2adfe626e846609867c47806e7782e6a.ppt
- Количество слайдов: 37
 Chemistry for Aggies or “Everythin’ I ever needed for CHEM 111, I learnt in HORT 100” A/H 100 G J. G. Mexal
Chemistry for Aggies or “Everythin’ I ever needed for CHEM 111, I learnt in HORT 100” A/H 100 G J. G. Mexal
 Chemistry for Aggies Stuff ya’ll need to know! • • • Woolworth’s 5 and 10 ¢ Metric units Fertilizer calculations Irrigation requirements p. H
Chemistry for Aggies Stuff ya’ll need to know! • • • Woolworth’s 5 and 10 ¢ Metric units Fertilizer calculations Irrigation requirements p. H
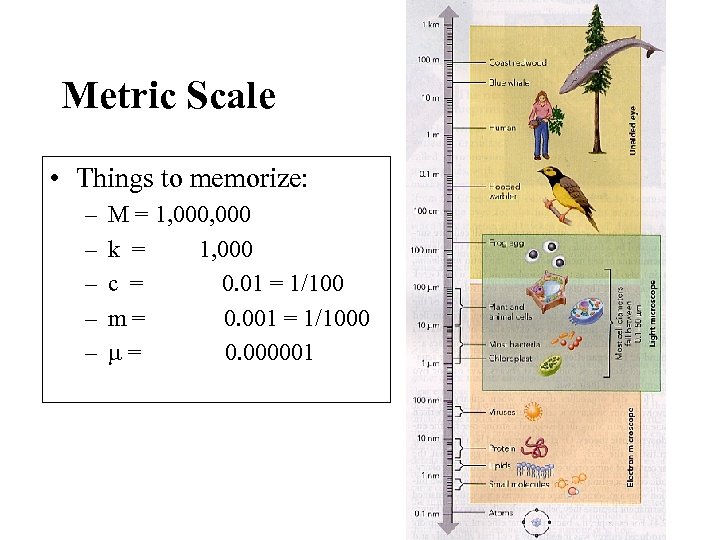 Metric Scale • Things to memorize: – – – M = 1, 000 k = 1, 000 c = 0. 01 = 1/100 m= 0. 001 = 1/1000 = 0. 000001
Metric Scale • Things to memorize: – – – M = 1, 000 k = 1, 000 c = 0. 01 = 1/100 m= 0. 001 = 1/1000 = 0. 000001
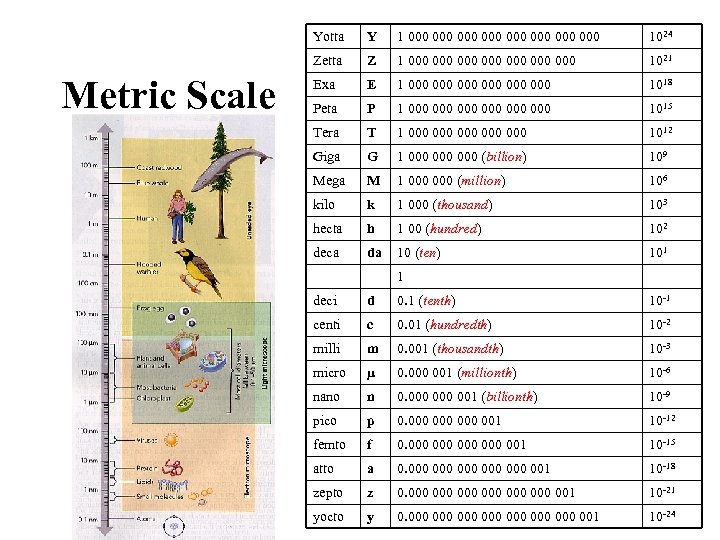 Yotta 1 000 000 1024 Zetta Metric Scale Y Z 1 000 000 1021 Exa E 1 000 000 000 1018 Peta P 1 000 000 000 1015 Tera T 1 000 000 000 1012 Giga G 1 000 000 (billion) 109 Mega M 1 000 (million) 106 kilo k 1 000 (thousand) 103 hecta h 1 00 (hundred) 102 deca da 10 (ten) 101 1 deci d 0. 1 (tenth) 10 -1 centi c 0. 01 (hundredth) 10 -2 milli m 0. 001 (thousandth) 10 -3 micro µ 0. 000 001 (millionth) 10 -6 nano n 0. 000 001 (billionth) 10 -9 pico p 0. 000 000 001 10 -12 femto f 0. 000 000 001 10 -15 atto a 0. 000 000 000 001 10 -18 zepto z 0. 000 000 000 001 10 -21 yocto y 0. 000 000 001 10 -24
Yotta 1 000 000 1024 Zetta Metric Scale Y Z 1 000 000 1021 Exa E 1 000 000 000 1018 Peta P 1 000 000 000 1015 Tera T 1 000 000 000 1012 Giga G 1 000 000 (billion) 109 Mega M 1 000 (million) 106 kilo k 1 000 (thousand) 103 hecta h 1 00 (hundred) 102 deca da 10 (ten) 101 1 deci d 0. 1 (tenth) 10 -1 centi c 0. 01 (hundredth) 10 -2 milli m 0. 001 (thousandth) 10 -3 micro µ 0. 000 001 (millionth) 10 -6 nano n 0. 000 001 (billionth) 10 -9 pico p 0. 000 000 001 10 -12 femto f 0. 000 000 001 10 -15 atto a 0. 000 000 000 001 10 -18 zepto z 0. 000 000 000 001 10 -21 yocto y 0. 000 000 001 10 -24
