5 Chemical_Kinetics.ppt
- Количество слайдов: 42
 Chemical thermodynamics
Chemical thermodynamics
 lesson plan 1. Types of Thermodynamic Systems 2. Internal Energy. The first law of thermodynamics 3. Enthalpy. Hess’s law 4. The Second and Third Laws of Thermodynamics 5. Gibbs Free Energy
lesson plan 1. Types of Thermodynamic Systems 2. Internal Energy. The first law of thermodynamics 3. Enthalpy. Hess’s law 4. The Second and Third Laws of Thermodynamics 5. Gibbs Free Energy
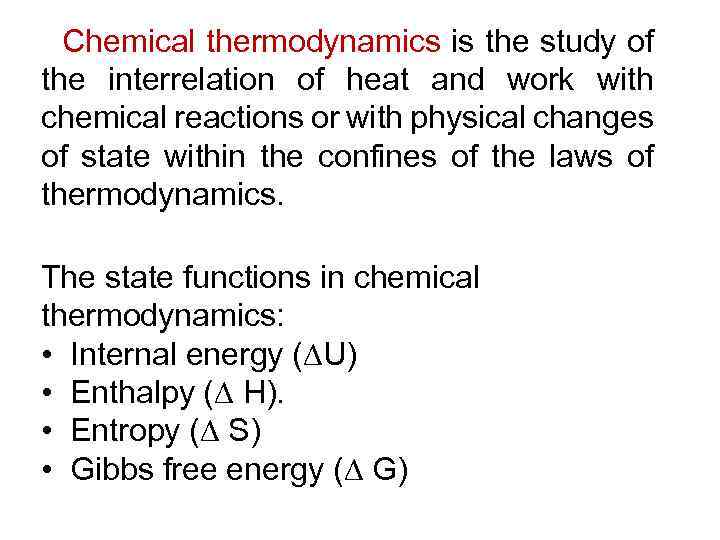 Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. The state functions in chemical thermodynamics: • Internal energy ( U) • Enthalpy ( H). • Entropy ( S) • Gibbs free energy ( G)
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. The state functions in chemical thermodynamics: • Internal energy ( U) • Enthalpy ( H). • Entropy ( S) • Gibbs free energy ( G)
 Types of Thermodynamic Systems Isolated System Closed System Isolated System: No matter or energy cross system boundaries. No work can be done on the system. Closed System: Energy can be exchanged but matter cannot. Open System: Free exchange across system boundaries.
Types of Thermodynamic Systems Isolated System Closed System Isolated System: No matter or energy cross system boundaries. No work can be done on the system. Closed System: Energy can be exchanged but matter cannot. Open System: Free exchange across system boundaries.
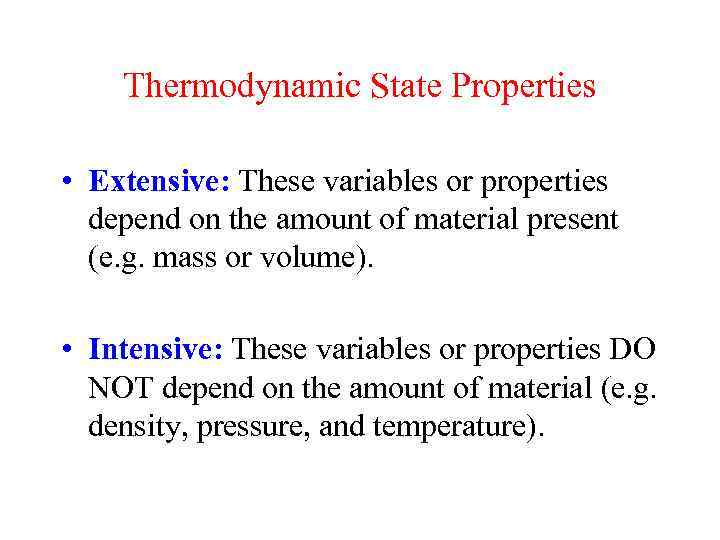 Thermodynamic State Properties • Extensive: These variables or properties depend on the amount of material present (e. g. mass or volume). • Intensive: These variables or properties DO NOT depend on the amount of material (e. g. density, pressure, and temperature).
Thermodynamic State Properties • Extensive: These variables or properties depend on the amount of material present (e. g. mass or volume). • Intensive: These variables or properties DO NOT depend on the amount of material (e. g. density, pressure, and temperature).
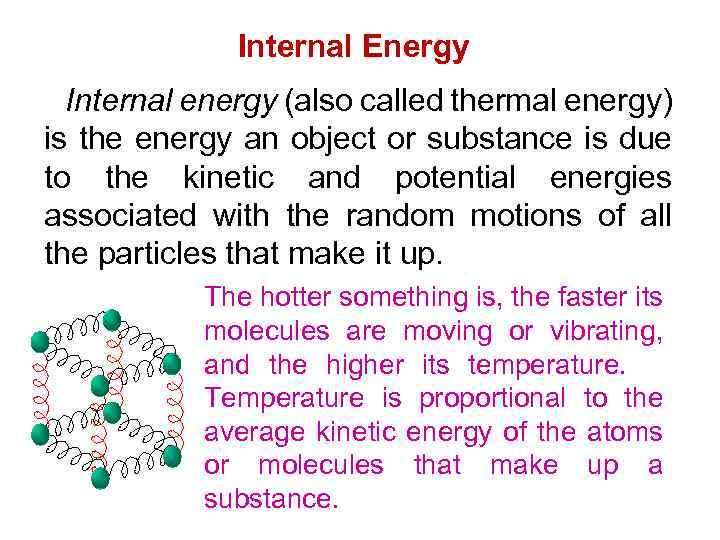 Internal Energy Internal energy (also called thermal energy) is the energy an object or substance is due to the kinetic and potential energies associated with the random motions of all the particles that make it up. The hotter something is, the faster its molecules are moving or vibrating, and the higher its temperature. Temperature is proportional to the average kinetic energy of the atoms or molecules that make up a substance.
Internal Energy Internal energy (also called thermal energy) is the energy an object or substance is due to the kinetic and potential energies associated with the random motions of all the particles that make it up. The hotter something is, the faster its molecules are moving or vibrating, and the higher its temperature. Temperature is proportional to the average kinetic energy of the atoms or molecules that make up a substance.
