1e032bf5cfefaba646607c5c40440027.ppt
- Количество слайдов: 18

Chapter 7 Thermodynamics of Living Systems Problems 7 – 10 Astrobiology: A Multidisciplinary Approach by Jonathan Lunine
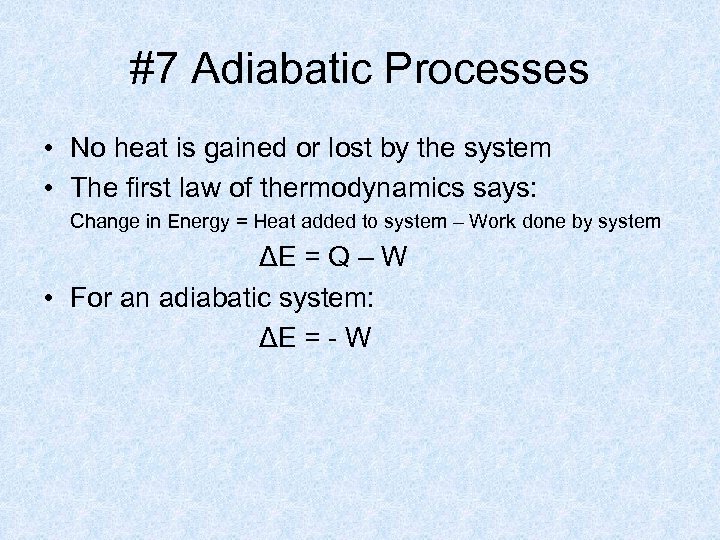
#7 Adiabatic Processes • No heat is gained or lost by the system • The first law of thermodynamics says: Change in Energy = Heat added to system – Work done by system ΔE = Q – W • For an adiabatic system: ΔE = - W
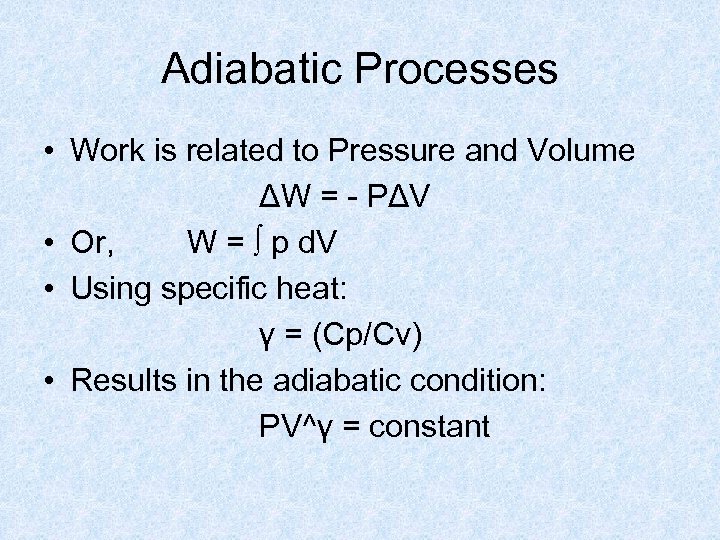
Adiabatic Processes • Work is related to Pressure and Volume ΔW = - PΔV • Or, W = ∫ p d. V • Using specific heat: γ = (Cp/Cv) • Results in the adiabatic condition: PV^γ = constant
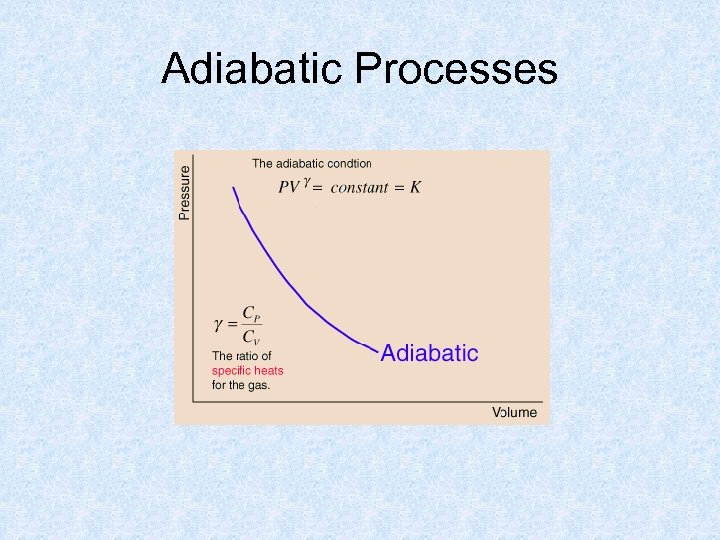
Adiabatic Processes

