Aromatic Hydrocarbons.pptx
- Количество слайдов: 24

Chapter 5 Aromatic Hydrocarbons Table of Contents Introduction 1. Benzene and Its Structure 2. Nomenclature of Aromatic Compounds 3. Isomerism in Benzene Derivative 4. Physical Properties of Benzene 5. Chemical Properties and Reactions of Benzene 6. Occurrence and Preparation of Benzene 7. Other Aromatic Hydrocarbons

Chapter 5 Aromatic Hydrocarbons Warm up • Tell about the discovery of structure of benzene. • How is different from alkenes? • Where are the aromatic compounds used?

Chapter 5 Introduction • Compounds having similar chemical properties to benzene are called aromatics. • The name aromatic is used for characteristic pleasant odor. • Aromatic hydrocarbons are also called arenes. • The simplest aromatic hydrocarbon is benzene. • The source of aromatic hydrocarbons are coke and petroleum.
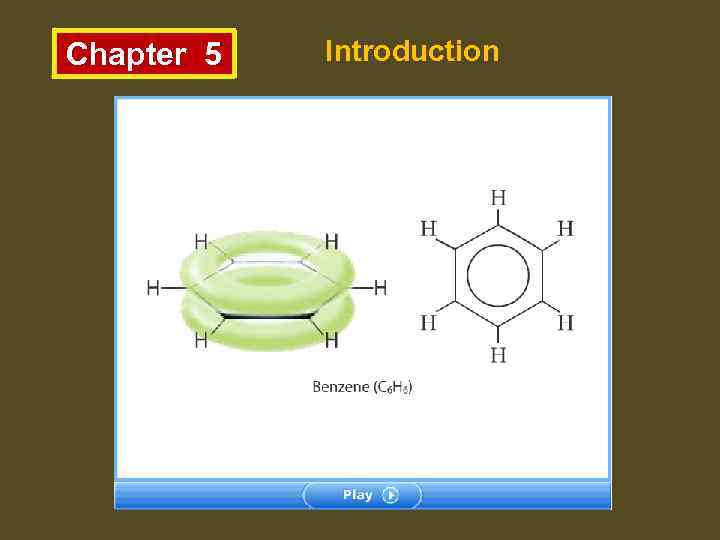
Chapter 5 Introduction

Chapter 5 Introduction
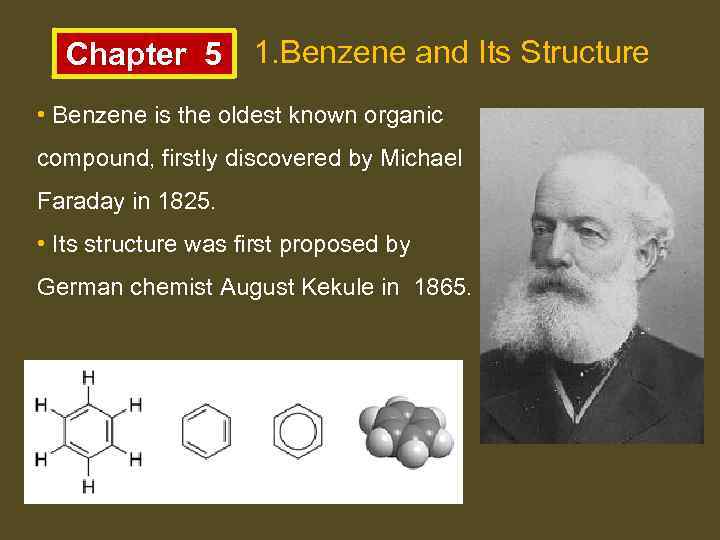
Chapter 5 1. Benzene and Its Structure • Benzene is the oldest known organic compound, firstly discovered by Michael Faraday in 1825. • Its structure was first proposed by German chemist August Kekule in 1865.
