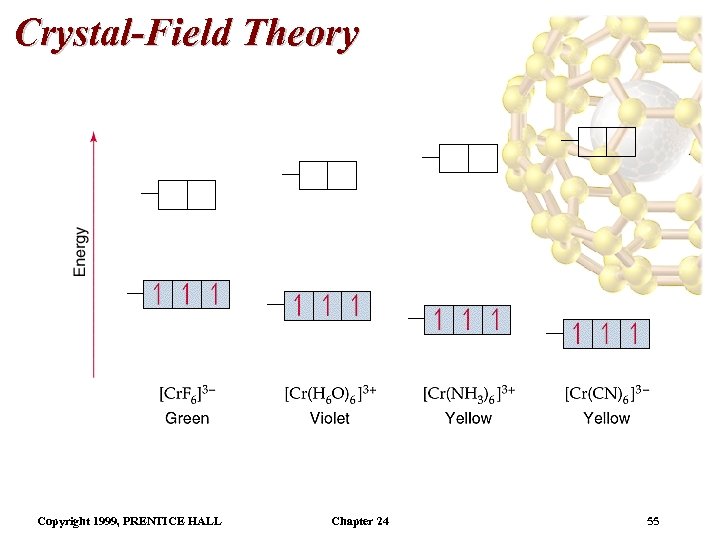
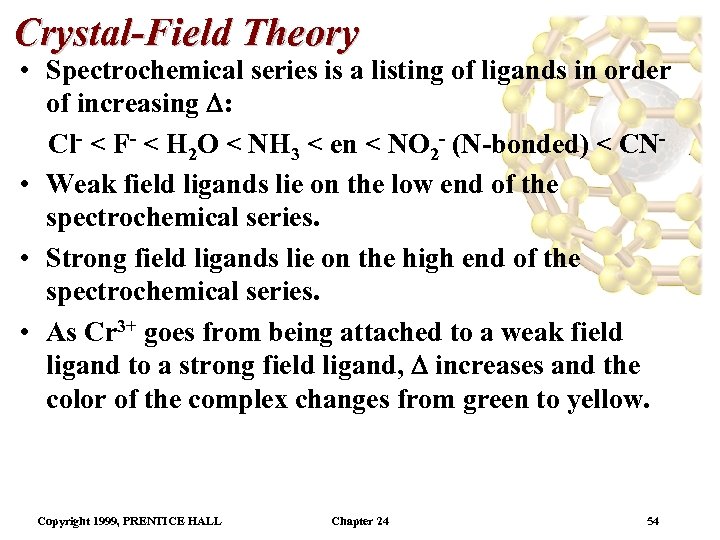 Crystal-Field Theory • Spectrochemical series is a listing of ligands in order of increasing : Cl- < F- < H 2 O < NH 3 < en < NO 2 - (N-bonded) < CN • Weak field ligands lie on the low end of the spectrochemical series. • Strong field ligands lie on the high end of the spectrochemical series. • As Cr 3+ goes from being attached to a weak field ligand to a strong field ligand, increases and the color of the complex changes from green to yellow. Copyright 1999, PRENTICE HALL Chapter 24 54
Crystal-Field Theory • Spectrochemical series is a listing of ligands in order of increasing : Cl- < F- < H 2 O < NH 3 < en < NO 2 - (N-bonded) < CN • Weak field ligands lie on the low end of the spectrochemical series. • Strong field ligands lie on the high end of the spectrochemical series. • As Cr 3+ goes from being attached to a weak field ligand to a strong field ligand, increases and the color of the complex changes from green to yellow. Copyright 1999, PRENTICE HALL Chapter 24 54
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 55
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 55
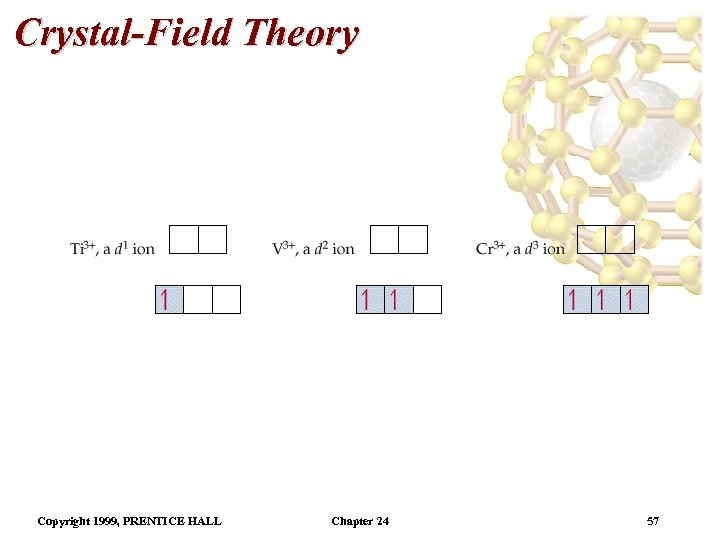
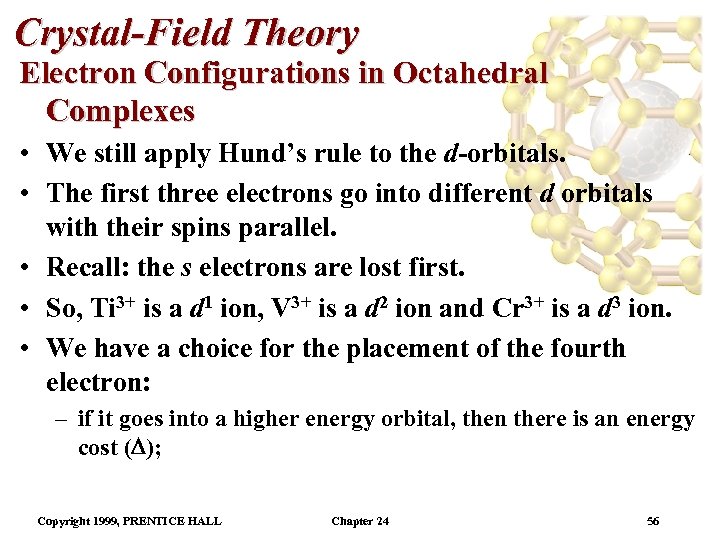 Crystal-Field Theory Electron Configurations in Octahedral Complexes • We still apply Hund’s rule to the d-orbitals. • The first three electrons go into different d orbitals with their spins parallel. • Recall: the s electrons are lost first. • So, Ti 3+ is a d 1 ion, V 3+ is a d 2 ion and Cr 3+ is a d 3 ion. • We have a choice for the placement of the fourth electron: – if it goes into a higher energy orbital, then there is an energy cost ( ); Copyright 1999, PRENTICE HALL Chapter 24 56
Crystal-Field Theory Electron Configurations in Octahedral Complexes • We still apply Hund’s rule to the d-orbitals. • The first three electrons go into different d orbitals with their spins parallel. • Recall: the s electrons are lost first. • So, Ti 3+ is a d 1 ion, V 3+ is a d 2 ion and Cr 3+ is a d 3 ion. • We have a choice for the placement of the fourth electron: – if it goes into a higher energy orbital, then there is an energy cost ( ); Copyright 1999, PRENTICE HALL Chapter 24 56
