6dfe16e9e74af05474ddd20e18abcc1f.ppt
- Количество слайдов: 43
 Chemistry 103 Lecture 4
Chemistry 103 Lecture 4
 Outline I. Matter Classified (CH 3) II. The Periodic Table (CH 4) - The “Atom” defined - History - Atomic Symbols - Atomic Mass
Outline I. Matter Classified (CH 3) II. The Periodic Table (CH 4) - The “Atom” defined - History - Atomic Symbols - Atomic Mass
 Density in Review • What mass of gold (density = 19. 3 g/cm 3) occupies the same volume as 80. 0 g of lithium (density = 0. 534 g/cm 3)?
Density in Review • What mass of gold (density = 19. 3 g/cm 3) occupies the same volume as 80. 0 g of lithium (density = 0. 534 g/cm 3)?
 Chemical vs Physical? ? ? • • • Popping a balloon Burning a marshmellow Snapping a twig Water evaporating from a lake Metabolizing a meal Frying an egg
Chemical vs Physical? ? ? • • • Popping a balloon Burning a marshmellow Snapping a twig Water evaporating from a lake Metabolizing a meal Frying an egg
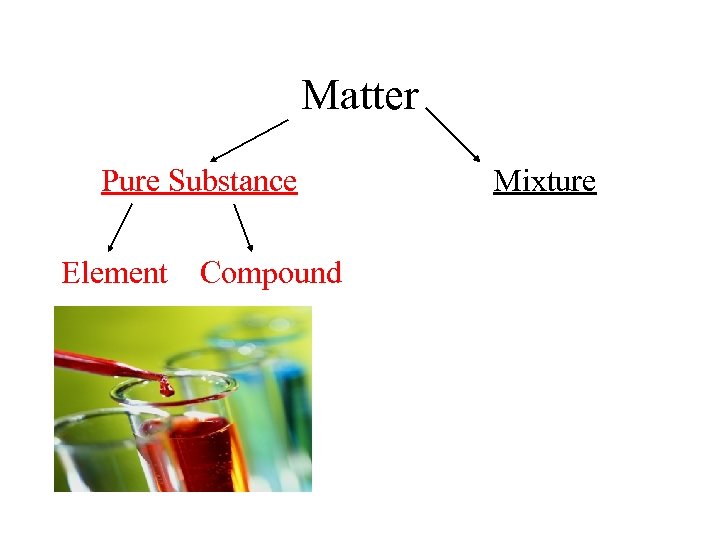 Matter Pure Substance Element Compound Mixture
Matter Pure Substance Element Compound Mixture
 Pure Substances: Compounds • Compounds – A chemical combination of 2 or more elements. – A pure substance that can be broken down into simpler substances by chemical means. Salt (Na. Cl) Table sugar (C 12 H 22 O 11) Water (H 2 O) NOTE: CO is different from Co
Pure Substances: Compounds • Compounds – A chemical combination of 2 or more elements. – A pure substance that can be broken down into simpler substances by chemical means. Salt (Na. Cl) Table sugar (C 12 H 22 O 11) Water (H 2 O) NOTE: CO is different from Co
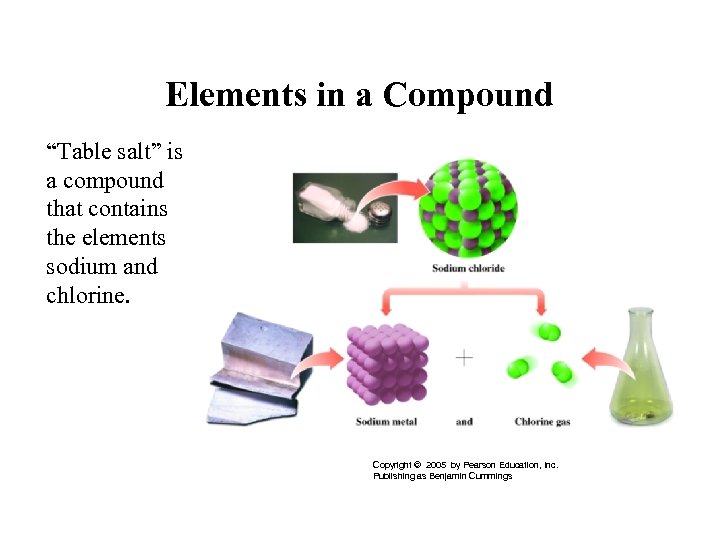 Elements in a Compound “Table salt” is a compound that contains the elements sodium and chlorine. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings
Elements in a Compound “Table salt” is a compound that contains the elements sodium and chlorine. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings
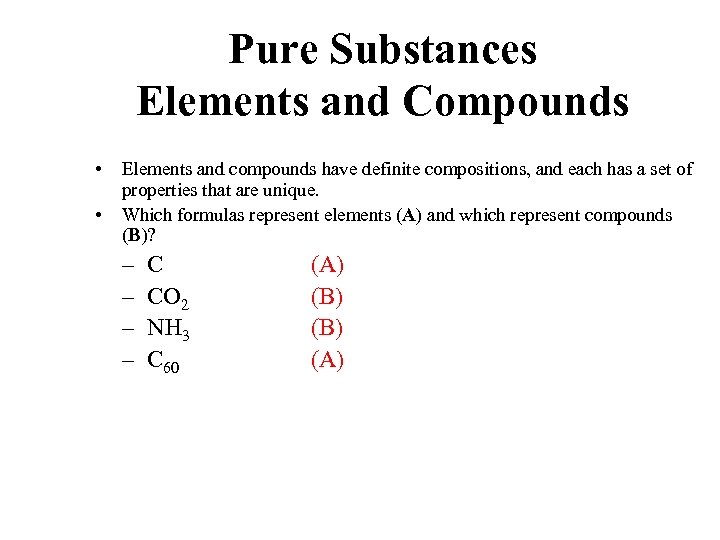 Pure Substances Elements and Compounds • • Elements and compounds have definite compositions, and each has a set of properties that are unique. Which formulas represent elements (A) and which represent compounds (B)? – – C CO 2 NH 3 C 60 (A) (B) (A)
Pure Substances Elements and Compounds • • Elements and compounds have definite compositions, and each has a set of properties that are unique. Which formulas represent elements (A) and which represent compounds (B)? – – C CO 2 NH 3 C 60 (A) (B) (A)
 Matter Pure Substance Element Compound Mixture
Matter Pure Substance Element Compound Mixture
 MATTER • Mixtures: a physical combination of two or more pure substances in which each substance retains its own chemical identity
MATTER • Mixtures: a physical combination of two or more pure substances in which each substance retains its own chemical identity
 Mixturesof: A mixture is a type of matter that consists • Two or more substances that are physically mixed, not chemically combined • Two or more substances in different proportions • Substances that can be separated by physical methods Example: Pasta and water can be separated with a strainer.
