69d3d02cce37f3ce837b440ba7769117.ppt
- Количество слайдов: 100
Chapter 6 Chain Copolymerization : Don’t be confused with the Step Polymerization → not copolymerization Chain Coplymerization : Chain Polymerization in which two monomers are simultaneously polymerized, producing copolymer Multi-component Coplymerization : Three or more monomers Terpolymerization : Three monomers
6 -1 General Considerations 6 -1 a Importance of Chain Copolymerization ① Reactivity of monomers ⇒ from copolymerization studies ② To tailor-make a polymer product not by homopolymerization ⇒ ∵ one product ex) PS : 8 million PS related product 1/3 ~ homo 2/3 ~ copolymer (SAN, ABS, …) 6 -1 b Types of Copolymers Random, Alternating Copolymer Block Copolymer Graft Copolymer
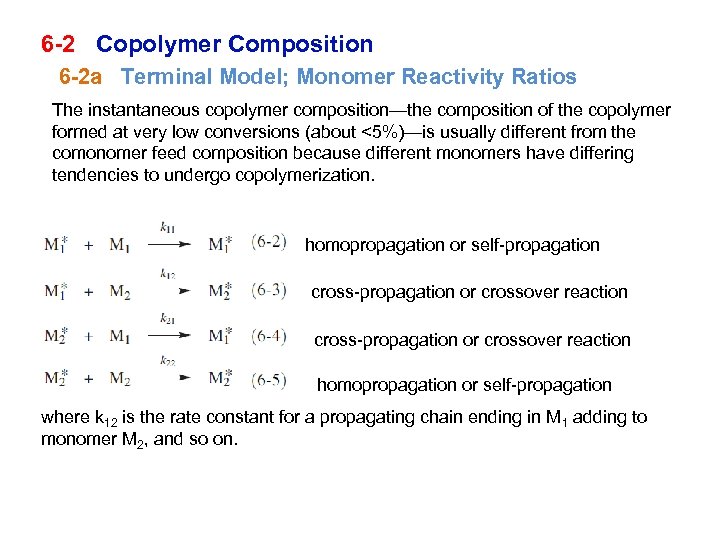
6 -2 Copolymer Composition 6 -2 a Terminal Model; Monomer Reactivity Ratios The instantaneous copolymer composition—the composition of the copolymer formed at very low conversions (about <5%)—is usually different from the comonomer feed composition because different monomers have differing tendencies to undergo copolymerization. homopropagation or self-propagation cross-propagation or crossover reaction homopropagation or self-propagation where k 12 is the rate constant for a propagating chain ending in M 1 adding to monomer M 2, and so on.
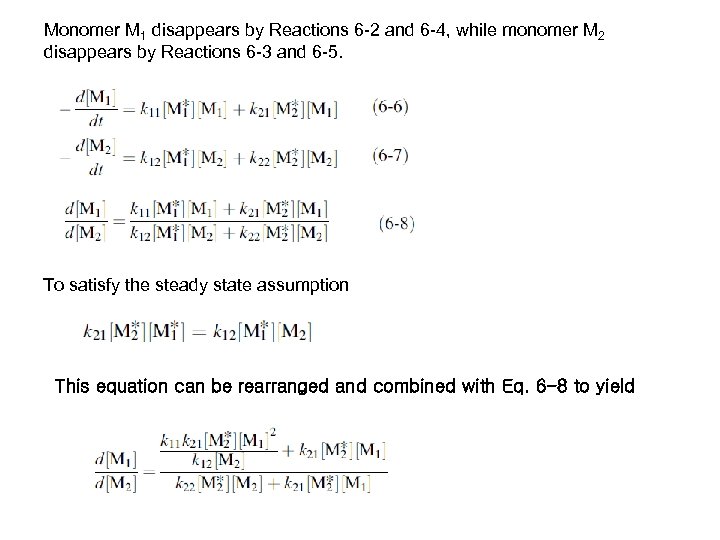
Monomer M 1 disappears by Reactions 6 -2 and 6 -4, while monomer M 2 disappears by Reactions 6 -3 and 6 -5. To satisfy the steady state assumption This equation can be rearranged and combined with Eq. 6 -8 to yield
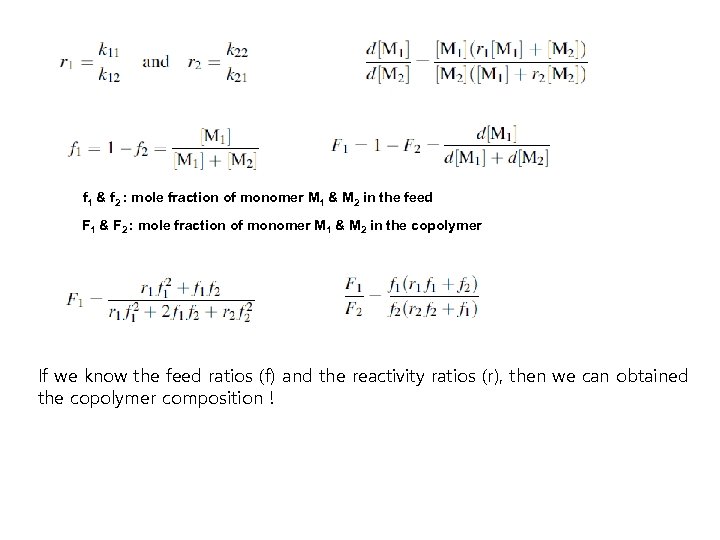
f 1 & f 2 : mole fraction of monomer M 1 & M 2 in the feed F 1 & F 2 : mole fraction of monomer M 1 & M 2 in the copolymer If we know the feed ratios (f) and the reactivity ratios (r), then we can obtained the copolymer composition !
![6 -2 b Statistical Derivation of Copolymerization Equation and + Divided by [M 1*] 6 -2 b Statistical Derivation of Copolymerization Equation and + Divided by [M 1*]](https://present5.com/presentation/69d3d02cce37f3ce837b440ba7769117/image-6.jpg)
6 -2 b Statistical Derivation of Copolymerization Equation and + Divided by [M 1*] and k 12 Using the same method
=
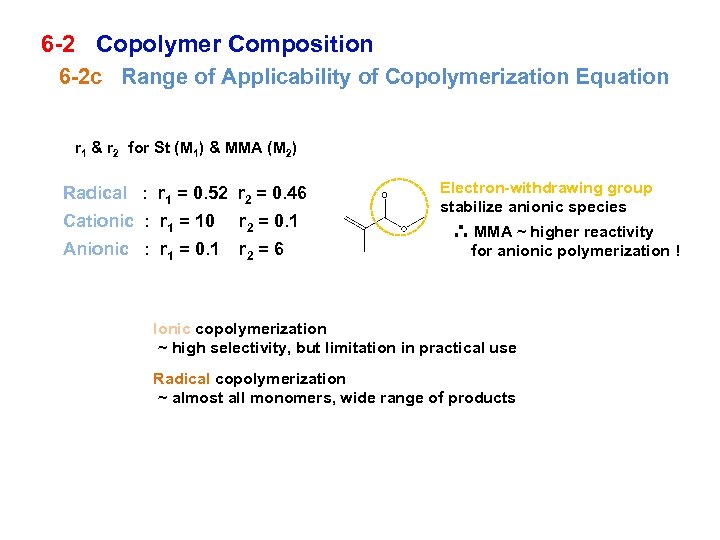
6 -2 Copolymer Composition 6 -2 c Range of Applicability of Copolymerization Equation r 1 & r 2 for St (M 1) & MMA (M 2) Radical : r 1 = 0. 52 r 2 = 0. 46 Cationic : r 1 = 10 r 2 = 0. 1 Anionic : r 1 = 0. 1 r 2 = 6 Electron-withdrawing group stabilize anionic species ∴ MMA ~ higher reactivity for anionic polymerization ! Ionic copolymerization ~ high selectivity, but limitation in practical use Radical copolymerization ~ almost all monomers, wide range of products
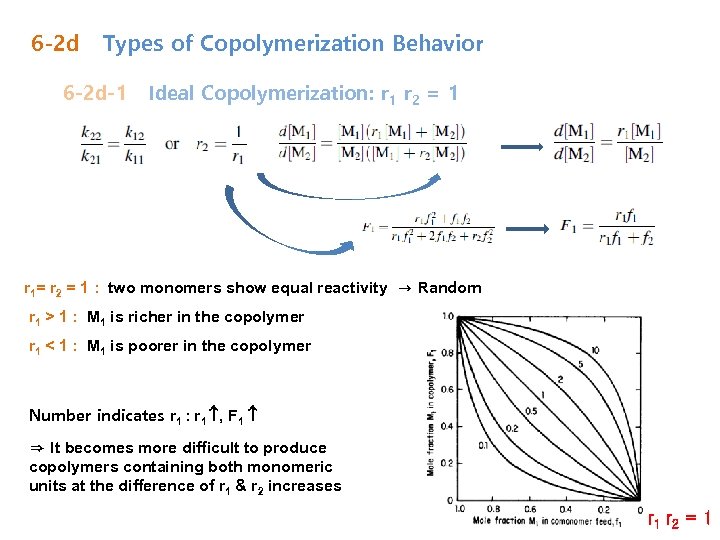
6 -2 d Types of Copolymerization Behavior 6 -2 d-1 Ideal Copolymerization: r 1 r 2 = 1 r 1= r 2 = 1 : two monomers show equal reactivity → Random r 1 > 1 : M 1 is richer in the copolymer r 1 < 1 : M 1 is poorer in the copolymer Number indicates r 1 : r 1 , F 1 ⇒ It becomes more difficult to produce copolymers containing both monomeric units at the difference of r 1 & r 2 increases r 1 r 2 = 1
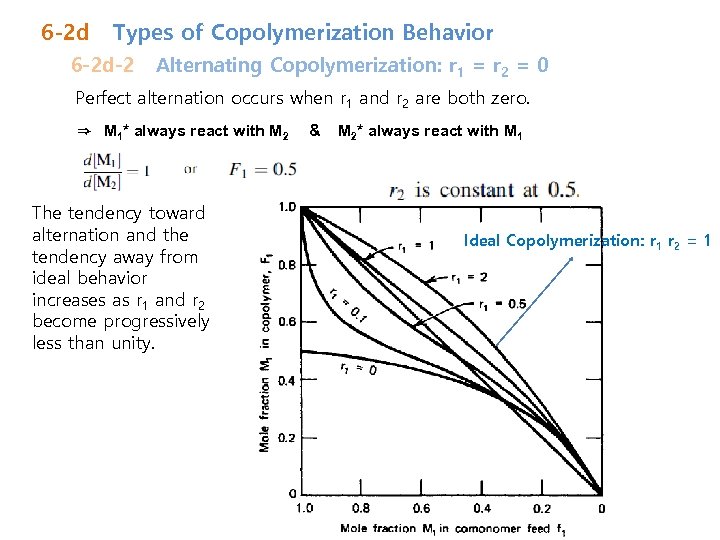
6 -2 d Types of Copolymerization Behavior 6 -2 d-2 Alternating Copolymerization: r 1 = r 2 = 0 Perfect alternation occurs when r 1 and r 2 are both zero. ⇒ M 1* always react with M 2 The tendency toward alternation and the tendency away from ideal behavior increases as r 1 and r 2 become progressively less than unity. & M 2* always react with M 1 Ideal Copolymerization: r 1 r 2 = 1
* Azeotropic copolymerization ⇒ the copolymer and the feed compositions are the same ∴ d[M 1]/d[M 2] = [M 1]/[M 2] from and * consecutive homopolymerization Monomer M 1 tends to homopolymerize until it is consumed; monomer M 2 will subsequently homopolymerize. Ex) styrene-vinyl acetate with monomer reactivity ratios of 55 and 0. 01 • Block Copolymerization r 1 > 1, r 2 > 1
6 -2 e Variation of Copolymer Composition with Conversion Until now instantaneous copolymer composition → at low degree of conversion (<5%) → cannot be applied to the true systems * ∴ We need the composition at a function of conversion Copolymer composition as a function of conversion M moles of the two monomers Copolymer formed is richer in monomer M 1 (i. e. , F 1 > f 1) When d M moles of monomers are copolymerized, the copolymer has F 1 d M moles of monomer 1, and the feed will contain moles of monomer 1. A material balance for monomer 1
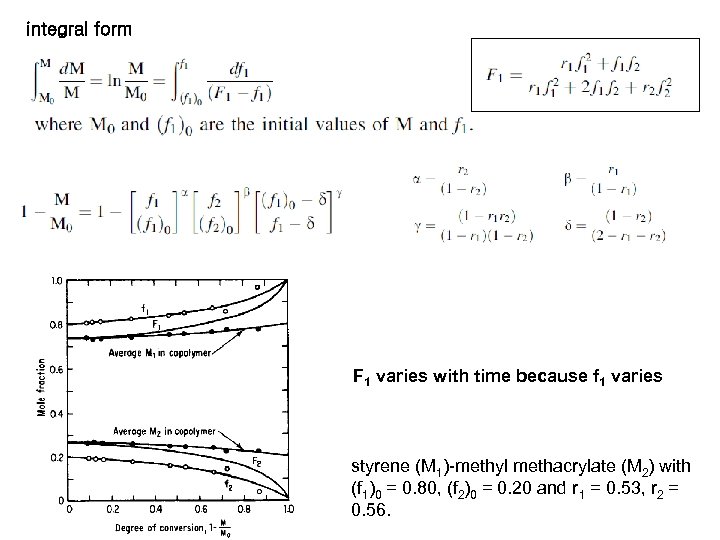
integral form F 1 varies with time because f 1 varies styrene (M 1)-methyl methacrylate (M 2) with (f 1)0 = 0. 80, (f 2)0 = 0. 20 and r 1 = 0. 53, r 2 = 0. 56.
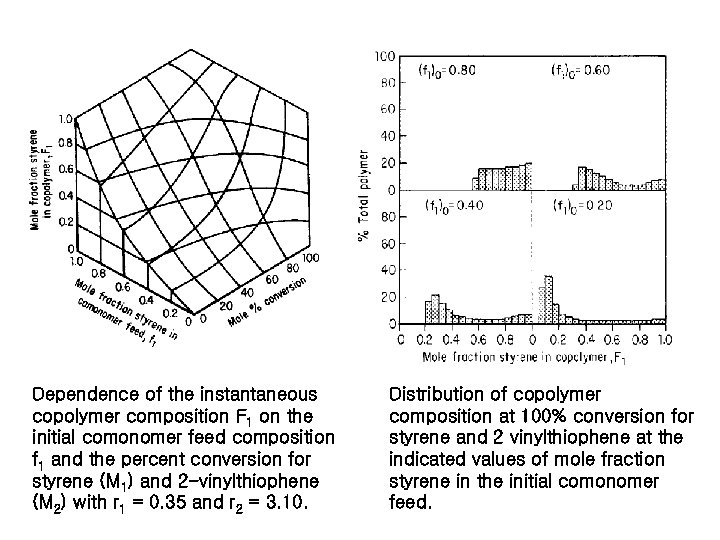
Dependence of the instantaneous copolymer composition F 1 on the initial comonomer feed composition f 1 and the percent conversion for styrene (M 1) and 2 -vinylthiophene (M 2) with r 1 = 0. 35 and r 2 = 3. 10. Distribution of copolymer composition at 100% conversion for styrene and 2 vinylthiophene at the indicated values of mole fraction styrene in the initial comonomer feed.