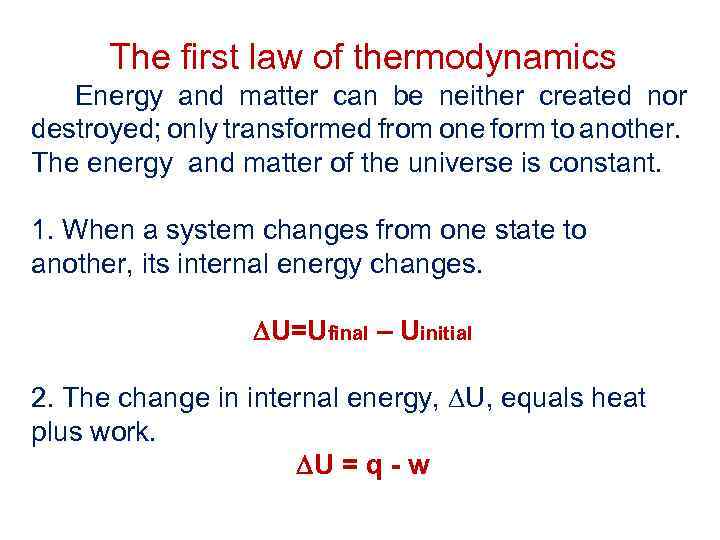 The first law of thermodynamics Energy and matter can be neither created nor destroyed; only transformed from one form to another. The energy and matter of the universe is constant. 1. When a system changes from one state to another, its internal energy changes. U=Ufinal – Uinitial 2. The change in internal energy, U, equals heat plus work. U = q - w
The first law of thermodynamics Energy and matter can be neither created nor destroyed; only transformed from one form to another. The energy and matter of the universe is constant. 1. When a system changes from one state to another, its internal energy changes. U=Ufinal – Uinitial 2. The change in internal energy, U, equals heat plus work. U = q - w
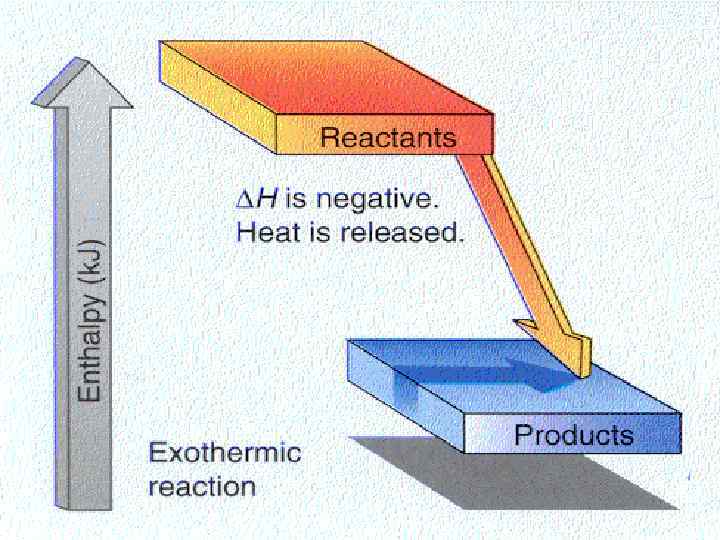
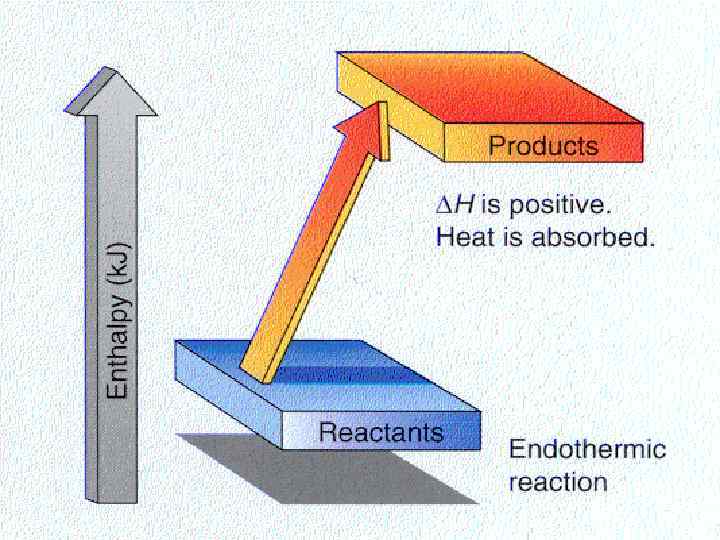
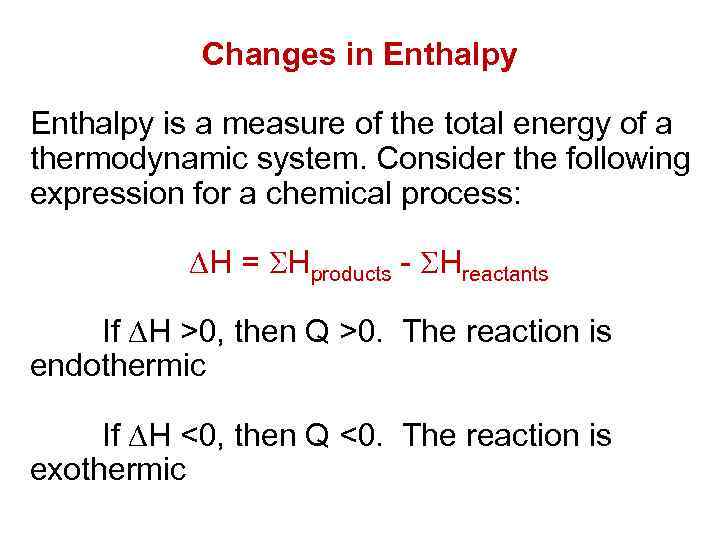 Changes in Enthalpy is a measure of the total energy of a thermodynamic system. Consider the following expression for a chemical process: H = Hproducts - Hreactants If H >0, then Q >0. The reaction is endothermic If H <0, then Q <0. The reaction is exothermic
Changes in Enthalpy is a measure of the total energy of a thermodynamic system. Consider the following expression for a chemical process: H = Hproducts - Hreactants If H >0, then Q >0. The reaction is endothermic If H <0, then Q <0. The reaction is exothermic
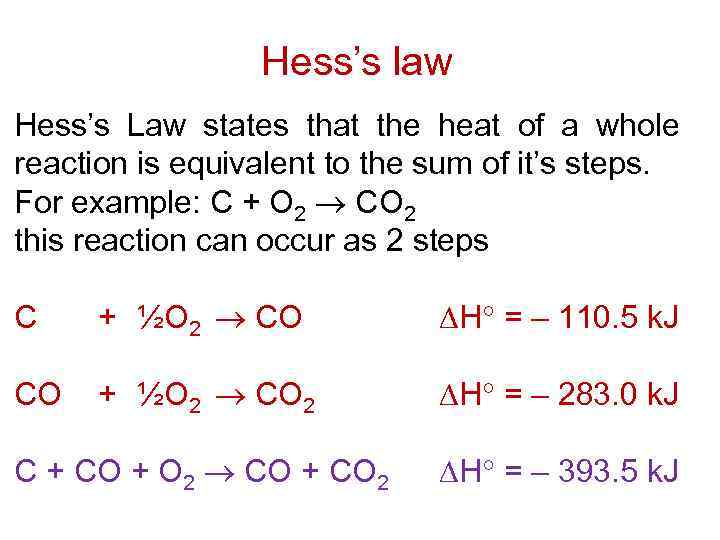 Hess’s law Hess’s Law states that the heat of a whole reaction is equivalent to the sum of it’s steps. For example: C + O 2 CO 2 this reaction can occur as 2 steps C + ½O 2 CO H = – 110. 5 k. J CO + ½O 2 CO 2 H = – 283. 0 k. J C + CO + O 2 CO + CO 2 H = – 393. 5 k. J
Hess’s law Hess’s Law states that the heat of a whole reaction is equivalent to the sum of it’s steps. For example: C + O 2 CO 2 this reaction can occur as 2 steps C + ½O 2 CO H = – 110. 5 k. J CO + ½O 2 CO 2 H = – 283. 0 k. J C + CO + O 2 CO + CO 2 H = – 393. 5 k. J
