Energy.pptx
- Количество слайдов: 34

Chapter 3 Energy, Catalysis and Biosynthesis Arnat Balabiyev Ph. D Student Arizona State University
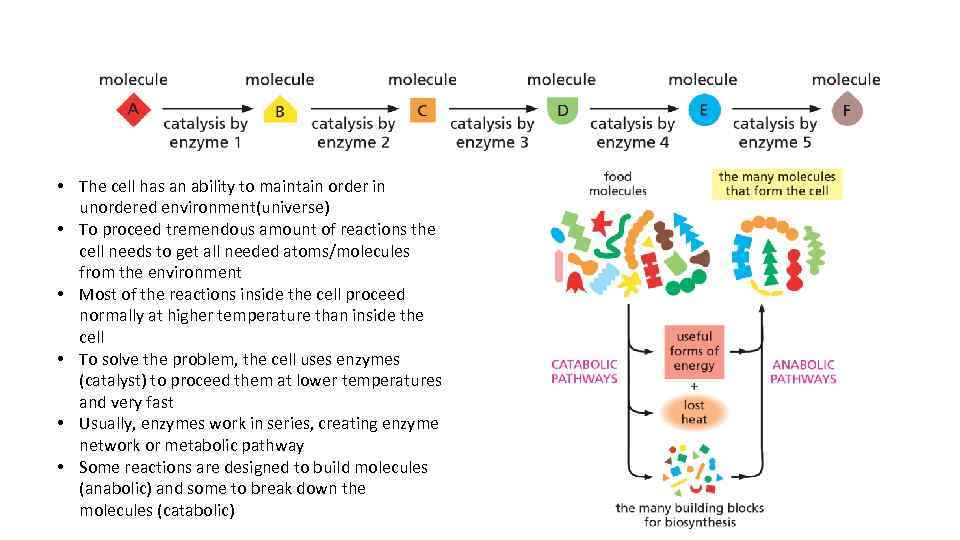
• The cell has an ability to maintain order in unordered environment(universe) • To proceed tremendous amount of reactions the cell needs to get all needed atoms/molecules from the environment • Most of the reactions inside the cell proceed normally at higher temperature than inside the cell • To solve the problem, the cell uses enzymes (catalyst) to proceed them at lower temperatures and very fast • Usually, enzymes work in series, creating