0201ee0bd87f002264df31389d0b300b.ppt
- Количество слайдов: 82
 CHEM 106 Fundamentals I Measurement: Units & Standards Measurement, Conversions & Calculations Dr. Ron Rusay
CHEM 106 Fundamentals I Measurement: Units & Standards Measurement, Conversions & Calculations Dr. Ron Rusay
 Chemistry & STEAM Fundamentals I Measurement, Conversions & Calculations Dr. Ron Rusay
Chemistry & STEAM Fundamentals I Measurement, Conversions & Calculations Dr. Ron Rusay
 CHEM 106 Basic Measurements: for stuff that you can see or sense • LENGTH, WIDTH, HEIGHT, (DIAMETER) • TIME • VOLUME (occupied space) • TEMPERATURE • MASS (weight) • Qualitative vs. Quantitative • Eg. Qualitative: Old (sloth dung) vs. Young (you? ) • Quantitative: 38, 000 year old (dung) vs. a 20 year old (you? )
CHEM 106 Basic Measurements: for stuff that you can see or sense • LENGTH, WIDTH, HEIGHT, (DIAMETER) • TIME • VOLUME (occupied space) • TEMPERATURE • MASS (weight) • Qualitative vs. Quantitative • Eg. Qualitative: Old (sloth dung) vs. Young (you? ) • Quantitative: 38, 000 year old (dung) vs. a 20 year old (you? )
 Units of Measure
Units of Measure
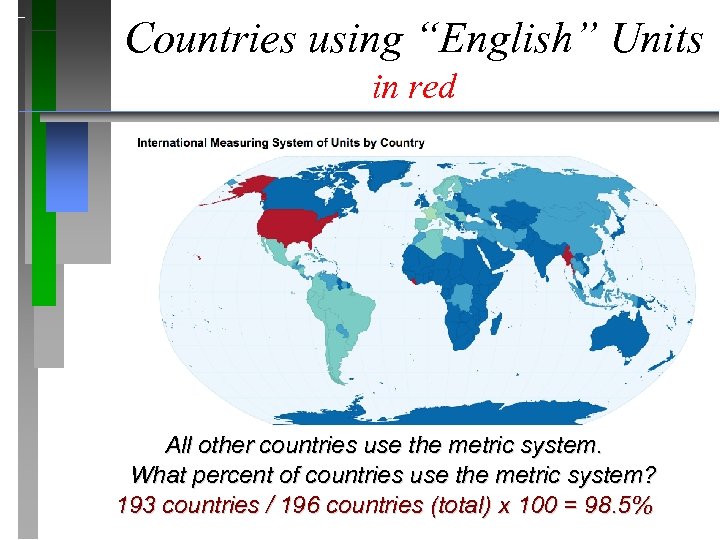 Countries using “English” Units in red All other countries use the metric system. What percent of countries use the metric system? 193 countries / 196 countries (total) x 100 = 98. 5%
Countries using “English” Units in red All other countries use the metric system. What percent of countries use the metric system? 193 countries / 196 countries (total) x 100 = 98. 5%
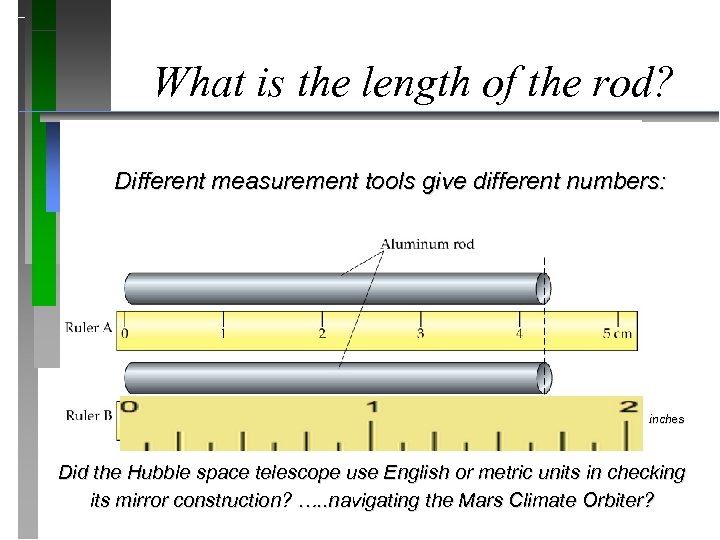 What is the length of the rod? Different measurement tools give different numbers: inches Did the Hubble space telescope use English or metric units in checking its mirror construction? …. . navigating the Mars Climate Orbiter?
What is the length of the rod? Different measurement tools give different numbers: inches Did the Hubble space telescope use English or metric units in checking its mirror construction? …. . navigating the Mars Climate Orbiter?
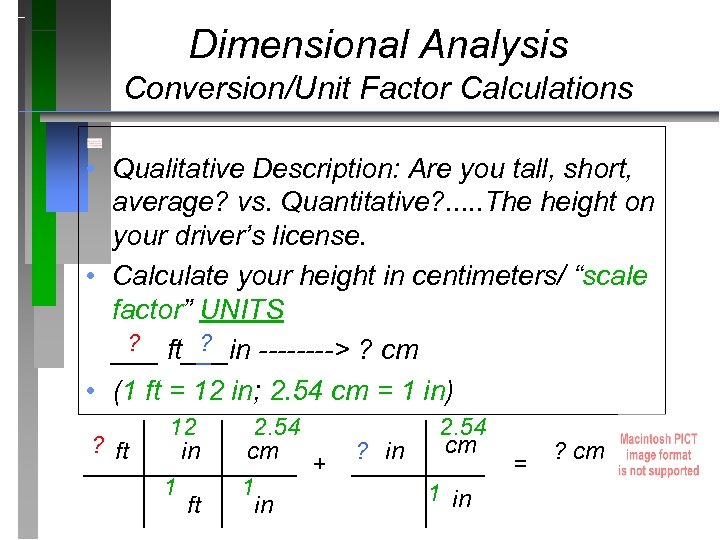 Dimensional Analysis Conversion/Unit Factor Calculations • Qualitative Description: Are you tall, short, average? vs. Quantitative? . . . The height on your driver’s license. • Calculate your height in centimeters/ “scale factor” UNITS ? ? ___ ft___in ----> ? cm • (1 ft = 12 in; 2. 54 cm = 1 in) 12 2. 54 cm ? ft in ? cm cm ________ + _____ = 1 1 1 in ft in
Dimensional Analysis Conversion/Unit Factor Calculations • Qualitative Description: Are you tall, short, average? vs. Quantitative? . . . The height on your driver’s license. • Calculate your height in centimeters/ “scale factor” UNITS ? ? ___ ft___in ----> ? cm • (1 ft = 12 in; 2. 54 cm = 1 in) 12 2. 54 cm ? ft in ? cm cm ________ + _____ = 1 1 1 in ft in
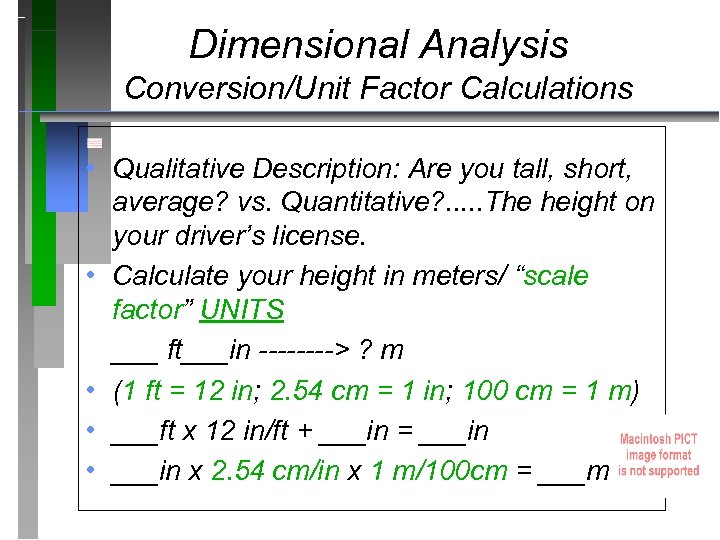 Dimensional Analysis Conversion/Unit Factor Calculations • Qualitative Description: Are you tall, short, average? vs. Quantitative? . . . The height on your driver’s license. • Calculate your height in meters/ “scale factor” UNITS ___ ft___in ----> ? m • (1 ft = 12 in; 2. 54 cm = 1 in; 100 cm = 1 m) • ___ft x 12 in/ft + ___in = ___in • ___in x 2. 54 cm/in x 1 m/100 cm = ___m
Dimensional Analysis Conversion/Unit Factor Calculations • Qualitative Description: Are you tall, short, average? vs. Quantitative? . . . The height on your driver’s license. • Calculate your height in meters/ “scale factor” UNITS ___ ft___in ----> ? m • (1 ft = 12 in; 2. 54 cm = 1 in; 100 cm = 1 m) • ___ft x 12 in/ft + ___in = ___in • ___in x 2. 54 cm/in x 1 m/100 cm = ___m
 White Dwarf Stars After it was fixed! Did the Hubble space telescope use English or metric units in checking its mirror construction? …. BOTH …. . navigating the Mars Climate Orbiter? BOTH …. Orbiter was LOST!
White Dwarf Stars After it was fixed! Did the Hubble space telescope use English or metric units in checking its mirror construction? …. BOTH …. . navigating the Mars Climate Orbiter? BOTH …. Orbiter was LOST!
