9f981294b087e5000ea0a24197af1be4.ppt
- Количество слайдов: 160
Chapter 9 Molecular Geometry Bonding Theories
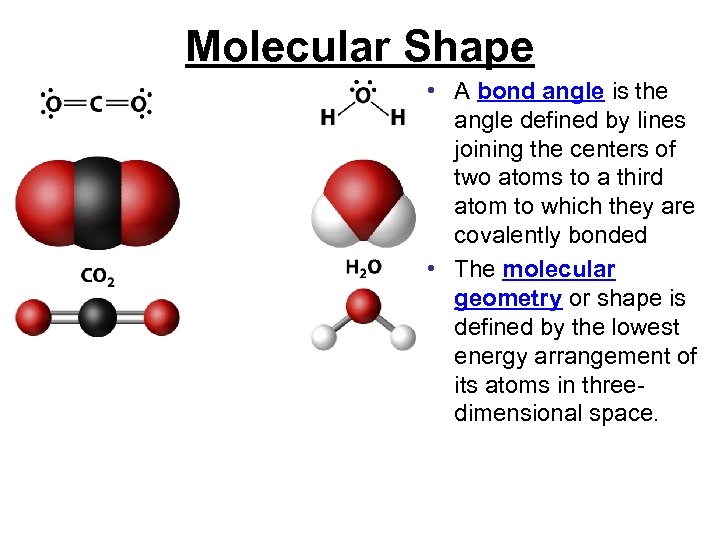
Molecular Shape • A bond angle is the angle defined by lines joining the centers of two atoms to a third atom to which they are covalently bonded • The molecular geometry or shape is defined by the lowest energy arrangement of its atoms in threedimensional space.

VSEPR Valence-Shell Electron-Pair Repulsion Theory The geometric arrangement of atoms bonded to a given atom is determined principally by minimizing electron pair repulsions of bonding and nonbonding electrons.
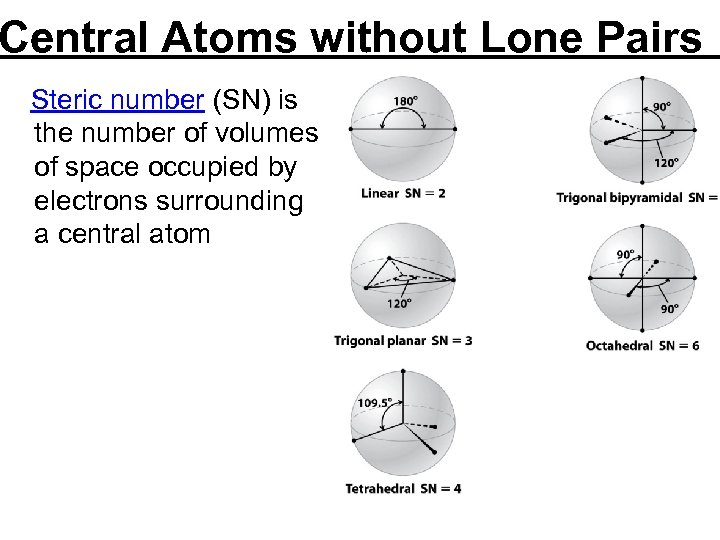
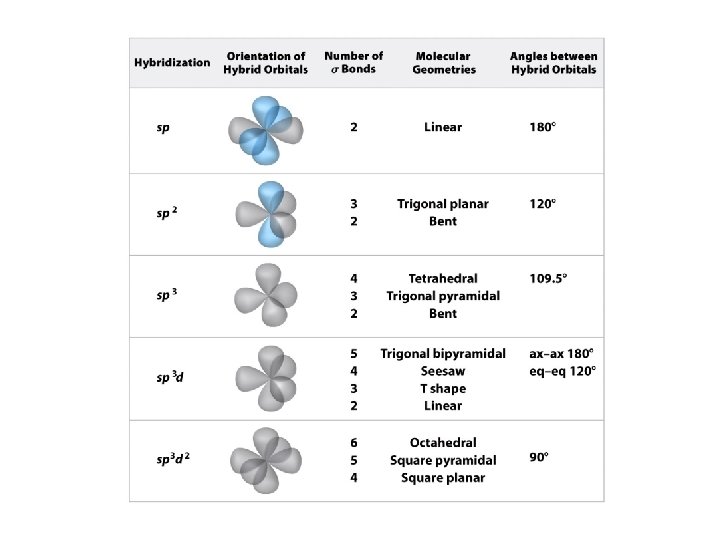
Central Atoms without Lone Pairs Steric number (SN) is the number of volumes of space occupied by electrons surrounding a central atom
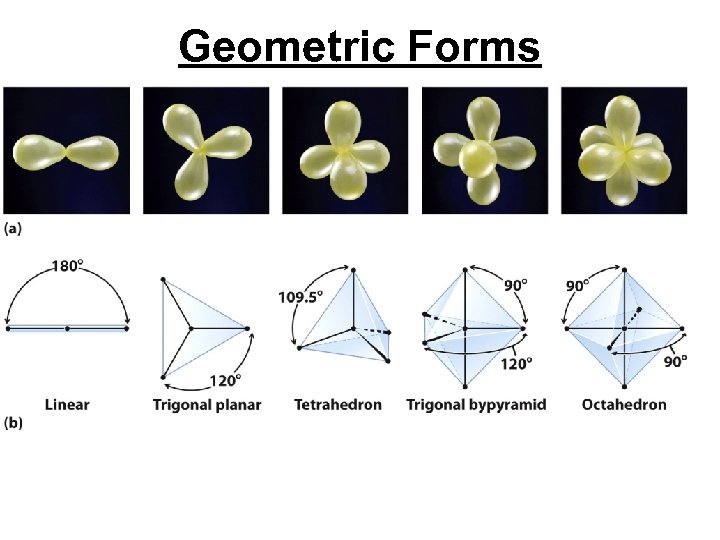
Geometric Forms
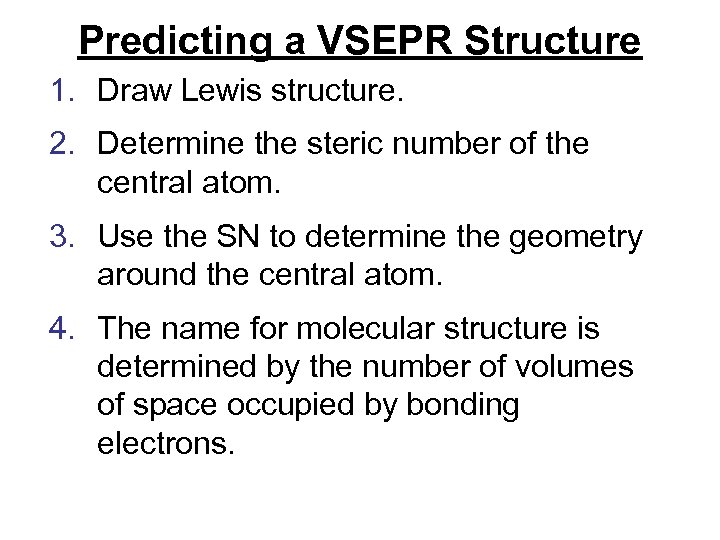
Predicting a VSEPR Structure 1. Draw Lewis structure. 2. Determine the steric number of the central atom. 3. Use the SN to determine the geometry around the central atom. 4. The name for molecular structure is determined by the number of volumes of space occupied by bonding electrons.
Examples • What is the molecular geometry of BF 3? • What is the molecular geometry of CH 4
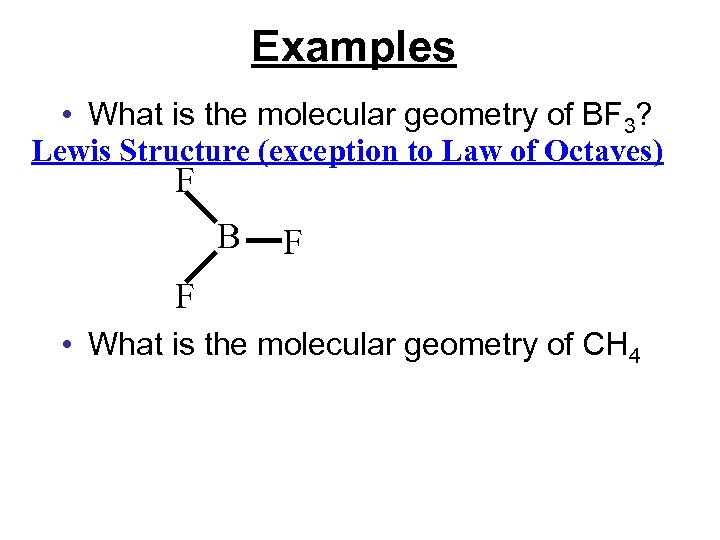
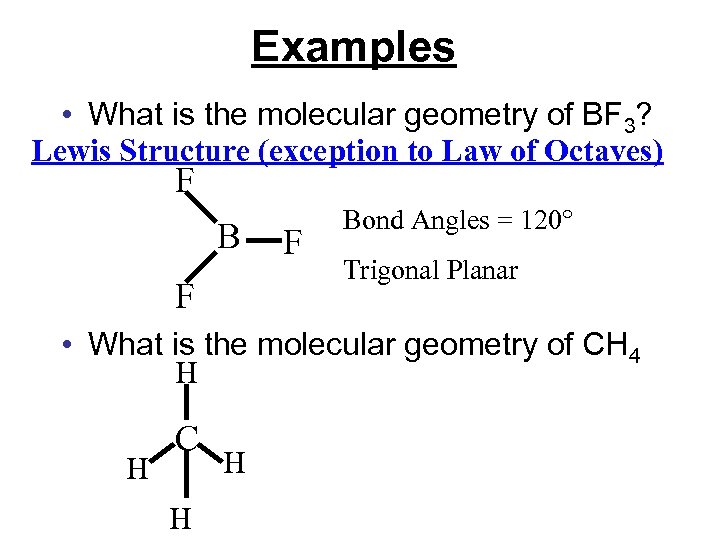
Examples • What is the molecular geometry of BF 3? Lewis Structure (exception to Law of Octaves) F B F F • What is the molecular geometry of CH 4
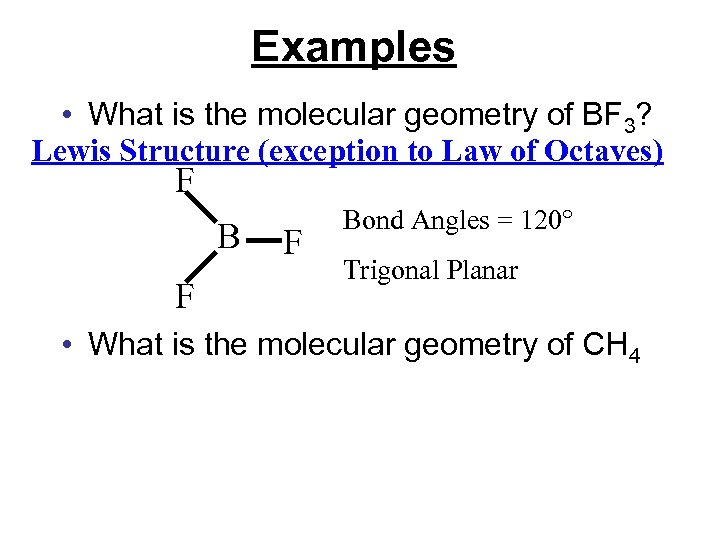
Examples • What is the molecular geometry of BF 3? Lewis Structure (exception to Law of Octaves) F B F F Bond Angles = 120° Trigonal Planar • What is the molecular geometry of CH 4
Examples • What is the molecular geometry of BF 3? Lewis Structure (exception to Law of Octaves) F B F F Bond Angles = 120° Trigonal Planar • What is the molecular geometry of CH 4 H H C H H
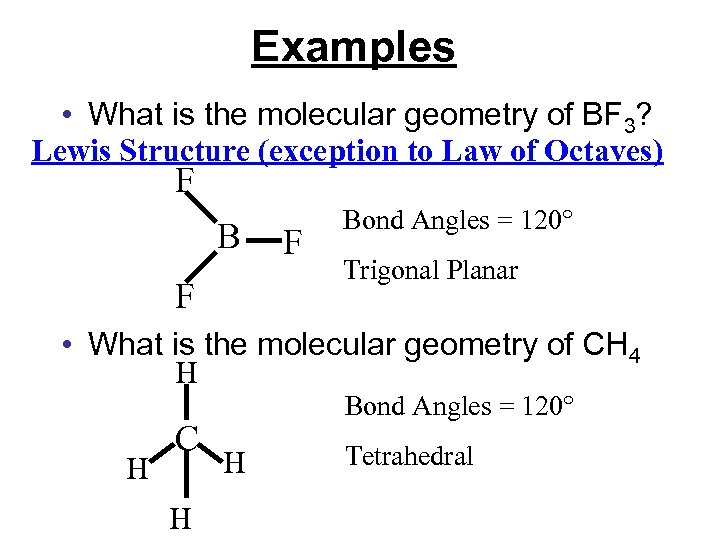
Examples • What is the molecular geometry of BF 3? Lewis Structure (exception to Law of Octaves) F B F F Bond Angles = 120° Trigonal Planar • What is the molecular geometry of CH 4 H H C H Bond Angles = 120° H Tetrahedral
Central Atoms with Lone Pairs • Electron-pair geometry describes the arrangement of atoms and lone pairs of electrons about a central atom. § The electron-pair geometry will always be one of the five geometries presented previously. • The molecular geometry in these molecules describes the shape of the atoms present (it excludes the lone pairs).
Lone Pairs • Lone pairs of electrons occupy more space around a central atom than do bonding electrons. • Lone pair-lone pair repulsion is the largest. • Lone pair-bonding pair repulsion is the next largest. • Bonding pair-bonding pair repulsion is the smallest. • In structures with lone pairs on the central atom, the bond angles are a little smaller than predicted based on the electron-pair geometry.
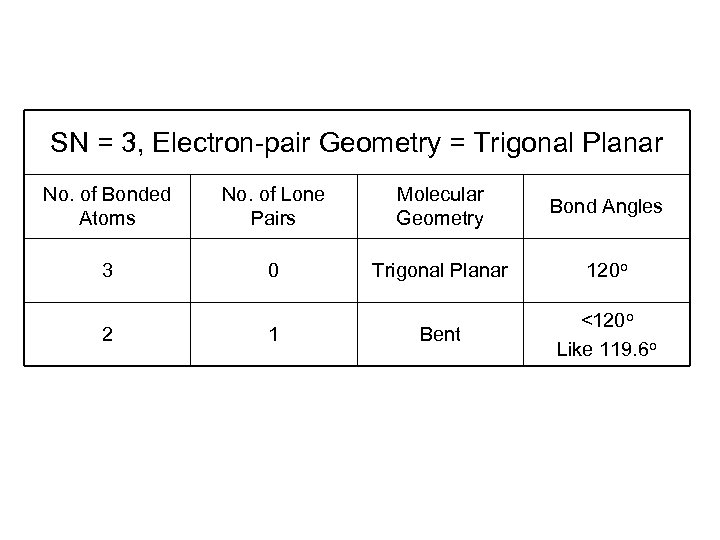
SN = 3, Electron-pair Geometry = Trigonal Planar No. of Bonded Atoms No. of Lone Pairs Molecular Geometry Bond Angles 3 0 Trigonal Planar 120 o Bent <120 o Like 119. 6 o 2 1
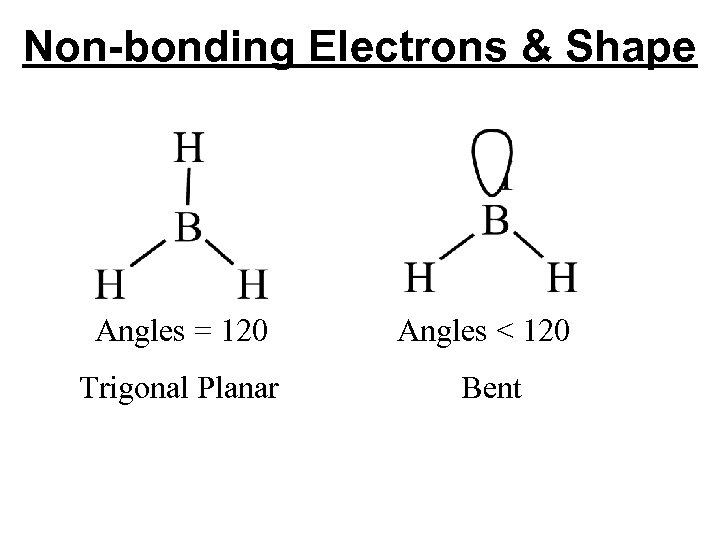
Non-bonding Electrons & Shape Angles = 120 Angles < 120 Trigonal Planar Bent
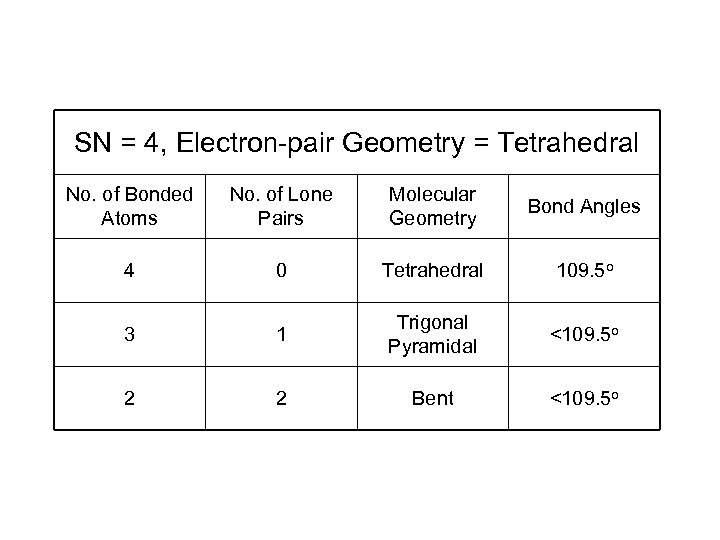
SN = 4, Electron-pair Geometry = Tetrahedral No. of Bonded Atoms No. of Lone Pairs Molecular Geometry Bond Angles 4 0 Tetrahedral 109. 5 o 3 1 Trigonal Pyramidal <109. 5 o 2 2 Bent <109. 5 o
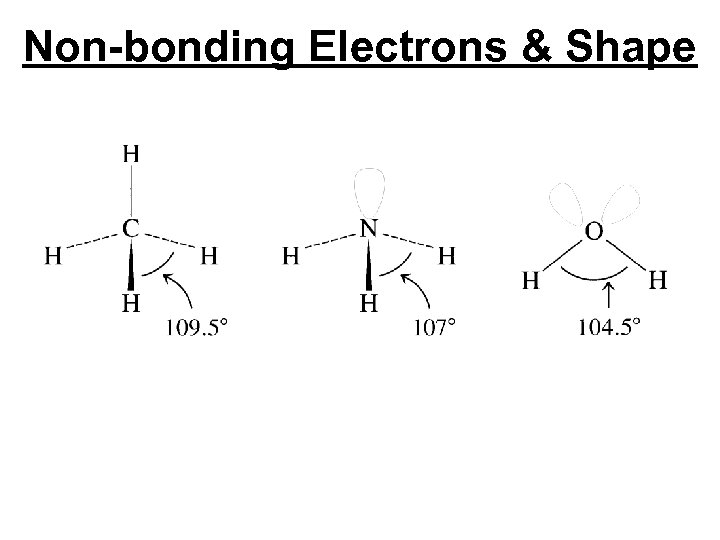
Non-bonding Electrons & Shape
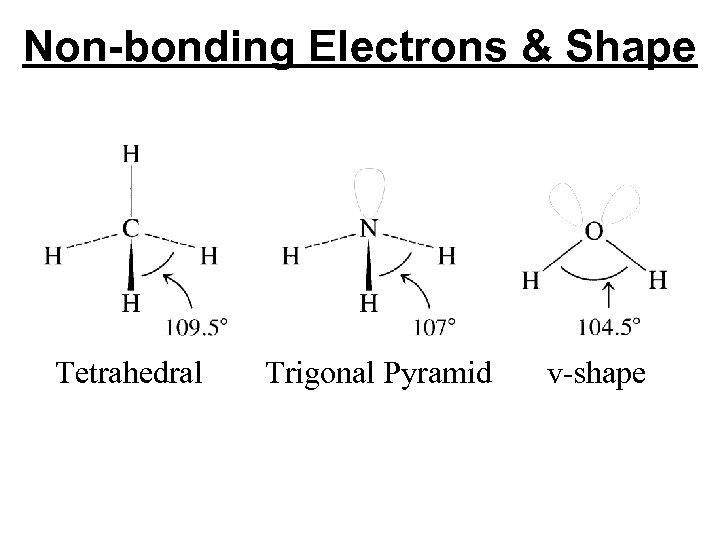
Non-bonding Electrons & Shape Tetrahedral Trigonal Pyramid v-shape
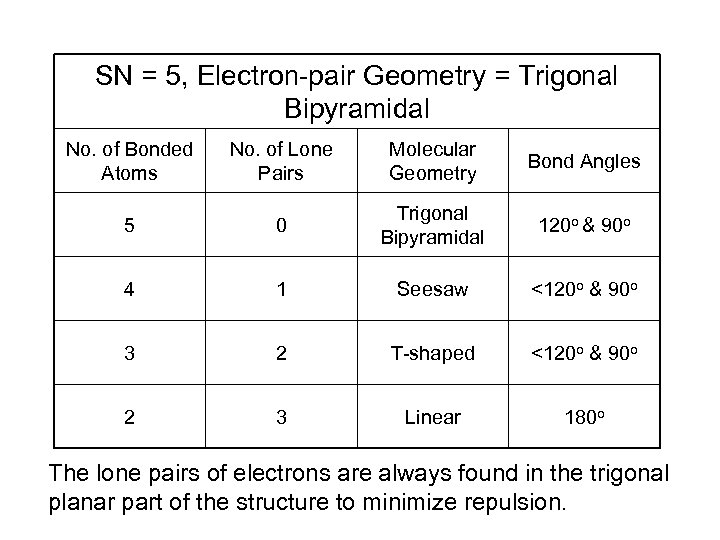
SN = 5, Electron-pair Geometry = Trigonal Bipyramidal No. of Bonded Atoms No. of Lone Pairs Molecular Geometry Bond Angles 5 0 Trigonal Bipyramidal 120 o & 90 o 4 1 Seesaw <120 o & 90 o 3 2 T-shaped <120 o & 90 o 2 3 Linear 180 o The lone pairs of electrons are always found in the trigonal planar part of the structure to minimize repulsion.
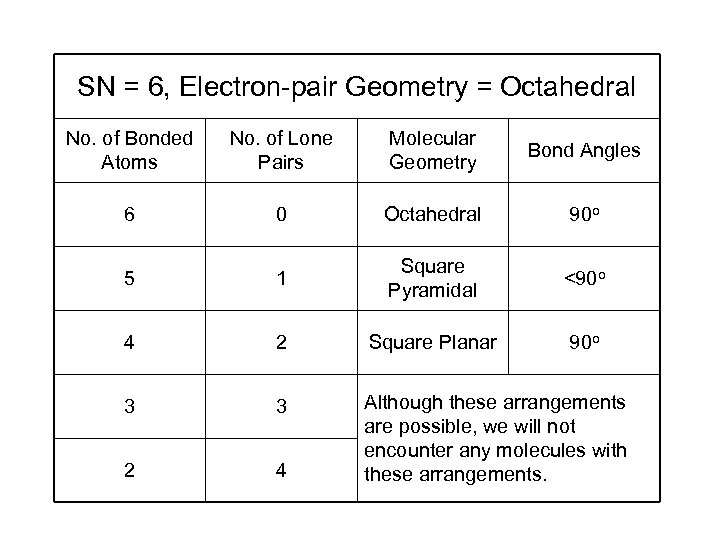
SN = 6, Electron-pair Geometry = Octahedral No. of Bonded Atoms No. of Lone Pairs Molecular Geometry Bond Angles 6 0 Octahedral 90 o 5 1 Square Pyramidal <90 o 4 2 Square Planar 90 o 3 3 2 4 Although these arrangements are possible, we will not encounter any molecules with these arrangements.
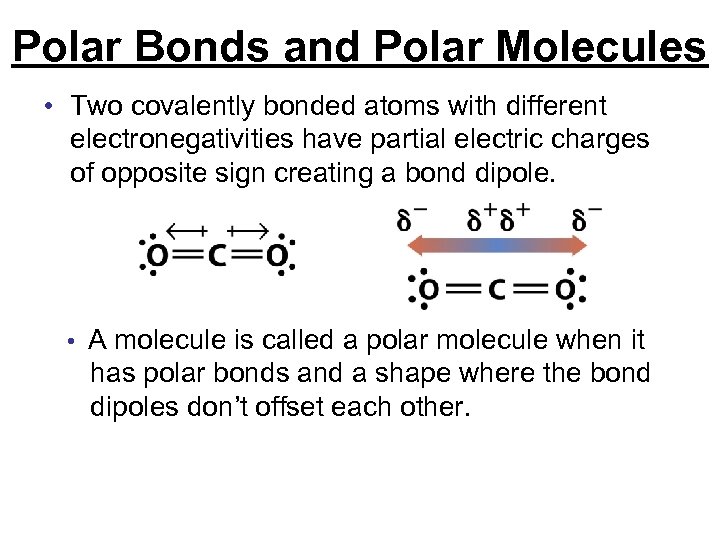
Polar Bonds and Polar Molecules • Two covalently bonded atoms with different electronegativities have partial electric charges of opposite sign creating a bond dipole. • A molecule is called a polar molecule when it has polar bonds and a shape where the bond dipoles don’t offset each other.
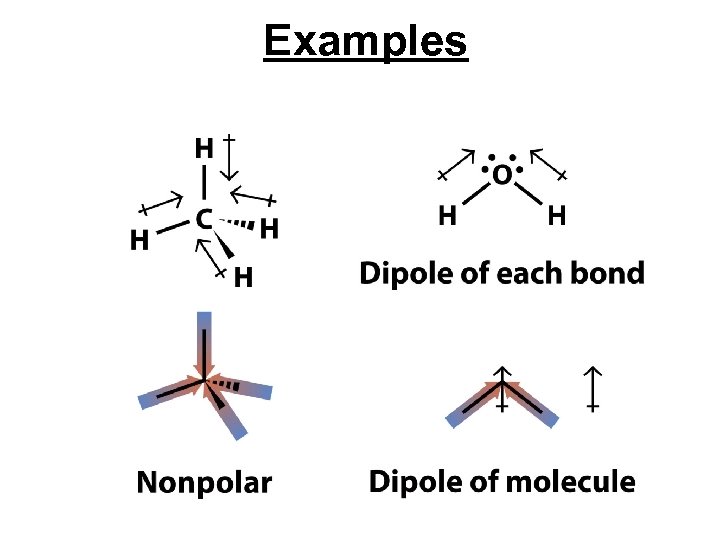
Examples
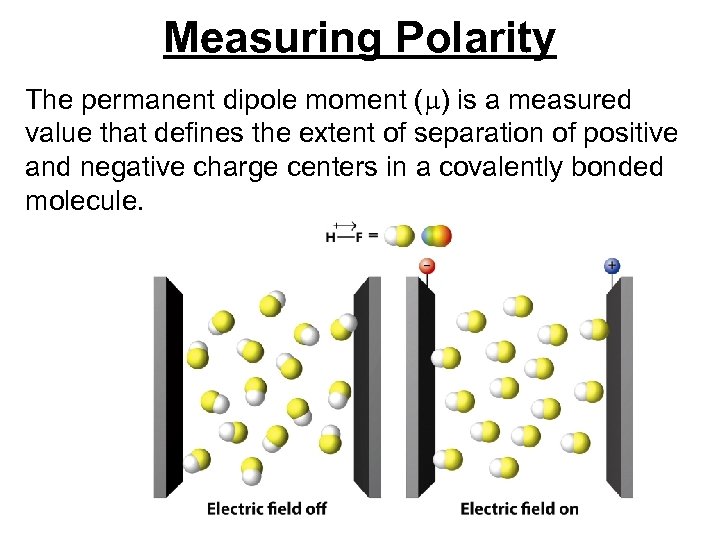
Measuring Polarity The permanent dipole moment ( ) is a measured value that defines the extent of separation of positive and negative charge centers in a covalently bonded molecule.

