826020baac2415cb946a3a245815f8cc.ppt
- Количество слайдов: 68
Chapter 5 - Chemical Bonding
1 Chemical compounds A compound is a substance that is made up of two ore more elements combined together chemically E. G. hydrogen gas burning in oxygen will result in formation of a compound of water. 2 H 2(g) + O 2 (g) 2 H 20(l) Compounds can be broken down into their elements. If an electric current is passed through water the compound splits into its elements of hydrogen and oxygen.

2. The octet rule • Elements will try to lose gain or share electrons to achieve 8 electrons in their outer shell. The octet rule states that when bonding occurs atoms tend to reach an electron arrangement with 8 electrons in the outermost energy level • This outermost energy level is also known as the valence shell.
Exceptions to the Octet Rule 1. Transition metals – they can have more or fewer than 8 electrons in outermost energy level 2. Elements near helium – tend to have 2 electrons in outer energy level rather than 8 in the noble gases.
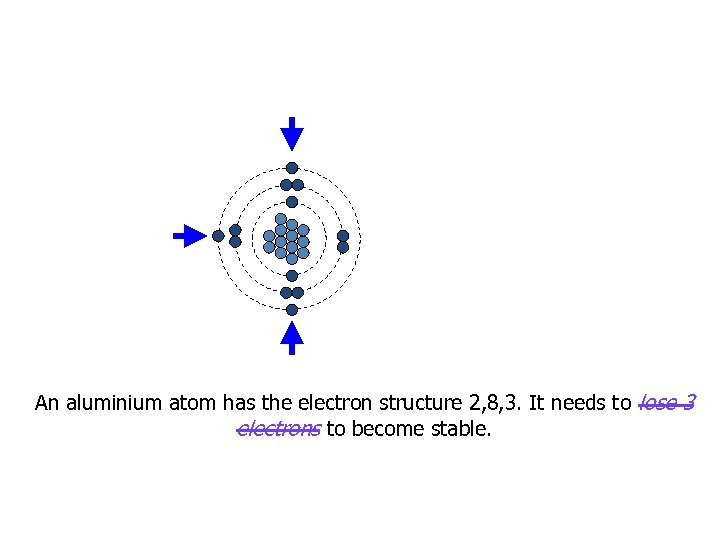
An aluminium atom has the electron structure 2, 8, 3. It needs to lose 3 electrons to become stable.
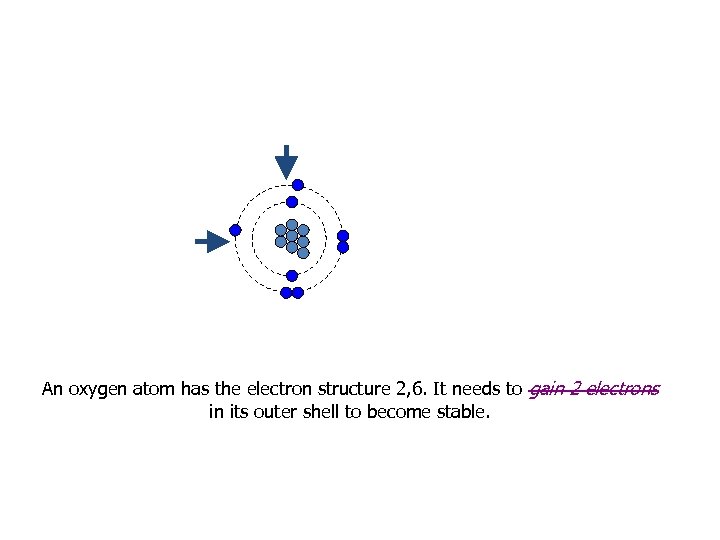
An oxygen atom has the electron structure 2, 6. It needs to gain 2 electrons in its outer shell to become stable.
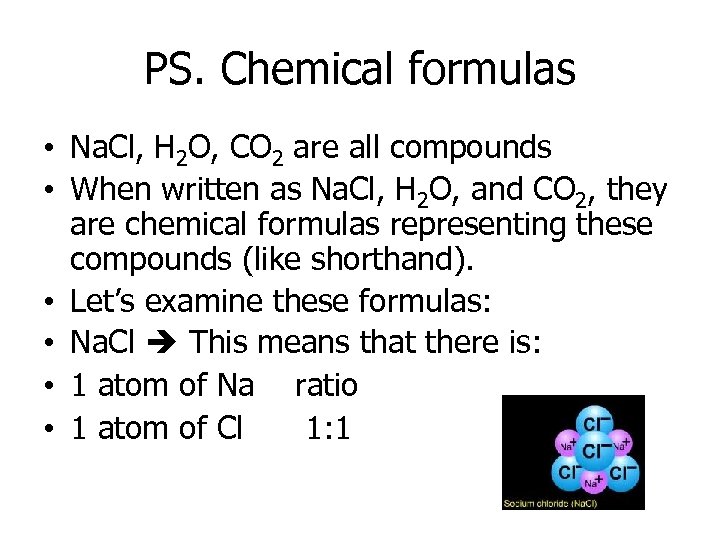
PS. Chemical formulas • Na. Cl, H 2 O, CO 2 are all compounds • When written as Na. Cl, H 2 O, and CO 2, they are chemical formulas representing these compounds (like shorthand). • Let’s examine these formulas: • Na. Cl This means that there is: • 1 atom of Na ratio • 1 atom of Cl 1: 1
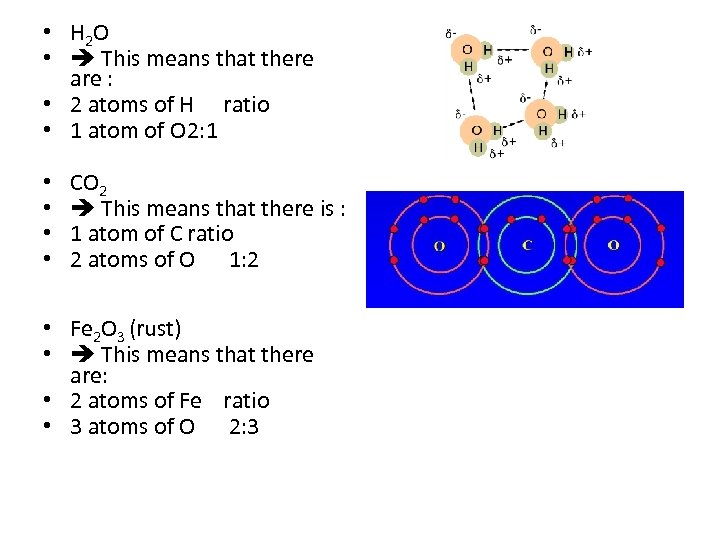
• H 2 O • This means that there are : • 2 atoms of H ratio • 1 atom of O 2: 1 • • CO 2 This means that there is : 1 atom of C ratio 2 atoms of O 1: 2 • Fe 2 O 3 (rust) • This means that there are: • 2 atoms of Fe ratio • 3 atoms of O 2: 3
3 Ionic Bonding An Ionic bond is the force of attraction between oppositely charged ions in a compound. Ionic bonds are always formed by the complete transfer of electrons from one atom to another An ion is a charged atom or groups of atom E. G. Na has 11 e- (E. C. = 2, 8, 1. ) When Na gives away this one e- it now has more protons than electrons so it has an overall positive charge. • Ionic bonds generally form between metals and non-metals.