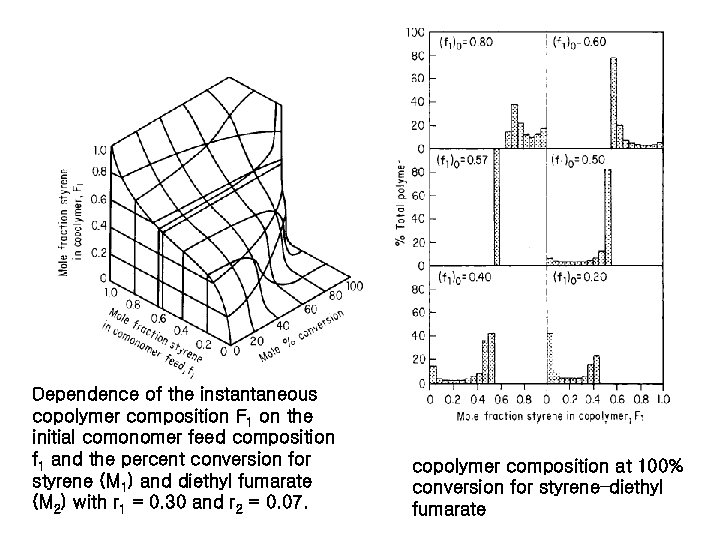
Dependence of the instantaneous copolymer composition F 1 on the initial comonomer feed composition f 1 and the percent conversion for styrene (M 1) and diethyl fumarate (M 2) with r 1 = 0. 30 and r 2 = 0. 07. copolymer composition at 100% conversion for styrene–diethyl fumarate
Three monomer Reactivity r > > r Co-Polymers from FRP Reaction time ~ life time of radical : Seconds
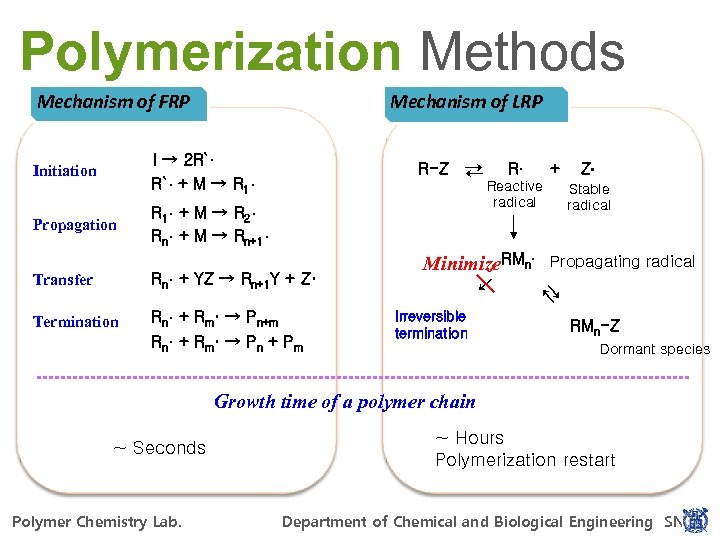
Polymerization Methods Mechanism of FRP Mechanism of LRP R`· + M → R 1· Propagation → R 1 · + M → R 2 · Rn· + M → Rn+1· Rn· + Rm· → Pn+m Rn· + R m · → P n + P m → Termination Reactive radical Minimize. RMn Rn· + YZ → Rn+1 Y + Z· + Z Stable radical Propagating radical → Transfer R → R-Z → I → 2 R`· Initiation Irreversible termination RMn-Z Dormant species Growth time of a polymer chain ~ Hours Polymerization restart ~ Seconds For Power. Point 97 -2010 Polymer Chemistry Lab. POLYMER CHEMISTRY LAB. Department of Chemical and Biological Engineering SNU
![Mechanism of FRP Mechanism of LRP R-Z R`· + M → R 1· [M]/[I], Mechanism of FRP Mechanism of LRP R-Z R`· + M → R 1· [M]/[I],](https://present5.com/presentation/69d3d02cce37f3ce837b440ba7769117/image-18.jpg)
Mechanism of FRP Mechanism of LRP R-Z R`· + M → R 1· [M]/[I], concentration, + M → R 2 · Rn· temperature, etc. + M → Rn+1· Minimize. RMn Rn· + R m · → P n + P m → Rn· + Rm· → Pn+m Reactive radical Z Stable radical M. W. Rn· + YZ → Rn+1 Y + Z· Termination + Propagating radical → Transfer R → M. W. Propagation → → I → 2 R`· Initiation Irreversible termination RMn-Z Dormant species [M]/[chain number] conversion (rxn time) Growth time of a polymer chain Time ~ Hours Polymerization restart ~ Seconds For Power. Point 97 -2010 Polymer Chemistry Lab. POLYMER CHEMISTRY LAB. Department of Chemical and Biological Engineering SNU
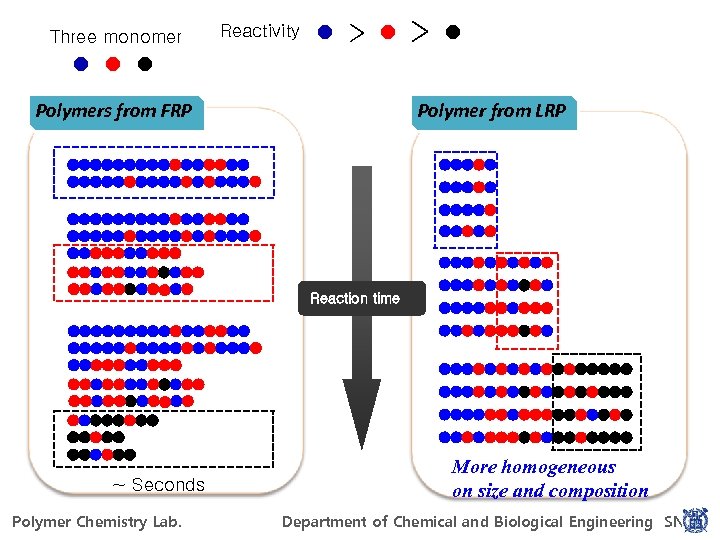
Three monomer Reactivity > Polymers from FRP > Polymer from LRP Reaction time More homogeneous on size and composition ~ Seconds For Power. Point 97 -2010 Polymer Chemistry Lab. POLYMER CHEMISTRY LAB. Department of Chemical and Biological Engineering SNU
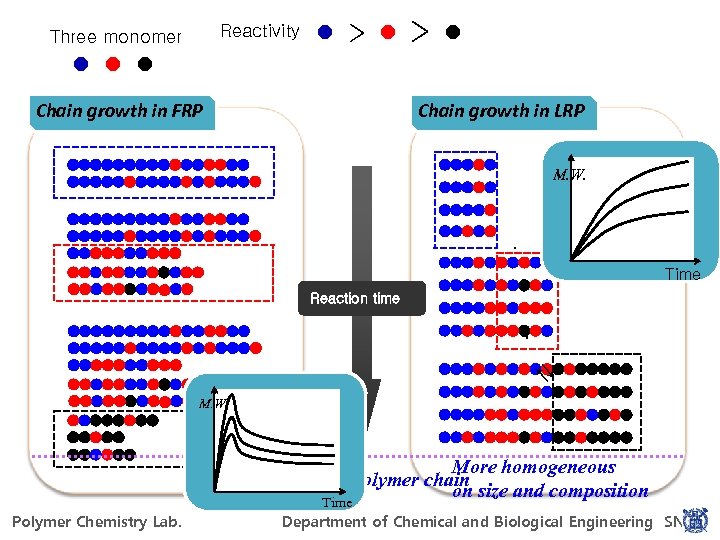
Reactivity Three monomer > Chain growth in FRP > Chain growth in LRP M. W. Time → → Reaction time M. W. For Power. Point 97 -2010 Polymer Chemistry Lab. More homogeneous Growth time of a polymer chain on size and composition Time POLYMER CHEMISTRY LAB. Department of Chemical and Biological Engineering SNU
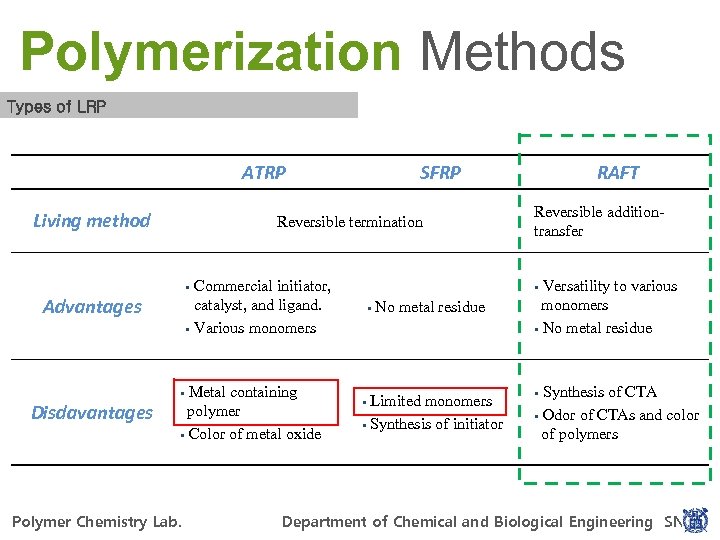
Polymerization Methods Types of LRP ATRP Living method SFRP Reversible termination Commercial initiator, catalyst, and ligand. § Various monomers § Advantages Metal containing polymer § Color of metal oxide § Disdavantages RAFT Reversible additiontransfer Versatility to various monomers § No metal residue § § No metal residue Limited monomers § Synthesis of initiator § Synthesis of CTA § Odor of CTAs and color of polymers § For Power. Point 97 -2010 POLYMER CHEMISTRY LAB. Polymer Chemistry Lab. POLYMER CHEMISTRY LAB. Department of Chemical and Biological Engineering SNU
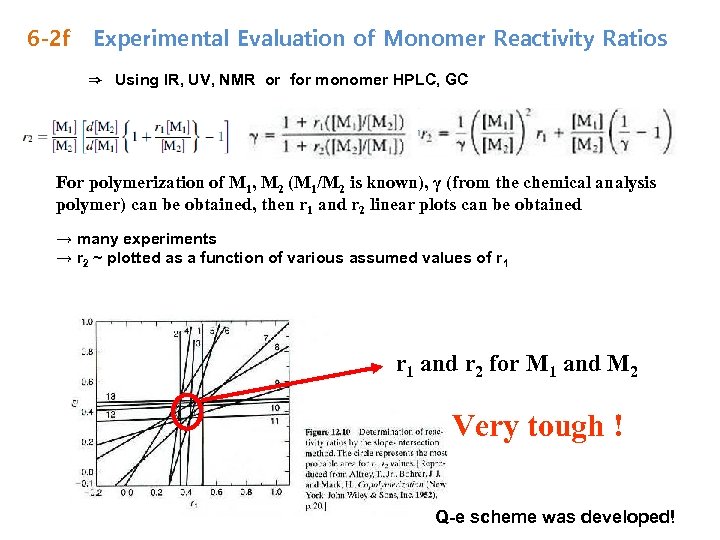
6 -2 f Experimental Evaluation of Monomer Reactivity Ratios ⇒ Using IR, UV, NMR or for monomer HPLC, GC For polymerization of M 1, M 2 (M 1/M 2 is known), γ (from the chemical analysis polymer) can be obtained, then r 1 and r 2 linear plots can be obtained → many experiments → r 2 ~ plotted as a function of various assumed values of r 1 and r 2 for M 1 and M 2 Very tough ! Q-e scheme was developed!
