0bc1edc5594f2f62e69771be89e9cae6.ppt
- Количество слайдов: 135
Chemistry 10/31/14 Bell Ringer 1. 2. 3. Complete a Bohr Model for the element Calcium What family are the elements in Group 18? What are the elements called that run left to right on the periodic table
Electron Configuration The how and why

You need out the following items: u Periodic Table that has been labled* u Composition Notebook for notes* u Electron Configuration Packet • Red indicates that you picked these up as you walked in • * indicates that you picked it up already

Today’s Date: October 31, 2014 Big Objective: CHEM 6 E: Express the arrangement of electrons in atoms through electron configurations and Lewis valence electron structures Daily Objectives: SWBAT define electron configuration SWBAT identify and draw orbitals SWBAT define the rules for electron configuration SWBAT write electron configurations

What We Know… u Answer the following questions on your handout: • Where are electrons located? – Outside the nucleus, on energy levels specifically • How many electrons can each shell hold? – 1 st – 2; 2 nd – 8; 3 rd – 18; 4 th – 32; 5 th – 50; 6 th - 72 • What is the outermost shell called? – Valence shell
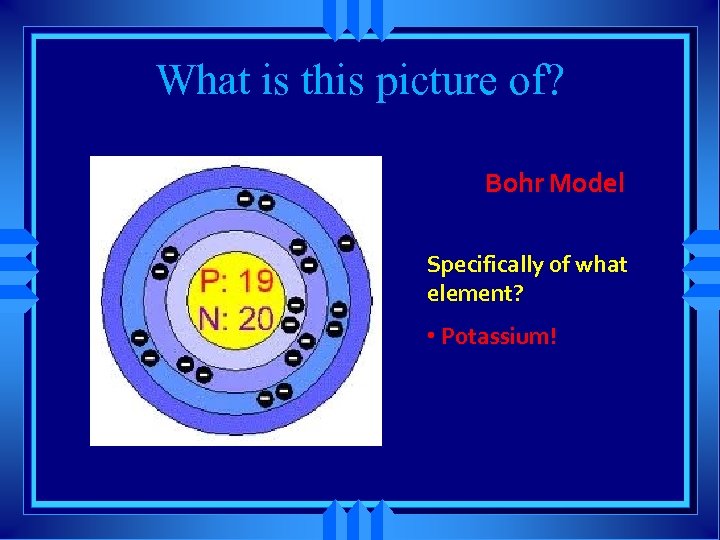
What is this picture of? Bohr Model Specifically of what element? • Potassium!
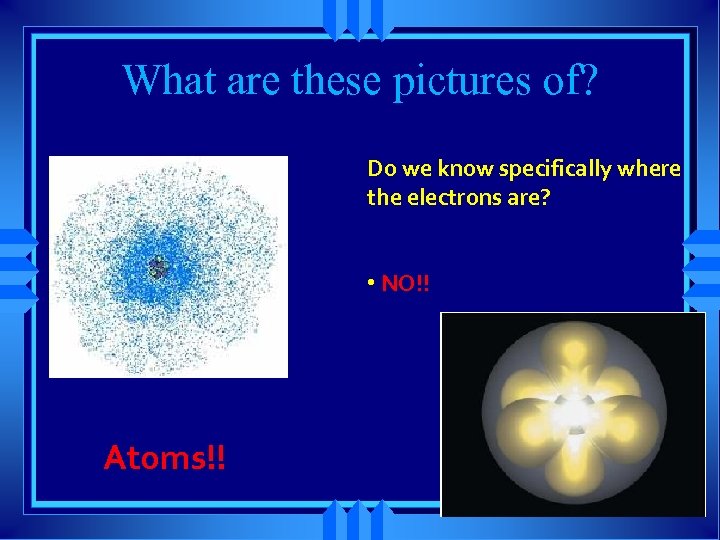
What are these pictures of? Do we know specifically where the electrons are? • NO!! Atoms!!
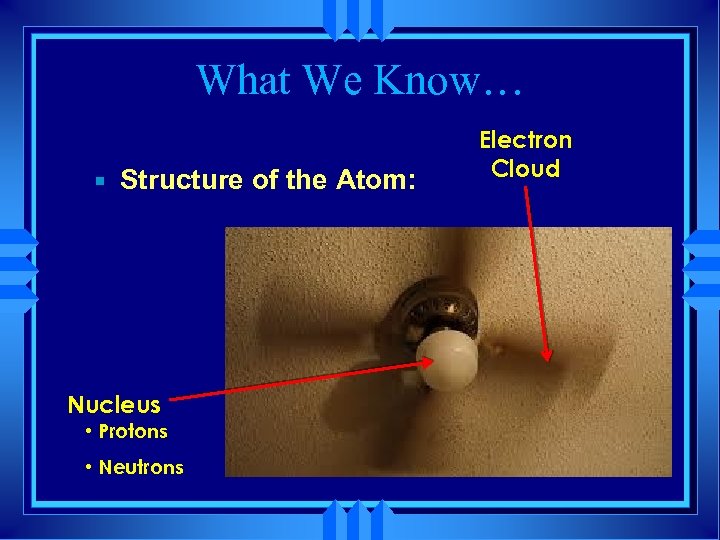
What We Know… Structure of the Atom: Nucleus • Protons • Neutrons Electron Cloud

What is an address? u The particulars of the place where someone lives or an organization is situated.

When we write an address, what do we normally include? u House # u Street u City u State u Zip Code
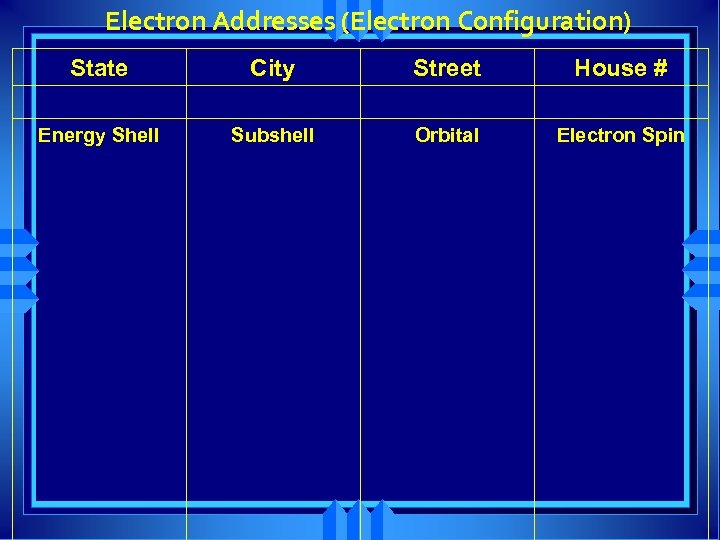
Electron Addresses (Electron Configuration) State City Street House # Energy Shell Subshell Orbital Electron Spin

What is electron configuration? u The arrangement of electrons in an atom is called the atom’s electron configuration

When we write electron addresses… u We • • go backwards! State (Energy Level) City (Subshell) Orbital Electron Spin
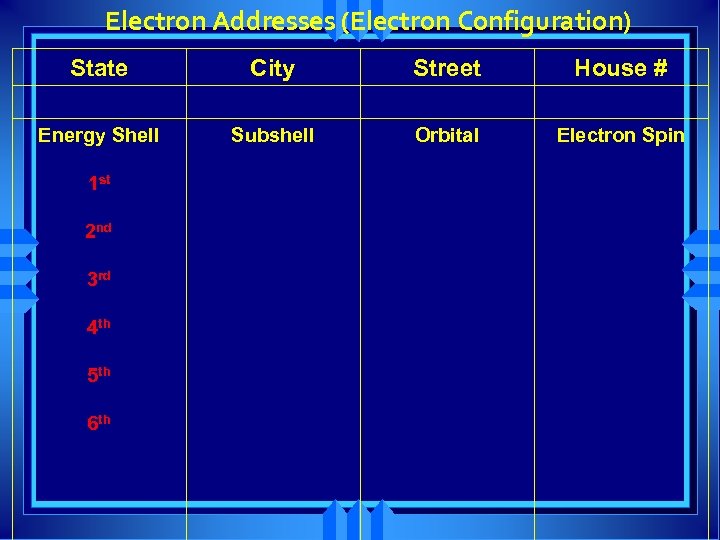
Electron Addresses (Electron Configuration) State City Street House # Energy Shell Subshell Orbital Electron Spin 1 st 2 nd 3 rd 4 th 5 th 6 th

Subshells Within each energy level are subshells. 4 types of subshells: s p d f Each subshell has a different shape.
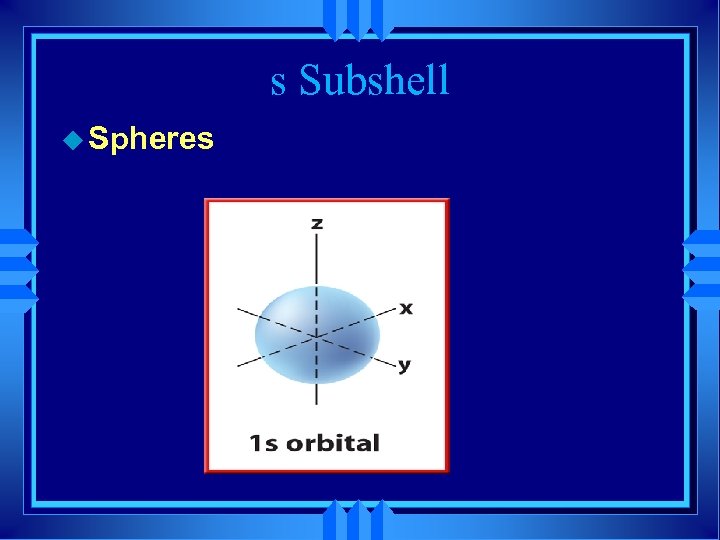
s Subshell u Spheres
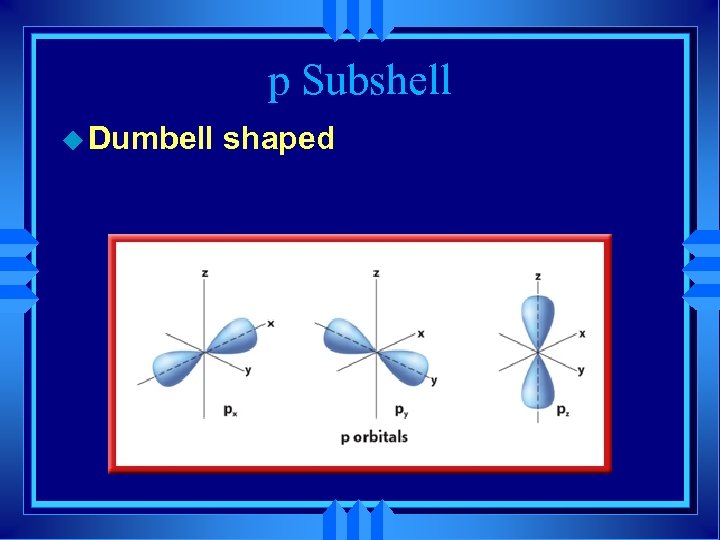
p Subshell u Dumbell shaped
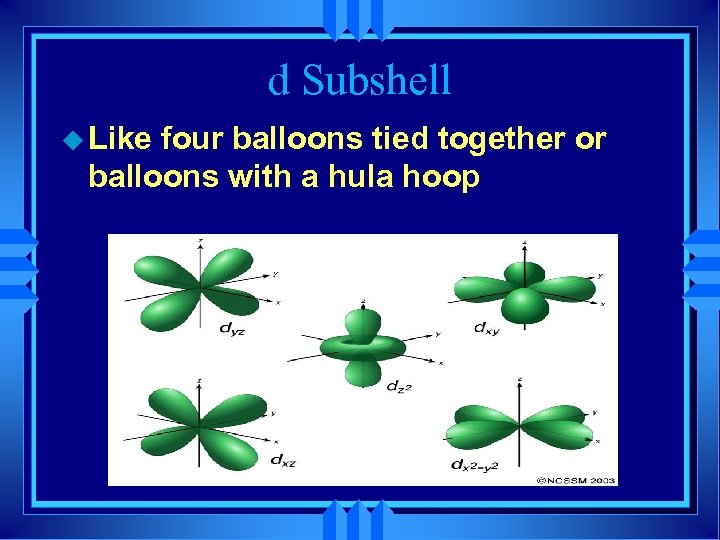
d Subshell u Like four balloons tied together or balloons with a hula hoop
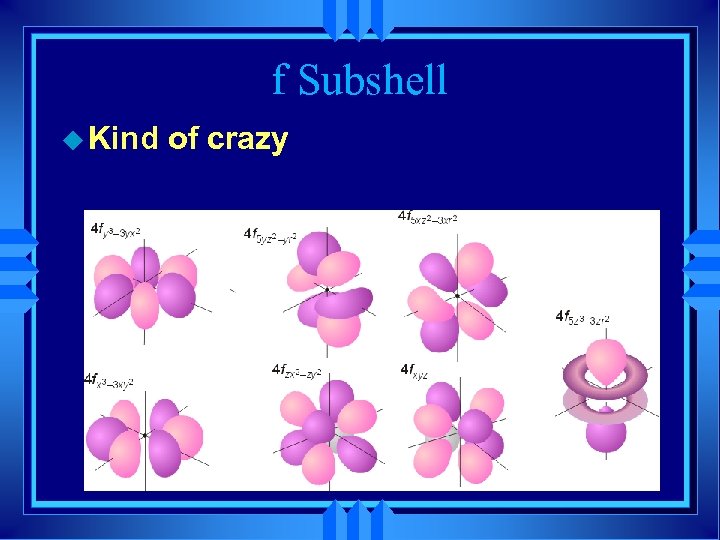
f Subshell u Kind of crazy

What are orbitals? u Orbitals are sublevels within energy levels; they are a region of probability in which the electron is found u Each subshell type has a certain number of orbitals. u Each orbital can hold 2 electrons.
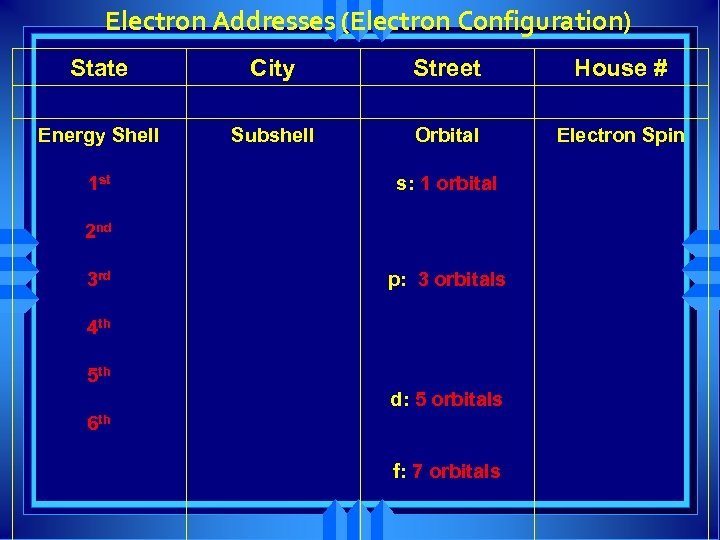
Electron Addresses (Electron Configuration) State City Street House # Energy Shell Subshell Orbital Electron Spin 1 st s: 1 orbital 2 nd 3 rd p: 3 orbitals 4 th 5 th d: 5 orbitals 6 th f: 7 orbitals

