dd3798950f634fb81dd9a991c6452c9e.ppt
- Количество слайдов: 33
CHEMISTRY
BIG IDEAS • The number of protons in the nucleus of an atom determines what the element is. • The way that electrons are organized around the nucleus of an atom determines how it interacts with other substances. • Atoms join together chemically to create strong bonds resulting in new substances called compounds.
Atomic Structure
Main Ideas: üAn atom is the smallest part of an element that can exist and still be identifiable as that element; building blocks of elements. üAn element a type of matter made of many identical atoms.

An atom is the smallest part of an element that can exist.
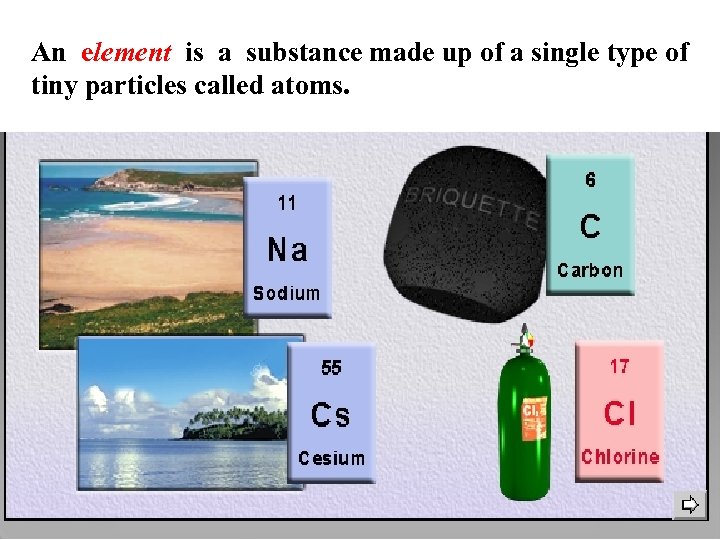
An element is a substance made up of a single type of tiny particles called atoms.

Meet the Elements Song- TMBG Elements Song- Tom Lehrer

What are atoms? -Atoms are the basic building blocks of matter that make up everyday objects. -A desk, the air, even you are made up of atoms! -There are 90 naturally occurring kinds of atoms.
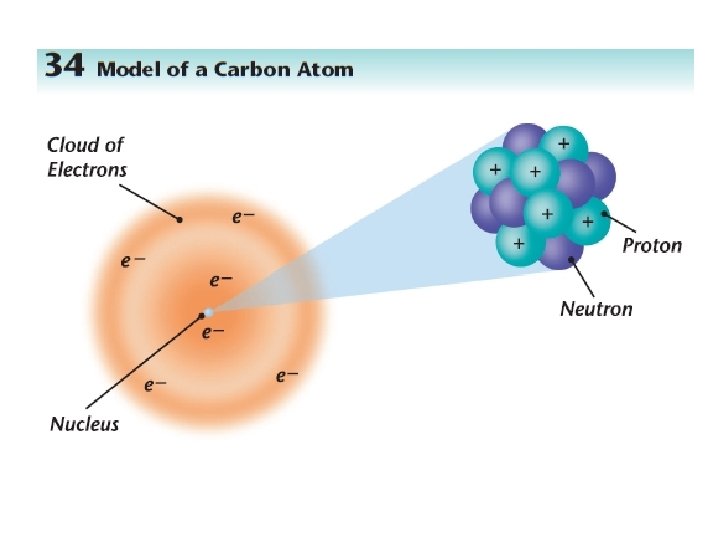
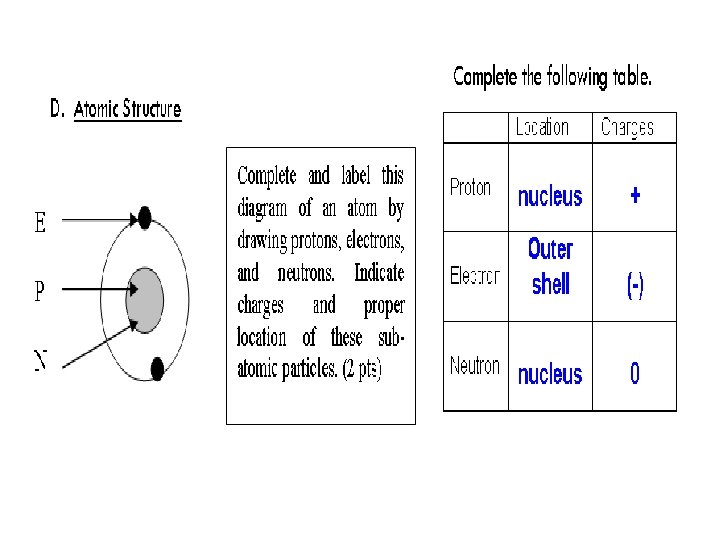


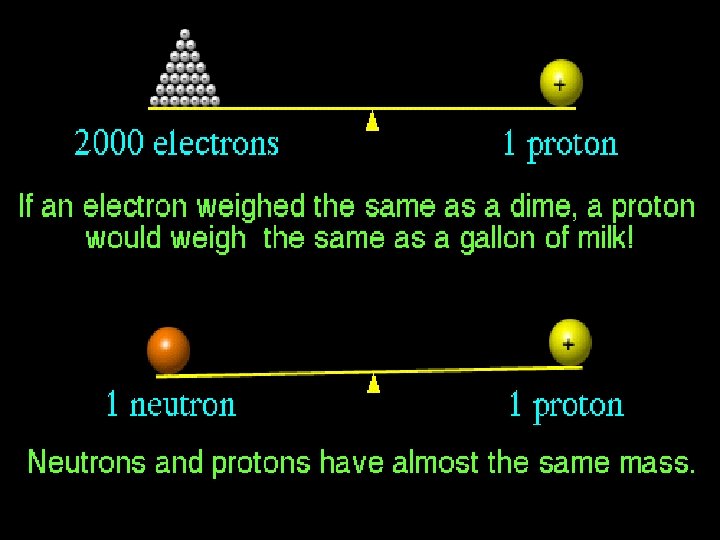