enzyme network or metabolic pathway • Some reactions are designed to build molecules (anabolic) and some to break down the molecules (catabolic)

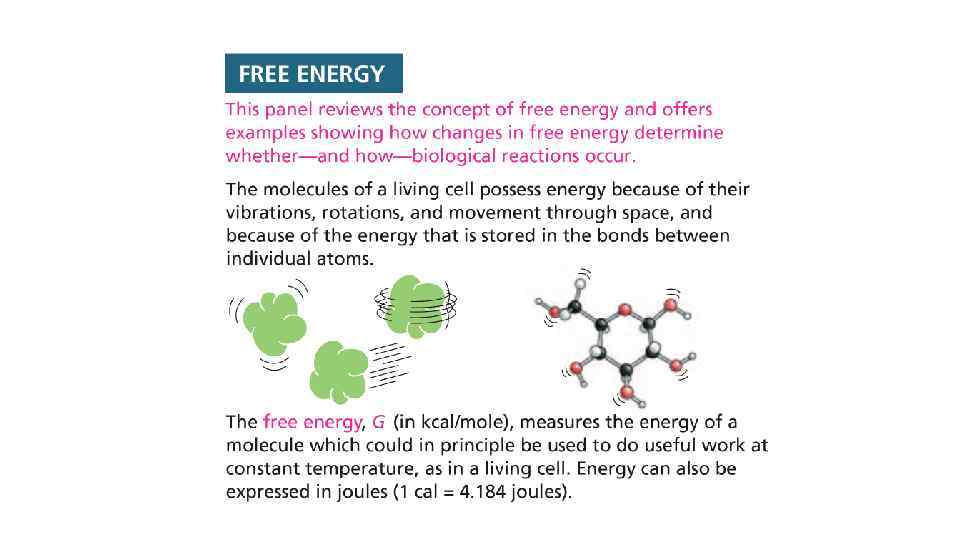
The use of energy by cells • Biological systems have to absorb nutrients from outside • The absorbed nutrients are then converted to different forms of energy • To create order inside the cell, heat should be released from the cell into the surrounding environment. • This creates more disorder outside of the cell • According to second law of thermodynamics the universe is constantly increasing the level of disorder
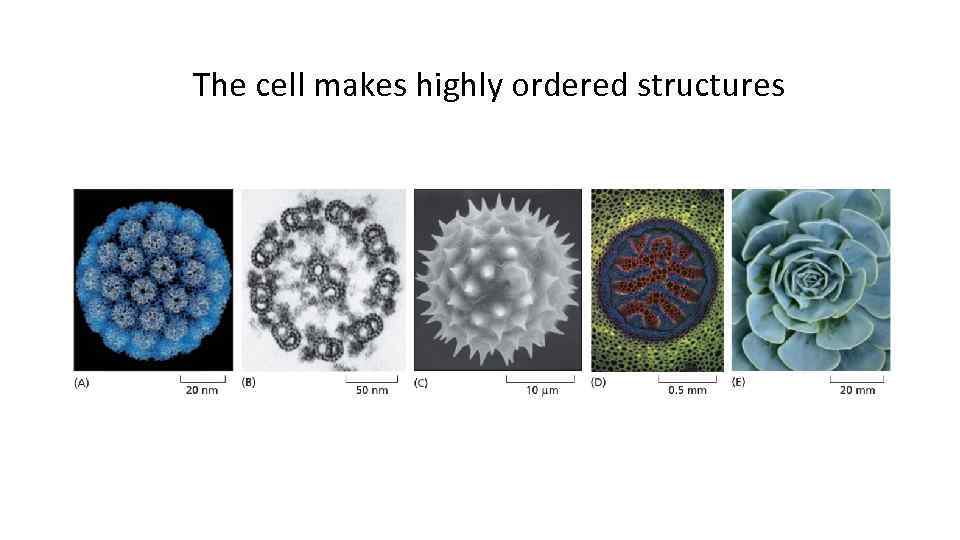
