865008b857d785a7779063f6739b64ef.ppt
- Количество слайдов: 22
 CHEMICAL UNIT OPERATIONS IN ENVIRONMENTAL ENGINEERING CHAPTER 2 NEUTRALIZATION Asst. Prof. Selami DEMİR
CHEMICAL UNIT OPERATIONS IN ENVIRONMENTAL ENGINEERING CHAPTER 2 NEUTRALIZATION Asst. Prof. Selami DEMİR
 p. H n A measure of free hydrogen ions (H+) in water n Minus logarithm of hydrogen ion activities. n Scales between 0 and 14 n With p. H < 7 being acidic n With p. H > 7 being alkaline n With p. H = 7 neutral n p. H of n Sulfuric acid = 0, orange juice = 3, acid rain = 4, cokes = 5, blood = 7. 4, sea water = 8, soap = 9, costic = 14 n p. OH of n Sulfuric acid = 14, orange juice = 11, acid rain = 10, cokes = 9, blood = 6. 6, sea water = 6, soap = 5, costic = 0
p. H n A measure of free hydrogen ions (H+) in water n Minus logarithm of hydrogen ion activities. n Scales between 0 and 14 n With p. H < 7 being acidic n With p. H > 7 being alkaline n With p. H = 7 neutral n p. H of n Sulfuric acid = 0, orange juice = 3, acid rain = 4, cokes = 5, blood = 7. 4, sea water = 8, soap = 9, costic = 14 n p. OH of n Sulfuric acid = 14, orange juice = 11, acid rain = 10, cokes = 9, blood = 6. 6, sea water = 6, soap = 5, costic = 0
 Acids and Bases (1/3) n Acids Substances that produce hydrogen ions (H+) in water when dissolved n Strongly dissociate in water strong acids (HCl, H 2 SO 4) n Weakly dissociate in water weak acids (CH 3 COOH, HCl. O) n Bases n Substances that produces hydroxide ions (OH-) in water when dissolved n Strongly dissociate in water strong bases (Na. OH) n Weakly dissociate in water weak bases (NH 3) n
