1974a8bd663ba94140c3deb845cf3a17.ppt
- Количество слайдов: 143

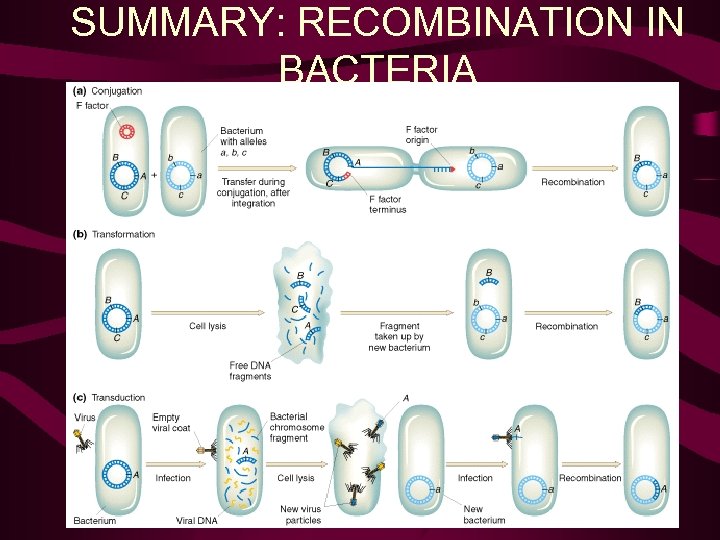
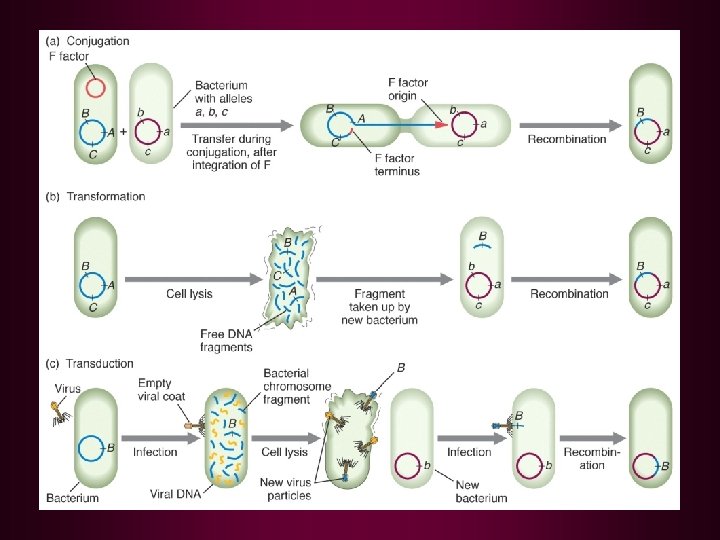
Chapter 9 The Genetics of Bacteria and Phages Fso far. . . recombination & mapping in eukaryotes Fnow. . . prokaryotes & viruses resolution F 3 ways to incorporate & recombine DNA in bacteria: 1. conjugation – plasmid-mediated transfer
Both bacteria and bacteriophages(Bacterial viruses) demonstrate mechanisms by which genetic recombination occurs, processes that can serve as the basis for genetic mapping As a result, both groups have been the subject of extensive analysis.
KEY CONCEPTS Ffertility factor (F) permits bacterial cells to transfer DNA to other bacteria cells through conjugation FF can be integrated or cytoplasmic Fwhen integrated, F can transfer host chromosome markers through conjugation Fbacteriophages can transfer DNA from one bacterial cell to another in two ways. . . Fgeneralized transduction is the transfer of randomly incorporated bacterial chromosome fragments Fspecialized transduction is the transfer of specific genes near phage integration sites Fthese methods of gene transfer facilitate construction of detailed maps of bacterial genomes

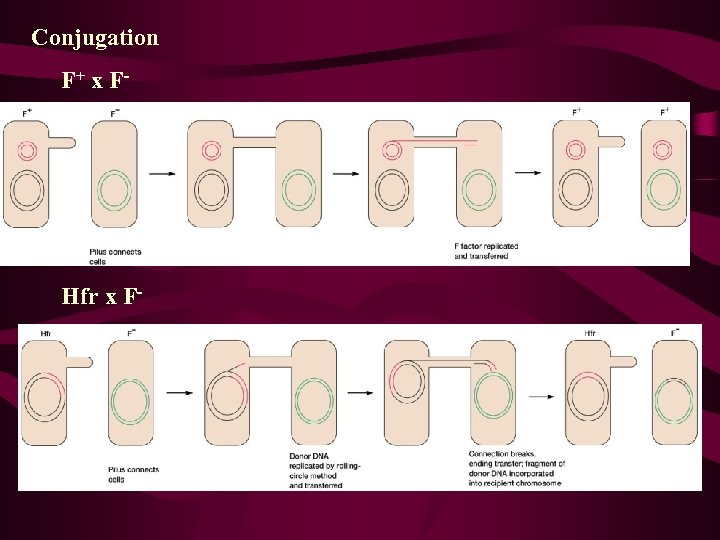
• Bacteria also contain extrachromosomal DNA in the form of plasmids, which can house a fertility factor that plays a key role in genetic recombination. • Some E. coli cells carry a circular plasmid called the sex factor, F. • Factor F can exist in a free state in the cytoplasm or it can be integrated into the circular bacterial chromosome. • In the nonintegrated state, F can pass into F-free cells during cell conjugation. • When F is integrated, the bacterial chromosome is transferred linearly to an F-free cell during conjugation.
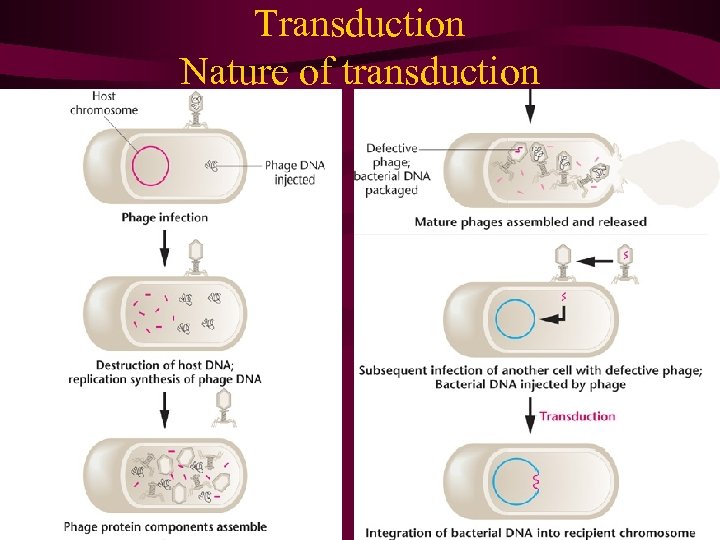
• Bacteriophages can transfer (transduce) DNA from one bacterial cell to another. • During generalized transduction, random chromosome fragments are incorporated into the heads of phages and transferred to other cells by infection. • During specialized transduction, specific genes near the phage-integration sites on the bacterial chromosome are mistakenly incorporated into the phage genome and transferred to other cells by infection. • DNA from the medium can enter a bacterial cell and integrate into the chromosome, thereby transforming the genotype. • The different methods of gene transfer in bacteria generate partial diploids that permit the study of recombination and gene interaction.