• Elements in group one lose one e- to from an ion with a positive charge • Group 2 – ion with 2 positive charges etc Note: Positive ions are also called cations Negative ions are called anions
How are ionic bonds formed? https: //www. youtube. com/watch? v=5 IJq. PU 11 n g. Y
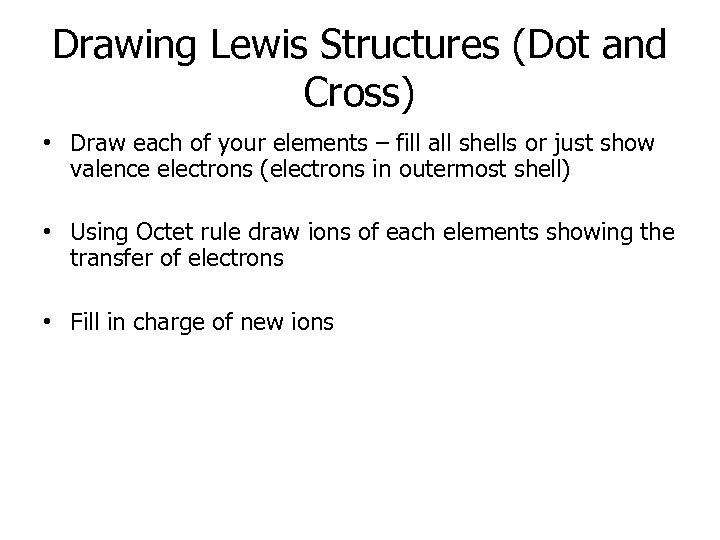
Drawing Lewis Structures (Dot and Cross) • Draw each of your elements – fill all shells or just show valence electrons (electrons in outermost shell) • Using Octet rule draw ions of each elements showing the transfer of electrons • Fill in charge of new ions
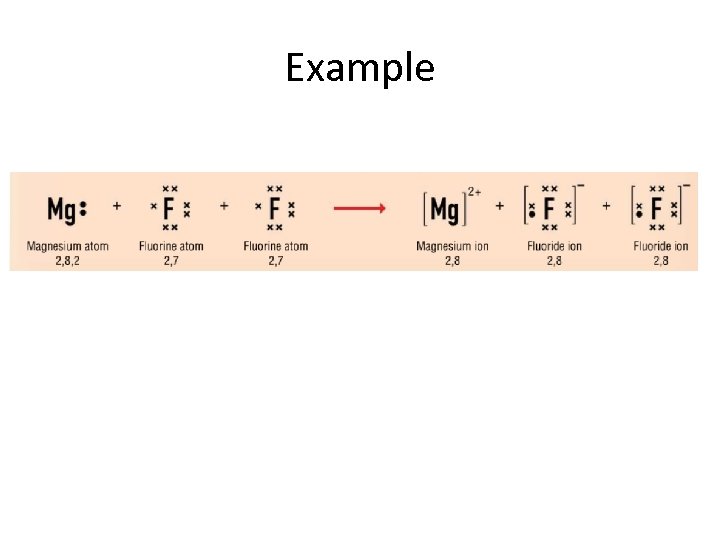
Example
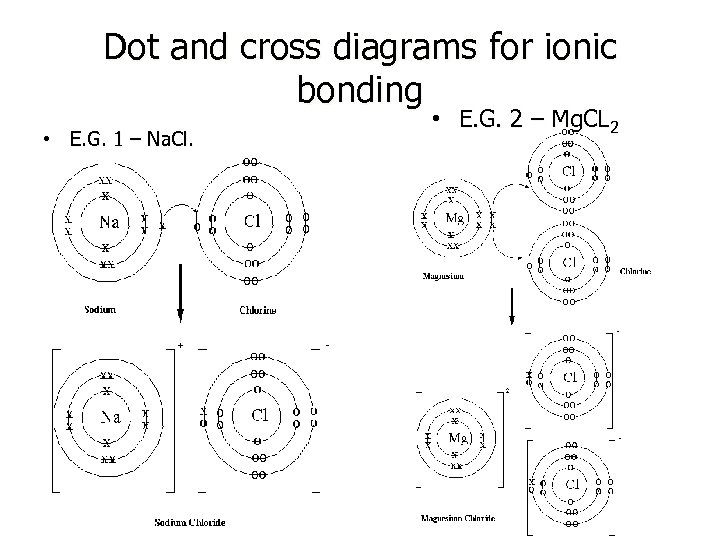
Dot and cross diagrams for ionic bonding • E. G. 1 – Na. Cl. • E. G. 2 – Mg. CL 2