The cell makes highly ordered structures

The universe (outside of the cell) tend to increase the disorder The term that describes the disorder is "ENTROPY" Higher the value of entropy, higher the level of disorder
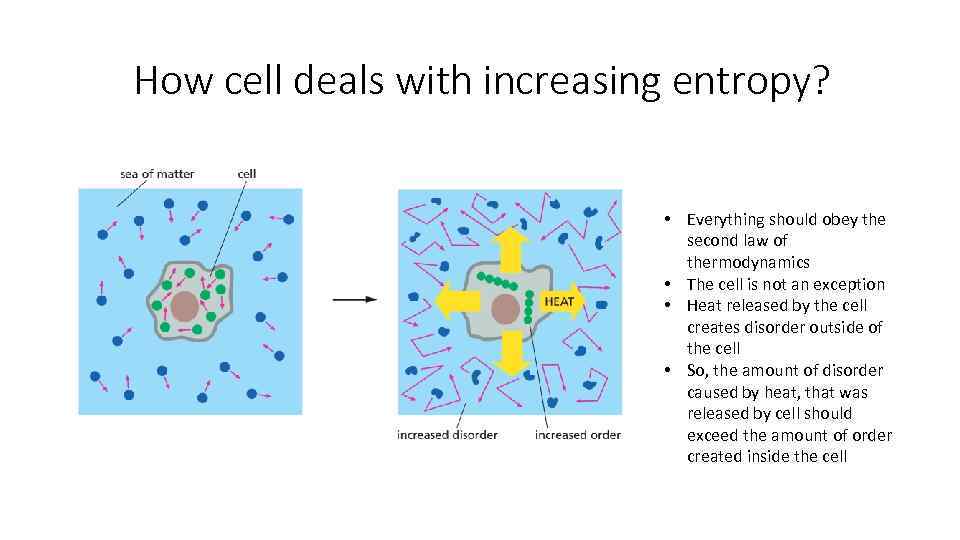
How cell deals with increasing entropy? • Everything should obey the second law of thermodynamics • The cell is not an exception • Heat released by the cell creates disorder outside of the cell • So, the amount of disorder caused by heat, that was released by cell should exceed the amount of order created inside the cell
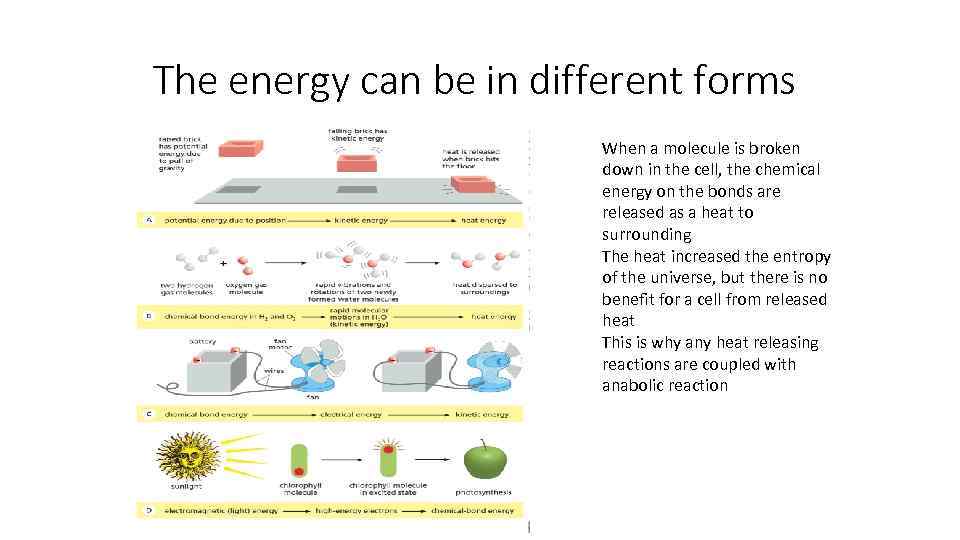