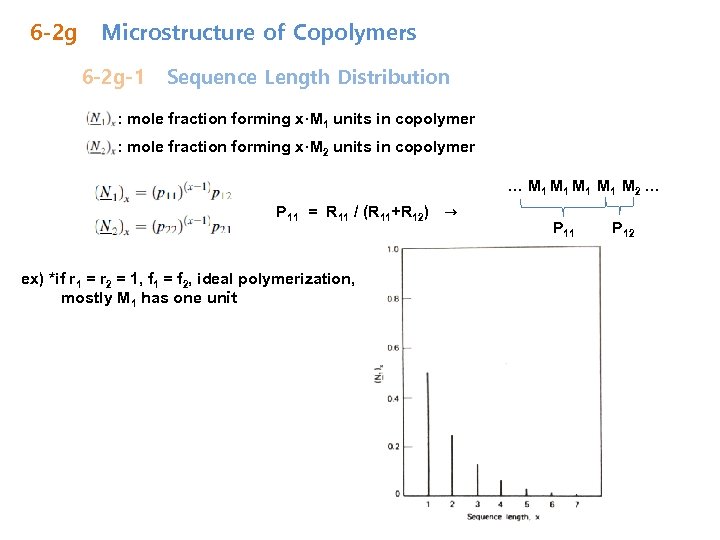
6 -2 g Microstructure of Copolymers 6 -2 g-1 Sequence Length Distribution : mole fraction forming x·M 1 units in copolymer : mole fraction forming x·M 2 units in copolymer … M 1 M 1 M 2 … P 11 = R 11 / (R 11+R 12) → ex) *if r 1 = r 2 = 1, f 1 = f 2, ideal polymerization, mostly M 1 has one unit P 11 P 12
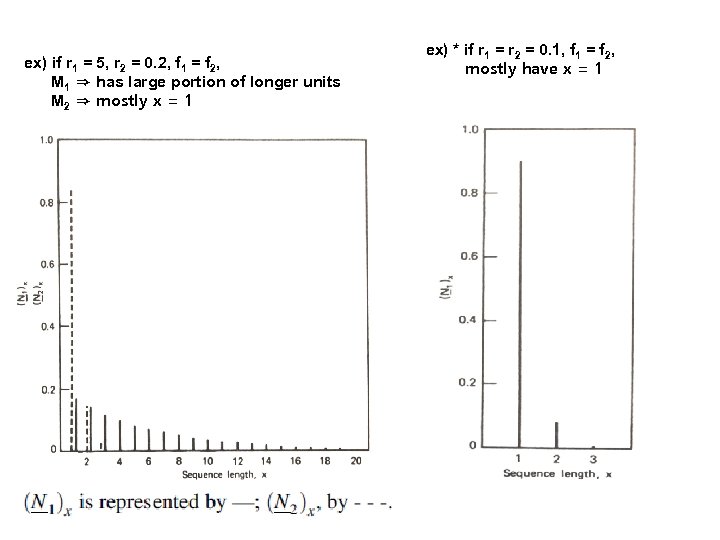
ex) if r 1 = 5, r 2 = 0. 2, f 1 = f 2, M 1 ⇒ has large portion of longer units M 2 ⇒ mostly x = 1 ex) * if r 1 = r 2 = 0. 1, f 1 = f 2, mostly have x = 1
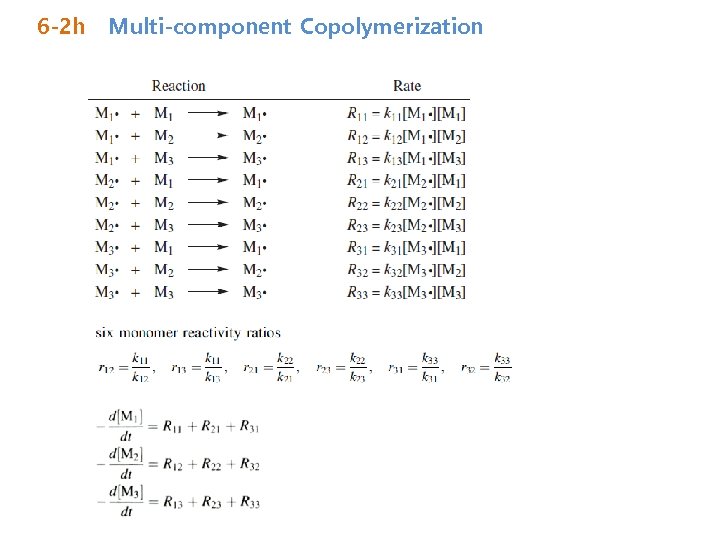
6 -2 h Multi-component Copolymerization
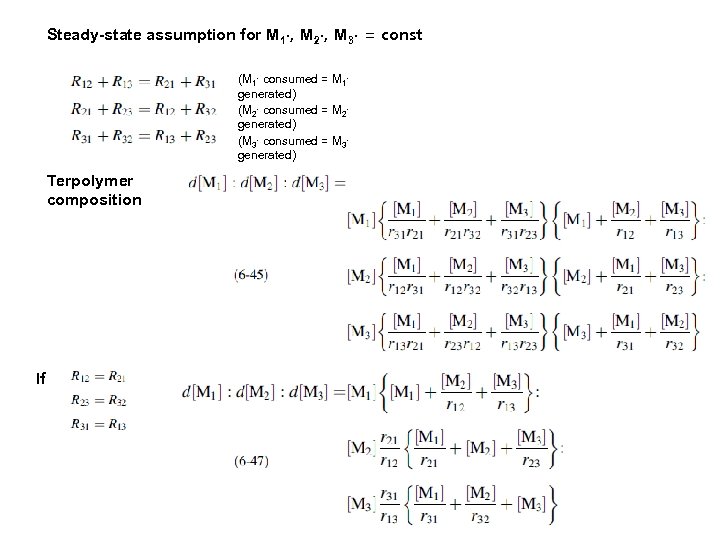
Steady-state assumption for M 1∙, M 2∙, M 3∙ = const (M 1∙ consumed = M 1∙ generated) (M 2∙ consumed = M 2∙ generated) (M 3∙ consumed = M 3∙ generated) Terpolymer composition If
6 -3 a Effect of Reaction Conditions 6 -3 a-1 Reaction Medium Solubility : r 1 & r 2 affected by reaction medium ex) bulk, emulsion, suspension, solvent M 1 = MMA, M 2 = N-vinyl carbazole in benzene, r 1 = 1. 80, r 2 = 0. 06 (copolymer is soluble) in Me. OH, r 1 = 0. 57, r 2 = 0. 75 (copolymer is microheterogeneous; N-vinyl carbazole is located preferentially around the copolymer propagating chain) Viscosity : M 1(St) & M 2(MMA) ~ bulk; less styrene (St has less mobility in the viscous medium)
p. H M 1 = acrylic acid, M 2 = acrylamide p. H = 2 ; r 1 = 0. 90, r 2 = 0. 25 p. H = 9 ; r 1 = 0. 30, r 2 = 0. 95 ∵ acrylic acid at p. H = 9 ⇒ acrylate ion (salt form) ~ lower reactivity due to the repulsion Polarity M 1 = polar monomer, M 2 = non-polar monomer in polar solvent, r 1↓ & r 2↑ (compared to bulk) ∵ high solubility of polar monomer in polar solvent Pressure P↑ ⇒ radical polymerization rate↑ ΔV 11 - ΔV 12 is small ⇒ r is not sensitive to pressure
6 -3 a-2 Temperature ⇒ r 1 & r 2 are relatively insensitive to temperature Ex) styrene-1, 3 -butadiene styrene–methyl methacrylate r 1 and r 2 0. 64 and 1. 4 at 5 o. C 0. 60 and 1. 8 at 45 o. C 0. 52 0. 59 and 0. 46 at 60 o and 0. 54 at 131 o. C. ∵ E (act. E) of radical propagation is small (radical is very reactive ~ don’t need much E) E 12 -E 11 ; even smaller than 10 Kcal/mol ※ T↑ ⇒ r → 1 ; selectivity↓ cf) initiation ⇒ generation of radical ~ need large E
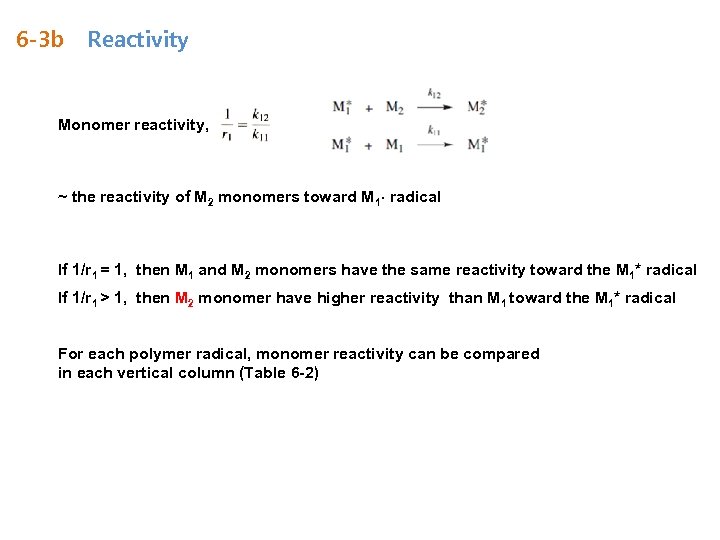
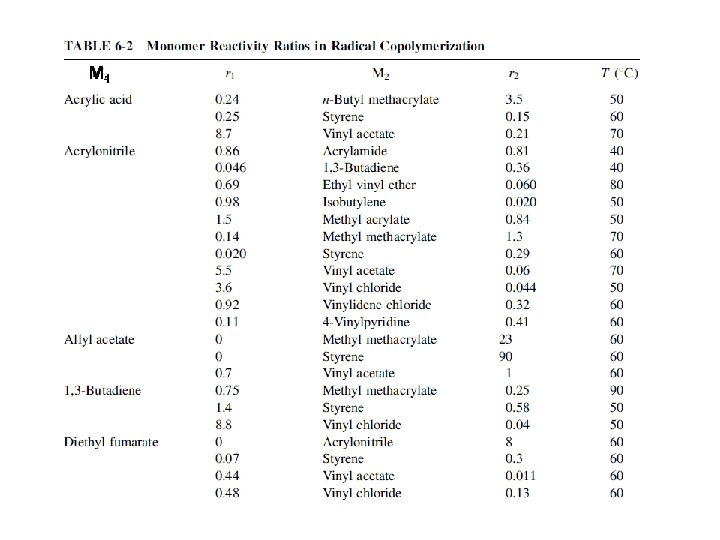
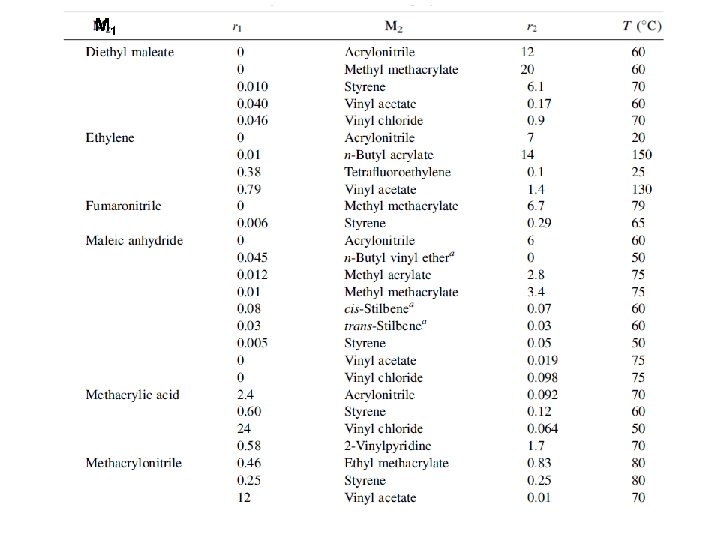
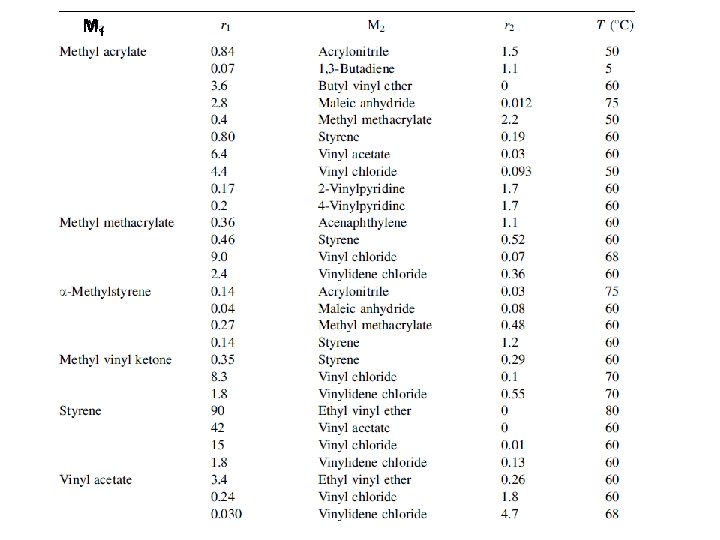
6 -3 b Reactivity Monomer reactivity, ~ the reactivity of M 2 monomers toward M 1∙ radical If 1/r 1 = 1, then M 1 and M 2 monomers have the same reactivity toward the M 1* radical If 1/r 1 > 1, then M 2 monomer have higher reactivity than M 1 toward the M 1* radical For each polymer radical, monomer reactivity can be compared in each vertical column (Table 6 -2)
M 1
M 1
M 1 6 -3 b Reactivity
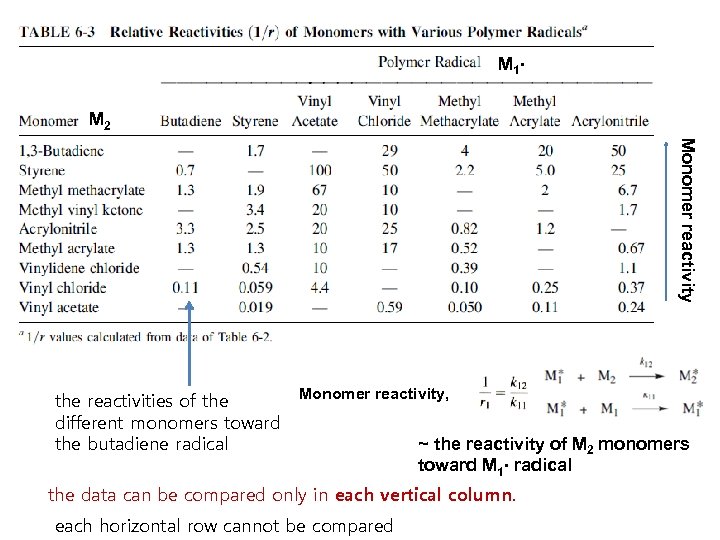
M 1 ∙ M 2 Monomer reactivity the reactivities of the different monomers toward the butadiene radical Monomer reactivity, ~ the reactivity of M 2 monomers toward M 1∙ radical the data can be compared only in each vertical column. each horizontal row cannot be compared
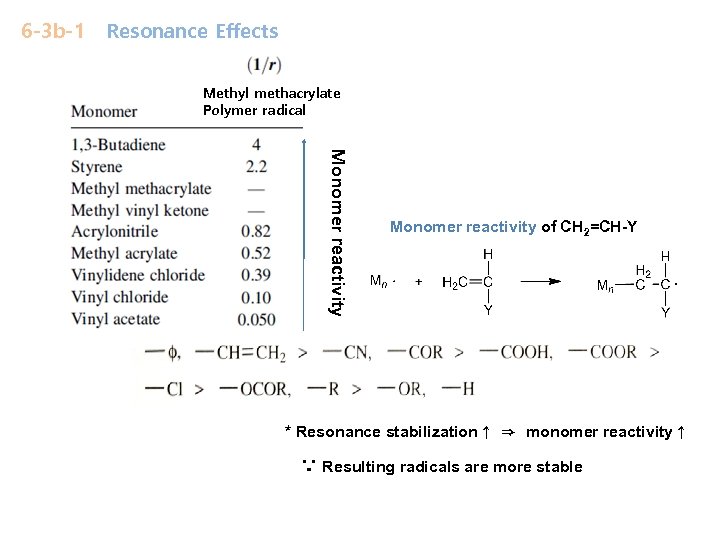
6 -3 b-1 Resonance Effects Methyl methacrylate Polymer radical Monomer reactivity of CH 2=CH-Y * Resonance stabilization↑ ⇒ monomer reactivity↑ ∵ Resulting radicals are more stable
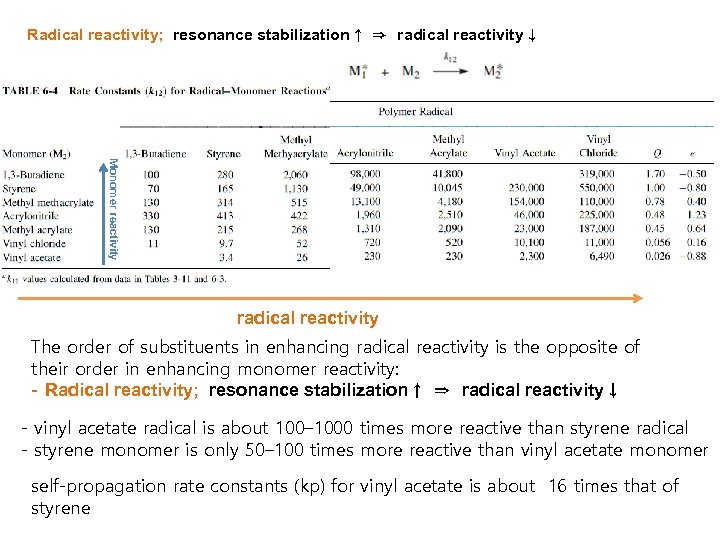
Radical reactivity; resonance stabilization↑ ⇒ radical reactivity↓ Monomer reactivity radical reactivity The order of substituents in enhancing radical reactivity is the opposite of their order in enhancing monomer reactivity: - Radical reactivity; resonance stabilization↑ ⇒ radical reactivity↓ - vinyl acetate radical is about 100– 1000 times more reactive than styrene radical - styrene monomer is only 50– 100 times more reactive than vinyl acetate monomer self-propagation rate constants (kp) for vinyl acetate is about 16 times that of styrene
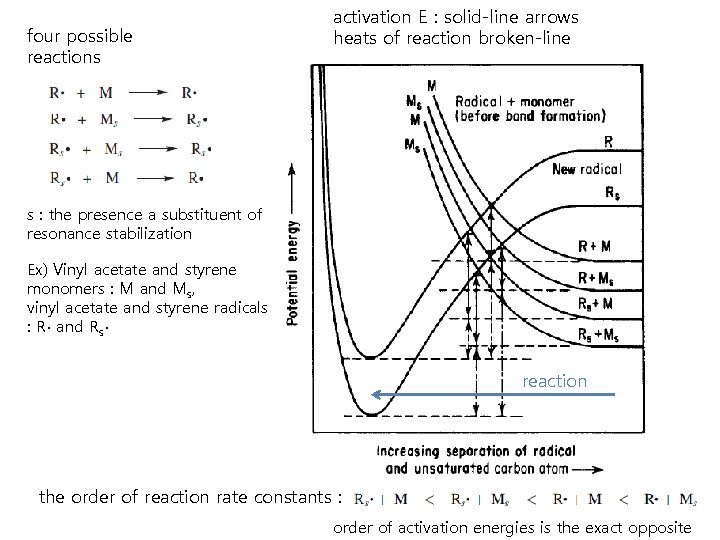
