21b2a897eff29f644b3adc43a30fe391.ppt
- Количество слайдов: 84
 Chapter 39 Plant Responses to Internal and External Signals Power. Point Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Chapter 39 Plant Responses to Internal and External Signals Power. Point Lectures for Biology, Seventh Edition Neil Campbell and Jane Reece Lectures by Chris Romero Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Overview: Stimuli and a Stationary Life • Plants, being rooted to the ground – Must respond to whatever environmental change comes their way Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Overview: Stimuli and a Stationary Life • Plants, being rooted to the ground – Must respond to whatever environmental change comes their way Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • For example, the bending of a grass seedling toward light – Begins with the plant sensing the direction, quantity, and color of the light Figure 39. 1 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• For example, the bending of a grass seedling toward light – Begins with the plant sensing the direction, quantity, and color of the light Figure 39. 1 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Concept 39. 1: Signal transduction pathways link signal reception to response • Plants have cellular receptors – That they use to detect important changes in their environment • For a stimulus to elicit a response – Certain cells must have an appropriate receptor Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Concept 39. 1: Signal transduction pathways link signal reception to response • Plants have cellular receptors – That they use to detect important changes in their environment • For a stimulus to elicit a response – Certain cells must have an appropriate receptor Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
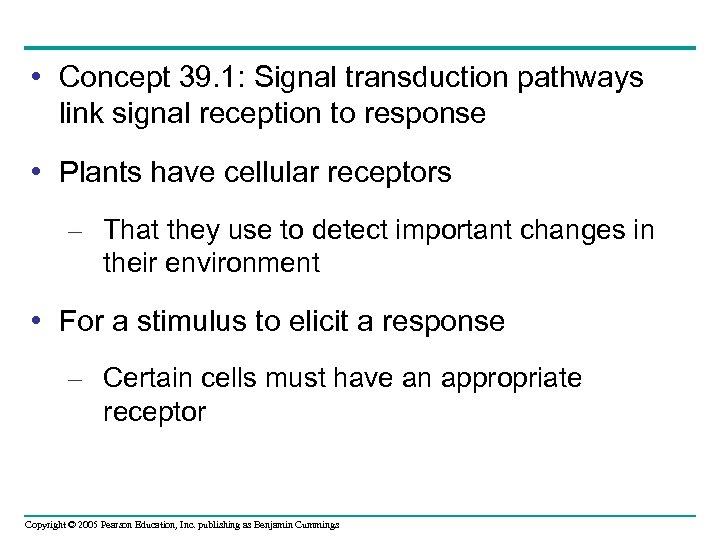
 • A potato left growing in darkness – Will produce shoots that do not appear healthy, and will lack elongated roots • These are morphological adaptations for growing in darkness – Collectively referred to as etiolation (a) Before exposure to light. A dark-grown potato has tall, spindly stems and nonexpanded leaves—morphological adaptations that enable the shoots to penetrate the soil. The roots are short, but there is little need for water absorption because little water is lost by the shoots. Figure 39. 2 a Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• A potato left growing in darkness – Will produce shoots that do not appear healthy, and will lack elongated roots • These are morphological adaptations for growing in darkness – Collectively referred to as etiolation (a) Before exposure to light. A dark-grown potato has tall, spindly stems and nonexpanded leaves—morphological adaptations that enable the shoots to penetrate the soil. The roots are short, but there is little need for water absorption because little water is lost by the shoots. Figure 39. 2 a Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
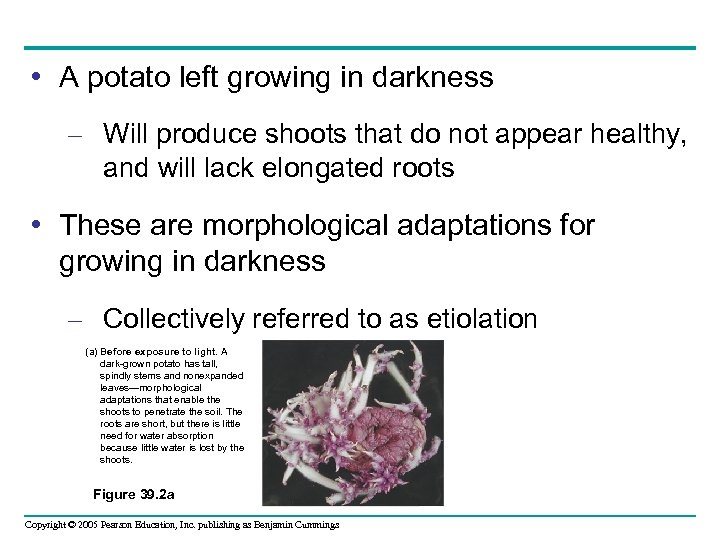
 • After the potato is exposed to light – The plant undergoes profound changes called deetiolation, in which shoots and roots grow normally (b) After a week’s exposure to natural daylight. The potato plant begins to resemble a typical plant with broad green leaves, short sturdy stems, and long roots. This transformation begins with the reception of light by a specific pigment, phytochrome. Figure 39. 2 b Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• After the potato is exposed to light – The plant undergoes profound changes called deetiolation, in which shoots and roots grow normally (b) After a week’s exposure to natural daylight. The potato plant begins to resemble a typical plant with broad green leaves, short sturdy stems, and long roots. This transformation begins with the reception of light by a specific pigment, phytochrome. Figure 39. 2 b Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
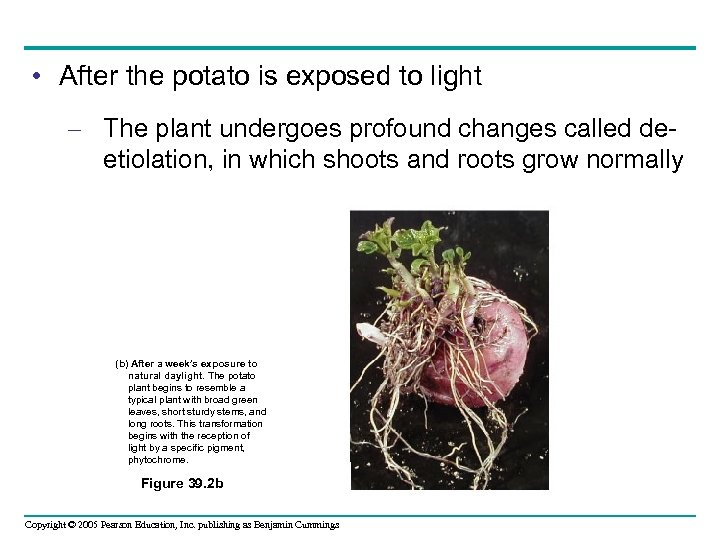
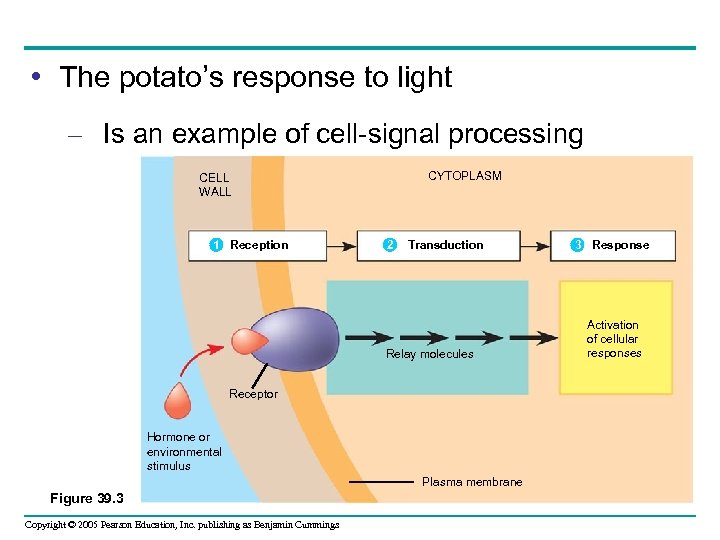 • The potato’s response to light – Is an example of cell-signal processing CELL WALL 1 Reception CYTOPLASM 2 Transduction Relay molecules Receptor Hormone or environmental stimulus Plasma membrane Figure 39. 3 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 3 Response Activation of cellular responses
• The potato’s response to light – Is an example of cell-signal processing CELL WALL 1 Reception CYTOPLASM 2 Transduction Relay molecules Receptor Hormone or environmental stimulus Plasma membrane Figure 39. 3 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 3 Response Activation of cellular responses
 Reception • Internal and external signals are detected by receptors – Proteins that change in response to specific stimuli Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Reception • Internal and external signals are detected by receptors – Proteins that change in response to specific stimuli Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Transduction • Second messengers – Transfer and amplify signals from receptors to proteins that cause specific responses Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Transduction • Second messengers – Transfer and amplify signals from receptors to proteins that cause specific responses Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
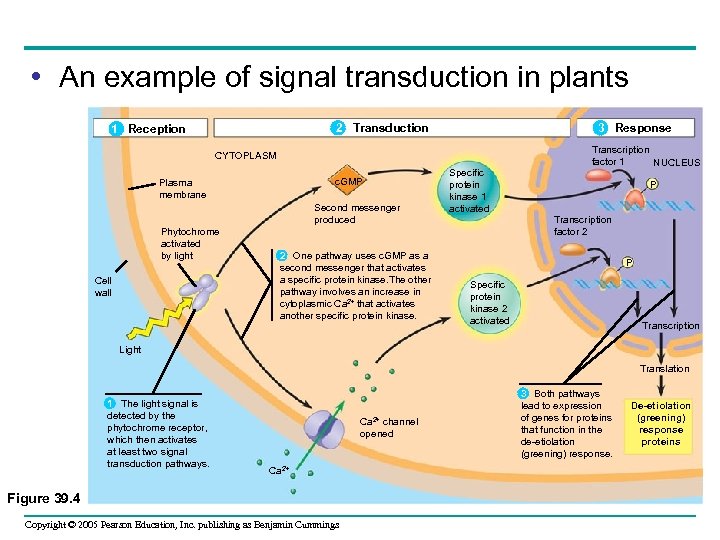 • An example of signal transduction in plants 2 Transduction 1 Reception 3 Response CYTOPLASM c. GMP Plasma membrane Second messenger produced Phytochrome activated by light Cell wall 2 One pathway uses c. GMP as a second messenger that activates a specific protein kinase. The other pathway involves an increase in cytoplasmic Ca 2+ that activates another specific protein kinase. Specific protein kinase 1 activated Transcription factor 1 NUCLEUS P Transcription factor 2 P Specific protein kinase 2 activated Transcription Light Translation 1 The light signal is detected by the phytochrome receptor, which then activates at least two signal transduction pathways. Ca 2+ channel opened Ca 2+ Figure 39. 4 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 3 Both pathways lead to expression of genes for proteins that function in the de-etiolation (greening) response. De-etiolation (greening) response proteins
• An example of signal transduction in plants 2 Transduction 1 Reception 3 Response CYTOPLASM c. GMP Plasma membrane Second messenger produced Phytochrome activated by light Cell wall 2 One pathway uses c. GMP as a second messenger that activates a specific protein kinase. The other pathway involves an increase in cytoplasmic Ca 2+ that activates another specific protein kinase. Specific protein kinase 1 activated Transcription factor 1 NUCLEUS P Transcription factor 2 P Specific protein kinase 2 activated Transcription Light Translation 1 The light signal is detected by the phytochrome receptor, which then activates at least two signal transduction pathways. Ca 2+ channel opened Ca 2+ Figure 39. 4 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 3 Both pathways lead to expression of genes for proteins that function in the de-etiolation (greening) response. De-etiolation (greening) response proteins
 Response • Ultimately, a signal transduction pathway – Leads to a regulation of one or more cellular activities • In most cases – These responses to stimulation involve the increased activity of certain enzymes Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Response • Ultimately, a signal transduction pathway – Leads to a regulation of one or more cellular activities • In most cases – These responses to stimulation involve the increased activity of certain enzymes Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Transcriptional Regulation • Transcription factors bind directly to specific regions of DNA – And control the transcription of specific genes Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Transcriptional Regulation • Transcription factors bind directly to specific regions of DNA – And control the transcription of specific genes Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Post-Translational Modification of Proteins • Post-translational modification – Involves the activation of existing proteins involved in the signal response Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Post-Translational Modification of Proteins • Post-translational modification – Involves the activation of existing proteins involved in the signal response Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 De-Etioloation (“Greening”) Proteins • Many enzymes that function in certain signal responses are involved in photosynthesis directly – While others are involved in supplying the chemical precursors necessary for chlorophyll production Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
