e8cde7ee17c7648956edcedce10cd305.ppt
- Количество слайдов: 114
 CHE-300 Review nomenclature syntheses reactions mechanisms
CHE-300 Review nomenclature syntheses reactions mechanisms
 Alkanes Alkyl halides Alcohols Ethers Alkenes conjugated dienes Alkynes Alicyclics Epoxides
Alkanes Alkyl halides Alcohols Ethers Alkenes conjugated dienes Alkynes Alicyclics Epoxides
 Alkanes Nomenclature Syntheses 1. reduction of alkene (addition of hydrogen) 2. reduction of an alkyl halide a) hydrolysis of a Grignard reagent b) with an active metal and acid 3. Corey-House Synthesis Reactions 1. halogenation 2. combustion (oxidation) 3. pyrolysis (cracking)
Alkanes Nomenclature Syntheses 1. reduction of alkene (addition of hydrogen) 2. reduction of an alkyl halide a) hydrolysis of a Grignard reagent b) with an active metal and acid 3. Corey-House Synthesis Reactions 1. halogenation 2. combustion (oxidation) 3. pyrolysis (cracking)
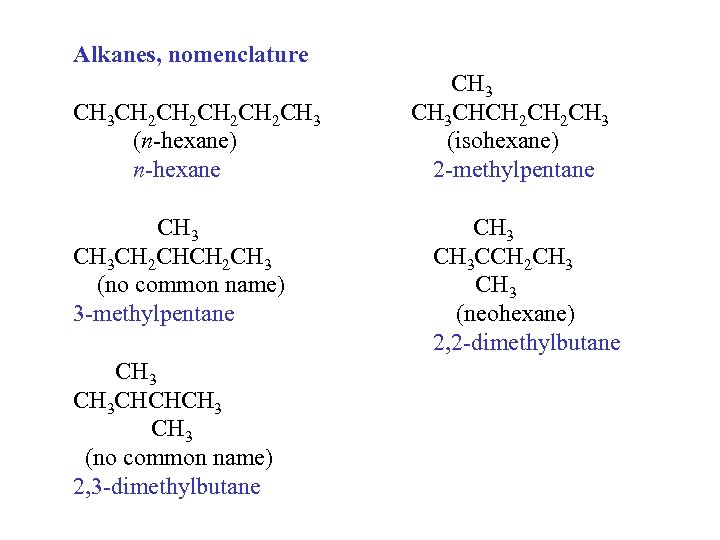 Alkanes, nomenclature CH 3 CH 2 CH 2 CH 3 (n-hexane) n-hexane CH 3 CH 2 CH 3 (no common name) 3 -methylpentane CH 3 CHCHCH 3 (no common name) 2, 3 -dimethylbutane CH 3 CHCH 2 CH 3 (isohexane) 2 -methylpentane CH 3 CCH 2 CH 3 (neohexane) 2, 2 -dimethylbutane
Alkanes, nomenclature CH 3 CH 2 CH 2 CH 3 (n-hexane) n-hexane CH 3 CH 2 CH 3 (no common name) 3 -methylpentane CH 3 CHCHCH 3 (no common name) 2, 3 -dimethylbutane CH 3 CHCH 2 CH 3 (isohexane) 2 -methylpentane CH 3 CCH 2 CH 3 (neohexane) 2, 2 -dimethylbutane
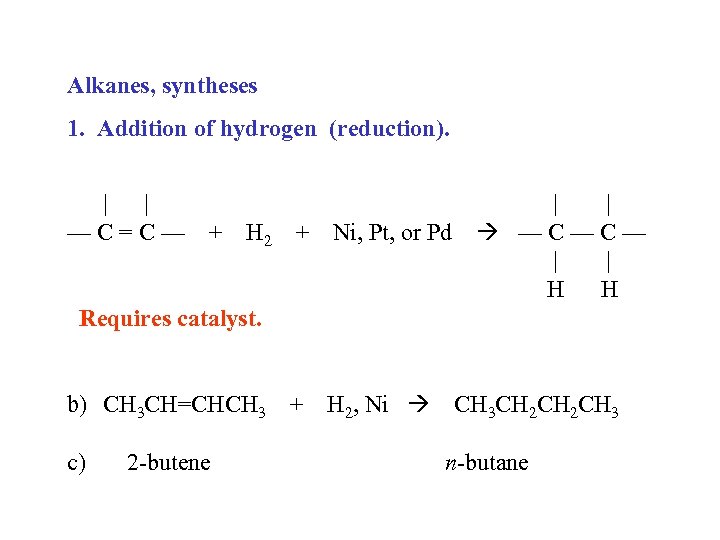 Alkanes, syntheses 1. Addition of hydrogen (reduction). | | —C=C— + H 2 + Ni, Pt, or Pd | | —C—C— | | H H Requires catalyst. b) CH 3 CH=CHCH 3 + H 2, Ni CH 3 CH 2 CH 3 c) 2 -butene n-butane
Alkanes, syntheses 1. Addition of hydrogen (reduction). | | —C=C— + H 2 + Ni, Pt, or Pd | | —C—C— | | H H Requires catalyst. b) CH 3 CH=CHCH 3 + H 2, Ni CH 3 CH 2 CH 3 c) 2 -butene n-butane
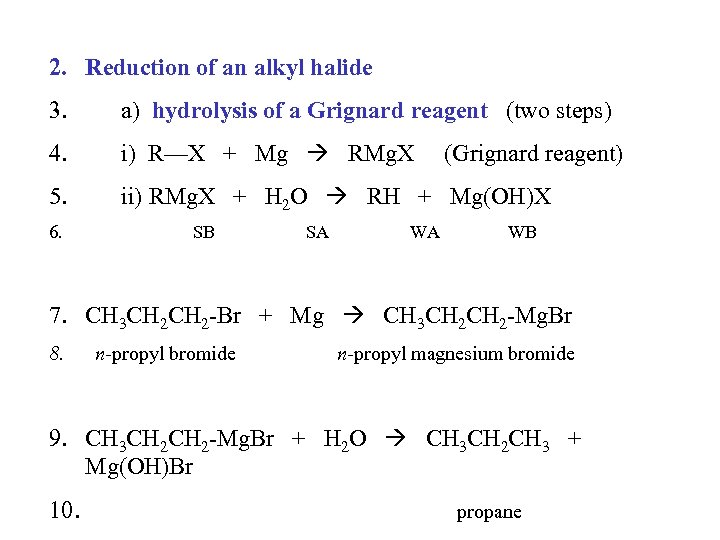 2. Reduction of an alkyl halide 3. a) hydrolysis of a Grignard reagent (two steps) 4. i) R—X + Mg RMg. X 5. ii) RMg. X + H 2 O RH + Mg(OH)X 6. SB SA WA (Grignard reagent) WB 7. CH 3 CH 2 -Br + Mg CH 3 CH 2 -Mg. Br 8. n-propyl bromide n-propyl magnesium bromide 9. CH 3 CH 2 -Mg. Br + H 2 O CH 3 CH 2 CH 3 + Mg(OH)Br 10. propane
2. Reduction of an alkyl halide 3. a) hydrolysis of a Grignard reagent (two steps) 4. i) R—X + Mg RMg. X 5. ii) RMg. X + H 2 O RH + Mg(OH)X 6. SB SA WA (Grignard reagent) WB 7. CH 3 CH 2 -Br + Mg CH 3 CH 2 -Mg. Br 8. n-propyl bromide n-propyl magnesium bromide 9. CH 3 CH 2 -Mg. Br + H 2 O CH 3 CH 2 CH 3 + Mg(OH)Br 10. propane
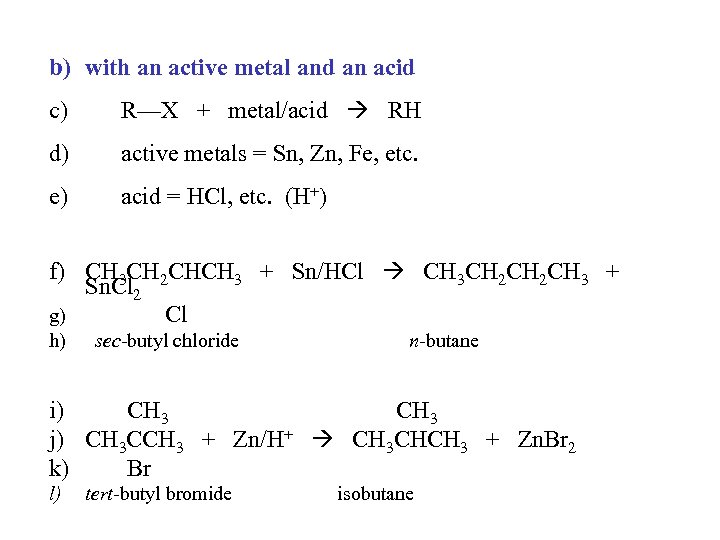 b) with an active metal and an acid c) R—X + metal/acid RH d) active metals = Sn, Zn, Fe, etc. e) acid = HCl, etc. (H+) f) CH 3 CH 2 CHCH 3 + Sn/HCl CH 3 CH 2 CH 3 + Sn. Cl 2 g) Cl h) sec-butyl chloride n-butane i) CH 3 j) CH 3 CCH 3 + Zn/H+ CH 3 CHCH 3 + Zn. Br 2 k) Br l) tert-butyl bromide isobutane
b) with an active metal and an acid c) R—X + metal/acid RH d) active metals = Sn, Zn, Fe, etc. e) acid = HCl, etc. (H+) f) CH 3 CH 2 CHCH 3 + Sn/HCl CH 3 CH 2 CH 3 + Sn. Cl 2 g) Cl h) sec-butyl chloride n-butane i) CH 3 j) CH 3 CCH 3 + Zn/H+ CH 3 CHCH 3 + Zn. Br 2 k) Br l) tert-butyl bromide isobutane
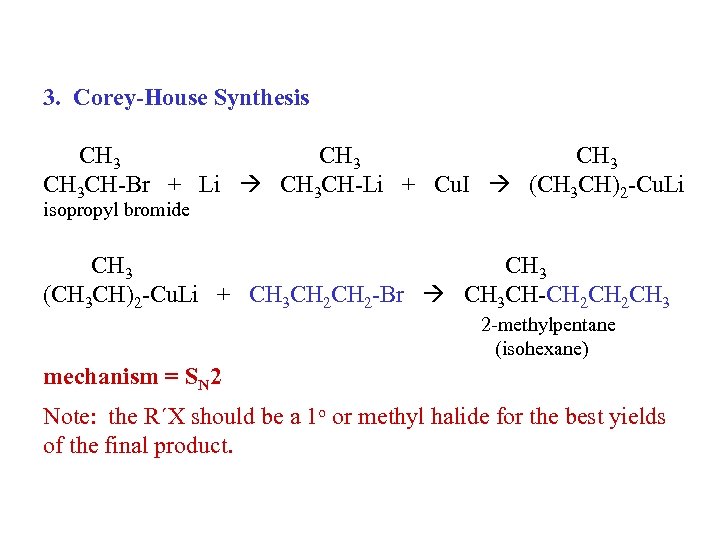 3. Corey-House Synthesis CH 3 CH-Br + Li CH 3 CH-Li + Cu. I (CH 3 CH)2 -Cu. Li isopropyl bromide CH 3 (CH 3 CH)2 -Cu. Li + CH 3 CH 2 -Br CH 3 CH-CH 2 CH 3 2 -methylpentane (isohexane) mechanism = SN 2 Note: the R´X should be a 1 o or methyl halide for the best yields of the final product.
3. Corey-House Synthesis CH 3 CH-Br + Li CH 3 CH-Li + Cu. I (CH 3 CH)2 -Cu. Li isopropyl bromide CH 3 (CH 3 CH)2 -Cu. Li + CH 3 CH 2 -Br CH 3 CH-CH 2 CH 3 2 -methylpentane (isohexane) mechanism = SN 2 Note: the R´X should be a 1 o or methyl halide for the best yields of the final product.
 Alkanes, reactions 1. Halogenation R-H + X 2, heat or hv R-X + HX a) heat or light required for reaction. b) X 2: Cl 2 > Br 2 I 2 c) yields mixtures d) H: 3 o > 2 o > 1 o > CH 4 e) bromine is more selective f) free radical substitution
Alkanes, reactions 1. Halogenation R-H + X 2, heat or hv R-X + HX a) heat or light required for reaction. b) X 2: Cl 2 > Br 2 I 2 c) yields mixtures d) H: 3 o > 2 o > 1 o > CH 4 e) bromine is more selective f) free radical substitution
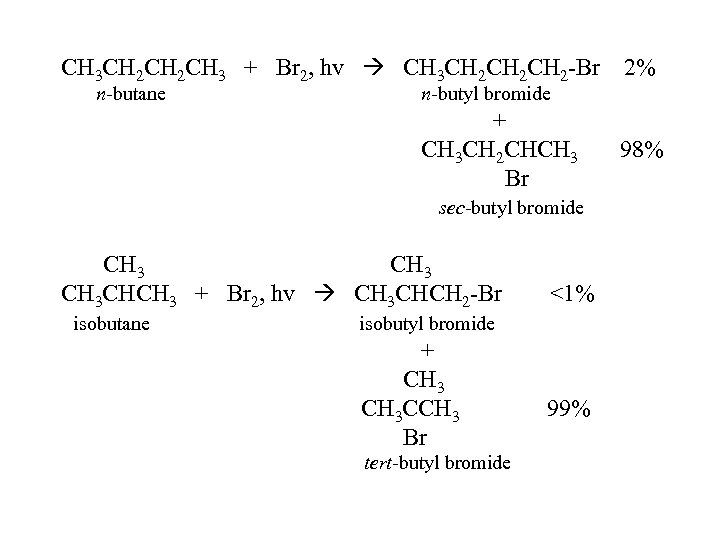 CH 3 CH 2 CH 3 + Br 2, hv CH 3 CH 2 CH 2 -Br n-butane n-butyl bromide + CH 3 CH 2 CHCH 3 Br sec-butyl bromide CH 3 CHCH 3 + Br 2, hv CH 3 CHCH 2 -Br isobutane 2% <1% isobutyl bromide + CH 3 CCH 3 Br tert-butyl bromide 99% 98%
CH 3 CH 2 CH 3 + Br 2, hv CH 3 CH 2 CH 2 -Br n-butane n-butyl bromide + CH 3 CH 2 CHCH 3 Br sec-butyl bromide CH 3 CHCH 3 + Br 2, hv CH 3 CHCH 2 -Br isobutane 2% <1% isobutyl bromide + CH 3 CCH 3 Br tert-butyl bromide 99% 98%
 Alkyl halides nomenclature syntheses 1. from alcohols a) HX b) PX 3 2. halogenation of certain alkanes 3. addition of hydrogen halides to alkenes 4. addition of halogens to alkenes 5. halide exchange for iodide reactions 1. nucleophilic substitution 2. dehydrohalogenation 3. formation of Grignard reagent 4. reduction
Alkyl halides nomenclature syntheses 1. from alcohols a) HX b) PX 3 2. halogenation of certain alkanes 3. addition of hydrogen halides to alkenes 4. addition of halogens to alkenes 5. halide exchange for iodide reactions 1. nucleophilic substitution 2. dehydrohalogenation 3. formation of Grignard reagent 4. reduction
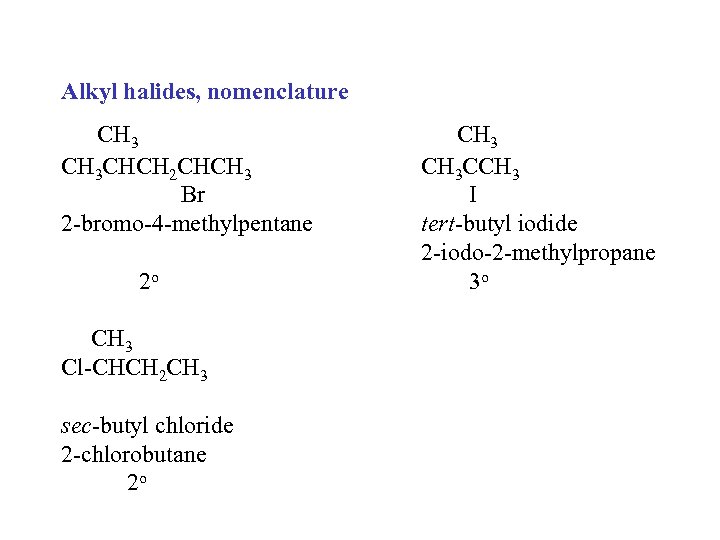 Alkyl halides, nomenclature CH 3 CHCH 2 CHCH 3 Br 2 -bromo-4 -methylpentane 2 o CH 3 Cl-CHCH 2 CH 3 sec-butyl chloride 2 -chlorobutane 2 o CH 3 CCH 3 I tert-butyl iodide 2 -iodo-2 -methylpropane 3 o
Alkyl halides, nomenclature CH 3 CHCH 2 CHCH 3 Br 2 -bromo-4 -methylpentane 2 o CH 3 Cl-CHCH 2 CH 3 sec-butyl chloride 2 -chlorobutane 2 o CH 3 CCH 3 I tert-butyl iodide 2 -iodo-2 -methylpropane 3 o
 Alkyl halides, syntheses 1. From alcohols a) With HX b) R-OH c) i) HX = HCl, HBr, HI d) ii) may be acid catalyzed (H+) + HX R-X + H 2 O e) iii) ROH: 3 o > 2 o > CH 3 > 1 o (3 o/2 o – SN 1; CH 3/1 o – SN 2) f) iv) rearrangements are possible except with most 1 o ROH
Alkyl halides, syntheses 1. From alcohols a) With HX b) R-OH c) i) HX = HCl, HBr, HI d) ii) may be acid catalyzed (H+) + HX R-X + H 2 O e) iii) ROH: 3 o > 2 o > CH 3 > 1 o (3 o/2 o – SN 1; CH 3/1 o – SN 2) f) iv) rearrangements are possible except with most 1 o ROH
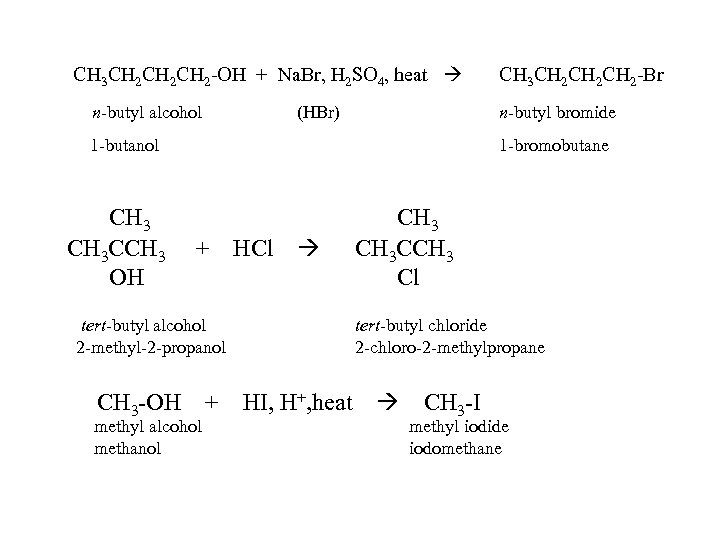 CH 3 CH 2 CH 2 -OH + Na. Br, H 2 SO 4, heat n-butyl alcohol (HBr) n-butyl bromide 1 -butanol CH 3 CCH 3 OH CH 3 CH 2 CH 2 -Br 1 -bromobutane + HCl tert-butyl alcohol 2 -methyl-2 -propanol CH 3 -OH + methyl alcohol methanol CH 3 CCH 3 Cl tert-butyl chloride 2 -chloro-2 -methylpropane HI, H+, heat CH 3 -I methyl iodide iodomethane
CH 3 CH 2 CH 2 -OH + Na. Br, H 2 SO 4, heat n-butyl alcohol (HBr) n-butyl bromide 1 -butanol CH 3 CCH 3 OH CH 3 CH 2 CH 2 -Br 1 -bromobutane + HCl tert-butyl alcohol 2 -methyl-2 -propanol CH 3 -OH + methyl alcohol methanol CH 3 CCH 3 Cl tert-butyl chloride 2 -chloro-2 -methylpropane HI, H+, heat CH 3 -I methyl iodide iodomethane
