d808e4967f6bc1f62c3787aaca6b7d7d.ppt
- Количество слайдов: 63
 Chapter 6 COUNTERCURRENT MULTISTAGE EXTRACTION II More Applications HETP, HTU, Capacity
Chapter 6 COUNTERCURRENT MULTISTAGE EXTRACTION II More Applications HETP, HTU, Capacity
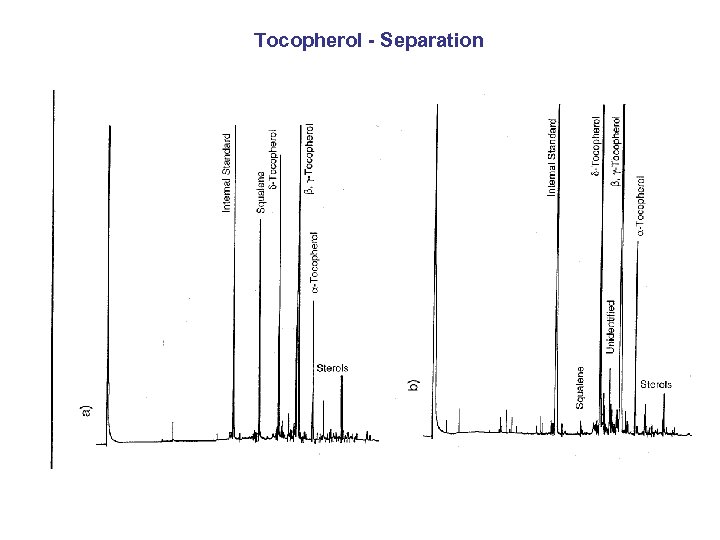 Tocopherol - Separation
Tocopherol - Separation
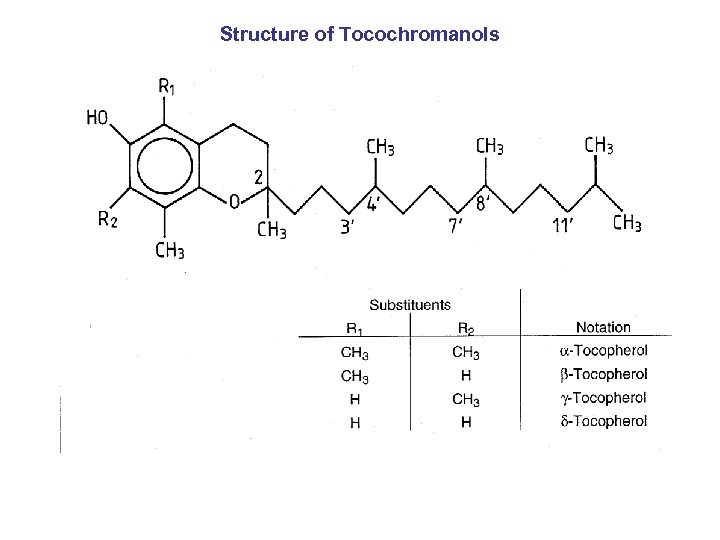 Structure of Tocochromanols
Structure of Tocochromanols
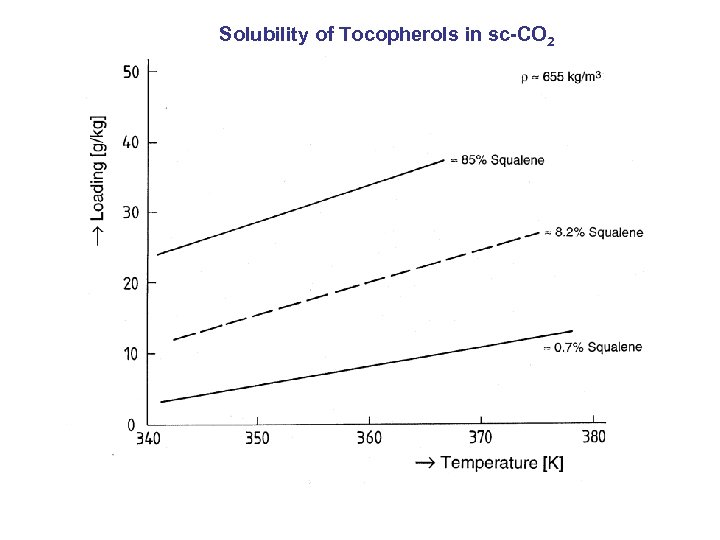 Solubility of Tocopherols in sc-CO 2
Solubility of Tocopherols in sc-CO 2
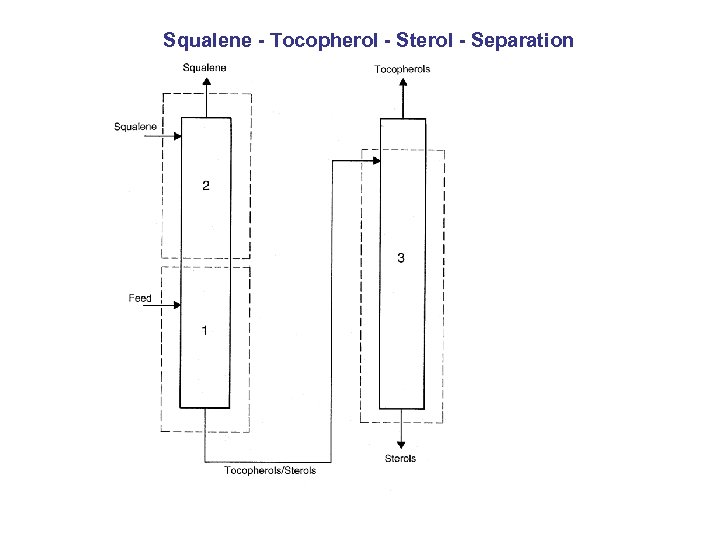 Squalene - Tocopherol - Sterol - Separation
Squalene - Tocopherol - Sterol - Separation
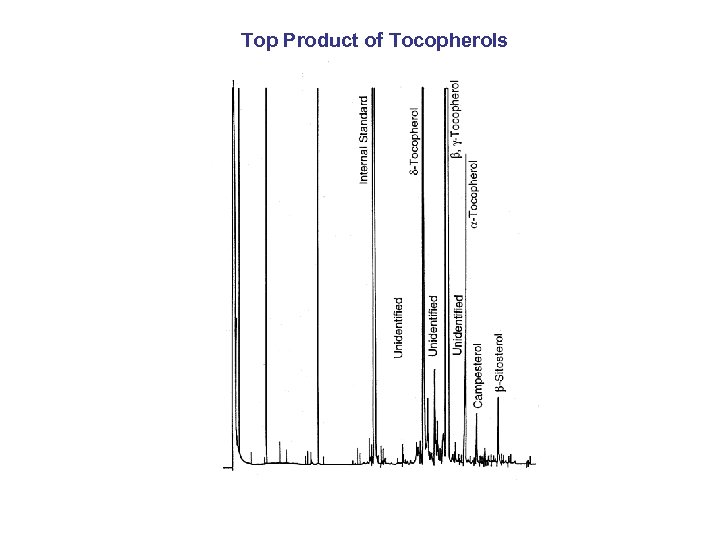 Top Product of Tocopherols
Top Product of Tocopherols
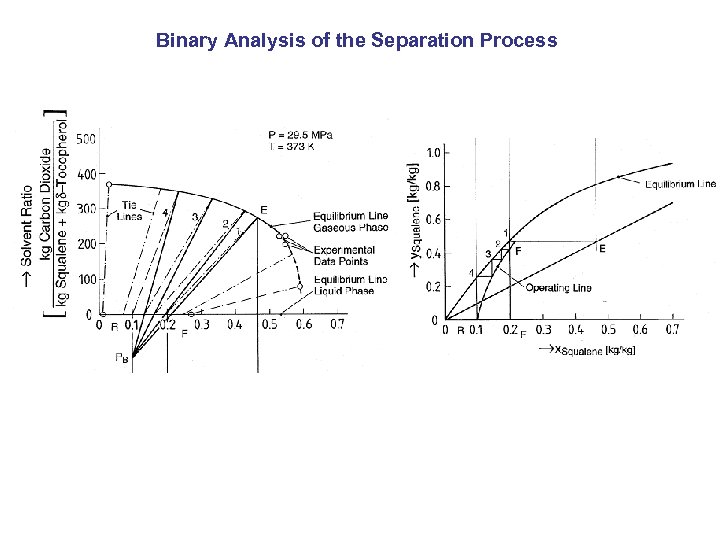 Binary Analysis of the Separation Process
Binary Analysis of the Separation Process
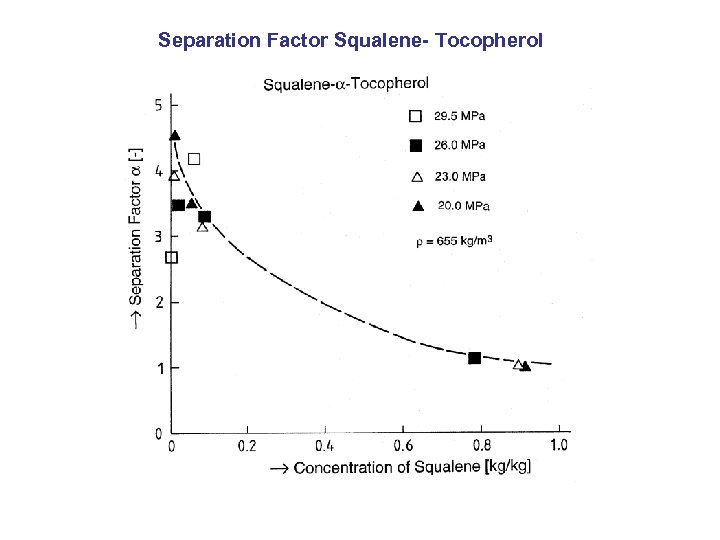 Separation Factor Squalene- Tocopherol
Separation Factor Squalene- Tocopherol
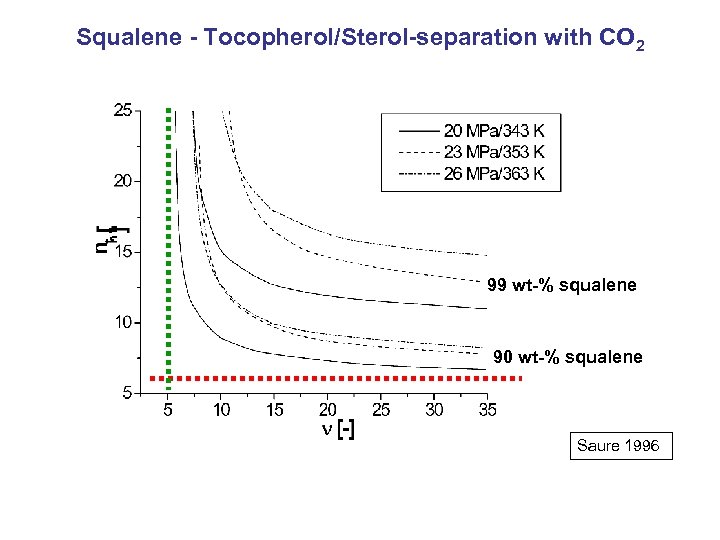 Squalene - Tocopherol/Sterol-separation with CO 2 99 wt-% squalene 90 wt-% squalene Saure 1996
Squalene - Tocopherol/Sterol-separation with CO 2 99 wt-% squalene 90 wt-% squalene Saure 1996
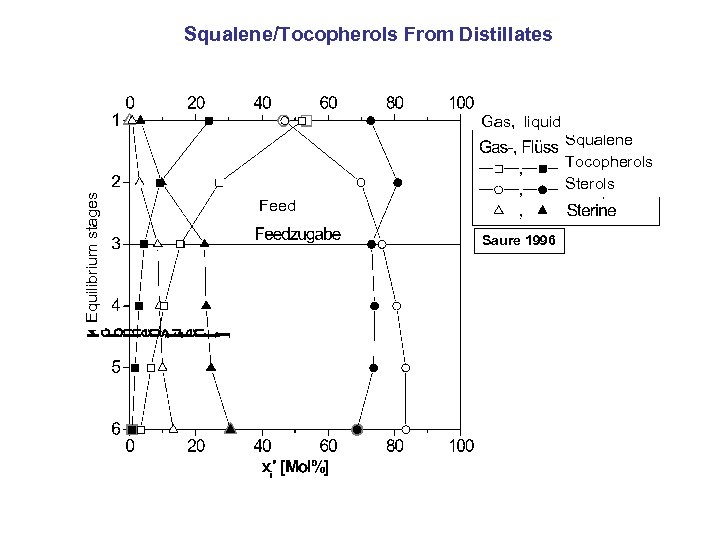 Equilibrium stages Squalene/Tocopherols From Distillates Gas, liquid Squalene Tocopherols Sterols Feed Saure 1996
Equilibrium stages Squalene/Tocopherols From Distillates Gas, liquid Squalene Tocopherols Sterols Feed Saure 1996
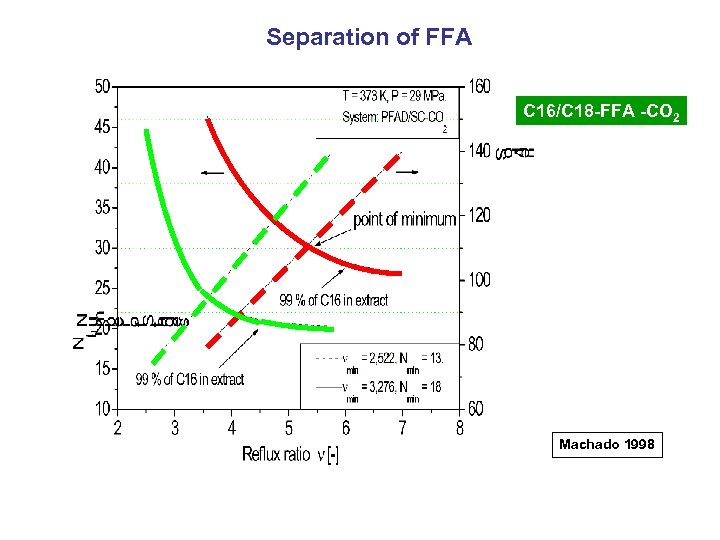 Separation of FFA C 16/C 18 -FFA -CO 2 Machado 1998
Separation of FFA C 16/C 18 -FFA -CO 2 Machado 1998
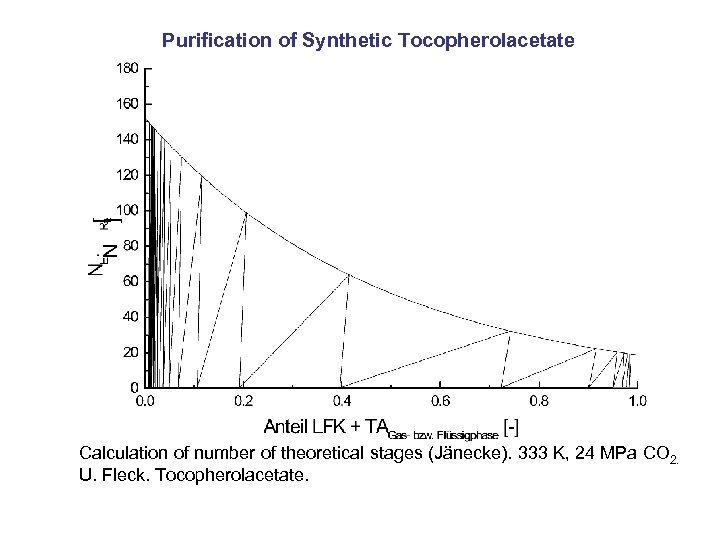 Purification of Synthetic Tocopherolacetate Calculation of number of theoretical stages (Jänecke). 333 K, 24 MPa CO 2. U. Fleck. Tocopherolacetate.