Mixturesof: A mixture is a type of matter that consists • Two or more substances that are physically mixed, not chemically combined • Two or more substances in different proportions • Substances that can be separated by physical methods Example: Pasta and water can be separated with a strainer.
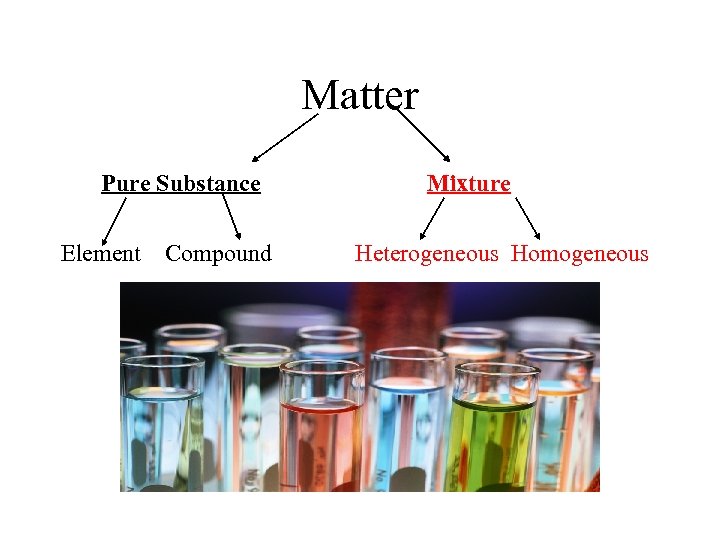 Matter Pure Substance Element Compound Mixture Heterogeneous Homogeneous
Matter Pure Substance Element Compound Mixture Heterogeneous Homogeneous
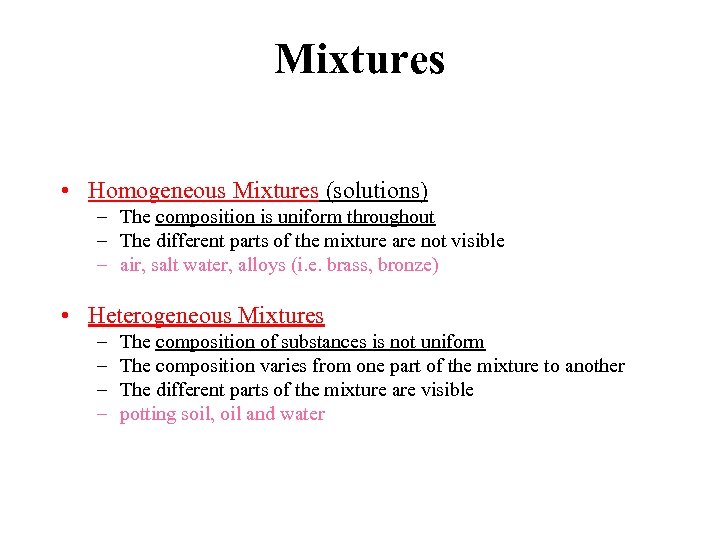 Mixtures • Homogeneous Mixtures (solutions) – The composition is uniform throughout – The different parts of the mixture are not visible – air, salt water, alloys (i. e. brass, bronze) • Heterogeneous Mixtures – – The composition of substances is not uniform The composition varies from one part of the mixture to another The different parts of the mixture are visible potting soil, oil and water
Mixtures • Homogeneous Mixtures (solutions) – The composition is uniform throughout – The different parts of the mixture are not visible – air, salt water, alloys (i. e. brass, bronze) • Heterogeneous Mixtures – – The composition of substances is not uniform The composition varies from one part of the mixture to another The different parts of the mixture are visible potting soil, oil and water
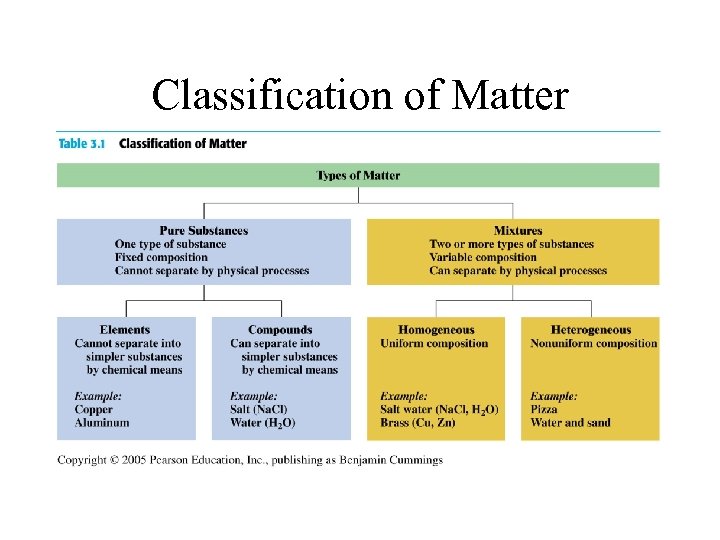 Classification of Matter
Classification of Matter
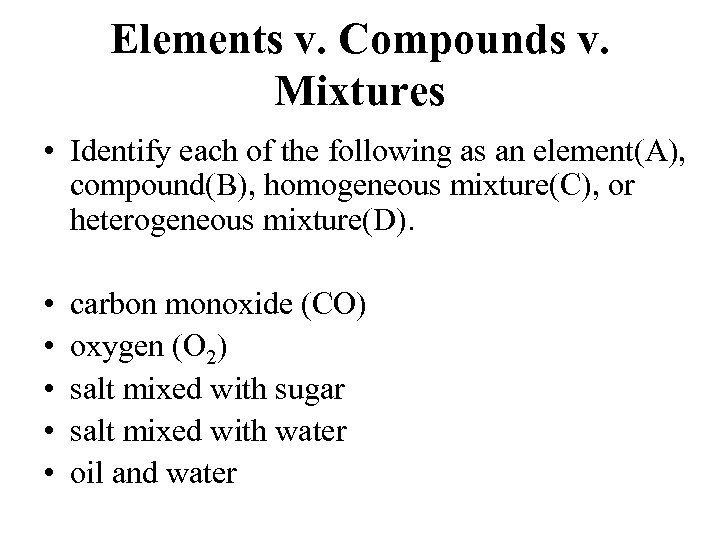 Elements v. Compounds v. Mixtures • Identify each of the following as an element(A), compound(B), homogeneous mixture(C), or heterogeneous mixture(D). • • • carbon monoxide (CO) oxygen (O 2) salt mixed with sugar salt mixed with water oil and water
Elements v. Compounds v. Mixtures • Identify each of the following as an element(A), compound(B), homogeneous mixture(C), or heterogeneous mixture(D). • • • carbon monoxide (CO) oxygen (O 2) salt mixed with sugar salt mixed with water oil and water
 A closer look at Elements & The Periodic Table
A closer look at Elements & The Periodic Table