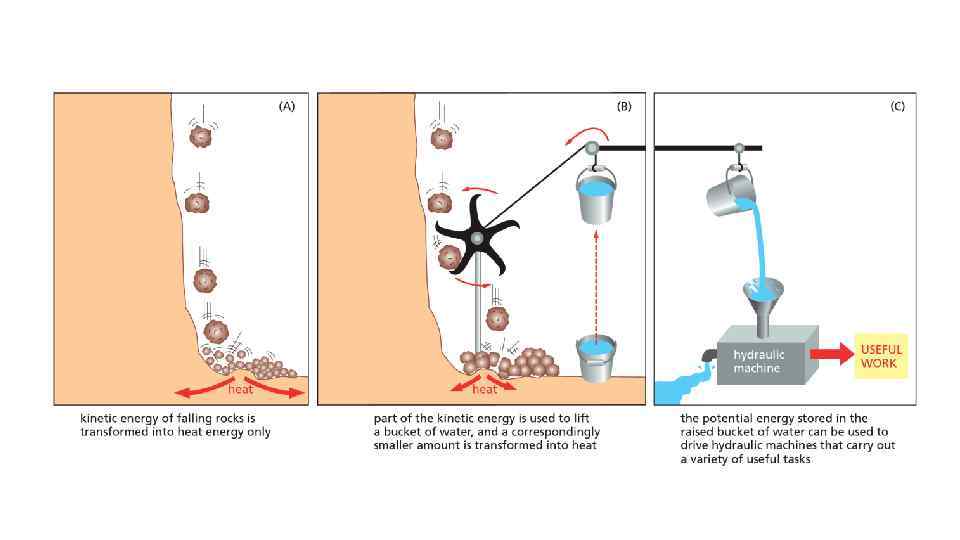
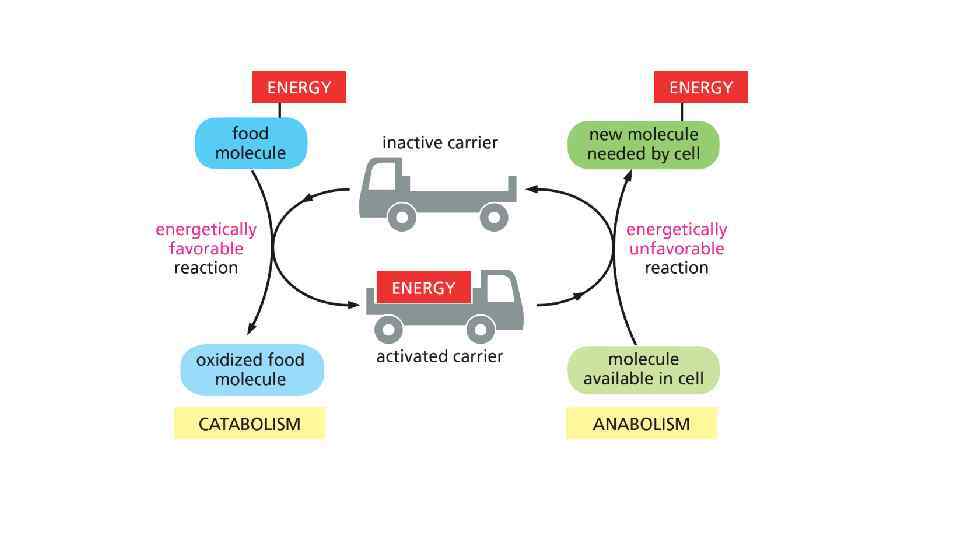
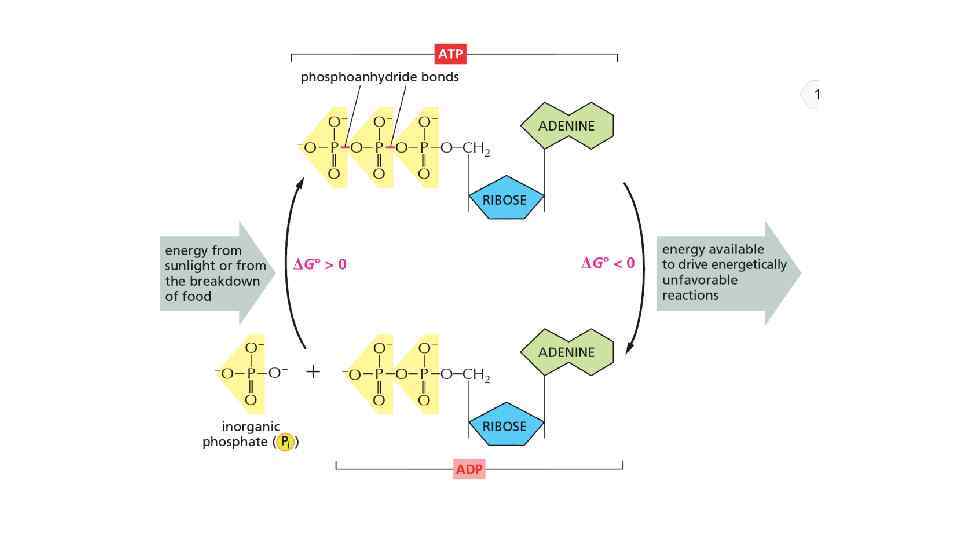
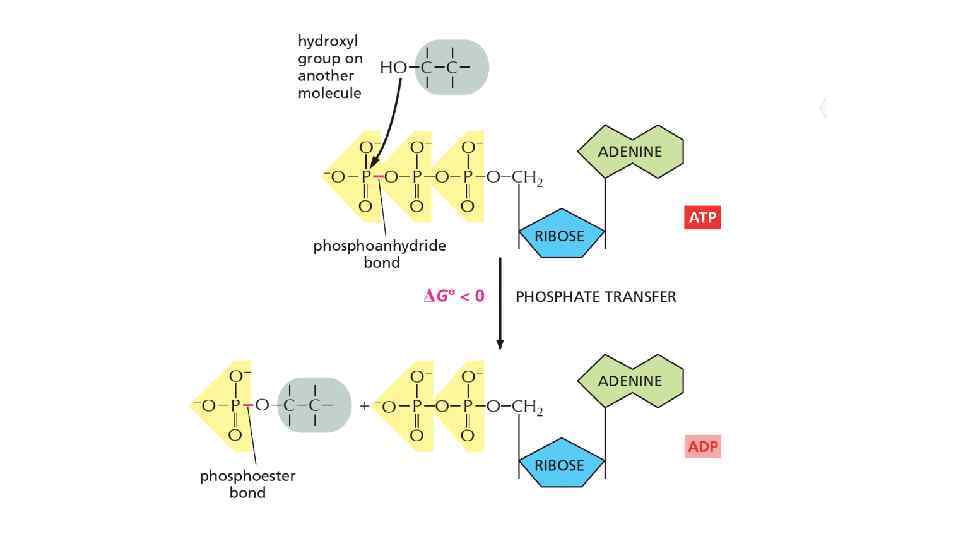
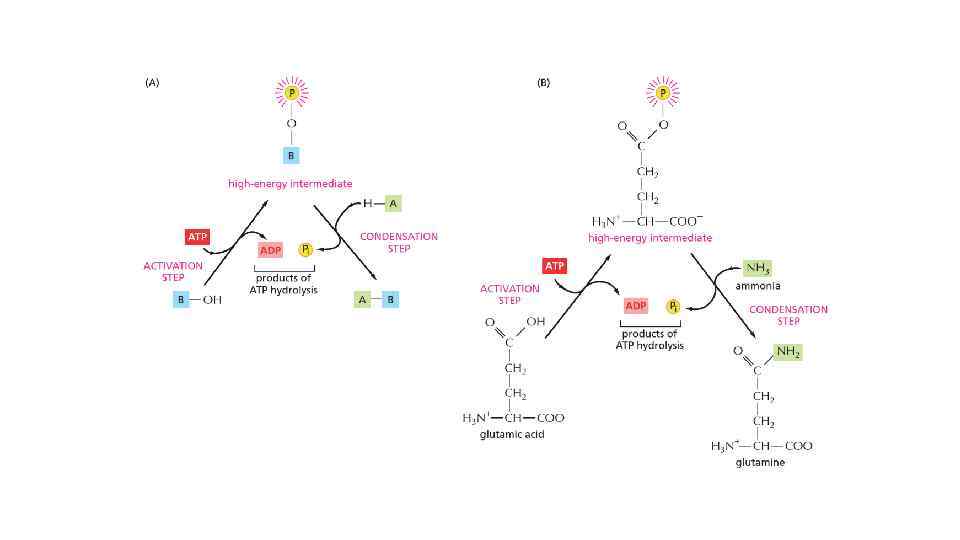
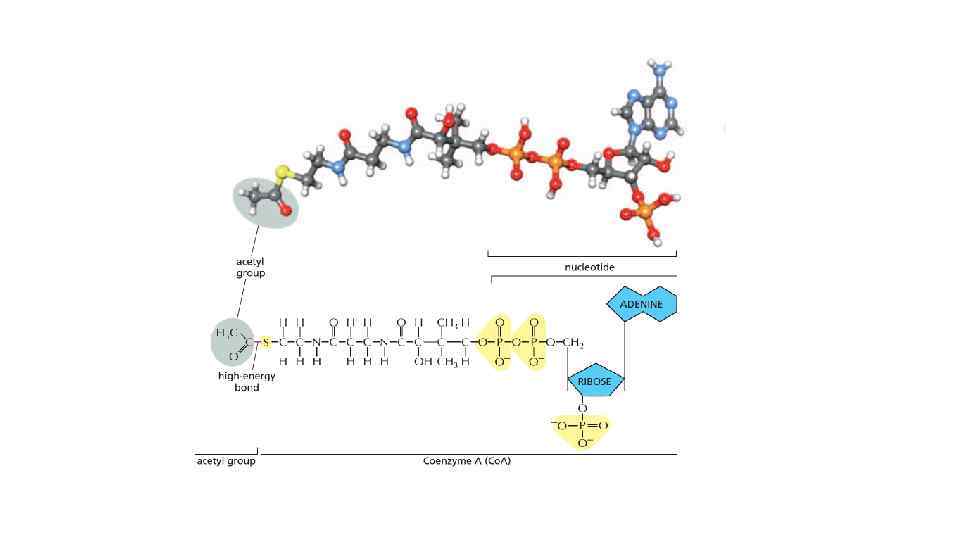
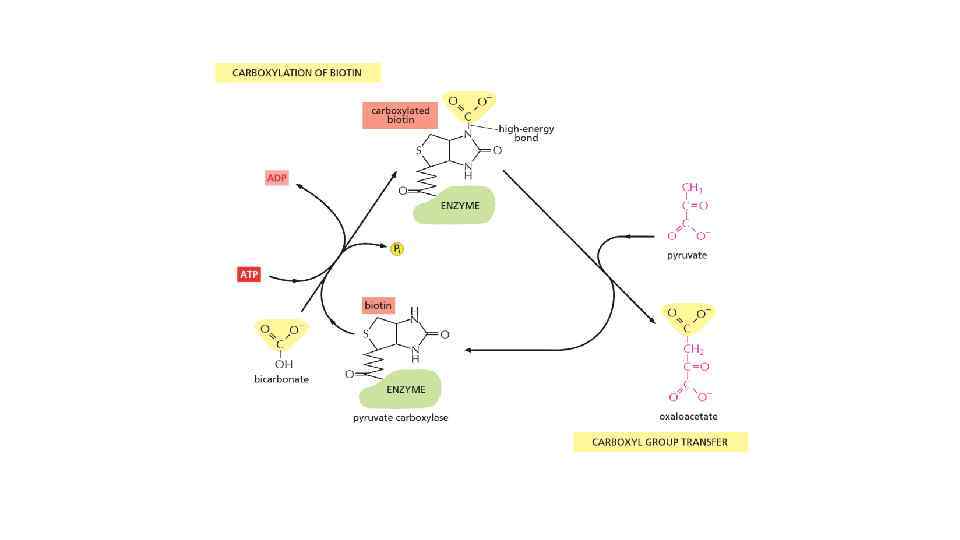
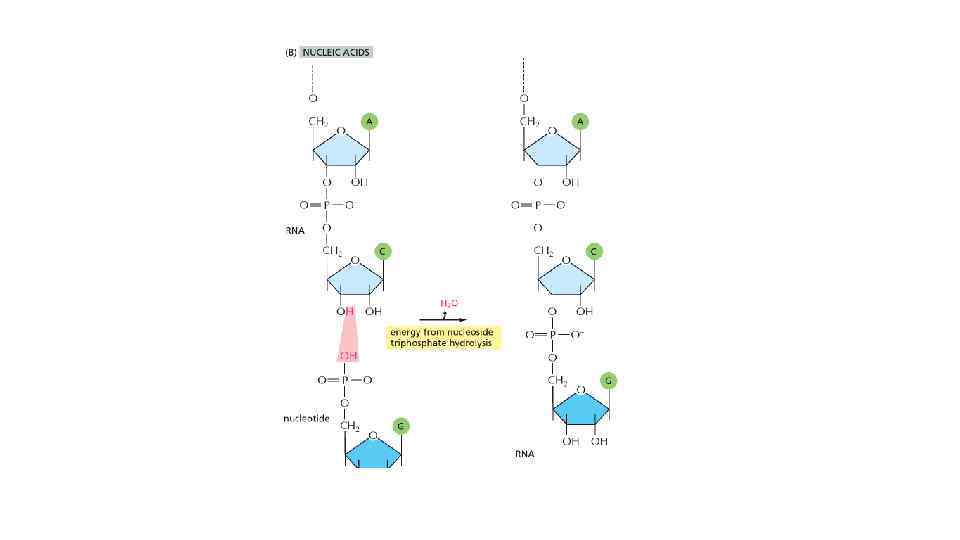
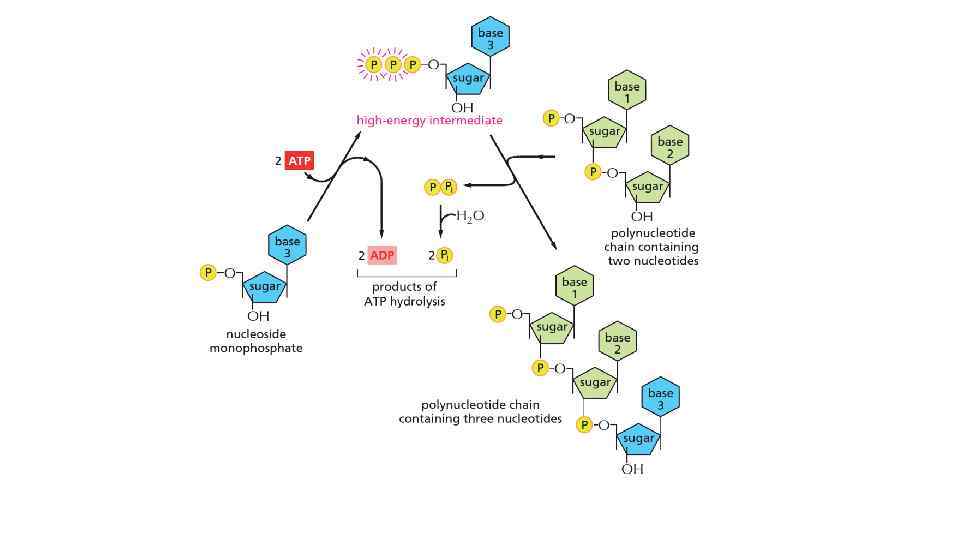
The energy can be in different forms When a molecule is broken down in the cell, the chemical energy on the bonds are released as a heat to surrounding The heat increased the entropy of the universe, but there is no benefit for a cell from released heat This is why any heat releasing reactions are coupled with anabolic reaction
