70c29892716afb523be65d00c045d0b6.ppt
- Количество слайдов: 39
 CHEM 205 Tutorial 0 chapter 1 http: //www. karentimberlake. com/chemodul. htm
CHEM 205 Tutorial 0 chapter 1 http: //www. karentimberlake. com/chemodul. htm
 Units of measurement
Units of measurement
 Measurement You are making a measurement when you ¨ Check you weight ¨ Read your watch ¨ Take your temperature ¨ Weigh an apple What kinds of measurements did you make today?
Measurement You are making a measurement when you ¨ Check you weight ¨ Read your watch ¨ Take your temperature ¨ Weigh an apple What kinds of measurements did you make today?
 Some Tools for Measurement How do you measure…. Temperature? Time? Volume? Weight?
Some Tools for Measurement How do you measure…. Temperature? Time? Volume? Weight?
 Measurement in Science In Science we ¨do experiments ¨measure quantities ¨use numbers to report measurements
Measurement in Science In Science we ¨do experiments ¨measure quantities ¨use numbers to report measurements
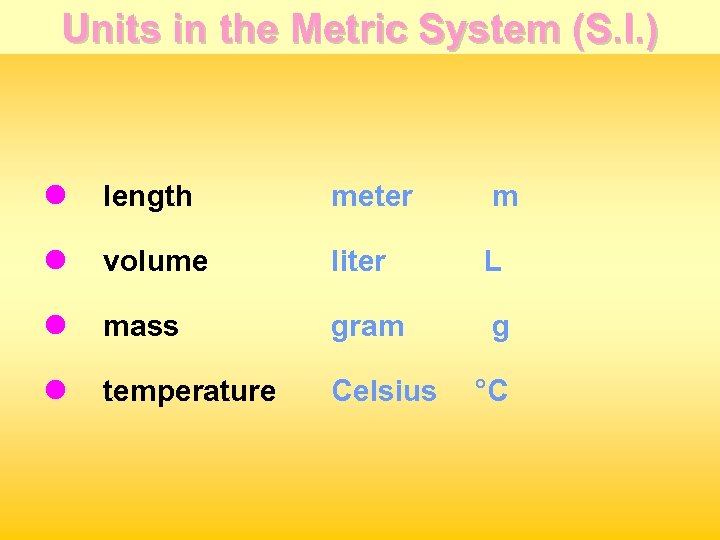 Units in the Metric System (S. I. ) l length meter m l volume liter L l mass gram g l temperature Celsius °C
Units in the Metric System (S. I. ) l length meter m l volume liter L l mass gram g l temperature Celsius °C
 Stating a Measurement In every measurement there is a NUMBER followed by a UNIT from measuring device The patient’s temperature is 102°F. The sack holds 5 lbs of potatoes. It is 8 miles from your house to school. The bottle holds 2 L of orange soda
Stating a Measurement In every measurement there is a NUMBER followed by a UNIT from measuring device The patient’s temperature is 102°F. The sack holds 5 lbs of potatoes. It is 8 miles from your house to school. The bottle holds 2 L of orange soda
 Significant Figures
Significant Figures
 Reading a measure First digit (known) =2 2. ? ? cm Second digit (known) = 0. 7 2. 7? cm Third digit (estimated) between 0. 05 - 0. 07 Length reported = 2. 74 cm or 2. 75 cm or 2. 73 cm
Reading a measure First digit (known) =2 2. ? ? cm Second digit (known) = 0. 7 2. 7? cm Third digit (estimated) between 0. 05 - 0. 07 Length reported = 2. 74 cm or 2. 75 cm or 2. 73 cm
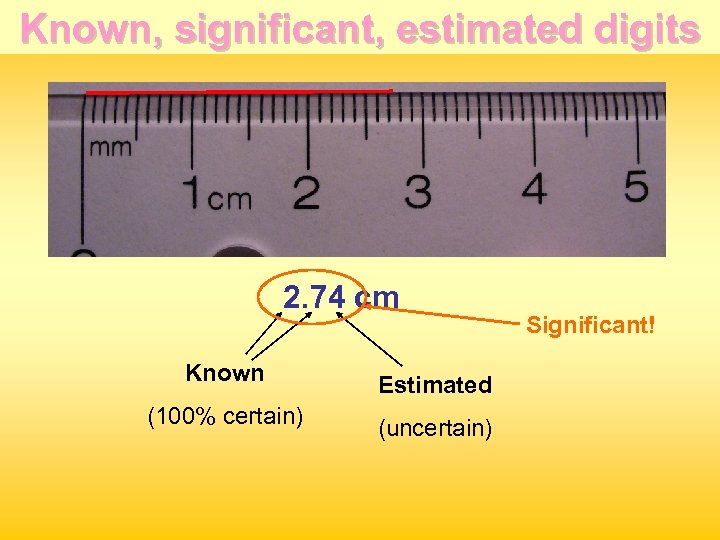 Known, significant, estimated digits 2. 74 cm Known Estimated (100% certain) (uncertain) Significant!
Known, significant, estimated digits 2. 74 cm Known Estimated (100% certain) (uncertain) Significant!
