8a2a1982dcb97d97ea16a2f4378f83cf.ppt
- Количество слайдов: 185
 CHE-302 Review
CHE-302 Review
 Nomenclature Syntheses Reactions Mechanisms Spectroscopy
Nomenclature Syntheses Reactions Mechanisms Spectroscopy
 Aromatic Hydrocarbons (Electrophilic Aromatic Substitution) Spectroscopy (infrared & H-nmr) Arenes Aldehydes & Ketones Carboxylic Acids Functional Derivatives of Carboxylic Acids Acid Chlorides, Anhydrides, Amides, Esters Carbanions Amines & Diazonium Salts Phenols
Aromatic Hydrocarbons (Electrophilic Aromatic Substitution) Spectroscopy (infrared & H-nmr) Arenes Aldehydes & Ketones Carboxylic Acids Functional Derivatives of Carboxylic Acids Acid Chlorides, Anhydrides, Amides, Esters Carbanions Amines & Diazonium Salts Phenols
 Mechanisms: Electrophilic Aromatic Substitution Nitration Sulfonation Halogenation Friedel-Crafts Alkylation & Acylation Nucleophilic Addition to Carbonyl, Acid Catalyzed Nucleophilic Acyl Substitution, Acid Catalyzed
Mechanisms: Electrophilic Aromatic Substitution Nitration Sulfonation Halogenation Friedel-Crafts Alkylation & Acylation Nucleophilic Addition to Carbonyl, Acid Catalyzed Nucleophilic Acyl Substitution, Acid Catalyzed
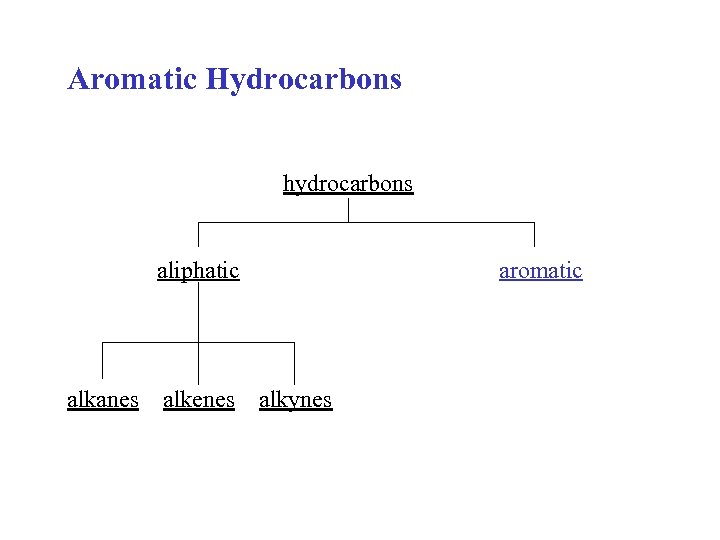 Aromatic Hydrocarbons hydrocarbons aliphatic alkanes alkenes aromatic alkynes
Aromatic Hydrocarbons hydrocarbons aliphatic alkanes alkenes aromatic alkynes
 Aliphatic compounds: open-chain compounds and ring compounds that are chemically similar to open-chain compounds. Alkanes, alkenes, alkynes, dienes, alicyclics, etc. Aromatic compounds: unsaturated ring compounds that are far more stable than they should be and resist the addition reactions typical of unsaturated aliphatic compounds. Benzene and related compounds.
Aliphatic compounds: open-chain compounds and ring compounds that are chemically similar to open-chain compounds. Alkanes, alkenes, alkynes, dienes, alicyclics, etc. Aromatic compounds: unsaturated ring compounds that are far more stable than they should be and resist the addition reactions typical of unsaturated aliphatic compounds. Benzene and related compounds.
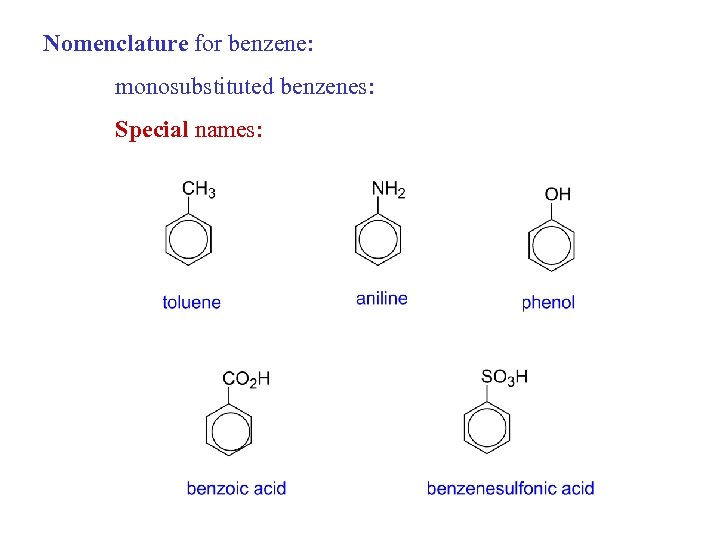 Nomenclature for benzene: monosubstituted benzenes: Special names:
Nomenclature for benzene: monosubstituted benzenes: Special names:

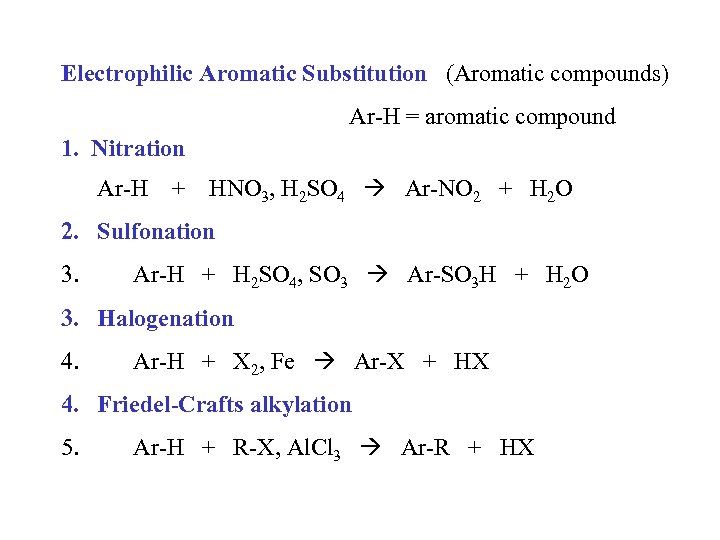 Electrophilic Aromatic Substitution (Aromatic compounds) Ar-H = aromatic compound 1. Nitration Ar-H + HNO 3, H 2 SO 4 Ar-NO 2 + H 2 O 2. Sulfonation 3. Ar-H + H 2 SO 4, SO 3 Ar-SO 3 H + H 2 O 3. Halogenation 4. Ar-H + X 2, Fe Ar-X + HX 4. Friedel-Crafts alkylation 5. Ar-H + R-X, Al. Cl 3 Ar-R + HX
Electrophilic Aromatic Substitution (Aromatic compounds) Ar-H = aromatic compound 1. Nitration Ar-H + HNO 3, H 2 SO 4 Ar-NO 2 + H 2 O 2. Sulfonation 3. Ar-H + H 2 SO 4, SO 3 Ar-SO 3 H + H 2 O 3. Halogenation 4. Ar-H + X 2, Fe Ar-X + HX 4. Friedel-Crafts alkylation 5. Ar-H + R-X, Al. Cl 3 Ar-R + HX
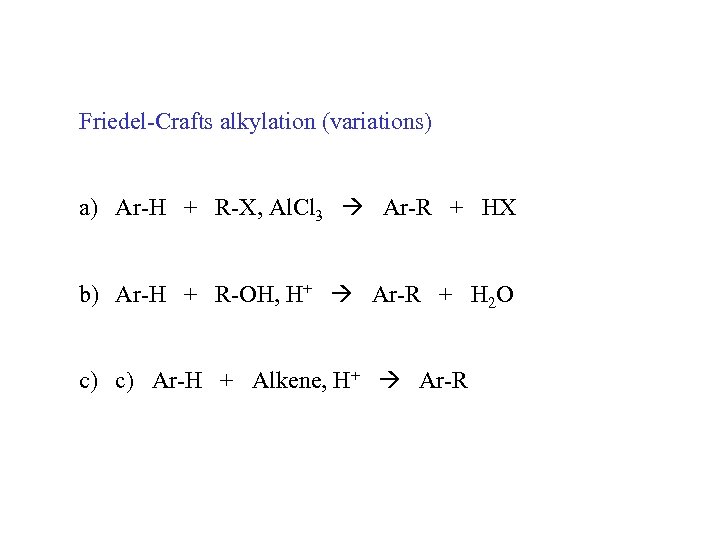 Friedel-Crafts alkylation (variations) a) Ar-H + R-X, Al. Cl 3 Ar-R + HX b) Ar-H + R-OH, H+ Ar-R + H 2 O c) c) Ar-H + Alkene, H+ Ar-R
Friedel-Crafts alkylation (variations) a) Ar-H + R-X, Al. Cl 3 Ar-R + HX b) Ar-H + R-OH, H+ Ar-R + H 2 O c) c) Ar-H + Alkene, H+ Ar-R
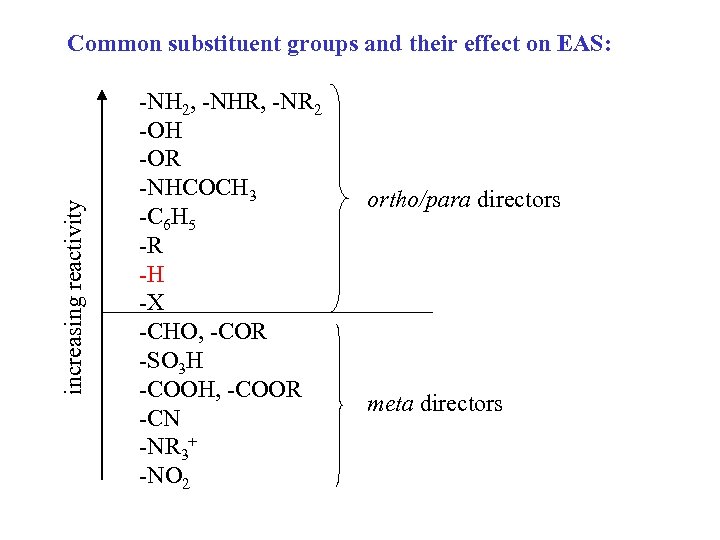 increasing reactivity Common substituent groups and their effect on EAS: -NH 2, -NHR, -NR 2 -OH -OR -NHCOCH 3 -C 6 H 5 -R -H -X -CHO, -COR -SO 3 H -COOH, -COOR -CN -NR 3+ -NO 2 ortho/para directors meta directors
increasing reactivity Common substituent groups and their effect on EAS: -NH 2, -NHR, -NR 2 -OH -OR -NHCOCH 3 -C 6 H 5 -R -H -X -CHO, -COR -SO 3 H -COOH, -COOR -CN -NR 3+ -NO 2 ortho/para directors meta directors
 If there is more than one group on the benzene ring: 1. The group that is more activating (higher on “the list”) will direct the next substitution. 2. You will get little or no substitution between groups that are meta- to each other.
If there is more than one group on the benzene ring: 1. The group that is more activating (higher on “the list”) will direct the next substitution. 2. You will get little or no substitution between groups that are meta- to each other.
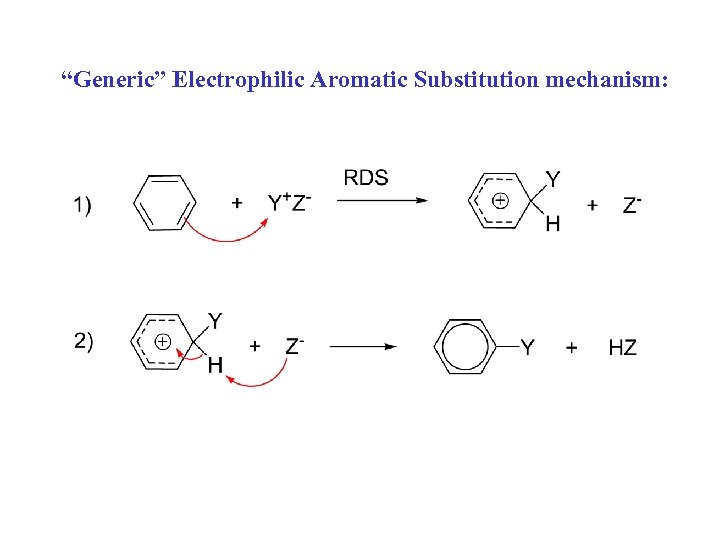 “Generic” Electrophilic Aromatic Substitution mechanism:
“Generic” Electrophilic Aromatic Substitution mechanism:
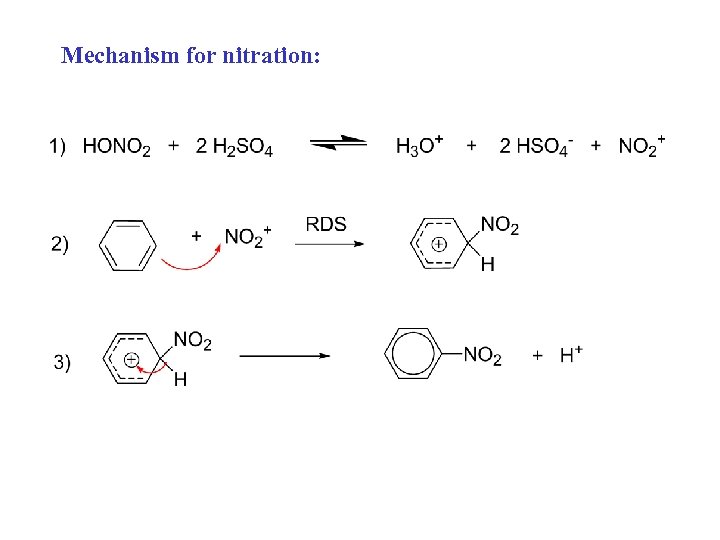 Mechanism for nitration:
Mechanism for nitration:
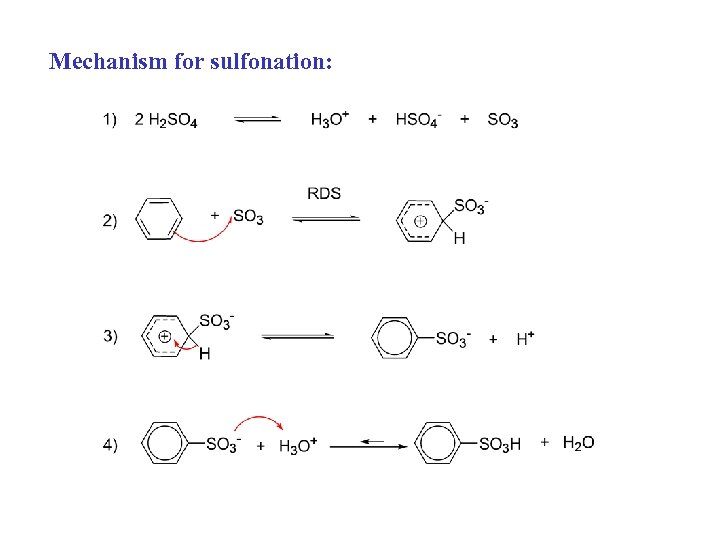 Mechanism for sulfonation:
Mechanism for sulfonation:
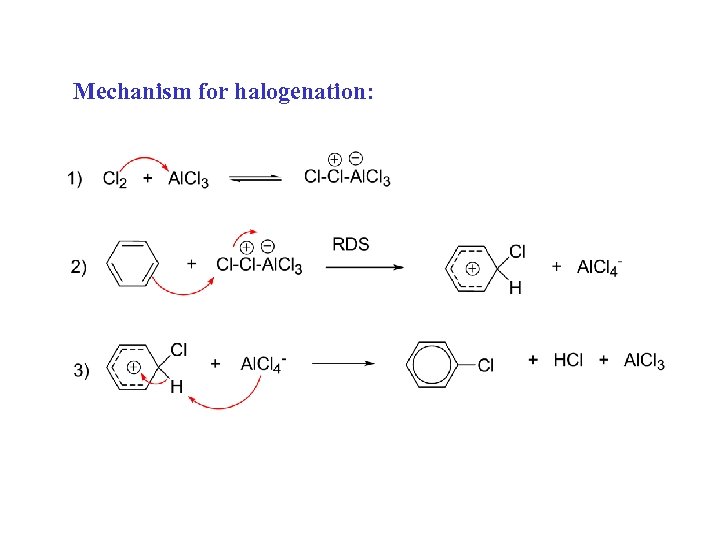 Mechanism for halogenation:
Mechanism for halogenation:
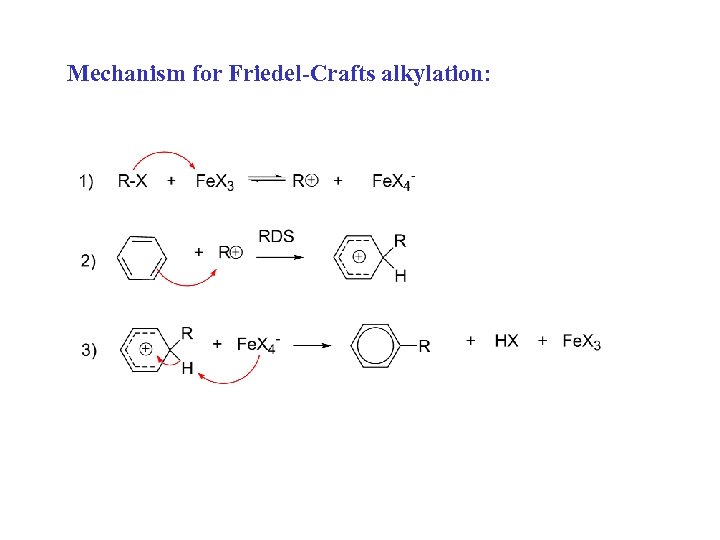 Mechanism for Friedel-Crafts alkylation:
Mechanism for Friedel-Crafts alkylation:
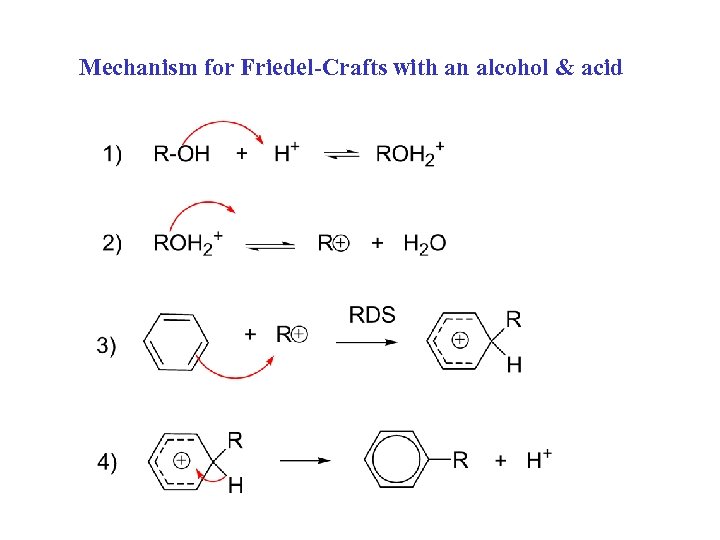 Mechanism for Friedel-Crafts with an alcohol & acid
Mechanism for Friedel-Crafts with an alcohol & acid
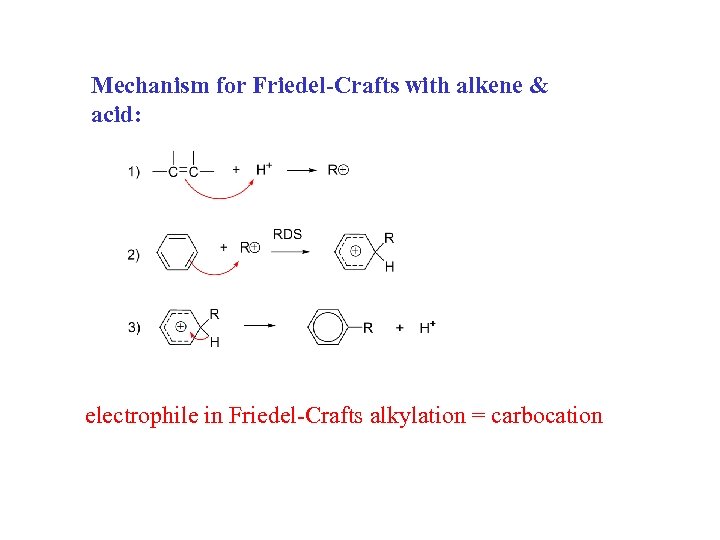 Mechanism for Friedel-Crafts with alkene & acid: electrophile in Friedel-Crafts alkylation = carbocation
Mechanism for Friedel-Crafts with alkene & acid: electrophile in Friedel-Crafts alkylation = carbocation
 Arenes alkylbenzenes alkenylbenzenes alkynylbenzenes etc.
Arenes alkylbenzenes alkenylbenzenes alkynylbenzenes etc.