four possible reactions activation E : solid-line arrows heats of reaction broken-line s : the presence a substituent of resonance stabilization Ex) Vinyl acetate and styrene monomers : M and Ms, vinyl acetate and styrene radicals : R∙ and Rs∙ reaction the order of reaction rate constants : order of activation energies is the exact opposite
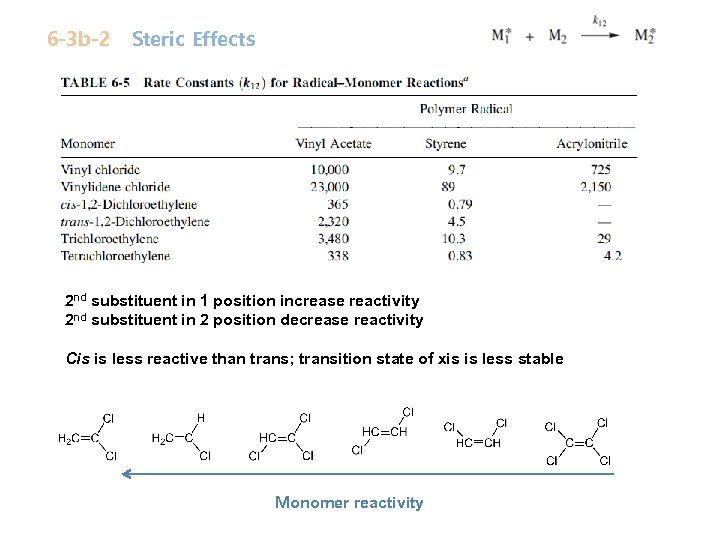
6 -3 b-2 Steric Effects 2 nd substituent in 1 position increase reactivity 2 nd substituent in 2 position decrease reactivity Cis is less reactive than trans; transition state of xis is less stable Monomer reactivity
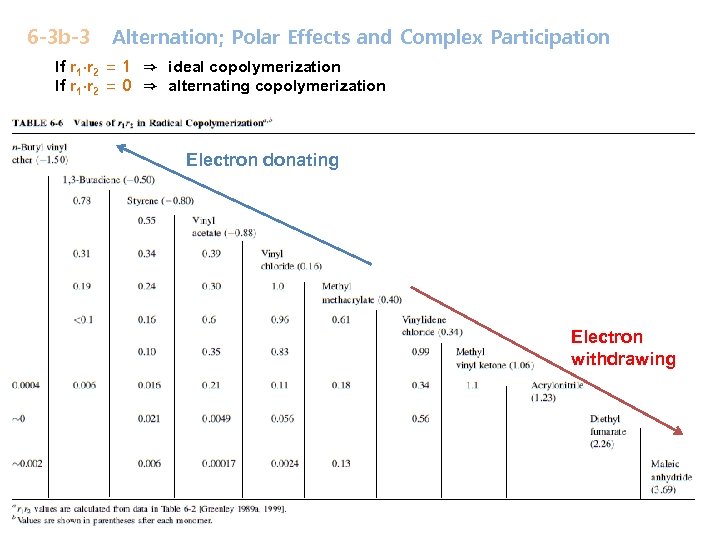
6 -3 b-3 Alternation; Polar Effects and Complex Participation If r 1∙r 2 = 1 ⇒ ideal copolymerization If r 1∙r 2 = 0 ⇒ alternating copolymerization Electron donating Electron withdrawing
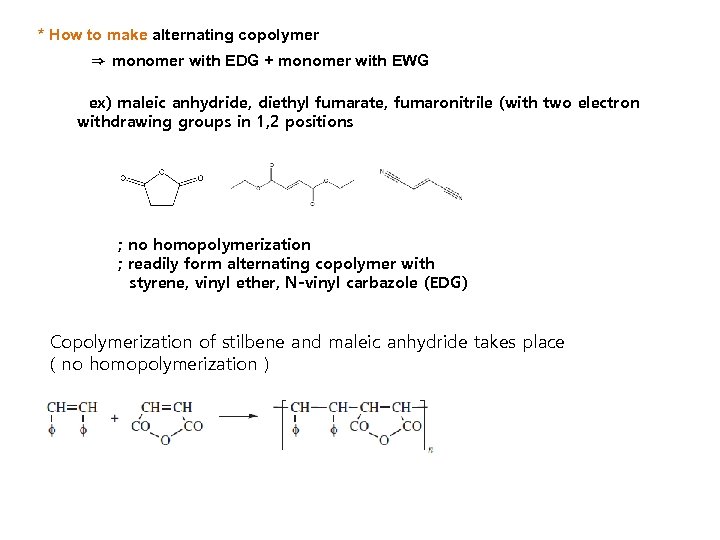
* How to make alternating copolymer ⇒ monomer with EDG + monomer with EWG ex) maleic anhydride, diethyl fumarate, fumaronitrile (with two electron withdrawing groups in 1, 2 positions ; no homopolymerization ; readily form alternating copolymer with styrene, vinyl ether, N-vinyl carbazole (EDG) Copolymerization of stilbene and maleic anhydride takes place ( no homopolymerization )
* Addition of Lewis acid (Zn. Cl 2, R 2 Al. Cl, Al. R 1. 5 Cl 1. 5) increase the tendency to form alternating copolymer ex) AN, MA, MMA, methyl vinyl ketone ; e- - acceptor monomer + propylene, isobutylene, VC, vinylidene chloride ; e- - donor monomer ⇒ without Lewis acid, no alternation! * (Lewis acid + e- - acceptor monomer) increase the electron accepting property
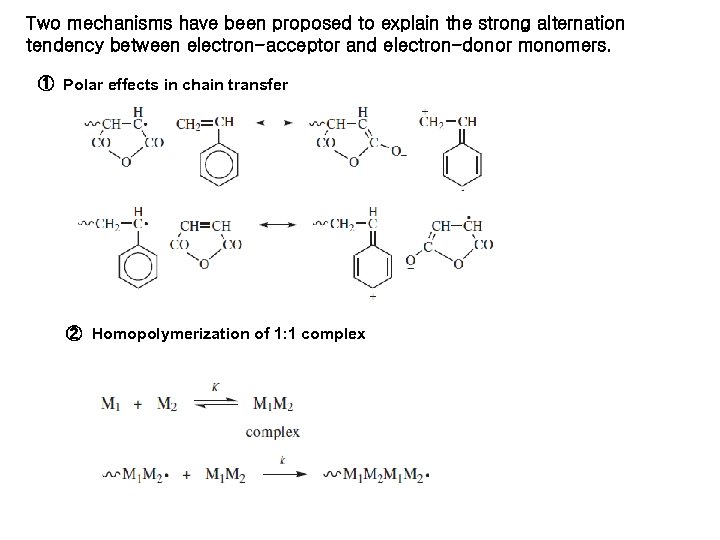
Two mechanisms have been proposed to explain the strong alternation tendency between electron-acceptor and electron-donor monomers. ① Polar effects in chain transfer ② Homopolymerization of 1: 1 complex
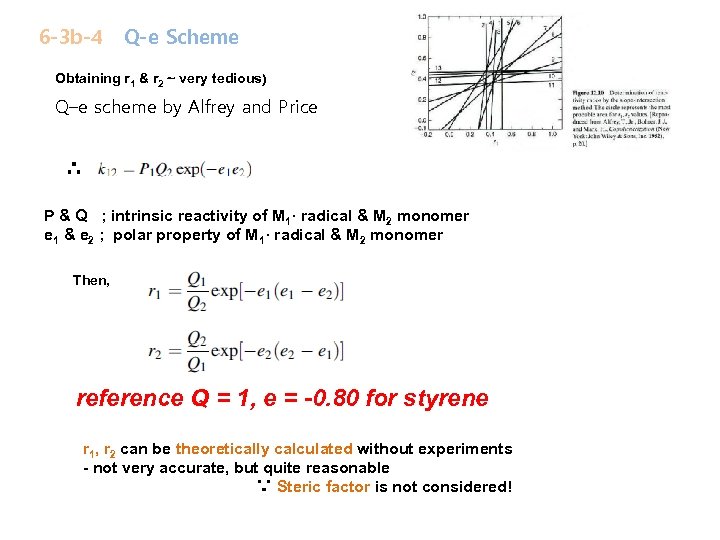
6 -3 b-4 Q-e Scheme Obtaining r 1 & r 2 ~ very tedious) Q–e scheme by Alfrey and Price ∴ P & Q ; intrinsic reactivity of M 1· radical & M 2 monomer e 1 & e 2 ; polar property of M 1· radical & M 2 monomer Then, reference Q = 1, e = -0. 80 for styrene r 1, r 2 can be theoretically calculated without experiments - not very accurate, but quite reasonable ∵ Steric factor is not considered!
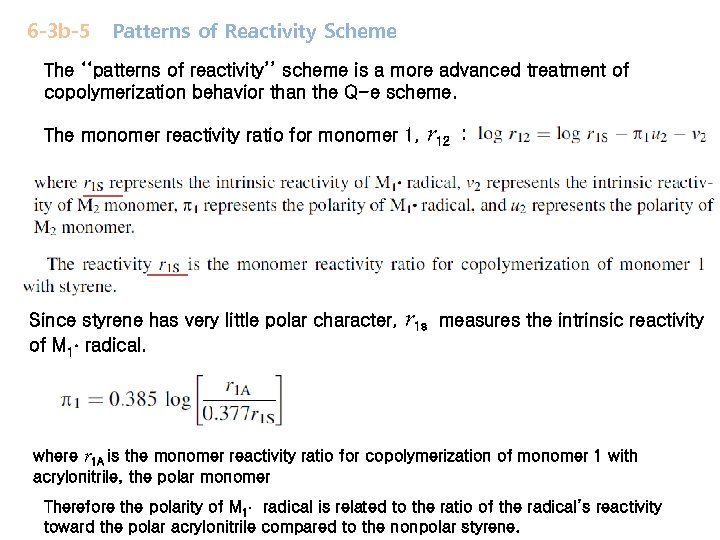
6 -3 b-5 Patterns of Reactivity Scheme The ‘‘patterns of reactivity’’ scheme is a more advanced treatment of copolymerization behavior than the Q-e scheme. The monomer reactivity ratio for monomer 1, Since styrene has very little polar character, of M 1 radical. r 12 r 1 s : measures the intrinsic reactivity where r 1 A is the monomer reactivity ratio for copolymerization of monomer 1 with acrylonitrile, the polar monomer Therefore the polarity of M 1 radical is related to the ratio of the radical’s reactivity toward the polar acrylonitrile compared to the nonpolar styrene.
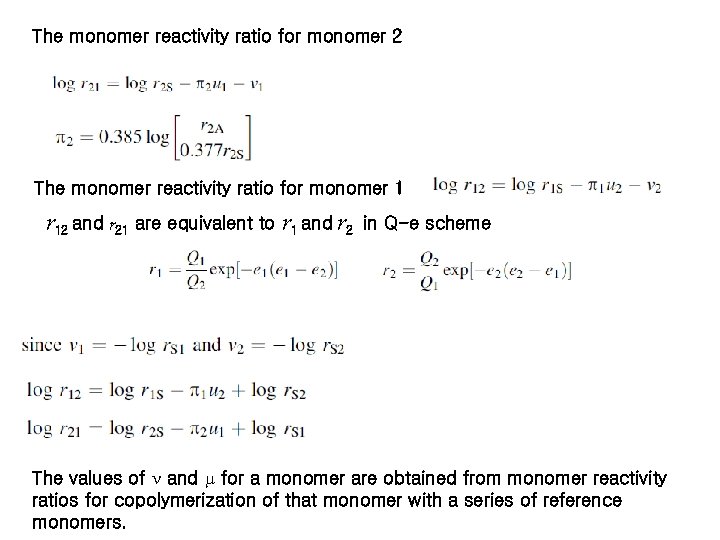
The monomer reactivity ratio for monomer 2 The monomer reactivity ratio for monomer 1 r 12 and r 21 are equivalent to r 1 and r 2 in Q-e scheme The values of and for a monomer are obtained from monomer reactivity ratios for copolymerization of that monomer with a series of reference monomers.
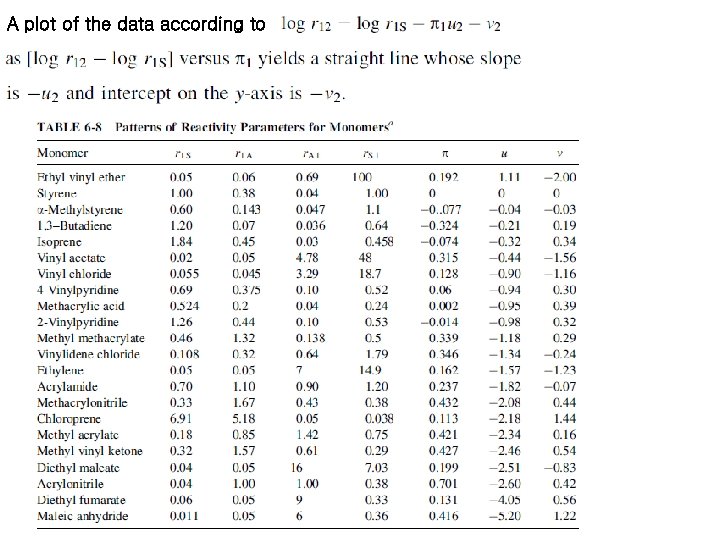
A plot of the data according to
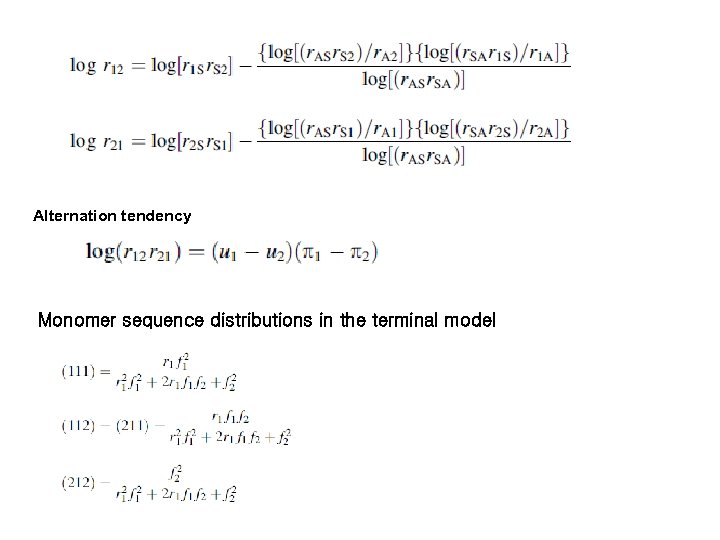
Alternation tendency Monomer sequence distributions in the terminal model
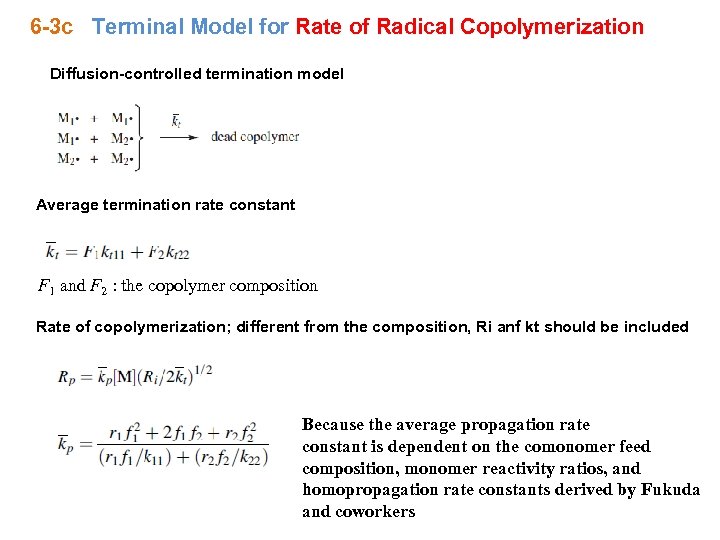
6 -3 c Terminal Model for Rate of Radical Copolymerization Diffusion-controlled termination model Average termination rate constant F 1 and F 2 : the copolymer composition Rate of copolymerization; different from the composition, Ri anf kt should be included Because the average propagation rate constant is dependent on the comonomer feed composition, monomer reactivity ratios, and homopropagation rate constants derived by Fukuda and coworkers
6 -4 Ionic Copolymerization ⇒ very limited # of monomers are possible compared with radical copolymerization * General rule ① r 1 · r 2 ~ 1 ② Similar reactivity of ionic species toward two radicals ∴ no alternation (see why alternation is possible in radical copolymerization) ③ Sensitivity of the r 1 · r 2 changed according to rxn condition change ⇒ in radical not much sensitive !