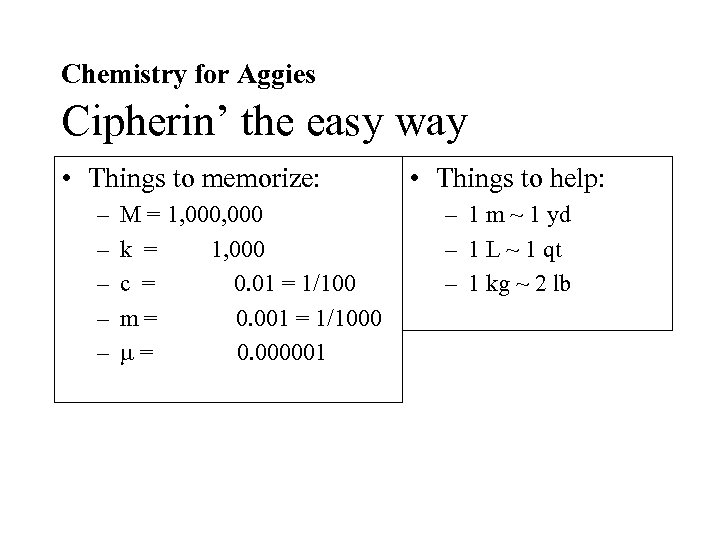 Chemistry for Aggies Cipherin’ the easy way • Things to memorize: – – – M = 1, 000 k = 1, 000 c = 0. 01 = 1/100 m= 0. 001 = 1/1000 = 0. 000001 • Things to help: – 1 m ~ 1 yd – 1 L ~ 1 qt – 1 kg ~ 2 lb
Chemistry for Aggies Cipherin’ the easy way • Things to memorize: – – – M = 1, 000 k = 1, 000 c = 0. 01 = 1/100 m= 0. 001 = 1/1000 = 0. 000001 • Things to help: – 1 m ~ 1 yd – 1 L ~ 1 qt – 1 kg ~ 2 lb
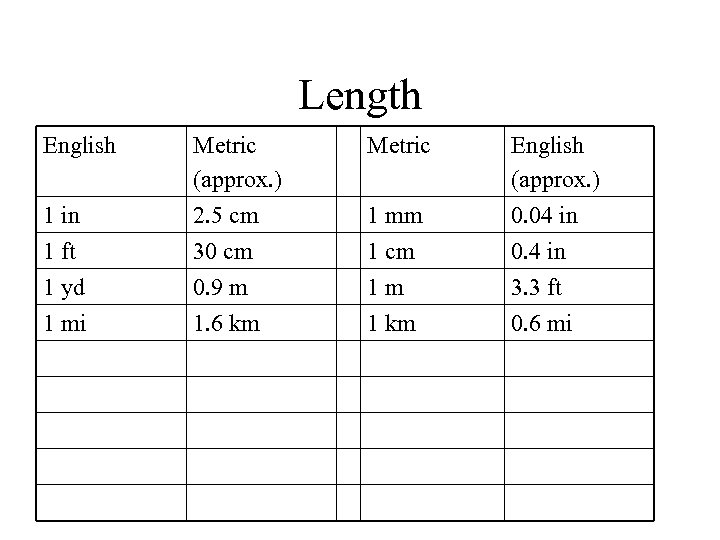 Length English Metric 1 in Metric (approx. ) 2. 5 cm 1 mm English (approx. ) 0. 04 in 1 ft 30 cm 1 cm 0. 4 in 1 yd 1 mi 0. 9 m 1. 6 km 1 m 1 km 3. 3 ft 0. 6 mi
Length English Metric 1 in Metric (approx. ) 2. 5 cm 1 mm English (approx. ) 0. 04 in 1 ft 30 cm 1 cm 0. 4 in 1 yd 1 mi 0. 9 m 1. 6 km 1 m 1 km 3. 3 ft 0. 6 mi
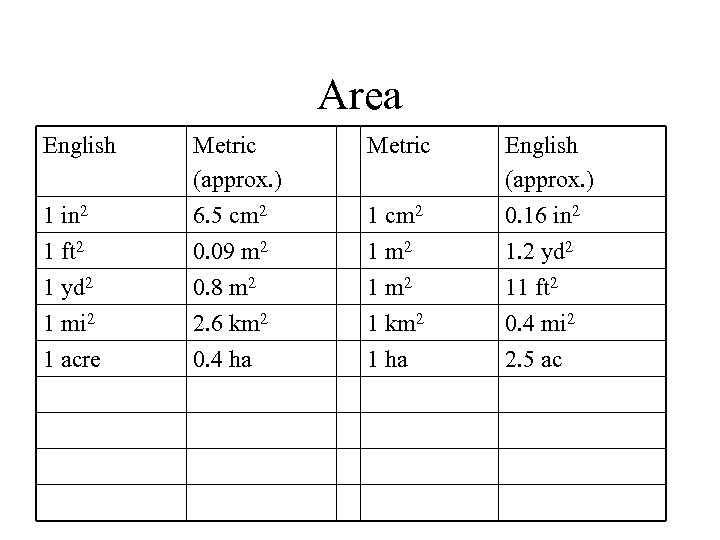 Area English Metric 1 in 2 Metric (approx. ) 6. 5 cm 2 1 cm 2 English (approx. ) 0. 16 in 2 1 ft 2 0. 09 m 2 1. 2 yd 2 1 mi 2 1 acre 0. 8 m 2 2. 6 km 2 0. 4 ha 1 m 2 1 km 2 1 ha 11 ft 2 0. 4 mi 2 2. 5 ac
Area English Metric 1 in 2 Metric (approx. ) 6. 5 cm 2 1 cm 2 English (approx. ) 0. 16 in 2 1 ft 2 0. 09 m 2 1. 2 yd 2 1 mi 2 1 acre 0. 8 m 2 2. 6 km 2 0. 4 ha 1 m 2 1 km 2 1 ha 11 ft 2 0. 4 mi 2 2. 5 ac
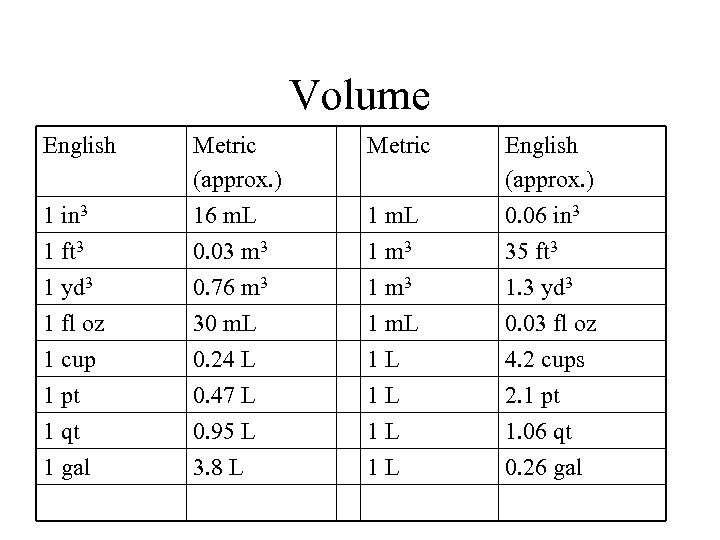 Volume English Metric 1 in 3 Metric (approx. ) 16 m. L 1 m. L English (approx. ) 0. 06 in 3 1 ft 3 0. 03 m 3 1 m 3 35 ft 3 1 yd 3 1 fl oz 1 cup 1 pt 1 qt 1 gal 0. 76 m 3 30 m. L 0. 24 L 0. 47 L 0. 95 L 3. 8 L 1 m 3 1 m. L 1 L 1 L 1. 3 yd 3 0. 03 fl oz 4. 2 cups 2. 1 pt 1. 06 qt 0. 26 gal
Volume English Metric 1 in 3 Metric (approx. ) 16 m. L 1 m. L English (approx. ) 0. 06 in 3 1 ft 3 0. 03 m 3 1 m 3 35 ft 3 1 yd 3 1 fl oz 1 cup 1 pt 1 qt 1 gal 0. 76 m 3 30 m. L 0. 24 L 0. 47 L 0. 95 L 3. 8 L 1 m 3 1 m. L 1 L 1 L 1. 3 yd 3 0. 03 fl oz 4. 2 cups 2. 1 pt 1. 06 qt 0. 26 gal
