ddeade502581f275314efd3c9b6d5045.ppt
- Количество слайдов: 55

Chemistry: Atoms First Second Edition Julia Burdge & Jason Overby Chapter 20 Nuclear Chemistry M. Stacey Thomson Pasco-Hernando State College Copyright (c) The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. 1
What Is Radioactivity? • Radioactivity is the release of tiny, highenergy particles or gamma rays from an atom • Particles are ejected from the nucleus Tro: Chemistry: A Molecular Approach 2
Nuclear Decay Some nuclei are unstable, and will, over time, emit particles and/or electromagnetic radiation until they become stable. The spontaneous emission of particles or electromagnetic radiation is known as radioactivity. All elements with Z > 83 are radioactive. Tro: Chemistry: A Molecular Approach 3
20. 1 Nuclei and Nuclear Reactions During a nuclear reaction, the products and reactants will contain different elements as the nuclei change. There are several types of particles or forms of electromagnetic radiation that may be emitted during a nuclear reaction. You should learn the names, symbols and the mass number and charge of each of the particles. 4
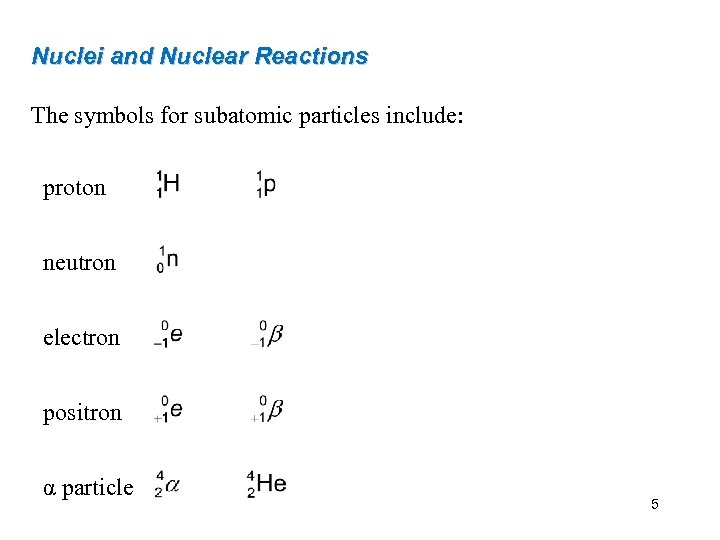
Nuclei and Nuclear Reactions The symbols for subatomic particles include: proton neutron electron positron α particle 5
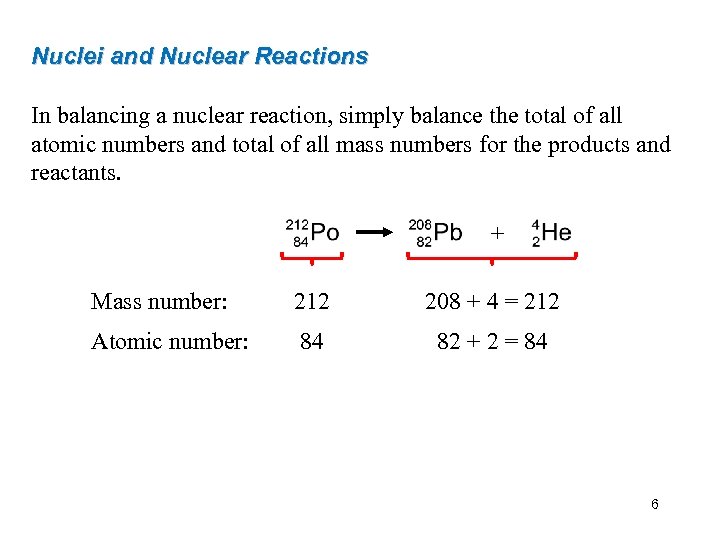
Nuclei and Nuclear Reactions In balancing a nuclear reaction, simply balance the total of all atomic numbers and total of all mass numbers for the products and reactants. + Mass number: 212 208 + 4 = 212 Atomic number: 84 82 + 2 = 84 6
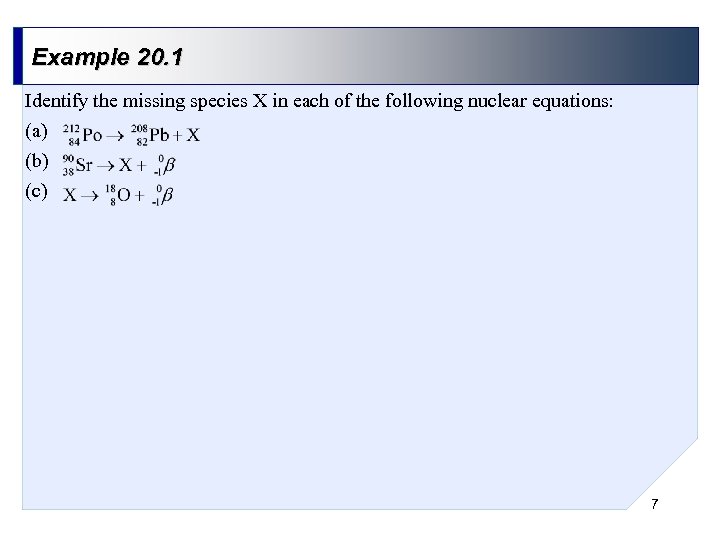
Example 20. 1 Identify the missing species X in each of the following nuclear equations: (a) (b) (c) 7