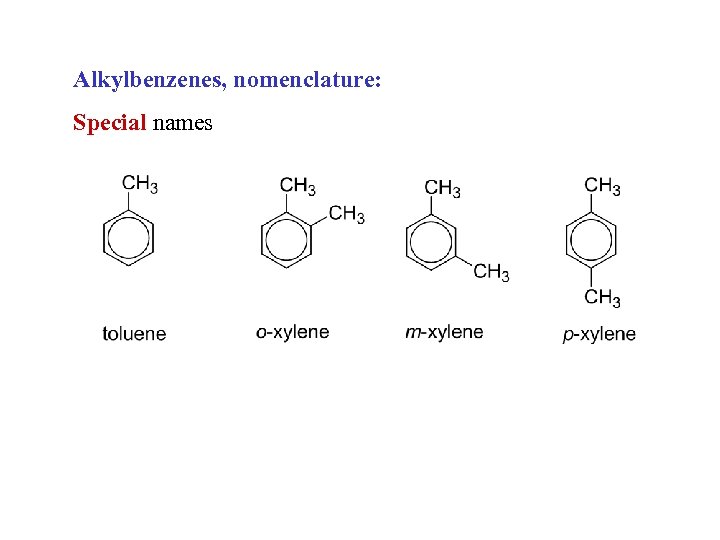 Alkylbenzenes, nomenclature: Special names
Alkylbenzenes, nomenclature: Special names
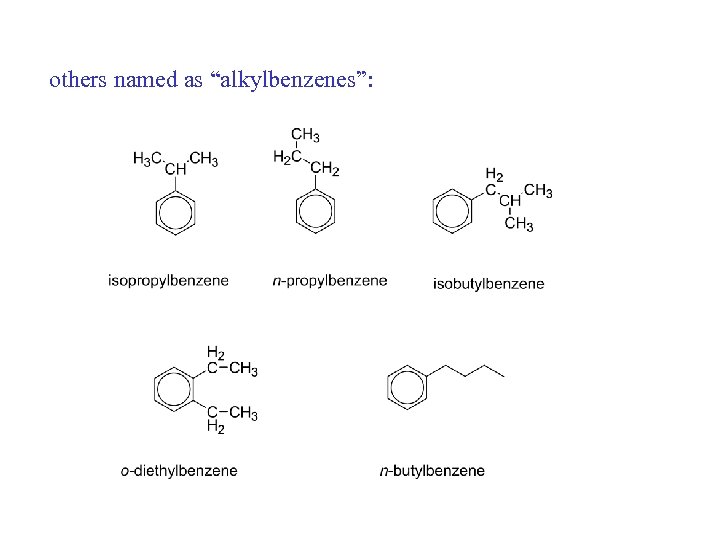 others named as “alkylbenzenes”:
others named as “alkylbenzenes”:
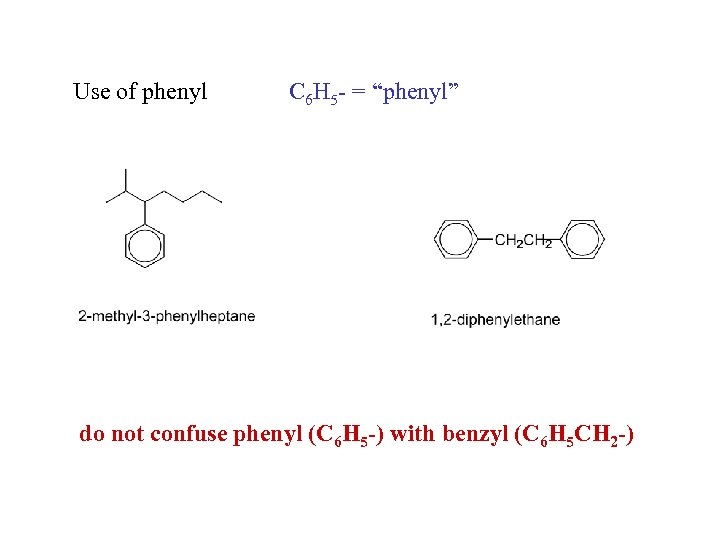 Use of phenyl C 6 H 5 - = “phenyl” do not confuse phenyl (C 6 H 5 -) with benzyl (C 6 H 5 CH 2 -)
Use of phenyl C 6 H 5 - = “phenyl” do not confuse phenyl (C 6 H 5 -) with benzyl (C 6 H 5 CH 2 -)
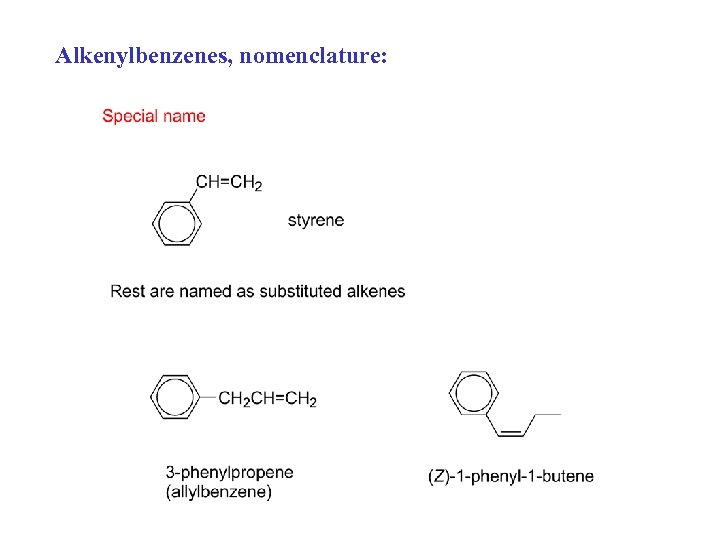 Alkenylbenzenes, nomenclature:
Alkenylbenzenes, nomenclature:
 Alkylbenzenes, syntheses: 1. Friedel-Crafts alkylation 2. Modification of a side chain: 3. a) addition of hydrogen to an alkene 4. b) reduction of an alkylhalide 5. i) hydrolysis of Grignard reagent 6. ii) active metal and acid 7. c) Corey-House synthesis
Alkylbenzenes, syntheses: 1. Friedel-Crafts alkylation 2. Modification of a side chain: 3. a) addition of hydrogen to an alkene 4. b) reduction of an alkylhalide 5. i) hydrolysis of Grignard reagent 6. ii) active metal and acid 7. c) Corey-House synthesis
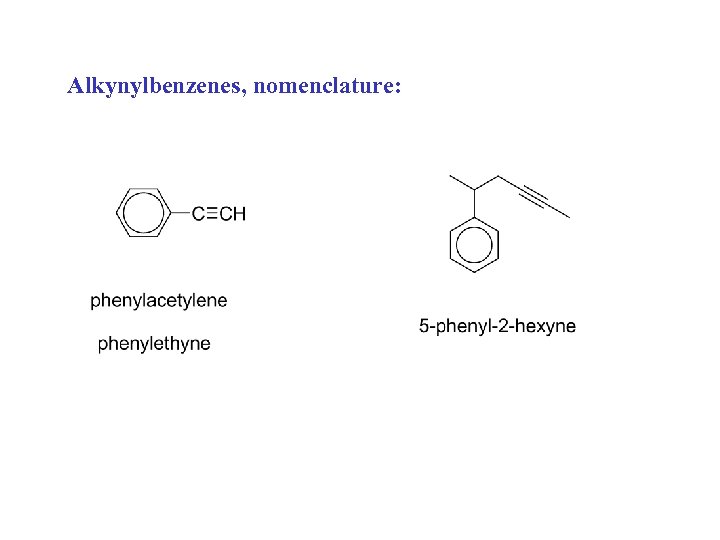 Alkynylbenzenes, nomenclature:
Alkynylbenzenes, nomenclature:
 Friedel-Crafts alkylation
Friedel-Crafts alkylation
 Friedel-Crafts limitations: a) Polyalkylation b) Possible rearrangement c) R-X cannot be Ar-X d) NR when the benzene ring is less reactive than bromobenzene e) NR with -NH 2, -NHR, -NR 2 groups
Friedel-Crafts limitations: a) Polyalkylation b) Possible rearrangement c) R-X cannot be Ar-X d) NR when the benzene ring is less reactive than bromobenzene e) NR with -NH 2, -NHR, -NR 2 groups
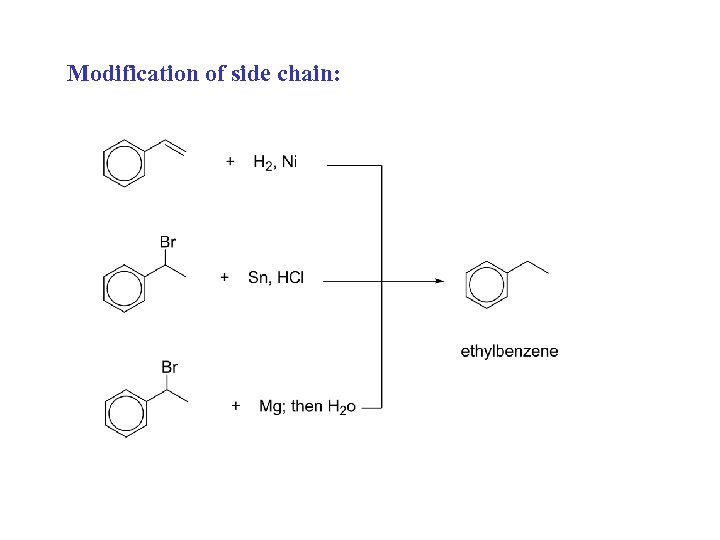 Modification of side chain:
Modification of side chain:
 Alkylbenzenes, reactions: 1. Reduction 2. Oxidation 3. EAS 4. a) nitration 5. b) sulfonation 6. c) halogenation 7. d) Friedel-Crafts alkylation 4. Side chain 5. free radical halogenation
Alkylbenzenes, reactions: 1. Reduction 2. Oxidation 3. EAS 4. a) nitration 5. b) sulfonation 6. c) halogenation 7. d) Friedel-Crafts alkylation 4. Side chain 5. free radical halogenation
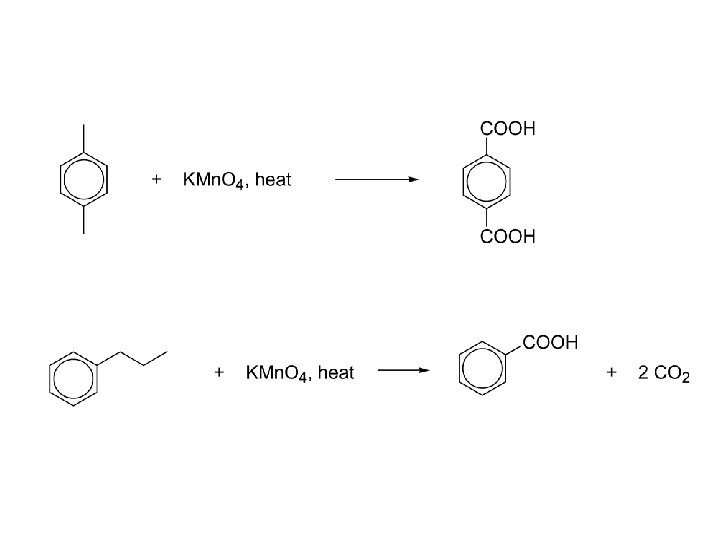
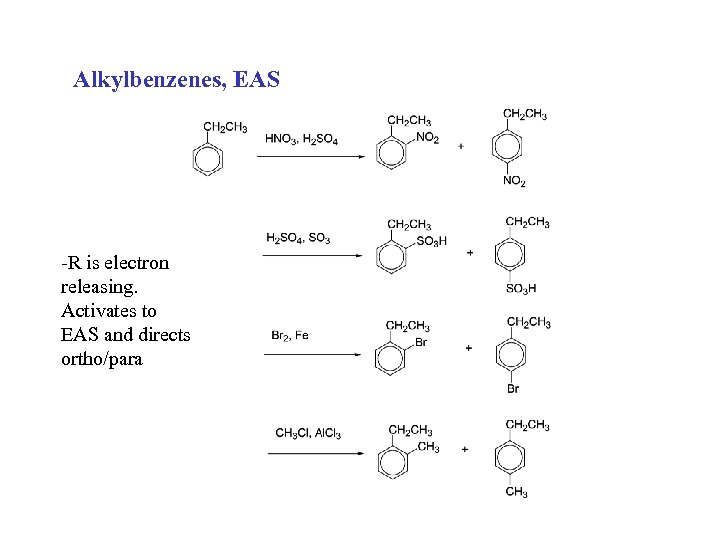 Alkylbenzenes, EAS -R is electron releasing. Activates to EAS and directs ortho/para
Alkylbenzenes, EAS -R is electron releasing. Activates to EAS and directs ortho/para
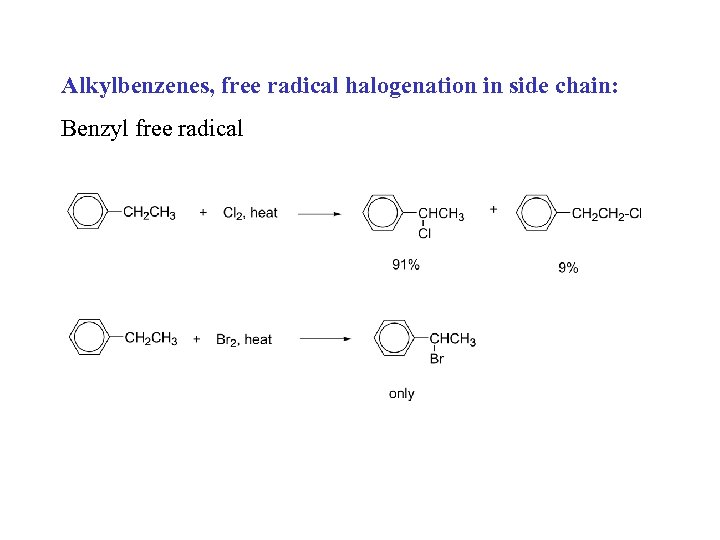 Alkylbenzenes, free radical halogenation in side chain: Benzyl free radical
Alkylbenzenes, free radical halogenation in side chain: Benzyl free radical
 Alkenylbenzenes, syntheses: 1. Modification of side chain: 2. a) dehydrohalogenation of alkyl halide 3. b) dehydration of alcohol 4. c) dehalogenation of vicinal dihalide 5. d) reduction of alkyne (2. Friedel-Crafts alkylation)
Alkenylbenzenes, syntheses: 1. Modification of side chain: 2. a) dehydrohalogenation of alkyl halide 3. b) dehydration of alcohol 4. c) dehalogenation of vicinal dihalide 5. d) reduction of alkyne (2. Friedel-Crafts alkylation)
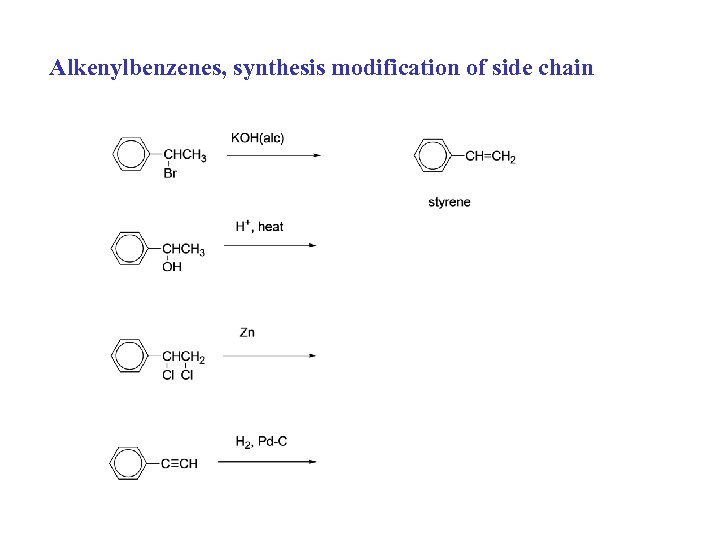 Alkenylbenzenes, synthesis modification of side chain
Alkenylbenzenes, synthesis modification of side chain
 Alkenylbenzenes, reactions: 1. Reduction 2. Oxidation 3. EAS 4. Side chain 5. a) add’n of H 2 j) oxymercuration 6. b) add’n of X 2 k) hydroboration 7. c) add’n of HX l) addition of free rad. 8. d) add’n of H 2 SO 4 carbenes m) add’n of 9. e) add’n of H 2 O n) epoxidation 10. f) add’n of X 2 & H 2 O o) hydroxylation 11. g) dimerization p) allylic halogenation 12. h) alkylation q) ozonolysis 13. i) dimerization r) vigorous oxidation
Alkenylbenzenes, reactions: 1. Reduction 2. Oxidation 3. EAS 4. Side chain 5. a) add’n of H 2 j) oxymercuration 6. b) add’n of X 2 k) hydroboration 7. c) add’n of HX l) addition of free rad. 8. d) add’n of H 2 SO 4 carbenes m) add’n of 9. e) add’n of H 2 O n) epoxidation 10. f) add’n of X 2 & H 2 O o) hydroxylation 11. g) dimerization p) allylic halogenation 12. h) alkylation q) ozonolysis 13. i) dimerization r) vigorous oxidation
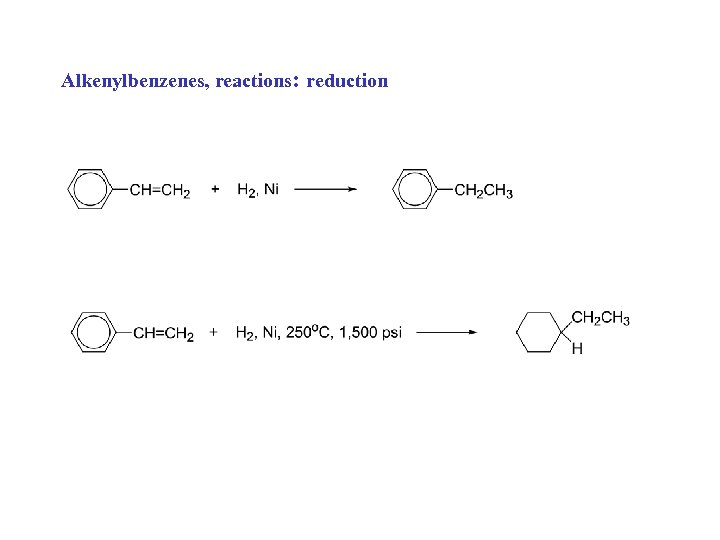 Alkenylbenzenes, reactions: reduction
Alkenylbenzenes, reactions: reduction
 Alkenylbenzenes, reactions oxidation
Alkenylbenzenes, reactions oxidation
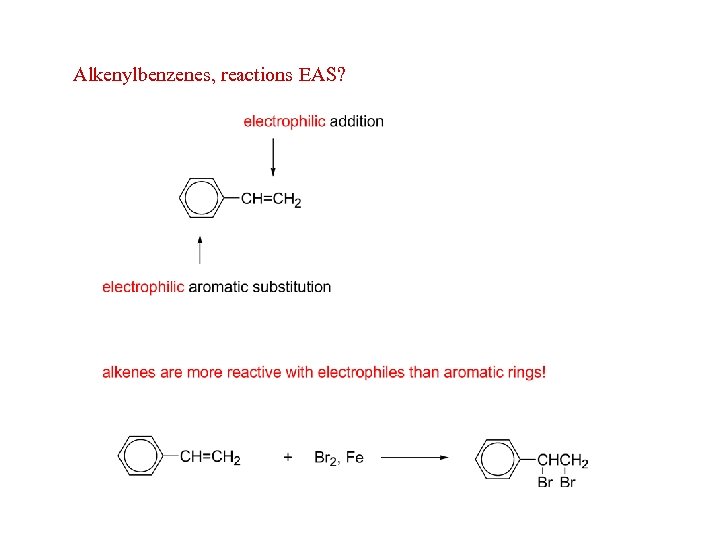 Alkenylbenzenes, reactions EAS?
Alkenylbenzenes, reactions EAS?
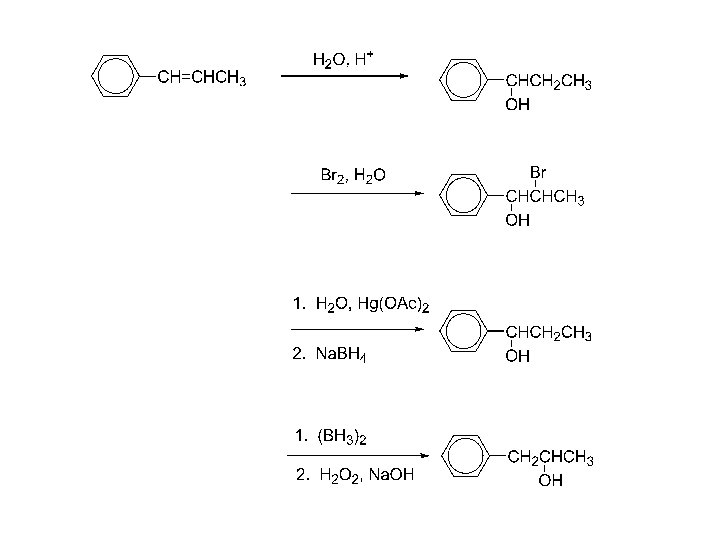
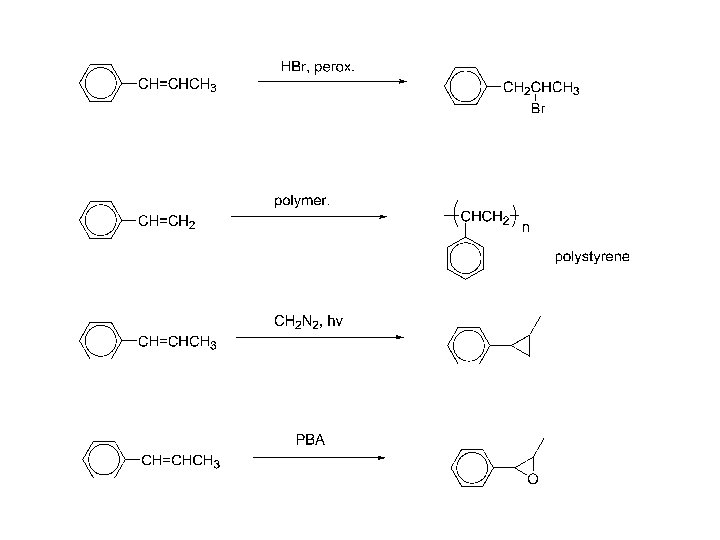
 100 syn-oxidation; make a model!
100 syn-oxidation; make a model!
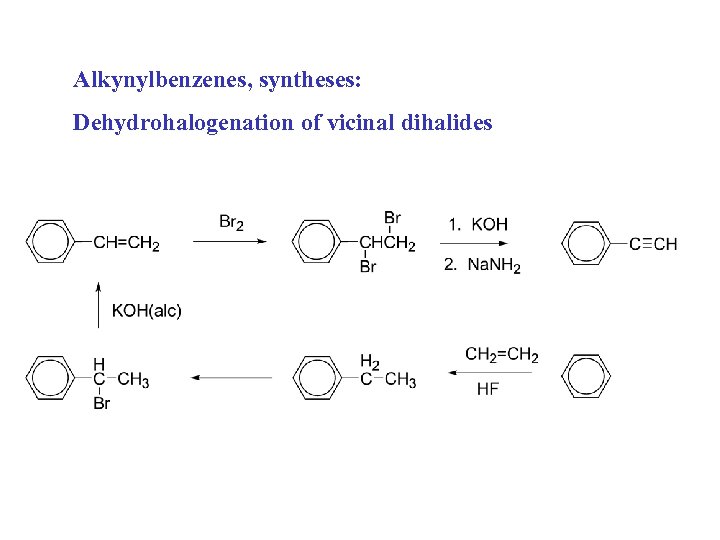 Alkynylbenzenes, syntheses: Dehydrohalogenation of vicinal dihalides
Alkynylbenzenes, syntheses: Dehydrohalogenation of vicinal dihalides
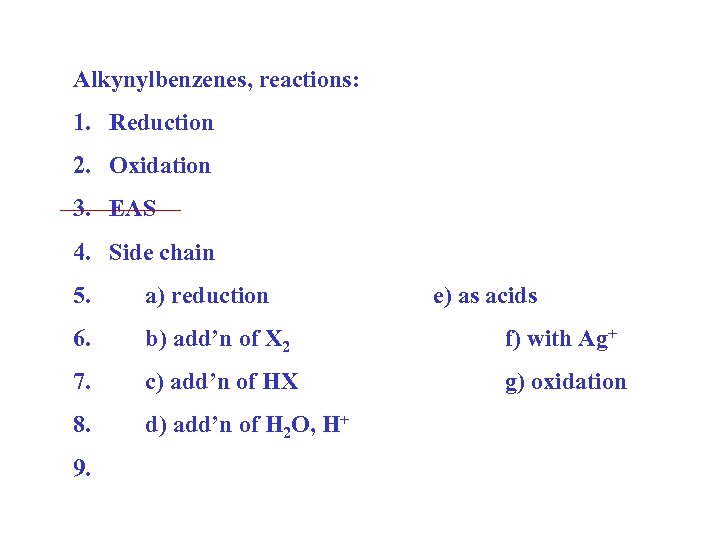 Alkynylbenzenes, reactions: 1. Reduction 2. Oxidation 3. EAS 4. Side chain 5. a) reduction 6. b) add’n of X 2 f) with Ag+ 7. c) add’n of HX g) oxidation 8. d) add’n of H 2 O, H+ 9. e) as acids
Alkynylbenzenes, reactions: 1. Reduction 2. Oxidation 3. EAS 4. Side chain 5. a) reduction 6. b) add’n of X 2 f) with Ag+ 7. c) add’n of HX g) oxidation 8. d) add’n of H 2 O, H+ 9. e) as acids
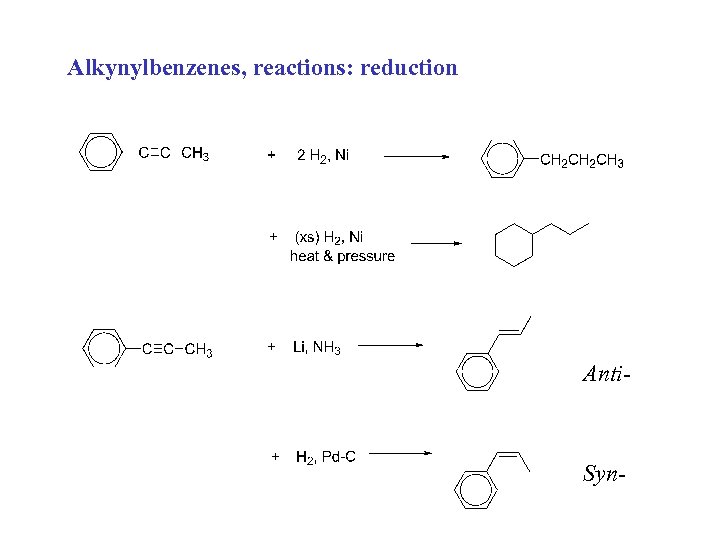 Alkynylbenzenes, reactions: reduction Anti- Syn-
Alkynylbenzenes, reactions: reduction Anti- Syn-
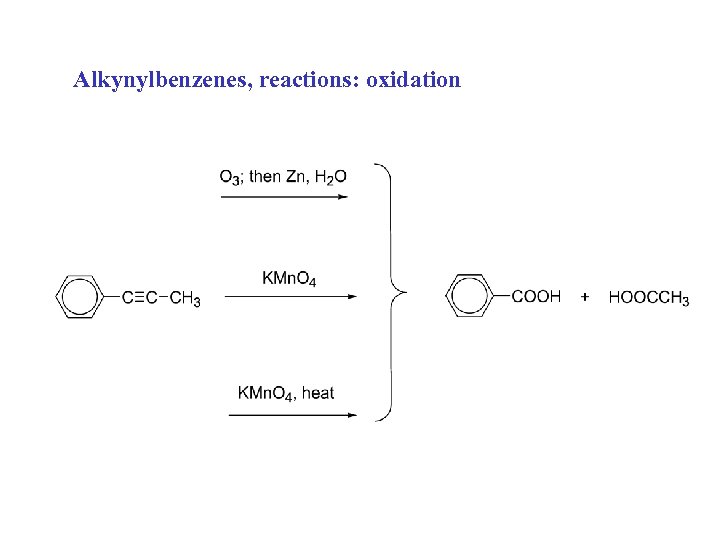 Alkynylbenzenes, reactions: oxidation
Alkynylbenzenes, reactions: oxidation
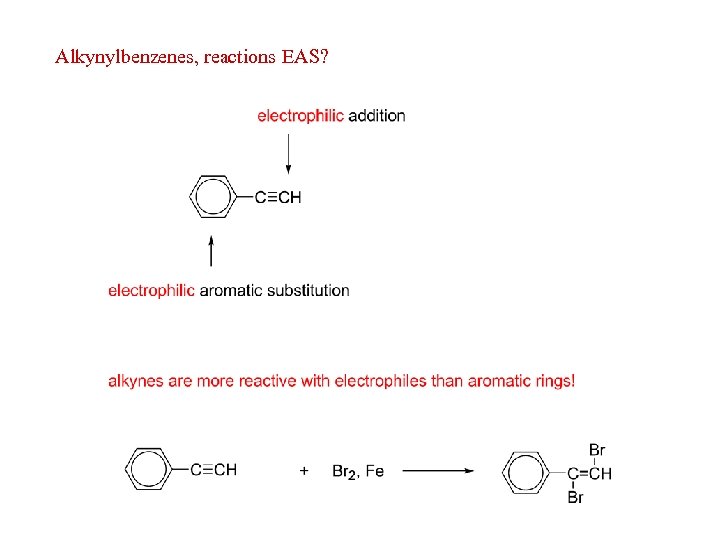 Alkynylbenzenes, reactions EAS?
Alkynylbenzenes, reactions EAS?