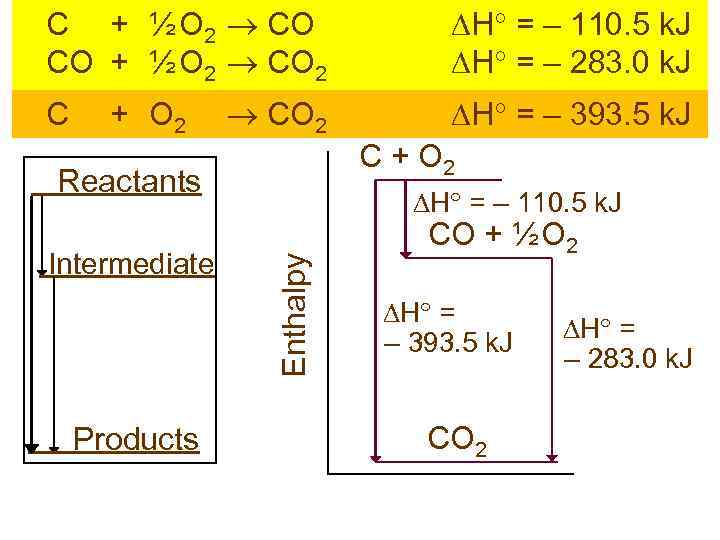 C + ½ O 2 CO CO + ½ O 2 CO 2 H = – 110. 5 k. J H = – 283. 0 k. J CO 2 H = – 393. 5 k. J C + O 2 Reactants Products Enthalpy Intermediate H = – 110. 5 k. J CO + ½ O 2 H = – 393. 5 k. J CO 2 H = – 283. 0 k. J
C + ½ O 2 CO CO + ½ O 2 CO 2 H = – 110. 5 k. J H = – 283. 0 k. J CO 2 H = – 393. 5 k. J C + O 2 Reactants Products Enthalpy Intermediate H = – 110. 5 k. J CO + ½ O 2 H = – 393. 5 k. J CO 2 H = – 283. 0 k. J
 The Second Law of Thermodynamics In any spontaneous process, there is always an increase in entropy of the universe Suniverse = Ssystem + Ssurroundings Suniverse > 0 for spontaneous rxn The Third Law of Thermodynamics The entropy of a perfect crystal at 0 kelvins is zero Suniverse = 0 at equilibrium
The Second Law of Thermodynamics In any spontaneous process, there is always an increase in entropy of the universe Suniverse = Ssystem + Ssurroundings Suniverse > 0 for spontaneous rxn The Third Law of Thermodynamics The entropy of a perfect crystal at 0 kelvins is zero Suniverse = 0 at equilibrium
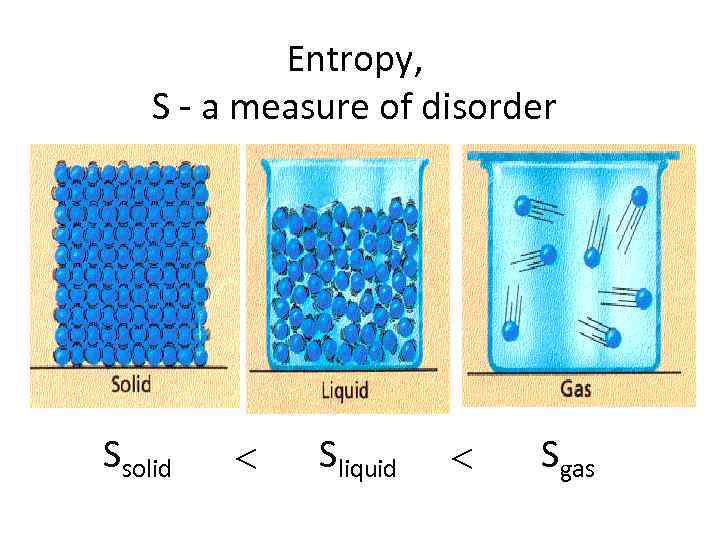 Entropy, S - a measure of disorder Ssolid Sliquid Sgas
Entropy, S - a measure of disorder Ssolid Sliquid Sgas
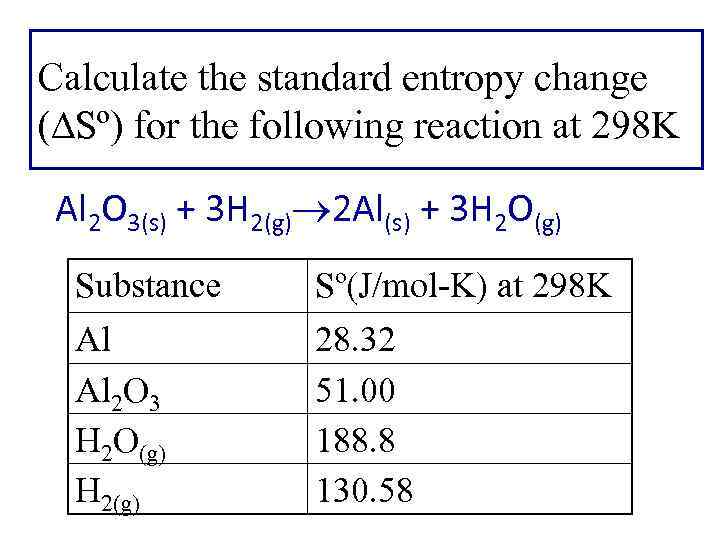 Calculate the standard entropy change ( Sº) for the following reaction at 298 K Al 2 O 3(s) + 3 H 2(g) 2 Al(s) + 3 H 2 O(g) Substance Al Al 2 O 3 H 2 O(g) H 2(g) Sº(J/mol-K) at 298 K 28. 32 51. 00 188. 8 130. 58
Calculate the standard entropy change ( Sº) for the following reaction at 298 K Al 2 O 3(s) + 3 H 2(g) 2 Al(s) + 3 H 2 O(g) Substance Al Al 2 O 3 H 2 O(g) H 2(g) Sº(J/mol-K) at 298 K 28. 32 51. 00 188. 8 130. 58