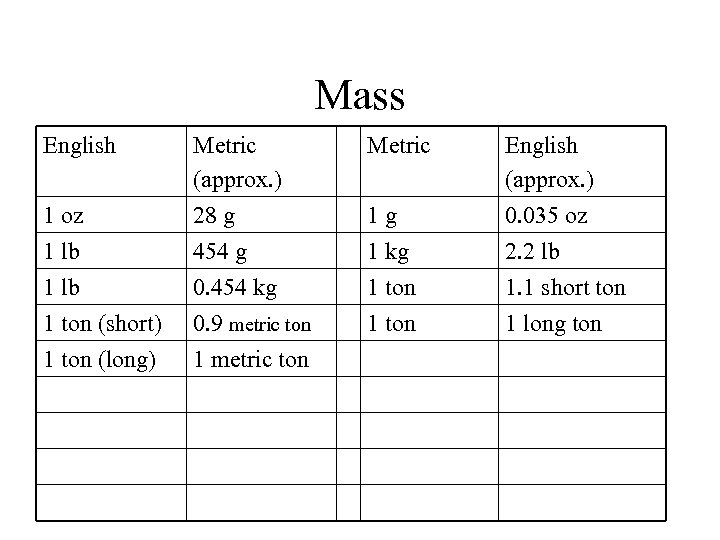 Mass English Metric 1 oz Metric (approx. ) 28 g 1 g English (approx. ) 0. 035 oz 1 lb 454 g 1 kg 2. 2 lb 1 ton (short) 1 ton (long) 0. 454 kg 0. 9 metric ton 1. 1 short ton 1 long ton
Mass English Metric 1 oz Metric (approx. ) 28 g 1 g English (approx. ) 0. 035 oz 1 lb 454 g 1 kg 2. 2 lb 1 ton (short) 1 ton (long) 0. 454 kg 0. 9 metric ton 1. 1 short ton 1 long ton
 Chemistry for Aggies Doin’ it the hard way • • How many square inches in a square yard? How many ounces in a gallon? How many square feet in an acre? The K-Mart parking lot is one acre in size and receives 1” or rain. How many gallons will run off into the sewer?
Chemistry for Aggies Doin’ it the hard way • • How many square inches in a square yard? How many ounces in a gallon? How many square feet in an acre? The K-Mart parking lot is one acre in size and receives 1” or rain. How many gallons will run off into the sewer?
 Chemistry for Aggies Why use metric units? • Metrics involves the judicious movement of zeros to determine the answer. There are no complicated conversions to learn. • Ro. T: If the answer looks right--it probably is! • The question you must always ask yourself is: “Does the answer make sense? ”
Chemistry for Aggies Why use metric units? • Metrics involves the judicious movement of zeros to determine the answer. There are no complicated conversions to learn. • Ro. T: If the answer looks right--it probably is! • The question you must always ask yourself is: “Does the answer make sense? ”
 Chemistry for Aggies Cipherin’ the easy way • How many cm 2 in a m 2? • How many m. L in a L? • The Wal. Mart parking lot is 1 ha in size and receives 1 cm of rain. How many L of water will run off into the sewer?