20. 2 Nuclear Stability Review the information from Chapter 2 on Nuclear stability. Principle factor for nuclear stability is neutron-to-proton ratio (n/p) Ø There are more stabile nuclei with 2, 8, 20, 50, 82, or 126 protons or neutrons Ø More with even #’s Ø All with atomic number > 83 are radioactive Ø All isotopes of Tc and Pm are radioactive 8
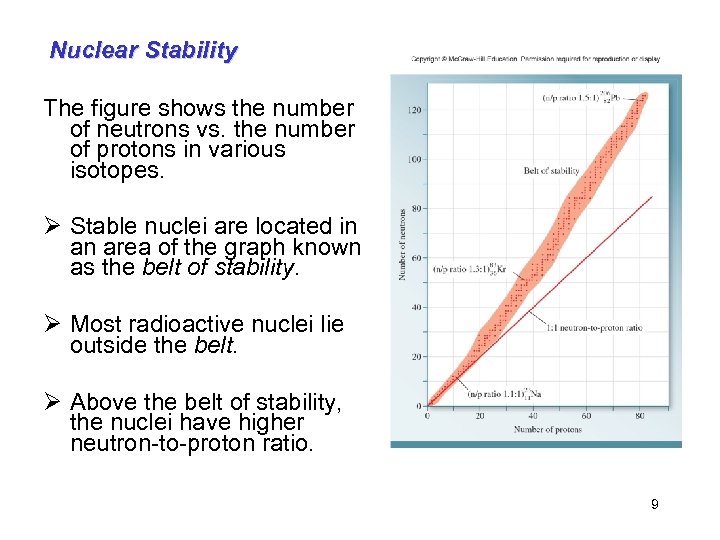
Nuclear Stability The figure shows the number of neutrons vs. the number of protons in various isotopes. Ø Stable nuclei are located in an area of the graph known as the belt of stability. Ø Most radioactive nuclei lie outside the belt. Ø Above the belt of stability, the nuclei have higher neutron-to-proton ratio. 9
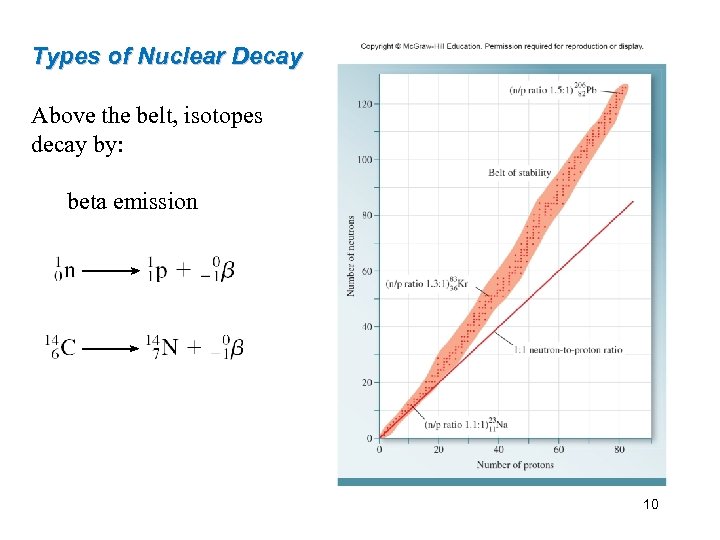
Types of Nuclear Decay Above the belt, isotopes decay by: beta emission 10
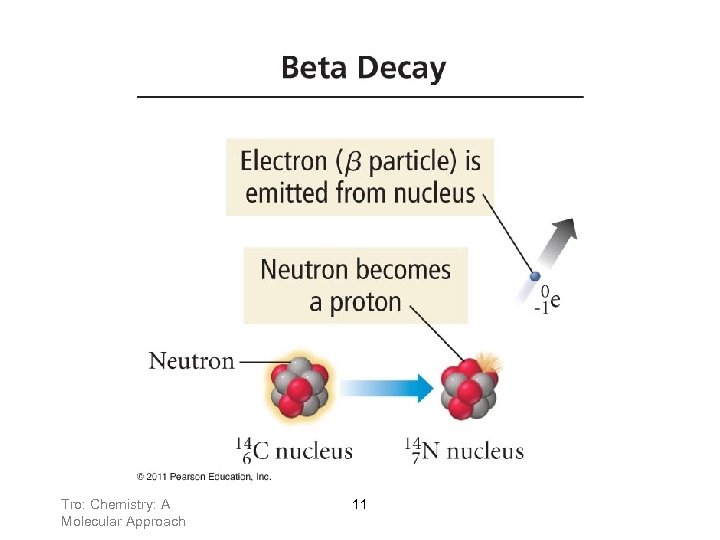
Tro: Chemistry: A Molecular Approach 11
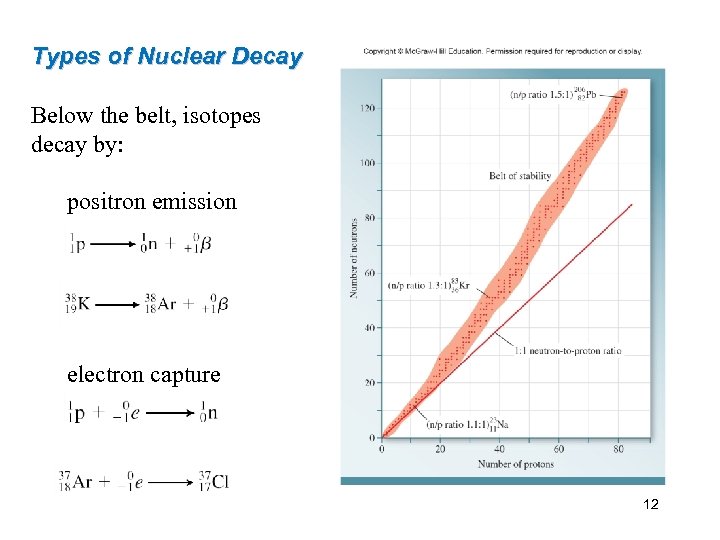
Types of Nuclear Decay Below the belt, isotopes decay by: positron emission electron capture 12
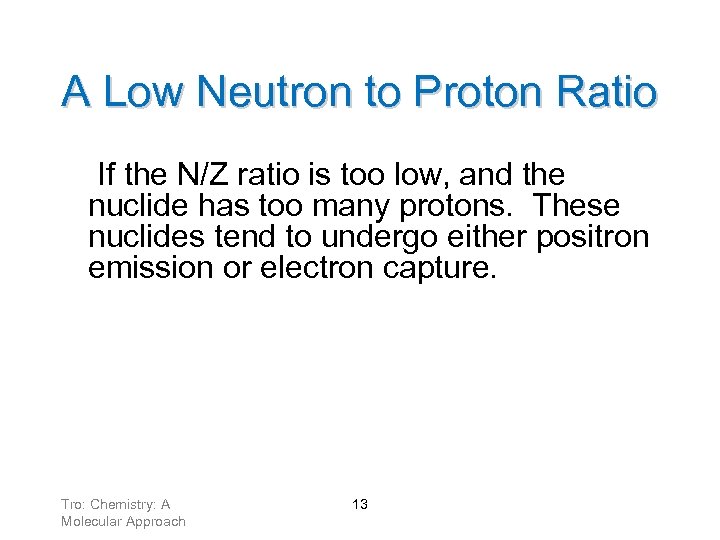
A Low Neutron to Proton Ratio If the N/Z ratio is too low, and the nuclide has too many protons. These nuclides tend to undergo either positron emission or electron capture. Tro: Chemistry: A Molecular Approach 13
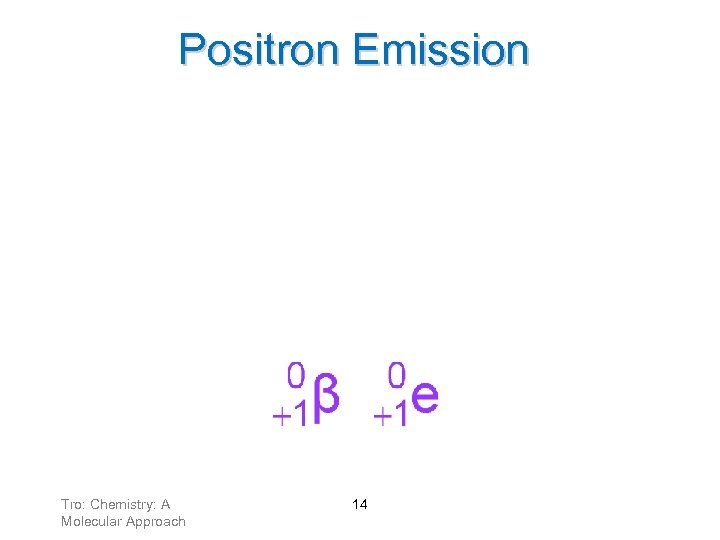
Positron Emission Positrons result from a proton changing into a neutron. A positron has a charge of +1 and negligible mass. ü anti-electron Tro: Chemistry: A Molecular Approach 14
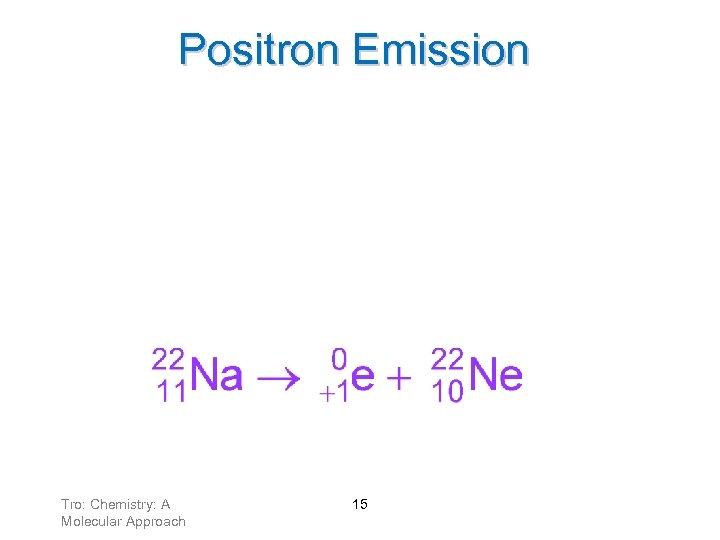
Positron Emission • When an atom loses a positron from the nucleus, its ü mass number remains the same ü atomic number decreases by 1 Tro: Chemistry: A Molecular Approach 15
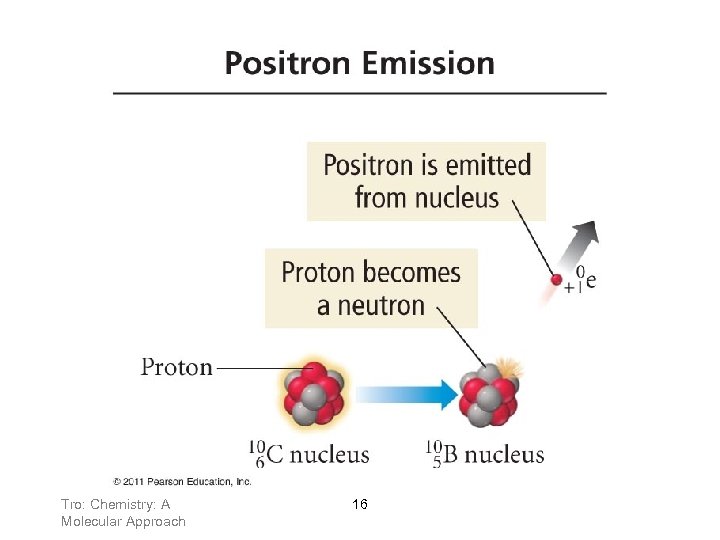
Tro: Chemistry: A Molecular Approach 16