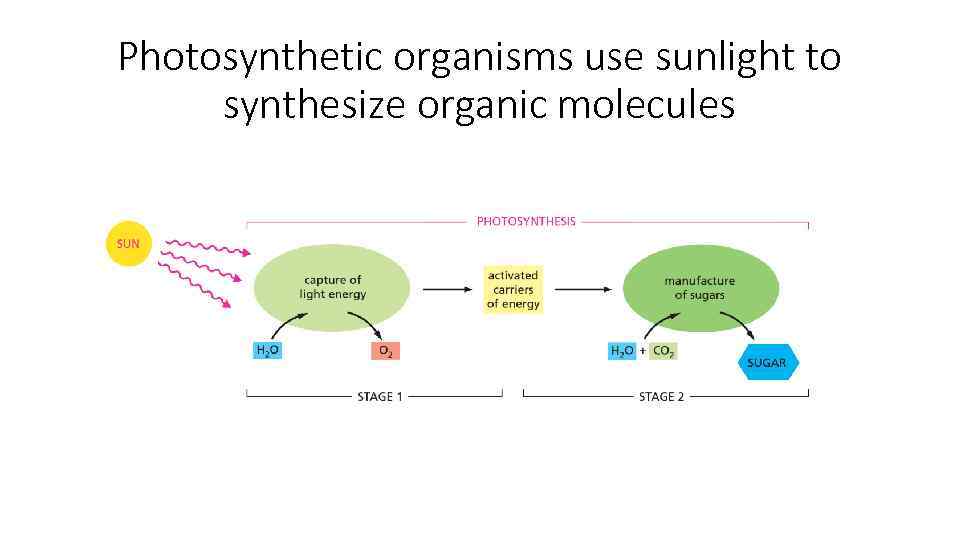
Photosynthetic organisms use sunlight to synthesize organic molecules
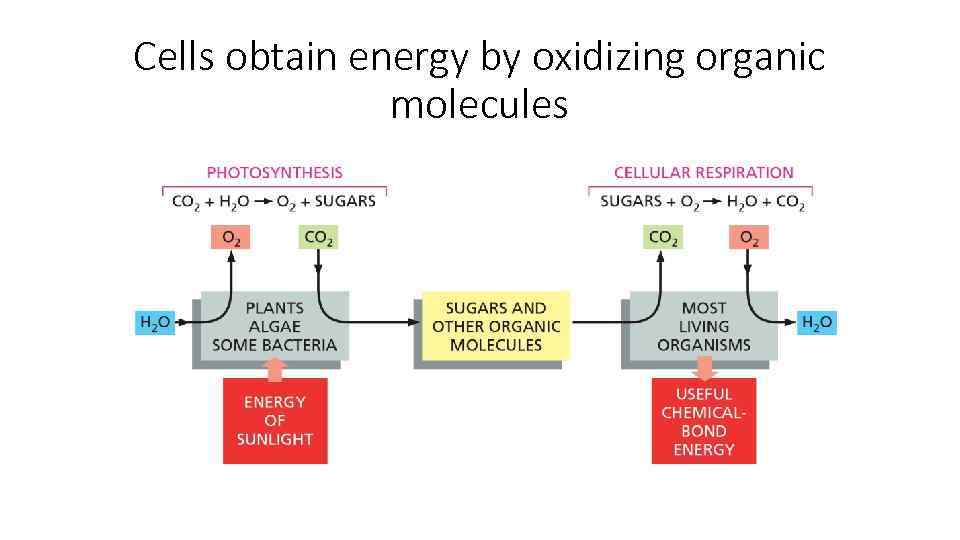
Cells obtain energy by oxidizing organic molecules
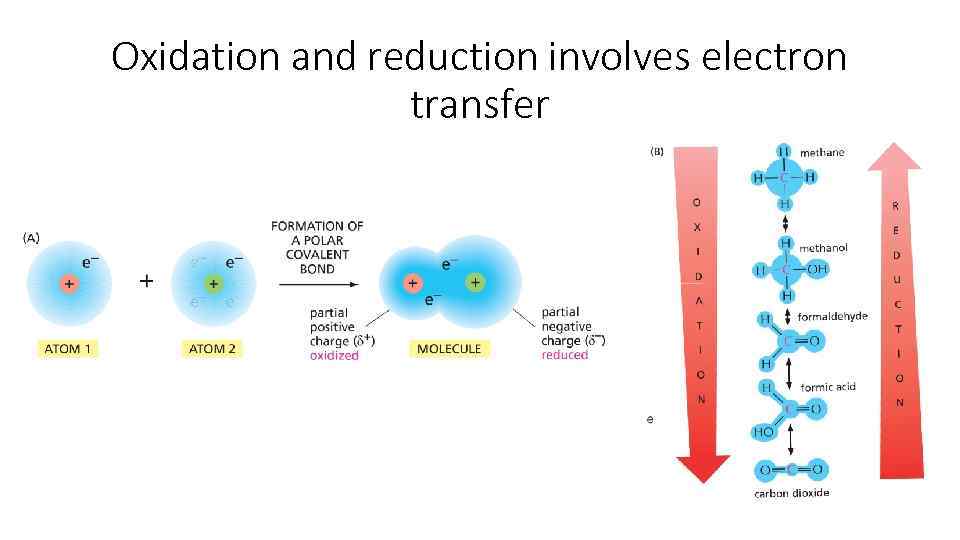
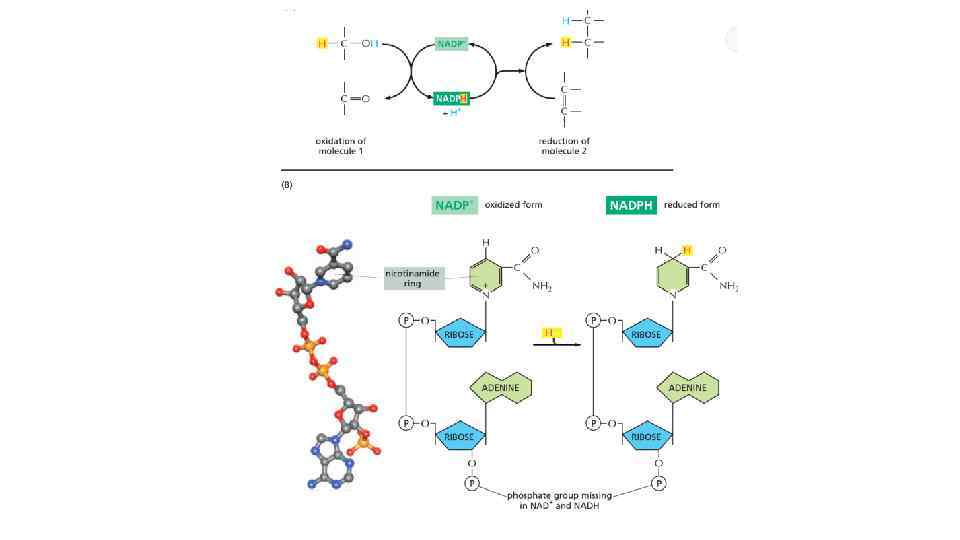
Oxidation and reduction involves electron transfer

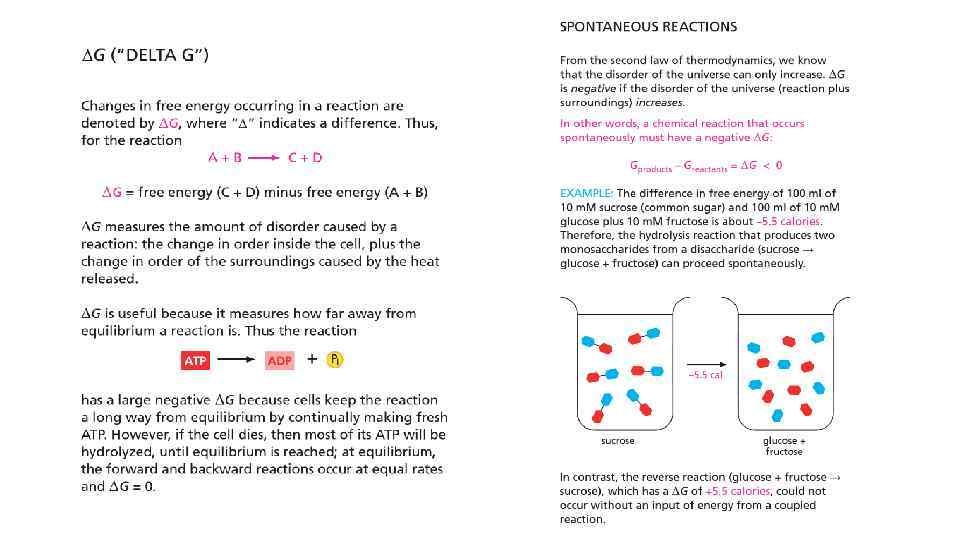
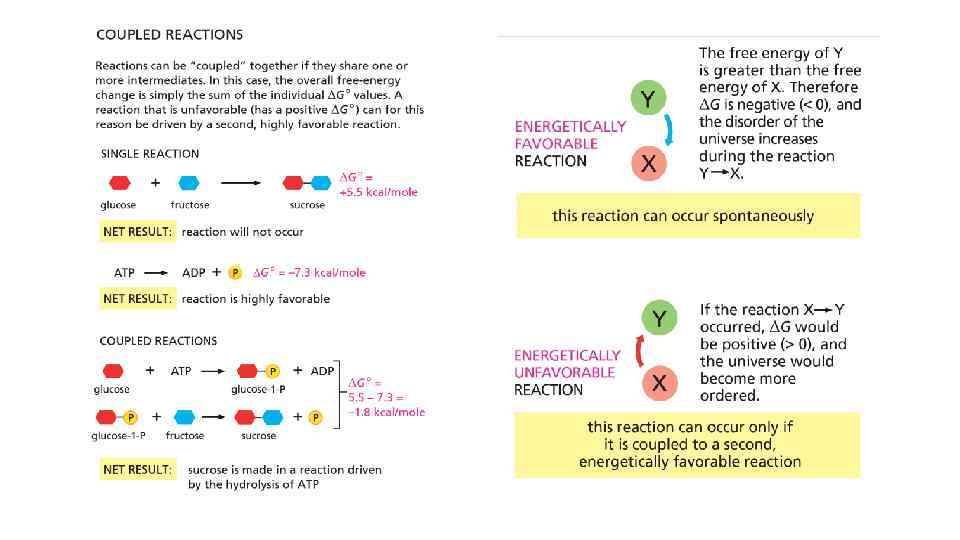
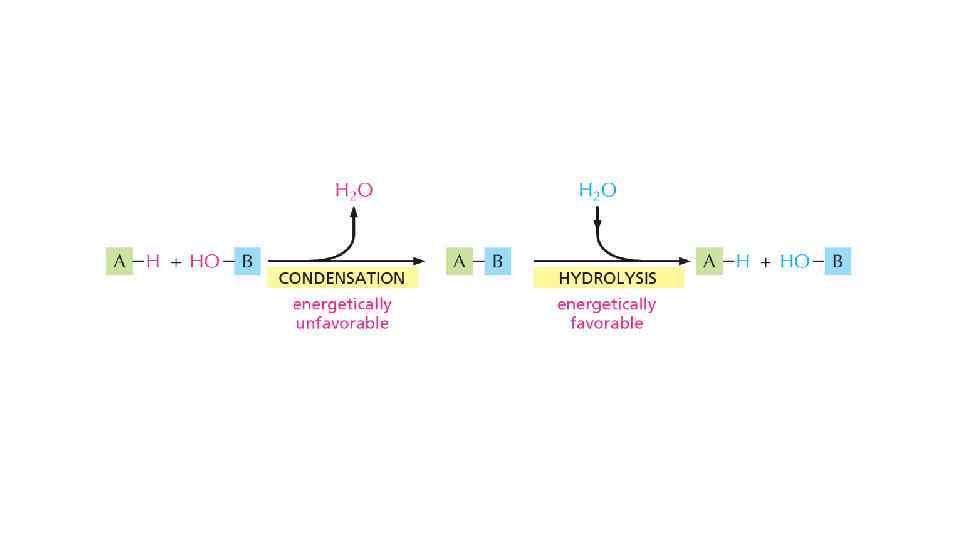