Purification of Synthetic Tocopherolacetate Calculation of number of theoretical stages (Jänecke). 333 K, 24 MPa CO 2. U. Fleck. Tocopherolacetate.
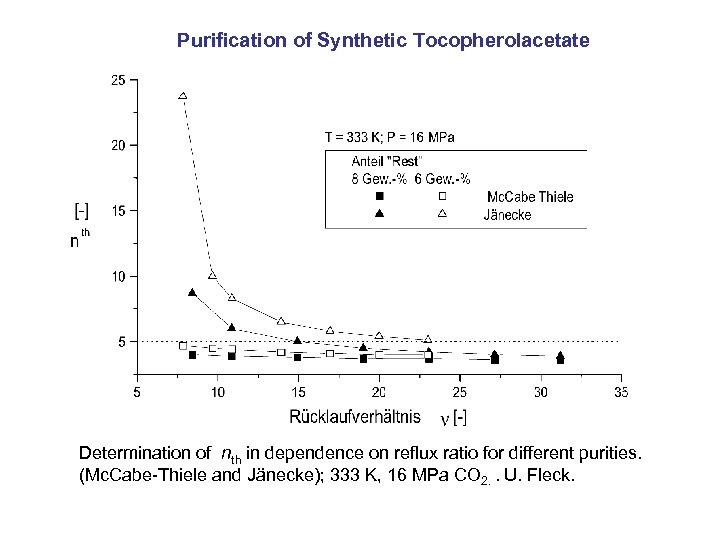 Purification of Synthetic Tocopherolacetate Determination of nth in dependence on reflux ratio for different purities. (Mc. Cabe-Thiele and Jänecke); 333 K, 16 MPa CO 2. . U. Fleck.
Purification of Synthetic Tocopherolacetate Determination of nth in dependence on reflux ratio for different purities. (Mc. Cabe-Thiele and Jänecke); 333 K, 16 MPa CO 2. . U. Fleck.
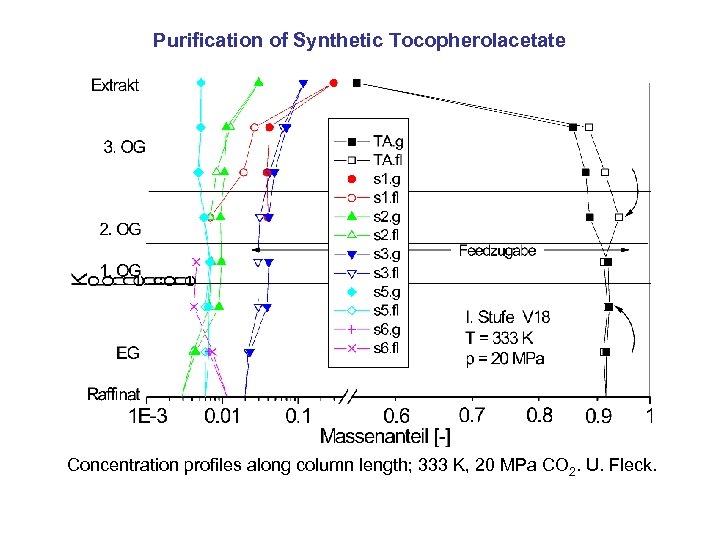 Purification of Synthetic Tocopherolacetate Concentration profiles along column length; 333 K, 20 MPa CO 2. U. Fleck.
Purification of Synthetic Tocopherolacetate Concentration profiles along column length; 333 K, 20 MPa CO 2. U. Fleck.
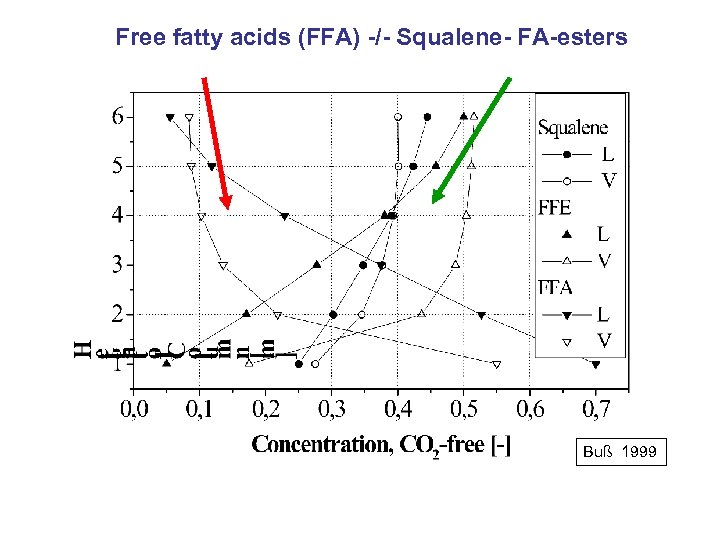 Free fatty acids (FFA) -/- Squalene- FA-esters Buß 1999
Free fatty acids (FFA) -/- Squalene- FA-esters Buß 1999
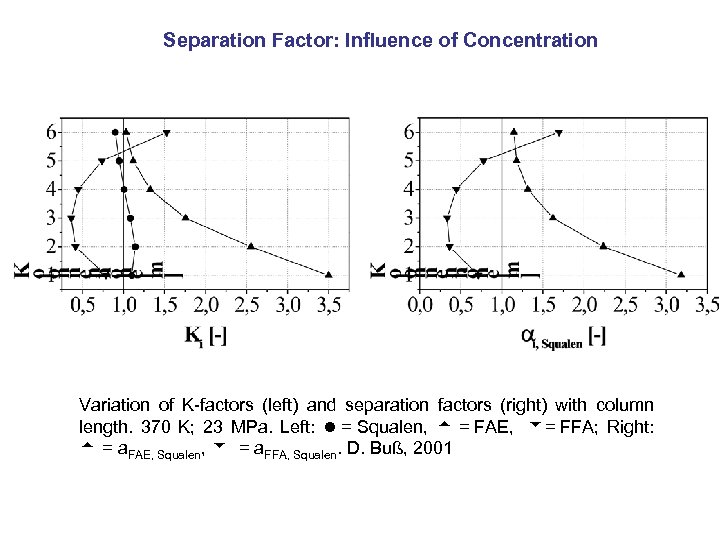 Separation Factor: Influence of Concentration Variation of K-factors (left) and separation factors (right) with column length. 370 K; 23 MPa. Left: = Squalen, = FAE, = FFA; Right: = a. FAE, Squalen, = a. FFA, Squalen. D. Buß, 2001
Separation Factor: Influence of Concentration Variation of K-factors (left) and separation factors (right) with column length. 370 K; 23 MPa. Left: = Squalen, = FAE, = FFA; Right: = a. FAE, Squalen, = a. FFA, Squalen. D. Buß, 2001
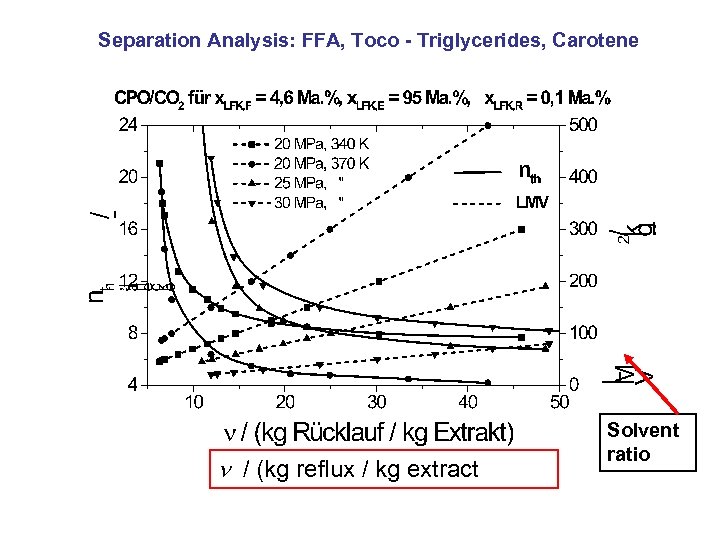 Separation Analysis: FFA, Toco - Triglycerides, Carotene / (kg reflux / kg extract Solvent ratio
Separation Analysis: FFA, Toco - Triglycerides, Carotene / (kg reflux / kg extract Solvent ratio
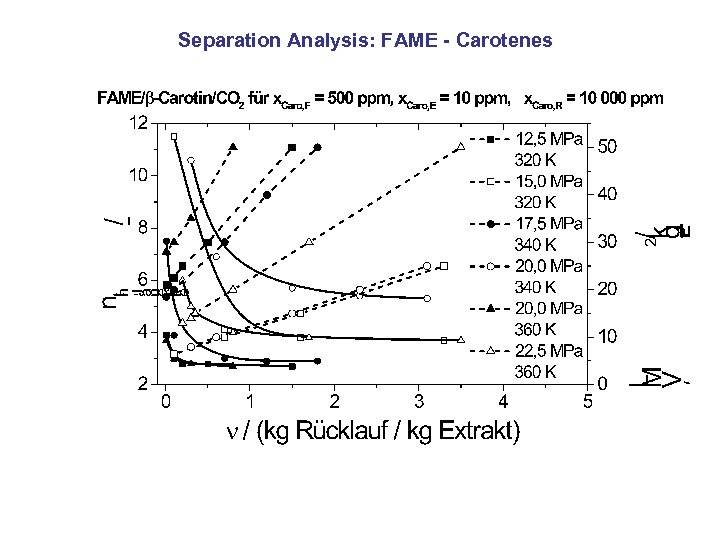 Separation Analysis: FAME - Carotenes
Separation Analysis: FAME - Carotenes
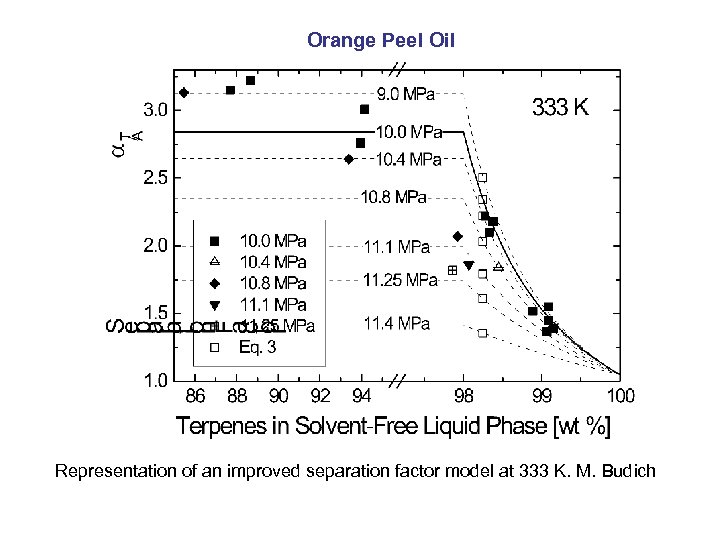 Orange Peel Oil Representation of an improved separation factor model at 333 K. M. Budich