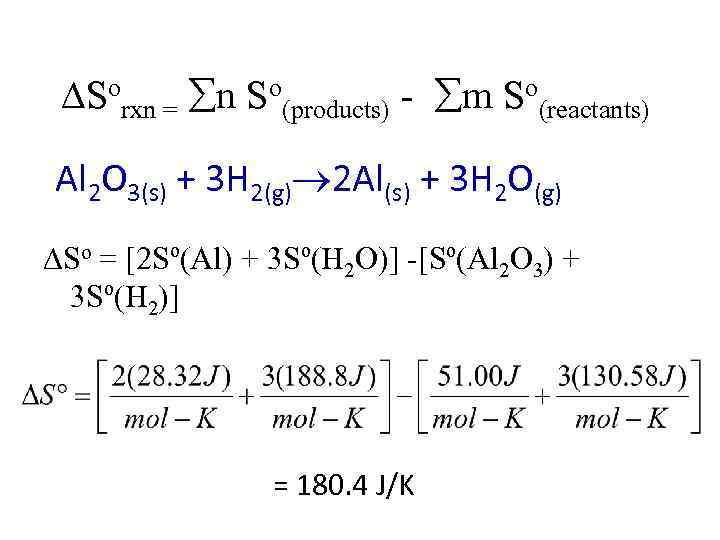 Sorxn = n So(products) - m So(reactants) Al 2 O 3(s) + 3 H 2(g) 2 Al(s) + 3 H 2 O(g) So = [2 Sº(Al) + 3 Sº(H 2 O)] -[Sº(Al 2 O 3) + 3 Sº(H 2)] = 180. 4 J/K
Sorxn = n So(products) - m So(reactants) Al 2 O 3(s) + 3 H 2(g) 2 Al(s) + 3 H 2 O(g) So = [2 Sº(Al) + 3 Sº(H 2 O)] -[Sº(Al 2 O 3) + 3 Sº(H 2)] = 180. 4 J/K
 Gibbs Free Energy G = H - T S Compares entropy, enthalpy and temperature If G 0 (is negative) the reaction is spontaneous • If G 0 (is positive) the reaction is nonspontaneous • If G =0 the reaction is at equilibrium •
Gibbs Free Energy G = H - T S Compares entropy, enthalpy and temperature If G 0 (is negative) the reaction is spontaneous • If G 0 (is positive) the reaction is nonspontaneous • If G =0 the reaction is at equilibrium •
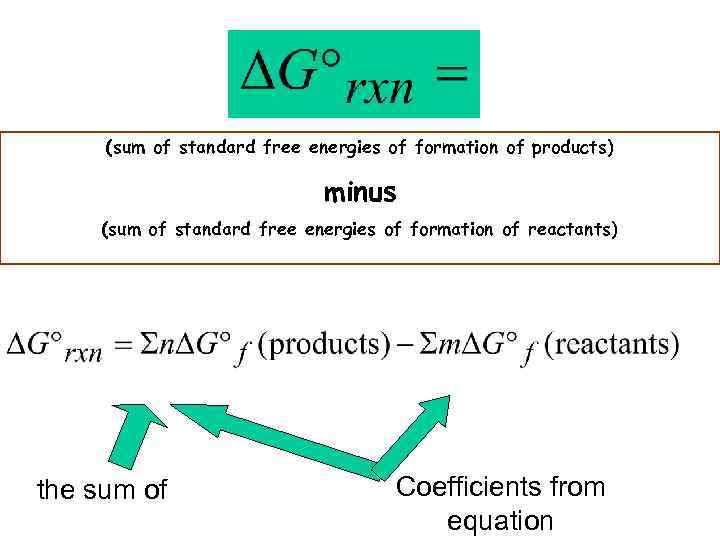 (sum of standard free energies of formation of products) minus (sum of standard free energies of formation of reactants) the sum of Coefficients from equation
(sum of standard free energies of formation of products) minus (sum of standard free energies of formation of reactants) the sum of Coefficients from equation
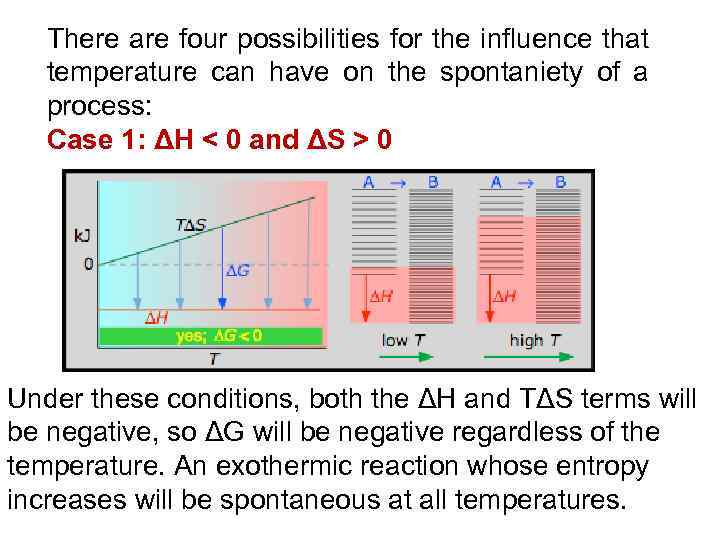 There are four possibilities for the influence that temperature can have on the spontaniety of a process: Case 1: ΔH < 0 and ΔS > 0 Under these conditions, both the ΔH and TΔS terms will be negative, so ΔG will be negative regardless of the temperature. An exothermic reaction whose entropy increases will be spontaneous at all temperatures.
There are four possibilities for the influence that temperature can have on the spontaniety of a process: Case 1: ΔH < 0 and ΔS > 0 Under these conditions, both the ΔH and TΔS terms will be negative, so ΔG will be negative regardless of the temperature. An exothermic reaction whose entropy increases will be spontaneous at all temperatures.