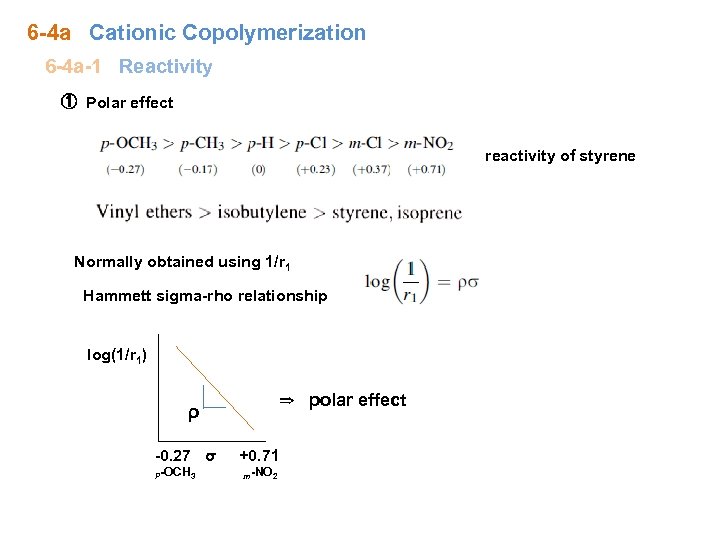
6 -4 a Cationic Copolymerization 6 -4 a-1 Reactivity ① Polar effect reactivity of styrene Normally obtained using 1/r 1 Hammett sigma-rho relationship log(1/r 1) ⇒ polar effect ρ -0. 27 σ P-OCH 3 +0. 71 m-NO 2
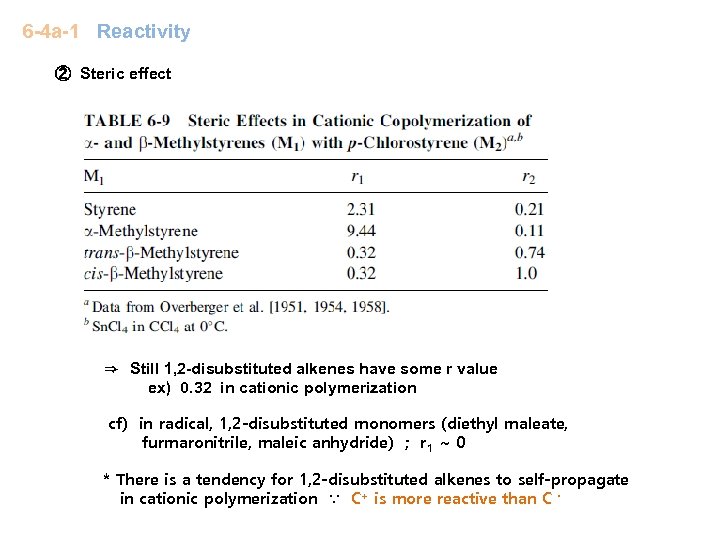
6 -4 a-1 Reactivity ② Steric effect ⇒ Still 1, 2 -disubstituted alkenes have some r value ex) 0. 32 in cationic polymerization cf) in radical, 1, 2 -disubstituted monomers (diethyl maleate, furmaronitrile, maleic anhydride) ; r 1 ~ 0 * There is a tendency for 1, 2 -disubstituted alkenes to self-propagate in cationic polymerization ∵ C+ is more reactive than C ·
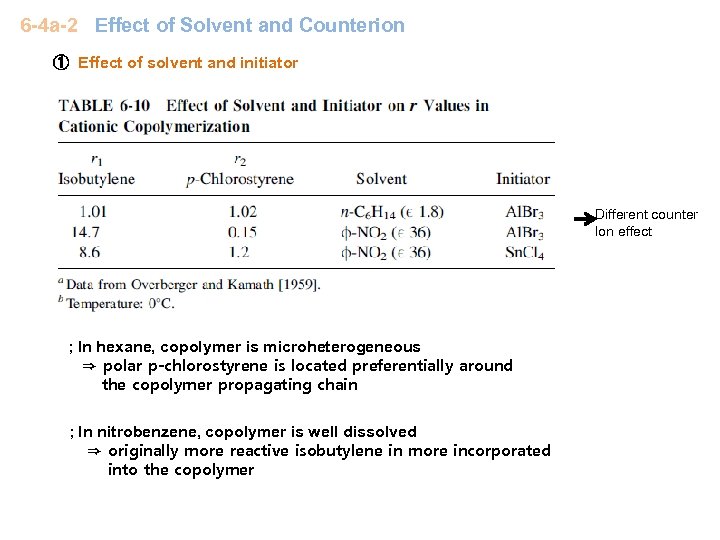
6 -4 a-2 Effect of Solvent and Counterion ① Effect of solvent and initiator Different counter Ion effect ; In hexane, copolymer is microheterogeneous ⇒ polar p-chlorostyrene is located preferentially around the copolymer propagating chain ; In nitrobenzene, copolymer is well dissolved ⇒ originally more reactive isobutylene in more incorporated into the copolymer
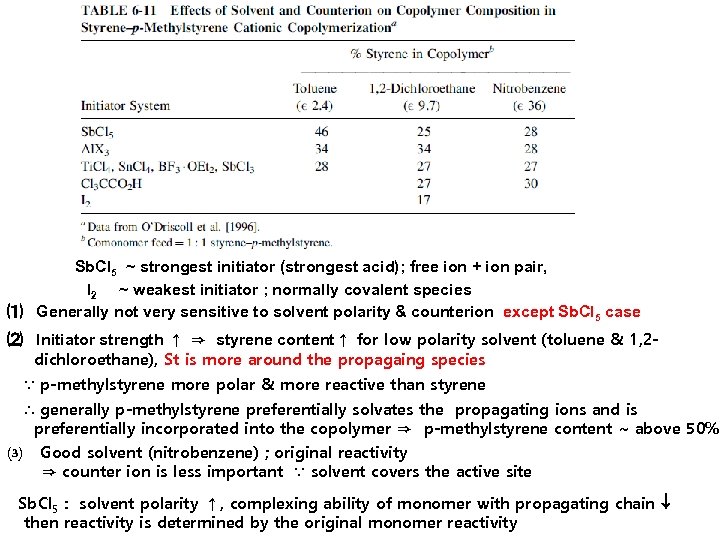
Sb. Cl 5 ~ strongest initiator (strongest acid); free ion + ion pair, l 2 ~ weakest initiator ; normally covalent species ⑴ Generally not very sensitive to solvent polarity & counterion except Sb. Cl 5 case ⑵ Initiator strength ↑ ⇒ styrene content↑ for low polarity solvent (toluene & 1, 2 dichloroethane), St is more around the propagaing species ∵ p-methylstyrene more polar & more reactive than styrene ∴ generally p-methylstyrene preferentially solvates the propagating ions and is preferentially incorporated into the copolymer ⇒ p-methylstyrene content ~ above 50% ⑶ Good solvent (nitrobenzene) ; original reactivity ⇒ counter ion is less important ∵ solvent covers the active site Sb. Cl 5 : solvent polarity ↑, complexing ability of monomer with propagating chain then reactivity is determined by the original monomer reactivity
6 -4 a-3 Effect of Temperature T↑⇒ r=1 ∵ r with smaller value increase faster with the increase of temperature No general trend ! ⇒ different amount of propagating species with different identities (free ion, ion pairs, covalent species)
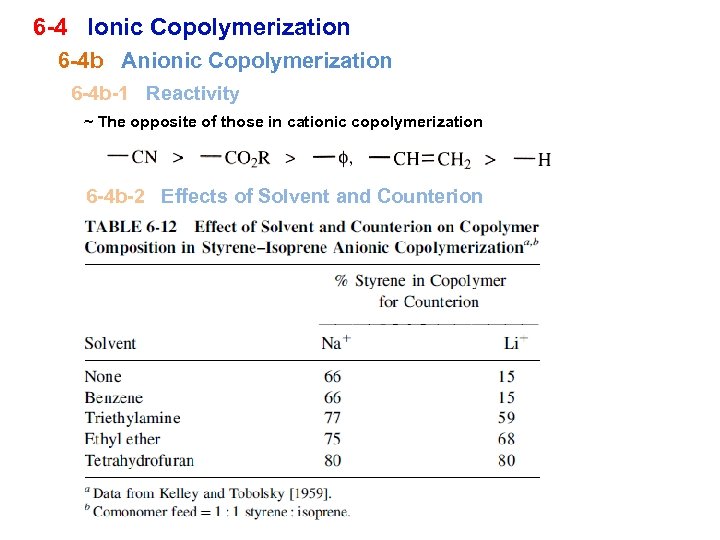
6 -4 Ionic Copolymerization 6 -4 b Anionic Copolymerization 6 -4 b-1 Reactivity ~ The opposite of those in cationic copolymerization 6 -4 b-2 Effects of Solvent and Counterion

① Lithium ion (tightly bound), large effect due to solvent ; poor solvent ~ less reactive, isoprene is rich ∵ isoprene is preferentially complexed with lithium good solvent ~ inherent reactivity more reactive styrene is rich in later copolymer ② Sodium ; larger, loosely bound ~ less effect due to solvent
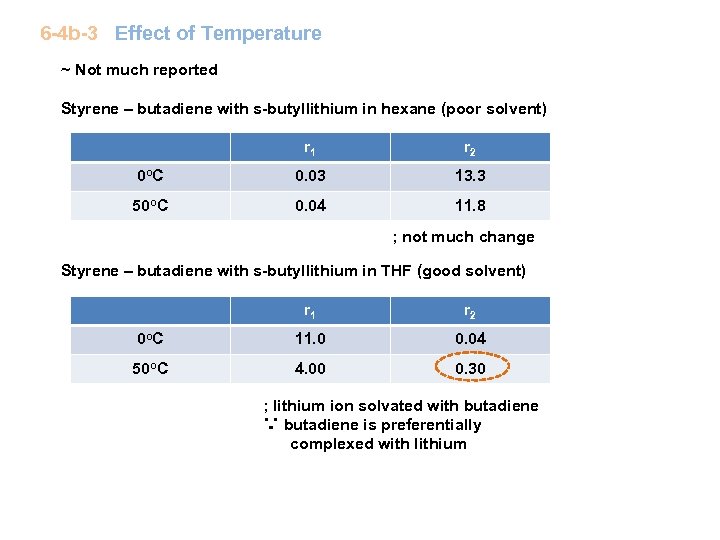
6 -4 b-3 Effect of Temperature ~ Not much reported Styrene – butadiene with s-butyllithium in hexane (poor solvent) r 1 r 2 0 o C 0. 03 13. 3 50 o. C 0. 04 11. 8 ; not much change Styrene – butadiene with s-butyllithium in THF (good solvent) r 1 r 2 0 o C 11. 0 0. 04 50 o. C 4. 00 0. 30 ; lithium ion solvated with butadiene ∵ butadiene is preferentially complexed with lithium
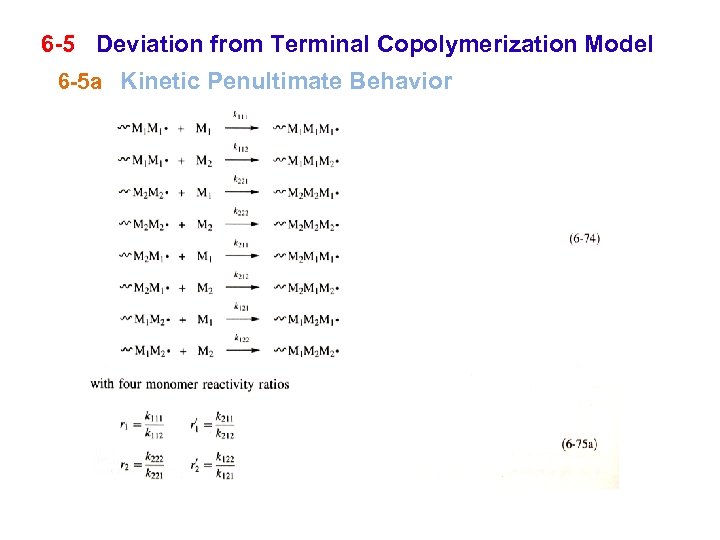
6 -5 Deviation from Terminal Copolymerization Model 6 -5 a Kinetic Penultimate Behavior
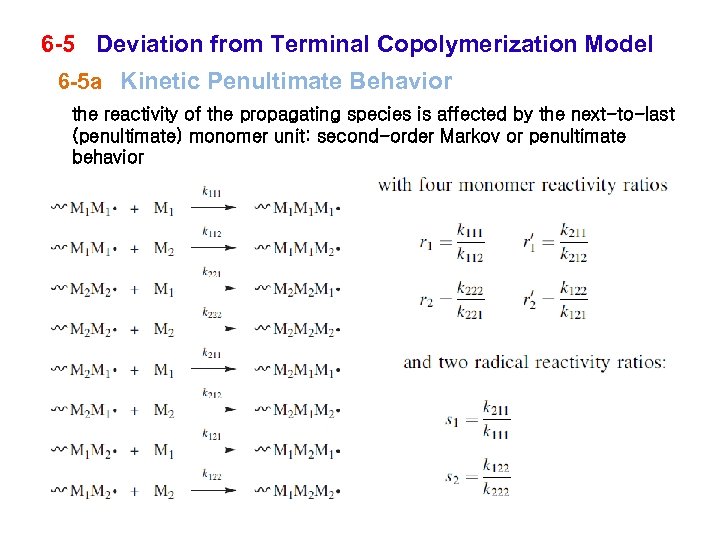
6 -5 Deviation from Terminal Copolymerization Model 6 -5 a Kinetic Penultimate Behavior the reactivity of the propagating species is affected by the next-to-last (penultimate) monomer unit: second-order Markov or penultimate behavior