Electron Capture Electron capture occurs when an inner orbital electron is pulled into the nucleus. A proton combines with the electron to make a neutron. This decreases the atomic number, and increases the N/Z ratio. Tro: Chemistry: A Molecular Approach 17
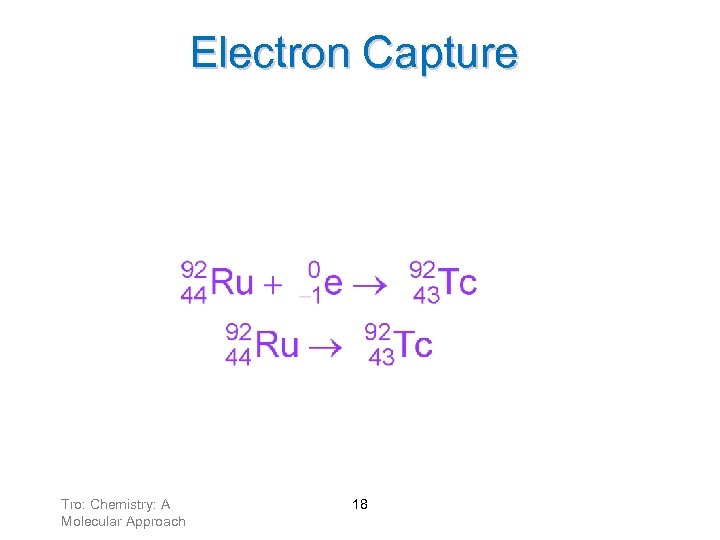
Electron Capture As a result of electron capture: ü mass number stays the same ü atomic number decreases by one Tro: Chemistry: A Molecular Approach 18
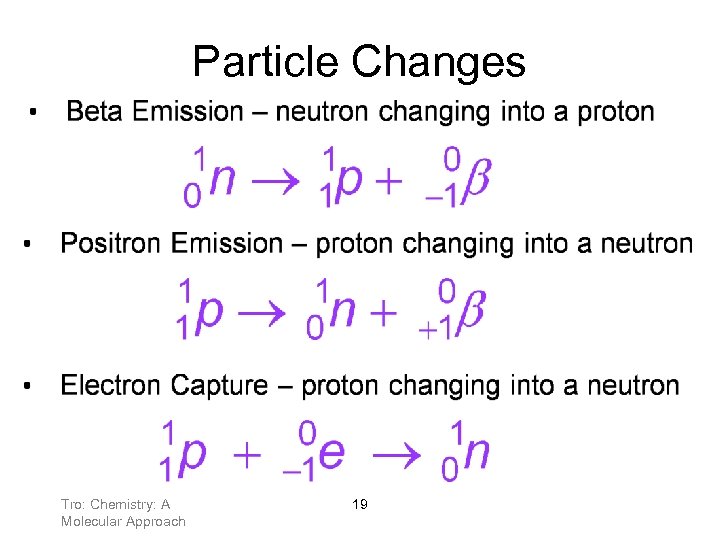
Particle Changes Tro: Chemistry: A Molecular Approach 19

Alpha (α) Emission Many nuclides that are too heavy to be stable (Z>83) undergo alpha emission. An particle contains 2 protons and 2 neutrons, and is the same as a helium nucleus. Tro: Chemistry: A Molecular Approach 20
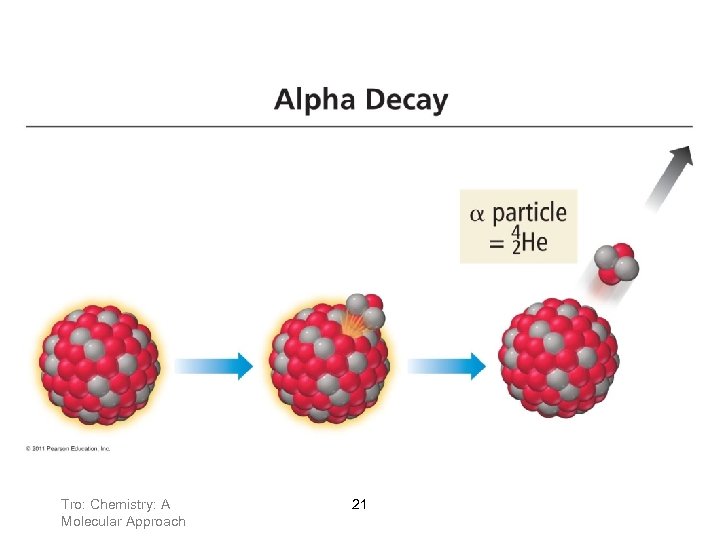
Tro: Chemistry: A Molecular Approach 21
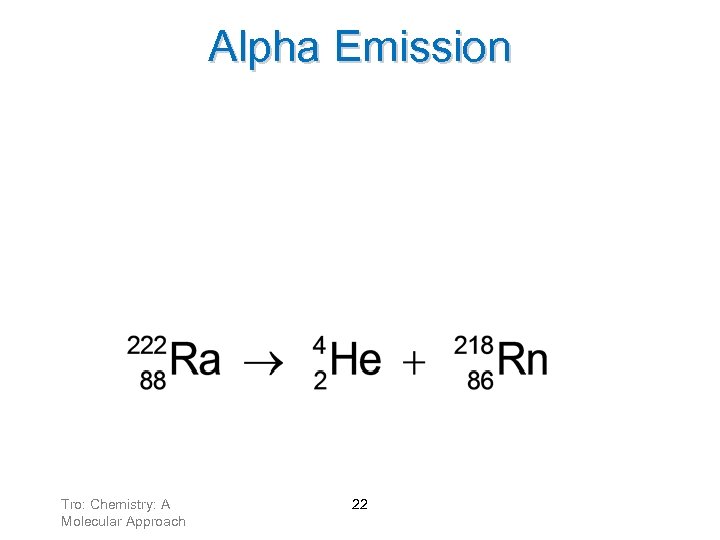
Alpha Emission Loss of an alpha particle means: ü atomic number decreases by 2 ü mass number decreases by 4 As a result, the N/Z ratio increases. Tro: Chemistry: A Molecular Approach 22
Gamma (γ) Emission During a nuclear reaction, high energy electromagnetic radiation, called gamma rays, is often emitted. Generally occurs after the nucleus undergoes some other type of decay and the remaining particles rearrange. Tro: Chemistry: A Molecular Approach 23
Gamma Emission • Gamma (g) rays are high energy photons of • • light No loss of particles from the nucleus No change in the composition of the nucleus ü same atomic number and mass number Tro: Chemistry: A Molecular Approach 24
Other Properties of Radioactivity • Radioactive rays can ionize matter ü cause uncharged matter to become charged ü basis of Geiger Counter and electroscope • Radioactive rays have high energy • Radioactive rays can penetrate matter • Radioactive rays cause phosphorescent chemicals to glow ü basis of scintillation counter Tro: Chemistry: A Molecular Approach 25