De-Etioloation (“Greening”) Proteins • Many enzymes that function in certain signal responses are involved in photosynthesis directly – While others are involved in supplying the chemical precursors necessary for chlorophyll production Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Concept 39. 2: Plant hormones help coordinate growth, development, and responses to stimuli • Hormones – Are chemical signals that coordinate the different parts of an organism Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Concept 39. 2: Plant hormones help coordinate growth, development, and responses to stimuli • Hormones – Are chemical signals that coordinate the different parts of an organism Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 The Discovery of Plant Hormones • Any growth response – That results in curvatures of whole plant organs toward or away from a stimulus is called a tropism – Is often caused by hormones Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
The Discovery of Plant Hormones • Any growth response – That results in curvatures of whole plant organs toward or away from a stimulus is called a tropism – Is often caused by hormones Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Charles Darwin and his son Francis – Conducted some of the earliest experiments on phototropism, a plant’s response to light, in the late 19 th century Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Charles Darwin and his son Francis – Conducted some of the earliest experiments on phototropism, a plant’s response to light, in the late 19 th century Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
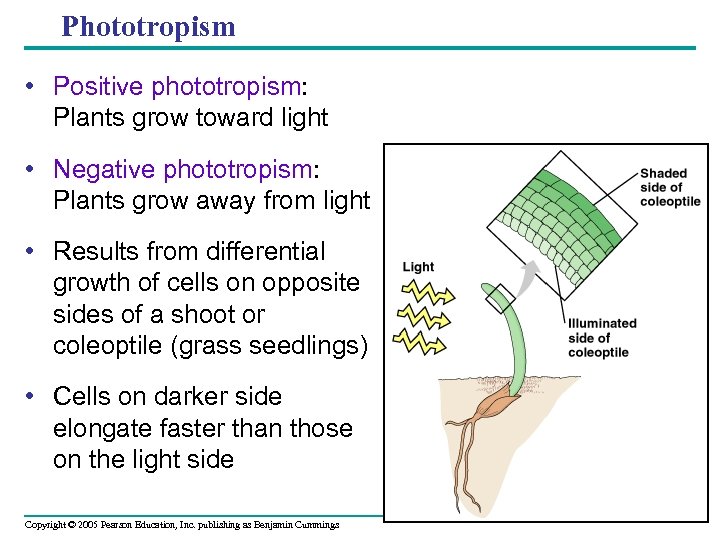 Phototropism • Positive phototropism: Plants grow toward light • Negative phototropism: Plants grow away from light • Results from differential growth of cells on opposite sides of a shoot or coleoptile (grass seedlings) • Cells on darker side elongate faster than those on the light side Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Phototropism • Positive phototropism: Plants grow toward light • Negative phototropism: Plants grow away from light • Results from differential growth of cells on opposite sides of a shoot or coleoptile (grass seedlings) • Cells on darker side elongate faster than those on the light side Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
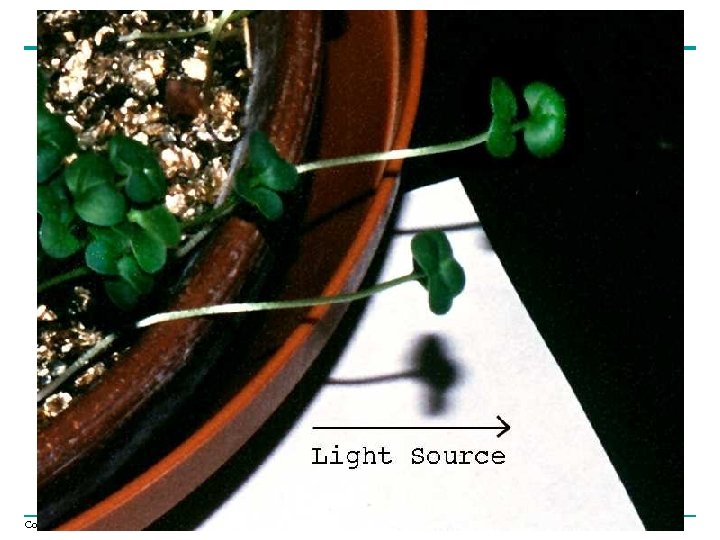
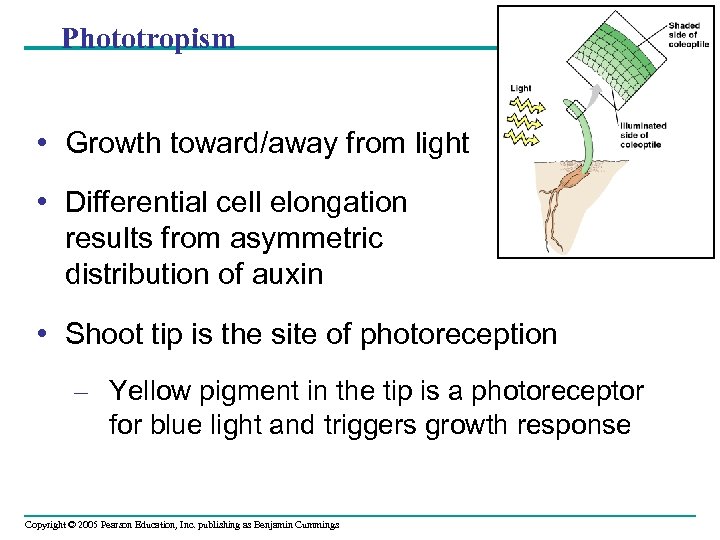 Phototropism • Growth toward/away from light • Differential cell elongation results from asymmetric distribution of auxin • Shoot tip is the site of photoreception – Yellow pigment in the tip is a photoreceptor for blue light and triggers growth response Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Phototropism • Growth toward/away from light • Differential cell elongation results from asymmetric distribution of auxin • Shoot tip is the site of photoreception – Yellow pigment in the tip is a photoreceptor for blue light and triggers growth response Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
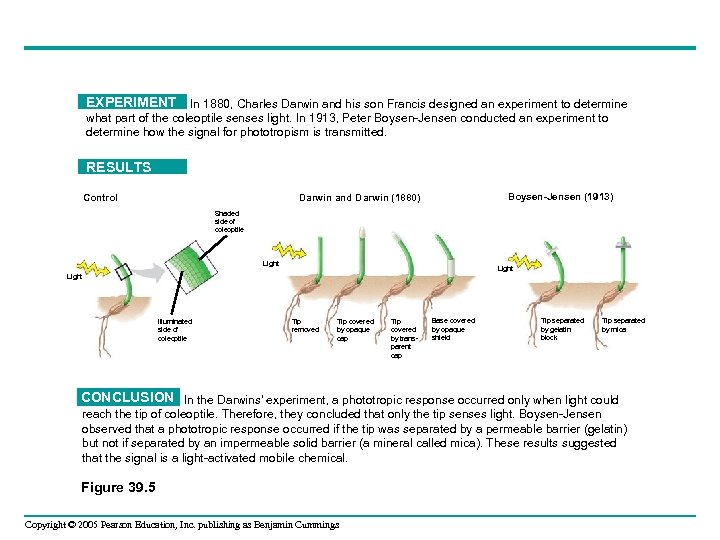 EXPERIMENT In 1880, Charles Darwin and his son Francis designed an experiment to determine what part of the coleoptile senses light. In 1913, Peter Boysen-Jensen conducted an experiment to determine how the signal for phototropism is transmitted. RESULTS Control Boysen-Jensen (1913) Darwin and Darwin (1880) Shaded side of coleoptile Light Illuminated side of coleoptile Tip removed Tip covered by opaque cap Tip covered by transparent cap Base covered by opaque shield Tip separated by gelatin block Tip separated by mica CONCLUSION In the Darwins’ experiment, a phototropic response occurred only when light could reach the tip of coleoptile. Therefore, they concluded that only the tip senses light. Boysen-Jensen observed that a phototropic response occurred if the tip was separated by a permeable barrier (gelatin) but not if separated by an impermeable solid barrier (a mineral called mica). These results suggested that the signal is a light-activated mobile chemical. Figure 39. 5 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
EXPERIMENT In 1880, Charles Darwin and his son Francis designed an experiment to determine what part of the coleoptile senses light. In 1913, Peter Boysen-Jensen conducted an experiment to determine how the signal for phototropism is transmitted. RESULTS Control Boysen-Jensen (1913) Darwin and Darwin (1880) Shaded side of coleoptile Light Illuminated side of coleoptile Tip removed Tip covered by opaque cap Tip covered by transparent cap Base covered by opaque shield Tip separated by gelatin block Tip separated by mica CONCLUSION In the Darwins’ experiment, a phototropic response occurred only when light could reach the tip of coleoptile. Therefore, they concluded that only the tip senses light. Boysen-Jensen observed that a phototropic response occurred if the tip was separated by a permeable barrier (gelatin) but not if separated by an impermeable solid barrier (a mineral called mica). These results suggested that the signal is a light-activated mobile chemical. Figure 39. 5 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
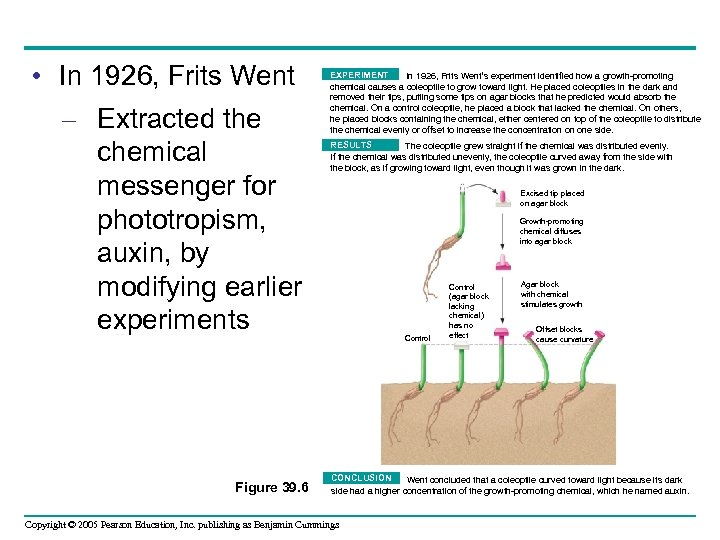 • In 1926, Frits Went – Extracted the chemical messenger for phototropism, auxin, by modifying earlier experiments Figure 39. 6 EXPERIMENT In 1926, Frits Went’s experiment identified how a growth-promoting chemical causes a coleoptile to grow toward light. He placed coleoptiles in the dark and removed their tips, putting some tips on agar blocks that he predicted would absorb the chemical. On a control coleoptile, he placed a block that lacked the chemical. On others, he placed blocks containing the chemical, either centered on top of the coleoptile to distribute the chemical evenly or offset to increase the concentration on one side. RESULTS The coleoptile grew straight if the chemical was distributed evenly. If the chemical was distributed unevenly, the coleoptile curved away from the side with the block, as if growing toward light, even though it was grown in the dark. Excised tip placed on agar block Growth-promoting chemical diffuses into agar block Control (agar block lacking chemical) has no effect Agar block with chemical stimulates growth Offset blocks cause curvature CONCLUSION Went concluded that a coleoptile curved toward light because its dark side had a higher concentration of the growth-promoting chemical, which he named auxin. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• In 1926, Frits Went – Extracted the chemical messenger for phototropism, auxin, by modifying earlier experiments Figure 39. 6 EXPERIMENT In 1926, Frits Went’s experiment identified how a growth-promoting chemical causes a coleoptile to grow toward light. He placed coleoptiles in the dark and removed their tips, putting some tips on agar blocks that he predicted would absorb the chemical. On a control coleoptile, he placed a block that lacked the chemical. On others, he placed blocks containing the chemical, either centered on top of the coleoptile to distribute the chemical evenly or offset to increase the concentration on one side. RESULTS The coleoptile grew straight if the chemical was distributed evenly. If the chemical was distributed unevenly, the coleoptile curved away from the side with the block, as if growing toward light, even though it was grown in the dark. Excised tip placed on agar block Growth-promoting chemical diffuses into agar block Control (agar block lacking chemical) has no effect Agar block with chemical stimulates growth Offset blocks cause curvature CONCLUSION Went concluded that a coleoptile curved toward light because its dark side had a higher concentration of the growth-promoting chemical, which he named auxin. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
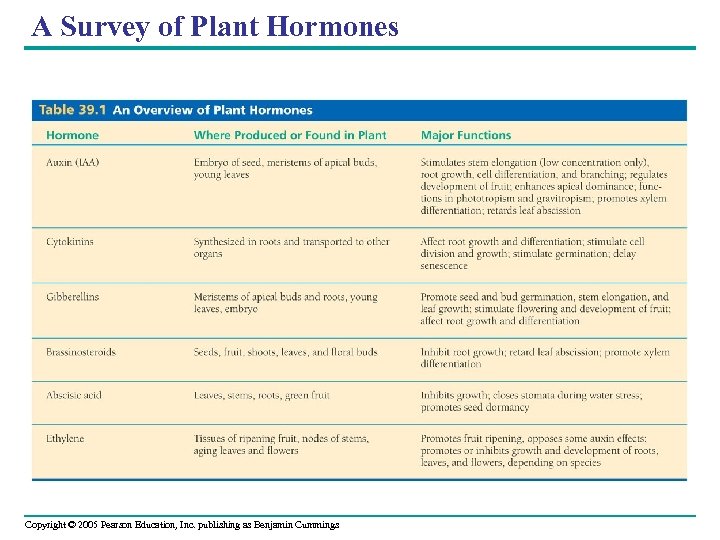 A Survey of Plant Hormones Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
A Survey of Plant Hormones Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • In general, hormones control plant growth and development – By affecting the division, elongation, and differentiation of cells • Plant hormones are produced in very low concentrations – But a minute amount can have a profound effect on the growth and development of a plant organ Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• In general, hormones control plant growth and development – By affecting the division, elongation, and differentiation of cells • Plant hormones are produced in very low concentrations – But a minute amount can have a profound effect on the growth and development of a plant organ Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Auxin • The term auxin – Is used for any chemical substance that promotes cell elongation in different target tissues • Promotes elongation of young developing shoots or coleoptiles • Major site of production is the apical meristem • Moves from the apex down to the zone of cell elongation – Polar transport is unidirectional and requires metabolic energy which is provided by chemiosmosis – Movement aided by differences in p. H between the acidic cell wall and the neutral cytoplasm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Auxin • The term auxin – Is used for any chemical substance that promotes cell elongation in different target tissues • Promotes elongation of young developing shoots or coleoptiles • Major site of production is the apical meristem • Moves from the apex down to the zone of cell elongation – Polar transport is unidirectional and requires metabolic energy which is provided by chemiosmosis – Movement aided by differences in p. H between the acidic cell wall and the neutral cytoplasm Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 The Role of Auxin in Cell Elongation • According to a model called the acid growth hypothesis – Proton pumps play a major role in the growth response of cells to auxin Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