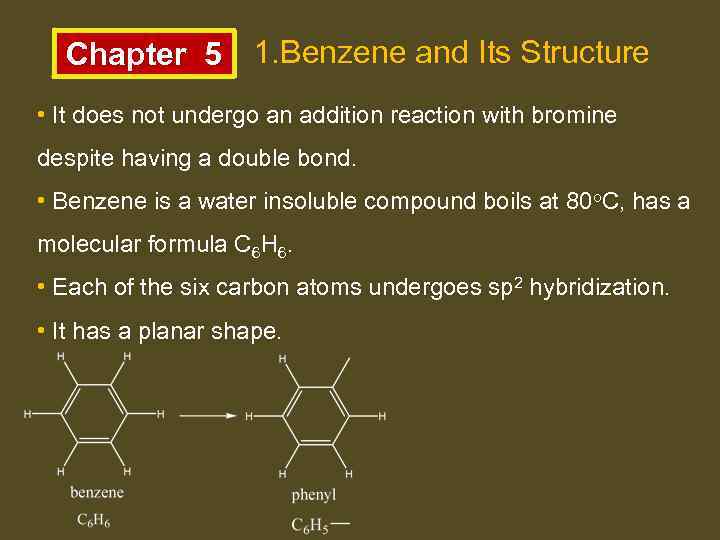
Chapter 5 1. Benzene and Its Structure • It does not undergo an addition reaction with bromine despite having a double bond. • Benzene is a water insoluble compound boils at 80 o. C, has a molecular formula C 6 H 6. • Each of the six carbon atoms undergoes sp 2 hybridization. • It has a planar shape.
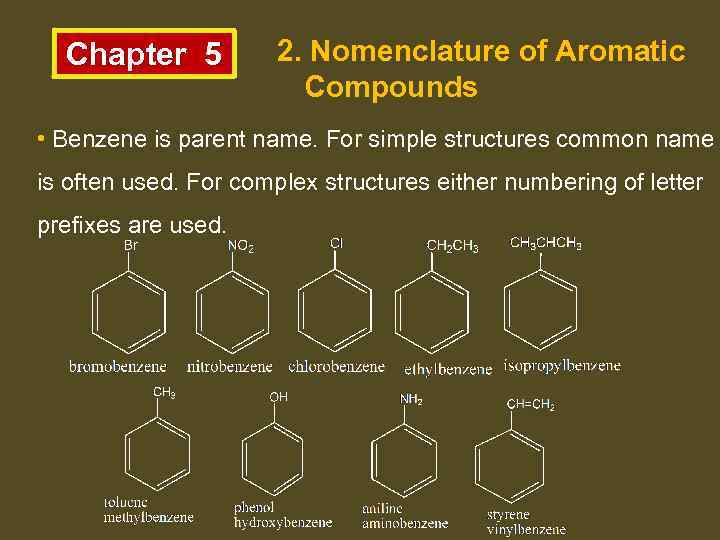
Chapter 5 2. Nomenclature of Aromatic Compounds • Benzene is parent name. For simple structures common name is often used. For complex structures either numbering of letter prefixes are used.
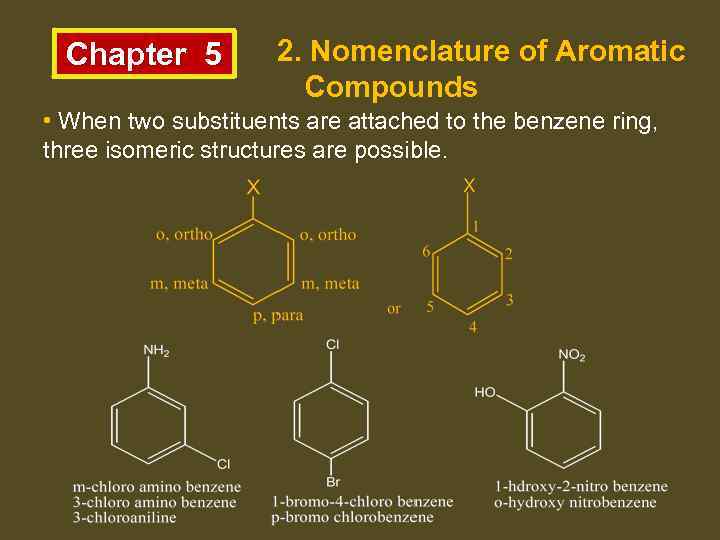
Chapter 5 2. Nomenclature of Aromatic Compounds • When two substituents are attached to the benzene ring, three isomeric structures are possible.