Acids and Bases (1/3) n Acids Substances that produce hydrogen ions (H+) in water when dissolved n Strongly dissociate in water strong acids (HCl, H 2 SO 4) n Weakly dissociate in water weak acids (CH 3 COOH, HCl. O) n Bases n Substances that produces hydroxide ions (OH-) in water when dissolved n Strongly dissociate in water strong bases (Na. OH) n Weakly dissociate in water weak bases (NH 3) n
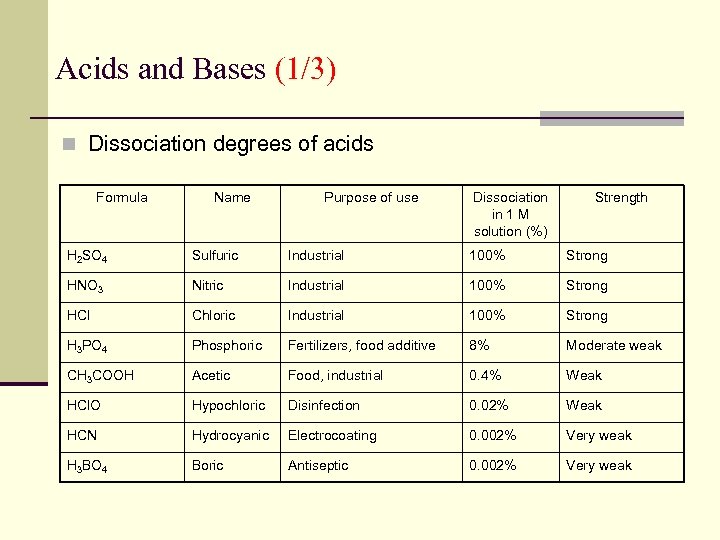 Acids and Bases (1/3) n Dissociation degrees of acids Formula Name Purpose of use Dissociation in 1 M solution (%) Strength H 2 SO 4 Sulfuric Industrial 100% Strong HNO 3 Nitric Industrial 100% Strong HCl Chloric Industrial 100% Strong H 3 PO 4 Phosphoric Fertilizers, food additive 8% Moderate weak CH 3 COOH Acetic Food, industrial 0. 4% Weak HCl. O Hypochloric Disinfection 0. 02% Weak HCN Hydrocyanic Electrocoating 0. 002% Very weak H 3 BO 4 Boric Antiseptic 0. 002% Very weak
Acids and Bases (1/3) n Dissociation degrees of acids Formula Name Purpose of use Dissociation in 1 M solution (%) Strength H 2 SO 4 Sulfuric Industrial 100% Strong HNO 3 Nitric Industrial 100% Strong HCl Chloric Industrial 100% Strong H 3 PO 4 Phosphoric Fertilizers, food additive 8% Moderate weak CH 3 COOH Acetic Food, industrial 0. 4% Weak HCl. O Hypochloric Disinfection 0. 02% Weak HCN Hydrocyanic Electrocoating 0. 002% Very weak H 3 BO 4 Boric Antiseptic 0. 002% Very weak