Valence Bond Theory • Hybridization is the mixing of atomic orbitals to generate new sets of orbitals that are then available to overlap and form covalent bonds with other atoms. • A hybrid atomic orbital is one of a set of equivalent orbitals about an atom created when specific atomic orbitals are mixed.

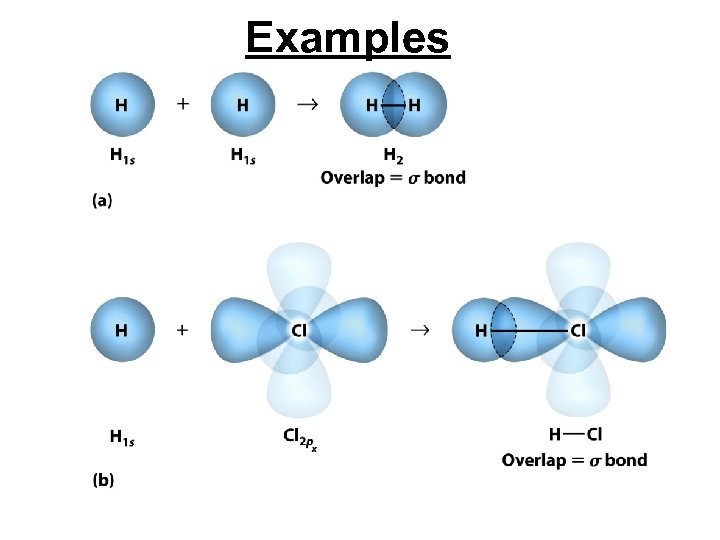
Valence-Bond Theory • Valence-bond theory assumes that covalent bonds form when orbitals on different atoms overlap or occupy the same region of space. • A sigma ( ) bond is a covalent bond in which the highest electron density lies between the two atoms along the bond axis connecting them.
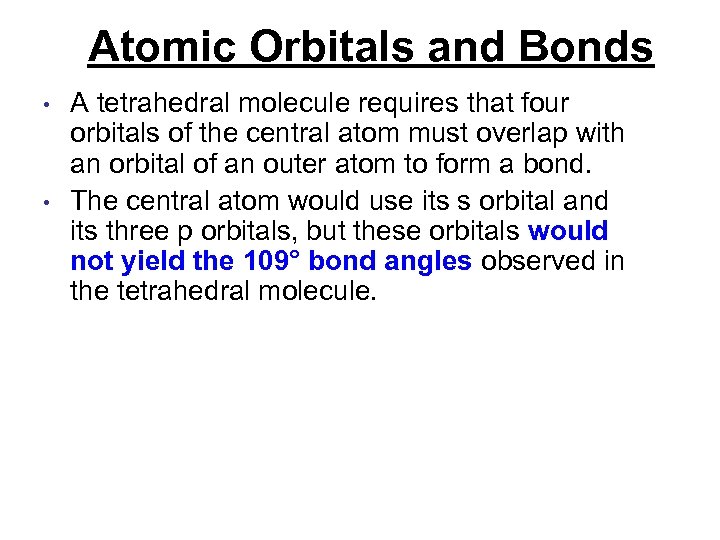
Atomic Orbitals and Bonds • • A tetrahedral molecule requires that four orbitals of the central atom must overlap with an orbital of an outer atom to form a bond. The central atom would use its s orbital and its three p orbitals, but these orbitals would not yield the 109° bond angles observed in the tetrahedral molecule.

Hybrid Orbitals You may have noticed that the electron pairs in molecules have different orientations in space compared to atomic orbitals. Wave equations mathematically generated volumes of space where electrons spend most of their time, but what about molecules?
Hybrid Orbitals You may have noticed that the electron pairs in molecules have different orientations in space compared to atomic orbitals. Wave equations mathematically generated volumes of space where electrons spend most of their time, but what about molecules? This brings us to the concept of hybrid orbitals, combinations of atomic orbitals, or molecular orbitals (from wave equations of electrons in molecules)

Hybrid Orbitals You may have noticed that the electron pairs in molecules have different orientations in space compared to atomic orbitals. Wave equations mathematically generated volumes of space where electrons spend most of their time, but what about molecules?
Hybrid Orbitals You may have noticed that the electron pairs in molecules have different orientations in space compared to atomic orbitals. Wave equations mathematically generated volumes of space where electrons spend most of their time, but what about molecules? This brings us to the concept of hybrid orbitals, combinations of atomic orbitals, or molecular orbitals (from wave equations of electrons in molecules)

Hybrid Orbitals Hybridization is a concept you might be familiar with. For example a grapefruit is a hybrid of what two fruits?

Hybrid Orbitals Hybridization is a concept you might be familiar with. For example a grapefruit is a hybrid of what two fruits?

Hybrid Orbitals Hybridization is a concept you might be familiar with. For example a grapefruit is a hybrid of what two fruits? Lemon and orange
Hybrid Orbitals How about a nectarine?

Hybrid Orbitals How about a nectarine? Plumb and a peach.

Hybrid Orbitals How about a nectarine? Plumb and a peach. Broccoaflower? Broccoli and cauliflower

Hybrid Orbitals How about a nectarine? Plumb and a peach. Broccoaflower? Broccoli and cauliflower And a Cocapoo?

Hybrid Orbitals How about a nectarine? Plumb and a peach. Broccoaflower? Broccoli and cauliflower And a Cocapoo? Cocker spaniel and poodle

Hybrid Orbitals On to Chemistry! How about an s-orbital and a porbital? Yes, sp orbital.

Hybrid Orbitals On to Chemistry! How about an s-orbital and a porbital? Yes, sp orbital. How about one s-orbital and two p-orbitals?

Hybrid Orbitals On to Chemistry! How about an s-orbital and a porbital? Yes, sp orbital. How about one s-orbital and two p-orbitals? Yes an sp 2 orbital.
Examples
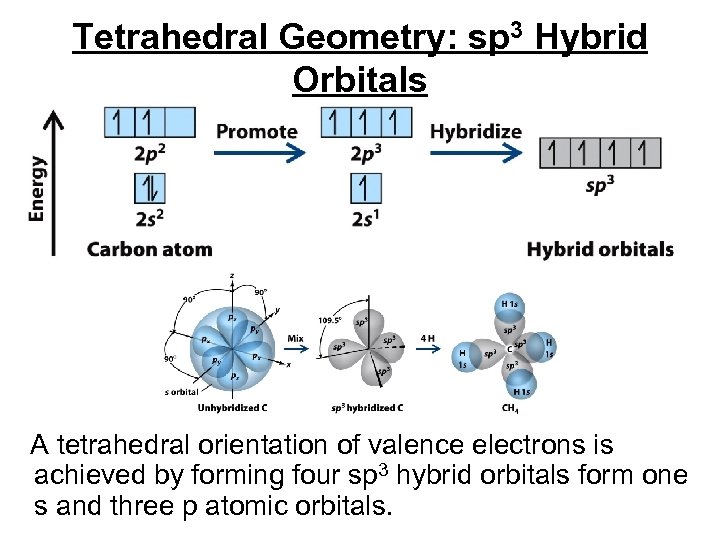
Tetrahedral Geometry: sp 3 Hybrid Orbitals A tetrahedral orientation of valence electrons is achieved by forming four sp 3 hybrid orbitals form one s and three p atomic orbitals.
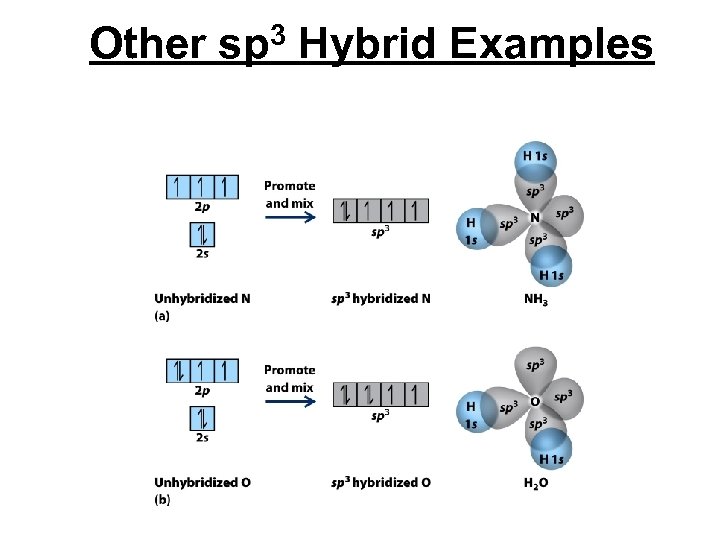
Other sp 3 Hybrid Examples
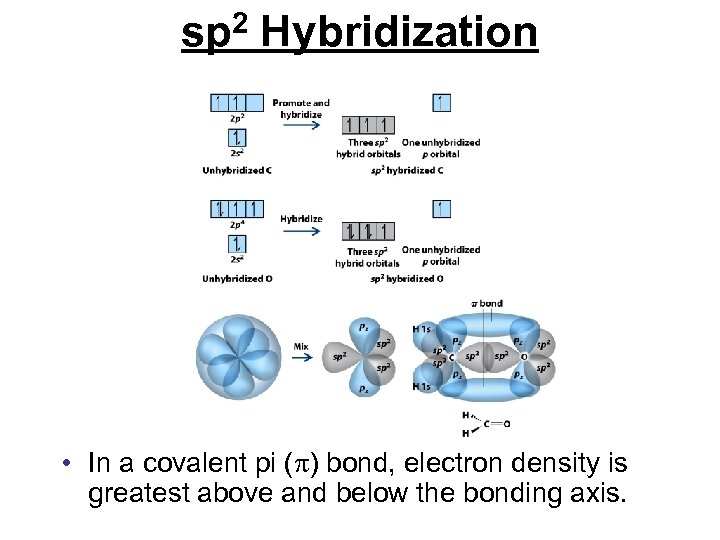
sp 2 Hybridization • In a covalent pi ( ) bond, electron density is greatest above and below the bonding axis.
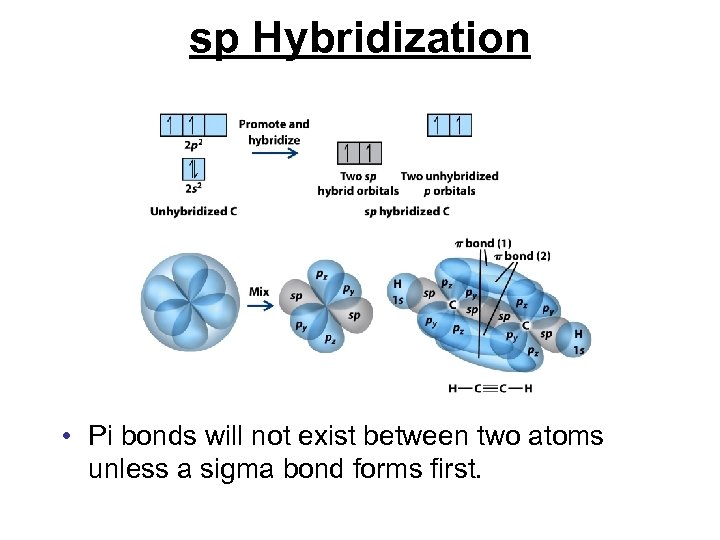
sp Hybridization • Pi bonds will not exist between two atoms unless a sigma bond forms first.
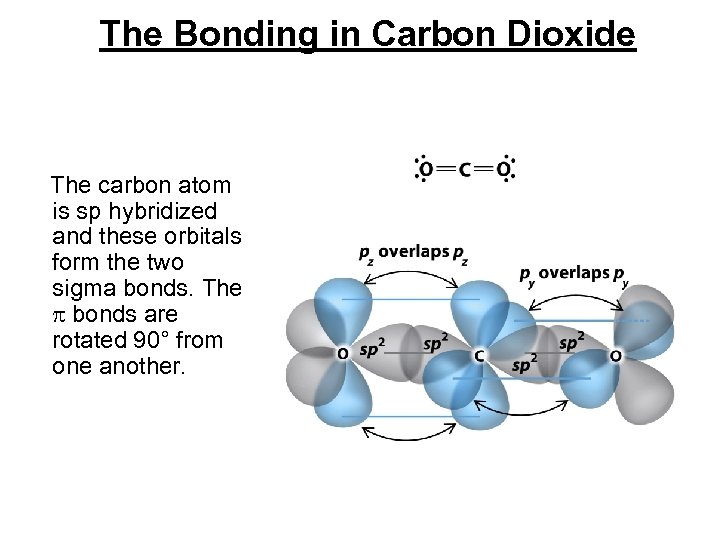
The Bonding in Carbon Dioxide The carbon atom is sp hybridized and these orbitals form the two sigma bonds. The bonds are rotated 90° from one another.
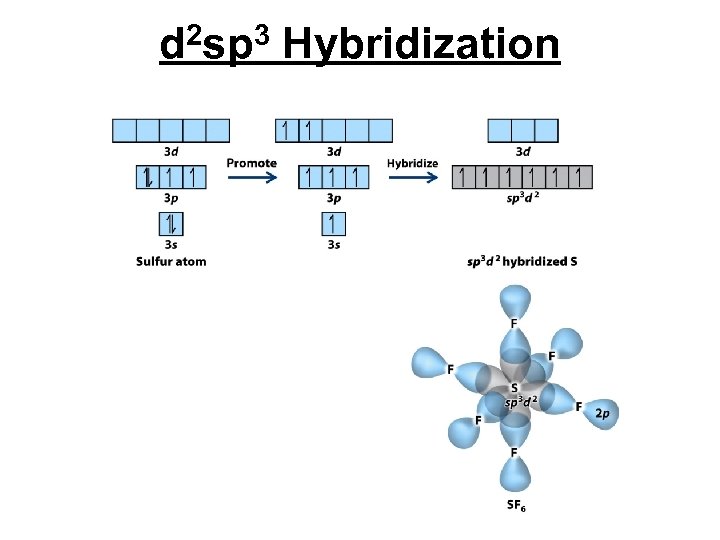
d 2 sp 3 Hybridization
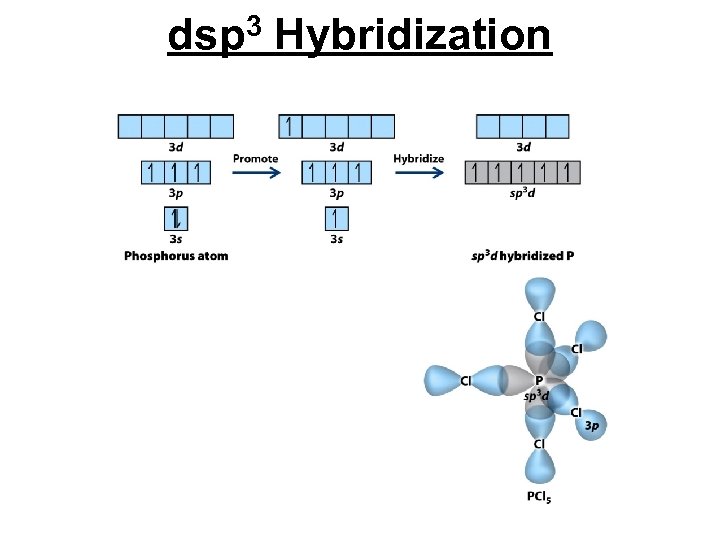
dsp 3 Hybridization
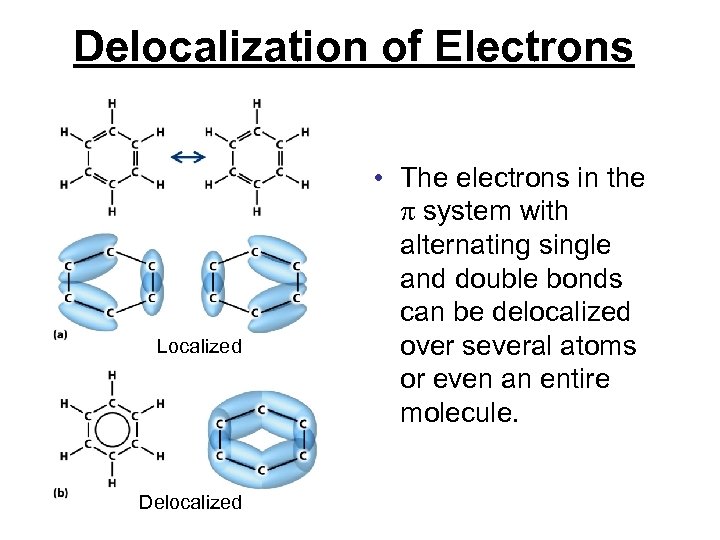
Delocalization of Electrons Localized Delocalized • The electrons in the system with alternating single and double bonds can be delocalized over several atoms or even an entire molecule.