Each subshell has a certain number of orbitals! us – 1 orbital up – 3 orbitals ud – 5 orbitals uf – 7 orbitals
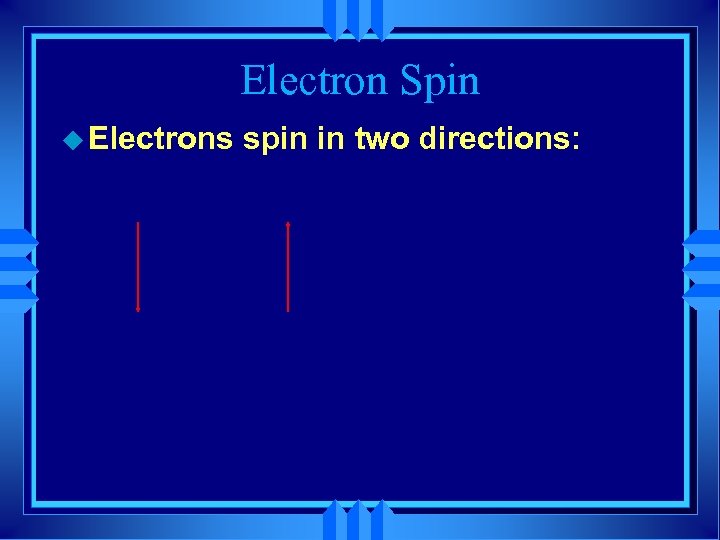
Electron Spin u Electrons spin in two directions:
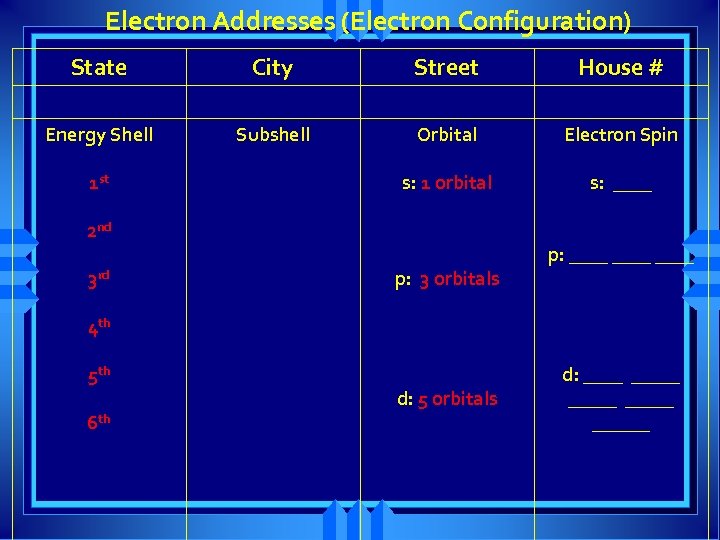
Electron Addresses (Electron Configuration) State City Street House # Energy Shell Subshell Orbital Electron Spin s: 1 orbital s: ____ 1 st 2 nd 3 rd p: 3 orbitals p: ____ 4 th 5 th 6 th d: 5 orbitals d: _____ ______

So when we write electron addresses… u We follow specific rules: • Energy Level – a number • Sublevel (orbital) – a letter (s, p, d, f) • Number of electrons u [energy level][orbital type][# of electrons]

Why? u The part of the atom another atom sees is the electron cloud. u More importantly the outside orbitals. u The orbitals fill up in a regular pattern. u The outside orbital electron configuration repeats. u The properties of atoms repeat.
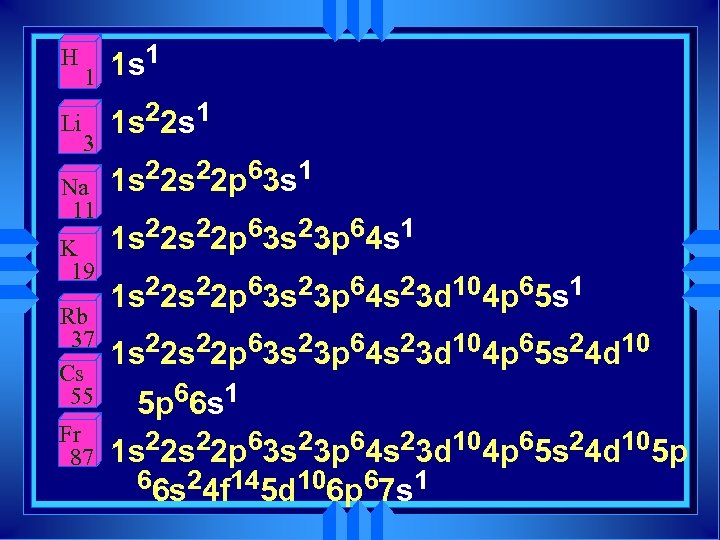
H Li 1 3 Na 11 K 19 Rb 37 Cs 55 Fr 87 1 s 1 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 1 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10 5 p 66 s 1 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 66 s 24 f 145 d 106 p 67 s 1

1 s 2 He 2 22 s 22 p 6 Ne 1 s 10 22 s 22 p 63 s 23 p 6 Ar 1 s 18 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 Kr 36 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 105 p 6 Xe 54 1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10 Rn 5 p 66 s 24 f 145 d 106 p 6 86
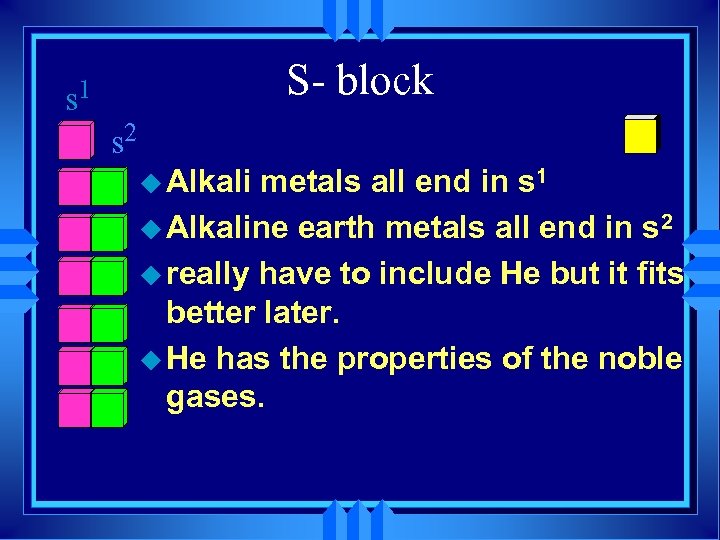
s 1 S- block s 2 u Alkali metals all end in s 1 u Alkaline earth metals all end in s 2 u really have to include He but it fits better later. u He has the properties of the noble gases.
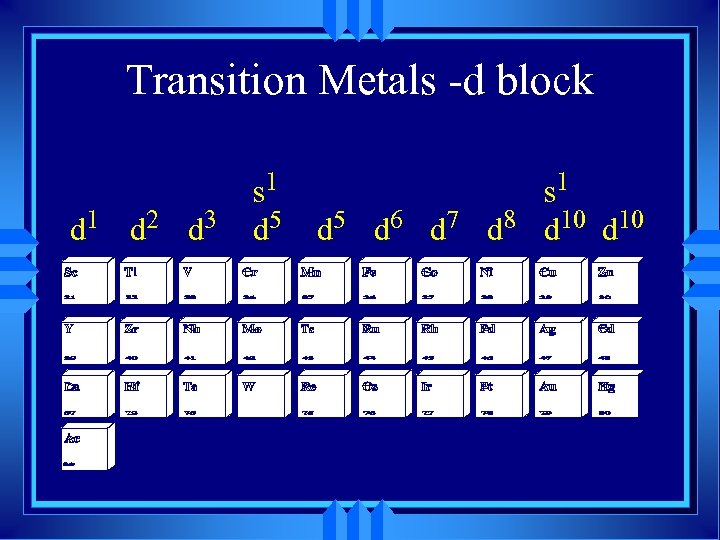
Transition Metals -d block d 1 d 2 d 3 s 1 d 5 d 6 d 7 d 8 d 10
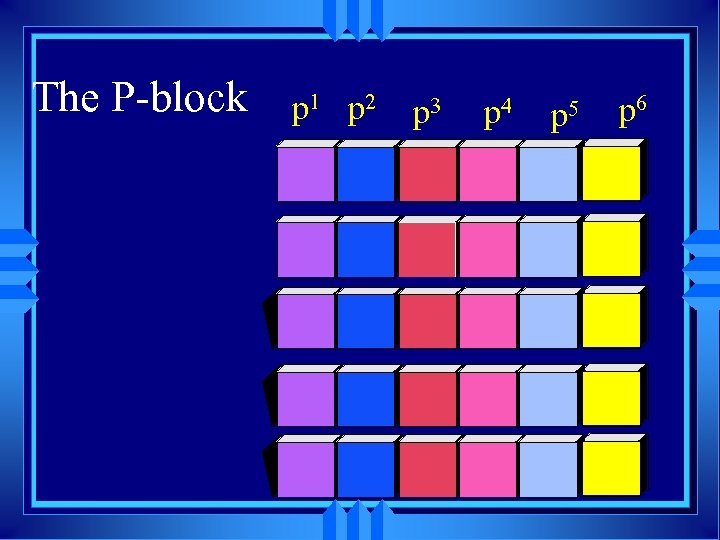
The P-block p 1 p 2 p 3 p 4 p 5 p 6
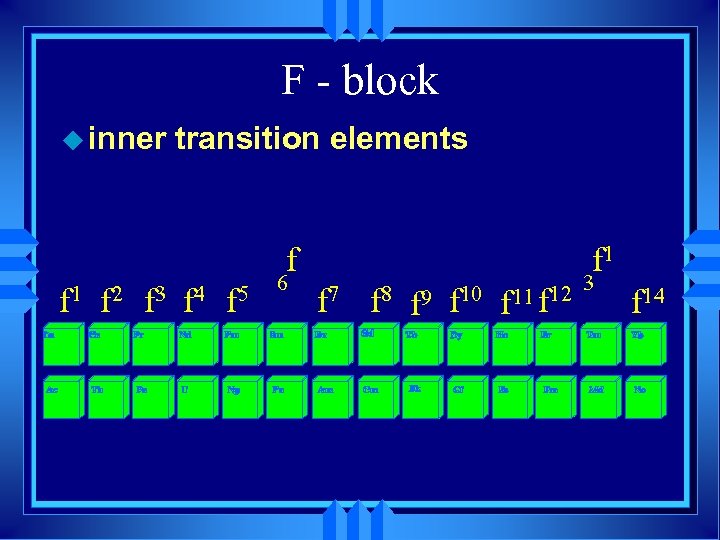
F - block u inner transition elements f f 1 f 2 f 3 f 4 f 5 6 f 1 f 7 f 8 f 9 f 10 f 11 f 12 3 f 14
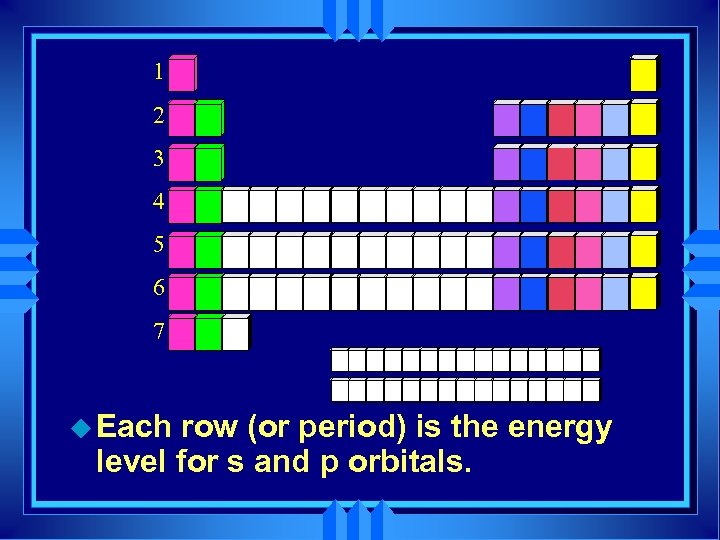
1 2 3 4 5 6 7 u Each row (or period) is the energy level for s and p orbitals.
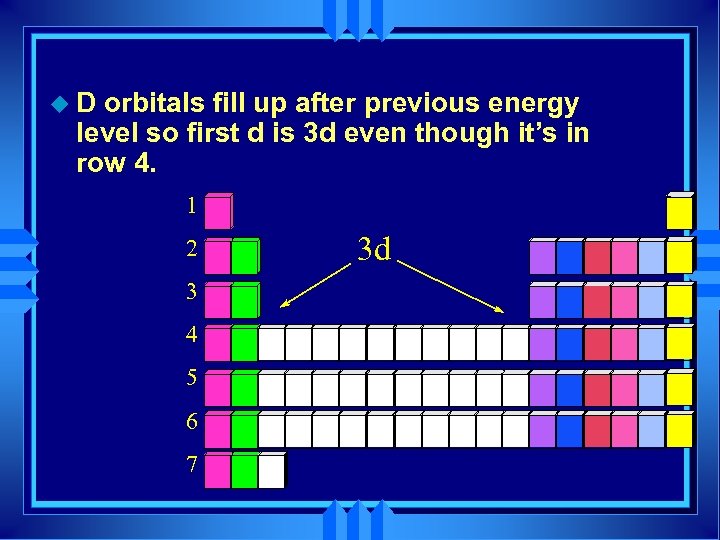
u. D orbitals fill up after previous energy level so first d is 3 d even though it’s in row 4. 1 2 3 4 5 6 7 3 d
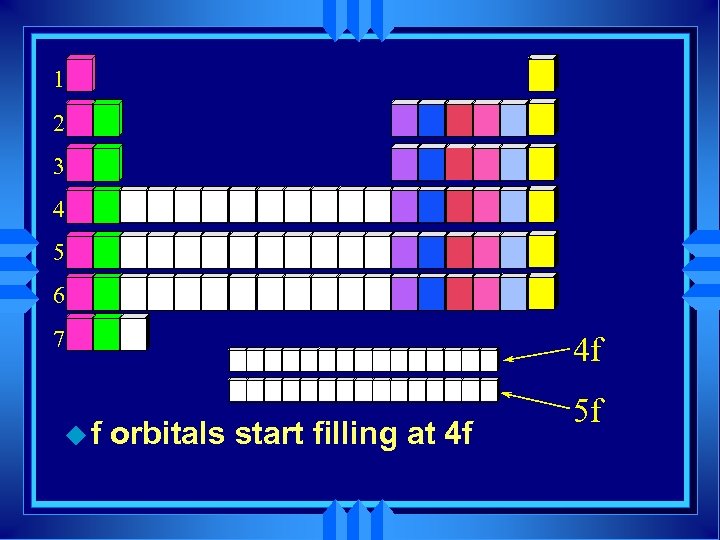
1 2 3 4 5 6 7 uf 4 f orbitals start filling at 4 f 5 f

Writing Electron configurations the easy way The Easy Way and Yes, there is an even easier way, shorthand

