Amines Amides and Amino Acids.pptx
- Количество слайдов: 26

Chapter 6 Table of Contents Amines, Amides and Amino Acids 1. Amines 2. Amides 3. Amino Acids 4. Peptide Formation and Proteins

Chapter 6 Amines, Amides and Amino Acids • List down some nitrogen based organic compounds with their properties. • What do you remember when you heard about words “amino acid”.
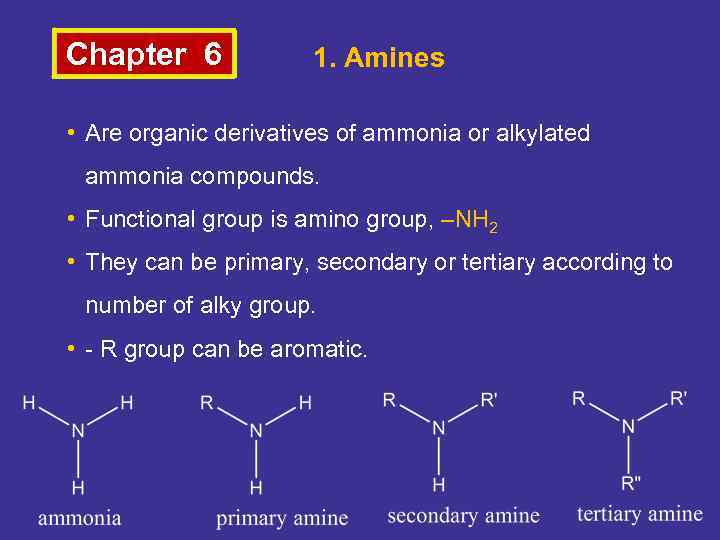
Chapter 6 1. Amines • Are organic derivatives of ammonia or alkylated ammonia compounds. • Functional group is amino group, –NH 2 • They can be primary, secondary or tertiary according to number of alky group. • - R group can be aromatic.
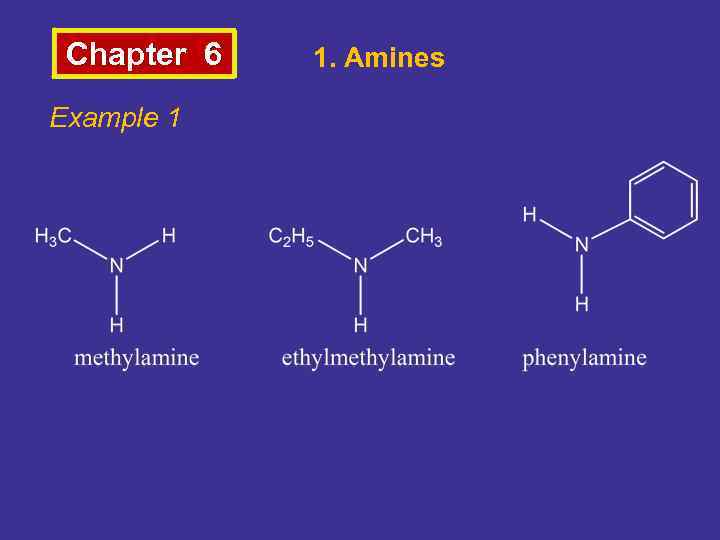
Chapter 6 Example 1 1. Amines
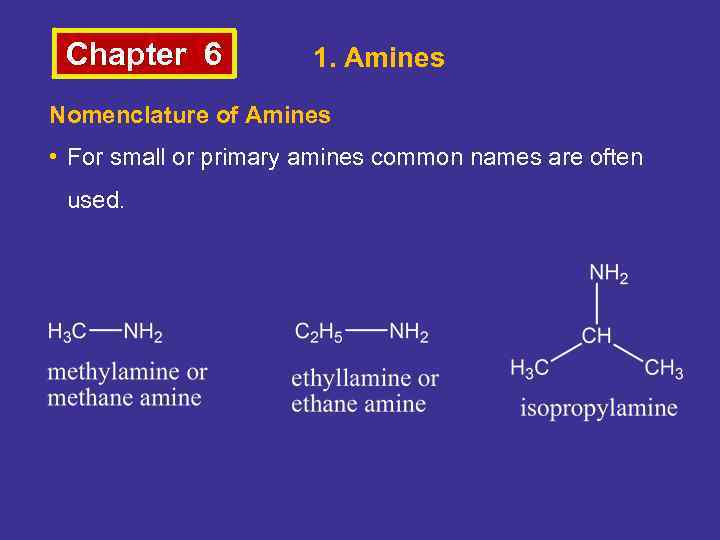
Chapter 6 1. Amines Nomenclature of Amines • For small or primary amines common names are often used.