Questions Draw dot and cross diagrams for the following • Li. F • Na 2 O • Mg. O • Al. Cl 3 • Mg. Cl 2
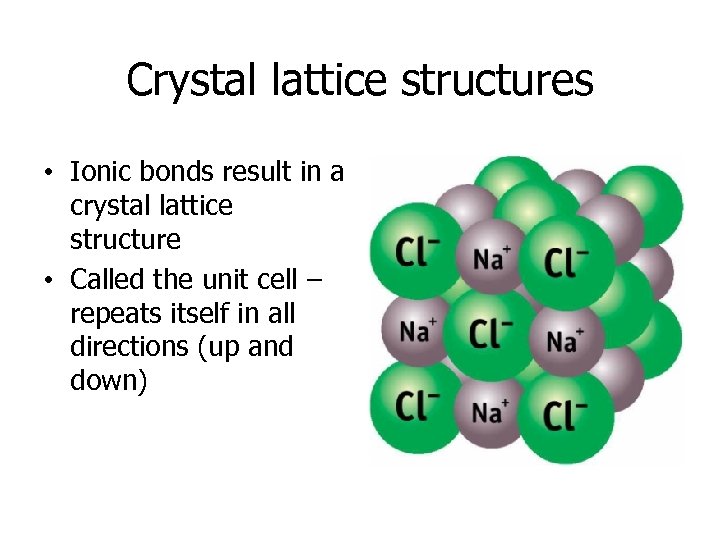
Crystal lattice structures • Ionic bonds result in a crystal lattice structure • Called the unit cell – repeats itself in all directions (up and down)

4. a Writing Formulas of Ionic Compounds • Usually metal and non-metal • Metals tendency to lose electrons and non metals gain • Ionic compound is neutral overall – need same no of positive and negative charges
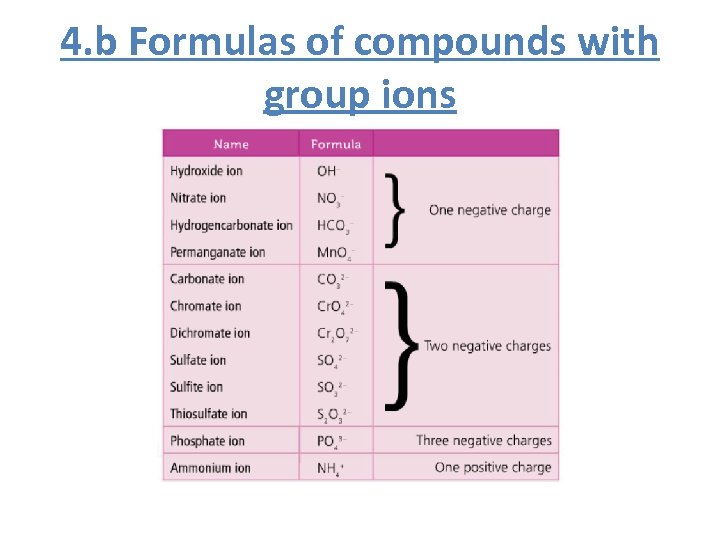
4. b Formulas of compounds with group ions

Quiz • Nitrate ion • NO 3 - • Sulfate ion • SO 42 - • Phosphate ion • PO 43 • NH 4+ • Ammonium ion
4. c Formulas containing transition metals It is not possible to predict the charges of -block ions They have variable valency d
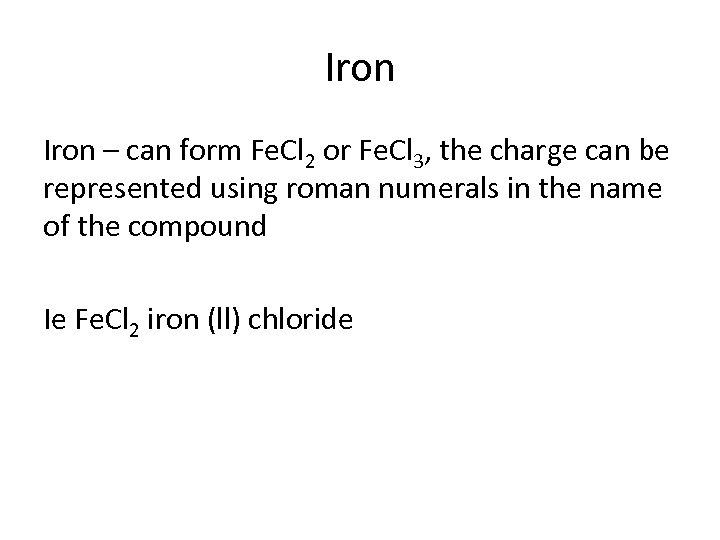
Iron – can form Fe. Cl 2 or Fe. Cl 3, the charge can be represented using roman numerals in the name of the compound Ie Fe. Cl 2 iron (ll) chloride

Copper Cu 2 O or Cu. O • Trend is also seen in manganese and chromium

Naming these compounds • -ide compound with 2 elements • -ate contains ocygen and 2 other elements
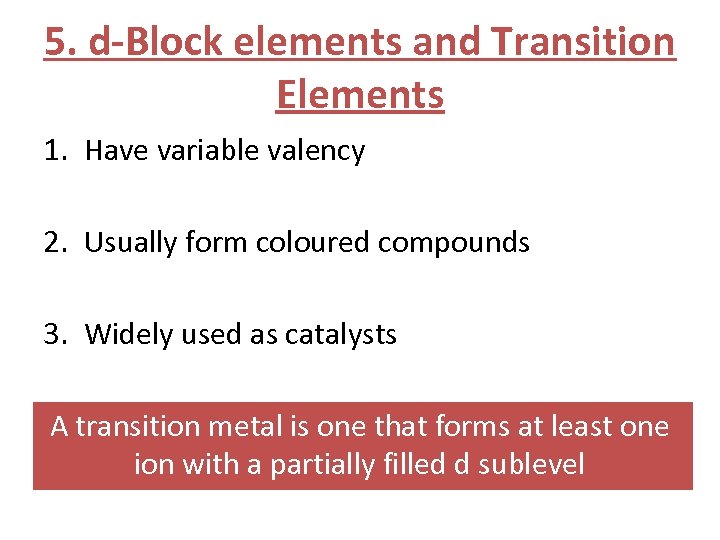
5. d-Block elements and Transition Elements 1. Have variable valency 2. Usually form coloured compounds 3. Widely used as catalysts A transition metal is one that forms at least one ion with a partially filled d sublevel