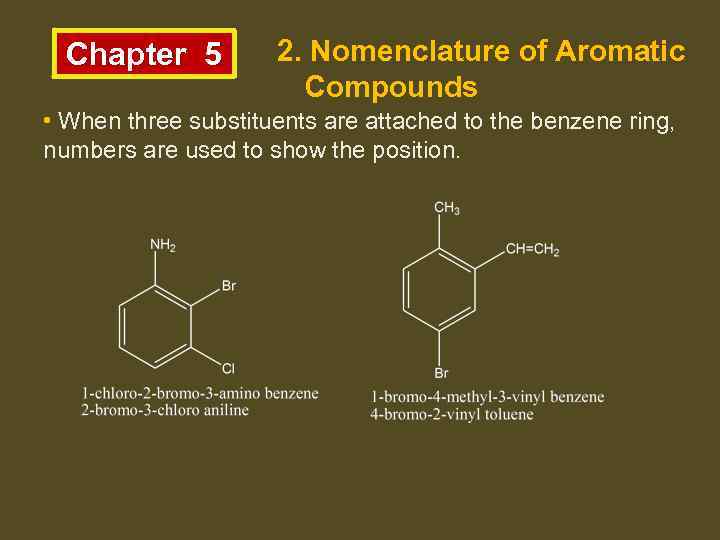
Chapter 5 2. Nomenclature of Aromatic Compounds • When three substituents are attached to the benzene ring, numbers are used to show the position.
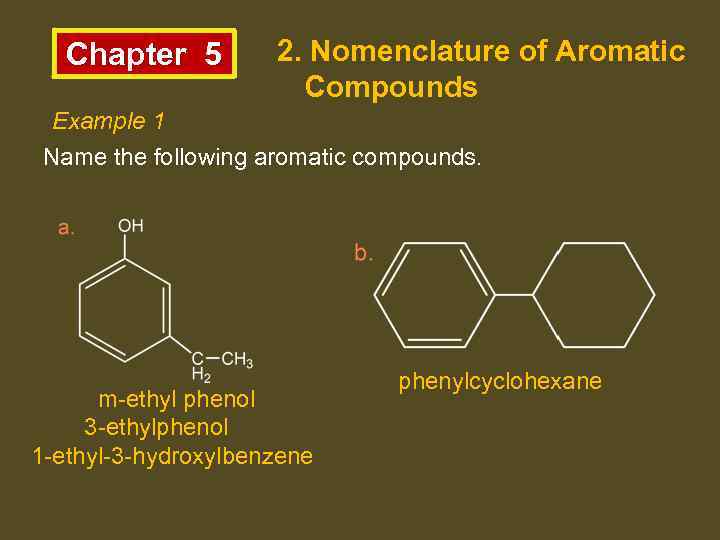
Chapter 5 2. Nomenclature of Aromatic Compounds Example 1 Name the following aromatic compounds. m-ethyl phenol 3 -ethylphenol 1 -ethyl-3 -hydroxylbenzene phenylcyclohexane
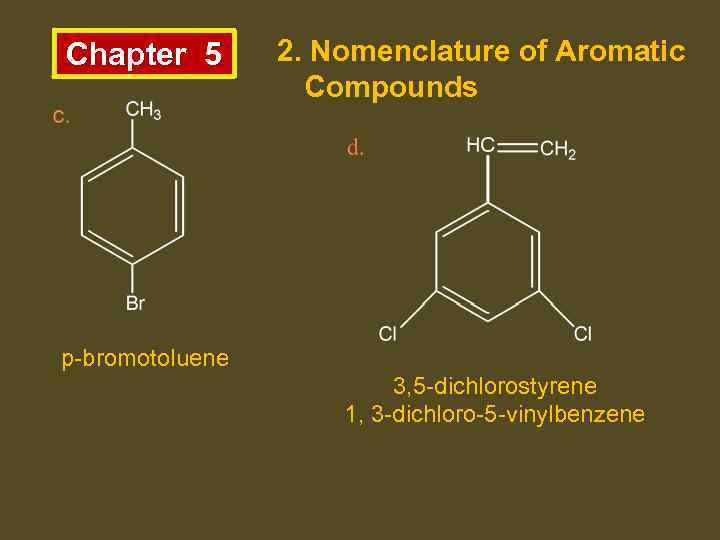
Chapter 5 2. Nomenclature of Aromatic Compounds p-bromotoluene 3, 5 -dichlorostyrene 1, 3 -dichloro-5 -vinylbenzene

Chapter 5 Example 2 2. Nomenclature of Aromatic Compounds Draw the structures of the following aromatic compounds. a. m-nitrotoluene b. m-dinitrobenzene c. 1, 3 -dichloro-5 -nitrobenzene d. nitrobenzene e. triphenylmethane