The Role of Auxin in Cell Elongation • According to a model called the acid growth hypothesis – Proton pumps play a major role in the growth response of cells to auxin Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
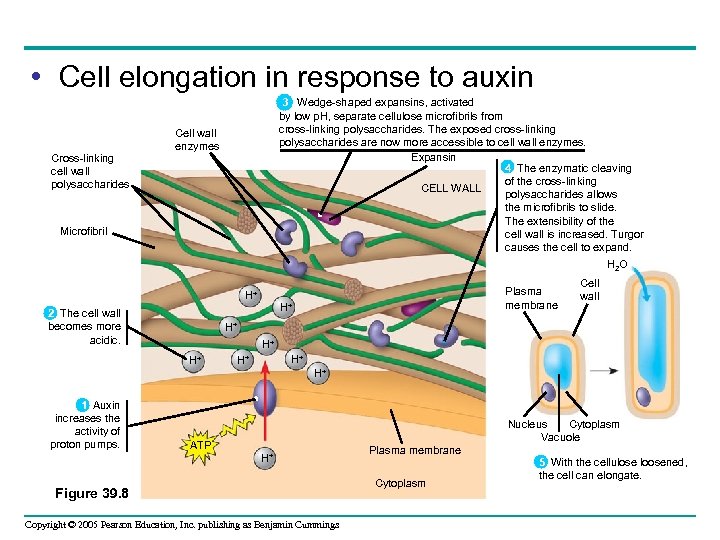 • Cell elongation in response to auxin Cross-linking cell wall polysaccharides 3 Wedge-shaped expansins, activated by low p. H, separate cellulose microfibrils from cross-linking polysaccharides. The exposed cross-linking polysaccharides are now more accessible to cell wall enzymes. Expansin 4 The enzymatic cleaving of the cross-linking CELL WALL polysaccharides allows the microfibrils to slide. The extensibility of the cell wall is increased. Turgor causes the cell to expand. Cell wall enzymes Microfibril H 2 O H+ 2 The cell wall becomes more acidic. Plasma membrane H+ Cell wall H+ H+ H+ 1 Auxin increases the activity of proton pumps. Cytoplasm Nucleus Vacuole ATP H+ Figure 39. 8 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Plasma membrane Cytoplasm 5 With the cellulose loosened, the cell can elongate.
• Cell elongation in response to auxin Cross-linking cell wall polysaccharides 3 Wedge-shaped expansins, activated by low p. H, separate cellulose microfibrils from cross-linking polysaccharides. The exposed cross-linking polysaccharides are now more accessible to cell wall enzymes. Expansin 4 The enzymatic cleaving of the cross-linking CELL WALL polysaccharides allows the microfibrils to slide. The extensibility of the cell wall is increased. Turgor causes the cell to expand. Cell wall enzymes Microfibril H 2 O H+ 2 The cell wall becomes more acidic. Plasma membrane H+ Cell wall H+ H+ H+ 1 Auxin increases the activity of proton pumps. Cytoplasm Nucleus Vacuole ATP H+ Figure 39. 8 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Plasma membrane Cytoplasm 5 With the cellulose loosened, the cell can elongate.
 Other functions of Auxin • Affects secondary growth by inducing vascular cambium cell division and differentiation of secondary xylem • Promotes formation of adventitious roots • Involved in the formation and branching of roots • Promotes fruit growth in many plants • Can be used as herbicides – 2, 4 -D—synthetic auxin which affects dicots selectively, allowing removal of broadleaf weeds from a lawn Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Other functions of Auxin • Affects secondary growth by inducing vascular cambium cell division and differentiation of secondary xylem • Promotes formation of adventitious roots • Involved in the formation and branching of roots • Promotes fruit growth in many plants • Can be used as herbicides – 2, 4 -D—synthetic auxin which affects dicots selectively, allowing removal of broadleaf weeds from a lawn Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Cytokinins • Cytokinins • Modified forms of adenine that stimulate cytokinesis • Function in several areas of plant growth – Stimulates cell division and differentiation – Apical dominance – Anti-aging hormones • Effect complemented or countered by auxin Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Cytokinins • Cytokinins • Modified forms of adenine that stimulate cytokinesis • Function in several areas of plant growth – Stimulates cell division and differentiation – Apical dominance – Anti-aging hormones • Effect complemented or countered by auxin Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Control of Cell Division and Differentiation • Cytokinins – Are produced in actively growing tissues such as roots, embryos, and fruits • Move from the roots to target tissues by moving up in the xylem sap • Stimulate production of RNA and protein involved with cell division • Works in conjunction with auxin – Stem parenchyma cells grown without cytokinins grow large but don’t divide – Cytokinins alone have no affect on cells – Cytokinins = auxin stimulate cell growth and division, but they remain an undifferentiated callus – Cytokinin > auxin causes shoot buds to develop from callus – Cytokinin < auxin causes roots to form Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Control of Cell Division and Differentiation • Cytokinins – Are produced in actively growing tissues such as roots, embryos, and fruits • Move from the roots to target tissues by moving up in the xylem sap • Stimulate production of RNA and protein involved with cell division • Works in conjunction with auxin – Stem parenchyma cells grown without cytokinins grow large but don’t divide – Cytokinins alone have no affect on cells – Cytokinins = auxin stimulate cell growth and division, but they remain an undifferentiated callus – Cytokinin > auxin causes shoot buds to develop from callus – Cytokinin < auxin causes roots to form Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
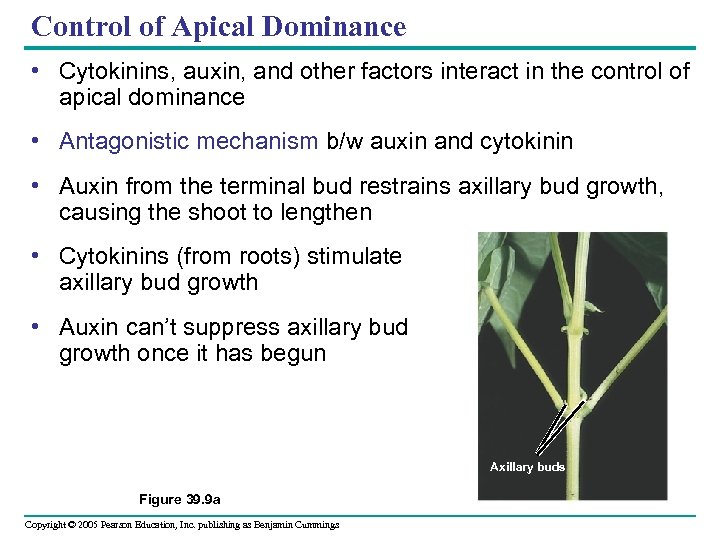 Control of Apical Dominance • Cytokinins, auxin, and other factors interact in the control of apical dominance • Antagonistic mechanism b/w auxin and cytokinin • Auxin from the terminal bud restrains axillary bud growth, causing the shoot to lengthen • Cytokinins (from roots) stimulate axillary bud growth • Auxin can’t suppress axillary bud growth once it has begun Axillary buds Figure 39. 9 a Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Control of Apical Dominance • Cytokinins, auxin, and other factors interact in the control of apical dominance • Antagonistic mechanism b/w auxin and cytokinin • Auxin from the terminal bud restrains axillary bud growth, causing the shoot to lengthen • Cytokinins (from roots) stimulate axillary bud growth • Auxin can’t suppress axillary bud growth once it has begun Axillary buds Figure 39. 9 a Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
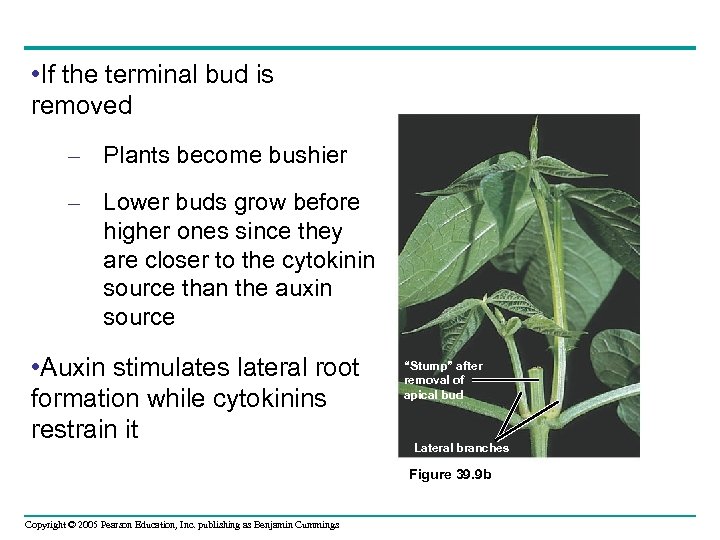 • If the terminal bud is removed – Plants become bushier – Lower buds grow before higher ones since they are closer to the cytokinin source than the auxin source • Auxin stimulates lateral root formation while cytokinins restrain it “Stump” after removal of apical bud Lateral branches Figure 39. 9 b Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• If the terminal bud is removed – Plants become bushier – Lower buds grow before higher ones since they are closer to the cytokinin source than the auxin source • Auxin stimulates lateral root formation while cytokinins restrain it “Stump” after removal of apical bud Lateral branches Figure 39. 9 b Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Anti-Aging Effects • Cytokinins retard the aging of some plant organs – By inhibiting protein breakdown, stimulating RNA and protein synthesis, and mobilizing nutrients from surrounding tissues – May slow leaf deterioration on plants since detached leaves dipped in a cytokinin solution stay green longer than without Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Anti-Aging Effects • Cytokinins retard the aging of some plant organs – By inhibiting protein breakdown, stimulating RNA and protein synthesis, and mobilizing nutrients from surrounding tissues – May slow leaf deterioration on plants since detached leaves dipped in a cytokinin solution stay green longer than without Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
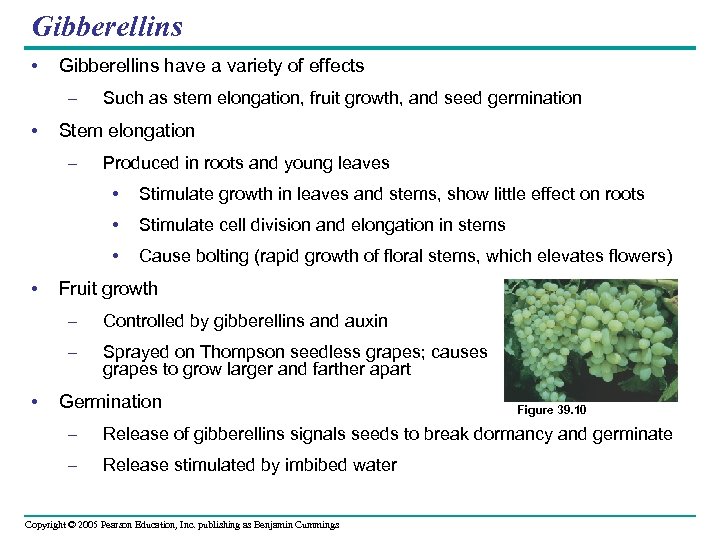 Gibberellins • Gibberellins have a variety of effects – • Such as stem elongation, fruit growth, and seed germination Stem elongation – Produced in roots and young leaves • • Stimulate cell division and elongation in stems • • Stimulate growth in leaves and stems, show little effect on roots Cause bolting (rapid growth of floral stems, which elevates flowers) Fruit growth – – • Controlled by gibberellins and auxin Sprayed on Thompson seedless grapes; causes grapes to grow larger and farther apart Germination Figure 39. 10 – Release of gibberellins signals seeds to break dormancy and germinate – Release stimulated by imbibed water Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Gibberellins • Gibberellins have a variety of effects – • Such as stem elongation, fruit growth, and seed germination Stem elongation – Produced in roots and young leaves • • Stimulate cell division and elongation in stems • • Stimulate growth in leaves and stems, show little effect on roots Cause bolting (rapid growth of floral stems, which elevates flowers) Fruit growth – – • Controlled by gibberellins and auxin Sprayed on Thompson seedless grapes; causes grapes to grow larger and farther apart Germination Figure 39. 10 – Release of gibberellins signals seeds to break dormancy and germinate – Release stimulated by imbibed water Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