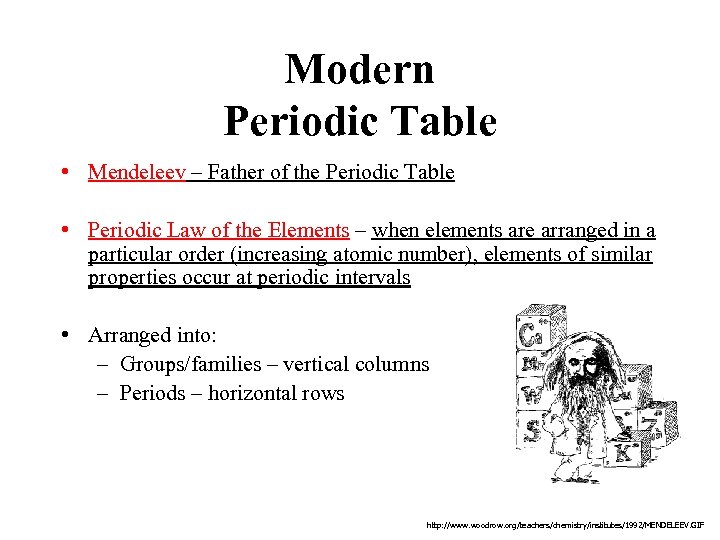 Modern Periodic Table • Mendeleev – Father of the Periodic Table • Periodic Law of the Elements – when elements are arranged in a particular order (increasing atomic number), elements of similar properties occur at periodic intervals • Arranged into: – Groups/families – vertical columns – Periods – horizontal rows http: //www. woodrow. org/teachers/chemistry/institutes/1992/MENDELEEV. GIF
Modern Periodic Table • Mendeleev – Father of the Periodic Table • Periodic Law of the Elements – when elements are arranged in a particular order (increasing atomic number), elements of similar properties occur at periodic intervals • Arranged into: – Groups/families – vertical columns – Periods – horizontal rows http: //www. woodrow. org/teachers/chemistry/institutes/1992/MENDELEEV. GIF
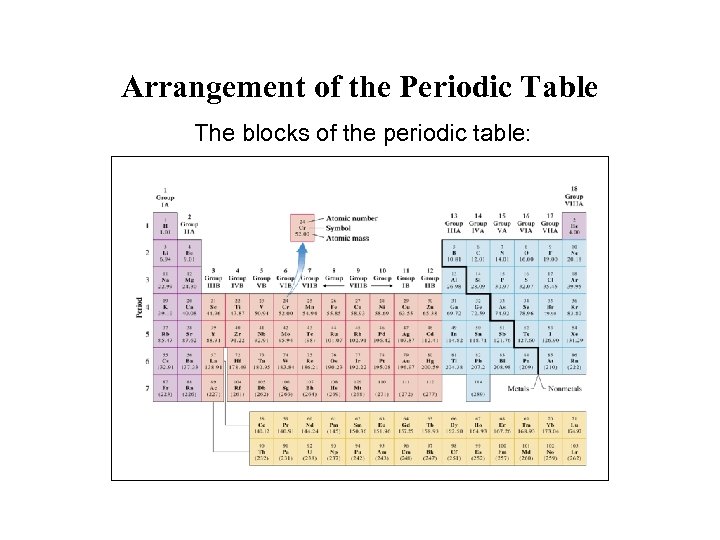 Arrangement of the Periodic Table The blocks of the periodic table:
Arrangement of the Periodic Table The blocks of the periodic table:
 The Periodic Table – All Stretched Out
The Periodic Table – All Stretched Out
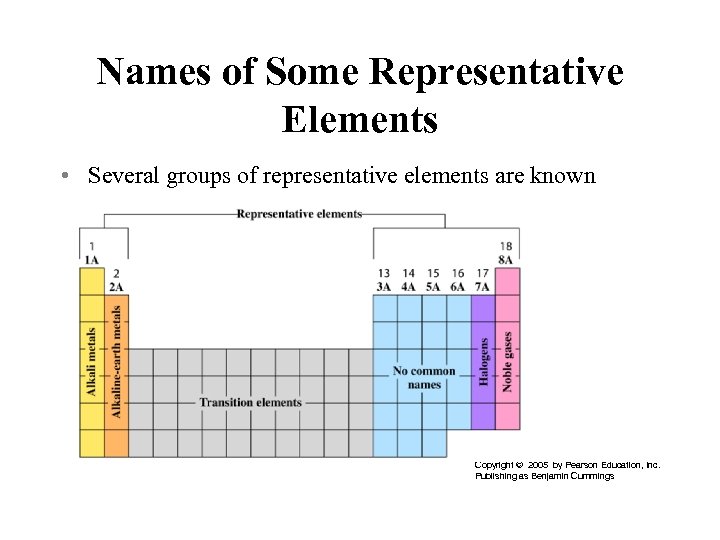 Names of Some Representative Elements • Several groups of representative elements are known by common names. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings
Names of Some Representative Elements • Several groups of representative elements are known by common names. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings
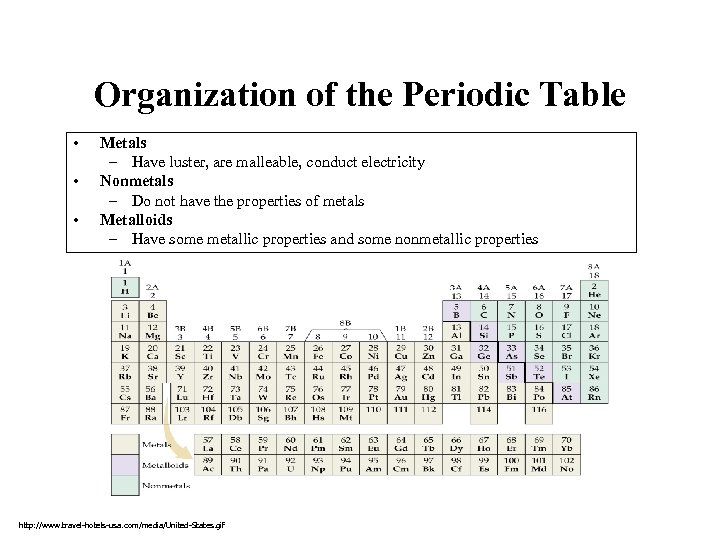 Organization of the Periodic Table • • • Metals – Have luster, are malleable, conduct electricity Nonmetals – Do not have the properties of metals Metalloids – Have some metallic properties and some nonmetallic properties http: //www. travel-hotels-usa. com/media/United-States. gif
Organization of the Periodic Table • • • Metals – Have luster, are malleable, conduct electricity Nonmetals – Do not have the properties of metals Metalloids – Have some metallic properties and some nonmetallic properties http: //www. travel-hotels-usa. com/media/United-States. gif
 A closer look still
A closer look still