Hybrid Orbital Notation In order to construct hybrid orbital notation, we need to separate the central atom from the surrounding electrons, usually the central atom is the largest, the most electronegative, or the one that there is one of.
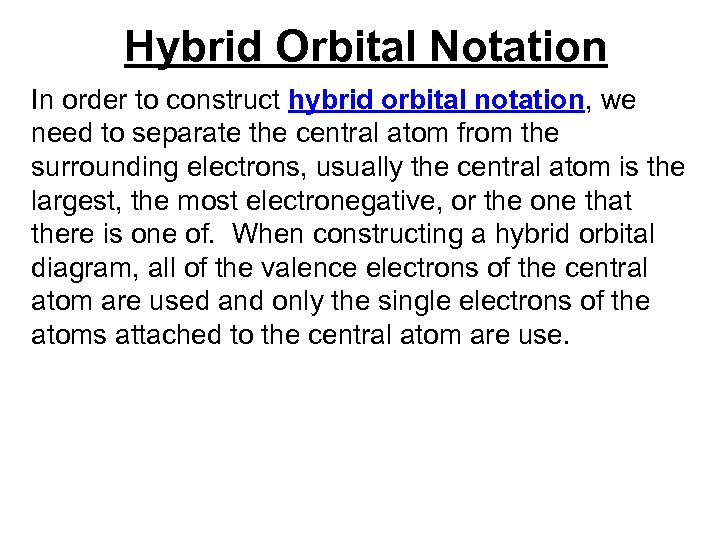
Hybrid Orbital Notation In order to construct hybrid orbital notation, we need to separate the central atom from the surrounding electrons, usually the central atom is the largest, the most electronegative, or the one that there is one of. When constructing a hybrid orbital diagram, all of the valence electrons of the central atom are used and only the single electrons of the atoms attached to the central atom are use.
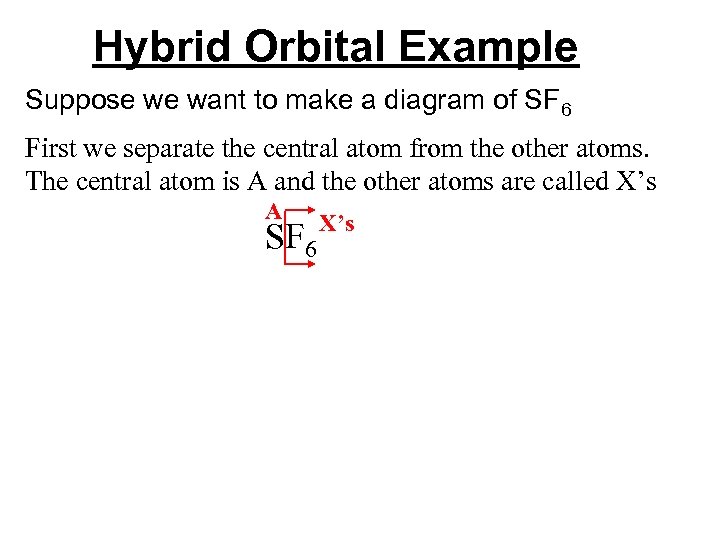
Hybrid Orbital Example Suppose we want to make a diagram of SF 6 First we separate the central atom from the other atoms. The central atom is A and the other atoms are called X’s A SF 6 X’s
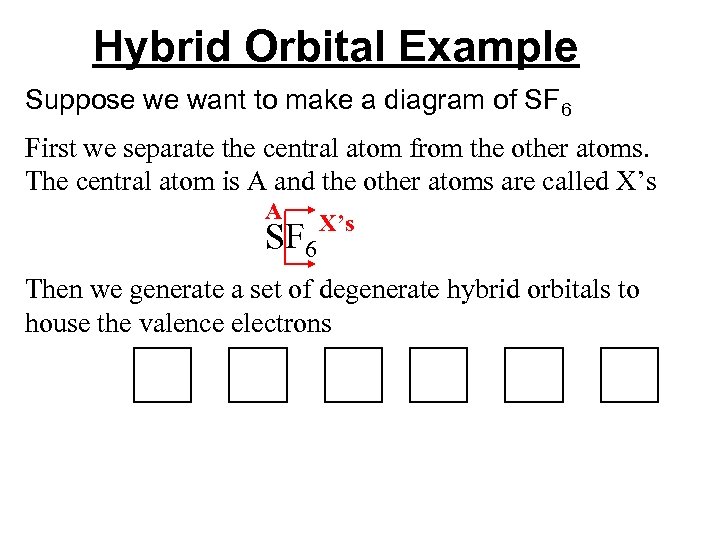
Hybrid Orbital Example Suppose we want to make a diagram of SF 6 First we separate the central atom from the other atoms. The central atom is A and the other atoms are called X’s A SF 6 X’s Then we generate a set of degenerate hybrid orbitals to house the valence electrons
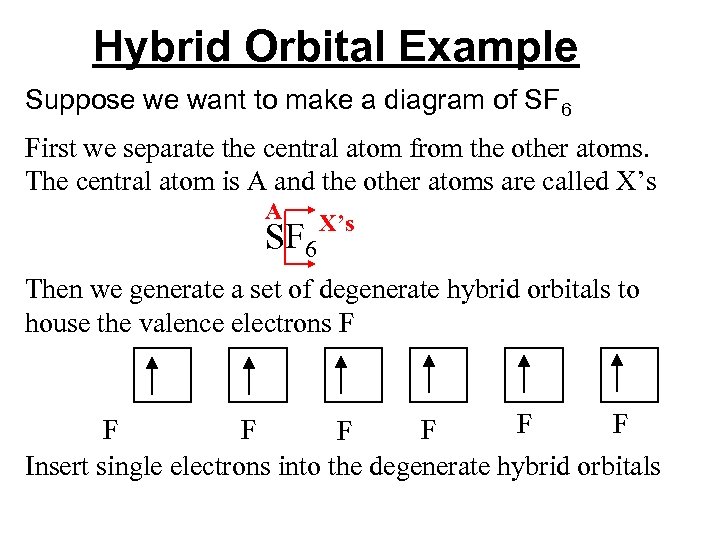
Hybrid Orbital Example Suppose we want to make a diagram of SF 6 First we separate the central atom from the other atoms. The central atom is A and the other atoms are called X’s A SF 6 X’s Then we generate a set of degenerate hybrid orbitals to house the valence electrons F F F F Insert single electrons into the degenerate hybrid orbitals
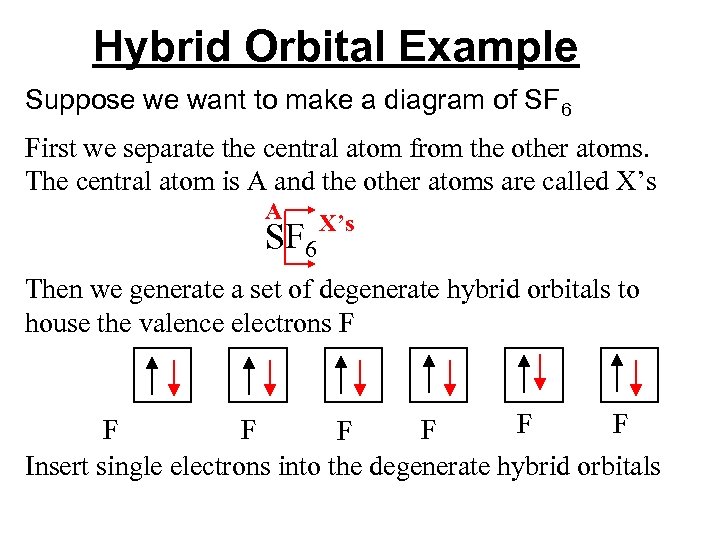
Hybrid Orbital Example Suppose we want to make a diagram of SF 6 First we separate the central atom from the other atoms. The central atom is A and the other atoms are called X’s A SF 6 X’s Then we generate a set of degenerate hybrid orbitals to house the valence electrons F F F F Insert single electrons into the degenerate hybrid orbitals
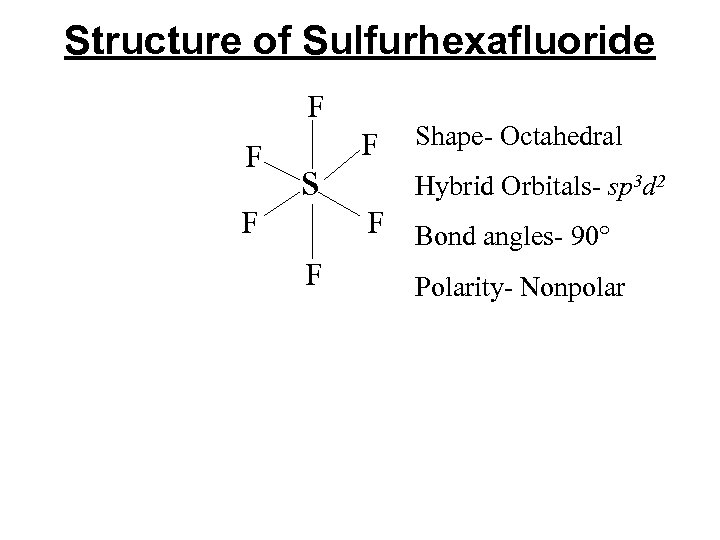
Structure of Sulfurhexafluoride F F F s F F Shape- Octahedral Hybrid Orbitals- sp 3 d 2 F Bond angles- 90° Polarity- Nonpolar
Noble Gas Compounds Critics of the hybrid orbital theory argued that the hybrid orbital theory suggests that compounds of Noble gases should exist or be made. In 1962 Neil Bartlett created a compound of xenon, platinum and fluorine. Today there are now several hundred Noble gas compounds known. University of British Columbia
Noble Gas Compounds Practice Xe. O 2 Kr. F 4 Xe. O 2 F 22+
Problems with Bonding Theories • Lewis structure and valence bond theory help us understand the bonding capacities of elements. • VSEPR and valence bond theories account for the observed molecular geometries. • None of these models enables us to explain why O 2 is attracted to a magnetic field while N 2 is repelled slightly.
Molecular Orbital (MO) Theory • The wave functions of atomic orbitals of atoms are combined to create molecular orbitals (MOs) in molecules. § Each MO is associated with an entire molecule, not just a single atom. MOs are spread out, or delocalized over all the atoms in a molecule.

Types of MOs • Electrons in bonding orbitals serve to hold atoms together in molecules by increasing the electron density between nuclear centers. • Electrons in antibonding orbitals in a molecule destabilize the molecule because they do not increase the electron density between nuclear centers.