Chemistry for Aggies Cipherin’ the easy way • How many cm 2 in a m 2? • How many m. L in a L? • The Wal. Mart parking lot is 1 ha in size and receives 1 cm of rain. How many L of water will run off into the sewer?
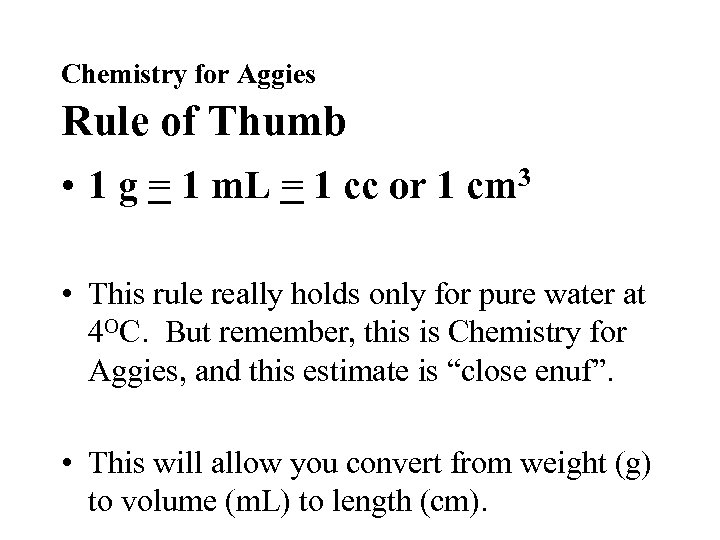 Chemistry for Aggies Rule of Thumb • 1 g = 1 m. L = 1 cc or 1 cm 3 • This rule really holds only for pure water at 4 OC. But remember, this is Chemistry for Aggies, and this estimate is “close enuf”. • This will allow you convert from weight (g) to volume (m. L) to length (cm).
Chemistry for Aggies Rule of Thumb • 1 g = 1 m. L = 1 cc or 1 cm 3 • This rule really holds only for pure water at 4 OC. But remember, this is Chemistry for Aggies, and this estimate is “close enuf”. • This will allow you convert from weight (g) to volume (m. L) to length (cm).
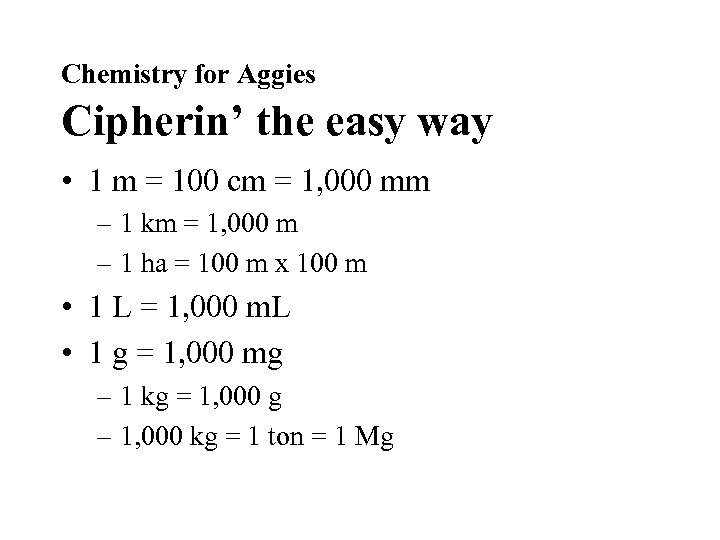 Chemistry for Aggies Cipherin’ the easy way • 1 m = 100 cm = 1, 000 mm – 1 km = 1, 000 m – 1 ha = 100 m x 100 m • 1 L = 1, 000 m. L • 1 g = 1, 000 mg – 1 kg = 1, 000 g – 1, 000 kg = 1 ton = 1 Mg
Chemistry for Aggies Cipherin’ the easy way • 1 m = 100 cm = 1, 000 mm – 1 km = 1, 000 m – 1 ha = 100 m x 100 m • 1 L = 1, 000 m. L • 1 g = 1, 000 mg – 1 kg = 1, 000 g – 1, 000 kg = 1 ton = 1 Mg
 Chemistry for Aggies Cipherin’ the easy way • How many cm 2 in a m 2? • Answer: = 1 m = 100 cm = 1 m x 1 m = 100 cm x 100 cm = 1 m 2 = 10, 000 cm 2
Chemistry for Aggies Cipherin’ the easy way • How many cm 2 in a m 2? • Answer: = 1 m = 100 cm = 1 m x 1 m = 100 cm x 100 cm = 1 m 2 = 10, 000 cm 2
 Chemistry for Aggies Cipherin’ the easy way • How many m. L in a L? • Answer: = 1 L = 1, 000 m. L
Chemistry for Aggies Cipherin’ the easy way • How many m. L in a L? • Answer: = 1 L = 1, 000 m. L
 Chemistry for Aggies Cipherin’ the easy way • The Wal. Mart parking lot is 1 ha in size and receives 1 cm or rain. How many L of water will run off into the sewer? • Answer:
Chemistry for Aggies Cipherin’ the easy way • The Wal. Mart parking lot is 1 ha in size and receives 1 cm or rain. How many L of water will run off into the sewer? • Answer:
 Chemistry for Aggies Cipherin’ the easy way • The Wal. Mart parking lot is 1 ha in size and receives 1 cm or rain. How many L of water will run off into the sewer? • Answer: * 1 ha = 100 m x 100 m = (100 m x 100 cm/m) x (100 m x 100 cm/m) = 100, 000 cm 2 * 1 cm of rain on 100, 000 cm 2 = 100, 000 cm 3 * 100, 000 m. L x 1 L/ 1, 000 m. L = 100, 000 L
Chemistry for Aggies Cipherin’ the easy way • The Wal. Mart parking lot is 1 ha in size and receives 1 cm or rain. How many L of water will run off into the sewer? • Answer: * 1 ha = 100 m x 100 m = (100 m x 100 cm/m) x (100 m x 100 cm/m) = 100, 000 cm 2 * 1 cm of rain on 100, 000 cm 2 = 100, 000 cm 3 * 100, 000 m. L x 1 L/ 1, 000 m. L = 100, 000 L
 Chemistry for Aggies Woolworth’s 5 and 10 ¢ • To estimate the temperature in OC, beginning at the freezing point of water (32 OF and 0 OC), the Celsius scale changes about 5 O for every 10 O change in the Fahrenheit scale.