 Measurement & Units SI units & common units in General Chemistry • Quantitative vs. Qualitative • MASS (Chem 106: gram; SI: kg; other mg) • LENGTH (Chem 106: cm & mm; SI: m; other km) • TEMPERATURE (Celsius & Kelvin; SI: K) • VOLUME (Chem 106: m. L; SI: Liter; other d. L) • CHEMICAL AMOUNT: mole (mol); SI: (mol); other (mmol)
Measurement & Units SI units & common units in General Chemistry • Quantitative vs. Qualitative • MASS (Chem 106: gram; SI: kg; other mg) • LENGTH (Chem 106: cm & mm; SI: m; other km) • TEMPERATURE (Celsius & Kelvin; SI: K) • VOLUME (Chem 106: m. L; SI: Liter; other d. L) • CHEMICAL AMOUNT: mole (mol); SI: (mol); other (mmol)
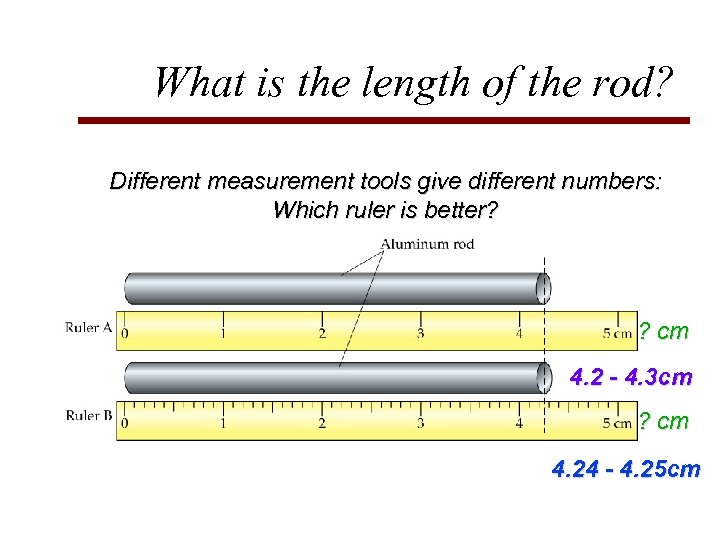
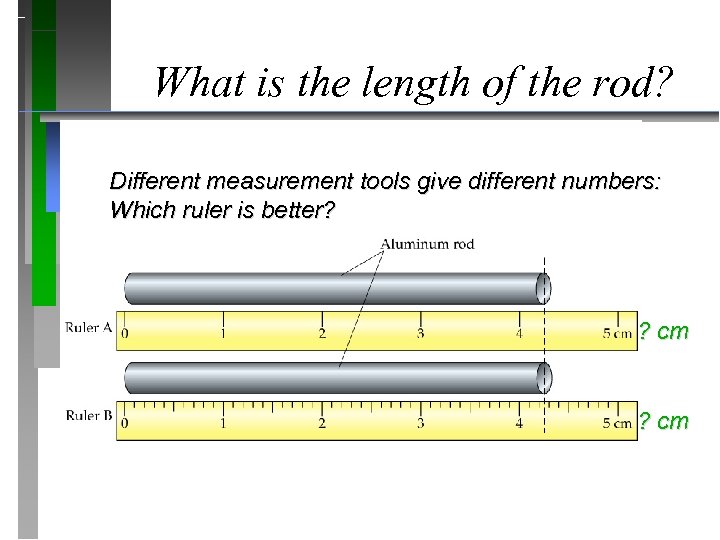 What is the length of the rod? Different measurement tools give different numbers: Which ruler is better? ? cm
What is the length of the rod? Different measurement tools give different numbers: Which ruler is better? ? cm
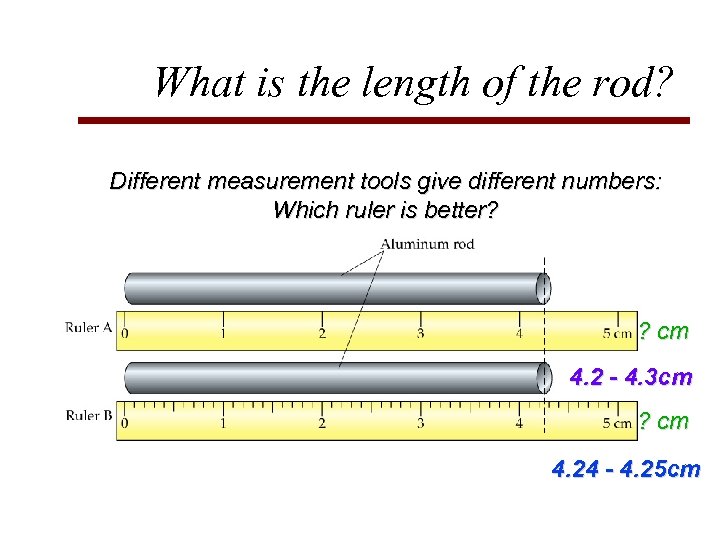 What is the length of the rod? Different measurement tools give different numbers: Which ruler is better? ? cm 4. 2 - 4. 3 cm ? cm 4. 24 - 4. 25 cm
What is the length of the rod? Different measurement tools give different numbers: Which ruler is better? ? cm 4. 2 - 4. 3 cm ? cm 4. 24 - 4. 25 cm
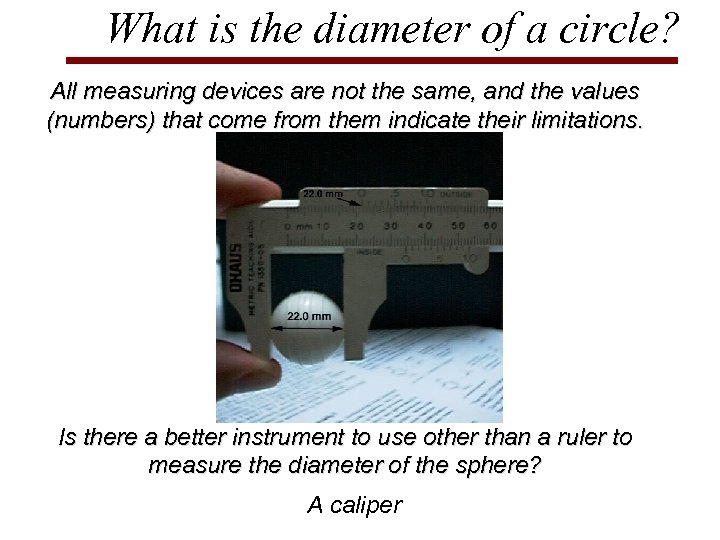 What is the diameter of a circle? All measuring devices are not the same, and the values (numbers) that come from them indicate their limitations. Is there a better instrument to use other than a ruler to measure the diameter of the sphere? A caliper
What is the diameter of a circle? All measuring devices are not the same, and the values (numbers) that come from them indicate their limitations. Is there a better instrument to use other than a ruler to measure the diameter of the sphere? A caliper
 Mass Determination (Weighing Devices: Balances)
Mass Determination (Weighing Devices: Balances)
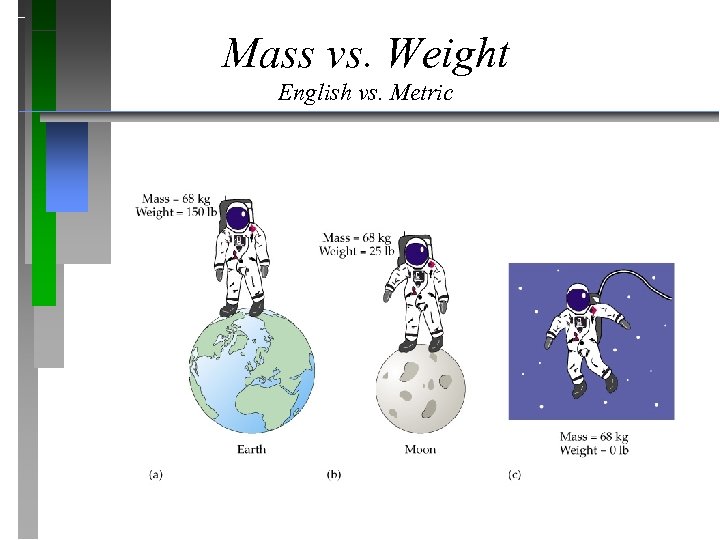 Mass vs. Weight English vs. Metric
Mass vs. Weight English vs. Metric