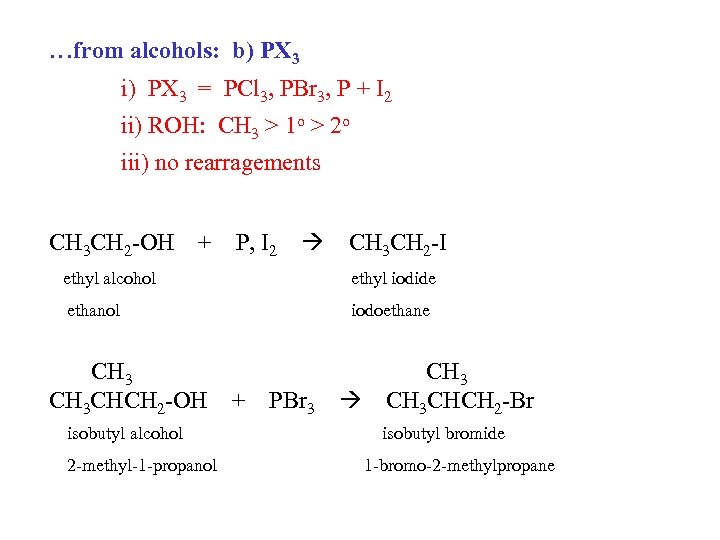 …from alcohols: b) PX 3 i) PX 3 = PCl 3, PBr 3, P + I 2 ii) ROH: CH 3 > 1 o > 2 o iii) no rearragements CH 3 CH 2 -OH + P, I 2 CH 3 CH 2 -I ethyl alcohol ethyl iodide ethanol iodoethane CH 3 CHCH 2 -OH isobutyl alcohol 2 -methyl-1 -propanol + PBr 3 CH 3 CHCH 2 -Br isobutyl bromide 1 -bromo-2 -methylpropane
…from alcohols: b) PX 3 i) PX 3 = PCl 3, PBr 3, P + I 2 ii) ROH: CH 3 > 1 o > 2 o iii) no rearragements CH 3 CH 2 -OH + P, I 2 CH 3 CH 2 -I ethyl alcohol ethyl iodide ethanol iodoethane CH 3 CHCH 2 -OH isobutyl alcohol 2 -methyl-1 -propanol + PBr 3 CH 3 CHCH 2 -Br isobutyl bromide 1 -bromo-2 -methylpropane
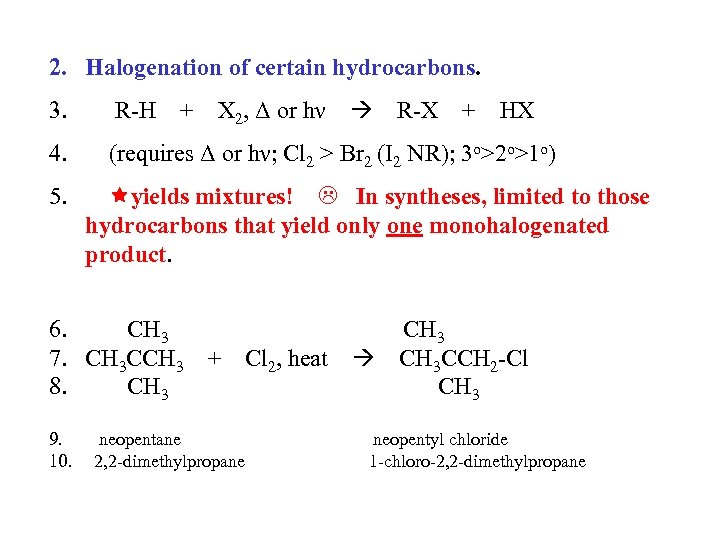 2. Halogenation of certain hydrocarbons. 3. R-H 4. (requires Δ or hν; Cl 2 > Br 2 (I 2 NR); 3 o>2 o>1 o) 5. + X 2, Δ or hν R-X + HX yields mixtures! In syntheses, limited to those hydrocarbons that yield only one monohalogenated product. 6. CH 3 7. CH 3 CCH 3 + Cl 2, heat CH 3 CCH 2 -Cl 8. CH 3 9. 10. neopentane 2, 2 -dimethylpropane neopentyl chloride 1 -chloro-2, 2 -dimethylpropane
2. Halogenation of certain hydrocarbons. 3. R-H 4. (requires Δ or hν; Cl 2 > Br 2 (I 2 NR); 3 o>2 o>1 o) 5. + X 2, Δ or hν R-X + HX yields mixtures! In syntheses, limited to those hydrocarbons that yield only one monohalogenated product. 6. CH 3 7. CH 3 CCH 3 + Cl 2, heat CH 3 CCH 2 -Cl 8. CH 3 9. 10. neopentane 2, 2 -dimethylpropane neopentyl chloride 1 -chloro-2, 2 -dimethylpropane
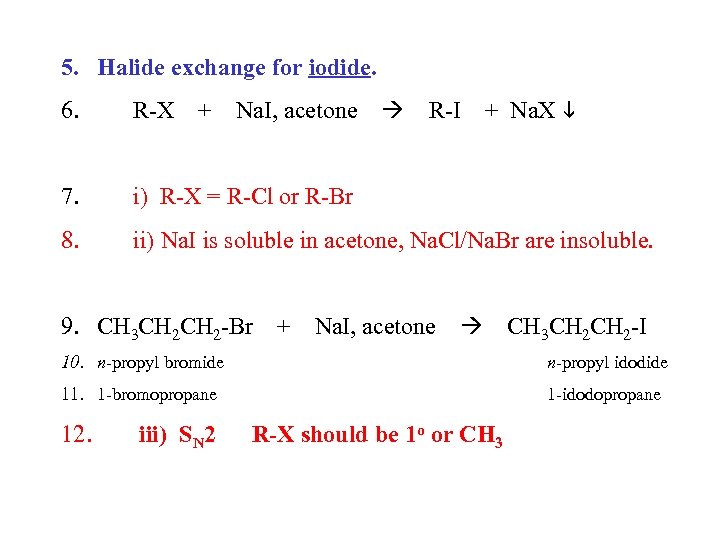 5. Halide exchange for iodide. + Na. X 6. R-X 7. i) R-X = R-Cl or R-Br 8. ii) Na. I is soluble in acetone, Na. Cl/Na. Br are insoluble. + Na. I, acetone R-I 9. CH 3 CH 2 -Br + Na. I, acetone CH 3 CH 2 -I 10. n-propyl bromide n-propyl idodide 11. 1 -bromopropane 1 -idodopropane 12. iii) SN 2 R-X should be 1 o or CH 3
5. Halide exchange for iodide. + Na. X 6. R-X 7. i) R-X = R-Cl or R-Br 8. ii) Na. I is soluble in acetone, Na. Cl/Na. Br are insoluble. + Na. I, acetone R-I 9. CH 3 CH 2 -Br + Na. I, acetone CH 3 CH 2 -I 10. n-propyl bromide n-propyl idodide 11. 1 -bromopropane 1 -idodopropane 12. iii) SN 2 R-X should be 1 o or CH 3
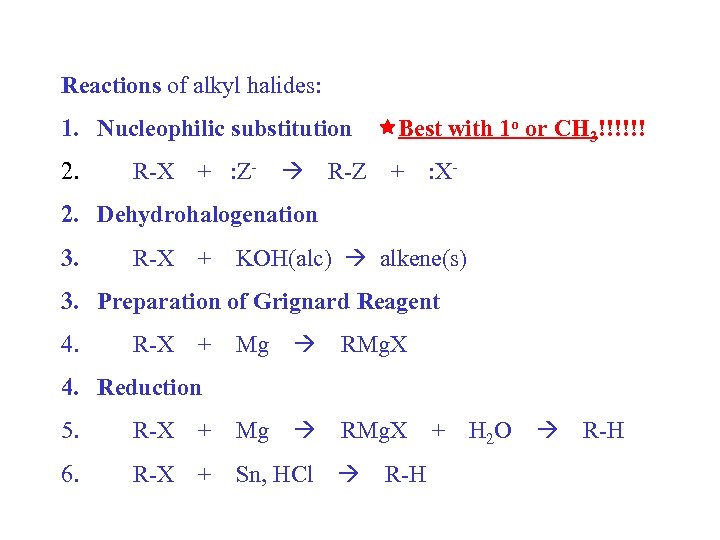 Reactions of alkyl halides: 1. Nucleophilic substitution Best with 1 o or CH 3!!!!!! 2. R-X + : Z- R-Z + : X- 2. Dehydrohalogenation 3. R-X + KOH(alc) alkene(s) 3. Preparation of Grignard Reagent 4. R-X + Mg RMg. X 4. Reduction 5. R-X + Mg 6. R-X + Sn, HCl R-H + H 2 O R-H
Reactions of alkyl halides: 1. Nucleophilic substitution Best with 1 o or CH 3!!!!!! 2. R-X + : Z- R-Z + : X- 2. Dehydrohalogenation 3. R-X + KOH(alc) alkene(s) 3. Preparation of Grignard Reagent 4. R-X + Mg RMg. X 4. Reduction 5. R-X + Mg 6. R-X + Sn, HCl R-H + H 2 O R-H
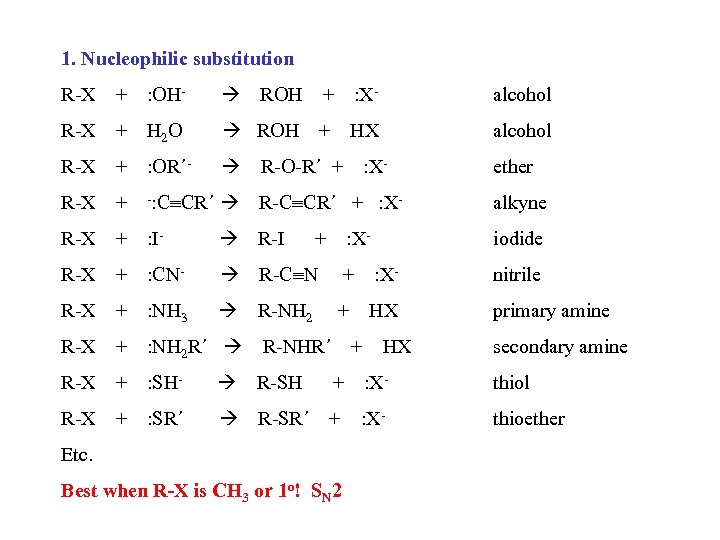 1. Nucleophilic substitution R-X + : OH- ROH + : X- alcohol R-X + H 2 O ROH + HX alcohol R-X + : OR´- R-O-R´ + R-X + -: C CR´ R-C CR´ + : X- alkyne R-X + : I- R-I iodide R-X + : CN- R-C N + : X- nitrile R-X + : NH 3 R-NH 2 + HX primary amine R-X + : NH 2 R´ R-X + : SH- R-SH + : X- thiol R-X + : SR´ R-SR´ + : X- thioether + : X: X- R-NHR´ + Etc. Best when R-X is CH 3 or 1 o! SN 2 HX ether secondary amine
1. Nucleophilic substitution R-X + : OH- ROH + : X- alcohol R-X + H 2 O ROH + HX alcohol R-X + : OR´- R-O-R´ + R-X + -: C CR´ R-C CR´ + : X- alkyne R-X + : I- R-I iodide R-X + : CN- R-C N + : X- nitrile R-X + : NH 3 R-NH 2 + HX primary amine R-X + : NH 2 R´ R-X + : SH- R-SH + : X- thiol R-X + : SR´ R-SR´ + : X- thioether + : X: X- R-NHR´ + Etc. Best when R-X is CH 3 or 1 o! SN 2 HX ether secondary amine
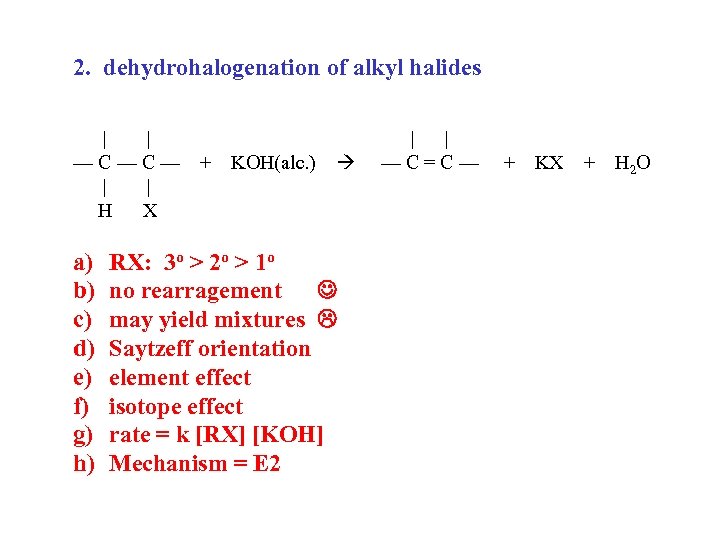 2. dehydrohalogenation of alkyl halides | | —C—C— | | H X a) b) c) d) e) f) g) h) + KOH(alc. ) RX: 3 o > 2 o > 1 o no rearragement may yield mixtures Saytzeff orientation element effect isotope effect rate = k [RX] [KOH] Mechanism = E 2 | | —C=C— + KX + H 2 O
2. dehydrohalogenation of alkyl halides | | —C—C— | | H X a) b) c) d) e) f) g) h) + KOH(alc. ) RX: 3 o > 2 o > 1 o no rearragement may yield mixtures Saytzeff orientation element effect isotope effect rate = k [RX] [KOH] Mechanism = E 2 | | —C=C— + KX + H 2 O
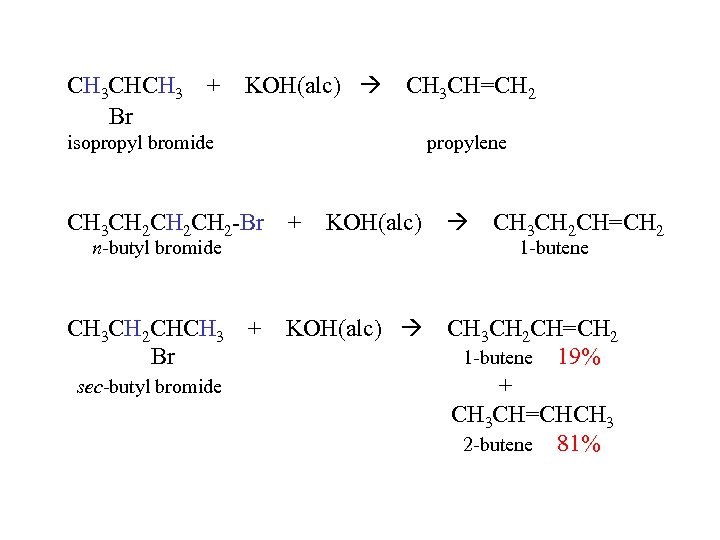 CH 3 CHCH 3 + Br KOH(alc) CH 3 CH=CH 2 isopropyl bromide propylene CH 3 CH 2 CH 2 -Br + KOH(alc) CH 3 CH 2 CHCH 3 + Br KOH(alc) n-butyl bromide sec-butyl bromide CH 3 CH 2 CH=CH 2 1 -butene 19% + CH 3 CH=CHCH 3 2 -butene 81%
CH 3 CHCH 3 + Br KOH(alc) CH 3 CH=CH 2 isopropyl bromide propylene CH 3 CH 2 CH 2 -Br + KOH(alc) CH 3 CH 2 CHCH 3 + Br KOH(alc) n-butyl bromide sec-butyl bromide CH 3 CH 2 CH=CH 2 1 -butene 19% + CH 3 CH=CHCH 3 2 -butene 81%
 1. 3. preparation of Grignard reagent 2. CH 3 CH 2 -Br + Mg CH 3 CH 2 -Mg. Br 3. n-propyl bromide n-propyl magnesium bromide 4. reduction 1. CH 3 CH 2 -Br + Mg CH 3 CH 2 -Mg. Br 2. CH 3 CH 2 -Mg. Br + H 2 O CH 3 CH 2 CH 3 + Mg(OH)Br 3. propane 4. CH 3 CH 2 CHCH 3 + Sn/HCl CH 3 CH 2 CH 3 + Sn. Cl 2 5. Cl 6. sec-butyl chloride n-butane
1. 3. preparation of Grignard reagent 2. CH 3 CH 2 -Br + Mg CH 3 CH 2 -Mg. Br 3. n-propyl bromide n-propyl magnesium bromide 4. reduction 1. CH 3 CH 2 -Br + Mg CH 3 CH 2 -Mg. Br 2. CH 3 CH 2 -Mg. Br + H 2 O CH 3 CH 2 CH 3 + Mg(OH)Br 3. propane 4. CH 3 CH 2 CHCH 3 + Sn/HCl CH 3 CH 2 CH 3 + Sn. Cl 2 5. Cl 6. sec-butyl chloride n-butane
 Alcohols nomenclature syntheses 1. oxymercuration-demercuration 2. hydroboration-oxidation 3. 4. hydrolysis of some alkyl halides reactions 1. HX 2. PX 3 3. dehydration 4. as acids 5. ester formation 6. oxidation
Alcohols nomenclature syntheses 1. oxymercuration-demercuration 2. hydroboration-oxidation 3. 4. hydrolysis of some alkyl halides reactions 1. HX 2. PX 3 3. dehydration 4. as acids 5. ester formation 6. oxidation
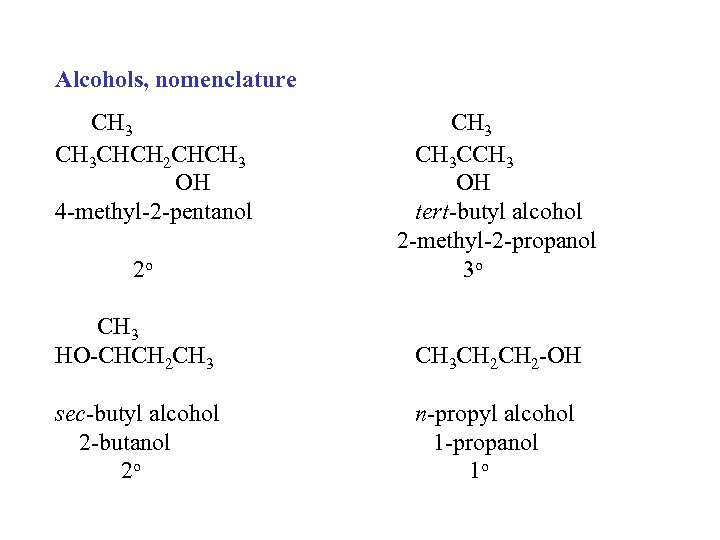 Alcohols, nomenclature CH 3 CHCH 2 CHCH 3 OH 4 -methyl-2 -pentanol 2 o CH 3 CCH 3 OH tert-butyl alcohol 2 -methyl-2 -propanol 3 o CH 3 HO-CHCH 2 CH 3 CH 2 -OH sec-butyl alcohol 2 -butanol 2 o n-propyl alcohol 1 -propanol 1 o
Alcohols, nomenclature CH 3 CHCH 2 CHCH 3 OH 4 -methyl-2 -pentanol 2 o CH 3 CCH 3 OH tert-butyl alcohol 2 -methyl-2 -propanol 3 o CH 3 HO-CHCH 2 CH 3 CH 2 -OH sec-butyl alcohol 2 -butanol 2 o n-propyl alcohol 1 -propanol 1 o
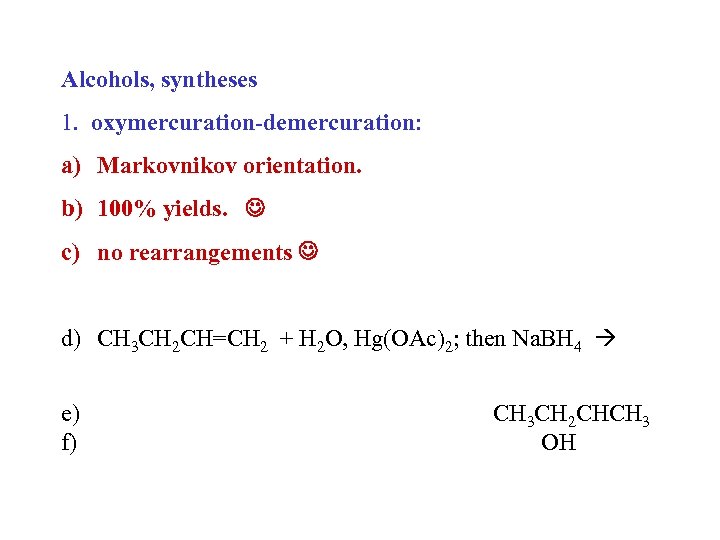 Alcohols, syntheses 1. oxymercuration-demercuration: a) Markovnikov orientation. b) 100% yields. c) no rearrangements d) CH 3 CH 2 CH=CH 2 + H 2 O, Hg(OAc)2; then Na. BH 4 e) f) CH 3 CH 2 CHCH 3 OH
Alcohols, syntheses 1. oxymercuration-demercuration: a) Markovnikov orientation. b) 100% yields. c) no rearrangements d) CH 3 CH 2 CH=CH 2 + H 2 O, Hg(OAc)2; then Na. BH 4 e) f) CH 3 CH 2 CHCH 3 OH
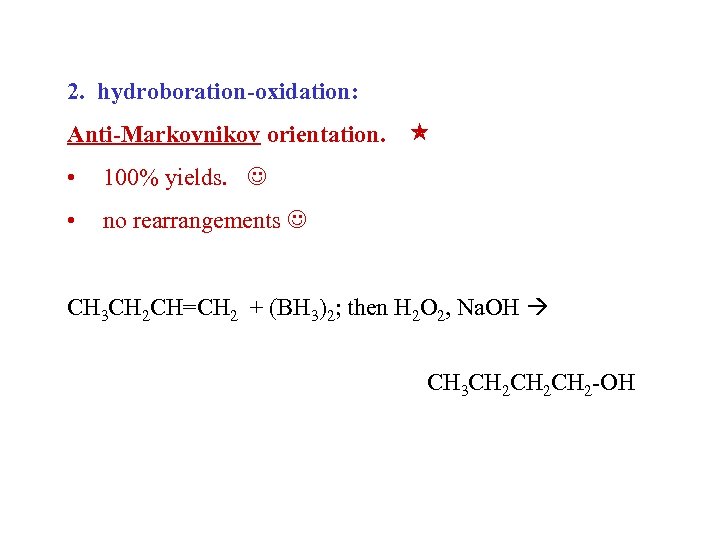 2. hydroboration-oxidation: Anti-Markovnikov orientation. • 100% yields. • no rearrangements CH 3 CH 2 CH=CH 2 + (BH 3)2; then H 2 O 2, Na. OH CH 3 CH 2 CH 2 -OH
2. hydroboration-oxidation: Anti-Markovnikov orientation. • 100% yields. • no rearrangements CH 3 CH 2 CH=CH 2 + (BH 3)2; then H 2 O 2, Na. OH CH 3 CH 2 CH 2 -OH
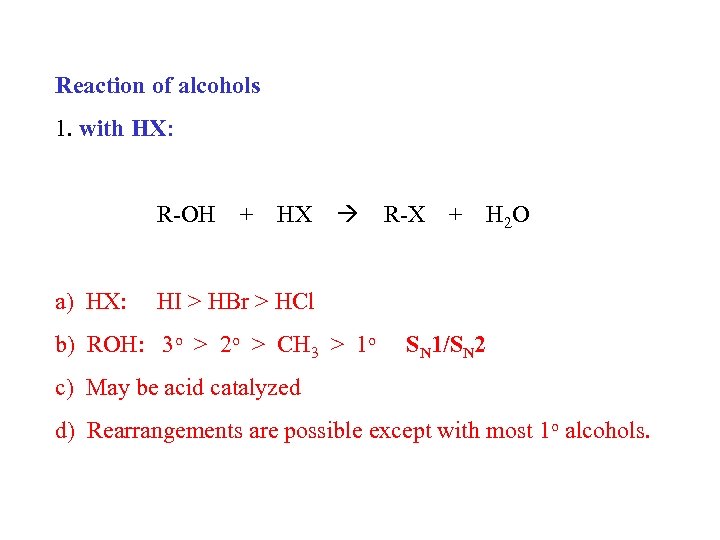 Reaction of alcohols 1. with HX: R-OH a) HX: + HX R-X + H 2 O HI > HBr > HCl b) ROH: 3 o > 2 o > CH 3 > 1 o SN 1/SN 2 c) May be acid catalyzed d) Rearrangements are possible except with most 1 o alcohols.
Reaction of alcohols 1. with HX: R-OH a) HX: + HX R-X + H 2 O HI > HBr > HCl b) ROH: 3 o > 2 o > CH 3 > 1 o SN 1/SN 2 c) May be acid catalyzed d) Rearrangements are possible except with most 1 o alcohols.
