Protein.pptx
- Количество слайдов: 50
Chapter 4 Protein Structure and Function Arnat Balabiyev Ph. D Candidate Arizona State University
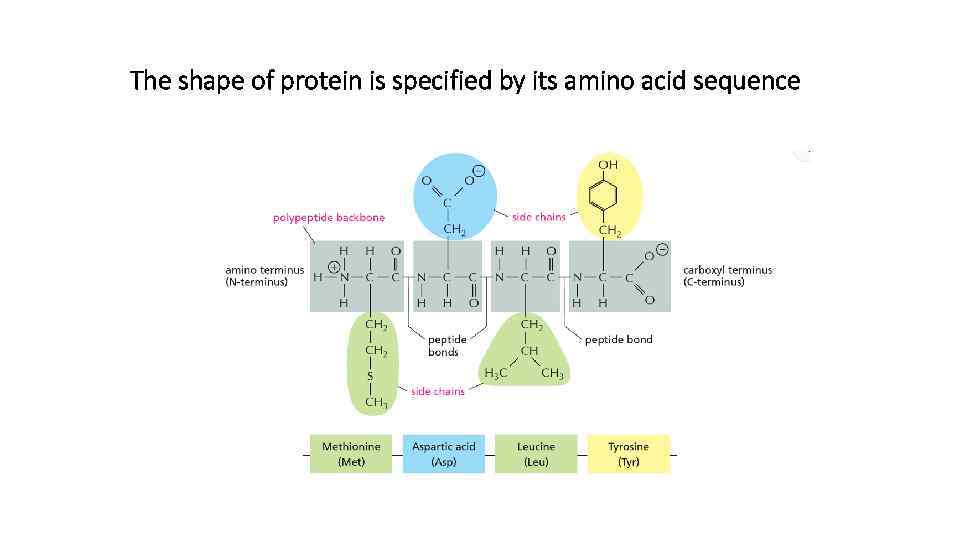
The shape of protein is specified by its amino acid sequence
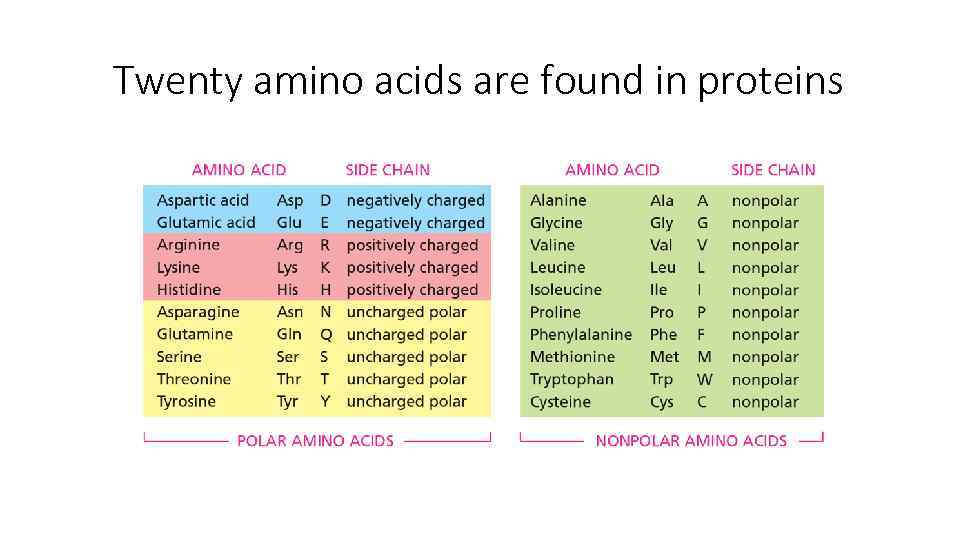
Twenty amino acids are found in proteins
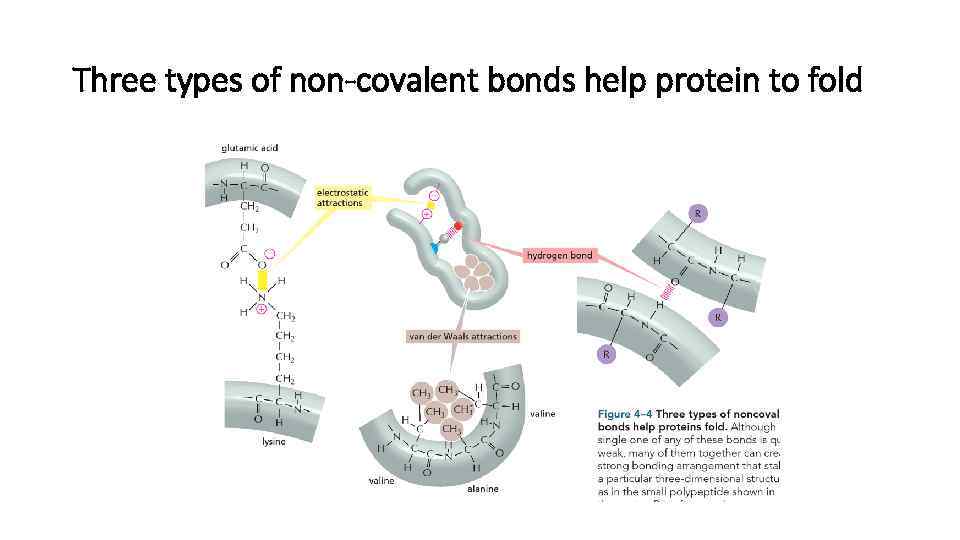
Three types of non-covalent bonds help protein to fold
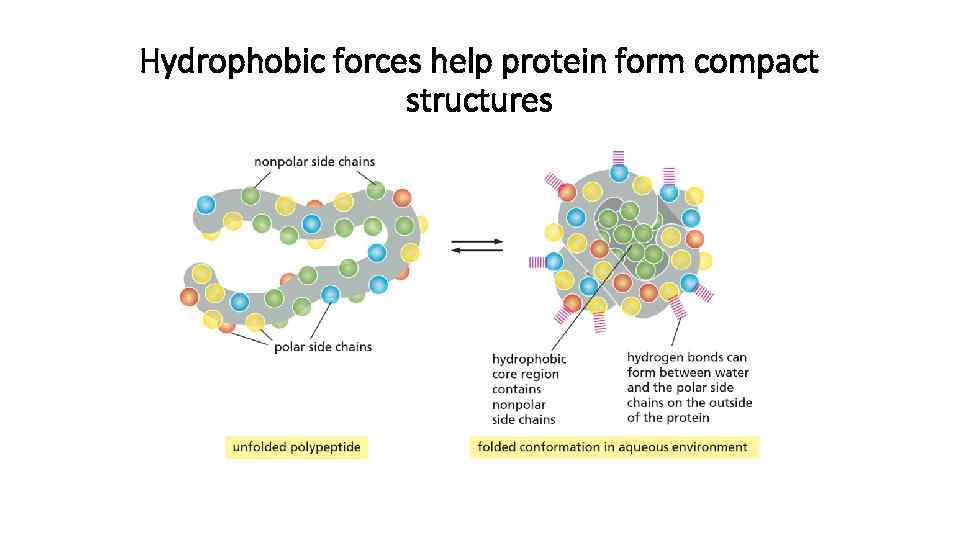
Hydrophobic forces help protein form compact structures
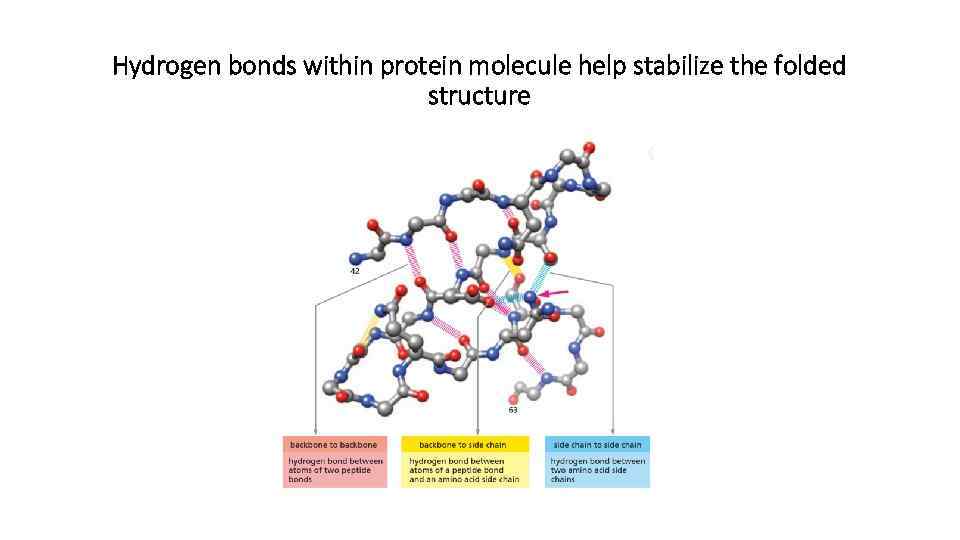
Hydrogen bonds within protein molecule help stabilize the folded structure
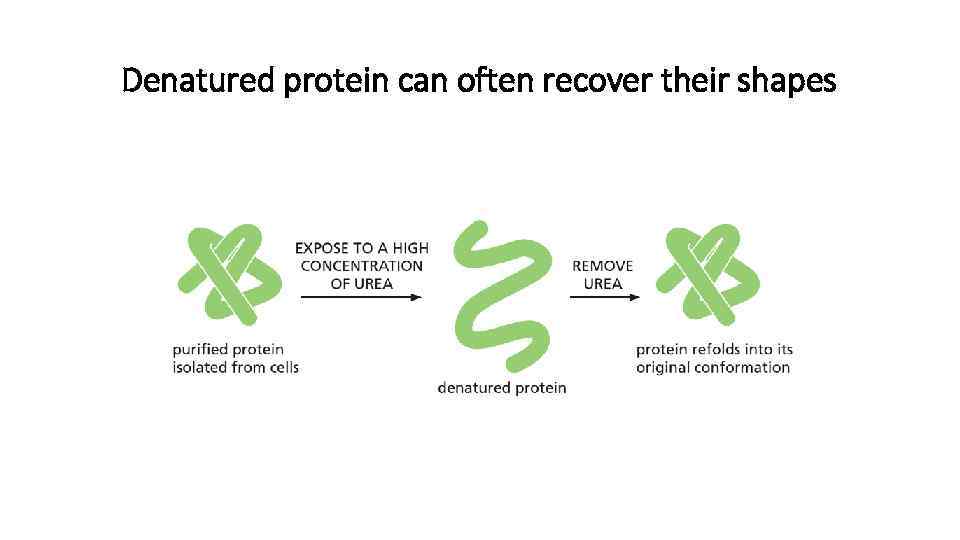
Denatured protein can often recover their shapes
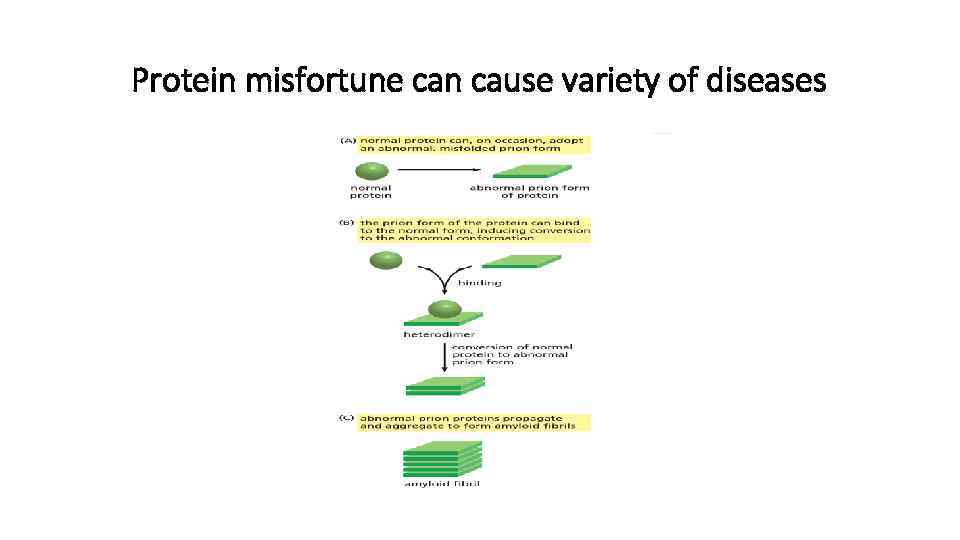