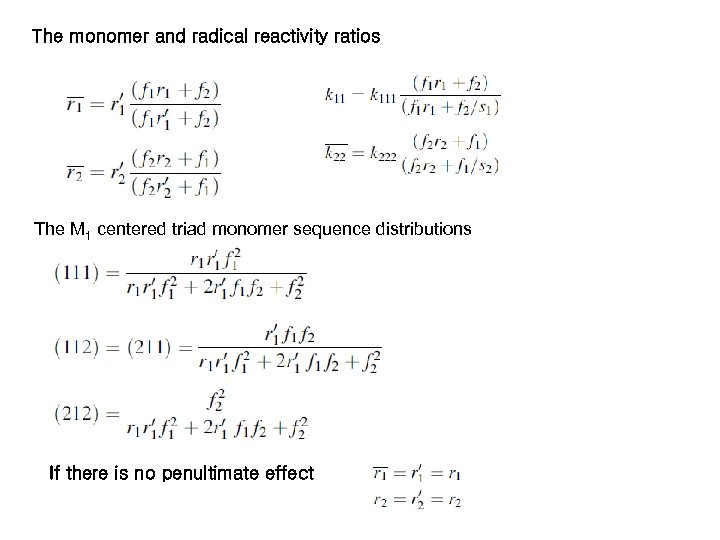
The monomer and radical reactivity ratios The M 1 centered triad monomer sequence distributions If there is no penultimate effect
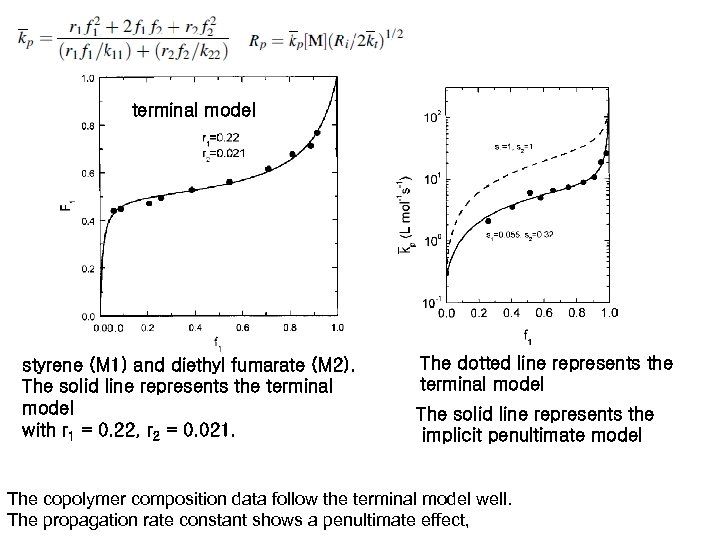
terminal model styrene (M 1) and diethyl fumarate (M 2). The solid line represents the terminal model with r 1 = 0. 22, r 2 = 0. 021. The dotted line represents the terminal model The solid line represents the implicit penultimate model The copolymer composition data follow the terminal model well. The propagation rate constant shows a penultimate effect,
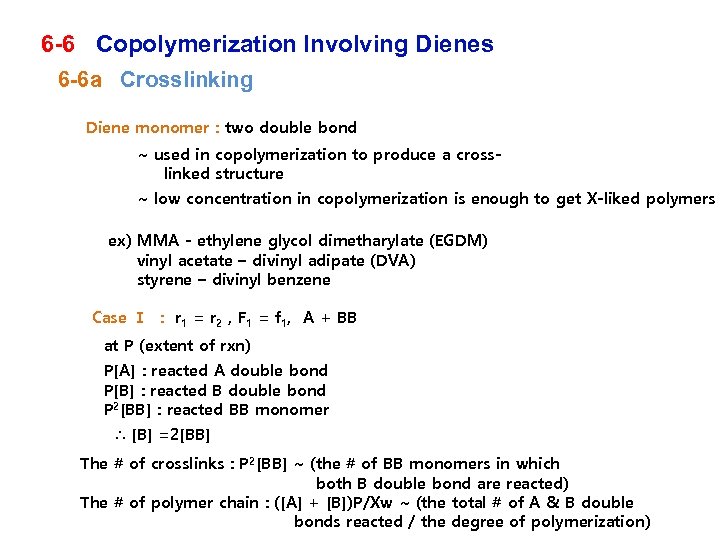
6 -6 Copolymerization Involving Dienes 6 -6 a Crosslinking Diene monomer : two double bond ~ used in copolymerization to produce a crosslinked structure ~ low concentration in copolymerization is enough to get X-liked polymers ex) MMA - ethylene glycol dimetharylate (EGDM) vinyl acetate – divinyl adipate (DVA) styrene – divinyl benzene Case Ⅰ : r 1 = r 2 , F 1 = f 1, A + BB at P (extent of rxn) P[A] : reacted A double bond P[B] : reacted B double bond P 2[BB] : reacted BB monomer ∴ [B] =2[BB] The # of crosslinks : P 2[BB] ~ (the # of BB monomers in which both B double bond are reacted) The # of polymer chain : ([A] + [B])P/Xw ~ (the total # of A & B double bonds reacted / the degree of polymerization)
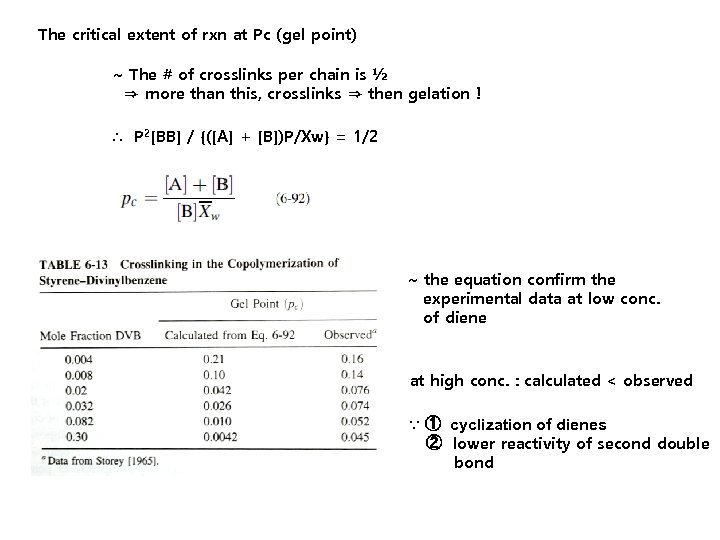
The critical extent of rxn at Pc (gel point) ~ The # of crosslinks per chain is ½ ⇒ more than this, crosslinks ⇒ then gelation ! ∴ P 2[BB] / {([A] + [B])P/Xw} = 1/2 ~ the equation confirm the experimental data at low conc. of diene at high conc. : calculated < observed ∵ ① cyclization of dienes ② lower reactivity of second double bond
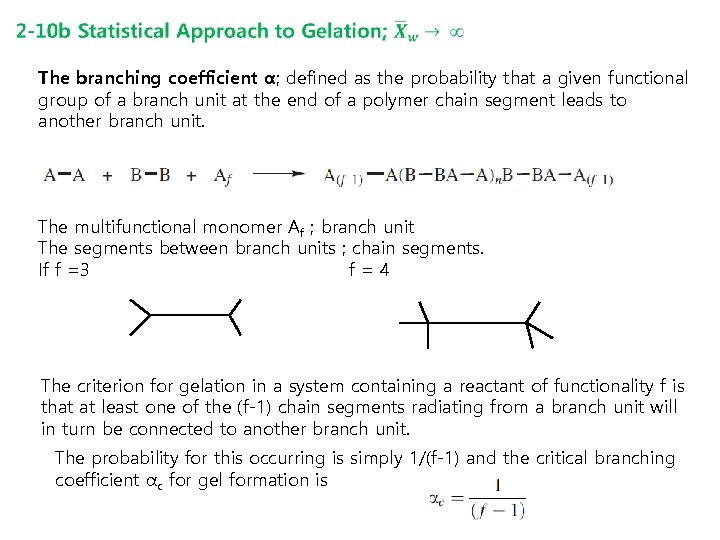
The branching coefficient α; defined as the probability that a given functional group of a branch unit at the end of a polymer chain segment leads to another branch unit. The multifunctional monomer Af ; branch unit The segments between branch units ; chain segments. If f =3 f=4 The criterion for gelation in a system containing a reactant of functionality f is that at least one of the (f-1) chain segments radiating from a branch unit will in turn be connected to another branch unit. The probability for this occurring is simply 1/(f-1) and the critical branching coefficient αc for gel formation is
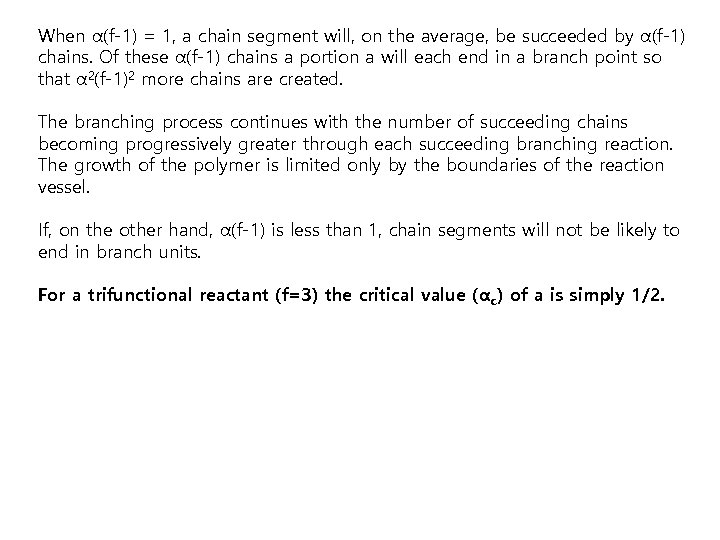
When α(f-1) = 1, a chain segment will, on the average, be succeeded by α(f-1) chains. Of these α(f-1) chains a portion a will each end in a branch point so that α 2(f-1)2 more chains are created. The branching process continues with the number of succeeding chains becoming progressively greater through each succeeding branching reaction. The growth of the polymer is limited only by the boundaries of the reaction vessel. If, on the other hand, α(f-1) is less than 1, chain segments will not be likely to end in branch units. For a trifunctional reactant (f=3) the critical value (αc) of a is simply 1/2.
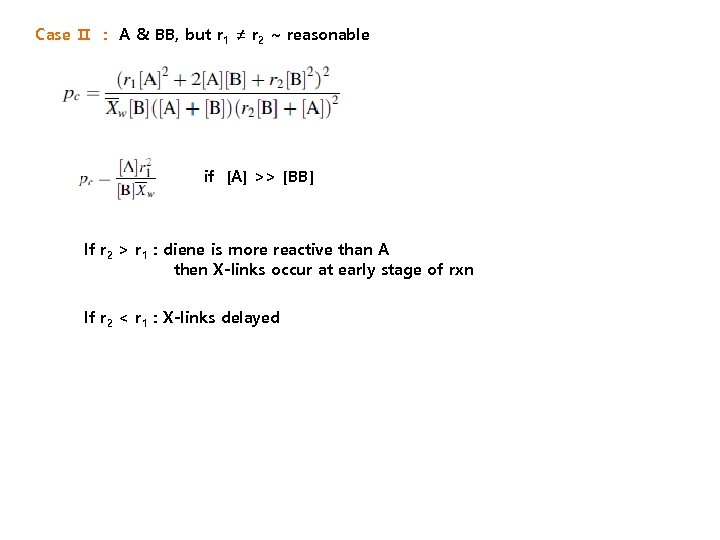
Case Ⅱ : A & BB, but r 1 ≠ r 2 ~ reasonable if [A] >> [BB] If r 2 > r 1 : diene is more reactive than A then X-links occur at early stage of rxn If r 2 < r 1 : X-links delayed
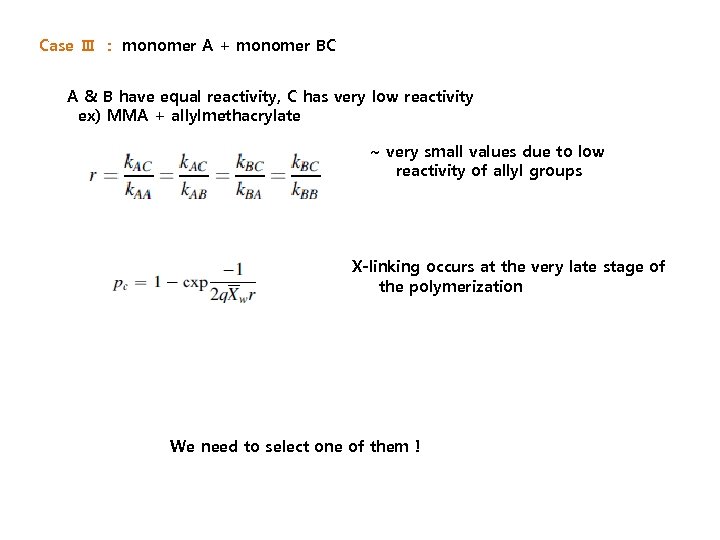
Case Ⅲ : monomer A + monomer BC A & B have equal reactivity, C has very low reactivity ex) MMA + allylmethacrylate ~ very small values due to low reactivity of allyl groups X-linking occurs at the very late stage of the polymerization We need to select one of them !