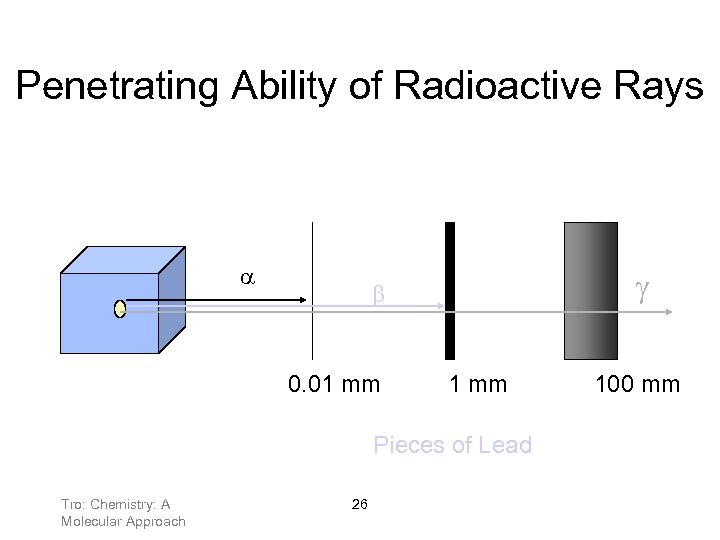
Penetrating Ability of Radioactive Rays g b 0. 01 mm Pieces of Lead Tro: Chemistry: A Molecular Approach 26 100 mm

Ionizing Ability of Radiation Highly energetic radiation interacts with molecules and atoms by ionizing them. This can have serious biological effects on cells in living systems. Cell damage, or abnormal cell replication can occur. α particles are highly ionizing, but not very penetrating. They can be stopped by a sheet of paper, clothing, or air. As a result, they are not very damaging unless ingested or breathed into the lungs. β particles have lower ionizing power, but are more penetrating. A sheet of metal or a thick piece of wood will stop them. Tro: Chemistry: A Molecular Approach 27
Ionizing Ability of Radiation γ rays have the lowest ionizing power, but are the most penetrating. Several inches of lead or slabs of concrete are needed to stop gamma rays. Tro: Chemistry: A Molecular Approach 28
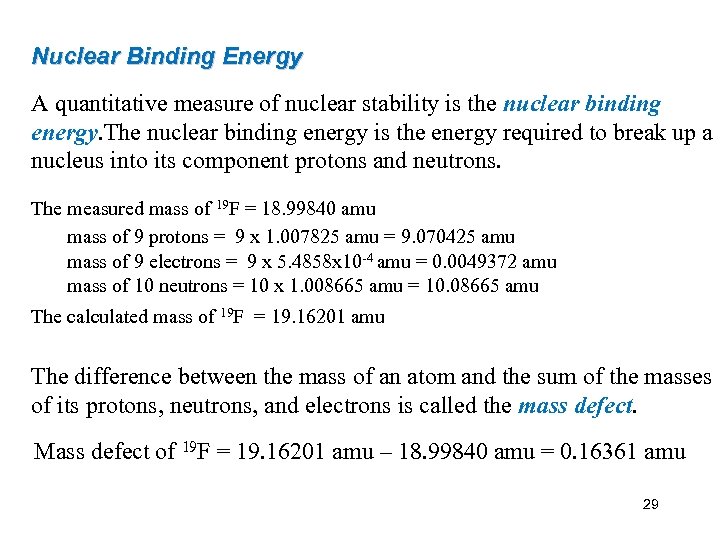
Nuclear Binding Energy A quantitative measure of nuclear stability is the nuclear binding energy. The nuclear binding energy is the energy required to break up a nucleus into its component protons and neutrons. The measured mass of 19 F = 18. 99840 amu mass of 9 protons = 9 x 1. 007825 amu = 9. 070425 amu mass of 9 electrons = 9 x 5. 4858 x 10 -4 amu = 0. 0049372 amu mass of 10 neutrons = 10 x 1. 008665 amu = 10. 08665 amu The calculated mass of 19 F = 19. 16201 amu The difference between the mass of an atom and the sum of the masses of its protons, neutrons, and electrons is called the mass defect. Mass defect of 19 F = 19. 16201 amu – 18. 99840 amu = 0. 16361 amu 29
