ed91e7c6e7ed40e52db9cce7f2692511.ppt
- Количество слайдов: 85
 CHAPTER 9 THE MOLE © 2013 Marshall Cavendish International (Singapore) Private Limited
CHAPTER 9 THE MOLE © 2013 Marshall Cavendish International (Singapore) Private Limited
 Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 2
Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 2
 9. 1 Relative Atomic Mass Learning Outcome By the end of this section, you should be able to: • define relative atomic mass (Ar). 3
9. 1 Relative Atomic Mass Learning Outcome By the end of this section, you should be able to: • define relative atomic mass (Ar). 3
 9. 1 Relative Atomic Mass An atom is so tiny… How can we measure its mass? • Impractical to measure the actual mass of an atom • Mass of an atom is measured relative to a standard atom Carbon-12 4
9. 1 Relative Atomic Mass An atom is so tiny… How can we measure its mass? • Impractical to measure the actual mass of an atom • Mass of an atom is measured relative to a standard atom Carbon-12 4
 9. 1 Relative Atomic Mass (Ar) The relative atomic mass (Ar) of an atom is the average mass of one atom of that element compared to of the mass of one carbon-12 atom. Relative atomic = mass (Ar) average mass of one atom of the element mass of an atom of carbon-12 The relative atomic mass of carbon-12 is taken to be 12. 5
9. 1 Relative Atomic Mass (Ar) The relative atomic mass (Ar) of an atom is the average mass of one atom of that element compared to of the mass of one carbon-12 atom. Relative atomic = mass (Ar) average mass of one atom of the element mass of an atom of carbon-12 The relative atomic mass of carbon-12 is taken to be 12. 5
 9. 1 Relative Atomic Mass • is represented by the symbol Ar; • has NO unit; • of an element can be found in the Periodic Table. 24 Mg Here it is! Magnesium 12 6
9. 1 Relative Atomic Mass • is represented by the symbol Ar; • has NO unit; • of an element can be found in the Periodic Table. 24 Mg Here it is! Magnesium 12 6
 9. 1 Relative Atomic Masses of Some Elements Element hydrogen Relative atomic mass, Ar 1 carbon 12 oxygen 16 chlorine 35. 5 Some Ar values are not whole numbers. Why? 7
9. 1 Relative Atomic Masses of Some Elements Element hydrogen Relative atomic mass, Ar 1 carbon 12 oxygen 16 chlorine 35. 5 Some Ar values are not whole numbers. Why? 7
 9. 1 Relative Atomic Mass Why is the Ar of Chlorine Not a Whole Number? Recall: Most elements occur naturally as a mixture of isotopes. In nature, chlorine exists in two isotopic forms: Chlorine-35 75% Chlorine-37 25% Hence, Relative atomic mass of chlorine = (0. 75 × 35) + (0. 25 × 37) = 26. 25 + 9. 25 = 35. 5 8
9. 1 Relative Atomic Mass Why is the Ar of Chlorine Not a Whole Number? Recall: Most elements occur naturally as a mixture of isotopes. In nature, chlorine exists in two isotopic forms: Chlorine-35 75% Chlorine-37 25% Hence, Relative atomic mass of chlorine = (0. 75 × 35) + (0. 25 × 37) = 26. 25 + 9. 25 = 35. 5 8
 9. 1 Relative Atomic Mass of an Element • takes into account the relative abundance of all the isotopes of the element. The amount of an isotope in an element expressed in percentage. Therefore, the Ar values of some elements are not whole numbers. 9
9. 1 Relative Atomic Mass of an Element • takes into account the relative abundance of all the isotopes of the element. The amount of an isotope in an element expressed in percentage. Therefore, the Ar values of some elements are not whole numbers. 9
 Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 10
Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 10
 9. 2 Relative Molecular Mass and Relative Formula Mass Learning Outcomes By the end of this section, you should be able to: • define relative molecular mass (Mr); • calculate relative molecular mass or relative formula mass of a substance. 11
9. 2 Relative Molecular Mass and Relative Formula Mass Learning Outcomes By the end of this section, you should be able to: • define relative molecular mass (Mr); • calculate relative molecular mass or relative formula mass of a substance. 11
 9. 2 Relative Molecular Mass and Relative Formula Mass Relative Molecular Mass (Mr) The relative molecular mass (Mr) of a molecular substance is the average mass of one molecule of that element or compound compared to of the mass of one carbon-12 atom. Relative molecular mass (Mr) = average mass of one molecule of an element or a compound mass of an atom of carbon-12 12
9. 2 Relative Molecular Mass and Relative Formula Mass Relative Molecular Mass (Mr) The relative molecular mass (Mr) of a molecular substance is the average mass of one molecule of that element or compound compared to of the mass of one carbon-12 atom. Relative molecular mass (Mr) = average mass of one molecule of an element or a compound mass of an atom of carbon-12 12
 9. 2 Relative Molecular Mass and Relative Formula Mass Relative Molecular Mass • is represented by the symbol Mr; • is the sum of the Ar of all the atoms in a molecule; • has NO unit. 13
9. 2 Relative Molecular Mass and Relative Formula Mass Relative Molecular Mass • is represented by the symbol Mr; • is the sum of the Ar of all the atoms in a molecule; • has NO unit. 13