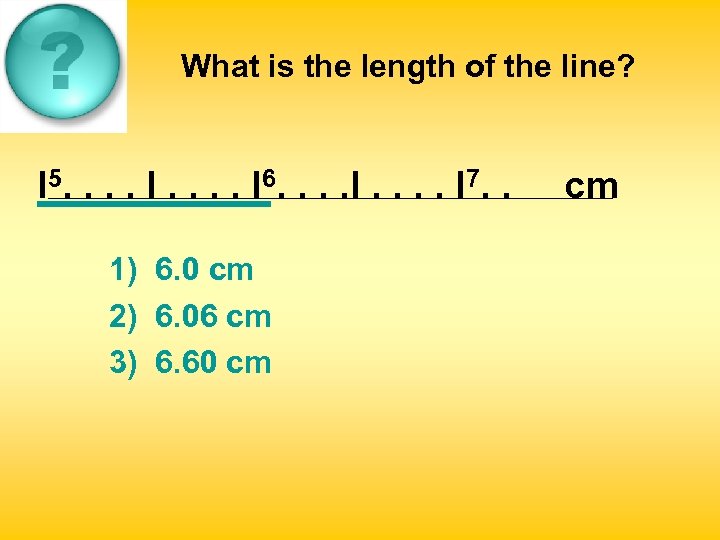 What is the length of the line? l 5. . . . I 6. . . . I 7. . 1) 6. 0 cm 2) 6. 06 cm 3) 6. 60 cm cm
What is the length of the line? l 5. . . . I 6. . . . I 7. . 1) 6. 0 cm 2) 6. 06 cm 3) 6. 60 cm cm
 Exact Numbers l Obtained when you count objects 2 soccer balls 1 watch 3 pizzas l Obtained from a defined relationship 1 foot = 12 inches 1 meters = 100 cm l Not obtained with measuring tools
Exact Numbers l Obtained when you count objects 2 soccer balls 1 watch 3 pizzas l Obtained from a defined relationship 1 foot = 12 inches 1 meters = 100 cm l Not obtained with measuring tools
 Classify each of the following as an exact (E) or a measured (M) number. A. ___Gold melts at 1064°C A. _M_Goldmelts at 1064°C B. ___1 yard = 3 feet B. _E_1 yard = 3 feet C. ___A red blood cell with diameter 6 x 10 -4 cm C. _M_Ared blood cell with diameter 6 x 10 -4 cm D. ___There were 6 hats on the shelf D. _E_There were 6 hats on the shelf E. ___A can of soda contains 355 m. L of soda E. _M_Acan of soda contains 355 m. L of soda
Classify each of the following as an exact (E) or a measured (M) number. A. ___Gold melts at 1064°C A. _M_Goldmelts at 1064°C B. ___1 yard = 3 feet B. _E_1 yard = 3 feet C. ___A red blood cell with diameter 6 x 10 -4 cm C. _M_Ared blood cell with diameter 6 x 10 -4 cm D. ___There were 6 hats on the shelf D. _E_There were 6 hats on the shelf E. ___A can of soda contains 355 m. L of soda E. _M_Acan of soda contains 355 m. L of soda
 Significant Figures in Calculations
Significant Figures in Calculations
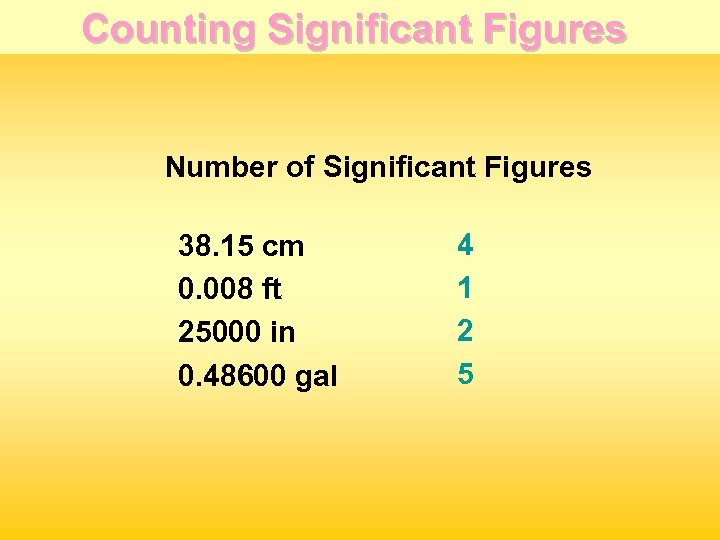 Counting Significant Figures Number of Significant Figures 38. 15 cm 0. 008 ft 25000 in 0. 48600 gal 4 1 2 5