Protein misfortune can cause variety of diseases
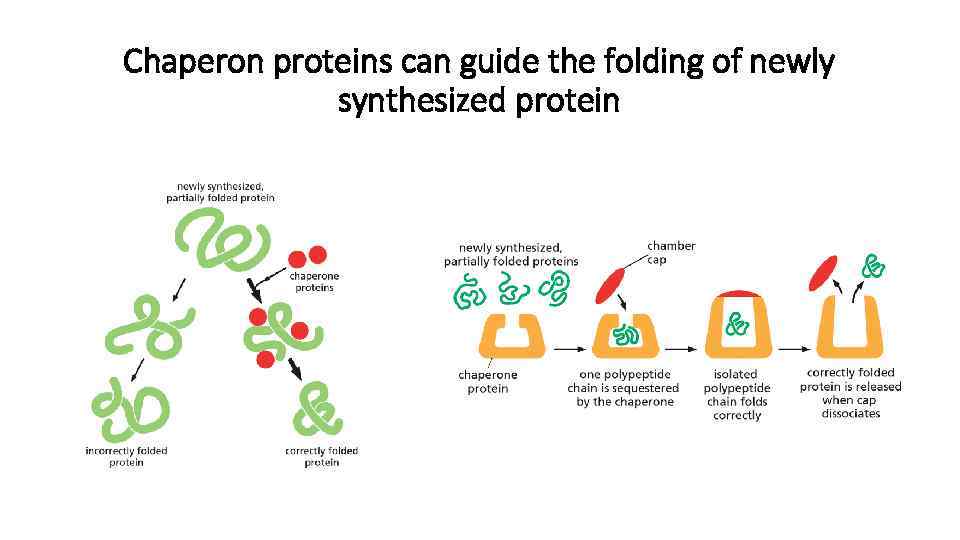
Chaperon proteins can guide the folding of newly synthesized protein
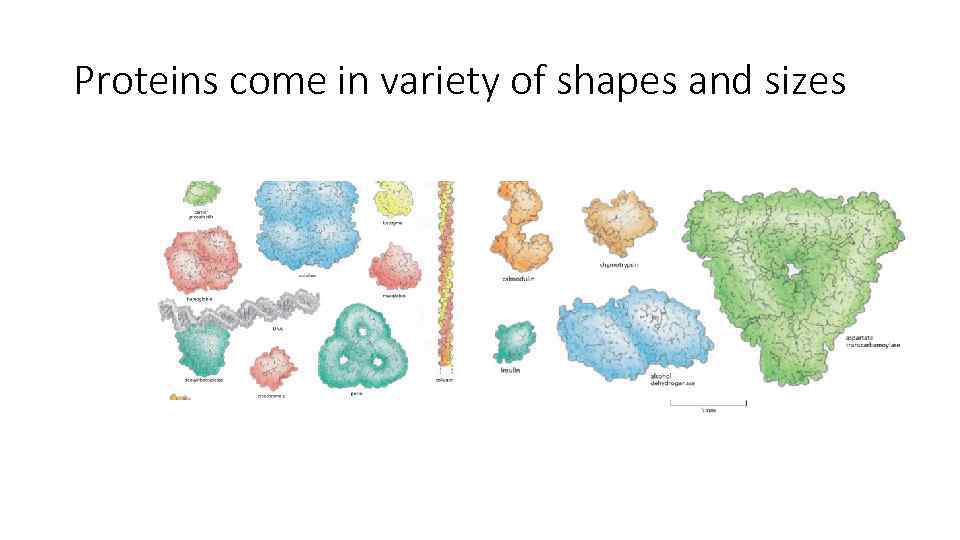
Proteins come in variety of shapes and sizes
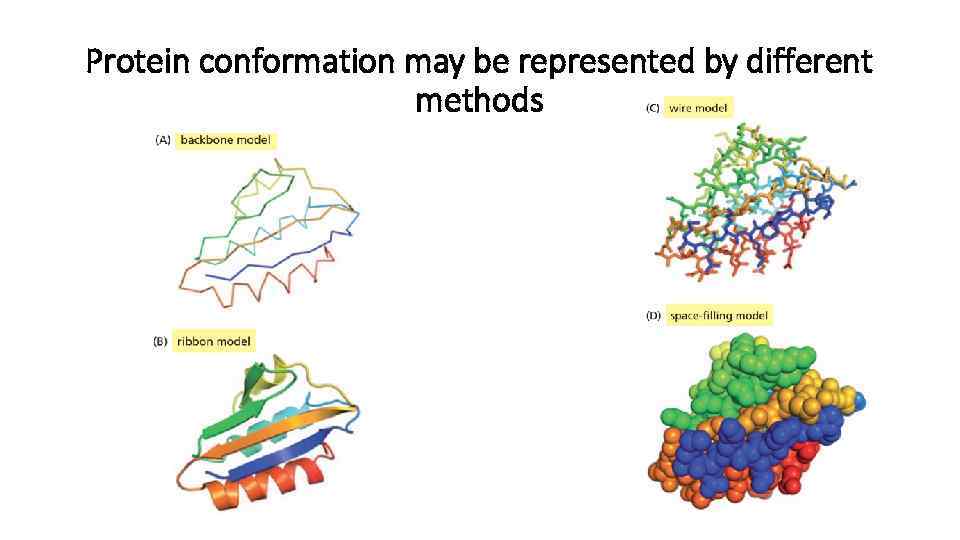
Protein conformation may be represented by different methods
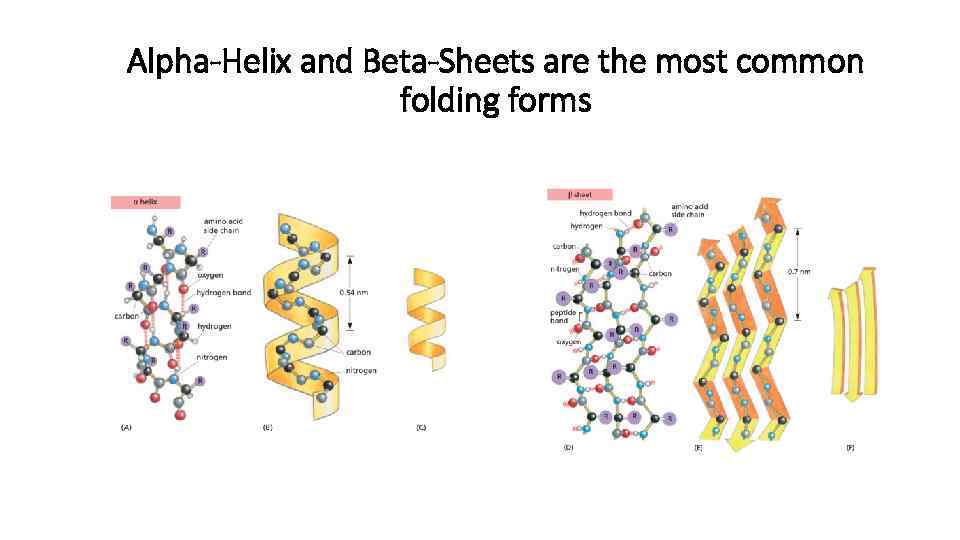
Alpha-Helix and Beta-Sheets are the most common folding forms
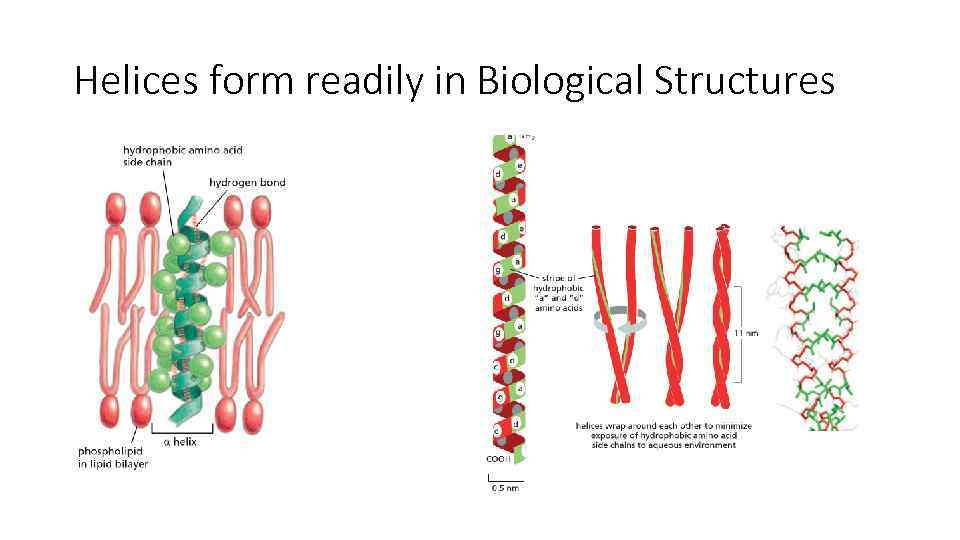
Helices form readily in Biological Structures
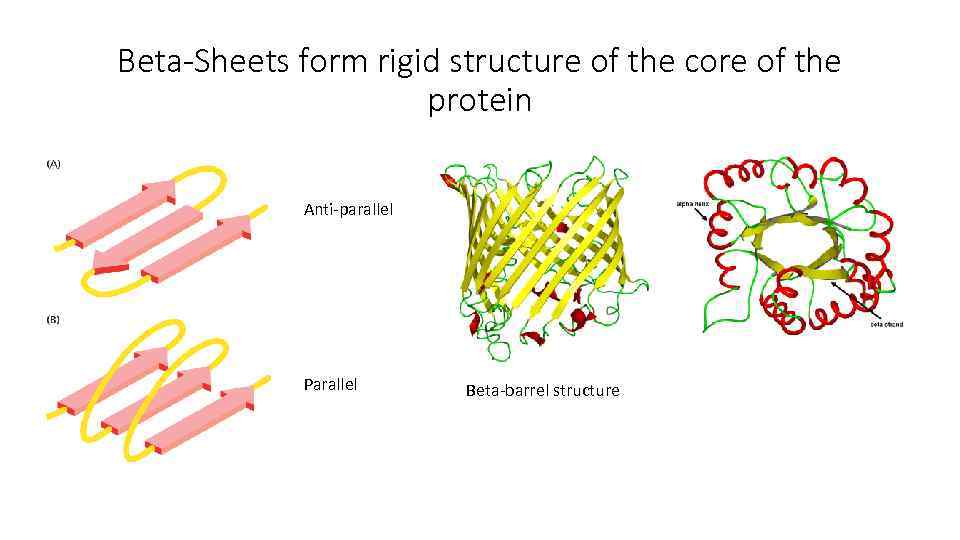
Beta-Sheets form rigid structure of the core of the protein Anti-parallel Parallel Beta-barrel structure
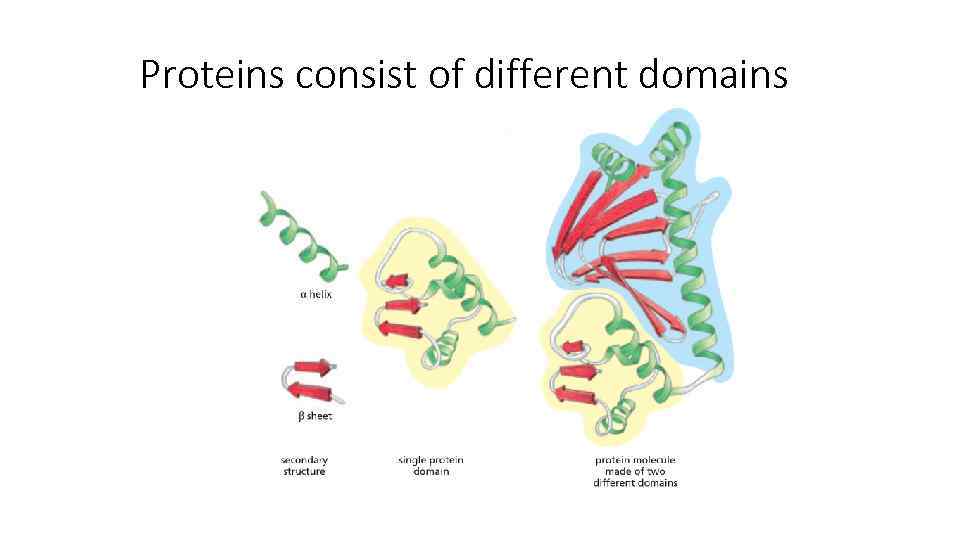
Proteins consist of different domains
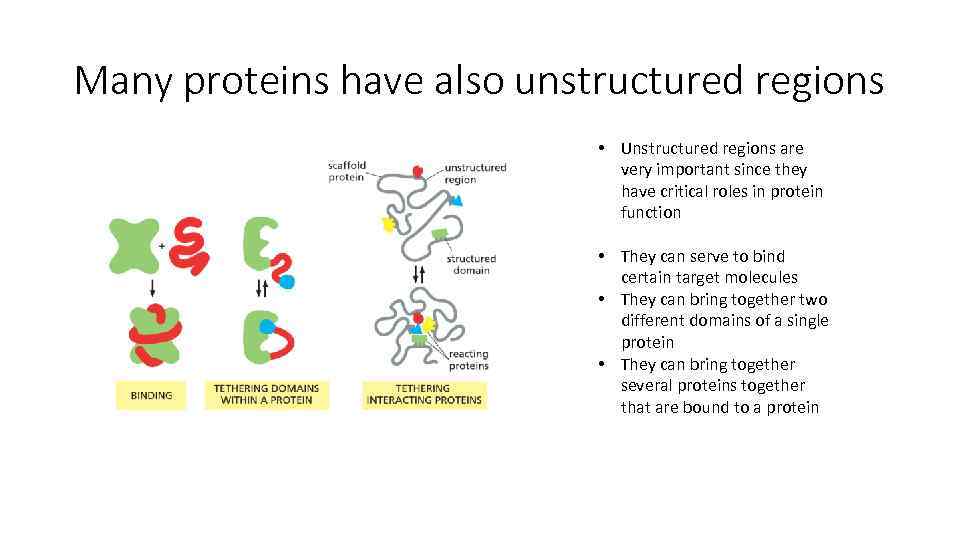