Free energy and catalysis • Enzymes obey 2 nd law of thermodynamics • Enzymes cannot force unfavorable reaction to proceed • To proceed unfavorable reaction enzymes need second, coupled favorable reaction to occur • Enzymes just speed up the reaction, brining up the molecules together
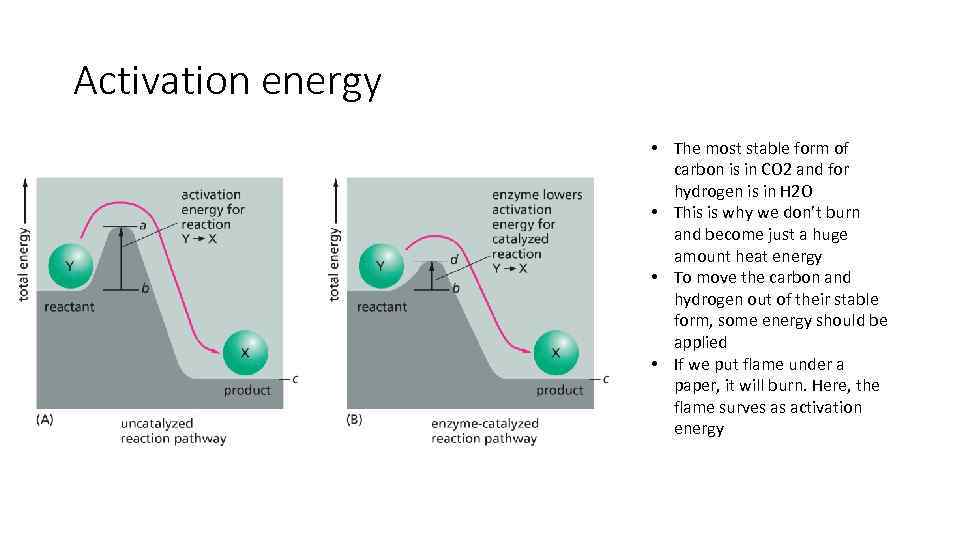
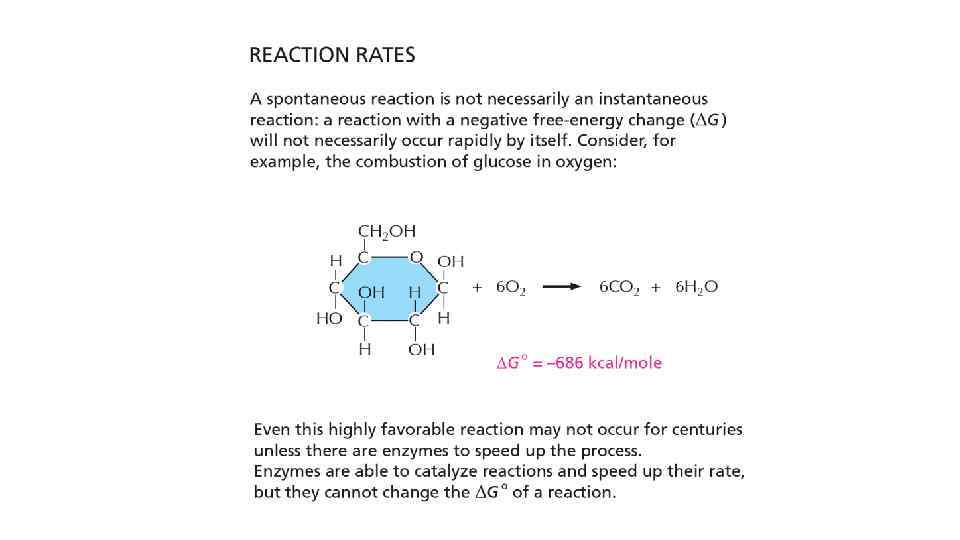
Activation energy • The most stable form of carbon is in CO 2 and for hydrogen is in H 2 O • This is why we don’t burn and become just a huge amount heat energy • To move the carbon and hydrogen out of their stable form, some energy should be applied • If we put flame under a paper, it will burn. Here, the flame surves as activation energy