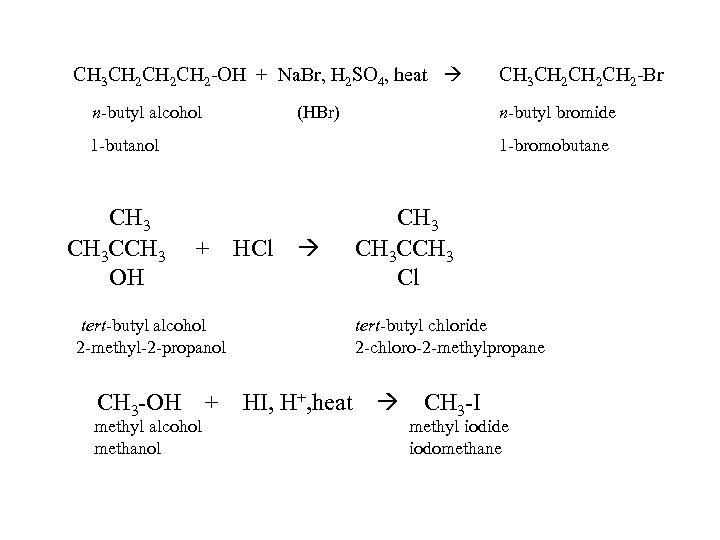 CH 3 CH 2 CH 2 -OH + Na. Br, H 2 SO 4, heat n-butyl alcohol (HBr) n-butyl bromide 1 -butanol CH 3 CCH 3 OH CH 3 CH 2 CH 2 -Br 1 -bromobutane + HCl tert-butyl alcohol 2 -methyl-2 -propanol CH 3 -OH + methyl alcohol methanol CH 3 CCH 3 Cl tert-butyl chloride 2 -chloro-2 -methylpropane HI, H+, heat CH 3 -I methyl iodide iodomethane
CH 3 CH 2 CH 2 -OH + Na. Br, H 2 SO 4, heat n-butyl alcohol (HBr) n-butyl bromide 1 -butanol CH 3 CCH 3 OH CH 3 CH 2 CH 2 -Br 1 -bromobutane + HCl tert-butyl alcohol 2 -methyl-2 -propanol CH 3 -OH + methyl alcohol methanol CH 3 CCH 3 Cl tert-butyl chloride 2 -chloro-2 -methylpropane HI, H+, heat CH 3 -I methyl iodide iodomethane
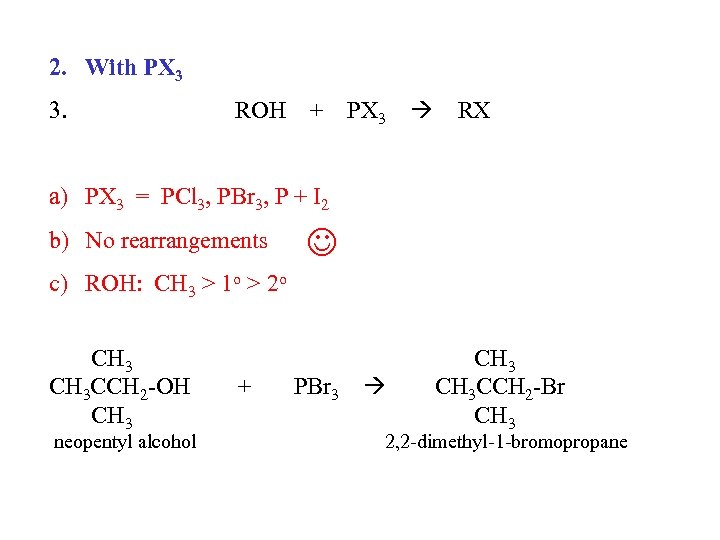 2. With PX 3 3. ROH + PX 3 RX a) PX 3 = PCl 3, PBr 3, P + I 2 b) No rearrangements c) ROH: CH 3 > 1 o > 2 o CH 3 CCH 2 -OH CH 3 neopentyl alcohol + PBr 3 CH 3 CCH 2 -Br CH 3 2, 2 -dimethyl-1 -bromopropane
2. With PX 3 3. ROH + PX 3 RX a) PX 3 = PCl 3, PBr 3, P + I 2 b) No rearrangements c) ROH: CH 3 > 1 o > 2 o CH 3 CCH 2 -OH CH 3 neopentyl alcohol + PBr 3 CH 3 CCH 2 -Br CH 3 2, 2 -dimethyl-1 -bromopropane
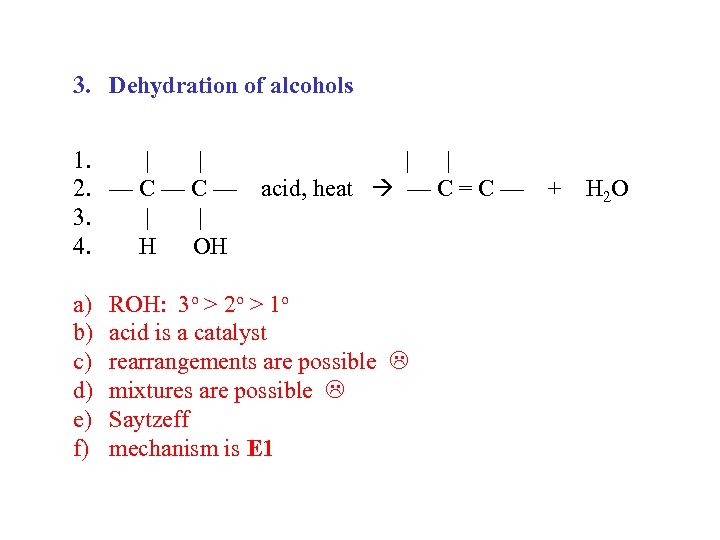 3. Dehydration of alcohols 1. | | 2. — C — acid, heat — C = C — + H 2 O 3. | | 4. H OH a) b) c) d) e) f) ROH: 3 o > 2 o > 1 o acid is a catalyst rearrangements are possible mixtures are possible Saytzeff mechanism is E 1
3. Dehydration of alcohols 1. | | 2. — C — acid, heat — C = C — + H 2 O 3. | | 4. H OH a) b) c) d) e) f) ROH: 3 o > 2 o > 1 o acid is a catalyst rearrangements are possible mixtures are possible Saytzeff mechanism is E 1
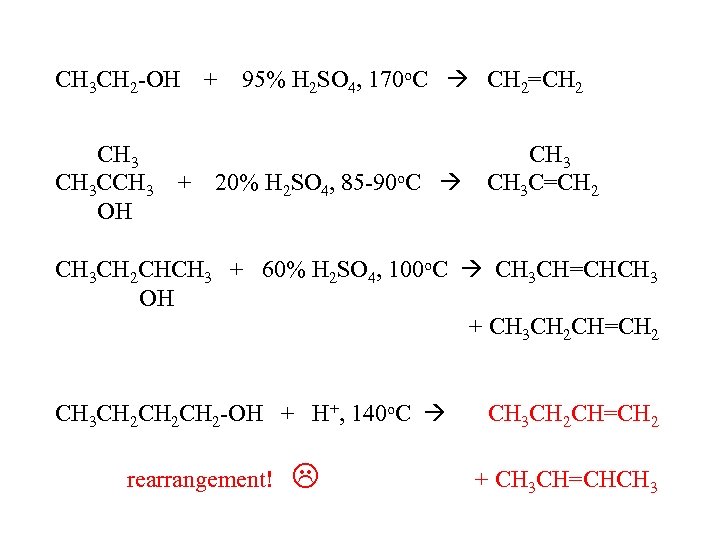 CH 3 CH 2 -OH CH 3 CCH 3 + OH + 95% H 2 SO 4, 170 o. C CH 2=CH 2 20% H 2 SO 4, 85 -90 o. C CH 3 C=CH 2 CH 3 CH 2 CHCH 3 + 60% H 2 SO 4, 100 o. C CH 3 CH=CHCH 3 OH + CH 3 CH 2 CH=CH 2 CH 3 CH 2 CH 2 -OH + H+, 140 o. C rearrangement! CH 3 CH 2 CH=CH 2 + CH 3 CH=CHCH 3
CH 3 CH 2 -OH CH 3 CCH 3 + OH + 95% H 2 SO 4, 170 o. C CH 2=CH 2 20% H 2 SO 4, 85 -90 o. C CH 3 C=CH 2 CH 3 CH 2 CHCH 3 + 60% H 2 SO 4, 100 o. C CH 3 CH=CHCH 3 OH + CH 3 CH 2 CH=CH 2 CH 3 CH 2 CH 2 -OH + H+, 140 o. C rearrangement! CH 3 CH 2 CH=CH 2 + CH 3 CH=CHCH 3
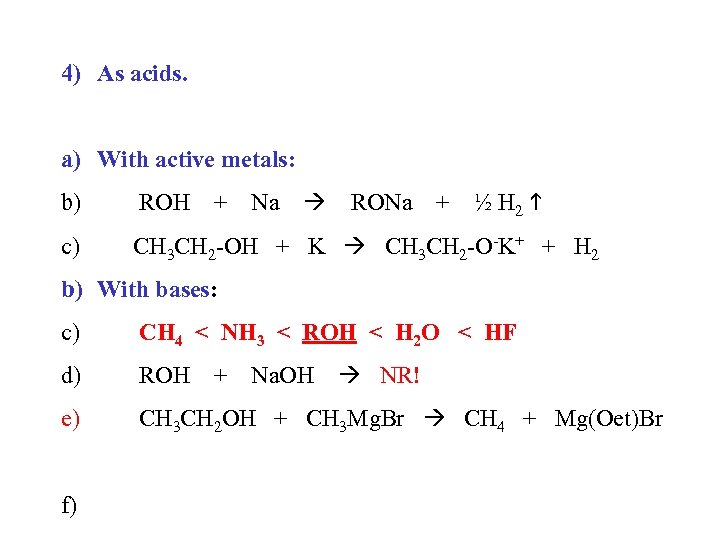 4) As acids. a) With active metals: ½ H 2 b) ROH c) CH 3 CH 2 -OH + K CH 3 CH 2 -O-K+ + H 2 + Na RONa + b) With bases: c) CH 4 < NH 3 < ROH < H 2 O < HF d) ROH e) CH 3 CH 2 OH + CH 3 Mg. Br CH 4 + Mg(Oet)Br f) + Na. OH NR!
4) As acids. a) With active metals: ½ H 2 b) ROH c) CH 3 CH 2 -OH + K CH 3 CH 2 -O-K+ + H 2 + Na RONa + b) With bases: c) CH 4 < NH 3 < ROH < H 2 O < HF d) ROH e) CH 3 CH 2 OH + CH 3 Mg. Br CH 4 + Mg(Oet)Br f) + Na. OH NR!
 5. Ester formation. 6. CH 3 CH 2 -OH + CH 3 CO 2 H, H+ 7. CH 3 CH 2 -OH + CH 3 COCl 8. CH 3 -OH CH 3 SO 2 Cl 9. + Esters are alkyl “salts” of acids. CH 3 CO 2 CH 2 CH 3 SO 3 CH 3 + HCl + + H 2 O HCl
5. Ester formation. 6. CH 3 CH 2 -OH + CH 3 CO 2 H, H+ 7. CH 3 CH 2 -OH + CH 3 COCl 8. CH 3 -OH CH 3 SO 2 Cl 9. + Esters are alkyl “salts” of acids. CH 3 CO 2 CH 2 CH 3 SO 3 CH 3 + HCl + + H 2 O HCl
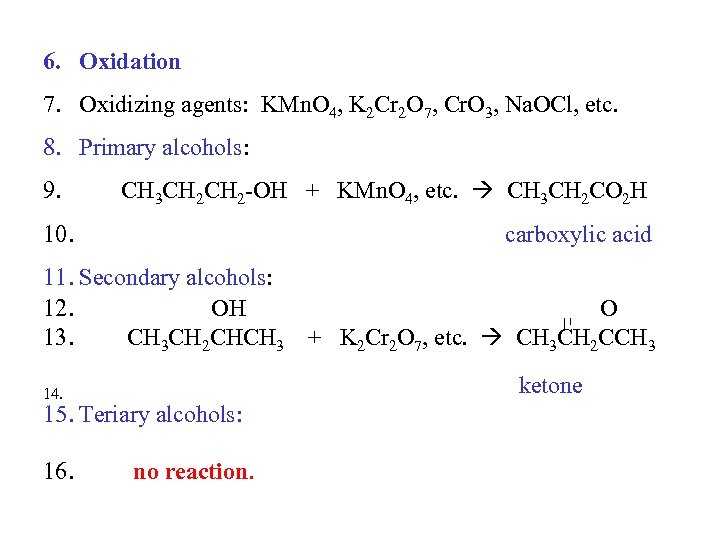 6. Oxidation 7. Oxidizing agents: KMn. O 4, K 2 Cr 2 O 7, Cr. O 3, Na. OCl, etc. 8. Primary alcohols: 9. CH 3 CH 2 -OH + KMn. O 4, etc. CH 3 CH 2 CO 2 H 10. carboxylic acid 11. Secondary alcohols: 12. OH O 13. CH 3 CH 2 CHCH 3 + K 2 Cr 2 O 7, etc. CH 3 CH 2 CCH 3 ketone 14. 15. Teriary alcohols: 16. no reaction.
6. Oxidation 7. Oxidizing agents: KMn. O 4, K 2 Cr 2 O 7, Cr. O 3, Na. OCl, etc. 8. Primary alcohols: 9. CH 3 CH 2 -OH + KMn. O 4, etc. CH 3 CH 2 CO 2 H 10. carboxylic acid 11. Secondary alcohols: 12. OH O 13. CH 3 CH 2 CHCH 3 + K 2 Cr 2 O 7, etc. CH 3 CH 2 CCH 3 ketone 14. 15. Teriary alcohols: 16. no reaction.
 Primary alcohols can also be oxidized to aldehydes: CH 3 CH 2 -OH + C 5 H 5 NHCr. O 3 Cl pyridinium chlorochromate CH 3 CH 2 CHO aldehyde or CH 3 CH 2 -OH + K 2 Cr 2 O 7, special conditions
Primary alcohols can also be oxidized to aldehydes: CH 3 CH 2 -OH + C 5 H 5 NHCr. O 3 Cl pyridinium chlorochromate CH 3 CH 2 CHO aldehyde or CH 3 CH 2 -OH + K 2 Cr 2 O 7, special conditions
 Ethers nomenclature syntheses 1. Williamson Synthesis 2. alkoxymercuration-demercuration reactions 1. acid cleavage
Ethers nomenclature syntheses 1. Williamson Synthesis 2. alkoxymercuration-demercuration reactions 1. acid cleavage
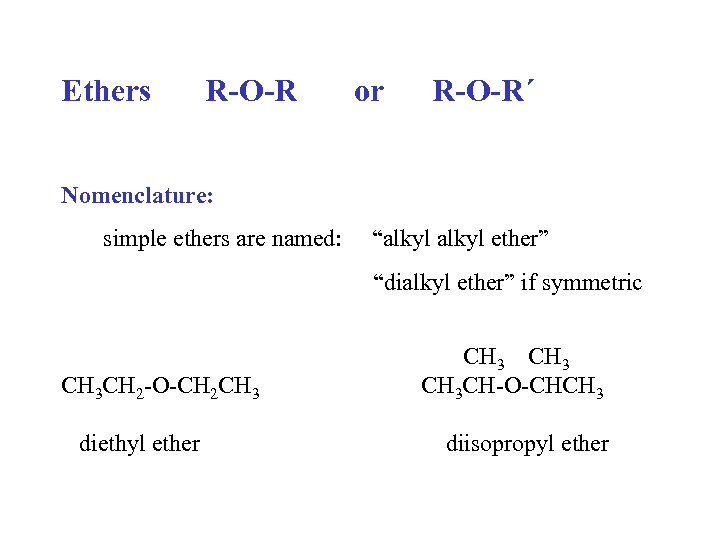 Ethers R-O-R or R-O-R´ Nomenclature: simple ethers are named: “alkyl ether” “dialkyl ether” if symmetric CH 3 CH 2 -O-CH 2 CH 3 diethyl ether CH 3 CH-O-CHCH 3 diisopropyl ether
Ethers R-O-R or R-O-R´ Nomenclature: simple ethers are named: “alkyl ether” “dialkyl ether” if symmetric CH 3 CH 2 -O-CH 2 CH 3 diethyl ether CH 3 CH-O-CHCH 3 diisopropyl ether
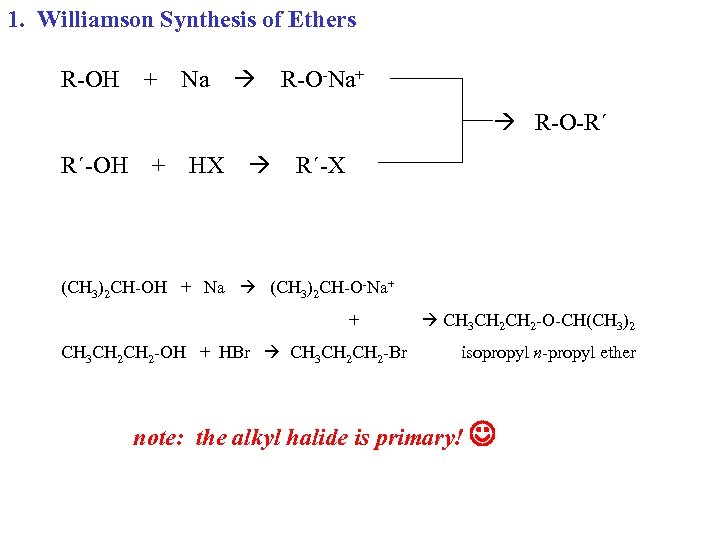 1. Williamson Synthesis of Ethers R-OH + Na R-O-Na+ R-O-R´ R´-OH + HX R´-X (CH 3)2 CH-OH + Na (CH 3)2 CH-O-Na+ + CH 3 CH 2 -OH + HBr CH 3 CH 2 CH 2 -O-CH(CH 3)2 isopropyl n-propyl ether note: the alkyl halide is primary!
1. Williamson Synthesis of Ethers R-OH + Na R-O-Na+ R-O-R´ R´-OH + HX R´-X (CH 3)2 CH-OH + Na (CH 3)2 CH-O-Na+ + CH 3 CH 2 -OH + HBr CH 3 CH 2 CH 2 -O-CH(CH 3)2 isopropyl n-propyl ether note: the alkyl halide is primary!
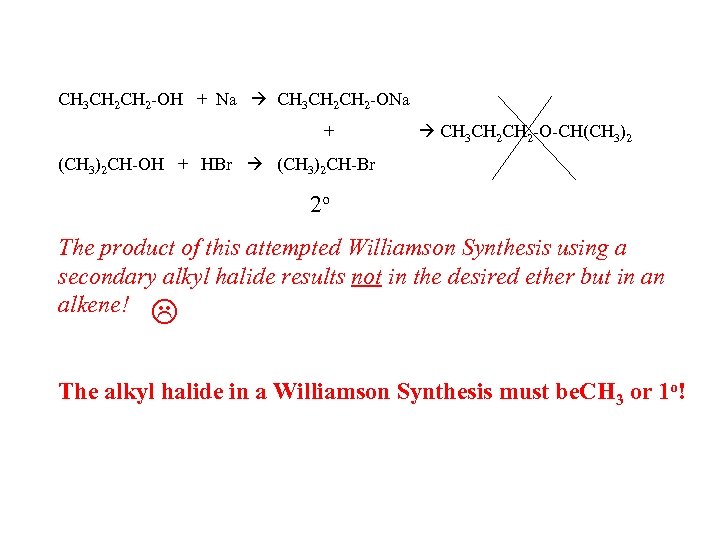 CH 3 CH 2 -OH + Na CH 3 CH 2 -ONa + CH 3 CH 2 -O-CH(CH 3)2 CH-OH + HBr (CH 3)2 CH-Br 2 o The product of this attempted Williamson Synthesis using a secondary alkyl halide results not in the desired ether but in an alkene! The alkyl halide in a Williamson Synthesis must be. CH 3 or 1 o!
CH 3 CH 2 -OH + Na CH 3 CH 2 -ONa + CH 3 CH 2 -O-CH(CH 3)2 CH-OH + HBr (CH 3)2 CH-Br 2 o The product of this attempted Williamson Synthesis using a secondary alkyl halide results not in the desired ether but in an alkene! The alkyl halide in a Williamson Synthesis must be. CH 3 or 1 o!
 2. alkoxymercuration-demercuration: a) Markovnikov orientation. b) 100% yields. c) no rearrangements d) CH 3 CH=CH 2 + CH 3 CHCH 3, Hg(TFA)2; then Na. BH 4 e) OH f) CH 3 g) CH 3 CH-OCHCH 3 h) i) diisopropyl ether j) k) Avoids the elimination with 2 o/3 o RX in Williamson Synthesis.
2. alkoxymercuration-demercuration: a) Markovnikov orientation. b) 100% yields. c) no rearrangements d) CH 3 CH=CH 2 + CH 3 CHCH 3, Hg(TFA)2; then Na. BH 4 e) OH f) CH 3 g) CH 3 CH-OCHCH 3 h) i) diisopropyl ether j) k) Avoids the elimination with 2 o/3 o RX in Williamson Synthesis.