MO Guidelines 1. The total number of MO formed equals the number of atomic orbitals used in the mixing process. 2. Orbitals with similar energy and shape mix more effectively than do those that are different. 3. Orbitals of different principal quantum numbers have different sizes and energies resulting in less effective mixing. 4. A MO can accommodate two electrons with opposite spin. 5. Electrons are placed in MO diagrams according to Hund’s rule.
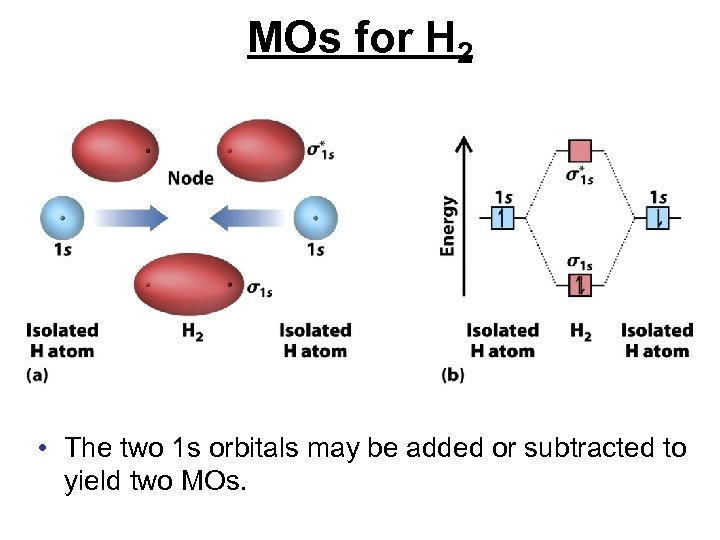
MOs for H 2 • The two 1 s orbitals may be added or subtracted to yield two MOs.
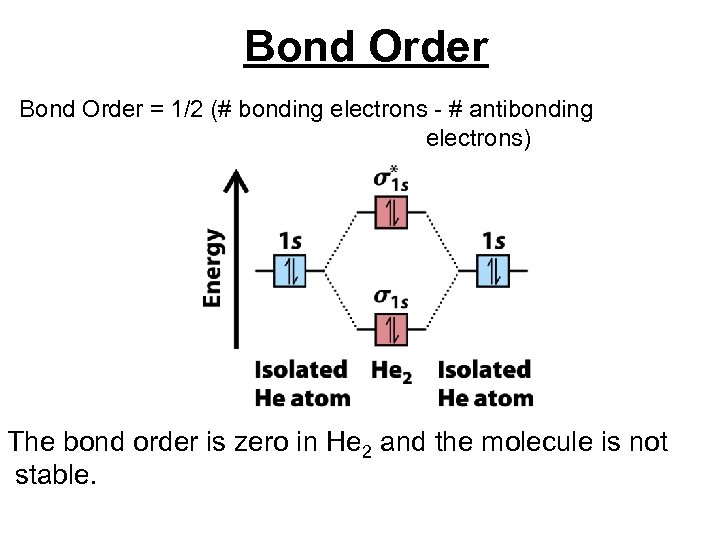
Bond Order = 1/2 (# bonding electrons - # antibonding electrons) The bond order is zero in He 2 and the molecule is not stable.
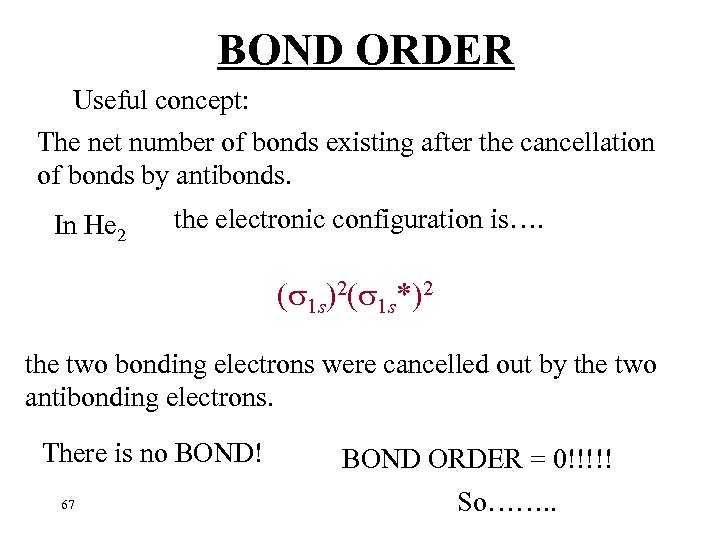
BOND ORDER Useful concept: The net number of bonds existing after the cancellation of bonds by antibonds. In He 2 the electronic configuration is…. ( 1 s)2( 1 s*)2 the two bonding electrons were cancelled out by the two antibonding electrons. There is no BOND! 67 BOND ORDER = 0!!!!! So……. .
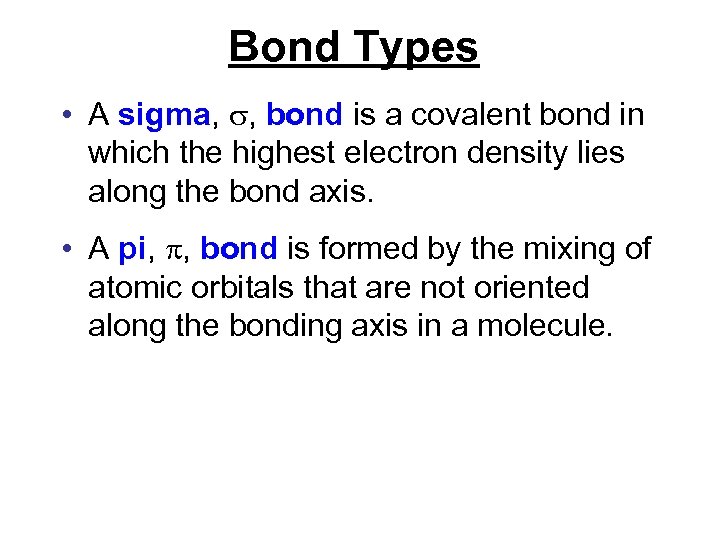
Bond Types • A sigma, , bond is a covalent bond in which the highest electron density lies along the bond axis. • A pi, , bond is formed by the mixing of atomic orbitals that are not oriented along the bonding axis in a molecule.
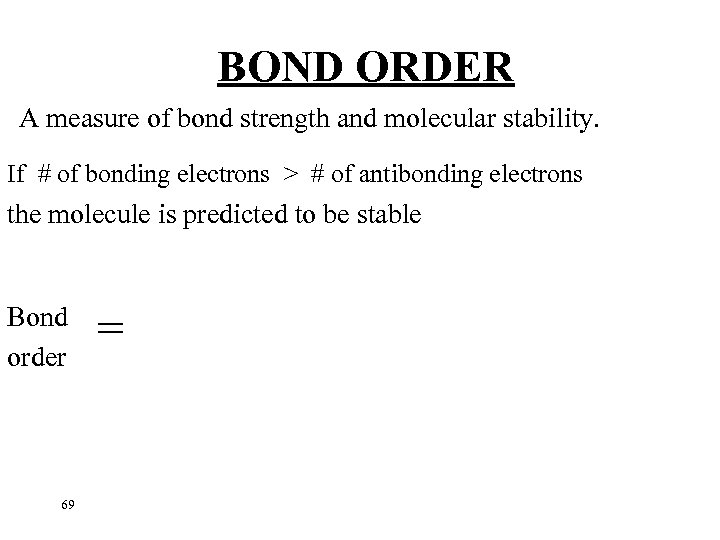
BOND ORDER A measure of bond strength and molecular stability. If # of bonding electrons > # of antibonding electrons the molecule is predicted to be stable Bond order 69 =
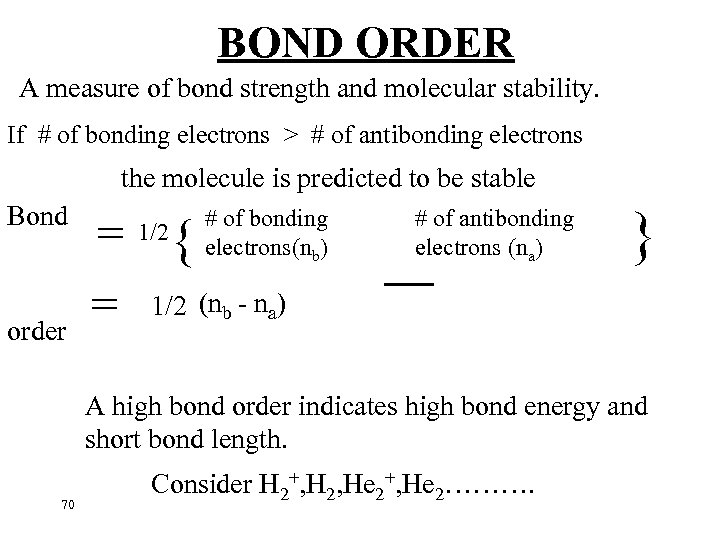
BOND ORDER A measure of bond strength and molecular stability. If # of bonding electrons > # of antibonding electrons the molecule is predicted to be stable Bond order # of bonding electrons(nb) = 1/2{ = 1/2 (n - n ) b a # of antibonding electrons (na) – } A high bond order indicates high bond energy and short bond length. 70 Consider H 2+, H 2, He 2+, He 2……….
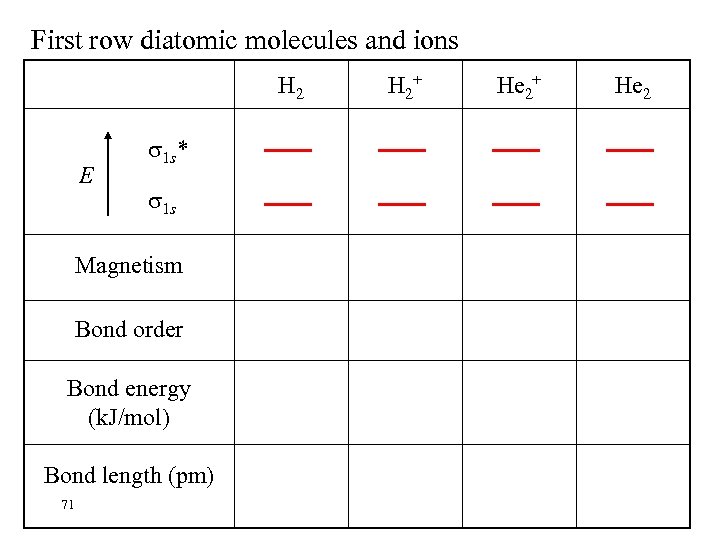
First row diatomic molecules and ions H 2 E 1 s* 1 s Magnetism Bond order Bond energy (k. J/mol) Bond length (pm) 71 H 2+ He 2
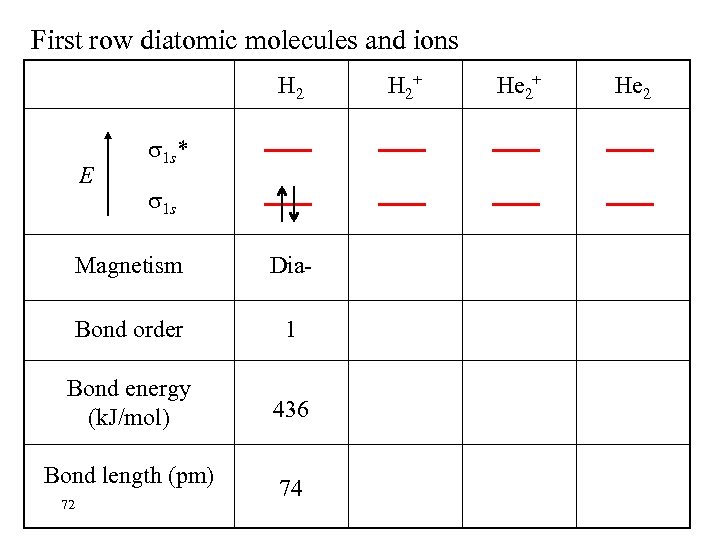
First row diatomic molecules and ions H 2 E 1 s* 1 s Magnetism Dia- Bond order 1 Bond energy (k. J/mol) 436 Bond length (pm) 72 74 H 2+ He 2
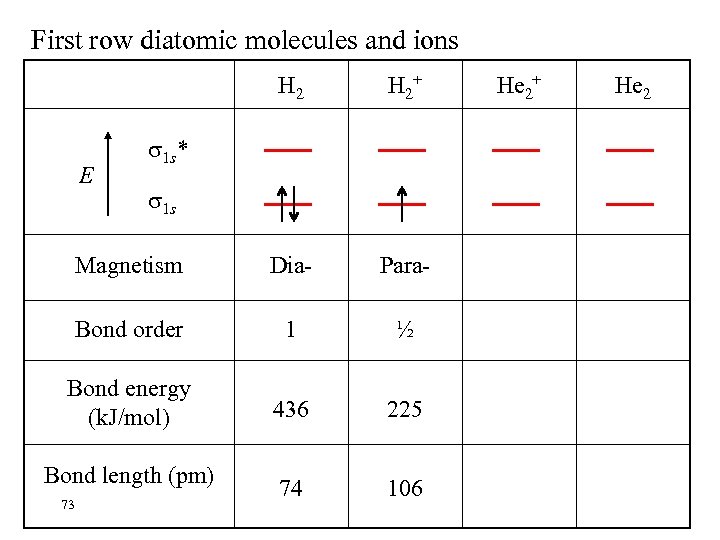
First row diatomic molecules and ions H 2+ Magnetism Dia- Para- Bond order 1 ½ Bond energy (k. J/mol) 436 225 74 106 E 1 s* 1 s Bond length (pm) 73 He 2+ He 2
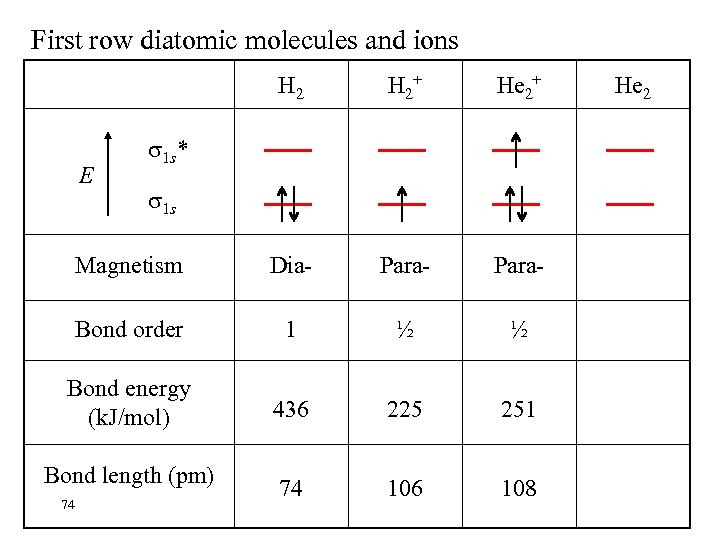
First row diatomic molecules and ions H 2+ He 2+ Magnetism Dia- Para- Bond order 1 ½ ½ Bond energy (k. J/mol) 436 225 251 74 106 108 E 1 s* 1 s Bond length (pm) 74 He 2
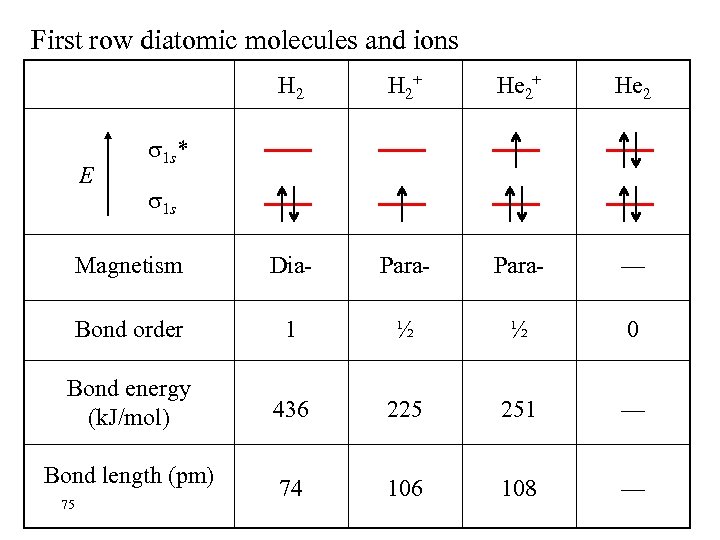
First row diatomic molecules and ions H 2+ He 2 Magnetism Dia- Para- — Bond order 1 ½ ½ 0 Bond energy (k. J/mol) 436 225 251 — 74 106 108 — E 1 s* 1 s Bond length (pm) 75

HOMONUCLEAR DIATOMICS Now look at second period…. . First is Li 2 Li : 1 s 22 s 1 Both the 1 s and 2 s overlap to produce bonding and anti-bonding orbitals. This is the energy level diagram…. . 76
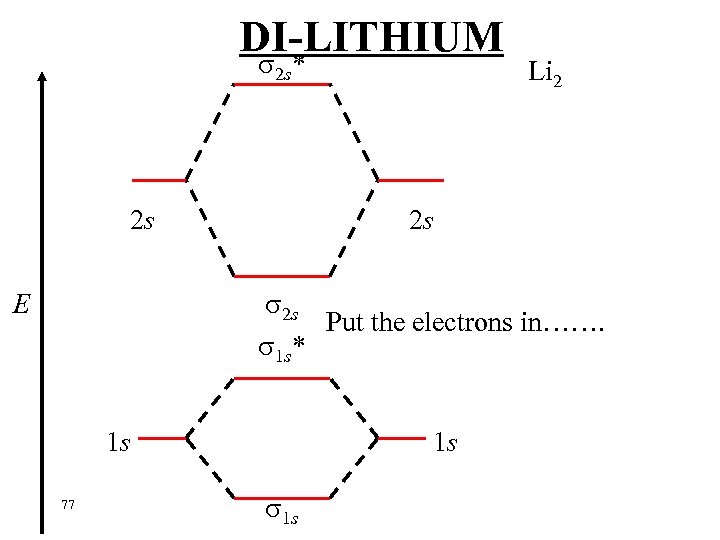
DI-LITHIUM * 2 s 2 s Li 2 2 s 2 s Put the electrons in……. 1 s* E 1 s 77 1 s 1 s
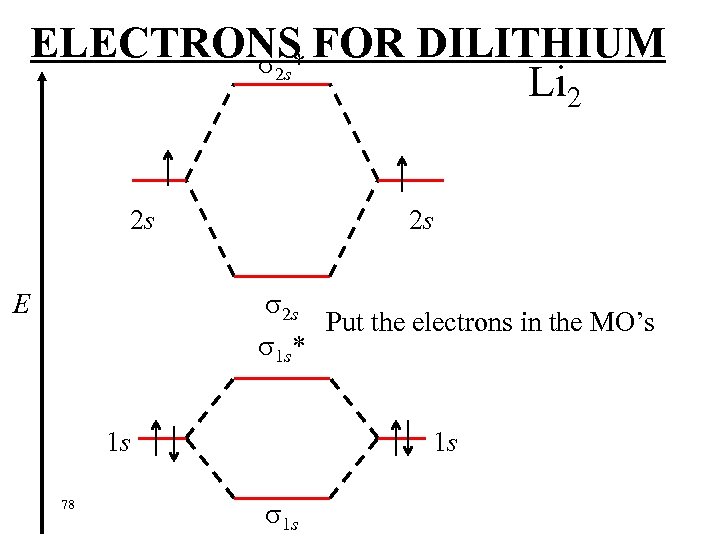
ELECTRONS FOR DILITHIUM 2 s* Li 2 2 s 2 s 2 s Put the electrons in the MO’s 1 s* E 1 s 78 1 s 1 s
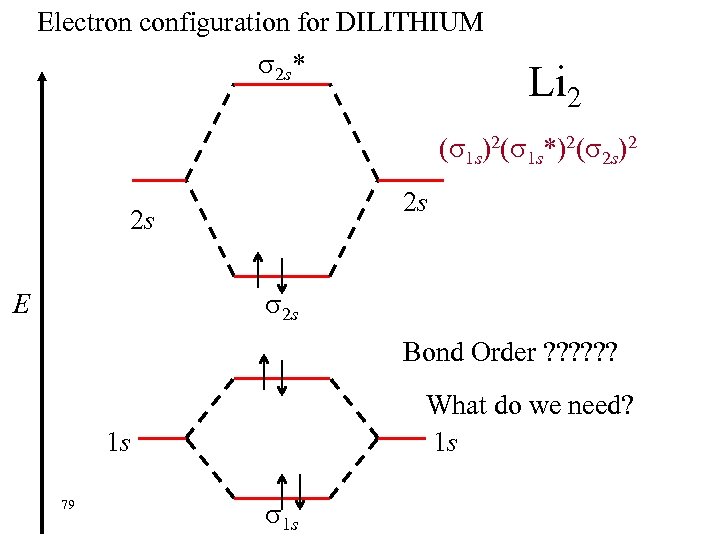
Electron configuration for DILITHIUM 2 s* Li 2 ( 1 s)2( 1 s*)2( 2 s)2 2 s 2 s 2 s E Bond Order ? ? ? What do we need? 1 s 1 s 79 1 s
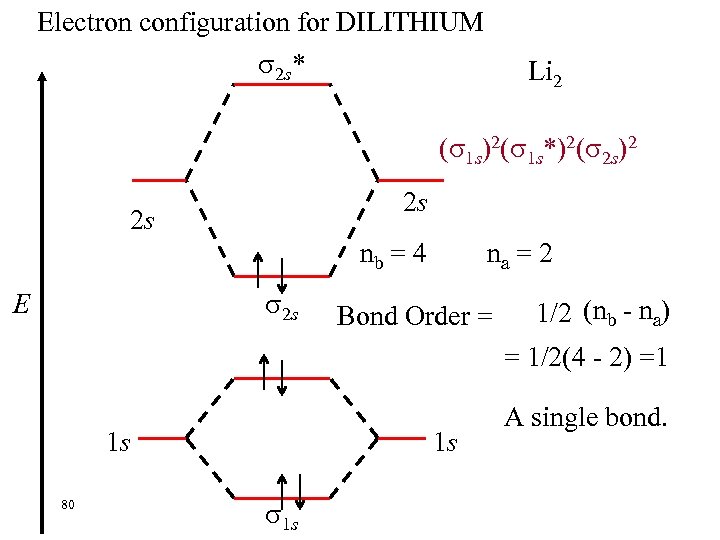
Electron configuration for DILITHIUM 2 s* Li 2 ( 1 s)2( 1 s*)2( 2 s)2 2 s 2 s na = 2 nb = 4 2 s E Bond Order = 1/2 (nb - na) = 1/2(4 - 2) =1 1 s 80 1 s 1 s A single bond.