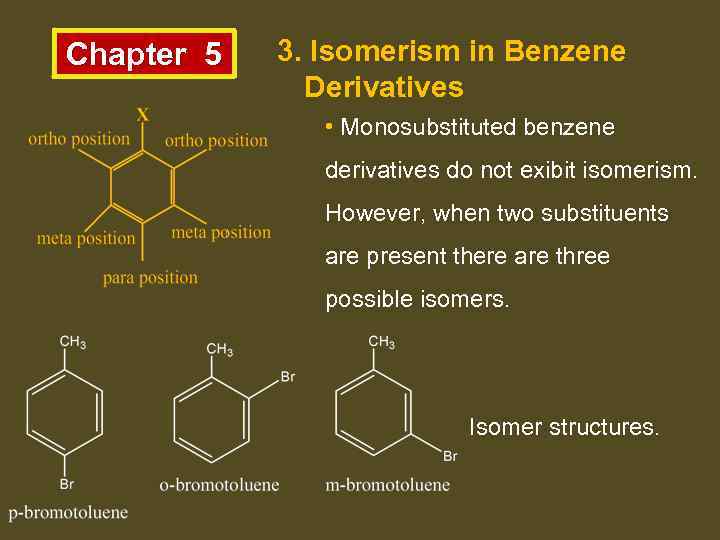
Chapter 5 3. Isomerism in Benzene Derivatives • Monosubstituted benzene derivatives do not exibit isomerism. However, when two substituents are present there are three possible isomers. Isomer structures.

Chapter 5 4. Physical Properties of Benzene • Colorless, poisonous liquid with a specific odor. • Dissolves in organic solvents such as ether, alcohol, acetone and acetic acid. • Good solvent for non-polar substances • Used to dissolve fats, resins, rubber, iodine and sulfur. • Carbon content is high, therefore, when burned, it gives a sooty flame.
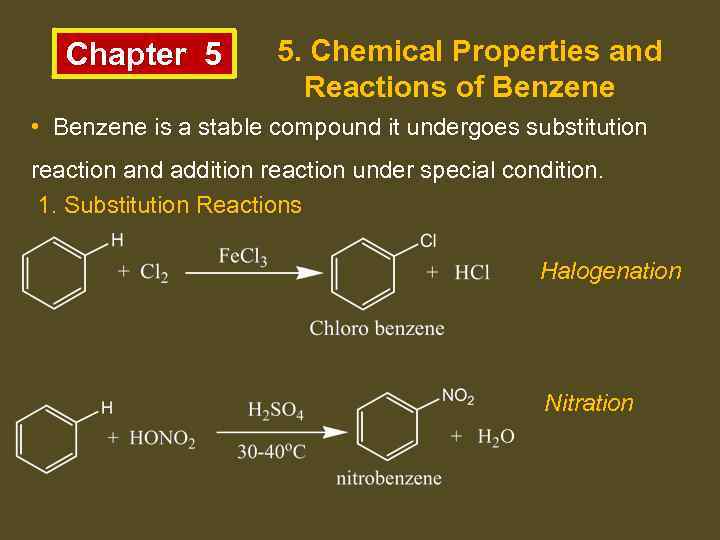
Chapter 5 5. Chemical Properties and Reactions of Benzene • Benzene is a stable compound it undergoes substitution reaction and addition reaction under special condition. 1. Substitution Reactions Halogenation Nitration