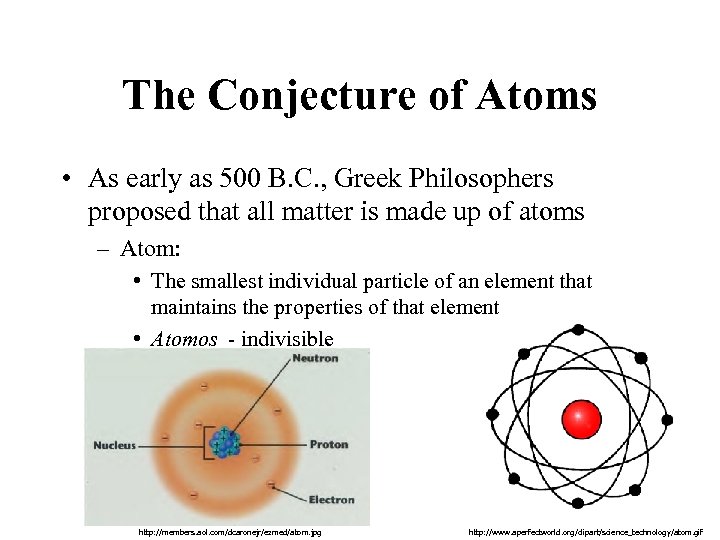 The Conjecture of Atoms • As early as 500 B. C. , Greek Philosophers proposed that all matter is made up of atoms – Atom: • The smallest individual particle of an element that maintains the properties of that element • Atomos - indivisible http: //members. aol. com/dcaronejr/ezmed/atom. jpg http: //www. aperfectworld. org/clipart/science_technology/atom. gif
The Conjecture of Atoms • As early as 500 B. C. , Greek Philosophers proposed that all matter is made up of atoms – Atom: • The smallest individual particle of an element that maintains the properties of that element • Atomos - indivisible http: //members. aol. com/dcaronejr/ezmed/atom. jpg http: //www. aperfectworld. org/clipart/science_technology/atom. gif
 Dalton’s Law of Atomic Theory 1. All matter is composed of extremely small particles called atoms. 2. Atoms of a given element are identical in their physical and chemical properties. 3. Atoms of different elements differ in their physical and chemical properties. http: //www. kjemi. uio. no/software/dalton/graphics/john_dalton. gif
Dalton’s Law of Atomic Theory 1. All matter is composed of extremely small particles called atoms. 2. Atoms of a given element are identical in their physical and chemical properties. 3. Atoms of different elements differ in their physical and chemical properties. http: //www. kjemi. uio. no/software/dalton/graphics/john_dalton. gif
 Dalton’s Law of Atomic Theory 4. Atoms of different elements combine in simple, wholenumber ratios to form compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged but never created, destroyed, or changed. http: //www. kjemi. uio. no/software/dalton/graphics/john_dalton. gif
Dalton’s Law of Atomic Theory 4. Atoms of different elements combine in simple, wholenumber ratios to form compounds. 5. In chemical reactions, atoms are combined, separated, or rearranged but never created, destroyed, or changed. http: //www. kjemi. uio. no/software/dalton/graphics/john_dalton. gif
 Atom Defined • An atom is the smallest particle of an element that can exist and still have the properties of the element. The fundamental building block of matter.
Atom Defined • An atom is the smallest particle of an element that can exist and still have the properties of the element. The fundamental building block of matter.