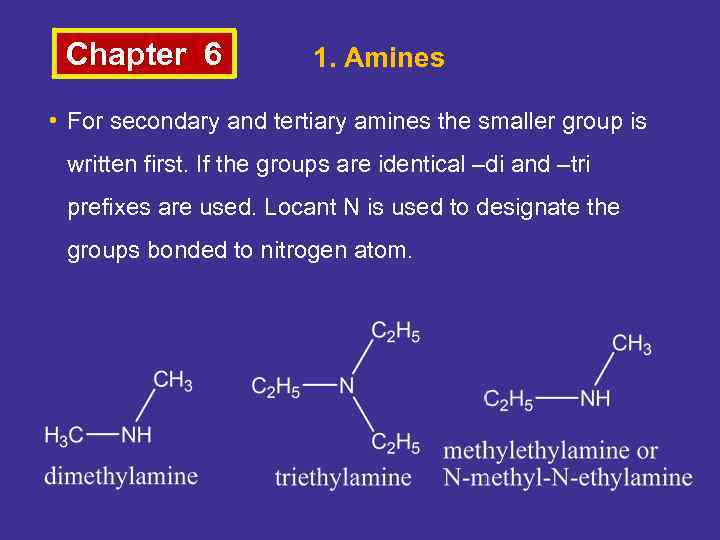
Chapter 6 1. Amines • For secondary and tertiary amines the smaller group is written first. If the groups are identical –di and –tri prefixes are used. Locant N is used to designate the groups bonded to nitrogen atom.
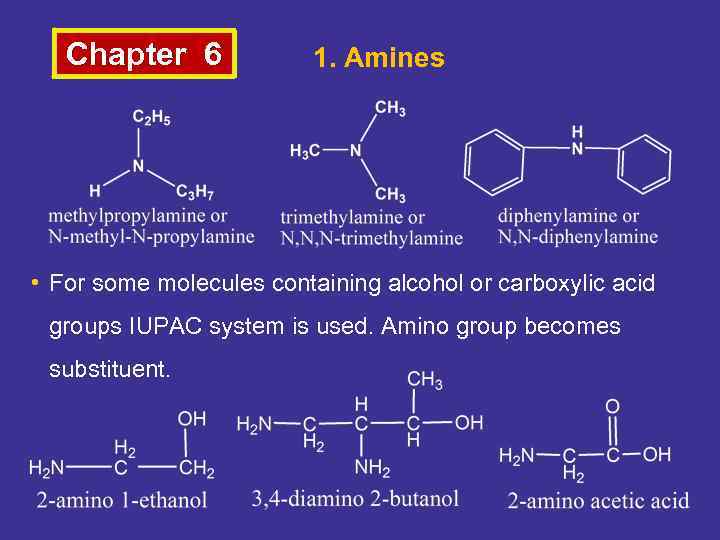
Chapter 6 1. Amines • For some molecules containing alcohol or carboxylic acid groups IUPAC system is used. Amino group becomes substituent.

Chapter 6 1. Amines Physical Properties of Amines • Small amines are gaseous and soluble in water. • Have bad smell like tainted fish. • Polar compounds. • Primary and secondary amines have hydrogen bond therefore their boiling points are higher than that of equivalent ether and alkanes. Tertiary amines do not have N-H bond so they have lower boiling points.

Chapter 6 1. Amines Chemical Properties of Amines • They are basic compounds, form hydrogen bonds upon dissolving in water. • Order of basic strength is R 3 N>R 2 NH>RNH 2>NH 3 Preparation of Amines • Reactions of ammonia with alkyl halides produce ammonium salts, and treating these ammonium salts with bases yields primary amines.