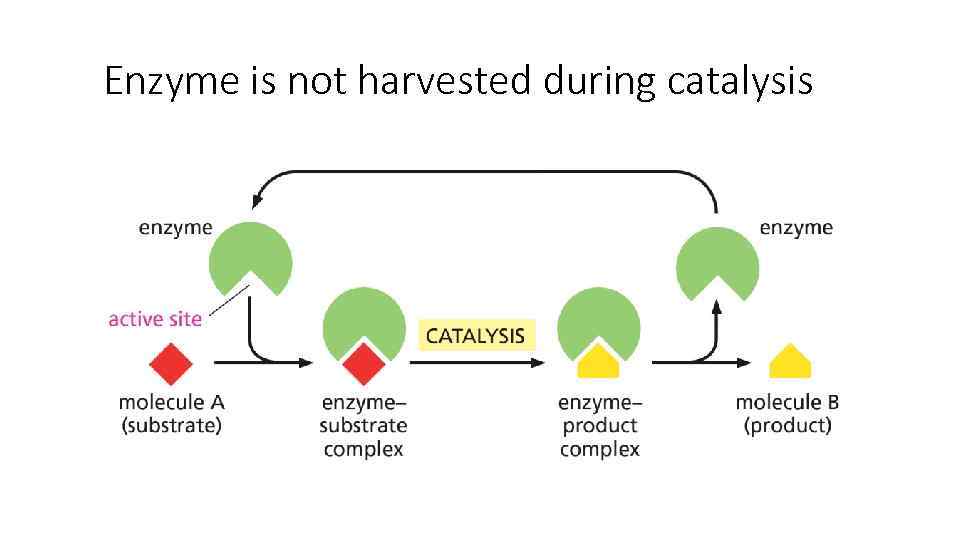
Enzyme is not harvested during catalysis
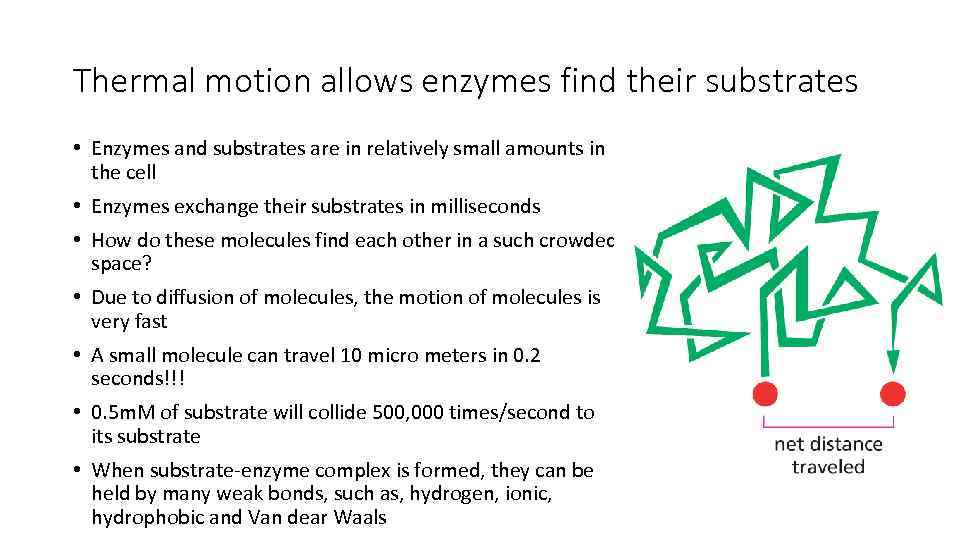
Thermal motion allows enzymes find their substrates • Enzymes and substrates are in relatively small amounts in the cell • Enzymes exchange their substrates in milliseconds • How do these molecules find each other in a such crowded space? • Due to diffusion of molecules, the motion of molecules is very fast • A small molecule can travel 10 micro meters in 0. 2 seconds!!! • 0. 5 m. M of substrate will collide 500, 000 times/second to its substrate • When substrate-enzyme complex is formed, they can be held by many weak bonds, such as, hydrogen, ionic, hydrophobic and Van dear Waals