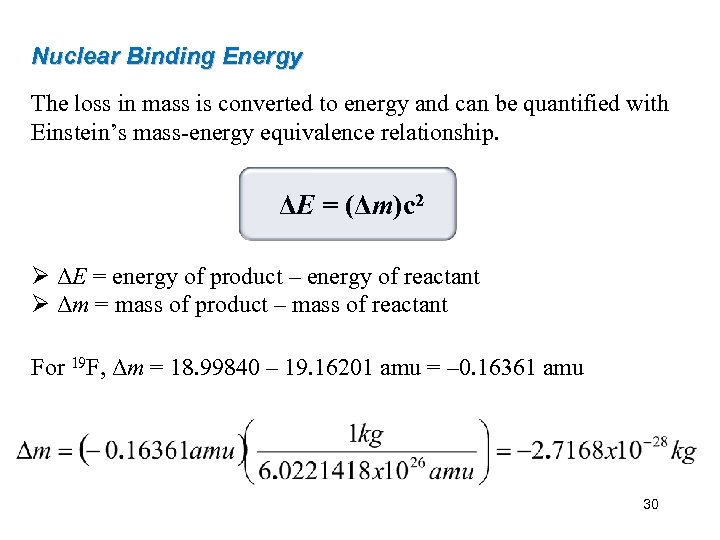
Nuclear Binding Energy The loss in mass is converted to energy and can be quantified with Einstein’s mass-energy equivalence relationship. ΔE = (Δm)c 2 Ø ΔE = energy of product – energy of reactant Ø Δm = mass of product – mass of reactant For 19 F, Δm = 18. 99840 – 19. 16201 amu = – 0. 16361 amu 30
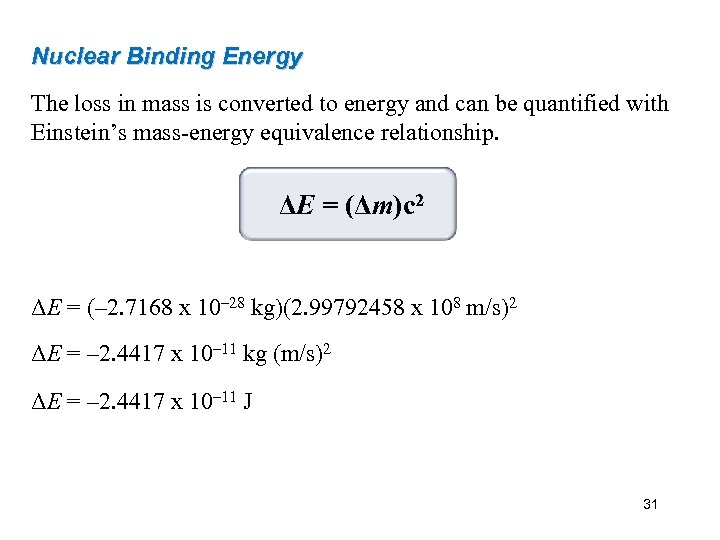
Nuclear Binding Energy The loss in mass is converted to energy and can be quantified with Einstein’s mass-energy equivalence relationship. ΔE = (Δm)c 2 ΔE = (– 2. 7168 x 10– 28 kg)(2. 99792458 x 108 m/s)2 ΔE = – 2. 4417 x 10– 11 kg (m/s)2 ΔE = – 2. 4417 x 10– 11 J 31
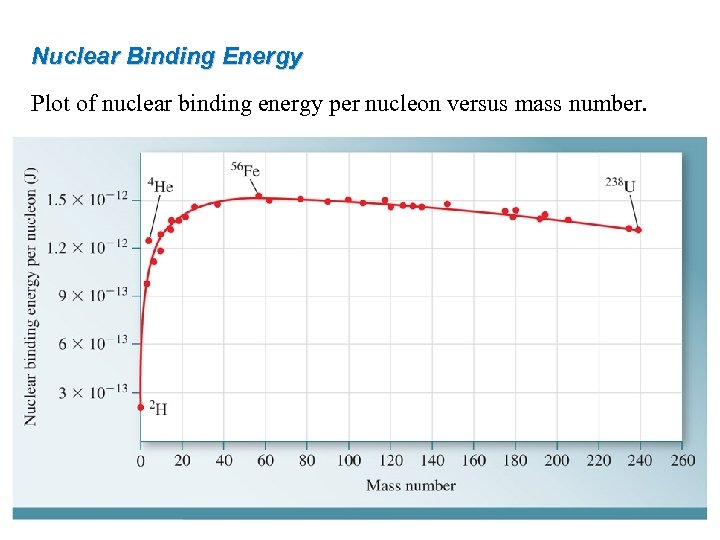
Nuclear Binding Energy Plot of nuclear binding energy per nucleon versus mass number. 32
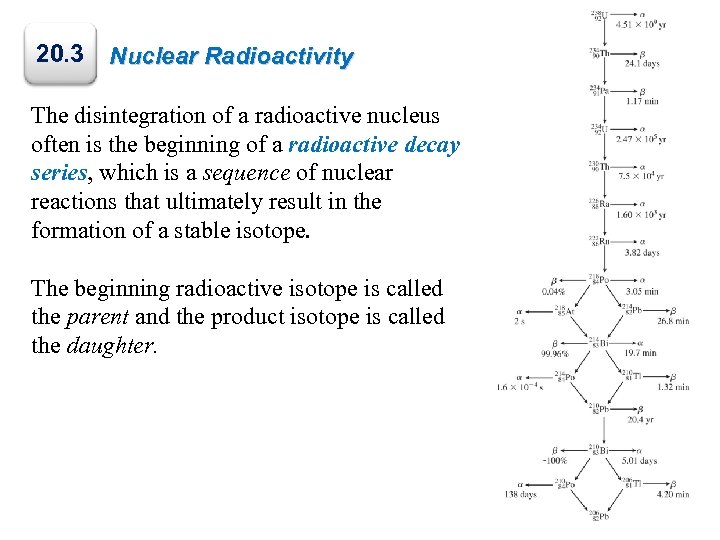
20. 3 Nuclear Radioactivity The disintegration of a radioactive nucleus often is the beginning of a radioactive decay series, which is a sequence of nuclear reactions that ultimately result in the formation of a stable isotope. The beginning radioactive isotope is called the parent and the product isotope is called the daughter. 33
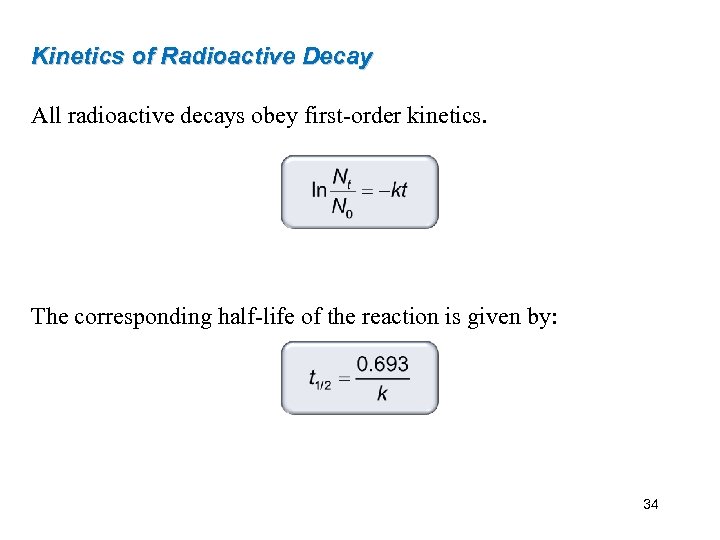
Kinetics of Radioactive Decay All radioactive decays obey first-order kinetics. The corresponding half-life of the reaction is given by: 34
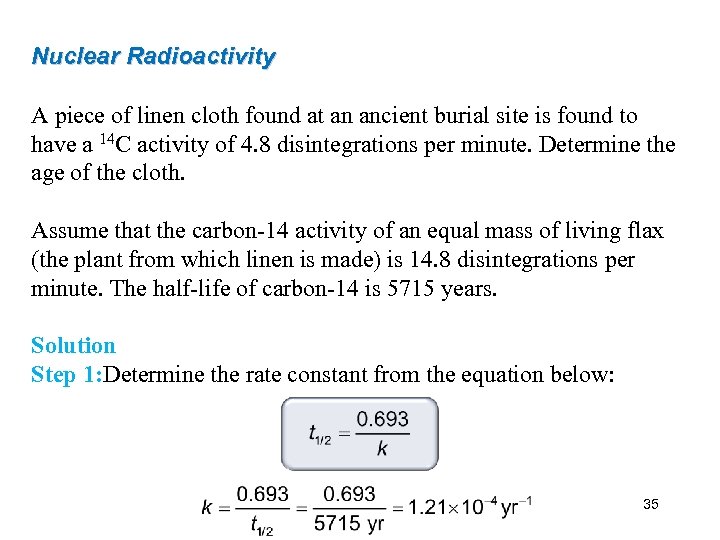
Nuclear Radioactivity A piece of linen cloth found at an ancient burial site is found to have a 14 C activity of 4. 8 disintegrations per minute. Determine the age of the cloth. Assume that the carbon-14 activity of an equal mass of living flax (the plant from which linen is made) is 14. 8 disintegrations per minute. The half-life of carbon-14 is 5715 years. Solution Step 1: Determine the rate constant from the equation below: 35