Counting Significant Figures Number of Significant Figures 38. 15 cm 0. 008 ft 25000 in 0. 48600 gal 4 1 2 5
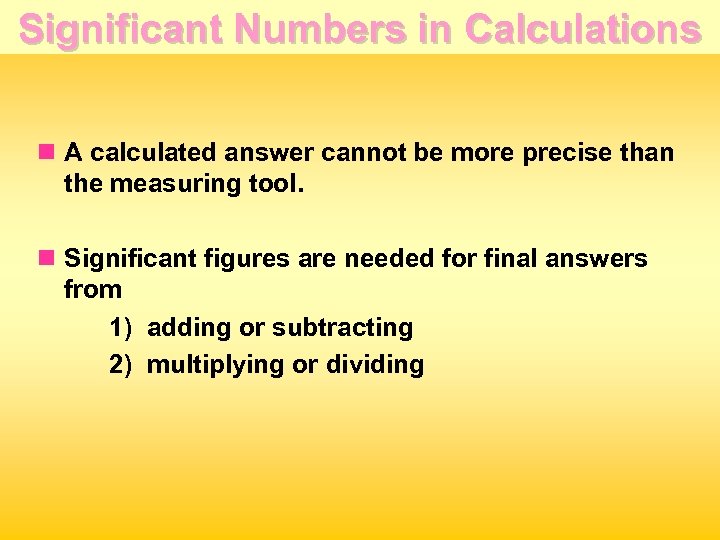 Significant Numbers in Calculations n A calculated answer cannot be more precise than the measuring tool. n Significant figures are needed for final answers from 1) adding or subtracting 2) multiplying or dividing
Significant Numbers in Calculations n A calculated answer cannot be more precise than the measuring tool. n Significant figures are needed for final answers from 1) adding or subtracting 2) multiplying or dividing
 Adding and Subtracting The answer has the same number of decimal places as the measurement with the fewest decimal places. 25. 2 ONE decimal place + 1. 34 TWO decimal places 26. 54 answer 26. 5 ONE decimal place
Adding and Subtracting The answer has the same number of decimal places as the measurement with the fewest decimal places. 25. 2 ONE decimal place + 1. 34 TWO decimal places 26. 54 answer 26. 5 ONE decimal place
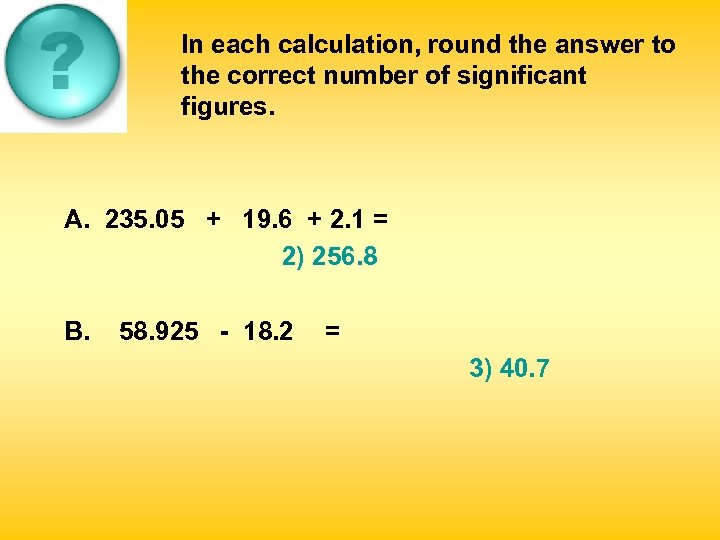 In each calculation, round the answer to the correct number of significant figures. A. 235. 05 + 19. 6 + 2. 1 = 1) 256. 75 2) 256. 8 B. 58. 925 - 18. 2 = 1) 40. 725 2) 40. 73 3) 257 3) 40. 7
In each calculation, round the answer to the correct number of significant figures. A. 235. 05 + 19. 6 + 2. 1 = 1) 256. 75 2) 256. 8 B. 58. 925 - 18. 2 = 1) 40. 725 2) 40. 73 3) 257 3) 40. 7
 Multiplying and Dividing Round (or add zeros) to the calculated answer until you have the same number of significant figures as the measurement with the fewest significant figures.
Multiplying and Dividing Round (or add zeros) to the calculated answer until you have the same number of significant figures as the measurement with the fewest significant figures.