Adiabatic Processes • Earth’s troposphere: the region of rising and falling packets of air. • Rising air experiences a drop in temperature even though no heat is lost to the outside. • The drop of temperature is due to the decrease of pressure higher in the atmosphere. • The energy is used to do the work of expanding the parcel of air. • Adiabatic cooling of rising air is the dominant cause of cloud formation.
# 8 State Functions • State functions describe the state of the system and are not dependent on how we arrived at the state. • Energy and Entropy are state functions. • Work and Heat refer to the transfer of energy; they are not state functions.
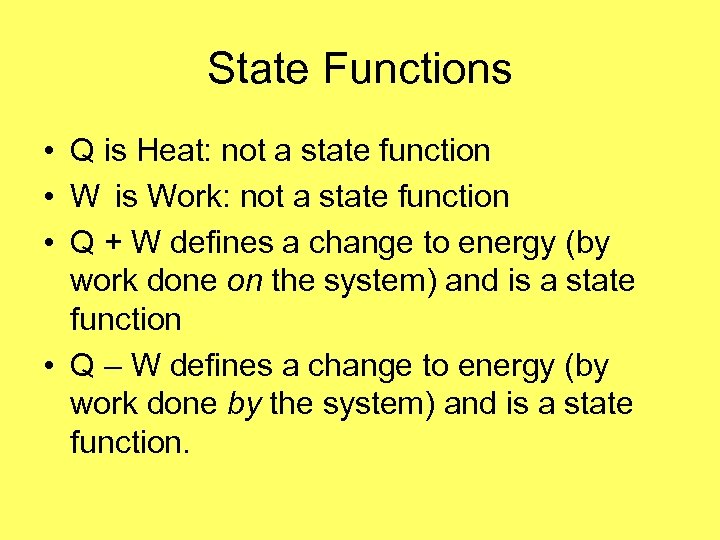
State Functions • Q is Heat: not a state function • W is Work: not a state function • Q + W defines a change to energy (by work done on the system) and is a state function • Q – W defines a change to energy (by work done by the system) and is a state function.

#9 Entropy of Poker • Ranking of Poker hands highest to lowest • • Five-of-a-kind Straight flush Four-of-a-kind Full house Straight Three-of-a-kind Two pairs One pair

Entropy of Poker • Entropy is a measure of disorder • Lower entropy means more order • Assume highest poker hand has highest order, lowest entropy • By rank then: c, a, d, b, e defines the hands in lowest to highest entropy (more order to less order).

10. Chain of Energy Exchanges • ATP conversion to ADP provides energy for cellular processes. • In skeletal muscle ATP is roughly ten times more abundant than ADP. • ATP is more abundant in skeletal muscle cells than in most cells. • ADP is “charged up” to ATP by the metabolism of carbohydrates. • A substantial amount of Gibbs free energy is available from the ATP to ADP conversion. • The Gibbs free energy describes how much energy is available to do work at cellular temperature and pressures.
Energy of Muscle Contraction • The major site of daily glucose consumption (75%) is the brain via aerobic pathways. Most of the remainder of is utilized by erythrocytes, skeletal muscle, and heart muscle. • The trypsin site of the myocin molecule in muscle acts as one hinge point involved in converting the chemical energy of ATP into the mechanical events of contraction and relaxation. • In non-contracting muscle, myocin exists in a high energy state (M*).