Electron configuration for DILITHIUM 2 s* Li 2 ( 1 s)2( 1 s*)2( 2 s)2 2 s 2 s na = 2 nb = 4 2 s E Note: The 1 s and 1 s* orbitals cancel! 1 s 81 1 s 1 s So…….
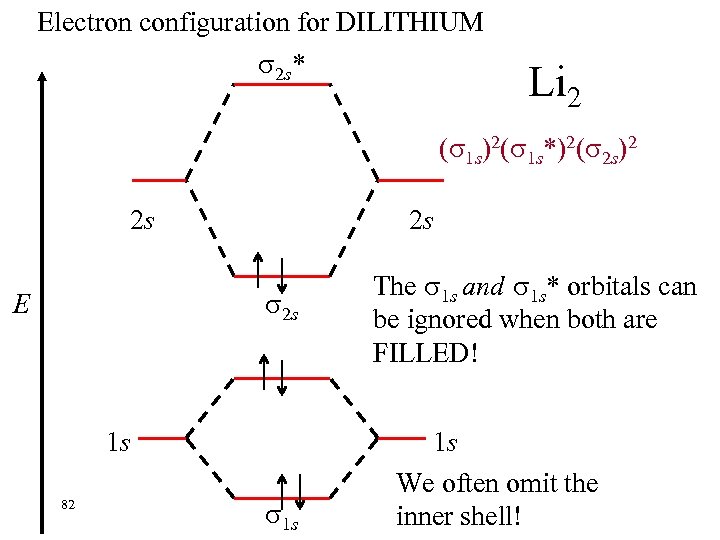
Electron configuration for DILITHIUM 2 s* Li 2 ( 1 s)2( 1 s*)2( 2 s)2 2 s 2 s 2 s E 1 s 82 1 s The 1 s and 1 s* orbitals can be ignored when both are FILLED! 1 s We often omit the inner shell!
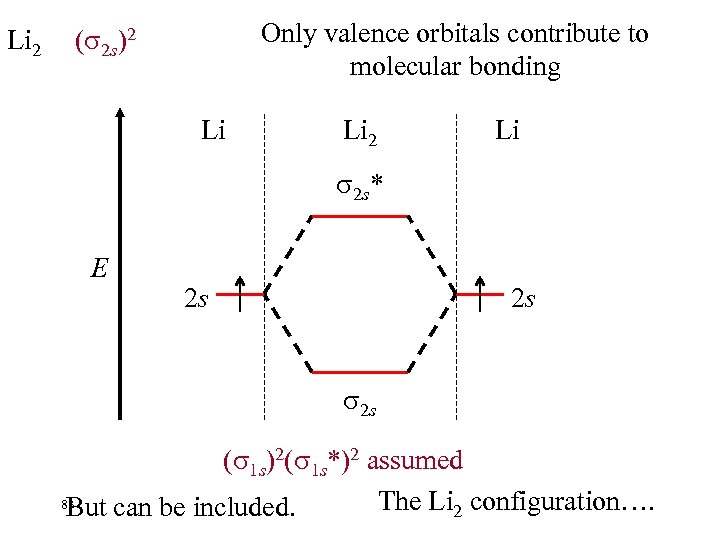
Li 2 Only valence orbitals contribute to molecular bonding ( 2 s)2 Li Li 2 Li 2 s* E 2 s 2 s 2 s ( 1 s)2( 1 s*)2 assumed 83 The Li 2 configuration…. But can be included.
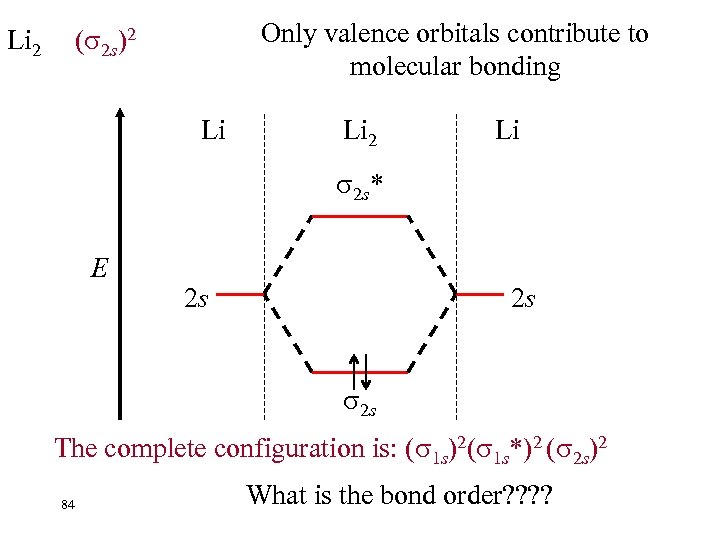
Li 2 Only valence orbitals contribute to molecular bonding ( 2 s)2 Li Li 2 Li 2 s* E 2 s 2 s 2 s The complete configuration is: ( 1 s)2( 1 s*)2 ( 2 s)2 84 What is the bond order? ?
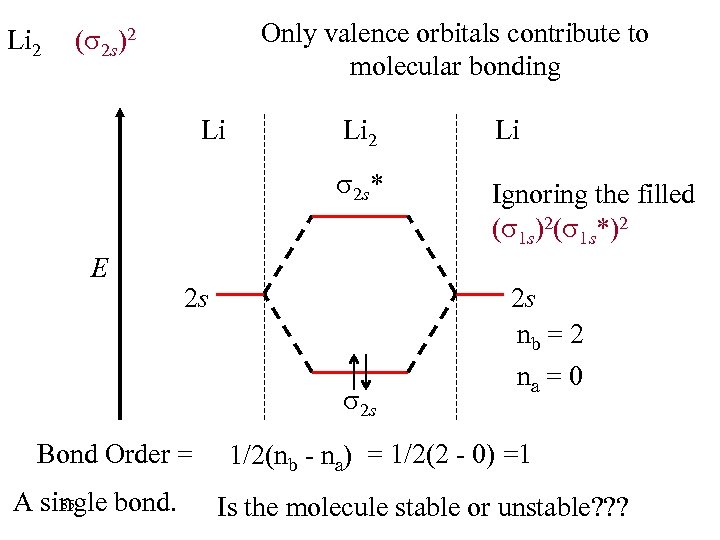
Li 2 Only valence orbitals contribute to molecular bonding ( 2 s)2 Li Li 2 s* E Li 2 Ignoring the filled ( 1 s)2( 1 s*)2 2 s 2 s Bond Order = 85 A single bond. 2 s nb = 2 na = 0 1/2(nb - na) = 1/2(2 - 0) =1 Is the molecule stable or unstable? ? ?
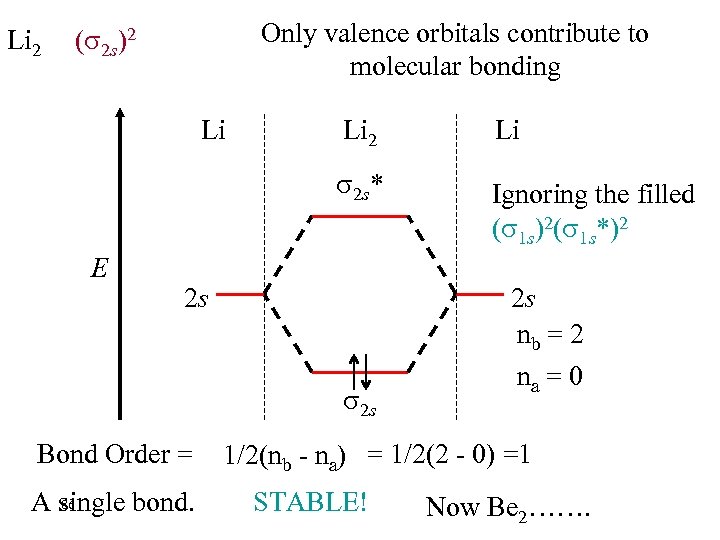
Li 2 Only valence orbitals contribute to molecular bonding ( 2 s)2 Li Li 2 s* E Li 2 Ignoring the filled ( 1 s)2( 1 s*)2 2 s 2 s Bond Order = 86 A single bond. 2 s nb = 2 na = 0 1/2(nb - na) = 1/2(2 - 0) =1 STABLE! Now Be 2…….
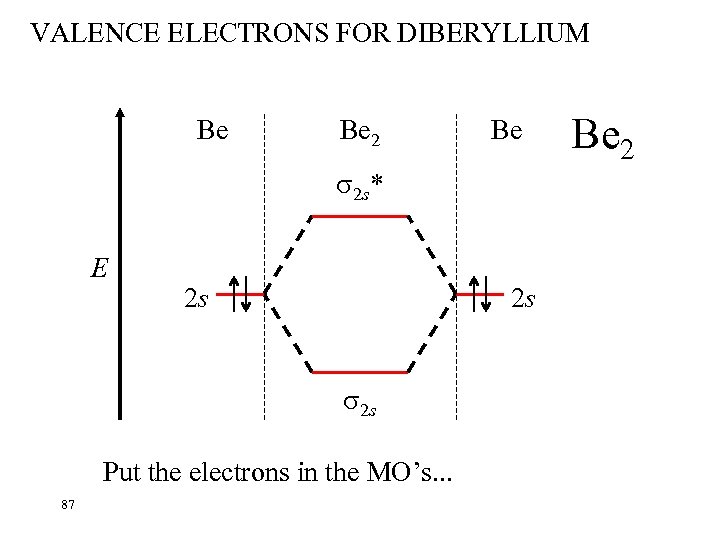
VALENCE ELECTRONS FOR DIBERYLLIUM Be Be 2 Be 2 s* E 2 s 2 s 2 s Put the electrons in the MO’s. . . 87 Be 2
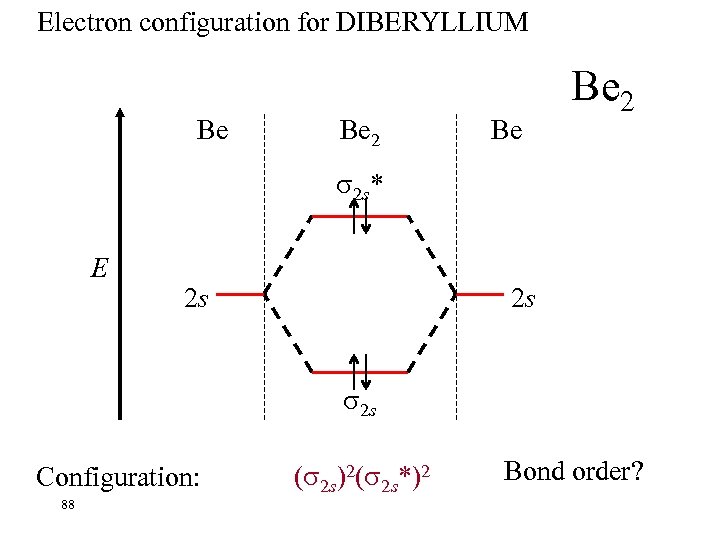
Electron configuration for DIBERYLLIUM Be Be 2 2 s* E 2 s 2 s 2 s Configuration: 88 ( 2 s)2( 2 s*)2 Bond order?
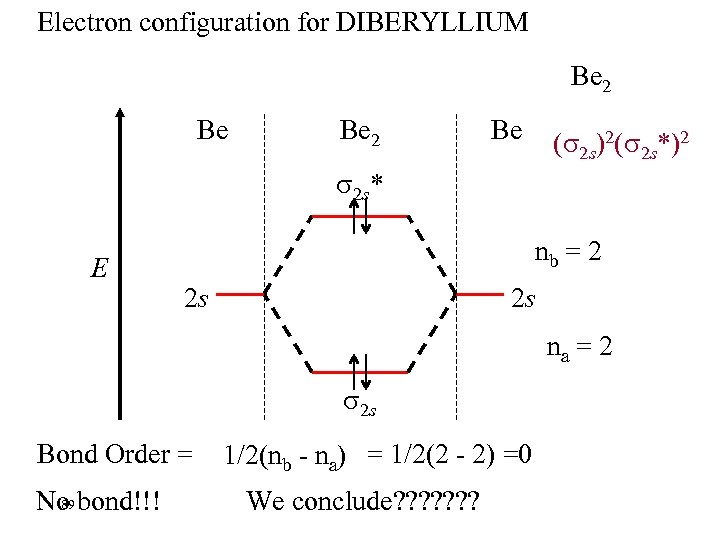
Electron configuration for DIBERYLLIUM Be 2 Be ( 2 s)2( 2 s*)2 2 s* E nb = 2 2 s 2 s na = 2 2 s Bond Order = 89 No bond!!! 1/2(nb - na) = 1/2(2 - 2) =0 We conclude? ? ? ?
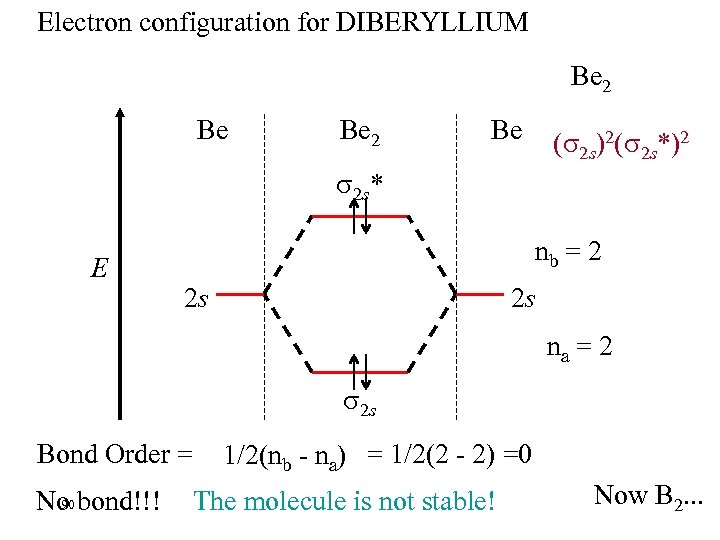
Electron configuration for DIBERYLLIUM Be 2 Be ( 2 s)2( 2 s*)2 2 s* E nb = 2 2 s 2 s na = 2 2 s Bond Order = 90 No bond!!! 1/2(nb - na) = 1/2(2 - 2) =0 The molecule is not stable! Now B 2. . .

B 2 The Boron atomic configuration is 1 s 22 p 1 So we expect B to use 2 p orbitals to form molecular orbitals. How do we do that? ? ? Combine them by ? ? ? Addition and subtraction…. 91 This is what they look like…….
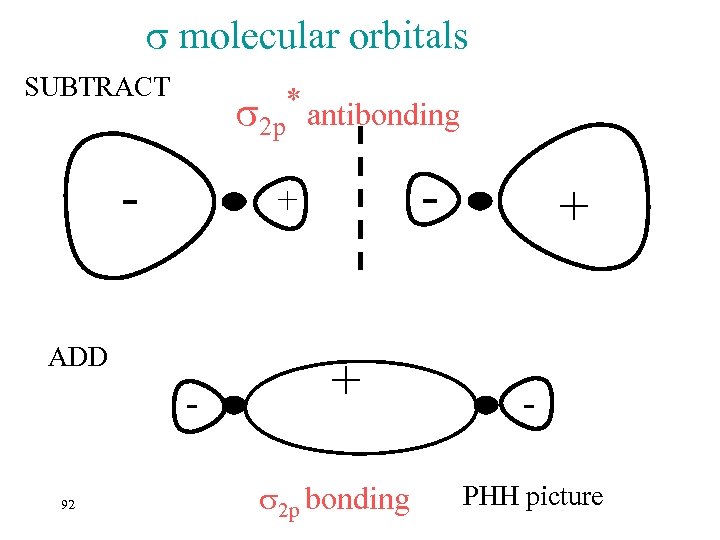
molecular orbitals SUBTRACT * antibonding 2 p ADD 92 - + + 2 p bonding + PHH picture
s-molecular orbitals PHH picture - 93 - + + - MO’s -
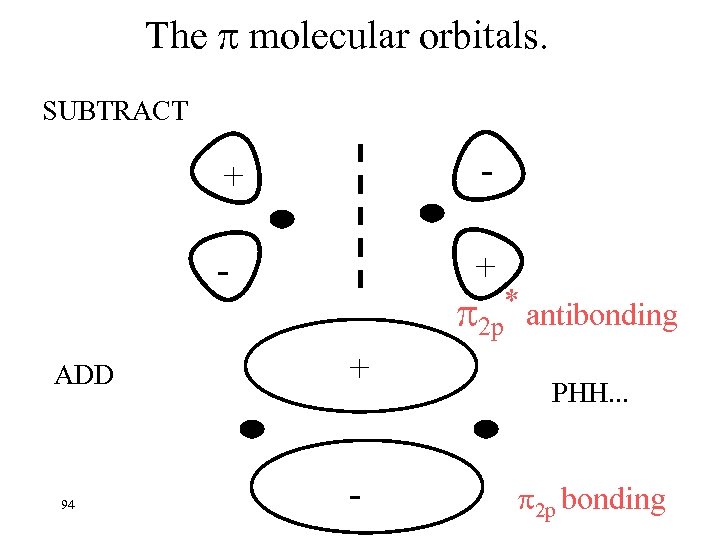
The molecular orbitals. SUBTRACT + ADD 94 + * antibonding 2 p + - PHH. . . 2 p bonding
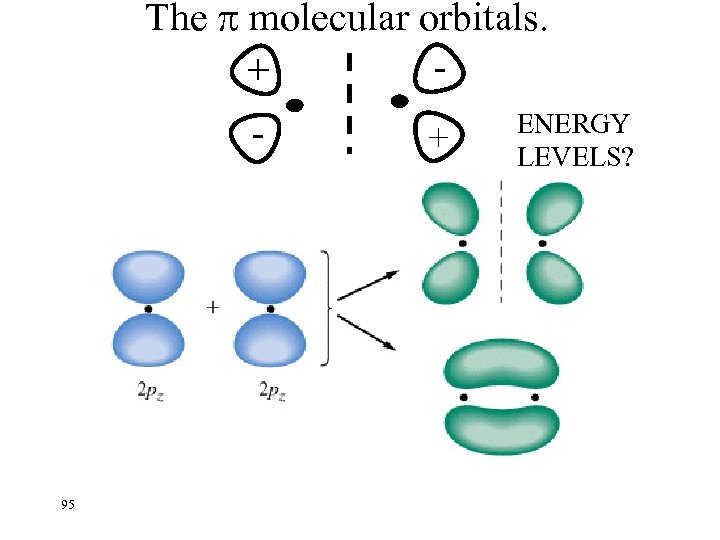
The molecular orbitals. + - + + - 95 ENERGY LEVELS?
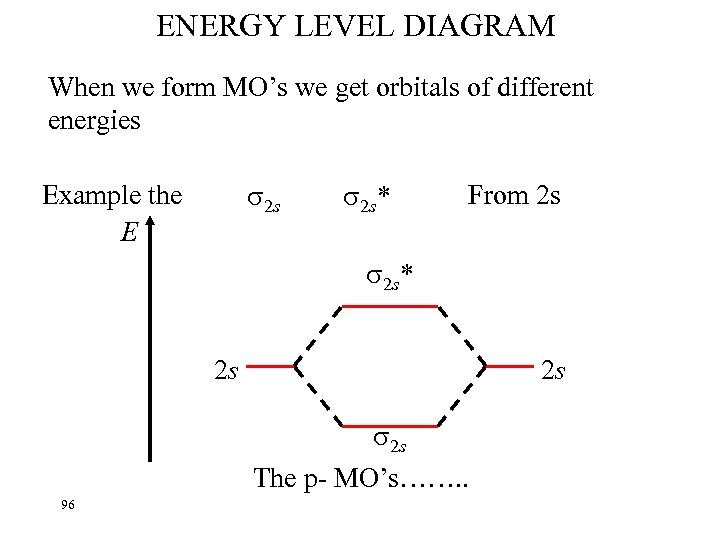
ENERGY LEVEL DIAGRAM When we form MO’s we get orbitals of different energies 2 s Example the E 2 s* From 2 s 2 s* 2 s 2 s 2 s The p- MO’s……. . 96
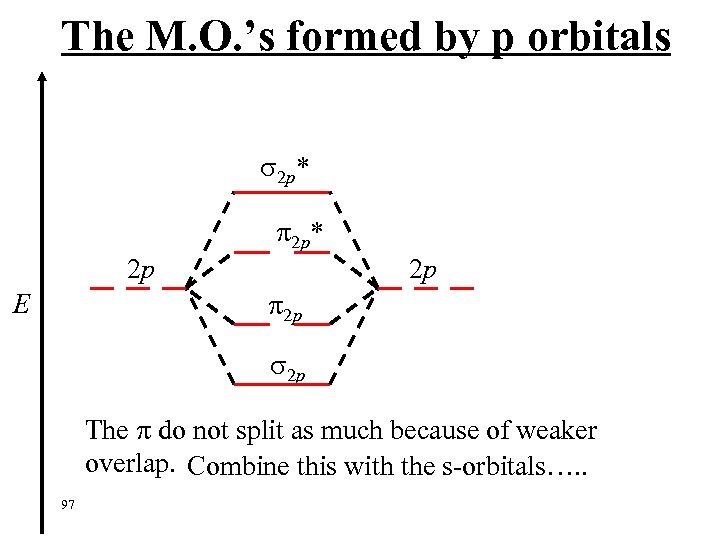
The M. O. ’s formed by p orbitals 2 p* 2 p 2 p 2 p E 2 p The do not split as much because of weaker overlap. Combine this with the s-orbitals…. . 97