Uses of ionic materials • Salt tablets are taken to replace lost salt in perspiration. • Brine is used to cure bacon in a preservation process. • Fluoridation of water supplies to prevent tooth decay.
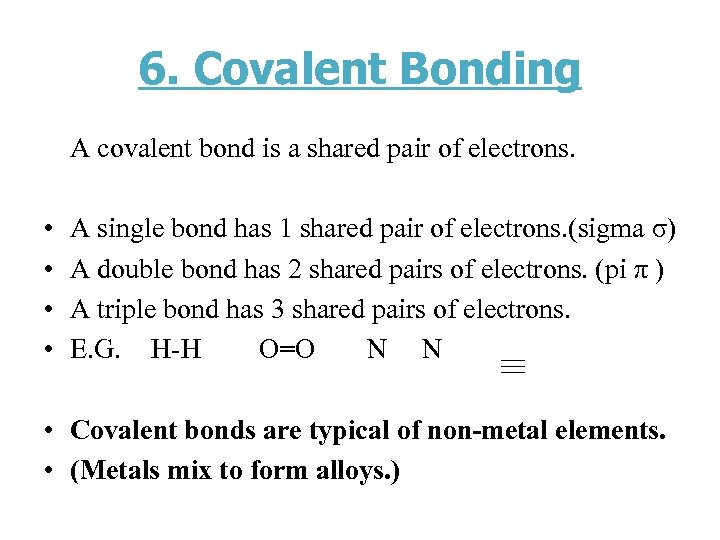
6. Covalent Bonding A covalent bond is a shared pair of electrons. • • A single bond has 1 shared pair of electrons. (sigma σ) A double bond has 2 shared pairs of electrons. (pi π ) A triple bond has 3 shared pairs of electrons. E. G. H-H O=O N N • Covalent bonds are typical of non-metal elements. • (Metals mix to form alloys. )
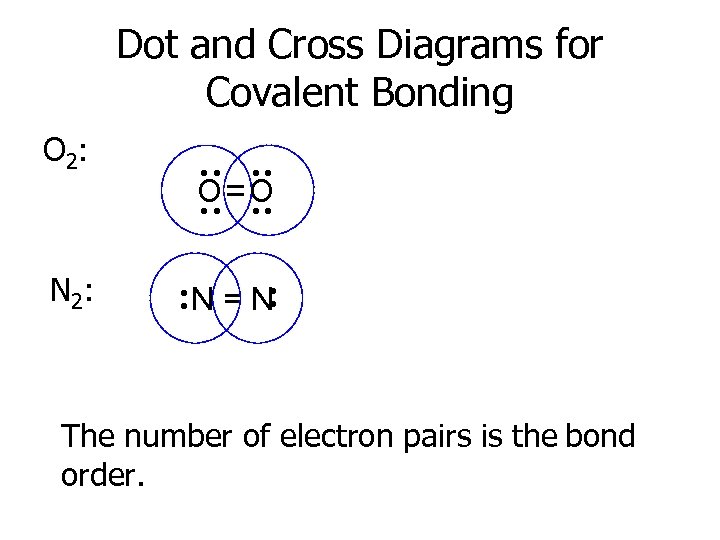
Dot and Cross Diagrams for Covalent Bonding O 2: · · O =O · · · N=N · · N 2: · · The number of electron pairs is the bond order.
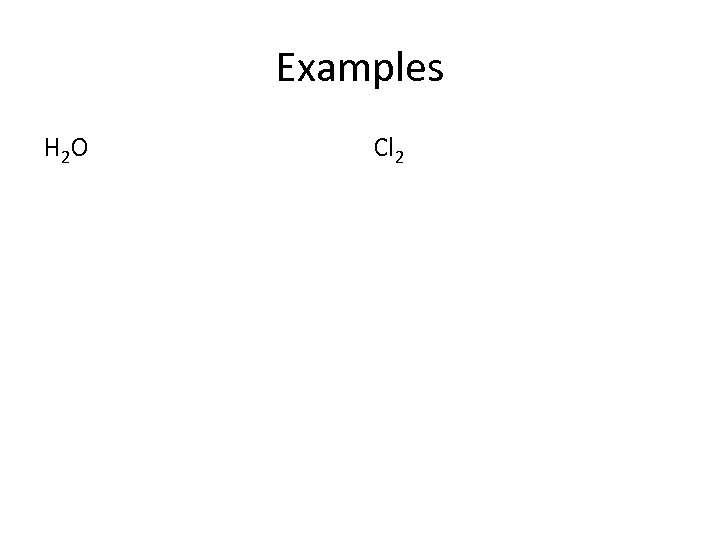
Examples H 2 O Cl 2
NH 3 H 2