Electron Configurations repeat u The shape of the periodic table is a representation of this repetition. u When we get to the end of the column the outermost energy level is full. u This is the basis for our shorthand.
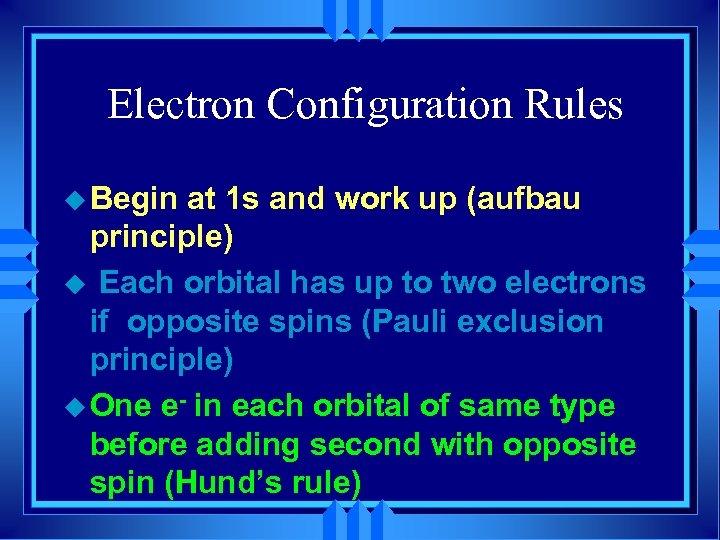
Electron Configuration Rules u Begin at 1 s and work up (aufbau principle) u Each orbital has up to two electrons if opposite spins (Pauli exclusion principle) u One e- in each orbital of same type before adding second with opposite spin (Hund’s rule)
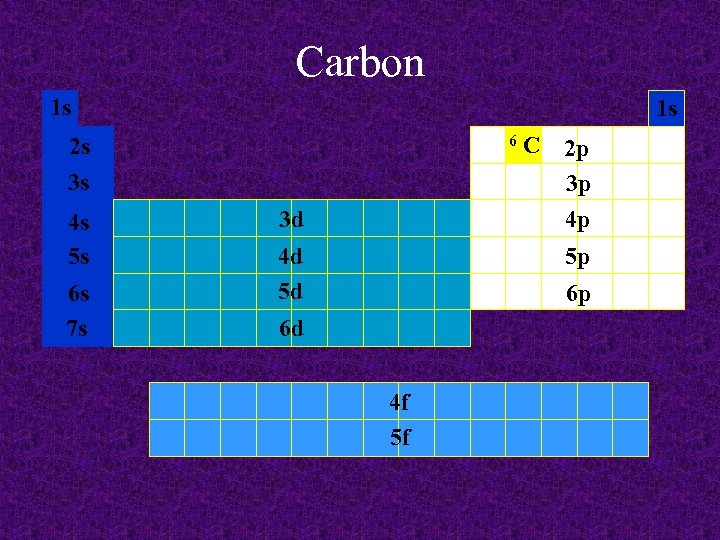
Carbon 1 s 1 s 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 4 d 5 d C 2 p 3 p 4 p 5 p 6 p 6 d 4 f 5 f
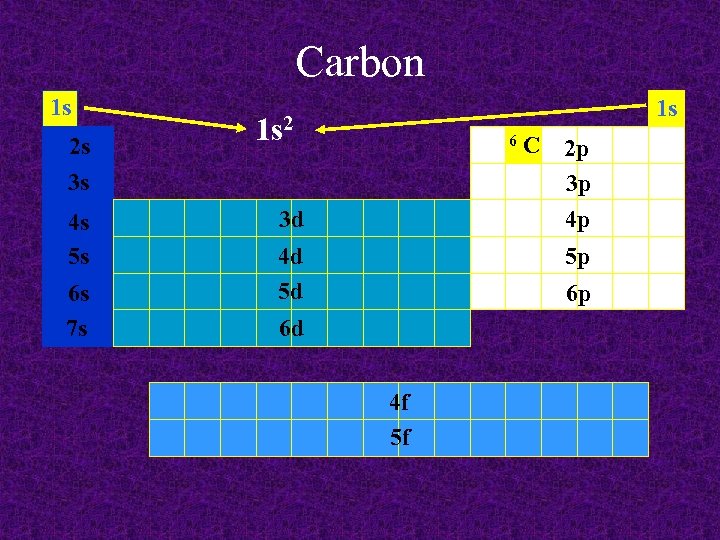
Carbon 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 1 s 2 6 3 d 4 d 5 d C 2 p 3 p 4 p 5 p 6 p 6 d 4 f 5 f
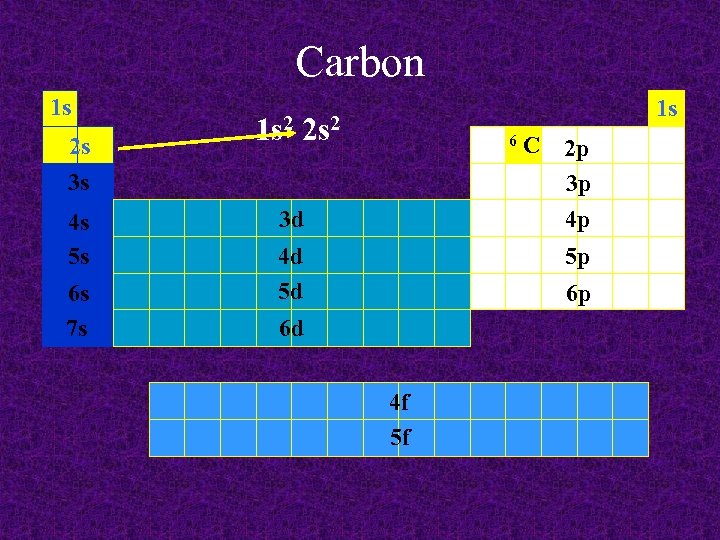
Carbon 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 2 s 2 6 3 d 4 d 5 d C 2 p 3 p 4 p 5 p 6 p 6 d 4 f 5 f
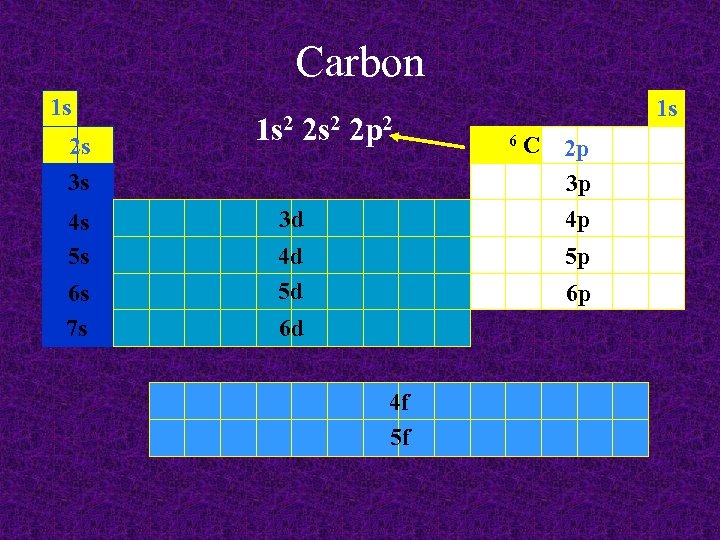
Carbon 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 2 2 p 2 3 d 4 d 5 d 1 s 6 C 2 p 3 p 4 p 5 p 6 p 6 d 4 f 5 f
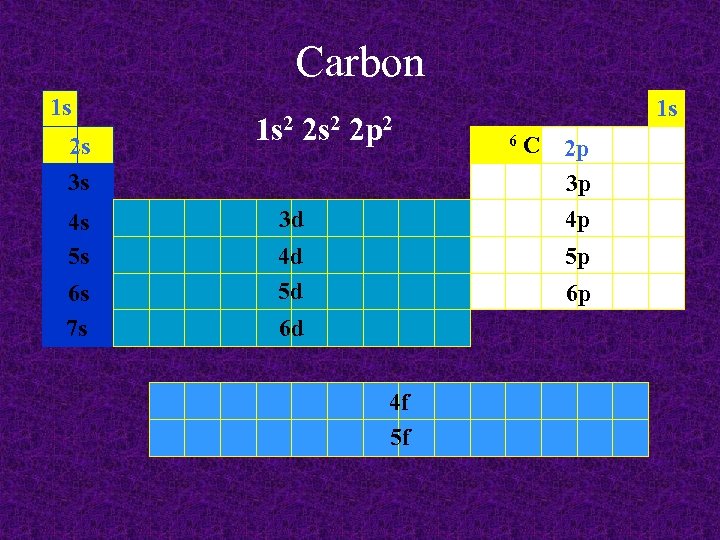
Carbon 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 2 2 p 2 3 d 4 d 5 d 1 s 6 C 2 p 3 p 4 p 5 p 6 p 6 d 4 f 5 f
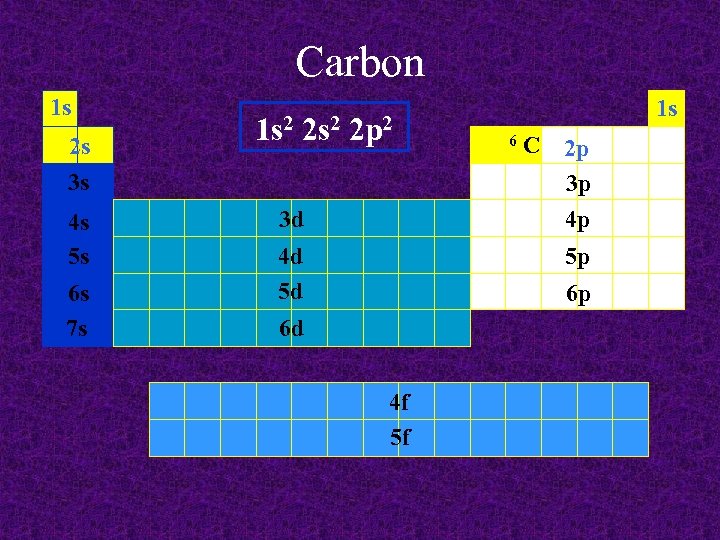
Carbon 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 2 2 p 2 3 d 4 d 5 d 1 s 6 C 2 p 3 p 4 p 5 p 6 p 6 d 4 f 5 f
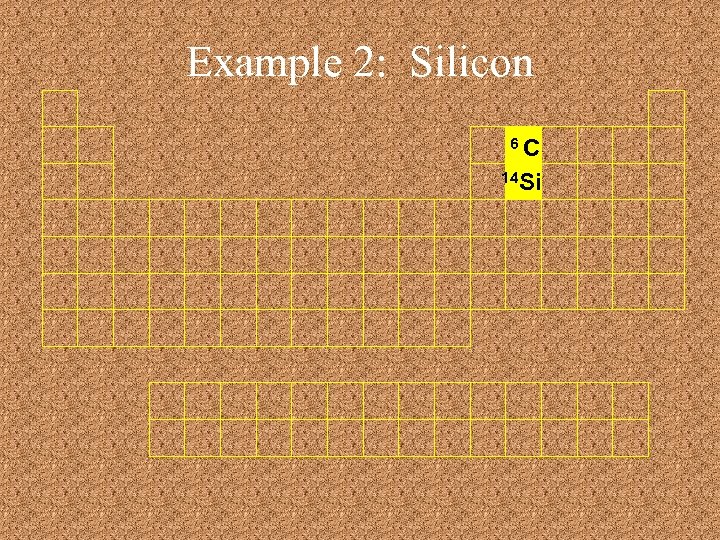
Example 2: Silicon 6 C 14 Si
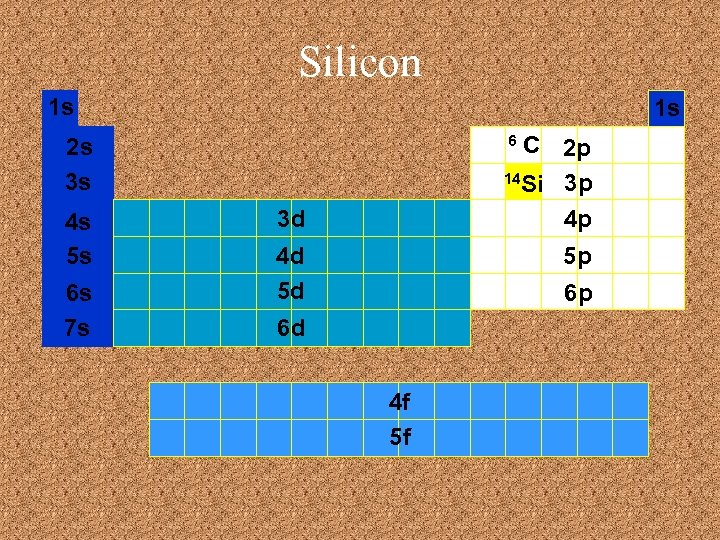
Silicon 1 s 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 4 d 5 d 5 p 6 p 6 d 4 f 5 f
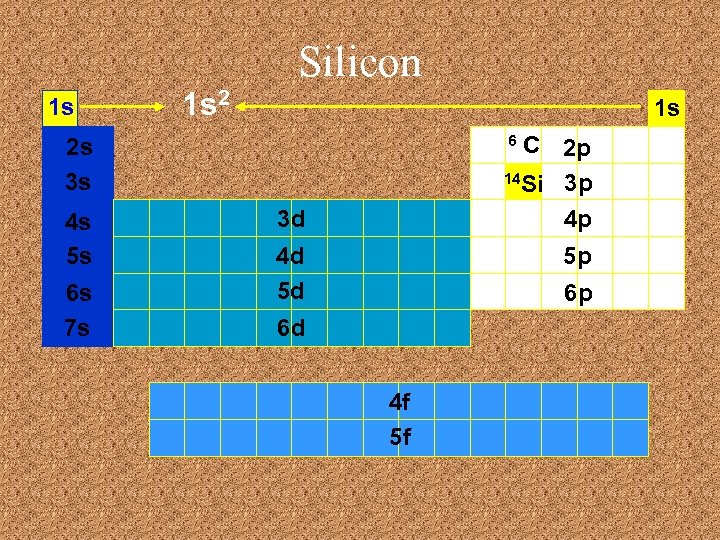
Silicon 1 s 1 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 4 d 5 d 5 p 6 p 6 d 4 f 5 f
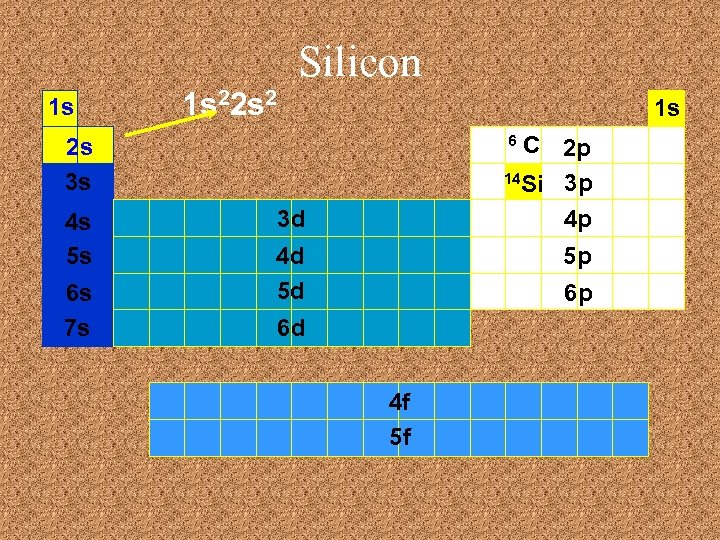
Silicon 1 s 1 s 22 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 4 d 5 d 5 p 6 p 6 d 4 f 5 f
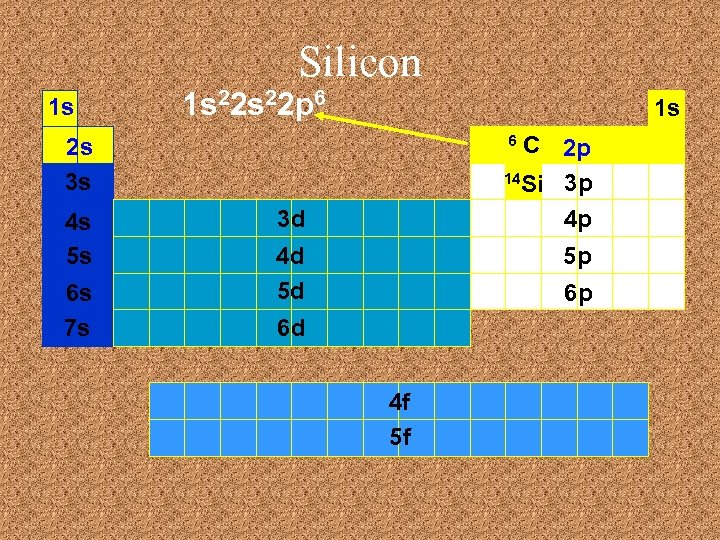
Silicon 1 s 1 s 22 p 6 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 4 d 5 d 5 p 6 p 6 d 4 f 5 f
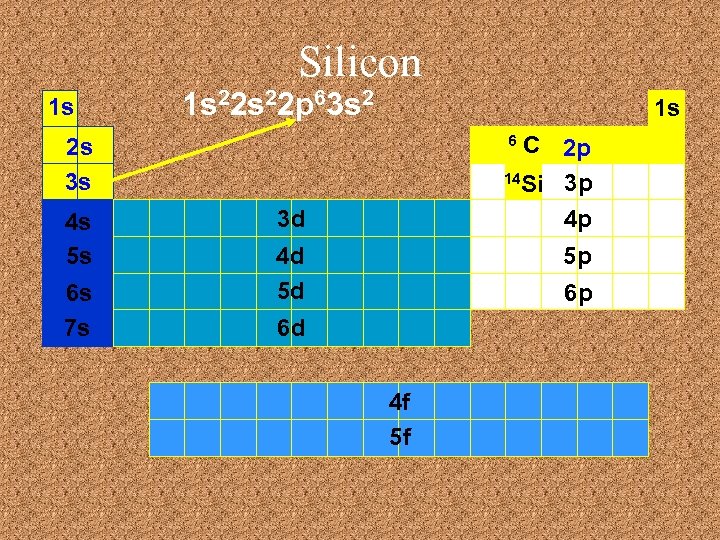
Silicon 1 s 1 s 22 p 63 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 4 d 5 d 5 p 6 p 6 d 4 f 5 f
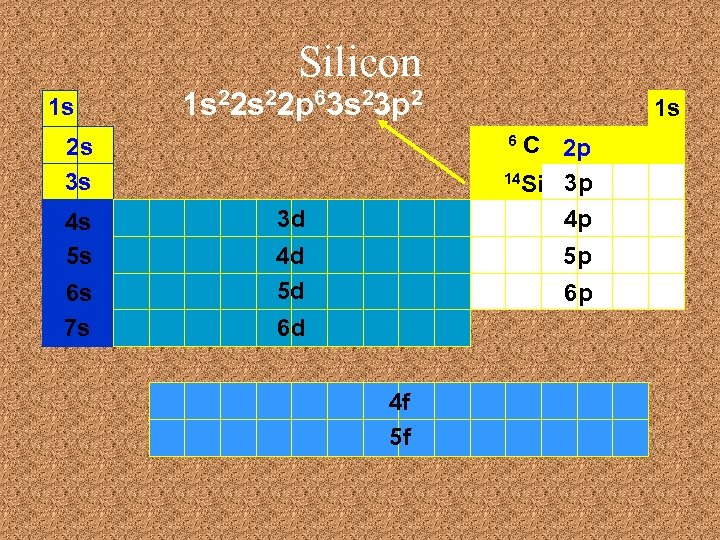
Silicon 1 s 1 s 22 p 63 s 23 p 2 C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 1 s 3 d 4 d 5 d 5 p 6 p 6 d 4 f 5 f
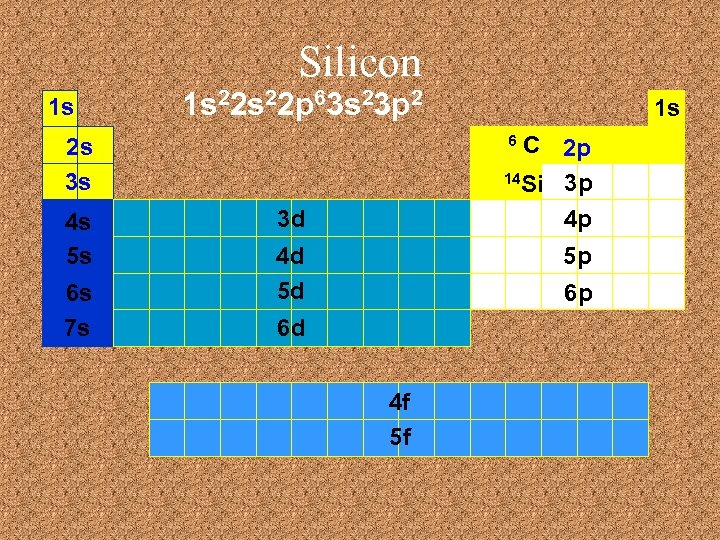
Silicon 1 s 1 s 22 p 63 s 23 p 2 C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 1 s 3 d 4 d 5 d 5 p 6 p 6 d 4 f 5 f
Example 3: Iron 6 C 14 Si 26 Fe
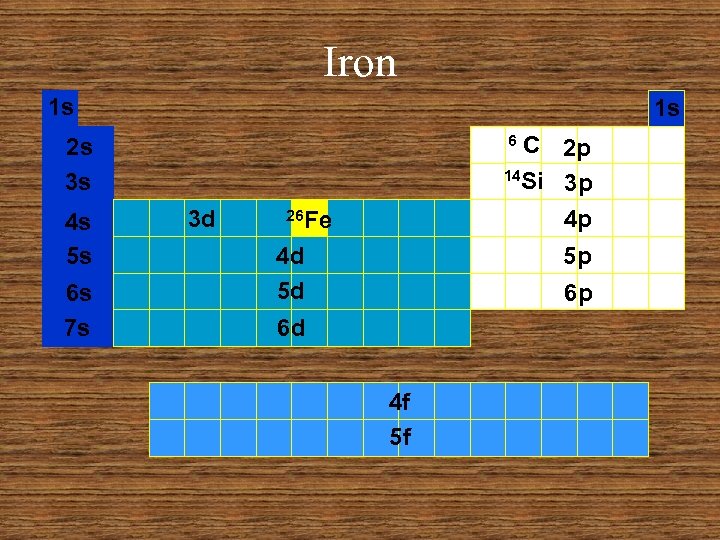