Energy of Muscle Contraction • The energy of ATP hydrolysis is what “cocks the trigger” of the muscle myocin. ATP + H 2 O <=> ADP + inorganic phosphate (Pi) • The formation of an actomyosin complex “pulls the trigger” releasing the stored energy. • ATP binds to myosin releasing actin in a very exergonic reaction (uses energy). • Hence, ATP is also required for muscle relaxation.
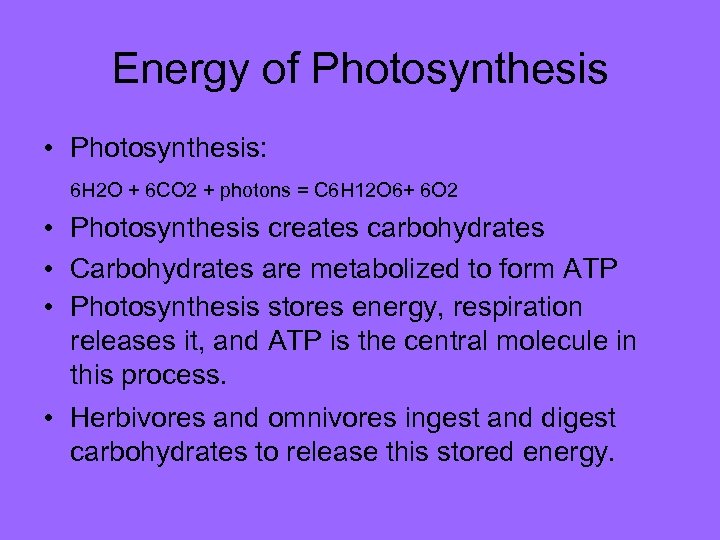
Energy of Photosynthesis • Photosynthesis: 6 H 2 O + 6 CO 2 + photons = C 6 H 12 O 6+ 6 O 2 • Photosynthesis creates carbohydrates • Carbohydrates are metabolized to form ATP • Photosynthesis stores energy, respiration releases it, and ATP is the central molecule in this process. • Herbivores and omnivores ingest and digest carbohydrates to release this stored energy.

Energy from the Sun • Hydrogen fusion in the Sun releases photons (and generates entropy). • Photons radiate to the Earth and are absorbed as heat. • Some radiated photons are captured by photosynthetic organisms. • Cells cannot operate as heat engines. They cannot use heat transfer to generate energy. • Photosynthetic cells convert sunlight to form chemical energy, which carries a much greater potential for doing work (an entropy per energy 10 times smaller) than a heat engine.
Photosynthetic Inefficiency • First law of thermodynamics • ΔE = Q + W • Second law of thermodynamics • There is no such thing as a perfectly efficient engine. • Plants are adapted to prevent excessive heating, but some energy is lost to heat during the photosynthetic process. • Chlorophyll does not absorb wavelengths well that would be directly converted to heat (in the red). • Pigments other than chlorophyll help manage the heat-generating photons.
Entropy in the Biome • We can see how the biome functions thermodynamically. • It requires a great deal of energy input in the form of sunlight. • The cumulative generation of entropy, first during the photosynthesis of glucose, and then through the respiration of glucose back into carbon dioxide and water guarantees that the entropy always increases. • This relentless increase in entropy, along with the conservation of energy (in terms of heat, internal energy and work) make up the two "laws" of thermodynamics.

Order from Chaos • Entropy is generally understood to signify an inherent tendency towards disorganization. • Does the Universe as a whole tend inexorably toward a state of entropy? – “Entropy is generated at a high rate in any system that exhibits self-organizing behavior. ” • In a state of non-equilibrium, order emerges. "Non-equilibrium brings order out of chaos. " – Ilya Prigogine

Chapter 7 References • Adiabatic Processes – – http: //daphne. palomar. edu/jthorngren/adiabatic_processes. htm http: //hyperphysics. phy-astr. gsu. edu/hbase/thermo/adiab. html http: //csep 10. phys. utk. edu/astr 161/lect/earth/atmosphere. html Halliday, D (2001). Fundamentals of Physics Sixth Ed. P 441 • Entropy of Poker – Frey, R. (1970). According to Hoyle, Fawcett Columbine, NY • Chain of Energy Exchanges – – http: //web. indstate. edu/thcme/mwking/muscle. html http: //www. life. uiuc. edu/crofts/bioph 354/atp_hydrolysis. html http: //www. rwc. uc. edu/koehler/biophys/8 f. html http: //www. marxist. com/science/arrowoftime. html
1e032bf5cfefaba646607c5c40440027.ppt