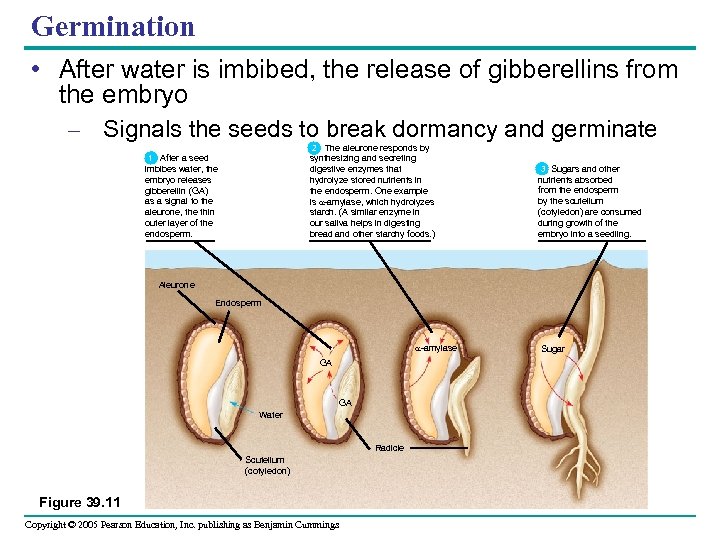 Germination • After water is imbibed, the release of gibberellins from the embryo – Signals the seeds to break dormancy and germinate 2 The aleurone responds by 2 synthesizing and secreting digestive enzymes that hydrolyze stored nutrients in the endosperm. One example is -amylase, which hydrolyzes starch. (A similar enzyme in our saliva helps in digesting bread and other starchy foods. ) 1 After a seed imbibes water, the embryo releases gibberellin (GA) as a signal to the aleurone, the thin outer layer of the endosperm. 3 Sugars and other nutrients absorbed from the endosperm by the scutellum (cotyledon) are consumed during growth of the embryo into a seedling. Aleurone Endosperm -amylase GA GA Water Radicle Scutellum (cotyledon) Figure 39. 11 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Sugar
Germination • After water is imbibed, the release of gibberellins from the embryo – Signals the seeds to break dormancy and germinate 2 The aleurone responds by 2 synthesizing and secreting digestive enzymes that hydrolyze stored nutrients in the endosperm. One example is -amylase, which hydrolyzes starch. (A similar enzyme in our saliva helps in digesting bread and other starchy foods. ) 1 After a seed imbibes water, the embryo releases gibberellin (GA) as a signal to the aleurone, the thin outer layer of the endosperm. 3 Sugars and other nutrients absorbed from the endosperm by the scutellum (cotyledon) are consumed during growth of the embryo into a seedling. Aleurone Endosperm -amylase GA GA Water Radicle Scutellum (cotyledon) Figure 39. 11 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Sugar
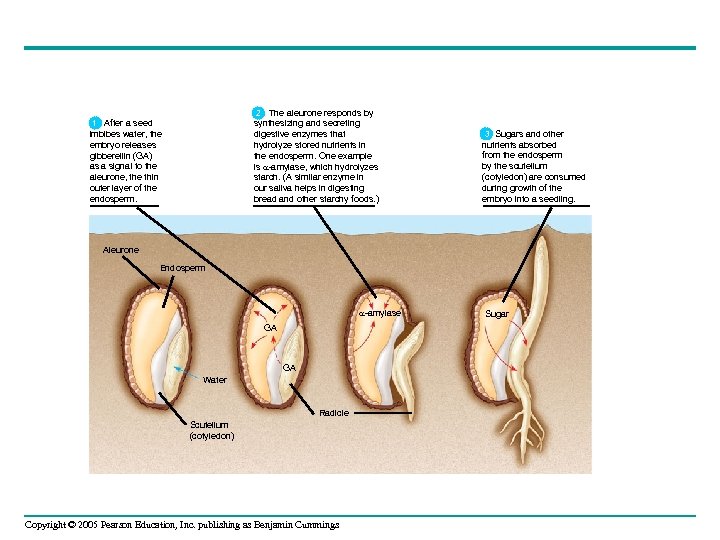 2 The aleurone responds by 2 synthesizing and secreting digestive enzymes that hydrolyze stored nutrients in the endosperm. One example is -amylase, which hydrolyzes starch. (A similar enzyme in our saliva helps in digesting bread and other starchy foods. ) 1 After a seed imbibes water, the embryo releases gibberellin (GA) as a signal to the aleurone, the thin outer layer of the endosperm. 3 Sugars and other nutrients absorbed from the endosperm by the scutellum (cotyledon) are consumed during growth of the embryo into a seedling. Aleurone Endosperm -amylase GA GA Water Radicle Scutellum (cotyledon) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Sugar
2 The aleurone responds by 2 synthesizing and secreting digestive enzymes that hydrolyze stored nutrients in the endosperm. One example is -amylase, which hydrolyzes starch. (A similar enzyme in our saliva helps in digesting bread and other starchy foods. ) 1 After a seed imbibes water, the embryo releases gibberellin (GA) as a signal to the aleurone, the thin outer layer of the endosperm. 3 Sugars and other nutrients absorbed from the endosperm by the scutellum (cotyledon) are consumed during growth of the embryo into a seedling. Aleurone Endosperm -amylase GA GA Water Radicle Scutellum (cotyledon) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Sugar
 Brassinosteroids • Brassinosteroids – Are similar to the sex hormones of animals – Induce cell elongation and division Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Brassinosteroids • Brassinosteroids – Are similar to the sex hormones of animals – Induce cell elongation and division Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Abscisic Acid • Two of the many effects of abscisic acid (ABA) are – Seed dormancy – Drought tolerance Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Abscisic Acid • Two of the many effects of abscisic acid (ABA) are – Seed dormancy – Drought tolerance Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Seed Dormancy • Ensures that the seed will germinate only when there are optimal conditions – Produced in the terminal bud and helps prepare plants for winter by suspending both primary and secondary growth • Directs leaf primordia to develop scales that protect dormant buds • Inhibits cell division in vascular cambium – At other times, seed dormancy proves advantageous • The ratio of ABA-gibberellins determines whether seeds remain dormant or germinate • In other plants, seeds germinate when ABA is washed out of the seeds (desert plants) or degraded by some other stimulus such as sunlight Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Seed Dormancy • Ensures that the seed will germinate only when there are optimal conditions – Produced in the terminal bud and helps prepare plants for winter by suspending both primary and secondary growth • Directs leaf primordia to develop scales that protect dormant buds • Inhibits cell division in vascular cambium – At other times, seed dormancy proves advantageous • The ratio of ABA-gibberellins determines whether seeds remain dormant or germinate • In other plants, seeds germinate when ABA is washed out of the seeds (desert plants) or degraded by some other stimulus such as sunlight Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Drought Tolerance • ABA is the primary internal signal – That enables plants to withstand drought – Acts as a stress hormone—closes stomata in times of water-stress and reduces transpiration water loss Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Drought Tolerance • ABA is the primary internal signal – That enables plants to withstand drought – Acts as a stress hormone—closes stomata in times of water-stress and reduces transpiration water loss Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Ethylene • Plants produce ethylene – In response to stresses such as drought, flooding, mechanical pressure, injury, and infection • Gaseous hormone that diffuses through air spaces b/w plant cells • High levels of auxin induce its release • Acts as a growth inhibitor Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Ethylene • Plants produce ethylene – In response to stresses such as drought, flooding, mechanical pressure, injury, and infection • Gaseous hormone that diffuses through air spaces b/w plant cells • High levels of auxin induce its release • Acts as a growth inhibitor Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
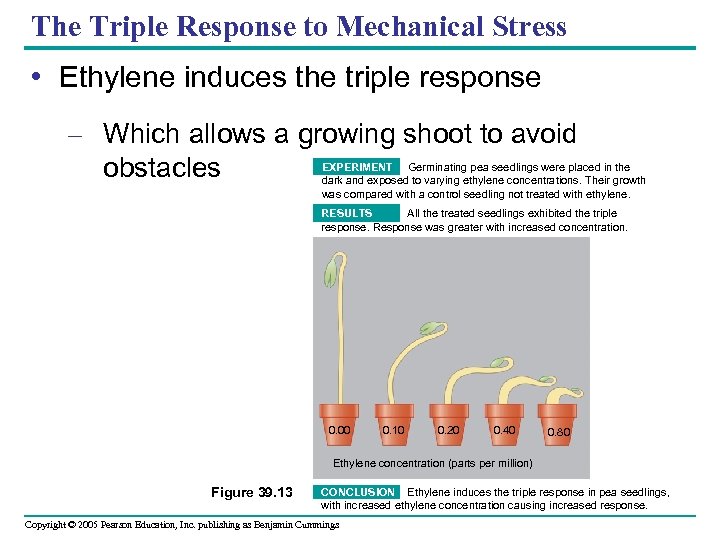 The Triple Response to Mechanical Stress • Ethylene induces the triple response – Which allows a growing shoot to avoid obstacles EXPERIMENT Germinating pea seedlings were placed in the dark and exposed to varying ethylene concentrations. Their growth was compared with a control seedling not treated with ethylene. RESULTS All the treated seedlings exhibited the triple response. Response was greater with increased concentration. 0. 00 0. 10 0. 20 0. 40 0. 80 Ethylene concentration (parts per million) Figure 39. 13 CONCLUSION Ethylene induces the triple response in pea seedlings, with increased ethylene concentration causing increased response. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
The Triple Response to Mechanical Stress • Ethylene induces the triple response – Which allows a growing shoot to avoid obstacles EXPERIMENT Germinating pea seedlings were placed in the dark and exposed to varying ethylene concentrations. Their growth was compared with a control seedling not treated with ethylene. RESULTS All the treated seedlings exhibited the triple response. Response was greater with increased concentration. 0. 00 0. 10 0. 20 0. 40 0. 80 Ethylene concentration (parts per million) Figure 39. 13 CONCLUSION Ethylene induces the triple response in pea seedlings, with increased ethylene concentration causing increased response. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Apoptosis: Programmed Cell Death • A burst of ethylene – Is associated with the programmed destruction of cells, organs, or whole plants Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Apoptosis: Programmed Cell Death • A burst of ethylene – Is associated with the programmed destruction of cells, organs, or whole plants Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
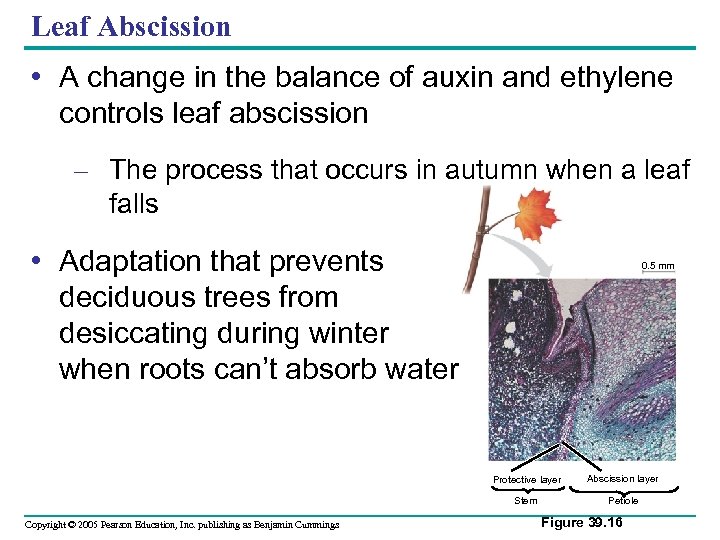 Leaf Abscission • A change in the balance of auxin and ethylene controls leaf abscission – The process that occurs in autumn when a leaf falls • Adaptation that prevents deciduous trees from desiccating during winter when roots can’t absorb water 0. 5 mm Protective layer Stem Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Abscission layer Petiole Figure 39. 16
Leaf Abscission • A change in the balance of auxin and ethylene controls leaf abscission – The process that occurs in autumn when a leaf falls • Adaptation that prevents deciduous trees from desiccating during winter when roots can’t absorb water 0. 5 mm Protective layer Stem Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Abscission layer Petiole Figure 39. 16