Orange Peel Oil Representation of an improved separation factor model at 333 K. M. Budich
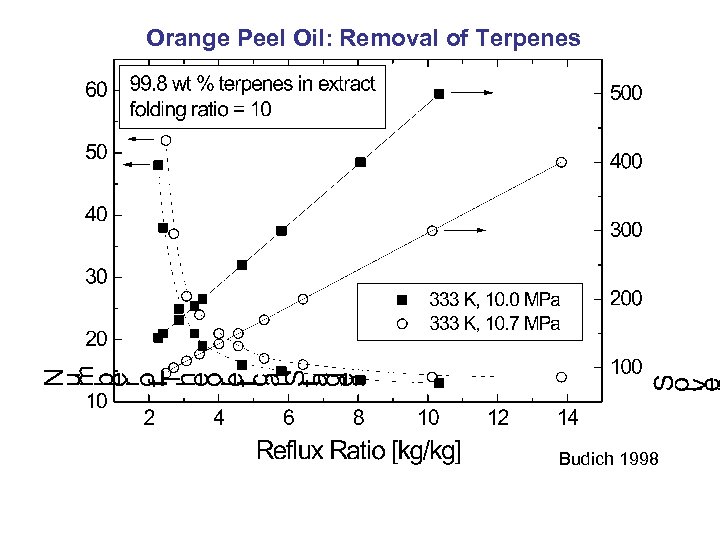 Orange Peel Oil: Removal of Terpenes Budich 1998
Orange Peel Oil: Removal of Terpenes Budich 1998
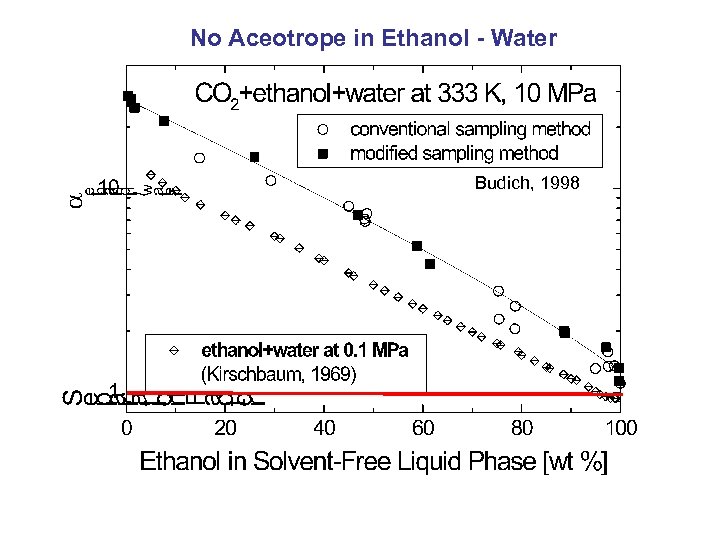 No Aceotrope in Ethanol - Water Budich, 1998
No Aceotrope in Ethanol - Water Budich, 1998
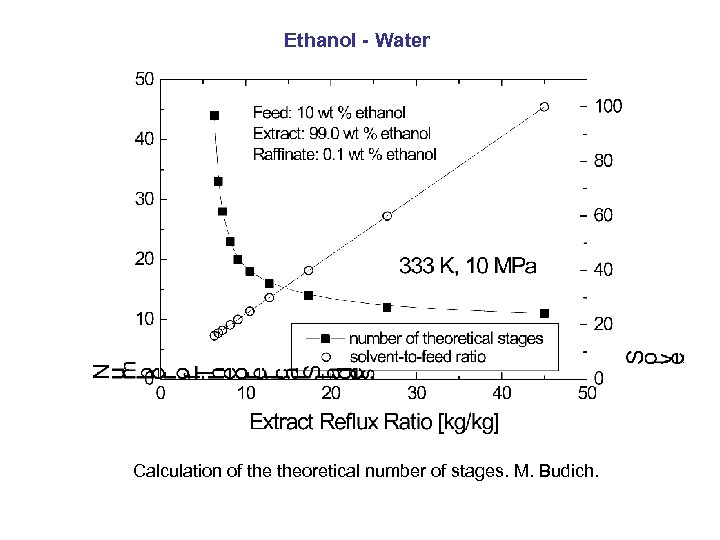 Ethanol - Water Calculation of theoretical number of stages. M. Budich.
Ethanol - Water Calculation of theoretical number of stages. M. Budich.
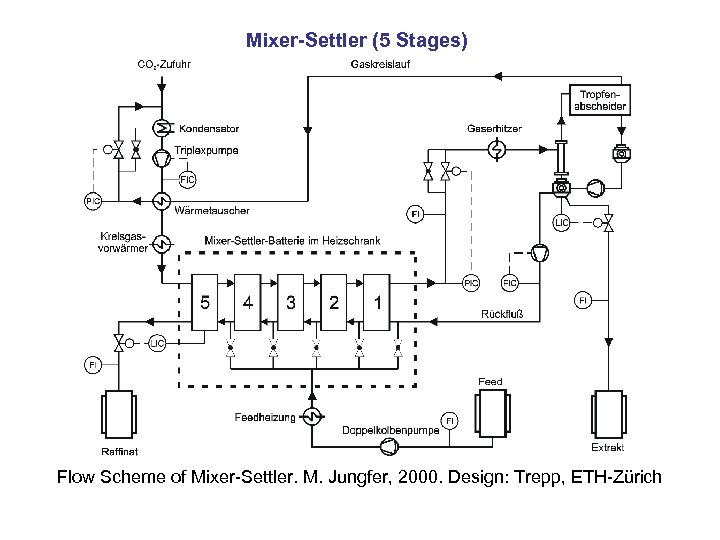 Mixer-Settler (5 Stages) Flow Scheme of Mixer-Settler. M. Jungfer, 2000. Design: Trepp, ETH-Zürich
Mixer-Settler (5 Stages) Flow Scheme of Mixer-Settler. M. Jungfer, 2000. Design: Trepp, ETH-Zürich
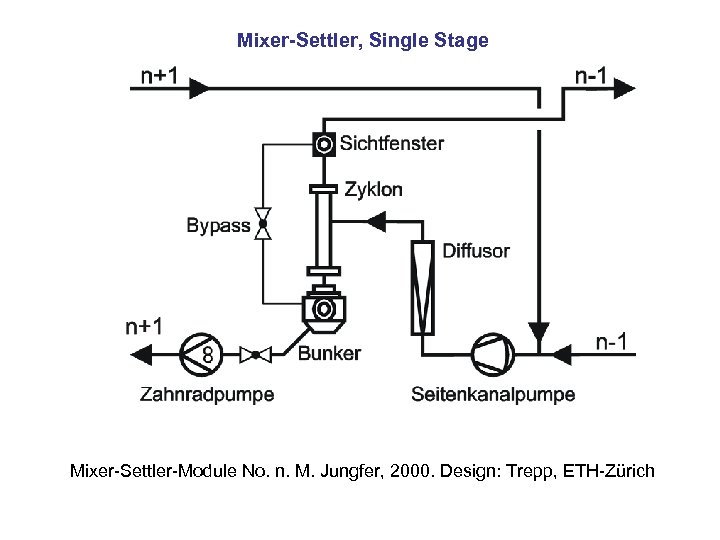 Mixer-Settler, Single Stage Mixer-Settler-Module No. n. M. Jungfer, 2000. Design: Trepp, ETH-Zürich
Mixer-Settler, Single Stage Mixer-Settler-Module No. n. M. Jungfer, 2000. Design: Trepp, ETH-Zürich
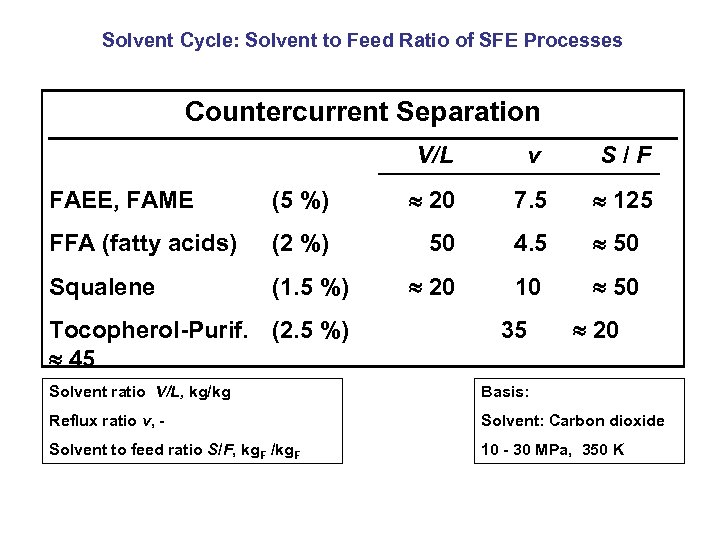 Solvent Cycle: Solvent to Feed Ratio of SFE Processes Countercurrent Separation V/L v S/F FAEE, FAME (5 %) 20 7. 5 125 FFA (fatty acids) (2 %) 50 4. 5 50 Squalene (1. 5 %) 20 10 50 Tocopherol-Purif. (2. 5 %) 45 35 20 Solvent ratio V/L, kg/kg Basis: Reflux ratio v, - Solvent: Carbon dioxide Solvent to feed ratio S/F, kg. F /kg. F 10 - 30 MPa, 350 K
Solvent Cycle: Solvent to Feed Ratio of SFE Processes Countercurrent Separation V/L v S/F FAEE, FAME (5 %) 20 7. 5 125 FFA (fatty acids) (2 %) 50 4. 5 50 Squalene (1. 5 %) 20 10 50 Tocopherol-Purif. (2. 5 %) 45 35 20 Solvent ratio V/L, kg/kg Basis: Reflux ratio v, - Solvent: Carbon dioxide Solvent to feed ratio S/F, kg. F /kg. F 10 - 30 MPa, 350 K
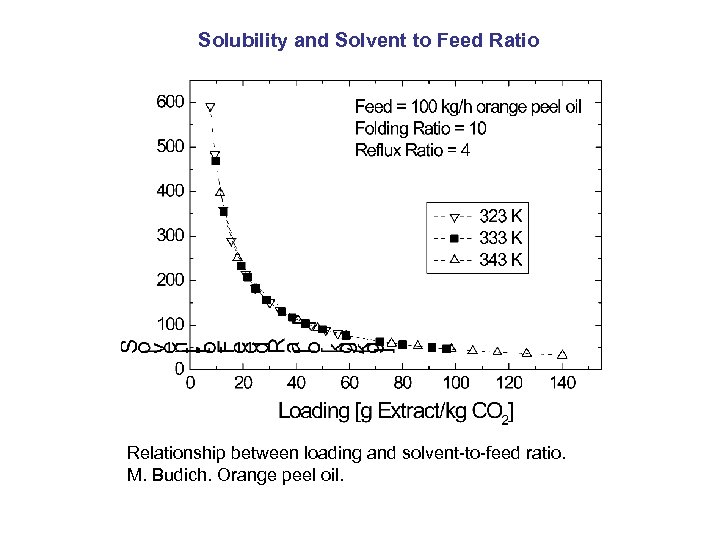 Solubility and Solvent to Feed Ratio Relationship between loading and solvent-to-feed ratio. M. Budich. Orange peel oil.