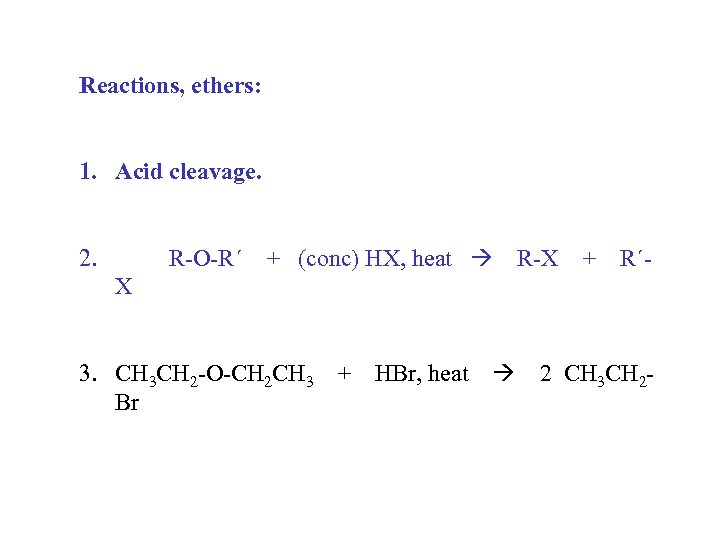 Reactions, ethers: 1. Acid cleavage. 2. R-O-R´ + (conc) HX, heat R-X + R´- X 3. CH 3 CH 2 -O-CH 2 CH 3 + HBr, heat 2 CH 3 CH 2 Br
Reactions, ethers: 1. Acid cleavage. 2. R-O-R´ + (conc) HX, heat R-X + R´- X 3. CH 3 CH 2 -O-CH 2 CH 3 + HBr, heat 2 CH 3 CH 2 Br
 Alkenes nomenclature syntheses 1. dehydrohalogenation of an alkyl halide 2. dehydration of an alcohol 3. dehalogenation of a vicinal dihalide 4. reduction of an alkyne reactions 1. addition of hydrogen 10. hydroboration-oxidation 2. addition of halogens 11. addition of free radicals 3. addition of hydrogen halides 12. polymerization 4. addition of sulfuric acid 13. addition of carbenes 5. addition of water 14. epoxidation 6. halohydrin formation 15. hydroxylation 7. dimerization 16. allylic halogenation 8. alkylation 17. ozonolysis 9. oxymercuration-demercuration 18. vigorous oxidation
Alkenes nomenclature syntheses 1. dehydrohalogenation of an alkyl halide 2. dehydration of an alcohol 3. dehalogenation of a vicinal dihalide 4. reduction of an alkyne reactions 1. addition of hydrogen 10. hydroboration-oxidation 2. addition of halogens 11. addition of free radicals 3. addition of hydrogen halides 12. polymerization 4. addition of sulfuric acid 13. addition of carbenes 5. addition of water 14. epoxidation 6. halohydrin formation 15. hydroxylation 7. dimerization 16. allylic halogenation 8. alkylation 17. ozonolysis 9. oxymercuration-demercuration 18. vigorous oxidation
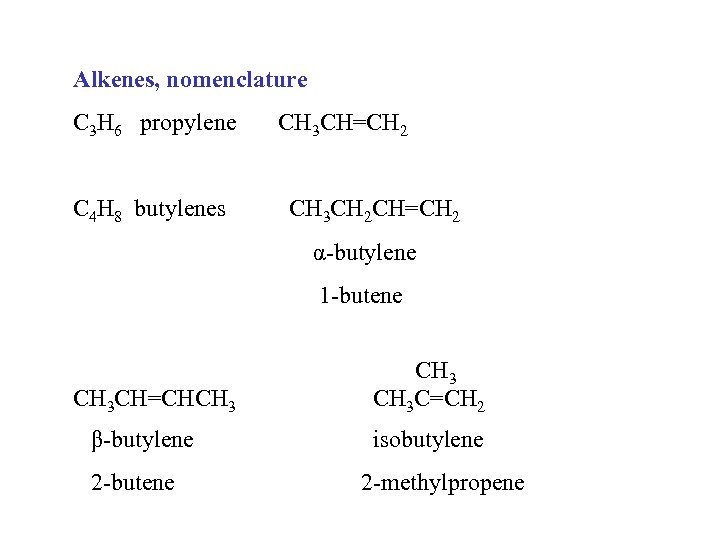 Alkenes, nomenclature C 3 H 6 propylene C 4 H 8 butylenes CH 3 CH=CH 2 CH 3 CH 2 CH=CH 2 α-butylene 1 -butene CH 3 CH=CHCH 3 β-butylene 2 -butene CH 3 C=CH 2 isobutylene 2 -methylpropene
Alkenes, nomenclature C 3 H 6 propylene C 4 H 8 butylenes CH 3 CH=CH 2 CH 3 CH 2 CH=CH 2 α-butylene 1 -butene CH 3 CH=CHCH 3 β-butylene 2 -butene CH 3 C=CH 2 isobutylene 2 -methylpropene
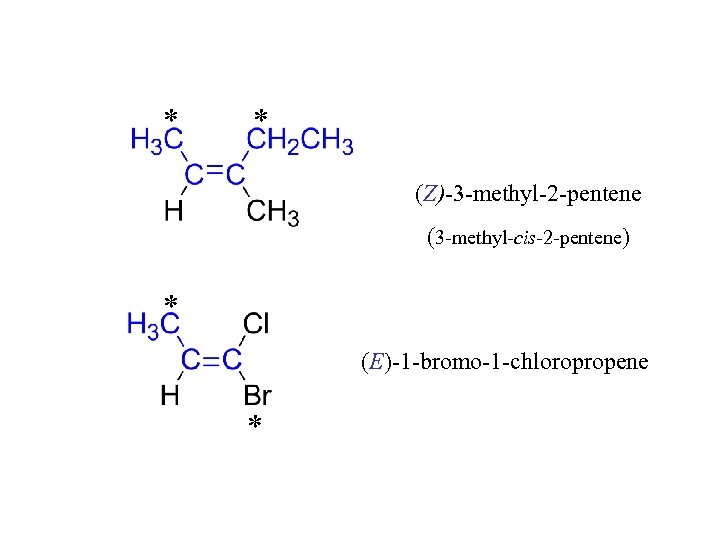 * * (Z)-3 -methyl-2 -pentene (3 -methyl-cis-2 -pentene) * (E)-1 -bromo-1 -chloropropene *
* * (Z)-3 -methyl-2 -pentene (3 -methyl-cis-2 -pentene) * (E)-1 -bromo-1 -chloropropene *
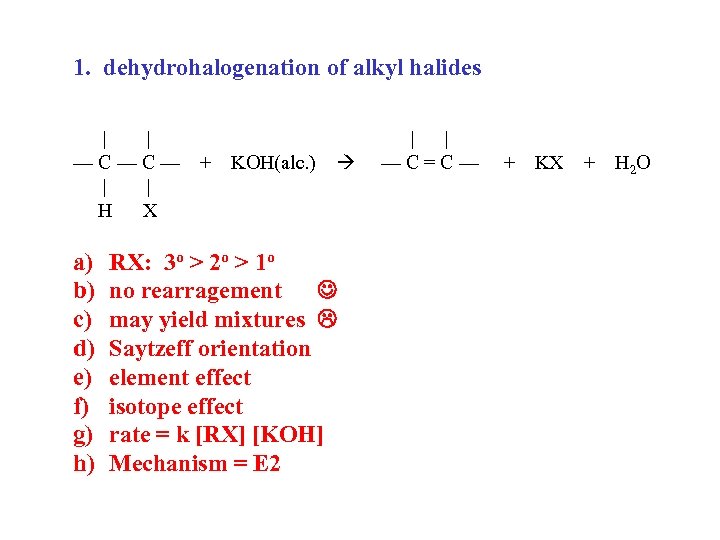 1. dehydrohalogenation of alkyl halides | | —C—C— | | H X a) b) c) d) e) f) g) h) + KOH(alc. ) RX: 3 o > 2 o > 1 o no rearragement may yield mixtures Saytzeff orientation element effect isotope effect rate = k [RX] [KOH] Mechanism = E 2 | | —C=C— + KX + H 2 O
1. dehydrohalogenation of alkyl halides | | —C—C— | | H X a) b) c) d) e) f) g) h) + KOH(alc. ) RX: 3 o > 2 o > 1 o no rearragement may yield mixtures Saytzeff orientation element effect isotope effect rate = k [RX] [KOH] Mechanism = E 2 | | —C=C— + KX + H 2 O
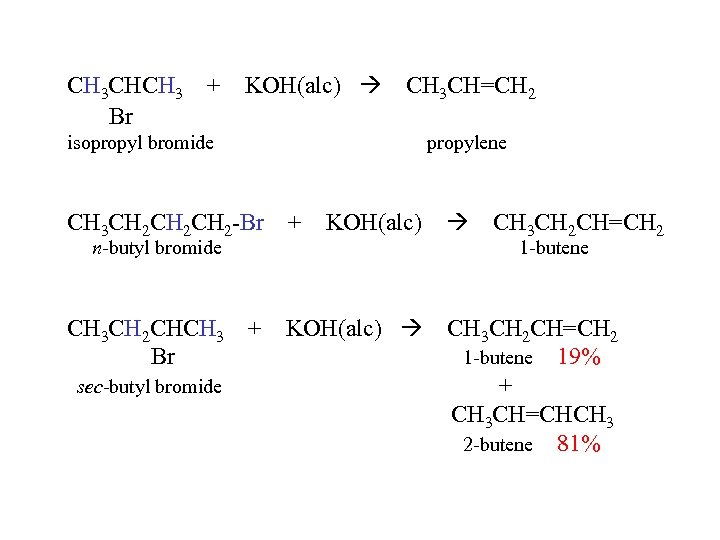 CH 3 CHCH 3 + Br KOH(alc) CH 3 CH=CH 2 isopropyl bromide propylene CH 3 CH 2 CH 2 -Br + KOH(alc) CH 3 CH 2 CHCH 3 + Br KOH(alc) n-butyl bromide sec-butyl bromide CH 3 CH 2 CH=CH 2 1 -butene 19% + CH 3 CH=CHCH 3 2 -butene 81%
CH 3 CHCH 3 + Br KOH(alc) CH 3 CH=CH 2 isopropyl bromide propylene CH 3 CH 2 CH 2 -Br + KOH(alc) CH 3 CH 2 CHCH 3 + Br KOH(alc) n-butyl bromide sec-butyl bromide CH 3 CH 2 CH=CH 2 1 -butene 19% + CH 3 CH=CHCH 3 2 -butene 81%
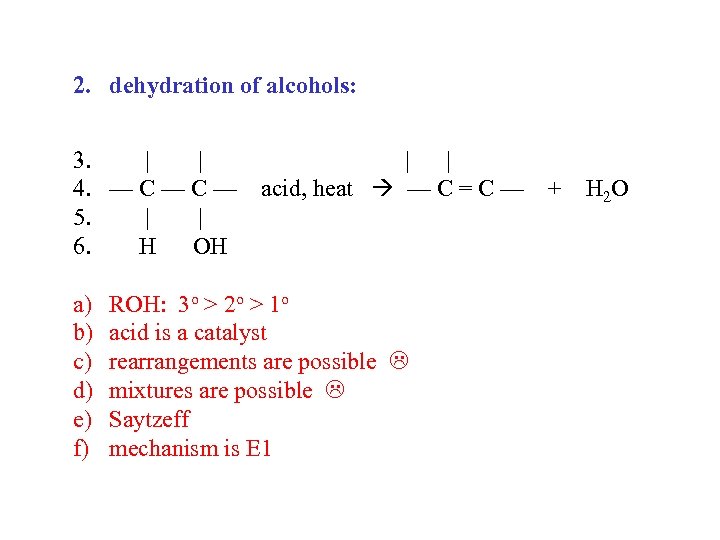 2. dehydration of alcohols: 3. | | 4. — C — acid, heat — C = C — + H 2 O 5. | | 6. H OH a) b) c) d) e) f) ROH: 3 o > 2 o > 1 o acid is a catalyst rearrangements are possible mixtures are possible Saytzeff mechanism is E 1
2. dehydration of alcohols: 3. | | 4. — C — acid, heat — C = C — + H 2 O 5. | | 6. H OH a) b) c) d) e) f) ROH: 3 o > 2 o > 1 o acid is a catalyst rearrangements are possible mixtures are possible Saytzeff mechanism is E 1
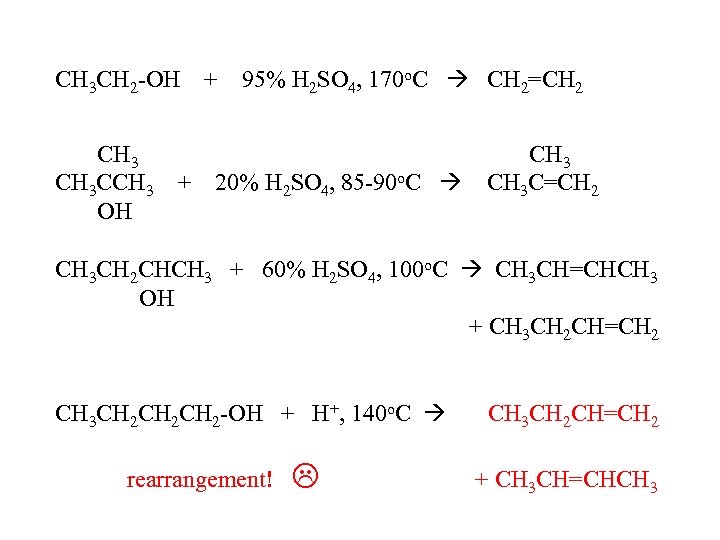 CH 3 CH 2 -OH CH 3 CCH 3 + OH + 95% H 2 SO 4, 170 o. C CH 2=CH 2 20% H 2 SO 4, 85 -90 o. C CH 3 C=CH 2 CH 3 CH 2 CHCH 3 + 60% H 2 SO 4, 100 o. C CH 3 CH=CHCH 3 OH + CH 3 CH 2 CH=CH 2 CH 3 CH 2 CH 2 -OH + H+, 140 o. C rearrangement! CH 3 CH 2 CH=CH 2 + CH 3 CH=CHCH 3
CH 3 CH 2 -OH CH 3 CCH 3 + OH + 95% H 2 SO 4, 170 o. C CH 2=CH 2 20% H 2 SO 4, 85 -90 o. C CH 3 C=CH 2 CH 3 CH 2 CHCH 3 + 60% H 2 SO 4, 100 o. C CH 3 CH=CHCH 3 OH + CH 3 CH 2 CH=CH 2 CH 3 CH 2 CH 2 -OH + H+, 140 o. C rearrangement! CH 3 CH 2 CH=CH 2 + CH 3 CH=CHCH 3
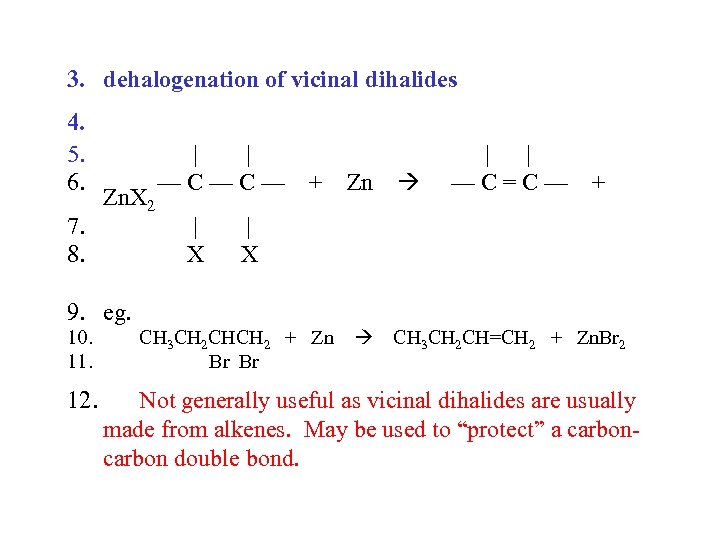 3. dehalogenation of vicinal dihalides 4. 5. 6. 7. 8. Zn. X 2 | | —C—C— | X + Zn | | —C=C— + | X 9. eg. 10. 11. 12. CH 3 CH 2 CHCH 2 + Zn Br Br CH 3 CH 2 CH=CH 2 + Zn. Br 2 Not generally useful as vicinal dihalides are usually made from alkenes. May be used to “protect” a carbon double bond.
3. dehalogenation of vicinal dihalides 4. 5. 6. 7. 8. Zn. X 2 | | —C—C— | X + Zn | | —C=C— + | X 9. eg. 10. 11. 12. CH 3 CH 2 CHCH 2 + Zn Br Br CH 3 CH 2 CH=CH 2 + Zn. Br 2 Not generally useful as vicinal dihalides are usually made from alkenes. May be used to “protect” a carbon double bond.
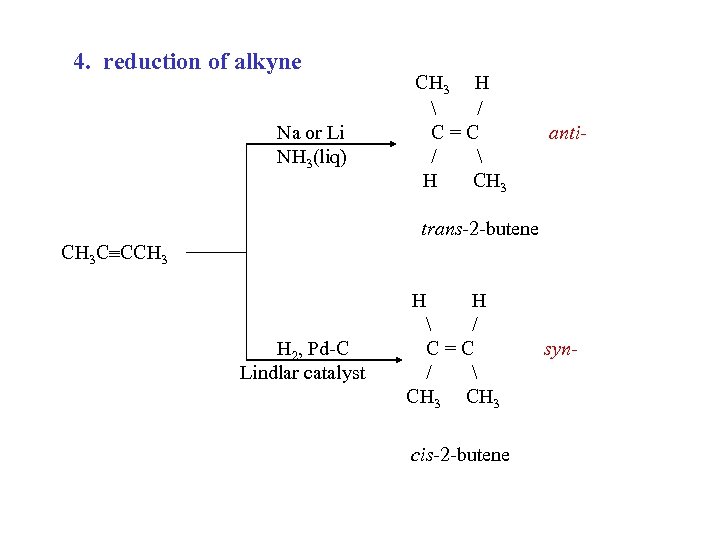 4. reduction of alkyne Na or Li NH 3(liq) CH 3 H / C=C / H CH 3 anti- trans-2 -butene CH 3 C CCH 3 H 2, Pd-C Lindlar catalyst H H / C=C / CH 3 cis-2 -butene syn-
4. reduction of alkyne Na or Li NH 3(liq) CH 3 H / C=C / H CH 3 anti- trans-2 -butene CH 3 C CCH 3 H 2, Pd-C Lindlar catalyst H H / C=C / CH 3 cis-2 -butene syn-
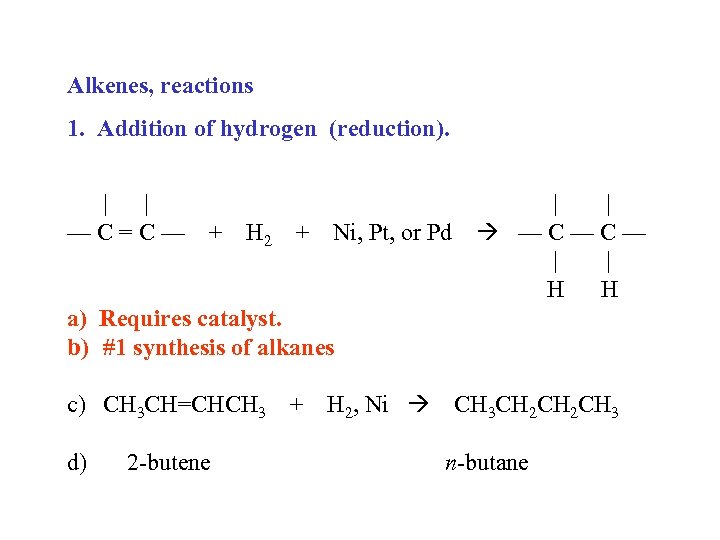 Alkenes, reactions 1. Addition of hydrogen (reduction). | | —C=C— + H 2 + Ni, Pt, or Pd | | —C—C— | | H H a) Requires catalyst. b) #1 synthesis of alkanes c) CH 3 CH=CHCH 3 + H 2, Ni CH 3 CH 2 CH 3 d) 2 -butene n-butane
Alkenes, reactions 1. Addition of hydrogen (reduction). | | —C=C— + H 2 + Ni, Pt, or Pd | | —C—C— | | H H a) Requires catalyst. b) #1 synthesis of alkanes c) CH 3 CH=CHCH 3 + H 2, Ni CH 3 CH 2 CH 3 d) 2 -butene n-butane