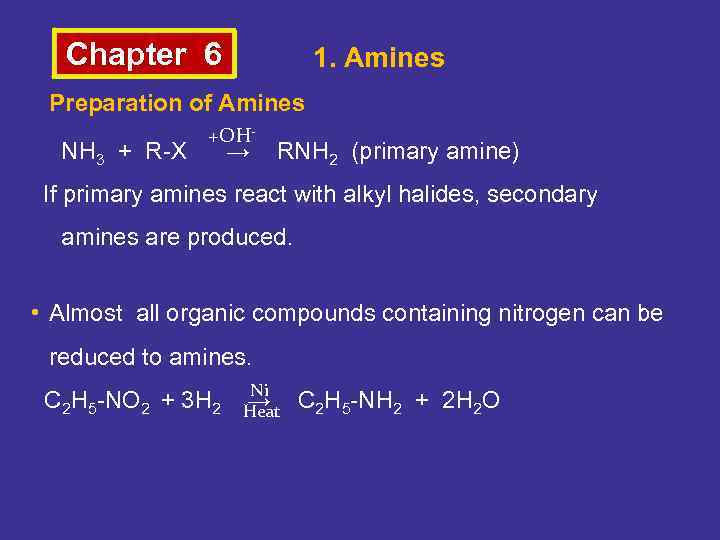
Chapter 6 1. Amines Preparation of Amines NH 3 + R-X +OH- → RNH 2 (primary amine) If primary amines react with alkyl halides, secondary amines are produced. • Almost all organic compounds containing nitrogen can be reduced to amines. C 2 H 5 -NO 2 + 3 H 2 Ni → C 2 H 5 -NH 2 + 2 H 2 O Heat
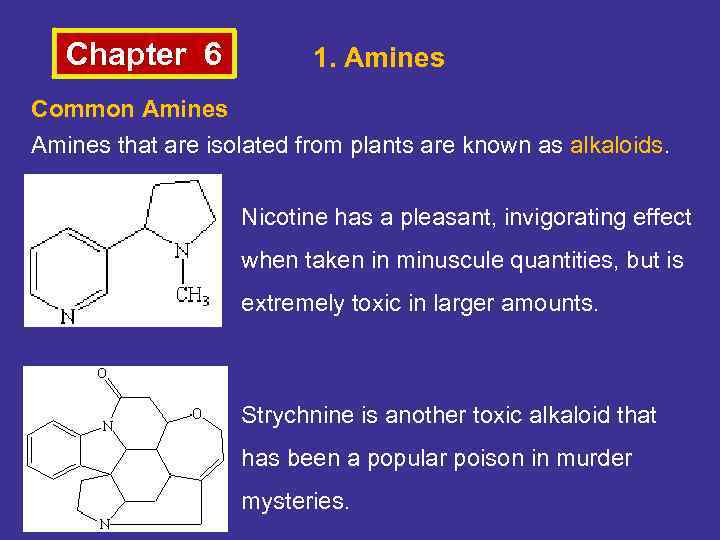
Chapter 6 1. Amines Common Amines that are isolated from plants are known as alkaloids. Nicotine has a pleasant, invigorating effect when taken in minuscule quantities, but is extremely toxic in larger amounts. Strychnine is another toxic alkaloid that has been a popular poison in murder mysteries.