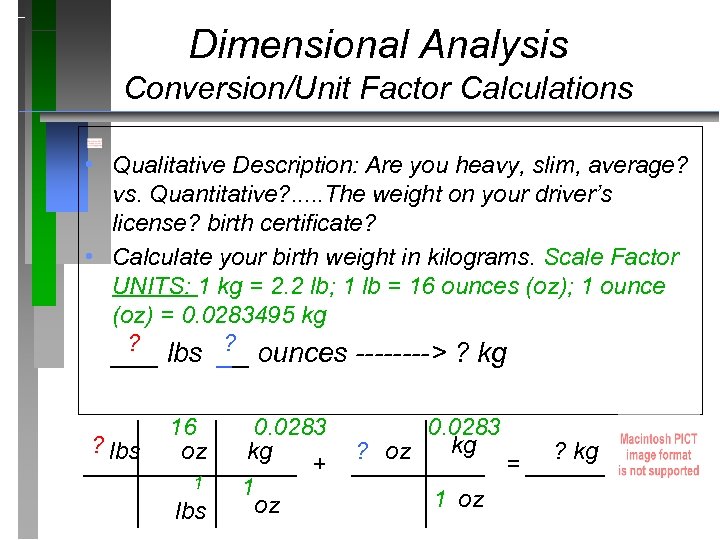 Dimensional Analysis Conversion/Unit Factor Calculations • Qualitative Description: Are you heavy, slim, average? vs. Quantitative? . . . The weight on your driver’s license? birth certificate? • Calculate your birth weight in kilograms. Scale Factor UNITS: 1 kg = 2. 2 lb; 1 lb = 16 ounces (oz); 1 ounce (oz) = 0. 0283495 kg ? ? ___ lbs __ ounces ----> ? kg 16 0. 0283 ? lbs kg oz ? kg kg ________ + _____ = ______ 1 1 1 oz lbs oz
Dimensional Analysis Conversion/Unit Factor Calculations • Qualitative Description: Are you heavy, slim, average? vs. Quantitative? . . . The weight on your driver’s license? birth certificate? • Calculate your birth weight in kilograms. Scale Factor UNITS: 1 kg = 2. 2 lb; 1 lb = 16 ounces (oz); 1 ounce (oz) = 0. 0283495 kg ? ? ___ lbs __ ounces ----> ? kg 16 0. 0283 ? lbs kg oz ? kg kg ________ + _____ = ______ 1 1 1 oz lbs oz
 Volume (Liquid Measurement Tools) (CHEM 106) METRIC UNITS: milliliter m. L m = milli L = liter
Volume (Liquid Measurement Tools) (CHEM 106) METRIC UNITS: milliliter m. L m = milli L = liter
 English Metric Comparisons
English Metric Comparisons
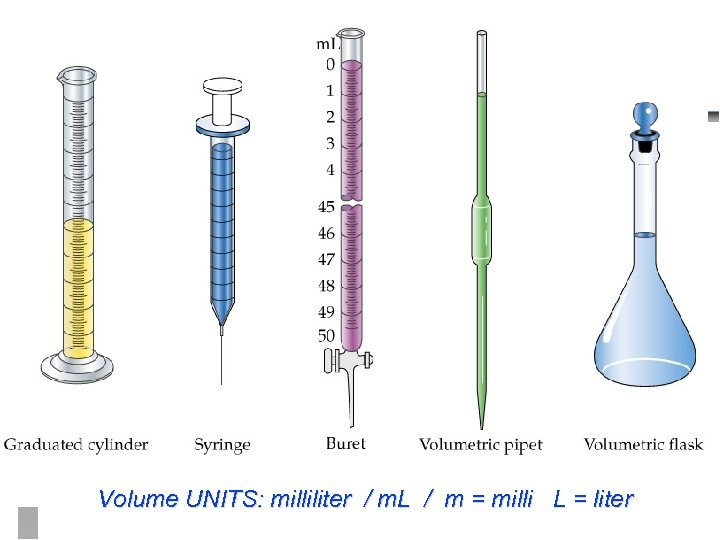 Volume UNITS: milliliter / m. L / m = milli L = liter
Volume UNITS: milliliter / m. L / m = milli L = liter
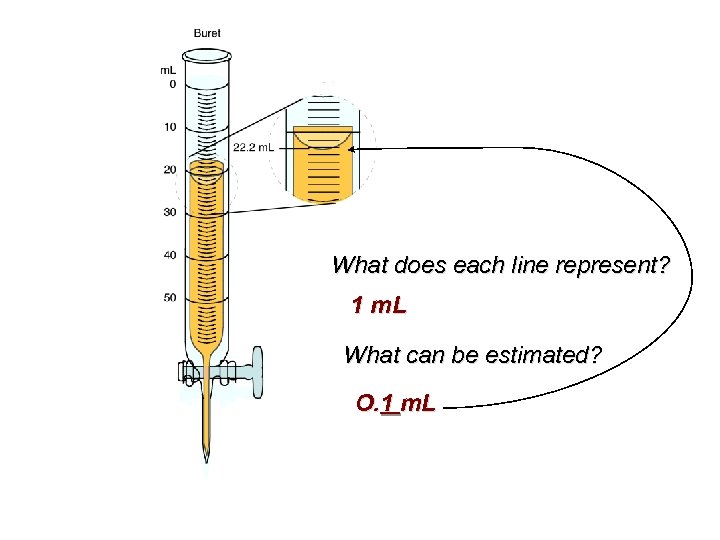 What does each line represent? 1 m. L What can be estimated? O. 1 m. L
What does each line represent? 1 m. L What can be estimated? O. 1 m. L
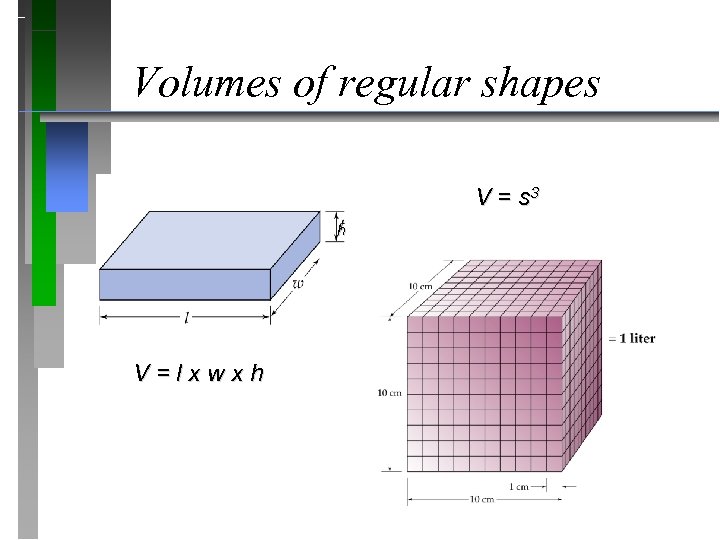 Volumes of regular shapes V = s 3 h V = l x w x h
Volumes of regular shapes V = s 3 h V = l x w x h
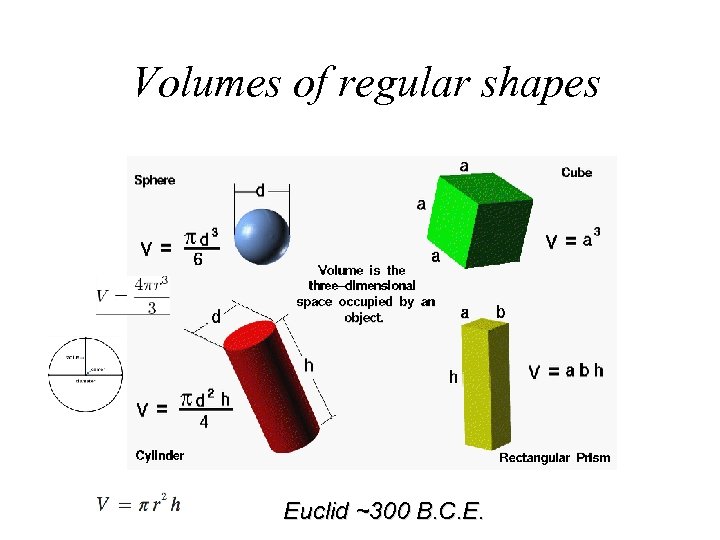 Volumes of regular shapes Euclid ~300 B. C. E.
Volumes of regular shapes Euclid ~300 B. C. E.
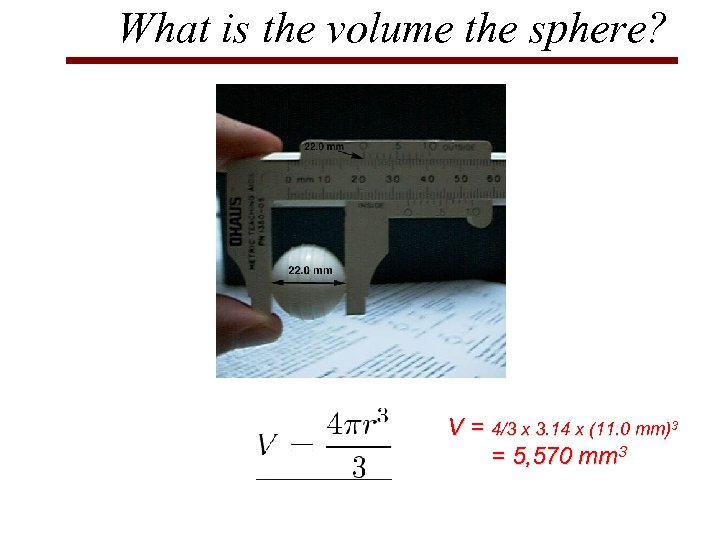 What is the volume the sphere? V = 4/3 x 3. 14 x (11. 0 mm)3 = 5, 570 mm 3
What is the volume the sphere? V = 4/3 x 3. 14 x (11. 0 mm)3 = 5, 570 mm 3
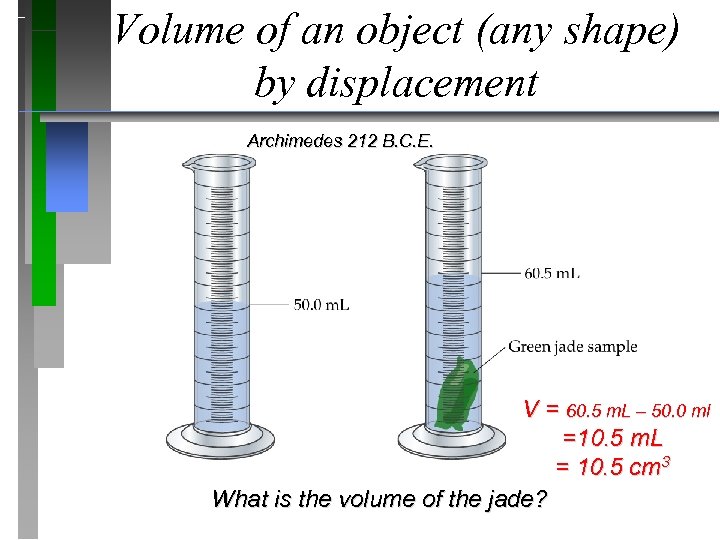 Volume of an object (any shape) by displacement Archimedes 212 B. C. E. V = 60. 5 m. L – 50. 0 ml =10. 5 m. L = 10. 5 cm 3 What is the volume of the jade?
Volume of an object (any shape) by displacement Archimedes 212 B. C. E. V = 60. 5 m. L – 50. 0 ml =10. 5 m. L = 10. 5 cm 3 What is the volume of the jade?