9. 1 Bacterial Mutation and Growth Genetic 9. 2 Recombination in Bacteria: Conjugation 9. 3 Rec Proteins and Bacterial Recombination 9. 4 F Factors and Plasmids 9. 5 Bacterial Transformation 9. 6 The Genetic Study of Bacteriophages Phage T 4: Structure and Life Cycle The Plaque Assay, Lysogeny 9. 7 Transduction: Virus-Mediated Bacterial. DNA Transfer The Lederberg-Zinder Experiment The Nature of Transduction and Mapping 9. 8 Intergenic Recombination and Mapping in Bacteriophages
• 9. 1 Bacterial Mutation and Growth Bacterial Phenotypes • To do genetics, we need phenotypic variation. • Prior to 1943 • The adaptation hypothesis, • spontaneous mutations Morphology/resistance/prototroph(autotroph)/ auxotroph and so on • fluctuation test,
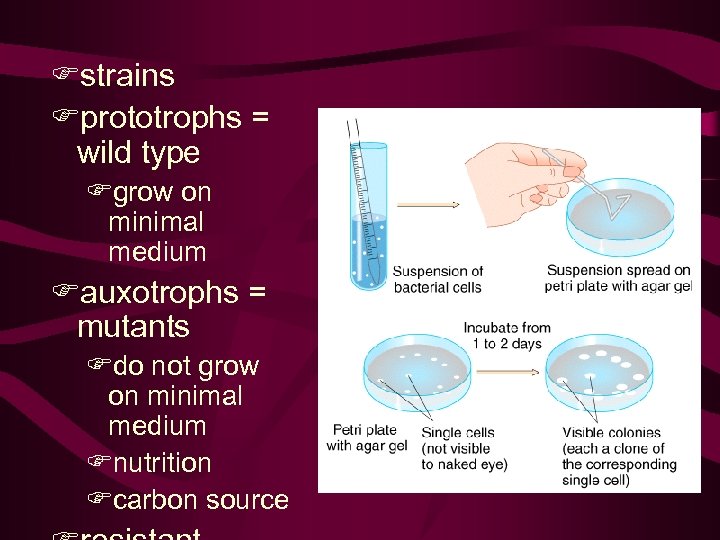
Fstrains Fprototrophs = wild type Fgrow on minimal medium Fauxotrophs = mutants Fdo not grow on minimal medium Fnutrition Fcarbon source
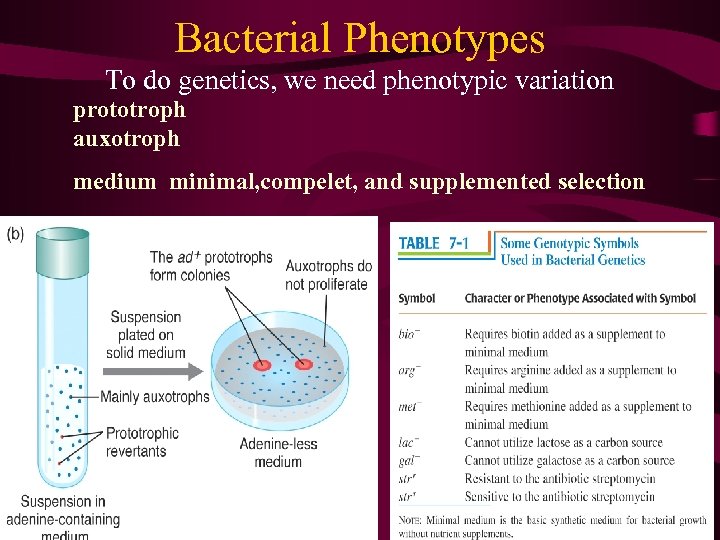
Bacterial Phenotypes To do genetics, we need phenotypic variation prototroph auxotroph medium minimal, compelet, and supplemented selection
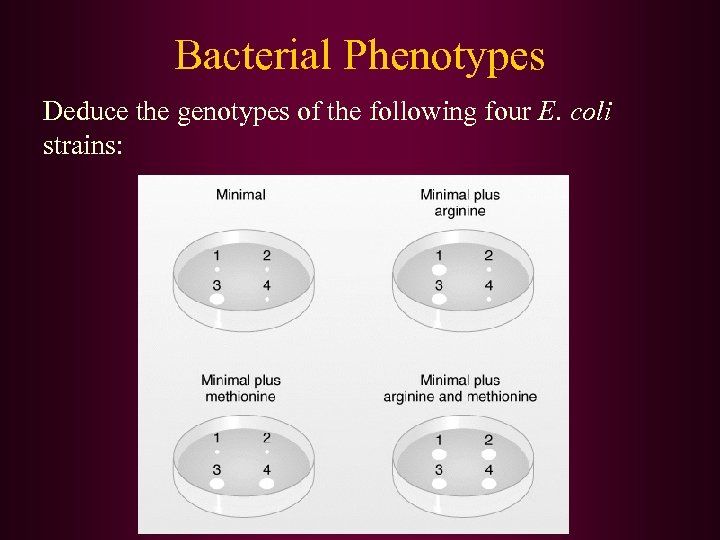
Bacterial Phenotypes Deduce the genotypes of the following four E. coli strains:
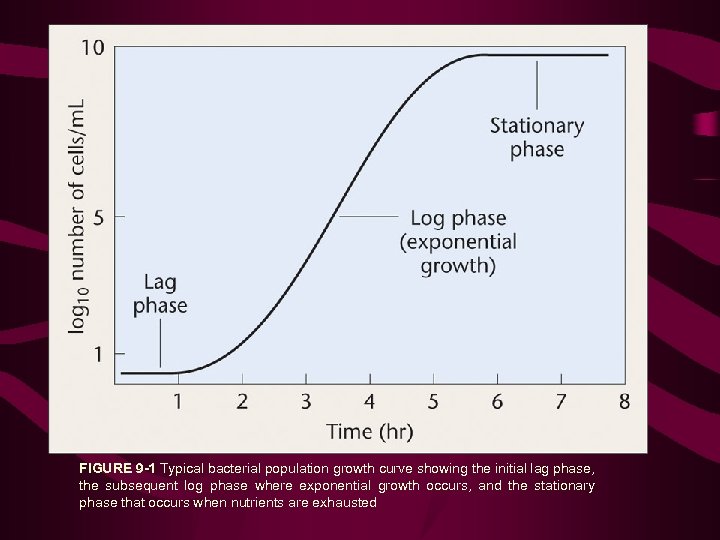
FIGURE 9 -1 Typical bacterial population growth curve showing the initial lag phase, the subsequent log phase where exponential growth occurs, and the stationary phase that occurs when nutrients are exhausted
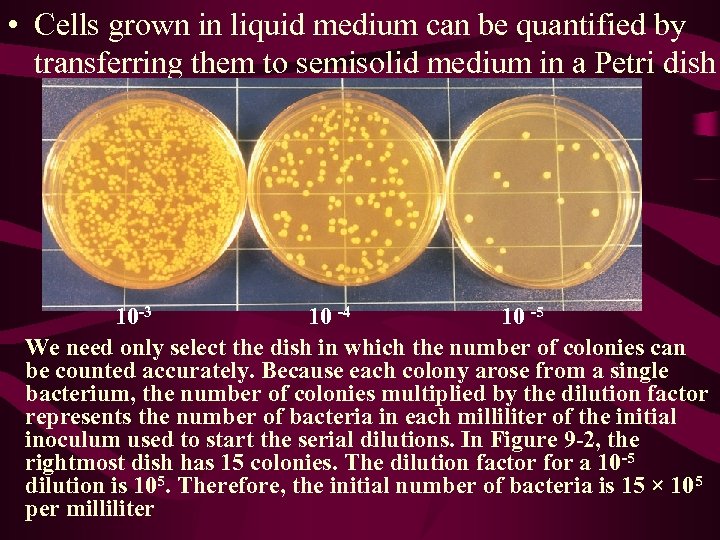
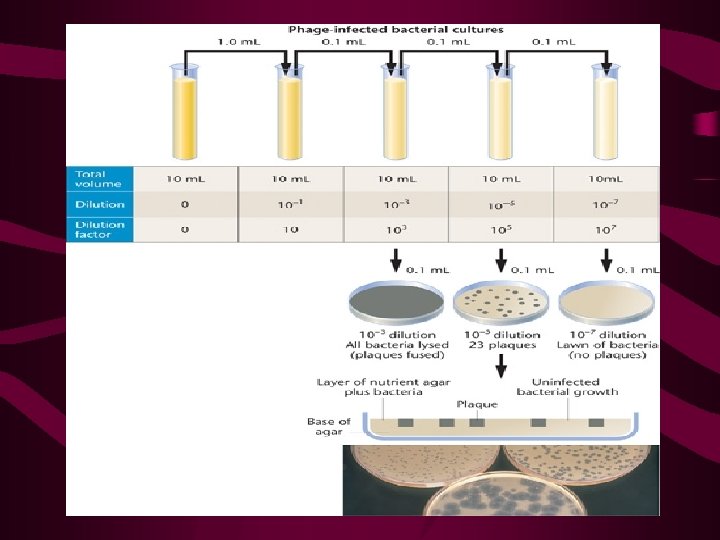
• Cells grown in liquid medium can be quantified by transferring them to semisolid medium in a Petri dish 10 -3 10 -4 10 -5 We need only select the dish in which the number of colonies can be counted accurately. Because each colony arose from a single bacterium, the number of colonies multiplied by the dilution factor represents the number of bacteria in each milliliter of the initial inoculum used to start the serial dilutions. In Figure 9 -2, the rightmost dish has 15 colonies. The dilution factor for a 10 -5 dilution is 105. Therefore, the initial number of bacteria is 15 × 105 per milliliter
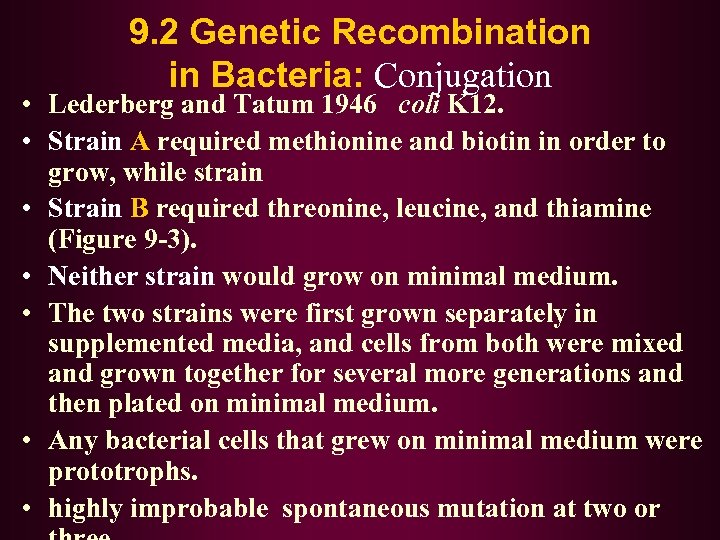
9. 2 Genetic Recombination in Bacteria: Conjugation • Lederberg and Tatum 1946 coli K 12. • Strain A required methionine and biotin in order to grow, while strain • Strain B required threonine, leucine, and thiamine (Figure 9 -3). • Neither strain would grow on minimal medium. • The two strains were first grown separately in supplemented media, and cells from both were mixed and grown together for several more generations and then plated on minimal medium. • Any bacterial cells that grew on minimal medium were prototrophs. • highly improbable spontaneous mutation at two or
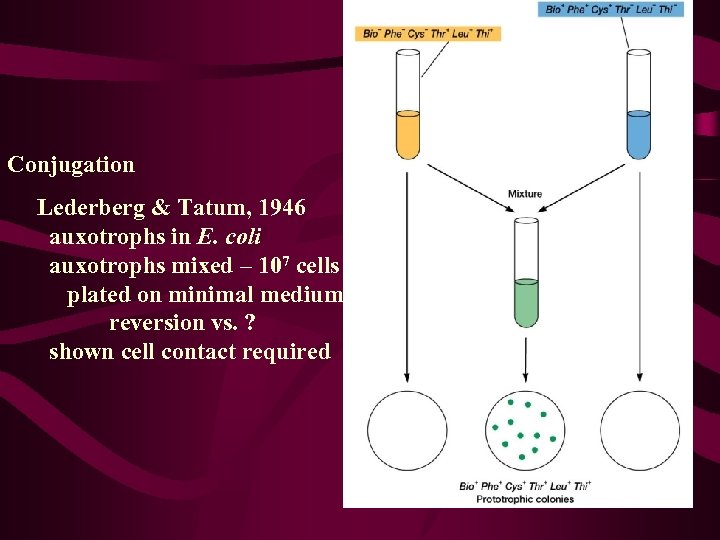
Conjugation Lederberg & Tatum, 1946 auxotrophs in E. coli auxotrophs mixed – 107 cells plated on minimal medium reversion vs. ? shown cell contact required
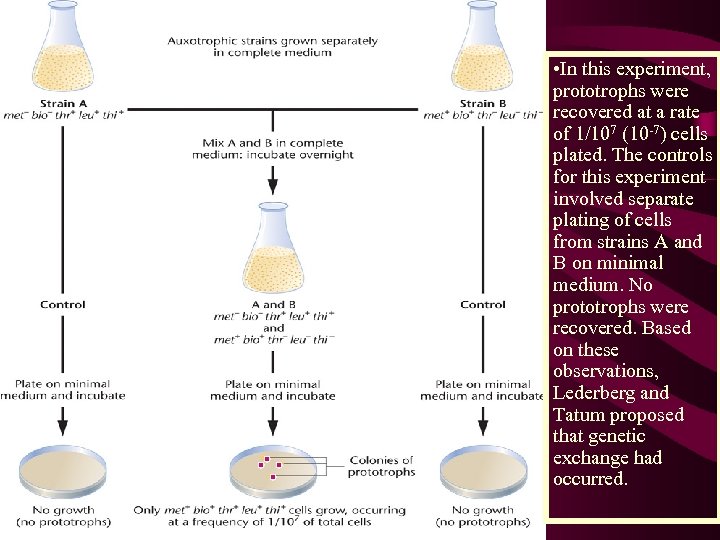
• In this experiment, prototrophs were recovered at a rate of 1/107 (10 -7) cells plated. The controls for this experiment involved separate plating of cells from strains A and B on minimal medium. No prototrophs were recovered. Based on these observations, Lederberg and Tatum proposed that genetic exchange had occurred.
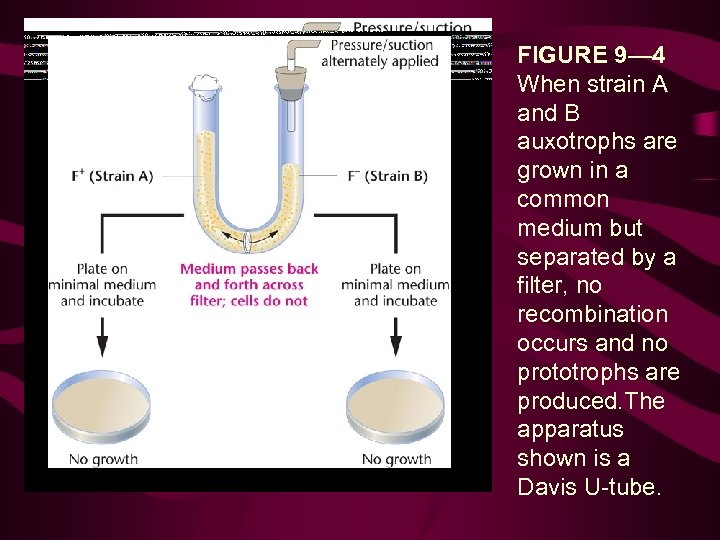
FIGURE 9— 4 When strain A and B auxotrophs are grown in a common medium but separated by a filter, no recombination occurs and no prototrophs are produced. The apparatus shown is a Davis U-tube.
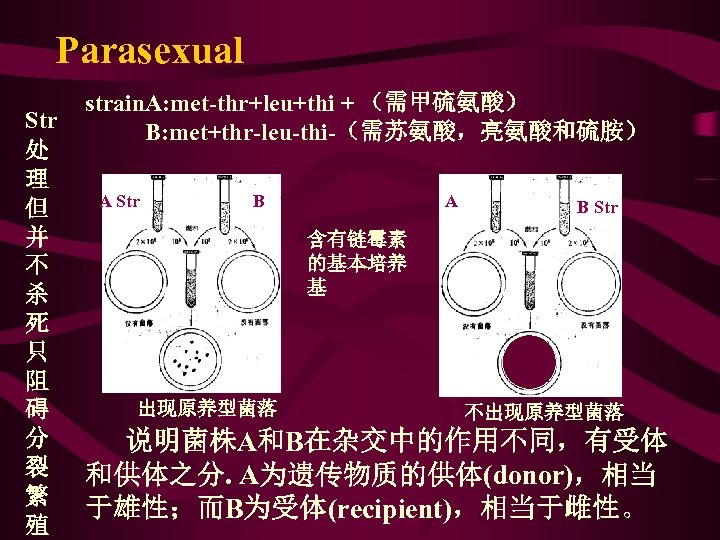
Parasexual Str 处 理 但 并 不 杀 死 只 阻 碍 分 裂 繁 殖 strain. A: met-thr+leu+thi + (需甲硫氨酸) B: met+thr-leu-thi-(需苏氨酸,亮氨酸和硫胺) A Str B A B Str 含有链霉素 的基本培养 基 出现原养型菌落 不出现原养型菌落 说明菌株A和B在杂交中的作用不同,有受体 和供体之分. A为遗传物质的供体(donor),相当 于雄性;而B为受体(recipient),相当于雌性。
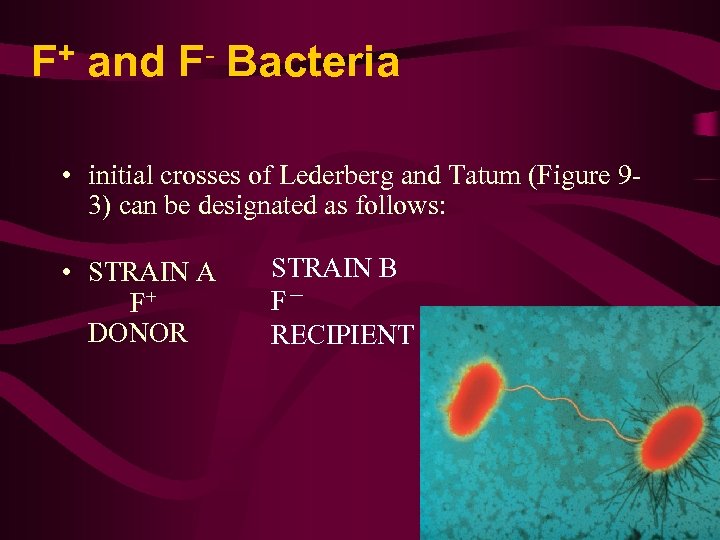
+ F and F Bacteria • initial crosses of Lederberg and Tatum (Figure 93) can be designated as follows: • STRAIN A F+ DONOR STRAIN B F- RECIPIENT
• Isolation of the F factor confirmed these conclusions. • Like the bacterial chromosome, though distinct from it, the F factor has been shown to consist of a circular, double-stranded DNA molecule, equivalent to about 2 percent of the bacterial chromosome (about 945, 000 nucleotide pairs). Contained in the F factor, among others, are 19 genes, the products of which are involved in the transfer of genetic information. These include those essential to the formation of the sex pilus. As we soon shall see, the F factor is in reality an autonomous genetic unit referred to as a plasmid. However, in our historical coverage of its discovery, we will continue to refer to it as a "factor. "
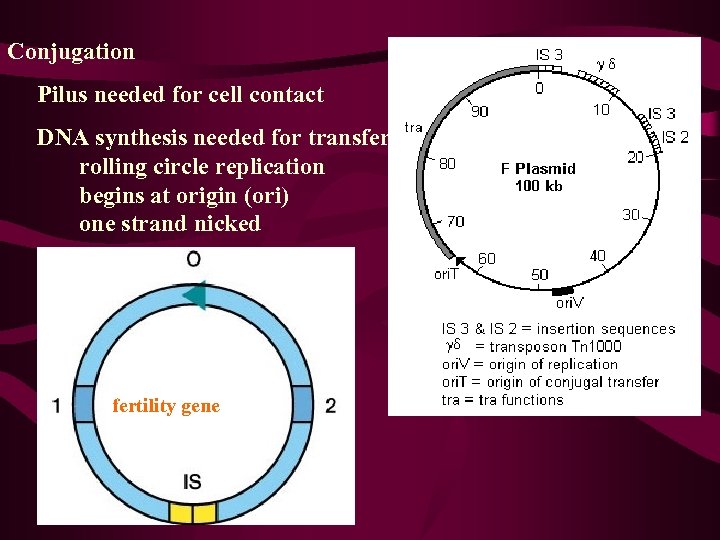
Conjugation Pilus needed for cell contact DNA synthesis needed for transfer rolling circle replication begins at origin (ori) one strand nicked fertility gene
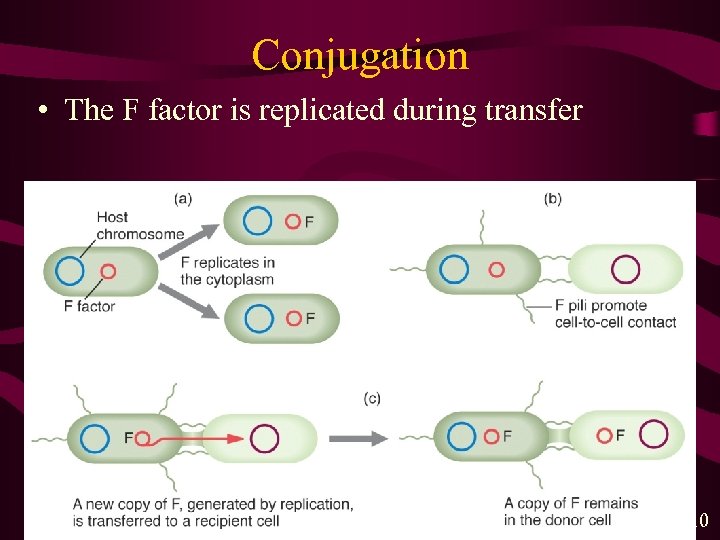
Conjugation • The F factor is replicated during transfer MGA 2 e Fig. 4 -10
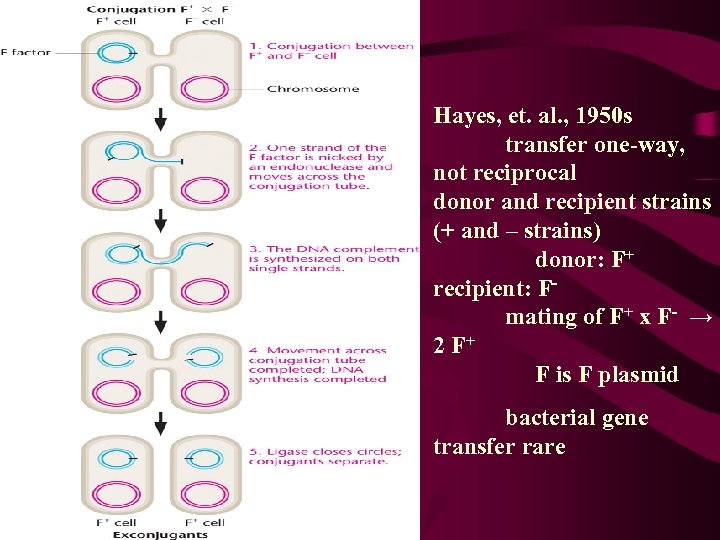
Hayes, et. al. , 1950 s transfer one-way, not reciprocal donor and recipient strains (+ and – strains) donor: F+ recipient: Fmating of F+ x F- → 2 F+ F is F plasmid bacterial gene transfer rare
• To summarize, an E. coli cell may or may not contain the F factor. When this factor is present, the cell is able to form a sex pilus and potentially serves as a donor of genetic information. During conjugation, a copy of the F factor is almost always transferred from the F+ cell to the F - recipient, converting it to the F+ state. The question remained as to exactly why such a low proportion of cells involved in these matings (10-7) also results in genetic recombination. The answer awaited further experimentation.
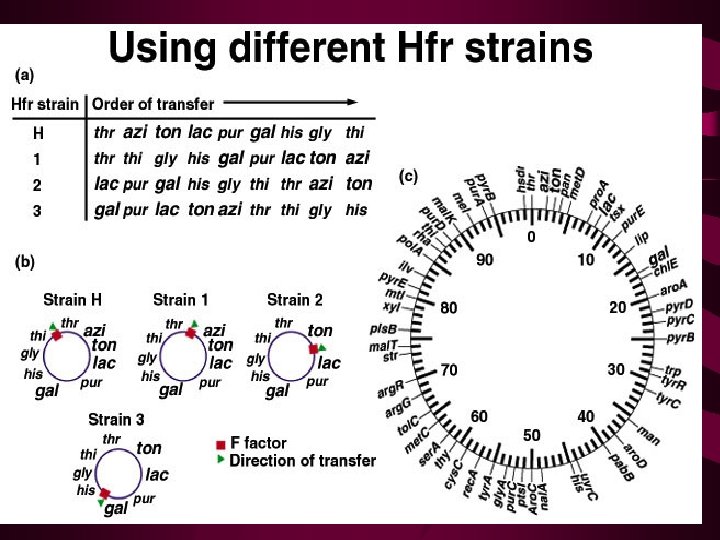
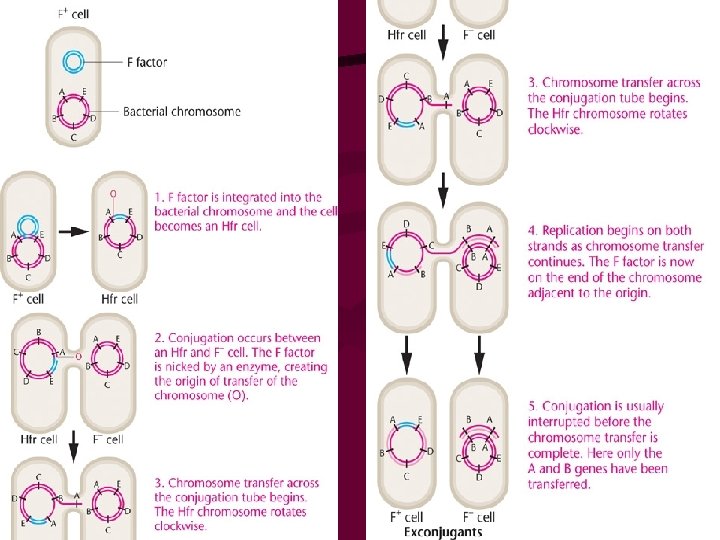
Hfr Bacteria and Chromosome Mapping • In 1950, Cavalli-Sforza treated an F+ strain of E. coli K 12 with nitrogen mustard, a chemical known to induce mutations. From these treated cells, he recovered a genetically altered strain of donor bacteria that underwent recombination at a rate of 1/104 (or 10-4), 1000 times more frequently than the original F+ strains. • In 1953, Hayes isolated another strain that demonstrated an elevated frequency. Both strains were designated Hfr, for high-frequency recombination. Because Hfr cells behave as donors, they are a special class of F+ cells.
• F+ × F- —>F+ (low rate of recombination) Hfr × F- —>F- (higher rate of recombination) • In the mid-1950 s, experimentation by Ellie Wollman and Francois Jacob explained the difference between Hfr and F+
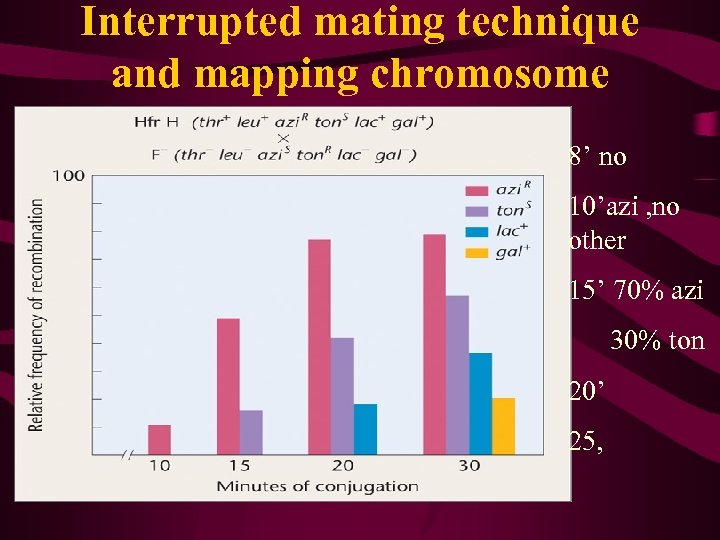
Interrupted mating technique and mapping chromosome 8’ no 10’azi , no other 15’ 70% azi 30% ton 20’ 25,
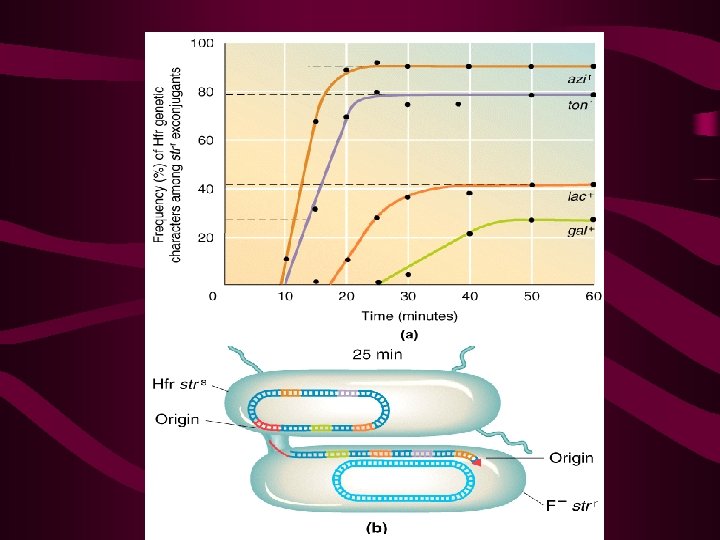
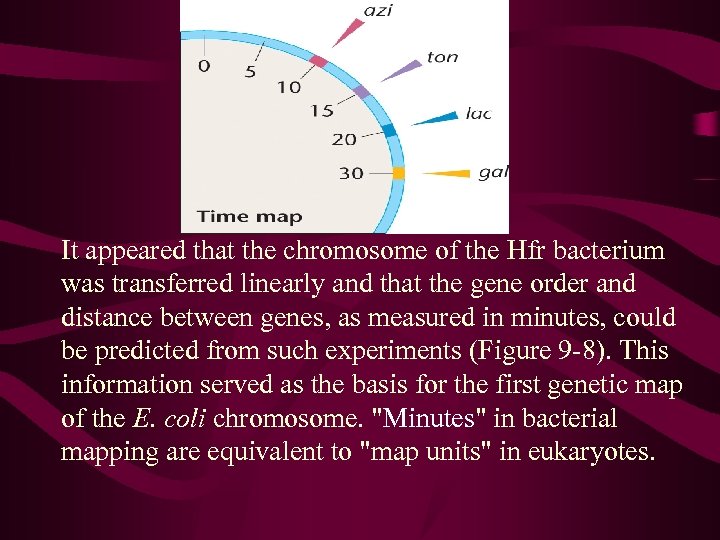
It appeared that the chromosome of the Hfr bacterium was transferred linearly and that the gene order and distance between genes, as measured in minutes, could be predicted from such experiments (Figure 9 -8). This information served as the basis for the first genetic map of the E. coli chromosome. "Minutes" in bacterial mapping are equivalent to "map units" in eukaryotes.
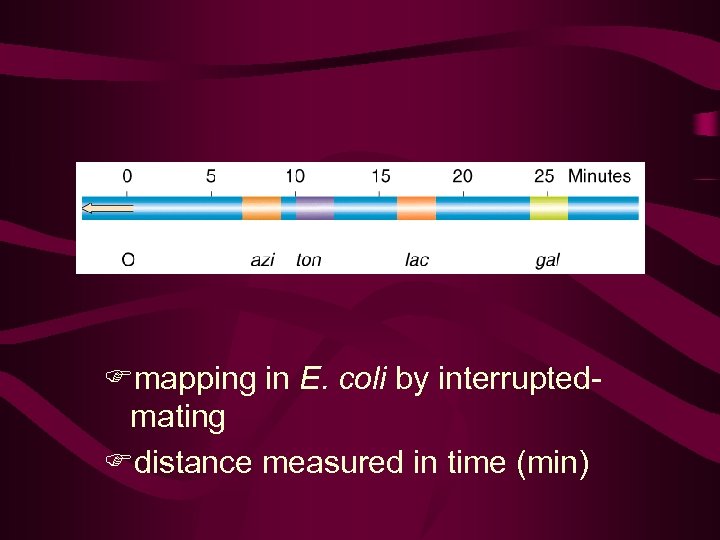
Fmapping in E. coli by interruptedmating Fdistance measured in time (min)
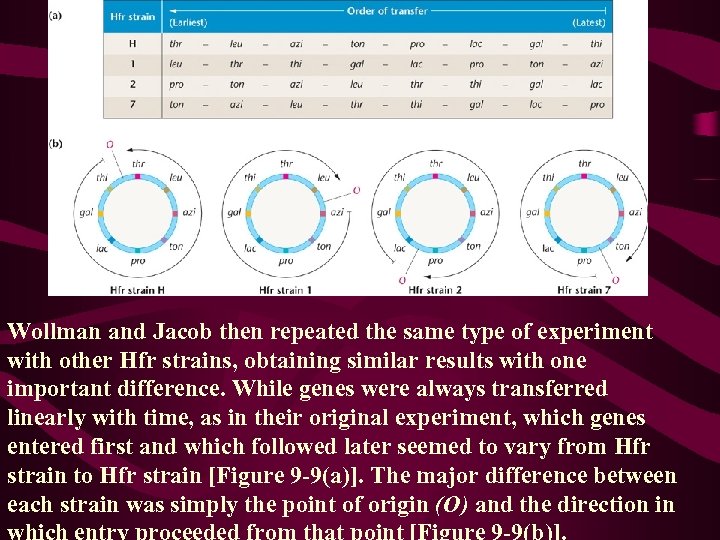
Wollman and Jacob then repeated the same type of experiment with other Hfr strains, obtaining similar results with one important difference. While genes were always transferred linearly with time, as in their original experiment, which genes entered first and which followed later seemed to vary from Hfr strain to Hfr strain [Figure 9 -9(a)]. The major difference between each strain was simply the point of origin (O) and the direction in
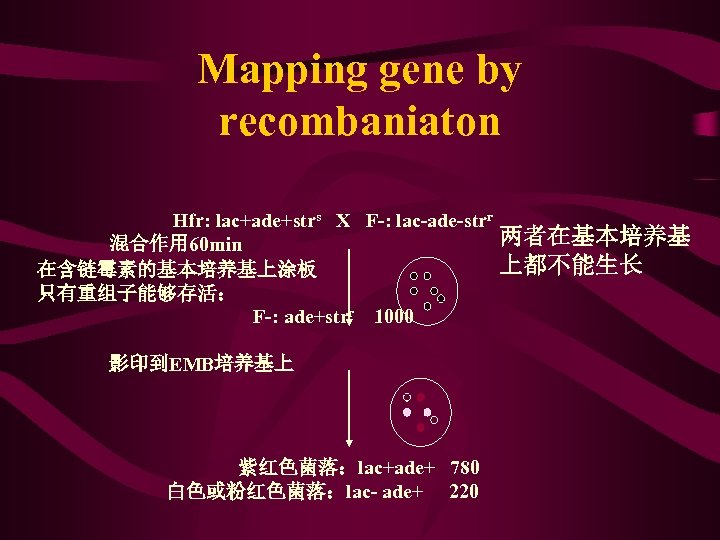
Mapping gene by recombaniaton Hfr: lac+ade+strs X F-: lac-ade-strr 混合作用 60 min 在含链霉素的基本培养基上涂板 只有重组子能够存活: F-: ade+strr 1000 影印到EMB培养基上 紫红色菌落:lac+ade+ 780 白色或粉红色菌落:lac- ade+ 220 两者在基本培养基 上都不能生长
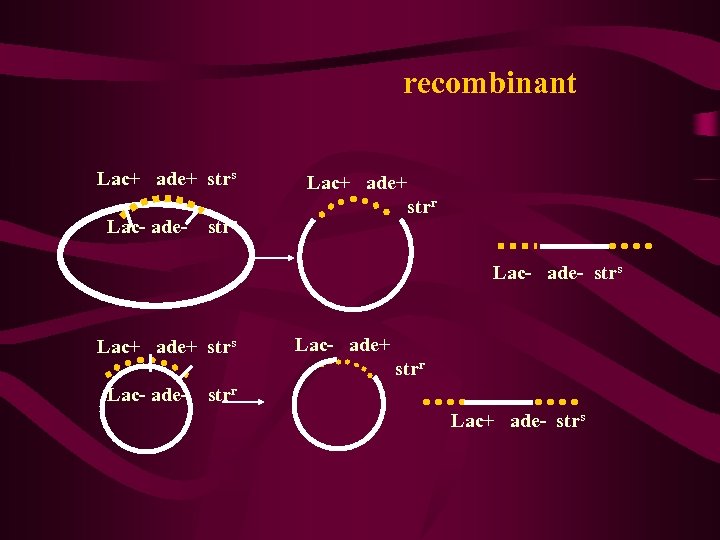
recombinant Lac+ ade+ strs Lac- ade- strr Lac+ ade+ strr Lac- ade- strs Lac+ ade+ strs Lac- ade+ strr Lac- ade- strr Lac+ ade- strs