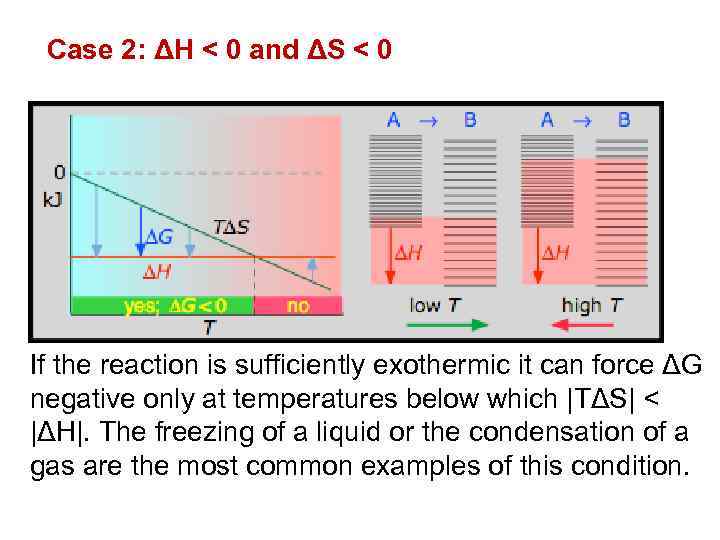 Case 2: ΔH < 0 and ΔS < 0 If the reaction is sufficiently exothermic it can force ΔG negative only at temperatures below which |TΔS| < |ΔH|. The freezing of a liquid or the condensation of a gas are the most common examples of this condition.
Case 2: ΔH < 0 and ΔS < 0 If the reaction is sufficiently exothermic it can force ΔG negative only at temperatures below which |TΔS| < |ΔH|. The freezing of a liquid or the condensation of a gas are the most common examples of this condition.
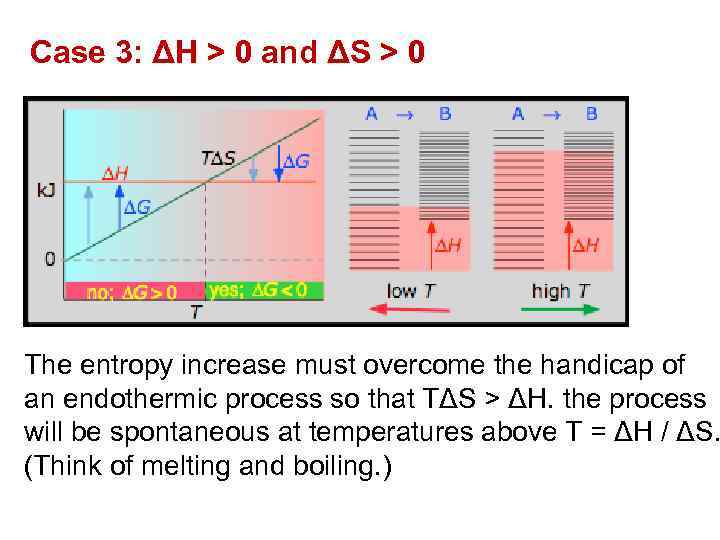 Case 3: ΔH > 0 and ΔS > 0 The entropy increase must overcome the handicap of an endothermic process so that TΔS > ΔH. the process will be spontaneous at temperatures above T = ΔH / ΔS. (Think of melting and boiling. )
Case 3: ΔH > 0 and ΔS > 0 The entropy increase must overcome the handicap of an endothermic process so that TΔS > ΔH. the process will be spontaneous at temperatures above T = ΔH / ΔS. (Think of melting and boiling. )
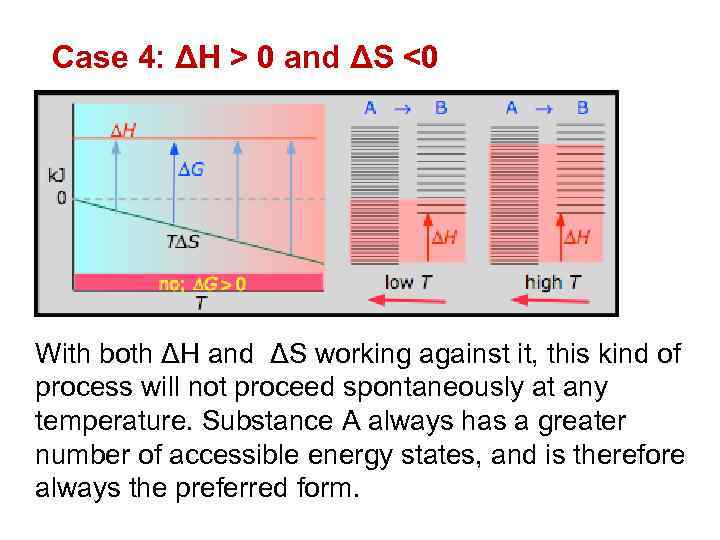 Case 4: ΔH > 0 and ΔS <0 With both ΔH and ΔS working against it, this kind of process will not proceed spontaneously at any temperature. Substance A always has a greater number of accessible energy states, and is therefore always the preferred form.
Case 4: ΔH > 0 and ΔS <0 With both ΔH and ΔS working against it, this kind of process will not proceed spontaneously at any temperature. Substance A always has a greater number of accessible energy states, and is therefore always the preferred form.
 Chemical Kinetics
Chemical Kinetics
 Chemical Kinetics Chemical kinetics is the study of the rates of chemical reactions. Reaction rate is the change in the concentration of a reactant or a product with time (M/s). A B [A] rate = t [A] = change in concentration of A over time period t [B] rate = t [B] = change in concentration of B over time period t Because [A] decreases with time, D[A] is negative.
Chemical Kinetics Chemical kinetics is the study of the rates of chemical reactions. Reaction rate is the change in the concentration of a reactant or a product with time (M/s). A B [A] rate = t [A] = change in concentration of A over time period t [B] rate = t [B] = change in concentration of B over time period t Because [A] decreases with time, D[A] is negative.
 Factors that Affect Reaction Rates 1. Physical state of the reactants – states that promote contact have faster rates; homogeneous vs. heterogeneous 2. Concentration of the reactants: conc. ↑, rate ↑ (or pressure for gases) 3. Temperature: temp. ↑, rate ↑ due to higher molecular energy and speed 4. Catalysts: rate ↑ by changing the mechanism and reaction energy 5. Other physical things like stirring and grinding solid reactants.
Factors that Affect Reaction Rates 1. Physical state of the reactants – states that promote contact have faster rates; homogeneous vs. heterogeneous 2. Concentration of the reactants: conc. ↑, rate ↑ (or pressure for gases) 3. Temperature: temp. ↑, rate ↑ due to higher molecular energy and speed 4. Catalysts: rate ↑ by changing the mechanism and reaction energy 5. Other physical things like stirring and grinding solid reactants.