Modified Molecular Orbital Diagram It should be noted that both sigma 1 s and sigma 2 p orbitals have similar shape and energy, thus mixing occurs lowering the sigma 1 s orbital and elevating the sigma 2 p orbital above the bonding pi molecular orbitals. This is illustrated on the next slide.
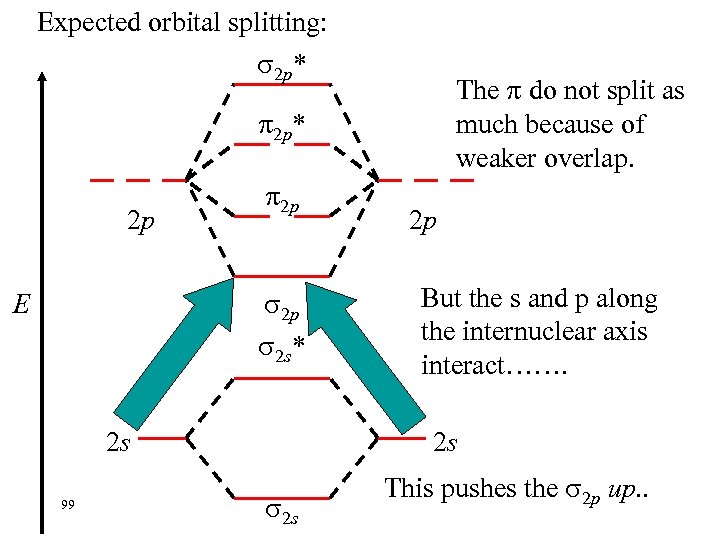
Expected orbital splitting: 2 p* The do not split as much because of weaker overlap. 2 p* 2 p 2 p 2 s* E 2 s 99 2 p But the s and p along the internuclear axis interact……. 2 s This pushes the 2 p up. .
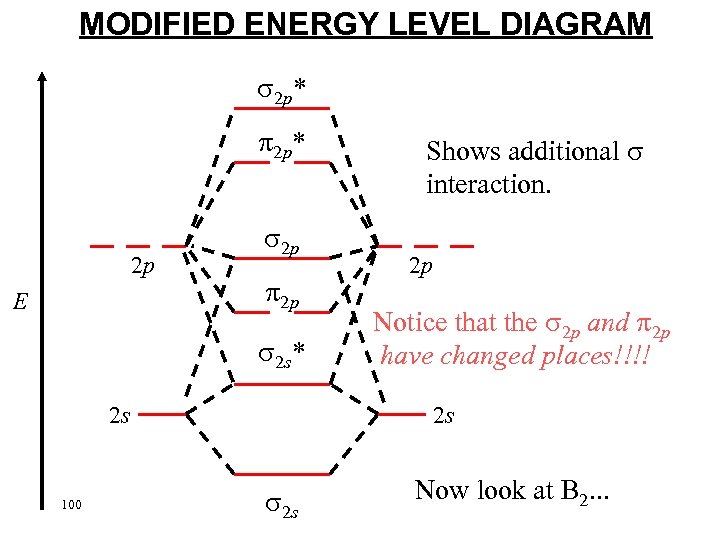
MODIFIED ENERGY LEVEL DIAGRAM 2 p* 2 p E 2 p 2 p 2 s* 2 s 100 Shows additional interaction. 2 p Notice that the 2 p and 2 p have changed places!!!! 2 s Now look at B 2. . .
![Electron configuration for B 2 2 p* B is [He] 2 s 22 p Electron configuration for B 2 2 p* B is [He] 2 s 22 p](https://present5.com/presentation/9f981294b087e5000ea0a24197af1be4/image-101.jpg)
Electron configuration for B 2 2 p* B is [He] 2 s 22 p 1 2 p* 2 p E 2 p 2 p 2 s* 2 s 101 2 p Place electrons from 2 s into 2 s and 2 s* 2 s
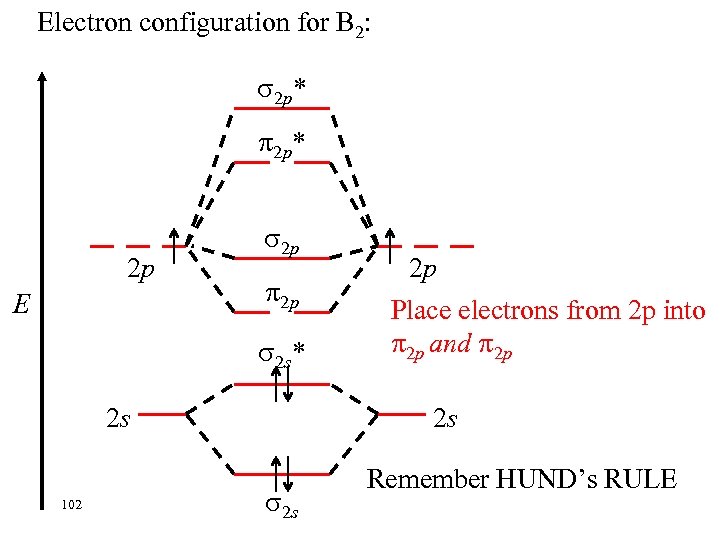
Electron configuration for B 2: 2 p* 2 p E 2 p 2 p 2 s* 2 s 102 2 p Place electrons from 2 p into 2 p and 2 p 2 s Remember HUND’s RULE
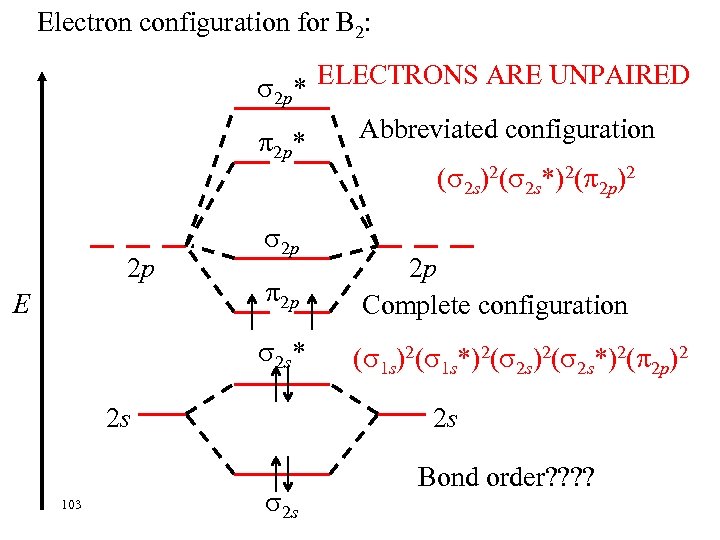
Electron configuration for B 2: ELECTRONS ARE UNPAIRED 2 p* Abbreviated configuration * 2 p ( 2 s)2( 2 s*)2( 2 p)2 2 p E 2 p 2 p 2 s* 2 s 103 2 p Complete configuration ( 1 s)2( 1 s*)2( 2 s*)2( 2 p)2 2 s Bond order? ?
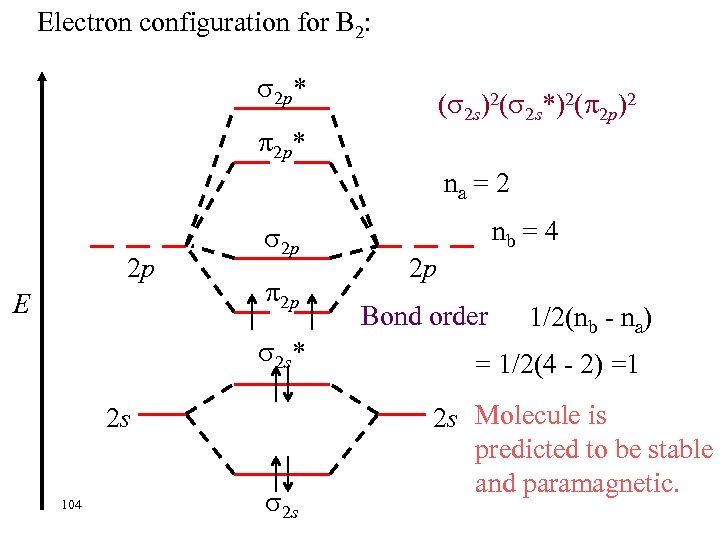
Electron configuration for B 2: 2 p* ( 2 s)2( 2 s*)2( 2 p)2 2 p* na = 2 2 p E 2 p 2 p 2 s* 2 s 104 2 s nb = 4 2 p Bond order 1/2(nb - na) = 1/2(4 - 2) =1 2 s Molecule is predicted to be stable and paramagnetic.
HOMONUCLEAR DIATOMICS Li 2 B 2 C 2 N 2 O 2 F 2 Which energy level diagram? ? ? The one with s and p interaction or the one without? We find………. 105
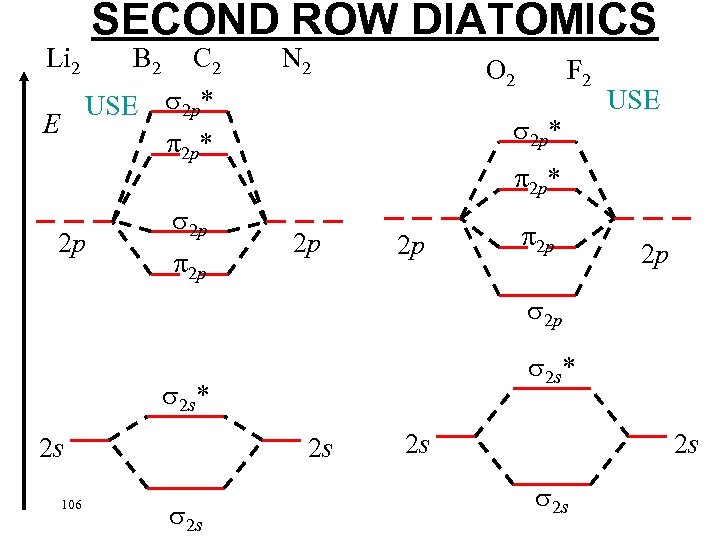
Li 2 SECOND ROW DIATOMICS C 2 USE 2 p* 2 p B 2 2 p 2 p N 2 O 2 F 2 2 p* USE 2 p* 2 p 2 p 2 p 2 s* 2 s 106 2 s 2 s 2 s
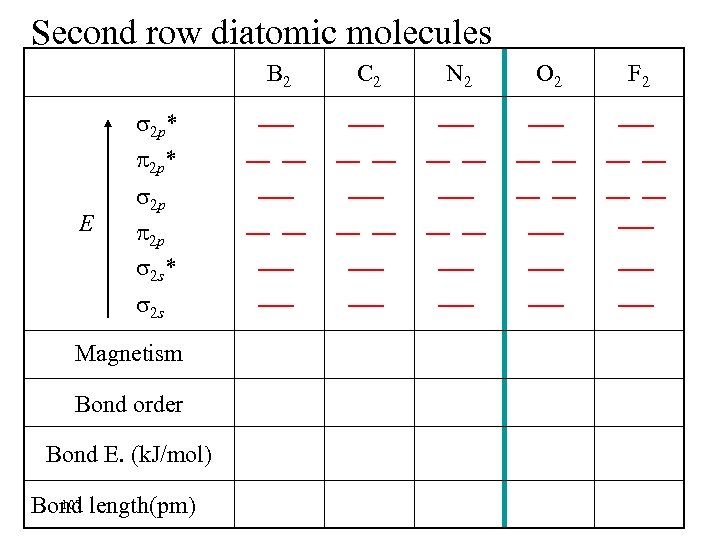
Second row diatomic molecules B 2 2 p* E 2 p 2 p 2 s* 2 s Magnetism Bond order Bond E. (k. J/mol) 107 Bond length(pm) C 2 N 2 O 2 F 2
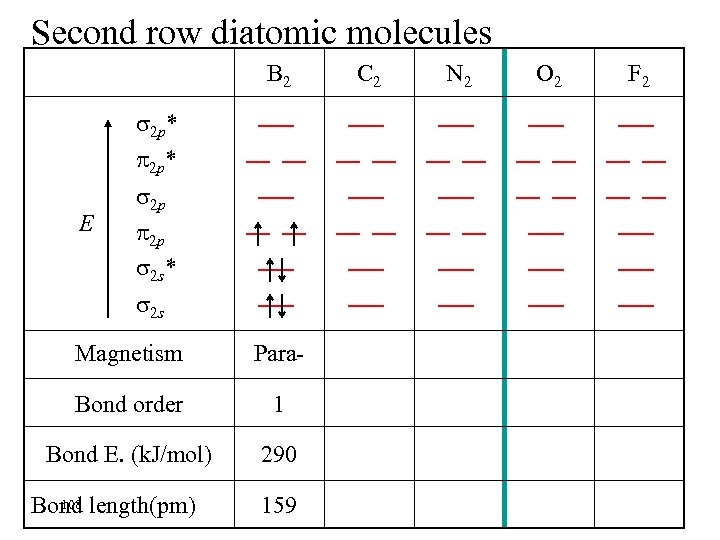
Second row diatomic molecules B 2 2 p* E 2 p 2 p 2 s* 2 s Magnetism Para- Bond order 1 Bond E. (k. J/mol) 290 108 Bond length(pm) 159 C 2 N 2 O 2 F 2
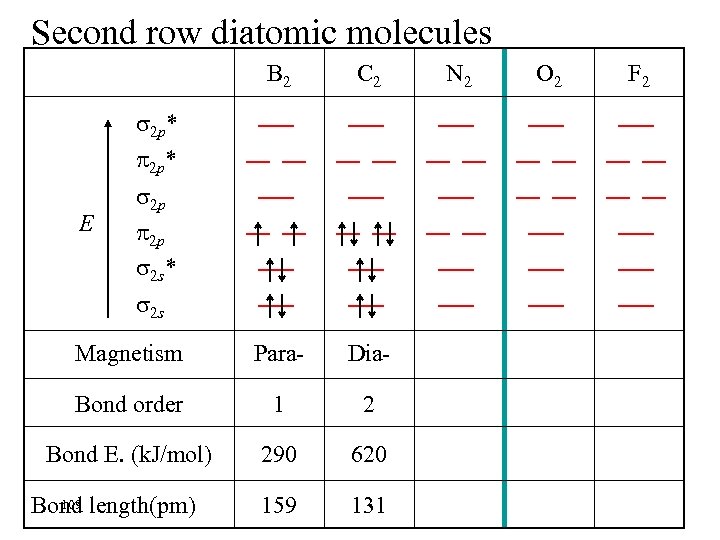
Second row diatomic molecules B 2 C 2 Magnetism Para- Dia- Bond order 1 2 Bond E. (k. J/mol) 290 620 159 131 2 p* E 2 p 2 p 2 s* 2 s 109 Bond length(pm) N 2 O 2 F 2
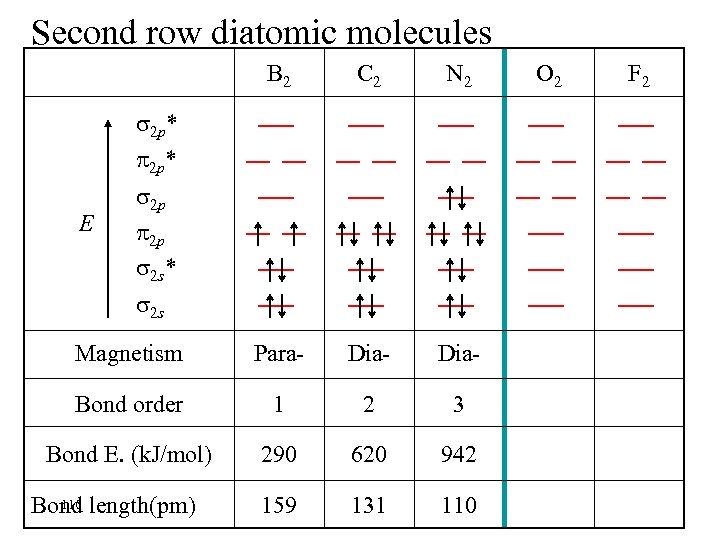
Second row diatomic molecules B 2 C 2 N 2 Magnetism Para- Dia- Bond order 1 2 3 Bond E. (k. J/mol) 290 620 942 159 131 110 2 p* E 2 p 2 p 2 s* 2 s 110 Bond length(pm) O 2 F 2
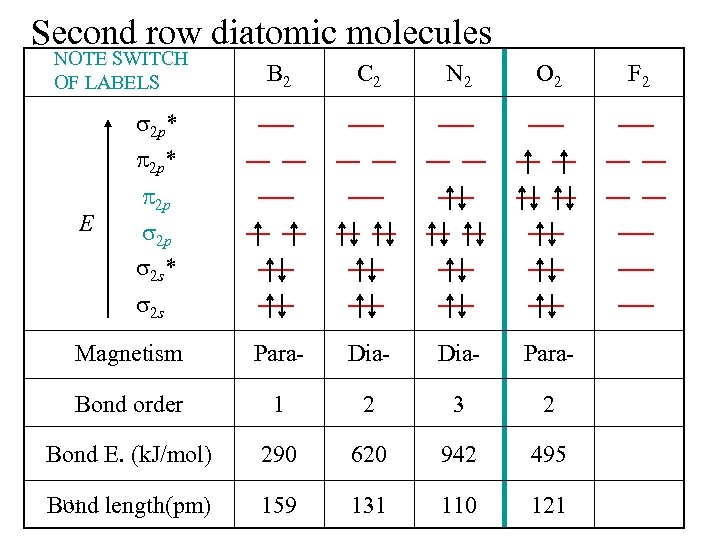
Second row diatomic molecules NOTE SWITCH OF LABELS B 2 C 2 N 2 O 2 Magnetism Para- Dia- Para- Bond order 1 2 3 2 Bond E. (k. J/mol) 290 620 942 495 111 Bond length(pm) 159 131 110 121 2 p* E 2 p 2 p 2 s* 2 s F 2
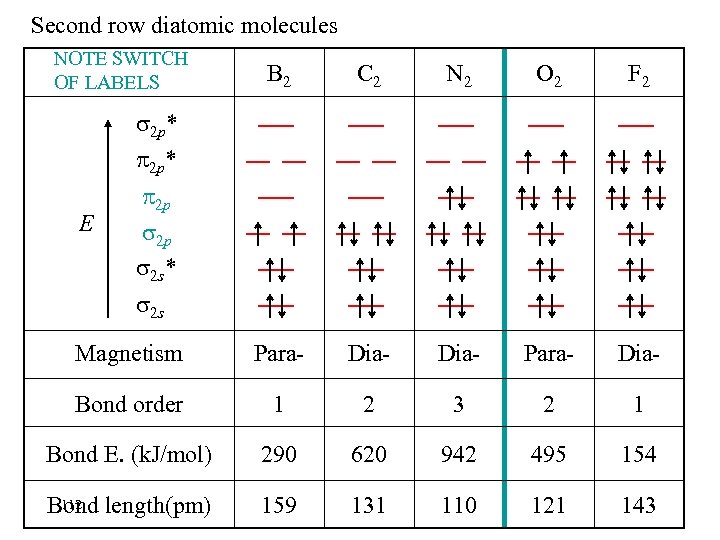
Second row diatomic molecules NOTE SWITCH OF LABELS B 2 C 2 N 2 O 2 F 2 Magnetism Para- Dia- Bond order 1 2 3 2 1 Bond E. (k. J/mol) 290 620 942 495 154 112 Bond length(pm) 159 131 110 121 143 2 p* E 2 p 2 p 2 s* 2 s

