 Chemistry Lecture #7 Volodimir Vreshch Ivano-Frankivsk
Chemistry Lecture #7 Volodimir Vreshch Ivano-Frankivsk
 1 Plane Introduction Solutions Definition Different types of concentration Conclusions
1 Plane Introduction Solutions Definition Different types of concentration Conclusions
 Solutions Solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. The solvent does the dissolving. The solution more or less takes on the characteristics of the solvent including its phase, and the solvent is commonly the major fraction of the mixture 2
Solutions Solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. The solvent does the dissolving. The solution more or less takes on the characteristics of the solvent including its phase, and the solvent is commonly the major fraction of the mixture 2
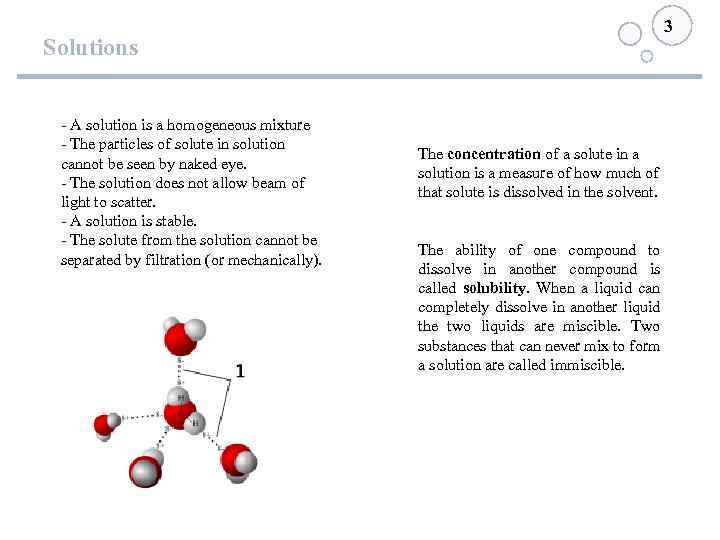 3 Solutions - A solution is a homogeneous mixture - The particles of solute in solution cannot be seen by naked eye. - The solution does not allow beam of light to scatter. - A solution is stable. - The solute from the solution cannot be separated by filtration (or mechanically). The concentration of a solute in a solution is a measure of how much of that solute is dissolved in the solvent. The ability of one compound to dissolve in another compound is called solubility. When a liquid can completely dissolve in another liquid the two liquids are miscible. Two substances that can never mix to form a solution are called immiscible.
3 Solutions - A solution is a homogeneous mixture - The particles of solute in solution cannot be seen by naked eye. - The solution does not allow beam of light to scatter. - A solution is stable. - The solute from the solution cannot be separated by filtration (or mechanically). The concentration of a solute in a solution is a measure of how much of that solute is dissolved in the solvent. The ability of one compound to dissolve in another compound is called solubility. When a liquid can completely dissolve in another liquid the two liquids are miscible. Two substances that can never mix to form a solution are called immiscible.
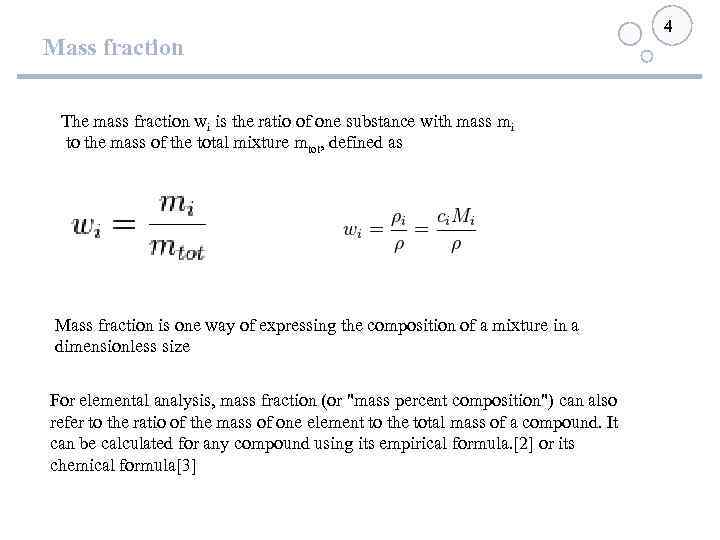 Mass fraction The mass fraction wi is the ratio of one substance with mass mi to the mass of the total mixture mtot, defined as Mass fraction is one way of expressing the composition of a mixture in a dimensionless size For elemental analysis, mass fraction (or "mass percent composition") can also refer to the ratio of the mass of one element to the total mass of a compound. It can be calculated for any compound using its empirical formula. [2] or its chemical formula[3] 4
Mass fraction The mass fraction wi is the ratio of one substance with mass mi to the mass of the total mixture mtot, defined as Mass fraction is one way of expressing the composition of a mixture in a dimensionless size For elemental analysis, mass fraction (or "mass percent composition") can also refer to the ratio of the mass of one element to the total mass of a compound. It can be calculated for any compound using its empirical formula. [2] or its chemical formula[3] 4
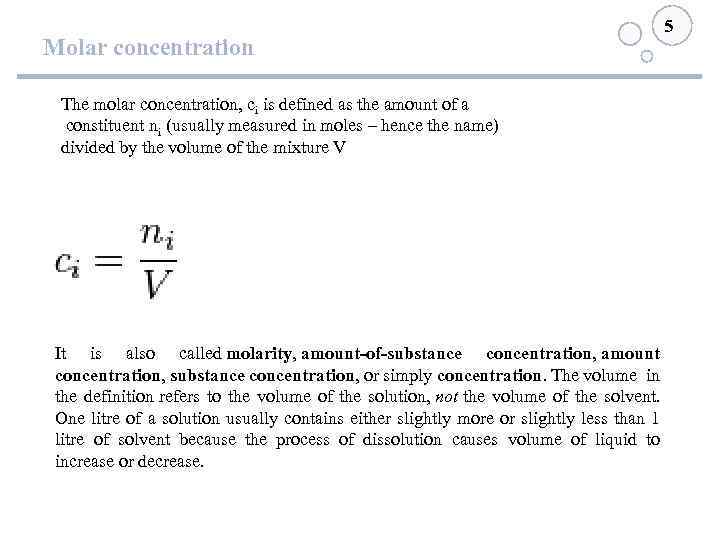 Molar concentration 5 The molar concentration, ci is defined as the amount of a constituent ni (usually measured in moles – hence the name) divided by the volume of the mixture V It is also called molarity, amount-of-substance concentration, amount concentration, substance concentration, or simply concentration. The volume in the definition refers to the volume of the solution, not the volume of the solvent. One litre of a solution usually contains either slightly more or slightly less than 1 litre of solvent because the process of dissolution causes volume of liquid to increase or decrease.
Molar concentration 5 The molar concentration, ci is defined as the amount of a constituent ni (usually measured in moles – hence the name) divided by the volume of the mixture V It is also called molarity, amount-of-substance concentration, amount concentration, substance concentration, or simply concentration. The volume in the definition refers to the volume of the solution, not the volume of the solvent. One litre of a solution usually contains either slightly more or slightly less than 1 litre of solvent because the process of dissolution causes volume of liquid to increase or decrease.
 Another well known formulas 6
Another well known formulas 6
 Examples of questions for the test 7
Examples of questions for the test 7