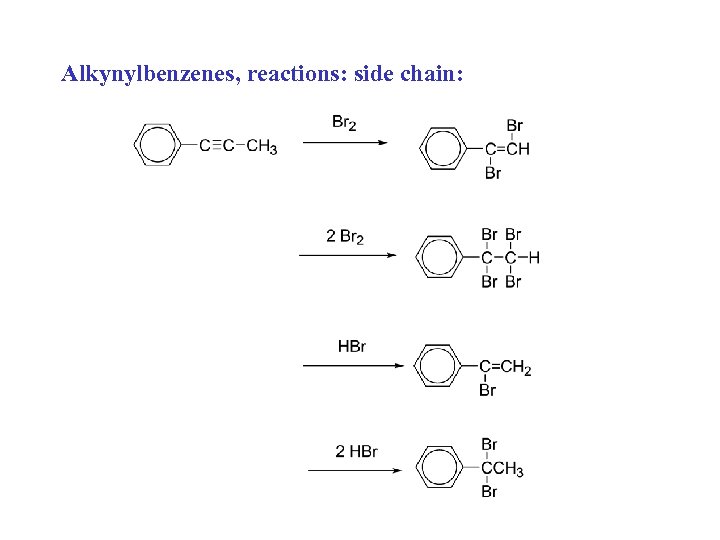 Alkynylbenzenes, reactions: side chain:
Alkynylbenzenes, reactions: side chain:
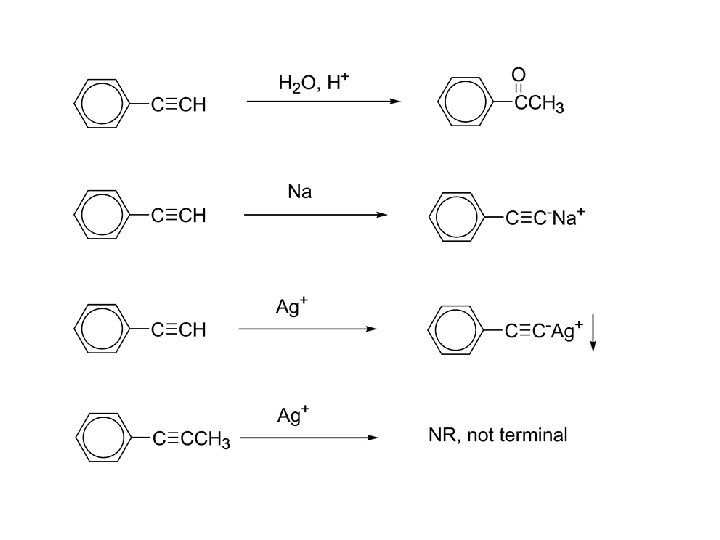
 Aldehydes and Ketones
Aldehydes and Ketones
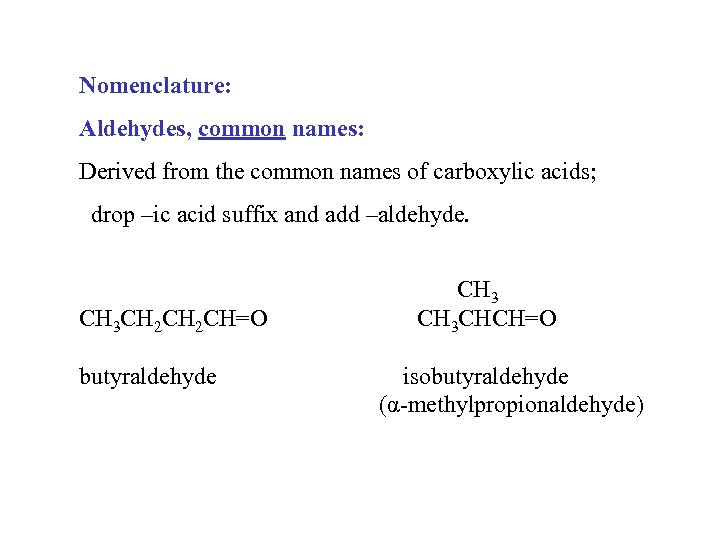 Nomenclature: Aldehydes, common names: Derived from the common names of carboxylic acids; drop –ic acid suffix and add –aldehyde. CH 3 CH 2 CH=O butyraldehyde CH 3 CHCH=O isobutyraldehyde (α-methylpropionaldehyde)
Nomenclature: Aldehydes, common names: Derived from the common names of carboxylic acids; drop –ic acid suffix and add –aldehyde. CH 3 CH 2 CH=O butyraldehyde CH 3 CHCH=O isobutyraldehyde (α-methylpropionaldehyde)
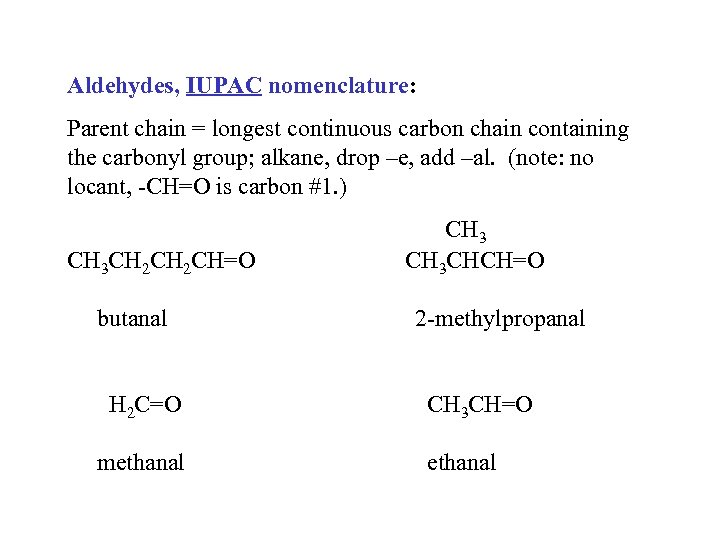 Aldehydes, IUPAC nomenclature: Parent chain = longest continuous carbon chain containing the carbonyl group; alkane, drop –e, add –al. (note: no locant, -CH=O is carbon #1. ) CH 3 CH 2 CH=O butanal H 2 C=O methanal CH 3 CHCH=O 2 -methylpropanal CH 3 CH=O ethanal
Aldehydes, IUPAC nomenclature: Parent chain = longest continuous carbon chain containing the carbonyl group; alkane, drop –e, add –al. (note: no locant, -CH=O is carbon #1. ) CH 3 CH 2 CH=O butanal H 2 C=O methanal CH 3 CHCH=O 2 -methylpropanal CH 3 CH=O ethanal
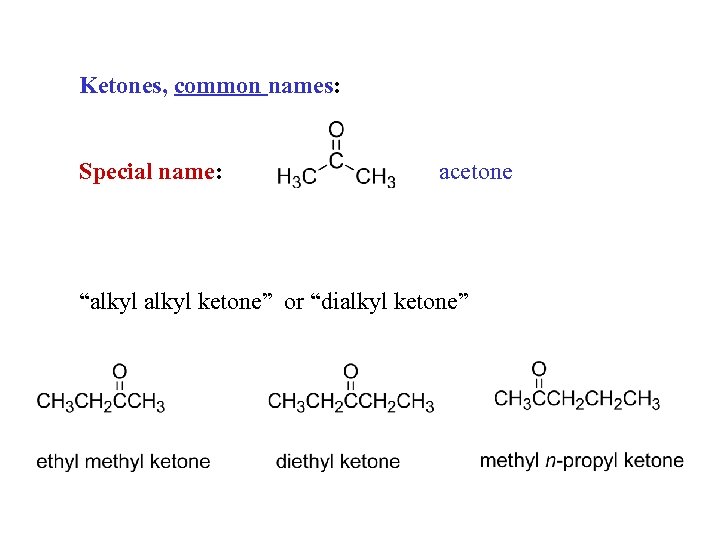 Ketones, common names: Special name: acetone “alkyl ketone” or “dialkyl ketone”
Ketones, common names: Special name: acetone “alkyl ketone” or “dialkyl ketone”
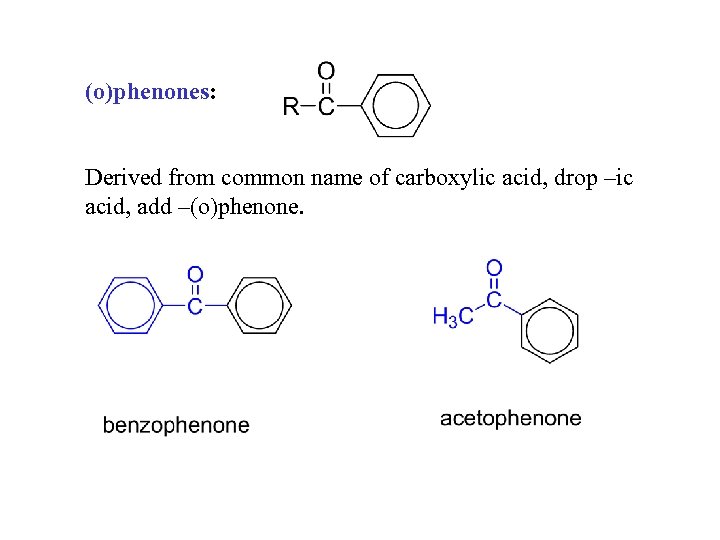 (o)phenones: Derived from common name of carboxylic acid, drop –ic acid, add –(o)phenone.
(o)phenones: Derived from common name of carboxylic acid, drop –ic acid, add –(o)phenone.
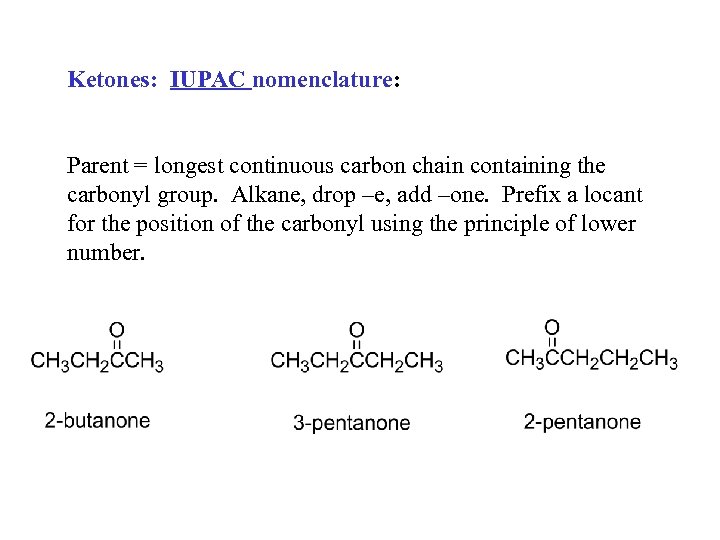 Ketones: IUPAC nomenclature: Parent = longest continuous carbon chain containing the carbonyl group. Alkane, drop –e, add –one. Prefix a locant for the position of the carbonyl using the principle of lower number.
Ketones: IUPAC nomenclature: Parent = longest continuous carbon chain containing the carbonyl group. Alkane, drop –e, add –one. Prefix a locant for the position of the carbonyl using the principle of lower number.
 Aldehydes, syntheses: 1. Oxidation of 1 o alcohols 2. Oxidation of methylaromatics 3. Reduction of acid chlorides 4. Ketones, syntheses: 5. Oxidation of 2 o alcohols 6. Friedel-Crafts acylation 7. Coupling of R 2 Cu. Li with acid chloride
Aldehydes, syntheses: 1. Oxidation of 1 o alcohols 2. Oxidation of methylaromatics 3. Reduction of acid chlorides 4. Ketones, syntheses: 5. Oxidation of 2 o alcohols 6. Friedel-Crafts acylation 7. Coupling of R 2 Cu. Li with acid chloride
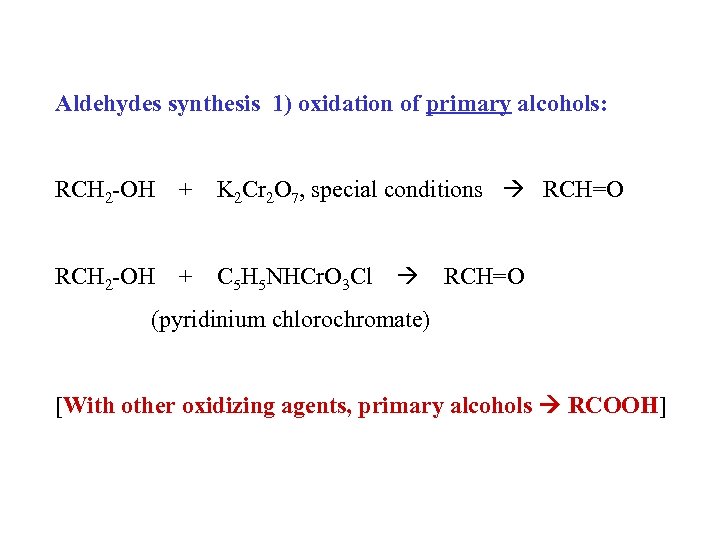 Aldehydes synthesis 1) oxidation of primary alcohols: RCH 2 -OH + K 2 Cr 2 O 7, special conditions RCH=O RCH 2 -OH + C 5 H 5 NHCr. O 3 Cl RCH=O (pyridinium chlorochromate) [With other oxidizing agents, primary alcohols RCOOH]
Aldehydes synthesis 1) oxidation of primary alcohols: RCH 2 -OH + K 2 Cr 2 O 7, special conditions RCH=O RCH 2 -OH + C 5 H 5 NHCr. O 3 Cl RCH=O (pyridinium chlorochromate) [With other oxidizing agents, primary alcohols RCOOH]
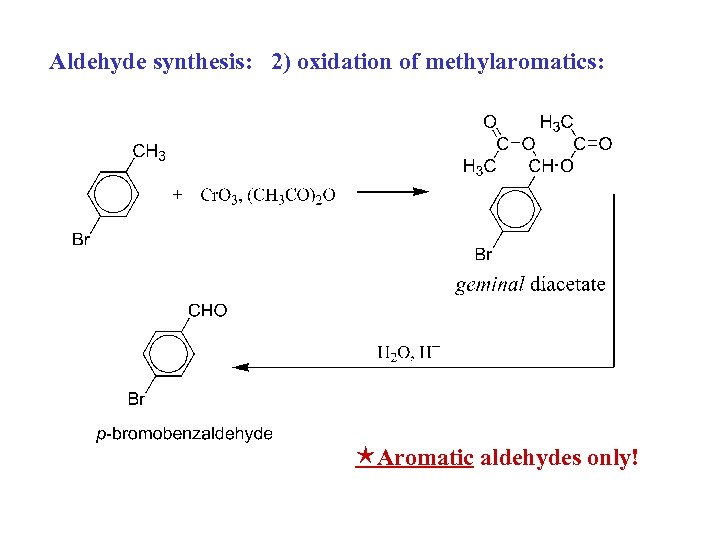 Aldehyde synthesis: 2) oxidation of methylaromatics: Aromatic aldehydes only!
Aldehyde synthesis: 2) oxidation of methylaromatics: Aromatic aldehydes only!
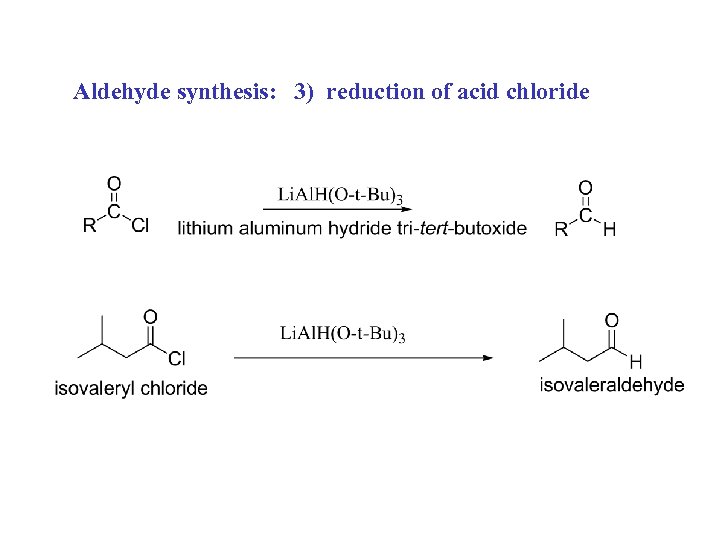 Aldehyde synthesis: 3) reduction of acid chloride
Aldehyde synthesis: 3) reduction of acid chloride
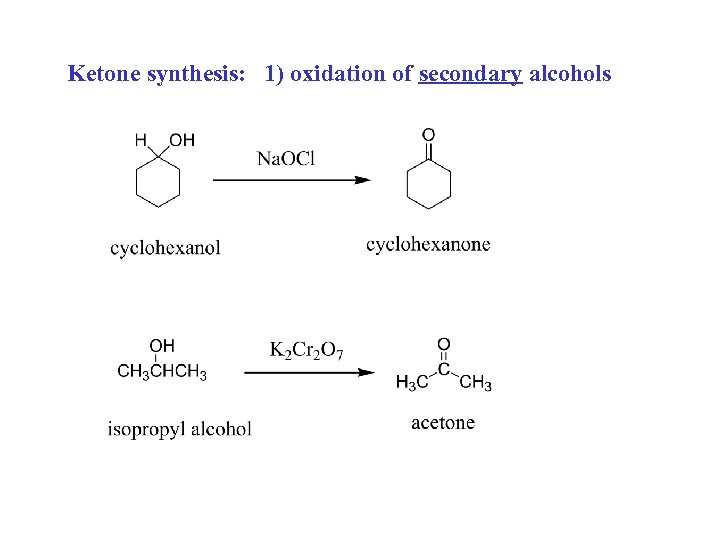 Ketone synthesis: 1) oxidation of secondary alcohols
Ketone synthesis: 1) oxidation of secondary alcohols
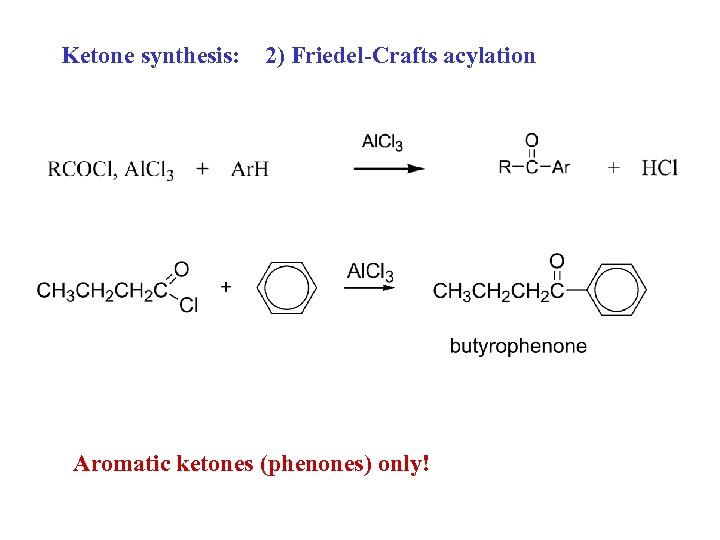 Ketone synthesis: 2) Friedel-Crafts acylation Aromatic ketones (phenones) only!
Ketone synthesis: 2) Friedel-Crafts acylation Aromatic ketones (phenones) only!
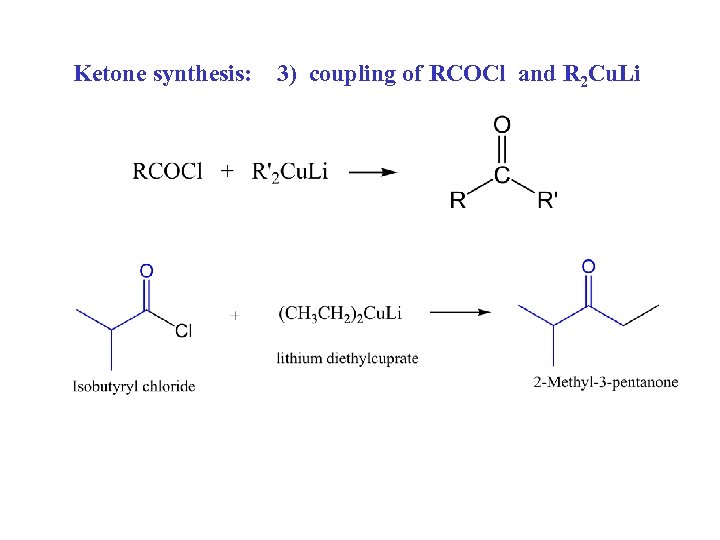 Ketone synthesis: 3) coupling of RCOCl and R 2 Cu. Li
Ketone synthesis: 3) coupling of RCOCl and R 2 Cu. Li
 Aldehydes & ketones, reactions: 1) Oxidation 2) Reduction 3) Addition of cyanide 4) Addition of derivatives of ammonia 5) Addition of alcohols 6) Cannizzaro reaction 7) Addition of Grignard reagents 8) 8) (Alpha-halogenation of ketones) 9) 9) (Addition of carbanions)
Aldehydes & ketones, reactions: 1) Oxidation 2) Reduction 3) Addition of cyanide 4) Addition of derivatives of ammonia 5) Addition of alcohols 6) Cannizzaro reaction 7) Addition of Grignard reagents 8) 8) (Alpha-halogenation of ketones) 9) 9) (Addition of carbanions)
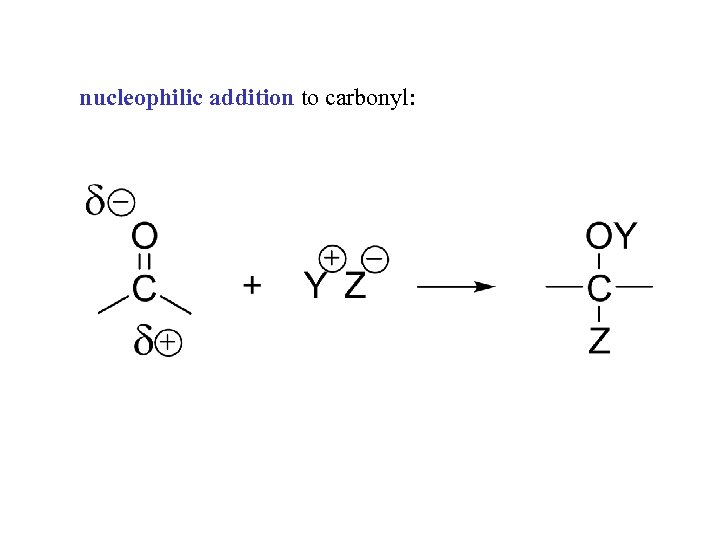 nucleophilic addition to carbonyl:
nucleophilic addition to carbonyl:
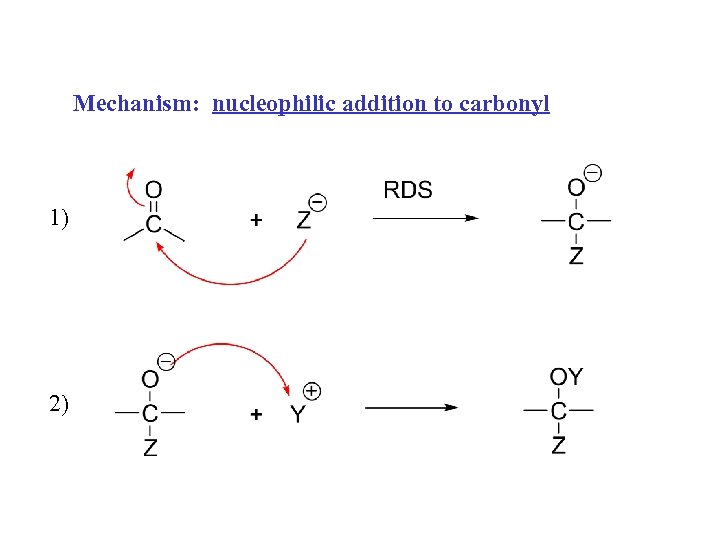 Mechanism: nucleophilic addition to carbonyl 1) 2)
Mechanism: nucleophilic addition to carbonyl 1) 2)
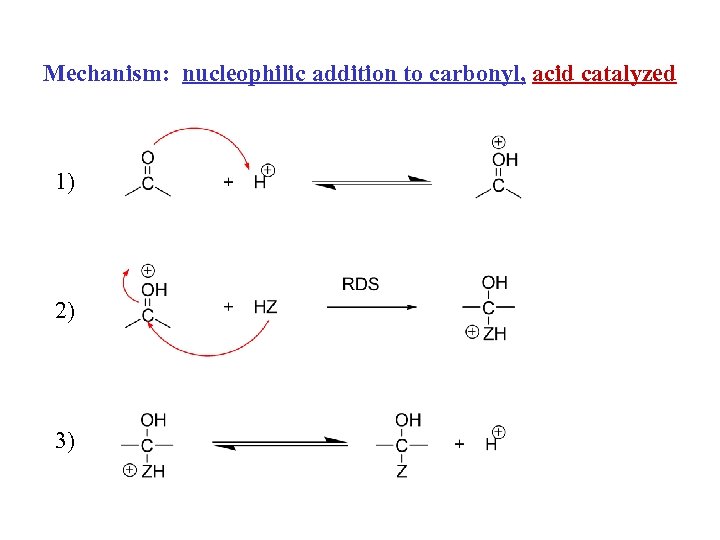 Mechanism: nucleophilic addition to carbonyl, acid catalyzed 1) 2) 3)
Mechanism: nucleophilic addition to carbonyl, acid catalyzed 1) 2) 3)
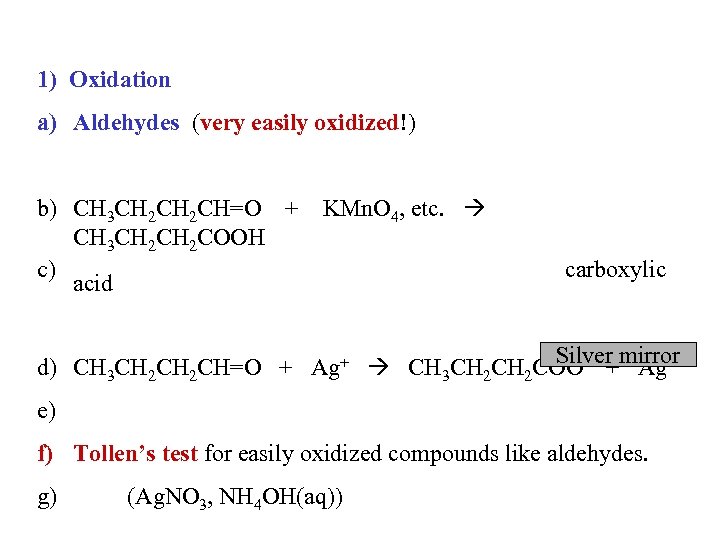 1) Oxidation a) Aldehydes (very easily oxidized!) b) CH 3 CH 2 CH=O + KMn. O 4, etc. CH 3 CH 2 COOH c) acid carboxylic Silver Ag d) CH 3 CH 2 CH=O + Ag+ CH 3 CH 2 COO- +mirror e) f) Tollen’s test for easily oxidized compounds like aldehydes. g) (Ag. NO 3, NH 4 OH(aq))