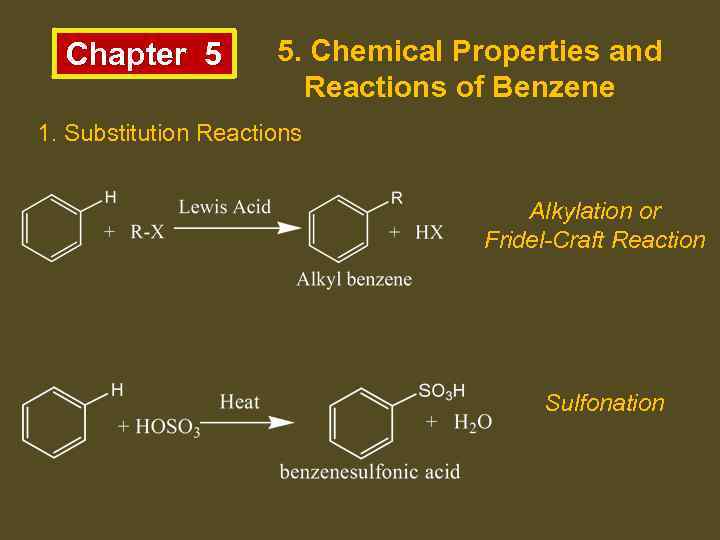
Chapter 5 5. Chemical Properties and Reactions of Benzene 1. Substitution Reactions Alkylation or Fridel-Craft Reaction Sulfonation
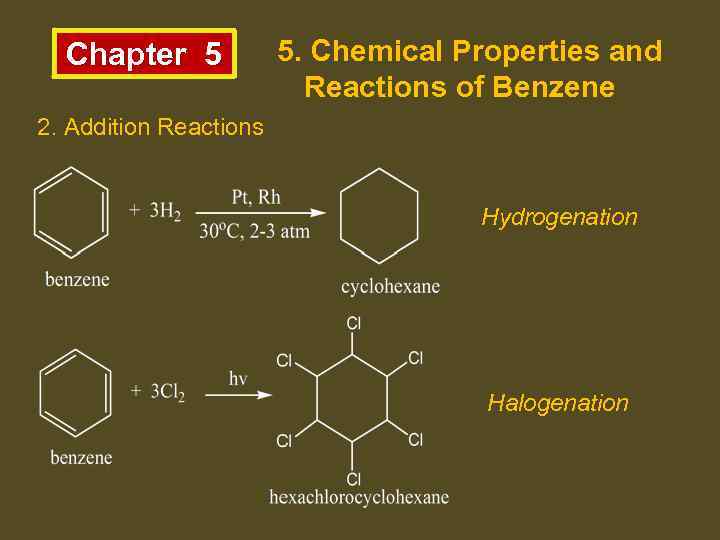
Chapter 5 5. Chemical Properties and Reactions of Benzene 2. Addition Reactions Hydrogenation Halogenation

Chapter 5 6. Occurrence and Preparation of Benzene • Main natural source of benzene is coal tar. It contains benzene, toluene, xylene, phenol, naphthalene and anthracene. • Also be produced from petroleum hydrocarbons by aromatization. • Alkanes can be dehydrogenated to produce benzene and its derivatives by heating them over catalysts.