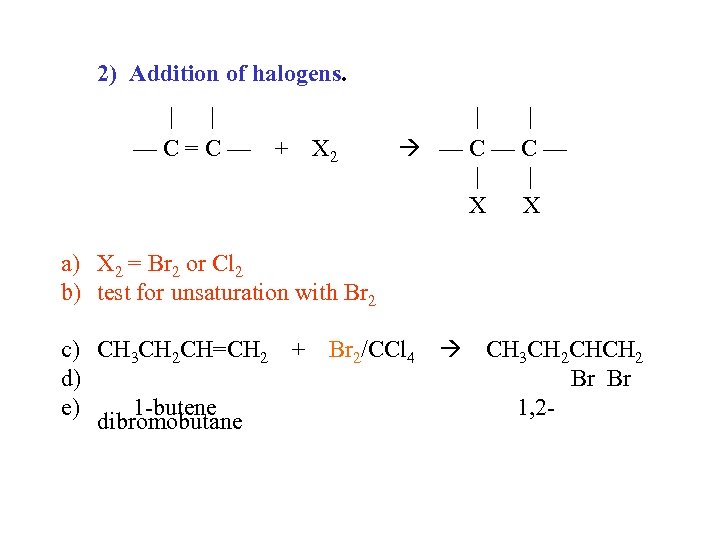 2) Addition of halogens. | | —C=C— + X 2 | | —C—C— | | X X a) X 2 = Br 2 or Cl 2 b) test for unsaturation with Br 2 c) CH 3 CH 2 CH=CH 2 + Br 2/CCl 4 CH 3 CH 2 CHCH 2 d) Br Br e) 1 -butene 1, 2 dibromobutane
2) Addition of halogens. | | —C=C— + X 2 | | —C—C— | | X X a) X 2 = Br 2 or Cl 2 b) test for unsaturation with Br 2 c) CH 3 CH 2 CH=CH 2 + Br 2/CCl 4 CH 3 CH 2 CHCH 2 d) Br Br e) 1 -butene 1, 2 dibromobutane
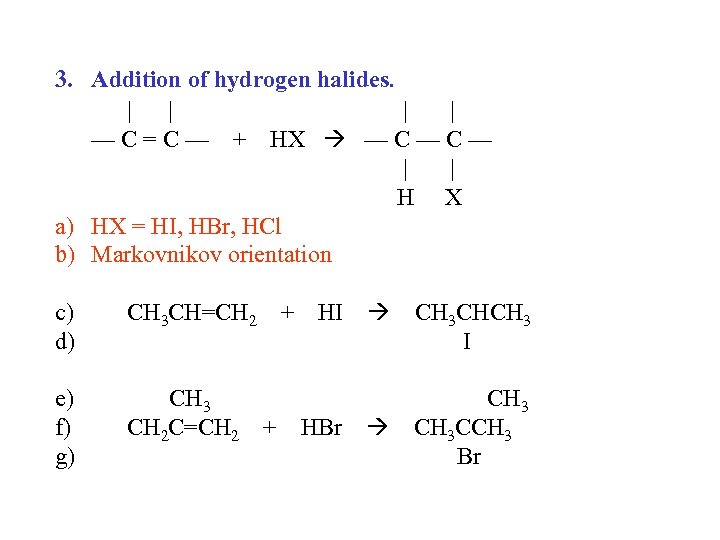 3. Addition of hydrogen halides. | | — C = C — + HX — C — | | H X a) HX = HI, HBr, HCl b) Markovnikov orientation c) d) CH 3 CH=CH 2 + e) f) g) CH 3 CH 2 C=CH 2 + HI HBr CH 3 CHCH 3 I CH 3 CCH 3 Br
3. Addition of hydrogen halides. | | — C = C — + HX — C — | | H X a) HX = HI, HBr, HCl b) Markovnikov orientation c) d) CH 3 CH=CH 2 + e) f) g) CH 3 CH 2 C=CH 2 + HI HBr CH 3 CHCH 3 I CH 3 CCH 3 Br
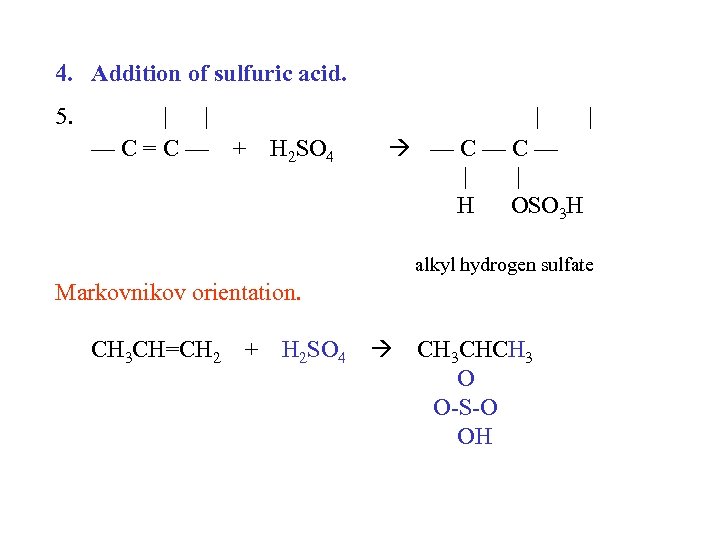 4. Addition of sulfuric acid. 5. | | —C=C— + H 2 SO 4 | | —C—C— | | H OSO 3 H alkyl hydrogen sulfate Markovnikov orientation. CH 3 CH=CH 2 + H 2 SO 4 CH 3 CHCH 3 O O-S-O OH
4. Addition of sulfuric acid. 5. | | —C=C— + H 2 SO 4 | | —C—C— | | H OSO 3 H alkyl hydrogen sulfate Markovnikov orientation. CH 3 CH=CH 2 + H 2 SO 4 CH 3 CHCH 3 O O-S-O OH
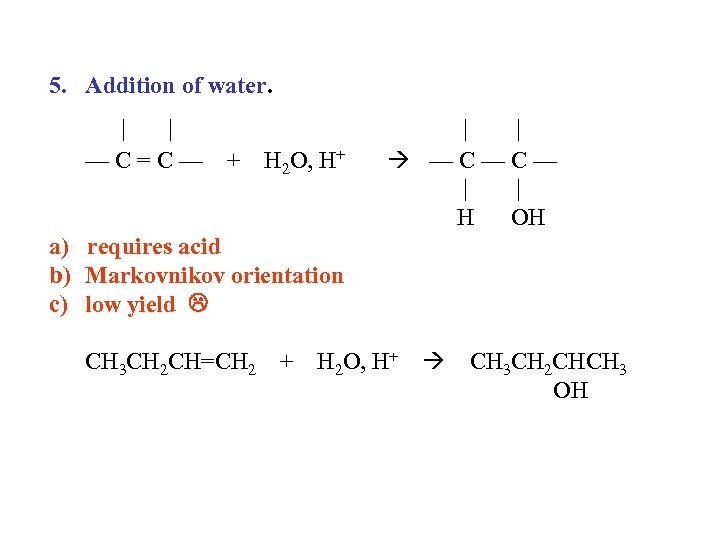 5. Addition of water. | | —C=C— + H 2 O, H+ | | —C—C— | | H OH a) requires acid b) Markovnikov orientation c) low yield CH 3 CH 2 CH=CH 2 + H 2 O, H+ CH 3 CH 2 CHCH 3 OH
5. Addition of water. | | —C=C— + H 2 O, H+ | | —C—C— | | H OH a) requires acid b) Markovnikov orientation c) low yield CH 3 CH 2 CH=CH 2 + H 2 O, H+ CH 3 CH 2 CHCH 3 OH
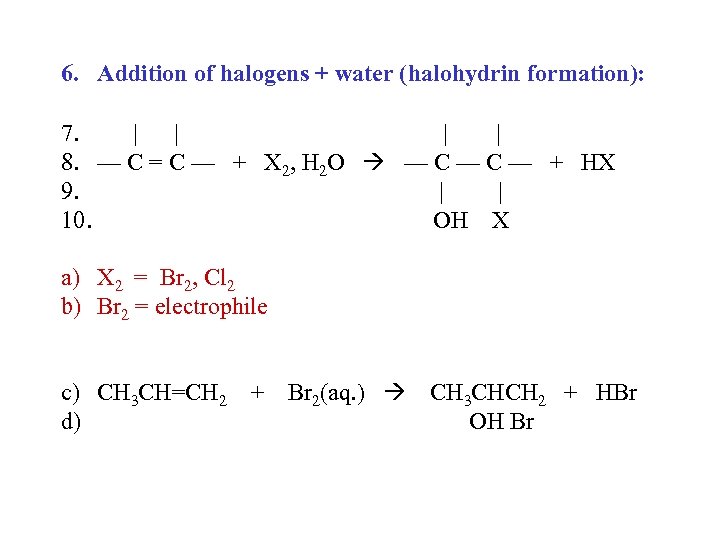 6. Addition of halogens + water (halohydrin formation): 7. | | 8. — C = C — + X 2, H 2 O — C — + HX 9. | | 10. OH X a) X 2 = Br 2, Cl 2 b) Br 2 = electrophile c) CH 3 CH=CH 2 + Br 2(aq. ) CH 3 CHCH 2 + HBr d) OH Br
6. Addition of halogens + water (halohydrin formation): 7. | | 8. — C = C — + X 2, H 2 O — C — + HX 9. | | 10. OH X a) X 2 = Br 2, Cl 2 b) Br 2 = electrophile c) CH 3 CH=CH 2 + Br 2(aq. ) CH 3 CHCH 2 + HBr d) OH Br
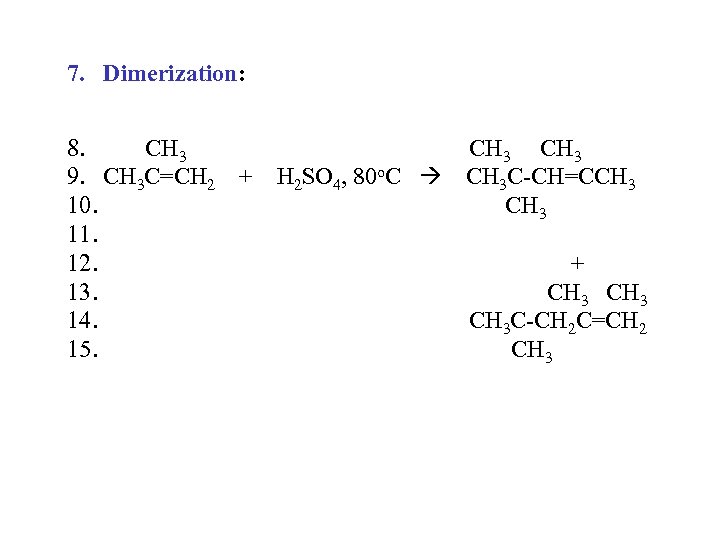 7. Dimerization: 8. CH 3 9. CH 3 C=CH 2 + H 2 SO 4, 80 o. C CH 3 C-CH=CCH 3 10. CH 3 11. 12. + 13. CH 3 14. CH 3 C-CH 2 C=CH 2 15. CH 3
7. Dimerization: 8. CH 3 9. CH 3 C=CH 2 + H 2 SO 4, 80 o. C CH 3 C-CH=CCH 3 10. CH 3 11. 12. + 13. CH 3 14. CH 3 C-CH 2 C=CH 2 15. CH 3
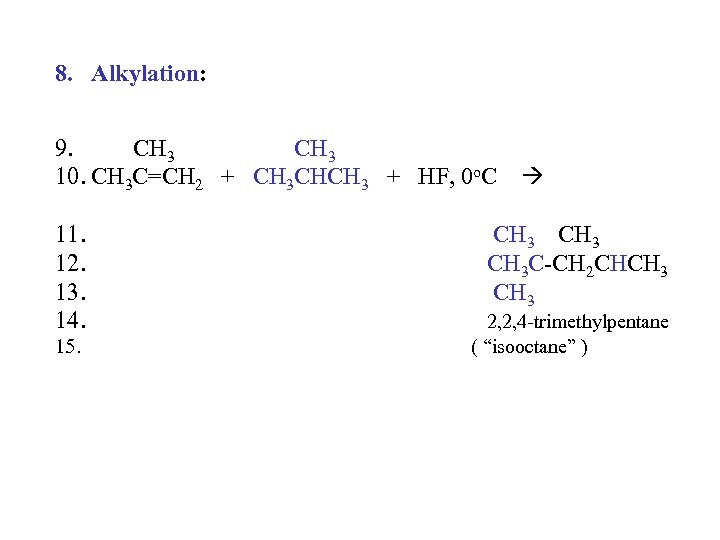 8. Alkylation: 9. CH 3 10. CH 3 C=CH 2 + CH 3 CHCH 3 + HF, 0 o. C 11. 12. 13. 14. 15. CH 3 C-CH 2 CHCH 3 2, 2, 4 -trimethylpentane ( “isooctane” )
8. Alkylation: 9. CH 3 10. CH 3 C=CH 2 + CH 3 CHCH 3 + HF, 0 o. C 11. 12. 13. 14. 15. CH 3 C-CH 2 CHCH 3 2, 2, 4 -trimethylpentane ( “isooctane” )
 9. oxymercuration-demercuration: 1. a) Markovnikov orientation. 2. b) 100% yields. 3. c) no rearrangements 4. CH 3 CH 2 CH=CH 2 + H 2 O, Hg(OAc)2; then Na. BH 4 5. 6. CH 3 CH 2 CHCH 3 OH
9. oxymercuration-demercuration: 1. a) Markovnikov orientation. 2. b) 100% yields. 3. c) no rearrangements 4. CH 3 CH 2 CH=CH 2 + H 2 O, Hg(OAc)2; then Na. BH 4 5. 6. CH 3 CH 2 CHCH 3 OH
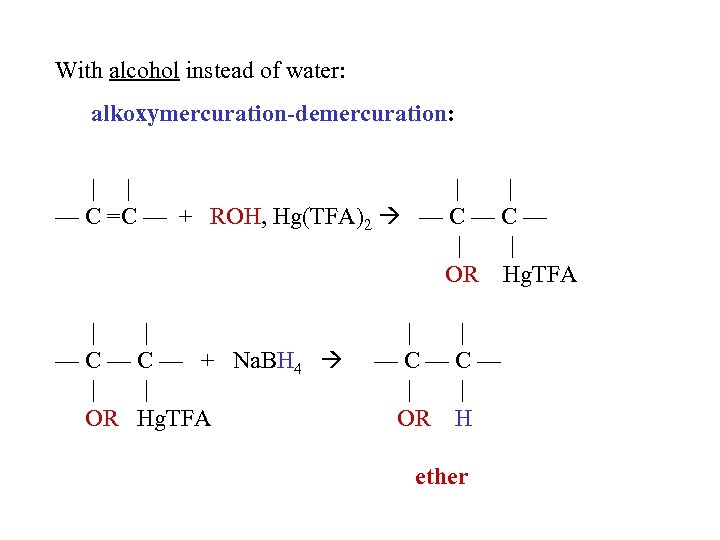 With alcohol instead of water: alkoxymercuration-demercuration: | | — C =C — + ROH, Hg(TFA)2 — C — | | OR Hg. TFA | | — C — + Na. BH 4 | | OR Hg. TFA | | —C—C— | | OR H ether
With alcohol instead of water: alkoxymercuration-demercuration: | | — C =C — + ROH, Hg(TFA)2 — C — | | OR Hg. TFA | | — C — + Na. BH 4 | | OR Hg. TFA | | —C—C— | | OR H ether
 10. hydroboration-oxidation: a) #2 synthesis of alcohols. b) Anti-Markovnikov orientation. c) 100% yields. d) no rearrangements e) CH 3 CH 2 CH=CH 2 + (BH 3)2; then H 2 O 2, Na. OH f) g) CH 3 CH 2 CH 2 -OH
10. hydroboration-oxidation: a) #2 synthesis of alcohols. b) Anti-Markovnikov orientation. c) 100% yields. d) no rearrangements e) CH 3 CH 2 CH=CH 2 + (BH 3)2; then H 2 O 2, Na. OH f) g) CH 3 CH 2 CH 2 -OH
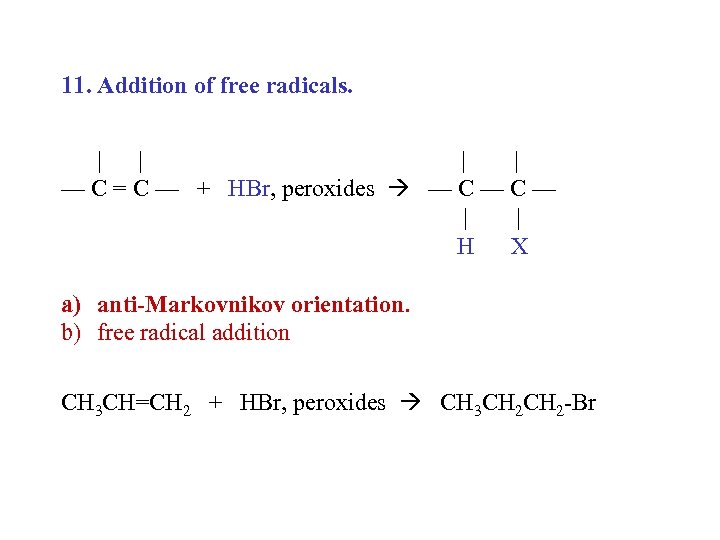 11. Addition of free radicals. | | — C = C — + HBr, peroxides — C — | | H X a) anti-Markovnikov orientation. b) free radical addition CH 3 CH=CH 2 + HBr, peroxides CH 3 CH 2 -Br
11. Addition of free radicals. | | — C = C — + HBr, peroxides — C — | | H X a) anti-Markovnikov orientation. b) free radical addition CH 3 CH=CH 2 + HBr, peroxides CH 3 CH 2 -Br
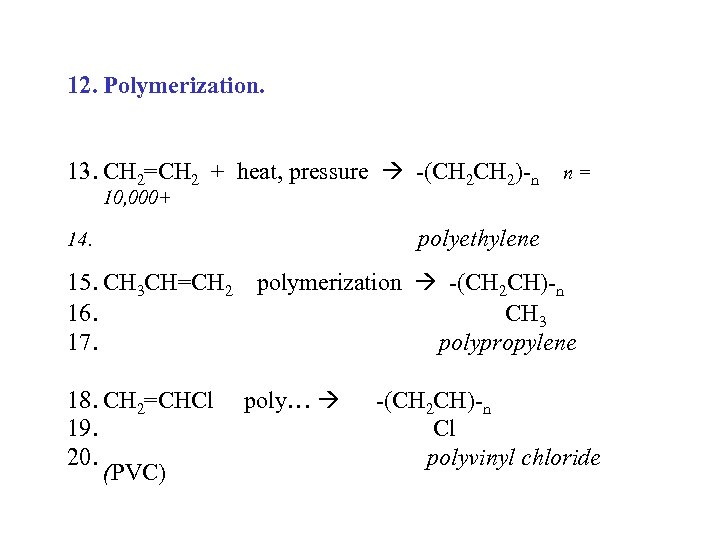 12. Polymerization. 13. CH 2=CH 2 + heat, pressure -(CH 2)-n n = 10, 000+ polyethylene 14. 15. CH 3 CH=CH 2 polymerization -(CH 2 CH)-n 16. CH 3 17. polypropylene 18. CH 2=CHCl 19. 20. (PVC) poly… -(CH 2 CH)-n Cl polyvinyl chloride
12. Polymerization. 13. CH 2=CH 2 + heat, pressure -(CH 2)-n n = 10, 000+ polyethylene 14. 15. CH 3 CH=CH 2 polymerization -(CH 2 CH)-n 16. CH 3 17. polypropylene 18. CH 2=CHCl 19. 20. (PVC) poly… -(CH 2 CH)-n Cl polyvinyl chloride
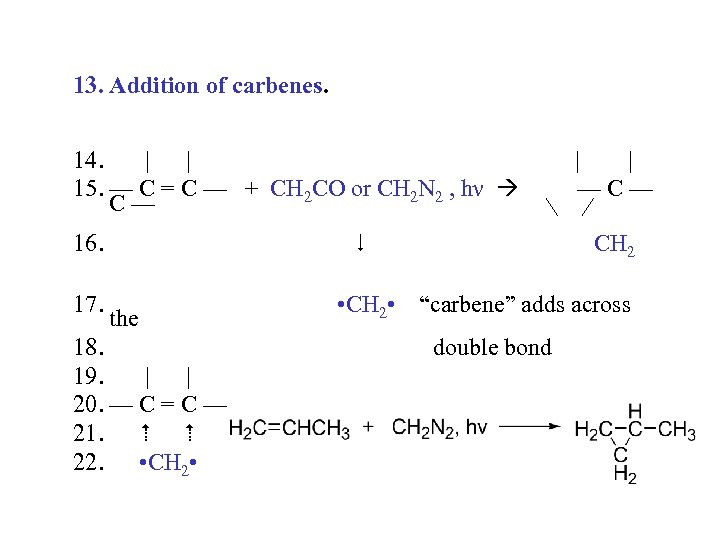 13. Addition of carbenes. 14. | | 15. — C = C — + CH 2 CO or CH 2 N 2 , hν C— 16. 17. • CH 2 • the 18. 19. | | 20. — C = C — 21. 22. • CH 2 • | | —C— CH 2 “carbene” adds across double bond
13. Addition of carbenes. 14. | | 15. — C = C — + CH 2 CO or CH 2 N 2 , hν C— 16. 17. • CH 2 • the 18. 19. | | 20. — C = C — 21. 22. • CH 2 • | | —C— CH 2 “carbene” adds across double bond
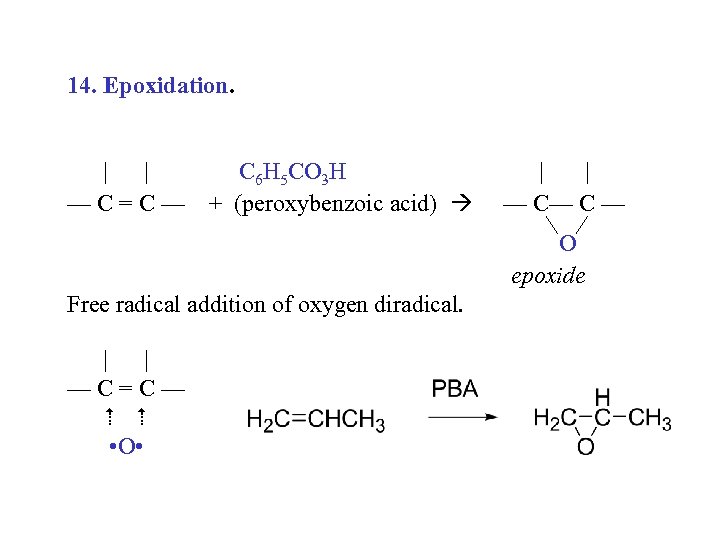 14. Epoxidation. | | —C=C— C 6 H 5 CO 3 H + (peroxybenzoic acid) | | — C— C — O epoxide Free radical addition of oxygen diradical. | | —C=C— • O •
14. Epoxidation. | | —C=C— C 6 H 5 CO 3 H + (peroxybenzoic acid) | | — C— C — O epoxide Free radical addition of oxygen diradical. | | —C=C— • O •