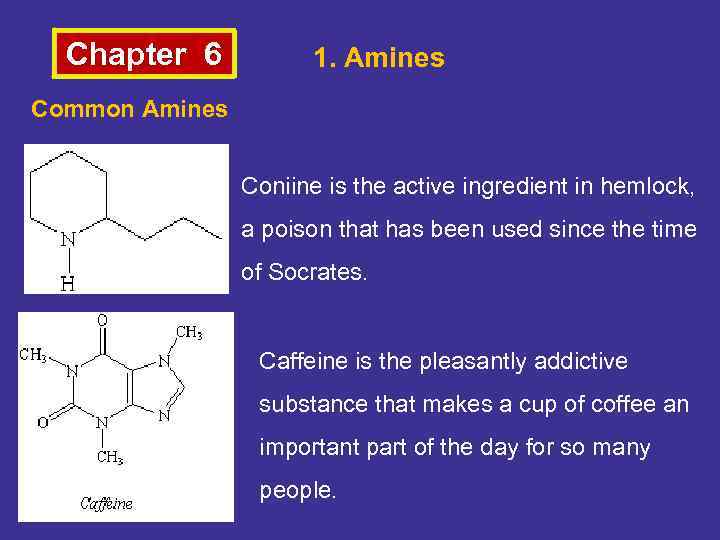
Chapter 6 1. Amines Common Amines Coniine is the active ingredient in hemlock, a poison that has been used since the time of Socrates. Caffeine is the pleasantly addictive substance that makes a cup of coffee an important part of the day for so many people.
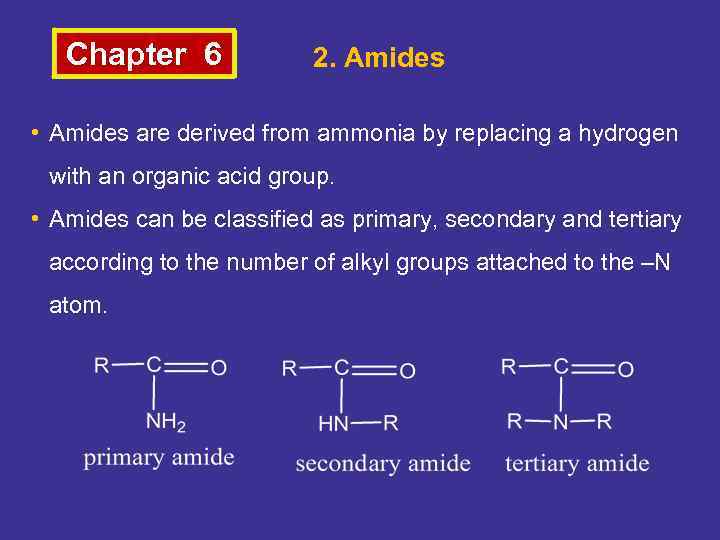
Chapter 6 2. Amides • Amides are derived from ammonia by replacing a hydrogen with an organic acid group. • Amides can be classified as primary, secondary and tertiary according to the number of alkyl groups attached to the –N atom.
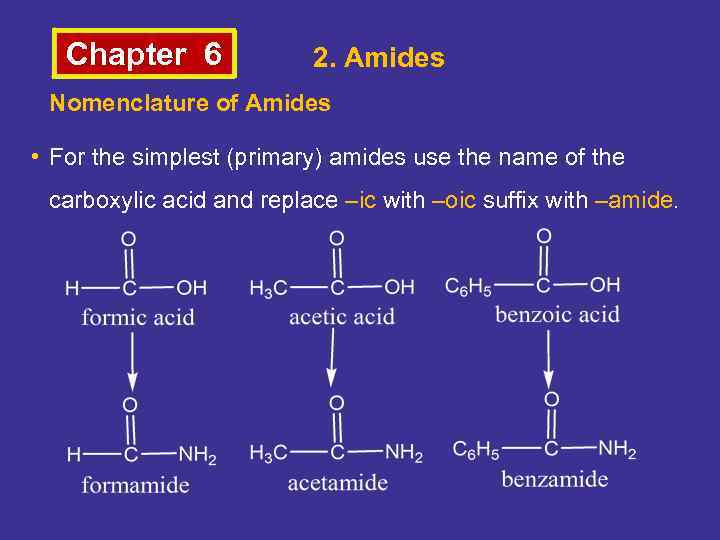
Chapter 6 2. Amides Nomenclature of Amides • For the simplest (primary) amides use the name of the carboxylic acid and replace –ic with –oic suffix with –amide.