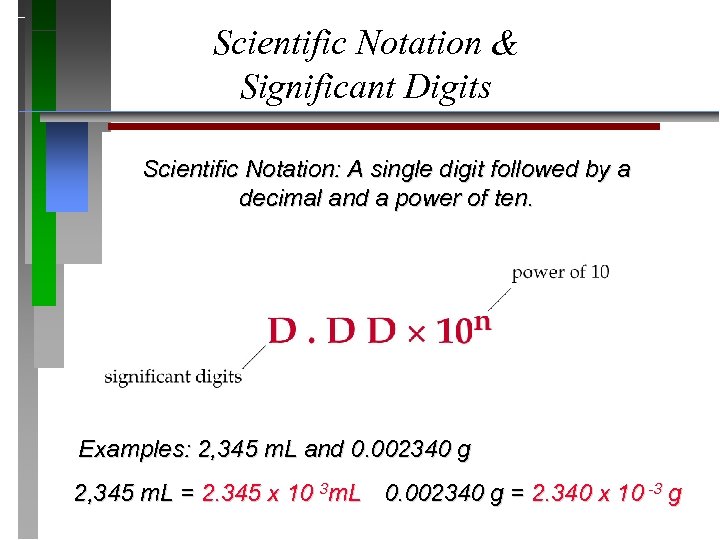 Scientific Notation & Significant Digits Scientific Notation: A single digit followed by a decimal and a power of ten. Examples: 2, 345 m. L and 0. 002340 g 2, 345 m. L = 2. 345 x 10 3 m. L 0. 002340 g = 2. 340 x 10 -3 g
Scientific Notation & Significant Digits Scientific Notation: A single digit followed by a decimal and a power of ten. Examples: 2, 345 m. L and 0. 002340 g 2, 345 m. L = 2. 345 x 10 3 m. L 0. 002340 g = 2. 340 x 10 -3 g
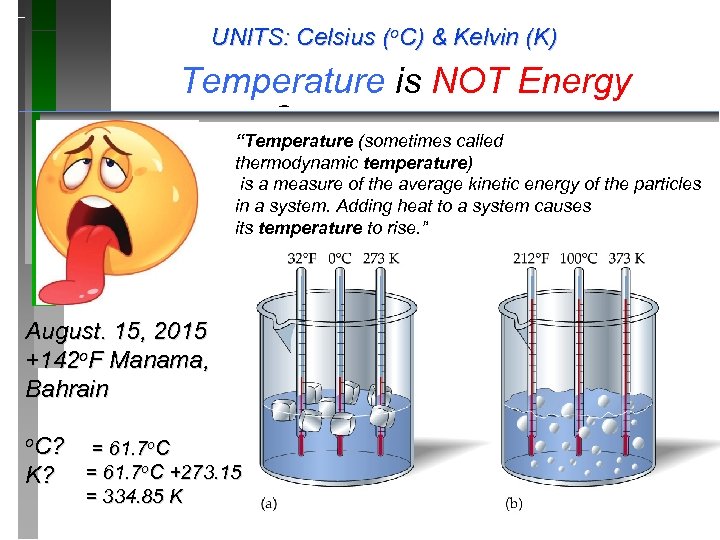 UNITS: Celsius (o. C) & Kelvin (K) Temperature is NOT Energy Temperature Scales “Temperature (sometimes called thermodynamic temperature) is a measure of the average kinetic energy of the particles in a system. Adding heat to a system causes its temperature to rise. ” August. 15, 2015 +142 o. F Manama, Bahrain o. C? K? = 61. 7 o. C +273. 15 = 334. 85 K
UNITS: Celsius (o. C) & Kelvin (K) Temperature is NOT Energy Temperature Scales “Temperature (sometimes called thermodynamic temperature) is a measure of the average kinetic energy of the particles in a system. Adding heat to a system causes its temperature to rise. ” August. 15, 2015 +142 o. F Manama, Bahrain o. C? K? = 61. 7 o. C +273. 15 = 334. 85 K
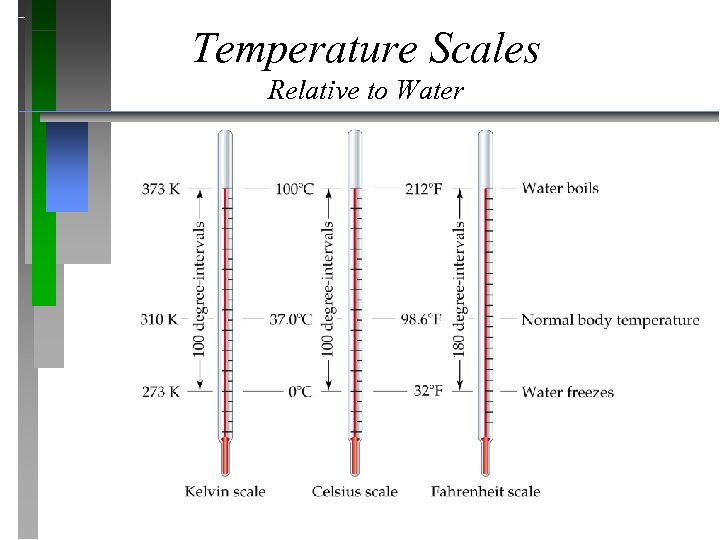 Temperature Scales Relative to Water
Temperature Scales Relative to Water
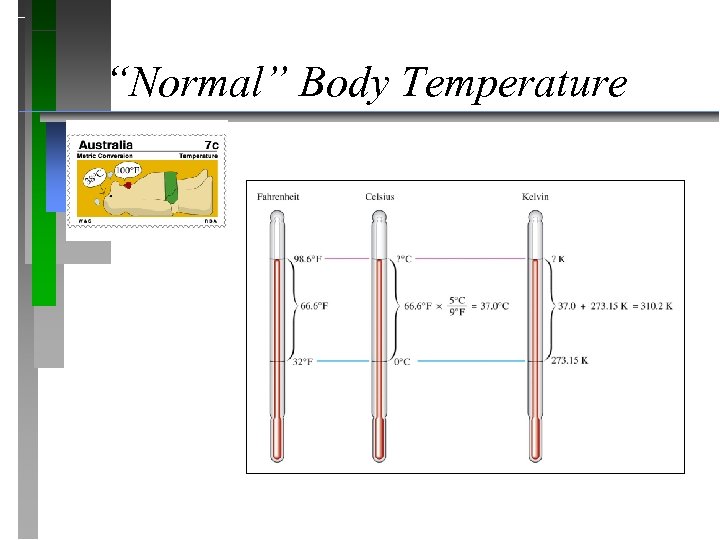 “Normal” Body Temperature
“Normal” Body Temperature
 QUESTION Dr. R. walks into class and claims, “It is very cold in here today. It feels like 242 K. ” If that were the temperature, would you agree that you would feel cold? What would that be in Celsius degrees? A. B. C. D. I agree, that would be 31°C. I agree, that would be – 31°C. I do not agree, that would be 515°C.
QUESTION Dr. R. walks into class and claims, “It is very cold in here today. It feels like 242 K. ” If that were the temperature, would you agree that you would feel cold? What would that be in Celsius degrees? A. B. C. D. I agree, that would be 31°C. I agree, that would be – 31°C. I do not agree, that would be 515°C.
 Answer Dr. R. walks into class and claims, “It is very cold in here today. It feels like 242 K. ” If that were the temperature, would you agree that you would feel cold? What would that be in Celsius degrees? A. B. C. D. I agree, that would be 31°C. I agree, that would be – 31°C. I do not agree, that would be 515°C. The formula to use is K = °C + 273. 15. Rearranged to yield K – 273. 15 = °C.
Answer Dr. R. walks into class and claims, “It is very cold in here today. It feels like 242 K. ” If that were the temperature, would you agree that you would feel cold? What would that be in Celsius degrees? A. B. C. D. I agree, that would be 31°C. I agree, that would be – 31°C. I do not agree, that would be 515°C. The formula to use is K = °C + 273. 15. Rearranged to yield K – 273. 15 = °C.
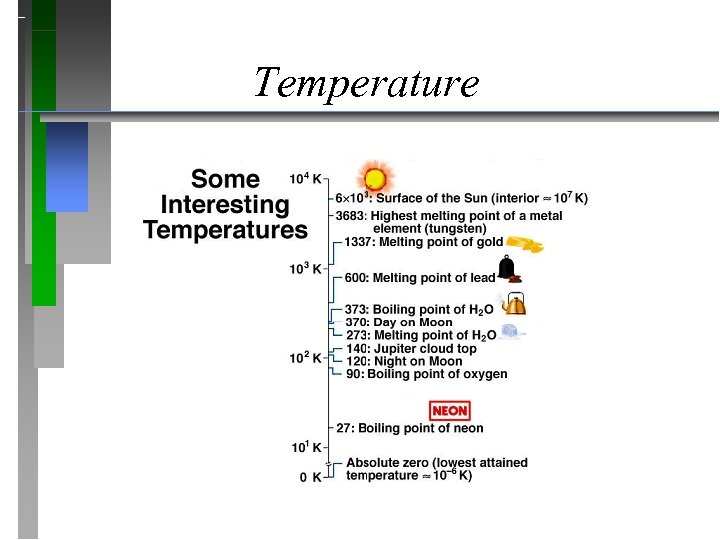 Temperature
Temperature
 QUESTION Identify the best match between the dimension or quantity and the unit that is most likely to be measured in Chem 106 lab. A) B) C) D) E) Dimension or Quantity Mass Length Volume Temperature Amount of substance Unit Kilogram Meter Milliliter Fahrenheit Megamole
QUESTION Identify the best match between the dimension or quantity and the unit that is most likely to be measured in Chem 106 lab. A) B) C) D) E) Dimension or Quantity Mass Length Volume Temperature Amount of substance Unit Kilogram Meter Milliliter Fahrenheit Megamole
 Answer Identify the best match between the dimension or quantity and the unit that is most likely to be measured in Chem 106 lab. A) B) C) D) E) Dimension or Quantity Mass Length Volume Temperature Amount of substance Unit Kilogram Meter Milliliter Fahrenheit Megamole
Answer Identify the best match between the dimension or quantity and the unit that is most likely to be measured in Chem 106 lab. A) B) C) D) E) Dimension or Quantity Mass Length Volume Temperature Amount of substance Unit Kilogram Meter Milliliter Fahrenheit Megamole
 Numbers & Measurement The Importance of Units ð Measurement - quantitative observation JOULE (J): consisting of 2 parts • • • Part 1 - number Part 2 – unit Relates to the instrument (tool) used for the measurement. Examples: • 20. 0 grams • 6. 63 joules / second • The heat required to raise the temperature of 1 g of water by 0. 24 K; 1 J = 0. 24 calories. [6] • The heat released as heat by a person at rest every 1/60 second (~17 ms); . [7] • The kinetic energy of a 50 kg (110 lb) human moving at 0. 43 mi/hr). • The amount of electricity required to light a 1 watt LED for 1 s.
Numbers & Measurement The Importance of Units ð Measurement - quantitative observation JOULE (J): consisting of 2 parts • • • Part 1 - number Part 2 – unit Relates to the instrument (tool) used for the measurement. Examples: • 20. 0 grams • 6. 63 joules / second • The heat required to raise the temperature of 1 g of water by 0. 24 K; 1 J = 0. 24 calories. [6] • The heat released as heat by a person at rest every 1/60 second (~17 ms); . [7] • The kinetic energy of a 50 kg (110 lb) human moving at 0. 43 mi/hr). • The amount of electricity required to light a 1 watt LED for 1 s.