重组频率的计算 lac-ade+ RFlac-ade = lac+ade++lac-ade+ *100% lac-ade+ = *100% + ade 220 = *100%=22 c. M 1000 用重组频率(RF)所测得的基因距离与用中断杂 交技术以时间为单位的基因距离基本上是成正比的, 大致是 1分钟相当于20%重组值,即: 1 min=20 c. M

大肠杆菌的遗传图谱 根据中断杂交实验和基因重组实验,以及其 他基因定位技术的结果,已经绘制出大肠杆菌的 环形遗传学图,即基因连锁图,图据单位为分钟, 以thr座位为起点(0分钟),总长度为 100分钟。 图中标出了常用的52个基因座位。。
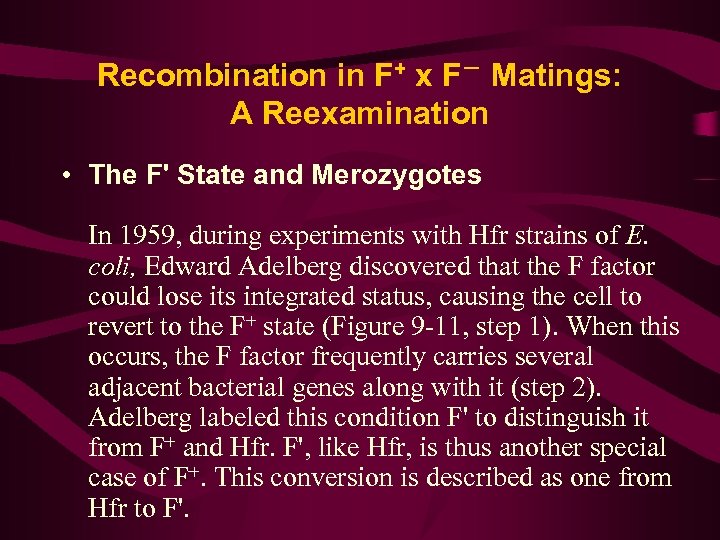
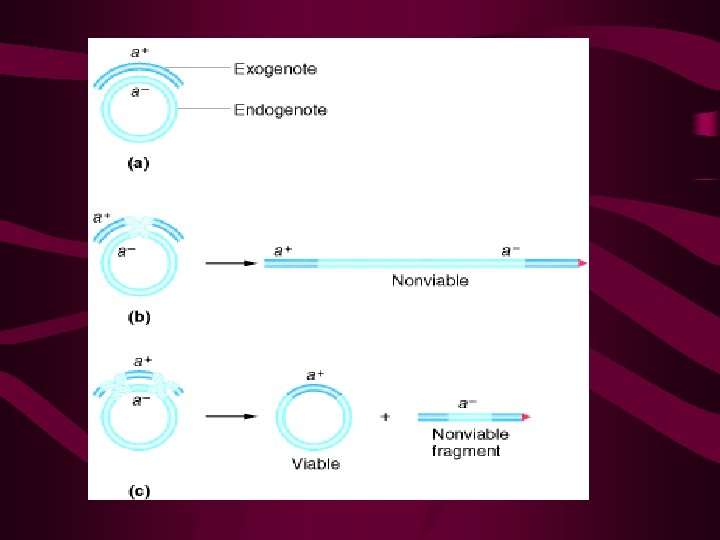
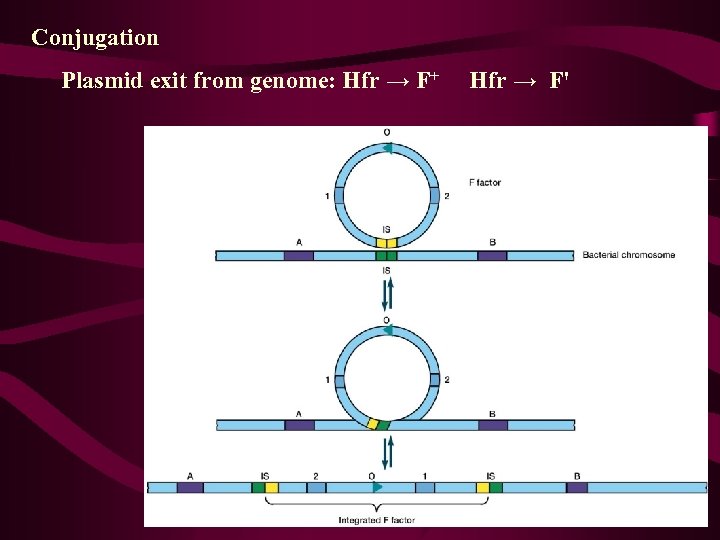
Recombination in F+ x F- Matings: A Reexamination • The F' State and Merozygotes In 1959, during experiments with Hfr strains of E. coli, Edward Adelberg discovered that the F factor could lose its integrated status, causing the cell to revert to the F+ state (Figure 9 -11, step 1). When this occurs, the F factor frequently carries several adjacent bacterial genes along with it (step 2). Adelberg labeled this condition F' to distinguish it from F+ and Hfr. F', like Hfr, is thus another special case of F+. This conversion is described as one from Hfr to F'.
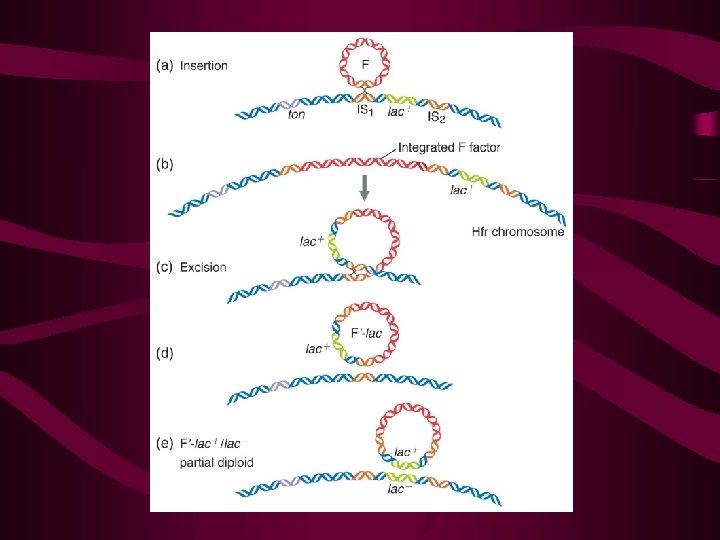

F’factor and sxe-duction 1. 1959年,Adel berg发现了一种新的F因 子,并称之为F’因子(F-prime factor), 这 是带有一小段细菌染色体基因的F因子。 它能使F-变成F’菌株,也能转移细菌基 因,形成部分二倍体,但是频率较Hfr为 低。 (如图) 2. 利用F’因子形成的部分二倍体,将供体细 胞基因导入受体的过程,叫做性导(sexduction or F’-duction)。

一、F´因子 • F+与Hfr两种菌株可以相互转变,也就是说F 因子既可以插入到染色体中去,形成Hfr菌株, 又可通过规则的交换和剪切,从染色体上完 整地游离下来形成F+菌株(图 6 -12),但是 偶尔也会出现不规则的环出,形成F因子携带 一段相邻的细菌染色体(图 6 -13)。这种带 有插入细菌基因的环状F因子是一个复制子, 这种新的F因子称为F´因子(F´-factor)。

• 已知F因子在细菌染色体上插入位置不同而构成 不同的Hfr品系,由此可形成不同的F´因子。 • 它们各自携带细菌的不同基因,有的F´因子携 带着干个基因的一大段细菌的染色体。 • F´与λdgal或λdbio颗粒不同,F´携带细菌的 基因,但并不减少本身的基因,如果本身的基 因丢失,转移就可能停止了。 • 此外F´因子也不存在蛋白质外壳包装的问题, 所以它的长度不为包装所限制,可以携带不同 长度的细菌DNA片段。 • F´因子和F+一样是能感染的,并把F因子转移给 F-细胞,同时也能转移细菌基因,其结果使F变成F´菌株,并形成部分二倍体。

二、性导 F´因子转移细菌基因不同于Hfr菌株。如比较下列两 个杂交结果: • (1)Hfr:thr+leu+strs×F-:thr-leu-strr • 结果:筛选出F-:thr+leu+strr重组子 • (2)F´:thr+leu+strs×F-:thr-leu-strr • 结果:筛选出F´:thr+leu+strr重组子 • 这两个杂交一个是Hfr菌株,另一个是来源于它的 F´菌株。杂交中把混合培养物涂布在含链霉素的 基本培养基上,第一个杂交选出的菌株都是F-,因 此不能将thr+leu+转入F-菌株,而第二个杂交选出 的菌株都带有活性的F´因子,能与其他F-菌株杂交, 并能将thr+leu+转入F-菌株,而且这些菌株也具有 F´因子,所以都是F+,仍具有感染能力。

• 产生F´因子的Hfr菌株仍保持单倍体状态, 当F´因子转入到受体细胞之后,由于引入 了供体细胞的部分基因,从而构成了部分 二倍体。如图 6 -13中F´lac+可转移到F-lac后构成F´lac+/F-lac-部分二倍体。这种利 用F因子将供体细胞的基因导人受体形成部 分二倍体的过程叫性导(sexduction或F´duction)。

• 性导在大肠杆菌的遗传学分析中十分有用。 这种部分二倍体如果不发生重组,那么 • ①F´因子自主复制,可在细菌细胞中延续下 去; • ②性导所形成的部分二倍体可用作不同突变 型之间的互补测验,以确定这两个突变型是 属于同一个基因或者是两个不同的基因; • ③观察由性导形成的杂合的部分二倍体中某 一性状的表现,可以确定这一性状的等位基 因中的显隐性关系;

• ④不同的F´因子带有不同的细菌DNA片段, 因此利用不同的F´因子性导可以测定不 同基因在一起性导的频率来作图。部分 二部体中也会出现重组,即F´因子所带 的供体细菌染色体同受体细菌染色体之 间的同源重组,如果发生单交换,就导 致F´整合形成Hfr品系,同时F´因子上所 携带的基因发生重组;如果双交换,则 形成F´品系,只是F´因子的细菌基因和 受体染色体上的等位基因之间发生互换。

三种致育因子F, F’,Hfr的关系是: (1). 有F因子的细菌为F+,没有F因子的为F-,具有致育因 子(F, F’或Hfr)的菌株就是雄性菌株(male strains) 。 (2). F因子可以整合到细菌染色体上,形成Hfr染色体。不 同的Hfr菌株F因子的整合位点不同。 (3). F因子又可以从Hfr染色体上剪切下来,产生F因子。 如果剪切不准确而带有一段细菌染色体,则称为F‘因 子。 (4). F因子很容易转移到F-细胞中, F+ × F- F+,但是供 体染色体的转移频率则很低, 重组频率很低。 (5). Hfr能以高频率把细菌染色体基因转移到F-细菌中,却 极少使F-变为F+(因为F因子位于Hfr染色体的最末端) (6). F‘因子的性质介于F+和Hfr之间,即可转移自身,又 可以转移细菌基因。但频率较低。
Conjugation F+ x F- Hfr x F-
Conjugation Plasmid exit from genome: Hfr → F+ Hfr → F'
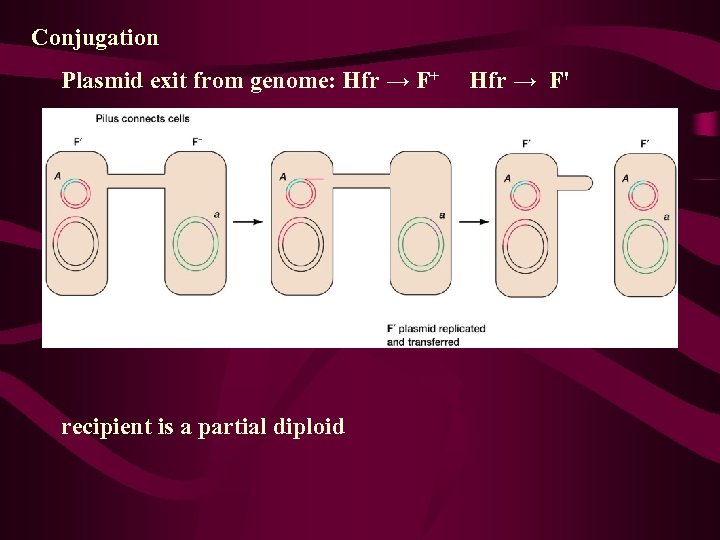
Conjugation Plasmid exit from genome: Hfr → F+ Hfr → F' recipient is a partial diploid

Recombination bringing new gene combinations together Eukaryotes - crossing over during meiosis reciprocal exchange Prokaryotes - transfer of genes from one cell to another one-way transfer of genes DNA transferred: exogenote recipient DNA: endogenote partial diploid may be formed Transformation Conjugation Transduction Limited transfer: one gene to a few genes closely related cells
9. 3 Rec Proteins and Bacterial Recombination • Once researchers established that a unidirectional transfer of DNA occurs between bacteria, they were interested in determining how the actual recombination event occurs in the recipient cell. Just how does the donor DNA replace the comparable region in the recipient chromosome? As with many systems, the biochemical mechanism by which recombination occurs was deciphered through genetic studies. Major insights were gained as a result of isolating a group of mutations representing rec genes.