Chemistry for Aggies Woolworth’s 5 and 10 ¢ • To estimate the temperature in OC, beginning at the freezing point of water (32 OF and 0 OC), the Celsius scale changes about 5 O for every 10 O change in the Fahrenheit scale.
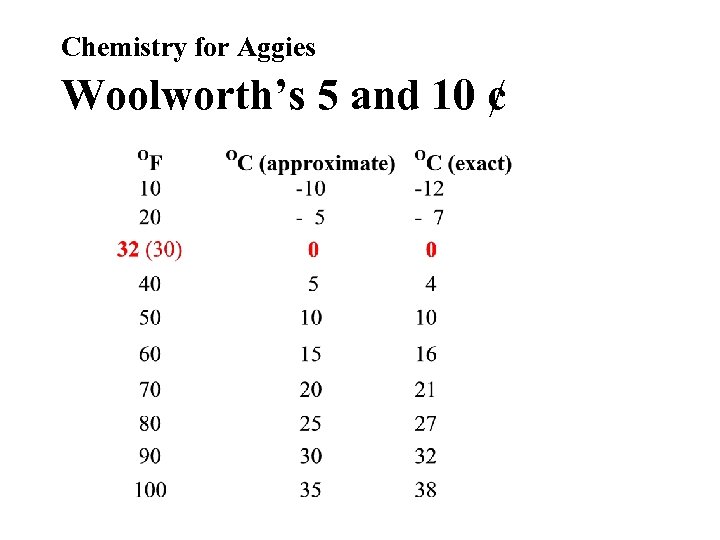 Chemistry for Aggies Woolworth’s 5 and 10 ¢
Chemistry for Aggies Woolworth’s 5 and 10 ¢
 Chemistry for Aggies More Cipherin’ the easy way • The Rio Grande has 300 ppm dissolved salts. If a farmer applies 5 cm of irrigation water to one ha, how may tons of salts is the farmer adding? • Answer:
Chemistry for Aggies More Cipherin’ the easy way • The Rio Grande has 300 ppm dissolved salts. If a farmer applies 5 cm of irrigation water to one ha, how may tons of salts is the farmer adding? • Answer:
 Chemistry for Aggies More Cipherin’ the easy way • The Rio Grande has 300 ppm dissolved salts. If a farmer applies 5 cm of irrigation water to one ha, how may tons of salts is the farmer adding? • Answer: Calculate the amount of water * 1 ha = 100 m x 100 m = (100 m x 100 cm/m) x (100 m x 100 cm/m) = 100, 000 cm 2 * 5 cm of water on 100, 000 cm 2 = 500, 000 cm 3 * 500, 000 m. L x 1 L/ 1, 000 m. L = 500, 000 L
Chemistry for Aggies More Cipherin’ the easy way • The Rio Grande has 300 ppm dissolved salts. If a farmer applies 5 cm of irrigation water to one ha, how may tons of salts is the farmer adding? • Answer: Calculate the amount of water * 1 ha = 100 m x 100 m = (100 m x 100 cm/m) x (100 m x 100 cm/m) = 100, 000 cm 2 * 5 cm of water on 100, 000 cm 2 = 500, 000 cm 3 * 500, 000 m. L x 1 L/ 1, 000 m. L = 500, 000 L
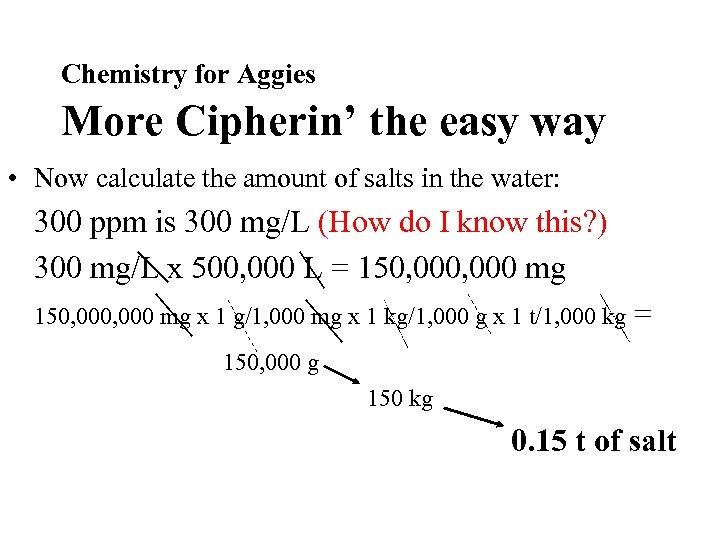 Chemistry for Aggies More Cipherin’ the easy way • Now calculate the amount of salts in the water: 300 ppm is 300 mg/L (How do I know this? ) 300 mg/L x 500, 000 L = 150, 000, 000 mg x 1 g/1, 000 mg x 1 kg/1, 000 g x 1 t/1, 000 kg = 150, 000 g 150 kg 0. 15 t of salt
Chemistry for Aggies More Cipherin’ the easy way • Now calculate the amount of salts in the water: 300 ppm is 300 mg/L (How do I know this? ) 300 mg/L x 500, 000 L = 150, 000, 000 mg x 1 g/1, 000 mg x 1 kg/1, 000 g x 1 t/1, 000 kg = 150, 000 g 150 kg 0. 15 t of salt
 Chemistry for Aggies %, ppm, mg/L • • % ppm ppb mg/L = parts/100 = parts/million = parts/billion (hazardous materials) = 0. 001 g/1, 000 g (or 1, 000 m. L) = 1 g/1, 000 g = ppm