Solubility and Solvent to Feed Ratio Relationship between loading and solvent-to-feed ratio. M. Budich. Orange peel oil.
 Means for reducing costs Enhance solubility in solvent: Pressure, temperature other solvent (C 3 H 8 vs. CO 2) Reduce energy for solvent cycle: low p for extract recovery
Means for reducing costs Enhance solubility in solvent: Pressure, temperature other solvent (C 3 H 8 vs. CO 2) Reduce energy for solvent cycle: low p for extract recovery
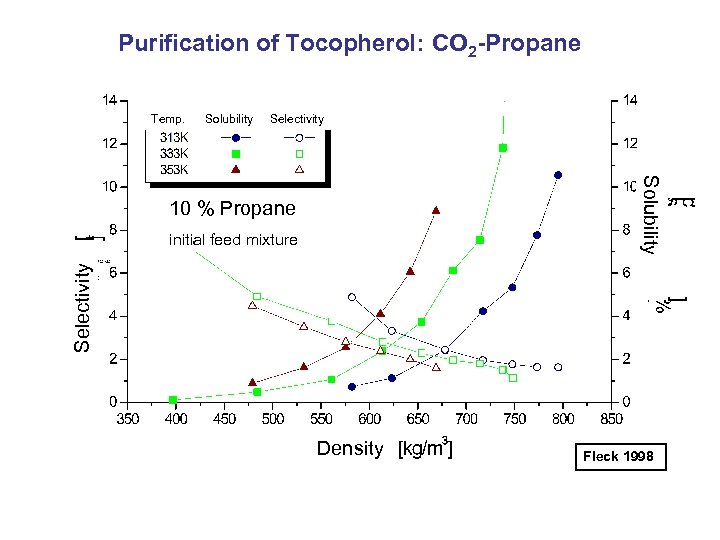 Purification of Tocopherol: CO 2 -Propane Temp. Solubility Selectivity initial feed mixture Solubility 10 % Propane Density Fleck 1998
Purification of Tocopherol: CO 2 -Propane Temp. Solubility Selectivity initial feed mixture Solubility 10 % Propane Density Fleck 1998
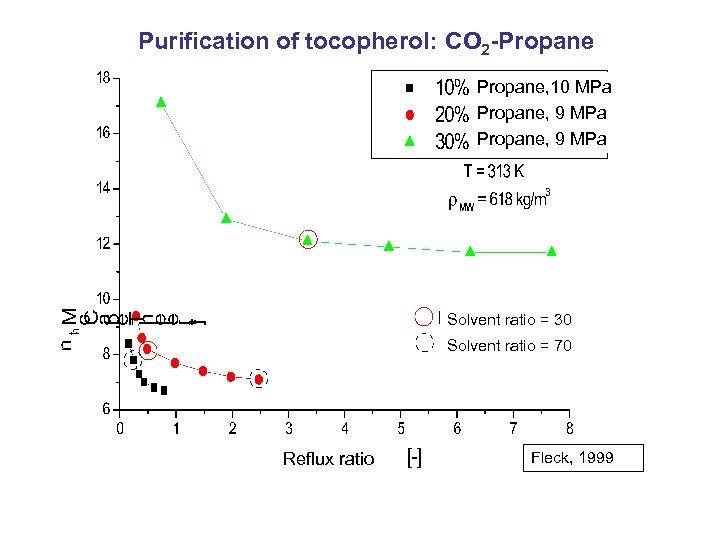 Purification of tocopherol: CO 2 -Propane, 10 MPa Propane, 9 MPa Solvent ratio = 30 Solvent ratio = 70 Reflux ratio Fleck, 1999
Purification of tocopherol: CO 2 -Propane, 10 MPa Propane, 9 MPa Solvent ratio = 30 Solvent ratio = 70 Reflux ratio Fleck, 1999
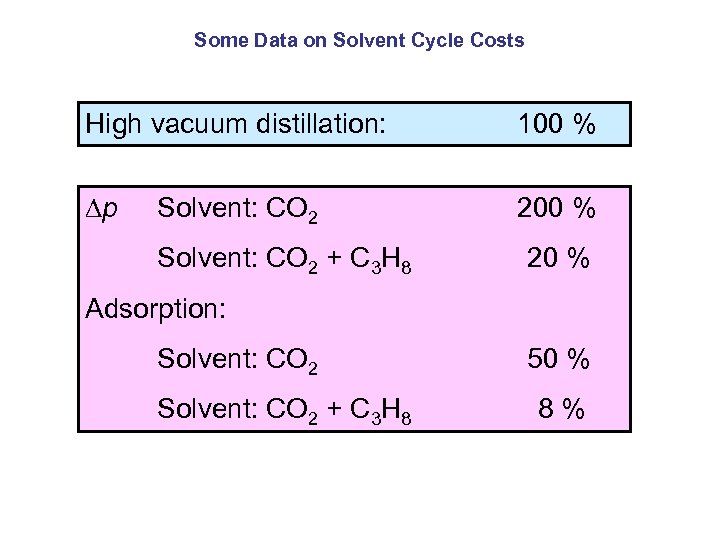 Some Data on Solvent Cycle Costs High vacuum distillation: 100 % p Solvent: CO 2 200 % Solvent: CO 2 + C 3 H 8 20 % Adsorption: Solvent: CO 2 50 % Solvent: CO 2 + C 3 H 8 8 %
Some Data on Solvent Cycle Costs High vacuum distillation: 100 % p Solvent: CO 2 200 % Solvent: CO 2 + C 3 H 8 20 % Adsorption: Solvent: CO 2 50 % Solvent: CO 2 + C 3 H 8 8 %
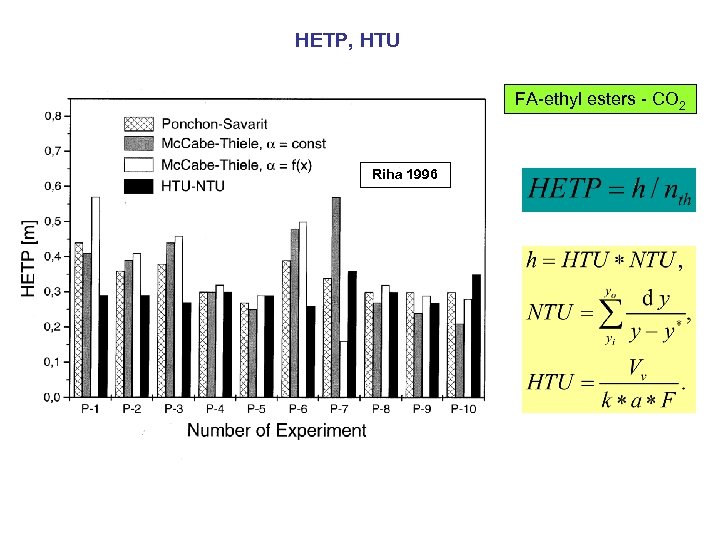 HETP, HTU FA-ethyl esters - CO 2 Riha 1996
HETP, HTU FA-ethyl esters - CO 2 Riha 1996
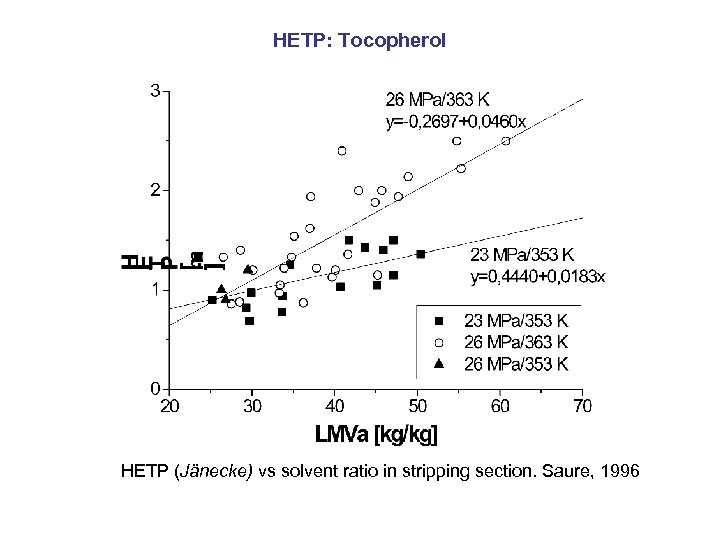 HETP: Tocopherol HETP (Jänecke) vs solvent ratio in stripping section. Saure, 1996
HETP: Tocopherol HETP (Jänecke) vs solvent ratio in stripping section. Saure, 1996
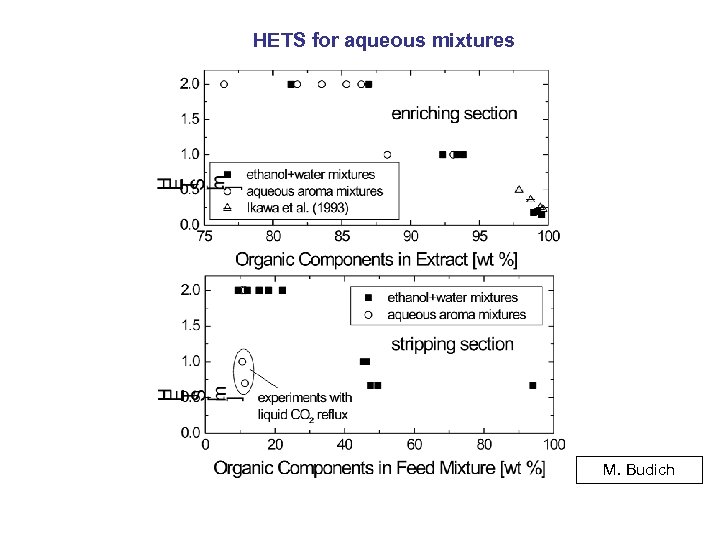 HETS for aqueous mixtures M. Budich
HETS for aqueous mixtures M. Budich
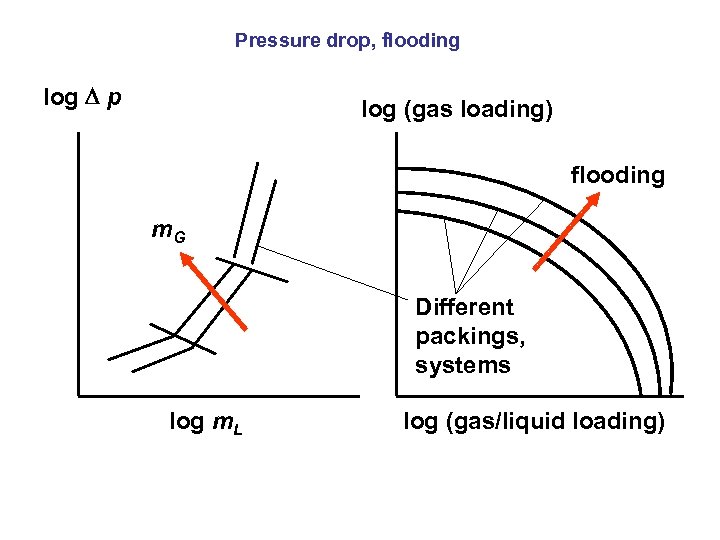 Pressure drop, flooding log p log (gas loading) flooding m. G Different packings, systems log m. L log (gas/liquid loading)
Pressure drop, flooding log p log (gas loading) flooding m. G Different packings, systems log m. L log (gas/liquid loading)