CH 4
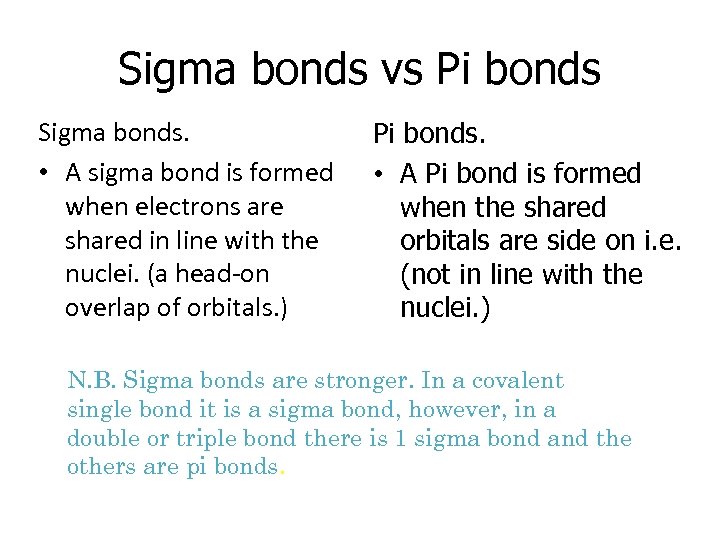
Sigma bonds vs Pi bonds Sigma bonds. • A sigma bond is formed when electrons are shared in line with the nuclei. (a head-on overlap of orbitals. ) Pi bonds. • A Pi bond is formed when the shared orbitals are side on i. e. (not in line with the nuclei. ) N. B. Sigma bonds are stronger. In a covalent single bond it is a sigma bond, however, in a double or triple bond there is 1 sigma bond and the others are pi bonds.
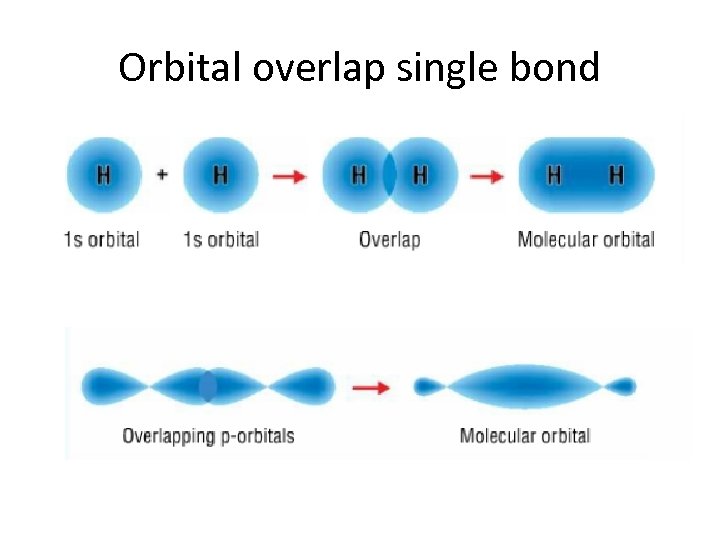
Orbital overlap single bond
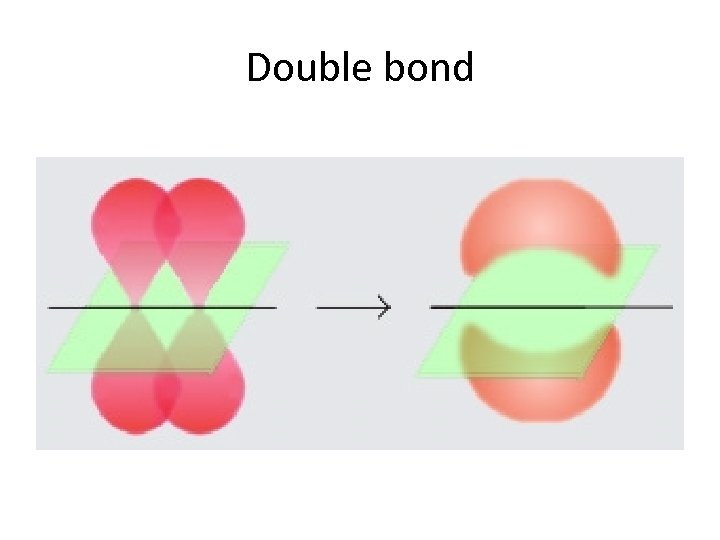
Double bond
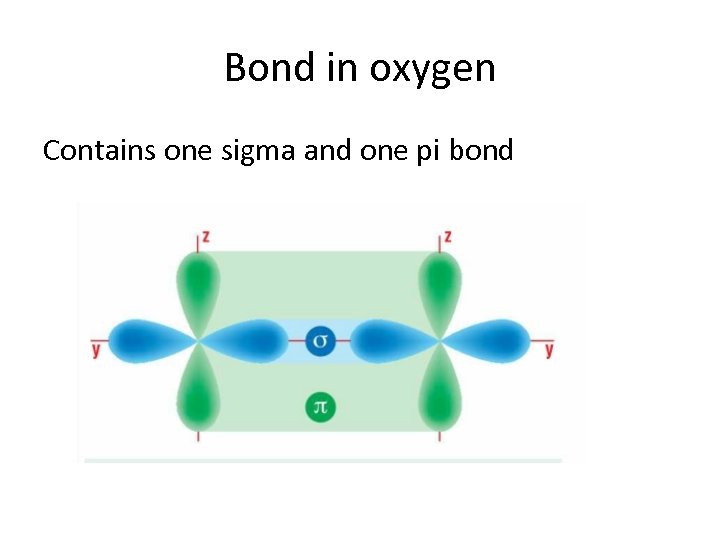
Bond in oxygen Contains one sigma and one pi bond