Iron 1 s 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f
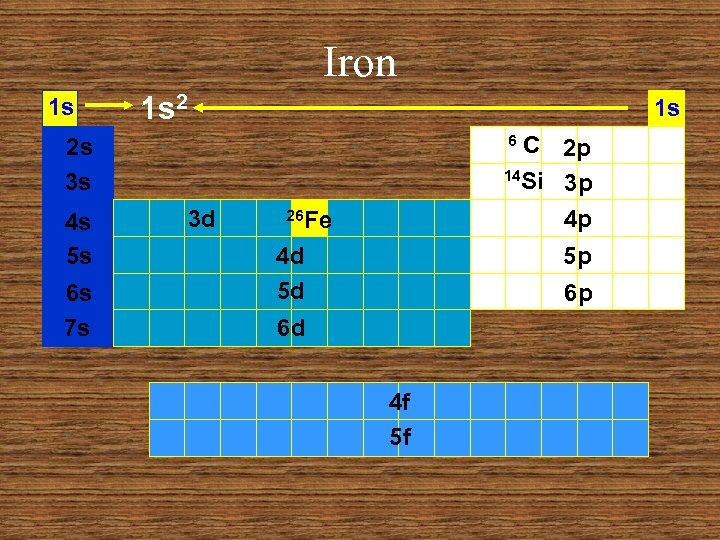
Iron 1 s 1 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f
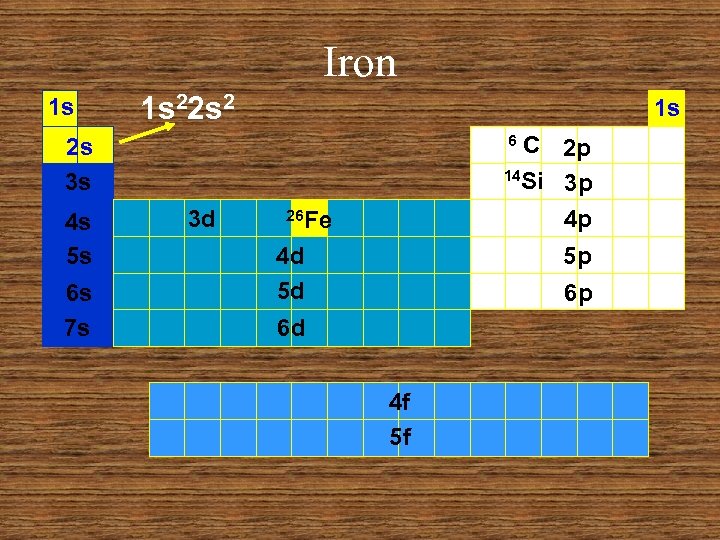
Iron 1 s 1 s 22 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f
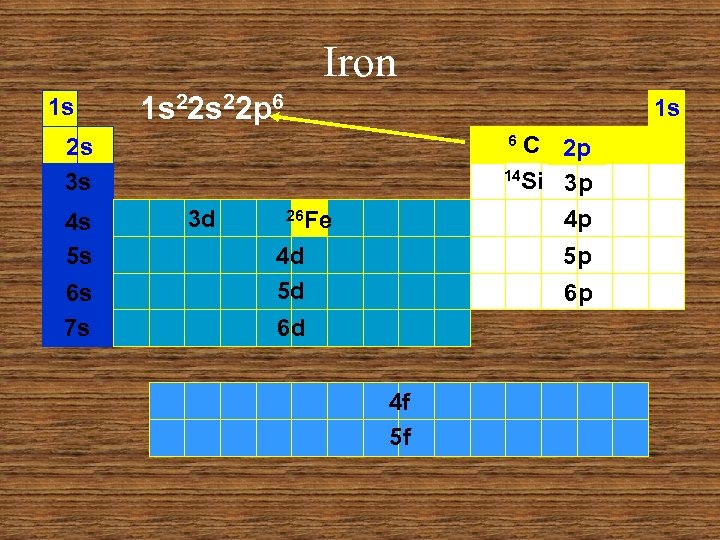
Iron 1 s 1 s 22 p 6 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f
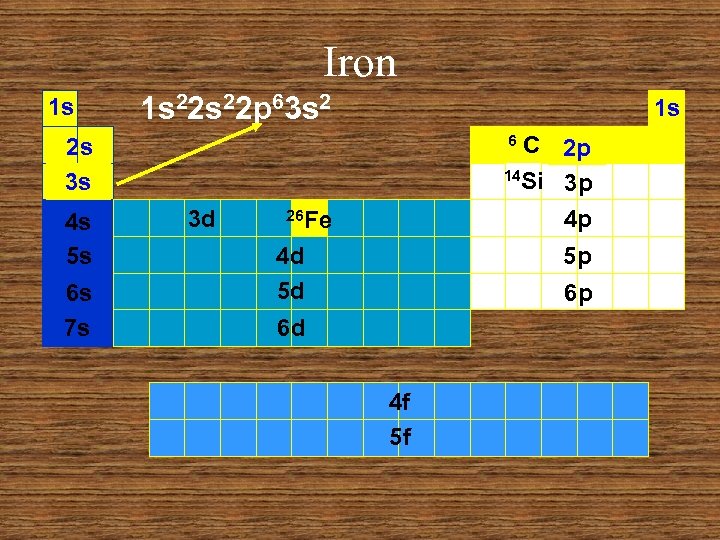
Iron 1 s 1 s 22 p 63 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f
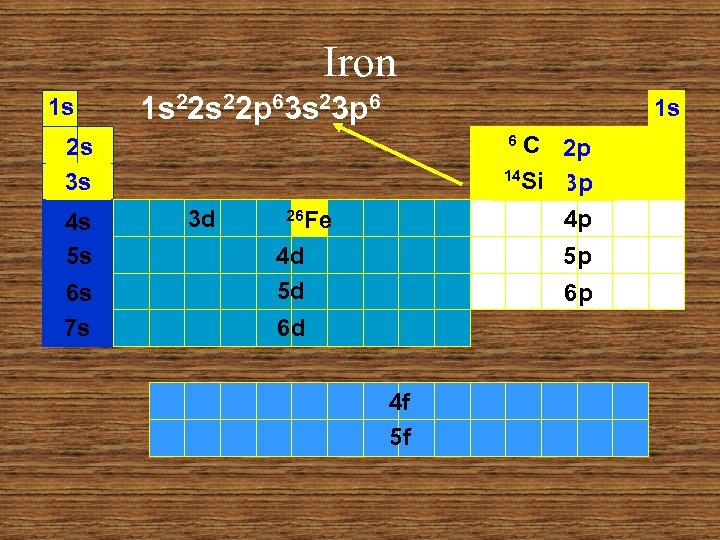
Iron 1 s 1 s 22 p 63 s 23 p 6 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f
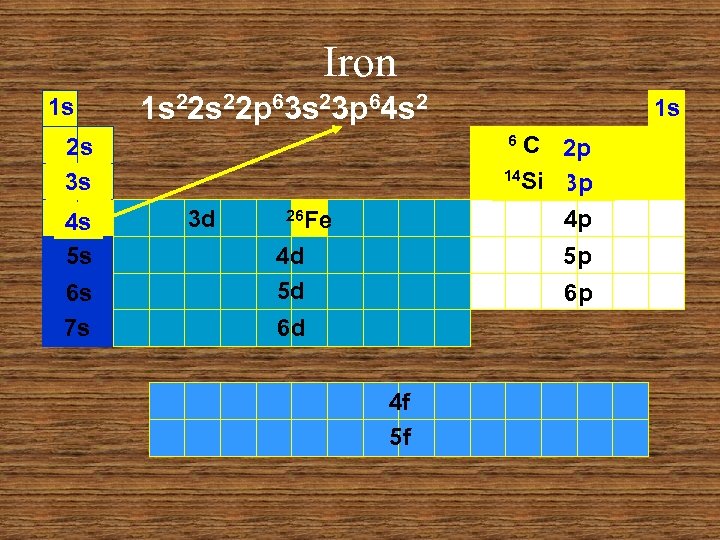
Iron 1 s 1 s 22 p 63 s 23 p 64 s 2 C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 1 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f
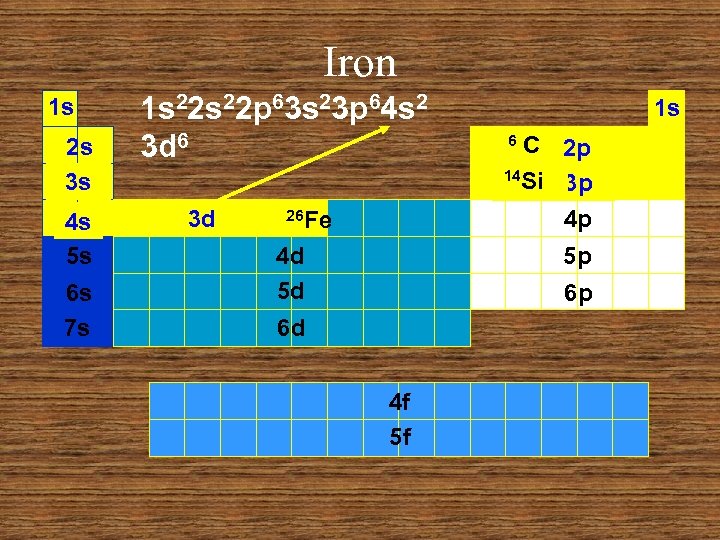
Iron 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 22 p 63 s 23 p 64 s 2 3 d 6 3 d 26 Fe 4 d 5 d 1 s C 2 p 14 Si 3 p 4 p 6 5 p 6 p 6 d 4 f 5 f
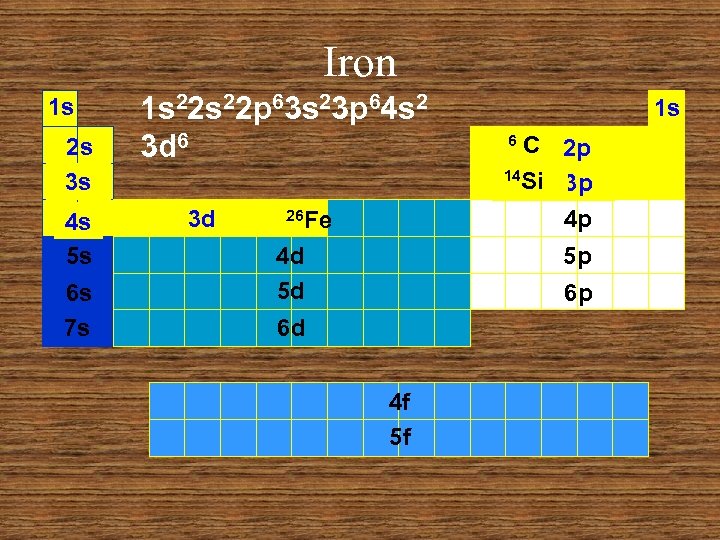
Iron 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 22 p 63 s 23 p 64 s 2 3 d 6 3 d 26 Fe 4 d 5 d 1 s C 2 p 14 Si 3 p 4 p 6 5 p 6 p 6 d 4 f 5 f
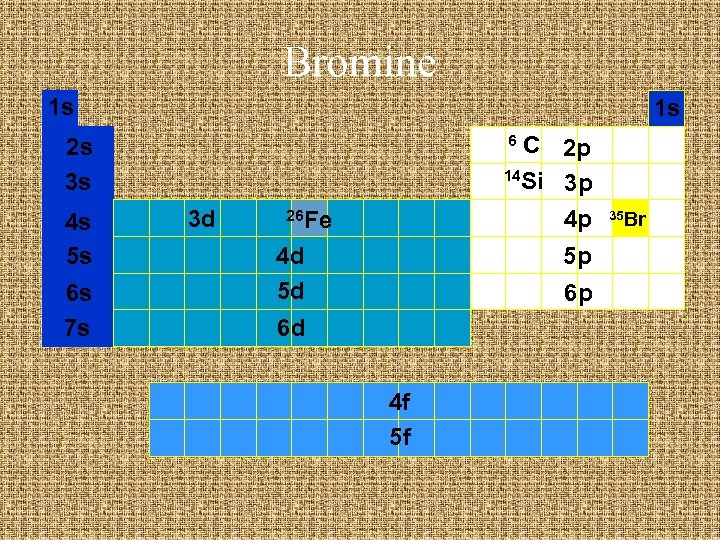
Bromine 1 s 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
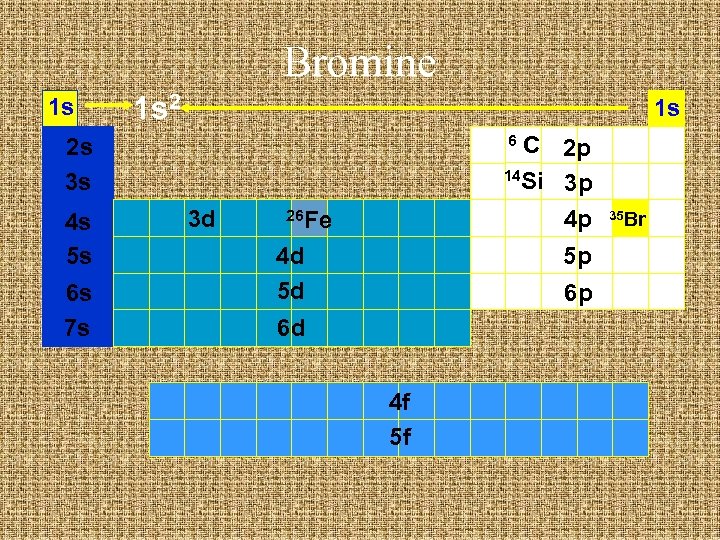
Bromine 1 s 1 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
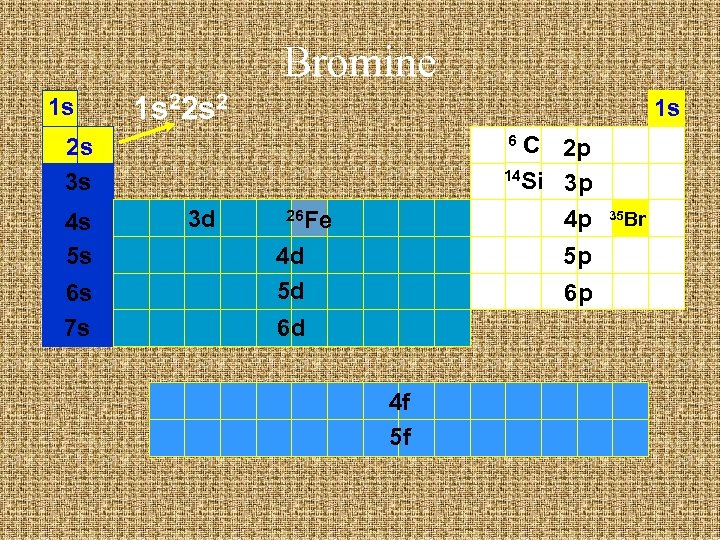
Bromine 1 s 1 s 22 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
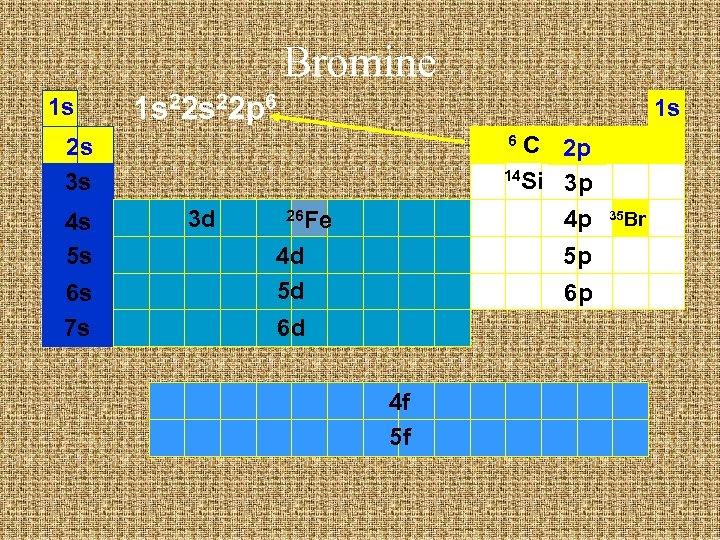
Bromine 1 s 1 s 22 p 6 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
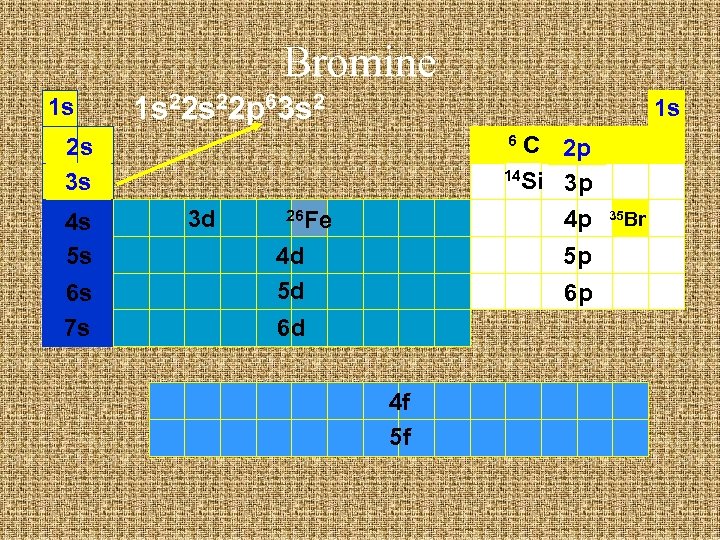
Bromine 1 s 1 s 22 p 63 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
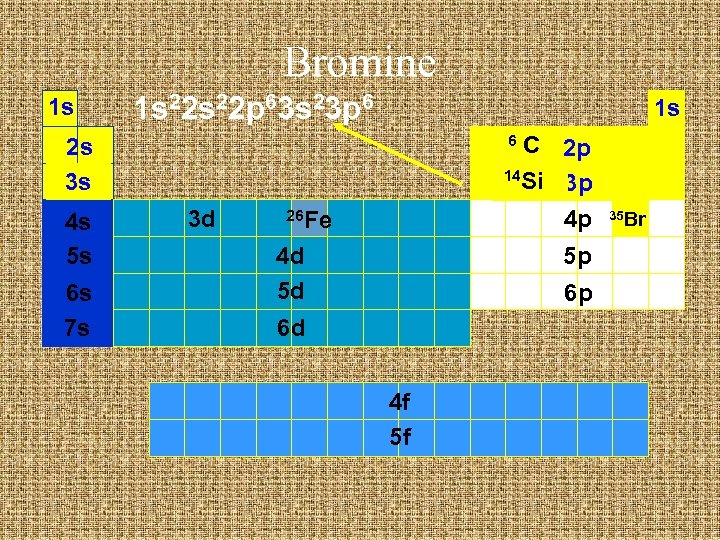
Bromine 1 s 1 s 22 p 63 s 23 p 6 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
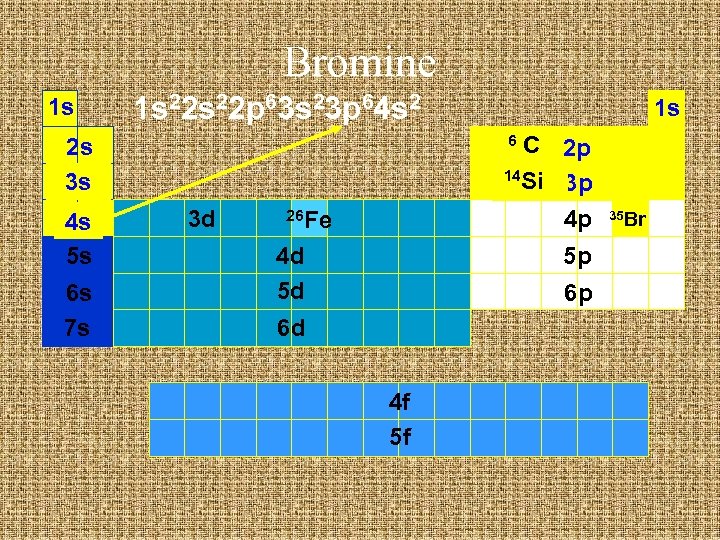
Bromine 1 s 1 s 22 p 63 s 23 p 64 s 2 C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 1 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
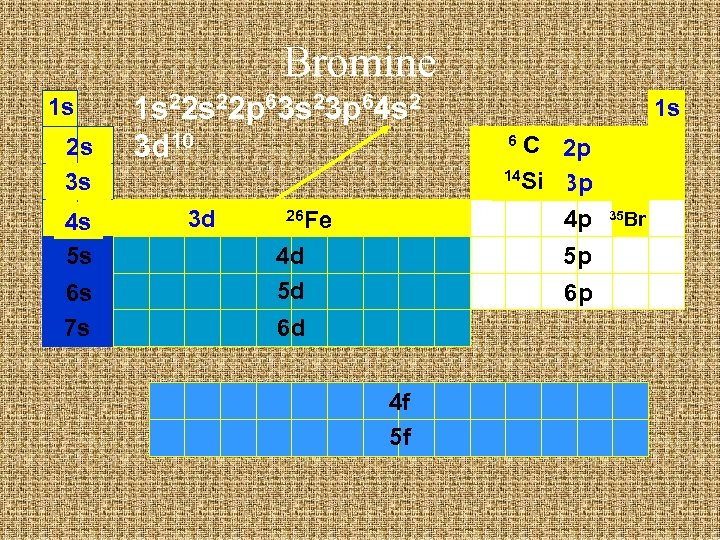
Bromine 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 22 p 63 s 23 p 64 s 2 3 d 10 3 d 26 Fe 4 d 5 d 1 s C 2 p 14 Si 3 p 4 p 6 5 p 6 p 6 d 4 f 5 f 35 Br
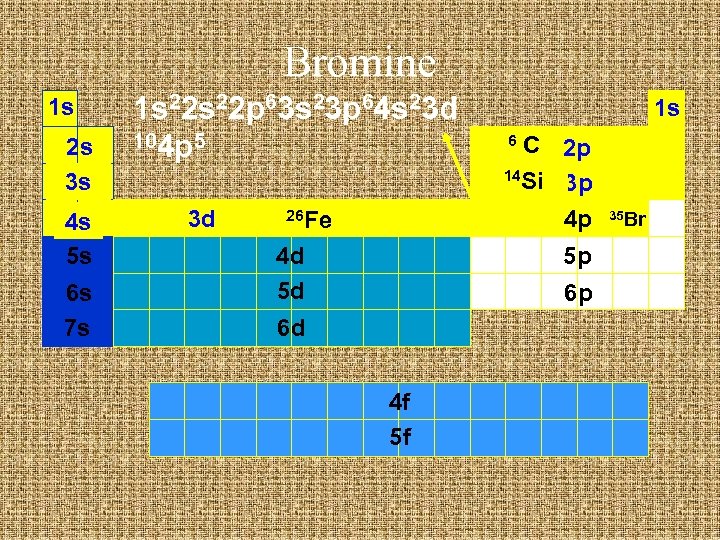
Bromine 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 22 p 63 s 23 p 64 s 23 d 104 p 5 3 d 26 Fe 4 d 5 d 1 s C 2 p 14 Si 3 p 4 p 6 5 p 6 p 6 d 4 f 5 f 35 Br
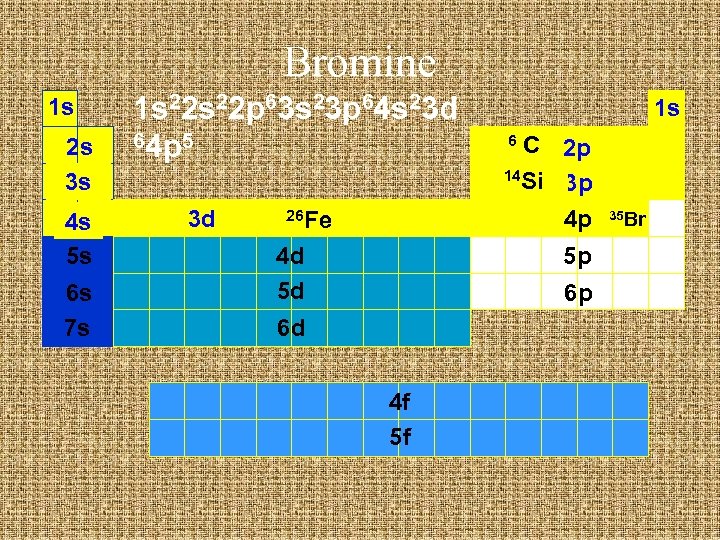
Bromine 1 s 2 s 3 s 4 s 5 s 6 s 7 s 1 s 22 p 63 s 23 p 64 s 23 d 64 p 5 3 d 26 Fe 4 d 5 d 1 s C 2 p 14 Si 3 p 4 p 6 5 p 6 p 6 d 4 f 5 f 35 Br