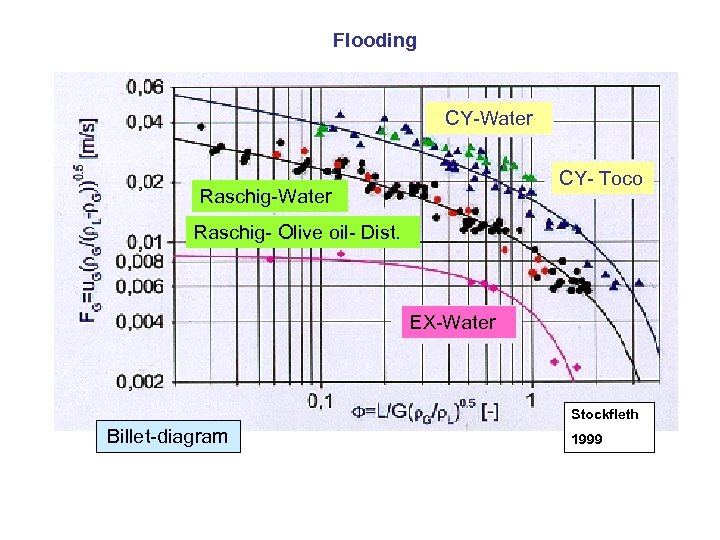 Flooding CY-Water CY- Toco Raschig-Water Raschig- Olive oil- Dist. EX-Water Stockfleth Billet-diagram 1999
Flooding CY-Water CY- Toco Raschig-Water Raschig- Olive oil- Dist. EX-Water Stockfleth Billet-diagram 1999
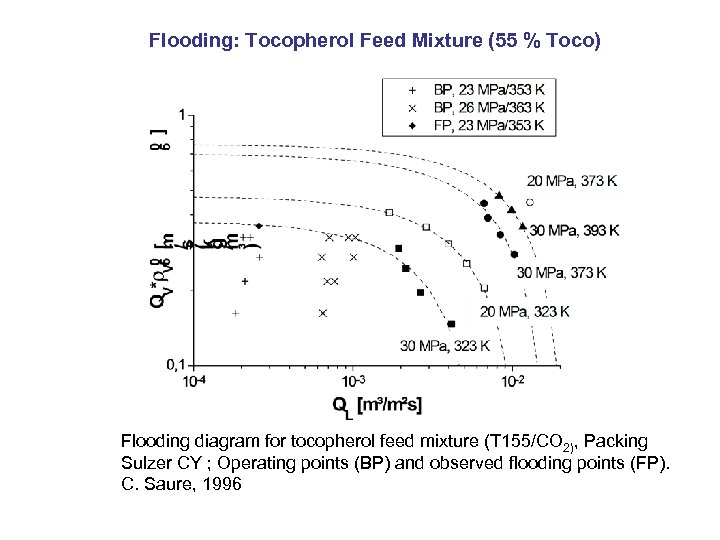 Flooding: Tocopherol Feed Mixture (55 % Toco) Flooding diagram for tocopherol feed mixture (T 155/CO 2), Packing Sulzer CY ; Operating points (BP) and observed flooding points (FP). C. Saure, 1996
Flooding: Tocopherol Feed Mixture (55 % Toco) Flooding diagram for tocopherol feed mixture (T 155/CO 2), Packing Sulzer CY ; Operating points (BP) and observed flooding points (FP). C. Saure, 1996
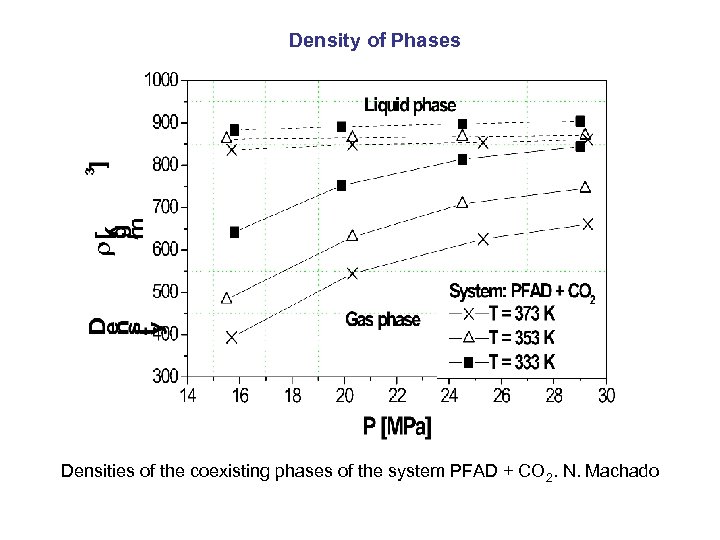 Density of Phases Densities of the coexisting phases of the system PFAD + CO 2. N. Machado
Density of Phases Densities of the coexisting phases of the system PFAD + CO 2. N. Machado
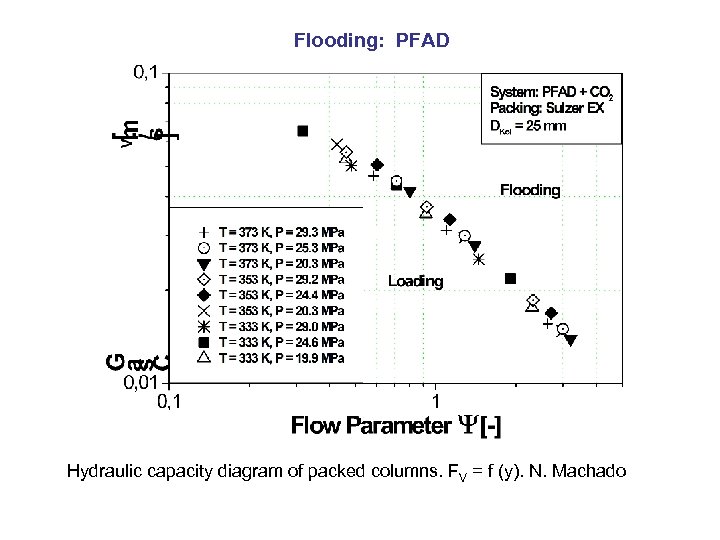 Flooding: PFAD Hydraulic capacity diagram of packed columns. FV = f (y). N. Machado
Flooding: PFAD Hydraulic capacity diagram of packed columns. FV = f (y). N. Machado
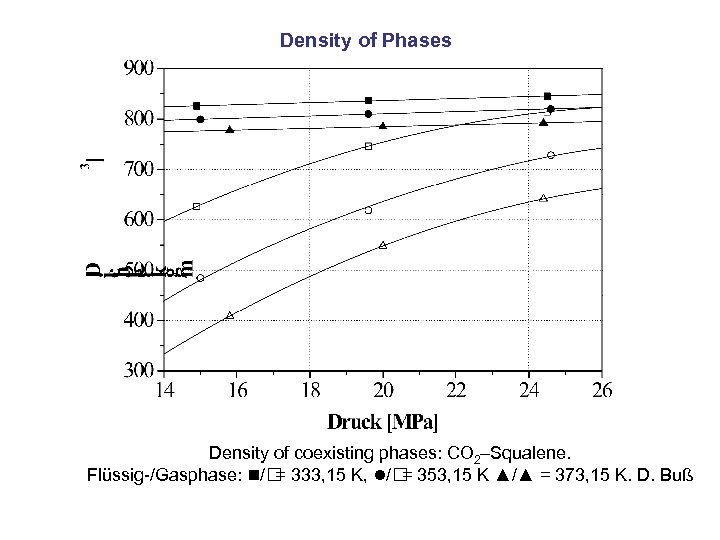 Density of Phases Density of coexisting phases: CO 2–Squalene. Flüssig-/Gasphase: / = 333, 15 K, / = 353, 15 K ▲/▲ = 373, 15 K. D. Buß
Density of Phases Density of coexisting phases: CO 2–Squalene. Flüssig-/Gasphase: / = 333, 15 K, / = 353, 15 K ▲/▲ = 373, 15 K. D. Buß
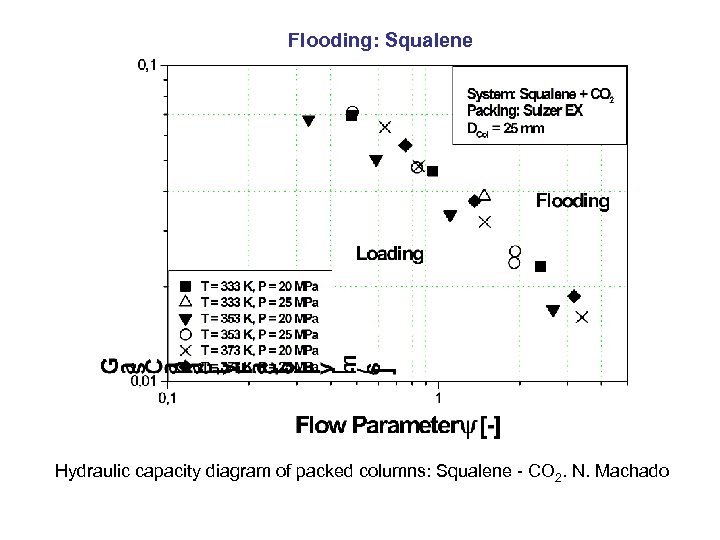 Flooding: Squalene Hydraulic capacity diagram of packed columns: Squalene - CO 2. N. Machado
Flooding: Squalene Hydraulic capacity diagram of packed columns: Squalene - CO 2. N. Machado
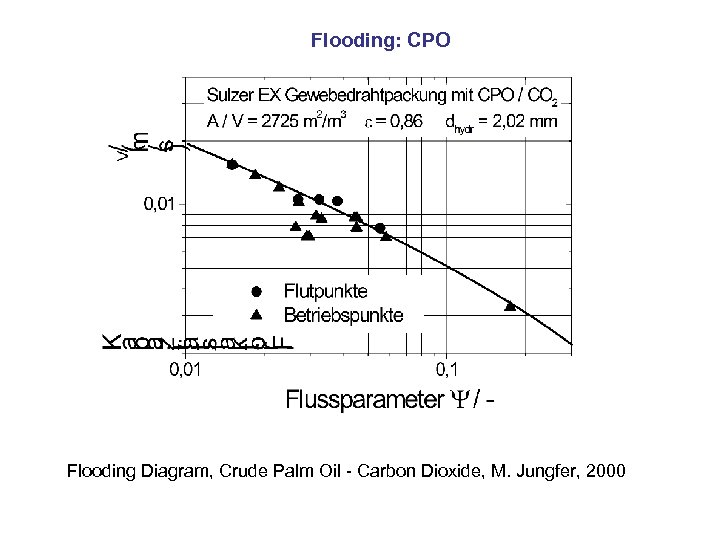 Flooding: CPO Flooding Diagram, Crude Palm Oil - Carbon Dioxide, M. Jungfer, 2000
Flooding: CPO Flooding Diagram, Crude Palm Oil - Carbon Dioxide, M. Jungfer, 2000
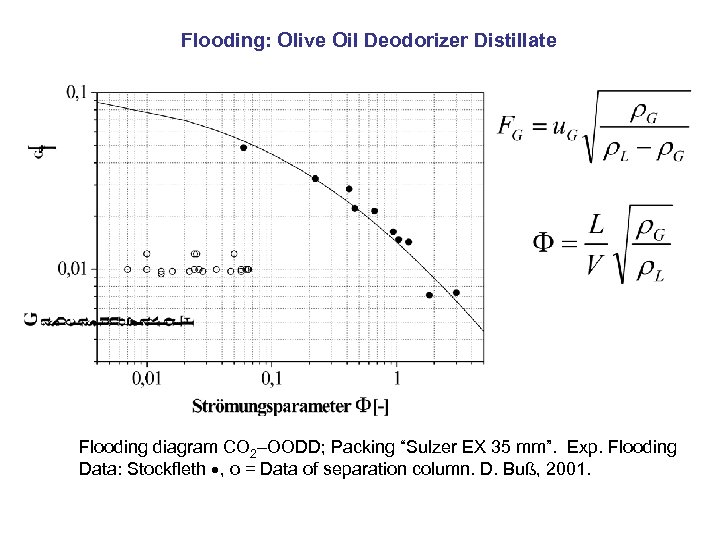 Flooding: Olive Oil Deodorizer Distillate Flooding diagram CO 2–OODD; Packing “Sulzer EX 35 mm”. Exp. Flooding Data: Stockfleth , o = Data of separation column. D. Buß, 2001.