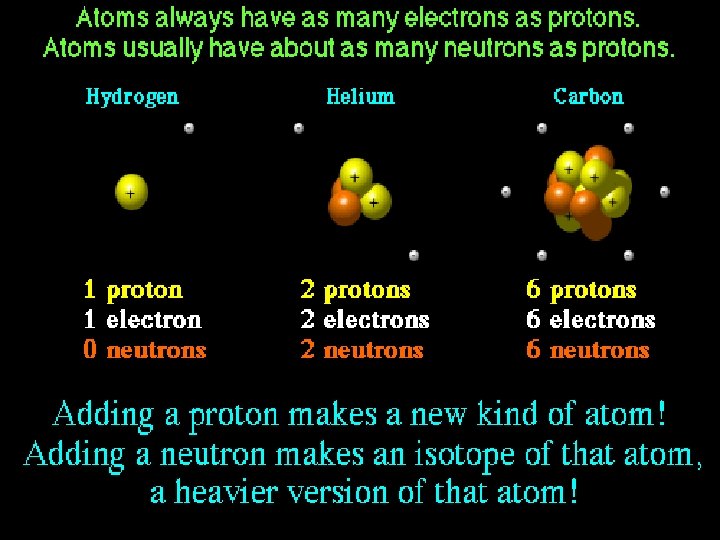
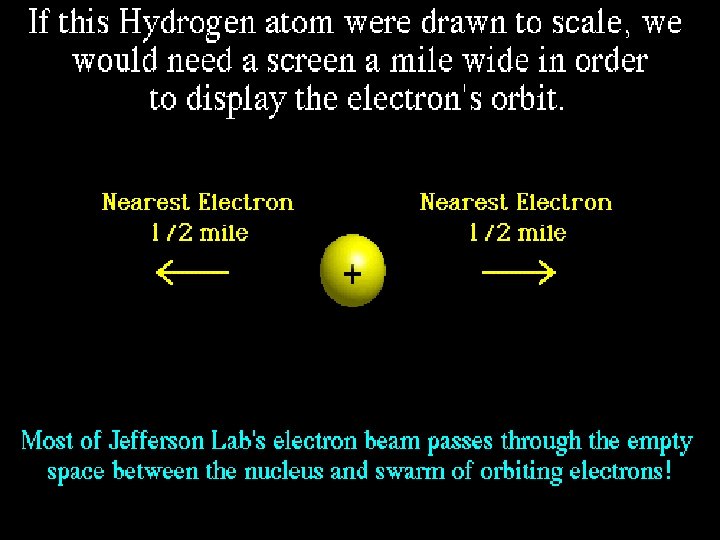

How do atoms get their identity? # of PROTONS = atomic number
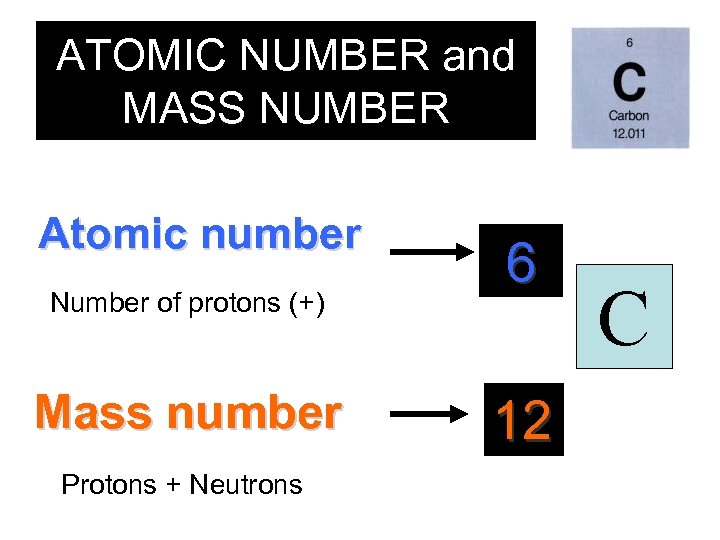
ATOMIC NUMBER and MASS NUMBER Atomic number Number of protons (+) Mass number Protons + Neutrons 6 12 C
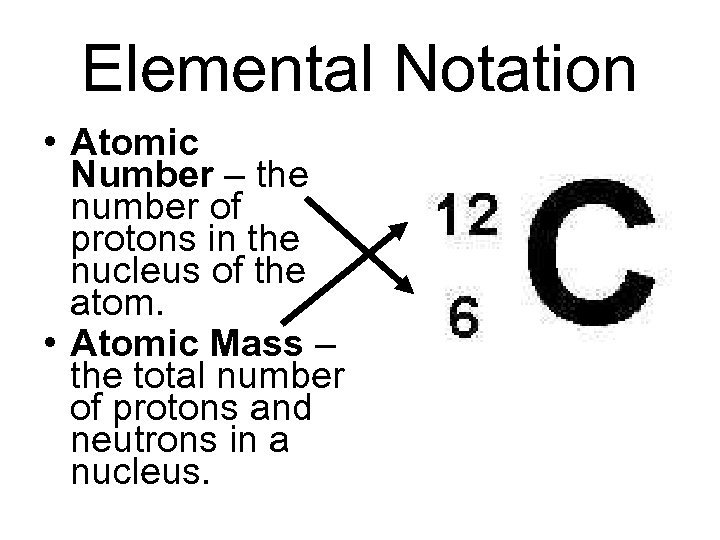
Elemental Notation • Atomic Number – the number of protons in the nucleus of the atom. • Atomic Mass – the total number of protons and neutrons in a nucleus.
ü Molecules are a single unit of 2+ atoms bonded together. üusually same type of atom üCompounds are many