1) Oxidation a) Aldehydes (very easily oxidized!) b) CH 3 CH 2 CH=O + KMn. O 4, etc. CH 3 CH 2 COOH c) acid carboxylic Silver Ag d) CH 3 CH 2 CH=O + Ag+ CH 3 CH 2 COO- +mirror e) f) Tollen’s test for easily oxidized compounds like aldehydes. g) (Ag. NO 3, NH 4 OH(aq))
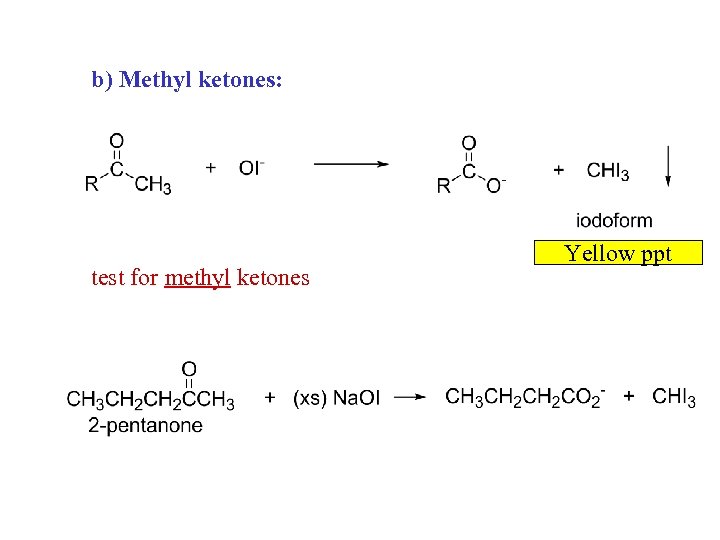 b) Methyl ketones: test for methyl ketones Yellow ppt
b) Methyl ketones: test for methyl ketones Yellow ppt
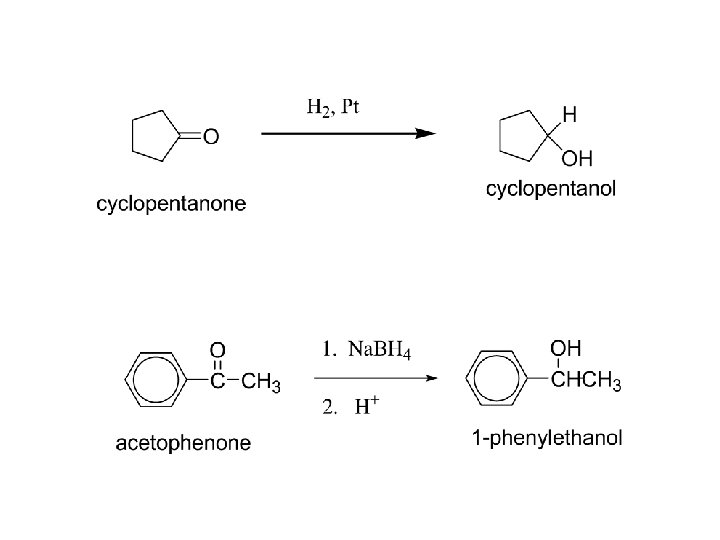
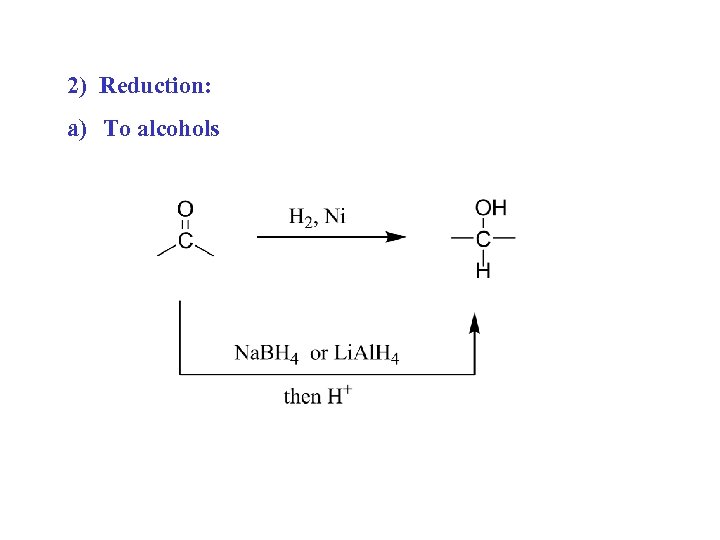 2) Reduction: a) To alcohols
2) Reduction: a) To alcohols
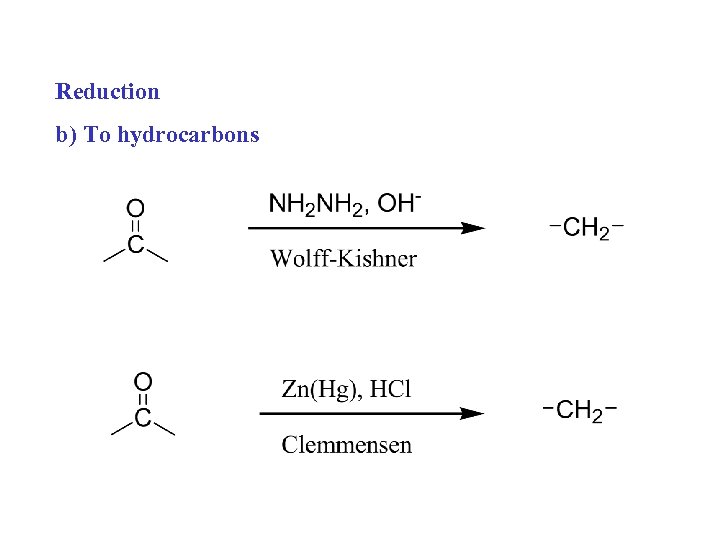 Reduction b) To hydrocarbons
Reduction b) To hydrocarbons
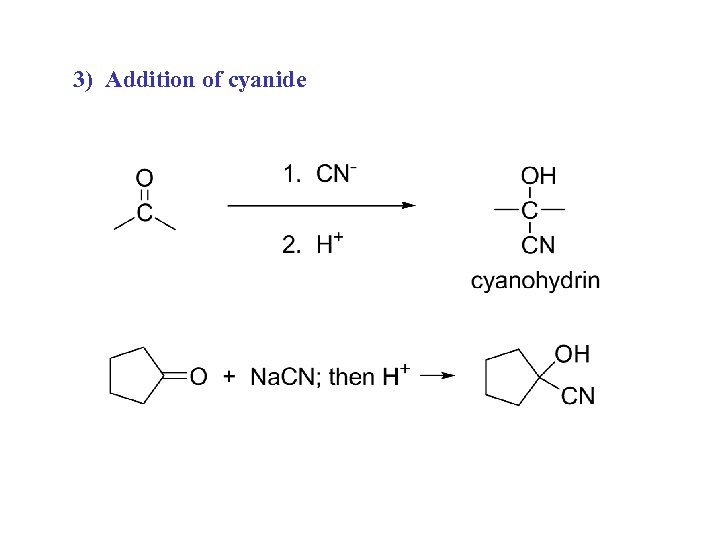 3) Addition of cyanide
3) Addition of cyanide
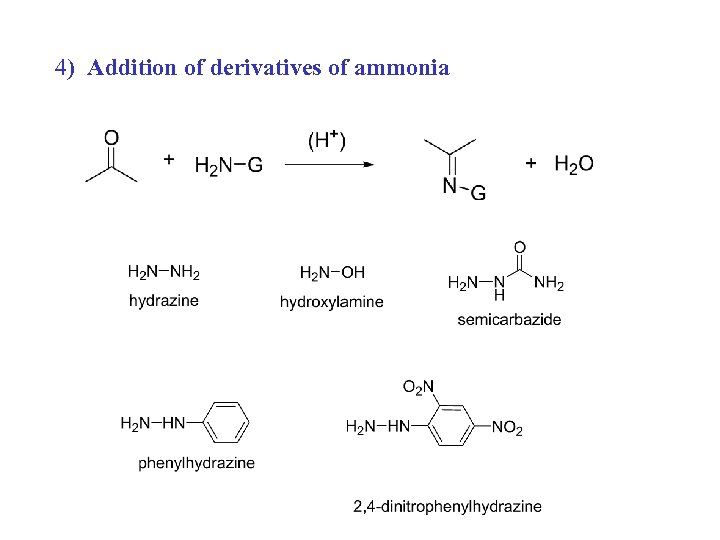 4) Addition of derivatives of ammonia
4) Addition of derivatives of ammonia

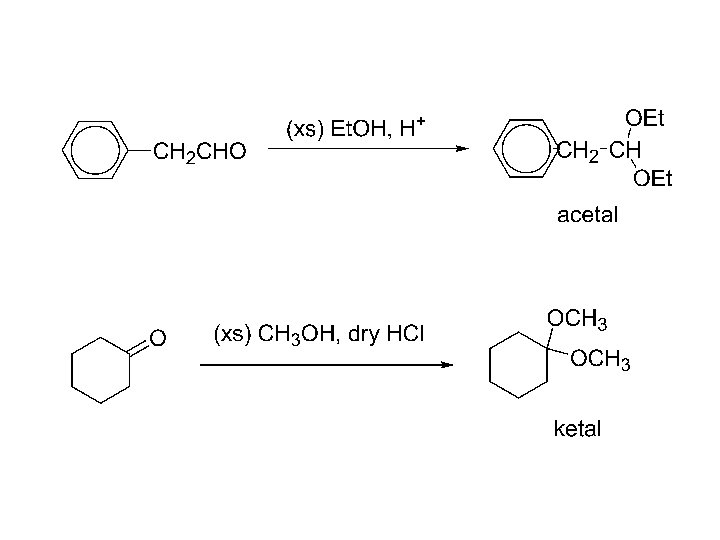
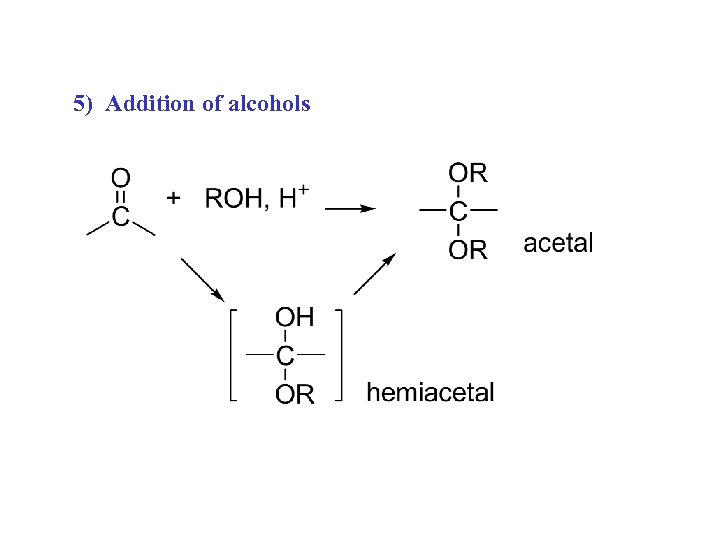 5) Addition of alcohols
5) Addition of alcohols
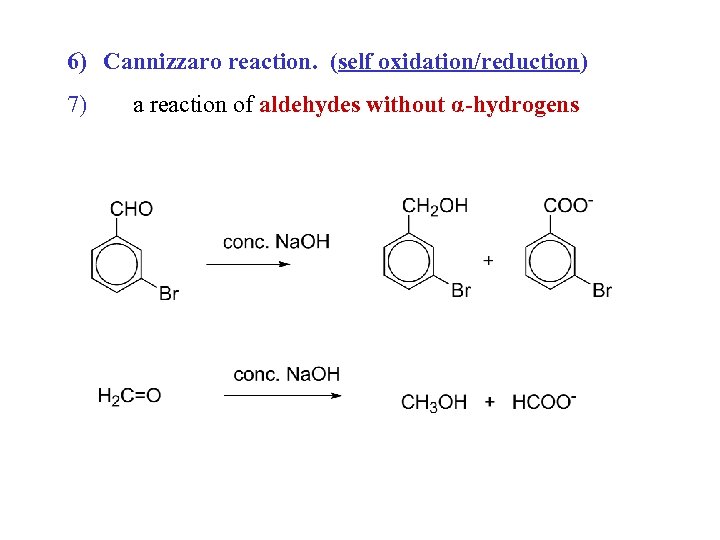 6) Cannizzaro reaction. (self oxidation/reduction) 7) a reaction of aldehydes without α-hydrogens
6) Cannizzaro reaction. (self oxidation/reduction) 7) a reaction of aldehydes without α-hydrogens
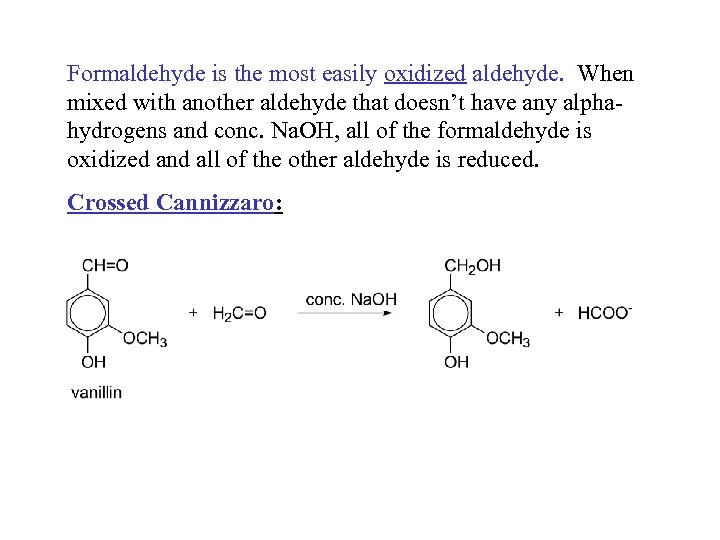 Formaldehyde is the most easily oxidized aldehyde. When mixed with another aldehyde that doesn’t have any alphahydrogens and conc. Na. OH, all of the formaldehyde is oxidized and all of the other aldehyde is reduced. Crossed Cannizzaro:
Formaldehyde is the most easily oxidized aldehyde. When mixed with another aldehyde that doesn’t have any alphahydrogens and conc. Na. OH, all of the formaldehyde is oxidized and all of the other aldehyde is reduced. Crossed Cannizzaro:
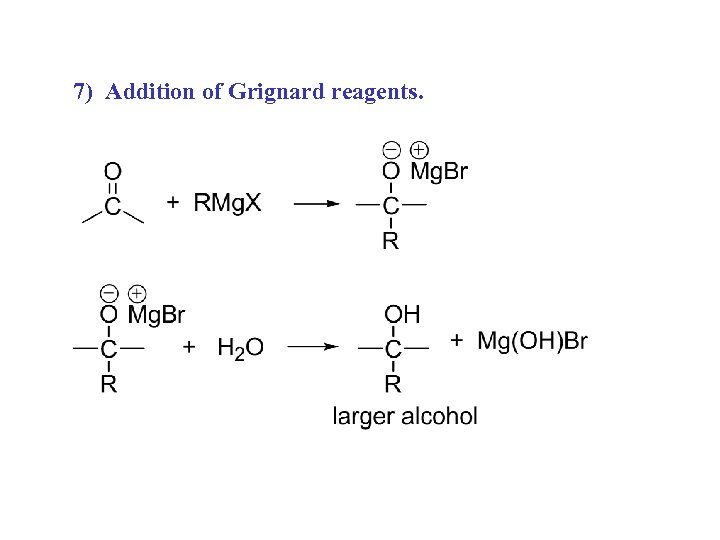 7) Addition of Grignard reagents.
7) Addition of Grignard reagents.
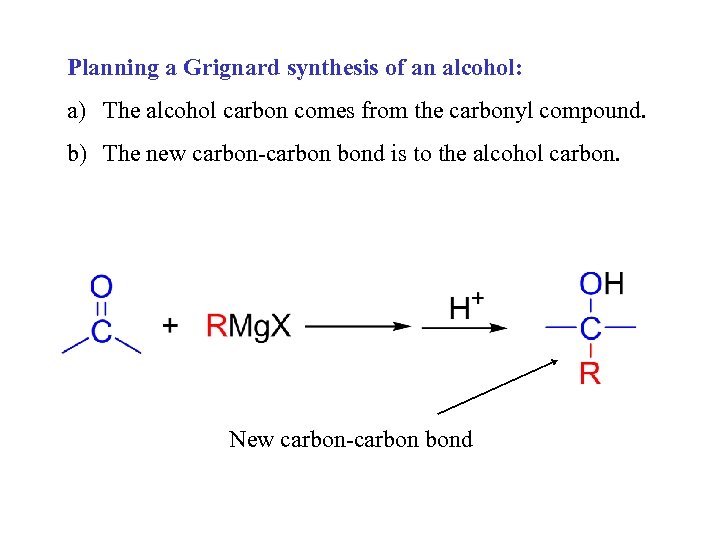 Planning a Grignard synthesis of an alcohol: a) The alcohol carbon comes from the carbonyl compound. b) The new carbon-carbon bond is to the alcohol carbon. New carbon-carbon bond
Planning a Grignard synthesis of an alcohol: a) The alcohol carbon comes from the carbonyl compound. b) The new carbon-carbon bond is to the alcohol carbon. New carbon-carbon bond
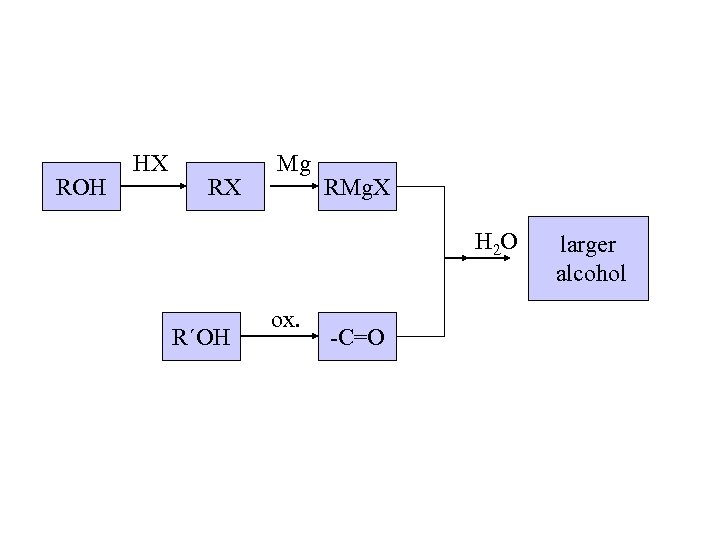 ROH HX RX Mg RMg. X H 2 O R´OH ox. -C=O larger alcohol
ROH HX RX Mg RMg. X H 2 O R´OH ox. -C=O larger alcohol
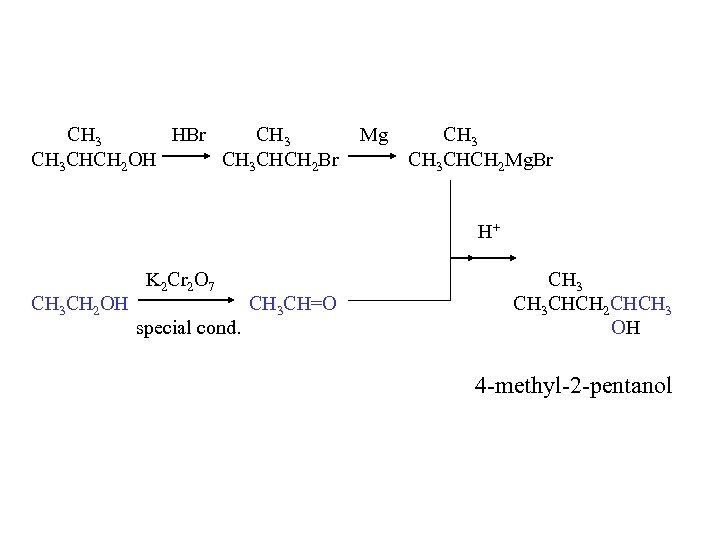 CH 3 HBr CH 3 CHCH 2 OH CH 3 CHCH 2 Br Mg CH 3 CHCH 2 Mg. Br H+ CH 3 CH 2 OH K 2 Cr 2 O 7 special cond. CH 3 CH=O CH 3 CHCH 2 CHCH 3 OH 4 -methyl-2 -pentanol
CH 3 HBr CH 3 CHCH 2 OH CH 3 CHCH 2 Br Mg CH 3 CHCH 2 Mg. Br H+ CH 3 CH 2 OH K 2 Cr 2 O 7 special cond. CH 3 CH=O CH 3 CHCH 2 CHCH 3 OH 4 -methyl-2 -pentanol
 Carboxylic Acids
Carboxylic Acids
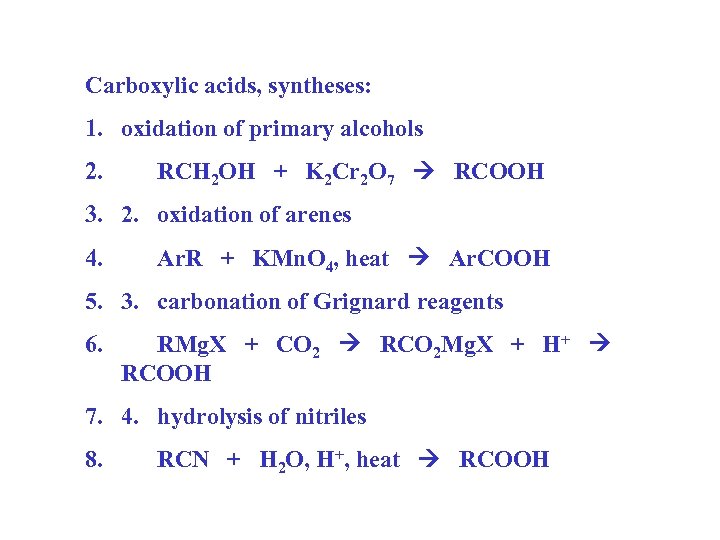 Carboxylic acids, syntheses: 1. oxidation of primary alcohols 2. RCH 2 OH + K 2 Cr 2 O 7 RCOOH 3. 2. oxidation of arenes 4. Ar. R + KMn. O 4, heat Ar. COOH 5. 3. carbonation of Grignard reagents 6. RMg. X + CO 2 RCO 2 Mg. X + H+ RCOOH 7. 4. hydrolysis of nitriles 8. RCN + H 2 O, H+, heat RCOOH
Carboxylic acids, syntheses: 1. oxidation of primary alcohols 2. RCH 2 OH + K 2 Cr 2 O 7 RCOOH 3. 2. oxidation of arenes 4. Ar. R + KMn. O 4, heat Ar. COOH 5. 3. carbonation of Grignard reagents 6. RMg. X + CO 2 RCO 2 Mg. X + H+ RCOOH 7. 4. hydrolysis of nitriles 8. RCN + H 2 O, H+, heat RCOOH
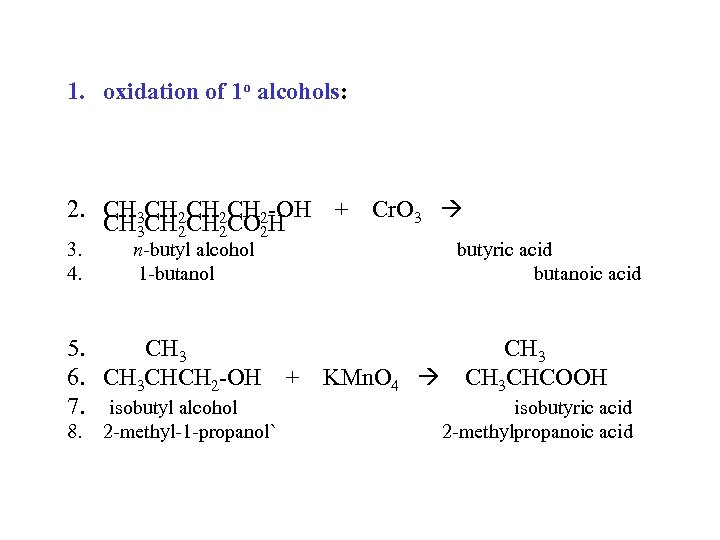 1. oxidation of 1 o alcohols: 2. CH 3 CH 2 CH 2 -OH + Cr. O 3 CH 3 CH 2 CO 2 H 3. 4. n-butyl alcohol 1 -butanol butyric acid butanoic acid 5. CH 3 6. CH 3 CHCH 2 -OH + KMn. O 4 CH 3 CHCOOH 7. isobutyl alcohol isobutyric acid 8. 2 -methyl-1 -propanol` 2 -methylpropanoic acid
1. oxidation of 1 o alcohols: 2. CH 3 CH 2 CH 2 -OH + Cr. O 3 CH 3 CH 2 CO 2 H 3. 4. n-butyl alcohol 1 -butanol butyric acid butanoic acid 5. CH 3 6. CH 3 CHCH 2 -OH + KMn. O 4 CH 3 CHCOOH 7. isobutyl alcohol isobutyric acid 8. 2 -methyl-1 -propanol` 2 -methylpropanoic acid
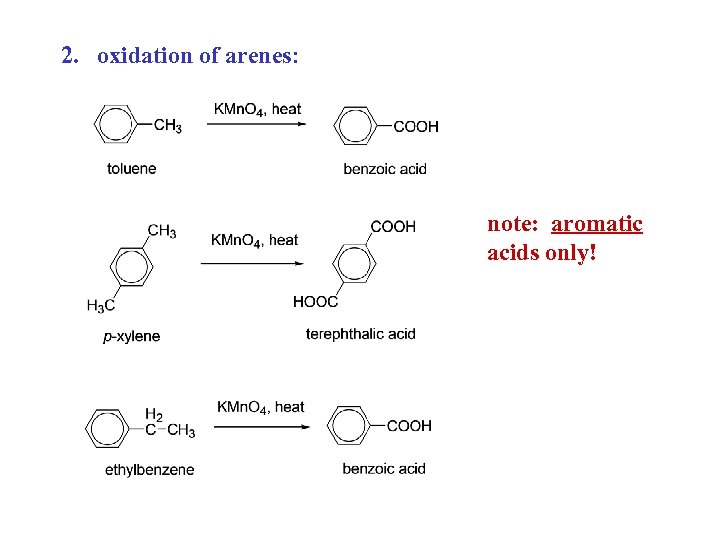 2. oxidation of arenes: note: aromatic acids only!
2. oxidation of arenes: note: aromatic acids only!
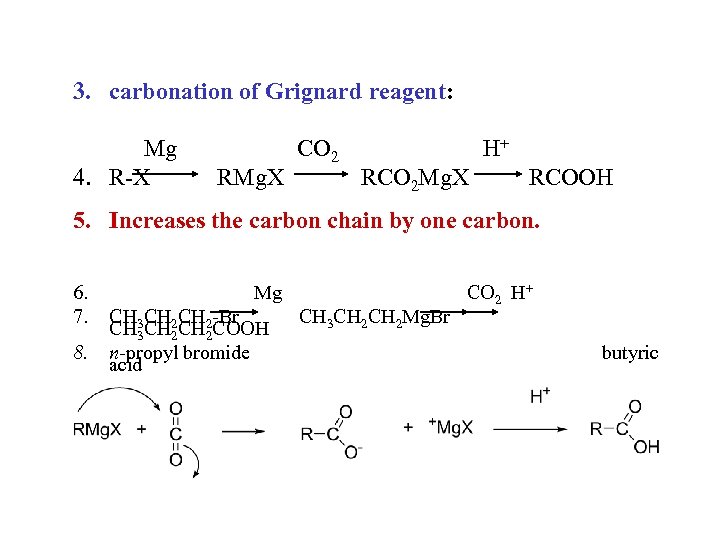 3. carbonation of Grignard reagent: Mg 4. R-X RMg. X CO 2 H+ RCO 2 Mg. X RCOOH 5. Increases the carbon chain by one carbon. 6. 7. 8. Mg CH 3 CH 2 -Br CH 3 CH 2 COOH n-propyl bromide acid CH 3 CH 2 Mg. Br CO 2 H+ butyric
3. carbonation of Grignard reagent: Mg 4. R-X RMg. X CO 2 H+ RCO 2 Mg. X RCOOH 5. Increases the carbon chain by one carbon. 6. 7. 8. Mg CH 3 CH 2 -Br CH 3 CH 2 COOH n-propyl bromide acid CH 3 CH 2 Mg. Br CO 2 H+ butyric
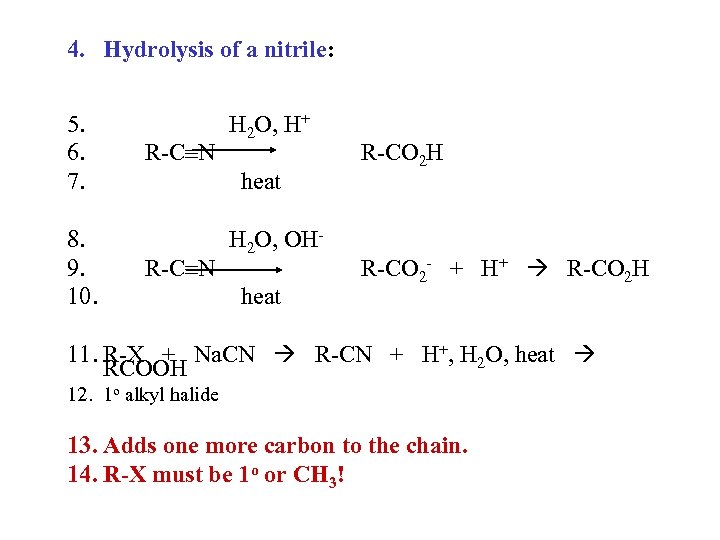 4. Hydrolysis of a nitrile: 5. 6. 7. 8. 9. 10. R-C N H 2 O, H+ heat R-C N H 2 O, OHheat R-CO 2 H R-CO 2 - + H+ R-CO 2 H 11. R-X + Na. CN R-CN + H+, H 2 O, heat RCOOH 12. 1 o alkyl halide 13. Adds one more carbon to the chain. 14. R-X must be 1 o or CH 3!