 Subatomic Particles Today, we know that atoms are made up of smaller, more fundamental particles called subatomic particles. Protons, Electrons & Neutrons
Subatomic Particles Today, we know that atoms are made up of smaller, more fundamental particles called subatomic particles. Protons, Electrons & Neutrons
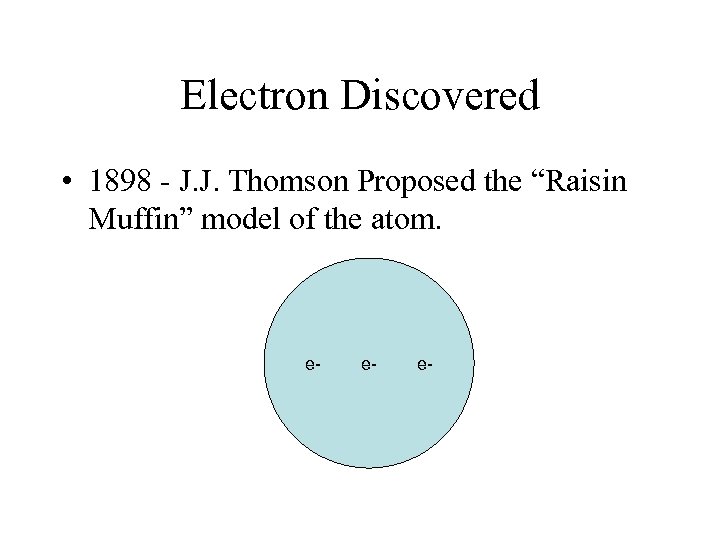 Electron Discovered • 1898 - J. J. Thomson Proposed the “Raisin Muffin” model of the atom. e- e- e-
Electron Discovered • 1898 - J. J. Thomson Proposed the “Raisin Muffin” model of the atom. e- e- e-
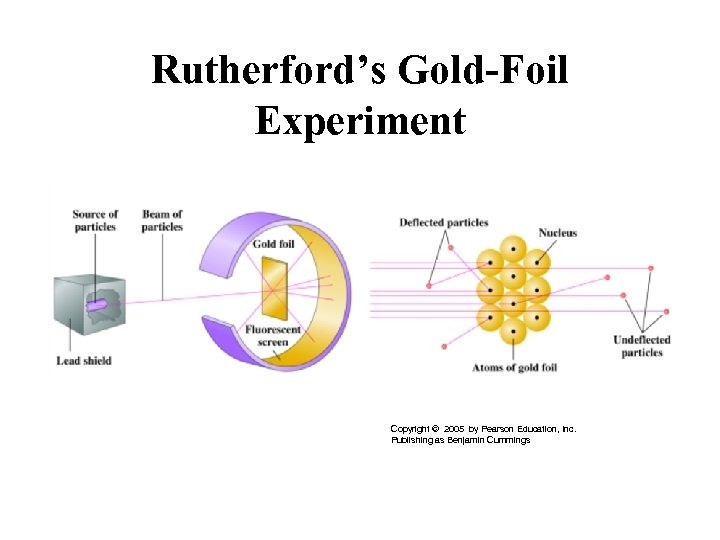 Rutherford’s Gold-Foil Experiment Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings
Rutherford’s Gold-Foil Experiment Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings
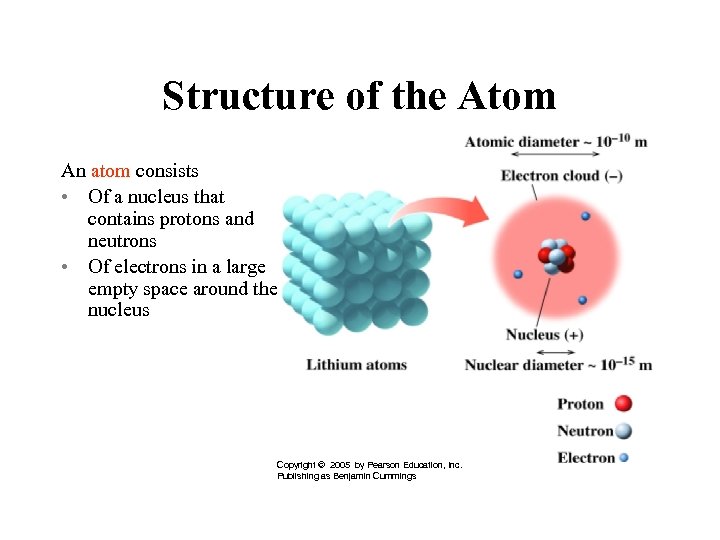 Structure of the Atom An atom consists • Of a nucleus that contains protons and neutrons • Of electrons in a large empty space around the nucleus Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings
Structure of the Atom An atom consists • Of a nucleus that contains protons and neutrons • Of electrons in a large empty space around the nucleus Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings
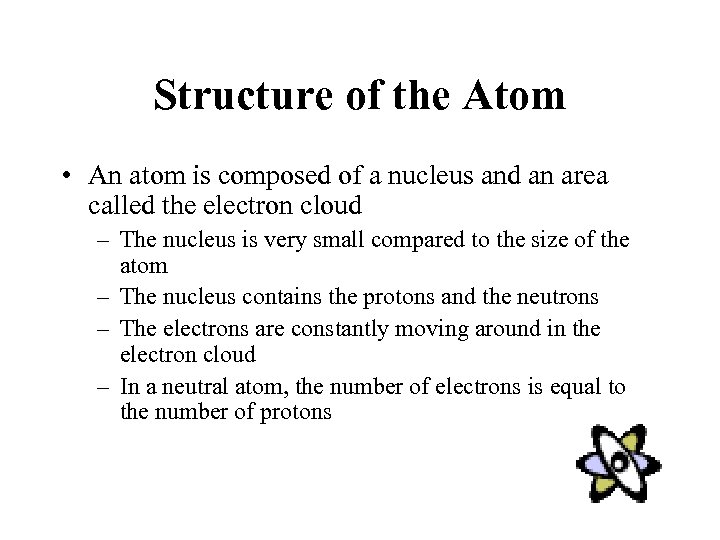 Structure of the Atom • An atom is composed of a nucleus and an area called the electron cloud – The nucleus is very small compared to the size of the atom – The nucleus contains the protons and the neutrons – The electrons are constantly moving around in the electron cloud – In a neutral atom, the number of electrons is equal to the number of protons