Many proteins have also unstructured regions • Unstructured regions are very important since they have critical roles in protein function • They can serve to bind certain target molecules • They can bring together two different domains of a single protein • They can bring together several proteins together that are bound to a protein

Only a few of many possible protein structures are useful • 20 amino acids are in protein structure • 4 amino acids peptide could form 20 x 20 x 20=160, 000 types of peptides • BUT!!! Not all of them are useful for a cell • Think of a protein that consist of 300 amino acids!! • 20^300 different types of proteins could exist
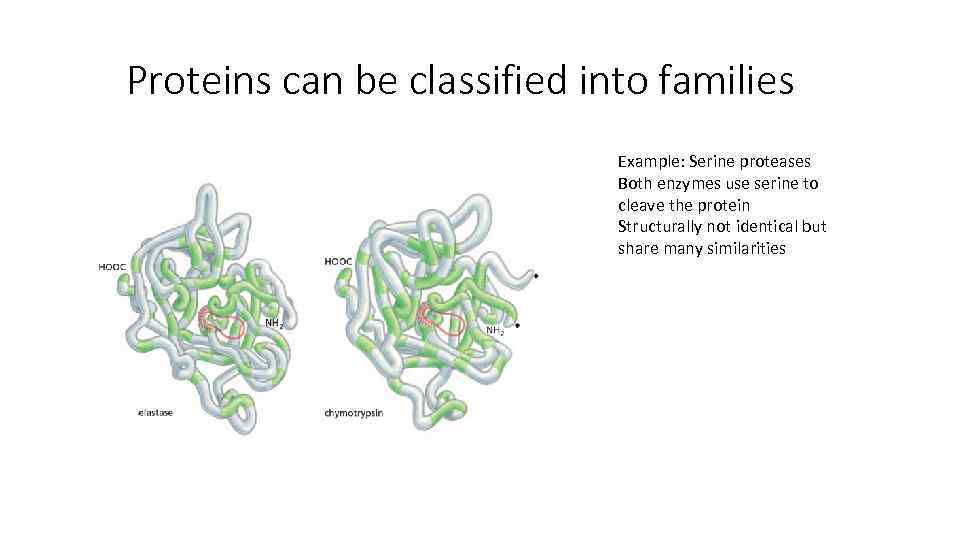
Proteins can be classified into families Example: Serine proteases Both enzymes use serine to cleave the protein Structurally not identical but share many similarities