4. Hydrolysis of a nitrile: 5. 6. 7. 8. 9. 10. R-C N H 2 O, H+ heat R-C N H 2 O, OHheat R-CO 2 H R-CO 2 - + H+ R-CO 2 H 11. R-X + Na. CN R-CN + H+, H 2 O, heat RCOOH 12. 1 o alkyl halide 13. Adds one more carbon to the chain. 14. R-X must be 1 o or CH 3!
 carboxylic acids, reactions: 1. as acids 2. conversion into functional derivatives 3. a) acid chlorides 4. b) esters 5. c) amides 3. reduction 4. alpha-halogenation 5. EAS
carboxylic acids, reactions: 1. as acids 2. conversion into functional derivatives 3. a) acid chlorides 4. b) esters 5. c) amides 3. reduction 4. alpha-halogenation 5. EAS
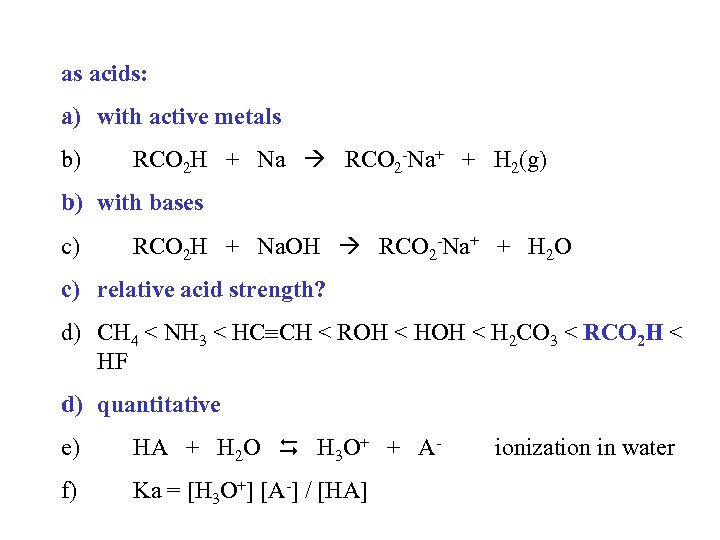 as acids: a) with active metals b) RCO 2 H + Na RCO 2 -Na+ + H 2(g) b) with bases c) RCO 2 H + Na. OH RCO 2 -Na+ + H 2 O c) relative acid strength? d) CH 4 < NH 3 < HC CH < ROH < H 2 CO 3 < RCO 2 H < HF d) quantitative e) HA + H 2 O H 3 O+ + A- f) Ka = [H 3 O+] [A-] / [HA] ionization in water
as acids: a) with active metals b) RCO 2 H + Na RCO 2 -Na+ + H 2(g) b) with bases c) RCO 2 H + Na. OH RCO 2 -Na+ + H 2 O c) relative acid strength? d) CH 4 < NH 3 < HC CH < ROH < H 2 CO 3 < RCO 2 H < HF d) quantitative e) HA + H 2 O H 3 O+ + A- f) Ka = [H 3 O+] [A-] / [HA] ionization in water
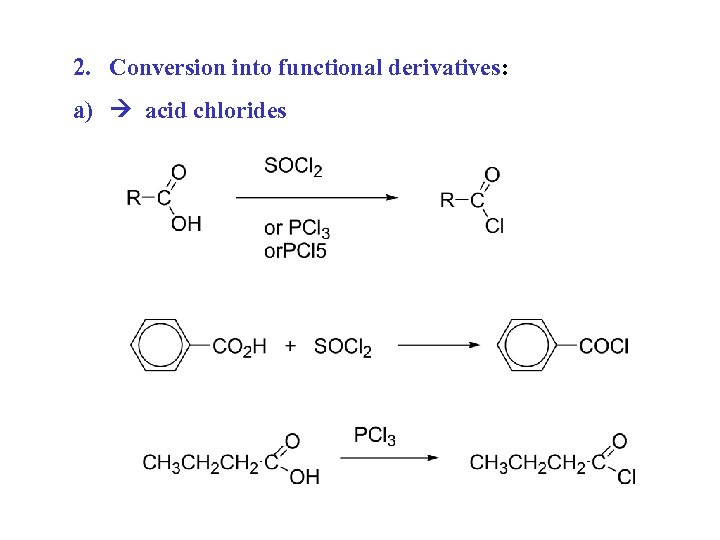 2. Conversion into functional derivatives: a) acid chlorides
2. Conversion into functional derivatives: a) acid chlorides
 b) esters c) “direct” esterification: d) RCOOH + R´OH RCO 2 R´ + H 2 O e) -reversible and often does not favor the ester f) -use an excess of the alcohol or acid to shift equilibrium g) -or remove the products to shift equilibrium to completion h) “indirect” esterification: i) RCOOH + PCl 3 RCOCl + R´OH RCO 2 R´ j) -convert the acid into the acid chloride first; not
b) esters c) “direct” esterification: d) RCOOH + R´OH RCO 2 R´ + H 2 O e) -reversible and often does not favor the ester f) -use an excess of the alcohol or acid to shift equilibrium g) -or remove the products to shift equilibrium to completion h) “indirect” esterification: i) RCOOH + PCl 3 RCOCl + R´OH RCO 2 R´ j) -convert the acid into the acid chloride first; not
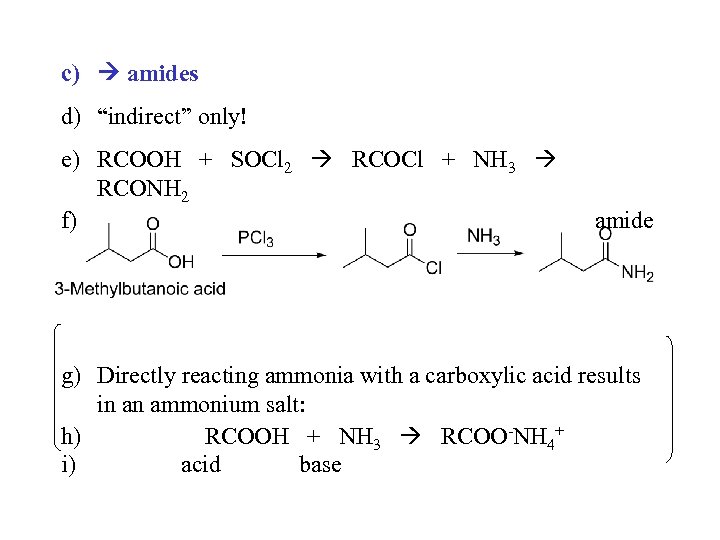 c) amides d) “indirect” only! e) RCOOH + SOCl 2 RCOCl + NH 3 RCONH 2 f) amide g) Directly reacting ammonia with a carboxylic acid results in an ammonium salt: h) RCOOH + NH 3 RCOO-NH 4+ i) acid base
c) amides d) “indirect” only! e) RCOOH + SOCl 2 RCOCl + NH 3 RCONH 2 f) amide g) Directly reacting ammonia with a carboxylic acid results in an ammonium salt: h) RCOOH + NH 3 RCOO-NH 4+ i) acid base
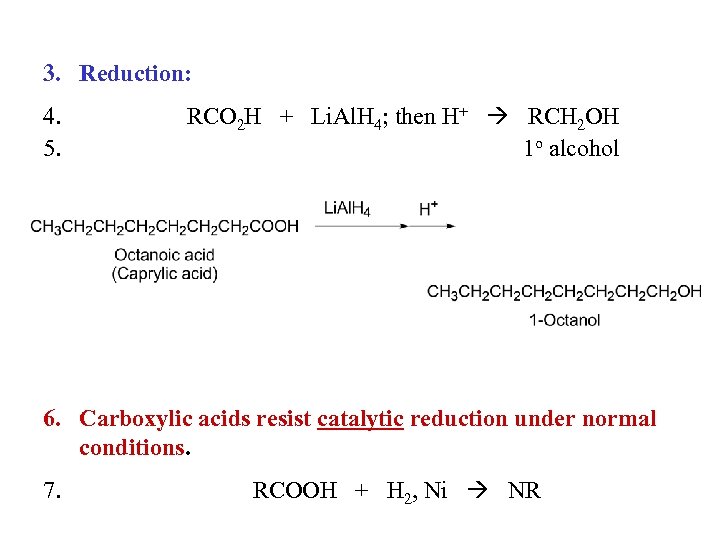 3. Reduction: 4. 5. RCO 2 H + Li. Al. H 4; then H+ RCH 2 OH 1 o alcohol 6. Carboxylic acids resist catalytic reduction under normal conditions. 7. RCOOH + H 2, Ni NR
3. Reduction: 4. 5. RCO 2 H + Li. Al. H 4; then H+ RCH 2 OH 1 o alcohol 6. Carboxylic acids resist catalytic reduction under normal conditions. 7. RCOOH + H 2, Ni NR
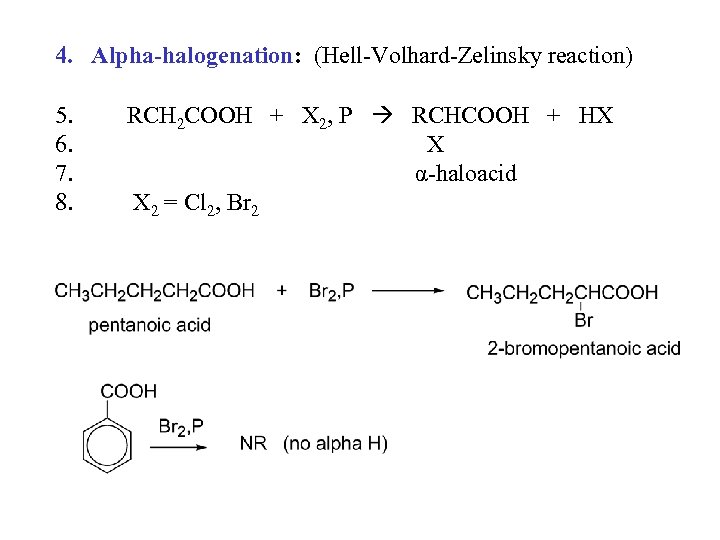 4. Alpha-halogenation: (Hell-Volhard-Zelinsky reaction) 5. 6. 7. 8. RCH 2 COOH + X 2, P RCHCOOH + HX X α-haloacid X 2 = Cl 2, Br 2
4. Alpha-halogenation: (Hell-Volhard-Zelinsky reaction) 5. 6. 7. 8. RCH 2 COOH + X 2, P RCHCOOH + HX X α-haloacid X 2 = Cl 2, Br 2
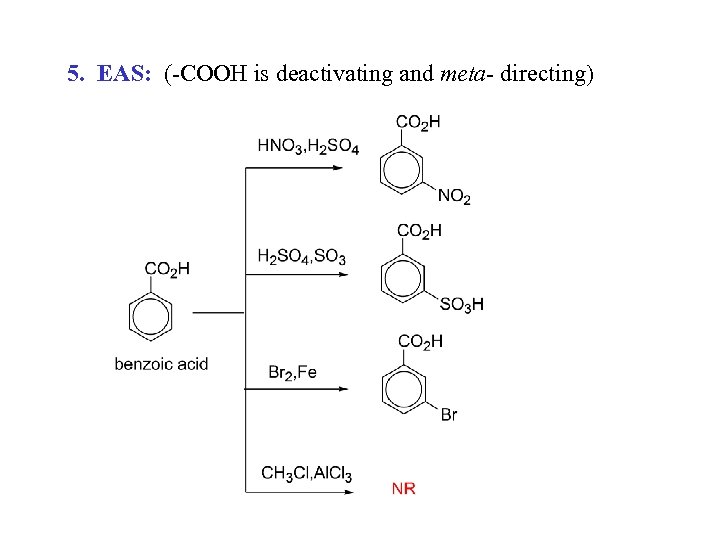 5. EAS: (-COOH is deactivating and meta- directing)
5. EAS: (-COOH is deactivating and meta- directing)
 Functional Derivatives of Carboxylic Acids
Functional Derivatives of Carboxylic Acids
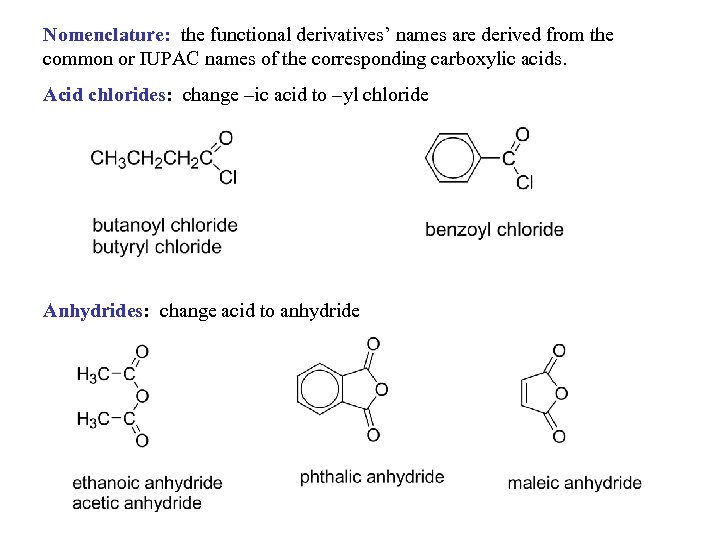 Nomenclature: the functional derivatives’ names are derived from the common or IUPAC names of the corresponding carboxylic acids. Acid chlorides: change –ic acid to –yl chloride Anhydrides: change acid to anhydride
Nomenclature: the functional derivatives’ names are derived from the common or IUPAC names of the corresponding carboxylic acids. Acid chlorides: change –ic acid to –yl chloride Anhydrides: change acid to anhydride
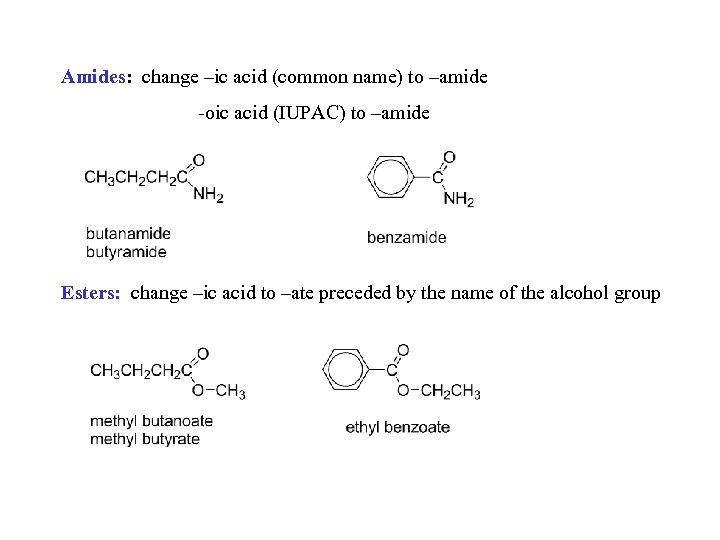 Amides: change –ic acid (common name) to –amide -oic acid (IUPAC) to –amide Esters: change –ic acid to –ate preceded by the name of the alcohol group
Amides: change –ic acid (common name) to –amide -oic acid (IUPAC) to –amide Esters: change –ic acid to –ate preceded by the name of the alcohol group
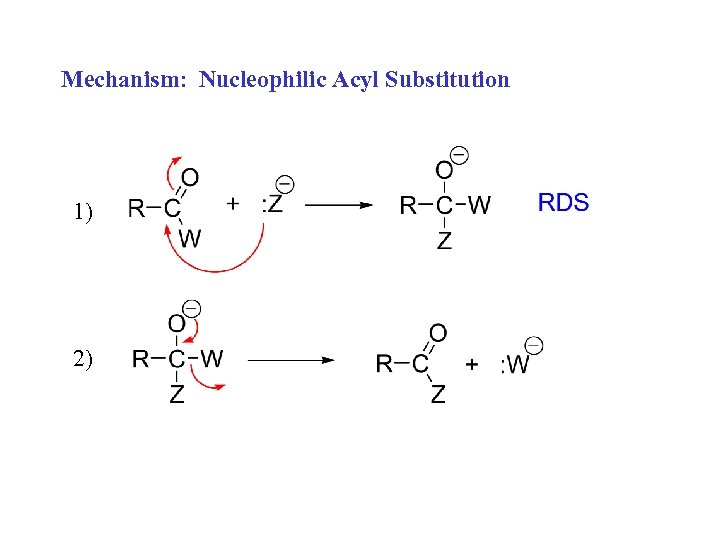 Mechanism: Nucleophilic Acyl Substitution 1) 2)
Mechanism: Nucleophilic Acyl Substitution 1) 2)
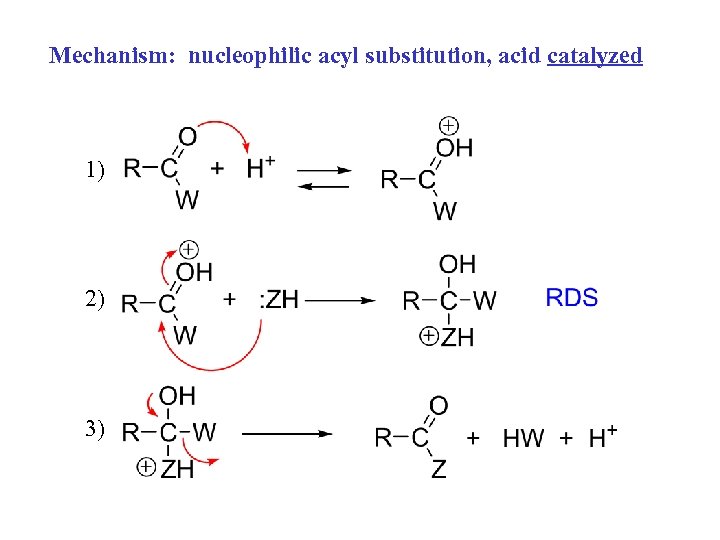 Mechanism: nucleophilic acyl substitution, acid catalyzed 1) 2) 3)
Mechanism: nucleophilic acyl substitution, acid catalyzed 1) 2) 3)
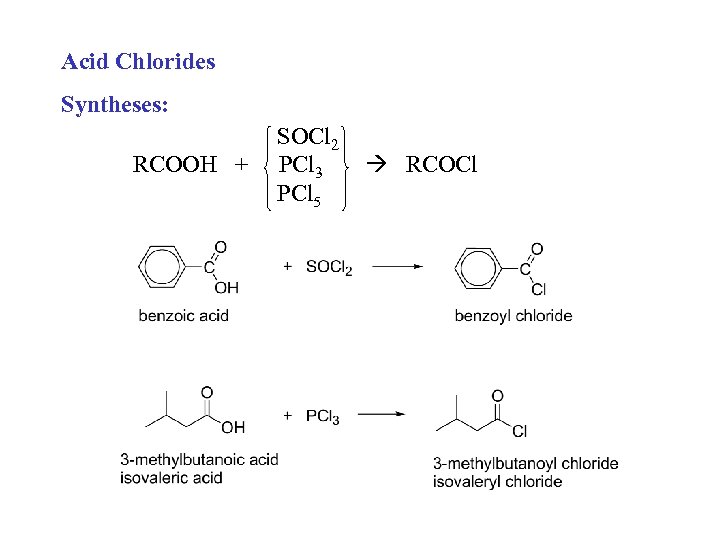 Acid Chlorides Syntheses: RCOOH + SOCl 2 PCl 3 PCl 5 RCOCl
Acid Chlorides Syntheses: RCOOH + SOCl 2 PCl 3 PCl 5 RCOCl
 Acid chlorides, reactions: 1. Conversion into acids and derivatives: 2. a) hydrolysis 3. b) ammonolysis 4. c) alcoholysis 2. Friedel-Crafts acylation 3. Coupling with lithium dialkylcopper 4. Reduction
Acid chlorides, reactions: 1. Conversion into acids and derivatives: 2. a) hydrolysis 3. b) ammonolysis 4. c) alcoholysis 2. Friedel-Crafts acylation 3. Coupling with lithium dialkylcopper 4. Reduction
 acid chlorides: conversion into acids and other derivatives
acid chlorides: conversion into acids and other derivatives
 acid chlorides: Friedel-Crafts acylation
acid chlorides: Friedel-Crafts acylation
 acid chlorides: coupling with lithium dialkylcopper
acid chlorides: coupling with lithium dialkylcopper
 acid chlorides: reduction to aldehydes
acid chlorides: reduction to aldehydes
 Anhydrides, syntheses: Buy the ones you want! Anhydrides, reactions: 1) Conversion into carboxylic acids and derivatives. 2) a) hydrolysis 3) b) ammonolysis 4) c) alcoholysis 5) 2) Friedel-Crafts acylation
Anhydrides, syntheses: Buy the ones you want! Anhydrides, reactions: 1) Conversion into carboxylic acids and derivatives. 2) a) hydrolysis 3) b) ammonolysis 4) c) alcoholysis 5) 2) Friedel-Crafts acylation
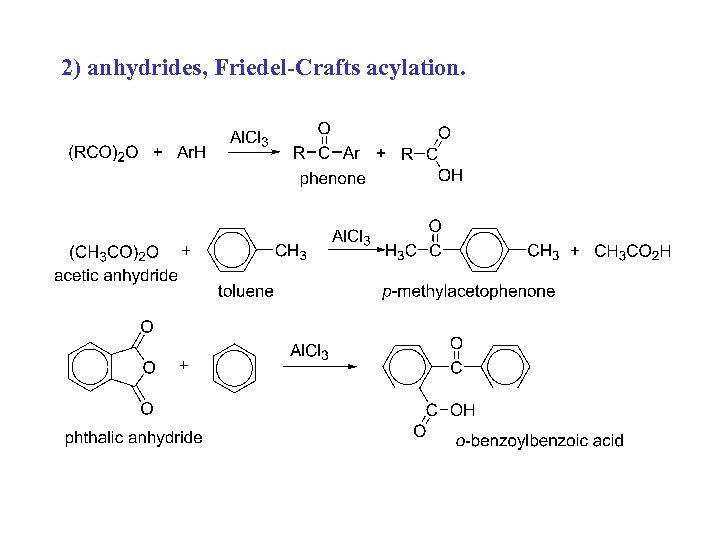 2) anhydrides, Friedel-Crafts acylation.
2) anhydrides, Friedel-Crafts acylation.
 Amides, synthesis: Indirectly via acid chlorides.
Amides, synthesis: Indirectly via acid chlorides.
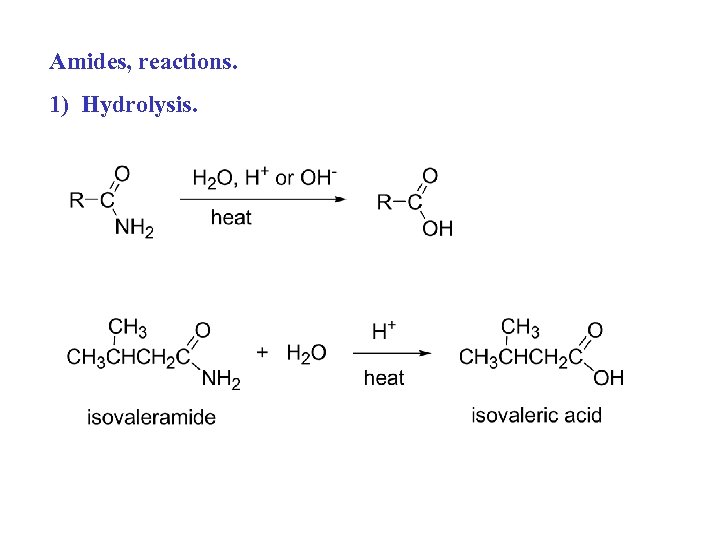 Amides, reactions. 1) Hydrolysis.
Amides, reactions. 1) Hydrolysis.