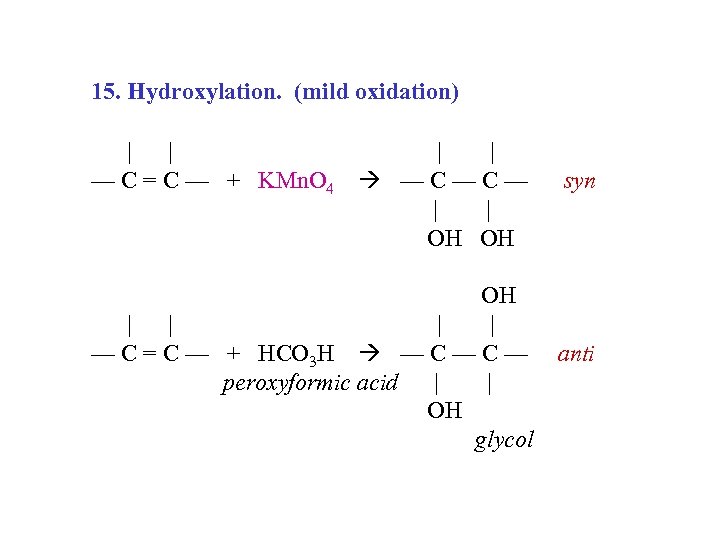 15. Hydroxylation. (mild oxidation) | | — C = C — + KMn. O 4 — C — | | OH OH syn OH | | — C = C — + HCO 3 H — C — anti peroxyformic acid | | OH glycol
15. Hydroxylation. (mild oxidation) | | — C = C — + KMn. O 4 — C — | | OH OH syn OH | | — C = C — + HCO 3 H — C — anti peroxyformic acid | | OH glycol
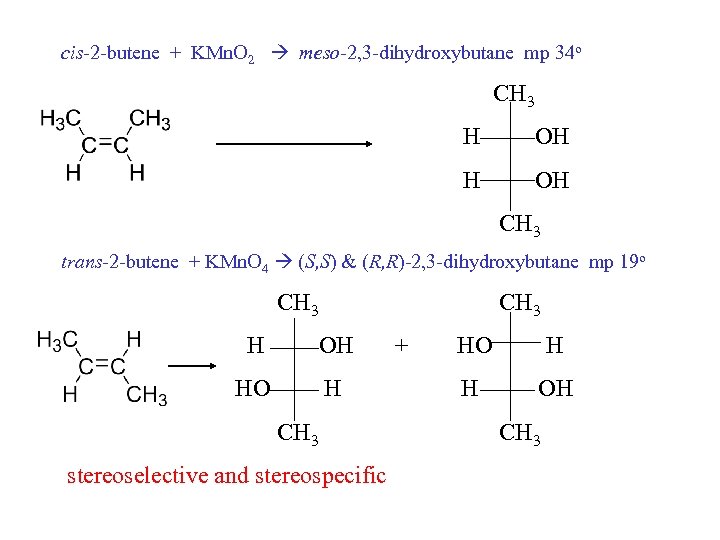 cis-2 -butene + KMn. O 2 meso-2, 3 -dihydroxybutane mp 34 o CH 3 H OH CH 3 trans-2 -butene + KMn. O 4 (S, S) & (R, R)-2, 3 -dihydroxybutane mp 19 o CH 3 H OH HO H CH 3 stereoselective and stereospecific + HO H H OH CH 3
cis-2 -butene + KMn. O 2 meso-2, 3 -dihydroxybutane mp 34 o CH 3 H OH CH 3 trans-2 -butene + KMn. O 4 (S, S) & (R, R)-2, 3 -dihydroxybutane mp 19 o CH 3 H OH HO H CH 3 stereoselective and stereospecific + HO H H OH CH 3
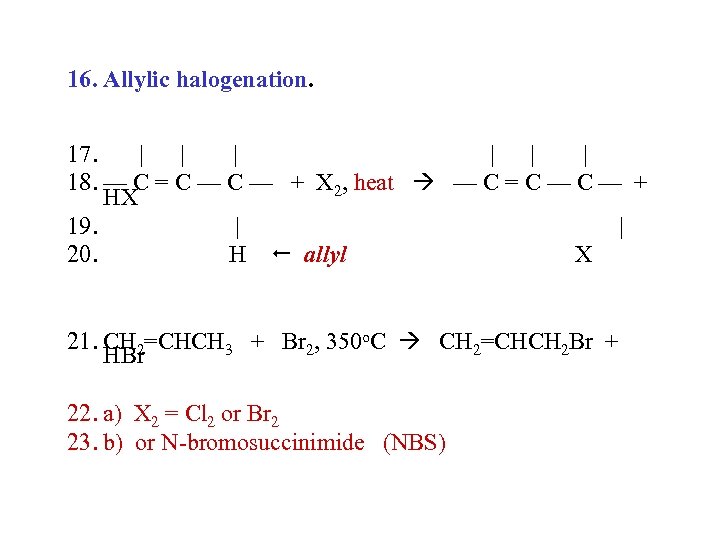 16. Allylic halogenation. 17. | | | 18. — C = C — + X 2, heat — C = C — + HX 19. | | 20. H allyl X 21. CH 2=CHCH 3 + Br 2, 350 o. C CH 2=CHCH 2 Br + HBr 22. a) X 2 = Cl 2 or Br 2 23. b) or N-bromosuccinimide (NBS)
16. Allylic halogenation. 17. | | | 18. — C = C — + X 2, heat — C = C — + HX 19. | | 20. H allyl X 21. CH 2=CHCH 3 + Br 2, 350 o. C CH 2=CHCH 2 Br + HBr 22. a) X 2 = Cl 2 or Br 2 23. b) or N-bromosuccinimide (NBS)
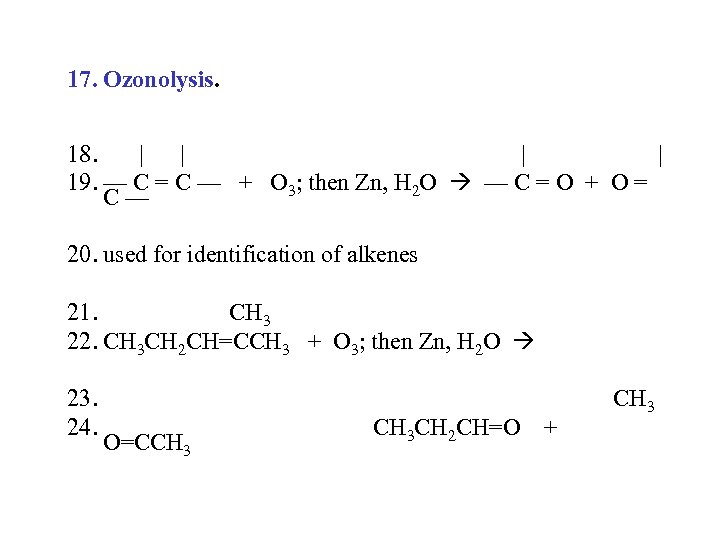 17. Ozonolysis. 18. | | 19. — C = C — + O 3; then Zn, H 2 O — C = O + O = C— 20. used for identification of alkenes 21. CH 3 22. CH 3 CH 2 CH=CCH 3 + O 3; then Zn, H 2 O 23. 24. O=CCH 3 CH 2 CH=O + CH 3
17. Ozonolysis. 18. | | 19. — C = C — + O 3; then Zn, H 2 O — C = O + O = C— 20. used for identification of alkenes 21. CH 3 22. CH 3 CH 2 CH=CCH 3 + O 3; then Zn, H 2 O 23. 24. O=CCH 3 CH 2 CH=O + CH 3
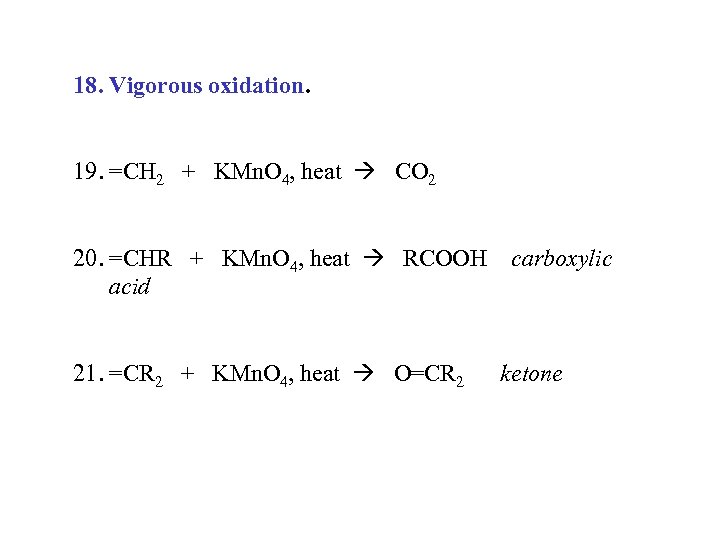 18. Vigorous oxidation. 19. =CH 2 + KMn. O 4, heat CO 2 20. =CHR + KMn. O 4, heat RCOOH carboxylic acid 21. =CR 2 + KMn. O 4, heat O=CR 2 ketone
18. Vigorous oxidation. 19. =CH 2 + KMn. O 4, heat CO 2 20. =CHR + KMn. O 4, heat RCOOH carboxylic acid 21. =CR 2 + KMn. O 4, heat O=CR 2 ketone
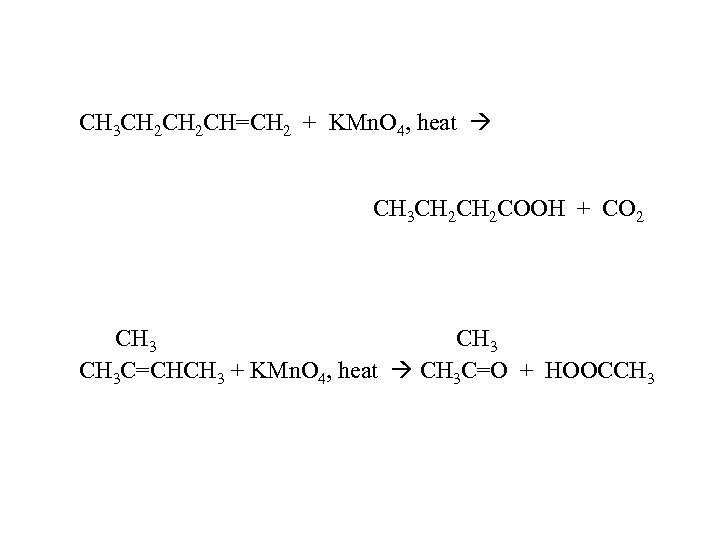 CH 3 CH 2 CH=CH 2 + KMn. O 4, heat CH 3 CH 2 COOH + CO 2 CH 3 C=CHCH 3 + KMn. O 4, heat CH 3 C=O + HOOCCH 3
CH 3 CH 2 CH=CH 2 + KMn. O 4, heat CH 3 CH 2 COOH + CO 2 CH 3 C=CHCH 3 + KMn. O 4, heat CH 3 C=O + HOOCCH 3
 Dienes nomenclature syntheses same as alkenes reactions same as alkenes special: conjugated dienes 1. more stable 2. preferred products of eliminations 3. give 1, 2 - & 1, 4 - addition products
Dienes nomenclature syntheses same as alkenes reactions same as alkenes special: conjugated dienes 1. more stable 2. preferred products of eliminations 3. give 1, 2 - & 1, 4 - addition products
 (cumulated dienes are not very stable and are rare) isolated dienes are as you would predict, undergo addition reactions with one or two moles… conjugated dienes are unusual in that they: 1) are more stable than predicted 2) are the preferred products of eliminations 3) give 1, 2 - plus 1, 4 -addition products
(cumulated dienes are not very stable and are rare) isolated dienes are as you would predict, undergo addition reactions with one or two moles… conjugated dienes are unusual in that they: 1) are more stable than predicted 2) are the preferred products of eliminations 3) give 1, 2 - plus 1, 4 -addition products
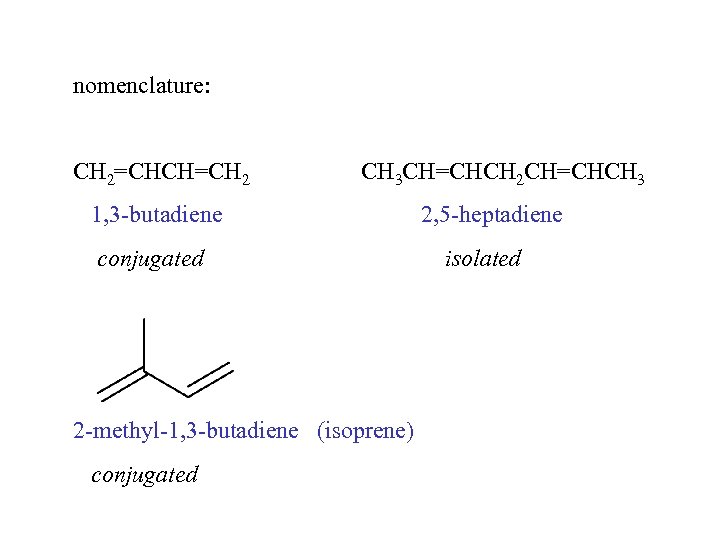 nomenclature: CH 2=CHCH=CH 2 CH 3 CH=CHCH 2 CH=CHCH 3 1, 3 -butadiene conjugated 2 -methyl-1, 3 -butadiene (isoprene) conjugated 2, 5 -heptadiene isolated
nomenclature: CH 2=CHCH=CH 2 CH 3 CH=CHCH 2 CH=CHCH 3 1, 3 -butadiene conjugated 2 -methyl-1, 3 -butadiene (isoprene) conjugated 2, 5 -heptadiene isolated
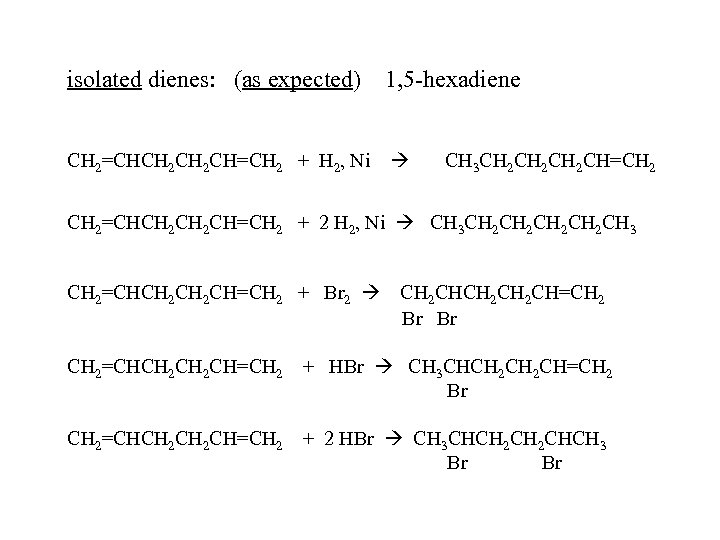 isolated dienes: (as expected) 1, 5 -hexadiene CH 2=CHCH 2 CH=CH 2 + H 2, Ni CH 3 CH 2 CH 2 CH=CH 2=CHCH 2 CH=CH 2 + 2 H 2, Ni CH 3 CH 2 CH 2 CH 3 CH 2=CHCH 2 CH=CH 2 + Br 2 CH 2 CH 2 CH=CH 2 Br Br CH 2=CHCH 2 CH=CH 2 + HBr CH 3 CHCH 2 CH=CH 2 Br CH 2=CHCH 2 CH=CH 2 + 2 HBr CH 3 CHCH 2 CHCH 3 Br Br
isolated dienes: (as expected) 1, 5 -hexadiene CH 2=CHCH 2 CH=CH 2 + H 2, Ni CH 3 CH 2 CH 2 CH=CH 2=CHCH 2 CH=CH 2 + 2 H 2, Ni CH 3 CH 2 CH 2 CH 3 CH 2=CHCH 2 CH=CH 2 + Br 2 CH 2 CH 2 CH=CH 2 Br Br CH 2=CHCH 2 CH=CH 2 + HBr CH 3 CHCH 2 CH=CH 2 Br CH 2=CHCH 2 CH=CH 2 + 2 HBr CH 3 CHCH 2 CHCH 3 Br Br
 conjugated dienes yield 1, 2 - plus 1, 4 -addition: CH 2=CHCH=CH 2 + H 2, Ni CH 3 CH 2 CH=CH 2 + CH 3 CH=CHCH 3 CH 2=CHCH=CH 2 + 2 H 2, Ni CH 3 CH 2 CH 3 CH 2=CHCH=CH 2 + Br 2 CH 2 CHCH=CH 2 + CH 2 CH=CHCH 2 Br Br CH 2=CHCH=CH 2 + HBr CH 3 CHCH=CH 2 + CH 3 CH=CHCH 2 Br Br peroxides CH 2=CHCH=CH 2 + HBr CH 2 CH=CHCH 3 + CH 2 CH=CH 2 Br Br
conjugated dienes yield 1, 2 - plus 1, 4 -addition: CH 2=CHCH=CH 2 + H 2, Ni CH 3 CH 2 CH=CH 2 + CH 3 CH=CHCH 3 CH 2=CHCH=CH 2 + 2 H 2, Ni CH 3 CH 2 CH 3 CH 2=CHCH=CH 2 + Br 2 CH 2 CHCH=CH 2 + CH 2 CH=CHCH 2 Br Br CH 2=CHCH=CH 2 + HBr CH 3 CHCH=CH 2 + CH 3 CH=CHCH 2 Br Br peroxides CH 2=CHCH=CH 2 + HBr CH 2 CH=CHCH 3 + CH 2 CH=CH 2 Br Br
 Alkynes nomenclature syntheses 1. dehydrohalogenation of vicinal dihalides 2. coupling of metal acetylides with alkyl halides reactions 1. reduction 2. addition of halogens 3. addition of hydrogen halides 4. addition of water 5. as acids 6. with Ag+ 7. oxidation
Alkynes nomenclature syntheses 1. dehydrohalogenation of vicinal dihalides 2. coupling of metal acetylides with alkyl halides reactions 1. reduction 2. addition of halogens 3. addition of hydrogen halides 4. addition of water 5. as acids 6. with Ag+ 7. oxidation
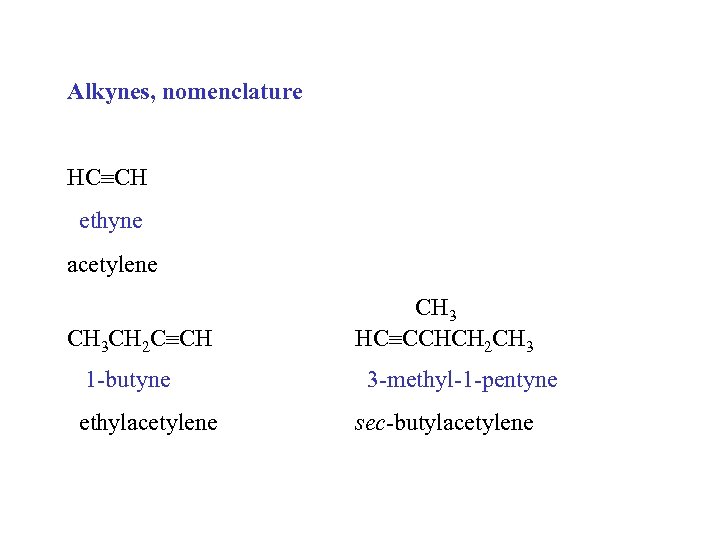 Alkynes, nomenclature HC CH ethyne acetylene CH 3 CH 2 C CH 1 -butyne ethylacetylene CH 3 HC CCHCH 2 CH 3 3 -methyl-1 -pentyne sec-butylacetylene