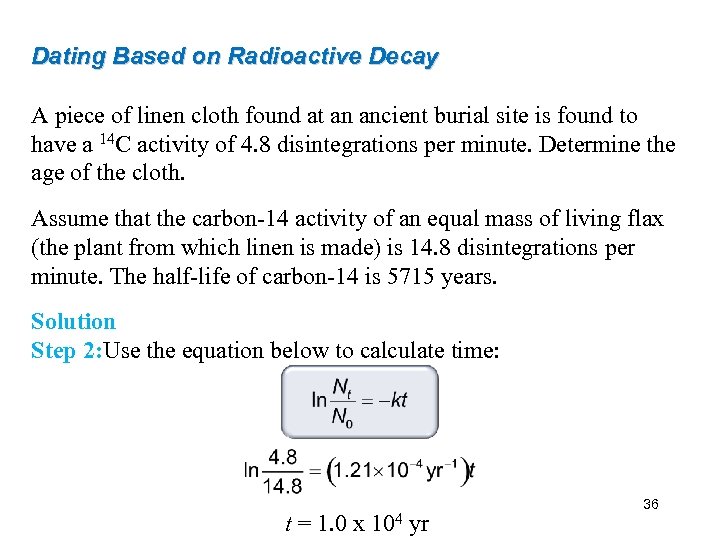
Dating Based on Radioactive Decay A piece of linen cloth found at an ancient burial site is found to have a 14 C activity of 4. 8 disintegrations per minute. Determine the age of the cloth. Assume that the carbon-14 activity of an equal mass of living flax (the plant from which linen is made) is 14. 8 disintegrations per minute. The half-life of carbon-14 is 5715 years. Solution Step 2: Use the equation below to calculate time: t = 1. 0 x 104 yr 36
Worked Example 20. 3 A wooden artifact is found to have a 14 C activity of 9. 1 disintegrations per second. Given that the 14 C activity of an equal mass of fresh-cut wood has a constant value of 15. 2 disintegrations per second, determine the age of the artifact. The half-life of carbon-14 is 5715 years. Strategy The activity of a radioactive sample is proportional to the number of radioactive nuclei. Thus, we can use ln([A]t/[A]0) = –kt with activity in place of concentration: 14 C activity in artifact ln 14 = –kt C activity in fresh-cut wood To determine k, though, we must solve t½ = 0. 693/k, using the value of t½ for carbon-14 (5715 years) given in the problem statement. 37
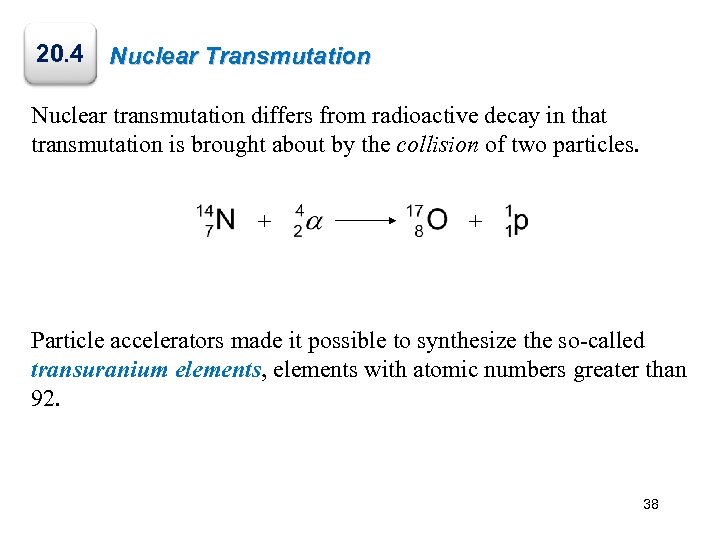
20. 4 Nuclear Transmutation Nuclear transmutation differs from radioactive decay in that transmutation is brought about by the collision of two particles. + + Particle accelerators made it possible to synthesize the so-called transuranium elements, elements with atomic numbers greater than 92. 38
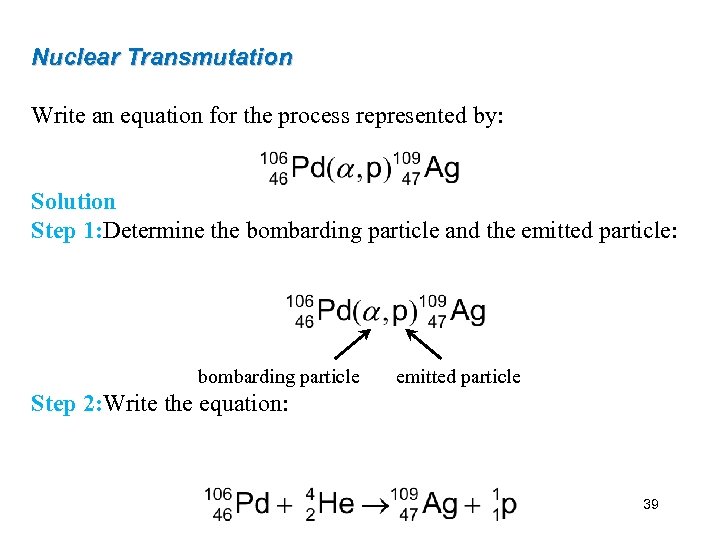
Nuclear Transmutation Write an equation for the process represented by: Solution Step 1: Determine the bombarding particle and the emitted particle: bombarding particle emitted particle Step 2: Write the equation: 39