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 57
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 57
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 58
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 58
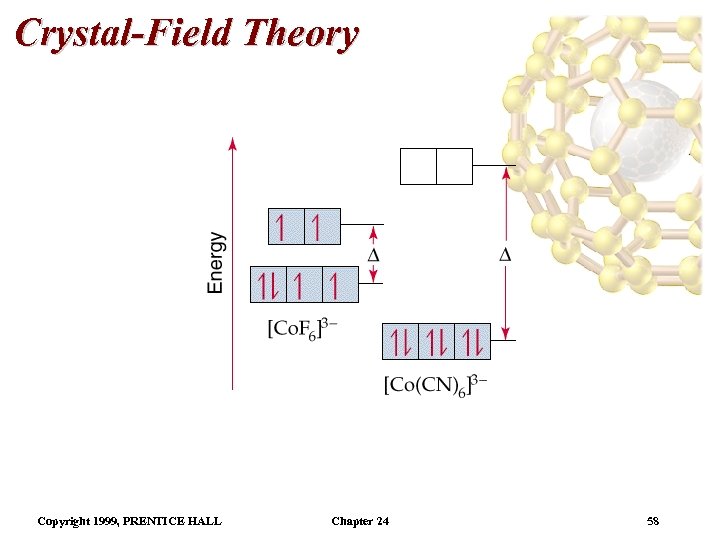
 Crystal-Field Theory Electron Configurations in Octahedral Complexes – if it goes into a lower energy orbital, there is a different energy cost (called the spin-pairing energy due to pairing an electron). • Weak field ligands tend to favor adding electrons to the higher energy orbitals (high spin complexes) because < pairing energy. • Strong field ligands tend to favor adding electrons to lower energy orbitals (low spin complexes) because > pairing energy. Copyright 1999, PRENTICE HALL Chapter 24 59
Crystal-Field Theory Electron Configurations in Octahedral Complexes – if it goes into a lower energy orbital, there is a different energy cost (called the spin-pairing energy due to pairing an electron). • Weak field ligands tend to favor adding electrons to the higher energy orbitals (high spin complexes) because < pairing energy. • Strong field ligands tend to favor adding electrons to lower energy orbitals (low spin complexes) because > pairing energy. Copyright 1999, PRENTICE HALL Chapter 24 59
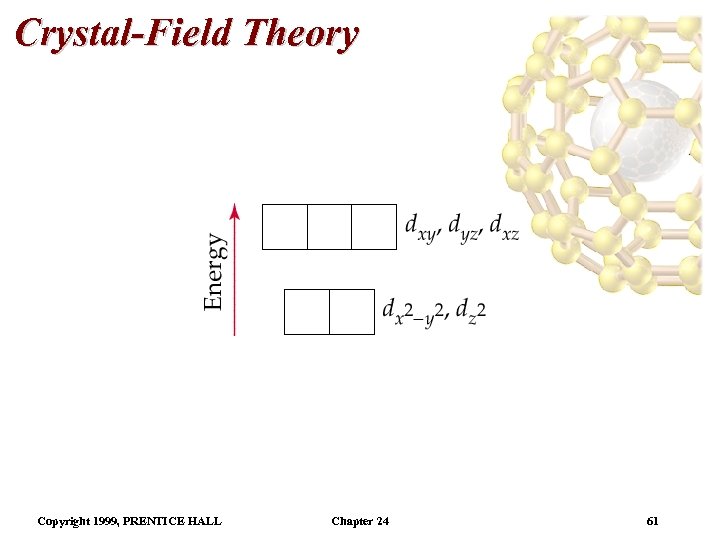
 Crystal-Field Theory Tetrahedral and Square-Planar Complexes • By using the same arguments as for the octahedral case, we can derive the relative orbital energies for d orbitals in a tetrahedral field. • The exact opposite of an octahedral field orbital arrangement is found (i. e. the dxy, dyz, and dxz orbitals are of lower energy than the and orbitals). • Because there are only 4 ligands, for a tetrahedral field is smaller than for an octahedral field (four ninths). • This causes all tetrahedral complexes to be high spin. Copyright 1999, PRENTICE HALL Chapter 24 60