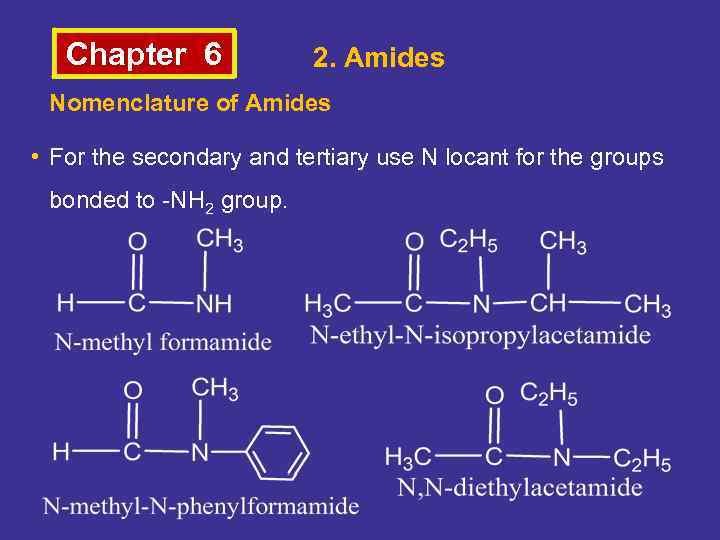
Chapter 6 2. Amides Nomenclature of Amides • For the secondary and tertiary use N locant for the groups bonded to -NH 2 group.

Chapter 6 2. Amides Properties of Amides • They have hydrogen bonds therefore their boiling points are relatively high. • They are water soluble compounds. • They do not react with acids and bases. Preparation of Amides • They can be prepared by the reaction of ammonia, primary amines or secondary amines with acid anhydrides, esters or acyl chlorides.
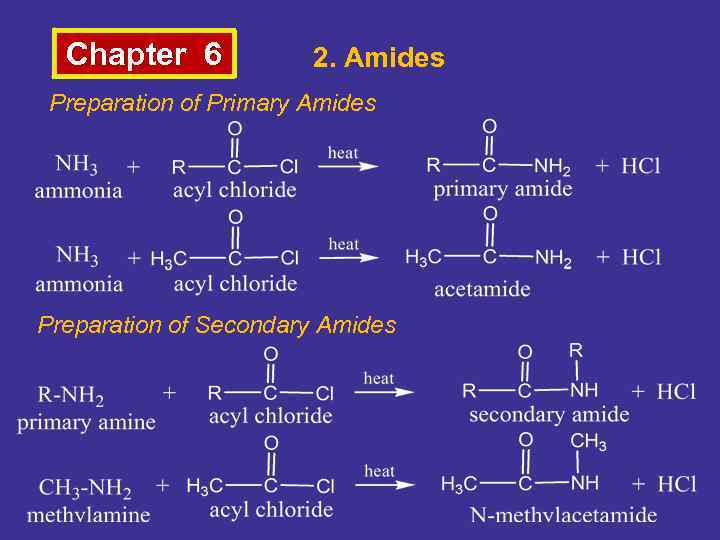
Chapter 6 2. Amides Preparation of Primary Amides Preparation of Secondary Amides
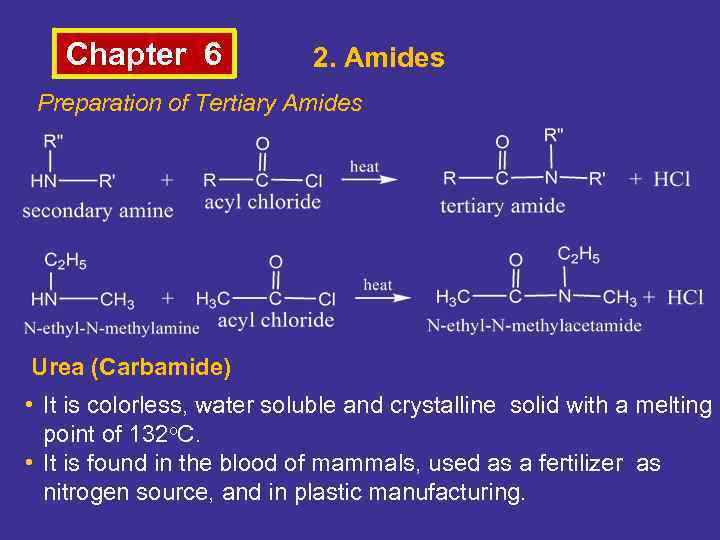
Chapter 6 2. Amides Preparation of Tertiary Amides Urea (Carbamide) • It is colorless, water soluble and crystalline solid with a melting point of 132 o. C. • It is found in the blood of mammals, used as a fertilizer as nitrogen source, and in plastic manufacturing.