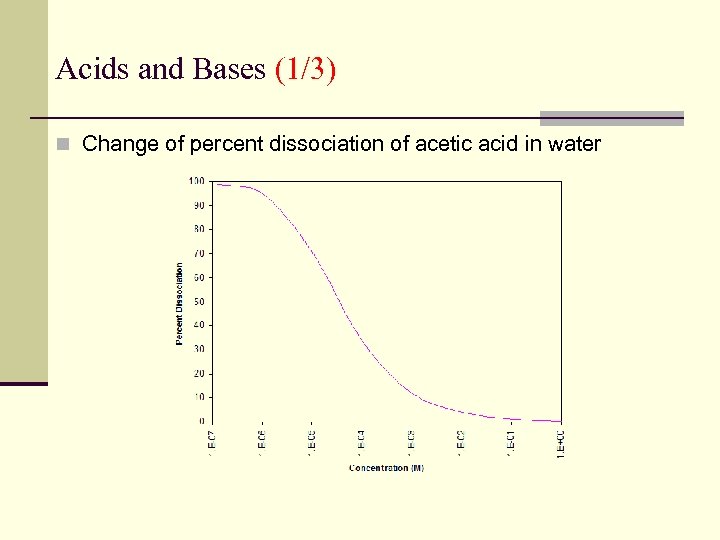 Acids and Bases (1/3) n Change of percent dissociation of acetic acid in water
Acids and Bases (1/3) n Change of percent dissociation of acetic acid in water
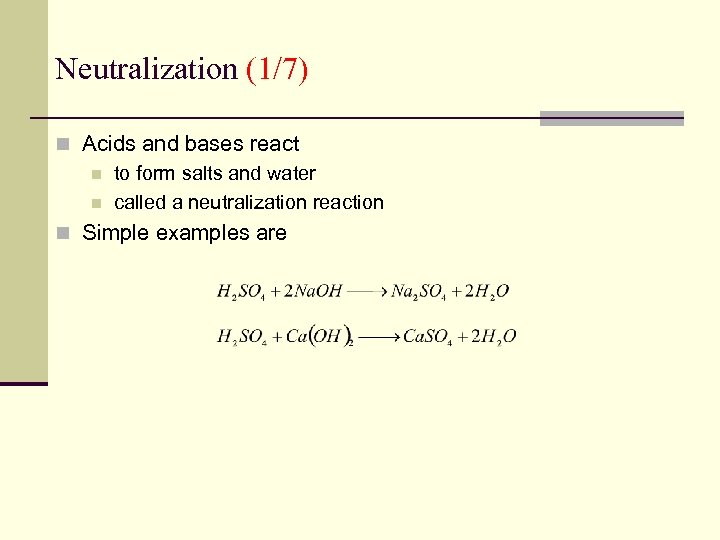 Neutralization (1/7) n Acids and bases react n to form salts and water n called a neutralization reaction n Simple examples are
Neutralization (1/7) n Acids and bases react n to form salts and water n called a neutralization reaction n Simple examples are
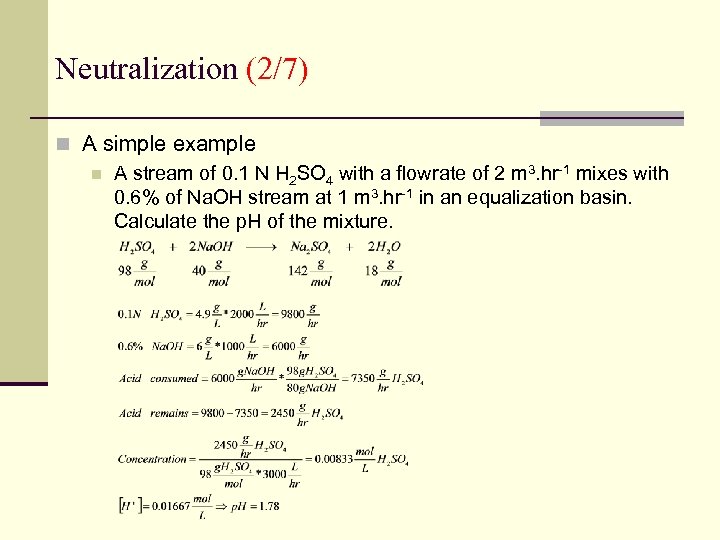 Neutralization (2/7) n A simple example n A stream of 0. 1 N H 2 SO 4 with a flowrate of 2 m 3. hr-1 mixes with 0. 6% of Na. OH stream at 1 m 3. hr-1 in an equalization basin. Calculate the p. H of the mixture.
Neutralization (2/7) n A simple example n A stream of 0. 1 N H 2 SO 4 with a flowrate of 2 m 3. hr-1 mixes with 0. 6% of Na. OH stream at 1 m 3. hr-1 in an equalization basin. Calculate the p. H of the mixture.
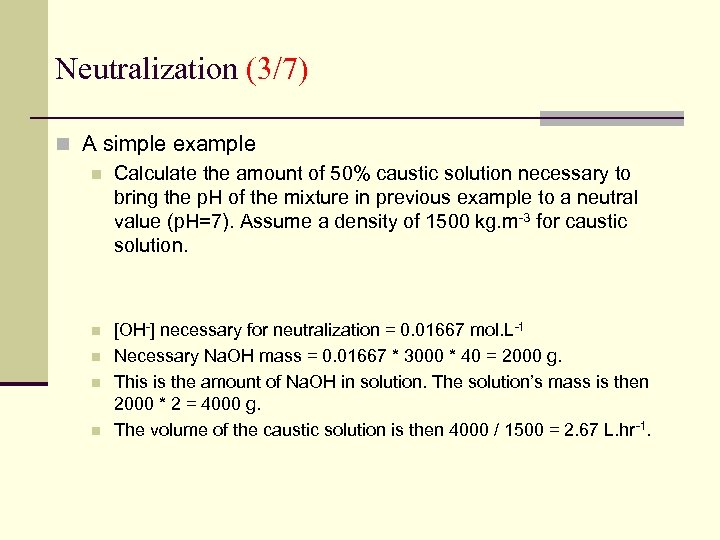 Neutralization (3/7) n A simple example n Calculate the amount of 50% caustic solution necessary to bring the p. H of the mixture in previous example to a neutral value (p. H=7). Assume a density of 1500 kg. m-3 for caustic solution. n n [OH-] necessary for neutralization = 0. 01667 mol. L-1 Necessary Na. OH mass = 0. 01667 * 3000 * 40 = 2000 g. This is the amount of Na. OH in solution. The solution’s mass is then 2000 * 2 = 4000 g. The volume of the caustic solution is then 4000 / 1500 = 2. 67 L. hr-1.