![A B time [A] rate = t [B] rate = t 13. 1 A B time [A] rate = t [B] rate = t 13. 1](https://present5.com/presentation/187015988_420570519/image-26.jpg) A B time [A] rate = t [B] rate = t 13. 1
A B time [A] rate = t [B] rate = t 13. 1
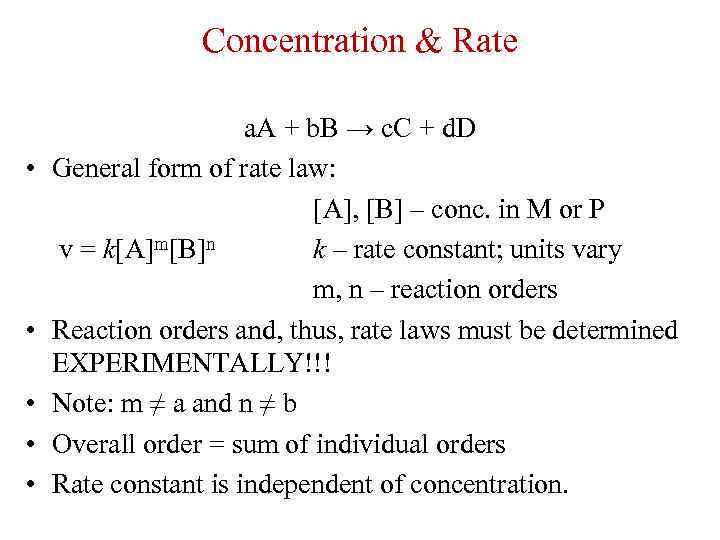 Concentration & Rate • • • a. A + b. B → c. C + d. D General form of rate law: [A], [B] – conc. in M or P v = k[A]m[B]n k – rate constant; units vary m, n – reaction orders Reaction orders and, thus, rate laws must be determined EXPERIMENTALLY!!! Note: m ≠ a and n ≠ b Overall order = sum of individual orders Rate constant is independent of concentration.
Concentration & Rate • • • a. A + b. B → c. C + d. D General form of rate law: [A], [B] – conc. in M or P v = k[A]m[B]n k – rate constant; units vary m, n – reaction orders Reaction orders and, thus, rate laws must be determined EXPERIMENTALLY!!! Note: m ≠ a and n ≠ b Overall order = sum of individual orders Rate constant is independent of concentration.
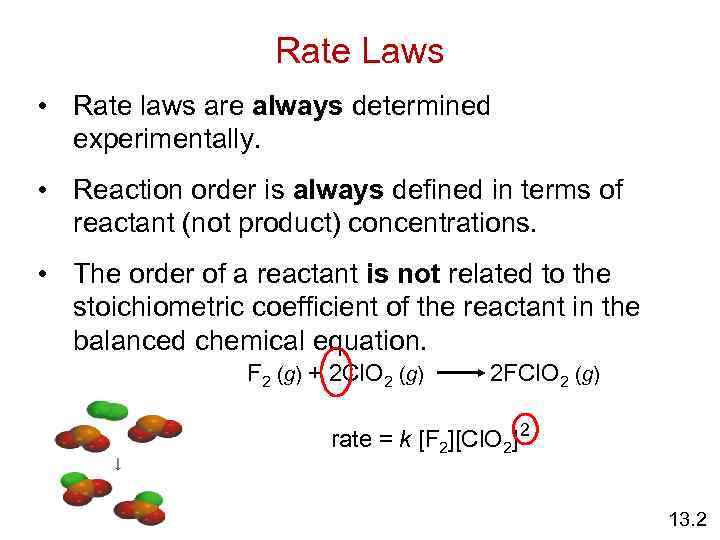 Rate Laws • Rate laws are always determined experimentally. • Reaction order is always defined in terms of reactant (not product) concentrations. • The order of a reactant is not related to the stoichiometric coefficient of the reactant in the balanced chemical equation. F 2 (g) + 2 Cl. O 2 (g) 2 FCl. O 2 (g) rate = k [F 2][Cl. O 2] 2 13. 2
Rate Laws • Rate laws are always determined experimentally. • Reaction order is always defined in terms of reactant (not product) concentrations. • The order of a reactant is not related to the stoichiometric coefficient of the reactant in the balanced chemical equation. F 2 (g) + 2 Cl. O 2 (g) 2 FCl. O 2 (g) rate = k [F 2][Cl. O 2] 2 13. 2
 Temperature and Rate • Generally, as temperature increases, so does reaction rate. • This is because k is temperature dependent.
Temperature and Rate • Generally, as temperature increases, so does reaction rate. • This is because k is temperature dependent.
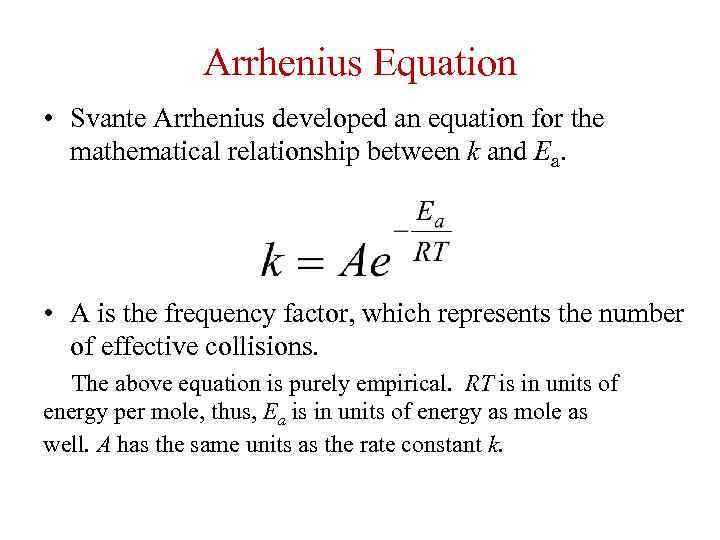 Arrhenius Equation • Svante Arrhenius developed an equation for the mathematical relationship between k and Ea. • A is the frequency factor, which represents the number of effective collisions. The above equation is purely empirical. RT is in units of energy per mole, thus, Ea is in units of energy as mole as well. A has the same units as the rate constant k.
Arrhenius Equation • Svante Arrhenius developed an equation for the mathematical relationship between k and Ea. • A is the frequency factor, which represents the number of effective collisions. The above equation is purely empirical. RT is in units of energy per mole, thus, Ea is in units of energy as mole as well. A has the same units as the rate constant k.