 Leaf Abscission • Prior to abscission-leaf’s essential elements are shunted to storage tissues in the stem • Environmental stimuli=shortening of days and cooler temperatures. • Mechanics controlled by change in balance of ethylene and auxin – auxin = cell sensitivity to ethylene; cells produce more ethylene which inhibits auxin production – Ethylene induces synthesis of enzymes that digest the polysaccharides in the cell walls, further weakening the abscission layer – Wind and weight cause the leaf to fall by causing a separation in the abscission layer – Before the leaf falls, a layer of cork forms a protective scar in the twig’s side of the abscission layer—prevents pathogens from entering the plant Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Leaf Abscission • Prior to abscission-leaf’s essential elements are shunted to storage tissues in the stem • Environmental stimuli=shortening of days and cooler temperatures. • Mechanics controlled by change in balance of ethylene and auxin – auxin = cell sensitivity to ethylene; cells produce more ethylene which inhibits auxin production – Ethylene induces synthesis of enzymes that digest the polysaccharides in the cell walls, further weakening the abscission layer – Wind and weight cause the leaf to fall by causing a separation in the abscission layer – Before the leaf falls, a layer of cork forms a protective scar in the twig’s side of the abscission layer—prevents pathogens from entering the plant Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Fruit Ripening • A burst of ethylene production in the fruit – Triggers the ripening process • Ethylene triggers senescence and the aging cells release more ethylene – Breakdown of cell walls and loss of chlorophyll – Signal to ripen spreads from fruit to fruit since ethylene is a gas Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Fruit Ripening • A burst of ethylene production in the fruit – Triggers the ripening process • Ethylene triggers senescence and the aging cells release more ethylene – Breakdown of cell walls and loss of chlorophyll – Signal to ripen spreads from fruit to fruit since ethylene is a gas Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Responses to light are critical for plant success • Light cues many key events in plant growth and development • Effects of light on plant morphology – Are what plant biologists call photomorphogenesis Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Responses to light are critical for plant success • Light cues many key events in plant growth and development • Effects of light on plant morphology – Are what plant biologists call photomorphogenesis Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Plants not only detect the presence of light – But also its direction, intensity, and wavelength (color) • A graph called an action spectrum – Depicts the relative response of a process to different wavelengths of light Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Plants not only detect the presence of light – But also its direction, intensity, and wavelength (color) • A graph called an action spectrum – Depicts the relative response of a process to different wavelengths of light Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
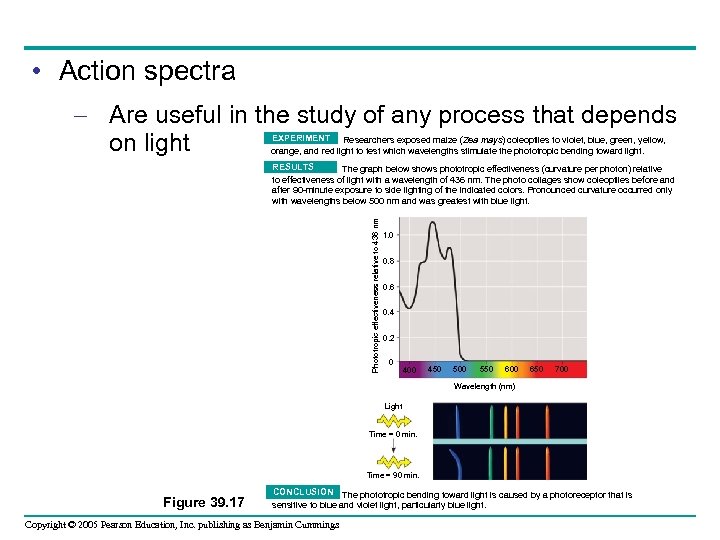 • Action spectra – Are useful in the study of any process that depends on light EXPERIMENT Researchers exposed maize (Zea mays) coleoptiles to violet, blue, green, yellow, orange, and red light to test which wavelengths stimulate the phototropic bending toward light. Phototropic effectiveness relative to 436 nm RESULTS The graph below shows phototropic effectiveness (curvature per photon) relative to effectiveness of light with a wavelength of 436 nm. The photo collages show coleoptiles before and after 90 -minute exposure to side lighting of the indicated colors. Pronounced curvature occurred only with wavelengths below 500 nm and was greatest with blue light. 1. 0 0. 8 0. 6 0. 4 0. 2 0 400 450 500 550 600 650 700 Wavelength (nm) Light Time = 0 min. Time = 90 min. Figure 39. 17 CONCLUSION The phototropic bending toward light is caused by a photoreceptor that is sensitive to blue and violet light, particularly blue light. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Action spectra – Are useful in the study of any process that depends on light EXPERIMENT Researchers exposed maize (Zea mays) coleoptiles to violet, blue, green, yellow, orange, and red light to test which wavelengths stimulate the phototropic bending toward light. Phototropic effectiveness relative to 436 nm RESULTS The graph below shows phototropic effectiveness (curvature per photon) relative to effectiveness of light with a wavelength of 436 nm. The photo collages show coleoptiles before and after 90 -minute exposure to side lighting of the indicated colors. Pronounced curvature occurred only with wavelengths below 500 nm and was greatest with blue light. 1. 0 0. 8 0. 6 0. 4 0. 2 0 400 450 500 550 600 650 700 Wavelength (nm) Light Time = 0 min. Time = 90 min. Figure 39. 17 CONCLUSION The phototropic bending toward light is caused by a photoreceptor that is sensitive to blue and violet light, particularly blue light. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Research on action spectra and absorption spectra of pigments – Led to the identification of two major classes of light receptors: blue-light photoreceptors and phytochromes Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Research on action spectra and absorption spectra of pigments – Led to the identification of two major classes of light receptors: blue-light photoreceptors and phytochromes Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Blue-Light Photoreceptors • Various blue-light photoreceptors – Control hypocotyl elongation, stomatal opening, and phototropism Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Blue-Light Photoreceptors • Various blue-light photoreceptors – Control hypocotyl elongation, stomatal opening, and phototropism Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Phytochromes as Photoreceptors • Phytochromes – Regulate many of a plant’s responses to light throughout its life Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Phytochromes as Photoreceptors • Phytochromes – Regulate many of a plant’s responses to light throughout its life Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Phytochromes and Seed Germination • Studies of seed germination – Led to the discovery of phytochromes Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Phytochromes and Seed Germination • Studies of seed germination – Led to the discovery of phytochromes Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • In the 1930 s, scientists at the U. S. Department of Agriculture – Determined the action spectrum for lightinduced germination of lettuce seeds Dark (control) Dark Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• In the 1930 s, scientists at the U. S. Department of Agriculture – Determined the action spectrum for lightinduced germination of lettuce seeds Dark (control) Dark Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
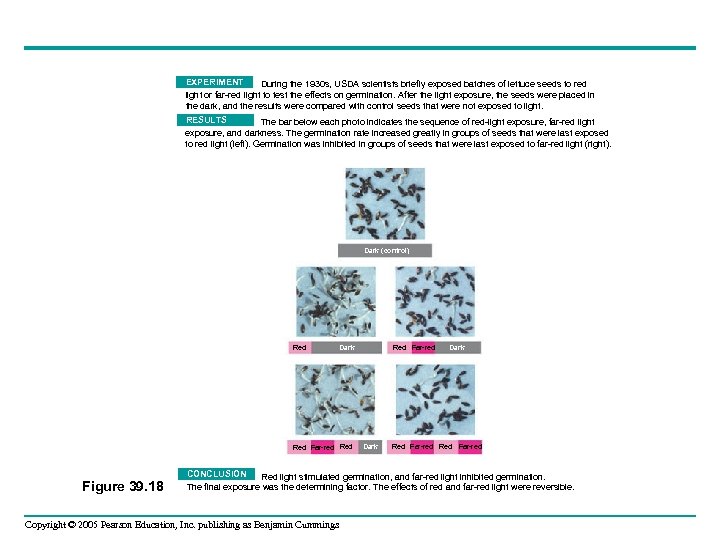 EXPERIMENT During the 1930 s, USDA scientists briefly exposed batches of lettuce seeds to red light or far-red light to test the effects on germination. After the light exposure, the seeds were placed in the dark, and the results were compared with control seeds that were not exposed to light. RESULTS The bar below each photo indicates the sequence of red-light exposure, far-red light exposure, and darkness. The germination rate increased greatly in groups of seeds that were last exposed to red light (left). Germination was inhibited in groups of seeds that were last exposed to far-red light (right). Dark (control) Red Dark Red Far-red Red Figure 39. 18 Red Far-red Dark Red Far-red CONCLUSION Red light stimulated germination, and far-red light inhibited germination. The final exposure was the determining factor. The effects of red and far-red light were reversible. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
EXPERIMENT During the 1930 s, USDA scientists briefly exposed batches of lettuce seeds to red light or far-red light to test the effects on germination. After the light exposure, the seeds were placed in the dark, and the results were compared with control seeds that were not exposed to light. RESULTS The bar below each photo indicates the sequence of red-light exposure, far-red light exposure, and darkness. The germination rate increased greatly in groups of seeds that were last exposed to red light (left). Germination was inhibited in groups of seeds that were last exposed to far-red light (right). Dark (control) Red Dark Red Far-red Red Figure 39. 18 Red Far-red Dark Red Far-red CONCLUSION Red light stimulated germination, and far-red light inhibited germination. The final exposure was the determining factor. The effects of red and far-red light were reversible. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