Neutralization (3/7) n A simple example n Calculate the amount of 50% caustic solution necessary to bring the p. H of the mixture in previous example to a neutral value (p. H=7). Assume a density of 1500 kg. m-3 for caustic solution. n n [OH-] necessary for neutralization = 0. 01667 mol. L-1 Necessary Na. OH mass = 0. 01667 * 3000 * 40 = 2000 g. This is the amount of Na. OH in solution. The solution’s mass is then 2000 * 2 = 4000 g. The volume of the caustic solution is then 4000 / 1500 = 2. 67 L. hr-1.
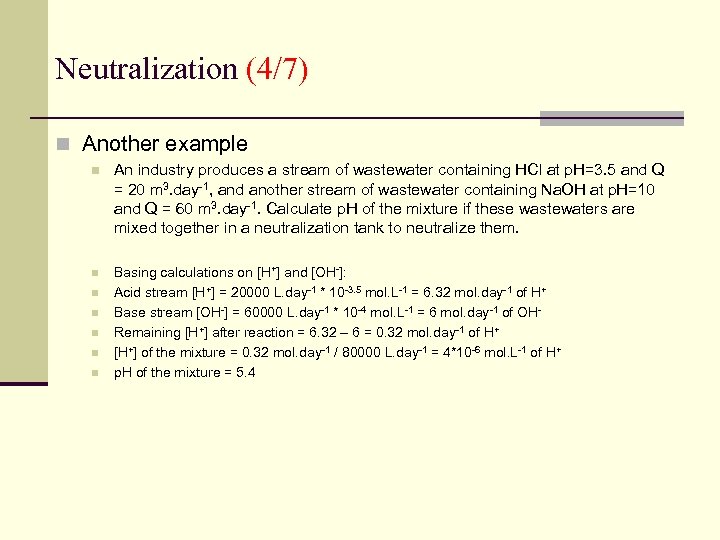 Neutralization (4/7) n Another example n An industry produces a stream of wastewater containing HCl at p. H=3. 5 and Q = 20 m 3. day-1, and another stream of wastewater containing Na. OH at p. H=10 and Q = 60 m 3. day-1. Calculate p. H of the mixture if these wastewaters are mixed together in a neutralization tank to neutralize them. n Basing calculations on [H+] and [OH-]: Acid stream [H+] = 20000 L. day-1 * 10 -3. 5 mol. L-1 = 6. 32 mol. day-1 of H+ Base stream [OH-] = 60000 L. day-1 * 10 -4 mol. L-1 = 6 mol. day-1 of OHRemaining [H+] after reaction = 6. 32 – 6 = 0. 32 mol. day-1 of H+ [H+] of the mixture = 0. 32 mol. day-1 / 80000 L. day-1 = 4*10 -6 mol. L-1 of H+ p. H of the mixture = 5. 4 n n n
Neutralization (4/7) n Another example n An industry produces a stream of wastewater containing HCl at p. H=3. 5 and Q = 20 m 3. day-1, and another stream of wastewater containing Na. OH at p. H=10 and Q = 60 m 3. day-1. Calculate p. H of the mixture if these wastewaters are mixed together in a neutralization tank to neutralize them. n Basing calculations on [H+] and [OH-]: Acid stream [H+] = 20000 L. day-1 * 10 -3. 5 mol. L-1 = 6. 32 mol. day-1 of H+ Base stream [OH-] = 60000 L. day-1 * 10 -4 mol. L-1 = 6 mol. day-1 of OHRemaining [H+] after reaction = 6. 32 – 6 = 0. 32 mol. day-1 of H+ [H+] of the mixture = 0. 32 mol. day-1 / 80000 L. day-1 = 4*10 -6 mol. L-1 of H+ p. H of the mixture = 5. 4 n n n
 Neutralization (5/7) n Highly acidic or alkaline wastewaters damage n Collection systems n n Treatment systems n n n p. H other than 6. 5 -8. 0 reduces growth rate of activated sludge Affects physical and chemical treatment units, too. Receiving bodies n n Lower than p. H 5. 5 causes corrosion Damages the ecosystem.