molecules; made of 2+ different elements (different types of atoms) that are chemically bonded together so elements have new properties (ex: Na. Cl) examples of differences between molecules and compounds
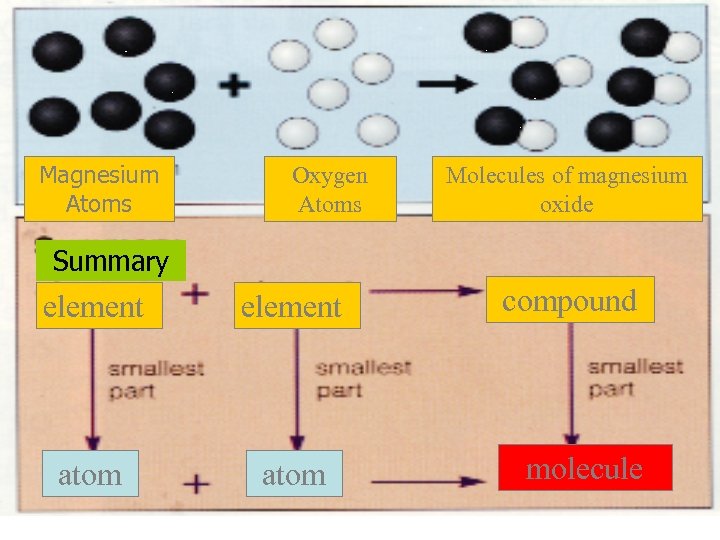
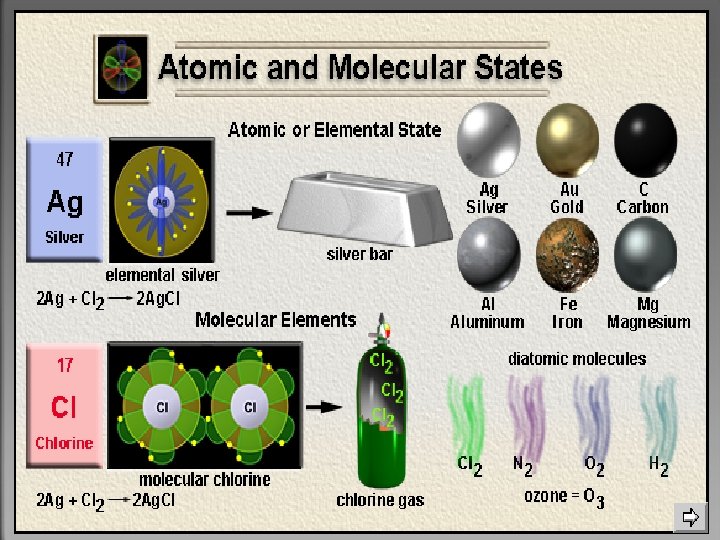
Magnesium Atoms Oxygen Atoms Molecules of magnesium oxide Summary element atom compound molecule
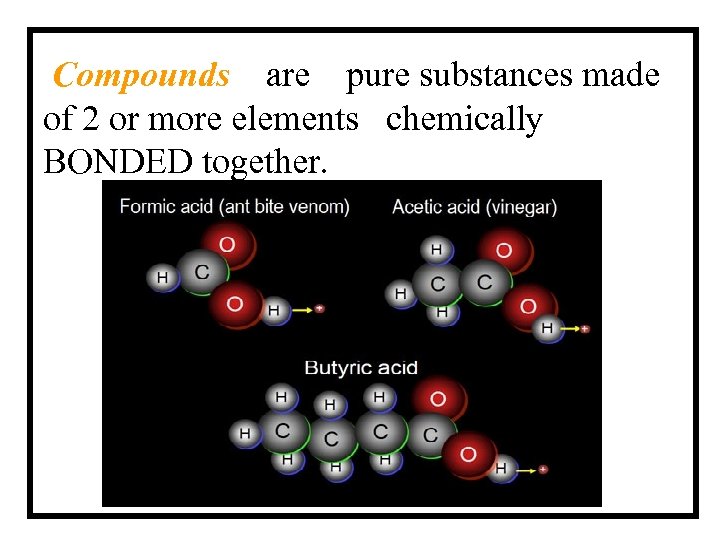
Compounds are pure substances made of 2 or more elements chemically BONDED together.
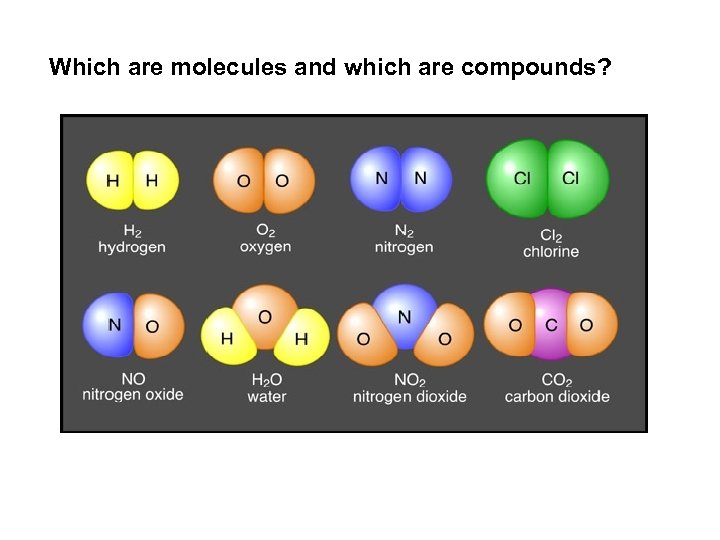
Which are molecules and which are compounds?