Chemistry for Aggies %, ppm, mg/L • • % ppm ppb mg/L = parts/100 = parts/million = parts/billion (hazardous materials) = 0. 001 g/1, 000 g (or 1, 000 m. L) = 1 g/1, 000 g = ppm
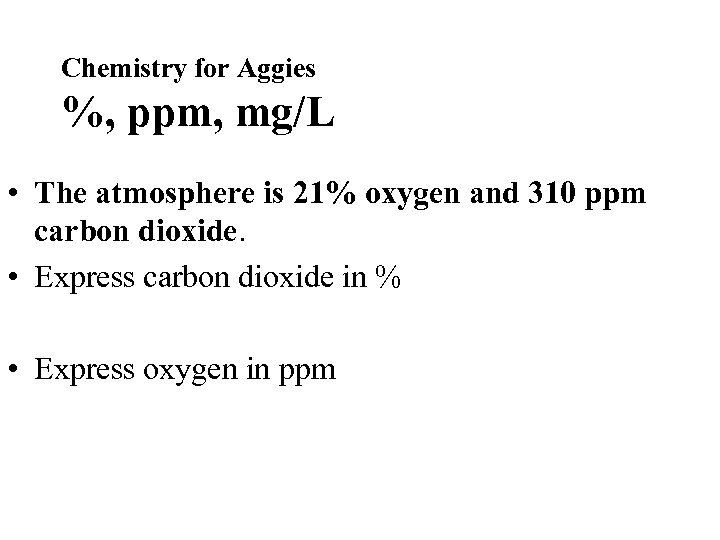 Chemistry for Aggies %, ppm, mg/L • The atmosphere is 21% oxygen and 310 ppm carbon dioxide. • Express carbon dioxide in % • Express oxygen in ppm
Chemistry for Aggies %, ppm, mg/L • The atmosphere is 21% oxygen and 310 ppm carbon dioxide. • Express carbon dioxide in % • Express oxygen in ppm
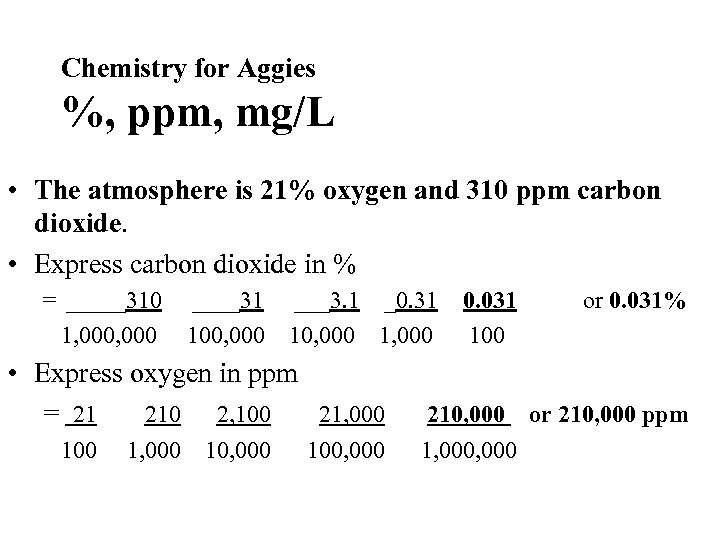 Chemistry for Aggies %, ppm, mg/L • The atmosphere is 21% oxygen and 310 ppm carbon dioxide. • Express carbon dioxide in % = _____310 1, 000 ____31 100, 000 ___3. 1 _0. 31 10, 000 1, 000 • Express oxygen in ppm = 21 210 2, 100 21, 000 100, 000 0. 031 100 or 0. 031% 210, 000 or 210, 000 ppm 1, 000
Chemistry for Aggies %, ppm, mg/L • The atmosphere is 21% oxygen and 310 ppm carbon dioxide. • Express carbon dioxide in % = _____310 1, 000 ____31 100, 000 ___3. 1 _0. 31 10, 000 1, 000 • Express oxygen in ppm = 21 210 2, 100 21, 000 100, 000 0. 031 100 or 0. 031% 210, 000 or 210, 000 ppm 1, 000
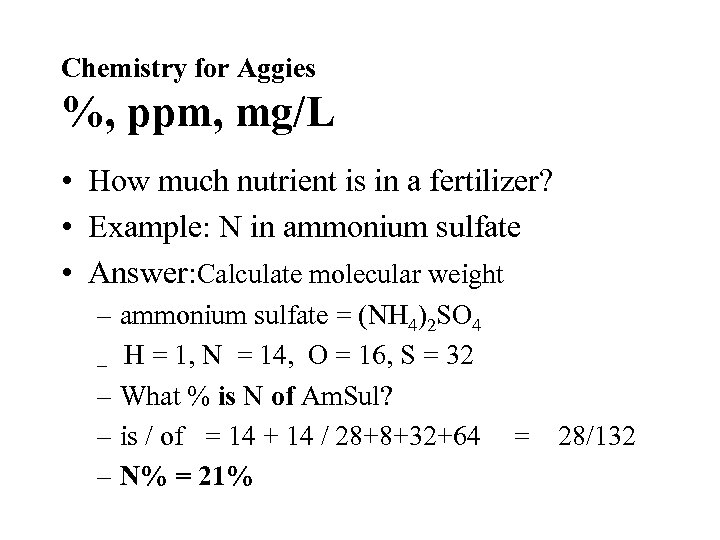 Chemistry for Aggies %, ppm, mg/L • How much nutrient is in a fertilizer? • Example: N in ammonium sulfate • Answer: Calculate molecular weight – ammonium sulfate = (NH 4)2 SO 4 – H = 1, N = 14, O = 16, S = 32 – What % is N of Am. Sul? – is / of = 14 + 14 / 28+8+32+64 – N% = 21% = 28/132
Chemistry for Aggies %, ppm, mg/L • How much nutrient is in a fertilizer? • Example: N in ammonium sulfate • Answer: Calculate molecular weight – ammonium sulfate = (NH 4)2 SO 4 – H = 1, N = 14, O = 16, S = 32 – What % is N of Am. Sul? – is / of = 14 + 14 / 28+8+32+64 – N% = 21% = 28/132
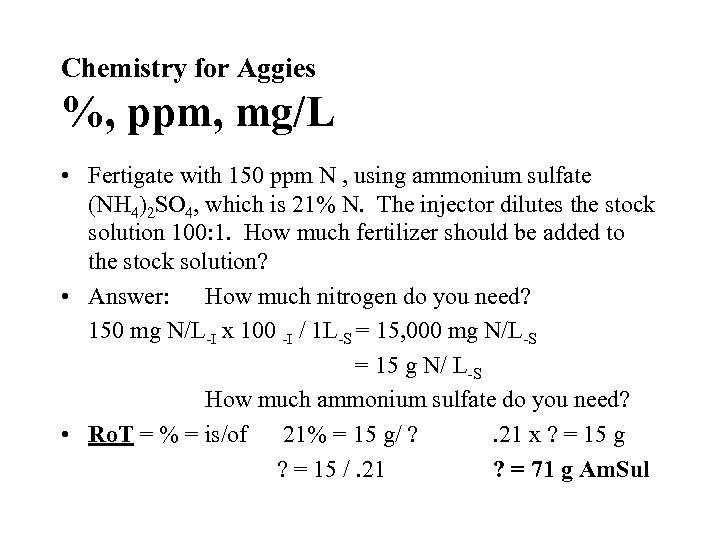 Chemistry for Aggies %, ppm, mg/L • Fertigate with 150 ppm N , using ammonium sulfate (NH 4)2 SO 4, which is 21% N. The injector dilutes the stock solution 100: 1. How much fertilizer should be added to the stock solution? • Answer: How much nitrogen do you need? 150 mg N/L-I x 100 -I / 1 L-S = 15, 000 mg N/L-S = 15 g N/ L-S How much ammonium sulfate do you need? • Ro. T = % = is/of 21% = 15 g/ ? . 21 x ? = 15 g ? = 15 /. 21 ? = 71 g Am. Sul