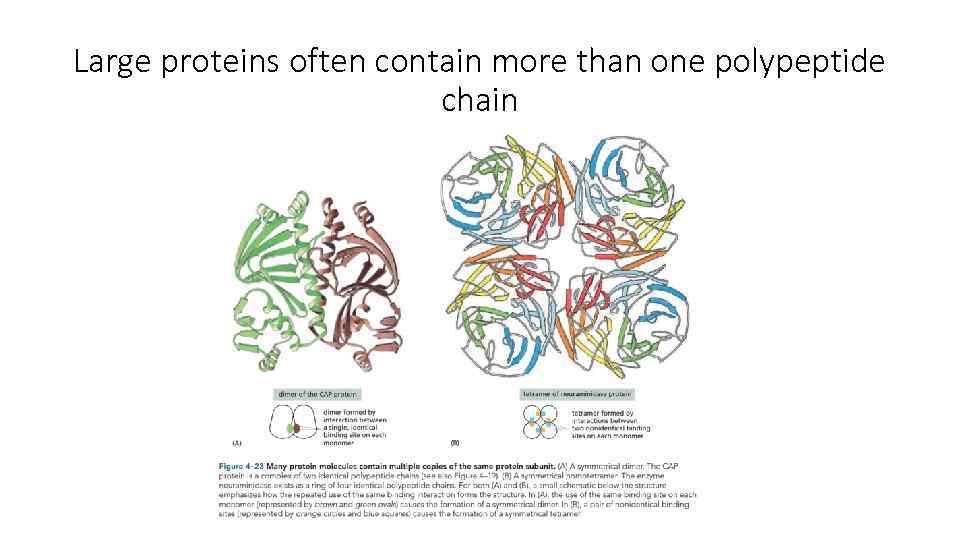
Large proteins often contain more than one polypeptide chain
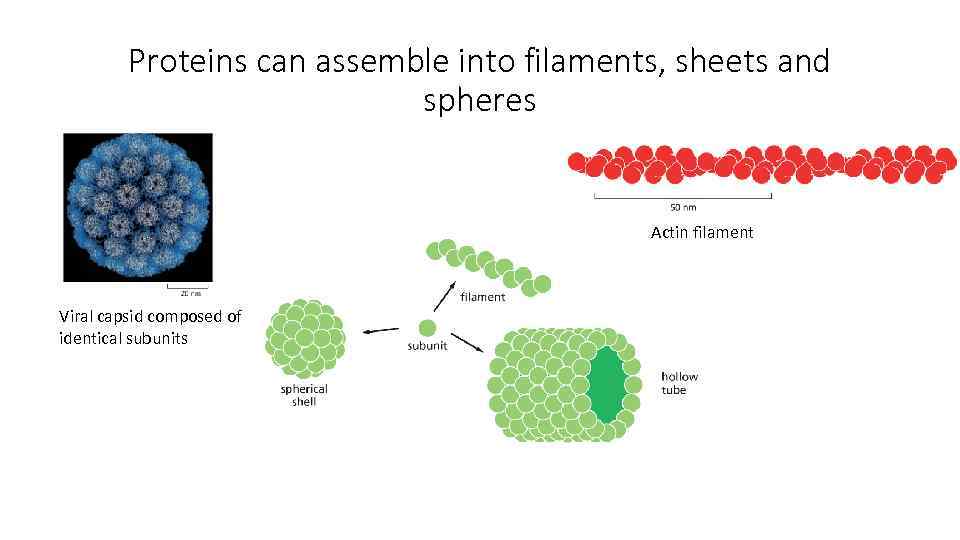
Proteins can assemble into filaments, sheets and spheres Actin filament Viral capsid composed of identical subunits
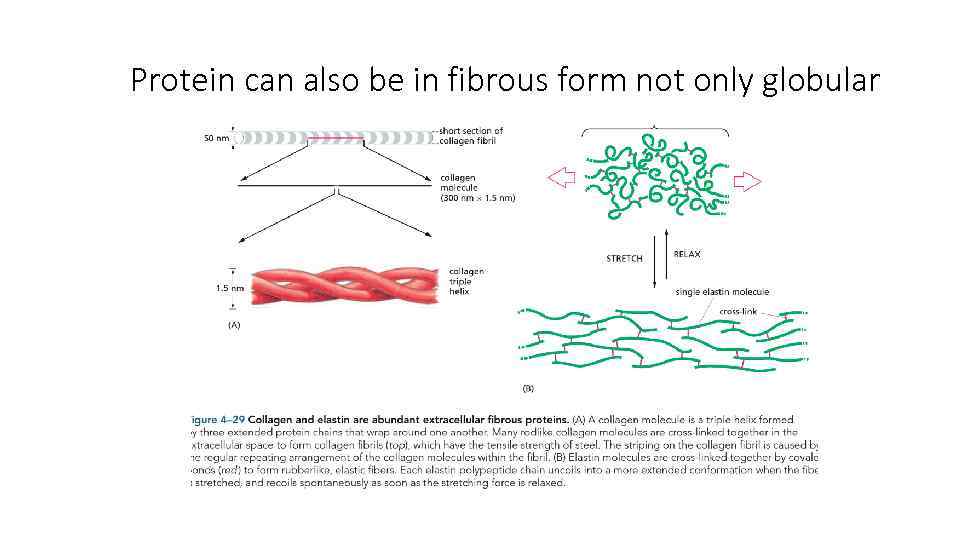
Protein can also be in fibrous form not only globular
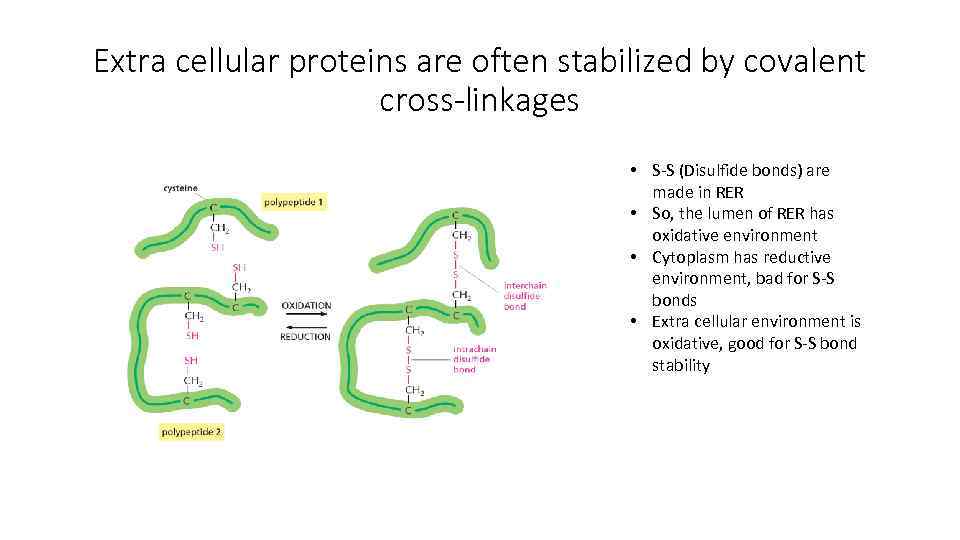
Extra cellular proteins are often stabilized by covalent cross-linkages • S-S (Disulfide bonds) are made in RER • So, the lumen of RER has oxidative environment • Cytoplasm has reductive environment, bad for S-S bonds • Extra cellular environment is oxidative, good for S-S bond stability
HOW PROTEINS WORK?