 9. 2 Relative Molecular Mass and Relative Formula Mass Calculating Relative Molecular Mass Example 1 Calculate the Mr of carbon dioxide. CO 2 Number of O atoms = 2 Ar of O = 16 Number of C atoms = 1 Ar of C = 12 Mr of carbon dioxide = (1 × 12) + (2 × 16) = 12 + 32 = 44 14
9. 2 Relative Molecular Mass and Relative Formula Mass Calculating Relative Molecular Mass Example 1 Calculate the Mr of carbon dioxide. CO 2 Number of O atoms = 2 Ar of O = 16 Number of C atoms = 1 Ar of C = 12 Mr of carbon dioxide = (1 × 12) + (2 × 16) = 12 + 32 = 44 14
 9. 2 Relative Molecular Mass and Relative Formula Mass Example 2 Calculate the Mr of ammonia. N H 3 Number of H atoms = 3 Ar of H = 1 Number of N atoms = 1 Ar of N = 14 Mr of ammonia = (1 × 14) + (3 × 1) = 14 + 3 = 17 15
9. 2 Relative Molecular Mass and Relative Formula Mass Example 2 Calculate the Mr of ammonia. N H 3 Number of H atoms = 3 Ar of H = 1 Number of N atoms = 1 Ar of N = 14 Mr of ammonia = (1 × 14) + (3 × 1) = 14 + 3 = 17 15
 9. 2 Relative Molecular Mass and Relative Formula Mass • is a more accurate way to refer to the Mr of ionic compounds; • is also represented by the symbol Mr; do not exist as molecules • has NO unit; • is calculated in exactly the same way as relative molecular mass. 16
9. 2 Relative Molecular Mass and Relative Formula Mass • is a more accurate way to refer to the Mr of ionic compounds; • is also represented by the symbol Mr; do not exist as molecules • has NO unit; • is calculated in exactly the same way as relative molecular mass. 16
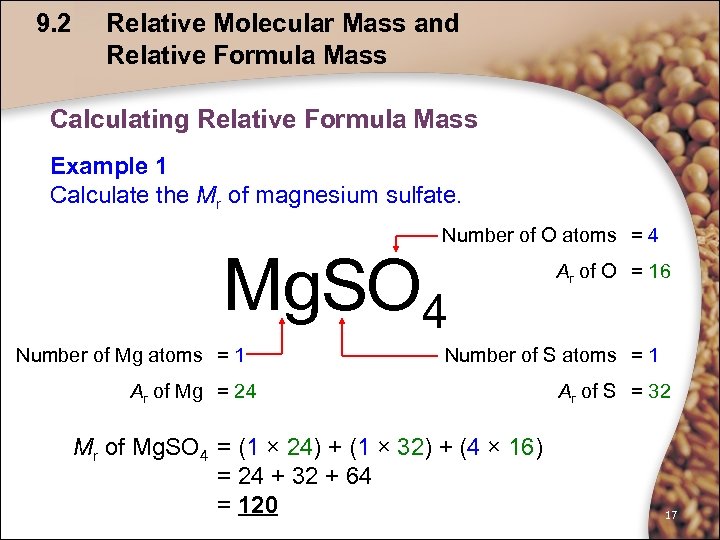 9. 2 Relative Molecular Mass and Relative Formula Mass Calculating Relative Formula Mass Example 1 Calculate the Mr of magnesium sulfate. Number of O atoms = 4 Mg. SO 4 Number of Mg atoms = 1 Ar of O = 16 Number of S atoms = 1 Ar of Mg = 24 Mr of Mg. SO 4 = (1 × 24) + (1 × 32) + (4 × 16) = 24 + 32 + 64 = 120 Ar of S = 32 17
9. 2 Relative Molecular Mass and Relative Formula Mass Calculating Relative Formula Mass Example 1 Calculate the Mr of magnesium sulfate. Number of O atoms = 4 Mg. SO 4 Number of Mg atoms = 1 Ar of O = 16 Number of S atoms = 1 Ar of Mg = 24 Mr of Mg. SO 4 = (1 × 24) + (1 × 32) + (4 × 16) = 24 + 32 + 64 = 120 Ar of S = 32 17
 9. 2 Relative Molecular Mass and Relative Formula Mass Example 2 Calculate the Mr of Cu. SO 4. 5 H 2 O. Denotes ‘plus’ in the calculation of Mr Denotes 5 molecules of water Cu. SO 4. 5 H 2 O Mr of Cu. SO 4. 5 H 2 O = (Mr of Cu. SO 4) + (5 × Mr of H 2 O) = (1 × 64) + (1 × 32) + (4 × 16) + (5 × 18) = 64 + 32 + 64 + 90 = 250 18
9. 2 Relative Molecular Mass and Relative Formula Mass Example 2 Calculate the Mr of Cu. SO 4. 5 H 2 O. Denotes ‘plus’ in the calculation of Mr Denotes 5 molecules of water Cu. SO 4. 5 H 2 O Mr of Cu. SO 4. 5 H 2 O = (Mr of Cu. SO 4) + (5 × Mr of H 2 O) = (1 × 64) + (1 × 32) + (4 × 16) + (5 × 18) = 64 + 32 + 64 + 90 = 250 18
 Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 19
Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 19
 9. 3 The Mole and Molar Mass Learning Outcomes By the end of this section, you should be able to: • convert number of particles into number of moles of particles and vice versa; • perform calculations involving the number of moles, mass and molar mass of a substance. 20
9. 3 The Mole and Molar Mass Learning Outcomes By the end of this section, you should be able to: • convert number of particles into number of moles of particles and vice versa; • perform calculations involving the number of moles, mass and molar mass of a substance. 20
 9. 3 The Mole and Molar Mass • For some items, it is easier to use different units so that we can count them easily. • E. g. A pair of shoes = 2 shoes A dozen pencils = 12 pencils A ream of paper = 500 sheets of paper 21
9. 3 The Mole and Molar Mass • For some items, it is easier to use different units so that we can count them easily. • E. g. A pair of shoes = 2 shoes A dozen pencils = 12 pencils A ream of paper = 500 sheets of paper 21
 9. 3 The Mole and Molar Mass Now, think of the atom. Can we count atoms? NO. . . ! TOO SMALL TOO MANY So we measure the quantity of atoms by a unit called. . . 22
9. 3 The Mole and Molar Mass Now, think of the atom. Can we count atoms? NO. . . ! TOO SMALL TOO MANY So we measure the quantity of atoms by a unit called. . . 22
 9. 3 The Mole and Molar Mass The Mole • is the unit of measurement for very small particles such as atoms, molecules, ions and electrons; • is represented by the symbol mol. So, what is this number? A mole of a substance contains the same number of particles as the number of atoms in 12 g of carbon-12. 23
9. 3 The Mole and Molar Mass The Mole • is the unit of measurement for very small particles such as atoms, molecules, ions and electrons; • is represented by the symbol mol. So, what is this number? A mole of a substance contains the same number of particles as the number of atoms in 12 g of carbon-12. 23
 9. 3 The Mole and Molar Mass Avogadro’s Number One mole of any substance contains 6 × 1023 particles. • • Atoms Molecules Ions Electrons The value 6 × 1023 is called Avogadro’s number. also known as Avogadro’s constant 24