Neutralization (5/7) n Highly acidic or alkaline wastewaters damage n Collection systems n n Treatment systems n n n p. H other than 6. 5 -8. 0 reduces growth rate of activated sludge Affects physical and chemical treatment units, too. Receiving bodies n n Lower than p. H 5. 5 causes corrosion Damages the ecosystem.
 Neutralization (6/7) n Neutralization process n is the removal of agressive properties of the wastewater before damaging the receiving bodies. n through treatment by adding bases to acidic wastewater and adding acids to alkaline wastewaters. n Discharge to receiving bodies to collection systems requires p. H 6 to p. H 9 in Turkey n How to neutralize wastewaters n n n Adding acids/bases to wastewater to bring p. H to neutral Passing the wastewater through lime beds. Mixing wastewaters with lime solutions, Na. OH, or Na 2 CO 3. Bringing wastewater in contact with combustion gases. Mixing wastewaters with H 2 SO 4 or HCl streams.
Neutralization (6/7) n Neutralization process n is the removal of agressive properties of the wastewater before damaging the receiving bodies. n through treatment by adding bases to acidic wastewater and adding acids to alkaline wastewaters. n Discharge to receiving bodies to collection systems requires p. H 6 to p. H 9 in Turkey n How to neutralize wastewaters n n n Adding acids/bases to wastewater to bring p. H to neutral Passing the wastewater through lime beds. Mixing wastewaters with lime solutions, Na. OH, or Na 2 CO 3. Bringing wastewater in contact with combustion gases. Mixing wastewaters with H 2 SO 4 or HCl streams.
 Neutralization (7/7) n Dominant parameters in neutralization n p. H of wastewater n Reaction rate n Components of wastewater. n Neutralization is affected by suspended solids and oil and grease content of wastewater n The cost of process includes n n n Tank construction Chemicals cost Personnel costs n The costs depends on n Treatment objectives Wastewater p. H and flowrate Reaction rates
Neutralization (7/7) n Dominant parameters in neutralization n p. H of wastewater n Reaction rate n Components of wastewater. n Neutralization is affected by suspended solids and oil and grease content of wastewater n The cost of process includes n n n Tank construction Chemicals cost Personnel costs n The costs depends on n Treatment objectives Wastewater p. H and flowrate Reaction rates
 Process Design n Could be designed as n Continous for Q > 380 m 3. d-1 or n Batch for lower flowrates n Could be of n Single stage or n n If 4 < p. H < 10 Multi stage n If p. H < 2 or p. H > 10 n Important considerations n n n n Reactor wall material Acid feeding systems capacity, mountage, availability, Need for skilled personnel Tank dimensions (retention time), Wastewater flow, Solubility of chemical to feed, Reaction products
Process Design n Could be designed as n Continous for Q > 380 m 3. d-1 or n Batch for lower flowrates n Could be of n Single stage or n n If 4 < p. H < 10 Multi stage n If p. H < 2 or p. H > 10 n Important considerations n n n n Reactor wall material Acid feeding systems capacity, mountage, availability, Need for skilled personnel Tank dimensions (retention time), Wastewater flow, Solubility of chemical to feed, Reaction products
 Chemical Feeding n The equipments n Solid dosing pumps (screws) n Adjustable dosing pumps n Slurry pumps n For Ca. O, Ca. CO 3, and Ca(OH)2 n Fed to a slurry tank with screw dosing system n Mixed with water n Fed to the wastewater flow in a channel or a neutralization tank n For Na. OH, KOH, and acids n Adjustable dosage pumps are used n Must be controlled automatically to avoid overdosing. n Automated p. H control is necessary. n to control dosing and slurry pumps
Chemical Feeding n The equipments n Solid dosing pumps (screws) n Adjustable dosing pumps n Slurry pumps n For Ca. O, Ca. CO 3, and Ca(OH)2 n Fed to a slurry tank with screw dosing system n Mixed with water n Fed to the wastewater flow in a channel or a neutralization tank n For Na. OH, KOH, and acids n Adjustable dosage pumps are used n Must be controlled automatically to avoid overdosing. n Automated p. H control is necessary. n to control dosing and slurry pumps
 Tank Geometry (1/2) n n Tank diameter should be equal to active depth Wastewater is introduced from top Wastewater is removed from the bottom Retention time n n At least 5 minutes 10 -30 minutes for lime neutralization n Multi staging for agressive wastewaters n Three stages with p. H ranges of n n n 0 -14 brief measurements are enough 2 -12 sensitivity is becoming a matter of concern 4 -10 must be sensitive n Dead time in control n Less than 5% of retention time.