Chemistry for Aggies %, ppm, mg/L • Fertigate with 150 ppm N , using ammonium sulfate (NH 4)2 SO 4, which is 21% N. The injector dilutes the stock solution 100: 1. How much fertilizer should be added to the stock solution? • Answer: How much nitrogen do you need? 150 mg N/L-I x 100 -I / 1 L-S = 15, 000 mg N/L-S = 15 g N/ L-S How much ammonium sulfate do you need? • Ro. T = % = is/of 21% = 15 g/ ? . 21 x ? = 15 g ? = 15 /. 21 ? = 71 g Am. Sul
 Chemistry for Aggies %, ppm, mg/L • Conclusion: You will need to add 71 g of ammonium sulfate per liter of stock solution to deliver 150 ppm N from your 1: 100 injection fertigater.
Chemistry for Aggies %, ppm, mg/L • Conclusion: You will need to add 71 g of ammonium sulfate per liter of stock solution to deliver 150 ppm N from your 1: 100 injection fertigater.
 More problems • A cabbage crop is 1. 5% N. If 1 ha yields 2, 000 boxes of 24 heads each weighing 0. 1 kg (DW), how much ammonium nitrate should be added to meet the minimum nitrogen requirement? – Hints: H = 1, N = 14, O = 16
More problems • A cabbage crop is 1. 5% N. If 1 ha yields 2, 000 boxes of 24 heads each weighing 0. 1 kg (DW), how much ammonium nitrate should be added to meet the minimum nitrogen requirement? – Hints: H = 1, N = 14, O = 16
 More problems • A homeowner irrigates the yard with city water (150 L/min). If his yard is 800 m 2 (40 m x 20 m), how long will it take to apply 1 cm?
More problems • A homeowner irrigates the yard with city water (150 L/min). If his yard is 800 m 2 (40 m x 20 m), how long will it take to apply 1 cm?
 More problems • Your roommate decides to kill you for eating all the Oreos. The only thing toxic in the house is some A-rest from your HORT 100 lab. If the plant growth regulator A-rest is toxic to humans at a rate of 5000 mg A-rest to 1 kg human body weight, what % A-rest will be found in your body at the time of death? • Alternatively, could he force feed you Oreos (sucrose LD 50 = 5, 440 mg/kg)?
More problems • Your roommate decides to kill you for eating all the Oreos. The only thing toxic in the house is some A-rest from your HORT 100 lab. If the plant growth regulator A-rest is toxic to humans at a rate of 5000 mg A-rest to 1 kg human body weight, what % A-rest will be found in your body at the time of death? • Alternatively, could he force feed you Oreos (sucrose LD 50 = 5, 440 mg/kg)?