9. 3 The Mole and Molar Mass Avogadro’s Number One mole of any substance contains 6 × 1023 particles. • • Atoms Molecules Ions Electrons The value 6 × 1023 is called Avogadro’s number. also known as Avogadro’s constant 24
 9. 3 The Mole and Molar Mass How Big is a Mole? • 1 mole of people will cover the whole surface of the Earth. • 1 mole of people standing on top of one another will reach beyond the Moon. • 1 mole of footballs will occupy the same volume as the Earth. • If you start to count now at 10 million atoms per second, you will take 2 billion years! 25
9. 3 The Mole and Molar Mass How Big is a Mole? • 1 mole of people will cover the whole surface of the Earth. • 1 mole of people standing on top of one another will reach beyond the Moon. • 1 mole of footballs will occupy the same volume as the Earth. • If you start to count now at 10 million atoms per second, you will take 2 billion years! 25
 9. 3 The Mole and Molar Mass Avogadro’s Number 1 mol of… contains… hydrogen atoms 6 × 1023 hydrogen atoms water 6 × 1023 water molecules Cu. SO 4 6 × 1023 formula units of Cu. SO 4 6 × 1023 Na+ ions An equal number of moles of substances contains an equal number of particles. 26
9. 3 The Mole and Molar Mass Avogadro’s Number 1 mol of… contains… hydrogen atoms 6 × 1023 hydrogen atoms water 6 × 1023 water molecules Cu. SO 4 6 × 1023 formula units of Cu. SO 4 6 × 1023 Na+ ions An equal number of moles of substances contains an equal number of particles. 26
 9. 3 The Mole and Molar Mass How do we convert between number of moles and number of particles? Number of moles = number of particles 6 × 1023 Example 1 How many moles of iron atoms contain 2. 7 × 1024 iron atoms? number of iron atoms Number of moles = of iron atoms 6 × 1023 2. 7 × 1024 = 6 × 1023 = 4. 5 mol 27
9. 3 The Mole and Molar Mass How do we convert between number of moles and number of particles? Number of moles = number of particles 6 × 1023 Example 1 How many moles of iron atoms contain 2. 7 × 1024 iron atoms? number of iron atoms Number of moles = of iron atoms 6 × 1023 2. 7 × 1024 = 6 × 1023 = 4. 5 mol 27
 9. 3 The Mole and Molar Mass Example 2 How many hydrogen atoms are there in three moles of hydrogen gas? • Hydrogen gas is made up of hydrogen molecules (H 2). • In one mole of H 2 molecules, there are two moles of H atoms. • In three moles of H 2 molecules, there are six moles of H 2 atoms. Number of hydrogen atoms = number of moles of hydrogen atoms × 6 × 1023 = 3. 6 × 1024 28
9. 3 The Mole and Molar Mass Example 2 How many hydrogen atoms are there in three moles of hydrogen gas? • Hydrogen gas is made up of hydrogen molecules (H 2). • In one mole of H 2 molecules, there are two moles of H atoms. • In three moles of H 2 molecules, there are six moles of H 2 atoms. Number of hydrogen atoms = number of moles of hydrogen atoms × 6 × 1023 = 3. 6 × 1024 28
 9. 3 The Mole and Molar Mass The Mole and Chemical Formulae H 2 O 1 molecule of H 2 O consists of 2 atoms of H and 1 atom of O consists of 2 mol of H atoms and 1 mol of O atoms Hence, 1 mol of H 2 O 6 × 1023 H 2 O molecules 2 × 6 × 1023 H atoms 6 × 1023 O atoms 29
9. 3 The Mole and Molar Mass The Mole and Chemical Formulae H 2 O 1 molecule of H 2 O consists of 2 atoms of H and 1 atom of O consists of 2 mol of H atoms and 1 mol of O atoms Hence, 1 mol of H 2 O 6 × 1023 H 2 O molecules 2 × 6 × 1023 H atoms 6 × 1023 O atoms 29
 9. 3 The Mole and Molar Mass The molar mass of an element is the mass of one mole of atoms of the element. Element Ar Molar mass/ (g/mol) What is the relationship 27 aluminium 27 carbon 12 12 neon 20 20 oxygen 16 16 between Ar and molar mass? 30
9. 3 The Mole and Molar Mass The molar mass of an element is the mass of one mole of atoms of the element. Element Ar Molar mass/ (g/mol) What is the relationship 27 aluminium 27 carbon 12 12 neon 20 20 oxygen 16 16 between Ar and molar mass? 30
 9. 3 The Mole and Molar Mass Molar mass of an element Molar mass of an ionic compound Relative atomic mass (Ar) in g URL Molar mass of a molecular substance Relative molecular mass (Mr) in g Relative formula mass (Mr) in g 31
9. 3 The Mole and Molar Mass Molar mass of an element Molar mass of an ionic compound Relative atomic mass (Ar) in g URL Molar mass of a molecular substance Relative molecular mass (Mr) in g Relative formula mass (Mr) in g 31
 9. 3 The Mole and Molar Mass Al Ar of Al = 27 Molar mass of Al = 27 g/mol H 2 O Ar of H 2 O = (2 × 1) + 16 = 18 Molar mass of H 2 O = 18 g/mol Na. Cl Ar of Na. Cl = 23 + 35. 5 = 58. 5 Molar mass of Na. Cl = 58. 5 g/mol 32
9. 3 The Mole and Molar Mass Al Ar of Al = 27 Molar mass of Al = 27 g/mol H 2 O Ar of H 2 O = (2 × 1) + 16 = 18 Molar mass of H 2 O = 18 g/mol Na. Cl Ar of Na. Cl = 23 + 35. 5 = 58. 5 Molar mass of Na. Cl = 58. 5 g/mol 32
 9. 3 The Mole and Molar Mass Calculations Involving the Mole and Molar Mass mass of the substance in g Number of moles = of a substance molar mass of the substance in g/mol Example 1 Find the number of moles in 0. 196 kg of iron. (Ar of Fe = 56) mass of iron in g Number of moles of iron = molar mass of iron in g/mol 0. 196 × 1000 56 = 3. 5 mol = 33