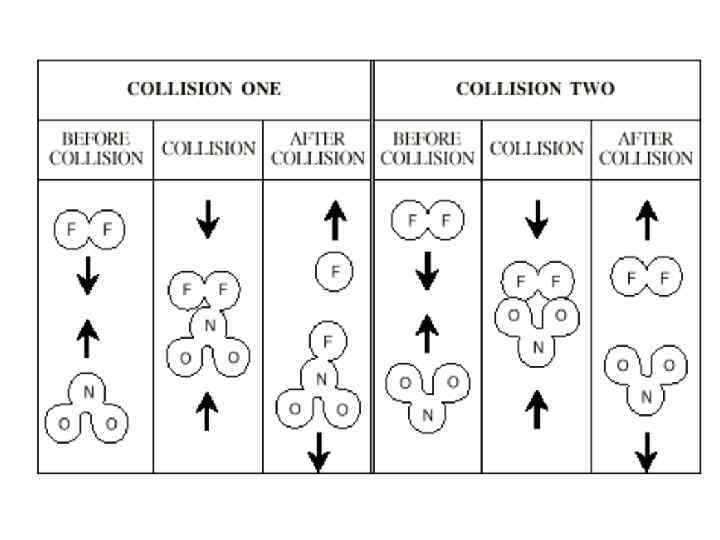
 Collision Theory -In order for two molecules to react, old chemical bonds have to be broken. That requires energy! -Collision theory considers that such energy is provided by kinetic energies of molecules upon their collisions. -Collision theory can qualitatively explain: 1) why the reaction rate, k, increases with increasing temperature 2) why only 1 of 1010 collisions results in reaction
Collision Theory -In order for two molecules to react, old chemical bonds have to be broken. That requires energy! -Collision theory considers that such energy is provided by kinetic energies of molecules upon their collisions. -Collision theory can qualitatively explain: 1) why the reaction rate, k, increases with increasing temperature 2) why only 1 of 1010 collisions results in reaction
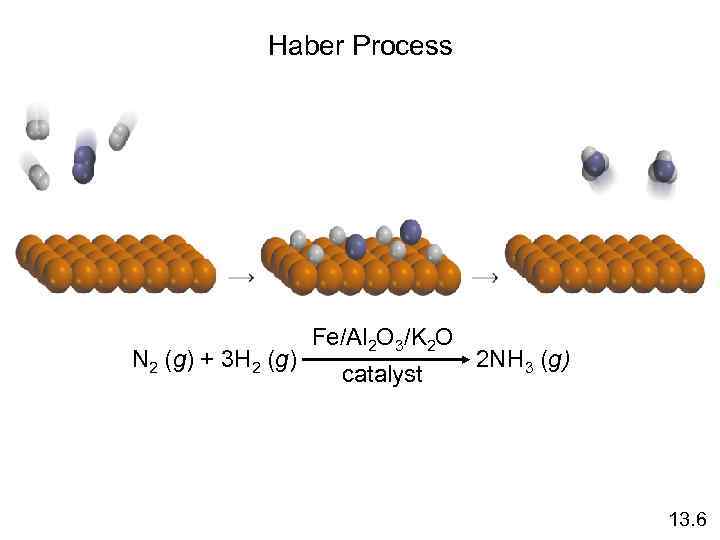 Haber Process N 2 (g) + 3 H 2 (g) Fe/Al 2 O 3/K 2 O catalyst 2 NH 3 (g) 13. 6
Haber Process N 2 (g) + 3 H 2 (g) Fe/Al 2 O 3/K 2 O catalyst 2 NH 3 (g) 13. 6
 Catalysis • Catalysts – increase the rate of a reaction without being consumed or changing chemically • Accomplished by lowering the activation energy and changing the reaction mechanism. • Heterogeneous vs. homogeneous catalysis • Examples: – Catalytic converter (p. 608) – Enzymes in the body (pp. 609 -611) – Ozone depletion (pp. 774 -775)
Catalysis • Catalysts – increase the rate of a reaction without being consumed or changing chemically • Accomplished by lowering the activation energy and changing the reaction mechanism. • Heterogeneous vs. homogeneous catalysis • Examples: – Catalytic converter (p. 608) – Enzymes in the body (pp. 609 -611) – Ozone depletion (pp. 774 -775)
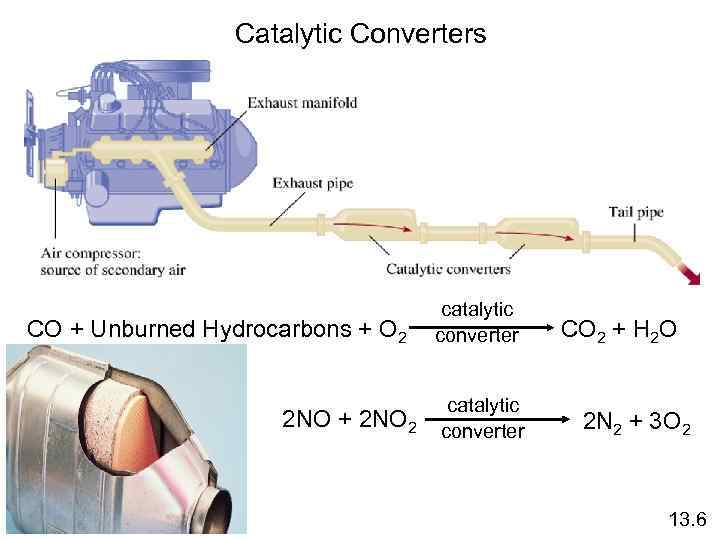 Catalytic Converters CO + Unburned Hydrocarbons + O 2 catalytic converter catalytic 2 NO + 2 NO 2 converter CO 2 + H 2 O 2 N 2 + 3 O 2 13. 6
Catalytic Converters CO + Unburned Hydrocarbons + O 2 catalytic converter catalytic 2 NO + 2 NO 2 converter CO 2 + H 2 O 2 N 2 + 3 O 2 13. 6
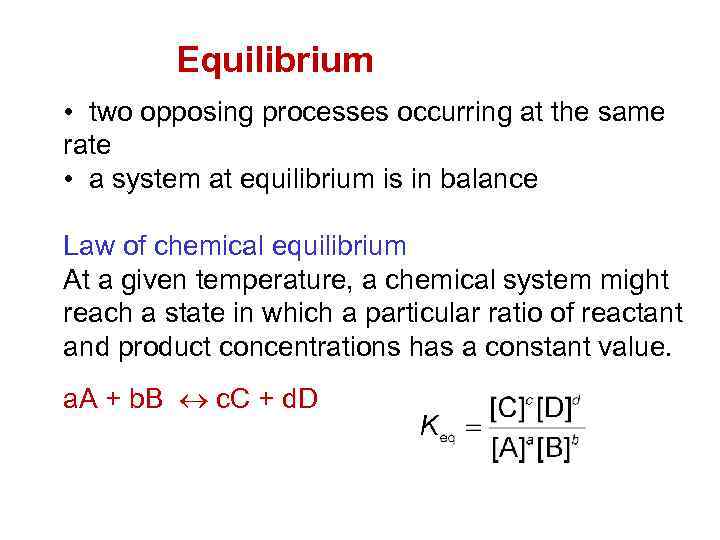 Equilibrium • two opposing processes occurring at the same rate • a system at equilibrium is in balance Law of chemical equilibrium At a given temperature, a chemical system might reach a state in which a particular ratio of reactant and product concentrations has a constant value. a. A + b. B c. C + d. D
Equilibrium • two opposing processes occurring at the same rate • a system at equilibrium is in balance Law of chemical equilibrium At a given temperature, a chemical system might reach a state in which a particular ratio of reactant and product concentrations has a constant value. a. A + b. B c. C + d. D