Draw dot and cross diagrams for… • HCL • CO 2 • NO 2

7. Characteristics of ionic and covalent bonds We will look at the following headings a. Hardness b. Melting and Boiling Points c. Conduction of electricity
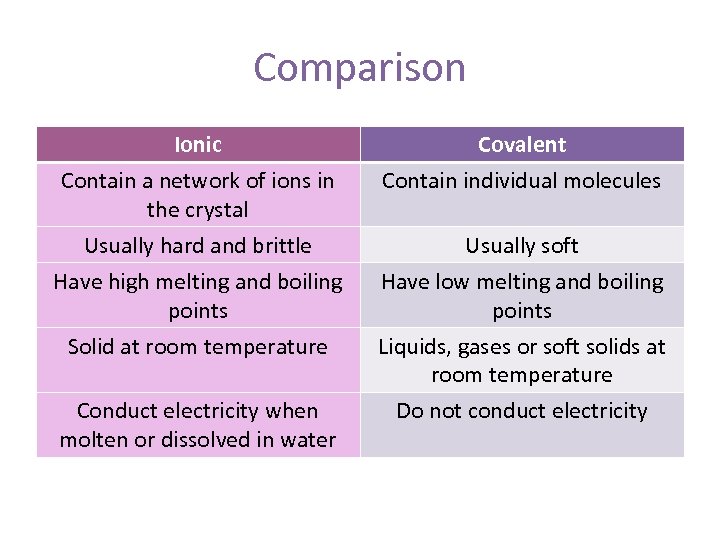
Comparison Ionic Covalent Contain a network of ions in the crystal Contain individual molecules Usually hard and brittle Usually soft Have high melting and boiling points Have low melting and boiling points Solid at room temperature Liquids, gases or soft solids at room temperature Conduct electricity when molten or dissolved in water Do not conduct electricity
8. Shapes of Covalent Molecules VSEPR Theory Valence Shell Electron Pair Repulsion Theory The shape of a molecule depends on the number of pairs of electrons around the central atom. • Electrons are negatively charged -> pairs repel each other and want to be as far apart as possible
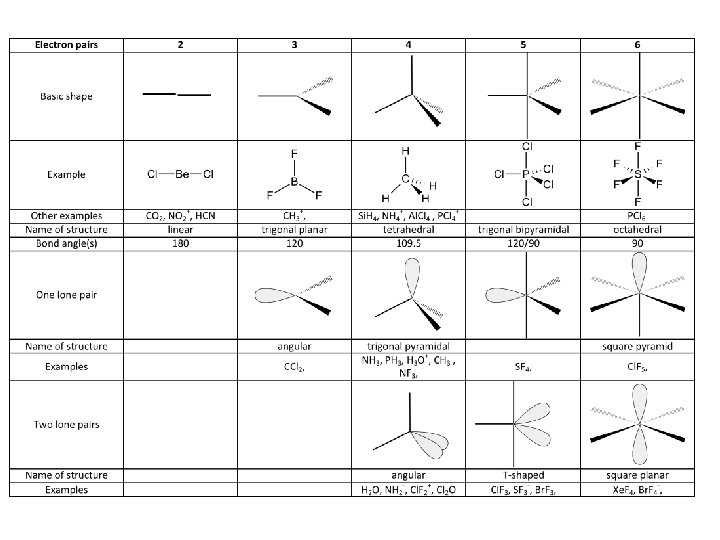
. • The following shapes will arise: Linear – one or two pairs of e- around the central atom. Bond angle is 180º Trigonal planar – Three pairs of e-, bond angle is 120º Tetrahedral – Four pairs of e-, bond angle is 109. 5º
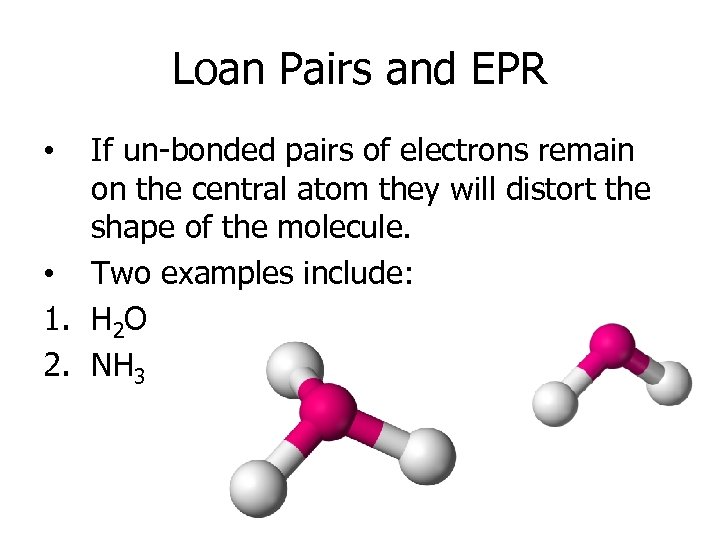
Loan Pairs and EPR If un-bonded pairs of electrons remain on the central atom they will distort the shape of the molecule. • Two examples include: 1. H 2 O 2. NH 3 •

Answering VSEPR questions 1. Check valence electron on central atom 2. How many electrons involved in bonding? 3. How many lone pairs 4. Assign shape and bond angle-diagram
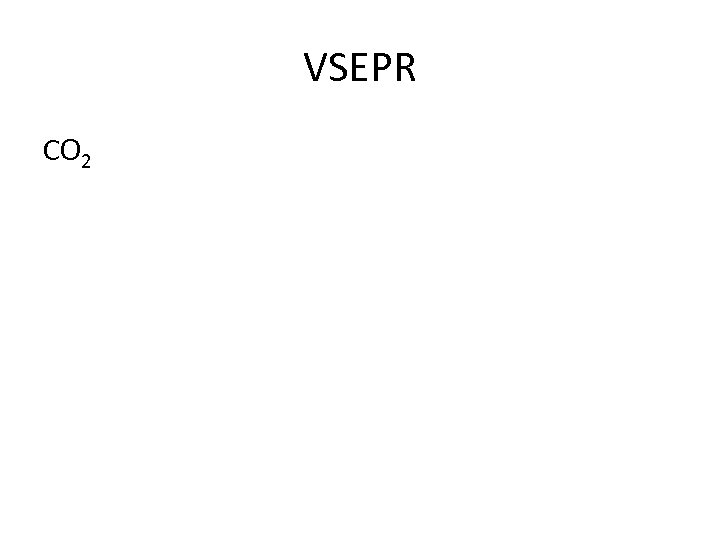
VSEPR CO 2

In a bond between two identical atoms the pair of electrons are shared equally chemists have found that in many bonds the pair of electrons are attracted to one of the atoms more than to the other.
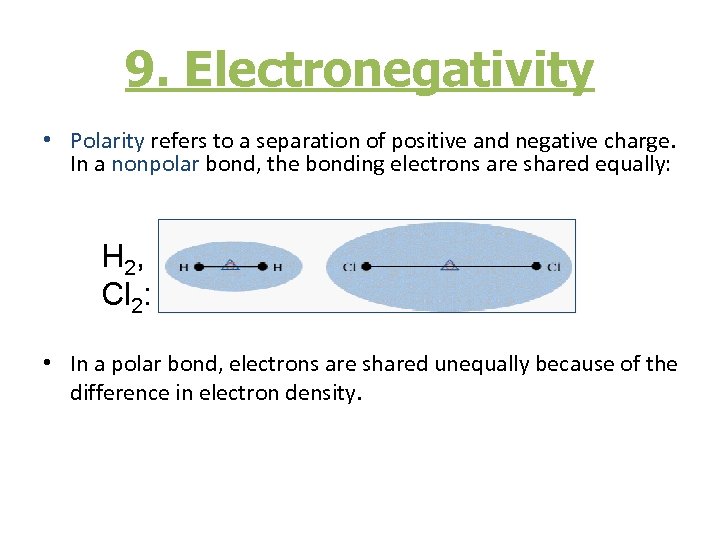
9. Electronegativity • Polarity refers to a separation of positive and negative charge. In a nonpolar bond, the bonding electrons are shared equally: H 2, Cl 2: • In a polar bond, electrons are shared unequally because of the difference in electron density.