9. 3 The Mole and Molar Mass Calculations Involving the Mole and Molar Mass mass of the substance in g Number of moles = of a substance molar mass of the substance in g/mol Example 1 Find the number of moles in 0. 196 kg of iron. (Ar of Fe = 56) mass of iron in g Number of moles of iron = molar mass of iron in g/mol 0. 196 × 1000 56 = 3. 5 mol = 33
 9. 3 The Mole and Molar Mass Example 2 Find the number of moles in 4. 25 g of ammonia (NH 3). (Ar: N = 14; H = 1) Molar mass of NH 3 = 14 + (3 × 1) = 17 g/mol mass of NH 3 in g Number of moles of NH 3 = molar mass of NH 3 in g/mol 4. 25 17 = 2. 5 mol = 34
9. 3 The Mole and Molar Mass Example 2 Find the number of moles in 4. 25 g of ammonia (NH 3). (Ar: N = 14; H = 1) Molar mass of NH 3 = 14 + (3 × 1) = 17 g/mol mass of NH 3 in g Number of moles of NH 3 = molar mass of NH 3 in g/mol 4. 25 17 = 2. 5 mol = 34
 9. 3 The Mole and Molar Mass Example 3 Find the number of moles in 20 g of magnesium oxide (Mg. O). (Ar: Mg = 24; O = 16) Molar mass of Mg. O = 24 + 16 = 40 g/mol mass of Mg. O in g molar mass of Mg. O in g/mol 20 = 40 = 0. 5 mol Number of moles of Mg. O = 35
9. 3 The Mole and Molar Mass Example 3 Find the number of moles in 20 g of magnesium oxide (Mg. O). (Ar: Mg = 24; O = 16) Molar mass of Mg. O = 24 + 16 = 40 g/mol mass of Mg. O in g molar mass of Mg. O in g/mol 20 = 40 = 0. 5 mol Number of moles of Mg. O = 35
 9. 3 The Mole and Molar Mass Relationship Between Number of Moles, Mass and Molar Mass mass in g Number of moles = of a substance molar mass in g/mol Mass = number of moles × molar mass number of moles molar mass 36
9. 3 The Mole and Molar Mass Relationship Between Number of Moles, Mass and Molar Mass mass in g Number of moles = of a substance molar mass in g/mol Mass = number of moles × molar mass number of moles molar mass 36
 9. 3 The Mole and Molar Mass Example 4 Find the mass of 0. 25 mol of calcium carbonate. (Ar: Ca = 40; C = 12; O = 16) Molar mass of Ca. CO 3 = (1 × 40) + (1 × 12) + (3 × 16) = 100 g/mol Mass of Ca. CO 3 = number of moles × molar mass = 0. 25 × 100 = 250 g 37
9. 3 The Mole and Molar Mass Example 4 Find the mass of 0. 25 mol of calcium carbonate. (Ar: Ca = 40; C = 12; O = 16) Molar mass of Ca. CO 3 = (1 × 40) + (1 × 12) + (3 × 16) = 100 g/mol Mass of Ca. CO 3 = number of moles × molar mass = 0. 25 × 100 = 250 g 37
 Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 38
Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 38
 9. 4 Percentage Composition of Compounds Learning Outcome By the end of this section, you should be able to: • determine the percentage composition of compounds from given data. 39
9. 4 Percentage Composition of Compounds Learning Outcome By the end of this section, you should be able to: • determine the percentage composition of compounds from given data. 39
 9. 4 Percentage Composition of Compounds Consider a pie… …how can we express how much meat there is in the pie? 1. Find out the mass of meat in the pie. 2. Express the mass of meat as a percentage mass of the entire pie. The same can be done with compounds. 40
9. 4 Percentage Composition of Compounds Consider a pie… …how can we express how much meat there is in the pie? 1. Find out the mass of meat in the pie. 2. Express the mass of meat as a percentage mass of the entire pie. The same can be done with compounds. 40
 9. 4 Percentage Composition of Compounds Finding the Percentage Composition of a Compound Percentage by mass of an element in a compound number of atoms of Ar of the element × the element in the formula = × 100% Mr of the compound 41
9. 4 Percentage Composition of Compounds Finding the Percentage Composition of a Compound Percentage by mass of an element in a compound number of atoms of Ar of the element × the element in the formula = × 100% Mr of the compound 41
 9. 4 Percentage Composition of Compounds Example 1 What is the percentage by mass of (a) hydrogen; (b) oxygen present in hydrogen peroxide (H 2 O 2)? Mr of H 2 O 2 = (2 × 1) + (2 × 16) = 34 (a) Percentage by mass of hydrogen in H 2 O 2 Ar of H × number of H atoms in the formula × 100% Mr of H 2 O 2 1× 2 = 34 × 100% = = 5. 9% 42
9. 4 Percentage Composition of Compounds Example 1 What is the percentage by mass of (a) hydrogen; (b) oxygen present in hydrogen peroxide (H 2 O 2)? Mr of H 2 O 2 = (2 × 1) + (2 × 16) = 34 (a) Percentage by mass of hydrogen in H 2 O 2 Ar of H × number of H atoms in the formula × 100% Mr of H 2 O 2 1× 2 = 34 × 100% = = 5. 9% 42
 9. 4 Percentage Composition of Compounds Example 1 (cont’d) What is the percentage by mass of (a) hydrogen; (b) oxygen present in hydrogen peroxide (H 2 O 2)? (b) Percentage by mass of oxygen in H 2 O 2 = 100% – 5. 9% = 94. 1% 43
9. 4 Percentage Composition of Compounds Example 1 (cont’d) What is the percentage by mass of (a) hydrogen; (b) oxygen present in hydrogen peroxide (H 2 O 2)? (b) Percentage by mass of oxygen in H 2 O 2 = 100% – 5. 9% = 94. 1% 43
 9. 4 Percentage Composition of Compounds The percentage composition of certain components (e. g. water of crystallisation) can also be calculated using the same method. Example 2 What is the percentage of water in copper(II) sulfate crystals (Cu. SO 4. 5 H 2 O)? Mr of Cu. SO 4. 5 H 2 O = 64 + 32 + (4 × 16) + (5 × 18) = 250 44