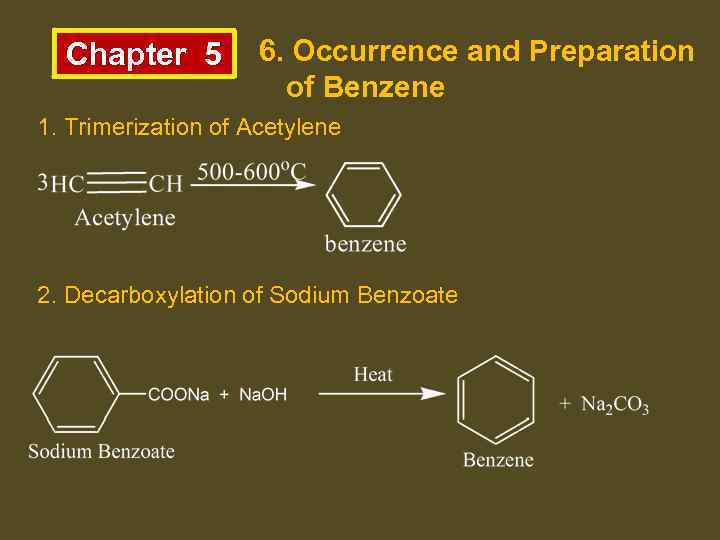
Chapter 5 6. Occurrence and Preparation of Benzene 1. Trimerization of Acetylene 2. Decarboxylation of Sodium Benzoate
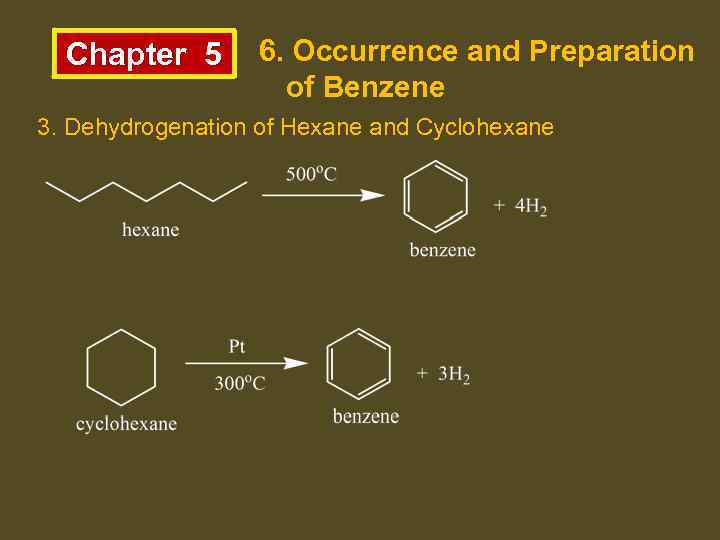
Chapter 5 6. Occurrence and Preparation of Benzene 3. Dehydrogenation of Hexane and Cyclohexane
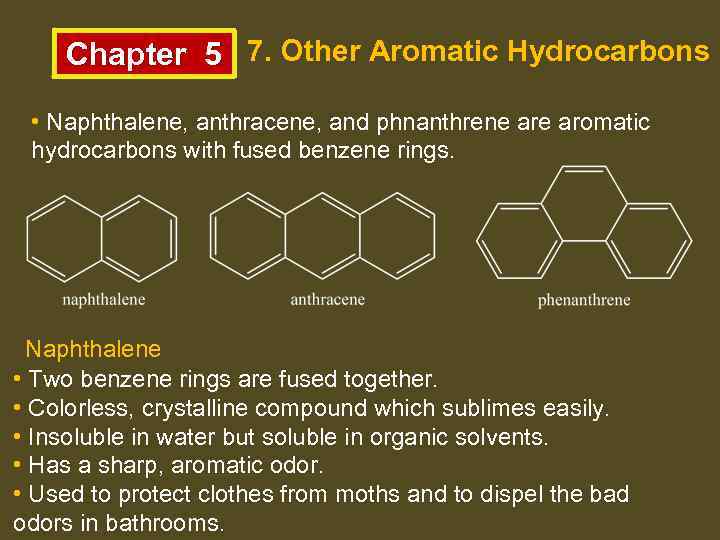
Chapter 5 7. Other Aromatic Hydrocarbons • Naphthalene, anthracene, and phnanthrene aromatic hydrocarbons with fused benzene rings. Naphthalene • Two benzene rings are fused together. • Colorless, crystalline compound which sublimes easily. • Insoluble in water but soluble in organic solvents. • Has a sharp, aromatic odor. • Used to protect clothes from moths and to dispel the bad odors in bathrooms.

Chapter 5 7. Other Aromatic Hydrocarbons Anthracene • Formed by fusing three benzene rings together in a linear geometry. • Colorless, crystalline compound used in the production of paints. • It is also used in wood preservatives, insecticides, and coating materials.

End of the chapter 5
Aromatic Hydrocarbons.pptx