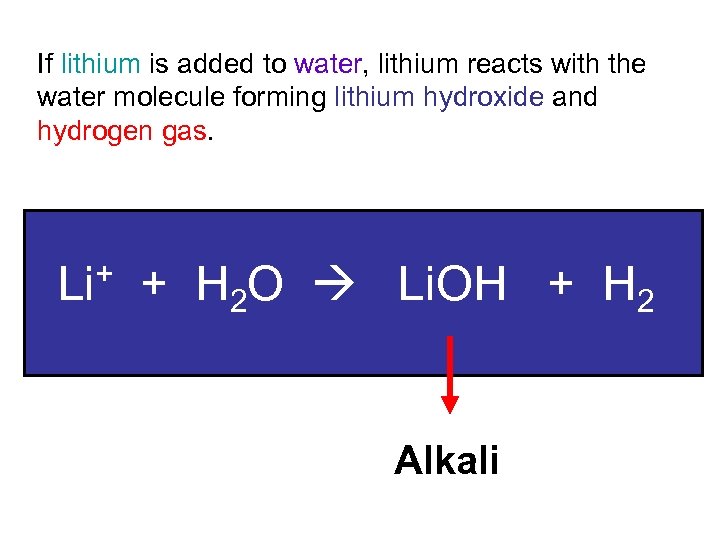
If lithium is added to water, lithium reacts with the water molecule forming lithium hydroxide and hydrogen gas. Li+ + H 2 O Li. OH + H 2 Alkali
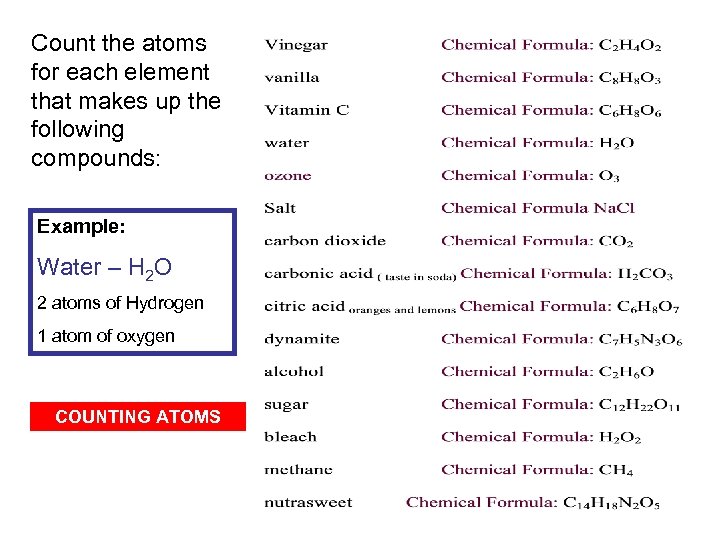
Count the atoms for each element that makes up the following compounds: Example: Water – H 2 O 2 atoms of Hydrogen 1 atom of oxygen COUNTING ATOMS
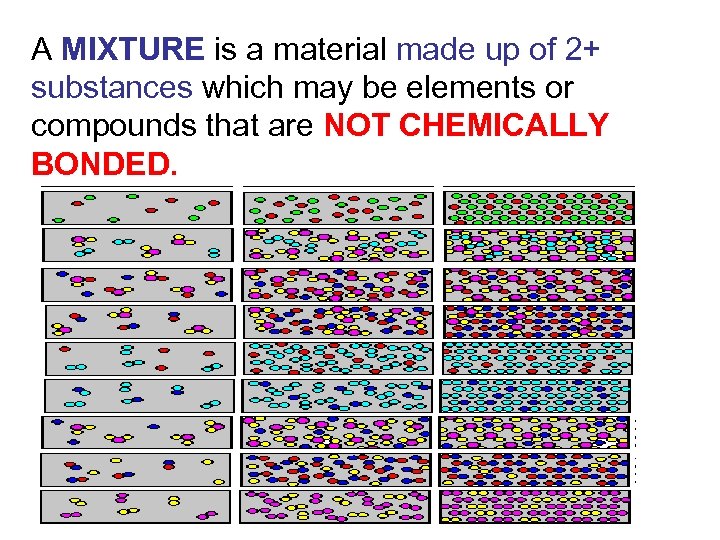
A MIXTURE is a material made up of 2+ substances which may be elements or compounds that are NOT CHEMICALLY BONDED.

Properties of Mixtures • The composition of a mixture is variable. • Each of its components retains its characteristic properties. • Its components are easily separated.