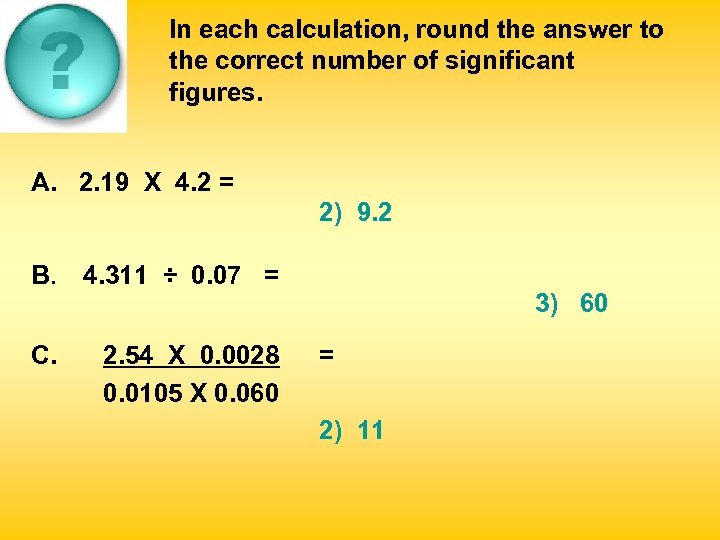 In each calculation, round the answer to the correct number of significant figures. A. 2. 19 X 4. 2 = 1) 9 B. 4. 311 ÷ 0. 07 = 1) 61. 58 C. 2. 54 X 0. 0028 0. 0105 X 0. 060 1) 11. 3 2) 9. 2 3) 9. 198 2) 62 3) 60 = 2) 11 3) 0. 041
In each calculation, round the answer to the correct number of significant figures. A. 2. 19 X 4. 2 = 1) 9 B. 4. 311 ÷ 0. 07 = 1) 61. 58 C. 2. 54 X 0. 0028 0. 0105 X 0. 060 1) 11. 3 2) 9. 2 3) 9. 198 2) 62 3) 60 = 2) 11 3) 0. 041
 Prefixes and Equalities
Prefixes and Equalities
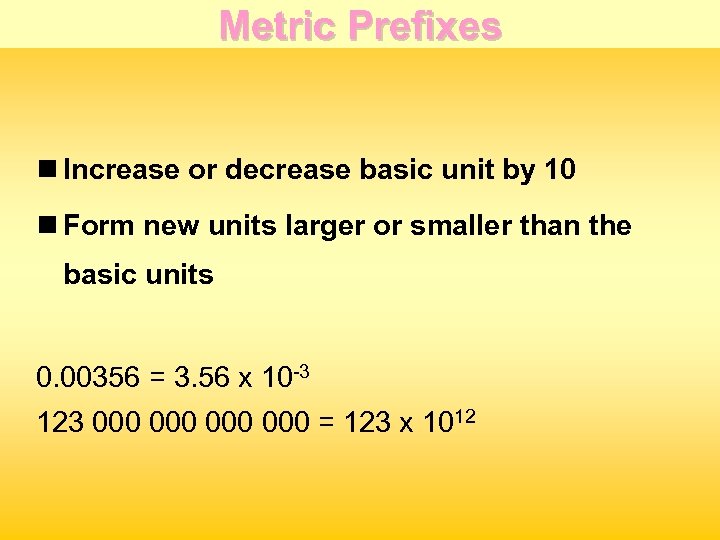 Metric Prefixes n Increase or decrease basic unit by 10 n Form new units larger or smaller than the basic units 0. 00356 = 3. 56 x 10 -3 123 000 000 = 123 x 1012
Metric Prefixes n Increase or decrease basic unit by 10 n Form new units larger or smaller than the basic units 0. 00356 = 3. 56 x 10 -3 123 000 000 = 123 x 1012
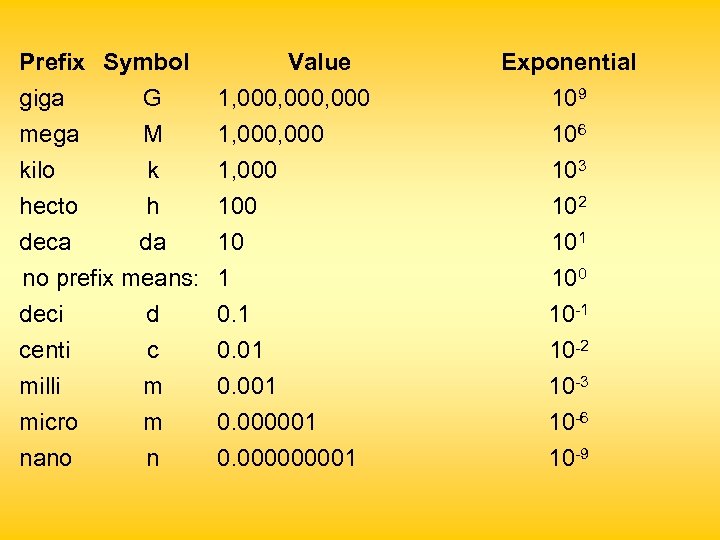 Prefix Symbol Value Exponential giga G 1, 000, 000 109 mega kilo M k 1, 000 106 103 100 10 1 0. 01 0. 000001 0. 00001 102 101 100 10 -1 10 -2 10 -3 10 -6 10 -9 hecto h deca da no prefix means: deci d centi c milli m micro m nano n
Prefix Symbol Value Exponential giga G 1, 000, 000 109 mega kilo M k 1, 000 106 103 100 10 1 0. 01 0. 000001 0. 00001 102 101 100 10 -1 10 -2 10 -3 10 -6 10 -9 hecto h deca da no prefix means: deci d centi c milli m micro m nano n