9. 4 Percentage Composition of Compounds The percentage composition of certain components (e. g. water of crystallisation) can also be calculated using the same method. Example 2 What is the percentage of water in copper(II) sulfate crystals (Cu. SO 4. 5 H 2 O)? Mr of Cu. SO 4. 5 H 2 O = 64 + 32 + (4 × 16) + (5 × 18) = 250 44
 9. 4 Percentage Composition of Compounds Example 2 (cont’d) What is the percentage of water in copper(II) sulfate crystals (Cu. SO 4. 5 H 2 O)? Percentage of water in Cu. SO 4. 5 H 2 O Mr of H 2 O × number of H 2 O molecules in the formula × 100% = Mr of Cu. SO 4. 5 H 2 O 18 × 5 = 250 × 100% = 36% 45
9. 4 Percentage Composition of Compounds Example 2 (cont’d) What is the percentage of water in copper(II) sulfate crystals (Cu. SO 4. 5 H 2 O)? Percentage of water in Cu. SO 4. 5 H 2 O Mr of H 2 O × number of H 2 O molecules in the formula × 100% = Mr of Cu. SO 4. 5 H 2 O 18 × 5 = 250 × 100% = 36% 45
 Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 46
Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 46
 9. 5 Empirical and Molecular Formulae Learning Outcome By the end of this section, you should be able to: • determine the empirical and molecular formulae of a compound from given data. 47
9. 5 Empirical and Molecular Formulae Learning Outcome By the end of this section, you should be able to: • determine the empirical and molecular formulae of a compound from given data. 47
 9. 5 Empirical and Molecular Formulae Empirical Formula • is the simplest formula of a compound; • shows the types of elements present in the compound; • shows the simplest ratio of the different types of atoms in the compound. 48
9. 5 Empirical and Molecular Formulae Empirical Formula • is the simplest formula of a compound; • shows the types of elements present in the compound; • shows the simplest ratio of the different types of atoms in the compound. 48
 9. 5 Empirical and Molecular Formulae Finding the Empirical Formula of a Compound 1. Find out the mass of the reactants taking part in the reaction. 2. Work out the relative numbers of moles (mole ratio) of the reactants used. 3. Find the formula of the compound using the mole ratio obtained. 49
9. 5 Empirical and Molecular Formulae Finding the Empirical Formula of a Compound 1. Find out the mass of the reactants taking part in the reaction. 2. Work out the relative numbers of moles (mole ratio) of the reactants used. 3. Find the formula of the compound using the mole ratio obtained. 49
 9. 5 Empirical and Molecular Formulae How can we find the empirical formula of magnesium oxide? Step 1: Find out the mass of the reactants taking part in the reaction. • Heat 1. 20 g magnesium strongly in a crucible. • Weigh the resulting magnesium oxide. Mass of magnesium reacted = 1. 20 g Mass of oxygen reacted = mass of magnesium oxide – mass of magnesium = 2. 00 – 1. 20 g = 0. 80 g 50
9. 5 Empirical and Molecular Formulae How can we find the empirical formula of magnesium oxide? Step 1: Find out the mass of the reactants taking part in the reaction. • Heat 1. 20 g magnesium strongly in a crucible. • Weigh the resulting magnesium oxide. Mass of magnesium reacted = 1. 20 g Mass of oxygen reacted = mass of magnesium oxide – mass of magnesium = 2. 00 – 1. 20 g = 0. 80 g 50
 9. 5 Empirical and Molecular Formulae Step 2: Work out the relative numbers of moles (mole ratio) of the reactants used. Element Magnesium Oxygen Mass/g 1. 20 0. 80 24 16 Relative Atomic Mass Number of Moles/mol 1. 20 24 = 0. 05 Mole ratio 0. 05 = 1 0. 80 16 = 0. 05 = 1 51
9. 5 Empirical and Molecular Formulae Step 2: Work out the relative numbers of moles (mole ratio) of the reactants used. Element Magnesium Oxygen Mass/g 1. 20 0. 80 24 16 Relative Atomic Mass Number of Moles/mol 1. 20 24 = 0. 05 Mole ratio 0. 05 = 1 0. 80 16 = 0. 05 = 1 51
 9. 5 Empirical and Molecular Formulae Step 3: Find the formula of the compound using the mole ratio obtained. From Step 2, mole ratio of Mg : O = 1 : 1. Thus, the empirical formula of magnesium oxide is Mg. O. 52
9. 5 Empirical and Molecular Formulae Step 3: Find the formula of the compound using the mole ratio obtained. From Step 2, mole ratio of Mg : O = 1 : 1. Thus, the empirical formula of magnesium oxide is Mg. O. 52
 9. 5 Empirical and Molecular Formulae Molecular Formula • Shows the exact number of atoms of each element in a molecule • For some compounds, the empirical formula accurately shows the number of atoms in the compound. • E. g. water Empirical formula = H 2 O Molecular formula = H 2 O 53
9. 5 Empirical and Molecular Formulae Molecular Formula • Shows the exact number of atoms of each element in a molecule • For some compounds, the empirical formula accurately shows the number of atoms in the compound. • E. g. water Empirical formula = H 2 O Molecular formula = H 2 O 53
 9. 5 Empirical and Molecular Formulae Molecular Formula • For other compounds, the empirical formula may not show the actual number of atoms in the compound. • E. g. phosphorus(V) oxide Empirical formula = P 2 O 5 Molecular formula = P 4 O 10 54