Electron Configuration Shorthand u Write the symbol of the noble gas before the element. u Then the rest of the electron configuration to the atom. u Aluminum - full configuration. u 1 s 22 p 63 s 23 p 1 u Ne is 1 s 22 p 6 u so Al is [Ne] 3 s 23 p 1
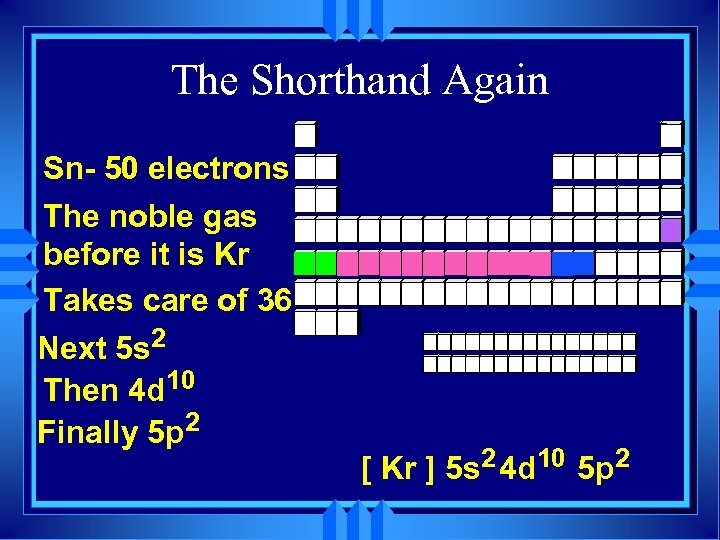
The Shorthand Again Sn- 50 electrons The noble gas before it is Kr Takes care of 36 Next 5 s 2 Then 4 d 10 Finally 5 p 2 [ Kr ] 5 s 2 4 d 10 5 p 2
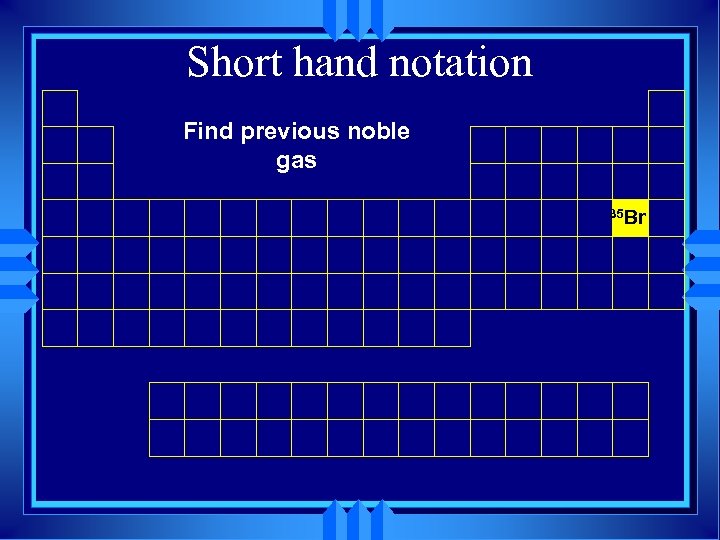
Short hand notation Find previous noble gas 35 Br
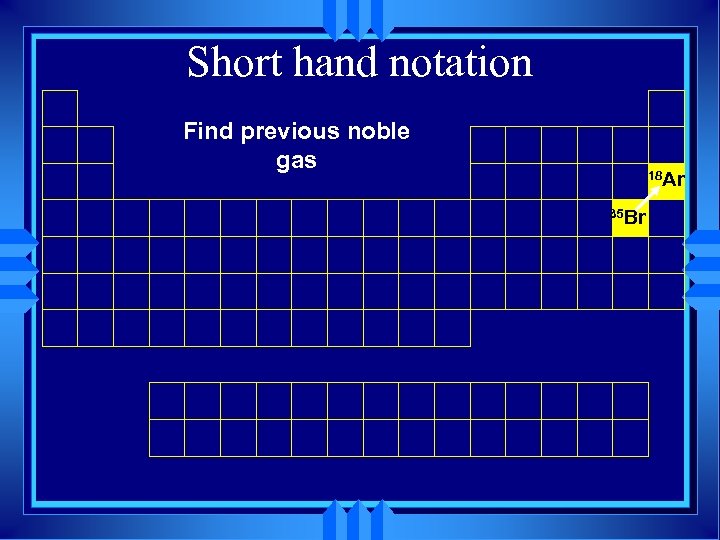
Short hand notation Find previous noble gas 18 Ar 35 Br
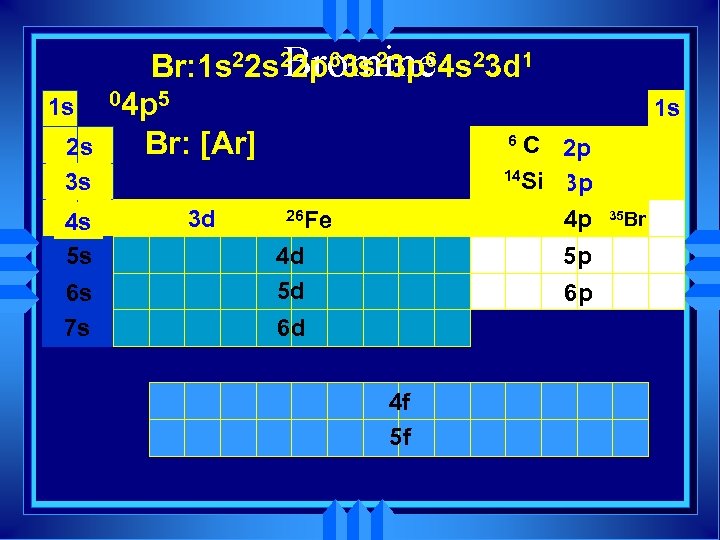
1 s 2 s 3 s 4 s 5 s 6 s 7 s Bromine Br: 1 s 22 p 63 s 23 p 64 s 23 d 1 04 p 5 6 C Br: [Ar] 3 d 1 s 2 p 14 Si 3 p 4 p 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
![Bromine 1 s [Ar] 4 s 2 1 s C 2 p 14 Si Bromine 1 s [Ar] 4 s 2 1 s C 2 p 14 Si](https://present5.com/presentation/0bc1edc5594f2f62e69771be89e9cae6/image-78.jpg)
Bromine 1 s [Ar] 4 s 2 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
![Bromine 1 s [Ar]4 s 23 d 10 1 s C 2 p 14 Bromine 1 s [Ar]4 s 23 d 10 1 s C 2 p 14](https://present5.com/presentation/0bc1edc5594f2f62e69771be89e9cae6/image-79.jpg)
Bromine 1 s [Ar]4 s 23 d 10 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br
![Bromine 1 s [Ar]4 s 23 d 104 p 5 1 s C 2 Bromine 1 s [Ar]4 s 23 d 104 p 5 1 s C 2](https://present5.com/presentation/0bc1edc5594f2f62e69771be89e9cae6/image-80.jpg)
Bromine 1 s [Ar]4 s 23 d 104 p 5 1 s C 2 p 14 Si 3 p 4 p 6 2 s 3 s 4 s 5 s 6 s 7 s 3 d 26 Fe 4 d 5 d 5 p 6 p 6 d 4 f 5 f 35 Br