Flooding: Olive Oil Deodorizer Distillate Flooding diagram CO 2–OODD; Packing “Sulzer EX 35 mm”. Exp. Flooding Data: Stockfleth , o = Data of separation column. D. Buß, 2001.
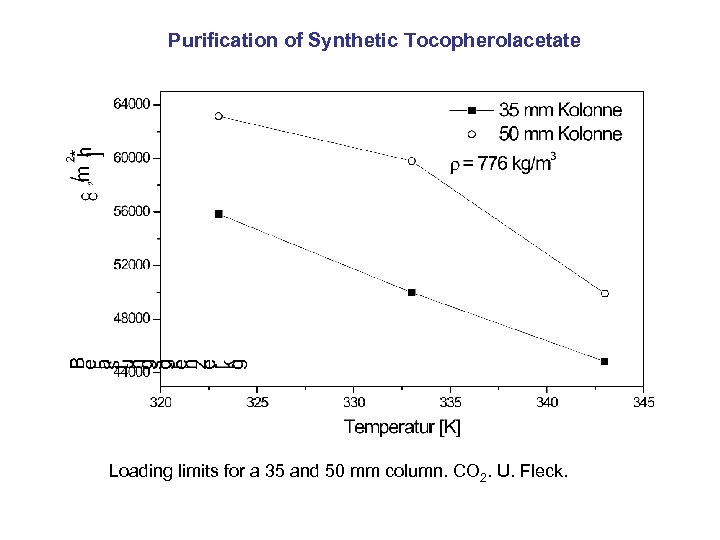 Purification of Synthetic Tocopherolacetate Loading limits for a 35 and 50 mm column. CO 2. U. Fleck.
Purification of Synthetic Tocopherolacetate Loading limits for a 35 and 50 mm column. CO 2. U. Fleck.
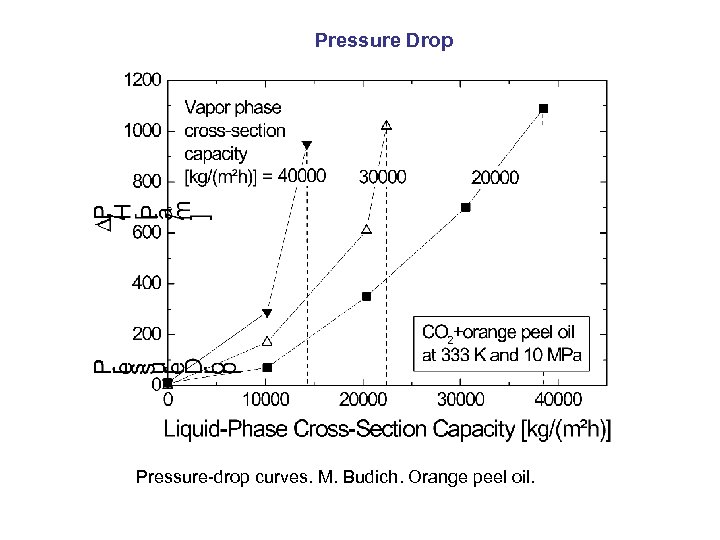 Pressure Drop Pressure-drop curves. M. Budich. Orange peel oil.
Pressure Drop Pressure-drop curves. M. Budich. Orange peel oil.
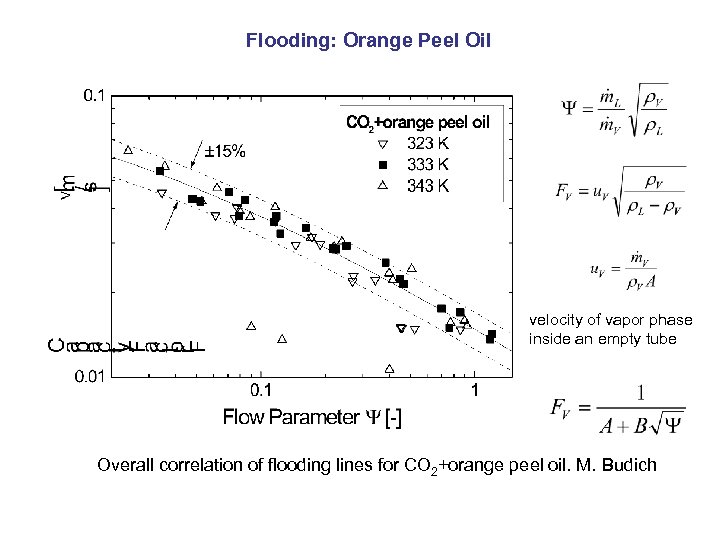 Flooding: Orange Peel Oil velocity of vapor phase inside an empty tube Overall correlation of flooding lines for CO 2+orange peel oil. M. Budich
Flooding: Orange Peel Oil velocity of vapor phase inside an empty tube Overall correlation of flooding lines for CO 2+orange peel oil. M. Budich
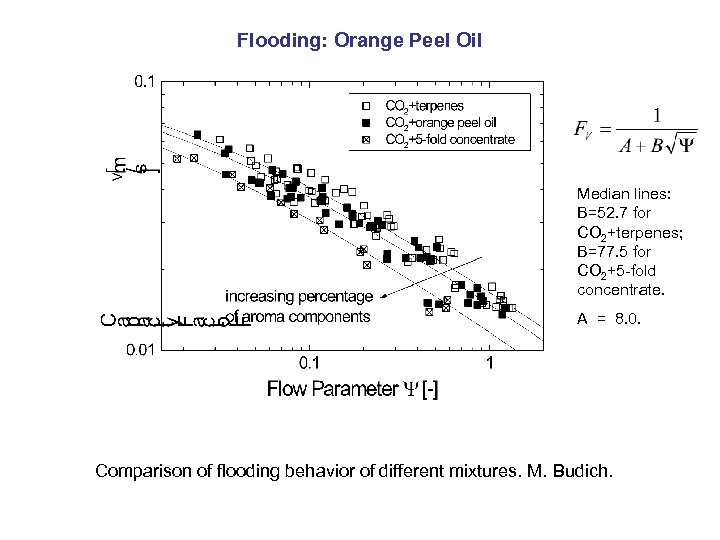 Flooding: Orange Peel Oil Median lines: B=52. 7 for CO 2+terpenes; B=77. 5 for CO 2+5 -fold concentrate. A = 8. 0. Comparison of flooding behavior of different mixtures. M. Budich.
Flooding: Orange Peel Oil Median lines: B=52. 7 for CO 2+terpenes; B=77. 5 for CO 2+5 -fold concentrate. A = 8. 0. Comparison of flooding behavior of different mixtures. M. Budich.
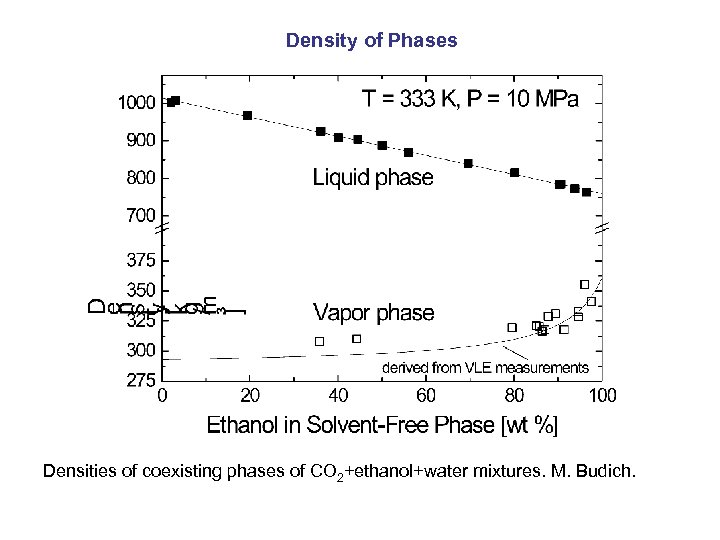 Density of Phases Densities of coexisting phases of CO 2+ethanol+water mixtures. M. Budich.
Density of Phases Densities of coexisting phases of CO 2+ethanol+water mixtures. M. Budich.
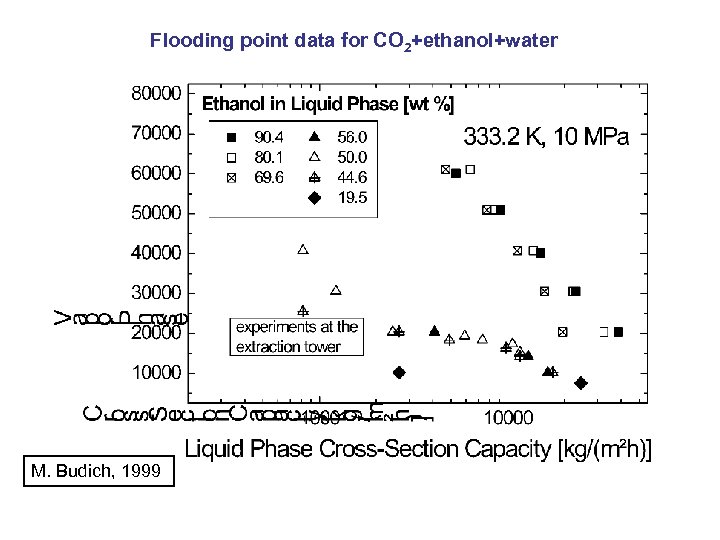 Flooding point data for CO 2+ethanol+water M. Budich, 1999
Flooding point data for CO 2+ethanol+water M. Budich, 1999
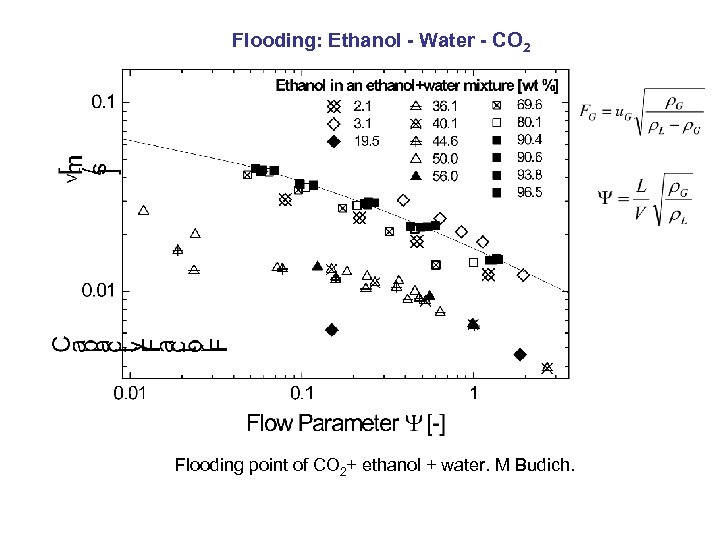 Flooding: Ethanol - Water - CO 2 Flooding point of CO 2+ ethanol + water. M Budich.
Flooding: Ethanol - Water - CO 2 Flooding point of CO 2+ ethanol + water. M Budich.