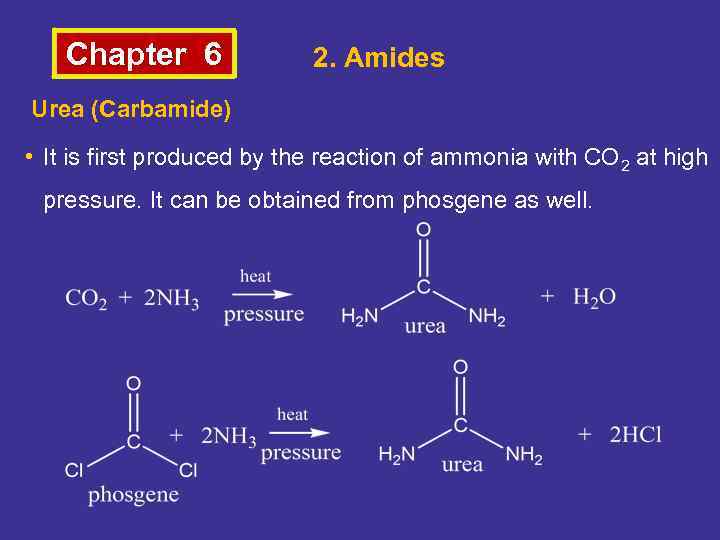
Chapter 6 2. Amides Urea (Carbamide) • It is first produced by the reaction of ammonia with CO 2 at high pressure. It can be obtained from phosgene as well.
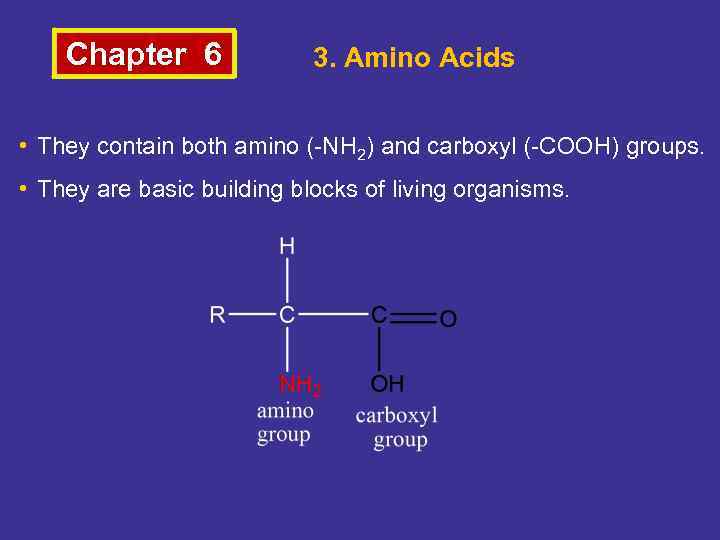
Chapter 6 3. Amino Acids • They contain both amino (-NH 2) and carboxyl (-COOH) groups. • They are basic building blocks of living organisms.
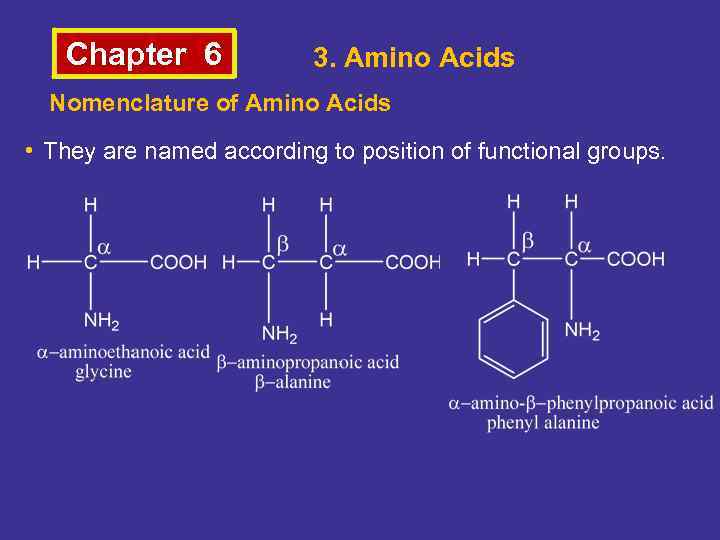
Chapter 6 3. Amino Acids Nomenclature of Amino Acids • They are named according to position of functional groups.