Which are mixtures and which are compounds? • • • soil ocean water (H 20 + Na. Cl) air (O 2 + N 2 + CO 2 + Ar) water (H 2 O) table salt (Na. Cl) Sugar (C 12 H 22 O 12)
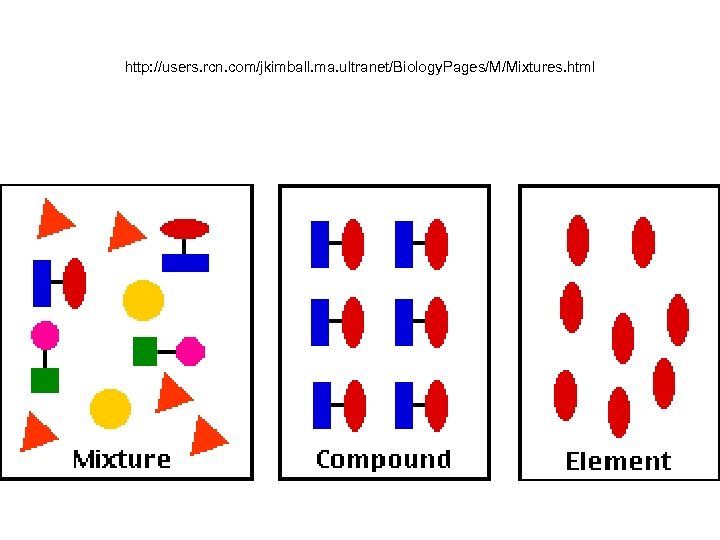
http: //users. rcn. com/jkimball. ma. ultranet/Biology. Pages/M/Mixtures. html
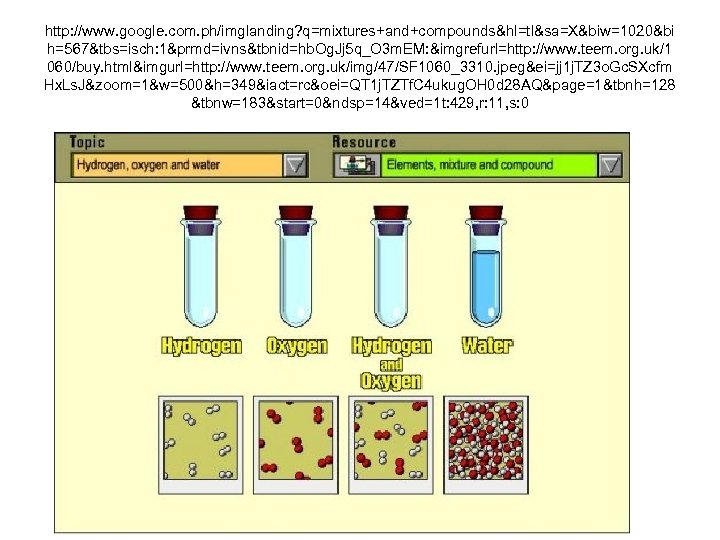
http: //www. google. com. ph/imglanding? q=mixtures+and+compounds&hl=tl&sa=X&biw=1020&bi h=567&tbs=isch: 1&prmd=ivns&tbnid=hb. Og. Jj 5 q_O 3 m. EM: &imgrefurl=http: //www. teem. org. uk/1 060/buy. html&imgurl=http: //www. teem. org. uk/img/47/SF 1060_3310. jpeg&ei=jj 1 j. TZ 3 o. Gc. SXcfm Hx. Ls. J&zoom=1&w=500&h=349&iact=rc&oei=QT 1 j. TZTf. C 4 ukug. OH 0 d 28 AQ&page=1&tbnh=128 &tbnw=183&start=0&ndsp=14&ved=1 t: 429, r: 11, s: 0

http: //www. google. com. ph/imglanding? q=mixtures+and+compounds&hl=tl&sa=X&biw=1020&bi h=567&tbs=isch: 1&prmd=ivns&tbnid=eet 6 v. XOJz 3 eq. M: &imgrefurl=http: //www. quantumtheatre. co. uk/BDks 2 web. html&imgurl=http: //www. quantumt heatre. co. uk/BDks 2 web_files/image 006. jpg&ei=QT 1 j. TZTf. C 4 ukug. OH 0 d 28 AQ&zoom=1&w=604 &h=292&iact=rc&oei=QT 1 j. TZTf. C 4 ukug. OH 0 d 28 AQ&page=1&tbnh=90&tbnw=187&start=0&nds p=14&ved=1 t: 429, r: 4, s: 0
Atom Molecule Mixture Compound Element
dd3798950f634fb81dd9a991c6452c9e.ppt