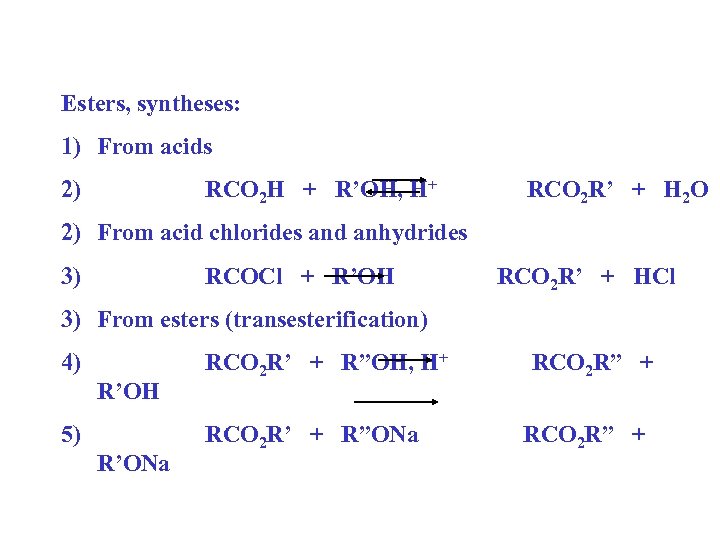 Esters, syntheses: 1) From acids 2) RCO 2 H + R’OH, H+ RCO 2 R’ + H 2 O 2) From acid chlorides and anhydrides 3) RCOCl + R’OH RCO 2 R’ + HCl 3) From esters (transesterification) 4) R’OH 5) R’ONa RCO 2 R’ + R”OH, H+ RCO 2 R’ + R”ONa RCO 2 R” +
Esters, syntheses: 1) From acids 2) RCO 2 H + R’OH, H+ RCO 2 R’ + H 2 O 2) From acid chlorides and anhydrides 3) RCOCl + R’OH RCO 2 R’ + HCl 3) From esters (transesterification) 4) R’OH 5) R’ONa RCO 2 R’ + R”OH, H+ RCO 2 R’ + R”ONa RCO 2 R” +
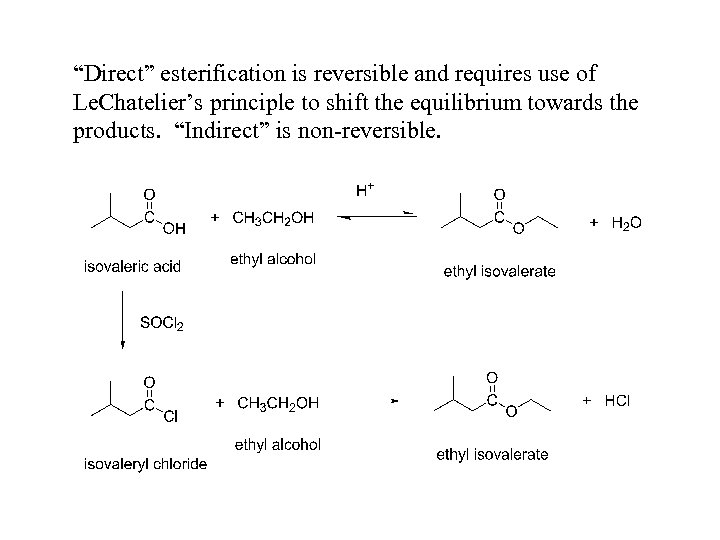 “Direct” esterification is reversible and requires use of Le. Chatelier’s principle to shift the equilibrium towards the products. “Indirect” is non-reversible.
“Direct” esterification is reversible and requires use of Le. Chatelier’s principle to shift the equilibrium towards the products. “Indirect” is non-reversible.
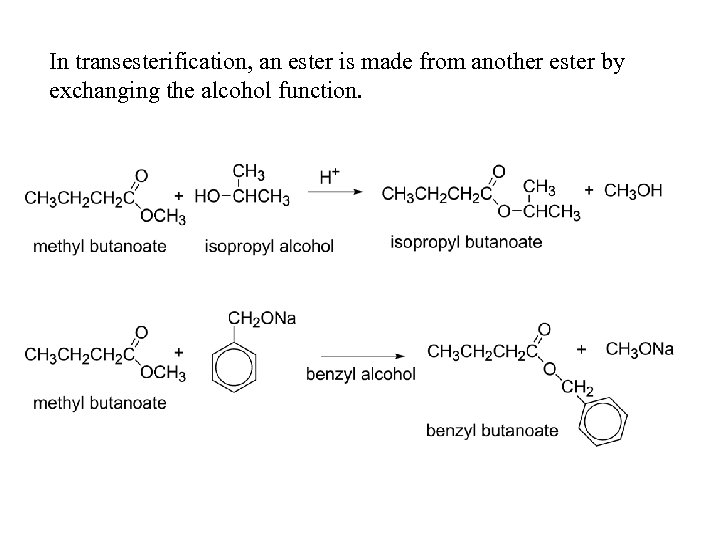 In transesterification, an ester is made from another ester by exchanging the alcohol function.
In transesterification, an ester is made from another ester by exchanging the alcohol function.
 Esters, reactions: 1) Conversion into acids and derivatives 2) a) hydrolysis 3) b) ammonolysis 4) c) alcoholysis 2) Reaction with Grignard reagents 3) Reduction 4) a) catalytic 5) b) chemical 6) 4) Claisen condensation
Esters, reactions: 1) Conversion into acids and derivatives 2) a) hydrolysis 3) b) ammonolysis 4) c) alcoholysis 2) Reaction with Grignard reagents 3) Reduction 4) a) catalytic 5) b) chemical 6) 4) Claisen condensation

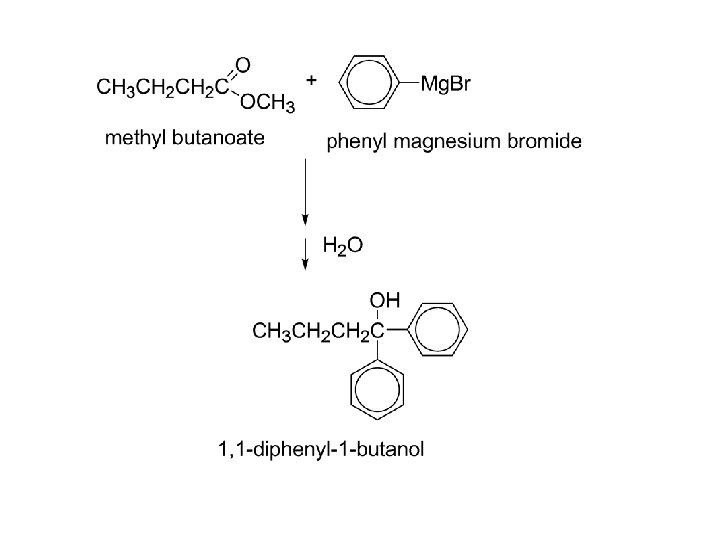
 Esters, reaction with Grignard reagents
Esters, reaction with Grignard reagents
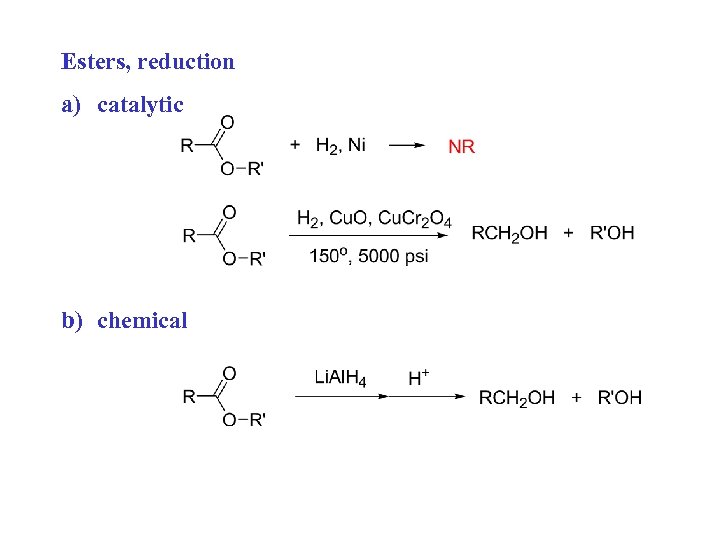 Esters, reduction a) catalytic b) chemical
Esters, reduction a) catalytic b) chemical
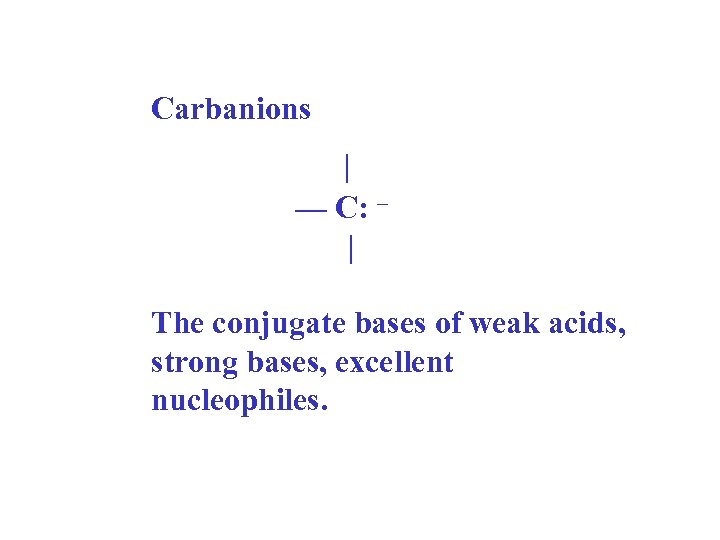 Carbanions | — C: – | The conjugate bases of weak acids, strong bases, excellent nucleophiles.
Carbanions | — C: – | The conjugate bases of weak acids, strong bases, excellent nucleophiles.
 1. Alpha-halogenation of ketones
1. Alpha-halogenation of ketones
 Carbanions. The conjugate bases of weak acids; strong bases, good nucleophiles. 1. enolate anions 2. organometallic compounds 3. ylides 4. cyanide 5. acetylides
Carbanions. The conjugate bases of weak acids; strong bases, good nucleophiles. 1. enolate anions 2. organometallic compounds 3. ylides 4. cyanide 5. acetylides
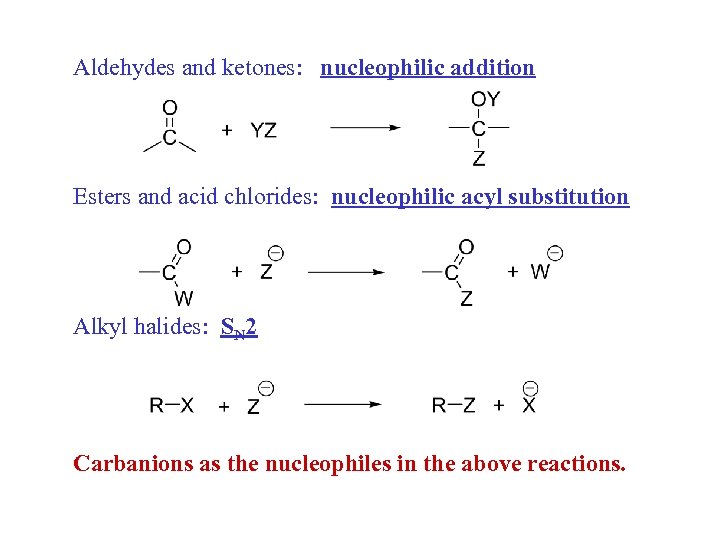 Aldehydes and ketones: nucleophilic addition Esters and acid chlorides: nucleophilic acyl substitution Alkyl halides: SN 2 Carbanions as the nucleophiles in the above reactions.
Aldehydes and ketones: nucleophilic addition Esters and acid chlorides: nucleophilic acyl substitution Alkyl halides: SN 2 Carbanions as the nucleophiles in the above reactions.
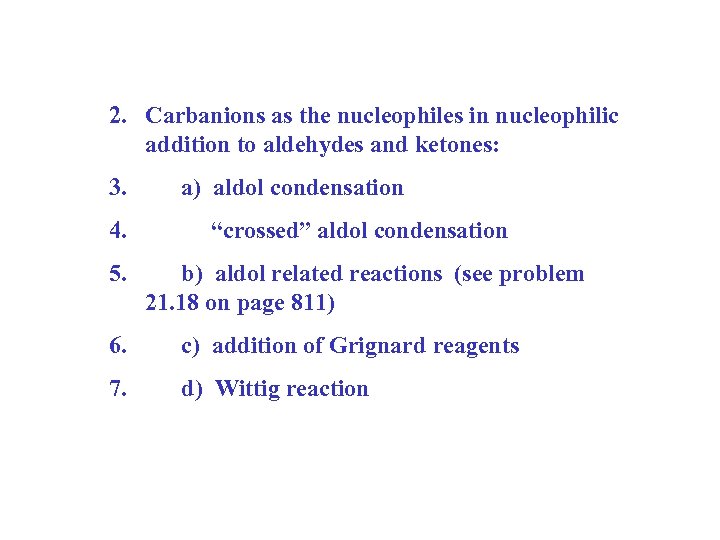 2. Carbanions as the nucleophiles in nucleophilic addition to aldehydes and ketones: 3. a) aldol condensation 4. “crossed” aldol condensation 5. b) aldol related reactions (see problem 21. 18 on page 811) 6. c) addition of Grignard reagents 7. d) Wittig reaction
2. Carbanions as the nucleophiles in nucleophilic addition to aldehydes and ketones: 3. a) aldol condensation 4. “crossed” aldol condensation 5. b) aldol related reactions (see problem 21. 18 on page 811) 6. c) addition of Grignard reagents 7. d) Wittig reaction
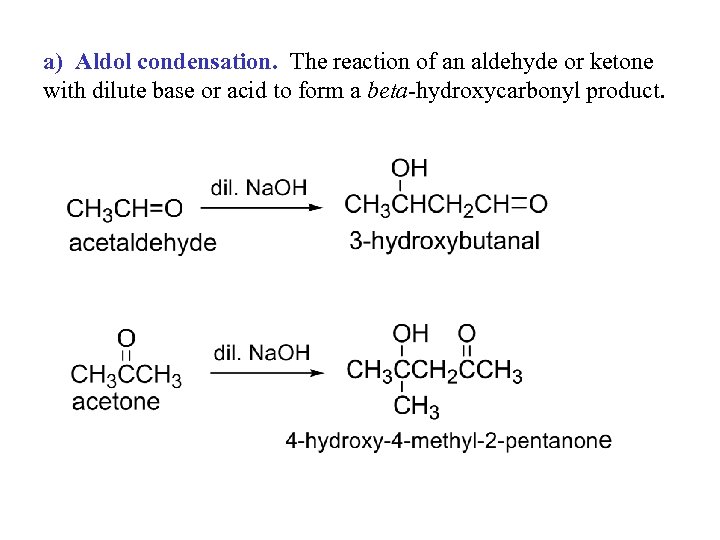 a) Aldol condensation. The reaction of an aldehyde or ketone with dilute base or acid to form a beta-hydroxycarbonyl product.
a) Aldol condensation. The reaction of an aldehyde or ketone with dilute base or acid to form a beta-hydroxycarbonyl product.
 nucleophilic addition by enolate ion.
nucleophilic addition by enolate ion.
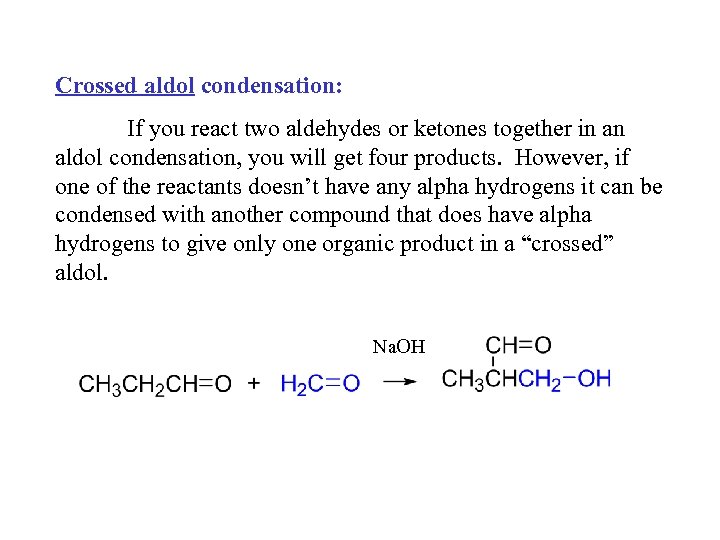 Crossed aldol condensation: If you react two aldehydes or ketones together in an aldol condensation, you will get four products. However, if one of the reactants doesn’t have any alpha hydrogens it can be condensed with another compound that does have alpha hydrogens to give only one organic product in a “crossed” aldol. Na. OH
Crossed aldol condensation: If you react two aldehydes or ketones together in an aldol condensation, you will get four products. However, if one of the reactants doesn’t have any alpha hydrogens it can be condensed with another compound that does have alpha hydrogens to give only one organic product in a “crossed” aldol. Na. OH
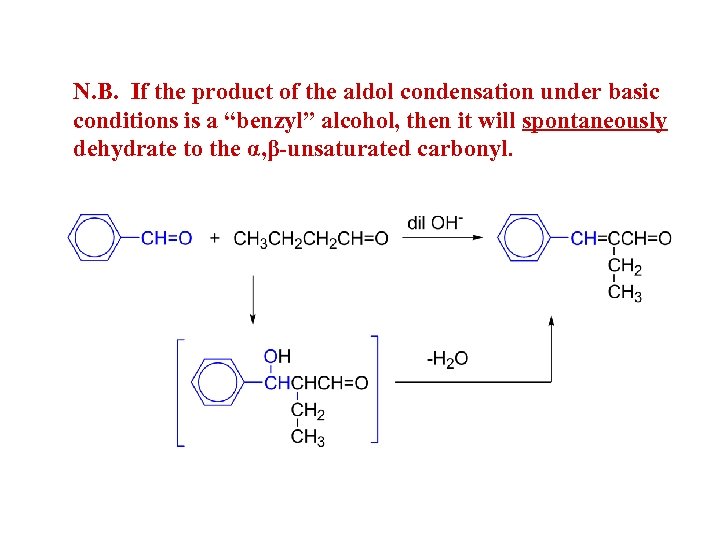 N. B. If the product of the aldol condensation under basic conditions is a “benzyl” alcohol, then it will spontaneously dehydrate to the α, β-unsaturated carbonyl.
N. B. If the product of the aldol condensation under basic conditions is a “benzyl” alcohol, then it will spontaneously dehydrate to the α, β-unsaturated carbonyl.
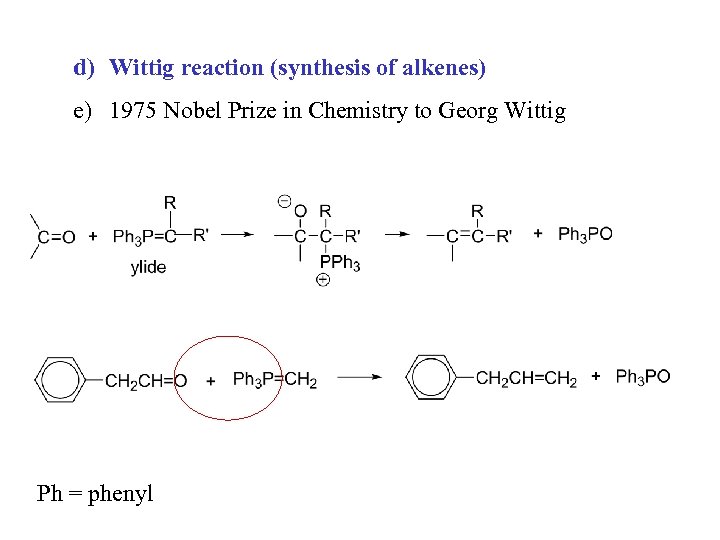 d) Wittig reaction (synthesis of alkenes) e) 1975 Nobel Prize in Chemistry to Georg Wittig Ph = phenyl
d) Wittig reaction (synthesis of alkenes) e) 1975 Nobel Prize in Chemistry to Georg Wittig Ph = phenyl
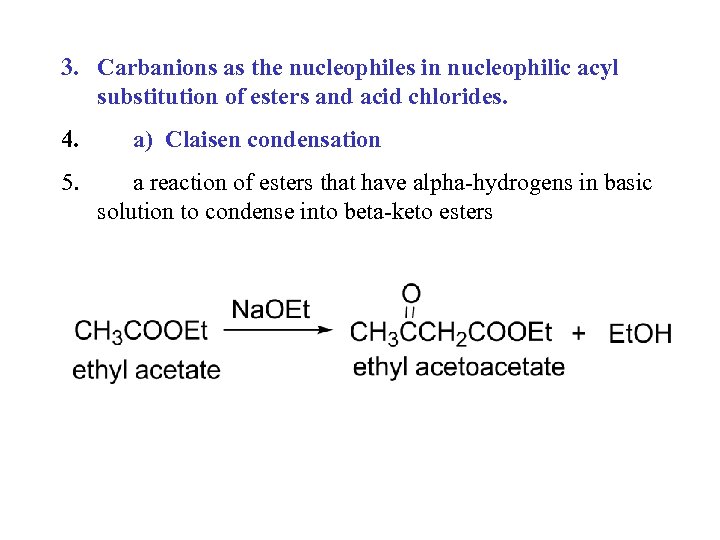 3. Carbanions as the nucleophiles in nucleophilic acyl substitution of esters and acid chlorides. 4. 5. a) Claisen condensation a reaction of esters that have alpha-hydrogens in basic solution to condense into beta-keto esters
3. Carbanions as the nucleophiles in nucleophilic acyl substitution of esters and acid chlorides. 4. 5. a) Claisen condensation a reaction of esters that have alpha-hydrogens in basic solution to condense into beta-keto esters
 Mechanism for the Claisen condensation:
Mechanism for the Claisen condensation:
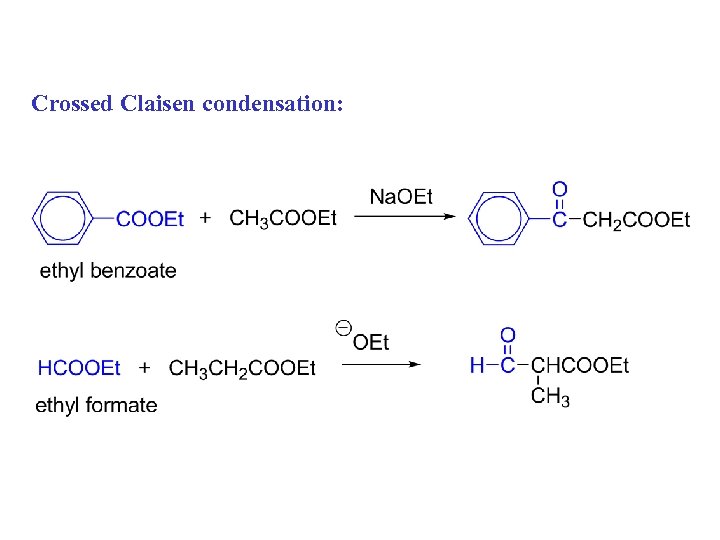 Crossed Claisen condensation:
Crossed Claisen condensation:
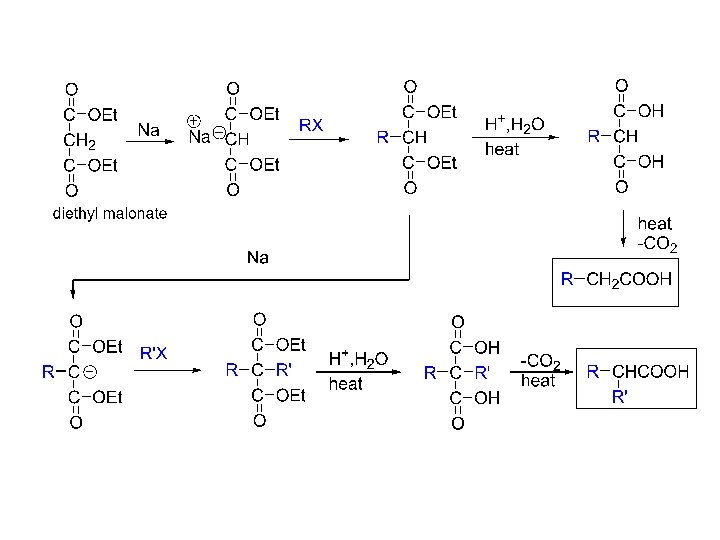
 Carbanions II Carbanions as nucleophiles in SN 2 reactions with alkyl halides. a) Malonate synthesis of carboxylic acids b) Acetoacetate synthesis of ketones c) 2 -oxazoline synthesis of esters/carboxylic acids d) Organoborane synthesis of acids/ketones e) Enamine synthesis of aldehydes/ketones
Carbanions II Carbanions as nucleophiles in SN 2 reactions with alkyl halides. a) Malonate synthesis of carboxylic acids b) Acetoacetate synthesis of ketones c) 2 -oxazoline synthesis of esters/carboxylic acids d) Organoborane synthesis of acids/ketones e) Enamine synthesis of aldehydes/ketones
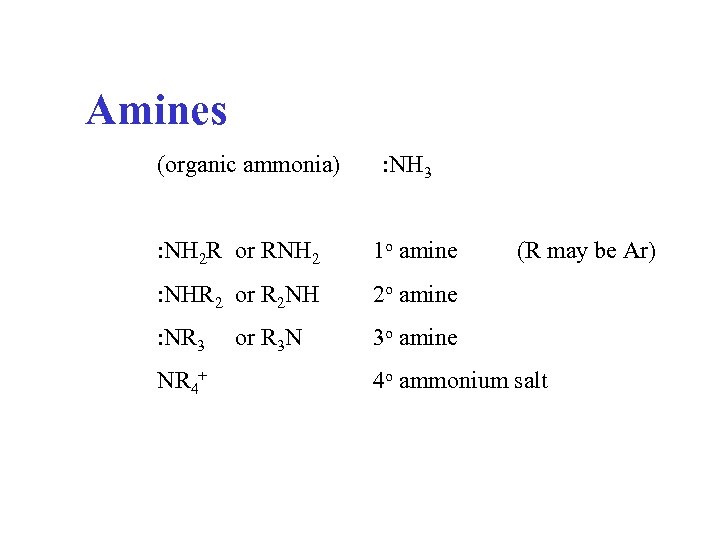 Amines (organic ammonia) : NH 3 : NH 2 R or RNH 2 1 o amine : NHR 2 or R 2 NH 2 o amine : NR 3 3 o amine NR 4+ or R 3 N (R may be Ar) 4 o ammonium salt
Amines (organic ammonia) : NH 3 : NH 2 R or RNH 2 1 o amine : NHR 2 or R 2 NH 2 o amine : NR 3 3 o amine NR 4+ or R 3 N (R may be Ar) 4 o ammonium salt
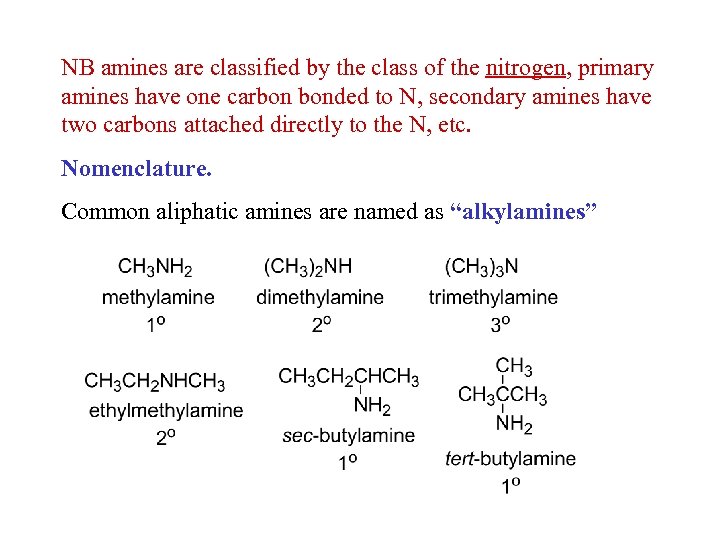 NB amines are classified by the class of the nitrogen, primary amines have one carbon bonded to N, secondary amines have two carbons attached directly to the N, etc. Nomenclature. Common aliphatic amines are named as “alkylamines”
NB amines are classified by the class of the nitrogen, primary amines have one carbon bonded to N, secondary amines have two carbons attached directly to the N, etc. Nomenclature. Common aliphatic amines are named as “alkylamines”

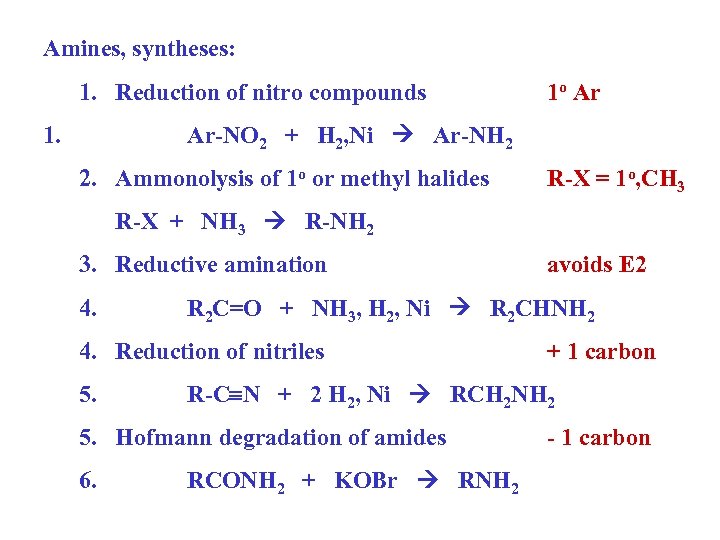 Amines, syntheses: 1. Reduction of nitro compounds 1. 1 o Ar Ar-NO 2 + H 2, Ni Ar-NH 2 2. Ammonolysis of 1 o or methyl halides R-X = 1 o, CH 3 R-X + NH 3 R-NH 2 3. Reductive amination 4. R 2 C=O + NH 3, H 2, Ni R 2 CHNH 2 4. Reduction of nitriles 5. + 1 carbon R-C N + 2 H 2, Ni RCH 2 NH 2 5. Hofmann degradation of amides 6. avoids E 2 RCONH 2 + KOBr RNH 2 - 1 carbon
Amines, syntheses: 1. Reduction of nitro compounds 1. 1 o Ar Ar-NO 2 + H 2, Ni Ar-NH 2 2. Ammonolysis of 1 o or methyl halides R-X = 1 o, CH 3 R-X + NH 3 R-NH 2 3. Reductive amination 4. R 2 C=O + NH 3, H 2, Ni R 2 CHNH 2 4. Reduction of nitriles 5. + 1 carbon R-C N + 2 H 2, Ni RCH 2 NH 2 5. Hofmann degradation of amides 6. avoids E 2 RCONH 2 + KOBr RNH 2 - 1 carbon
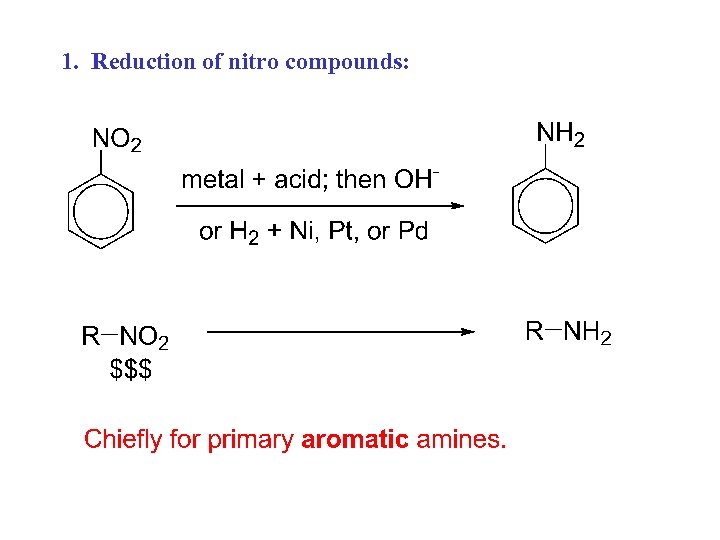 1. Reduction of nitro compounds:
1. Reduction of nitro compounds:
 2. Ammonolysis of 1 o or methyl halides.
2. Ammonolysis of 1 o or methyl halides.
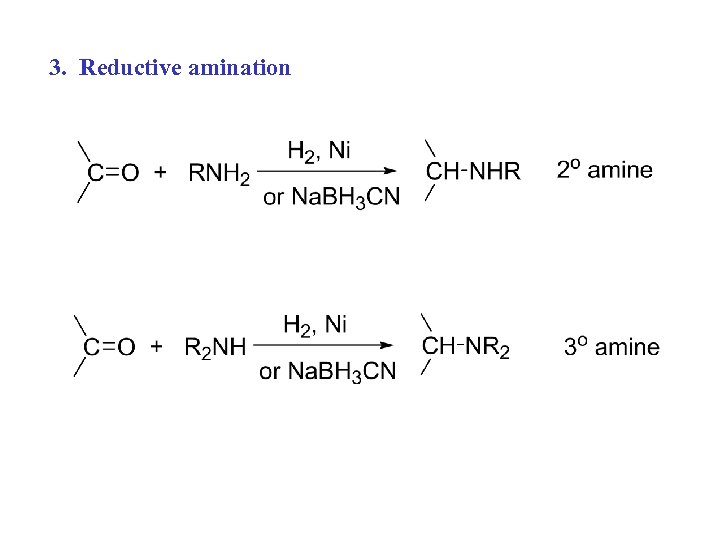
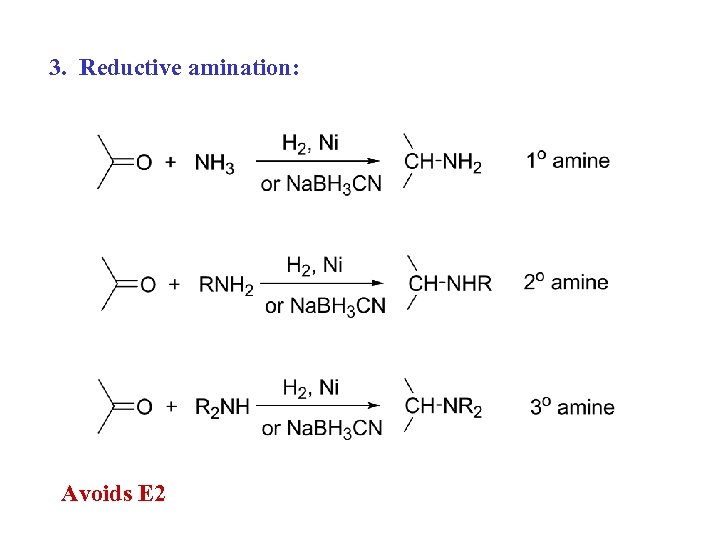 3. Reductive amination: Avoids E 2
3. Reductive amination: Avoids E 2
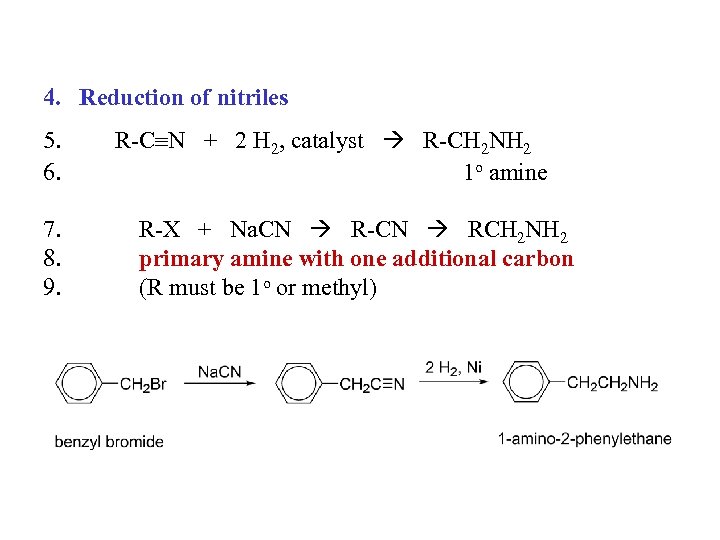 4. Reduction of nitriles 5. 6. 7. 8. 9. R-C N + 2 H 2, catalyst R-CH 2 NH 2 1 o amine R-X + Na. CN R-CN RCH 2 NH 2 primary amine with one additional carbon (R must be 1 o or methyl)
4. Reduction of nitriles 5. 6. 7. 8. 9. R-C N + 2 H 2, catalyst R-CH 2 NH 2 1 o amine R-X + Na. CN R-CN RCH 2 NH 2 primary amine with one additional carbon (R must be 1 o or methyl)
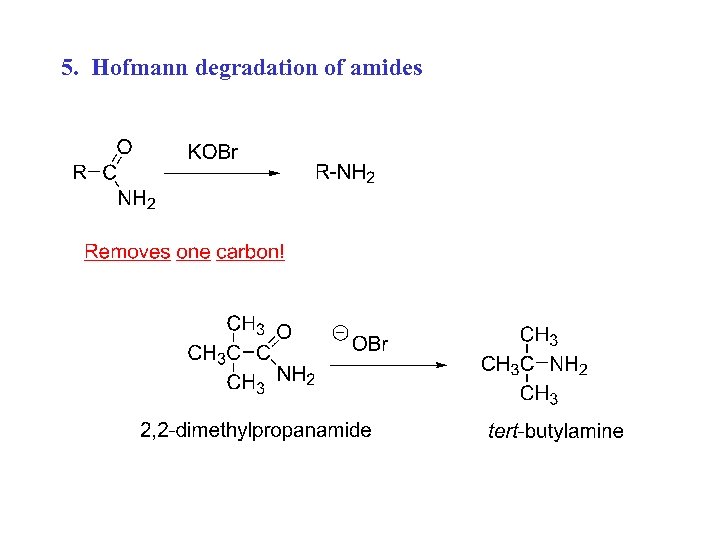 5. Hofmann degradation of amides
5. Hofmann degradation of amides
 Amine, reactions: 1. As bases 2. Alkylation 3. Reductive amination 4. Conversion into amides 5. EAS 6. Hofmann elimination from quarternary ammonium salts 7. Reactions with nitrous acid
Amine, reactions: 1. As bases 2. Alkylation 3. Reductive amination 4. Conversion into amides 5. EAS 6. Hofmann elimination from quarternary ammonium salts 7. Reactions with nitrous acid
 1. As bases 2. a) with acids 3. b) relative base strength 4. c) Kb 5. d) effect of groups on base strength
1. As bases 2. a) with acids 3. b) relative base strength 4. c) Kb 5. d) effect of groups on base strength
 2. Alkylation (ammonolysis of alkyl halides)
2. Alkylation (ammonolysis of alkyl halides)
 3. Reductive amination
3. Reductive amination
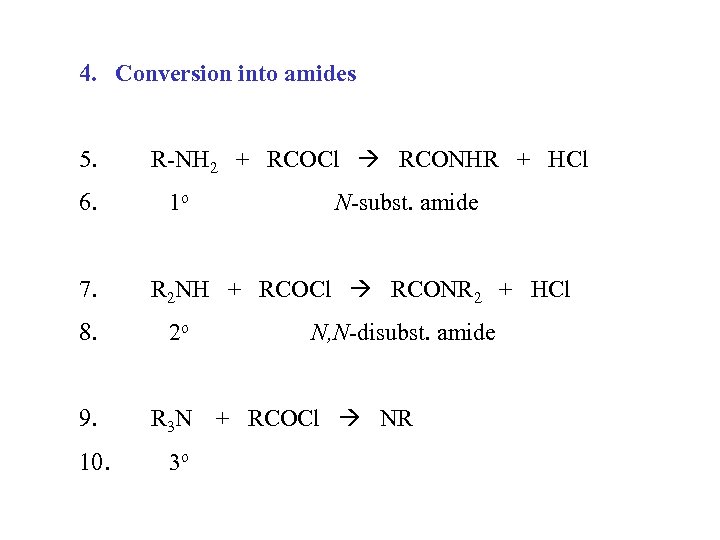 4. Conversion into amides 5. 6. 7. R-NH 2 + RCOCl RCONHR + HCl 1 o R 2 NH + RCOCl RCONR 2 + HCl 8. 2 o 9. R 3 N 10. N-subst. amide 3 o N, N-disubst. amide + RCOCl NR
4. Conversion into amides 5. 6. 7. R-NH 2 + RCOCl RCONHR + HCl 1 o R 2 NH + RCOCl RCONR 2 + HCl 8. 2 o 9. R 3 N 10. N-subst. amide 3 o N, N-disubst. amide + RCOCl NR
 5. EAS 6. -NH 2, -NHR, -NR 2 are powerful activating groups and ortho/para directors 7. a) nitration 8. b) sulfonation 9. c) halogenation 10. d) Friedel-Crafts alkylation 11. e) Friedel-Crafts acylation 12. f) coupling with diazonium salts 13. g) nitrosation
5. EAS 6. -NH 2, -NHR, -NR 2 are powerful activating groups and ortho/para directors 7. a) nitration 8. b) sulfonation 9. c) halogenation 10. d) Friedel-Crafts alkylation 11. e) Friedel-Crafts acylation 12. f) coupling with diazonium salts 13. g) nitrosation
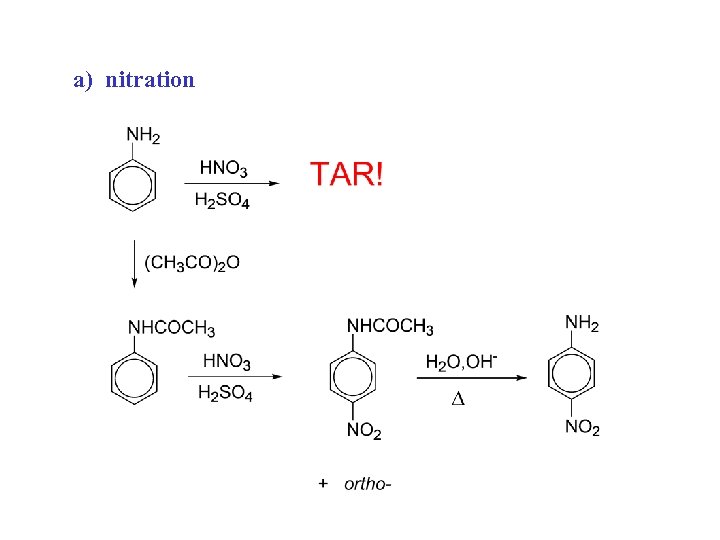 a) nitration
a) nitration
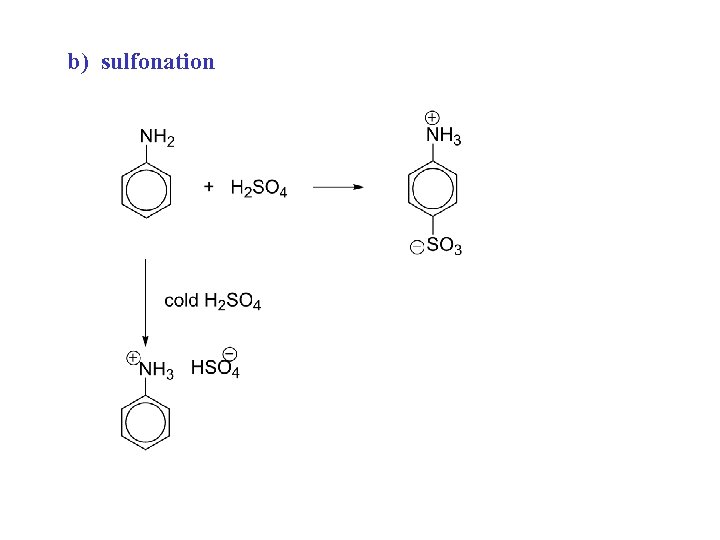 b) sulfonation
b) sulfonation
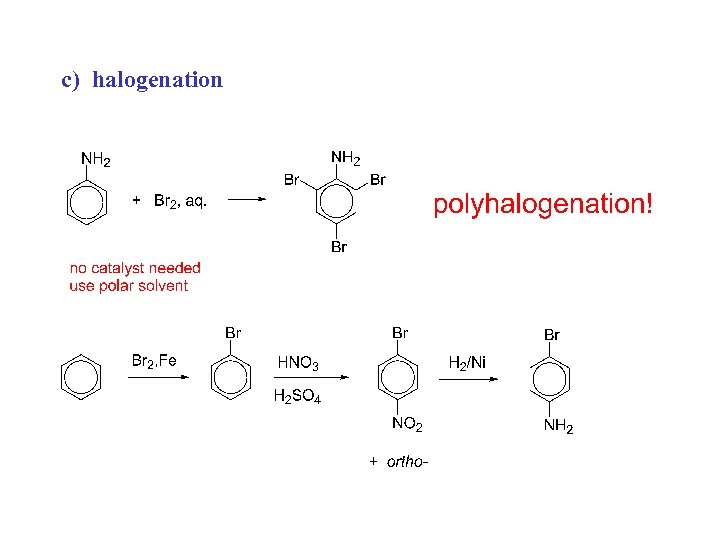 c) halogenation
c) halogenation
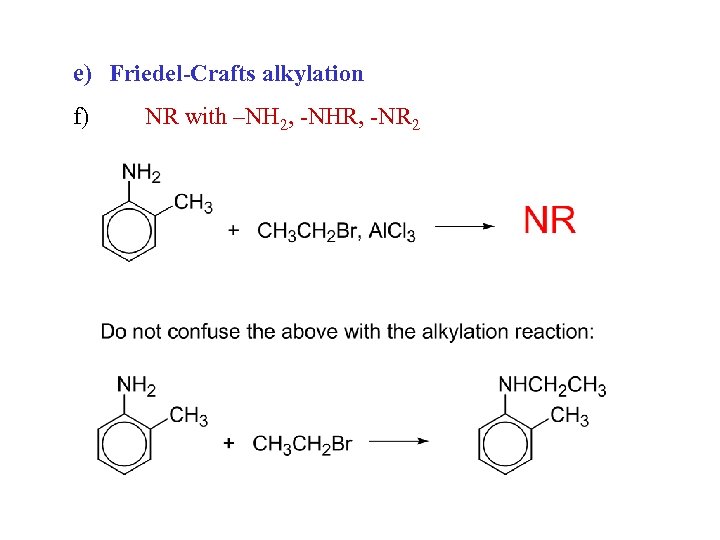 e) Friedel-Crafts alkylation f) NR with –NH 2, -NHR, -NR 2
e) Friedel-Crafts alkylation f) NR with –NH 2, -NHR, -NR 2
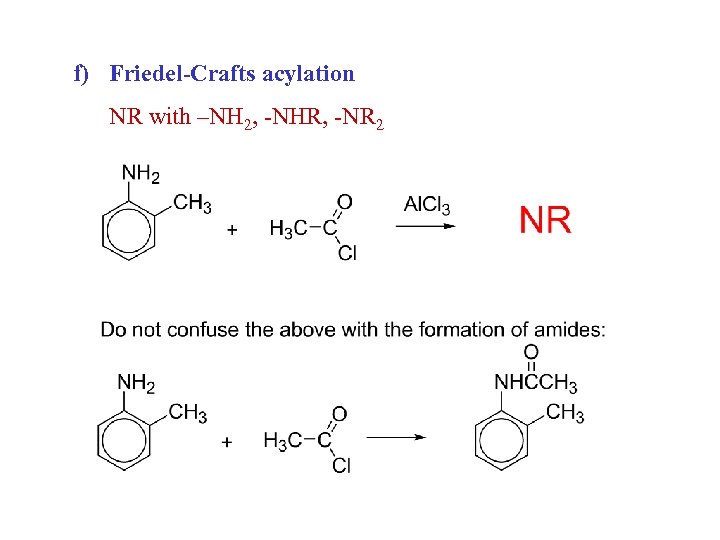 f) Friedel-Crafts acylation NR with –NH 2, -NHR, -NR 2
f) Friedel-Crafts acylation NR with –NH 2, -NHR, -NR 2
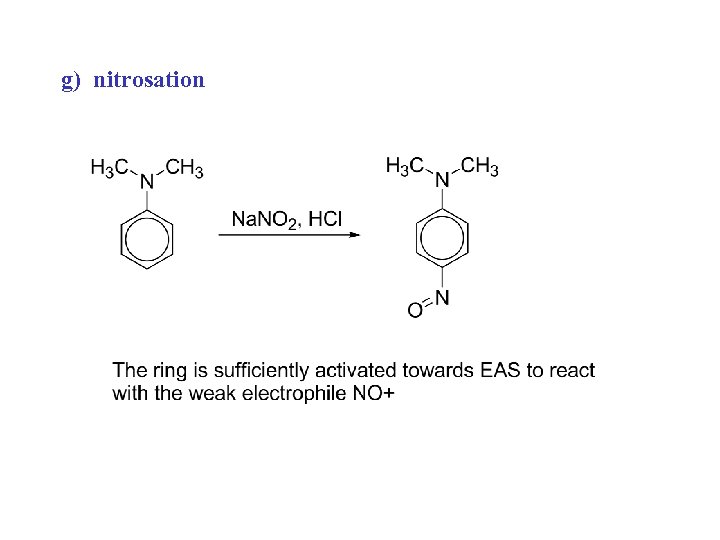 g) nitrosation
g) nitrosation
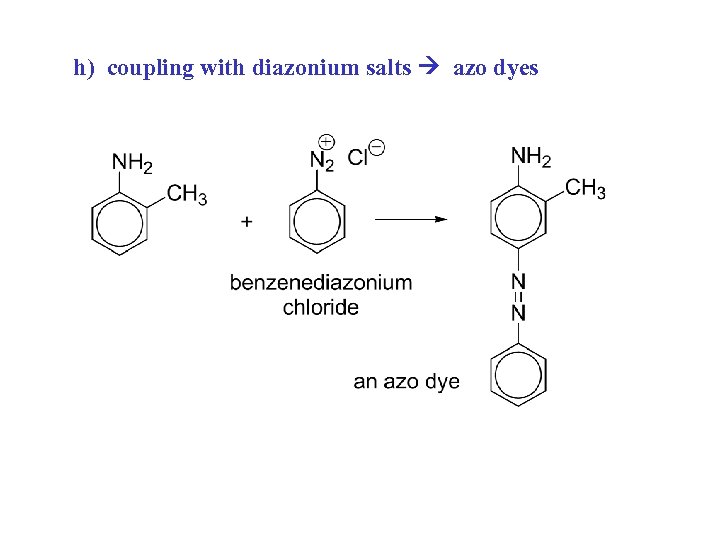 h) coupling with diazonium salts azo dyes
h) coupling with diazonium salts azo dyes
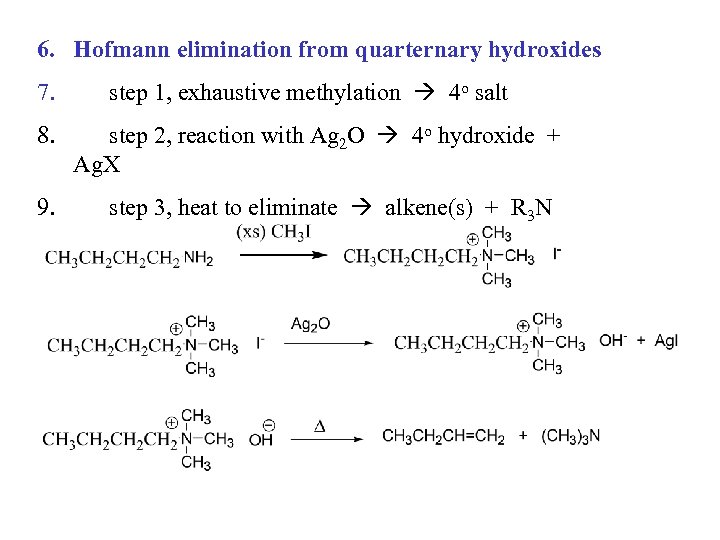 6. Hofmann elimination from quarternary hydroxides 7. 8. 9. step 1, exhaustive methylation 4 o salt step 2, reaction with Ag 2 O 4 o hydroxide + Ag. X step 3, heat to eliminate alkene(s) + R 3 N
6. Hofmann elimination from quarternary hydroxides 7. 8. 9. step 1, exhaustive methylation 4 o salt step 2, reaction with Ag 2 O 4 o hydroxide + Ag. X step 3, heat to eliminate alkene(s) + R 3 N
 7. Reactions with nitrous acid
7. Reactions with nitrous acid
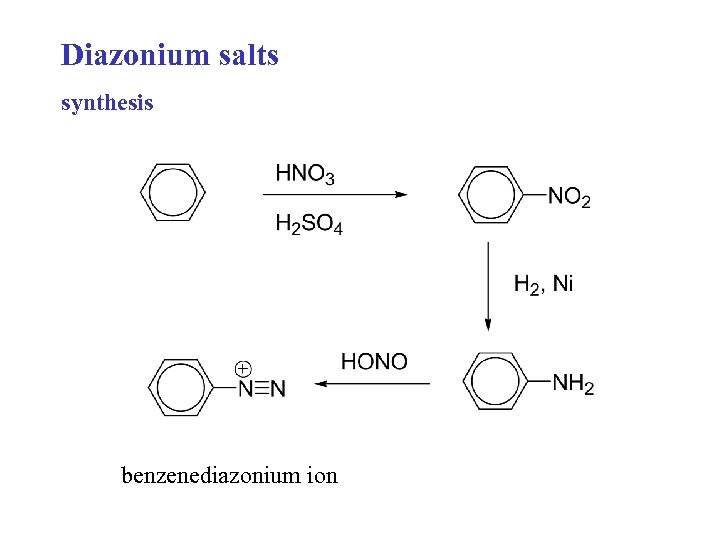 Diazonium salts synthesis benzenediazonium ion
Diazonium salts synthesis benzenediazonium ion
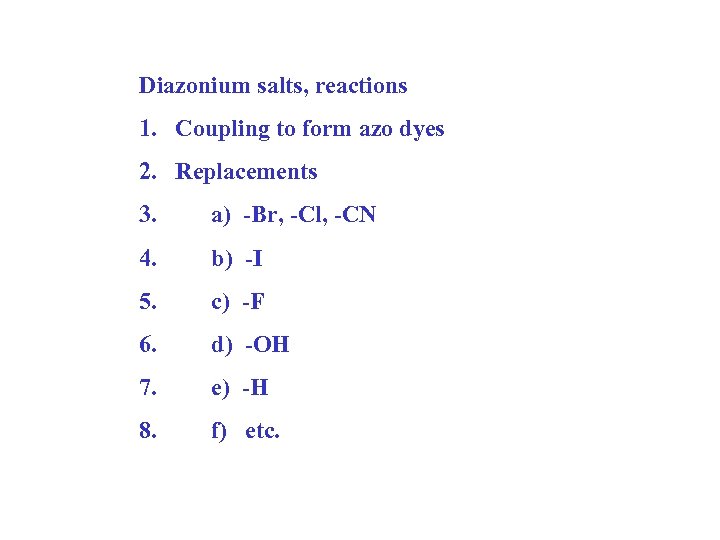 Diazonium salts, reactions 1. Coupling to form azo dyes 2. Replacements 3. a) -Br, -Cl, -CN 4. b) -I 5. c) -F 6. d) -OH 7. e) -H 8. f) etc.