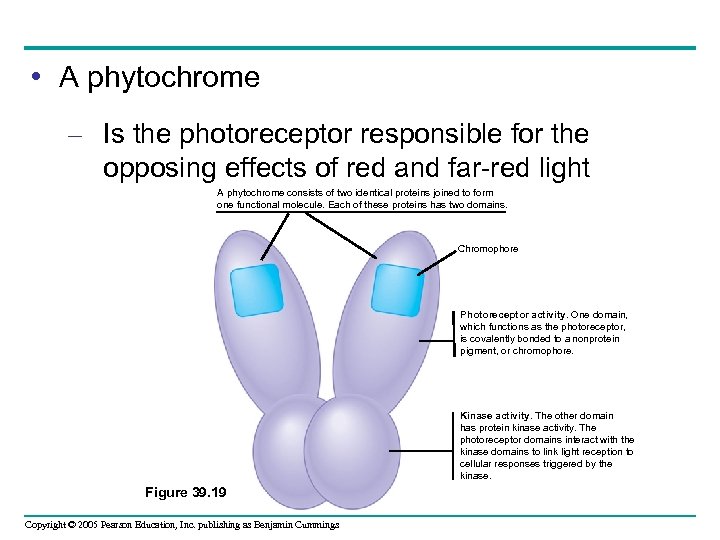 • A phytochrome – Is the photoreceptor responsible for the opposing effects of red and far-red light A phytochrome consists of two identical proteins joined to form one functional molecule. Each of these proteins has two domains. Chromophore Photoreceptor activity. One domain, which functions as the photoreceptor, is covalently bonded to a nonprotein pigment, or chromophore. Kinase activity. The other domain has protein kinase activity. The photoreceptor domains interact with the kinase domains to link light reception to cellular responses triggered by the kinase. Figure 39. 19 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• A phytochrome – Is the photoreceptor responsible for the opposing effects of red and far-red light A phytochrome consists of two identical proteins joined to form one functional molecule. Each of these proteins has two domains. Chromophore Photoreceptor activity. One domain, which functions as the photoreceptor, is covalently bonded to a nonprotein pigment, or chromophore. Kinase activity. The other domain has protein kinase activity. The photoreceptor domains interact with the kinase domains to link light reception to cellular responses triggered by the kinase. Figure 39. 19 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
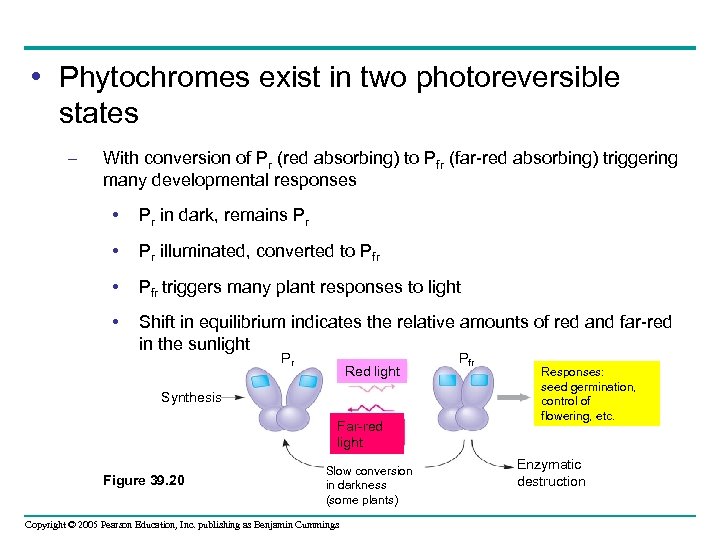 • Phytochromes exist in two photoreversible states – With conversion of Pr (red absorbing) to Pfr (far-red absorbing) triggering many developmental responses • Pr in dark, remains Pr • Pr illuminated, converted to Pfr • Pfr triggers many plant responses to light • Shift in equilibrium indicates the relative amounts of red and far-red in the sunlight Pr Red light Synthesis Far-red light Figure 39. 20 Slow conversion in darkness (some plants) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Pfr Responses: seed germination, control of flowering, etc. Enzymatic destruction
• Phytochromes exist in two photoreversible states – With conversion of Pr (red absorbing) to Pfr (far-red absorbing) triggering many developmental responses • Pr in dark, remains Pr • Pr illuminated, converted to Pfr • Pfr triggers many plant responses to light • Shift in equilibrium indicates the relative amounts of red and far-red in the sunlight Pr Red light Synthesis Far-red light Figure 39. 20 Slow conversion in darkness (some plants) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Pfr Responses: seed germination, control of flowering, etc. Enzymatic destruction
 Phytochromes and Shade Avoidance • The phytochrome system – Also provides the plant with information about the quality of light • In the “shade avoidance” response of a tree – The phytochrome ratio shifts in favor of Pr when a tree is shaded Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Phytochromes and Shade Avoidance • The phytochrome system – Also provides the plant with information about the quality of light • In the “shade avoidance” response of a tree – The phytochrome ratio shifts in favor of Pr when a tree is shaded Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Biological Clocks and Circadian Rhythms • Many plant processes oscillate during the day • Control circadian rhythms (cycle with frequency of 24 h) – Common in all eukaryotes • Oscillator is probably endogenous and set to a 24 h period daily by environmental signals • Most are cued to light-dark cycle resulting from the Earth’s rotation – May take days to reset once the cues change • Jet lag-lack of synchronization b/w internal clock and time zone • Research indicates that cyclic changes in levels of a protein form the basis for the internal clock Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Biological Clocks and Circadian Rhythms • Many plant processes oscillate during the day • Control circadian rhythms (cycle with frequency of 24 h) – Common in all eukaryotes • Oscillator is probably endogenous and set to a 24 h period daily by environmental signals • Most are cued to light-dark cycle resulting from the Earth’s rotation – May take days to reset once the cues change • Jet lag-lack of synchronization b/w internal clock and time zone • Research indicates that cyclic changes in levels of a protein form the basis for the internal clock Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
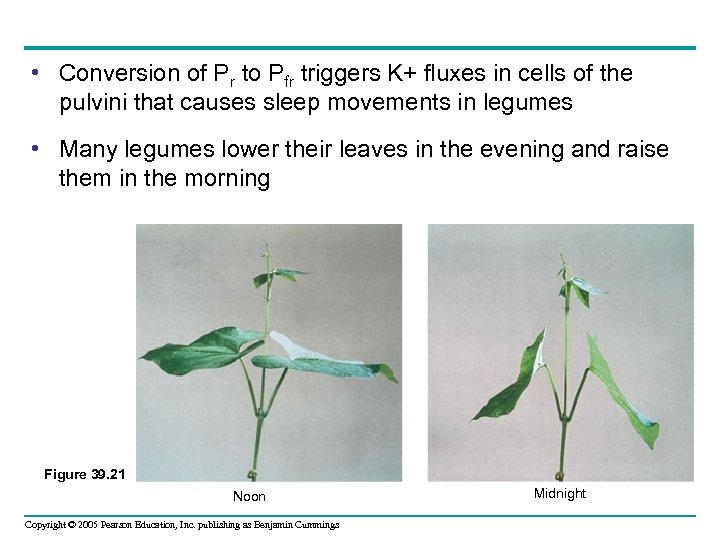 • Conversion of Pr to Pfr triggers K+ fluxes in cells of the pulvini that causes sleep movements in legumes • Many legumes lower their leaves in the evening and raise them in the morning Figure 39. 21 Noon Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Midnight
• Conversion of Pr to Pfr triggers K+ fluxes in cells of the pulvini that causes sleep movements in legumes • Many legumes lower their leaves in the evening and raise them in the morning Figure 39. 21 Noon Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Midnight
 The Effect of Light on the Biological Clock • Phytochrome conversion marks sunrise and sunset – Providing the biological clock with environmental cues Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
The Effect of Light on the Biological Clock • Phytochrome conversion marks sunrise and sunset – Providing the biological clock with environmental cues Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Photoperiodism and Responses to Seasons • Photoperiod, the relative lengths of night and day – Is the environmental stimulus plants use most often to detect the time of year • Photoperiodism – Is a physiological response to photoperiod Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Photoperiodism and Responses to Seasons • Photoperiod, the relative lengths of night and day – Is the environmental stimulus plants use most often to detect the time of year • Photoperiodism – Is a physiological response to photoperiod Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Photoperiodism and Control of Flowering • Some developmental processes, including flowering in many species – Requires a certain photoperiod • Night length controls flowering and other responses to photoperiod • Some flower after a single exposure to the proper photoperiod • Some require several successive days of the proper photoperiod to bloom • Can be interrupted by red light ( 660 nm) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Photoperiodism and Control of Flowering • Some developmental processes, including flowering in many species – Requires a certain photoperiod • Night length controls flowering and other responses to photoperiod • Some flower after a single exposure to the proper photoperiod • Some require several successive days of the proper photoperiod to bloom • Can be interrupted by red light ( 660 nm) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
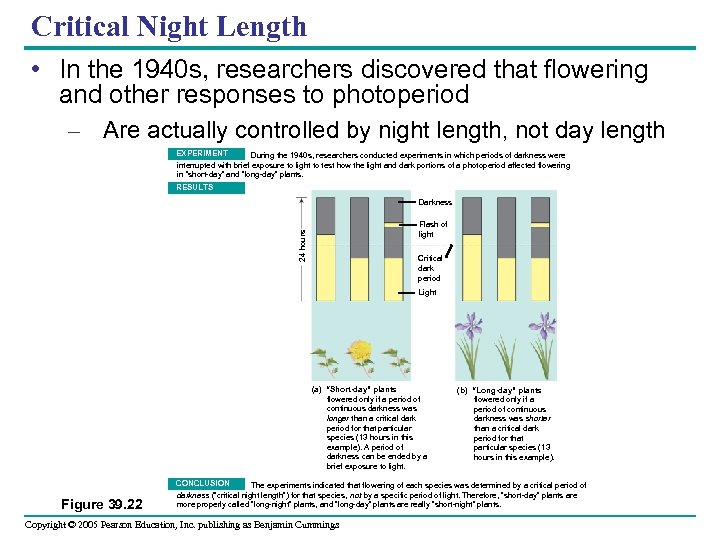 Critical Night Length • In the 1940 s, researchers discovered that flowering and other responses to photoperiod – Are actually controlled by night length, not day length EXPERIMENT During the 1940 s, researchers conducted experiments in which periods of darkness were interrupted with brief exposure to light to test how the light and dark portions of a photoperiod affected flowering in “short-day” and “long-day” plants. RESULTS Darkness 24 hours Flash of light Critical dark period Light (a) “Short-day” plants flowered only if a period of continuous darkness was longer than a critical dark period for that particular species (13 hours in this example). A period of darkness can be ended by a brief exposure to light. Figure 39. 22 (b) “Long-day” plants flowered only if a period of continuous darkness was shorter than a critical dark period for that particular species (13 hours in this example). CONCLUSION The experiments indicated that flowering of each species was determined by a critical period of darkness (“critical night length”) for that species, not by a specific period of light. Therefore, “short-day” plants are more properly called “long-night” plants, and “long-day” plants are really “short-night” plants. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Critical Night Length • In the 1940 s, researchers discovered that flowering and other responses to photoperiod – Are actually controlled by night length, not day length EXPERIMENT During the 1940 s, researchers conducted experiments in which periods of darkness were interrupted with brief exposure to light to test how the light and dark portions of a photoperiod affected flowering in “short-day” and “long-day” plants. RESULTS Darkness 24 hours Flash of light Critical dark period Light (a) “Short-day” plants flowered only if a period of continuous darkness was longer than a critical dark period for that particular species (13 hours in this example). A period of darkness can be ended by a brief exposure to light. Figure 39. 22 (b) “Long-day” plants flowered only if a period of continuous darkness was shorter than a critical dark period for that particular species (13 hours in this example). CONCLUSION The experiments indicated that flowering of each species was determined by a critical period of darkness (“critical night length”) for that species, not by a specific period of light. Therefore, “short-day” plants are more properly called “long-night” plants, and “long-day” plants are really “short-night” plants. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