9. 5 Empirical and Molecular Formulae Molecular Formula • For other compounds, the empirical formula may not show the actual number of atoms in the compound. • E. g. phosphorus(V) oxide Empirical formula = P 2 O 5 Molecular formula = P 4 O 10 54
 9. 5 Empirical and Molecular Formulae How is the empirical formula of a compound related to its molecular formula? • When the empirical formula and molecular formula are different, the molecular formula is always a multiple of the empirical formula. • We can find the molecular formula of a substance if we know the - empirical formula; - relative molecular mass. 55
9. 5 Empirical and Molecular Formulae How is the empirical formula of a compound related to its molecular formula? • When the empirical formula and molecular formula are different, the molecular formula is always a multiple of the empirical formula. • We can find the molecular formula of a substance if we know the - empirical formula; - relative molecular mass. 55
 9. 5 Empirical and Molecular Formulae How is the empirical formula of a compound related to its molecular formula? • If empirical formula = Ax. By, molecular formula = (Ax. By)n, where n = 1, 2, 3, etc. relative molecular mass n= relative mass from empirical formula 56
9. 5 Empirical and Molecular Formulae How is the empirical formula of a compound related to its molecular formula? • If empirical formula = Ax. By, molecular formula = (Ax. By)n, where n = 1, 2, 3, etc. relative molecular mass n= relative mass from empirical formula 56
 9. 5 Empirical and Molecular Formulae Example 1 The empirical formula of ethane is CH 3. Given that the relative molecular mass of ethane is 30, what is its molecular formula? Let the molecular formula of ethane be (CH 3)n. Relative mass of ethane from empirical formula = 12 + (3 × 1) = 15 relative molecular mass of ethane n = relative mass of ethane from empirical formula = 30 15 =2 Molecular formula mass of ethane = (CH 3)2 = C 2 H 6 57
9. 5 Empirical and Molecular Formulae Example 1 The empirical formula of ethane is CH 3. Given that the relative molecular mass of ethane is 30, what is its molecular formula? Let the molecular formula of ethane be (CH 3)n. Relative mass of ethane from empirical formula = 12 + (3 × 1) = 15 relative molecular mass of ethane n = relative mass of ethane from empirical formula = 30 15 =2 Molecular formula mass of ethane = (CH 3)2 = C 2 H 6 57
 Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 58
Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 58
 9. 6 Molar Volume of Gases Learning Outcome By the end of this section, you should be able to: • perform calculations involving molar gas volume and the number of moles of a gas. 59
9. 6 Molar Volume of Gases Learning Outcome By the end of this section, you should be able to: • perform calculations involving molar gas volume and the number of moles of a gas. 59
 9. 6 Molar Volume of Gases Molar Gas Volume Avogadro’s Law: Equal volumes of all gases, under the same temperature and pressure, contain the same number of particles. Do balloons of the same volume contain the same number of gaseous particles? Yes, they do! 60
9. 6 Molar Volume of Gases Molar Gas Volume Avogadro’s Law: Equal volumes of all gases, under the same temperature and pressure, contain the same number of particles. Do balloons of the same volume contain the same number of gaseous particles? Yes, they do! 60
 9. 6 Molar Volume of Gases Molar Gas Volume e. g. hydrogen, oxygen, nitrogen, carbon dioxide, methane, helium One mole of any gas occupies 24 dm 3 at room temperature and pressure. r. t. p. : 25°C, 1 atm 24 000 cm 3 24 dm 3 is called the molar volume of a gas at r. t. p. 61
9. 6 Molar Volume of Gases Molar Gas Volume e. g. hydrogen, oxygen, nitrogen, carbon dioxide, methane, helium One mole of any gas occupies 24 dm 3 at room temperature and pressure. r. t. p. : 25°C, 1 atm 24 000 cm 3 24 dm 3 is called the molar volume of a gas at r. t. p. 61
 9. 6 Molar Volume of Gases Molar Gas Volume Therefore, at room temperature and pressure (r. t. p. ), • 1 mol of oxygen occupies a volume of 24 dm 3. • 1 mol of hydrogen occupies a volume of 24 dm 3. • 1 mol of carbon dioxide occupies a volume of 24 dm 3. • 1 mol of methane occupies a volume of 24 dm 3. • 1 mol of helium occupies a volume of 24 dm 3. • 2 mol of oxygen occupies a volume of 48 dm 3. • 3 mol of nitrogen occupies a volume of 72 dm 3. 62
9. 6 Molar Volume of Gases Molar Gas Volume Therefore, at room temperature and pressure (r. t. p. ), • 1 mol of oxygen occupies a volume of 24 dm 3. • 1 mol of hydrogen occupies a volume of 24 dm 3. • 1 mol of carbon dioxide occupies a volume of 24 dm 3. • 1 mol of methane occupies a volume of 24 dm 3. • 1 mol of helium occupies a volume of 24 dm 3. • 2 mol of oxygen occupies a volume of 48 dm 3. • 3 mol of nitrogen occupies a volume of 72 dm 3. 62
 9. 6 Molar Volume of Gases Do balloons of the same mass have the number of particles? 0. 18 g O 2 0. 18 g H 2 0. 18 g CH 4 If each balloon here contains 0. 18 g of a different gas… 0. 18 g N 2 0. 18 g He 0. 18 g CO 2 63