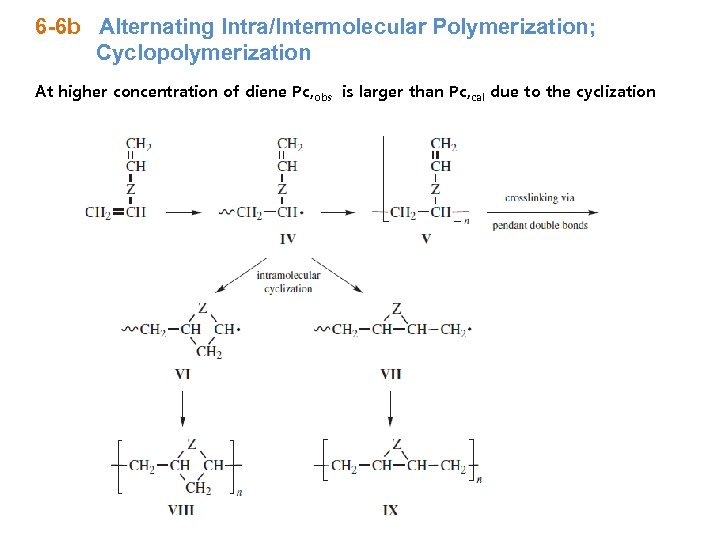
6 -6 b Alternating Intra/Intermolecular Polymerization; Cyclopolymerization At higher concentration of diene Pc, obs is larger than Pc, cal due to the cyclization
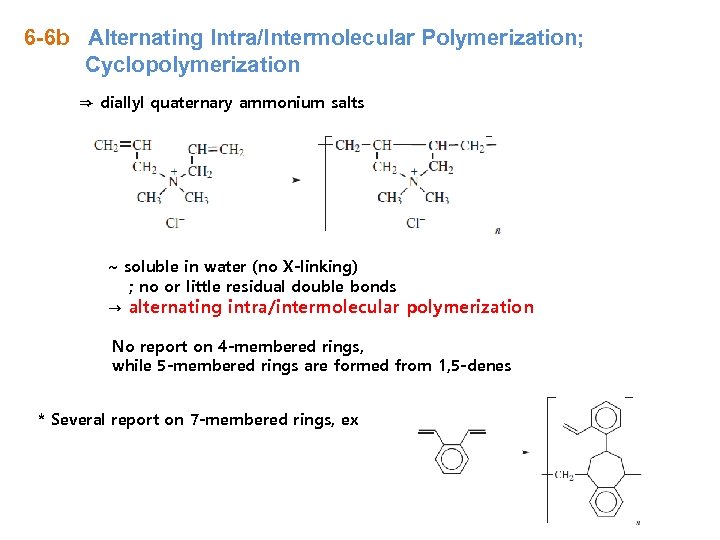
6 -6 b Alternating Intra/Intermolecular Polymerization; Cyclopolymerization ⇒ diallyl quaternary ammonium salts ~ soluble in water (no X-linking) ; no or little residual double bonds → alternating intra/intermolecular polymerization No report on 4 -membered rings, while 5 -membered rings are formed from 1, 5 -denes * Several report on 7 -membered rings, ex
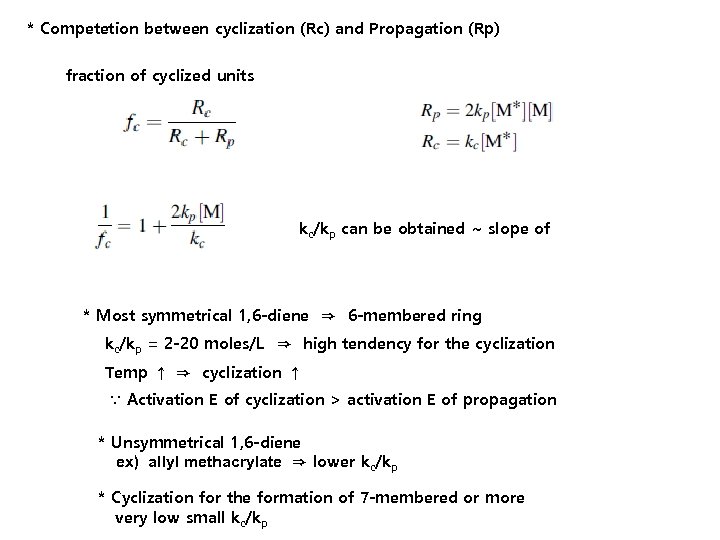
* Competetion between cyclization (Rc) and Propagation (Rp) fraction of cyclized units kc/kp can be obtained ~ slope of * Most symmetrical 1, 6 -diene ⇒ 6 -membered ring kc/kp = 2 -20 moles/L ⇒ high tendency for the cyclization Temp ↑ ⇒ cyclization ↑ ∵ Activation E of cyclization > activation E of propagation * Unsymmetrical 1, 6 -diene ex) allyl methacrylate ⇒ lower kc/kp * Cyclization for the formation of 7 -membered or more very low small kc/kp
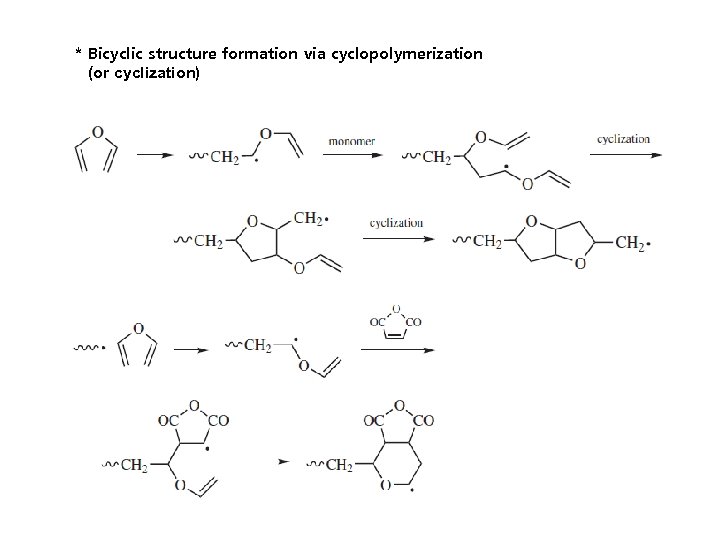
* Bicyclic structure formation via cyclopolymerization (or cyclization)
6 -6 c Interpenetrating Polymer Networks ⇒ Polymerization with cross-linking in the presence of another already cross-linked polymer ex) X-linked PU is swollen Add MMA, trimethylolpropane trimethacrylate, benzoyl peroxide ⇒ heating ⇒ combining properties of two different X-linked polymers
6 -7 Other Copolymerization Vinyl monomers + sulfur dioxide, SO 2 carbon monoxide, CO oxygen, O 2 no homopolymerization of SO 2, CO, O 2, while ⇒ Copolymerizations with alkenes are possible ⇒ ethylene + CO ⇒ alternating copolymer : polyketone The reaction with sulfur dioxide ① Ethylene, α-olefin, vinyl chloride, vinyl acetate : without strong EWG : 1: 1 alternating copolymer ② Styrene, butadiene : SO 2 unit is less than 50% in the copolymer : strong tendency for the ability of homopolymerization ③ AN, MMA : with strong EWG : no copolymerization
6 -8 Applications of Copolymerization 6 -8 a Styene ~ major styrene polymers are copolymer not homopolymer ; brittle i) SBR : styrene – butadiene copolymer ① 25% styrene + 75% butadiene ⇒ produced by emulsion polymerization ~ ~ similar to natural rubber (ex. Tensile strength) better ozone resistance & weatherability than natural rubber poorer resilience (탄성력) and greater heat buildup than natural rubber tire, hose, coated fabric, electrical insulator, belting ② 50~70% Styrene + 30~50% butadiene ~ latex paint ~ small amount of unsaturated carboxylic acid; backing material for carpet About 2 billion pounds per year are produced in the United States.

SBR은 Styrene과 Butadiene을 저온 유화 중합법으로 제조한 제품입니다. SBR 은 천연고무에 비해 품질이 균일하고 특히 내열성과 내마모성이 우수하며, 타 이어, 신발, 산업용품 등의 재료로 널리 사용되고 있습니다.

ii) SAN : styrene – AN ⇒ 10~40% AN content AN : intermolecular force, solvent resistence, permeability, improved tensile strength, raise upper use temperature ~ similar impact resistance Applications: houseware (refrigerator shelves and drawers, coffee mugs), packaging (bottle closures and sprayers), furniture (chair backs and shells), electronics (battery cases, cassette parts). About 200 million pounds per year are produced in US.