 Powers of Ten: Scale
Powers of Ten: Scale
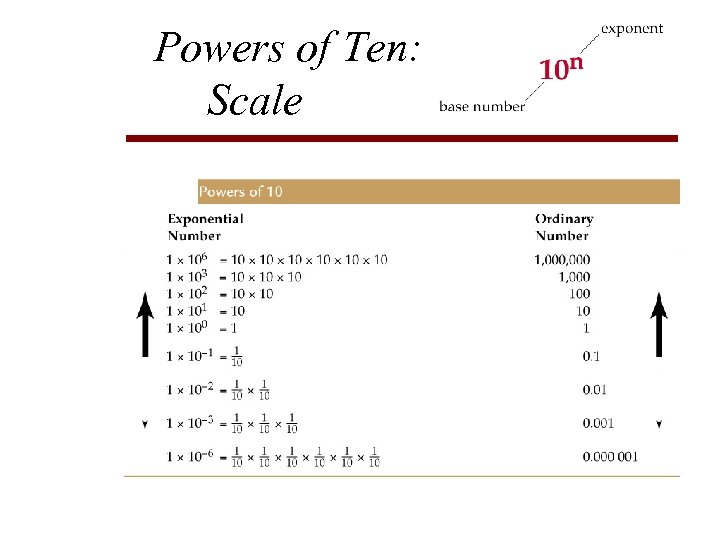
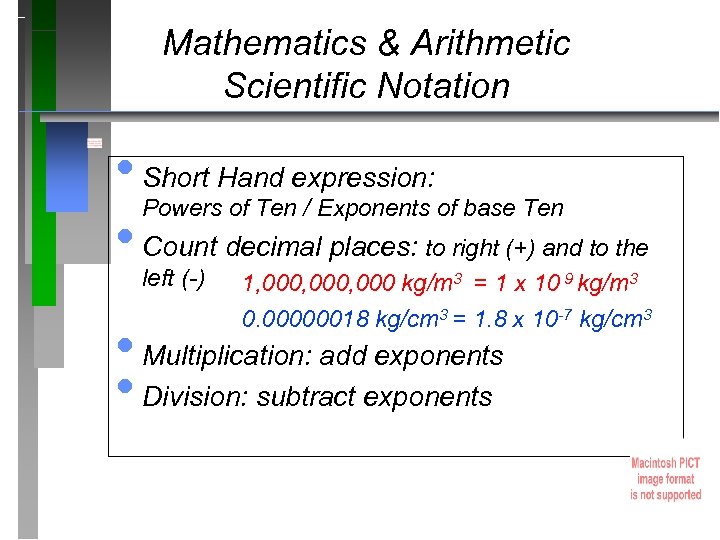 Mathematics & Arithmetic Scientific Notation • Short Hand expression: Powers of Ten / Exponents of base Ten • Count decimal places: to right (+) and to the left (-) 1, 000, 000 kg/m 3 = 1 x 10 9 kg/m 3 0. 00000018 kg/cm 3 = 1. 8 x 10 -7 kg/cm 3 • Multiplication: add exponents • Division: subtract exponents
Mathematics & Arithmetic Scientific Notation • Short Hand expression: Powers of Ten / Exponents of base Ten • Count decimal places: to right (+) and to the left (-) 1, 000, 000 kg/m 3 = 1 x 10 9 kg/m 3 0. 00000018 kg/cm 3 = 1. 8 x 10 -7 kg/cm 3 • Multiplication: add exponents • Division: subtract exponents
 Language describes scale (prefixes) Shorthand Prefixes Hella is a prefix associated with Northern California: UC Davis, UC Berkeley, LBL, LLNL & adopted by Google (2010) & Wolfram Alpha (2011) "hella-” = 10 27
Language describes scale (prefixes) Shorthand Prefixes Hella is a prefix associated with Northern California: UC Davis, UC Berkeley, LBL, LLNL & adopted by Google (2010) & Wolfram Alpha (2011) "hella-” = 10 27
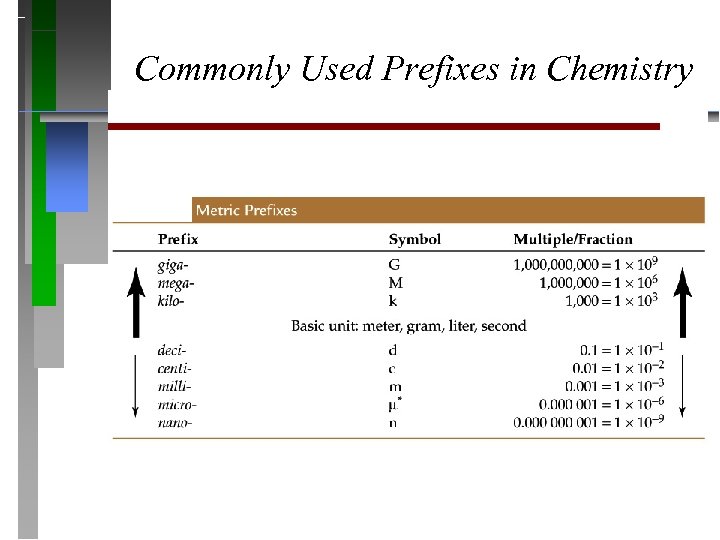 Commonly Used Prefixes in Chemistry
Commonly Used Prefixes in Chemistry
 QUESTION Select the correct relationship between these metric units of length or distance. A) 1 km = 100 m C) 1 nm = 109 m B) 1 mm = 10 cm D) 103 mm = 1 m
QUESTION Select the correct relationship between these metric units of length or distance. A) 1 km = 100 m C) 1 nm = 109 m B) 1 mm = 10 cm D) 103 mm = 1 m
 Answer Select the correct relationship between these metric units of length or distance. A) 1 km = 100 m C) 1 nm = 109 m B) 1 mm = 10 cm D) 103 mm = 1 m
Answer Select the correct relationship between these metric units of length or distance. A) 1 km = 100 m C) 1 nm = 109 m B) 1 mm = 10 cm D) 103 mm = 1 m
 QUESTION Coincidentally, a U. S. nickel has a mass of approximately 5 grams. If you had one dollar’s worth of nickels in your jean’s what would be the mass of the nickels in milligrams? A. B. C. D. 100 milligrams 50 milligrams 1, 000 milligrams 1000 milligrams (mg) = 1 gram (g)
QUESTION Coincidentally, a U. S. nickel has a mass of approximately 5 grams. If you had one dollar’s worth of nickels in your jean’s what would be the mass of the nickels in milligrams? A. B. C. D. 100 milligrams 50 milligrams 1, 000 milligrams 1000 milligrams (mg) = 1 gram (g)
 Answer Coincidentally, a U. S. nickel has a mass of approximately 5 grams. If you had one dollar’s worth of nickels in your jean’s what would be the mass of the nickels in milligrams? A. B. C. D. 100 milligrams 50 milligrams 1, 000 milligrams 100, 000 milligrams 20 nickels make up one dollar, then one dollar’s worth of nickels would have a mass of 5 g x 20=100 grams. Next, the conversion between grams and milligrams is done by multiplying by 1, 000 (because there are 1, 000 milligrams per 1 gram. ) Would the weight of the nickels pull your jean’s down off of your waist?
Answer Coincidentally, a U. S. nickel has a mass of approximately 5 grams. If you had one dollar’s worth of nickels in your jean’s what would be the mass of the nickels in milligrams? A. B. C. D. 100 milligrams 50 milligrams 1, 000 milligrams 100, 000 milligrams 20 nickels make up one dollar, then one dollar’s worth of nickels would have a mass of 5 g x 20=100 grams. Next, the conversion between grams and milligrams is done by multiplying by 1, 000 (because there are 1, 000 milligrams per 1 gram. ) Would the weight of the nickels pull your jean’s down off of your waist?
 Answer Coincidentally, a U. S. nickel has a mass of approximately 5 grams. If you had one dollar’s worth of nickels in your jean’s what would be the mass of the nickels in milligrams? A. B. C. D. 100 milligrams 50 milligrams 1, 000 milligrams 100, 000 milligrams 20 nickels make up one dollar, then one dollar’s worth of nickels would have a mass of 5 g x 20=100 grams. Next, the conversion between grams and milligrams is done by multiplying by 1, 000 (because there are 1, 000 milligrams per 1 gram. ) Would the weight of the nickels pull your jean’s down off of your waist? Likely not. 100 g = 100 g/454 g/lb equals 0. 22 lb ~ the weight of a quarter pounder
Answer Coincidentally, a U. S. nickel has a mass of approximately 5 grams. If you had one dollar’s worth of nickels in your jean’s what would be the mass of the nickels in milligrams? A. B. C. D. 100 milligrams 50 milligrams 1, 000 milligrams 100, 000 milligrams 20 nickels make up one dollar, then one dollar’s worth of nickels would have a mass of 5 g x 20=100 grams. Next, the conversion between grams and milligrams is done by multiplying by 1, 000 (because there are 1, 000 milligrams per 1 gram. ) Would the weight of the nickels pull your jean’s down off of your waist? Likely not. 100 g = 100 g/454 g/lb equals 0. 22 lb ~ the weight of a quarter pounder
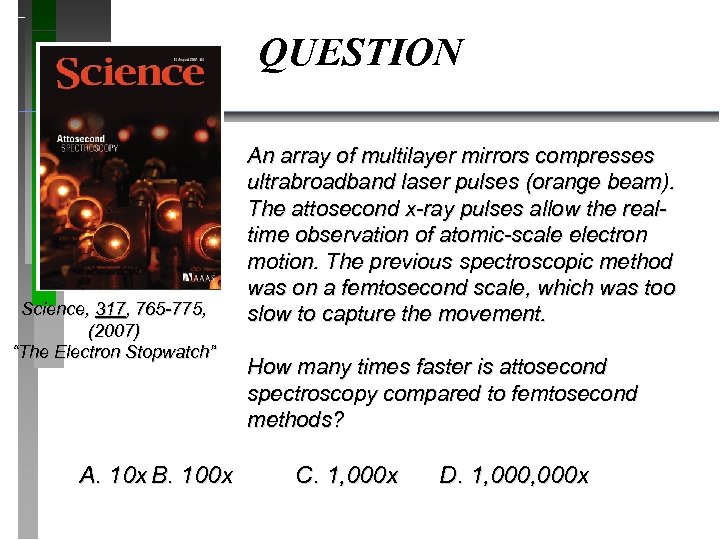 QUESTION Science, 317, 765 -775, (2007) “The Electron Stopwatch” A. 10 x B. 100 x An array of multilayer mirrors compresses ultrabroadband laser pulses (orange beam). The attosecond x-ray pulses allow the realtime observation of atomic-scale electron motion. The previous spectroscopic method was on a femtosecond scale, which was too slow to capture the movement. How many times faster is attosecond spectroscopy compared to femtosecond methods? C. 1, 000 x D. 1, 000 x
QUESTION Science, 317, 765 -775, (2007) “The Electron Stopwatch” A. 10 x B. 100 x An array of multilayer mirrors compresses ultrabroadband laser pulses (orange beam). The attosecond x-ray pulses allow the realtime observation of atomic-scale electron motion. The previous spectroscopic method was on a femtosecond scale, which was too slow to capture the movement. How many times faster is attosecond spectroscopy compared to femtosecond methods? C. 1, 000 x D. 1, 000 x
 QUESTION How many times faster is attosecond spectroscopy compared to femtosecond methods? Science, 317, 765 -775, (2007) “The Electron Stopwatch” A. 10 x B. 100 x C. 1, 000 x D. 1, 000 x
QUESTION How many times faster is attosecond spectroscopy compared to femtosecond methods? Science, 317, 765 -775, (2007) “The Electron Stopwatch” A. 10 x B. 100 x C. 1, 000 x D. 1, 000 x