QUESTION The molecule X+2 that has the molecular orbital configuration ( 1 s)2( 1 s*)2( 2 s*)2( 2 p)4 ( 2 p)2 ( 2 p*)1 is 1 B 2+ 2 C 2+ 3 N 2+ 4 O 2+ 5 F 2+ ANSWER…. . . 113
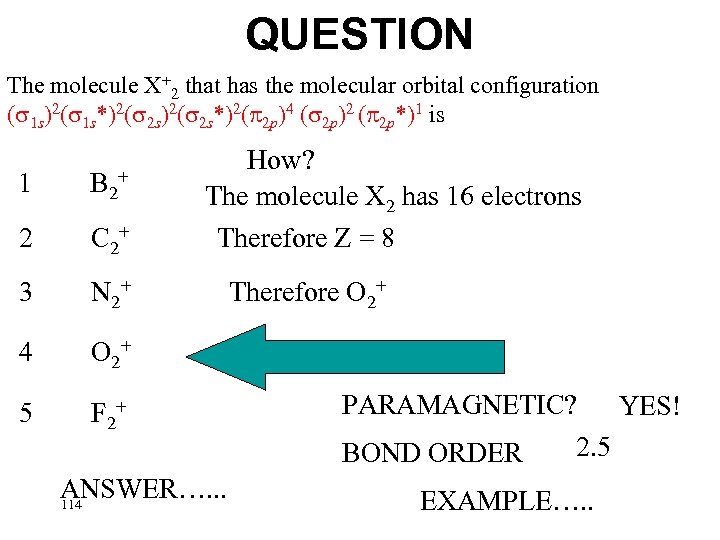
QUESTION The molecule X+2 that has the molecular orbital configuration ( 1 s)2( 1 s*)2( 2 s*)2( 2 p)4 ( 2 p)2 ( 2 p*)1 is 1 B 2+ 2 C 2+ 3 N 2+ 4 O 2+ 5 How? The molecule X 2 has 16 electrons Therefore Z = 8 F 2+ ANSWER…. . . 114 Therefore O 2+ PARAMAGNETIC? YES! 2. 5 BOND ORDER EXAMPLE…. .
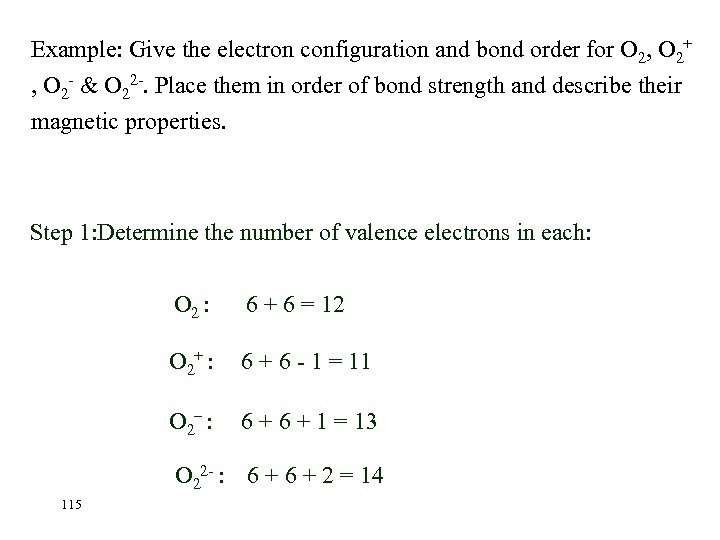
Example: Give the electron configuration and bond order for O 2, O 2+ , O 2 - & O 22 -. Place them in order of bond strength and describe their magnetic properties. Step 1: Determine the number of valence electrons in each: O 2 : 6 + 6 = 12 O 2+ : 6 + 6 - 1 = 11 O 2– : 6 + 1 = 13 O 22 - : 6 + 2 = 14 115
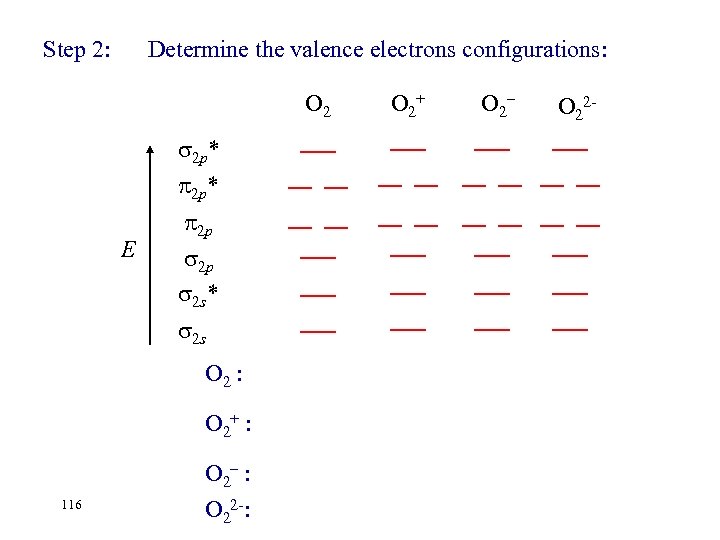
Step 2: Determine the valence electrons configurations: O 2 2 p* E 2 p 2 p 2 s* 2 s O 2 : O 2+ : 116 O 2– : O 22 -: O 2+ O 2– O 22 -
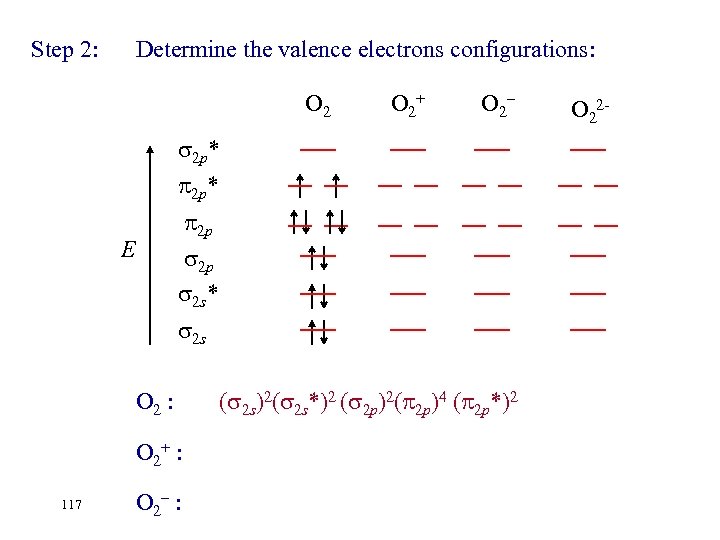
Step 2: Determine the valence electrons configurations: O 2+ O 2– 2 p* 2 p 2 p 2 s* E 2 s O 2 : O 2+ : 117 O 2– : ( 2 s)2( 2 s*)2 ( 2 p)2( 2 p)4 ( 2 p*)2 O 22 -
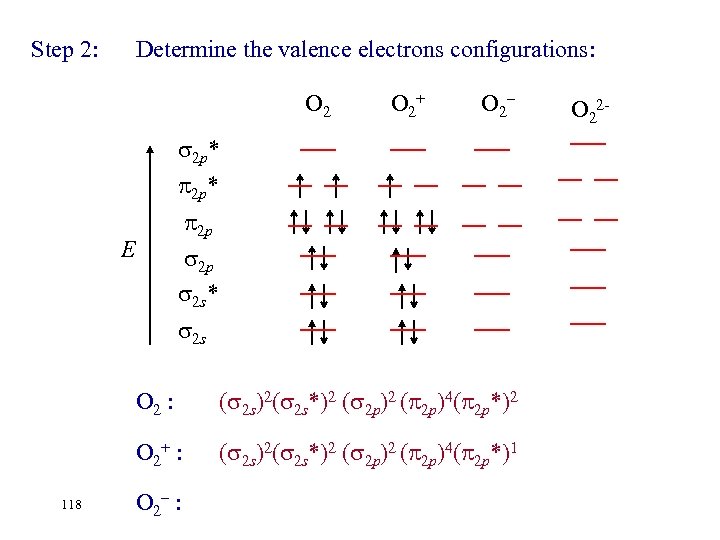
Step 2: Determine the valence electrons configurations: O 2+ O 2– 2 p* 2 p 2 p 2 s* E 2 s O 2 : O 2+ : 118 ( 2 s)2( 2 s*)2 ( 2 p)4( 2 p*)2 ( 2 s)2( 2 s*)2 ( 2 p)4( 2 p*)1 O 2– : O 22 -
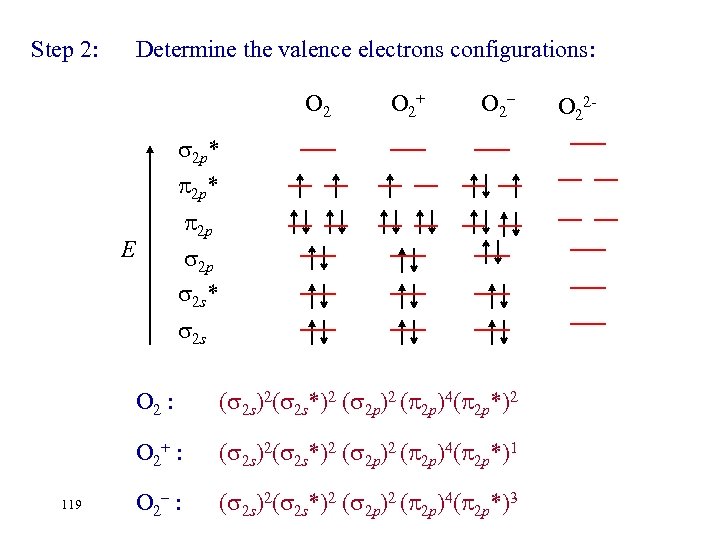
Step 2: Determine the valence electrons configurations: O 2+ O 2– 2 p* 2 p 2 p 2 s* E 2 s O 2 : O 2+ : 119 ( 2 s)2( 2 s*)2 ( 2 p)4( 2 p*)2 ( 2 s)2( 2 s*)2 ( 2 p)4( 2 p*)1 O 2– : ( 2 s)2( 2 s*)2 ( 2 p)4( 2 p*)3 O 22 -
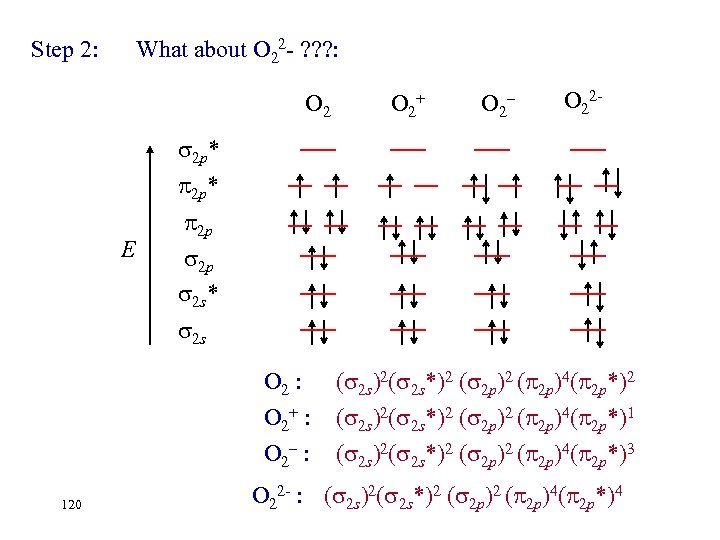
Step 2: What about O 22 - ? ? ? : O 2+ O 2– O 22 - 2 p* E 2 p 2 p 2 s* 2 s O 2 : O 2+ : O 2– : 120 ( 2 s)2( 2 s*)2 ( 2 p)4( 2 p*)2 ( 2 s)2( 2 s*)2 ( 2 p)4( 2 p*)1 ( 2 s)2( 2 s*)2 ( 2 p)4( 2 p*)3 O 22 - : ( 2 s)2( 2 s*)2 ( 2 p)4( 2 p*)4
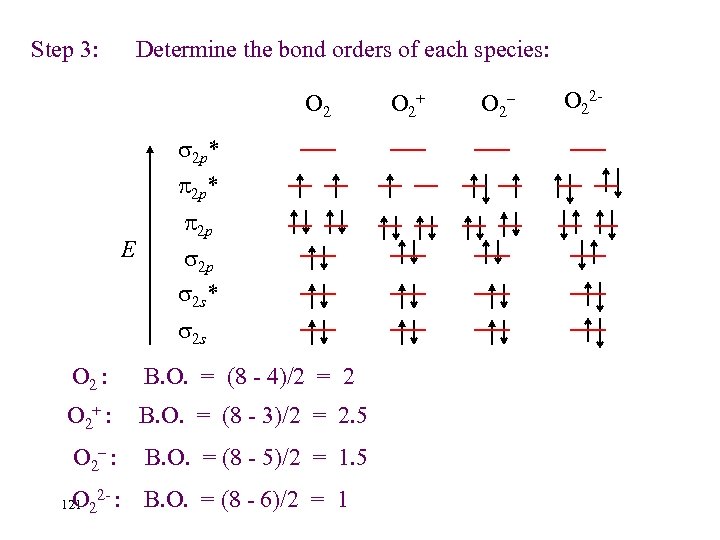
Step 3: Determine the bond orders of each species: O 2 2 p* E 2 p 2 p 2 s* 2 s O 2 : B. O. = (8 - 4)/2 = 2 O 2+ : B. O. = (8 - 3)/2 = 2. 5 O 2– : B. O. = (8 - 5)/2 = 1. 5 O B. O. = (8 - 6)/2 = 1 121 2 2 - : O 2+ O 2– O 22 -
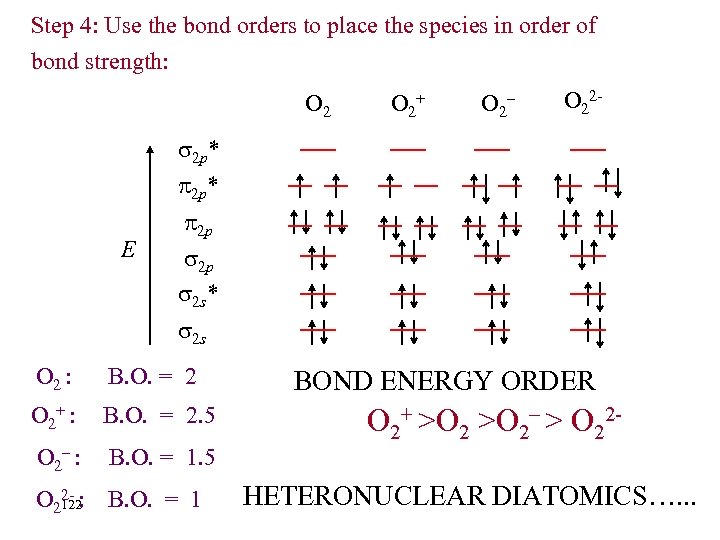
Step 4: Use the bond orders to place the species in order of bond strength: O 2+ O 2– O 22 - 2 p* E 2 p 2 p 2 s* 2 s O 2 : B. O. = 2 O 2+ : B. O. = 2. 5 O 2– : B. O. = 1. 5 O 22 - : B. O. = 1 122 BOND ENERGY ORDER O 2+ >O 2– > O 22 HETERONUCLEAR DIATOMICS…. . .
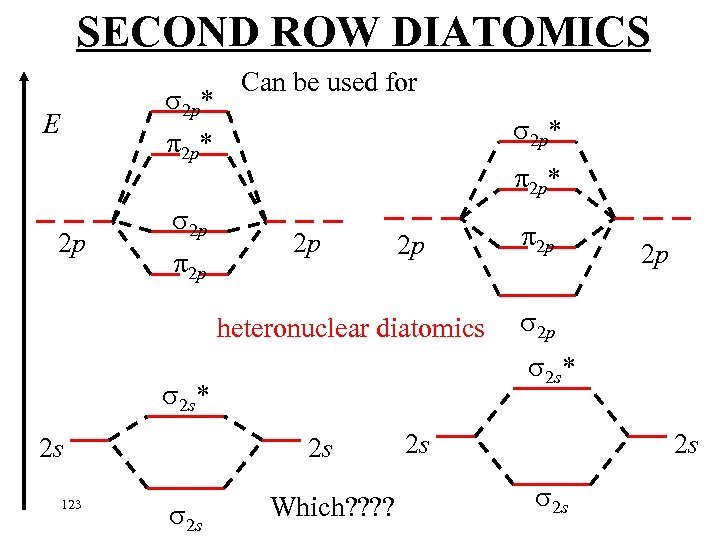
SECOND ROW DIATOMICS 2 p* E Can be used for 2 p* 2 p 2 p heteronuclear diatomics 123 2 s 2 s 2 p 2 s* 2 s 2 p Which? ? 2 s 2 s 2 s
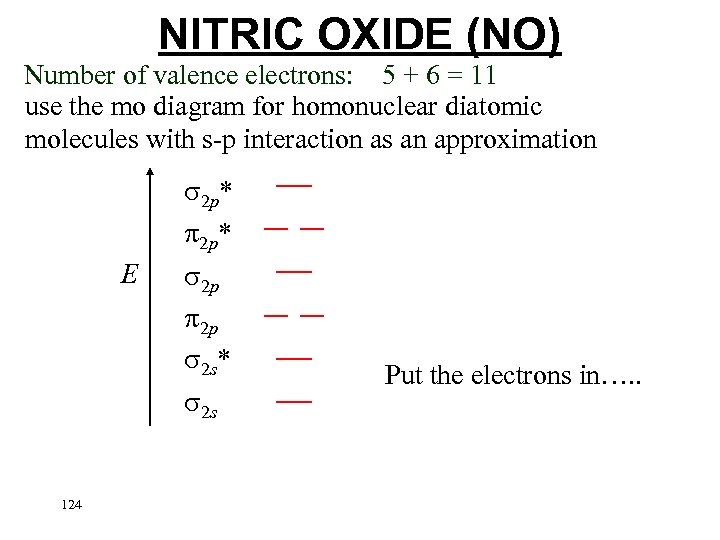
NITRIC OXIDE (NO) Number of valence electrons: 5 + 6 = 11 use the mo diagram for homonuclear diatomic molecules with s-p interaction as an approximation 2 p* E 2 p 2 p 2 s* 2 s 124 Put the electrons in…. .
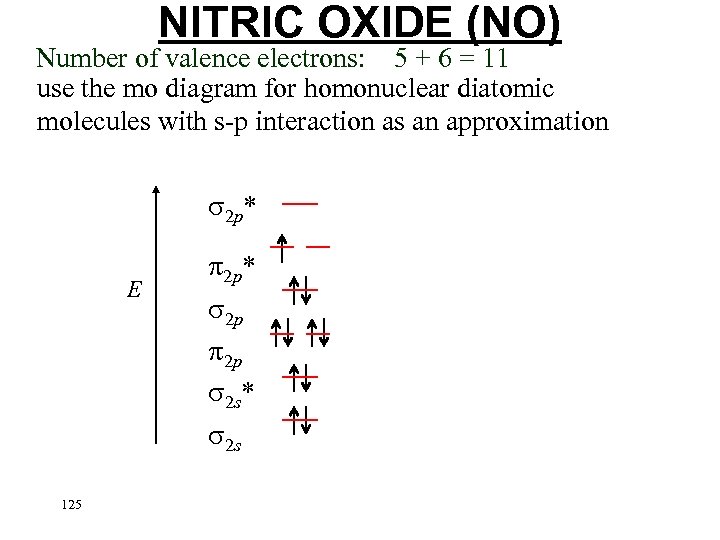
NITRIC OXIDE (NO) Number of valence electrons: 5 + 6 = 11 use the mo diagram for homonuclear diatomic molecules with s-p interaction as an approximation 2 p* E 2 p* 2 p 2 p 2 s* 2 s 125
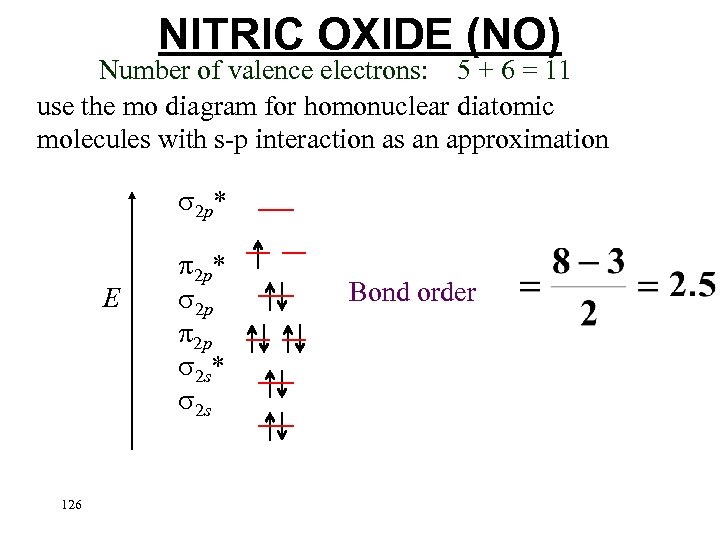
NITRIC OXIDE (NO) Number of valence electrons: 5 + 6 = 11 use the mo diagram for homonuclear diatomic molecules with s-p interaction as an approximation 2 p* E 126 2 p* 2 p 2 p 2 s* 2 s Bond order
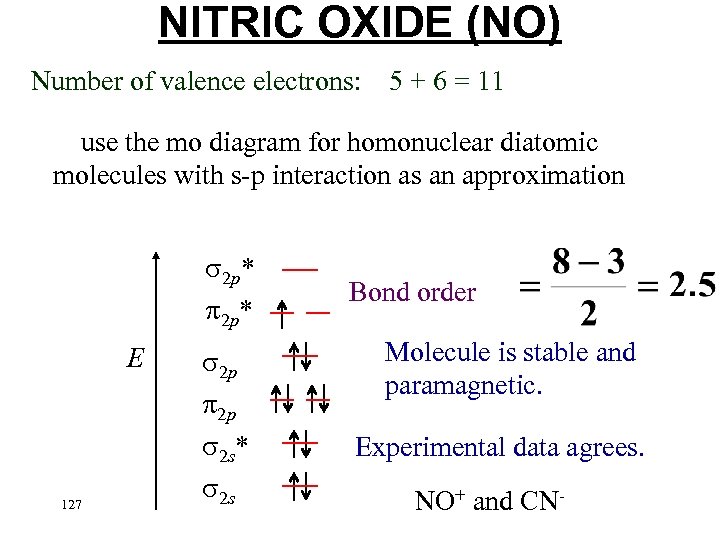
NITRIC OXIDE (NO) Number of valence electrons: 5 + 6 = 11 use the mo diagram for homonuclear diatomic molecules with s-p interaction as an approximation 2 p* E 127 2 p 2 p 2 s* 2 s Bond order Molecule is stable and paramagnetic. Experimental data agrees. NO+ and CN-

Localized/MO Combination Recalling the molecular orbital diagram for carbon we notice that the sigma and sigma antibonding fill before the pi bonds. Since this does not contribute to the bond order it makes sense that we can use localized bonding (line) to describe a sigma bond and use molecular orbitals on just the pi bonding electrons.