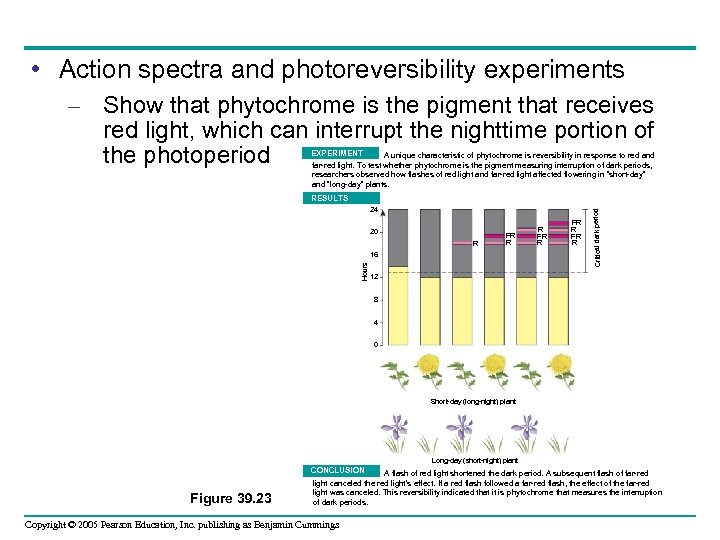 • Action spectra and photoreversibility experiments – Show that phytochrome is the pigment that receives red light, which can interrupt the nighttime portion of the photoperiod EXPERIMENT A unique characteristic of phytochrome is reversibility in response to red and far-red light. To test whether phytochrome is the pigment measuring interruption of dark periods, researchers observed how flashes of red light and far-red light affected flowering in “short-day” and “long-day” plants. 24 20 R FR R Hours 16 R FR R Critical dark period RESULTS 12 8 4 0 Short-day (long-night) plant Long-day (short-night) plant CONCLUSION Figure 39. 23 A flash of red light shortened the dark period. A subsequent flash of far-red light canceled the red light’s effect. If a red flash followed a far-red flash, the effect of the far-red light was canceled. This reversibility indicated that it is phytochrome that measures the interruption of dark periods. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Action spectra and photoreversibility experiments – Show that phytochrome is the pigment that receives red light, which can interrupt the nighttime portion of the photoperiod EXPERIMENT A unique characteristic of phytochrome is reversibility in response to red and far-red light. To test whether phytochrome is the pigment measuring interruption of dark periods, researchers observed how flashes of red light and far-red light affected flowering in “short-day” and “long-day” plants. 24 20 R FR R Hours 16 R FR R Critical dark period RESULTS 12 8 4 0 Short-day (long-night) plant Long-day (short-night) plant CONCLUSION Figure 39. 23 A flash of red light shortened the dark period. A subsequent flash of far-red light canceled the red light’s effect. If a red flash followed a far-red flash, the effect of the far-red light was canceled. This reversibility indicated that it is phytochrome that measures the interruption of dark periods. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 A Flowering Hormone? • The flowering signal, not yet chemically identified – Is called florigen, and it may be a hormone or a change in relative concentrations of multiple hormones • Leaves detect photoperiod while buds produce flowers • Florigen is produced in the leaves and moves to the buds • Appears to be the same hormone in both short-day and long-day plants Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
A Flowering Hormone? • The flowering signal, not yet chemically identified – Is called florigen, and it may be a hormone or a change in relative concentrations of multiple hormones • Leaves detect photoperiod while buds produce flowers • Florigen is produced in the leaves and moves to the buds • Appears to be the same hormone in both short-day and long-day plants Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
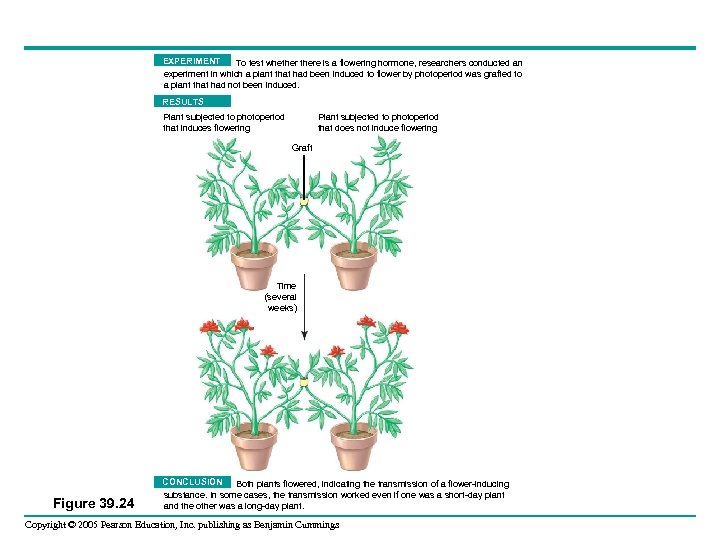 EXPERIMENT To test whethere is a flowering hormone, researchers conducted an experiment in which a plant that had been induced to flower by photoperiod was grafted to a plant that had not been induced. RESULTS Plant subjected to photoperiod that does not induce flowering Plant subjected to photoperiod that induces flowering Graft Time (several weeks) Figure 39. 24 CONCLUSION Both plants flowered, indicating the transmission of a flower-inducing substance. In some cases, the transmission worked even if one was a short-day plant and the other was a long-day plant. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
EXPERIMENT To test whethere is a flowering hormone, researchers conducted an experiment in which a plant that had been induced to flower by photoperiod was grafted to a plant that had not been induced. RESULTS Plant subjected to photoperiod that does not induce flowering Plant subjected to photoperiod that induces flowering Graft Time (several weeks) Figure 39. 24 CONCLUSION Both plants flowered, indicating the transmission of a flower-inducing substance. In some cases, the transmission worked even if one was a short-day plant and the other was a long-day plant. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Meristem Transition and Flowering • Whatever combination of environmental cues and internal signals is necessary for flowering to occur – The outcome is the transition of a bud’s meristem from a vegetative to a flowering state Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Meristem Transition and Flowering • Whatever combination of environmental cues and internal signals is necessary for flowering to occur – The outcome is the transition of a bud’s meristem from a vegetative to a flowering state Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Plants respond to a wide variety of stimuli other than light • Because of their immobility – Plants must adjust to a wide range of environmental circumstances through developmental and physiological mechanisms Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Plants respond to a wide variety of stimuli other than light • Because of their immobility – Plants must adjust to a wide range of environmental circumstances through developmental and physiological mechanisms Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Gravity • Response to gravity – Is known as gravitropism • Roots show positive gravitropism • Stems show negative gravitropism Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Gravity • Response to gravity – Is known as gravitropism • Roots show positive gravitropism • Stems show negative gravitropism Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
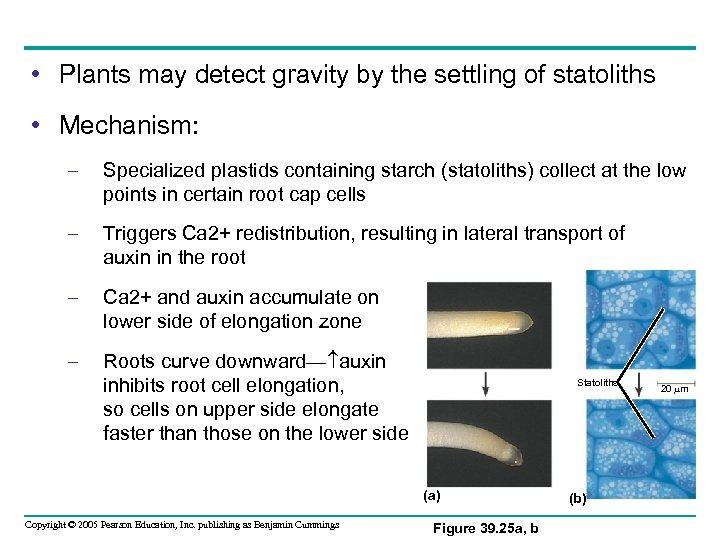 • Plants may detect gravity by the settling of statoliths • Mechanism: – Specialized plastids containing starch (statoliths) collect at the low points in certain root cap cells – Triggers Ca 2+ redistribution, resulting in lateral transport of auxin in the root – Ca 2+ and auxin accumulate on lower side of elongation zone – Roots curve downward— auxin inhibits root cell elongation, so cells on upper side elongate faster than those on the lower side Statoliths (a) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 39. 25 a, b (b) 20 m
• Plants may detect gravity by the settling of statoliths • Mechanism: – Specialized plastids containing starch (statoliths) collect at the low points in certain root cap cells – Triggers Ca 2+ redistribution, resulting in lateral transport of auxin in the root – Ca 2+ and auxin accumulate on lower side of elongation zone – Roots curve downward— auxin inhibits root cell elongation, so cells on upper side elongate faster than those on the lower side Statoliths (a) Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings Figure 39. 25 a, b (b) 20 m
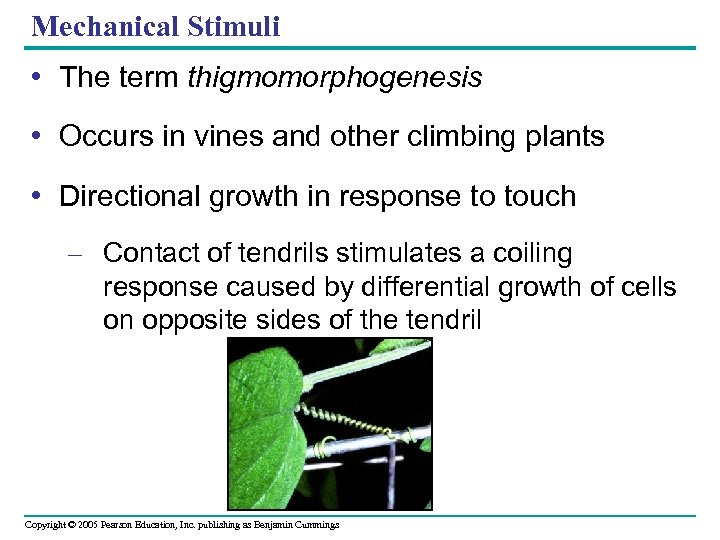 Mechanical Stimuli • The term thigmomorphogenesis • Occurs in vines and other climbing plants • Directional growth in response to touch – Contact of tendrils stimulates a coiling response caused by differential growth of cells on opposite sides of the tendril Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Mechanical Stimuli • The term thigmomorphogenesis • Occurs in vines and other climbing plants • Directional growth in response to touch – Contact of tendrils stimulates a coiling response caused by differential growth of cells on opposite sides of the tendril Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
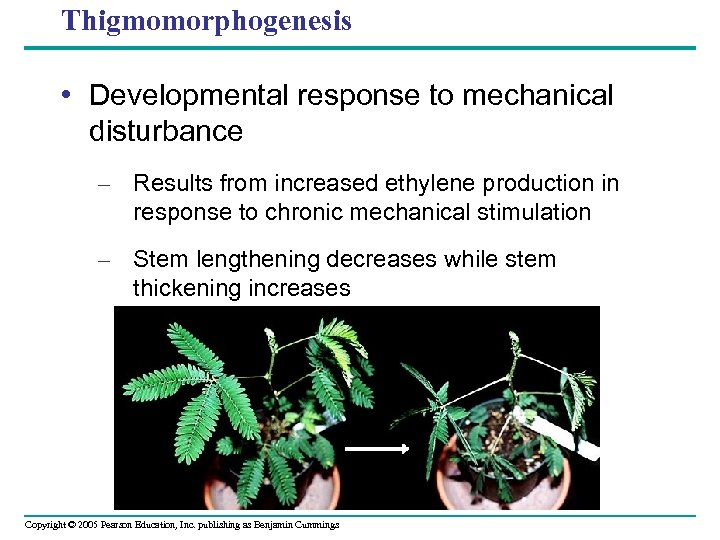 Thigmomorphogenesis • Developmental response to mechanical disturbance – Results from increased ethylene production in response to chronic mechanical stimulation – Stem lengthening decreases while stem thickening increases Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Thigmomorphogenesis • Developmental response to mechanical disturbance – Results from increased ethylene production in response to chronic mechanical stimulation – Stem lengthening decreases while stem thickening increases Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Rubbing the stems of young plants a couple of times daily – Results in plants that are shorter than controls Figure 39. 26 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Rubbing the stems of young plants a couple of times daily – Results in plants that are shorter than controls Figure 39. 26 Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
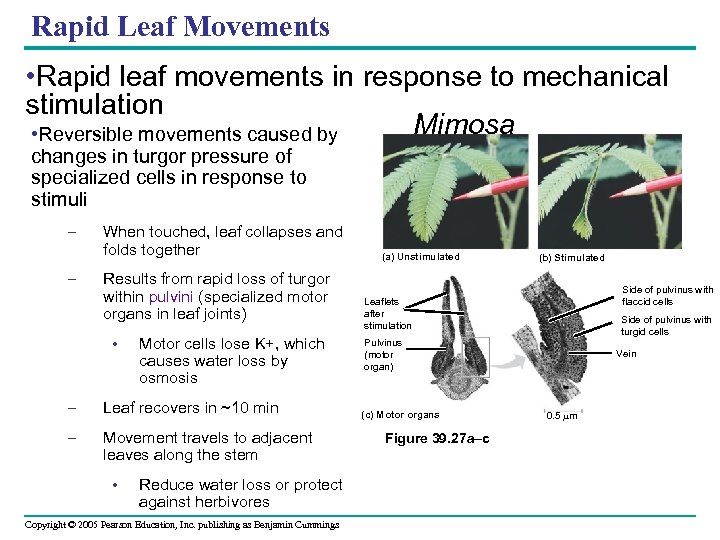 Rapid Leaf Movements • Rapid leaf movements in response to mechanical stimulation Mimosa • Reversible movements caused by changes in turgor pressure of specialized cells in response to stimuli – – When touched, leaf collapses and folds together Results from rapid loss of turgor within pulvini (specialized motor organs in leaf joints) • Motor cells lose K+, which causes water loss by osmosis – Leaf recovers in ~10 min – Movement travels to adjacent leaves along the stem • Reduce water loss or protect against herbivores Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings (a) Unstimulated (b) Stimulated Side of pulvinus with flaccid cells Leaflets after stimulation Side of pulvinus with turgid cells Pulvinus (motor organ) (c) Motor organs Figure 39. 27 a–c Vein 0. 5 m