• The first relevant observation in this case involved a series of mutant genes labeled rec. A, rec. B, rec. C, and rec. D. • The first mutant gene, rec. A, Rec. A protein diminished genetic recombination in bacteria 1000 -fold, nearly eliminating it altogether. • The other rec mutations Rec. BCD protein reduced recombination by about 100 times. Clearly, the normal wild-type products of these genes play some essential role in the process of recombination.
9. 4 F Factors and Plasmids • These characteristics place the F factor in the more general category of genetic structures called plasmids. These structures contain one or more genes—often, quite a few. Their replication depends on the same enzymes that replicate the chromosome of the host cell, and they are distributed to daughter cells along with the host chromosome during cell division. • Plasmids are generally classified according to the genetic information specified by their DNA. The F factor confers fertility and contains genes essential for sex pilus formation, upon which genetic recombination depends. Other examples of plasmids include the R and the Col plasmids.

• 染色体DNA作为细胞中的主要遗传因子,携带有 在所有生长条件下所必需的基因,这些基因有时 称之为“持家基因”(housekeeping gene),而质粒所 含的基因对宿主细胞一般是非必需的,只是在某 些特殊条件下,质粒能赋予宿主细胞以特殊的机 能,从而使宿主得到生长优势。例如抗药性质粒 和降解性质粒能使宿主细胞在具有相应药物或化 学毒物的环境中生存,而且在细胞分裂时恒定的 传递给子代细胞。 • 根据质粒所编码的功能和赋予宿主的表型效 应,可将其分为各种不同的类型:

1. 致育因子(Fertility factor,F因子) 又称F质粒,其大小约 100 kb,这是最早发现的一种与大肠 杆菌的有性生殖现象(接合作用)有关的质粒。携带F质粒的菌株 称为F+菌株(相当 于雄性),无F质粒的菌株称为F-菌株(相当于 雌性)。F质粒整合到宿主细胞染色体上的菌株称之为高频重组 菌株(high frequence recombination, 简称Hfr)。由于F因子能以 游离状态(F+)和以与染色体相结合的状态(Hfr)存在于细胞中, 所以又称之为附加体(episome)。 F质粒在大肠杆菌的接合作用 (conjugation)中起主要作用。当Hfr菌株上的F因子通过重组回 复成自主状态时,有时可将其相邻的染色体基因一起切割下来, 而成为携带某一染色体基因的F因子,例如F-lac、F-gal、Fpro等。因此将这些携带不同基因的F因子统称为F′,带有这些 F′因子的菌株也常用F′表示。

2. 抗性因子(Resistance factor,R因子) • 这是另一类普遍而重要的质粒,主要包括抗药性和 抗重金属二大类,简称R质粒。带有抗药性因子的细菌 有时对于几种抗生素或其他药物呈现抗性。例如R 1质粒 (94 kb)可使宿主对下列五种药物具有抗性:氯霉素 (Chlorampenicol, Cm)、链霉素(Streptomycin, Sm)、磺 胺(Sulfonamide, Su)、氨苄青霉素(Ampicillin, Ap)和卡 那霉素(Kanamycin, Km),并且负责这些抗性的基因是 成簇地存在于R 1抗性质粒上。 • 许多R质粒能使宿主细胞对许多金属离子呈现抗性, 包括碲(Te 6+)、砷(As 3+) 、汞(Hg 2+)、镍(Ni 2+)、钴 (Co 2+)、银(Ag+)、镉(Cd 2+)等。 在肠道细菌中发现的 R质粒,约有25%是抗汞离子的,而铜绿假单胞菌中约 占 75%。

• 3. Col质粒 • 因这类质粒首先发现于大肠杆菌中 而得名,该质粒含有编码大肠菌素的基 因,大肠菌素是一种细菌蛋白,只杀死 近缘且不含Col质粒的菌株,而宿主不 受其产生的细菌素的影响。由G+细菌 产生的细菌素通常也是由质粒基因编码, 有些甚至有商业价值,例如一种乳酸细 菌产生的细菌素Nisin. A能强烈抑制某些 G+细菌的生长,而被用于食品 业的 保藏。

4. 毒性质粒(virulence plasmid • 现在越来越多的证据表明,许多致病菌的致病性是由其所携带的质 粒引起的,这些质粒具有编码毒素的基因,例如产毒素大肠杆菌是引起 人类和动物腹泻的主要病原菌之一,其中许多菌株含有为一种或多种肠 毒素编码的质粒。有些使昆虫 致病乃至死亡的细菌毒素也是由质粒编码 的,苏云金杆菌产生的毒素是这种类型的典型例子 。研究表明,苏云金 杆菌含有编码δ内毒素(伴孢晶体中)的质粒,伴孢晶体的结构基因及调节 基因位于质粒上。 • 此外,目前广泛应用于转基因植物载体的是一种经过人 改造后的 Ti质粒(tumor-inducing-plasmid)(见第十章),Ti质粒是引起双子叶植物 冠瘿瘤的致病因子,其宿主是一种根癌土壤杆菌(Agrobacterium tumefaciens)。是引起植物冠瘿瘤的致病因子,其机制是Ti质粒上的一段 特殊DNA片段转移至植物细胞内并整合其染色体上,导致细胞无控制的 瘤状增生,合成正常植物所没有的冠瘿碱(opines)化合物,该DNA片段 称为T-DNA其上含有三个致癌基因。 发根土壤杆菌 (Agrobacterium rhizogenes)引起许多双子叶植物患毛根瘤,而致病因子是该菌所含的Ri 质粒。Ri质粒在功能上与Ti质粒有广泛的同源性,也有一段特殊的DNA 片段(T-DNA),在侵染过程中,能转移进植物基因组,也可用于转基因 植物载体。

5. 代谢质粒(Metabolic plasmid) • 这类质粒上携带有能降解某些基质的酶的基因,含有 这类质粒的细菌,特别是假单胞菌,能将复杂的有机化 合物降解成能被其作为碳源和能源利用的简单形式。尤 其是对一些有毒化合物,如芳香簇化合物(苯)、农药(2, 4 dichlorophenox yacetic acid)、辛烷和樟脑等的降解,在 环境保护方面具有重要的意义(见第十一章)。因此这类质 粒也常被称为降解质粒,每一种具体的质粒常以其降解 的底物而命名。如樟脑质粒 (camphor, CAM)、辛烷质粒 (octadecane, OCT)、二甲苯质粒(xylene, XYL)等。 • 此外,代谢质粒中还包括一些能编码固氮功能的质粒。 例如根瘤菌中与结瘤(nod)和固氮(fix)有关的所有基因均 位于共生质粒中。放线菌中也已发现许多大的线型质粒( ~ 500 kb以上) 含有抗生素合成的基因。

6. 隐秘质粒(cryptic plasmid) • 以上所讨论的质粒类型均具有某种可检测的遗传表型, 但隐秘质粒不显示任何表型效应,它们的存在只有通过物 理的方法,例如用凝胶电泳检测细胞抽提液等方法才能发 现。他们存在的生物学意义,目前几乎不了解。酵母的 2μm质粒不授予宿主任何表型效应,也属于隐秘型质粒。 • 除了根据质粒赋予宿主的遗传表型将质粒分成不同类 型外,还可根据质粒的拷贝数、宿主范围等将质粒分成不 同类型。例如:有些质粒在每个宿主细胞中可以有10 -100 个拷贝,称为高拷贝数(high copy number)质粒,另一些质 粒在每个细胞中只有1 -4个拷贝,为低拷贝数(Low copy number)质粒。前者又称松弛型质粒(relaxed plasmid),后 者又称严谨型质粒(stringent plasmid)。此外,还有一些质 粒的复制起始点(origin of replication)较特异,只能在一种 特定的宿主细胞中复制,称为窄宿主范围质粒(narrow host range plasmid);复制起始点不太特异,可以在许多种细菌 中复制,称为广宿主范围质粒(broad host range plasmid)。 能整合进染色体而随染色体的复制而进行复制的质粒又称 附加体(episome)。
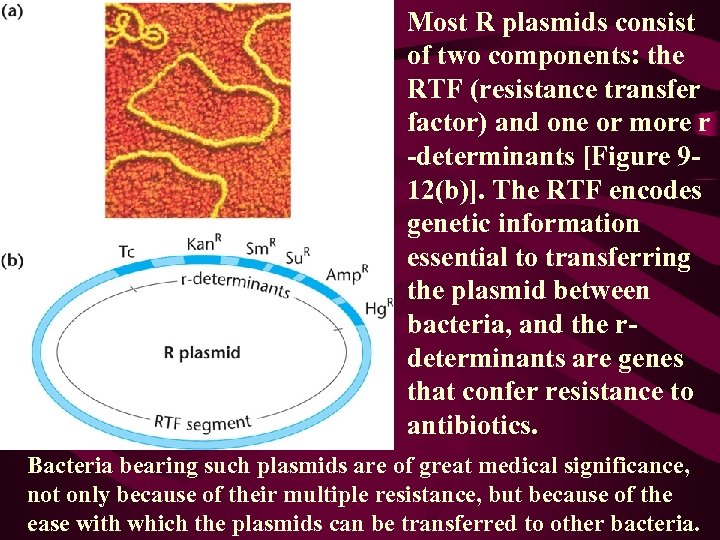
Most R plasmids consist of two components: the RTF (resistance transfer factor) and one or more r -determinants [Figure 912(b)]. The RTF encodes genetic information essential to transferring the plasmid between bacteria, and the rdeterminants are genes that confer resistance to antibiotics. Bacteria bearing such plasmids are of great medical significance, not only because of their multiple resistance, but because of the ease with which the plasmids can be transferred to other bacteria.
• While RTFs are similar to a variety of plasmids from different bacterial species, r-determinants, each of which is specific for resistance to one class of antibiotic, vary widely. • Sometimes, a bacterial cell contains r-determinant plasmids but no RTF. Such a cell is resistant but cannot transfer the genetic information for resistance to recipient cells. • The most commonly studied plasmids, however, contain the RTF as well as one or more rdeterminants. Resistance to tetracycline, streptomycin, ampicillin, sulfonamide, kanamycin, and chloramphenicol are most frequently encountered. Sometimes these occur in a single plasmid, conferring multiple resistance to several antibiotics [Figure 9 -12(b)].

The Col plasmid, Col. El, derived from E. coli, is clearly distinct from R plasmids. It encodes one or more proteins that are highly toxic to bacterial strains that do not harbor the same plasmid. These proteins, called colicins, can kill neighboring bacteria. Bacteria that carry the plasmid are said to be colicinogenic. Present in 10 -20 copies per cell, a gene in the plasmid encodes an immunity protein that protects the host cell from the toxin. Unlike an R plasmid, the Col plasmid is not usually transmissible to other cells.
Bacterial Gene Transfer • Conjugation • Transformation • Transduction Fconversion of one genotype to another by uptake of exogenous DNA Ftransformation principle – demonstrated that DNA was responsible for inherited differences in polysaccharide character of S. pneumoniae
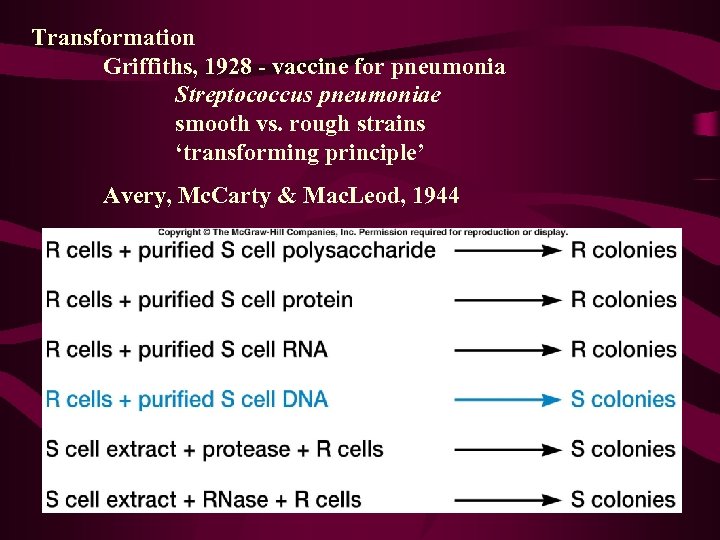
Transformation Griffiths, 1928 - vaccine for pneumonia Streptococcus pneumoniae smooth vs. rough strains ‘transforming principle’ Avery, Mc. Carty & Mac. Leod, 1944
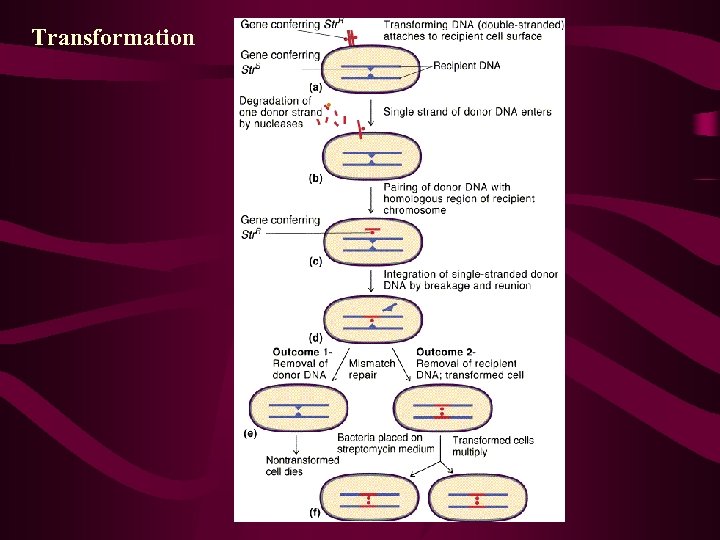
9. 5 Bacterial Transformation • Transformation also provides a mechanism for recombining genetic information in some bacteria. In transformation, small pieces of extracellular DNA are taken up by a living bacterium, ultimately leading to a stable genetic change in the recipient cell. We are interested in transformation in this chapter because, in those bacterial species where it occurs, the process can be used to map bacterial genes, although in a more limited way than conjugation.

l从Avery等(1944年)发现肺炎双球菌的转化作用 后,在其他属的细菌中也发现有这种转化作用,说 明在细菌中转化作用是一种十分普遍的现象。不过 一般说从一个供体菌株分离出来的DNA与另一受体 菌株活细胞接触,大约只有1%的受体细菌细胞可 吸收外源DNA,并发生遗传转化。转化频率低的原 因可能是: l(一)受体细菌细胞壁并非任何区域都允许外源 DNA片段通过,而只是在特定区域形成临时性通道, 因此将这一区域称为受体部位(receptor site), 而在受体细胞表面这一部位的数目是有限的;