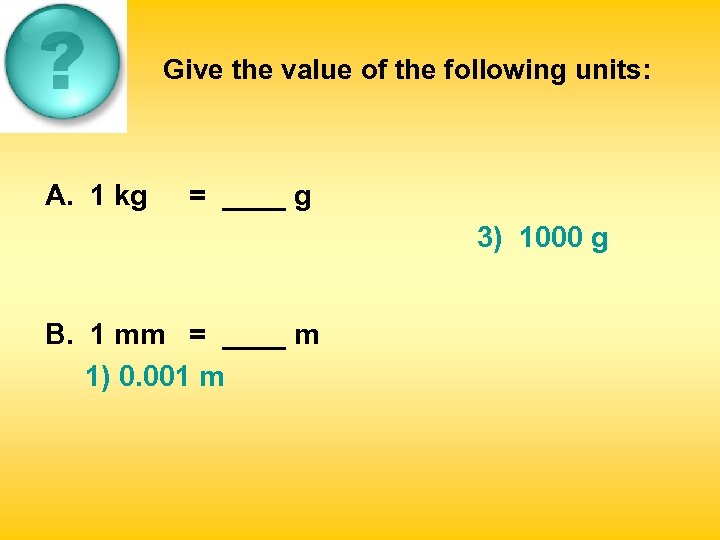 Give the value of the following units: A. 1 kg = ____ g 1) 10 g 2) 100 g 3) 1000 g B. 1 mm = ____ m 1) 0. 001 m 2) 0. 01 m 3) 0. 1 m
Give the value of the following units: A. 1 kg = ____ g 1) 10 g 2) 100 g 3) 1000 g B. 1 mm = ____ m 1) 0. 001 m 2) 0. 01 m 3) 0. 1 m
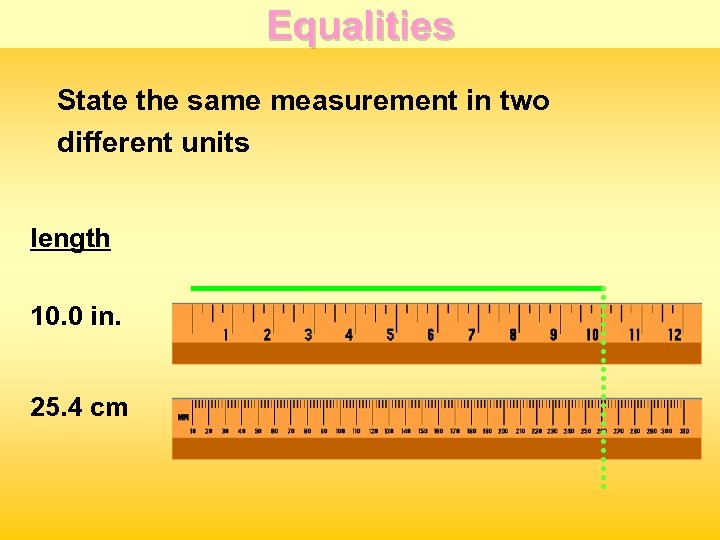 Equalities State the same measurement in two different units length 10. 0 in. 25. 4 cm
Equalities State the same measurement in two different units length 10. 0 in. 25. 4 cm
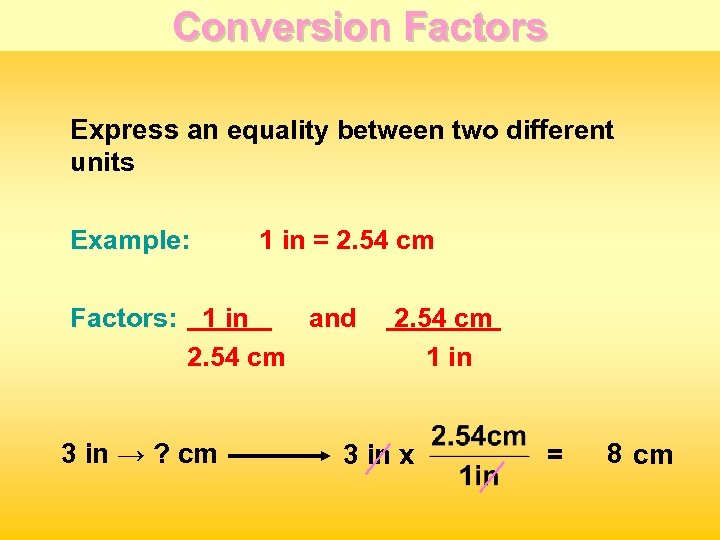 Conversion Factors Express an equality between two different units Example: 1 in = 2. 54 cm Factors: 1 in and 2. 54 cm 3 in → ? cm 2. 54 cm 1 in 3 in x 8 = 7. 62 cm
Conversion Factors Express an equality between two different units Example: 1 in = 2. 54 cm Factors: 1 in and 2. 54 cm 3 in → ? cm 2. 54 cm 1 in 3 in x 8 = 7. 62 cm
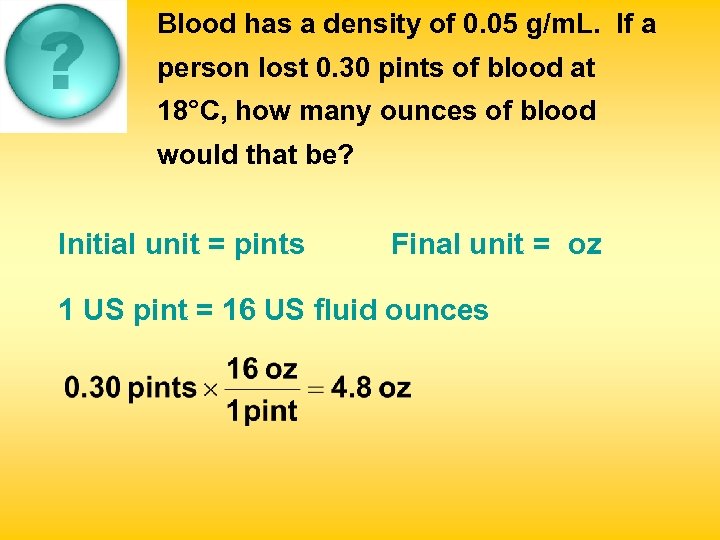 Blood has a density of 0. 05 g/m. L. If a person lost 0. 30 pints of blood at 18°C, how many ounces of blood would that be? Initial unit = pints Final unit = oz 1 US pint = 16 US fluid ounces
Blood has a density of 0. 05 g/m. L. If a person lost 0. 30 pints of blood at 18°C, how many ounces of blood would that be? Initial unit = pints Final unit = oz 1 US pint = 16 US fluid ounces