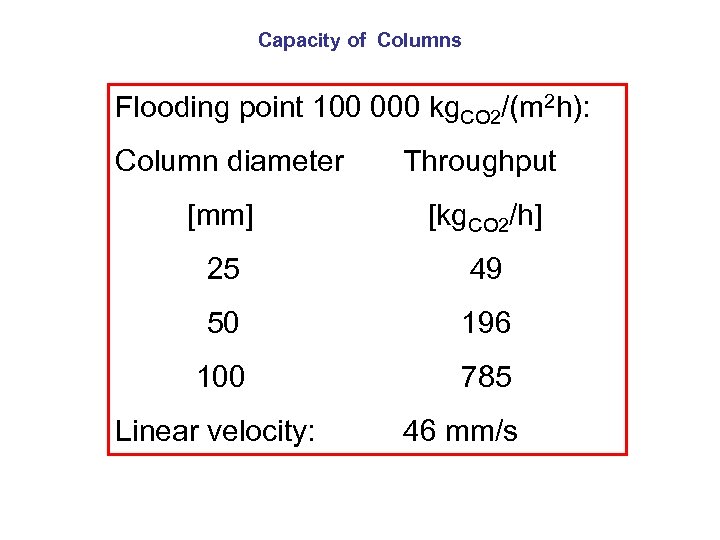 Capacity of Columns Flooding point 100 000 kg. CO 2/(m 2 h): Column diameter [mm] Throughput [kg. CO 2/h] 25 49 50 196 100 785 Linear velocity: 46 mm/s
Capacity of Columns Flooding point 100 000 kg. CO 2/(m 2 h): Column diameter [mm] Throughput [kg. CO 2/h] 25 49 50 196 100 785 Linear velocity: 46 mm/s
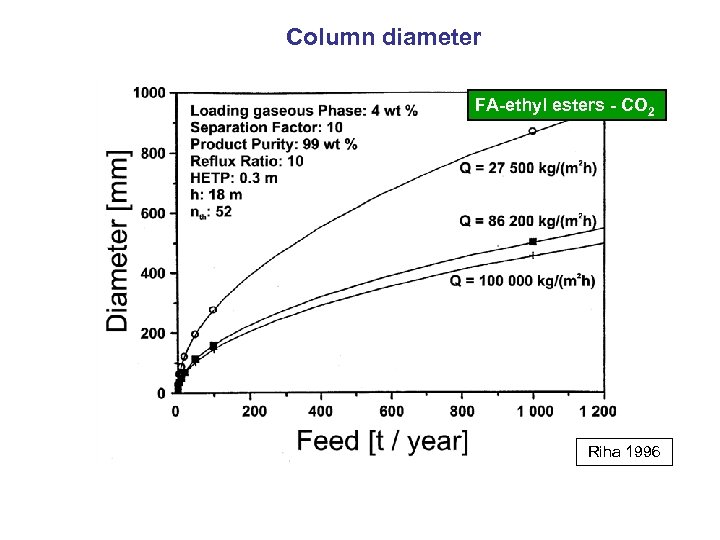 Column diameter FA-ethyl esters - CO 2 Riha 1996
Column diameter FA-ethyl esters - CO 2 Riha 1996
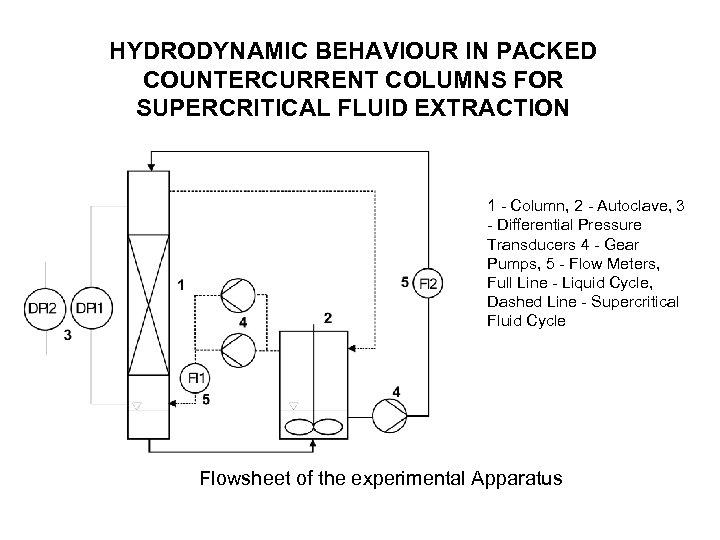 HYDRODYNAMIC BEHAVIOUR IN PACKED COUNTERCURRENT COLUMNS FOR SUPERCRITICAL FLUID EXTRACTION 1 - Column, 2 - Autoclave, 3 - Differential Pressure Transducers 4 - Gear Pumps, 5 - Flow Meters, Full Line - Liquid Cycle, Dashed Line - Supercritical Fluid Cycle Flowsheet of the experimental Apparatus
HYDRODYNAMIC BEHAVIOUR IN PACKED COUNTERCURRENT COLUMNS FOR SUPERCRITICAL FLUID EXTRACTION 1 - Column, 2 - Autoclave, 3 - Differential Pressure Transducers 4 - Gear Pumps, 5 - Flow Meters, Full Line - Liquid Cycle, Dashed Line - Supercritical Fluid Cycle Flowsheet of the experimental Apparatus
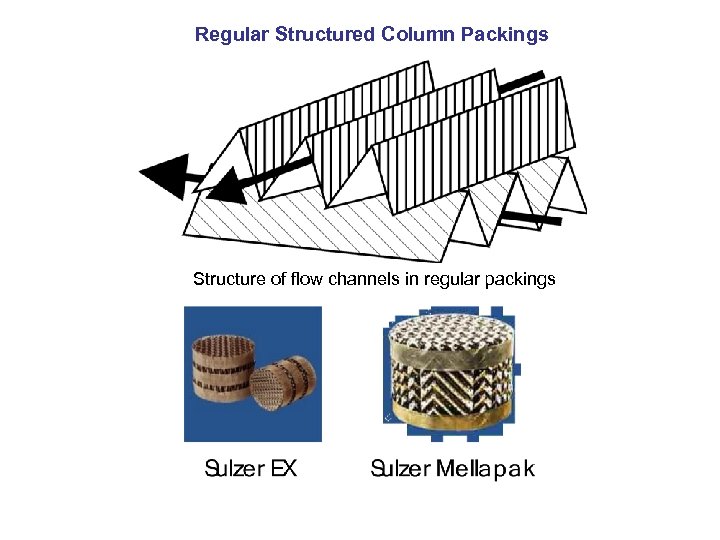 Regular Structured Column Packings Structure of flow channels in regular packings
Regular Structured Column Packings Structure of flow channels in regular packings
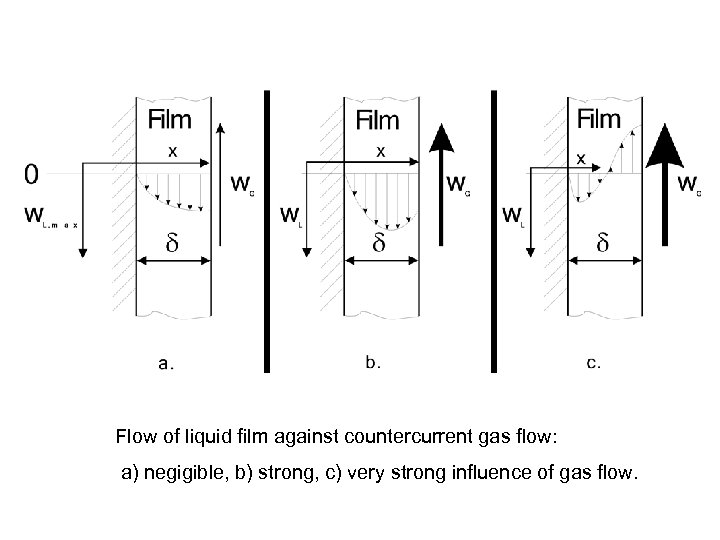 Flow of liquid film against countercurrent gas flow: a) negigible, b) strong, c) very strong influence of gas flow.
Flow of liquid film against countercurrent gas flow: a) negigible, b) strong, c) very strong influence of gas flow.
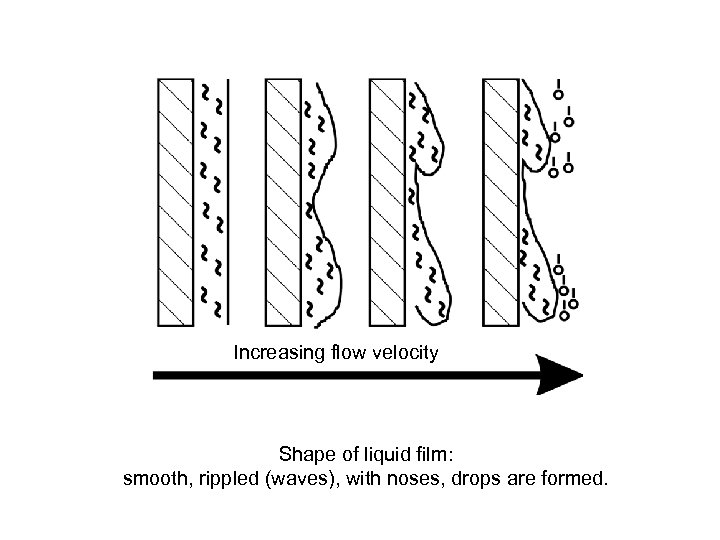 Increasing flow velocity Shape of liquid film: smooth, rippled (waves), with noses, drops are formed.
Increasing flow velocity Shape of liquid film: smooth, rippled (waves), with noses, drops are formed.
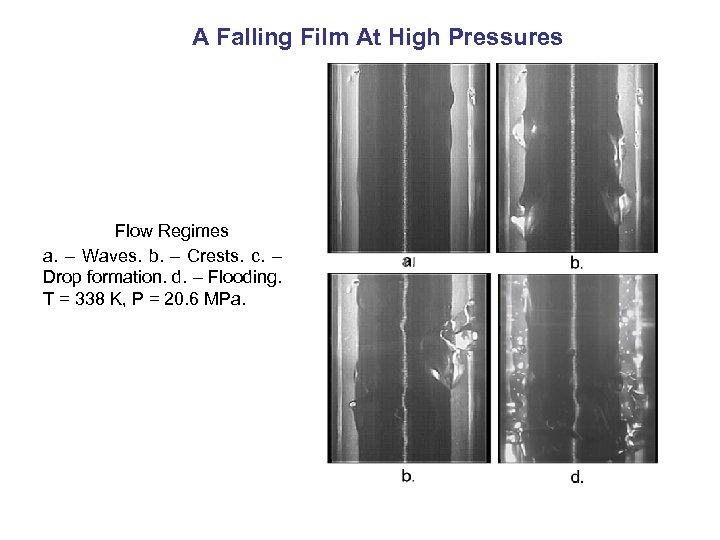 A Falling Film At High Pressures Flow Regimes a. – Waves. b. – Crests. c. – Drop formation. d. – Flooding. T = 338 K, P = 20. 6 MPa.
A Falling Film At High Pressures Flow Regimes a. – Waves. b. – Crests. c. – Drop formation. d. – Flooding. T = 338 K, P = 20. 6 MPa.