Tank Geometry (1/2) n n Tank diameter should be equal to active depth Wastewater is introduced from top Wastewater is removed from the bottom Retention time n n At least 5 minutes 10 -30 minutes for lime neutralization n Multi staging for agressive wastewaters n Three stages with p. H ranges of n n n 0 -14 brief measurements are enough 2 -12 sensitivity is becoming a matter of concern 4 -10 must be sensitive n Dead time in control n Less than 5% of retention time.
 Tank Geometry (2/2) n A mixer should be included n n n Vertical mixers if volume is less than 4 cubic meters Blade mixers if volume is more than 4 cubic meters Do not use horizontal paddle wheels. n Area of mixer 0. 25 to 0. 40 of tank surface area. n Mixer speed n 7. 5 m/sec if volume is less than 4 cubic meters n 3. 6 m/sec if volume is more than 4 cubic meters n
Tank Geometry (2/2) n A mixer should be included n n n Vertical mixers if volume is less than 4 cubic meters Blade mixers if volume is more than 4 cubic meters Do not use horizontal paddle wheels. n Area of mixer 0. 25 to 0. 40 of tank surface area. n Mixer speed n 7. 5 m/sec if volume is less than 4 cubic meters n 3. 6 m/sec if volume is more than 4 cubic meters n
 Chemicals (1/4) n Chosen depending on n the p. H of wastewater n availability of chemicals n cost n Products of neutralization (sludge) n Most commonly n Lime n Ca(OH)2 n Limestone (Ca. CO 3) n Sodium hydroxide n Sodium carbonate n Carbon dioxide n Sulfuric acid n Potassium hydroxide n Chloric acid
Chemicals (1/4) n Chosen depending on n the p. H of wastewater n availability of chemicals n cost n Products of neutralization (sludge) n Most commonly n Lime n Ca(OH)2 n Limestone (Ca. CO 3) n Sodium hydroxide n Sodium carbonate n Carbon dioxide n Sulfuric acid n Potassium hydroxide n Chloric acid
 Chemicals (2/4) n For alkaline wastewaters n Sulfuric acid is the most common n Chloric acid n n All chloride salts are soluble. Carbon dioxide n n Lower cost Possibility of forming precipitates with calcium in wastewater Prevents overdosing For acidic wastewaters n Should be careful about alkalinity factor n n Sodium hydroxide n n n High alkalinity factor Expensive Lime compounds n n n amount of chemical that has equivalent alkalinity with unit Ca. O Cheaper Low solubility problems with feeding Precipitates with sulfuric acid sludge handling problems Forming precipitates in piping and chennels Sodium carbonate n n low-moderate alkalinity factor Highly soluble
Chemicals (2/4) n For alkaline wastewaters n Sulfuric acid is the most common n Chloric acid n n All chloride salts are soluble. Carbon dioxide n n Lower cost Possibility of forming precipitates with calcium in wastewater Prevents overdosing For acidic wastewaters n Should be careful about alkalinity factor n n Sodium hydroxide n n n High alkalinity factor Expensive Lime compounds n n n amount of chemical that has equivalent alkalinity with unit Ca. O Cheaper Low solubility problems with feeding Precipitates with sulfuric acid sludge handling problems Forming precipitates in piping and chennels Sodium carbonate n n low-moderate alkalinity factor Highly soluble
 Chemicals (3/4) n Preparation of slaked lime n 900 – 1250 kg. m-3 n Fed as slurry in water n Slurry contains up to 45% solids n The p. H of saturated solution (1. 2 g/L) is 12. 5 n Commercially available lime contains 70 -96% Ca. O n Not suggested if less than 88% n A slurry tank is included in the system n The slurry is prepared and fed to the system via slurry pumps
Chemicals (3/4) n Preparation of slaked lime n 900 – 1250 kg. m-3 n Fed as slurry in water n Slurry contains up to 45% solids n The p. H of saturated solution (1. 2 g/L) is 12. 5 n Commercially available lime contains 70 -96% Ca. O n Not suggested if less than 88% n A slurry tank is included in the system n The slurry is prepared and fed to the system via slurry pumps