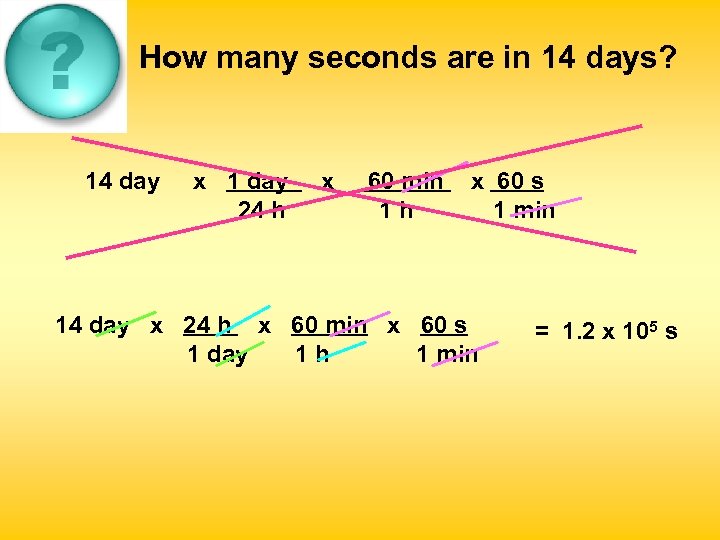 How many seconds are in 14 days? 14 day x 1 day 24 h x 60 min 1 h x 60 s 1 min 14 day x 24 h x 60 min x 60 s 1 day 1 h 1 min = 1. 2 x 105 s
How many seconds are in 14 days? 14 day x 1 day 24 h x 60 min 1 h x 60 s 1 min 14 day x 24 h x 60 min x 60 s 1 day 1 h 1 min = 1. 2 x 105 s
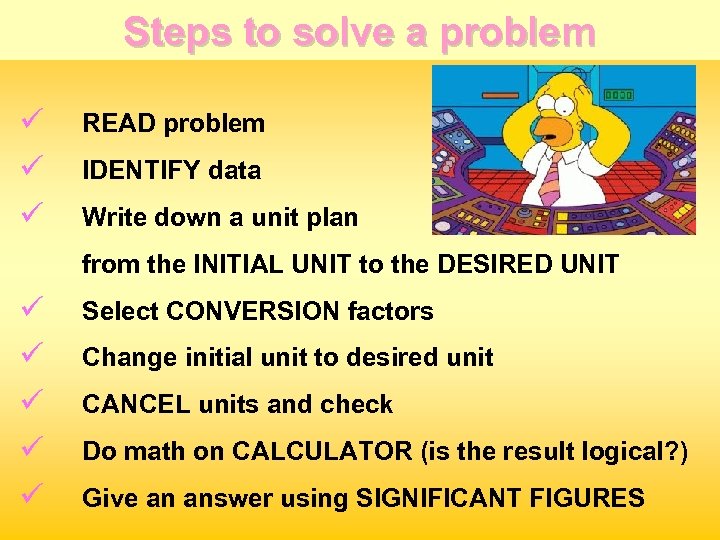 Steps to solve a problem ü ü ü READ problem IDENTIFY data Write down a unit plan from the INITIAL UNIT to the DESIRED UNIT ü ü ü Select CONVERSION factors Change initial unit to desired unit CANCEL units and check Do math on CALCULATOR (is the result logical? ) Give an answer using SIGNIFICANT FIGURES
Steps to solve a problem ü ü ü READ problem IDENTIFY data Write down a unit plan from the INITIAL UNIT to the DESIRED UNIT ü ü ü Select CONVERSION factors Change initial unit to desired unit CANCEL units and check Do math on CALCULATOR (is the result logical? ) Give an answer using SIGNIFICANT FIGURES
 Measuring Temperature
Measuring Temperature
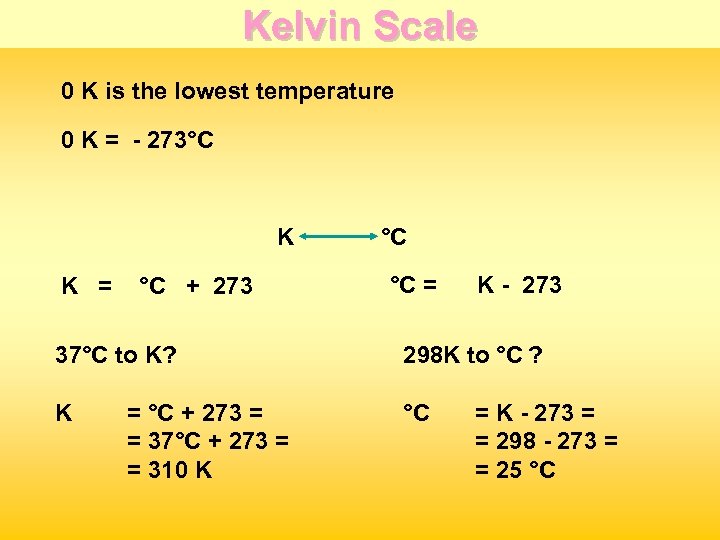 Kelvin Scale 0 K is the lowest temperature 0 K = - 273°C K K = °C + 273 °C °C = K - 273 37°C to K? 298 K to °C ? K °C = °C + 273 = = 37°C + 273 = = 310 K = K - 273 = = 298 - 273 = = 25 °C
Kelvin Scale 0 K is the lowest temperature 0 K = - 273°C K K = °C + 273 °C °C = K - 273 37°C to K? 298 K to °C ? K °C = °C + 273 = = 37°C + 273 = = 310 K = K - 273 = = 298 - 273 = = 25 °C