9. 6 Molar Volume of Gases Do balloons of the same mass have the number of particles? 0. 18 g O 2 0. 18 g H 2 0. 18 g CH 4 If each balloon here contains 0. 18 g of a different gas… 0. 18 g N 2 0. 18 g He 0. 18 g CO 2 63
 9. 6 Molar Volume of Gases . . . they will have a different number of particles. 5. 4 × 1022 H 2 molecules 3. 4 × 1021 O 2 molecules 1021 6. 8 × 1021 CH 4 molecules 3. 9 × N 2 molecules 2. 7 × 1022 He atoms 2. 5 × 1021 CO 2 molecules Equal masses of gases do NOT • have the same number of moles; • occupy the same volume. 64
9. 6 Molar Volume of Gases . . . they will have a different number of particles. 5. 4 × 1022 H 2 molecules 3. 4 × 1021 O 2 molecules 1021 6. 8 × 1021 CH 4 molecules 3. 9 × N 2 molecules 2. 7 × 1022 He atoms 2. 5 × 1021 CO 2 molecules Equal masses of gases do NOT • have the same number of moles; • occupy the same volume. 64
 9. 6 Molar Volume of Gases The Mole and Molar Volume volume of gas in dm 3 Number of moles of gas = 24 dm 3 Number of moles of gas = volume of gas in cm 3 24 000 cm 3 Example 1 Calculate the number of moles of carbon dioxide in 3 dm 3 of gas at r. t. p. volume of CO 2 in dm 3 Number of moles of CO 2 = 24 dm 3 3 = 24 = 0. 125 mol 65
9. 6 Molar Volume of Gases The Mole and Molar Volume volume of gas in dm 3 Number of moles of gas = 24 dm 3 Number of moles of gas = volume of gas in cm 3 24 000 cm 3 Example 1 Calculate the number of moles of carbon dioxide in 3 dm 3 of gas at r. t. p. volume of CO 2 in dm 3 Number of moles of CO 2 = 24 dm 3 3 = 24 = 0. 125 mol 65
 9. 6 Molar Volume of Gases Relationship Between Volume of Gas, Number of Moles and Molar Gas Volume Number of moles of gas = = volume of gas in dm 3 24 dm 3 volume of gas in cm 3 24 000 cm 3 Volume of gas = number of moles of gas × 24 dm 3 = number of moles of gas × 24 000 cm 3 volume of gas (dm 3) number of moles 24 dm 3 or volume of gas (cm 3) number of moles 24 000 cm 3 66
9. 6 Molar Volume of Gases Relationship Between Volume of Gas, Number of Moles and Molar Gas Volume Number of moles of gas = = volume of gas in dm 3 24 dm 3 volume of gas in cm 3 24 000 cm 3 Volume of gas = number of moles of gas × 24 dm 3 = number of moles of gas × 24 000 cm 3 volume of gas (dm 3) number of moles 24 dm 3 or volume of gas (cm 3) number of moles 24 000 cm 3 66
 9. 6 Molar Volume of Gases Example 2 Calculate the volume (in cm 3) of 0. 75 mol of methane at r. t. p. Volume of CH 4 in cm 3 = number of moles of CH 4 × 24 000 cm 3 = 0. 75 × 24 000 = 18 000 cm 3 67
9. 6 Molar Volume of Gases Example 2 Calculate the volume (in cm 3) of 0. 75 mol of methane at r. t. p. Volume of CH 4 in cm 3 = number of moles of CH 4 × 24 000 cm 3 = 0. 75 × 24 000 = 18 000 cm 3 67
 9. 6 Molar Volume of Gases 2 ways to calculate the number of moles of a gas: Number of moles of gas mass of gas in g molar mass of gas in g/mol volume of gas in dm 3 24 dm 3 68
9. 6 Molar Volume of Gases 2 ways to calculate the number of moles of a gas: Number of moles of gas mass of gas in g molar mass of gas in g/mol volume of gas in dm 3 24 dm 3 68
 9. 6 Molar Volume of Gases Example 3 What is the volume (in dm 3) of 4 g of oxygen gas at r. t. p. ? mass of O 2 in g molar mass of O 2 in g/mol 4 = 32 = 0. 125 mol Number of moles of O 2 = Volume of O 2 in dm 3 = number of moles of O 2 × 24 dm 3 = 0. 125 × 24 = 3 dm 3 69
9. 6 Molar Volume of Gases Example 3 What is the volume (in dm 3) of 4 g of oxygen gas at r. t. p. ? mass of O 2 in g molar mass of O 2 in g/mol 4 = 32 = 0. 125 mol Number of moles of O 2 = Volume of O 2 in dm 3 = number of moles of O 2 × 24 dm 3 = 0. 125 × 24 = 3 dm 3 69
 9. 6 Molar Volume of Gases Example 4 What is the mass of 12 dm 3 of carbon dioxide at r. t. p. ? Number of moles of CO 2 = = volume of O 2 in dm 3 12 24 24 dm 3 = 0. 5 mol Mass of CO 2 = number of moles of CO 2 × molar mass of CO 2 = 0. 5 × [(1 × 12) + (2 × 16)] = 0. 5 × 44 = 22 g 70
9. 6 Molar Volume of Gases Example 4 What is the mass of 12 dm 3 of carbon dioxide at r. t. p. ? Number of moles of CO 2 = = volume of O 2 in dm 3 12 24 24 dm 3 = 0. 5 mol Mass of CO 2 = number of moles of CO 2 × molar mass of CO 2 = 0. 5 × [(1 × 12) + (2 × 16)] = 0. 5 × 44 = 22 g 70
 Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 71
Chapter 9 The Mole 9. 1 Relative Atomic Mass 9. 2 Relative Molecular Mass and Relative Formula Mass 9. 3 The Mole and Molar Mass 9. 4 Percentage Composition of Compounds 9. 5 Empirical and Molecular Formulae 9. 6 Molar Volume of Gases 9. 7 The Concentration of a Solution 71
 9. 7 The Concentration of a Solution Learning Outcome By the end of this section, you should be able to: • perform calculations involving the concentration of a solution (g/dm 3 or mol/dm 3). 72
9. 7 The Concentration of a Solution Learning Outcome By the end of this section, you should be able to: • perform calculations involving the concentration of a solution (g/dm 3 or mol/dm 3). 72
 9. 7 The Concentration of a Solution Solute and Solvent Recall: solution solute solvent Solid to be dissolved Liquid in which the solute dissolves solvent solution 73
9. 7 The Concentration of a Solution Solute and Solvent Recall: solution solute solvent Solid to be dissolved Liquid in which the solute dissolves solvent solution 73