Alkynes, nomenclature HC CH ethyne acetylene CH 3 CH 2 C CH 1 -butyne ethylacetylene CH 3 HC CCHCH 2 CH 3 3 -methyl-1 -pentyne sec-butylacetylene
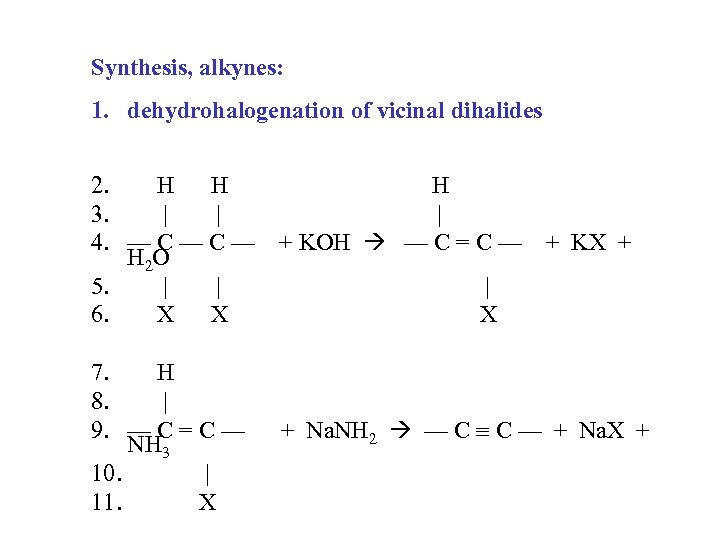 Synthesis, alkynes: 1. dehydrohalogenation of vicinal dihalides 2. H H H 3. | | | 4. — C — + KOH — C = C — + KX + H 2 O 5. | | | 6. X X X 7. H 8. | 9. — C = C — NH 3 10. | 11. X + Na. NH 2 — C C — + Na. X +
Synthesis, alkynes: 1. dehydrohalogenation of vicinal dihalides 2. H H H 3. | | | 4. — C — + KOH — C = C — + KX + H 2 O 5. | | | 6. X X X 7. H 8. | 9. — C = C — NH 3 10. | 11. X + Na. NH 2 — C C — + Na. X +
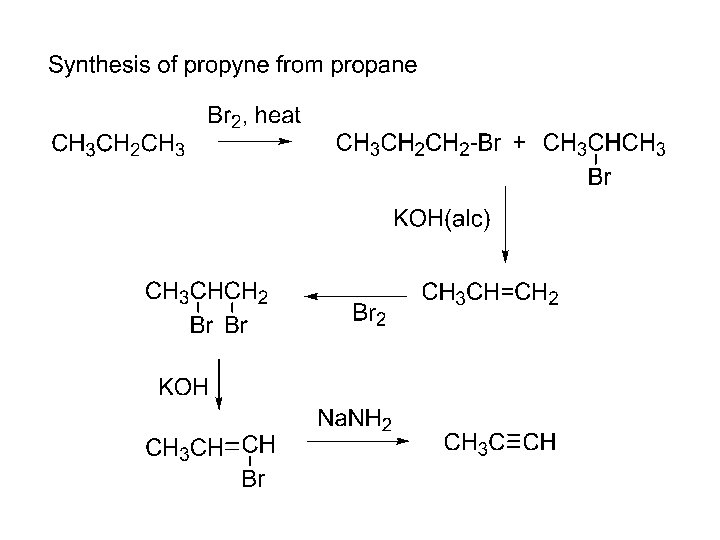
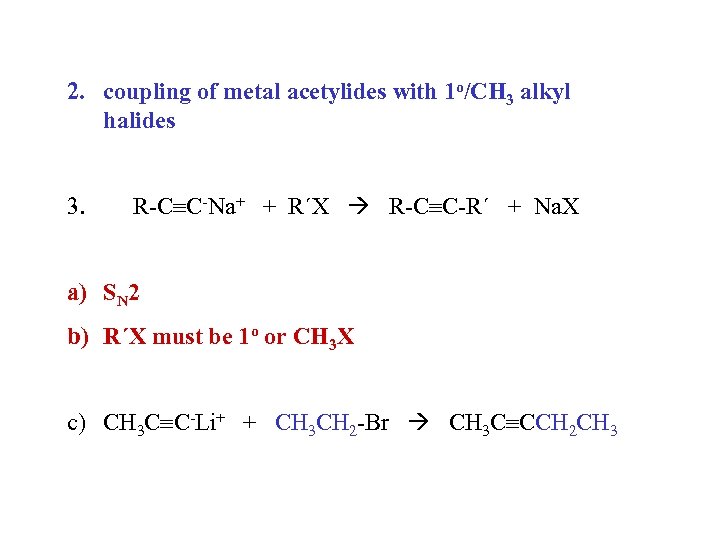 2. coupling of metal acetylides with 1 o/CH 3 alkyl halides 3. R-C C-Na+ + R´X R-C C-R´ + Na. X a) SN 2 b) R´X must be 1 o or CH 3 X c) CH 3 C C-Li+ + CH 3 CH 2 -Br CH 3 C CCH 2 CH 3
2. coupling of metal acetylides with 1 o/CH 3 alkyl halides 3. R-C C-Na+ + R´X R-C C-R´ + Na. X a) SN 2 b) R´X must be 1 o or CH 3 X c) CH 3 C C-Li+ + CH 3 CH 2 -Br CH 3 C CCH 2 CH 3
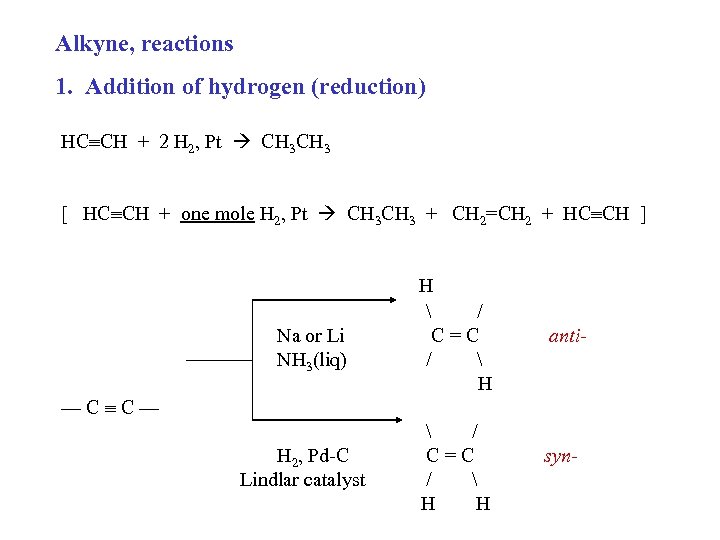 Alkyne, reactions 1. Addition of hydrogen (reduction) HC CH + 2 H 2, Pt CH 3 [ HC CH + one mole H 2, Pt CH 3 + CH 2=CH 2 + HC CH ] Na or Li NH 3(liq) —C C— H 2, Pd-C Lindlar catalyst H / C=C / H H anti- syn-
Alkyne, reactions 1. Addition of hydrogen (reduction) HC CH + 2 H 2, Pt CH 3 [ HC CH + one mole H 2, Pt CH 3 + CH 2=CH 2 + HC CH ] Na or Li NH 3(liq) —C C— H 2, Pd-C Lindlar catalyst H / C=C / H H anti- syn-
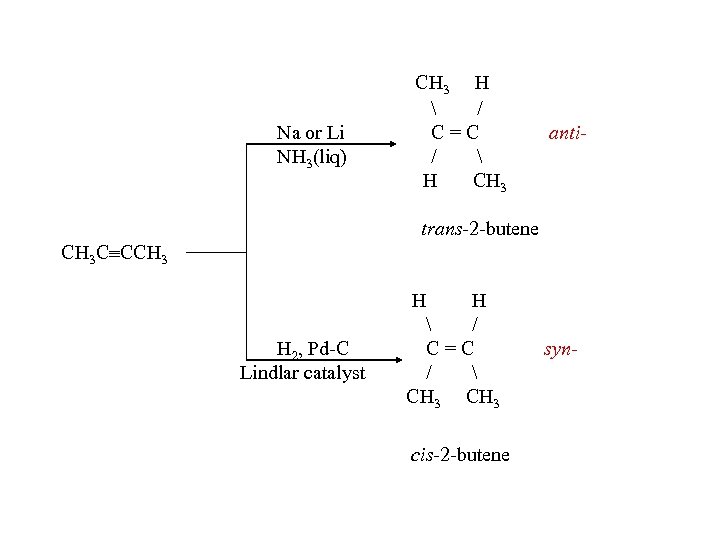 Na or Li NH 3(liq) CH 3 H / C=C / H CH 3 anti- trans-2 -butene CH 3 C CCH 3 H 2, Pd-C Lindlar catalyst H H / C=C / CH 3 cis-2 -butene syn-
Na or Li NH 3(liq) CH 3 H / C=C / H CH 3 anti- trans-2 -butene CH 3 C CCH 3 H 2, Pd-C Lindlar catalyst H H / C=C / CH 3 cis-2 -butene syn-
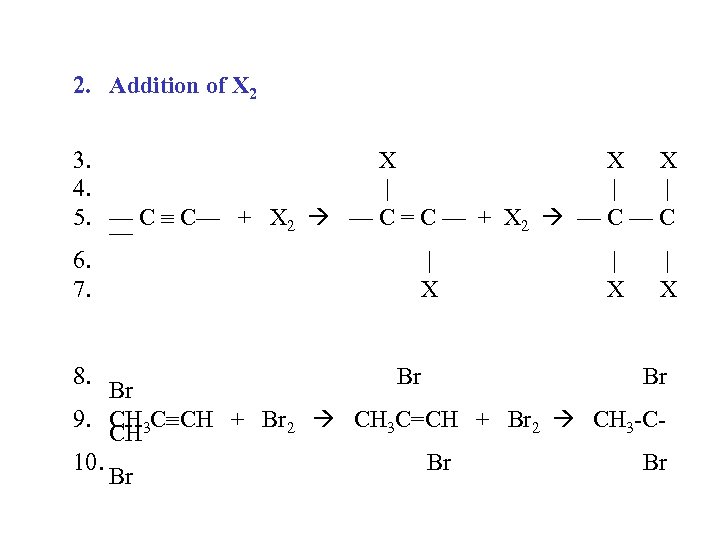 2. Addition of X 2 3. X X X 4. | | | 5. — C C— + X 2 — C = C — + X 2 — C — 6. | | | 7. X X X 8. Br Br Br 9. CH 3 C CH + Br 2 CH 3 C=CH + Br 2 CH 3 -CCH 10. Br Br Br
2. Addition of X 2 3. X X X 4. | | | 5. — C C— + X 2 — C = C — + X 2 — C — 6. | | | 7. X X X 8. Br Br Br 9. CH 3 C CH + Br 2 CH 3 C=CH + Br 2 CH 3 -CCH 10. Br Br Br
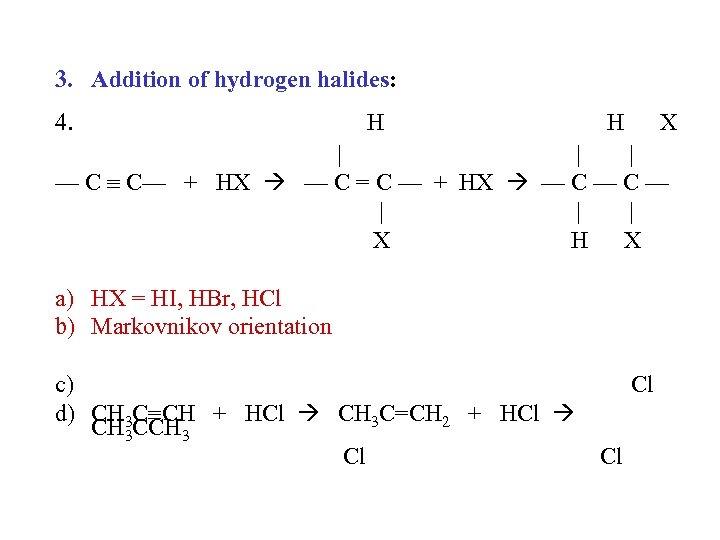 3. Addition of hydrogen halides: 4. H H X | | | — C C— + HX — C = C — + HX — C — | | | X H X a) HX = HI, HBr, HCl b) Markovnikov orientation c) d) CH 3 C CH + HCl CH 3 C=CH 2 + HCl CH 3 CCH 3 Cl Cl Cl
3. Addition of hydrogen halides: 4. H H X | | | — C C— + HX — C = C — + HX — C — | | | X H X a) HX = HI, HBr, HCl b) Markovnikov orientation c) d) CH 3 C CH + HCl CH 3 C=CH 2 + HCl CH 3 CCH 3 Cl Cl Cl
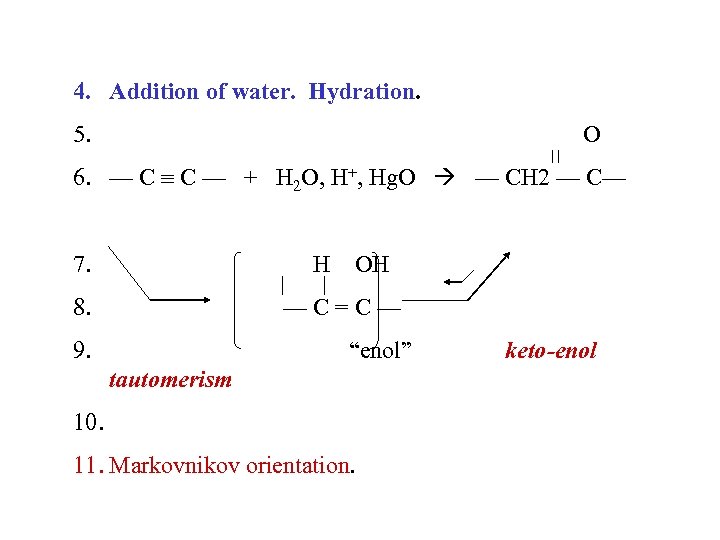 4. Addition of water. Hydration. 5. O 6. — C C — + H 2 O, H+, Hg. O — CH 2 — C— 7. H 8. OH —C=C— 9. “enol” tautomerism 10. 11. Markovnikov orientation. keto-enol
4. Addition of water. Hydration. 5. O 6. — C C — + H 2 O, H+, Hg. O — CH 2 — C— 7. H 8. OH —C=C— 9. “enol” tautomerism 10. 11. Markovnikov orientation. keto-enol
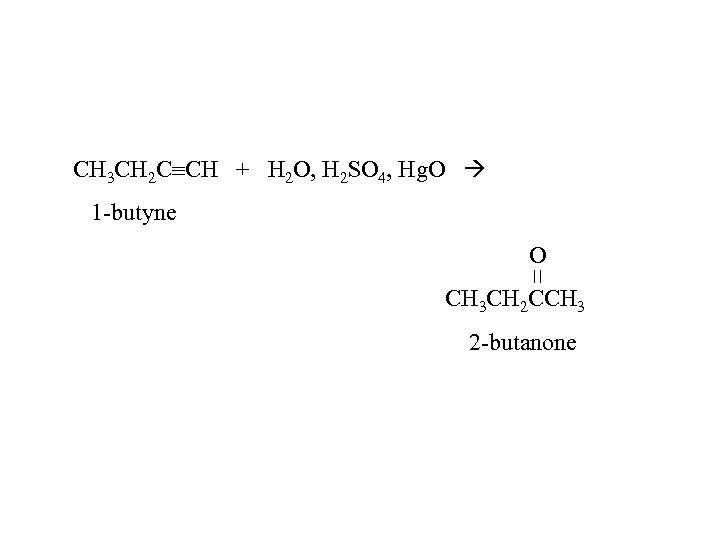 CH 3 CH 2 C CH + H 2 O, H 2 SO 4, Hg. O 1 -butyne O CH 3 CH 2 CCH 3 2 -butanone
CH 3 CH 2 C CH + H 2 O, H 2 SO 4, Hg. O 1 -butyne O CH 3 CH 2 CCH 3 2 -butanone
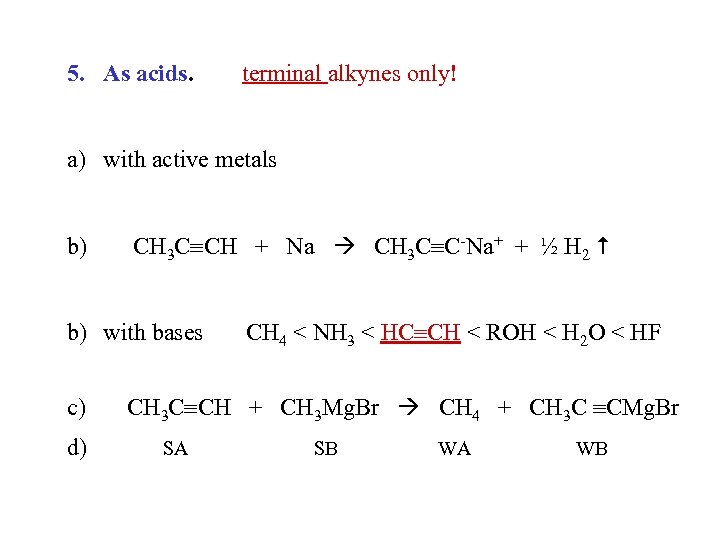 5. As acids. terminal alkynes only! a) with active metals b) CH 3 C CH + Na CH 3 C C-Na+ + ½ H 2 b) with bases c) d) CH 4 < NH 3 < HC CH < ROH < H 2 O < HF CH 3 C CH + CH 3 Mg. Br CH 4 + CH 3 C CMg. Br SA SB WA WB
5. As acids. terminal alkynes only! a) with active metals b) CH 3 C CH + Na CH 3 C C-Na+ + ½ H 2 b) with bases c) d) CH 4 < NH 3 < HC CH < ROH < H 2 O < HF CH 3 C CH + CH 3 Mg. Br CH 4 + CH 3 C CMg. Br SA SB WA WB
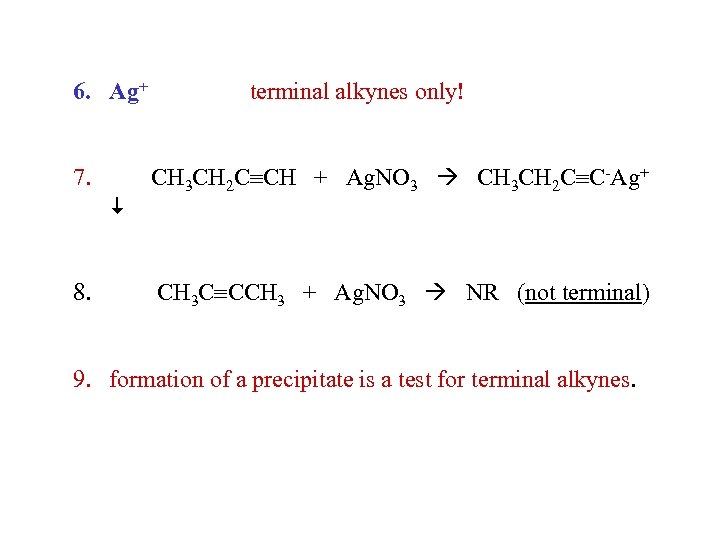 6. Ag+ 7. 8. terminal alkynes only! CH 3 CH 2 C CH + Ag. NO 3 CH 3 CH 2 C C-Ag+ CH 3 C CCH 3 + Ag. NO 3 NR (not terminal) 9. formation of a precipitate is a test for terminal alkynes.
6. Ag+ 7. 8. terminal alkynes only! CH 3 CH 2 C CH + Ag. NO 3 CH 3 CH 2 C C-Ag+ CH 3 C CCH 3 + Ag. NO 3 NR (not terminal) 9. formation of a precipitate is a test for terminal alkynes.
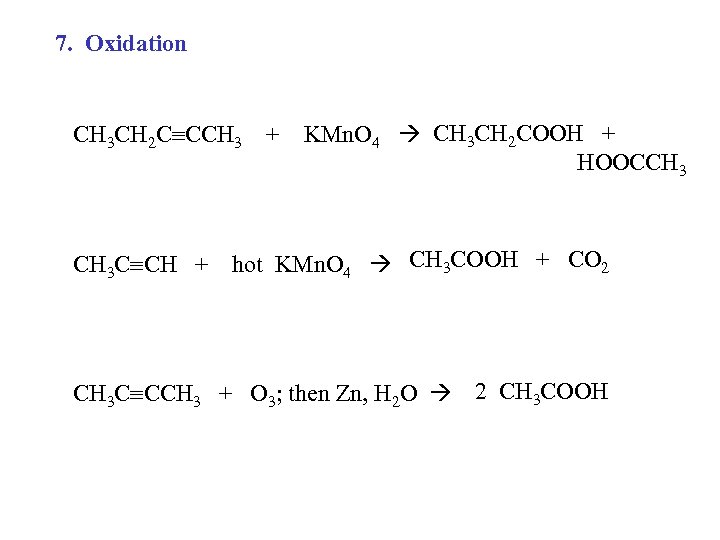 7. Oxidation CH 3 CH 2 C CCH 3 + CH 3 C CH + KMn. O 4 CH 3 CH 2 COOH + HOOCCH 3 hot KMn. O 4 CH 3 COOH + CO 2 CH 3 C CCH 3 + O 3; then Zn, H 2 O 2 CH 3 COOH
7. Oxidation CH 3 CH 2 C CCH 3 + CH 3 C CH + KMn. O 4 CH 3 CH 2 COOH + HOOCCH 3 hot KMn. O 4 CH 3 COOH + CO 2 CH 3 C CCH 3 + O 3; then Zn, H 2 O 2 CH 3 COOH
 Alicyclics nomenclature syntheses like alkanes, alkenes, alcohols, etc. reactions as expected exceptions: cyclopropane/cyclobutane
Alicyclics nomenclature syntheses like alkanes, alkenes, alcohols, etc. reactions as expected exceptions: cyclopropane/cyclobutane
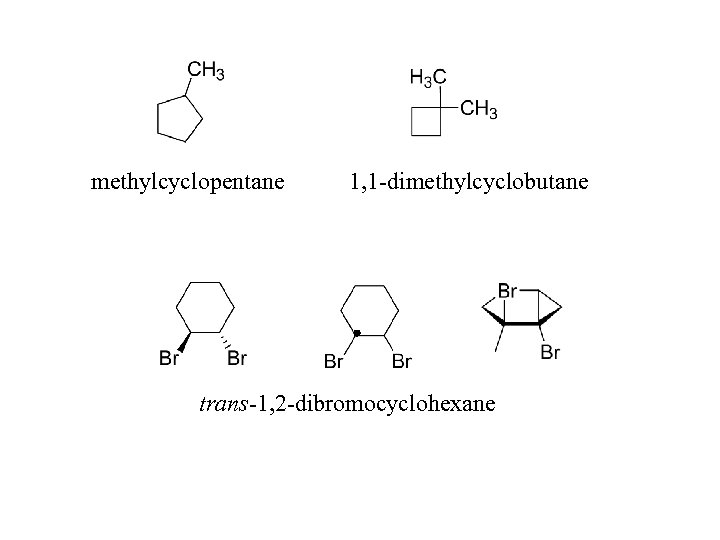 methylcyclopentane 1, 1 -dimethylcyclobutane trans-1, 2 -dibromocyclohexane
methylcyclopentane 1, 1 -dimethylcyclobutane trans-1, 2 -dibromocyclohexane
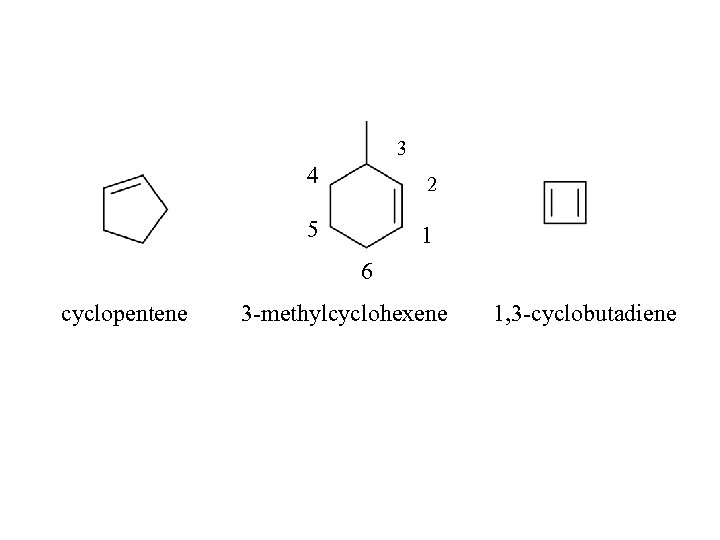 3 4 2 5 1 6 cyclopentene 3 -methylcyclohexene 1, 3 -cyclobutadiene
3 4 2 5 1 6 cyclopentene 3 -methylcyclohexene 1, 3 -cyclobutadiene
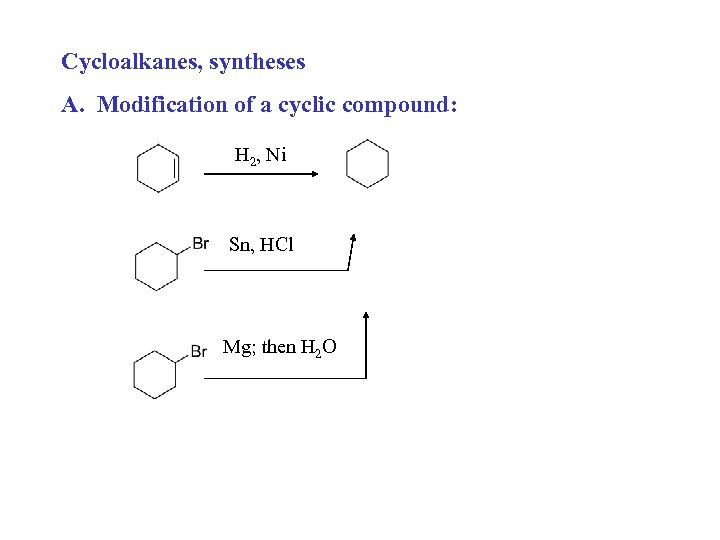 Cycloalkanes, syntheses A. Modification of a cyclic compound: H 2, Ni Sn, HCl Mg; then H 2 O
Cycloalkanes, syntheses A. Modification of a cyclic compound: H 2, Ni Sn, HCl Mg; then H 2 O
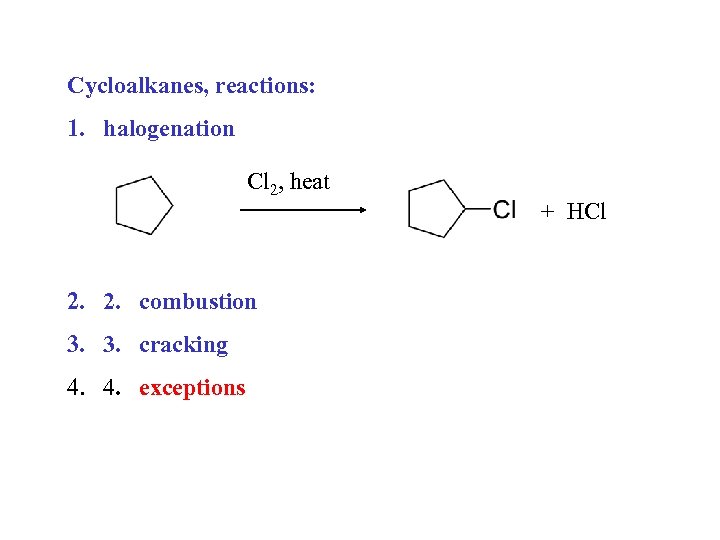 Cycloalkanes, reactions: 1. halogenation Cl 2, heat 2. 2. combustion 3. 3. cracking 4. 4. exceptions + HCl
Cycloalkanes, reactions: 1. halogenation Cl 2, heat 2. 2. combustion 3. 3. cracking 4. 4. exceptions + HCl
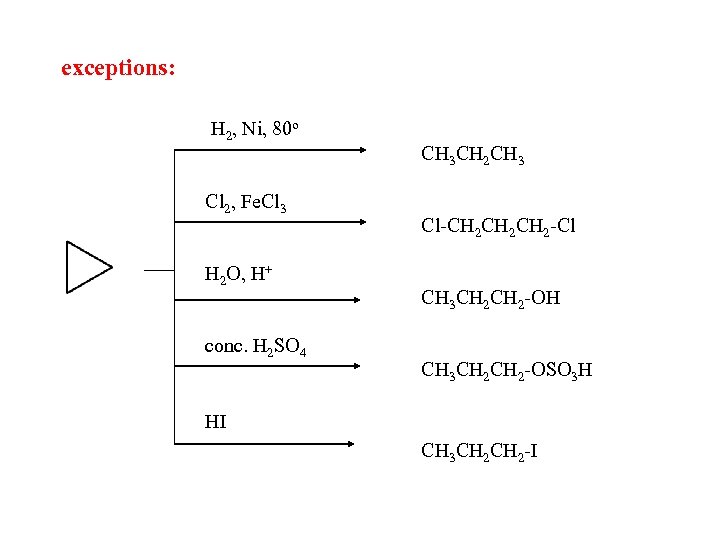 exceptions: H 2, Ni, 80 o Cl 2, Fe. Cl 3 H 2 O, H+ conc. H 2 SO 4 CH 3 CH 2 CH 3 Cl-CH 2 CH 2 -Cl CH 3 CH 2 -OH CH 3 CH 2 -OSO 3 H HI CH 3 CH 2 -I