 Density
Density
 Density compares the mass of an object to its volume Note: 1 m. L = 1 cm 3
Density compares the mass of an object to its volume Note: 1 m. L = 1 cm 3
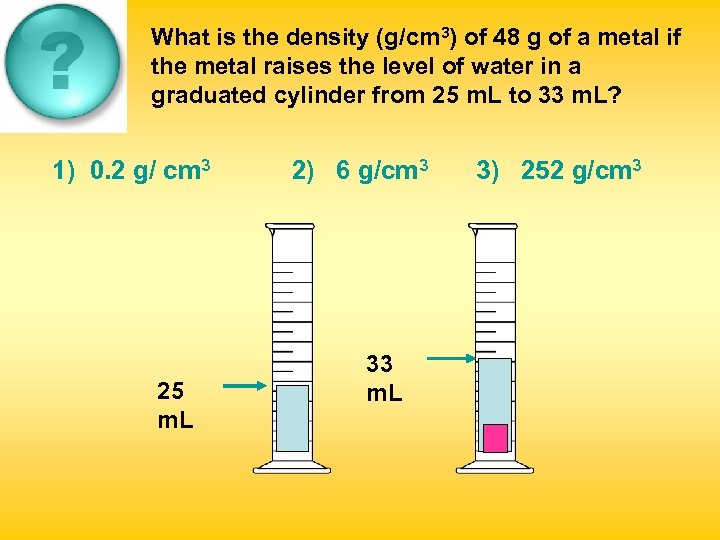 What is the density (g/cm 3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L? 1) 0. 2 g/ cm 3 25 m. L 2) 6 g/cm 3 33 m. L 3) 252 g/cm 3
What is the density (g/cm 3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L? 1) 0. 2 g/ cm 3 25 m. L 2) 6 g/cm 3 33 m. L 3) 252 g/cm 3
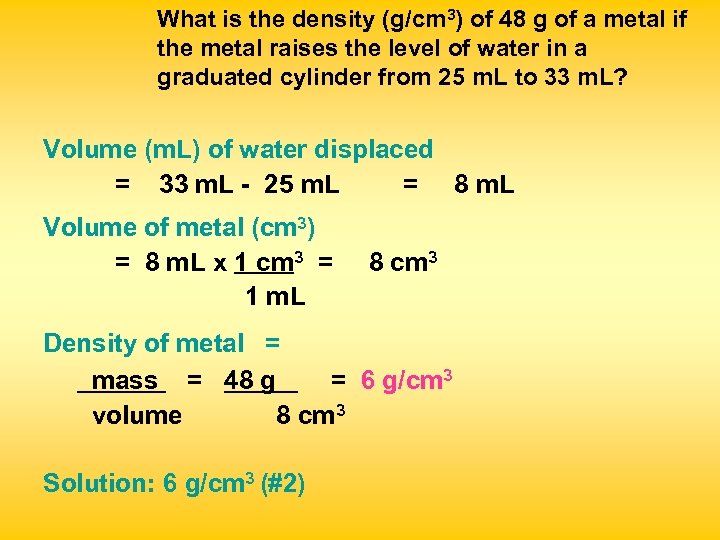 What is the density (g/cm 3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L? Volume (m. L) of water displaced = 33 m. L - 25 m. L = 8 m. L Volume of metal (cm 3) = 8 m. L x 1 cm 3 = 1 m. L 8 cm 3 Density of metal = mass = 48 g = 6 g/cm 3 volume 8 cm 3 Solution: 6 g/cm 3 (#2)
What is the density (g/cm 3) of 48 g of a metal if the metal raises the level of water in a graduated cylinder from 25 m. L to 33 m. L? Volume (m. L) of water displaced = 33 m. L - 25 m. L = 8 m. L Volume of metal (cm 3) = 8 m. L x 1 cm 3 = 1 m. L 8 cm 3 Density of metal = mass = 48 g = 6 g/cm 3 volume 8 cm 3 Solution: 6 g/cm 3 (#2)
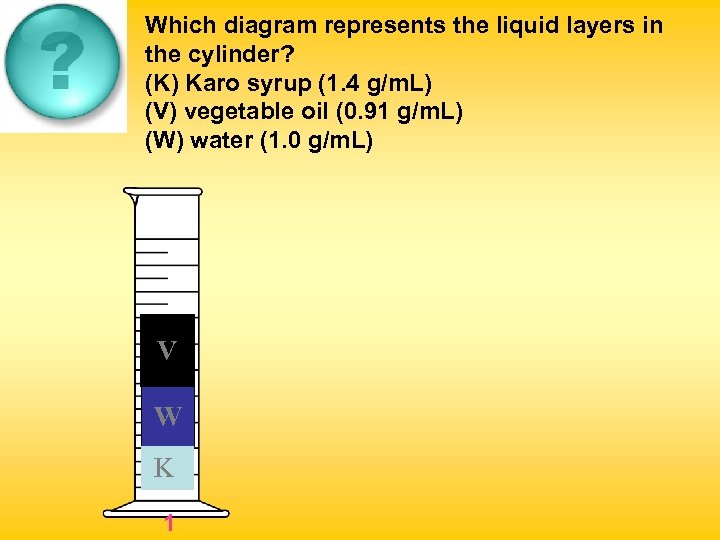 Which diagram represents the liquid layers in the cylinder? (K) Karo syrup (1. 4 g/m. L) (V) vegetable oil (0. 91 g/m. L) (W) water (1. 0 g/m. L) V W K 1 W K K V V W 2 3