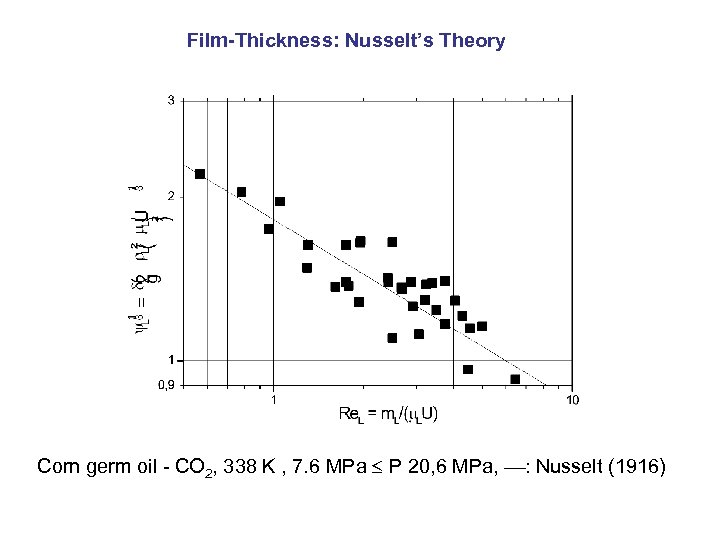 Film-Thickness: Nusselt’s Theory Corn germ oil - CO 2, 338 K , 7. 6 MPa P 20, 6 MPa, : Nusselt (1916)
Film-Thickness: Nusselt’s Theory Corn germ oil - CO 2, 338 K , 7. 6 MPa P 20, 6 MPa, : Nusselt (1916)
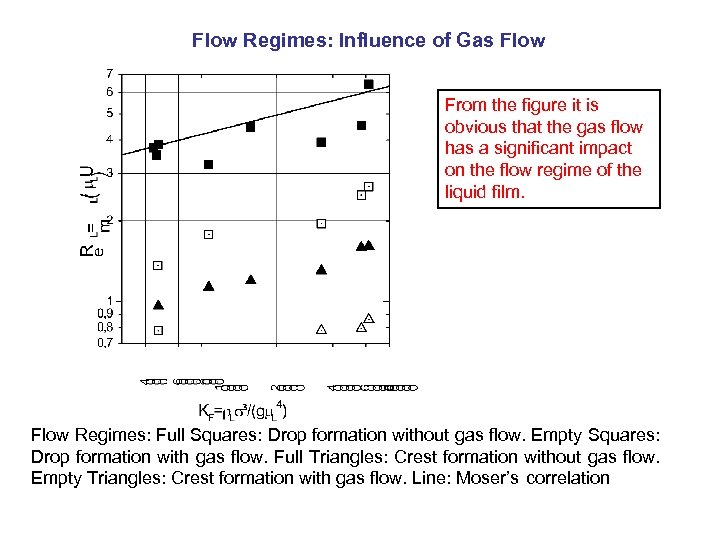 Flow Regimes: Influence of Gas Flow From the figure it is obvious that the gas flow has a significant impact on the flow regime of the liquid film. Flow Regimes: Full Squares: Drop formation without gas flow. Empty Squares: Drop formation with gas flow. Full Triangles: Crest formation without gas flow. Empty Triangles: Crest formation with gas flow. Line: Moser’s correlation
Flow Regimes: Influence of Gas Flow From the figure it is obvious that the gas flow has a significant impact on the flow regime of the liquid film. Flow Regimes: Full Squares: Drop formation without gas flow. Empty Squares: Drop formation with gas flow. Full Triangles: Crest formation without gas flow. Empty Triangles: Crest formation with gas flow. Line: Moser’s correlation
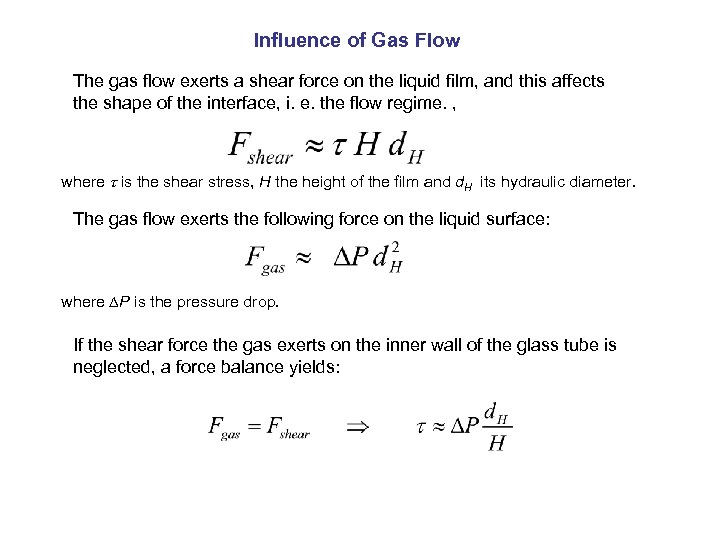 Influence of Gas Flow The gas flow exerts a shear force on the liquid film, and this affects the shape of the interface, i. e. the flow regime. , where is the shear stress, H the height of the film and d. H its hydraulic diameter. The gas flow exerts the following force on the liquid surface: where P is the pressure drop. If the shear force the gas exerts on the inner wall of the glass tube is neglected, a force balance yields:
Influence of Gas Flow The gas flow exerts a shear force on the liquid film, and this affects the shape of the interface, i. e. the flow regime. , where is the shear stress, H the height of the film and d. H its hydraulic diameter. The gas flow exerts the following force on the liquid surface: where P is the pressure drop. If the shear force the gas exerts on the inner wall of the glass tube is neglected, a force balance yields:
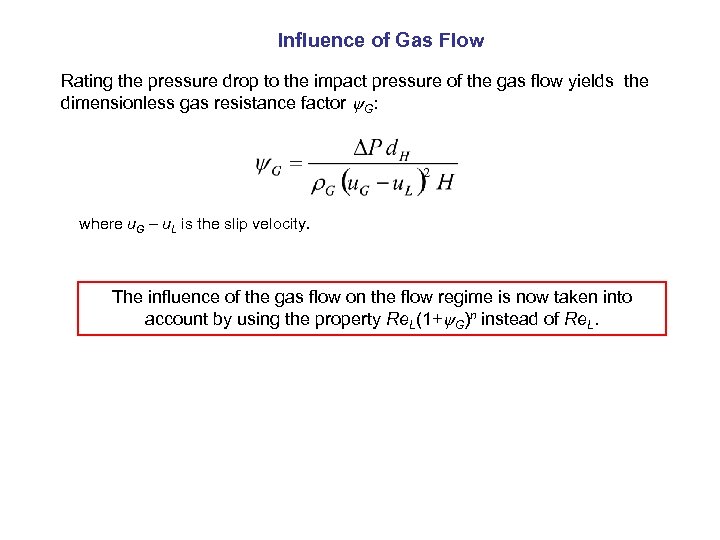 Influence of Gas Flow Rating the pressure drop to the impact pressure of the gas flow yields the dimensionless gas resistance factor G: where u. G – u. L is the slip velocity. The influence of the gas flow on the flow regime is now taken into account by using the property Re. L(1+ G)n instead of Re. L.
Influence of Gas Flow Rating the pressure drop to the impact pressure of the gas flow yields the dimensionless gas resistance factor G: where u. G – u. L is the slip velocity. The influence of the gas flow on the flow regime is now taken into account by using the property Re. L(1+ G)n instead of Re. L.
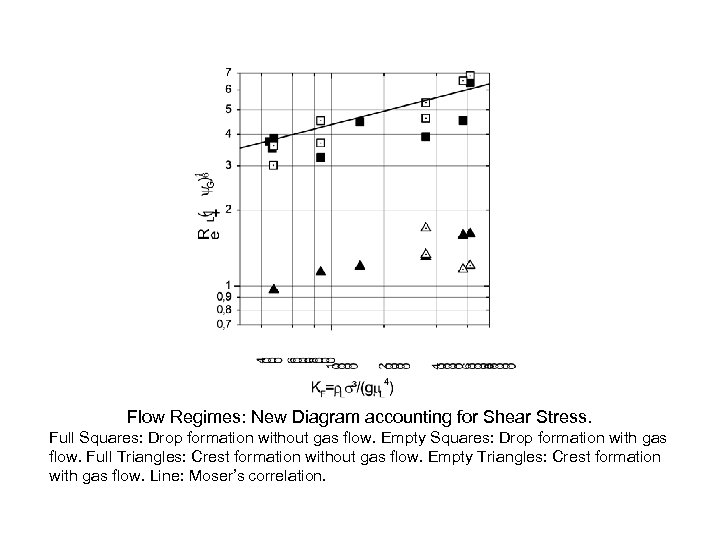 Flow Regimes: New Diagram accounting for Shear Stress. Full Squares: Drop formation without gas flow. Empty Squares: Drop formation with gas flow. Full Triangles: Crest formation without gas flow. Empty Triangles: Crest formation with gas flow. Line: Moser’s correlation.
Flow Regimes: New Diagram accounting for Shear Stress. Full Squares: Drop formation without gas flow. Empty Squares: Drop formation with gas flow. Full Triangles: Crest formation without gas flow. Empty Triangles: Crest formation with gas flow. Line: Moser’s correlation.
![Flooding Correlation of the flooding points according to Wallis [10]: G. B Wallis, (1969), Flooding Correlation of the flooding points according to Wallis [10]: G. B Wallis, (1969),](https://present5.com/presentation/d808e4967f6bc1f62c3787aaca6b7d7d/image-62.jpg) Flooding Correlation of the flooding points according to Wallis [10]: G. B Wallis, (1969), One-Dimensional Two-Phase Flow, Mc. Graw-Hill, New York With u. L for the superficial liquid velocity and the fractional void volume which is unity for a falling film column but smaller than unity for packed columns. j. G* and j. L* are modified Froude-Numbers rating the respective impact pressure to the difference between liquid head and buoyancy. For the correlation of the data displayed, the values K 1=0, 4222 and K 2=1, 1457 with a standard deviation of 19%.
Flooding Correlation of the flooding points according to Wallis [10]: G. B Wallis, (1969), One-Dimensional Two-Phase Flow, Mc. Graw-Hill, New York With u. L for the superficial liquid velocity and the fractional void volume which is unity for a falling film column but smaller than unity for packed columns. j. G* and j. L* are modified Froude-Numbers rating the respective impact pressure to the difference between liquid head and buoyancy. For the correlation of the data displayed, the values K 1=0, 4222 and K 2=1, 1457 with a standard deviation of 19%.
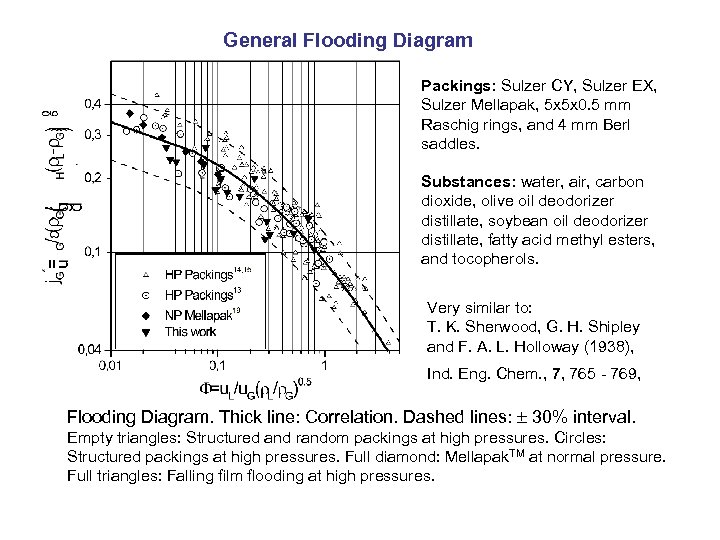 General Flooding Diagram Packings: Sulzer CY, Sulzer EX, Sulzer Mellapak, 5 x 5 x 0. 5 mm Raschig rings, and 4 mm Berl saddles. Substances: water, air, carbon dioxide, olive oil deodorizer distillate, soybean oil deodorizer distillate, fatty acid methyl esters, and tocopherols. Very similar to: T. K. Sherwood, G. H. Shipley and F. A. L. Holloway (1938), Ind. Eng. Chem. , 7, 765 - 769, Flooding Diagram. Thick line: Correlation. Dashed lines: 30% interval. Empty triangles: Structured and random packings at high pressures. Circles: Structured packings at high pressures. Full diamond: Mellapak. TM at normal pressure. Full triangles: Falling film flooding at high pressures.
General Flooding Diagram Packings: Sulzer CY, Sulzer EX, Sulzer Mellapak, 5 x 5 x 0. 5 mm Raschig rings, and 4 mm Berl saddles. Substances: water, air, carbon dioxide, olive oil deodorizer distillate, soybean oil deodorizer distillate, fatty acid methyl esters, and tocopherols. Very similar to: T. K. Sherwood, G. H. Shipley and F. A. L. Holloway (1938), Ind. Eng. Chem. , 7, 765 - 769, Flooding Diagram. Thick line: Correlation. Dashed lines: 30% interval. Empty triangles: Structured and random packings at high pressures. Circles: Structured packings at high pressures. Full diamond: Mellapak. TM at normal pressure. Full triangles: Falling film flooding at high pressures.