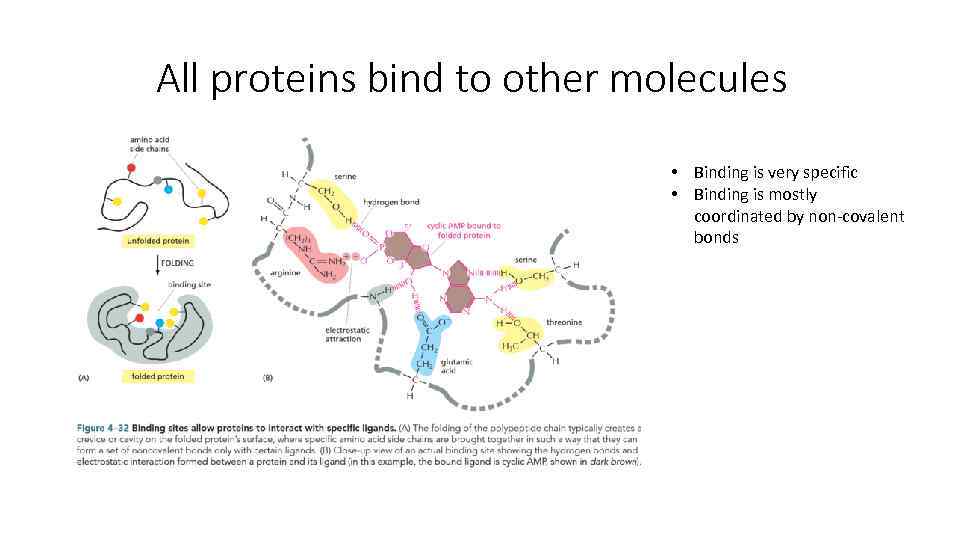
All proteins bind to other molecules • Binding is very specific • Binding is mostly coordinated by non-covalent bonds
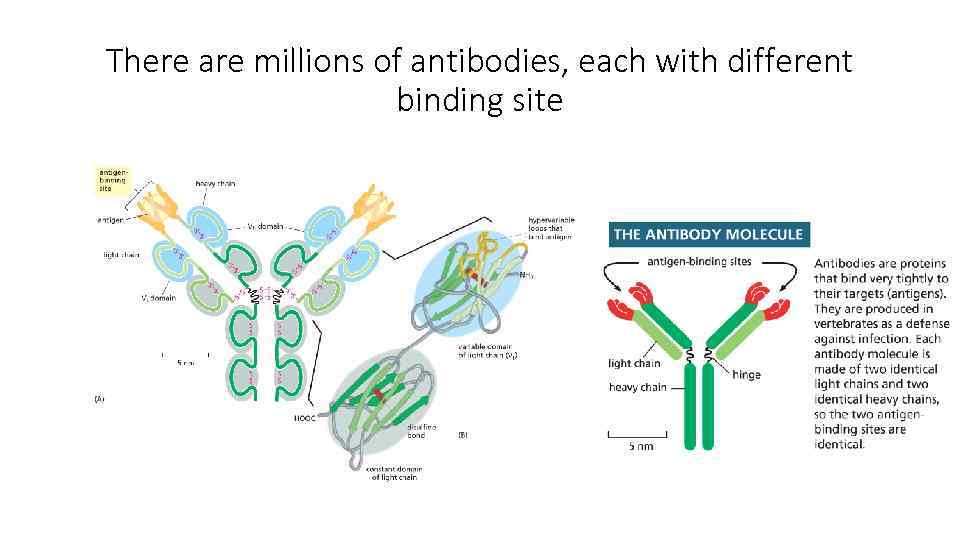
There are millions of antibodies, each with different binding site
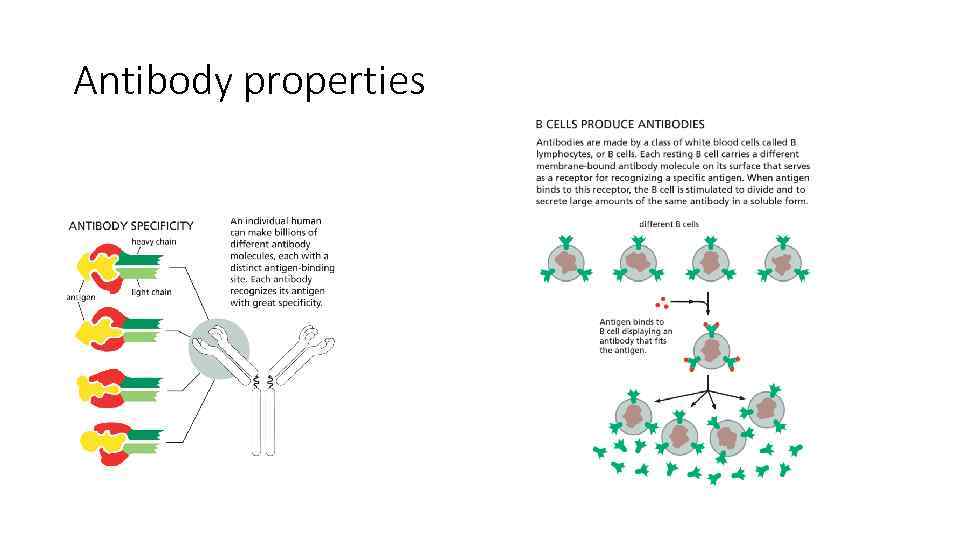
Antibody properties
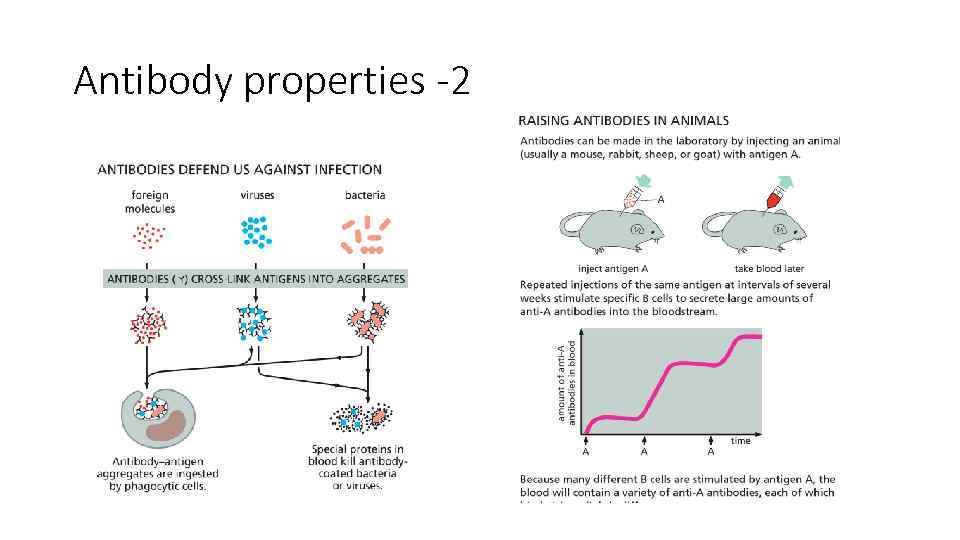
Antibody properties -2
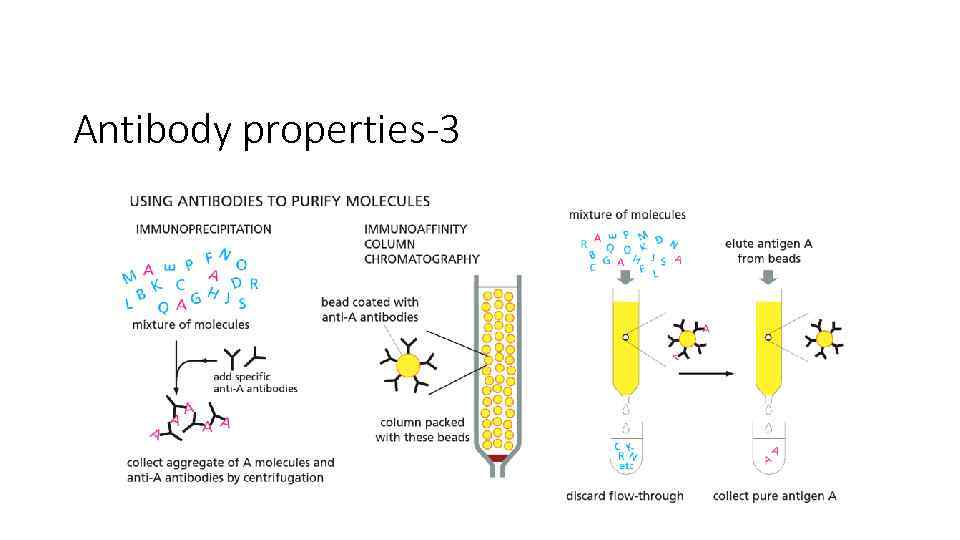
Antibody properties-3

Antibody properties-4
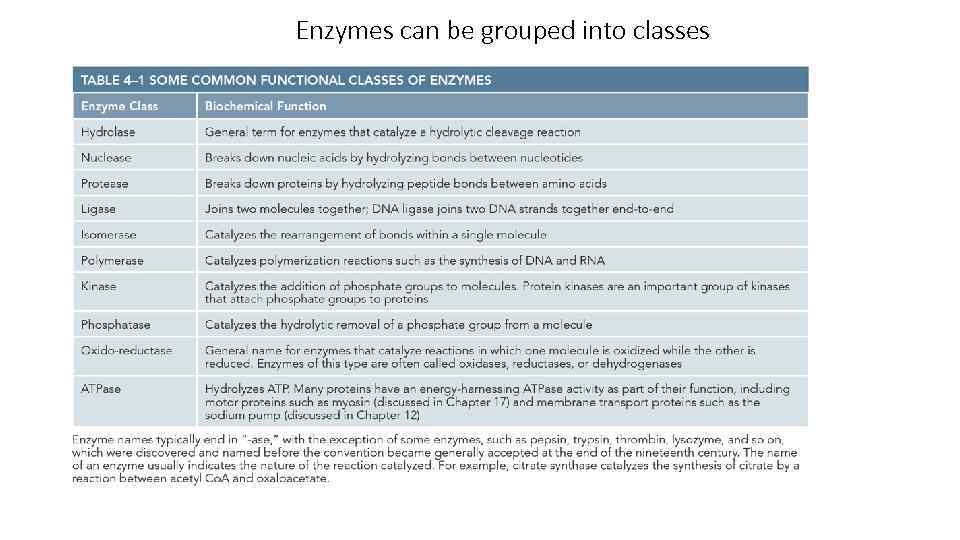
Enzymes can be grouped into classes
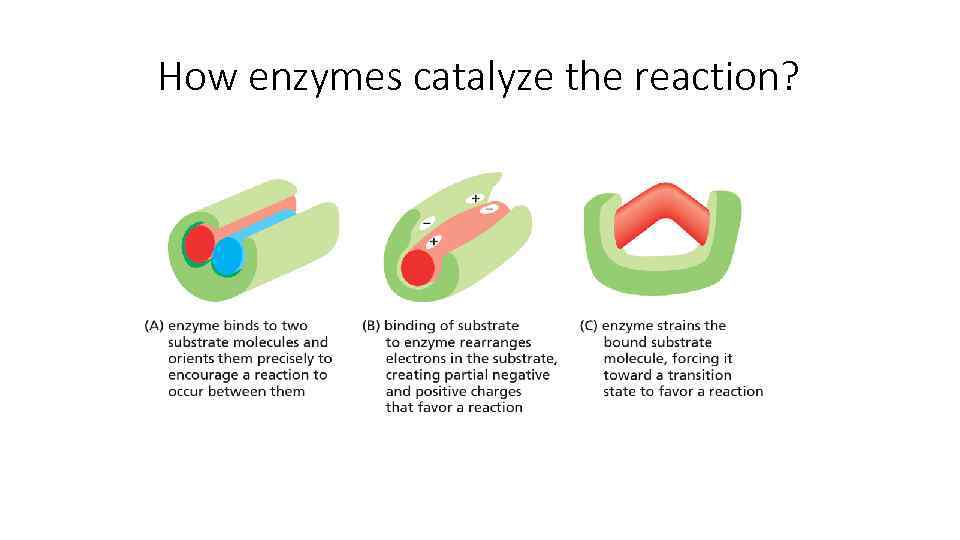
How enzymes catalyze the reaction?