Which diagram represents the liquid layers in the cylinder? (K) Karo syrup (1. 4 g/m. L) (V) vegetable oil (0. 91 g/m. L) (W) water (1. 0 g/m. L) V W K 1 W K K V V W 2 3
 Density as Conversion Factor A substance has a density of 3. 8 g/m. L. Density Equality = 3. 8 g/m. L 3. 8 g = 1 m. L = 1 cm 3 Conversion factors:
Density as Conversion Factor A substance has a density of 3. 8 g/m. L. Density Equality = 3. 8 g/m. L 3. 8 g = 1 m. L = 1 cm 3 Conversion factors:
 The density of octane, a component of gasoline, is 0. 702 g/m. L. What is the mass, in kg, of 875 m. L of octane? 1) 0. 614 kg 2) 614 kg 3) 1. 25 kg
The density of octane, a component of gasoline, is 0. 702 g/m. L. What is the mass, in kg, of 875 m. L of octane? 1) 0. 614 kg 2) 614 kg 3) 1. 25 kg
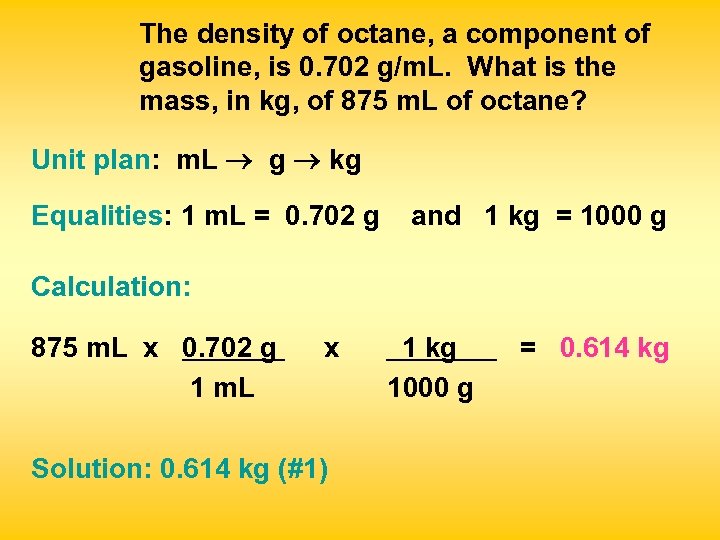 The density of octane, a component of gasoline, is 0. 702 g/m. L. What is the mass, in kg, of 875 m. L of octane? Unit plan: m. L g kg Equalities: 1 m. L = 0. 702 g and 1 kg = 1000 g Calculation: 875 m. L x 0. 702 g 1 m. L x Solution: 0. 614 kg (#1) 1 kg 1000 g = 0. 614 kg
The density of octane, a component of gasoline, is 0. 702 g/m. L. What is the mass, in kg, of 875 m. L of octane? Unit plan: m. L g kg Equalities: 1 m. L = 0. 702 g and 1 kg = 1000 g Calculation: 875 m. L x 0. 702 g 1 m. L x Solution: 0. 614 kg (#1) 1 kg 1000 g = 0. 614 kg