Localized/MO Combination Consider ozone, O 3, we can use lines to connect the three oxygen atoms together and do a molecular orbital diagram on just the unhybridized p-orbitals. Then we arrange three combinations of p-orbitals in increasing potential energy order by counting the nodes. Potential energy is inversely proportional to the number of nodes.
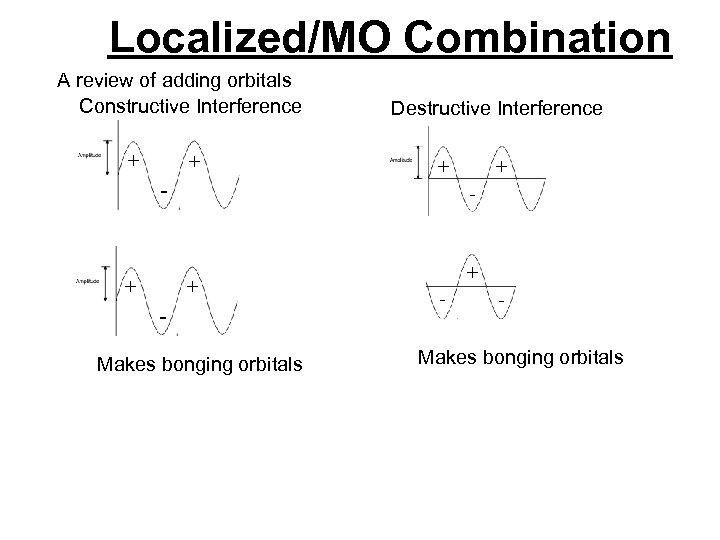
Localized/MO Combination A review of adding orbitals Constructive Interference + + - + Destructive Interference + + - + Makes bonging orbitals + - - Makes bonging orbitals
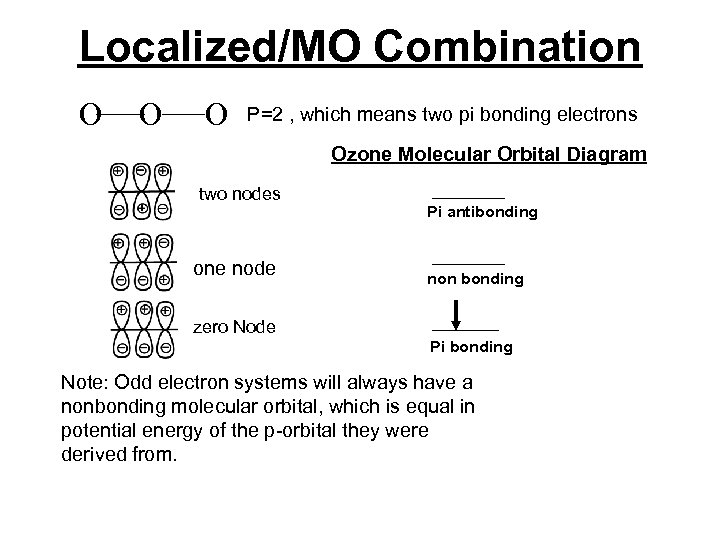
Localized/MO Combination O O O P=2 , which means two pi bonding electrons Ozone Molecular Orbital Diagram two nodes one node Pi antibonding non bonding zero Node Pi bonding Note: Odd electron systems will always have a nonbonding molecular orbital, which is equal in potential energy of the p-orbital they were derived from.
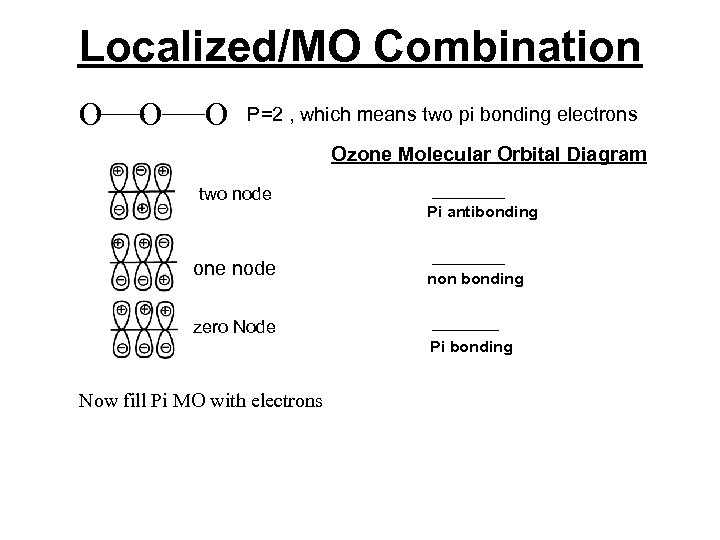
Localized/MO Combination O O O P=2 , which means two pi bonding electrons Ozone Molecular Orbital Diagram two node one node Pi antibonding non bonding zero Node Pi bonding Now fill Pi MO with electrons
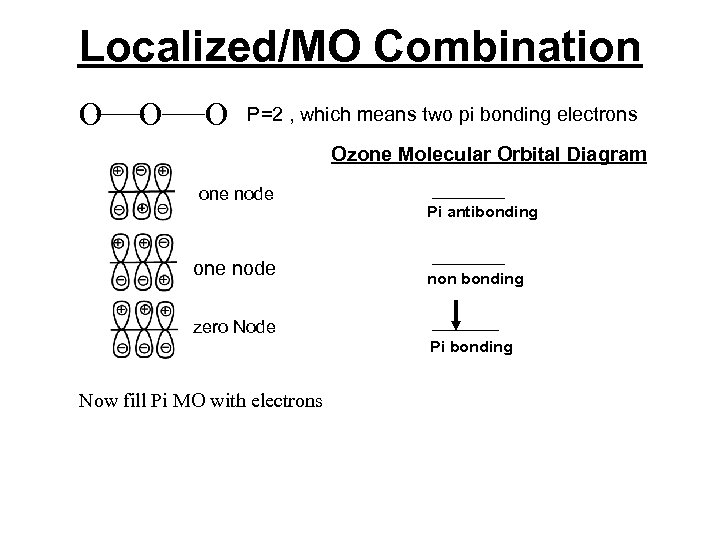
Localized/MO Combination O O O P=2 , which means two pi bonding electrons Ozone Molecular Orbital Diagram one node Pi antibonding non bonding zero Node Pi bonding Now fill Pi MO with electrons
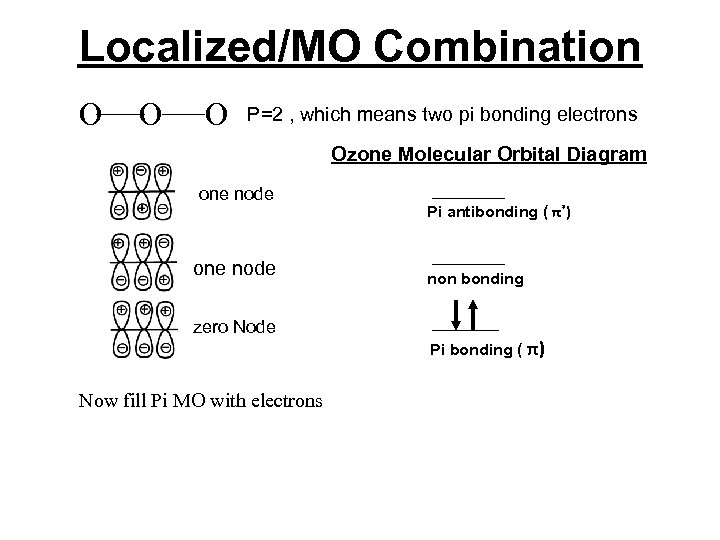
Localized/MO Combination O O O P=2 , which means two pi bonding electrons Ozone Molecular Orbital Diagram one node zero Node Now fill Pi MO with electrons Pi antibonding ( π*) non bonding Pi bonding ( π)
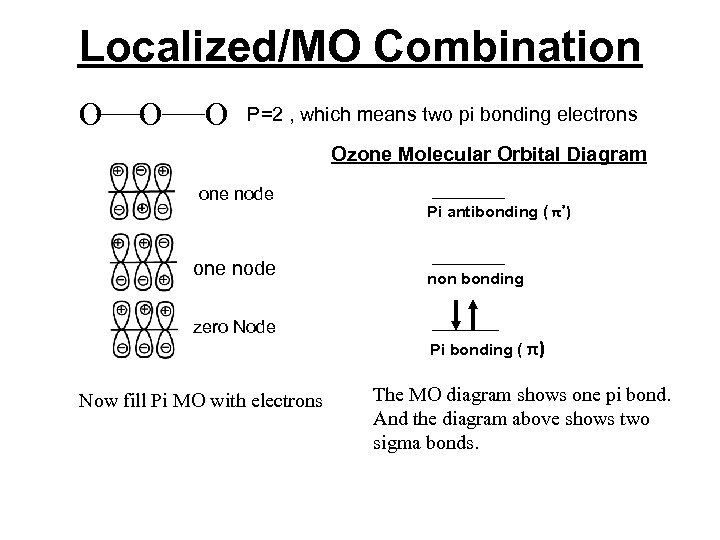
Localized/MO Combination O O O P=2 , which means two pi bonding electrons Ozone Molecular Orbital Diagram one node zero Node Now fill Pi MO with electrons Pi antibonding ( π*) non bonding Pi bonding ( π) The MO diagram shows one pi bond. And the diagram above shows two sigma bonds.
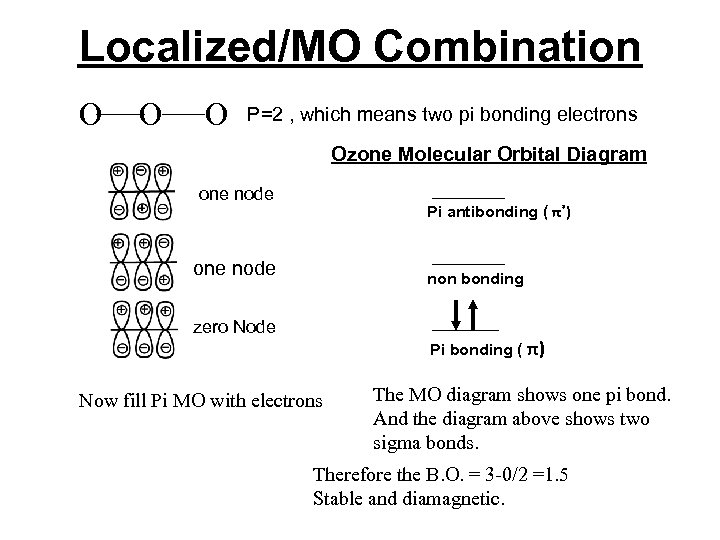
Localized/MO Combination O O O P=2 , which means two pi bonding electrons Ozone Molecular Orbital Diagram one node Pi antibonding ( π*) one node non bonding zero Node Pi bonding ( π) Now fill Pi MO with electrons The MO diagram shows one pi bond. And the diagram above shows two sigma bonds. Therefore the B. O. = 3 -0/2 =1. 5 Stable and diamagnetic.
Comparison of Theories • MO theory may provide the most complete picture of covalent bonding, but it is also the most difficult to apply to large molecules and it does not account for molecular shape.

The End
Chem. Tour: Partial Charges and Bond Dipoles Click to launch animation PC | Mac Students learn that covalent bonds often include unequal distribution of electrons leading to partial charges on atoms, bond dipole moments, and molecule polarity. Interactive Practice Exercises ask students to calculate dipole moments of polar molecule.

Chem. Tour: Greenhouse Effect Click to launch animation PC | Mac This unit explores how excess carbon dioxide and CFCs in the atmosphere contribute to global warming.
Chem. Tour: Vibrational Modes Click to launch animation PC | Mac This tutorial illustrates the three vibrational modes: bending, symmetric stretching, and asymmetric stretching. Students learn that molecules can absorb specific wavelengths of infrared radiation by converting this energy into molecular vibrations.

Chem. Tour: Hybridization Click to launch animation PC | Mac This tutorial animates the formation of hybrid orbitals from individual s and p orbitals, shows examples of their geometry, and describes how they can produce single, double, and triple bonds. Includes Practice Exercises.
Chem. Tour: Chemistry of the Upper Atmosphere Click to launch animation PC | Mac This Chem. Tour examines how particles of the upper atmosphere absorb and emit electromagnetic radiation.

Chem. Tour: Molecular Orbitals Click to launch animation PC | Mac This animated tutorial offers a patient explanation of molecular orbital theory, an alternative to the bonding theory depicted by Lewis dot structures. Includes Practice Exercises.
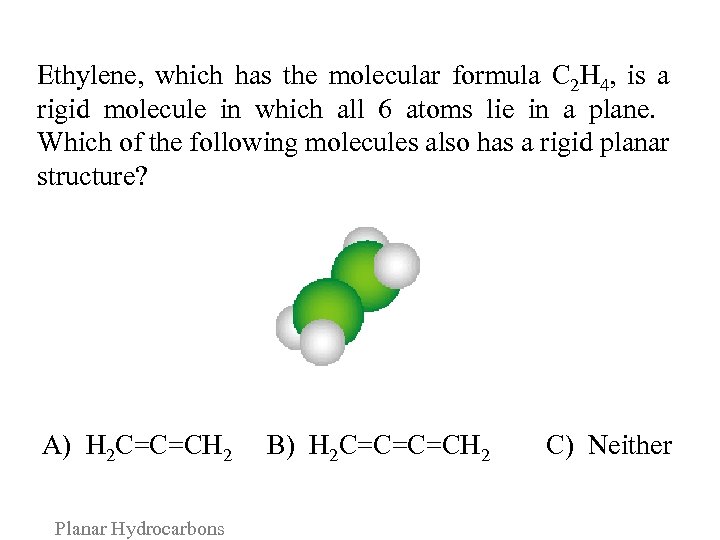
Ethylene, which has the molecular formula C 2 H 4, is a rigid molecule in which all 6 atoms lie in a plane. Which of the following molecules also has a rigid planar structure? A) H 2 C=C=CH 2 B) H 2 C=C=C=CH 2 Planar Hydrocarbons C) Neither

Consider the following arguments for each answer and vote again: A. A combination of 3 carbons and 4 hydrogens can form the rigid planar molecule H 2 C=C=CH 2. B. The orientations of the π bonds in H 2 C=C=C=CH 2 alternate in such a way as to create a planar structure. C. The hybridization of the atomic orbitals on the carbons prevents the retention of a planar structure in molecules longer than C 2 H 4. Planar Hydrocarbons
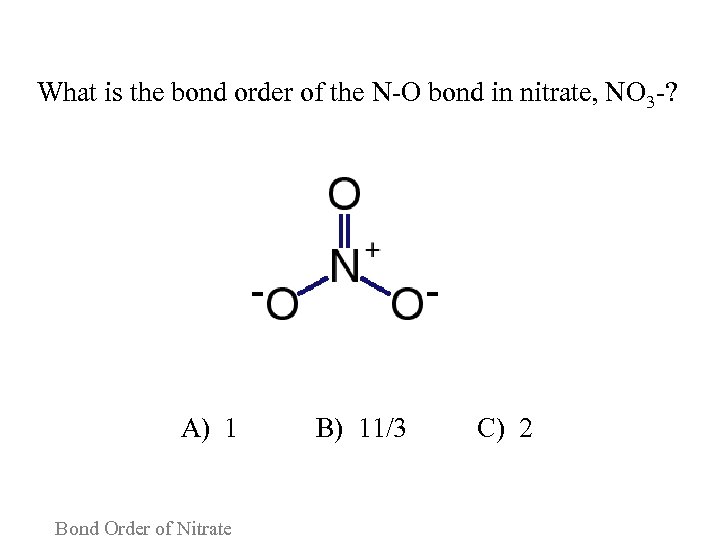
What is the bond order of the N-O bond in nitrate, NO 3 -? A) 1 B) 11/3 C) 2 Bond Order of Nitrate

Consider the following arguments for each answer and vote again: A. The majority of the bonds in NO 3 - are single bonds, so the bond order is 1. B. The N-O bond is twice as likely to be a single bond as it is to be a double bond, so the bond order should be 11/3. C. The bond order is dictated by the strongest bond, which in NO 3 - is a double bond. Bond Order of Nitrate
Which of the following species is not paramagnetic in its ground state? A) NO+ B) NO C) NO- Bond Order of Nitrate

Consider the following arguments for each answer and vote again: A. NO+ is isoelectronic with N 2, which has no unpaired electrons and hence is not paramagnetic. B. NO has no electrical charge and thus cannot be paramagnetic. C. By pairing an additional electron with the one unpaired electron in NO, a diamagnetic anion, NO-, is formed. Bond Order of Nitrate
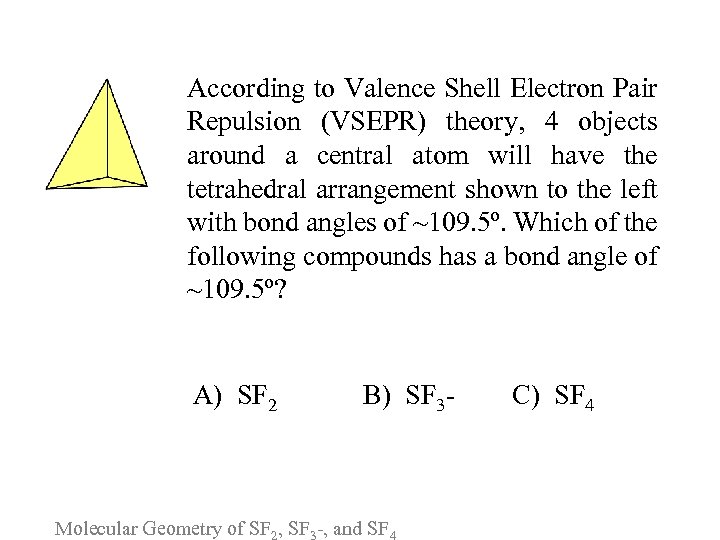
According to Valence Shell Electron Pair Repulsion (VSEPR) theory, 4 objects around a central atom will have the tetrahedral arrangement shown to the left with bond angles of ~109. 5º. Which of the following compounds has a bond angle of ~109. 5º? A) SF 2 B) SF 3 - Molecular Geometry of SF , SF -, and SF C) SF 4

Please consider the following arguments for each answer and vote again: A. SF 2 consists of a sulfur atom surrounded by 2 lone electron pairs and bonded to 2 fluorine atoms, therefore, it has an approximately tetrahedral bond angle. B. The tetrahedral VSEPR arrangement of SF 3 - is formed by a sulfur atom surrounded by 3 fluorine atoms and by the additional electron (from the negative charge). C. Sulfur tetrafluoride is the only molecule with a central atom (sulfur) surrounded by 4 additional atoms (4 fluorines) and so is the only molecule with a bond angle of ~109. 5º. Molecular Geometry of SF , SF -, and SF
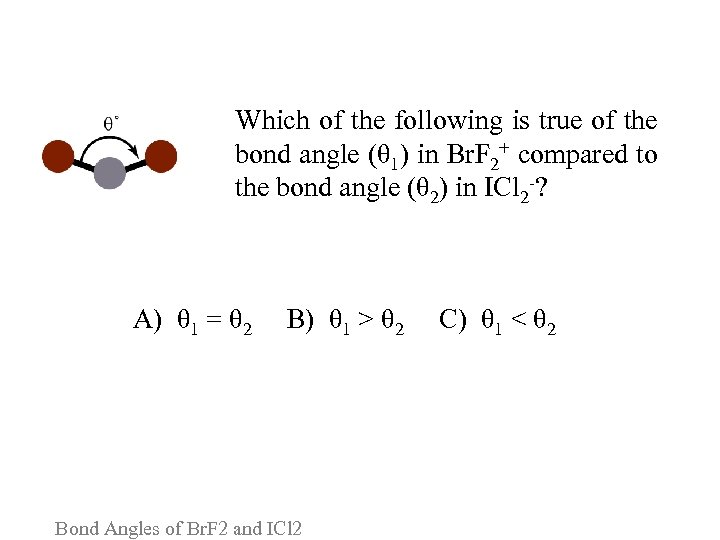
Which of the following is true of the bond angle (θ 1) in Br. F 2+ compared to the bond angle (θ 2) in ICl 2 -? A) θ 1 = θ 2 B) θ 1 > θ 2 C) θ 1 < θ 2 Bond Angles of Br. F 2 and ICl 2

Please consider the following arguments for each answer and vote again: A. Both Br. F 2+ and ICl 2 - consist of a central halogen atom bonded to two halogen atoms, and therefore should have the same arrangement of atoms. B. ICl 2 - has 1 more lone pair of electrons than Br. F 2+, which forces the chlorine atoms closer together. C. ICl 2 -, with 3 lone pairs, is linear whereas Br. F 2+, with 2 lone pairs, is bent. Bond Angles of Br. F 2 and ICl 2
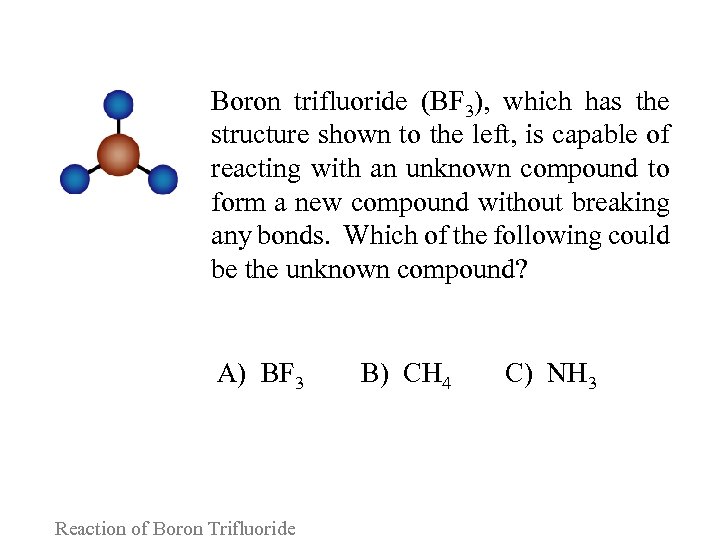
Boron trifluoride (BF 3), which has the structure shown to the left, is capable of reacting with an unknown compound to form a new compound without breaking any bonds. Which of the following could be the unknown compound? A) BF 3 Reaction of Boron Trifluoride B) CH 4 C) NH 3

Please consider the following arguments for each answer and vote again: A. BF 3 can dimerize to BF 3 -BF 3 by forming a boron single bond. B. By forming a boron-carbon bond, the carbon atom in CH 4 will increase its steric number to 5, thus expanding its octet to compensate for boron's incomplete octet. C. The nitrogen lone electron pair can form a nitrogenboron bond yielding BF 3 -NH 3, isoelectronic with CH 3 -CH 3. Reaction of Boron Trifluoride
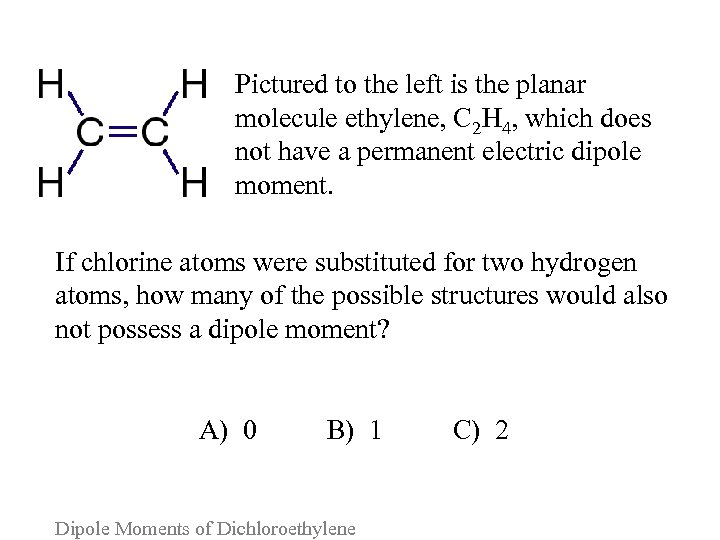
Pictured to the left is the planar molecule ethylene, C 2 H 4, which does not have a permanent electric dipole moment. If chlorine atoms were substituted for two hydrogen atoms, how many of the possible structures would also not possess a dipole moment? A) 0 B) 1 C) 2 Dipole Moments of Dichloroethylene

Consider the following arguments for each answer and vote again: A. Chlorine atoms always draw electron density away from carbon atoms, so all possible structures will possess a dipole moment. B. Only if the chlorine atoms are diagonally opposite will the two carbon-chlorine dipole moments cancel each other. C. So long as the two chlorine atoms are on different carbon atoms, no permanent dipole moment will form. Dipole Moments of Dichloroethylene
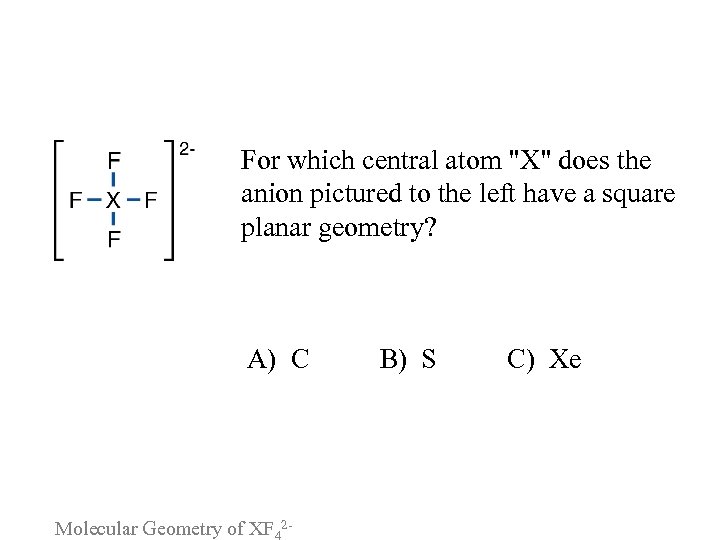
For which central atom "X" does the anion pictured to the left have a square planar geometry? A) C B) S C) Xe Molecular Geometry of XF 2 -

Please consider the following arguments for each answer and vote again: A. CF 42 - forms a structure in which the 4 fluorine atoms form a square plane with one negative charge on either side of the plane. B. With 2 lone electron pairs on the sulfur in SF 42 -, its steric number is 6. C. To maximize fluorine-fluorine distances, the 4 fluorine atoms in Xe. F 42 - will lie in a plane. Molecular Geometry of XF 2 -
9f981294b087e5000ea0a24197af1be4.ppt