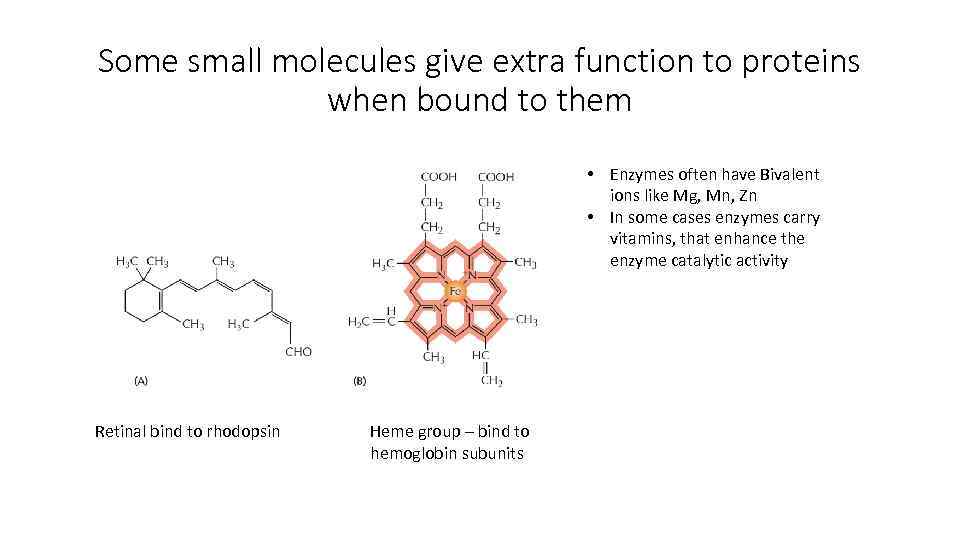
Some small molecules give extra function to proteins when bound to them • Enzymes often have Bivalent ions like Mg, Mn, Zn • In some cases enzymes carry vitamins, that enhance the enzyme catalytic activity Retinal bind to rhodopsin Heme group – bind to hemoglobin subunits
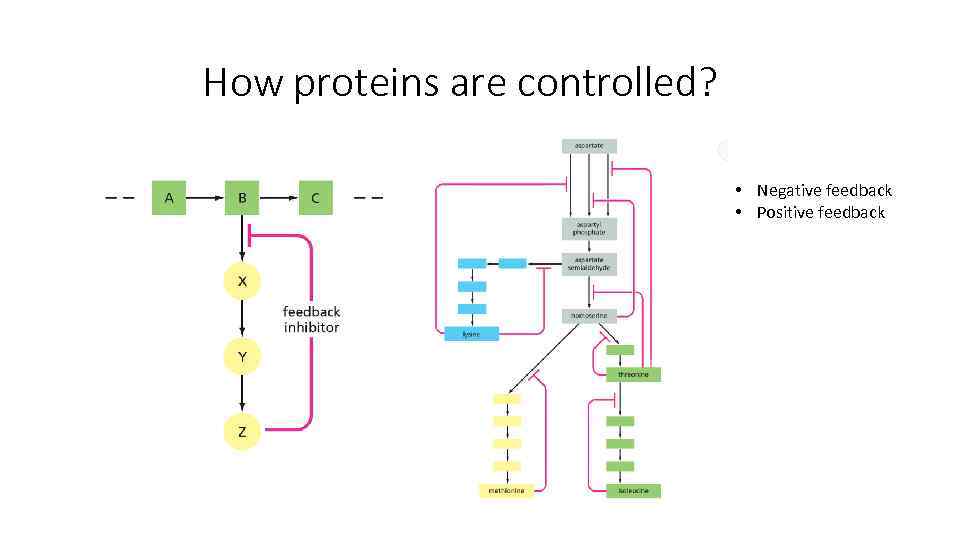
How proteins are controlled? • Negative feedback • Positive feedback
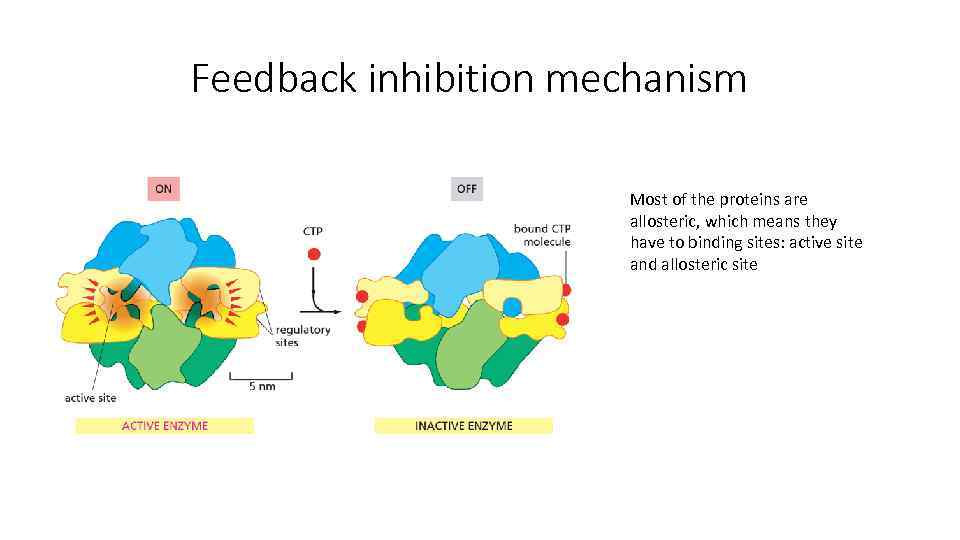
Feedback inhibition mechanism Most of the proteins are allosteric, which means they have to binding sites: active site and allosteric site
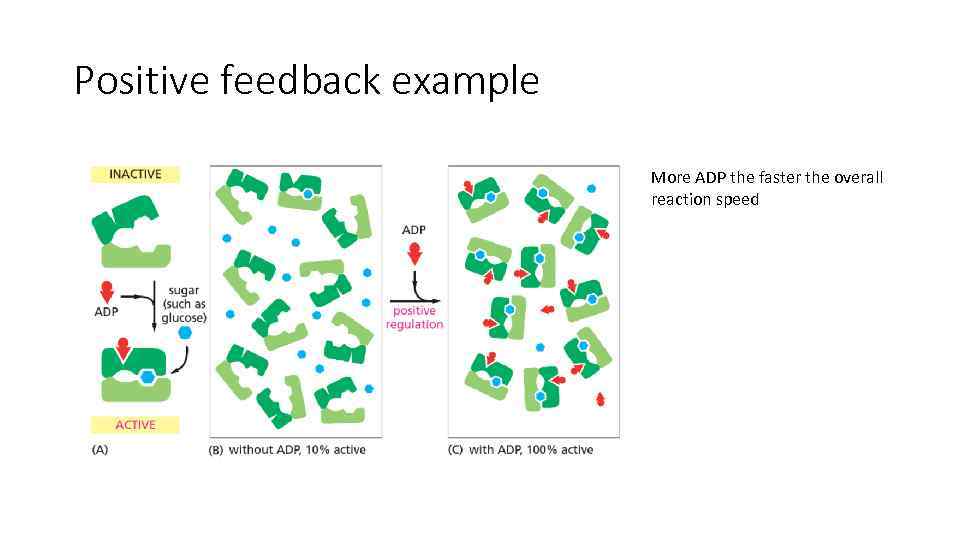
Positive feedback example More ADP the faster the overall reaction speed
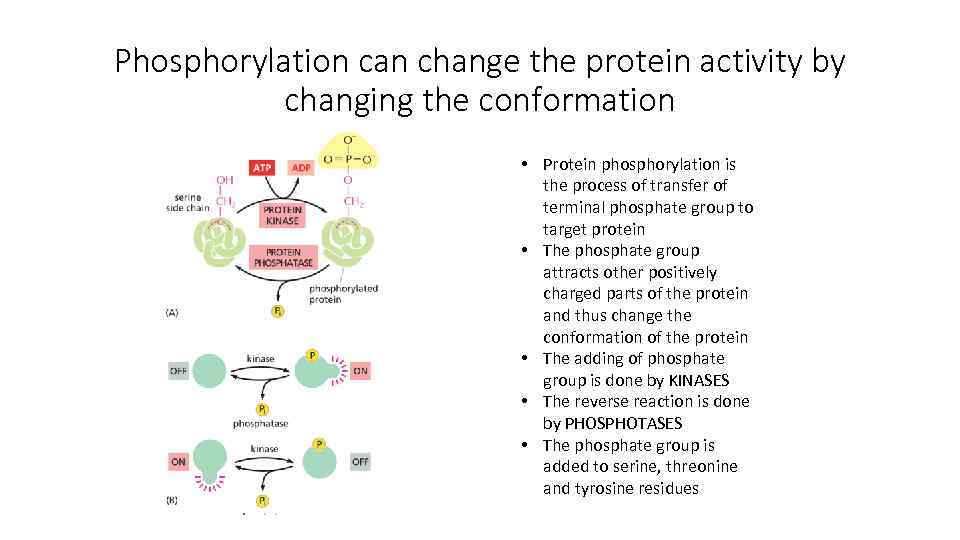