• 二)外源DNA进人除受体部位允许通过外还必 须有酶或蛋白质分子以及能量等的协同作用。 实验证明,利用某些影响酶的因素如阻碍蛋白 质形成的氯霉素(chloramphenical)和阻碍 能量产生的二硝基苯(dinitrophenol)可抑 制转化作用。显然外源DNA只有在酶促旺盛的 受体部位进人。这种能接受外源DNA分子并被 转化的细菌细胞称为感受态细胞(competence cell),而促进转化作用的酶或蛋白质分子称 为感受态因子(competence factor)。
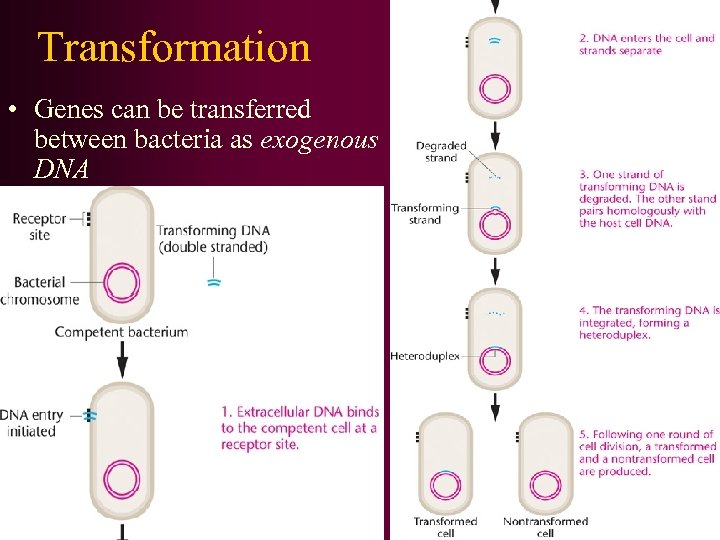
Transformation • Genes can be transferred between bacteria as exogenous DNA
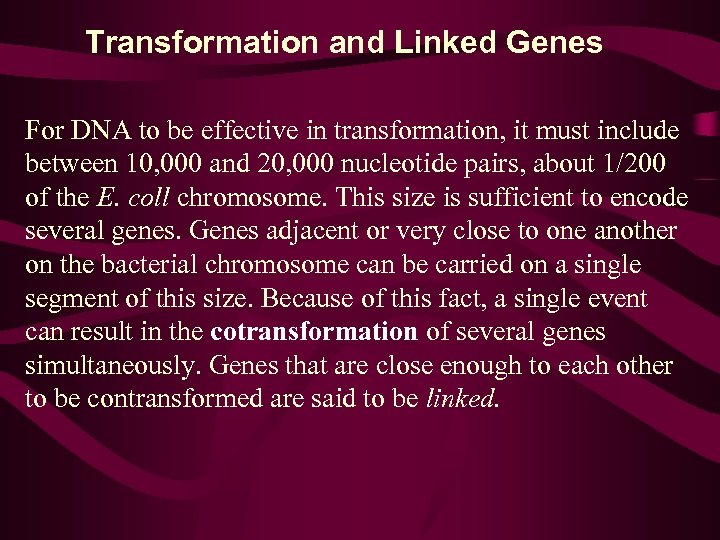
Transformation and Linked Genes For DNA to be effective in transformation, it must include between 10, 000 and 20, 000 nucleotide pairs, about 1/200 of the E. coll chromosome. This size is sufficient to encode several genes. Genes adjacent or very close to one another on the bacterial chromosome can be carried on a single segment of this size. Because of this fact, a single event can result in the cotransformation of several genes simultaneously. Genes that are close enough to each other to be contransformed are said to be linked.
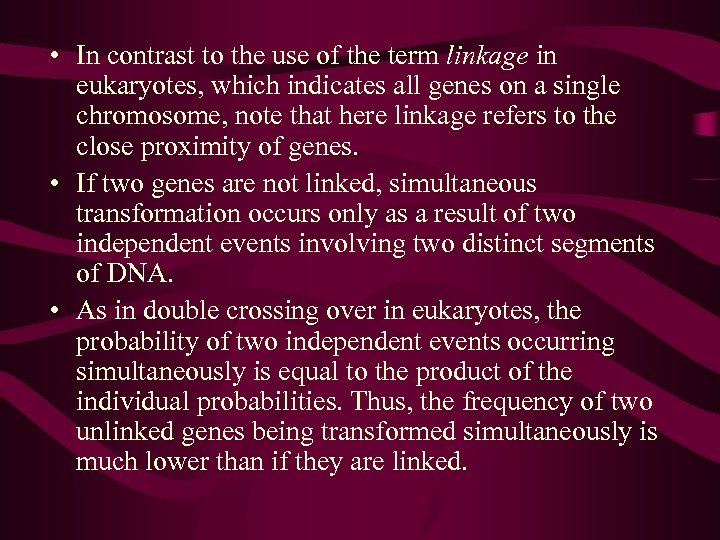
• In contrast to the use of the term linkage in eukaryotes, which indicates all genes on a single chromosome, note that here linkage refers to the close proximity of genes. • If two genes are not linked, simultaneous transformation occurs only as a result of two independent events involving two distinct segments of DNA. • As in double crossing over in eukaryotes, the probability of two independent events occurring simultaneously is equal to the product of the individual probabilities. Thus, the frequency of two unlinked genes being transformed simultaneously is much lower than if they are linked.
Transformation
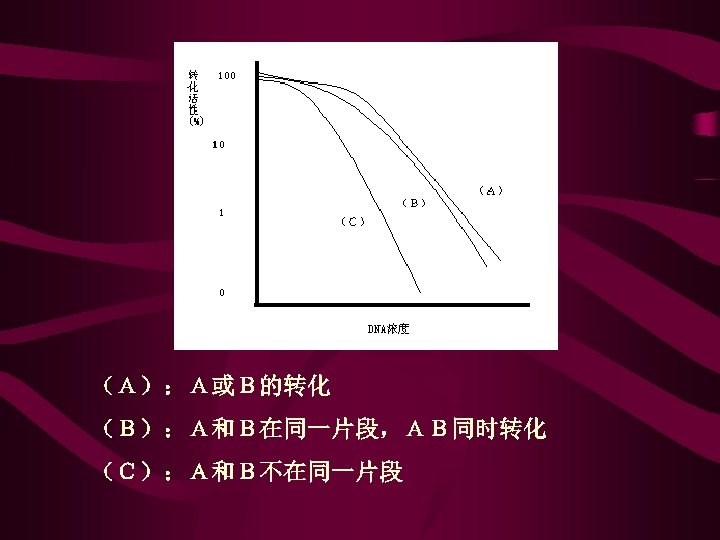
(A):A或B的转化 (B):A和B在同一片段,AB同时转化 (C):A和B不在同一片段

• 转化时供体细菌DNA断裂成小片段,这些片段平均长度 约为 20 000个核苷酸对,外源DNA片段进入受体后可以 和受体染色体形成部分二倍体,有可能发生重组,从 而使受体细胞发生稳定性的遗传转化。转化过程包括 几个连续的阶段: • ①双链DNA分子和细胞表面受体部位进行可逆性结合; ②供体DNA片段被吸入受体细胞,并要防止受体DNA酶 的破坏; • ③供体DNA进人受体后,立即从双链DNA转变成单链DNA, 其中一条单链被降解; • ④未被降解的一条单链DNA部分地或整个地插入受体细 胞的DNA链中,与同源区段形成杂合的DNA分子; • ⑤杂合DNA经复制、分离以后,形成一个受体亲代类型 的DNA和一个供体与受体DNA结合的杂种双链DNA,从而 导致基因重组形成各种类型的转化子(transformant )。

• 转化中的供体DNA片段往往可以携带若干个基因,这 些连锁基因可以同时转化,当然同时转化的基因不一 定都是连锁的,因为两个不连锁的不同DNA片段有可 能被同一个细菌细胞所吸收。但是可以区别这两个基 因是连锁的还是独立遗传的,一个可靠的证据是观察 DNA浓度降低时的转化频率的改变。如果A和B是连锁 的,那么当DNA浓度下降时,AB同时转化频率下降和A 或B转化频率下降程度相同;如果A和B并不连锁,那 么AB转化频率下降将远远超过A或B转化频率下降的程 度,这是因为在较低的浓度范围内,转化频率和转化 DNA的浓度成正比关系。如两个基因在同一DNA分子上, 那么浓度降低10倍时,两个基因同时转化的频率也将 减少 10倍;但是如果两个基因在不同的DNA片段上, 那么DNA浓度下降10倍时,两个基因同时转化的概率 将减少 100倍,而不是 10倍,由此可以确定A和B之间 是否连锁。确定连锁关系后就可通过转化实验进一步 作图
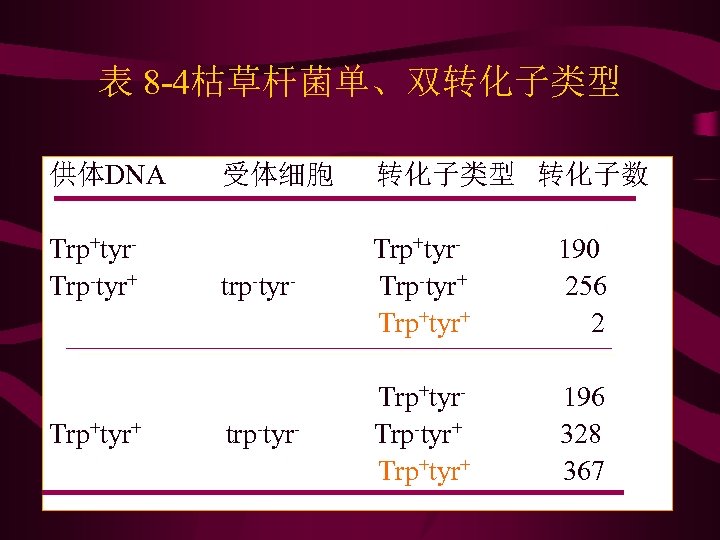
表 8 -4枯草杆菌单、双转化子类型 供体DNA Trp+tyr. Trp-tyr+ Trp+tyr+ 受体细胞 转化子类型 转化子数 trp-tyr- Trp+tyr. Trp-tyr+ Trp+tyr+ 190 256 2 trp-tyr- Trp+tyr. Trp-tyr+ Trp+tyr+ 196 328 367
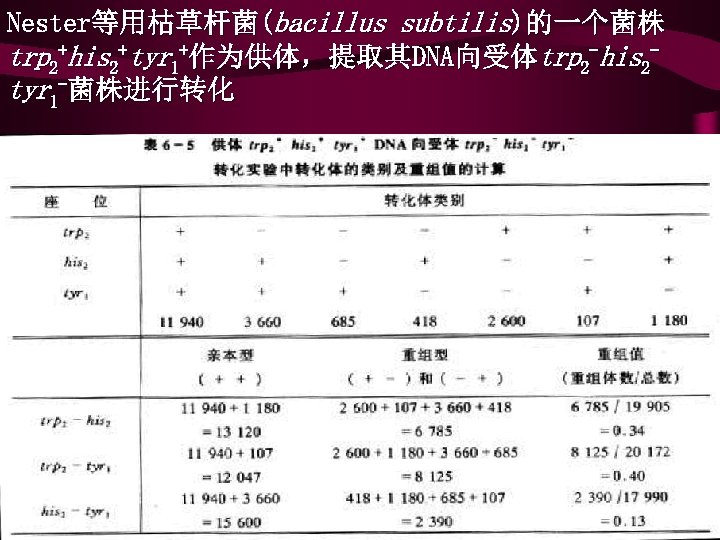
Nester等用枯草杆菌(bacillus subtilis)的一个菌株 trp 2+his 2+tyr 1+作为供体,提取其DNA向受体trp 2 -his 2 tyr 1 -菌株进行转化
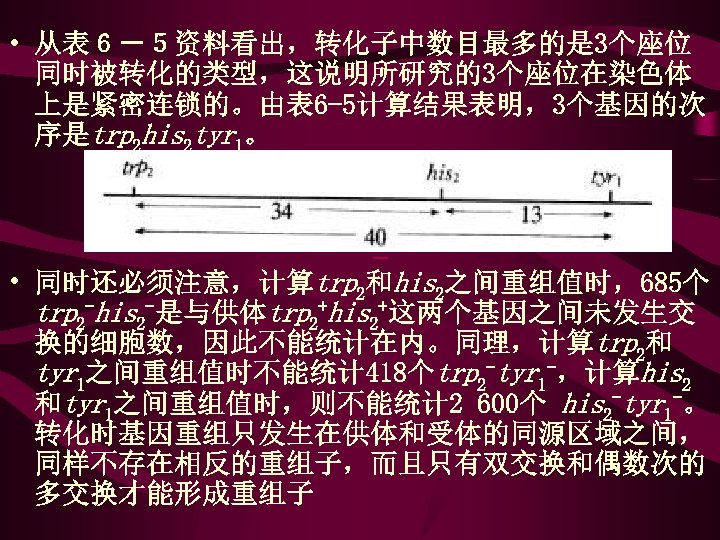
• 从表6-5资料看出,转化子中数目最多的是 3个座位 同时被转化的类型,这说明所研究的3个座位在染色体 上是紧密连锁的。由表 6 -5计算结果表明,3个基因的次 序是trp 2 his 2 tyr 1。 • • 同时还必须注意,计算trp 2和his 2之间重组值时,685个 trp 2 -his 2 -是与供体trp 2+his 2+这两个基因之间未发生交 换的细胞数,因此不能统计在内。同理,计算trp 2和 tyr 1之间重组值时不能统计 418个trp 2 -tyr 1 -,计算his 2 和tyr 1之间重组值时,则不能统计 2 600个 his 2 -tyr 1 -。 转化时基因重组只发生在供体和受体的同源区域之间, 同样不存在相反的重组子,而且只有双交换和偶数次的 多交换才能形成重组子
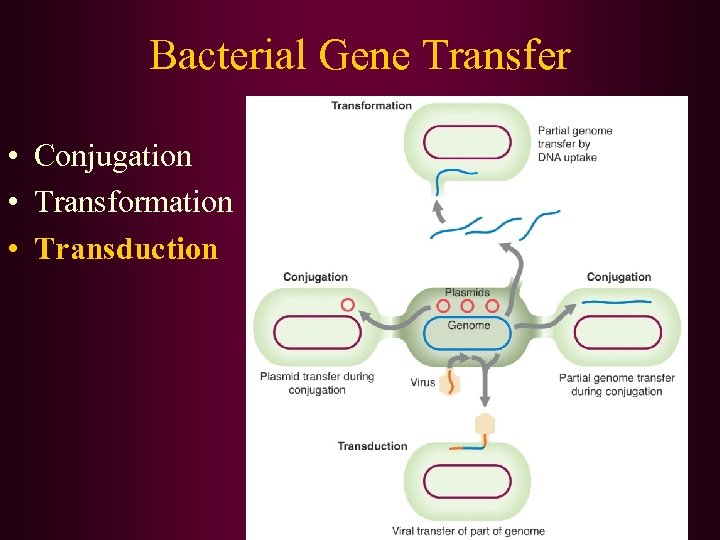
Bacterial Gene Transfer • Conjugation • Transformation • Transduction
9. 6 The Genetic Study of Bacteriophages Bacteria & Phage Genetics
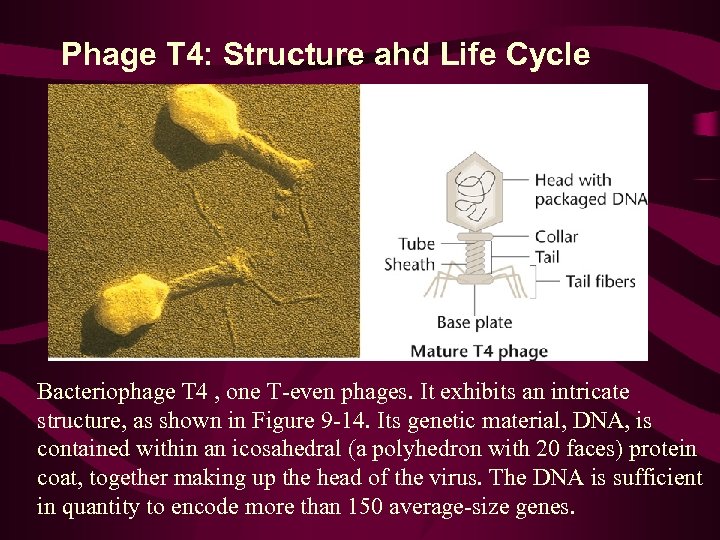
Phage T 4: Structure ahd Life Cycle Bacteriophage T 4 , one T-even phages. It exhibits an intricate structure, as shown in Figure 9 -14. Its genetic material, DNA, is contained within an icosahedral (a polyhedron with 20 faces) protein coat, together making up the head of the virus. The DNA is sufficient in quantity to encode more than 150 average-size genes.
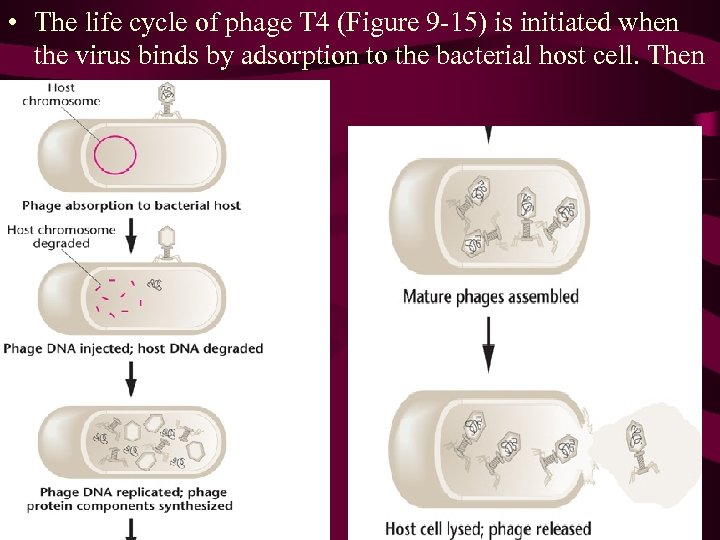
• The life cycle of phage T 4 (Figure 9 -15) is initiated when the virus binds by adsorption to the bacterial host cell. Then