exceptions: H 2, Ni, 80 o Cl 2, Fe. Cl 3 H 2 O, H+ conc. H 2 SO 4 CH 3 CH 2 CH 3 Cl-CH 2 CH 2 -Cl CH 3 CH 2 -OH CH 3 CH 2 -OSO 3 H HI CH 3 CH 2 -I
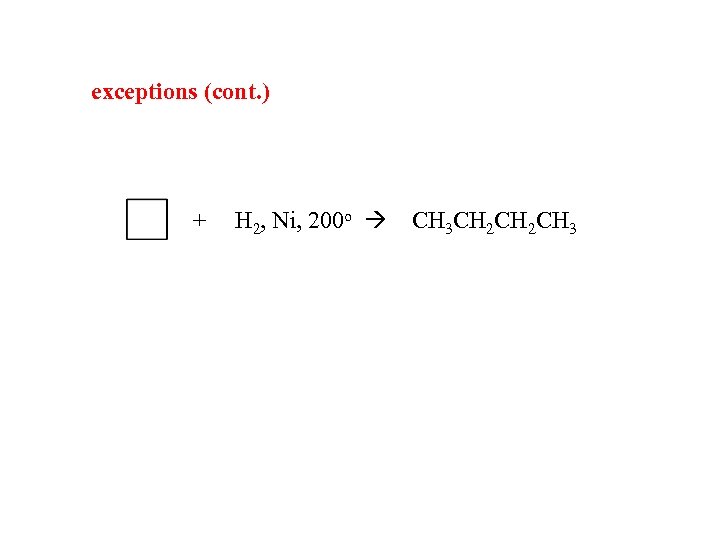 exceptions (cont. ) + H 2, Ni, 200 o CH 3 CH 2 CH 3
exceptions (cont. ) + H 2, Ni, 200 o CH 3 CH 2 CH 3
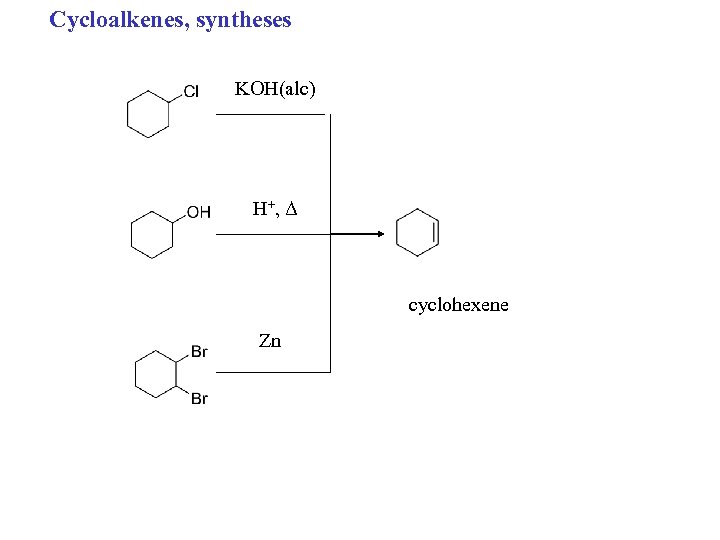 Cycloalkenes, syntheses KOH(alc) H+ , Δ cyclohexene Zn
Cycloalkenes, syntheses KOH(alc) H+ , Δ cyclohexene Zn
 Cycloalkenes, reactions: 1. addition of H 2 10. hydroboration-oxid. 2. addition of X 2 11. addition of free radicals 3. addition of HX 12. polymerization 4. addition of H 2 SO 4 13. addition of carbenes 5. addition of H 2 O, H+ 14. epoxidation 6. addition of X 2 + H 2 O 15. hydroxylation 7. dimerization 16. allylic halogenation 8. alkylation 17. ozonolysis 9. oxymerc-demerc. 18. vigorous oxidation
Cycloalkenes, reactions: 1. addition of H 2 10. hydroboration-oxid. 2. addition of X 2 11. addition of free radicals 3. addition of HX 12. polymerization 4. addition of H 2 SO 4 13. addition of carbenes 5. addition of H 2 O, H+ 14. epoxidation 6. addition of X 2 + H 2 O 15. hydroxylation 7. dimerization 16. allylic halogenation 8. alkylation 17. ozonolysis 9. oxymerc-demerc. 18. vigorous oxidation
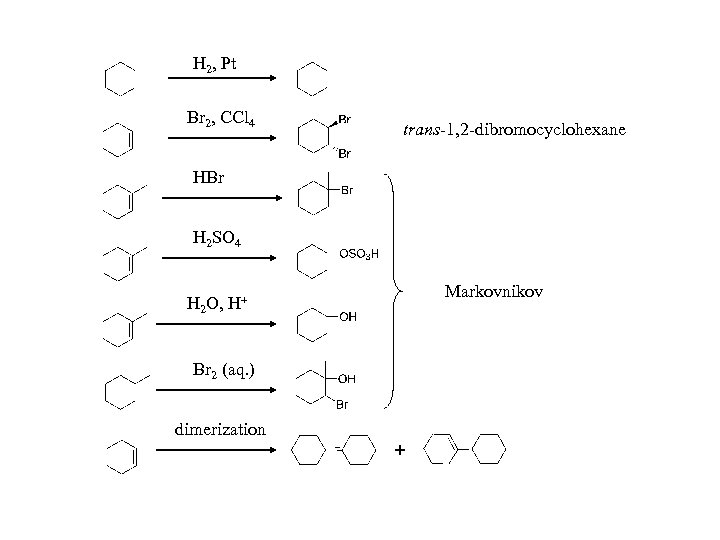 H 2, Pt Br 2, CCl 4 trans-1, 2 -dibromocyclohexane HBr H 2 SO 4 H 2 O, H+ Br 2 (aq. ) dimerization Markovnikov
H 2, Pt Br 2, CCl 4 trans-1, 2 -dibromocyclohexane HBr H 2 SO 4 H 2 O, H+ Br 2 (aq. ) dimerization Markovnikov
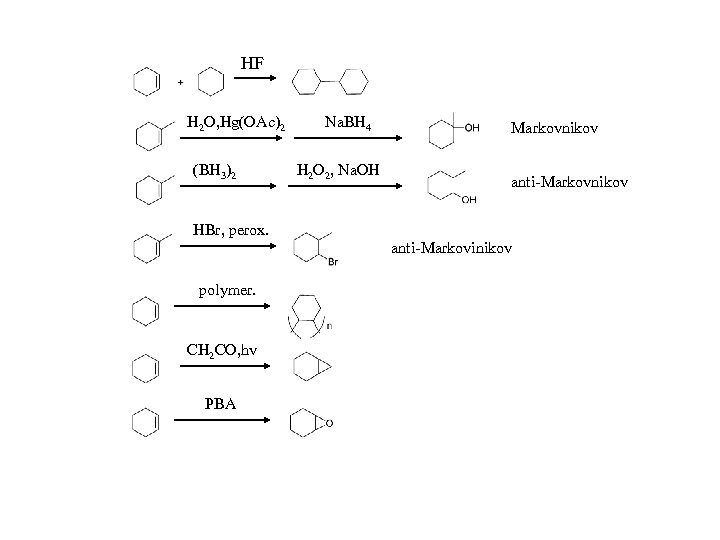 HF H 2 O, Hg(OAc)2 (BH 3)2 Na. BH 4 H 2 O 2, Na. OH Markovnikov anti-Markovnikov HBr, perox. anti-Markovinikov polymer. CH 2 CO, hv PBA
HF H 2 O, Hg(OAc)2 (BH 3)2 Na. BH 4 H 2 O 2, Na. OH Markovnikov anti-Markovnikov HBr, perox. anti-Markovinikov polymer. CH 2 CO, hv PBA
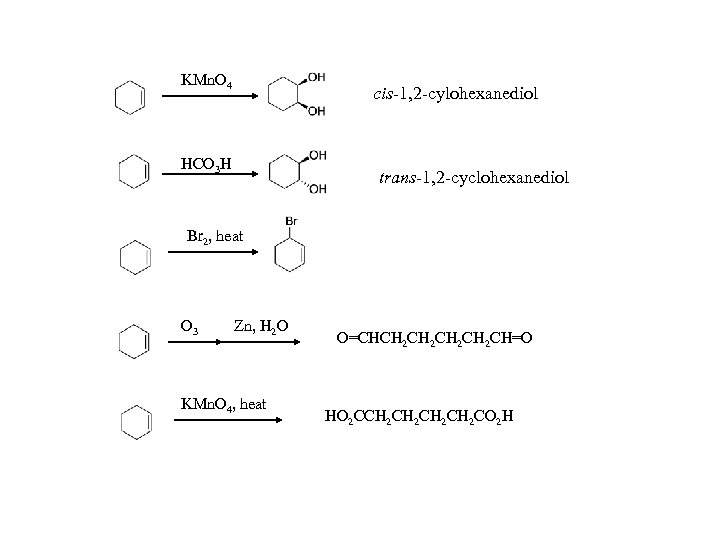 KMn. O 4 cis-1, 2 -cylohexanediol HCO 3 H trans-1, 2 -cyclohexanediol Br 2, heat O 3 Zn, H 2 O KMn. O 4, heat O=CHCH 2 CH 2 CH=O HO 2 CCH 2 CH 2 CO 2 H
KMn. O 4 cis-1, 2 -cylohexanediol HCO 3 H trans-1, 2 -cyclohexanediol Br 2, heat O 3 Zn, H 2 O KMn. O 4, heat O=CHCH 2 CH 2 CH=O HO 2 CCH 2 CH 2 CO 2 H
 Epoxides nomenclature syntheses 1. epoxidation of alkenes reactions 1. addition of acids 2. addition of bases
Epoxides nomenclature syntheses 1. epoxidation of alkenes reactions 1. addition of acids 2. addition of bases
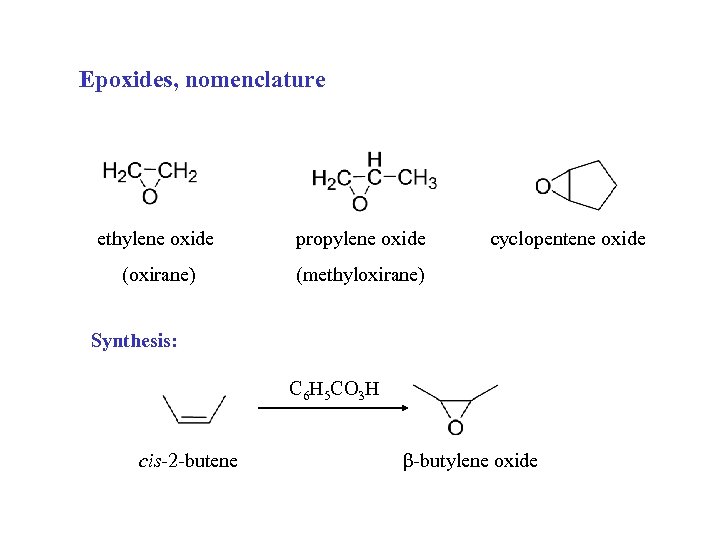 Epoxides, nomenclature ethylene oxide propylene oxide (oxirane) cyclopentene oxide (methyloxirane) Synthesis: C 6 H 5 CO 3 H cis-2 -butene β-butylene oxide
Epoxides, nomenclature ethylene oxide propylene oxide (oxirane) cyclopentene oxide (methyloxirane) Synthesis: C 6 H 5 CO 3 H cis-2 -butene β-butylene oxide
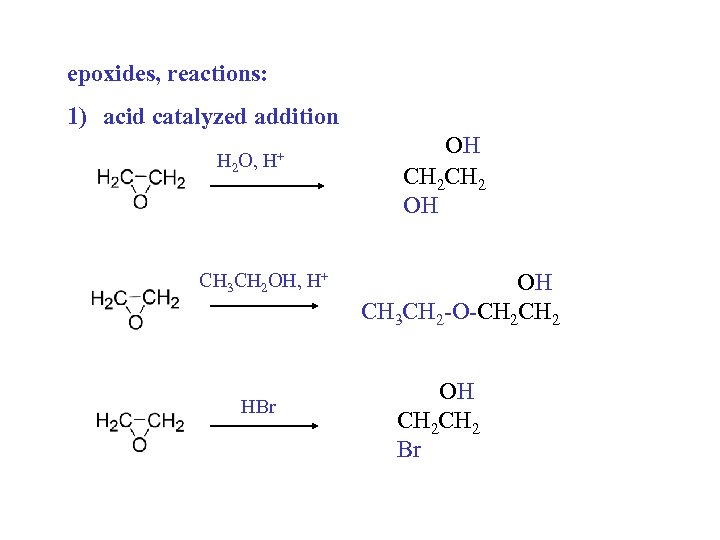 epoxides, reactions: 1) acid catalyzed addition H 2 O, H+ CH 3 CH 2 OH, H+ HBr OH CH 2 OH OH CH 3 CH 2 -O-CH 2 OH CH 2 Br
epoxides, reactions: 1) acid catalyzed addition H 2 O, H+ CH 3 CH 2 OH, H+ HBr OH CH 2 OH OH CH 3 CH 2 -O-CH 2 OH CH 2 Br
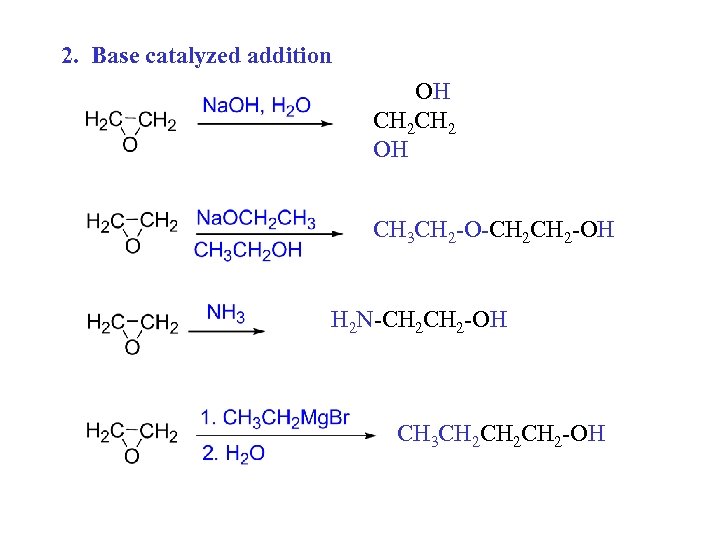 2. Base catalyzed addition OH CH 2 OH CH 3 CH 2 -O-CH 2 -OH H 2 N-CH 2 -OH CH 3 CH 2 CH 2 -OH
2. Base catalyzed addition OH CH 2 OH CH 3 CH 2 -O-CH 2 -OH H 2 N-CH 2 -OH CH 3 CH 2 CH 2 -OH
 Mechanisms: Free radical substitution SN 2 SN 1 E 2 E 1 ionic electrophilic addition free radical electrophilic addition Memorize (all steps, curved arrow formalism, RDS) and know which reactions go by these mechanisms!
Mechanisms: Free radical substitution SN 2 SN 1 E 2 E 1 ionic electrophilic addition free radical electrophilic addition Memorize (all steps, curved arrow formalism, RDS) and know which reactions go by these mechanisms!
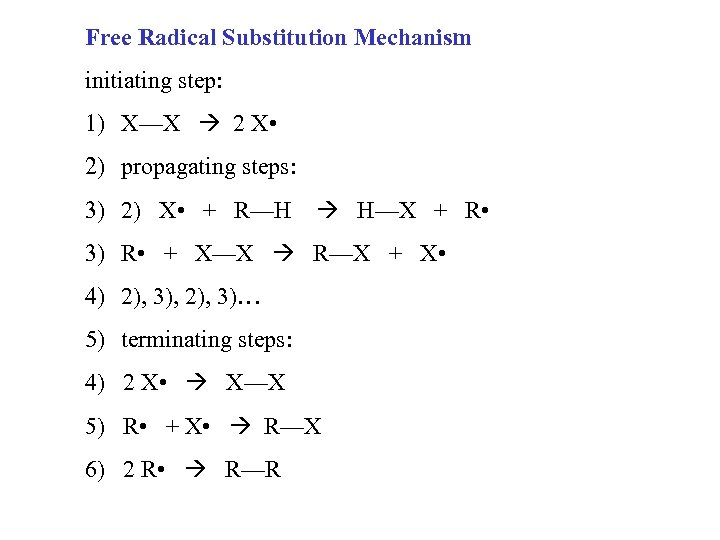 Free Radical Substitution Mechanism initiating step: 1) X—X 2 X • 2) propagating steps: 3) 2) X • + R—H H—X + R • 3) R • + X—X R—X + X • 4) 2), 3), 2), 3)… 5) terminating steps: 4) 2 X • X—X 5) R • + X • R—X 6) 2 R • R—R
Free Radical Substitution Mechanism initiating step: 1) X—X 2 X • 2) propagating steps: 3) 2) X • + R—H H—X + R • 3) R • + X—X R—X + X • 4) 2), 3), 2), 3)… 5) terminating steps: 4) 2 X • X—X 5) R • + X • R—X 6) 2 R • R—R
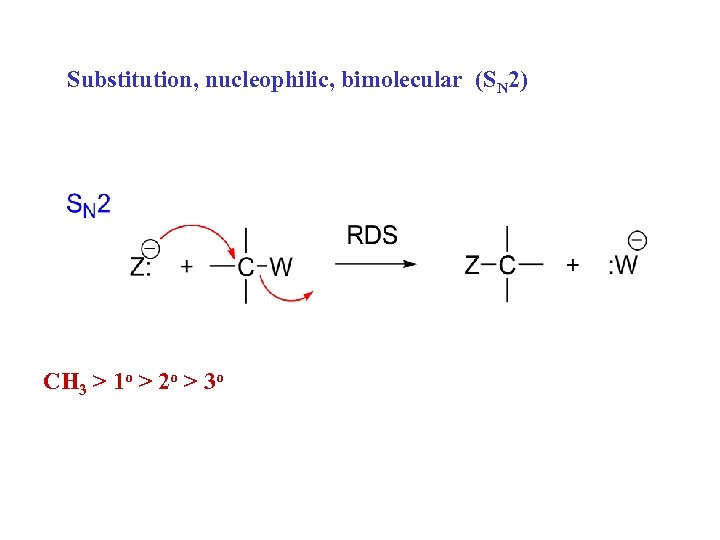 Substitution, nucleophilic, bimolecular (SN 2) CH 3 > 1 o > 2 o > 3 o
Substitution, nucleophilic, bimolecular (SN 2) CH 3 > 1 o > 2 o > 3 o
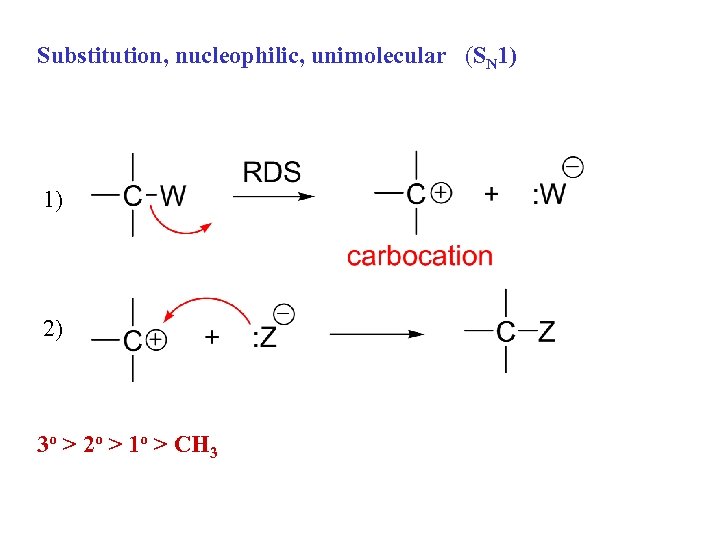 Substitution, nucleophilic, unimolecular (SN 1) 1) 2) 3 o > 2 o > 1 o > CH 3
Substitution, nucleophilic, unimolecular (SN 1) 1) 2) 3 o > 2 o > 1 o > CH 3
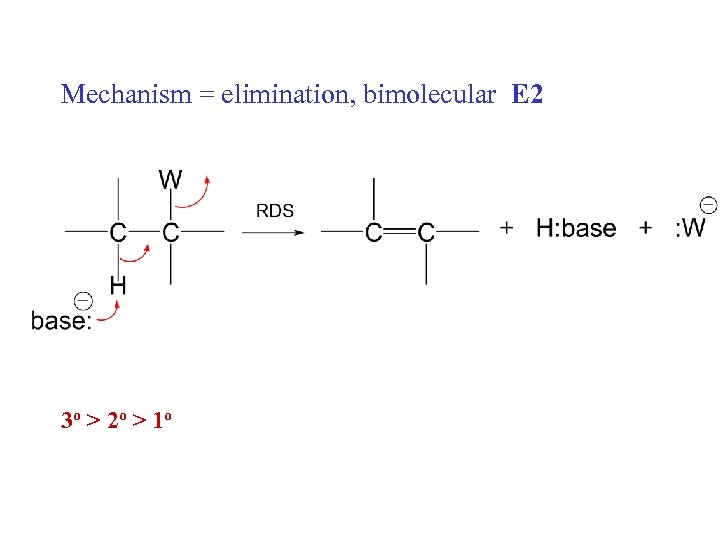 Mechanism = elimination, bimolecular E 2 3 o > 2 o > 1 o
Mechanism = elimination, bimolecular E 2 3 o > 2 o > 1 o
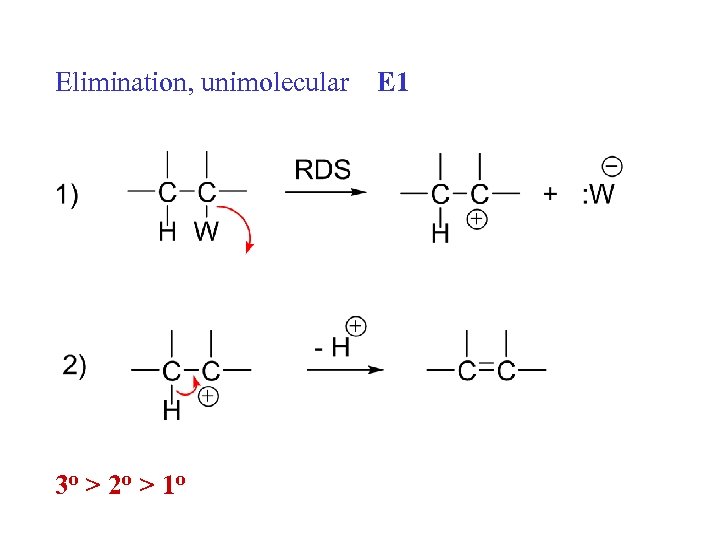 Elimination, unimolecular 3 o > 2 o > 1 o E 1
Elimination, unimolecular 3 o > 2 o > 1 o E 1
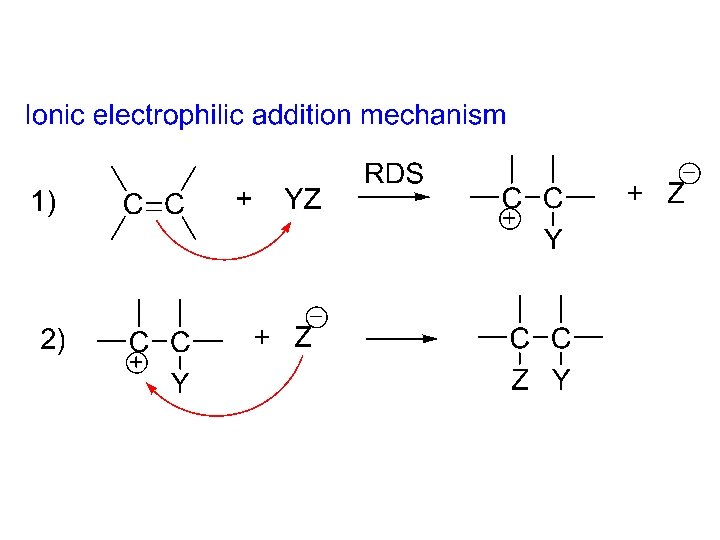
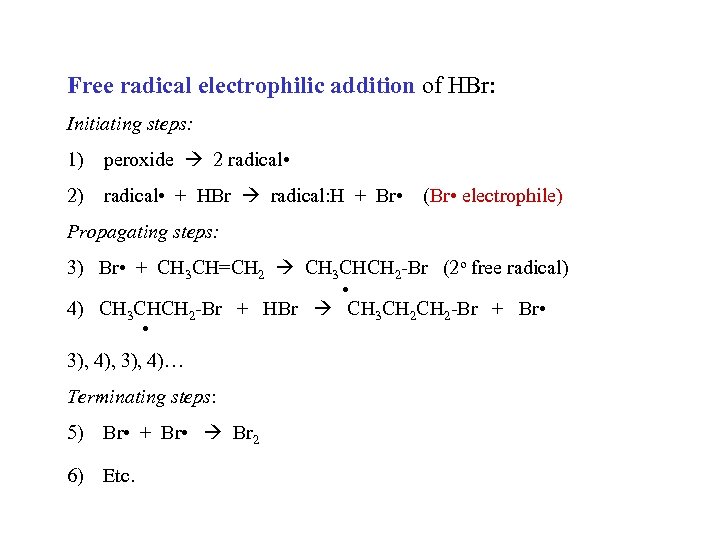 Free radical electrophilic addition of HBr: Initiating steps: 1) peroxide 2 radical • 2) radical • + HBr radical: H + Br • (Br • electrophile) Propagating steps: 3) Br • + CH 3 CH=CH 2 CH 3 CHCH 2 -Br (2 o free radical) • 4) CH 3 CHCH 2 -Br + HBr CH 3 CH 2 -Br + Br • • 3), 4), 3), 4)… Terminating steps: 5) Br • + Br • Br 2 6) Etc.
Free radical electrophilic addition of HBr: Initiating steps: 1) peroxide 2 radical • 2) radical • + HBr radical: H + Br • (Br • electrophile) Propagating steps: 3) Br • + CH 3 CH=CH 2 CH 3 CHCH 2 -Br (2 o free radical) • 4) CH 3 CHCH 2 -Br + HBr CH 3 CH 2 -Br + Br • • 3), 4), 3), 4)… Terminating steps: 5) Br • + Br • Br 2 6) Etc.