iii) ABS : Acrylonitrile–butadiene–styrene polymers - combine the properties of SAN with greatly improved resistance to impact. - produced by emulsion, suspension, or bulk copolymerization of styrene–acrylonitrile in the presence of a rubber. - About 2 billion pounds per year of ABS are produced in the United States. - Applications housewares (refrigerator doors, sewing machine and hair-dryer housings, luggage, furniture frames, margarine tubs) housing and construction (pipe, conduit, fittings, bathtubs and shower stalls), transportation (automotive instrument panels, light housings, grilles) business machine housings (telephone, calculator), recreation (golf clubs, boat hulls, camper top or shell, snowmobile shroud).
iv) HIPS (high-impact polystyrene) and GPPS : general purpose polystyrene ~ rubber (normally(*1, 3 -butdiene))+ styrene ; polymerization in the presence of rubber ~ cheaper than ABS, similar application
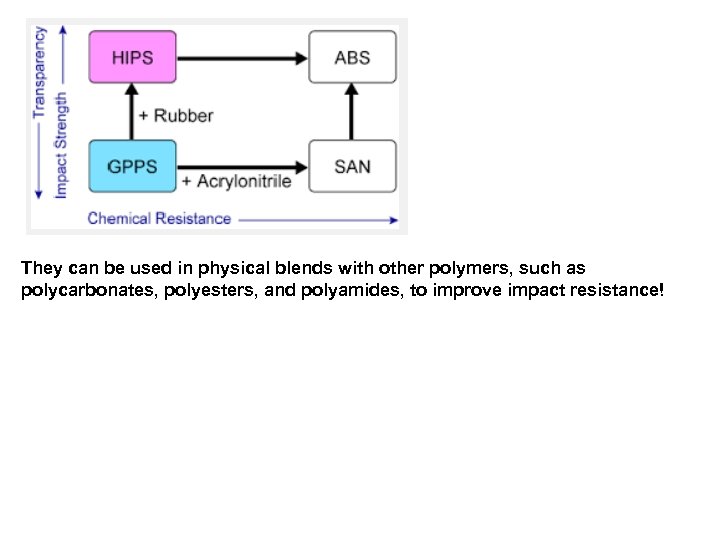
They can be used in physical blends with other polymers, such as polycarbonates, polyesters, and polyamides, to improve impact resistance!

6 -8 b Ethylene ~ LDPE (radical), HDPE (coordination) i) EVA : ethylene - vinyl acetate copolymer more than 1 billion pounds in the United States ~ VA ↑ ⇒ crystallinity ↓, Tm ↓, chemical resistance ↓ optical clarity ↑, impact and stress crack resistence ↑ flexibility ↑, adhesion ↑ ~ 2 -18% VA : clean rap, frozen food package, coating on aluminum foil ~ 20 -30% VA : blends with parafin wax carpet backing, hot-melt adhesive
iv) EVOH : Hydrolysis of EVA copolymers yields ethylene–vinyl alcohol copolymers ~ excellent barrier polymer ~ food container The poor moisture resistance of EVOH is overcome by coating, coextrusion, and lamination with other substrates.
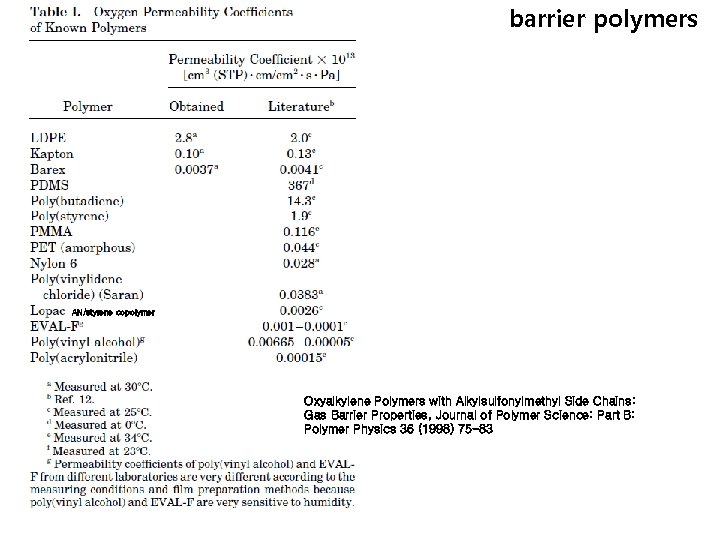
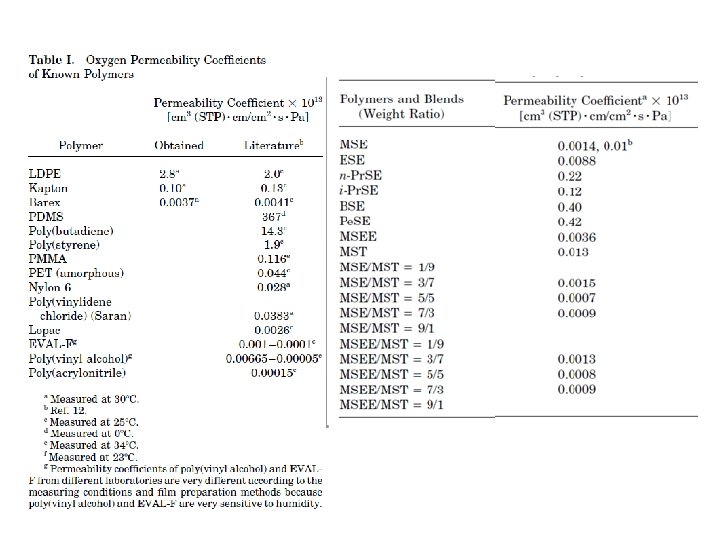
barrier polymers AN/styrene copolymer Oxyalkylene Polymers with Alkylsulfonylmethyl Side Chains: Gas Barrier Properties, Journal of Polymer Science: Part B: Polymer Physics 36 (1998) 75 -83
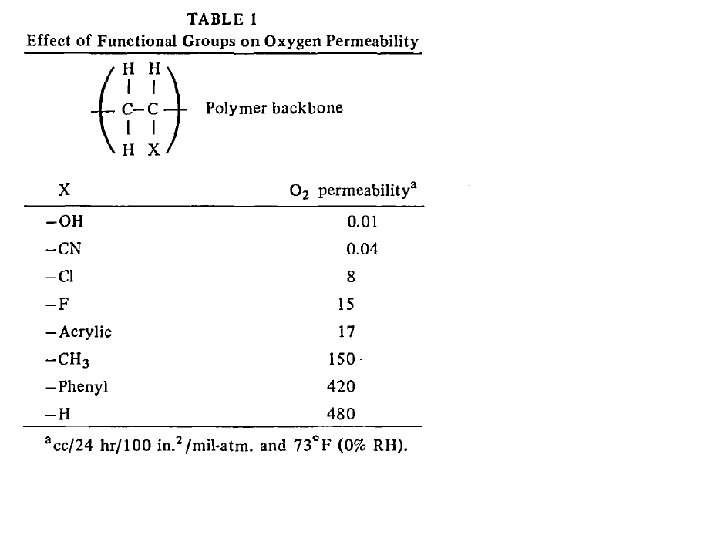
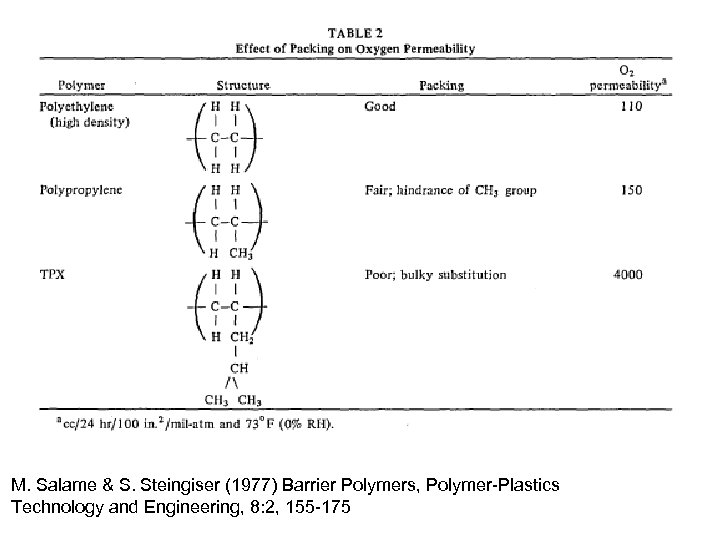
M. Salame & S. Steingiser (1977) Barrier Polymers, Polymer-Plastics Technology and Engineering, 8: 2, 155 -175
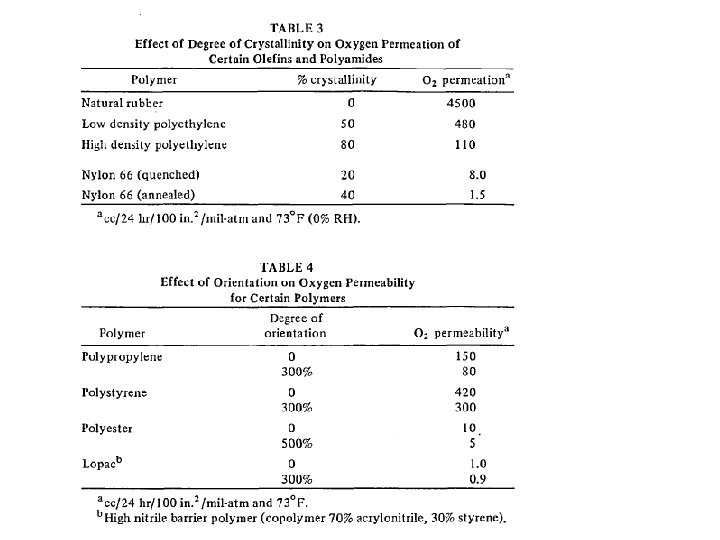
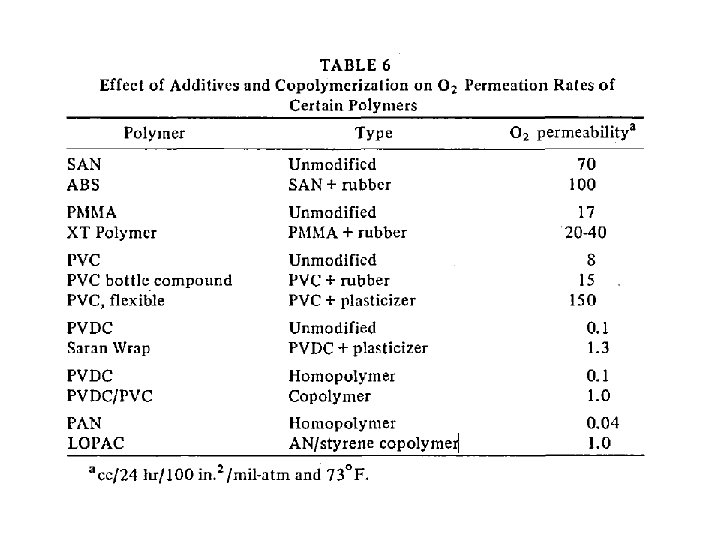
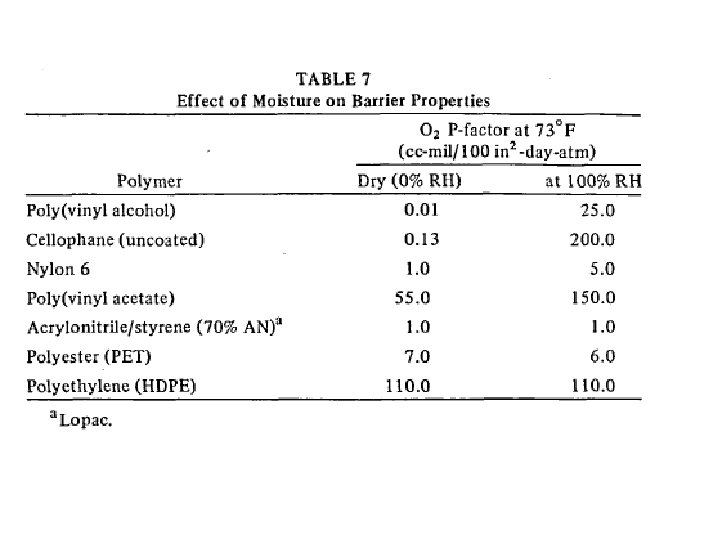
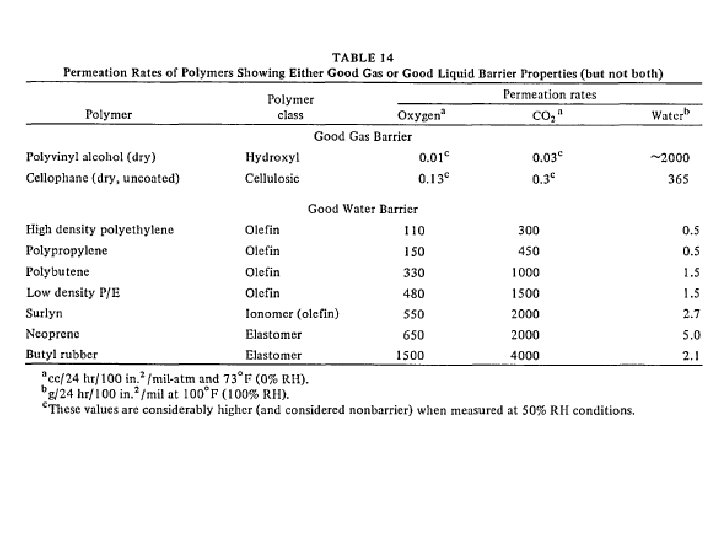
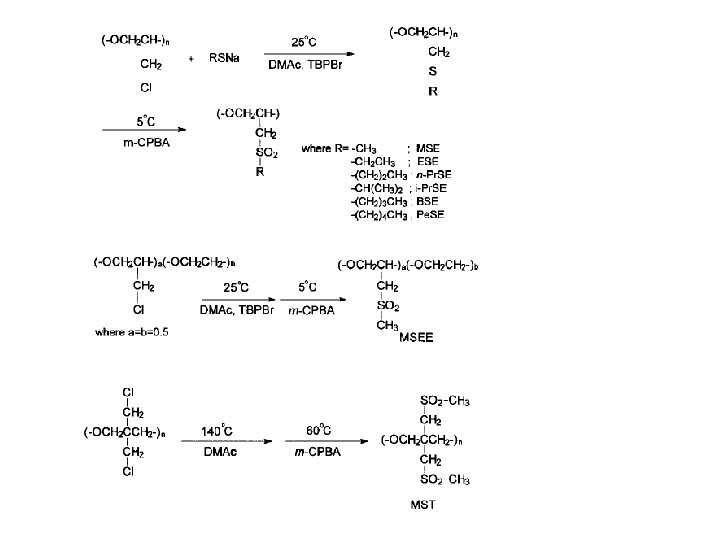
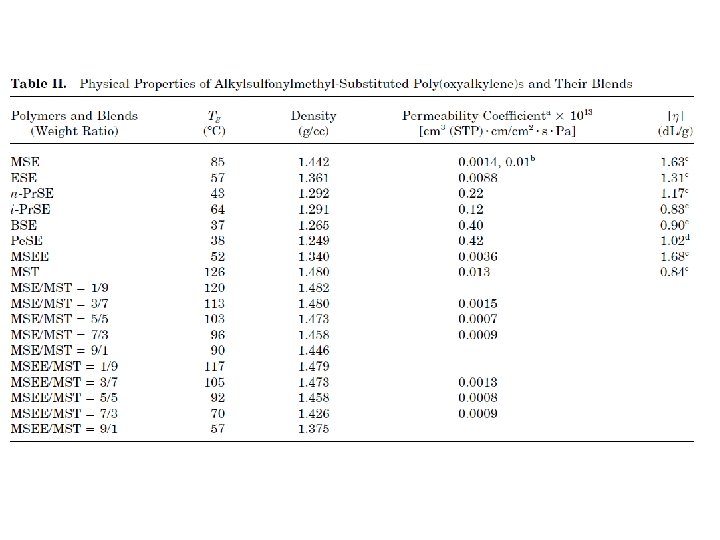
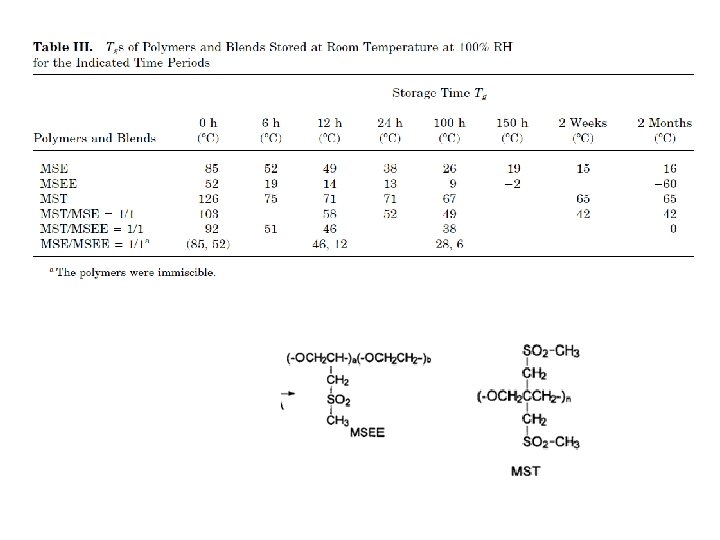
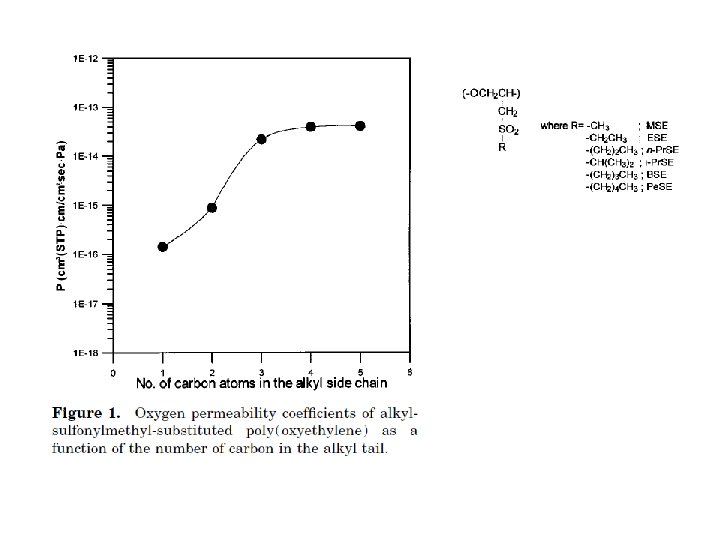
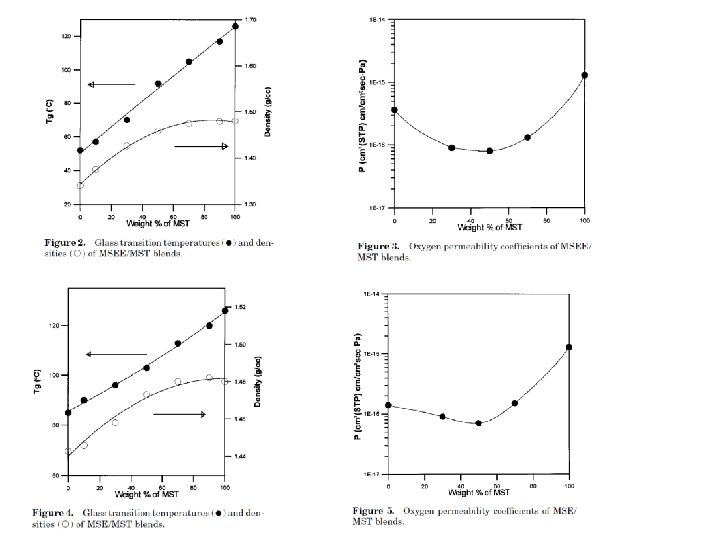
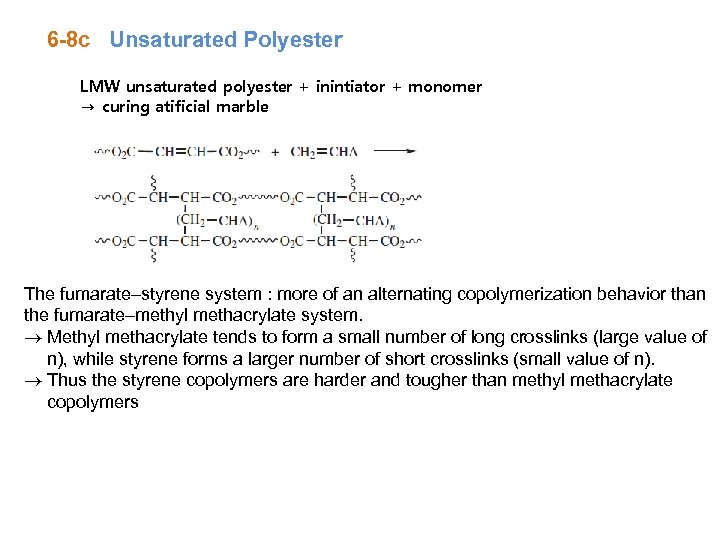
6 -8 c Unsaturated Polyester LMW unsaturated polyester + inintiator + monomer → curing atificial marble The fumarate–styrene system : more of an alternating copolymerization behavior than the fumarate–methyl methacrylate system. ® Methyl methacrylate tends to form a small number of long crosslinks (large value of n), while styrene forms a larger number of short crosslinks (small value of n). ® Thus the styrene copolymers are harder and tougher than methyl methacrylate copolymers
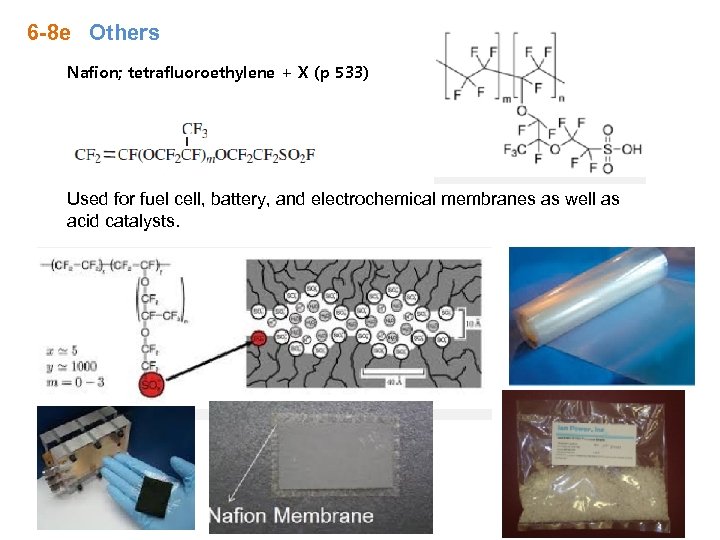
6 -8 e Others Nafion; tetrafluoroethylene + X (p 533) Used for fuel cell, battery, and electrochemical membranes as well as acid catalysts.

Nitrile rubber (NBR): a copolymer of 1, 3 -butadiene with 20– 40% acrylonitrile, - oil resistance. - More than 150 million pounds are produced annually in the United States. - Applications: fuel tanks, gasoline hoses, and creamery equipment.

Nitrile resin : copolymerizing acrylonitrile with about 20– 30% styrene or methyl methacrylate in the presence of NBR or SBR rubber -a blend of the graft terpolymer and homo-copolymer. Applications: extruded and blow-molded containers for household, automotive, and non beverage foods (spices, vitamins, candy).
69d3d02cce37f3ce837b440ba7769117.ppt