Crystal-Field Theory Tetrahedral and Square-Planar Complexes • By using the same arguments as for the octahedral case, we can derive the relative orbital energies for d orbitals in a tetrahedral field. • The exact opposite of an octahedral field orbital arrangement is found (i. e. the dxy, dyz, and dxz orbitals are of lower energy than the and orbitals). • Because there are only 4 ligands, for a tetrahedral field is smaller than for an octahedral field (four ninths). • This causes all tetrahedral complexes to be high spin. Copyright 1999, PRENTICE HALL Chapter 24 60
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 61
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 61
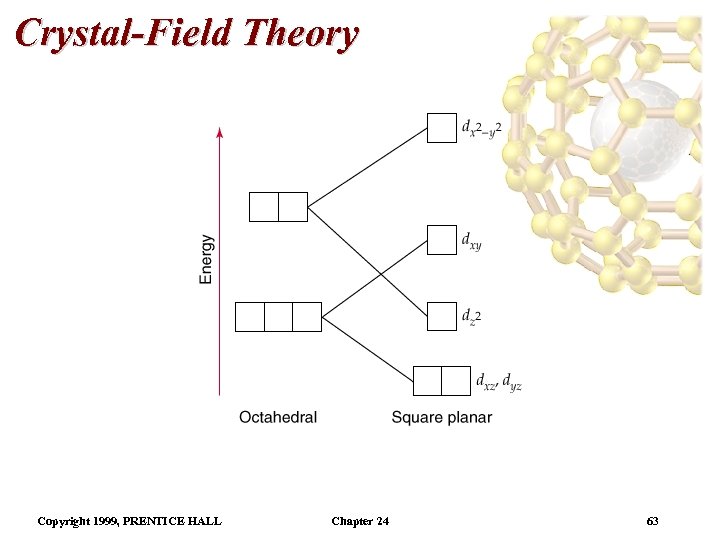
 Crystal-Field Theory Tetrahedral and Square-Planar Complexes • Square planar complexes can be thought of as follows: start with an octahedral complex and remove two ligands along the z-axis. • As a consequence the four planar ligands are drawn in towards the metal. • Relative to the octahedral field, the orbital is greatly lowered in energy, the dyz, and dxz orbitals lowered in energy, the dxy, and orbitals are raised in energy. • Most d 8 metal ions for square planar complexes. – the majority of complexes are low spin (i. e. diamagnetic). – Examples: Pd 2+, Pt 2+, Ir+, and Au 3+. Copyright 1999, PRENTICE HALL Chapter 24 62
Crystal-Field Theory Tetrahedral and Square-Planar Complexes • Square planar complexes can be thought of as follows: start with an octahedral complex and remove two ligands along the z-axis. • As a consequence the four planar ligands are drawn in towards the metal. • Relative to the octahedral field, the orbital is greatly lowered in energy, the dyz, and dxz orbitals lowered in energy, the dxy, and orbitals are raised in energy. • Most d 8 metal ions for square planar complexes. – the majority of complexes are low spin (i. e. diamagnetic). – Examples: Pd 2+, Pt 2+, Ir+, and Au 3+. Copyright 1999, PRENTICE HALL Chapter 24 62
 Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 63
Crystal-Field Theory Copyright 1999, PRENTICE HALL Chapter 24 63
 Chemistry of Coordination Compounds End of Chapter 24 Copyright 1999, PRENTICE HALL Chapter 24 64
Chemistry of Coordination Compounds End of Chapter 24 Copyright 1999, PRENTICE HALL Chapter 24 64