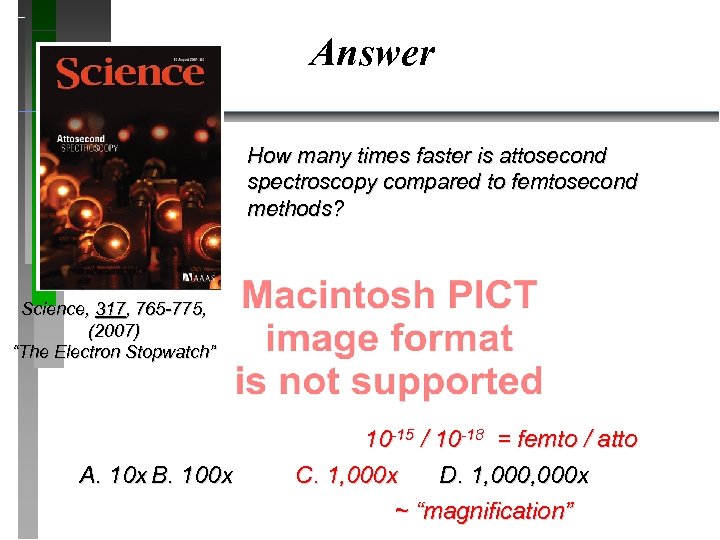 Answer How many times faster is attosecond spectroscopy compared to femtosecond methods? Science, 317, 765 -775, (2007) “The Electron Stopwatch” A. 10 x B. 100 x 10 -15 / 10 -18 = femto / atto C. 1, 000 x D. 1, 000 x ~ “magnification”
Answer How many times faster is attosecond spectroscopy compared to femtosecond methods? Science, 317, 765 -775, (2007) “The Electron Stopwatch” A. 10 x B. 100 x 10 -15 / 10 -18 = femto / atto C. 1, 000 x D. 1, 000 x ~ “magnification”
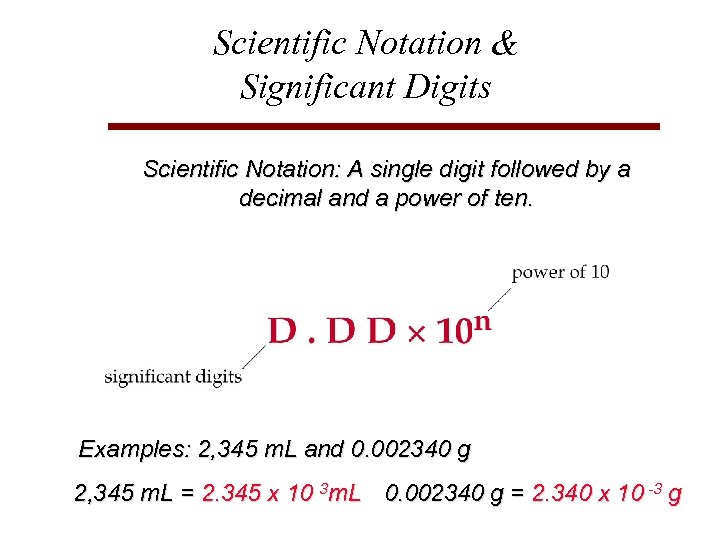 Scientific Notation & Significant Digits Scientific Notation: A single digit followed by a decimal and a power of ten. Examples: 2, 345 m. L and 0. 002340 g 2, 345 m. L = 2. 345 x 10 3 m. L 0. 002340 g = 2. 340 x 10 -3 g
Scientific Notation & Significant Digits Scientific Notation: A single digit followed by a decimal and a power of ten. Examples: 2, 345 m. L and 0. 002340 g 2, 345 m. L = 2. 345 x 10 3 m. L 0. 002340 g = 2. 340 x 10 -3 g
 Numbers • Expressing a number correctly is determined by the method used in the measurement! • How many numbers should I include? Significant Digits (Figures) Consider: the exactness of the measured value • Short Hand expression translates the number: Scientific Notation
Numbers • Expressing a number correctly is determined by the method used in the measurement! • How many numbers should I include? Significant Digits (Figures) Consider: the exactness of the measured value • Short Hand expression translates the number: Scientific Notation
 What is the length of the rod? Different measurement tools give different numbers: Which ruler is better? ? cm 4. 2 - 4. 3 cm ? cm 4. 24 - 4. 25 cm
What is the length of the rod? Different measurement tools give different numbers: Which ruler is better? ? cm 4. 2 - 4. 3 cm ? cm 4. 24 - 4. 25 cm
 Reporting Numbers Rules for Significant Digits (Figures) ð Nonzero integers always count as significant figures. ð 3456 g has how many sig figs? • Expressed in scientific notation?
Reporting Numbers Rules for Significant Digits (Figures) ð Nonzero integers always count as significant figures. ð 3456 g has how many sig figs? • Expressed in scientific notation?
 Reporting Numbers Rules for Significant Digits (Figures) ð Nonzero integers always count as significant figures. ð 3456 g has how many sig figs? ð 4 sig figs. • Expressed in scientific notation? 3. 456 x 10 3 g
Reporting Numbers Rules for Significant Digits (Figures) ð Nonzero integers always count as significant figures. ð 3456 g has how many sig figs? ð 4 sig figs. • Expressed in scientific notation? 3. 456 x 10 3 g
 Reporting Numbers Rules for Significant (Digits) Figures ð Exact numbers (unit, conversion or scale factors) can have an infinite number of significant figures. ð 1 liter = 1, 000. ml, exactly ð 1 inch = 2. 54 cm, exactly
Reporting Numbers Rules for Significant (Digits) Figures ð Exact numbers (unit, conversion or scale factors) can have an infinite number of significant figures. ð 1 liter = 1, 000. ml, exactly ð 1 inch = 2. 54 cm, exactly
 Zeros « Leading zeros do not count as significant figures. ð 0. 0486 m. L has how many sig figs? • Number expressed in scientific notation?
Zeros « Leading zeros do not count as significant figures. ð 0. 0486 m. L has how many sig figs? • Number expressed in scientific notation?
 Zeros « Leading zeros do not count as significant figures. ð 0. 0486 m. L has how many sig figs? ð 3 sig figs. • Number expressed in scientific notation? 4. 86 x 10 -2 m. L
Zeros « Leading zeros do not count as significant figures. ð 0. 0486 m. L has how many sig figs? ð 3 sig figs. • Number expressed in scientific notation? 4. 86 x 10 -2 m. L
 Zeros ð ð ð Captive zeros always count as significant figures. 16. 07 cm has how many sig figs? Number expressed in scientific notation?
Zeros ð ð ð Captive zeros always count as significant figures. 16. 07 cm has how many sig figs? Number expressed in scientific notation?
 Zeros ð ð ð Captive zeros always count as significant figures. 16. 07 cm has how many sig figs? ð 4 sig figs. Number expressed in scientific 1. 607 x 10 1 cm notation?
Zeros ð ð ð Captive zeros always count as significant figures. 16. 07 cm has how many sig figs? ð 4 sig figs. Number expressed in scientific 1. 607 x 10 1 cm notation?
 Zeros ð ð Trailing zeros are significant only if the number contains a decimal point. 9. 300 kg has how many sig figs? • Number expressed in scientific notation?
Zeros ð ð Trailing zeros are significant only if the number contains a decimal point. 9. 300 kg has how many sig figs? • Number expressed in scientific notation?
 Zeros ð ð Trailing zeros are significant only if the number contains a decimal point. 9. 300 kg has how many sig figs? ð 4 sig figs. • Number expressed in scientific 9. 300 kg notation?
Zeros ð ð Trailing zeros are significant only if the number contains a decimal point. 9. 300 kg has how many sig figs? ð 4 sig figs. • Number expressed in scientific 9. 300 kg notation?
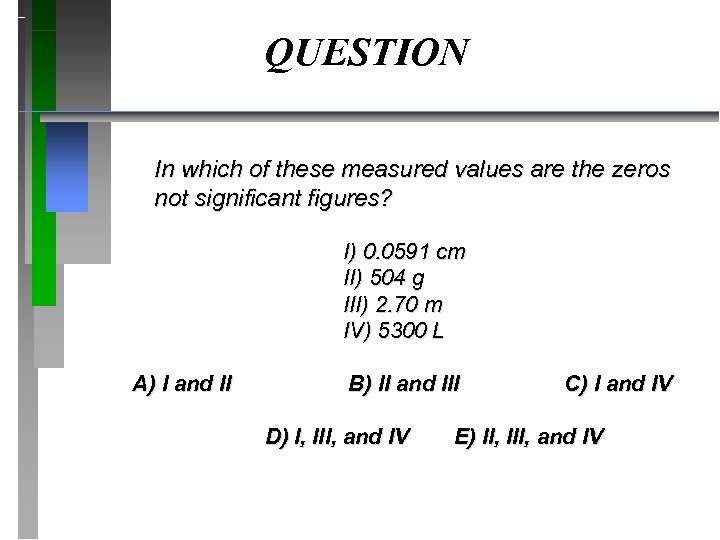 QUESTION In which of these measured values are the zeros not significant figures? I) 0. 0591 cm II) 504 g III) 2. 70 m IV) 5300 L A) I and II B) II and III D) I, III, and IV C) I and IV E) II, III, and IV
QUESTION In which of these measured values are the zeros not significant figures? I) 0. 0591 cm II) 504 g III) 2. 70 m IV) 5300 L A) I and II B) II and III D) I, III, and IV C) I and IV E) II, III, and IV
 Answer In which of these measured values are the zeros not significant figures? I) 0. 0591 cm II) 504 g III) 2. 70 m IV) 5300 L A) I and II B) II and III D) I, III, and IV C) I and IV E) II, III, and IV
Answer In which of these measured values are the zeros not significant figures? I) 0. 0591 cm II) 504 g III) 2. 70 m IV) 5300 L A) I and II B) II and III D) I, III, and IV C) I and IV E) II, III, and IV
 QUESTION Which one of the following does NOT represent four significant digits? A. B. C. D. 0. 07100 mg 0. 7010 mg 0. 0710 mg
QUESTION Which one of the following does NOT represent four significant digits? A. B. C. D. 0. 07100 mg 0. 7010 mg 0. 0710 mg
 Answer Which one of the following does NOT represent four significant digits? A. B. C. D. 0. 07100 mg 0. 7010 mg 0. 0710 mg The zero in front of the decimal point is not a part of any measurement, the next zero is a place holder, and the last zero is part of the measurement and significant. Therefore 3 sig figs.