The Plaque Assay • Bacteriophages and other viruses a critical role in our understanding of molecular genetics. • Often, over 1010 viruses are produced per milliliter of culture medium. Many genetic studies rely on the ability to quantify the number of phages produced following infection under specific culture conditions. The technique used routinely is called the plaque assay. This assay is shown in Figure 9 -16, where actual plaque morphology is also shown.
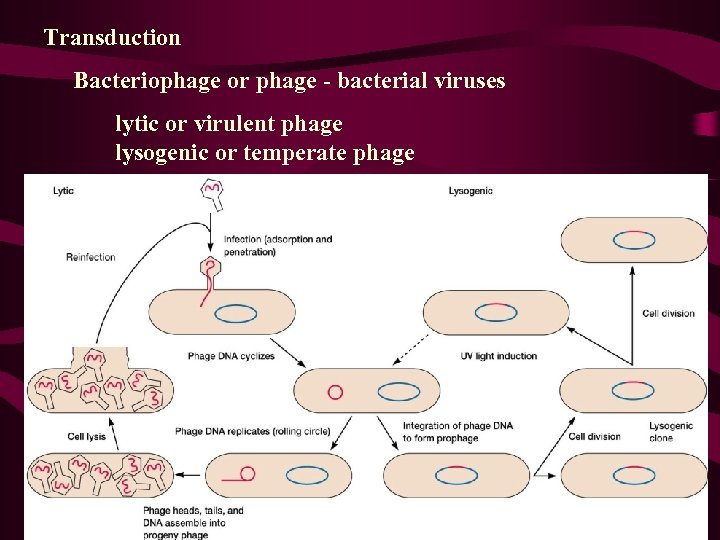
Transduction Bacteriophage or phage - bacterial viruses lytic or virulent phage lysogenic or temperate phage
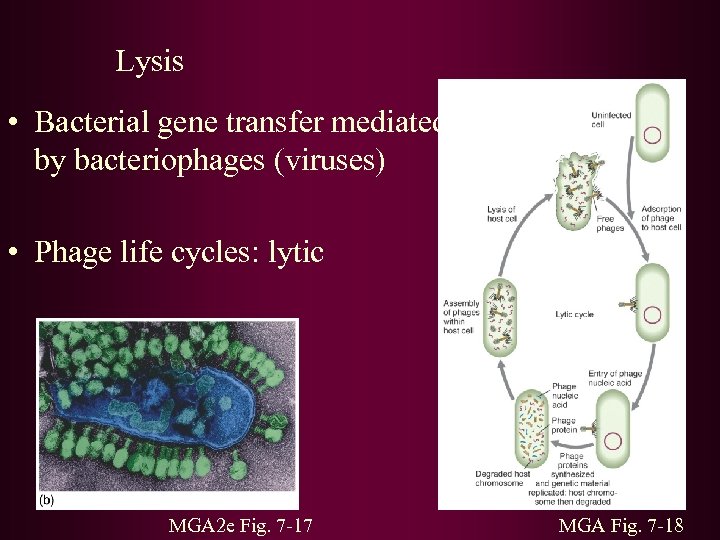
Lysis • Bacterial gene transfer mediated by bacteriophages (viruses) • Phage life cycles: lytic MGA 2 e Fig. 7 -17 MGA Fig. 7 -18
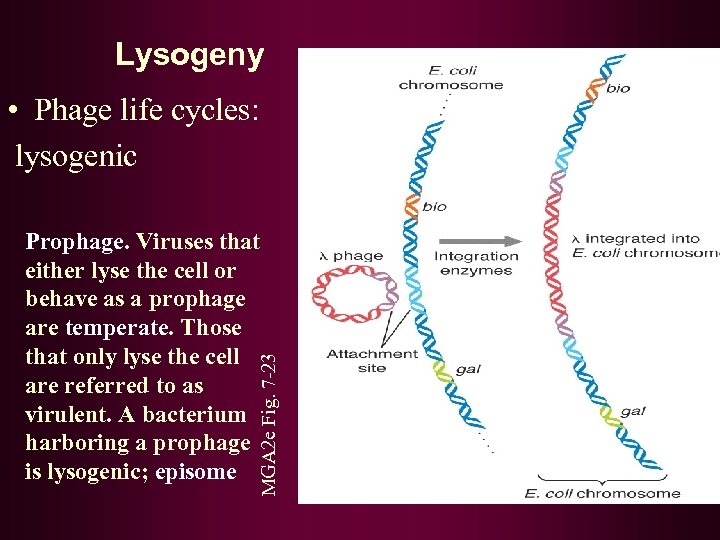
Lysogeny • Phage life cycles: lysogenic MGA 2 e Fig. 7 -23 Prophage. Viruses that either lyse the cell or behave as a prophage are temperate. Those that only lyse the cell are referred to as virulent. A bacterium harboring a prophage is lysogenic; episome
9. 7 Transduction: Virus-Mediated Bacterial DNA Transfer • In 1952, Norton Zinder and Joshua Lederberg were investigating possible recombination in the bacterium Salmonella typhimurium鼠伤寒沙门氏 杆菌. Although they recovered prototrophs from mixed cultures of two different auxotrophic strains, subsequent investigations revealed that recombination was occurring in a manner different from that attributable to the presence of an F factor, as in E. coli. What they discovered was a process of bacterial recombination mediated by bacteriophages and now called transduction.

The Lederberg-Zinder Experiment • Lederberg and Zinder mixed the Salmonella auxotrophic strains LA-22 and LA-2 together and, when the mixture was plated on minimal medium, they recovered prototrophic cells. • LA-22 (phe - trp - met+ his+) ), • LA-2 (phe+ trp+ met- his-). • Prototrophs (phe+ trp+ met+ his+) were recovered at a rate of about 1/105 (or 10-5) cells.
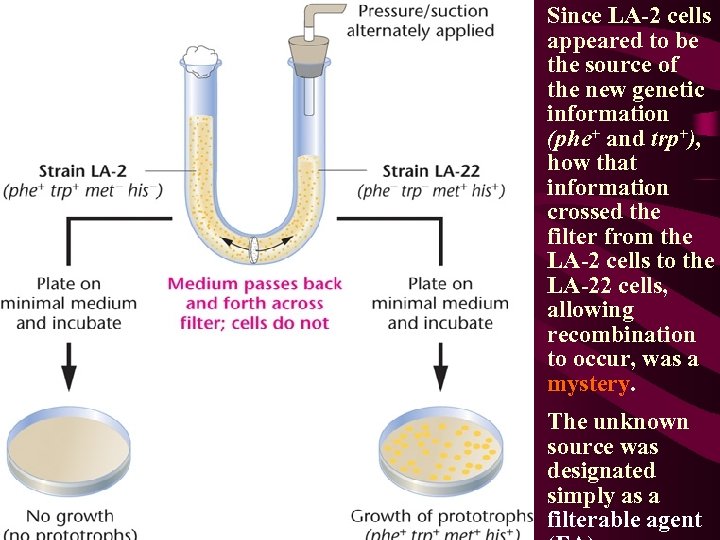
Since LA-2 cells appeared to be the source of the new genetic information (phe+ and trp+), how that information crossed the filter from the LA-2 cells to the LA-22 cells, allowing recombination to occur, was a mystery. The unknown source was designated simply as a filterable agent

• Three subsequent observations were useful in identifying the FA: • 1. The FA was produced by the LA-2 cells only when they were grown in association with LA-22 cells. If LA-2 cells were grown independently and that culture medium was then added to LA-22 cells, recombination did not occur. Therefore, LA-22 cells play some role in the production of FA by LA-2 cells and do so only when the two share common growth medium. • 2. The presence of DNase, which enzymatically digests DNA, did not render the FA ineffective. Therefore, the FA is not naked DNA, ruling out transformation. • 3. The FA could not pass across the filter of the Davis U-tube when the pore size was reduced below the size of bacteriophages.
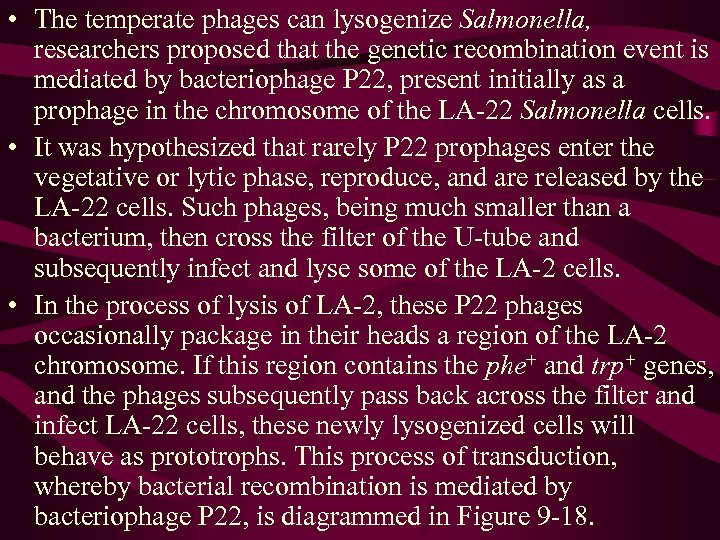
• The temperate phages can lysogenize Salmonella, researchers proposed that the genetic recombination event is mediated by bacteriophage P 22, present initially as a prophage in the chromosome of the LA-22 Salmonella cells. • It was hypothesized that rarely P 22 prophages enter the vegetative or lytic phase, reproduce, and are released by the LA-22 cells. Such phages, being much smaller than a bacterium, then cross the filter of the U-tube and subsequently infect and lyse some of the LA-2 cells. • In the process of lysis of LA-2, these P 22 phages occasionally package in their heads a region of the LA-2 chromosome. If this region contains the phe+ and trp+ genes, and the phages subsequently pass back across the filter and infect LA-22 cells, these newly lysogenized cells will behave as prototrophs. This process of transduction, whereby bacterial recombination is mediated by bacteriophage P 22, is diagrammed in Figure 9 -18.
Transduction Nature of transduction
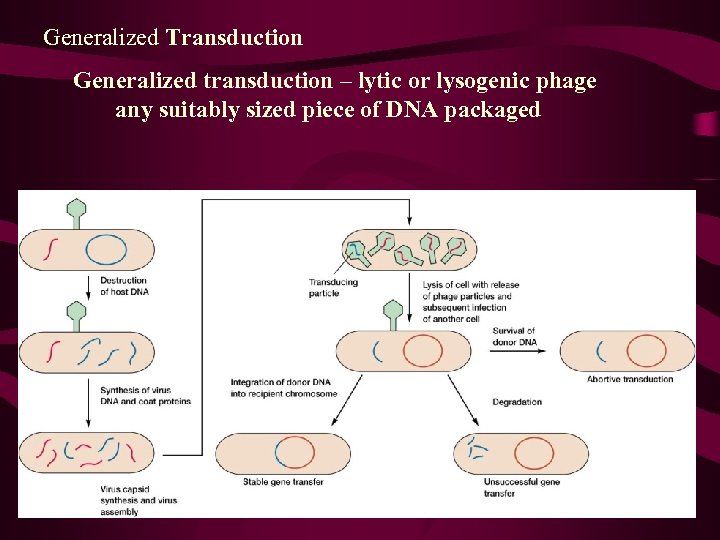
Generalized Transduction Generalized transduction – lytic or lysogenic phage any suitably sized piece of DNA packaged
Specialised transduction Again this represents the transfer of bacterial DNA from one bacterial cell to another via phage particles. However instead of random packaging of bacterial DNA this form of gene exchange results from imprecise excision of an integrated phage (prophage) integrated in to the bacterial chromosome at a site known as the att site. Consequently only genes flanking the integration or att site are carried in the phage transducing particle, and accompany phage genes (by accident).

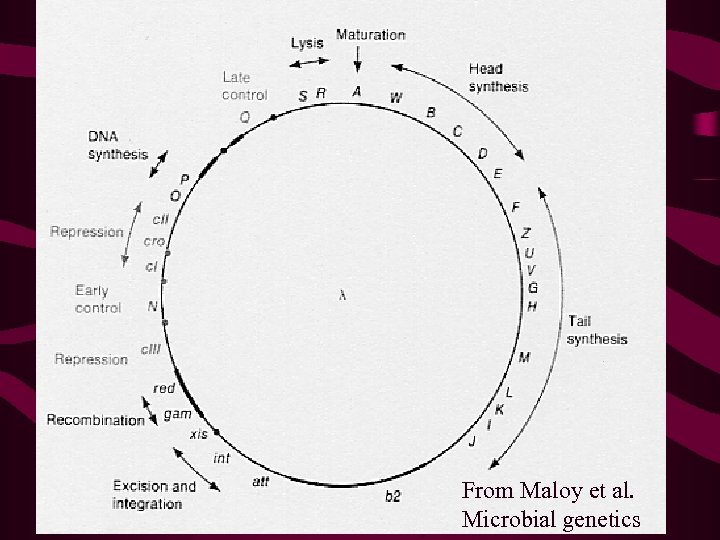
Lambda life cycle Following lambda infection there are 2 alternatives: 1. Lytic 2. Circular ds l DNA is replicated many fold via the 3. rolling circle mechanism, l gene products are made and 4. phage particle are assembled before the cell is 5. lysed and the viral particles released. 6. 7. 8. 9. 2. Lysogeny The l genome becomes integrated in to the bacterial genome at a unique site, the att site, by a site-specific recombination event (non-homologous recombination). 10. Behaves as a piece of chromosomal DNA, and is
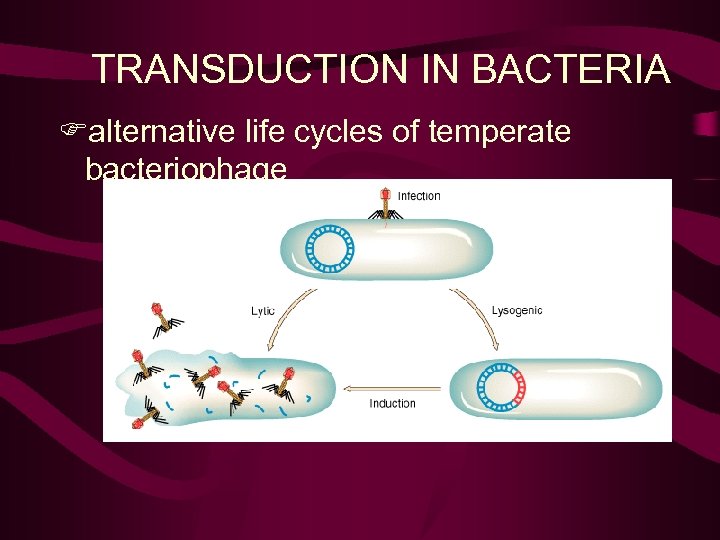
TRANSDUCTION IN BACTERIA Falternative life cycles of temperate bacteriophage