Electronegativity is the relative attraction that an atom in a molecule has for the shared pairs of electrons in a covalent bond

Hydrogen and Chlorine Electrons attracted to chlorine more than to hydrogen [ bigger , but more +ve nucleus] therefore the electrons spend more time near the chlorine than near the hydrogen this gives the chlorine a slightly negative charge [δ- delta minus] it gives the hydrogen a slightly positive charge [δ+ delta plus]
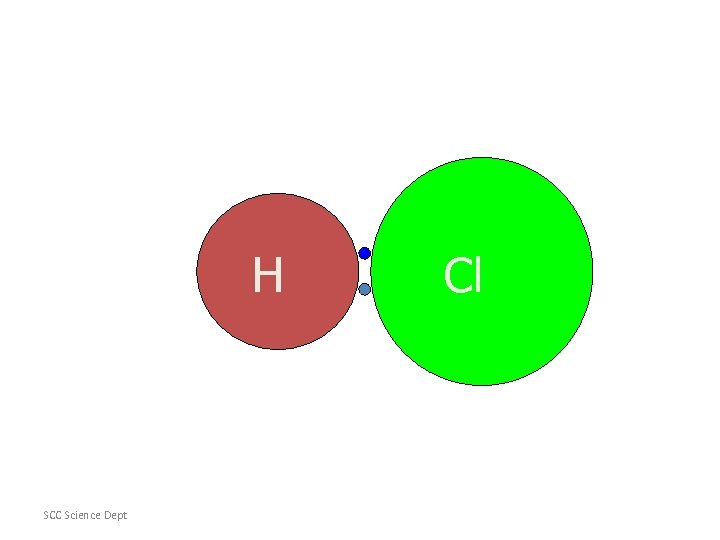
H SCC Science Dept Cl
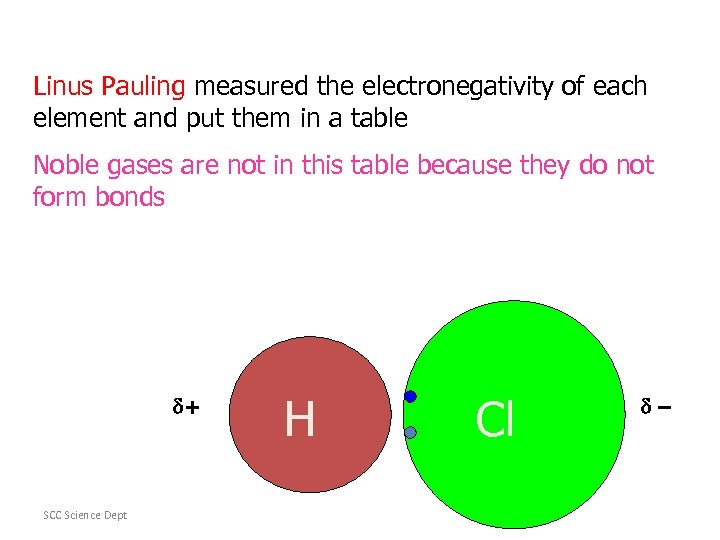
Linus Pauling measured the electronegativity of each element and put them in a table Noble gases are not in this table because they do not form bonds SCC Science Dept + H Cl –

Trends in Electronegativity • Down a group: electronegativity decreases 1. An extra level of electrons shields the outer e- from the nucleus. 2. An increase in atomic radius. • Across a period: Electronegativity increases 1. Increase in the size of nucleus (nuclear charge increases). 2. The atomic radius decreases.

EN Differences and Bond Type 1. 0 to 0. 4 : Pure Covalent Bond 2. 0. 4 but < 1. 7 : Polar Covalent Bond 3. = or > 1. 7 : Ionic Bond
Inter and Intra Molecular Forces • The force of attraction between ions is stronger than between molecules. • Inter: are forces between molecules. • Intra: are forces within a molecule. • There are 3 kinds of forces that can attract molecules together

1. Van der Waal’s: • These are the weakest forces caused by the movement of e- within a molecule. The electrons move randomly within the bond so at 1 point in time they are nearer to 1 atom than the other. This induces a temporary dipole force. • Temporary dipoles will result in increased boiling points. • The greater number of e- in a molecule the greater the number of temporary dipoles.

2. Dipole-dipole: • The positively charged end of a polar molecule is attracted to the negative end of another molecule. • The dipoles in this case are permanent. • As a result they are stronger than Van der Waal’s forces.

3. Hydrogen Bonding • When H is bonded to F, O or N these elements are sufficiently electronegative to make the bond polar. • • H has only 1 e- in its atom, a strong partial positive charge will result. This means it is very strongly attracted to the negative atom and as a result H 2 O is a liquid at room temperature with a fairly high boiling point.

Experiments on Bonding • Qualitative tests for the anions CO 32 -, HCO 3 -, SO 42 -, SO 32 -, Cl-, NO 3 - and PO 43 - in aqueous solution. • Theory: Reactions of anions with certain reagents to produce characteristic coloured precipitates or other easily identifiable results are employed to identify or confirm the presence of these anions in aqueous solution and to distinguish the anions from one another.