Structure of the Atom • An atom is composed of a nucleus and an area called the electron cloud – The nucleus is very small compared to the size of the atom – The nucleus contains the protons and the neutrons – The electrons are constantly moving around in the electron cloud – In a neutral atom, the number of electrons is equal to the number of protons
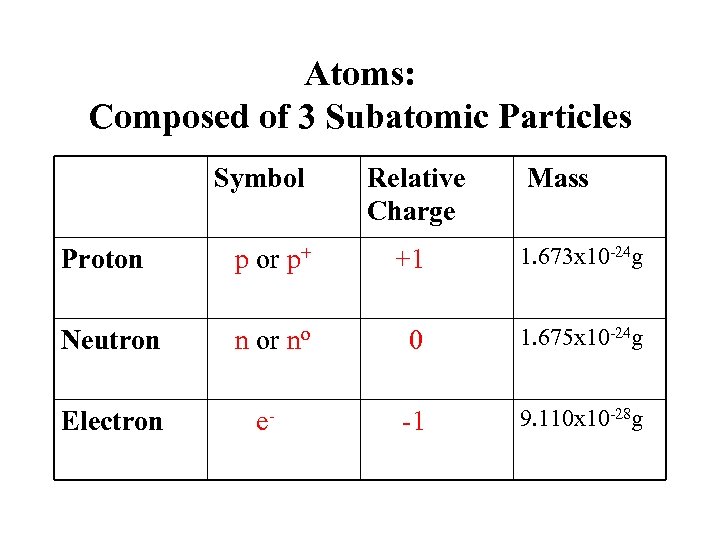 Atoms: Composed of 3 Subatomic Particles Symbol Relative Charge Mass Proton p or p+ +1 1. 673 x 10 -24 g Neutron n or no 0 1. 675 x 10 -24 g Electron e- -1 9. 110 x 10 -28 g
Atoms: Composed of 3 Subatomic Particles Symbol Relative Charge Mass Proton p or p+ +1 1. 673 x 10 -24 g Neutron n or no 0 1. 675 x 10 -24 g Electron e- -1 9. 110 x 10 -28 g
 The Atom • The nucleus of an atom carried most of the mass. A matchbox full of material of such density would weigh 2. 5 billion tons
The Atom • The nucleus of an atom carried most of the mass. A matchbox full of material of such density would weigh 2. 5 billion tons
 The Atom If an atom were scaled upwards in size so that the nuclei was 2 cm in diameter, the atom would have a diameter twice the length of a football field
The Atom If an atom were scaled upwards in size so that the nuclei was 2 cm in diameter, the atom would have a diameter twice the length of a football field
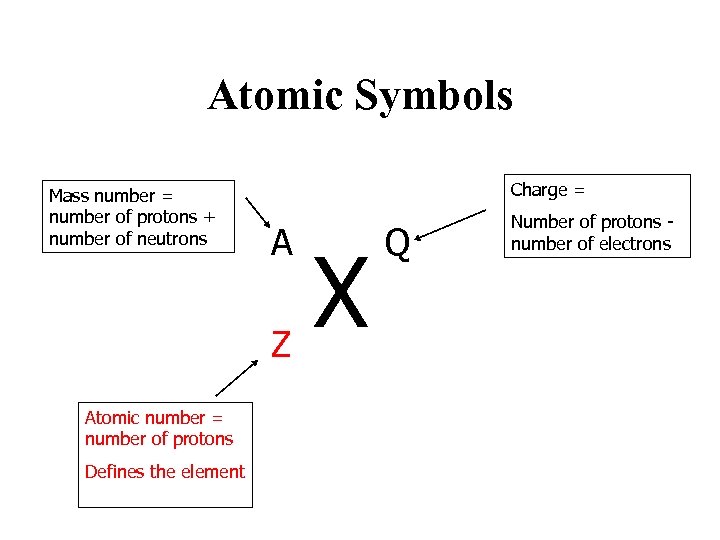 Atomic Symbols Mass number = number of protons + number of neutrons Charge = A Z Atomic number = number of protons Defines the element X Q Number of protons number of electrons
Atomic Symbols Mass number = number of protons + number of neutrons Charge = A Z Atomic number = number of protons Defines the element X Q Number of protons number of electrons