Answer Which one of the following does NOT represent four significant digits? A. B. C. D. 0. 07100 mg 0. 7010 mg 0. 0710 mg The zero in front of the decimal point is not a part of any measurement, the next zero is a place holder, and the last zero is part of the measurement and significant. Therefore 3 sig figs.
 Mathematics & Arithmetic • Relative to method(s) of measurement • Short Hand expression: Scientific Notation • Numbers : How many to include? Quantitative vs. Qualitative • Addition/Subtraction. . . • Multiplication/Division. . . • What is “significant”? . . . Rounding Off • http: //www. chemteam. info/Sig. Figs. Fable. html
Mathematics & Arithmetic • Relative to method(s) of measurement • Short Hand expression: Scientific Notation • Numbers : How many to include? Quantitative vs. Qualitative • Addition/Subtraction. . . • Multiplication/Division. . . • What is “significant”? . . . Rounding Off • http: //www. chemteam. info/Sig. Figs. Fable. html
 Computational Rules • Addition/Subtraction: Answer expressed • to the least number of decimal places of the figures in the process Multiplication/Division: Answer expressed to the least number of significant figures
Computational Rules • Addition/Subtraction: Answer expressed • to the least number of decimal places of the figures in the process Multiplication/Division: Answer expressed to the least number of significant figures
 Addition ð Four students were each asked to measure a piece of wire and provide a total length for the four pieces. ð Report the result correctly: 16. 346 cm
Addition ð Four students were each asked to measure a piece of wire and provide a total length for the four pieces. ð Report the result correctly: 16. 346 cm
 QUESTION If you were unloading a 23. 50 kg box of books from your car and a “friend” added two more 482 gram chemistry books, how much in kg and using the rules for significant digits, would you be lifting? A. B. C. D. 23. 98 kg 24. 464 kg 24. 46 kg 24. 5 kg
QUESTION If you were unloading a 23. 50 kg box of books from your car and a “friend” added two more 482 gram chemistry books, how much in kg and using the rules for significant digits, would you be lifting? A. B. C. D. 23. 98 kg 24. 464 kg 24. 46 kg 24. 5 kg
 Answer If you were unloading a 23. 50 kg box of books from your car and a “friend” added two more 482 gram chemistry books, how much in kg and using the rules for significant digits, would you be lifting? A. B. C. D. 23. 98 kg 24. 464 kg 24. 46 kg 24. 5 kg The 482 grams of mass must be doubled (to include both books) and 482 grams is 0. 482 kg. When adding measurements the answer has the same number of decimal places as the fewest decimal places in the calculation. Therefore the answer has 2 decimal places.
Answer If you were unloading a 23. 50 kg box of books from your car and a “friend” added two more 482 gram chemistry books, how much in kg and using the rules for significant digits, would you be lifting? A. B. C. D. 23. 98 kg 24. 464 kg 24. 46 kg 24. 5 kg The 482 grams of mass must be doubled (to include both books) and 482 grams is 0. 482 kg. When adding measurements the answer has the same number of decimal places as the fewest decimal places in the calculation. Therefore the answer has 2 decimal places.
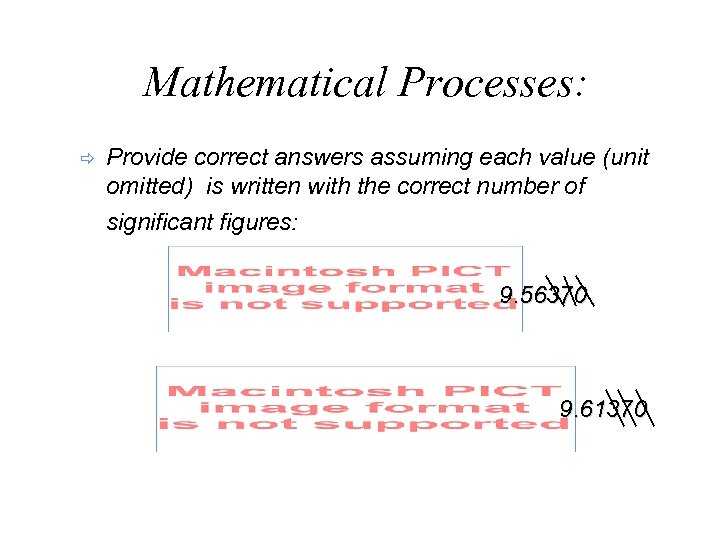 Mathematical Processes: ð Provide correct answers assuming each value (unit omitted) is written with the correct number of significant figures: 9. 56370 9. 61370
Mathematical Processes: ð Provide correct answers assuming each value (unit omitted) is written with the correct number of significant figures: 9. 56370 9. 61370
 QUESTION The average mass of a certain brand of vitamin C tablets is 253 mg. What is the mass of three such tablets rounded to the proper number of significant digits? A. B. C. D. 0. 760 grams 0. 7590 grams 0. 253 grams
QUESTION The average mass of a certain brand of vitamin C tablets is 253 mg. What is the mass of three such tablets rounded to the proper number of significant digits? A. B. C. D. 0. 760 grams 0. 7590 grams 0. 253 grams
 Answer The average mass of a certain brand of vitamin C tablets is 253 mg. What is the mass of three such tablets rounded to the proper number of significant digits? A. B. C. D. 0. 760 grams 0. 7590 grams 0. 253 grams 3 tablets 253 milligrams = 759 milligrams, then dividing by 1, 000 converts the milligrams to grams. Note three is a count of the number of objects, not a measured quantity and 759 retains the same number of significant digits as the least found in related measurements.
Answer The average mass of a certain brand of vitamin C tablets is 253 mg. What is the mass of three such tablets rounded to the proper number of significant digits? A. B. C. D. 0. 760 grams 0. 7590 grams 0. 253 grams 3 tablets 253 milligrams = 759 milligrams, then dividing by 1, 000 converts the milligrams to grams. Note three is a count of the number of objects, not a measured quantity and 759 retains the same number of significant digits as the least found in related measurements.
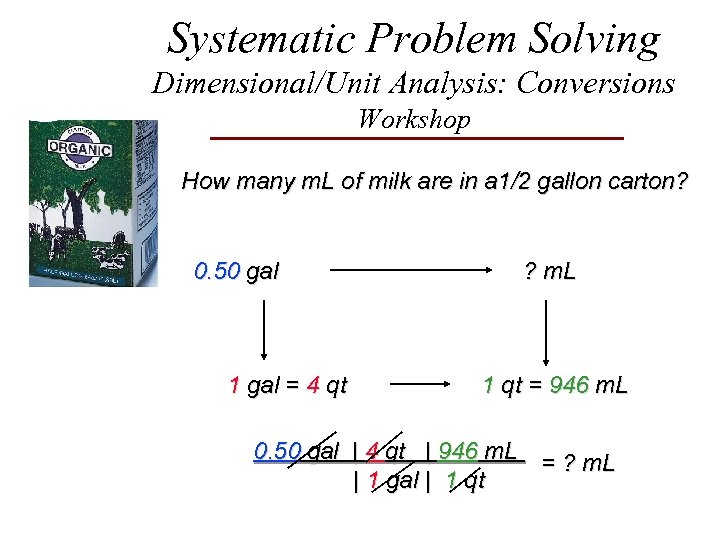 Systematic Problem Solving Dimensional/Unit Analysis: Conversions Workshop How many m. L of milk are in a 1/2 gallon carton? 0. 50 gal 1 gal = 4 qt ? m. L 1 qt = 946 m. L 0. 50 gal | 4 qt | 946 m. L = ? m. L | 1 gal | 1 qt
Systematic Problem Solving Dimensional/Unit Analysis: Conversions Workshop How many m. L of milk are in a 1/2 gallon carton? 0. 50 gal 1 gal = 4 qt ? m. L 1 qt = 946 m. L 0. 50 gal | 4 qt | 946 m. L = ? m. L | 1 gal | 1 qt
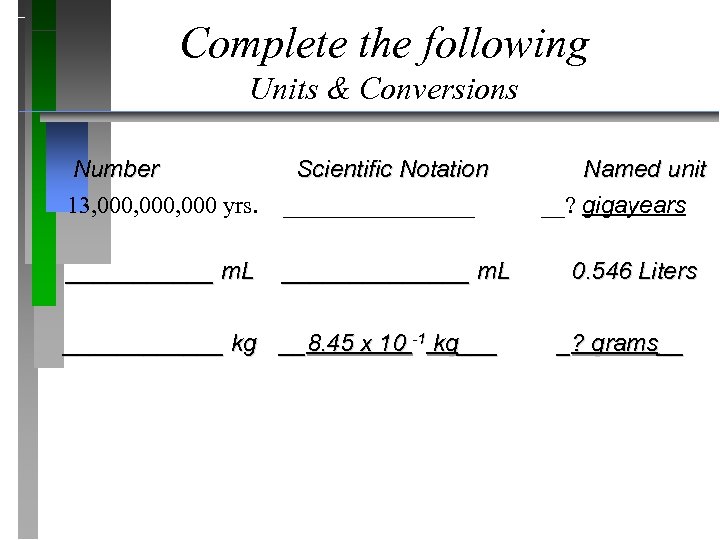 Complete the following Units & Conversions Number Scientific Notation Named unit 13, 000, 000 yrs. ________ __? gigayears ______ m. L _______ m. L 0. 546 Liters ______ kg __8. 45 x 10 -1 kg___ _? grams__
Complete the following Units & Conversions Number Scientific Notation Named unit 13, 000, 000 yrs. ________ __? gigayears ______ m. L _______ m. L 0. 546 Liters ______ kg __8. 45 x 10 -1 kg___ _? grams__
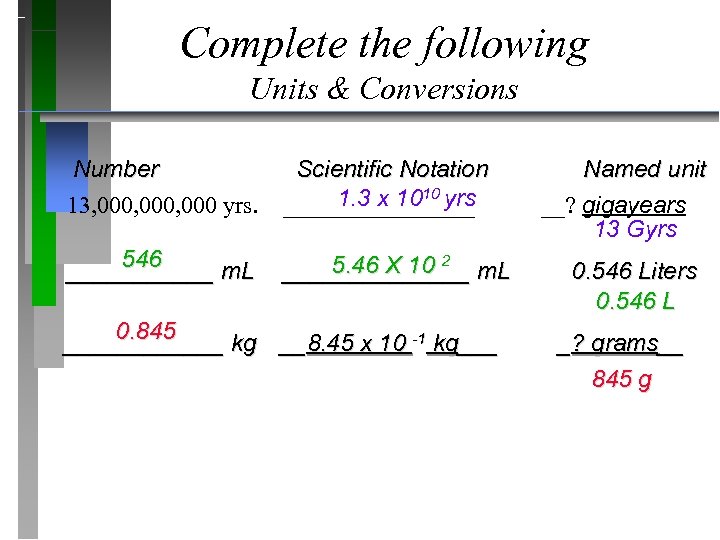 Complete the following Units & Conversions Number Scientific Notation Named unit 1. 3 x 1010 yrs 13, 000, 000 yrs. ________ __? gigayears 13 Gyrs 546 5. 46 X 10 2 ______ m. L _______ m. L 0. 546 Liters 0. 546 L 0. 845 ______ kg __8. 45 x 10 -1 kg___ _? grams__ 845 g