 9. 7 The Concentration of a Solution • is given by the amount of a solute dissolved in 1 dm 3 of a solution; in g or mol • can be expressed in – – gram per dm 3 (g/dm 3); mole per dm 3 (mol/dm 3). also known as molar concentration 74
9. 7 The Concentration of a Solution • is given by the amount of a solute dissolved in 1 dm 3 of a solution; in g or mol • can be expressed in – – gram per dm 3 (g/dm 3); mole per dm 3 (mol/dm 3). also known as molar concentration 74
 9. 7 The Concentration of a Solution Concentration in g/dm 3 = Concentration in mass of solute in g volume of solution in dm 3 mol/dm 3 = number of moles of solute volume of solution in dm 3 Mass of solute (g) = concentration (g/dm 3) × volume (dm 3) Number of moles of solute = concentration (mol/dm 3) × volume (dm 3) 75
9. 7 The Concentration of a Solution Concentration in g/dm 3 = Concentration in mass of solute in g volume of solution in dm 3 mol/dm 3 = number of moles of solute volume of solution in dm 3 Mass of solute (g) = concentration (g/dm 3) × volume (dm 3) Number of moles of solute = concentration (mol/dm 3) × volume (dm 3) 75
 9. 7 The Concentration of a Solution Relationship Between Concentration in mol/dm 3 and Concentration in g/dm 3 Concentration in mol/dm 3 number of moles of solute = volume of solution in dm 3 can also be expressed as 76
9. 7 The Concentration of a Solution Relationship Between Concentration in mol/dm 3 and Concentration in g/dm 3 Concentration in mol/dm 3 number of moles of solute = volume of solution in dm 3 can also be expressed as 76
 9. 7 The Concentration of a Solution Calculating the Concentration of a Solution Example 1 A solution of sodium hydroxide contains 9. 6 g of sodium hydroxide in 100 cm 3 of solution. Find the concentration of the solution in (a) g/dm 3; (b) mol/dm 3. 77
9. 7 The Concentration of a Solution Calculating the Concentration of a Solution Example 1 A solution of sodium hydroxide contains 9. 6 g of sodium hydroxide in 100 cm 3 of solution. Find the concentration of the solution in (a) g/dm 3; (b) mol/dm 3. 77
 9. 7 The Concentration of a Solution Example 1 (cont’d) (a) Mass of sodium hydroxide (Na. OH) = 9. 6 g Volume of solution in dm 3 = 1000 = 0. 1 dm 3 mass of Na. OH in g Concentration of Na. OH = volume of solution in dm 3 3 in g/dm 9. 6 = 0. 1 = 96 g/dm 3 78
9. 7 The Concentration of a Solution Example 1 (cont’d) (a) Mass of sodium hydroxide (Na. OH) = 9. 6 g Volume of solution in dm 3 = 1000 = 0. 1 dm 3 mass of Na. OH in g Concentration of Na. OH = volume of solution in dm 3 3 in g/dm 9. 6 = 0. 1 = 96 g/dm 3 78
 9. 7 The Concentration of a Solution Example 1 (cont’d) (b) Molar mass of Na. OH = 23 + 16 + 1 = 40 Number of moles of Na. OH = mass of Na. OH molar mass of Na. OH 9. 6 = 40 = 0. 24 mol Volume of solution in dm 3 = 0. 1 dm 3 number of moles of Na. OH Concentration of = 3 volume of solution in dm 3 Na. OH in mol/dm 0. 24 = 0. 1 = 2. 4 mol/dm 3 79
9. 7 The Concentration of a Solution Example 1 (cont’d) (b) Molar mass of Na. OH = 23 + 16 + 1 = 40 Number of moles of Na. OH = mass of Na. OH molar mass of Na. OH 9. 6 = 40 = 0. 24 mol Volume of solution in dm 3 = 0. 1 dm 3 number of moles of Na. OH Concentration of = 3 volume of solution in dm 3 Na. OH in mol/dm 0. 24 = 0. 1 = 2. 4 mol/dm 3 79
 9. 7 The Concentration of a Solution Example 1 (cont’d) (b) Alternatively, Concentration of Na. OH in mol/dm 3 = = concentration in g/dm 3 molar mass 96 (23 + 16 + 1) = 2. 4 mol/dm 3 80
9. 7 The Concentration of a Solution Example 1 (cont’d) (b) Alternatively, Concentration of Na. OH in mol/dm 3 = = concentration in g/dm 3 molar mass 96 (23 + 16 + 1) = 2. 4 mol/dm 3 80
 9. 7 The Concentration of a Solution Example 2 The concentration of a solution of hydrochloric acid is 73 g/dm 3. (a) What the concentration of this acid in mol/dm 3? (b) How many moles of HCl are there in 250 cm 3 of this solution? 81
9. 7 The Concentration of a Solution Example 2 The concentration of a solution of hydrochloric acid is 73 g/dm 3. (a) What the concentration of this acid in mol/dm 3? (b) How many moles of HCl are there in 250 cm 3 of this solution? 81
 9. 7 The Concentration of a Solution Example 2 (cont’d) (a) Molar mass of HCl = 1 + 35. 5 = 36. 5 g/mol 3 Concentration in concentration in g/dm = mol/dm 3 molar mass 73 = 36. 5 = 2 mol/dm 3 82
9. 7 The Concentration of a Solution Example 2 (cont’d) (a) Molar mass of HCl = 1 + 35. 5 = 36. 5 g/mol 3 Concentration in concentration in g/dm = mol/dm 3 molar mass 73 = 36. 5 = 2 mol/dm 3 82
 9. 7 The Concentration of a Solution Example 2 (cont’d) (b) Volume of solution in dm 3 = 250 1000 = 0. 25 dm 3 Number of moles of HCl in 250 cm 3 of solution = concentration in mol/dm 3 × volume in dm 3 = 2 × 0. 25 = 0. 5 mol 83
9. 7 The Concentration of a Solution Example 2 (cont’d) (b) Volume of solution in dm 3 = 250 1000 = 0. 25 dm 3 Number of moles of HCl in 250 cm 3 of solution = concentration in mol/dm 3 × volume in dm 3 = 2 × 0. 25 = 0. 5 mol 83
 Chapter 9 The Mole Concept Map 84
Chapter 9 The Mole Concept Map 84
 Chapter 9 The Mole Concept Map 85
Chapter 9 The Mole Concept Map 85