More shorthand examples = 1 s 22 p 63 s 23 p 64 s 23 d 104 p 2 u Ge = [Ar] 4 s 23 d 104 p 2 u Hf=1 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 2 4 d 105 p 66 s 24 f 145 d 2 u Hf=[Xe]6 s 24 f 145 d 2 u Ge

Electron Energy Levels

Electron Energy Levels 1 s __

Electron Energy Levels 2 s __ 1 s __
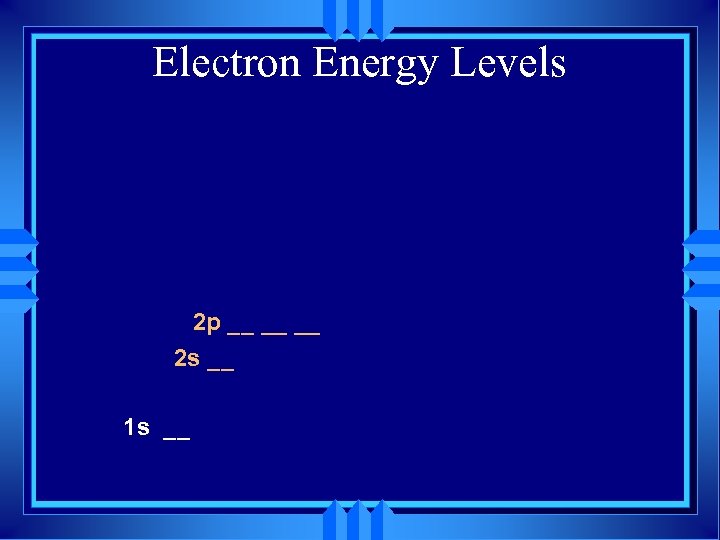
Electron Energy Levels 2 p __ __ __ 2 s __ 1 s __
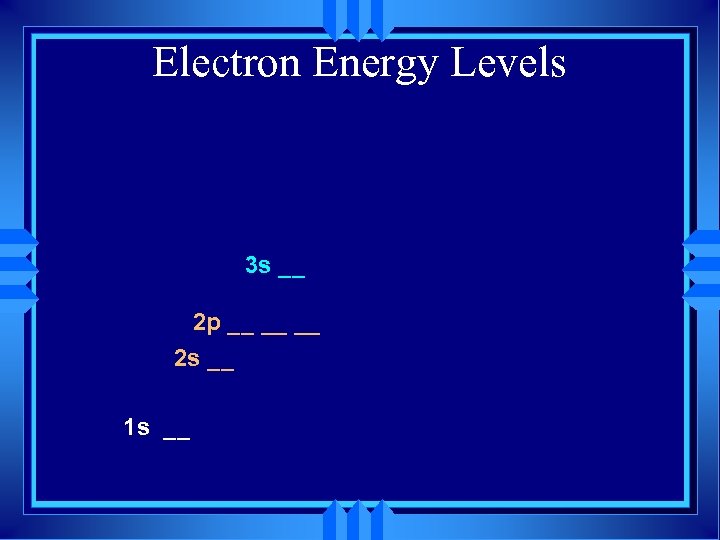
Electron Energy Levels 3 s __ 2 p __ __ __ 2 s __ 1 s __
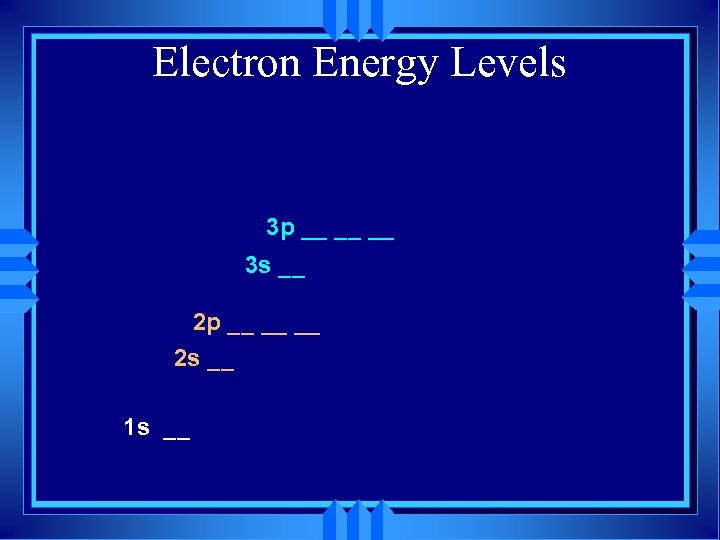
Electron Energy Levels 3 p __ __ __ 3 s __ 2 p __ __ __ 2 s __ 1 s __
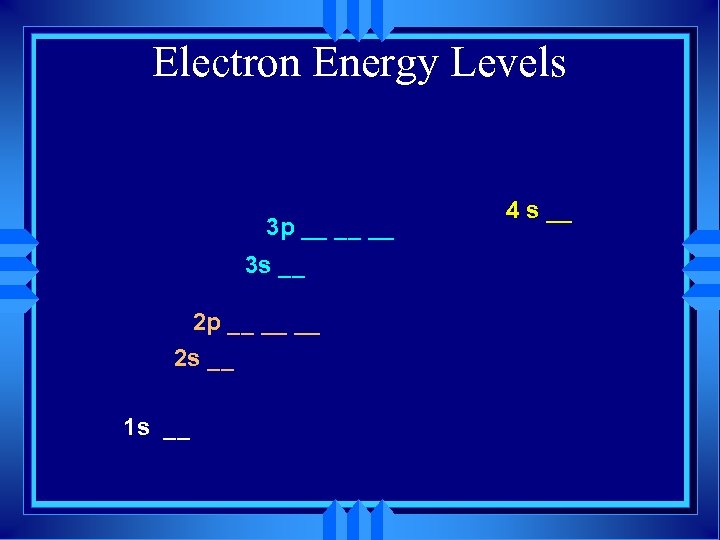
Electron Energy Levels 3 p __ __ __ 3 s __ 2 p __ __ __ 2 s __ 1 s __ 4 s __
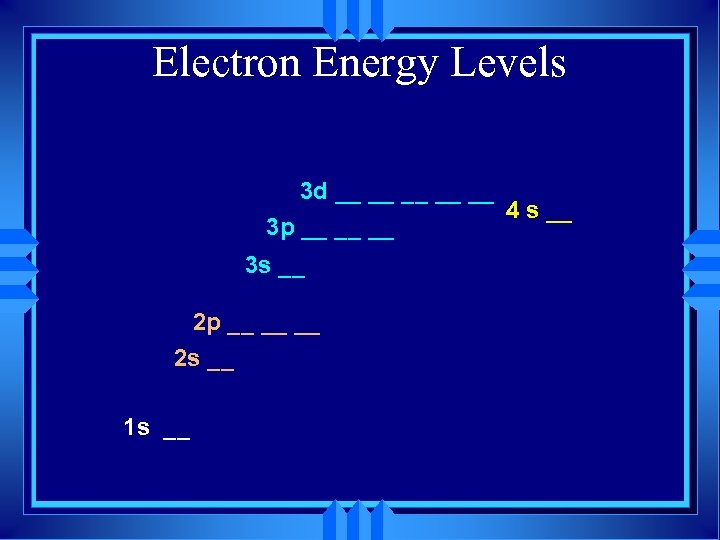
Electron Energy Levels 3 d __ __ __ 4 s __ 3 p __ __ __ 3 s __ 2 p __ __ __ 2 s __ 1 s __
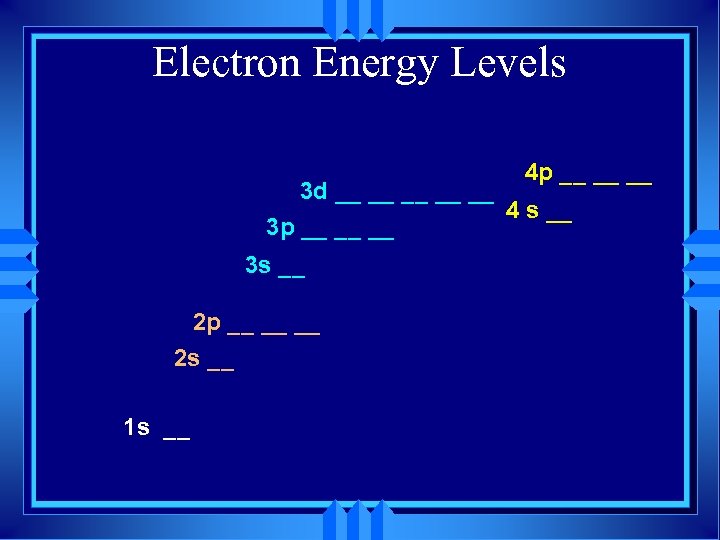
Electron Energy Levels 4 p __ __ __ 3 d __ __ __ 4 s __ 3 p __ __ __ 3 s __ 2 p __ __ __ 2 s __ 1 s __
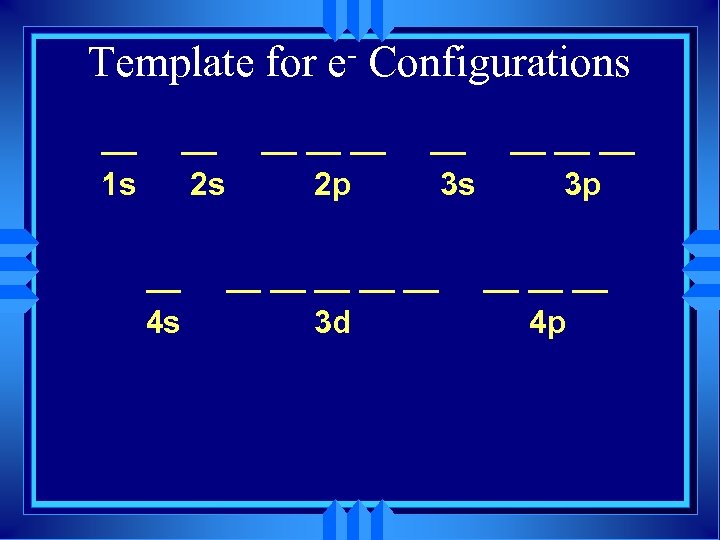
Template for __ 1 s __ 2 s __ 4 s e Configurations __ __ __ 2 p __ 3 s __ __ __ 3 d __ __ __ 3 p __ __ __ 4 p
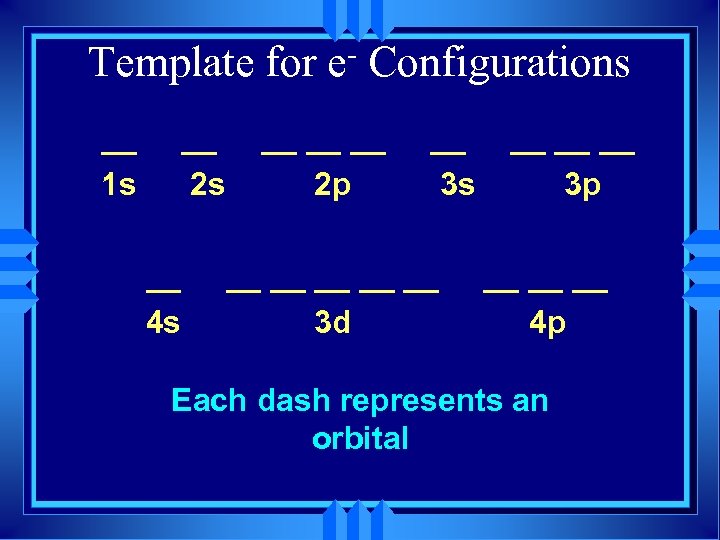
Template for __ 1 s __ 2 s __ 4 s e Configurations __ __ __ 2 p __ 3 s __ __ __ 3 d __ __ __ 3 p __ __ __ 4 p Each dash represents an orbital
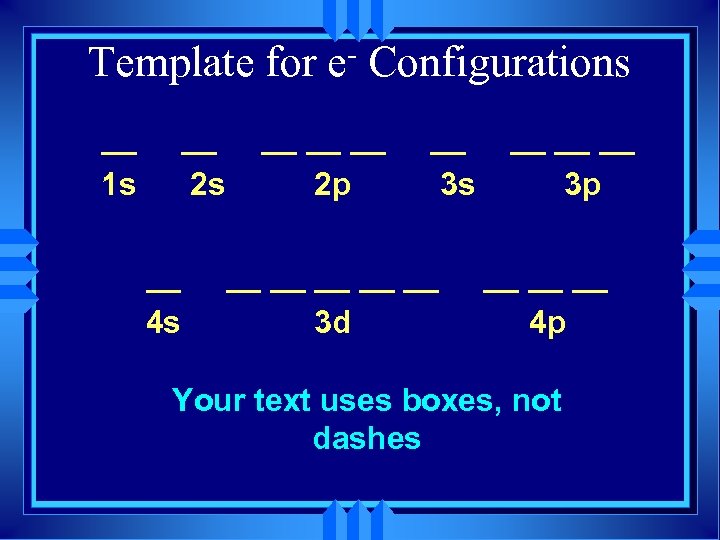
Template for __ 1 s __ 2 s __ 4 s e Configurations __ __ __ 2 p __ 3 s __ __ __ 3 d __ __ __ 3 p __ __ __ 4 p Your text uses boxes, not dashes
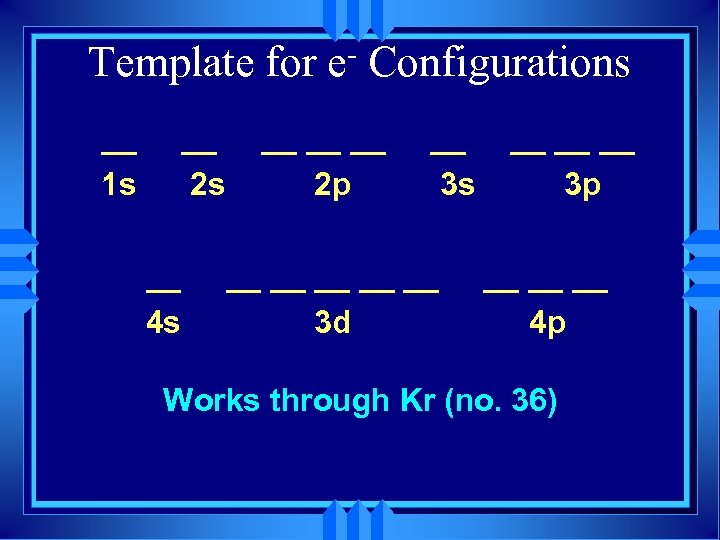
Template for __ 1 s __ 2 s __ 4 s e Configurations __ __ __ 2 p __ 3 s __ __ __ 3 d __ __ __ 3 p __ __ __ 4 p Works through Kr (no. 36)