• (a) To test for the carbonate and hydrogencarbonate anions. • NB: Wear your safety glasses. • 1. Add 2 cm 3 of sodium carbonate solution to a clean test tube labelled A, and 2 cm 3 of sodium hydrogencarbonate solution to a clean test tube labelled B. • 2. Using a dropping pipette add 2 cm 3 of dilute hydrochloric acid to each test tube. Record your observations.
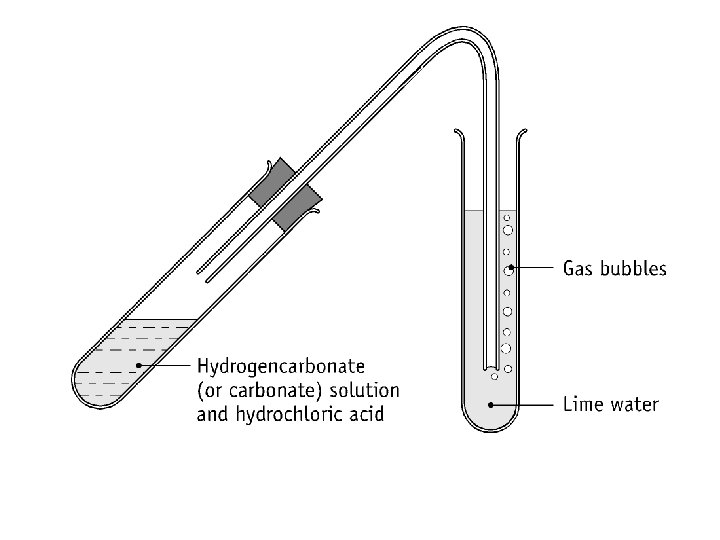

• Add 2 cm 3 of sodium carbonate solution to a clean test tube labelled C, and 2 cm 3 of sodium hydrogencarbonate solution to a clean test tube labelled D. Using a dropping pipette add 2 cm 3 of magnesium sulphate solution to each. Record your observations. • Carefully heat the contents of test tubes labelled C and D at the end of the last step.

• (b) To test for the sulphate and sulphite anions • Add 2 cm 3 of sodium sulfate solution to a clean test tube labelled A and 2 cm 3 of sodium sulfite solution to a clean test tube labelled B. • Using a dropping pipette add 1 cm 3 of barium chloride solution to each. Record your observations.

• Add 2 cm 3 of dilute hydrochloric acid to the contents of the test tubes labelled A and B at the end of the last step. Record your observations.

• (c) To test for the chloride anion • Add 2 cm 3 of sodium chloride solution to a clean test tube. Using a dropping pipette add a few drops of silver nitrate solution. Record your observations • Add 2 cm 3 of dilute ammonia solution to the contents of the test tubes at the end.

• (d) To test for the nitrate anion • Add 2 cm 3 of potassium nitrate solution to a clean test tube. Using a dropping pipette, add 3 cm 3 of cold saturated iron(II) sulfate solution. • Using a dropping pipette, carefully add 2 cm 3 of concentrated sulfuric acid slowly down the wall of the test tube. Do not mix the contents of the test tube. Record your observations.

• • • (e) To test for the phosphate anion Add 2 cm 3 of disodium hydrogenphosphate(V) solution to a clean test tube. Using a dropping pipette add approximately 6 cm 3 of the clear ammonium molybdate reagent to the test tube. Warm gently by placing in a beaker of water at a temperature not exceeding 40 C. Record any observations. Add an equal volume of ammonia solution to the contents of the test tube at the end.
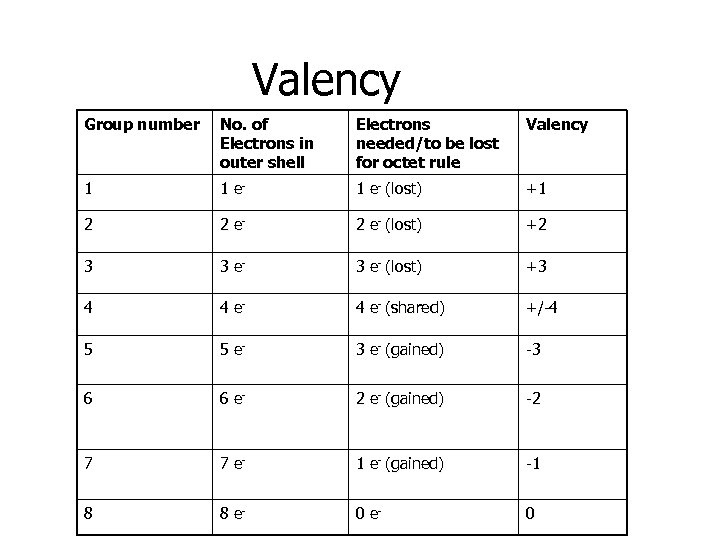
Valency Group number No. of Electrons in outer shell Electrons needed/to be lost for octet rule Valency 1 1 e- (lost) +1 2 2 e- (lost) +2 3 3 e- (lost) +3 4 4 e- (shared) +/-4 5 5 e- 3 e- (gained) -3 6 6 e- 2 e- (gained) -2 7 7 e- 1 e- (gained) -1 8 8 e- 0
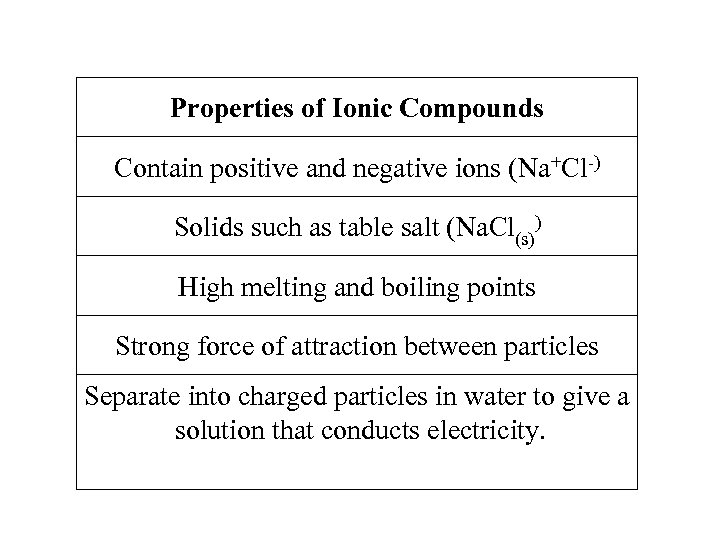
Properties of Ionic Compounds Contain positive and negative ions (Na+Cl-) Solids such as table salt (Na. Cl(s)) High melting and boiling points Strong force of attraction between particles Separate into charged particles in water to give a solution that conducts electricity.
826020baac2415cb946a3a245815f8cc.ppt