Rapid Leaf Movements • Rapid leaf movements in response to mechanical stimulation Mimosa • Reversible movements caused by changes in turgor pressure of specialized cells in response to stimuli – – When touched, leaf collapses and folds together Results from rapid loss of turgor within pulvini (specialized motor organs in leaf joints) • Motor cells lose K+, which causes water loss by osmosis – Leaf recovers in ~10 min – Movement travels to adjacent leaves along the stem • Reduce water loss or protect against herbivores Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings (a) Unstimulated (b) Stimulated Side of pulvinus with flaccid cells Leaflets after stimulation Side of pulvinus with turgid cells Pulvinus (motor organ) (c) Motor organs Figure 39. 27 a–c Vein 0. 5 m
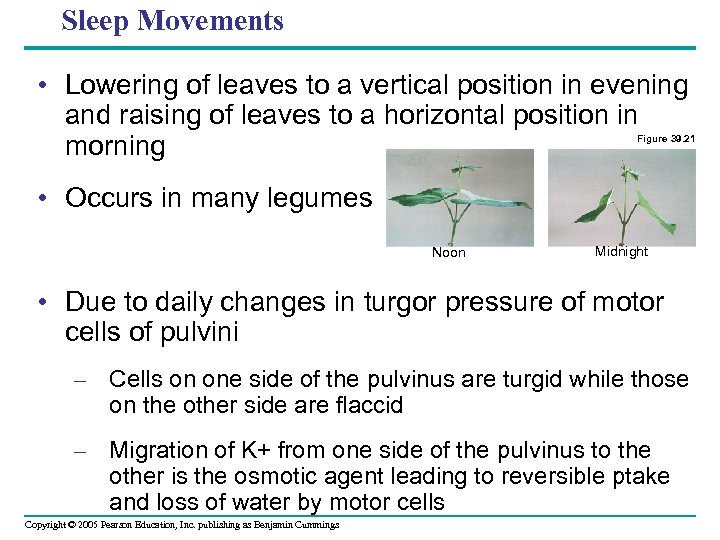 Sleep Movements • Lowering of leaves to a vertical position in evening and raising of leaves to a horizontal position in morning Figure 39. 21 • Occurs in many legumes Noon Midnight • Due to daily changes in turgor pressure of motor cells of pulvini – Cells on one side of the pulvinus are turgid while those on the other side are flaccid – Migration of K+ from one side of the pulvinus to the other is the osmotic agent leading to reversible ptake and loss of water by motor cells Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Sleep Movements • Lowering of leaves to a vertical position in evening and raising of leaves to a horizontal position in morning Figure 39. 21 • Occurs in many legumes Noon Midnight • Due to daily changes in turgor pressure of motor cells of pulvini – Cells on one side of the pulvinus are turgid while those on the other side are flaccid – Migration of K+ from one side of the pulvinus to the other is the osmotic agent leading to reversible ptake and loss of water by motor cells Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Environmental Stresses • Environmental stresses – Have a potentially adverse effect on a plant’s survival, growth, and reproduction – Can have a devastating impact on crop yields in agriculture Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Environmental Stresses • Environmental stresses – Have a potentially adverse effect on a plant’s survival, growth, and reproduction – Can have a devastating impact on crop yields in agriculture Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Drought-Water Stress • During drought – Plants respond to water deficit by reducing transpiration – Guard cells lose turgor and the stomata close when a leaf faces a water deficit – Mesophyll cells in the leaf synthesize and release abscisic acid which helps keep stomata closed – Growth of young leaves is inhibited by a water deficit since cell expansion is a turgor- dependent process (reduces leaf surface area) – Roots reduce growth • Drying soil inhibits growth of shallow roots • Deeper roots surrounded by moist soil continue to grow Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Drought-Water Stress • During drought – Plants respond to water deficit by reducing transpiration – Guard cells lose turgor and the stomata close when a leaf faces a water deficit – Mesophyll cells in the leaf synthesize and release abscisic acid which helps keep stomata closed – Growth of young leaves is inhibited by a water deficit since cell expansion is a turgor- dependent process (reduces leaf surface area) – Roots reduce growth • Drying soil inhibits growth of shallow roots • Deeper roots surrounded by moist soil continue to grow Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
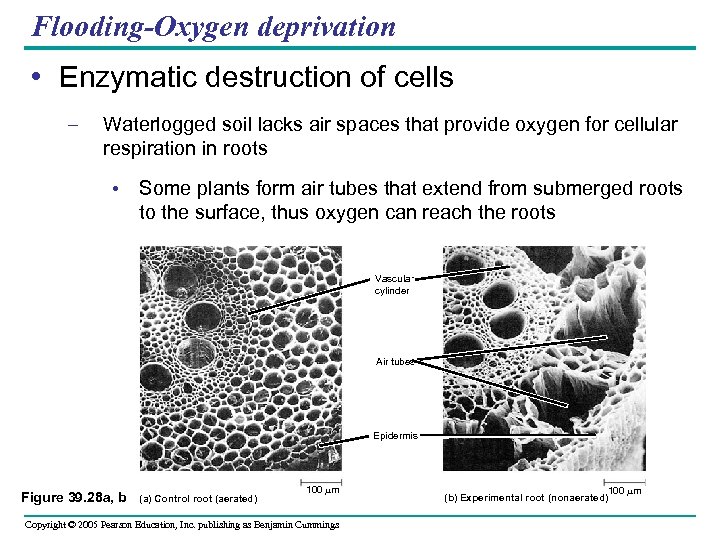 Flooding-Oxygen deprivation • Enzymatic destruction of cells – Waterlogged soil lacks air spaces that provide oxygen for cellular respiration in roots • Some plants form air tubes that extend from submerged roots to the surface, thus oxygen can reach the roots Vascular cylinder Air tubes Epidermis Figure 39. 28 a, b (a) Control root (aerated) 100 m Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 100 m (b) Experimental root (nonaerated)
Flooding-Oxygen deprivation • Enzymatic destruction of cells – Waterlogged soil lacks air spaces that provide oxygen for cellular respiration in roots • Some plants form air tubes that extend from submerged roots to the surface, thus oxygen can reach the roots Vascular cylinder Air tubes Epidermis Figure 39. 28 a, b (a) Control root (aerated) 100 m Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings 100 m (b) Experimental root (nonaerated)
 Salt Stress • Plants respond to salt stress by producing solutes tolerated at high concentrations – Lower the water potential of the soil solution causing a water deficit – roots lose water – Toxic effect on plant at high concentrations • Selective permeability to such solutes limits water intake – Produce compatible solutes to keep water potential of cells more negative than the soil solution without admitting toxic quantities of salt Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Salt Stress • Plants respond to salt stress by producing solutes tolerated at high concentrations – Lower the water potential of the soil solution causing a water deficit – roots lose water – Toxic effect on plant at high concentrations • Selective permeability to such solutes limits water intake – Produce compatible solutes to keep water potential of cells more negative than the soil solution without admitting toxic quantities of salt Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Heat Stress • Transpiration reduces effects of heat stress – Evaporative cooling keeps temperature of leaf 3 -10 C lower than ambient temperature – Continues as long as stomata stay open—will close if needed to reduce water loss • Produce heat-shock proteins when exposed to excessive temperatures – Serve as temporary supports which help other proteins fold into their functional conformations – Help enzymes and other proteins maintain their conformation, thus preventing denaturation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Heat Stress • Transpiration reduces effects of heat stress – Evaporative cooling keeps temperature of leaf 3 -10 C lower than ambient temperature – Continues as long as stomata stay open—will close if needed to reduce water loss • Produce heat-shock proteins when exposed to excessive temperatures – Serve as temporary supports which help other proteins fold into their functional conformations – Help enzymes and other proteins maintain their conformation, thus preventing denaturation Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Cold Stress • Altering lipid composition of membranes – Fluidity of cell membranes decreases • Lipids become locked into crystalline structures causing a loss of fluidity • Solute transport and membrane protein function are adversely affected by loss of fluidity – Plants alter the lipid composition of their membranes • Proportion of unsaturated fatty acids increase— shape reduces crystal formation and maintains fluidity at lower temps. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Cold Stress • Altering lipid composition of membranes – Fluidity of cell membranes decreases • Lipids become locked into crystalline structures causing a loss of fluidity • Solute transport and membrane protein function are adversely affected by loss of fluidity – Plants alter the lipid composition of their membranes • Proportion of unsaturated fatty acids increase— shape reduces crystal formation and maintains fluidity at lower temps. Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 • Plants defend themselves against herbivores and pathogens • Plants counter external threats – With defense systems that deter herbivory and prevent infection or combat pathogens Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
• Plants defend themselves against herbivores and pathogens • Plants counter external threats – With defense systems that deter herbivory and prevent infection or combat pathogens Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
 Defenses Against Herbivores • Herbivory, animals eating plants – Is a stress that plants face in any ecosystem • Plants counter excessive herbivory – With physical defenses such as thorns – With chemical defenses such as distasteful or toxic compounds Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings
Defenses Against Herbivores • Herbivory, animals eating plants – Is a stress that plants face in any ecosystem • Plants counter excessive herbivory – With physical defenses such as thorns – With chemical defenses such as distasteful or toxic compounds Copyright © 2005 Pearson Education, Inc. publishing as Benjamin Cummings