l DNA • has a genome size of 50 kb, with 48 genes. • is a circular ds DNA molecule, and is replicated largely (but not totally) by rolling circle replication. • Linear l DNA produced by rolling circle mechanism and cleavage, form circular molecules via ss complementary DNA termini 12 nt in length, which are known as cos (cohesive) sites. • DNA is contained within a phage particle, and can only replicate by infecting cells, and exploiting host DNA replication proteins.
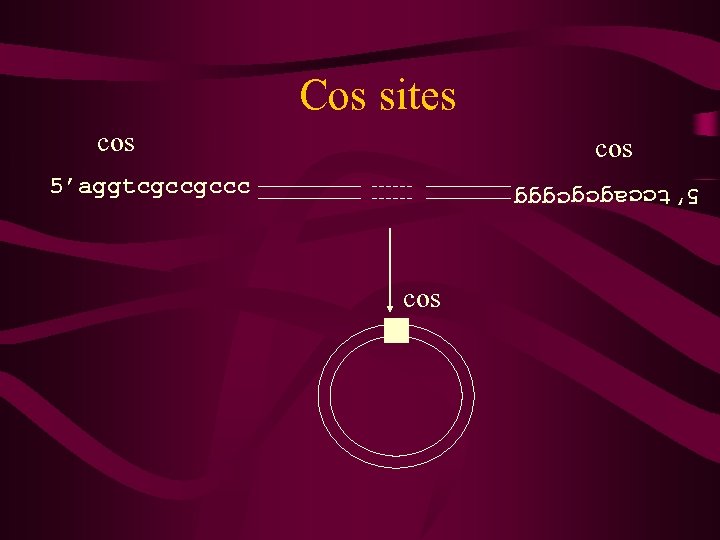
Cos sites cos 5’aggtcgccgccc 5’tccagcgcggg cos
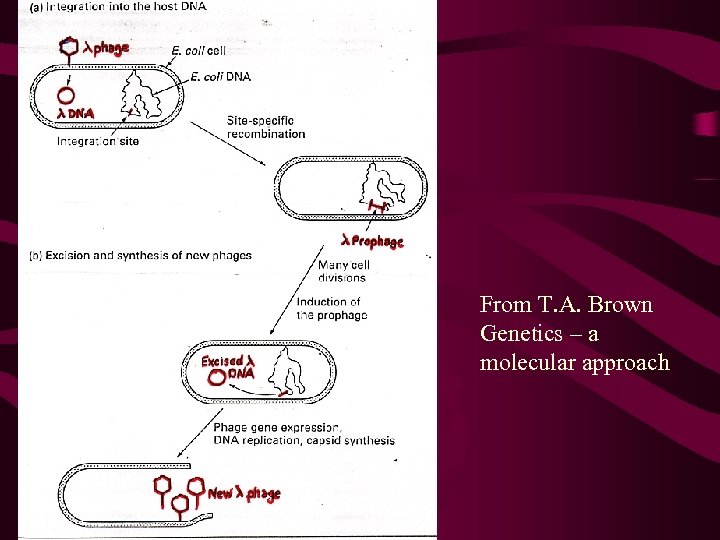
From T. A. Brown Genetics – a molecular approach
The l genome Genes with related function (lysis, integration, excision, synthesis of head and tail, etc are clustered together. Repressor proteins involved in the choice between lysis or lysogeny are l c. I and l cro. The balance of these two gene products determines whether the phage goes through lysis or lysogeny. The DNA is injected into the cell as a linear molecule. This DNA circularises via the cos sites, to initially replicate by a q (theta) structure (like a bacterial chrm), and then moves over to the rolling circle mechanism described earlier.
From Maloy et al. Microbial genetics
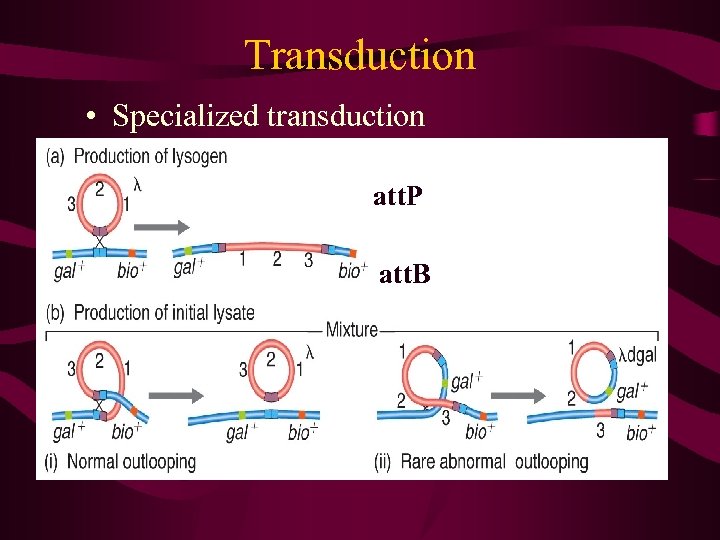
Transduction • Specialized transduction att. P att. B
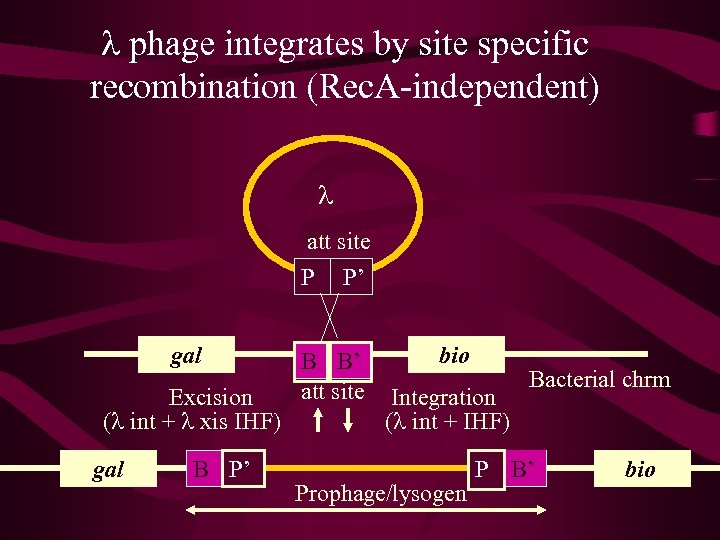
l phage integrates by site specific recombination (Rec. A-independent) l att site P P’ gal Excision (l int + l xis IHF) gal B P’ B B’ att site bio Integration (l int + IHF) Prophage/lysogen Bacterial chrm P B’ bio
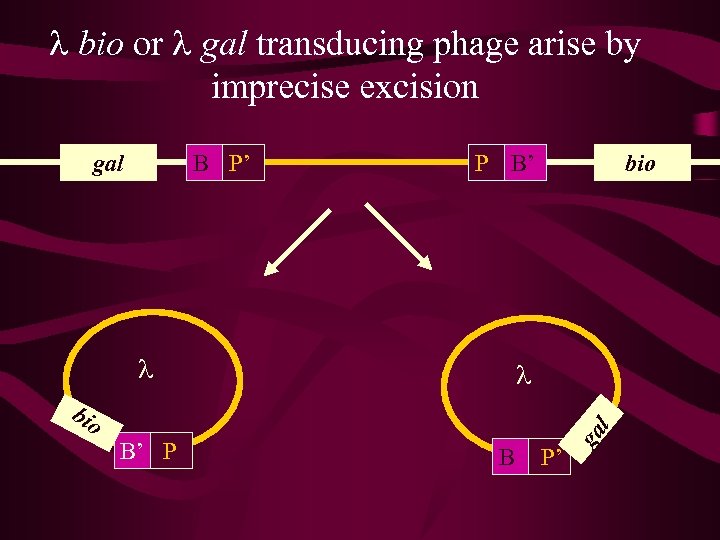
l bio or l gal transducing phage arise by imprecise excision B P’ P B’ l bio B’ P B P’ ga l gal
l bio or l gal transducing phage Due to imprecise excision these two types of phage carry a very small part of the adjacent flanking bacterial genome, either to the left or right of the att site. These phage can recombine into the genome via phage – mediated integration or homologous recombination. Given that these events are a feature of l integrating into a unique site it is not useful for general experiments on gene mapping, but has provided a useful insight into the lysis vs lysogeny phase of lambda development, and gene regulation in general.
l lysogens/prophage bail out of cells undergoing genetic stress UV-irradiation of cells carrying l lysogens/prophage results in the phage exiting the cell by the lysis stage of development. The lysogenic state is maintained by the l c. I repressor, which is related to the lex. A repressor. The l c. I repressor also undergoes auto lysis in the presence of activated Rec. A protein
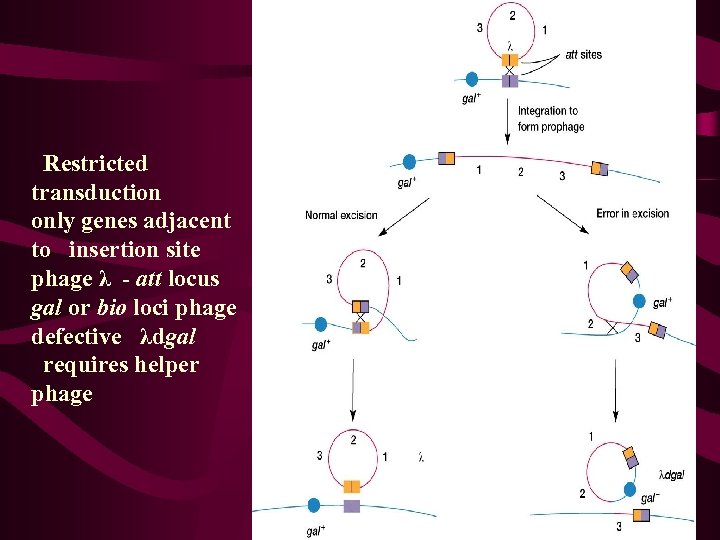
Restricted transduction only genes adjacent to insertion site phage λ - att locus gal or bio loci phage defective λdgal requires helper phage
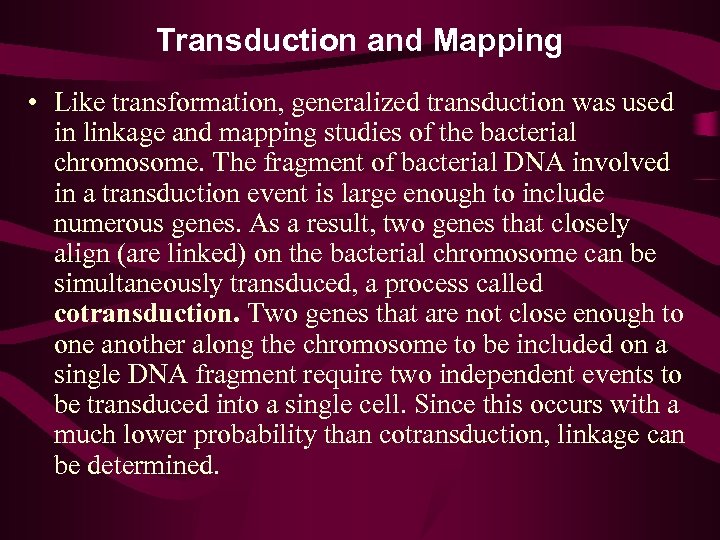
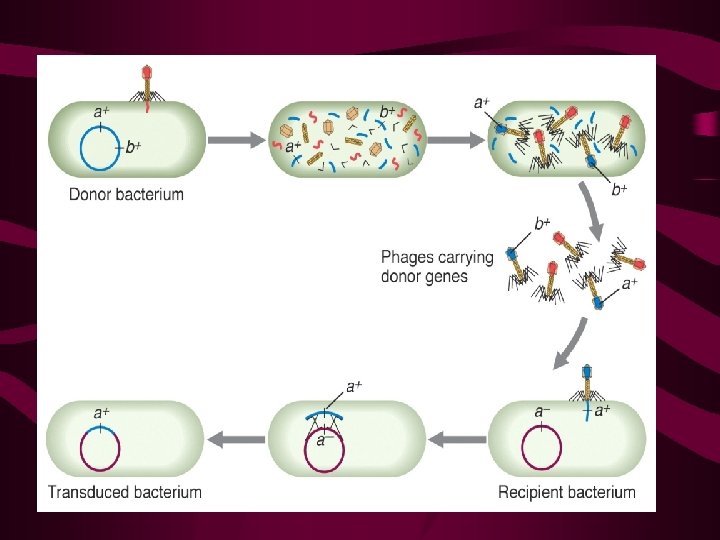
Transduction and Mapping • Like transformation, generalized transduction was used in linkage and mapping studies of the bacterial chromosome. The fragment of bacterial DNA involved in a transduction event is large enough to include numerous genes. As a result, two genes that closely align (are linked) on the bacterial chromosome can be simultaneously transduced, a process called cotransduction. Two genes that are not close enough to one another along the chromosome to be included on a single DNA fragment require two independent events to be transduced into a single cell. Since this occurs with a much lower probability than cotransduction, linkage can be determined.
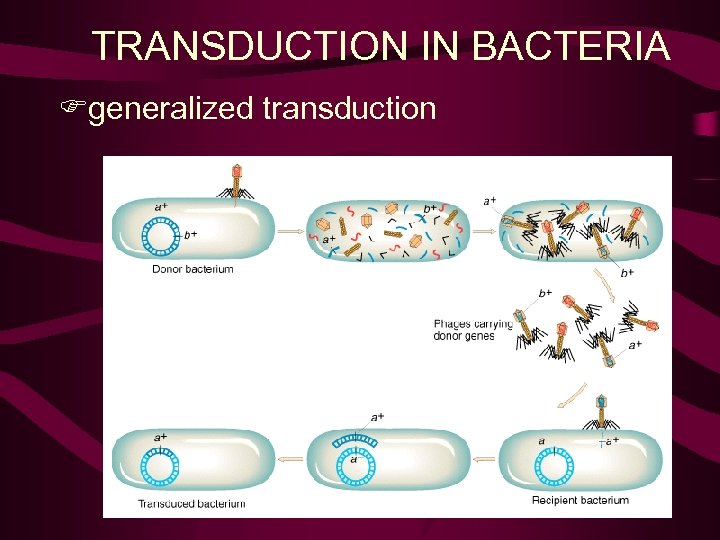
TRANSDUCTION IN BACTERIA Fgeneralized transduction
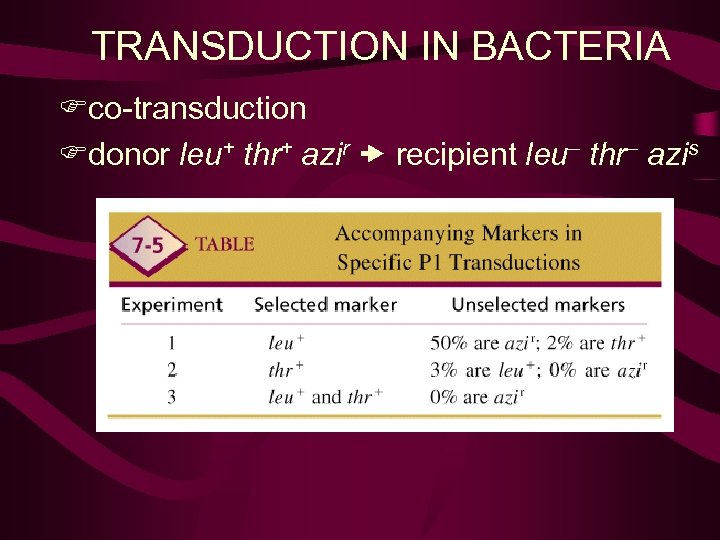
TRANSDUCTION IN BACTERIA Fco-transduction Fdonor leu+ thr+ azir recipient leu– thr– azis
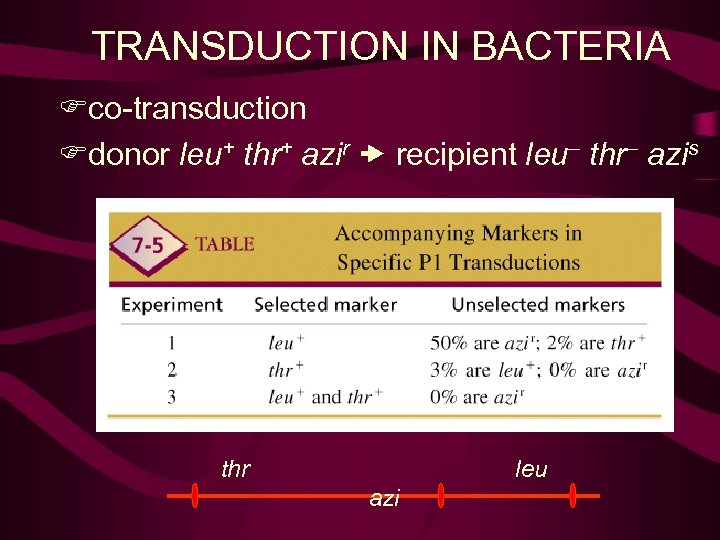
TRANSDUCTION IN BACTERIA Fco-transduction Fdonor leu+ thr+ azir recipient leu– thr– azis thr leu azi

• 普遍性转导频率很低,一般只有0. 3%左右的噬菌体是转导噬菌 体,因此尽管任何基因都有等同的转导机会,但是对每一个基 因来说转导频率是有限的,如沙门氏菌染色体大约有2 000~ 3 000个基因,但是能装入噬菌体头部的DNA充其量只约为一噬菌 体基因组大小,即沙门氏菌的20~ 30个基因,相当于沙门氏菌 染色体的1%。而在制备物中又只有0. 3%左右的噬菌体颗粒是误 装宿主DNA,因此对任一基因的转导频率约为 0. 3%× 1%=3× 10 -5。 • 如果细菌的两个基因之间距离大于噬菌体的染色体长度,一般 不能同时进行转导,除非携带不同基因颗粒同时感染同一细菌 细胞,而这种频率仅为 10 -5× 10 -5=10 -10。所以如果两个基因始 终是一起转导或同时转导频率较高,那么就足以证明这两个基 因是连锁的。两个基因同时转导的现象称为共转导或称并发转 导(cotransduction),两个基因共转导频率愈高,表明两个 基因连锁愈紧密,相反,共转导频率愈低,则表明这两个基因 距离愈远。正是利用这一原理,转导曾广泛地应用于细菌染色 体基因作图。

• 如作双因子转导(two-factor transduction)实 验就是每次观察两个基因的转导,通过每两个基因 之间的共转导频率就可以确定这些基因在染色体上 的次序。若要分析 3个基因则需做 3次双因子转导实 验,才能确定这3个基因的次序。假定这 3次实验 结果为: • ①a基因和b基因共转导频率高; • ②a和c基因的共转导频率也高; • ③b和c基因的共转导频率很低,那么这 3个基因的 次序就应为b、a、c。

• 通过3因子转导可以得到不同类型的转导子及其频 率,显然频率最低的一类转导子是最难于转导的, 因为它的产生需要同时发生的交换次数最多。这种 转导子的3个基因中,两边的应为供体基因,中间 的为受体基因。 • 例如大肠杆菌trp. A+sup. C+pyr. F+细胞作供体, • trp. A-sup. C-pyr. F-作为受体, • 由P 1噬菌体作为载体进行转导。这里trp. A代表色氨 酸(tryptophan)合成的基因,sup. C代表赭石突变 抑制基因(ochre-suppressor gene),pyr. F代表 嘧啶(pyrimidine)生物合成的基因。最初选择的 是sup. C+转导子,然后检查sup. C+转导子中其他两个 基因被转导的情况,得到的转导子类型和数目如下:

• • l) sup. C+ trp. A+ pyr. F+ 36 (2) sup. C+ trp. A+ pyr. F- 114 (3) sup. C+ trp. A- pyr. F+ 0 (4) sup. C+ trp. A- pyr. F- 453 603 第(3)类基因型转导子频率最低(等于零), 由此可见3个基因的次序是sup. C trp. A pyr. F, 因为这类重组子必须同时发生4次交换才能 产生(图 6 -15(3))。
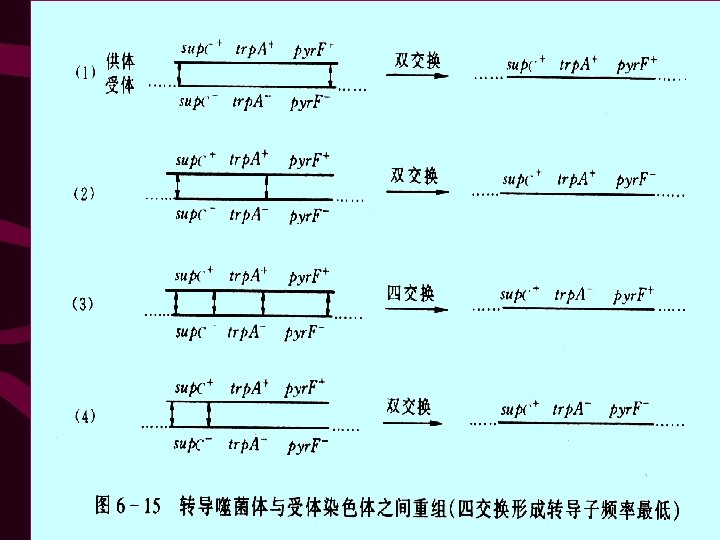