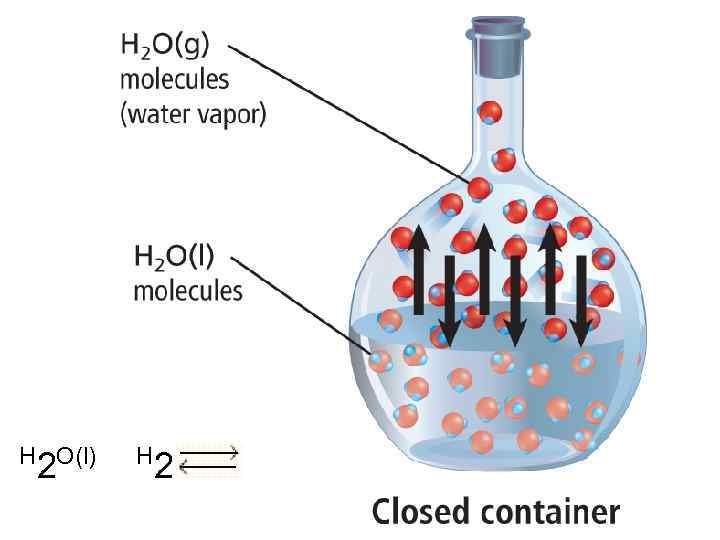 H O(l) 2 H O(g) 2
H O(l) 2 H O(g) 2
 Equilibrium constant (Keq) numerical value of the ratio of product to reactant concentrations constant only at a specific temperature Keq > 1: Products are favored at equilibrium Keq < 1: Reactants are favored at equilibrium A system at equilibrium must: • take place in closed • system • temperature remain constant • all reactants and products are present (both reactions can occur)
Equilibrium constant (Keq) numerical value of the ratio of product to reactant concentrations constant only at a specific temperature Keq > 1: Products are favored at equilibrium Keq < 1: Reactants are favored at equilibrium A system at equilibrium must: • take place in closed • system • temperature remain constant • all reactants and products are present (both reactions can occur)
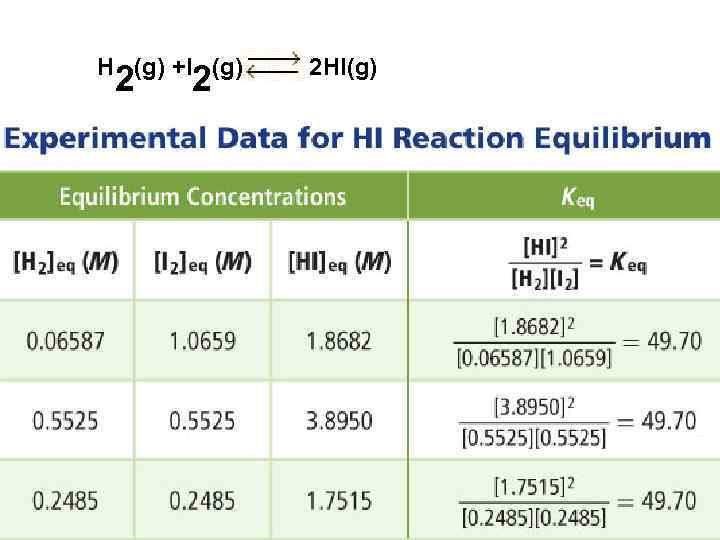 H (g) +I (g) 2 2 2 HI(g)
H (g) +I (g) 2 2 2 HI(g)
 Le Chatelier’s Principle If a stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress. Used to predict how a equilibrium system will react to changes in concentration, pressure (volume) and temperature.
Le Chatelier’s Principle If a stress is applied to a system at equilibrium, the system shifts in the direction that relieves the stress. Used to predict how a equilibrium system will react to changes in concentration, pressure (volume) and temperature.
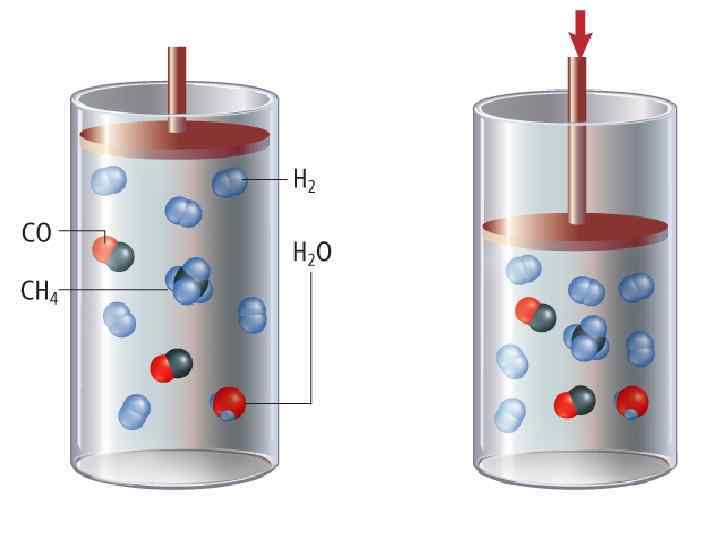
 Changes in Concentration 1. Reverse rxn speeds up. 2. Over time, reverse rxn slows down and forward rxn speeds up. 3. At equilibrium, forward and reverse rxns occur at same rate, new equilibrium position established. Equilibrium has shifted left; value of Keq unchanged. Changes in Pressure (Volume) If volume is decreased concentrations of all gaseous substances increases. Side with fewer moles of gas increases more. Changes in Temperature Review: If H is negative rxn is exothermic heat written as product If H is positive rxn is endothermic heat written as reactant
Changes in Concentration 1. Reverse rxn speeds up. 2. Over time, reverse rxn slows down and forward rxn speeds up. 3. At equilibrium, forward and reverse rxns occur at same rate, new equilibrium position established. Equilibrium has shifted left; value of Keq unchanged. Changes in Pressure (Volume) If volume is decreased concentrations of all gaseous substances increases. Side with fewer moles of gas increases more. Changes in Temperature Review: If H is negative rxn is exothermic heat written as product If H is positive rxn is endothermic heat written as reactant