Phosphorylation can change the protein activity by changing the conformation • Protein phosphorylation is the process of transfer of terminal phosphate group to target protein • The phosphate group attracts other positively charged parts of the protein and thus change the conformation of the protein • The adding of phosphate group is done by KINASES • The reverse reaction is done by PHOSPHOTASES • The phosphate group is added to serine, threonine and tyrosine residues
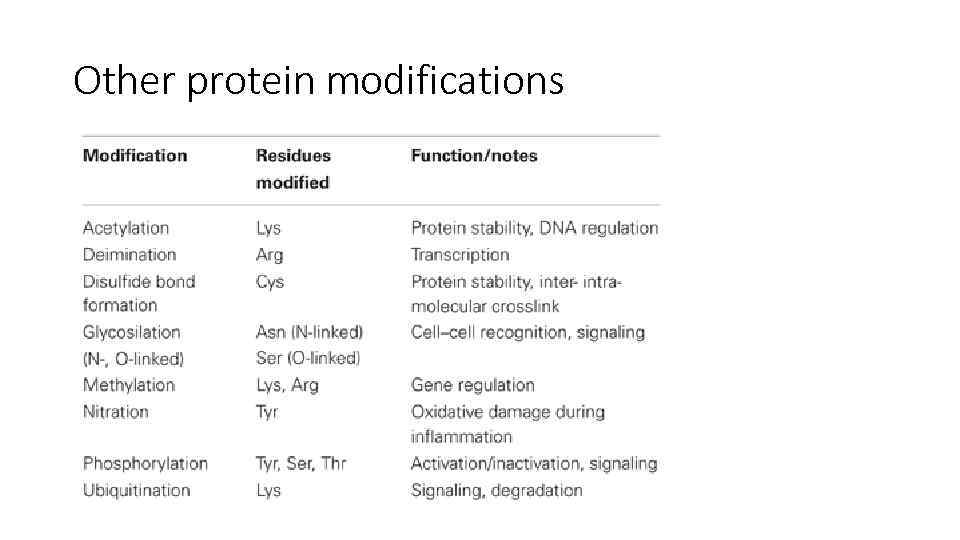
Other protein modifications
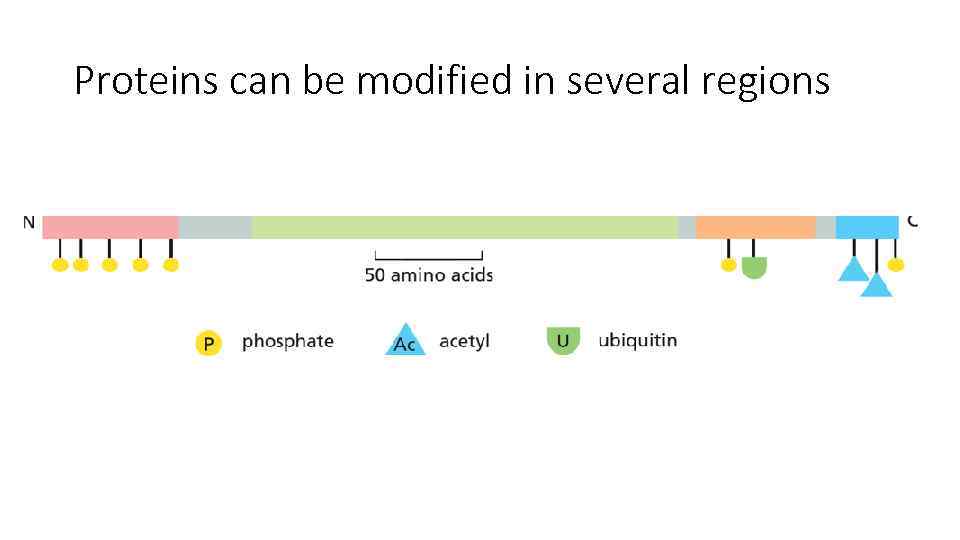
Proteins can be modified in several regions
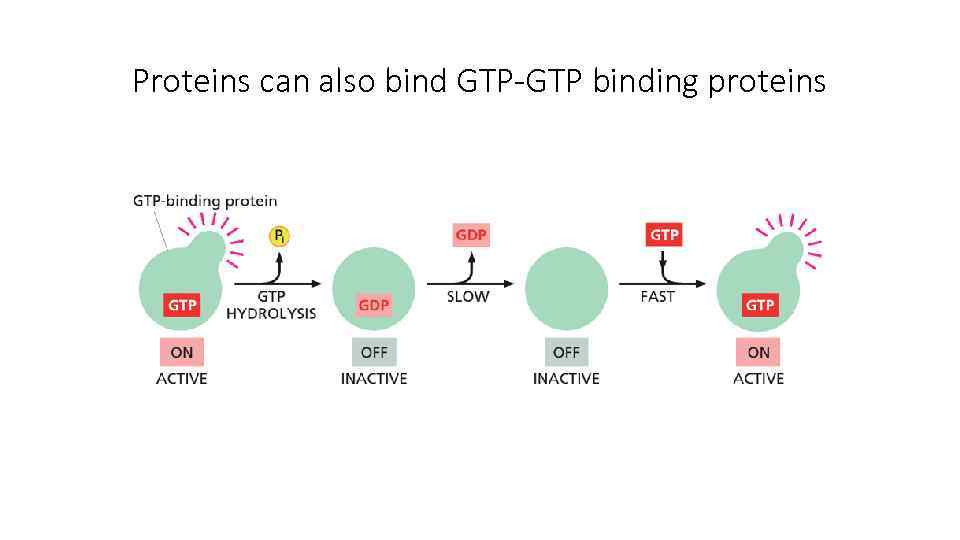
Proteins can also bind GTP-GTP binding proteins
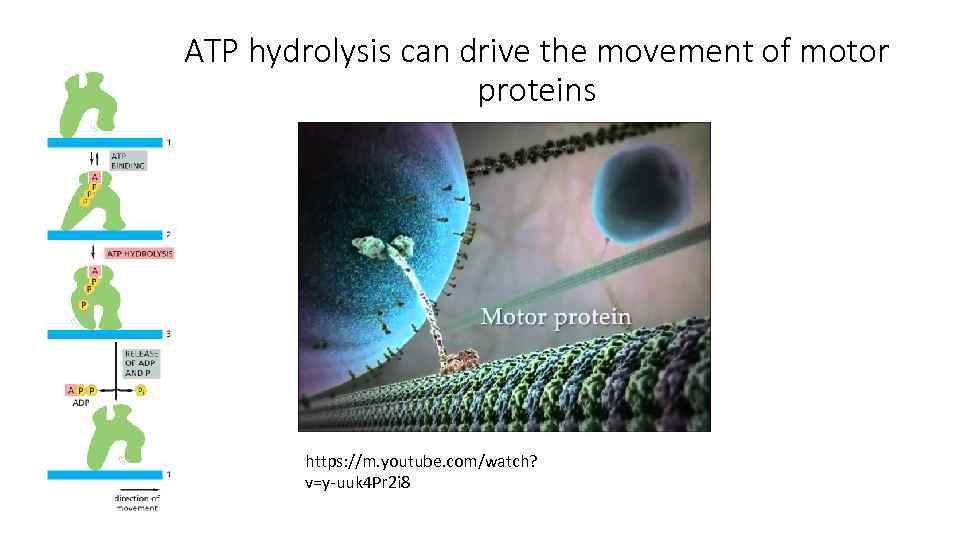
ATP hydrolysis can drive the movement of motor proteins https: //m. youtube. com/watch? v=y-uuk 4 Pr 2 i 8
How proteins are studied?