Chapter 6 3. Amino Acids Properties of Amino Acids • Because there are both amino and carboxyl groups in amino acids they are amphoteric substances. (have both acidic and basic characters) • They form metal salts with basic solutions. • They behave as bases towards acidic solutions. • In solid state, they form Zwitterion.
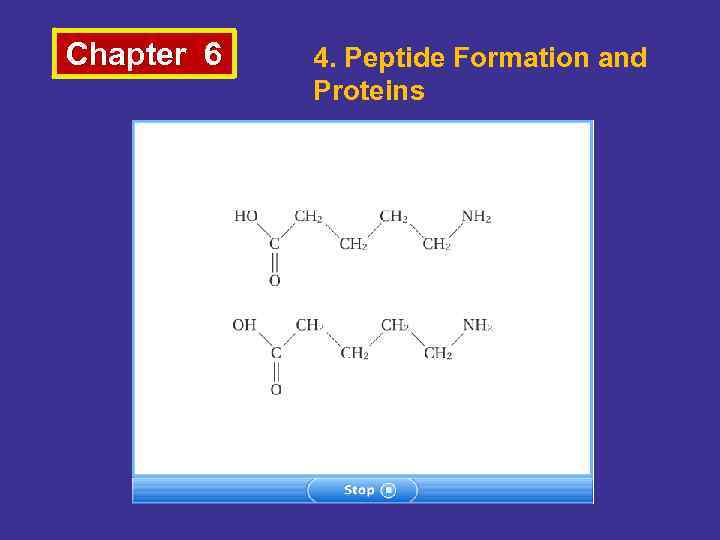
Chapter 6 4. Peptide Formation and Proteins • Proteins are the largest and most complicated molecules that exist in the cells of living organisms. • They are polymers of amino acid units These units are bonded together by a peptide linkage. • Peptides are amides formed by the reaction of amino groups with the carboxyl groups of amino acids. • According to number of amino acid unit they are classified as dipeptides, tripeptides and polypeptides.
Chapter 6 4. Peptide Formation and Proteins
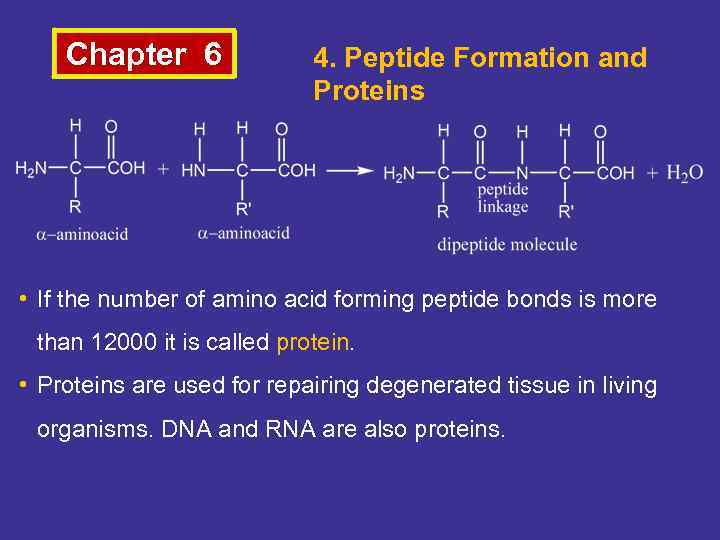
Chapter 6 4. Peptide Formation and Proteins • If the number of amino acid forming peptide bonds is more than 12000 it is called protein. • Proteins are used for repairing degenerated tissue in living organisms. DNA and RNA are also proteins.

End of the chapter 6
Amines Amides and Amino Acids.pptx