Worked Example 20. 5 Write the balanced nuclear equation for the reaction represented by where d represents a deuterium nucleus. Strategy The species written first is a reactant. The species written last is a product. Within the parentheses, the bombarding particle (a reactant) is written first, followed by the emitted particle (a product). Solution The bombarding and emitted particles are represented by respectively. and , Think About It Check your work by summing the mass numbers and atomic numbers on both sides of the equation. 40
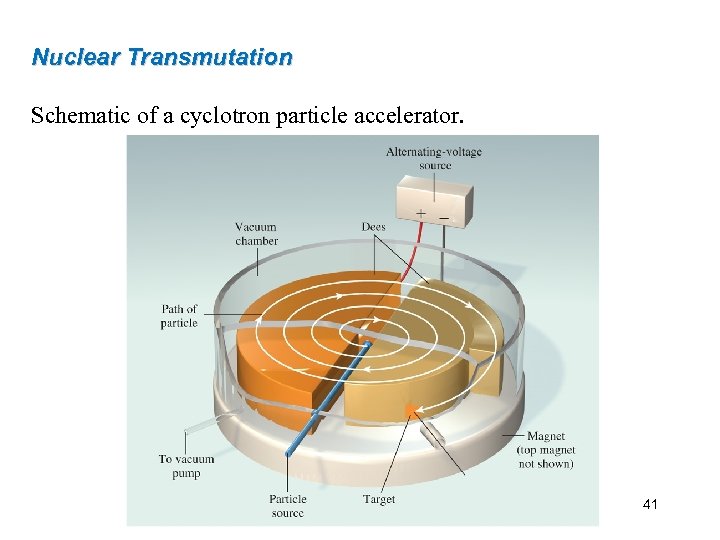
Nuclear Transmutation Schematic of a cyclotron particle accelerator. 41

Nuclear Transmutation Section of a particle accelerator. 42
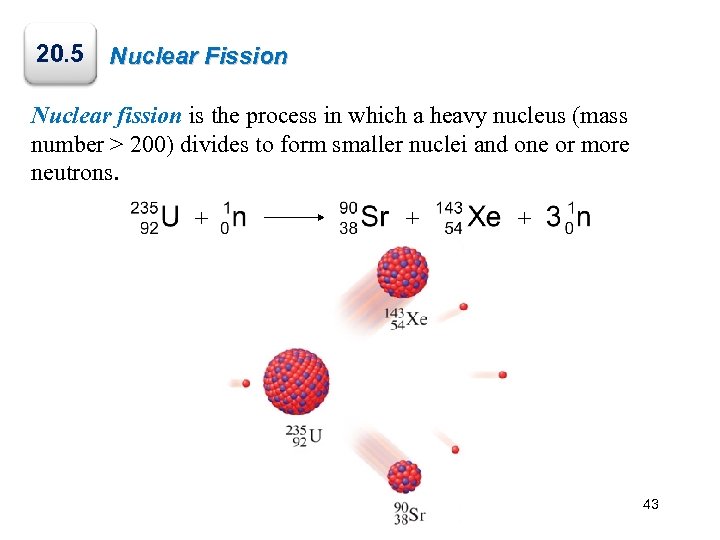
20. 5 Nuclear Fission Nuclear fission is the process in which a heavy nucleus (mass number > 200) divides to form smaller nuclei and one or more neutrons. + + + 43
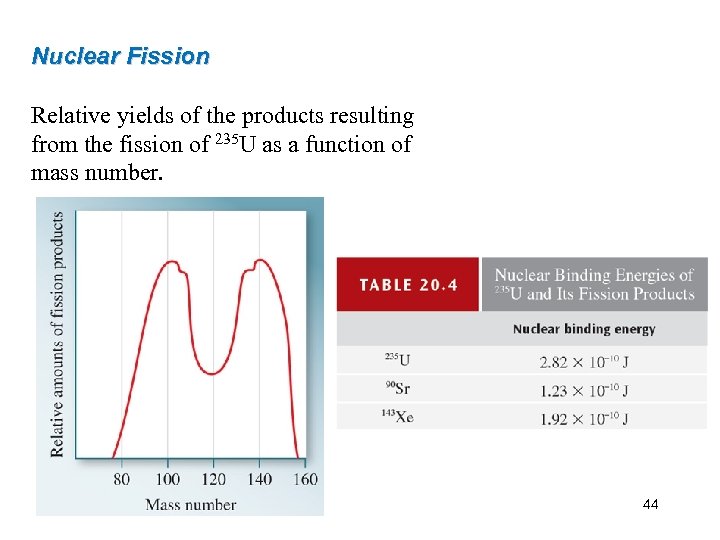
Nuclear Fission Relative yields of the products resulting from the fission of 235 U as a function of mass number. 44
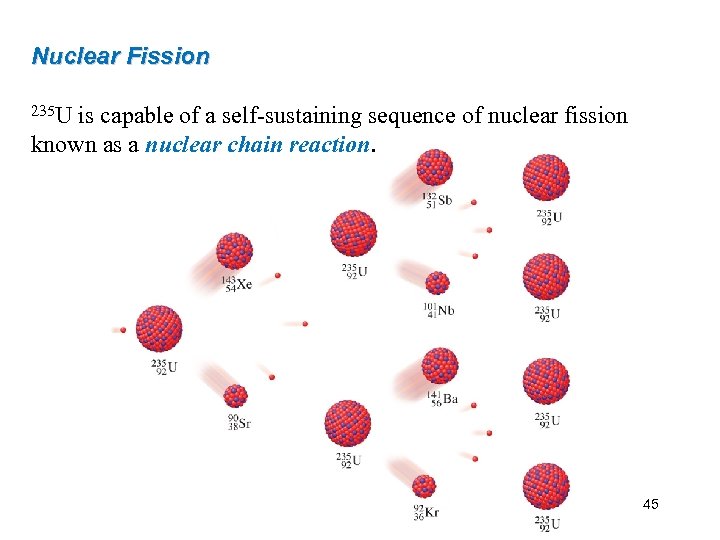
Nuclear Fission 235 U is capable of a self-sustaining sequence of nuclear fission known as a nuclear chain reaction. 45
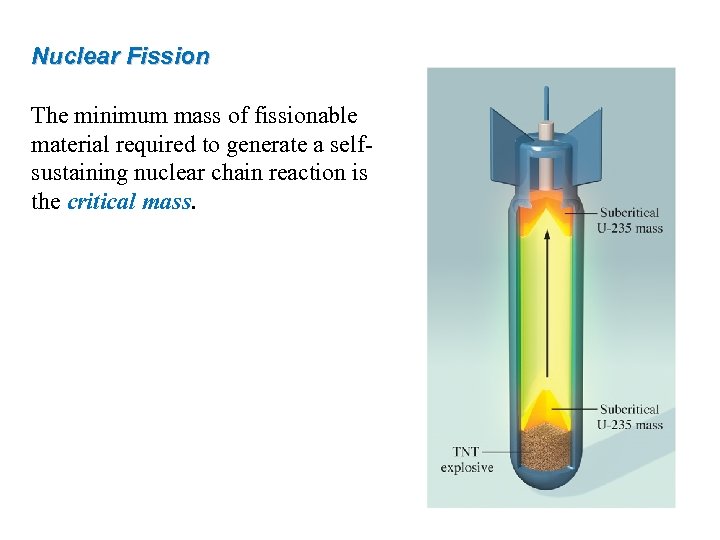
Nuclear Fission The minimum mass of fissionable material required to generate a selfsustaining nuclear chain reaction is the critical mass. 46
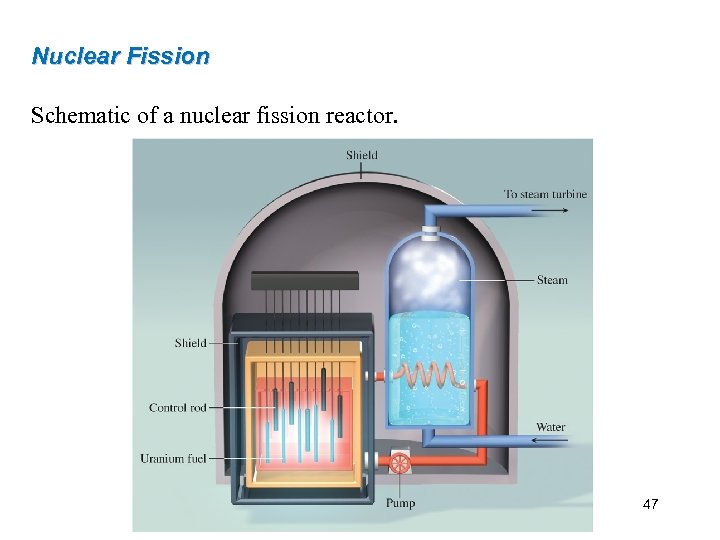
Nuclear Fission Schematic of a nuclear fission reactor. 47
20. 6 Nuclear Fusion Nuclear fusion is the process of combining small nuclei into larger ones. The following reactions are believed to take place in the sun: Because fusion reactions take place at very high temperatures, they are often called thermonuclear reactions. 48