Carbon Example __ 1 s __ 2 s __ 4 s __ __ __ 2 p __ 3 s __ __ __ 3 d __ __ __ 3 p __ __ __ 4 p

Carbon Example __ 1 s __ 2 s __ 4 s __ __ __ 2 p __ 3 s __ __ __ 3 d 6 electrons needed __ __ __ 3 p __ __ __ 4 p

Carbon Example __ 1 s __ 2 s __ 4 s __ __ __ 2 p __ 3 s __ __ __ 3 d 6 electrons needed 1 electron added __ __ __ 3 p __ __ __ 4 p 1 s 1 H

Carbon Example __ 1 s __ 2 s __ 4 s __ __ __ 2 p __ 3 s __ __ __ 3 d 6 electrons needed 2 electrons added __ __ __ 3 p __ __ __ 4 p 1 s 2 He
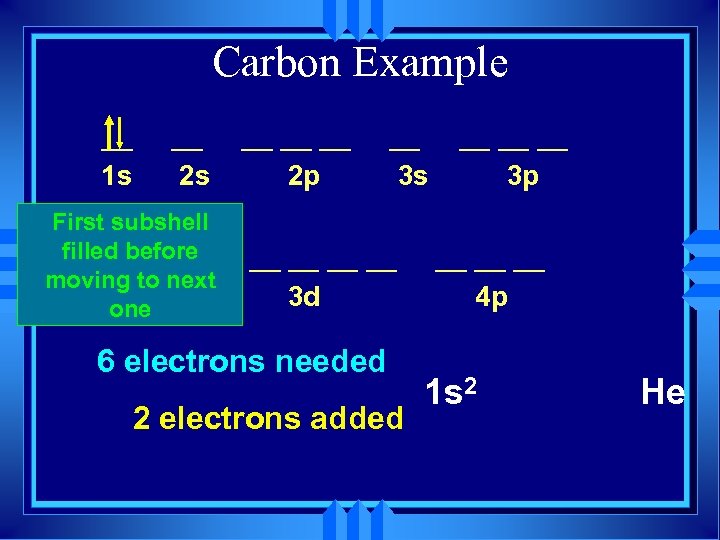
Carbon Example __ 1 s __ 2 s __ __ __ 2 p __ 3 s First subshell filled before __ __ __ moving to next 4 s 3 d one 6 electrons needed 2 electrons added __ __ __ 3 p __ __ __ 4 p 1 s 2 He

Carbon Example __ 1 s __ 2 s __ 4 s __ __ __ 2 p __ 3 s __ __ __ 3 d 6 electrons needed 3 electrons added __ __ __ 3 p __ __ __ 4 p 1 s 22 s 1 Li

Carbon Example __ 1 s __ 2 s __ 4 s __ __ __ 2 p __ 3 s __ __ __ 3 d 6 electrons needed 4 electrons added __ __ __ 3 p __ __ __ 4 p 1 s 22 s 2 Be

Carbon Example __ 1 s __ 2 s __ 4 s __ __ __ 2 p __ 3 s __ __ __ 3 d 6 electrons needed __ __ __ 3 p __ __ __ 4 p 1 s 22 p 1 5 electrons added B

Carbon Example __ 1 s __ 2 s __ 4 s __ __ __ 2 p __ 3 s __ __ __ 3 d 6 electrons needed __ __ __ 3 p __ __ __ 4 p 1 s 22 p 2 6 electrons added C

Carbon Example __ 1 s __ 2 s __ __ __ 2 p __ 3 s One e- in each orbital of __ __ __ a __ subshell before 4 s any of opposite 3 d spins added 6 electrons needed __ __ __ 3 p __ __ __ 4 p 1 s 22 p 2 6 electrons added C

Lewis Dot Structure Valence Electrons Only that can bond one atom with another.
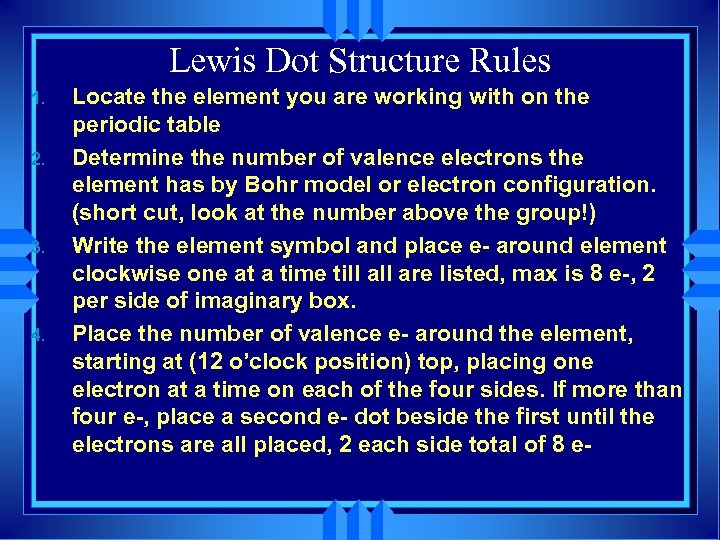
Lewis Dot Structure Rules 1. 2. 3. 4. Locate the element you are working with on the periodic table Determine the number of valence electrons the element has by Bohr model or electron configuration. (short cut, look at the number above the group!) Write the element symbol and place e- around element clockwise one at a time till are listed, max is 8 e-, 2 per side of imaginary box. Place the number of valence e- around the element, starting at (12 o’clock position) top, placing one electron at a time on each of the four sides. If more than four e-, place a second e- dot beside the first until the electrons are all placed, 2 each side total of 8 e-
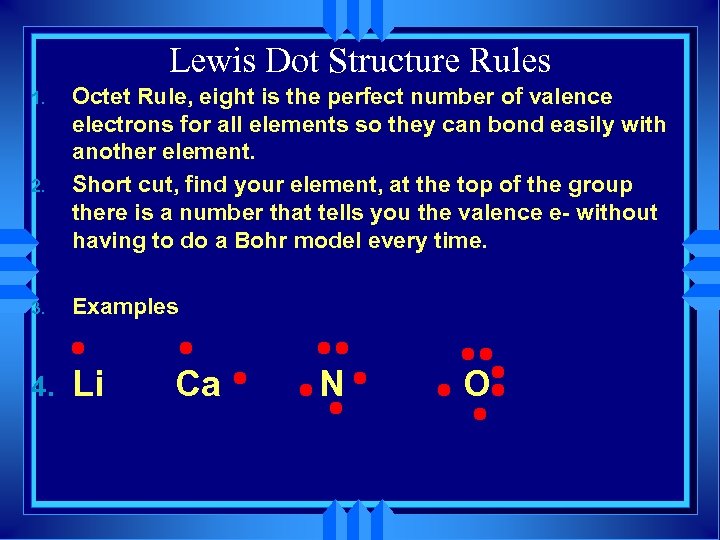
Lewis Dot Structure Rules 1. 2. Octet Rule, eight is the perfect number of valence electrons for all elements so they can bond easily with another element. Short cut, find your element, at the top of the group there is a number that tells you the valence e- without having to do a Bohr model every time. 3. Examples 4. Li Ca N O
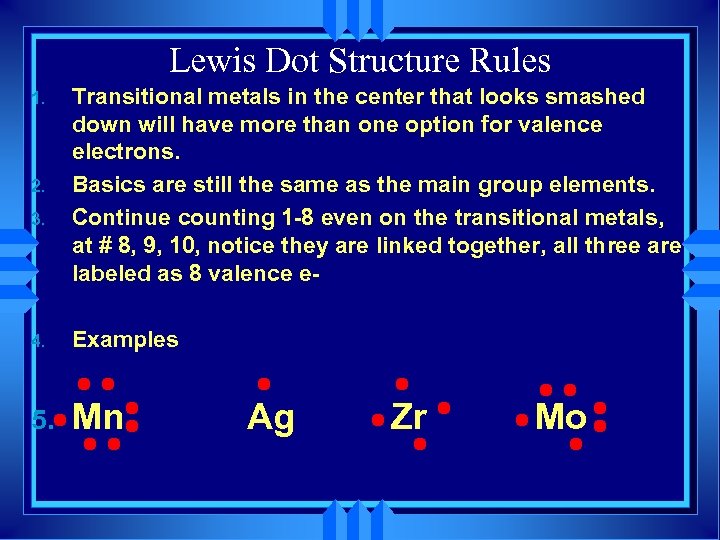
Lewis Dot Structure Rules 1. 2. 3. Transitional metals in the center that looks smashed down will have more than one option for valence electrons. Basics are still the same as the main group elements. Continue counting 1 -8 even on the transitional metals, at # 8, 9, 10, notice they are linked together, all three are labeled as 8 valence e- 4. Examples 5. Mn Ag Zr Mo

Atomic Size u First problem where do you start measuring. u The electron cloud doesn’t have a definite edge. u They get around this by measuring more than 1 atom at a time.