Complete the following Units & Conversions Number Scientific Notation Named unit 1. 3 x 1010 yrs 13, 000, 000 yrs. ________ __? gigayears 13 Gyrs 546 5. 46 X 10 2 ______ m. L _______ m. L 0. 546 Liters 0. 546 L 0. 845 ______ kg __8. 45 x 10 -1 kg___ _? grams__ 845 g
 Numbers & Measurement Units & Conversions Crash Course: Hank & John Green Unit Conversion & Significant Figures (11: 23 min: sec) https: //www. youtube. com/watch? v=h. Qp. Q 0 hx. VNTg&list=PL 8 d. Puua. Lj. Xt. P Hzz. Yu. Wy 6 f. YEa. X 9 m. QQ 8 o. Gr&index=2
Numbers & Measurement Units & Conversions Crash Course: Hank & John Green Unit Conversion & Significant Figures (11: 23 min: sec) https: //www. youtube. com/watch? v=h. Qp. Q 0 hx. VNTg&list=PL 8 d. Puua. Lj. Xt. P Hzz. Yu. Wy 6 f. YEa. X 9 m. QQ 8 o. Gr&index=2
 Worksheet Units, Measurements, & Conversions
Worksheet Units, Measurements, & Conversions
 World’s fastest computer (2001) (12 Tera. FLOPS/second) Tera- = ? 12 (trillion) Tera- = 10 Cost = ~$ 30, 000 (2001 dollars) http: //www. llnl. gov/asci-scrapbook/
World’s fastest computer (2001) (12 Tera. FLOPS/second) Tera- = ? 12 (trillion) Tera- = 10 Cost = ~$ 30, 000 (2001 dollars) http: //www. llnl. gov/asci-scrapbook/
 World’s fastest computer (2016) (33. 86 Peta. FLOPS) Peta- = 10 15 (quadrillion); 1, 000 trillion Peta = ? 2014 Tianhe-2, aka Milky Way, Guangzhou, China Estimated Cost: >$100 million (2016 dollars)
World’s fastest computer (2016) (33. 86 Peta. FLOPS) Peta- = 10 15 (quadrillion); 1, 000 trillion Peta = ? 2014 Tianhe-2, aka Milky Way, Guangzhou, China Estimated Cost: >$100 million (2016 dollars)
 Floating Point Operations Flops • Use a clock or watch with a second hand or read out and note the seconds. • Begin, counting 1+1. • Keep repeating the counting of 1+1 and keep track of how many times that you have repeated 1+1. • Stop when you reach 30 seconds on the timekeeping device, note your total of times that you have repeated 1+1. This is the number of Flops per 30 seconds.
Floating Point Operations Flops • Use a clock or watch with a second hand or read out and note the seconds. • Begin, counting 1+1. • Keep repeating the counting of 1+1 and keep track of how many times that you have repeated 1+1. • Stop when you reach 30 seconds on the timekeeping device, note your total of times that you have repeated 1+1. This is the number of Flops per 30 seconds.
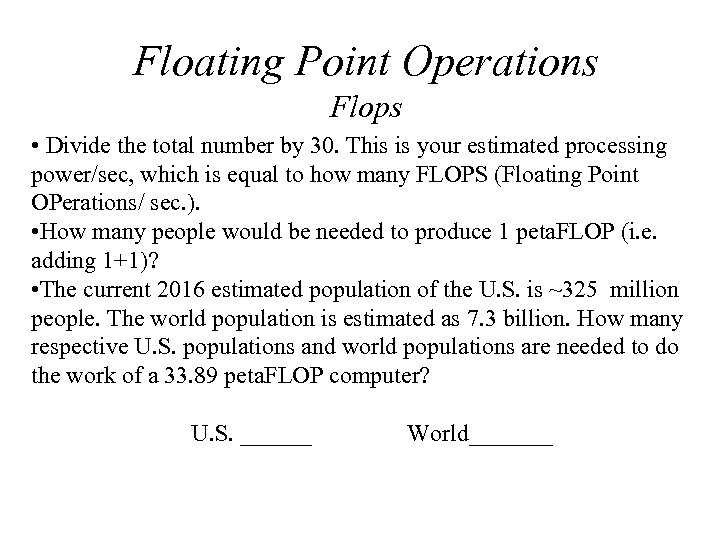 Floating Point Operations Flops • Divide the total number by 30. This is your estimated processing power/sec, which is equal to how many FLOPS (Floating Point OPerations/ sec. ). • How many people would be needed to produce 1 peta. FLOP (i. e. adding 1+1)? • The current 2016 estimated population of the U. S. is ~325 million people. The world population is estimated as 7. 3 billion. How many respective U. S. populations and world populations are needed to do the work of a 33. 89 peta. FLOP computer? U. S. ______ World_______
Floating Point Operations Flops • Divide the total number by 30. This is your estimated processing power/sec, which is equal to how many FLOPS (Floating Point OPerations/ sec. ). • How many people would be needed to produce 1 peta. FLOP (i. e. adding 1+1)? • The current 2016 estimated population of the U. S. is ~325 million people. The world population is estimated as 7. 3 billion. How many respective U. S. populations and world populations are needed to do the work of a 33. 89 peta. FLOP computer? U. S. ______ World_______
 Measurements: Scaling/ Problem Solving Powers of Ten (Exponents: 10 x) ð ð Scaling: U. S. Population in 2003 vs. 2016 • 1 birth every 12 seconds (2003) vs. 8 seconds (2016) • 1 death every 20 seconds (2003) vs. 10 seconds in (2016) • 1 new immigrant every 21 s (2003) vs. 29 s (2016) Considering the data, what is the net effect on the U. S. population in 2016…. Is it growing or declining? . . . faster or slower than 2003? … by what percent vs. 2016? The U. S. census population was 321, 442, 019 on July 4, 2015. Calculate what it is today considering the data. 365 days/yr x 24 hr/day x 60 min/hr x 60 sec/min = 31, 536, 000 sec/yr
Measurements: Scaling/ Problem Solving Powers of Ten (Exponents: 10 x) ð ð Scaling: U. S. Population in 2003 vs. 2016 • 1 birth every 12 seconds (2003) vs. 8 seconds (2016) • 1 death every 20 seconds (2003) vs. 10 seconds in (2016) • 1 new immigrant every 21 s (2003) vs. 29 s (2016) Considering the data, what is the net effect on the U. S. population in 2016…. Is it growing or declining? . . . faster or slower than 2003? … by what percent vs. 2016? The U. S. census population was 321, 442, 019 on July 4, 2015. Calculate what it is today considering the data. 365 days/yr x 24 hr/day x 60 min/hr x 60 sec/min = 31, 536, 000 sec/yr
 Measurements: Scaling/ Problem Solving Powers of Ten (Exponents: 10 x) ð ð ð ð ð Today? 365 days/yr x 24 hr/day x 60 min/hr x 60 sec/min = 31, 536, 000 sec/yr 3. 1536 x 10 7 sec/yr ( 1 new person /8 sec + 1 people /29 sec - 1 people /10 sec) 3. 1536 x 107 sec/yr ( 1/8 + 1/29 – 1/10) people /sec 3. 1536 x 107 sec/yr ( 0. 0833 + 0. 0476 - 0. 05) people/sec 3. 1536 x 107 sec/yr x 0. 0809 people/sec 3. 1536 x 10 7 sec/yr x 8. 09 x 10 -2 people/sec 25. 5 x 10 5 people / yr = 2. 55 x 10 6 people
Measurements: Scaling/ Problem Solving Powers of Ten (Exponents: 10 x) ð ð ð ð ð Today? 365 days/yr x 24 hr/day x 60 min/hr x 60 sec/min = 31, 536, 000 sec/yr 3. 1536 x 10 7 sec/yr ( 1 new person /8 sec + 1 people /29 sec - 1 people /10 sec) 3. 1536 x 107 sec/yr ( 1/8 + 1/29 – 1/10) people /sec 3. 1536 x 107 sec/yr ( 0. 0833 + 0. 0476 - 0. 05) people/sec 3. 1536 x 107 sec/yr x 0. 0809 people/sec 3. 1536 x 10 7 sec/yr x 8. 09 x 10 -2 people/sec 25. 5 x 10 5 people / yr = 2. 55 x 10 6 people
 Floating Point Operations Cost of 100+ Tera. Flops (2004)? • What is the cost? • $250, 000 ($ 250 x 10 6) • What do you get? 220 mph = 3. 66 miles/sec = 366 trillion miles >1 million trips to Mars!! An electrical impulse between neurons travels at 220 miles per hour. If each Flop was 1 impulse how far would 100 Tera. Flops travel?
Floating Point Operations Cost of 100+ Tera. Flops (2004)? • What is the cost? • $250, 000 ($ 250 x 10 6) • What do you get? 220 mph = 3. 66 miles/sec = 366 trillion miles >1 million trips to Mars!! An electrical impulse between neurons travels at 220 miles per hour. If each Flop was 1 impulse how far would 100 Tera. Flops travel?