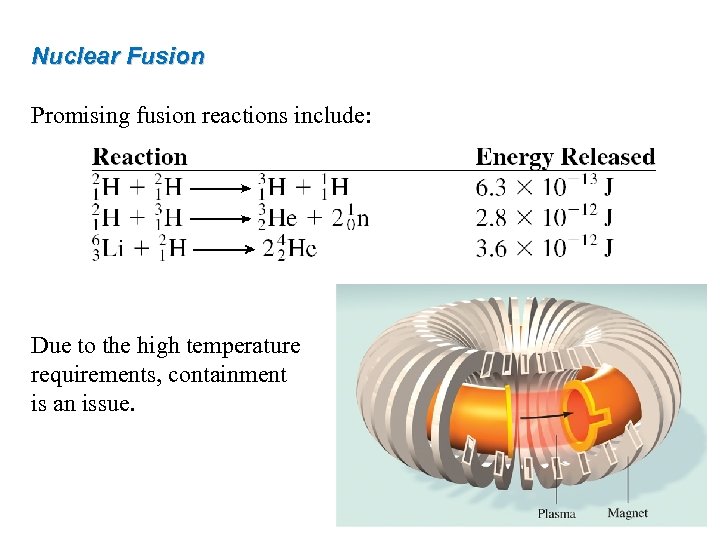
Nuclear Fusion Promising fusion reactions include: Due to the high temperature requirements, containment is an issue. 49
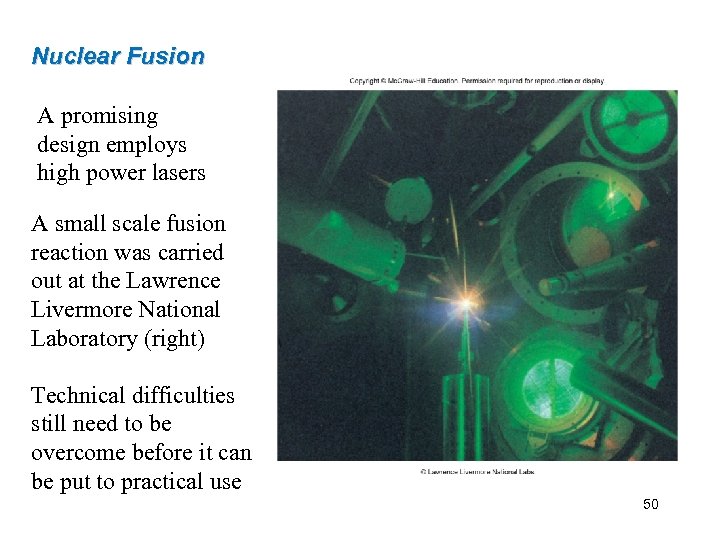
Nuclear Fusion A promising design employs high power lasers A small scale fusion reaction was carried out at the Lawrence Livermore National Laboratory (right) Technical difficulties still need to be overcome before it can be put to practical use 50
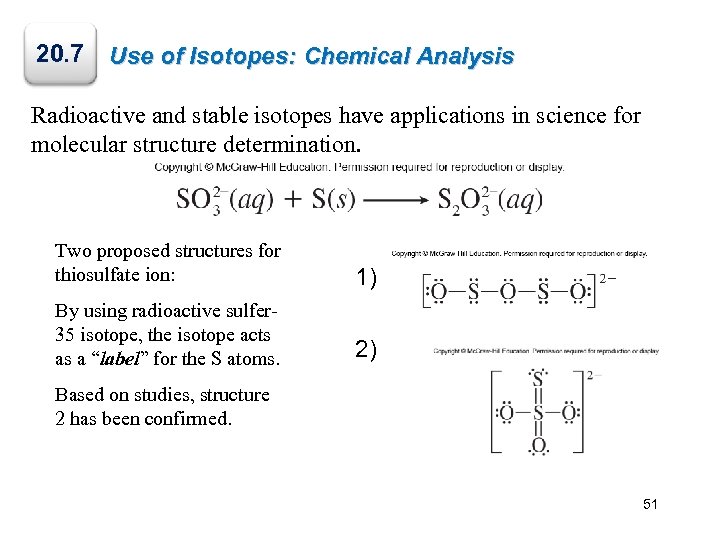
20. 7 Use of Isotopes: Chemical Analysis Radioactive and stable isotopes have applications in science for molecular structure determination. Two proposed structures for thiosulfate ion: 1) By using radioactive sulfer 35 isotope, the isotope acts as a “label” for the S atoms. 2) Based on studies, structure 2 has been confirmed. 51
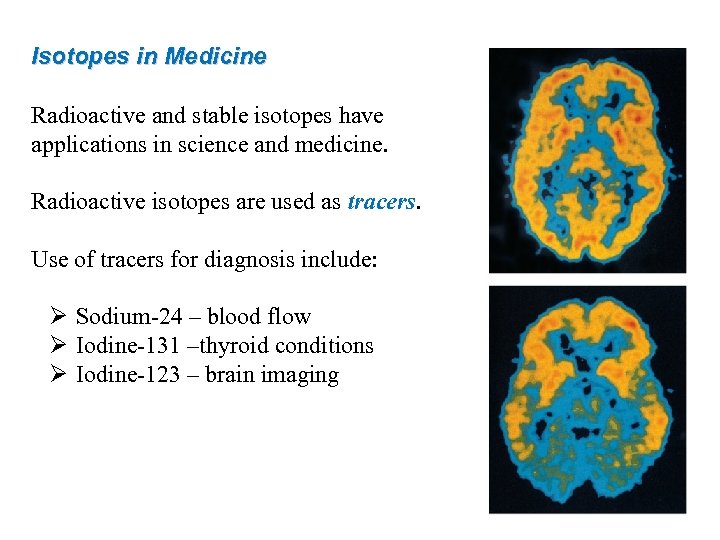
Isotopes in Medicine Radioactive and stable isotopes have applications in science and medicine. Radioactive isotopes are used as tracers. Use of tracers for diagnosis include: Ø Sodium-24 – blood flow Ø Iodine-131 –thyroid conditions Ø Iodine-123 – brain imaging 52
20. 8 Biological Effects of Radiation The fundamental unit of radioactivity is the curie (Ci) 1 Cu = 3. 70 x 1010 disintegrations per second. 53
Biological Effects of Radiation A common unit for the absorbed dose of radiation is the rad (radiation absorbed dose). 1 rad = 1 x 10 5 J/g of tissue irradiated The rem (roentgen equivalent for man) is determined from the number of rads: Number of rems = number of rads x 1 RBE = the relative biological effectivness. 54
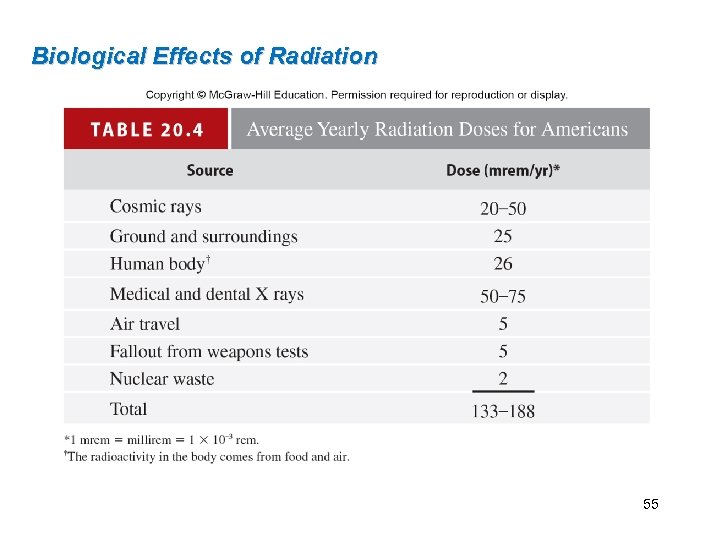
Biological Effects of Radiation 55
ddeade502581f275314efd3c9b6d5045.ppt