• 从图 6 -15可见:①第三类基因型的转导子是很难发生 的,由此类转导子基因型可以看出这 3个基因排列次 序是sup. C trp. A pyr. F;②sup. C+和trp. A+在第一、二类 转导子中是共转导,而在第三、四类转导子中不是共 转导,所以这两个基因共转导频率为 36+114/603=0. 25 ;③sup. C+和pyr. F+仅在第一类转导子中共转导,所以 共转导频率为 36/603=0. 06。如果两个基因紧密连锁, 它们就有可能经常在一起转导,共转导频率将接近于1 。如果两个基因从未或者几乎未包含在同一转导的DNA 片段中,那么它们的共转导频率接近于或等于0。利 用这种关系可以求出同一染色体上两个基因之间的物 理距离。经过一系列推导得到以下的计算公式:d=L(1 - 3 X ) • 公式中:d:同一染色体上两个基因之间的图距 • L:转导DNA的平均长度(约为一个噬菌体基因组大小) x:两个基因共转导的频率。
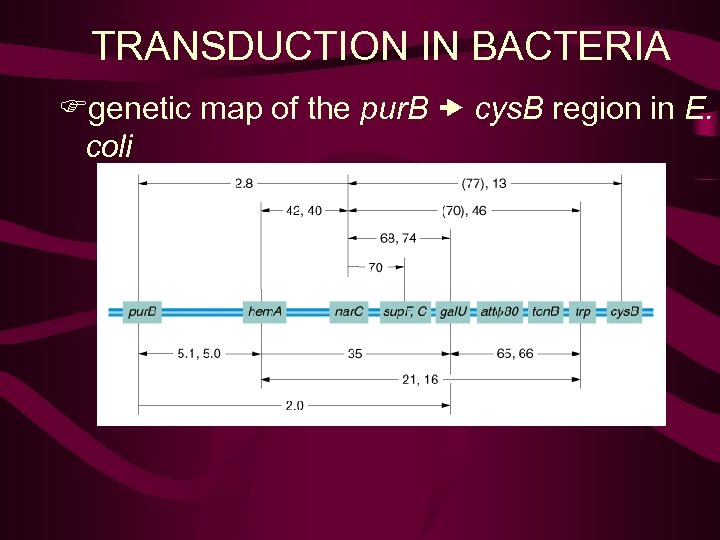
TRANSDUCTION IN BACTERIA Fgenetic map of the pur. B cys. B region in E. coli
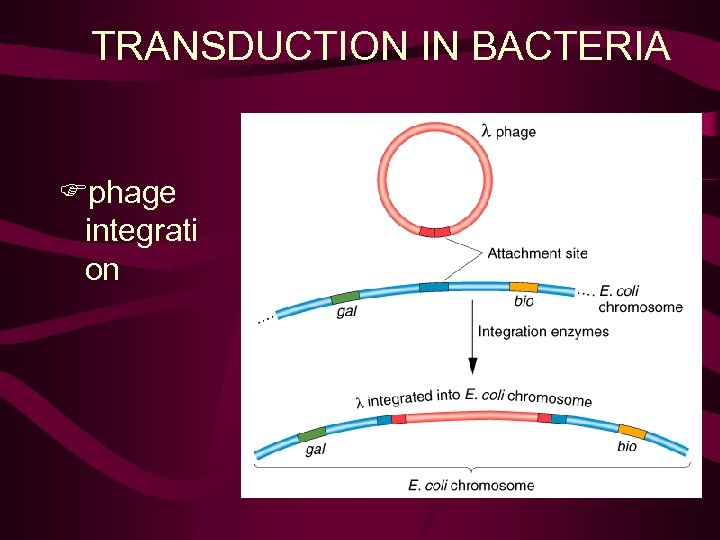
TRANSDUCTION IN BACTERIA Fphage integrati on
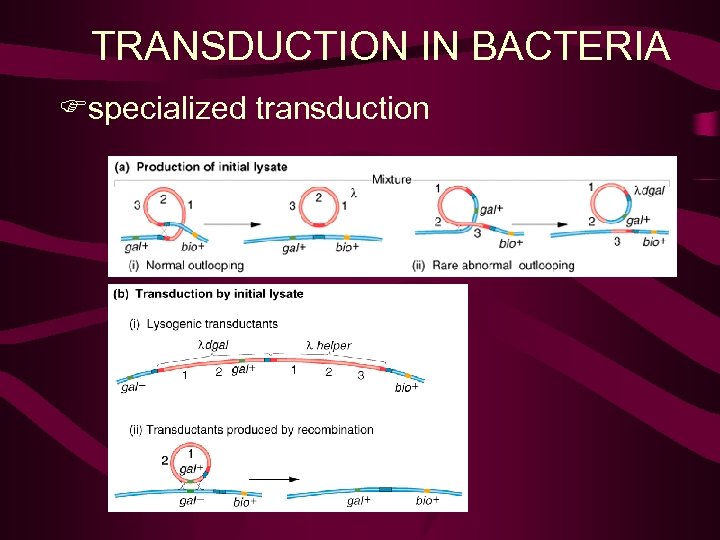
TRANSDUCTION IN BACTERIA Fspecialized transduction
TRANSDUCTION IN BACTERIA Ftransduction: phage acquire host genes and transfer them to other bacterial cells Fgeneralized transduction: transfers any host gene; and occurs when phage randomly package host DNA Fspecialized transduction: faulty separation of prophage (phage incorporated into host genome); new phage contains adjacent host genes only
SUMMARY: RECOMBINATION IN BACTERIA

P 1 Phage transduction Use P 1 phage to infect and lyse a Leu+ Phe+ Tyr+ host cell. Collect phage supernatant. Use this phage supernatant to infect a leu. A 6 phe. D 6 tyr. K 6 host cell. Allow infection to take place for 20 mins than add 0. 1 M sodium citrate (to prevent re-infection later). Plate infected cells on minimal media supplemented with phenylalanine and tyrosine, i. e. select for Leu+ transductants. Then test the Leu+ transductants on suitable minimal media for testing the status of the phe and tyr genes
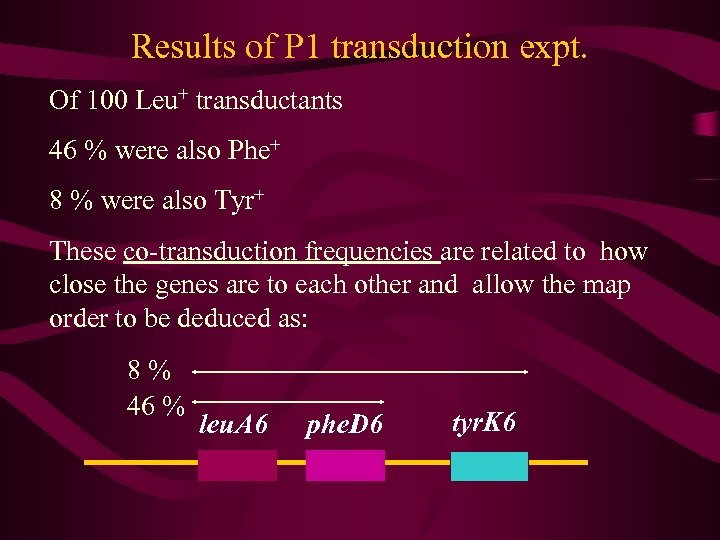
Results of P 1 transduction expt. Of 100 Leu+ transductants 46 % were also Phe+ 8 % were also Tyr+ These co-transduction frequencies are related to how close the genes are to each other and allow the map order to be deduced as: 8% 46 % leu. A 6 phe. D 6 tyr. K 6

• By concentrating on two or three linked genes, transduction studies can also determine the precise order of these genes. The closer linked genes are to each other, the greater the frequency of cotransduction. Mapping studies involving three closely aligned genes can thus be executed, and the analysis of such an experiment is predicated on the same rationale underlying other mapping techniques.
9. 8 Intergenic Recombination and Mapping in Bacteriophages • Around 1947, several research teams demonstrated that genetic recombination can be detected in bacteriophages. This led to the discovery that gene mapping can be performed in these viruses. Such studies relied on finding numerous phage mutations that could be visualized or assayed. As in bacteria and eukaryotes, these mutations allow genes to be identified and followed in mapping experiments. Thus, before considering recombination and mapping in these bacterial viruses, we briefly introduce several of the mutations that were studied.
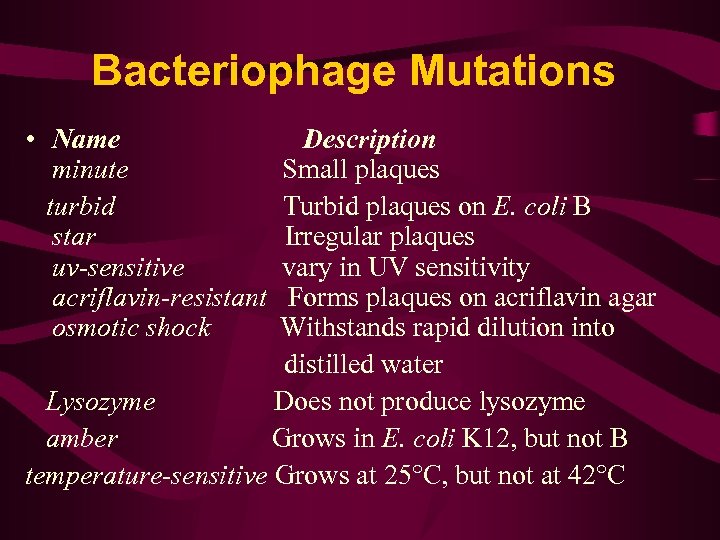
Bacteriophage Mutations • Name minute turbid star uv-sensitive acriflavin-resistant osmotic shock Description Small plaques Turbid plaques on E. coli B Irregular plaques vary in UV sensitivity Forms plaques on acriflavin agar Withstands rapid dilution into distilled water Lysozyme Does not produce lysozyme amber Grows in E. coli K 12, but not B temperature-sensitive Grows at 25°C, but not at 42°C
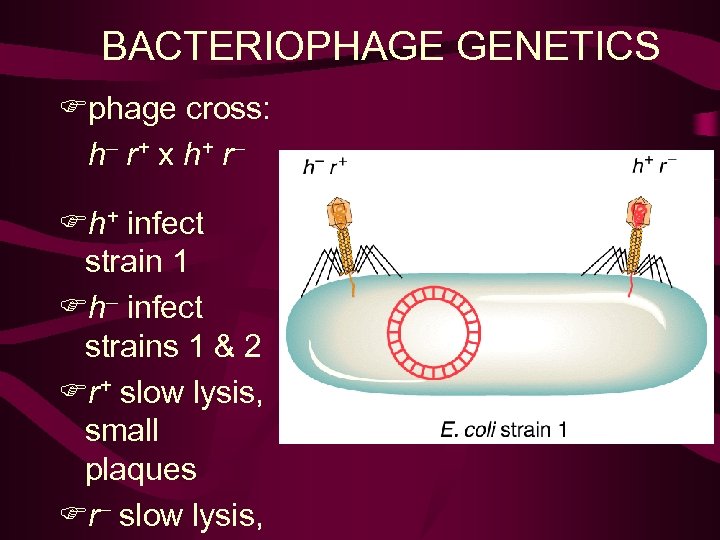
BACTERIOPHAGE GENETICS Fphage cross: h– r + x h+ r – Fh+ infect strain 1 Fh– infect strains 1 & 2 Fr+ slow lysis, small plaques Fr– slow lysis,
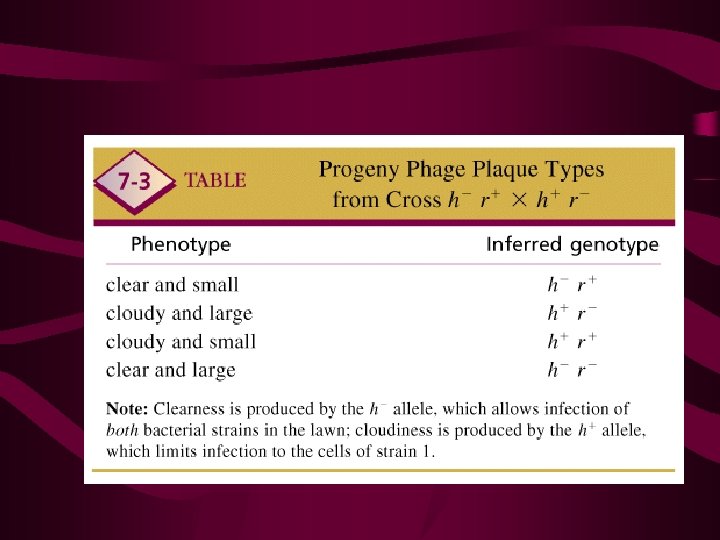
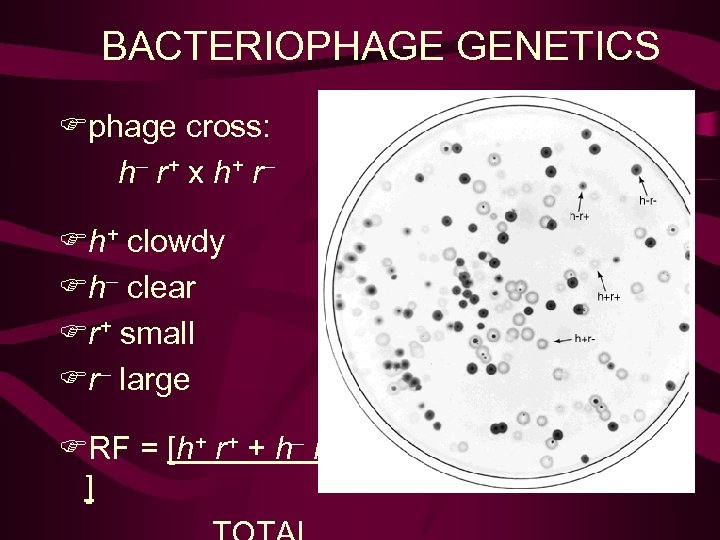
BACTERIOPHAGE GENETICS Fphage cross: h– r + x h+ r – Fh+ clowdy Fh– clear Fr+ small Fr– large FRF = [h+ r+ + h– r– ]

1). 噬菌斑形态突变型 • r+ 小噬菌斑, 边缘模糊: 2个以上噬菌体同时 侵袭时, 出现溶菌阻碍现象(lysis inhibition). • r 大噬菌斑, 边缘清晰: 快速溶菌(rapid lysis), 无溶菌阻碍现象(lysis inhibition) 2). 宿主范围突变型 E. coli B-2 E. coli B+E. coli B-2 h+ + - 半透明噬菌斑 h + 透明噬菌斑

• T 2噬菌体两对性状的重组(两点测验): 野生型 h+ 突变型h 宿主范围 E Coli B B和B-2 不能B-2 混合培养物—— 半透明斑 透明班 野生型r+ 突变型r 筮菌斑形态 小筮菌斑 大筮菌斑 1 mm 2 mm
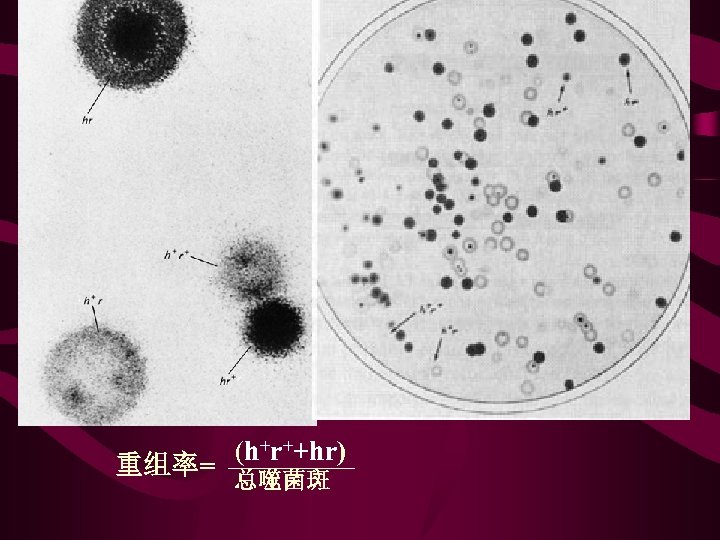
(h+r++hr) 重组率= 总噬菌斑
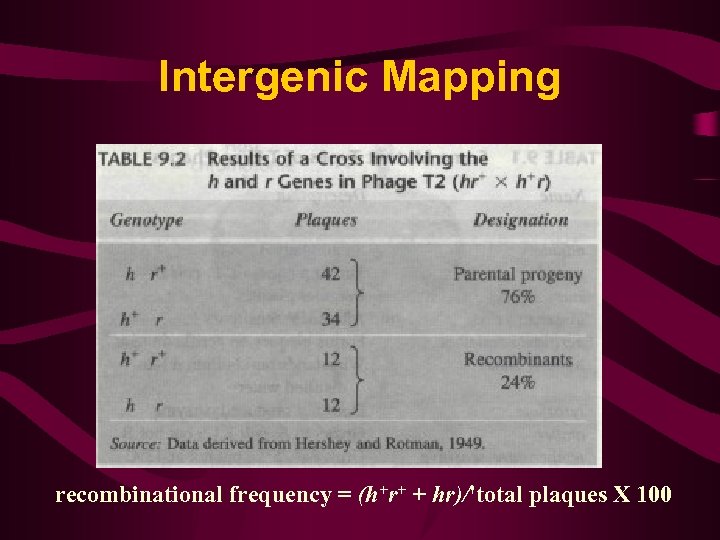
Intergenic Mapping recombinational frequency = (h+r+ + hr)/'total plaques X 100
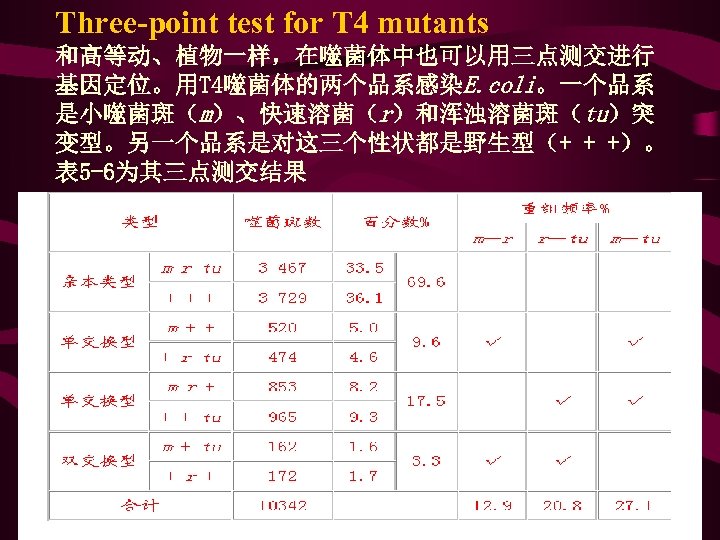
Three-point test for T 4 mutants 和高等动、植物一样,在噬菌体中也可以用三点测交进行 基因定位。用T 4噬菌体的两个品系感染E. coli。一个品系 是小噬菌斑(m)、快速溶菌(r)和浑浊溶菌斑(tu)突 变型。另一个品系是对这三个性状都是野生型(+ + +)。 表 5 -6为其三点测交结果
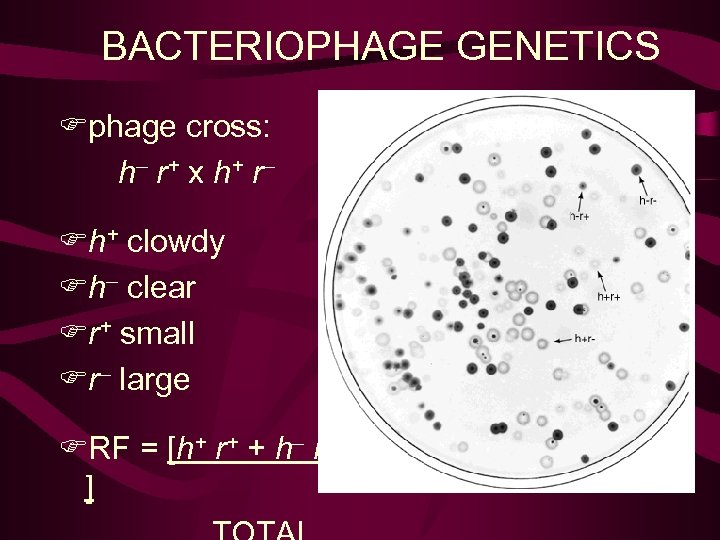
BACTERIOPHAGE GENETICS Fphage cross: h– r + x h+ r – Fh+ clowdy Fh– clear Fr+ small Fr– large FRF = [h+ r+ + h– r– ]
1974a8bd663ba94140c3deb845cf3a17.ppt