 More problems • How much ammonium nitrate should be applied to fertilize a crop at the 35 kg N/ha rate. Ammonium nitrate = NH 4 NO 3, N = 14, H = 1, O = 16, S = 32, K = 39. • How much hydrogen are you applying?
More problems • How much ammonium nitrate should be applied to fertilize a crop at the 35 kg N/ha rate. Ammonium nitrate = NH 4 NO 3, N = 14, H = 1, O = 16, S = 32, K = 39. • How much hydrogen are you applying?
 More problems • A farmer applies 40 t dry cow manure to 1 ha. If the manure contains 1% N and 7% salts (Ca, Mg, K, Na, etc): • How much N is added to the field? • How much total salt is added? Hint: 1 t = 1, 000 kg, 1 ha = 100 m 2 x 100 m 2
More problems • A farmer applies 40 t dry cow manure to 1 ha. If the manure contains 1% N and 7% salts (Ca, Mg, K, Na, etc): • How much N is added to the field? • How much total salt is added? Hint: 1 t = 1, 000 kg, 1 ha = 100 m 2 x 100 m 2
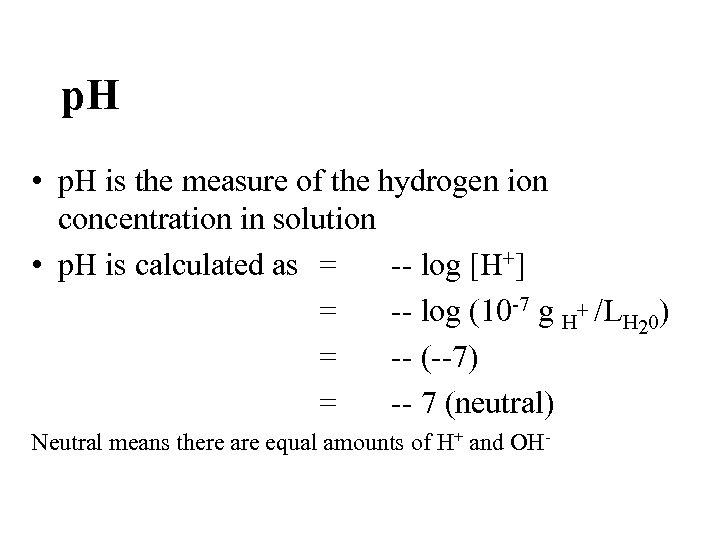 p. H • p. H is the measure of the hydrogen ion concentration in solution • p. H is calculated as = -- log [H+] = -- log (10 -7 g H+ /LH 20) = -- (--7) = -- 7 (neutral) Neutral means there are equal amounts of H+ and OH-
p. H • p. H is the measure of the hydrogen ion concentration in solution • p. H is calculated as = -- log [H+] = -- log (10 -7 g H+ /LH 20) = -- (--7) = -- 7 (neutral) Neutral means there are equal amounts of H+ and OH-
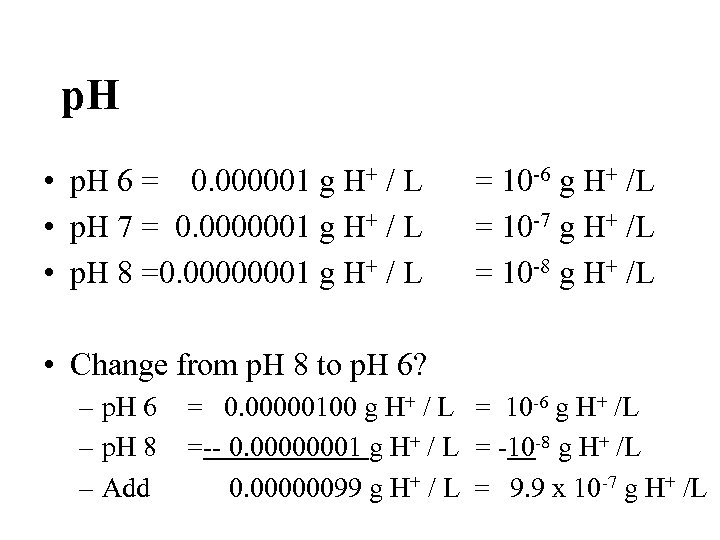 p. H • p. H 6 = 0. 000001 g H+ / L • p. H 7 = 0. 0000001 g H+ / L • p. H 8 =0. 00000001 g H+ / L = 10 -6 g H+ /L = 10 -7 g H+ /L = 10 -8 g H+ /L • Change from p. H 8 to p. H 6? – p. H 6 – p. H 8 – Add = 0. 00000100 g H+ / L = 10 -6 g H+ /L =-- 0. 00000001 g H+ / L = -10 -8 g H+ /L 0. 00000099 g H+ / L = 9. 9 x 10 -7 g H+ /L
p. H • p. H 6 = 0. 000001 g H+ / L • p. H 7 = 0. 0000001 g H+ / L • p. H 8 =0. 00000001 g H+ / L = 10 -6 g H+ /L = 10 -7 g H+ /L = 10 -8 g H+ /L • Change from p. H 8 to p. H 6? – p. H 6 – p. H 8 – Add = 0. 00000100 g H+ / L = 10 -6 g H+ /L =-- 0. 00000001 g H+ / L = -10 -8 g H+ /L 0. 00000099 g H+ / L = 9. 9 x 10 -7 g H+ /L
 p. H • Therefore, to change 1 L of p. H 8 solution to p. H 6, you need to add 0. 00000099 g H+ or add 1 L of p. H 6. 004 solution.
p. H • Therefore, to change 1 L of p. H 8 solution to p. H 6, you need to add 0. 00000099 g H+ or add 1 L of p. H 6. 004 solution.