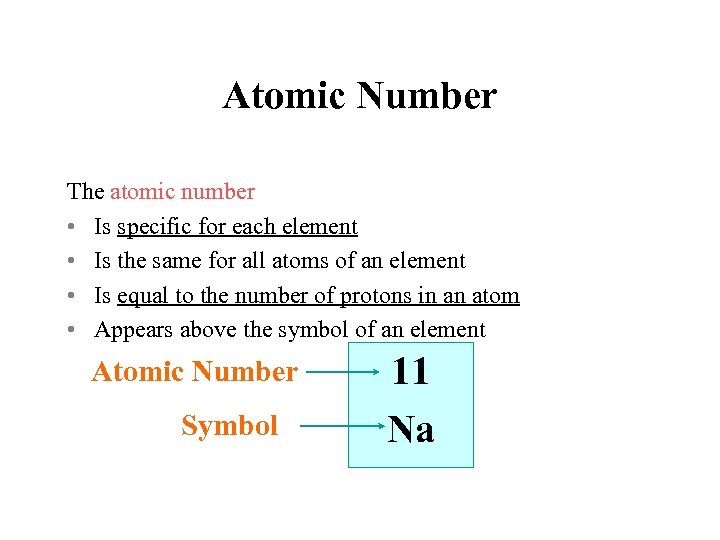 Atomic Number The atomic number • Is specific for each element • Is the same for all atoms of an element • Is equal to the number of protons in an atom • Appears above the symbol of an element Atomic Number Symbol 11 Na
Atomic Number The atomic number • Is specific for each element • Is the same for all atoms of an element • Is equal to the number of protons in an atom • Appears above the symbol of an element Atomic Number Symbol 11 Na
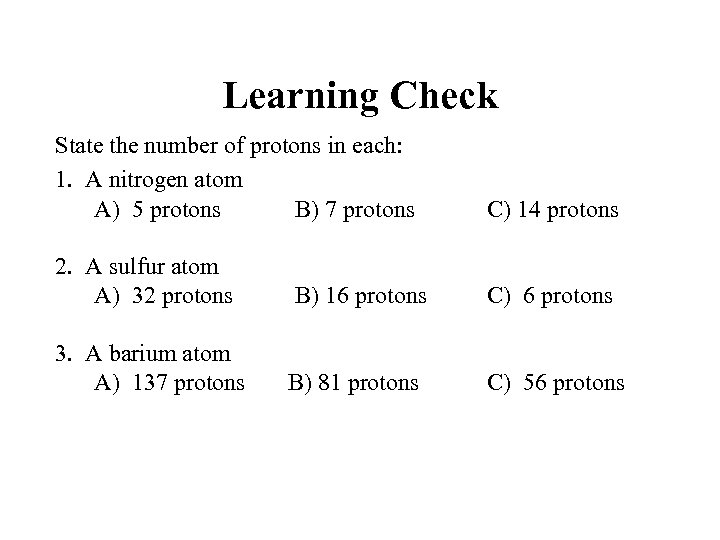 Learning Check State the number of protons in each: 1. A nitrogen atom A) 5 protons B) 7 protons C) 14 protons 2. A sulfur atom A) 32 protons C) 6 protons 3. A barium atom A) 137 protons B) 16 protons B) 81 protons C) 56 protons
Learning Check State the number of protons in each: 1. A nitrogen atom A) 5 protons B) 7 protons C) 14 protons 2. A sulfur atom A) 32 protons C) 6 protons 3. A barium atom A) 137 protons B) 16 protons B) 81 protons C) 56 protons
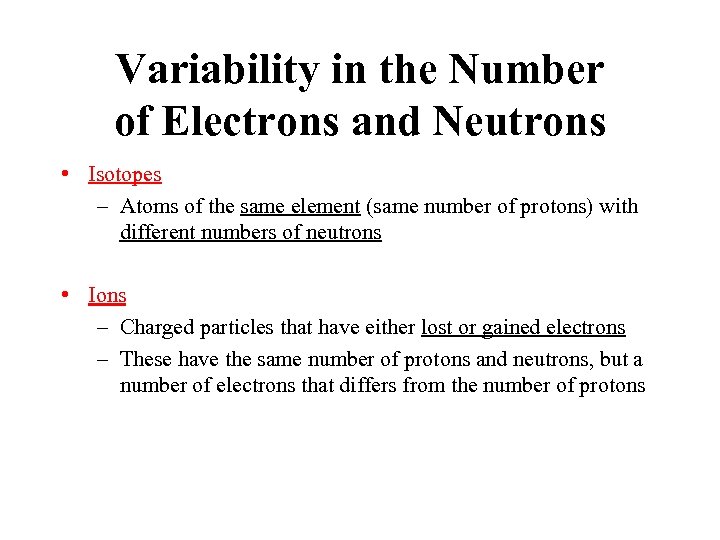 Variability in the Number of Electrons and Neutrons • Isotopes – Atoms of the same element (same number of protons) with different numbers of neutrons • Ions – Charged particles that have either lost or gained electrons – These have the same number of protons and neutrons, but a number of electrons that differs from the number of protons
Variability in the Number of Electrons and Neutrons • Isotopes – Atoms of the same element (same number of protons) with different numbers of neutrons • Ions – Charged particles that have either lost or gained electrons – These have the same number of protons and neutrons, but a number of electrons that differs from the number of protons
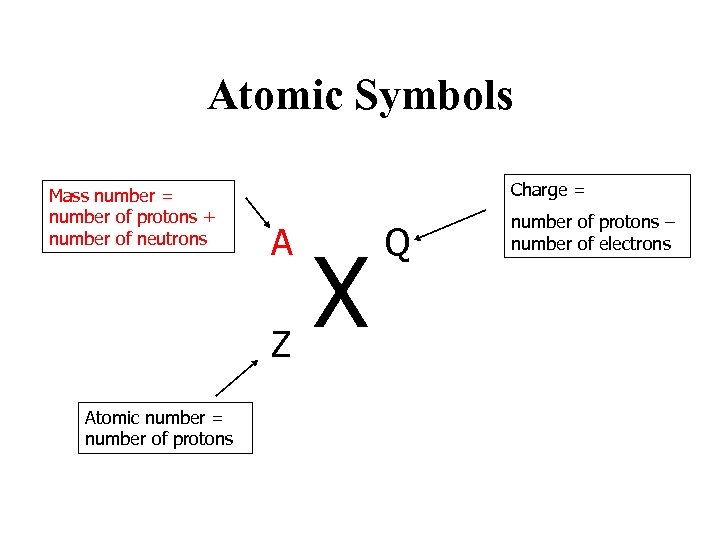 Atomic Symbols Mass number = number of protons + number of neutrons Charge = A Z Atomic number = number of protons X Q number of protons – number of electrons