Atomic Size } Radius u. Atomic Radius = half the distance between two nuclei of a diatomic molecule.

Trends in Atomic Size u. Influenced by two factors. u. Energy Level u. Higher energy level is further away. u. Charge on nucleus u. More charge pulls electrons in closer.
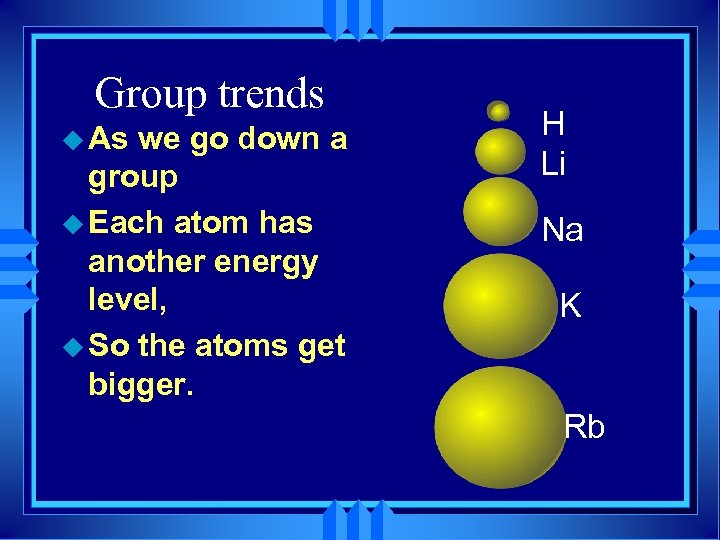
Group trends u As we go down a group u Each atom has another energy level, u So the atoms get bigger. H Li Na K Rb
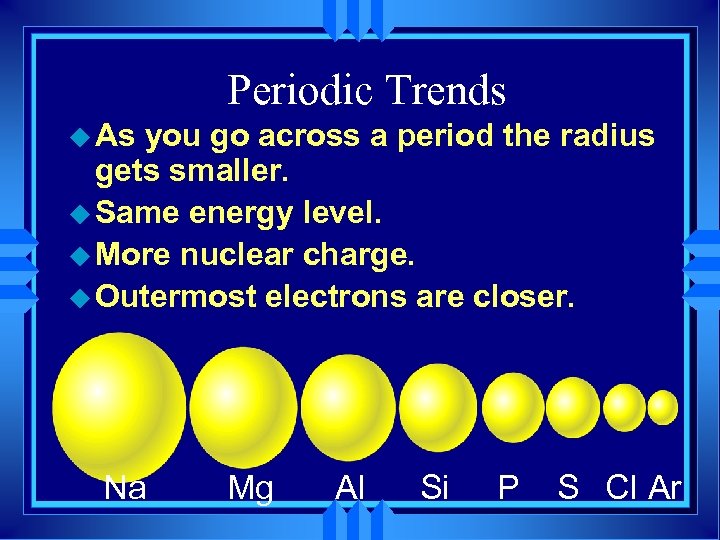
Periodic Trends u As you go across a period the radius gets smaller. u Same energy level. u More nuclear charge. u Outermost electrons are closer. Na Mg Al Si P S Cl Ar
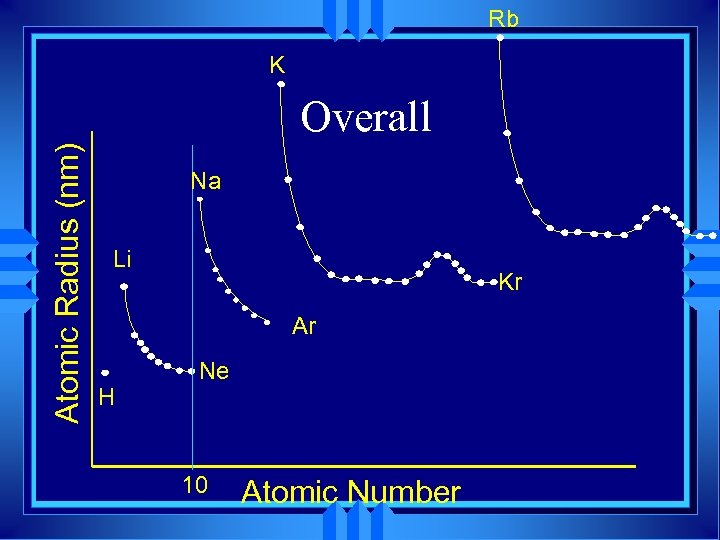
Rb K Atomic Radius (nm) Overall Na Li Kr Ar H Ne 10 Atomic Number

Ionization Energy u The amount of energy required to completely remove an electron from a gaseous atom. u Removing one electron makes a +1 ion. u The energy required is called the first ionization energy.

Ionization Energy u The second ionization energy is the energy required to remove the second electron. u Always greater than first IE. u The third IE is the energy required to remove a third electron. u Greater than 1 st of 2 nd IE.
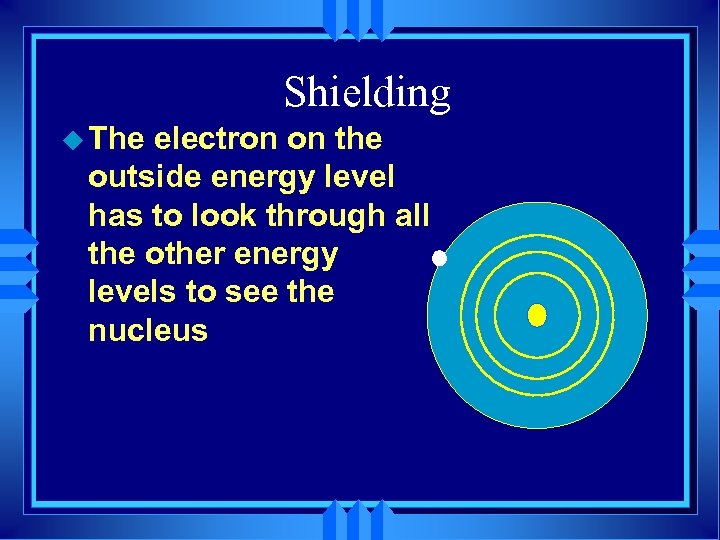
Shielding u The electron on the outside energy level has to look through all the other energy levels to see the nucleus
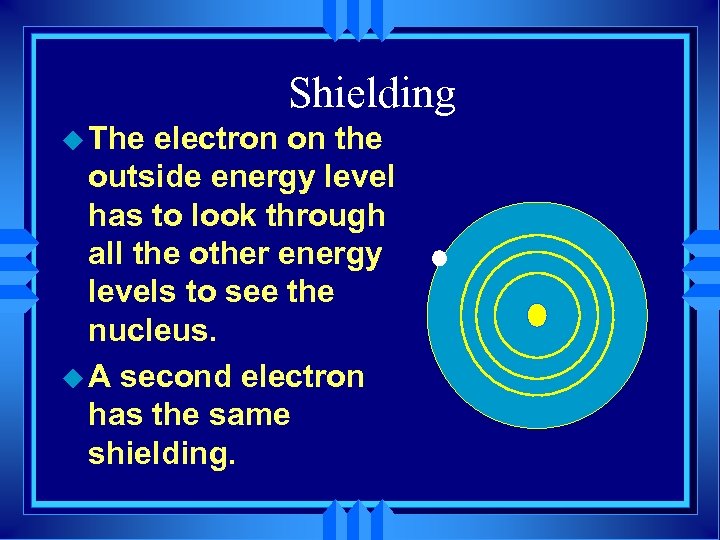
Shielding u The electron on the outside energy level has to look through all the other energy levels to see the nucleus. u A second electron has the same shielding.

Group trends u. As you go down a group first IE decreases because u. The electron is further away. u. More shielding.

Periodic trends u All the atoms in the same period have the same energy level. u Same shielding. u Increasing nuclear charge u So IE generally increases from left to right. u Exceptions at full and 1/2 fill orbitals.

Driving Force u Full Energy Levels are very low energy. u Noble Gases have full orbitals. u Atoms behave in ways to achieve noble gas configuration.

3 rd IE the same logic s 2 p 1 atoms have an low 3 rd IE. u Atoms in the aluminum family form + 3 ions. u 2 nd IE and 3 rd IE are always higher than 1 st IE!!! u Using

Electron Affinity u The energy change assciated with adding an electron to a gaseous atom. u Easiest to add to group 7 A. u Gets them to full energy level. u Increase from left to right atoms become smaller, with greater nuclear charge. u Decrease as we go down a group.

Ionic Size u Cations form by losing electrons. u Cations are smaller that the atom they come from. u Metals form cations. u Cations of representative elements have noble gas configuration.

Ionic size u Anions form by gaining electrons. u Anions are bigger that the atom they come from. u Nonmetals form anions. u Anions of representative elements have noble gas configuration.

Configuration of Ions u Ions always have noble gas configuration. u Na is 1 s 12 s 22 p 63 s 1 u Forms a +1 ion - 1 s 12 s 22 p 6 u Same configuration as neon. u Metals form ions with the configuration of the noble gas before them - they lose electrons.

Configuration of Ions u Non-metals form ions by gaining electrons to achieve noble gas configuration. u They end up with the configuration of the noble gas after them.
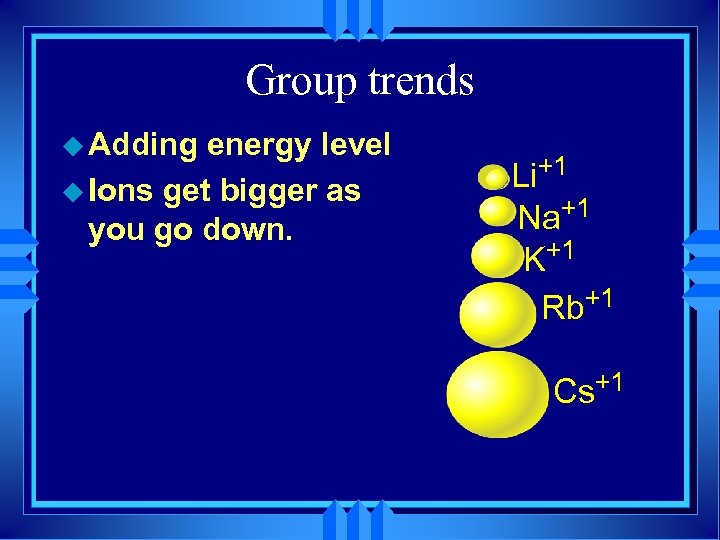
Group trends u Adding energy level u Ions get bigger as you go down. Li+1 Na+1 K+1 Rb+1 Cs+1
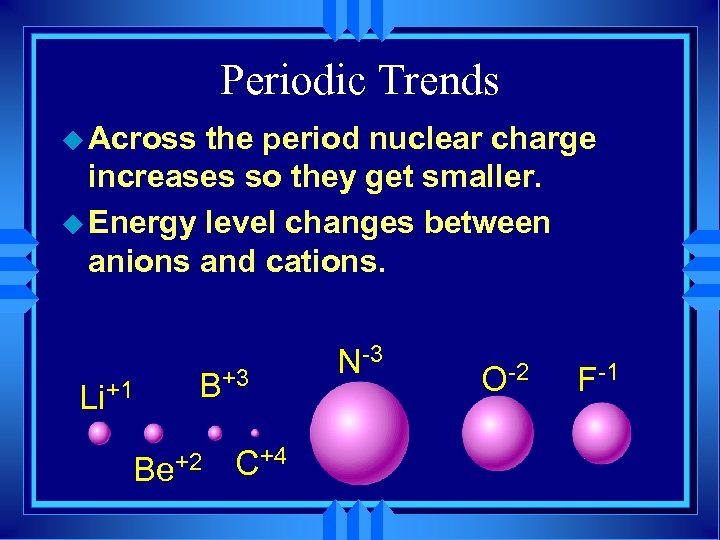
Periodic Trends u Across the period nuclear charge increases so they get smaller. u Energy level changes between anions and cations. Li+1 B+3 Be+2 C+4 N-3 O-2 F-1

Electronegativity

Electronegativity u The tendency for an atom to attract electrons to itself when it is chemically combined with another element. u How fair it shares. u Big electronegativity means it pulls the electron toward it. u Atoms with large negative electron affinity have larger electronegativity.

Group Trend u The further down a group the farther the electron is away and the more electrons an atom has. u More willing to share. u Low electronegativity.

Periodic Trend u Metals are at the left end. u They let their electrons go easily u Low electronegativity u At the right end are the nonmetals. u They want more electrons. u Try to take them away. u High electronegativity.
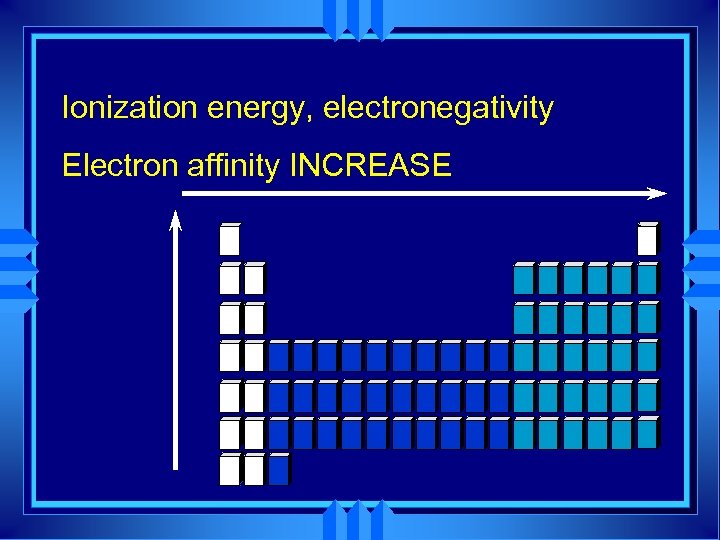
Ionization energy, electronegativity Electron affinity INCREASE
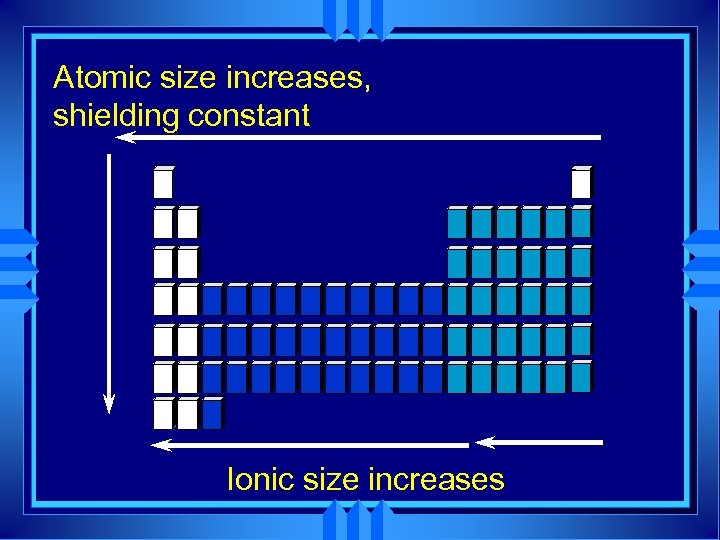
Atomic size increases, shielding constant Ionic size increases
0bc1edc5594f2f62e69771be89e9cae6.ppt