Diazonium salts, reactions 1. Coupling to form azo dyes 2. Replacements 3. a) -Br, -Cl, -CN 4. b) -I 5. c) -F 6. d) -OH 7. e) -H 8. f) etc.
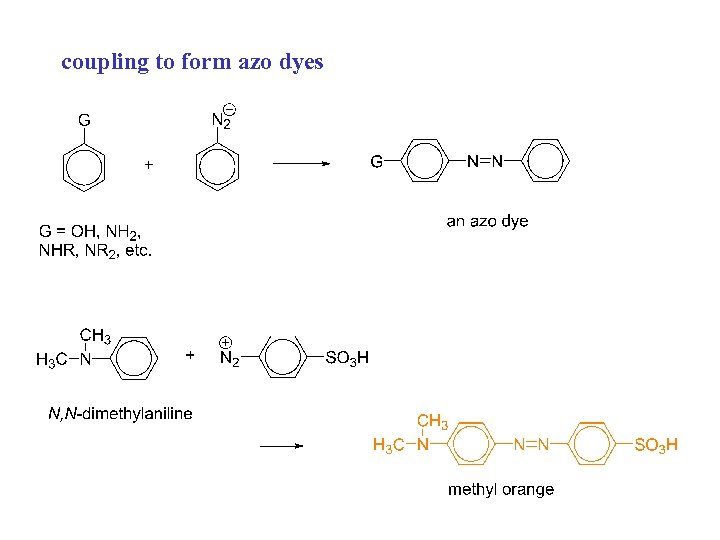 coupling to form azo dyes
coupling to form azo dyes
 Phenols Ar-OH Phenols are compounds with an –OH group attached to an aromatic carbon. Although they share the same functional group with alcohols, where the –OH group is attached to an aliphatic carbon, the chemistry of phenols is very different from that of alcohols.
Phenols Ar-OH Phenols are compounds with an –OH group attached to an aromatic carbon. Although they share the same functional group with alcohols, where the –OH group is attached to an aliphatic carbon, the chemistry of phenols is very different from that of alcohols.
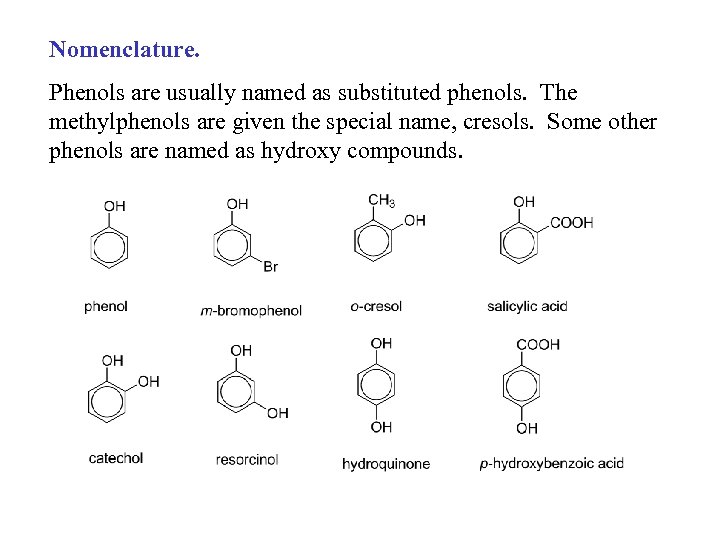 Nomenclature. Phenols are usually named as substituted phenols. The methylphenols are given the special name, cresols. Some other phenols are named as hydroxy compounds.
Nomenclature. Phenols are usually named as substituted phenols. The methylphenols are given the special name, cresols. Some other phenols are named as hydroxy compounds.
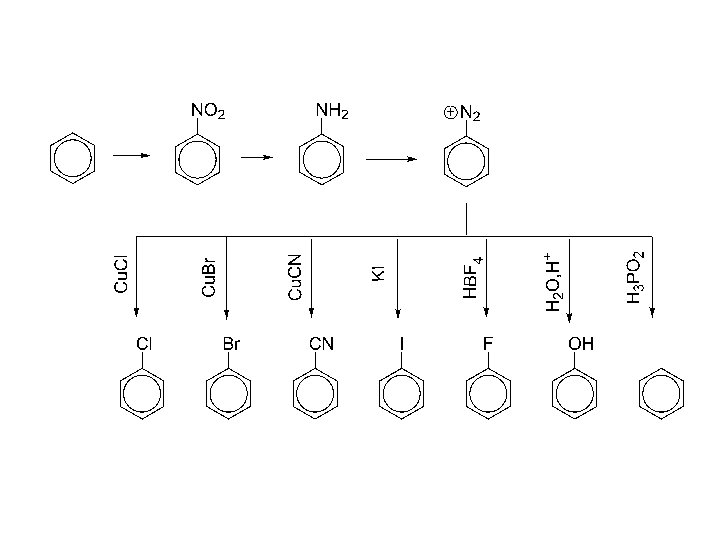
 phenols, syntheses: 1. From diazonium salts 2. 2. Alkali fusion of sulfonates
phenols, syntheses: 1. From diazonium salts 2. 2. Alkali fusion of sulfonates
 phenols, reactions: 1. as acids 2. ester formation 3. ether formation 4. EAS 5. a) nitration 6. b) sulfonation 7. c) halogenation 8. d) Friedel-Crafts alkylation i) Reimer-Tiemann 9. e) Friedel-Crafts acylation f) nitrosation g) coupling with diaz. salts h) Kolbe
phenols, reactions: 1. as acids 2. ester formation 3. ether formation 4. EAS 5. a) nitration 6. b) sulfonation 7. c) halogenation 8. d) Friedel-Crafts alkylation i) Reimer-Tiemann 9. e) Friedel-Crafts acylation f) nitrosation g) coupling with diaz. salts h) Kolbe
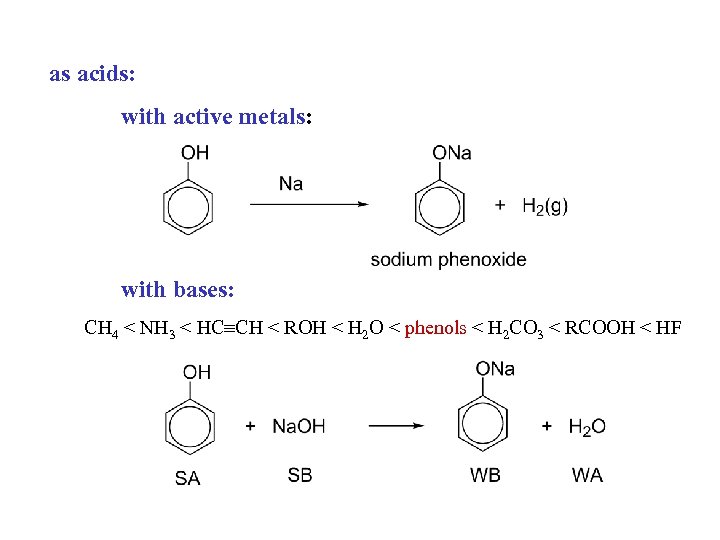 as acids: with active metals: with bases: CH 4 < NH 3 < HC CH < ROH < H 2 O < phenols < H 2 CO 3 < RCOOH < HF
as acids: with active metals: with bases: CH 4 < NH 3 < HC CH < ROH < H 2 O < phenols < H 2 CO 3 < RCOOH < HF
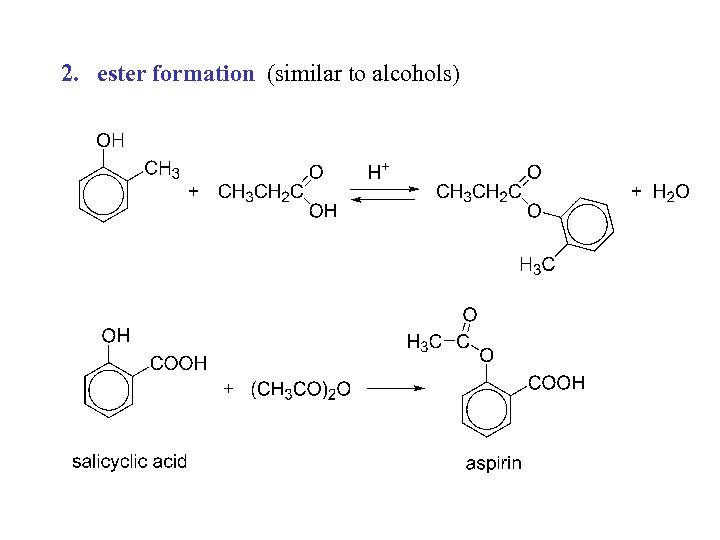 2. ester formation (similar to alcohols)
2. ester formation (similar to alcohols)
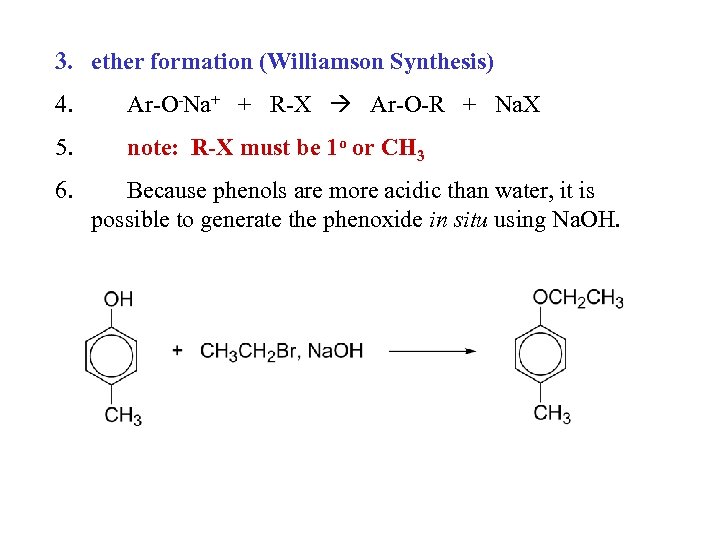 3. ether formation (Williamson Synthesis) 4. Ar-O-Na+ + R-X Ar-O-R + Na. X 5. note: R-X must be 1 o or CH 3 6. Because phenols are more acidic than water, it is possible to generate the phenoxide in situ using Na. OH.
3. ether formation (Williamson Synthesis) 4. Ar-O-Na+ + R-X Ar-O-R + Na. X 5. note: R-X must be 1 o or CH 3 6. Because phenols are more acidic than water, it is possible to generate the phenoxide in situ using Na. OH.
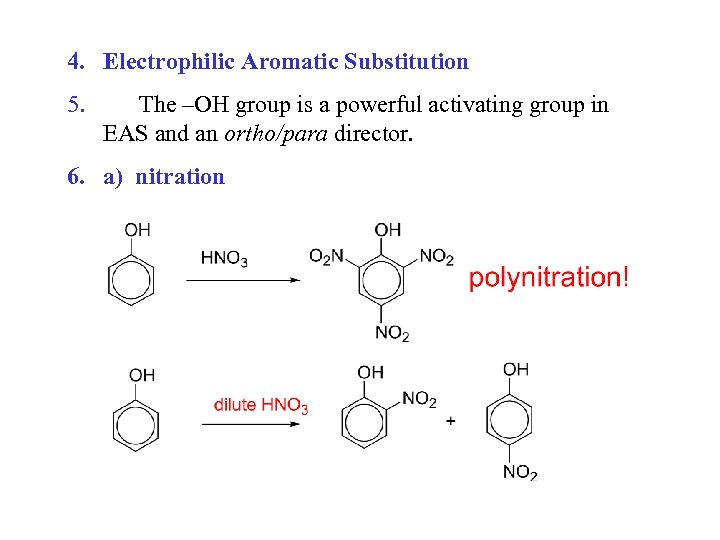 4. Electrophilic Aromatic Substitution 5. The –OH group is a powerful activating group in EAS and an ortho/para director. 6. a) nitration
4. Electrophilic Aromatic Substitution 5. The –OH group is a powerful activating group in EAS and an ortho/para director. 6. a) nitration
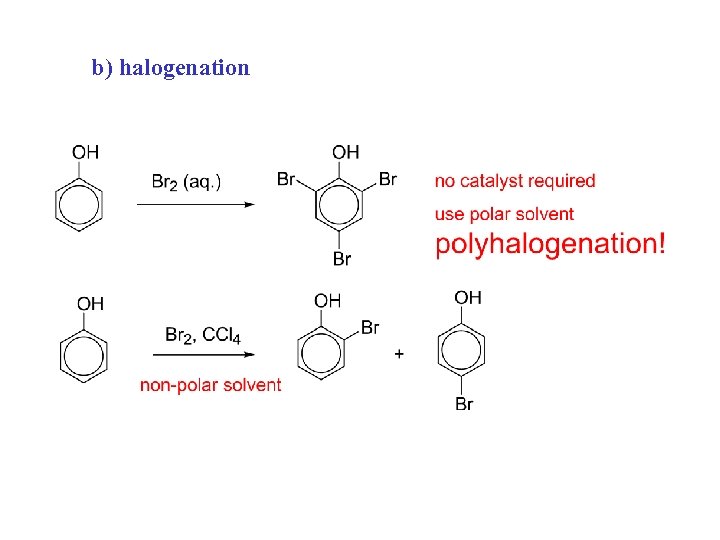 b) halogenation
b) halogenation
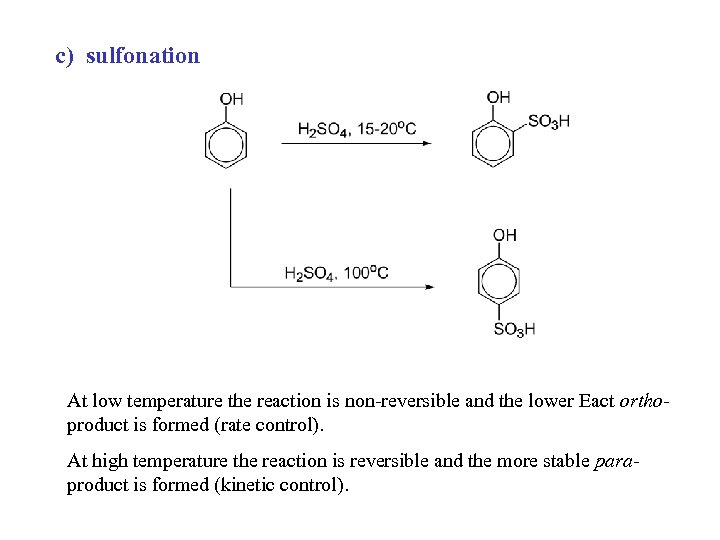 c) sulfonation At low temperature the reaction is non-reversible and the lower Eact orthoproduct is formed (rate control). At high temperature the reaction is reversible and the more stable paraproduct is formed (kinetic control).
c) sulfonation At low temperature the reaction is non-reversible and the lower Eact orthoproduct is formed (rate control). At high temperature the reaction is reversible and the more stable paraproduct is formed (kinetic control).
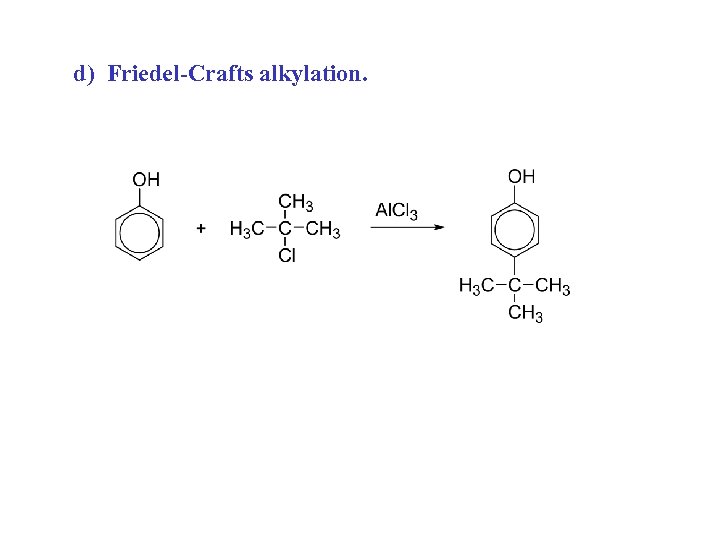 d) Friedel-Crafts alkylation.
d) Friedel-Crafts alkylation.
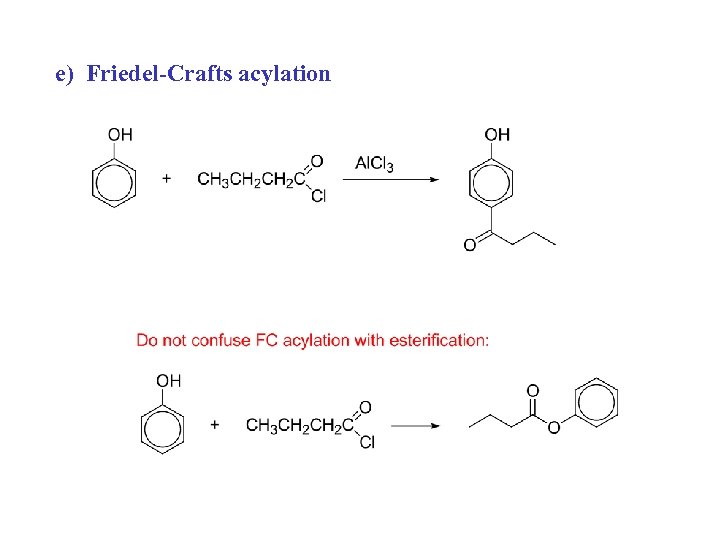 e) Friedel-Crafts acylation
e) Friedel-Crafts acylation
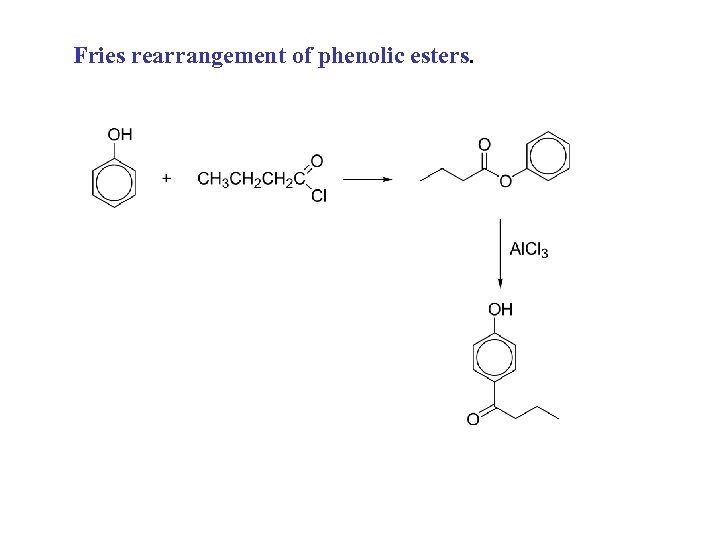 Fries rearrangement of phenolic esters.
Fries rearrangement of phenolic esters.
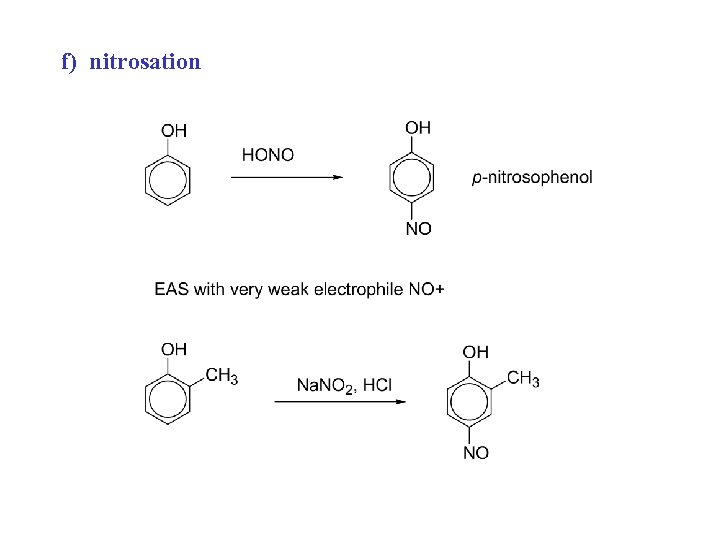 f) nitrosation
f) nitrosation
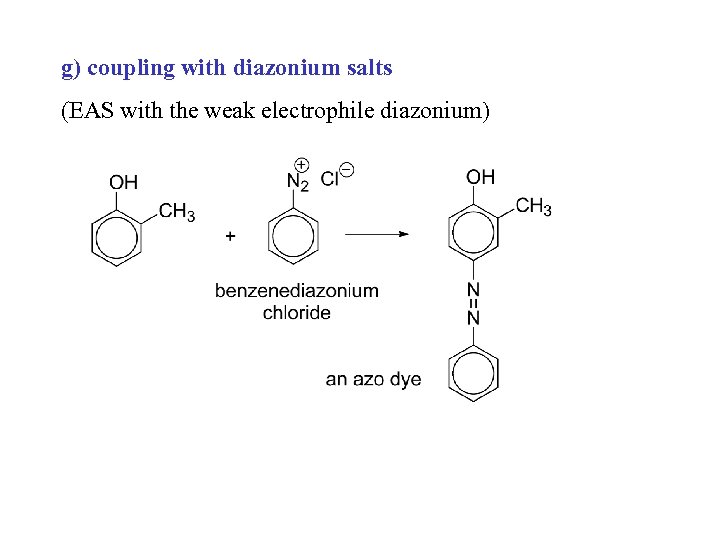 g) coupling with diazonium salts (EAS with the weak electrophile diazonium)
g) coupling with diazonium salts (EAS with the weak electrophile diazonium)
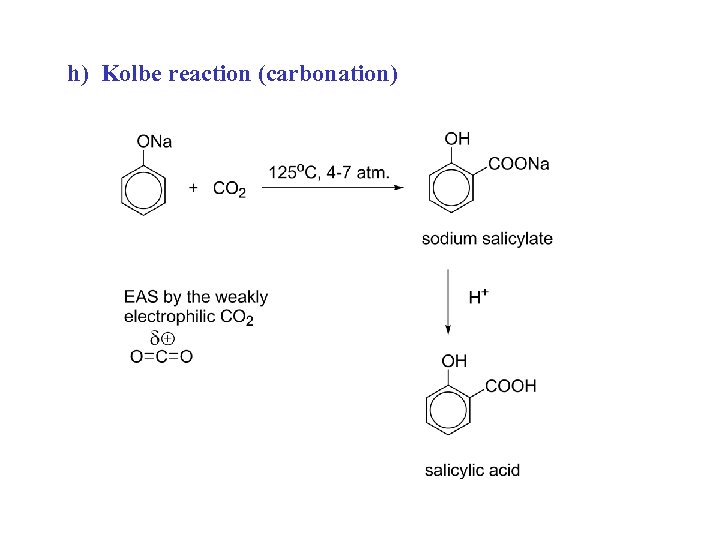 h) Kolbe reaction (carbonation)
h) Kolbe reaction (carbonation)
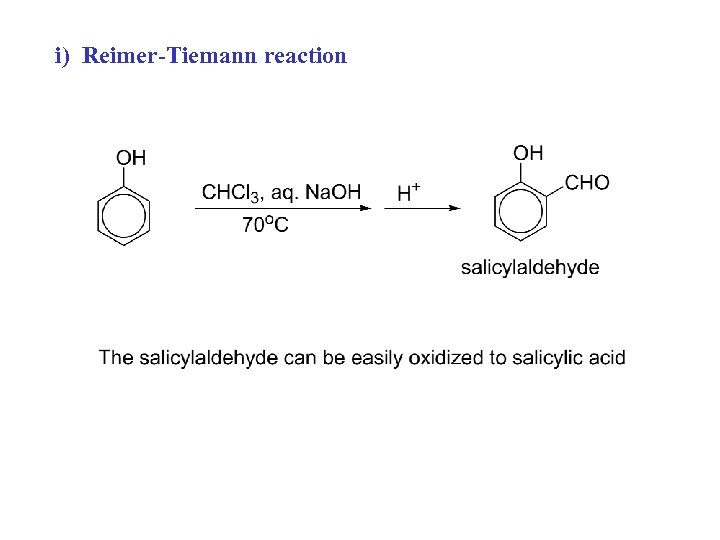 i) Reimer-Tiemann reaction
i) Reimer-Tiemann reaction
 Nomenclature Syntheses Reactions Mechanisms Spectroscopy
Nomenclature Syntheses Reactions Mechanisms Spectroscopy
 Aromatic Hydrocarbons (Electrophilic Aromatic Substitution) Spectroscopy (infrared & H-nmr) Arenes Aldehydes & Ketones Carboxylic Acids Functional Derivatives of Carboxylic Acids Acid Chlorides, Anhydrides, Amides, Esters Carbanions Amines & Diazonium Salts Phenols
Aromatic Hydrocarbons (Electrophilic Aromatic Substitution) Spectroscopy (infrared & H-nmr) Arenes Aldehydes & Ketones Carboxylic Acids Functional Derivatives of Carboxylic Acids Acid Chlorides, Anhydrides, Amides, Esters Carbanions Amines & Diazonium Salts Phenols
 Mechanisms: Electrophilic Aromatic Substitution Nitration Sulfonation Halogenation Friedel-Crafts Alkylation & Acylation Nucleophilic Addition to Carbonyl, Acid Catalyzed Nucleophilic Acyl Substitution, Acid Catalyzed
Mechanisms: Electrophilic Aromatic Substitution Nitration Sulfonation Halogenation Friedel-Crafts Alkylation & Acylation Nucleophilic Addition to Carbonyl, Acid Catalyzed Nucleophilic Acyl Substitution, Acid Catalyzed