Atomic Symbols Mass number = number of protons + number of neutrons Charge = A Z Atomic number = number of protons X Q number of protons – number of electrons
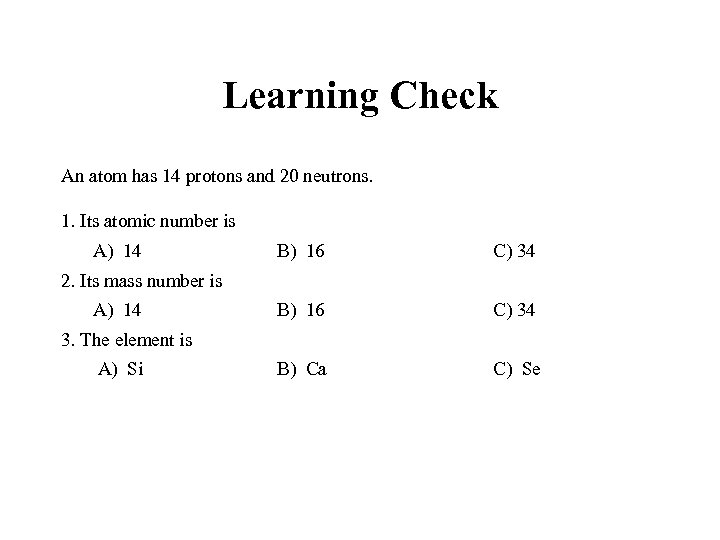 Learning Check An atom has 14 protons and 20 neutrons. 1. Its atomic number is A) 14 B) 16 C) 34 B) Ca C) Se 2. Its mass number is A) 14 3. The element is A) Si
Learning Check An atom has 14 protons and 20 neutrons. 1. Its atomic number is A) 14 B) 16 C) 34 B) Ca C) Se 2. Its mass number is A) 14 3. The element is A) Si
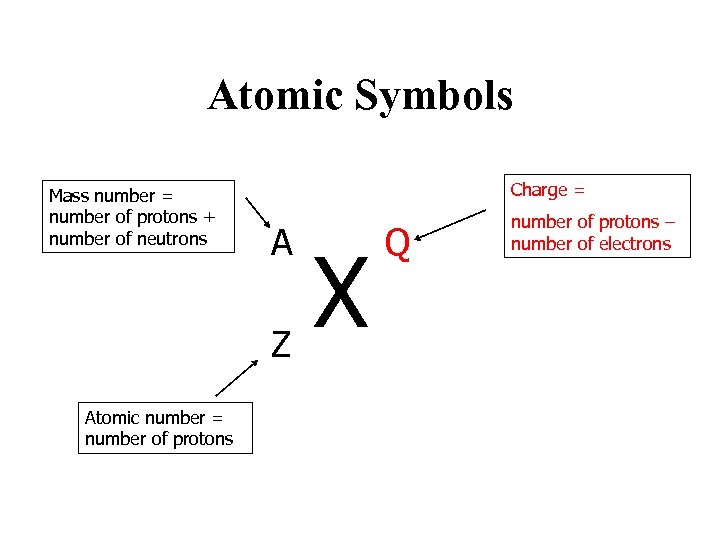 Atomic Symbols Mass number = number of protons + number of neutrons Charge = A Z Atomic number = number of protons X Q number of protons – number of electrons
Atomic Symbols Mass number = number of protons + number of neutrons Charge = A Z Atomic number = number of protons X Q number of protons – number of electrons
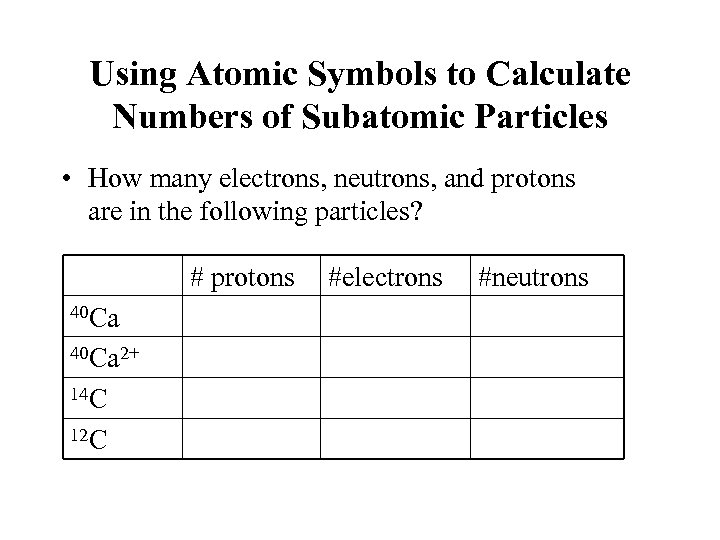 Using Atomic Symbols to Calculate Numbers of Subatomic Particles • How many electrons, neutrons, and protons are in the following particles? # protons 40 Ca 2+ 14 C 12 C #electrons #neutrons
Using Atomic Symbols to Calculate Numbers of Subatomic Particles • How many electrons, neutrons, and protons are in the following particles? # protons 40 Ca 2+ 14 C 12 C #electrons #neutrons
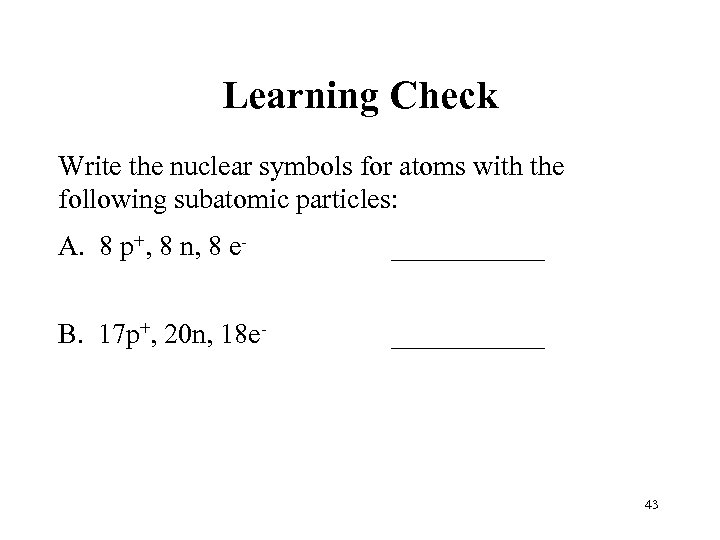 Learning Check Write the nuclear symbols for atoms with the following subatomic particles: A. 8 p+, 8 n, 8 e- ______ B. 17 p+, 20 n, 18 e- ______ 43
Learning Check Write the nuclear symbols for atoms with the following subatomic particles: A. 8 p+, 8 n, 8 e- ______ B. 17 p+, 20 n, 18 e- ______ 43