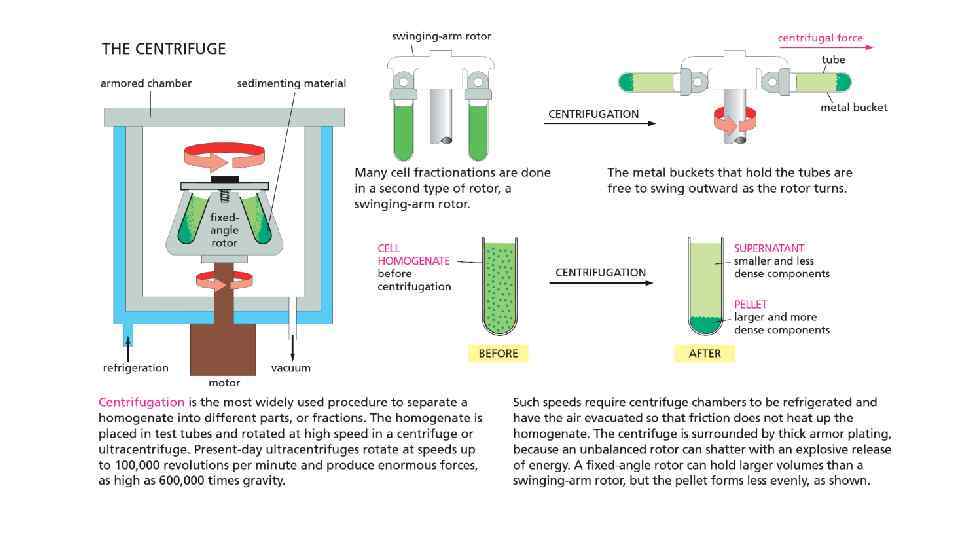
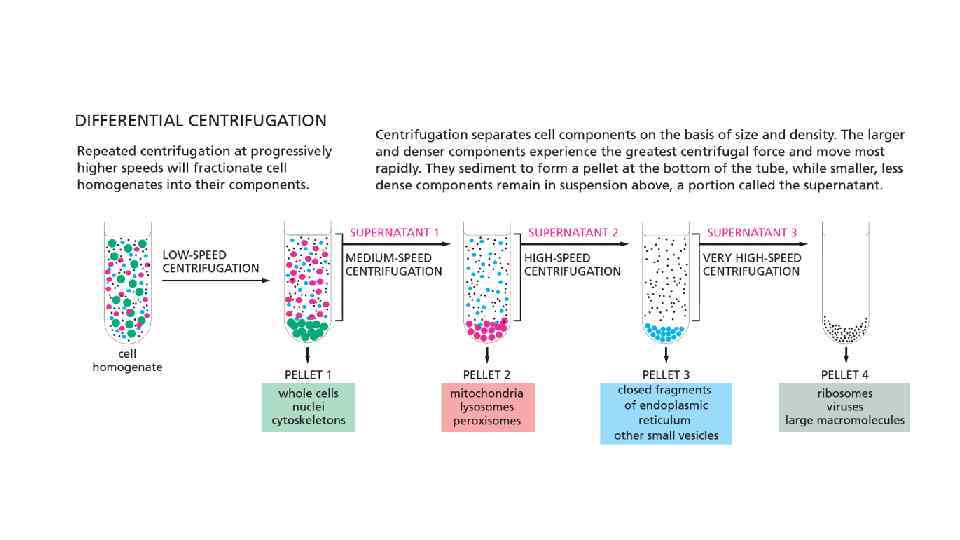
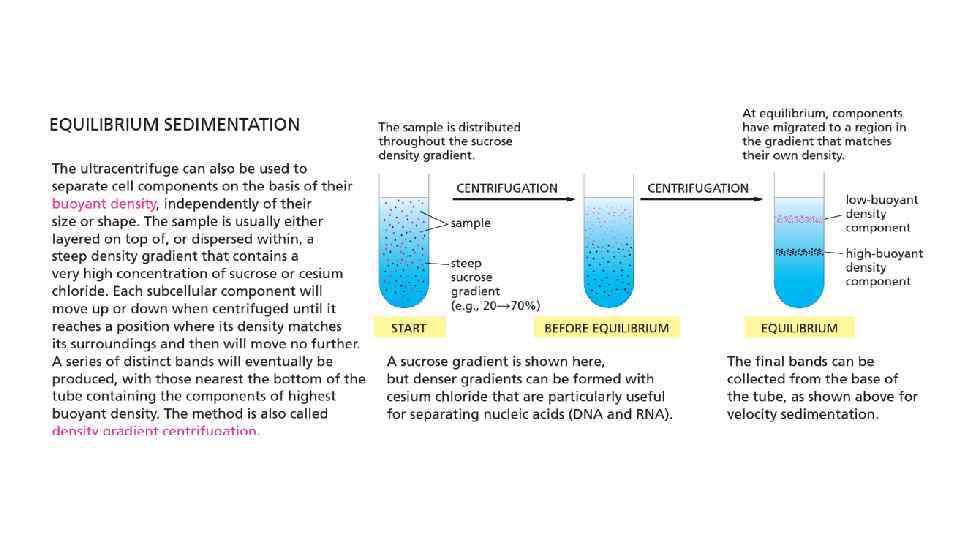
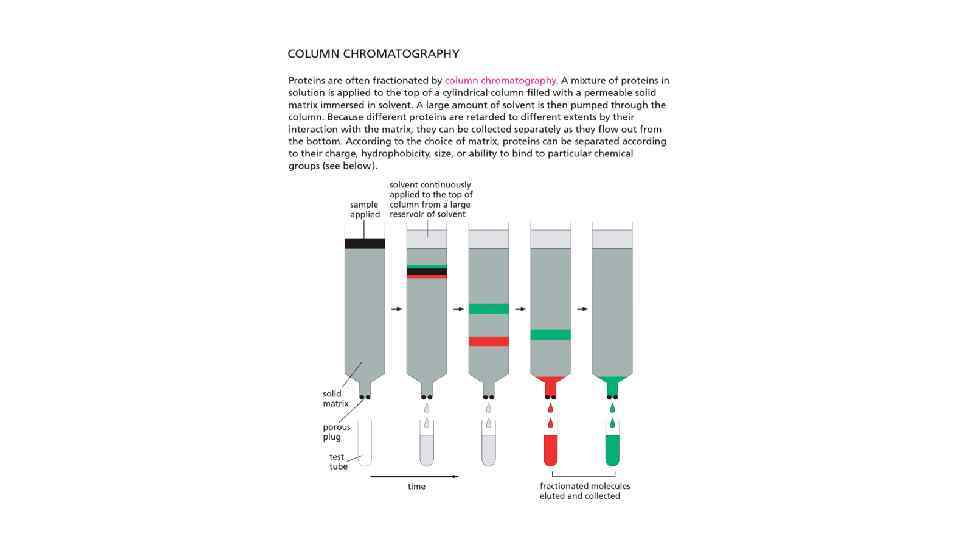
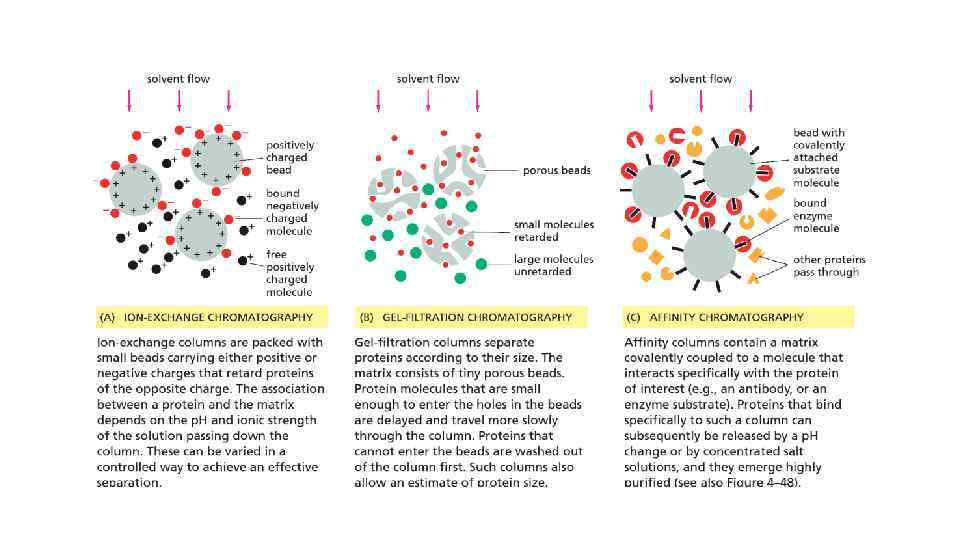
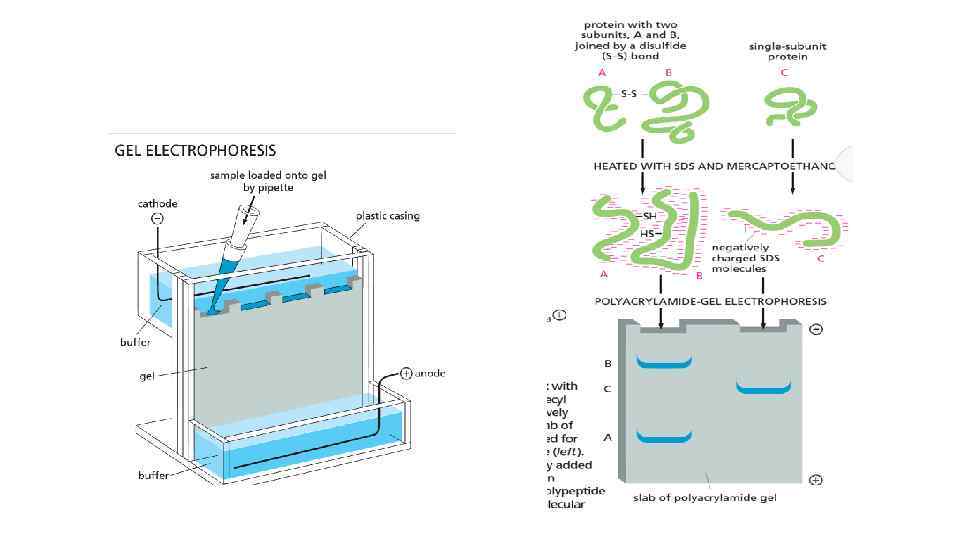


Cell Biology Chapter 4
Protein.pptx