 Chemicals (4/4) n Preparation of dolomitic lime n One of the most commonly used n Commercially available in 55 -58% Ca. O & 37 -41% Mg. O n Reactions with acids are slower, requires longer retention times
Chemicals (4/4) n Preparation of dolomitic lime n One of the most commonly used n Commercially available in 55 -58% Ca. O & 37 -41% Mg. O n Reactions with acids are slower, requires longer retention times
 Limestone-bed Neutralization (1/2) n One of the most commonly used processes n n n Does not require preparation of slurries Can employ granules or larger stones of lime Limestone beds are employed and acidic wastewaters are passed through Allows use of upflow and downflow reactors Unfortunately surface of limestone is coated with calcium sulfate n Load no more than 0. 6% of sulfuric acid to prevent coating Carbon dioxide is a product of neutralization reaction For lower p. Hs of wastewater, amount of carbon dioxide formed interferes with the process. System requires continuous care and control Can work with high surface loadings (40. 7 L/min/m 2) Requires a pre-treatment step for removing solids Requires a final sedimentation unit for removing solids of limestone Recycling is shown to increase the efficiency
Limestone-bed Neutralization (1/2) n One of the most commonly used processes n n n Does not require preparation of slurries Can employ granules or larger stones of lime Limestone beds are employed and acidic wastewaters are passed through Allows use of upflow and downflow reactors Unfortunately surface of limestone is coated with calcium sulfate n Load no more than 0. 6% of sulfuric acid to prevent coating Carbon dioxide is a product of neutralization reaction For lower p. Hs of wastewater, amount of carbon dioxide formed interferes with the process. System requires continuous care and control Can work with high surface loadings (40. 7 L/min/m 2) Requires a pre-treatment step for removing solids Requires a final sedimentation unit for removing solids of limestone Recycling is shown to increase the efficiency
 Limestone-bed Neutralization (2/2) n One of the most commonly used processes n n n Does not require preparation of slurries Can employ granules or larger stones of lime Limestone beds are employed and acidic wastewaters are passed through Allows use of upflow and downflow reactors Unfortunately surface of limestone is coated with calcium sulfate n Load no more than 0. 6% of sulfuric acid to prevent coating Carbon dioxide is a product of neutralization reaction For lower p. Hs of wastewater, amount of carbon dioxide formed interferes with the process. System requires continuous care and control Can work with high surface loadings (40. 7 L/min/m 2) Requires a pre-treatment step for removing solids Requires a final sedimentation unit for removing solids of limestone Recycling is shown to increase the efficiency
Limestone-bed Neutralization (2/2) n One of the most commonly used processes n n n Does not require preparation of slurries Can employ granules or larger stones of lime Limestone beds are employed and acidic wastewaters are passed through Allows use of upflow and downflow reactors Unfortunately surface of limestone is coated with calcium sulfate n Load no more than 0. 6% of sulfuric acid to prevent coating Carbon dioxide is a product of neutralization reaction For lower p. Hs of wastewater, amount of carbon dioxide formed interferes with the process. System requires continuous care and control Can work with high surface loadings (40. 7 L/min/m 2) Requires a pre-treatment step for removing solids Requires a final sedimentation unit for removing solids of limestone Recycling is shown to increase the efficiency