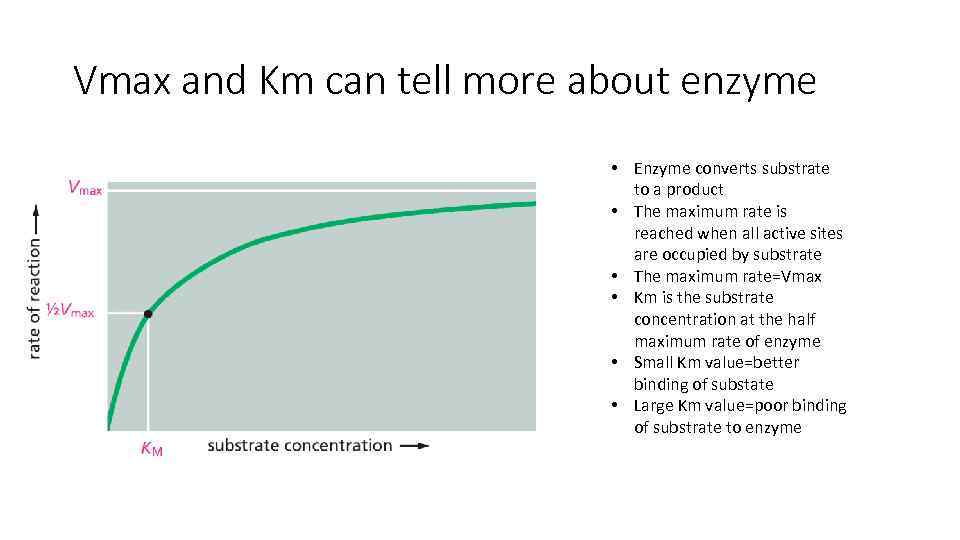
Vmax and Km can tell more about enzyme • Enzyme converts substrate to a product • The maximum rate is reached when all active sites are occupied by substrate • The maximum rate=Vmax • Km is the substrate concentration at the half maximum rate of enzyme • Small Km value=better binding of substate • Large Km value=poor binding of substrate to enzyme
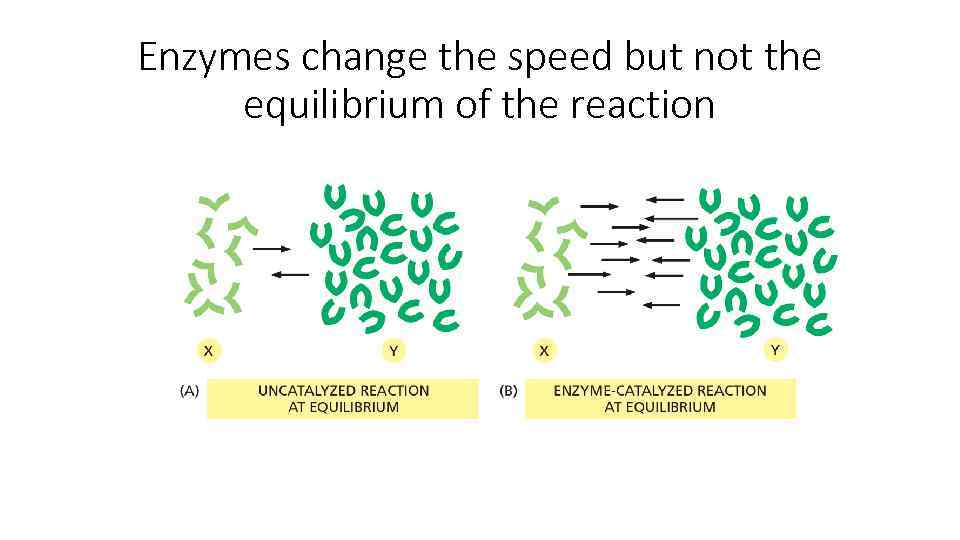
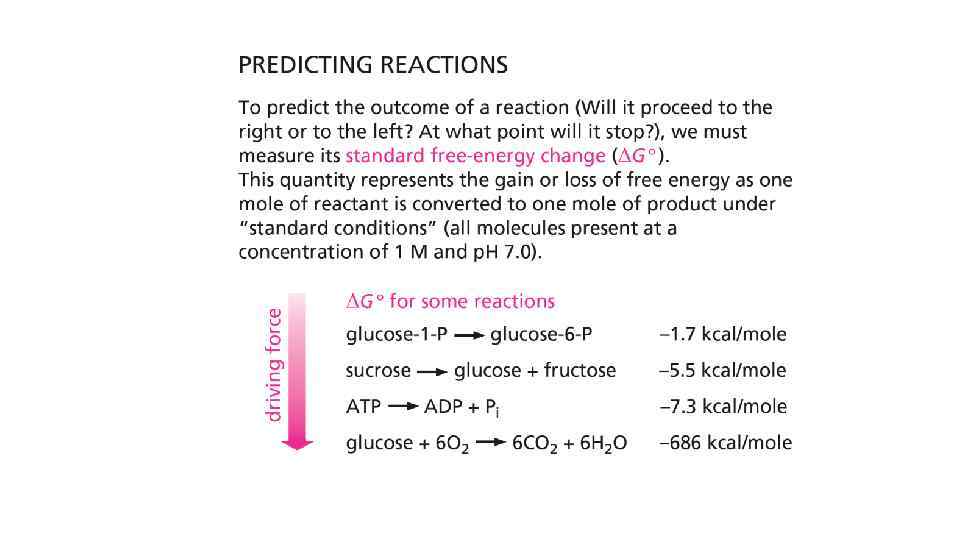
Enzymes change the speed but not the equilibrium of the reaction
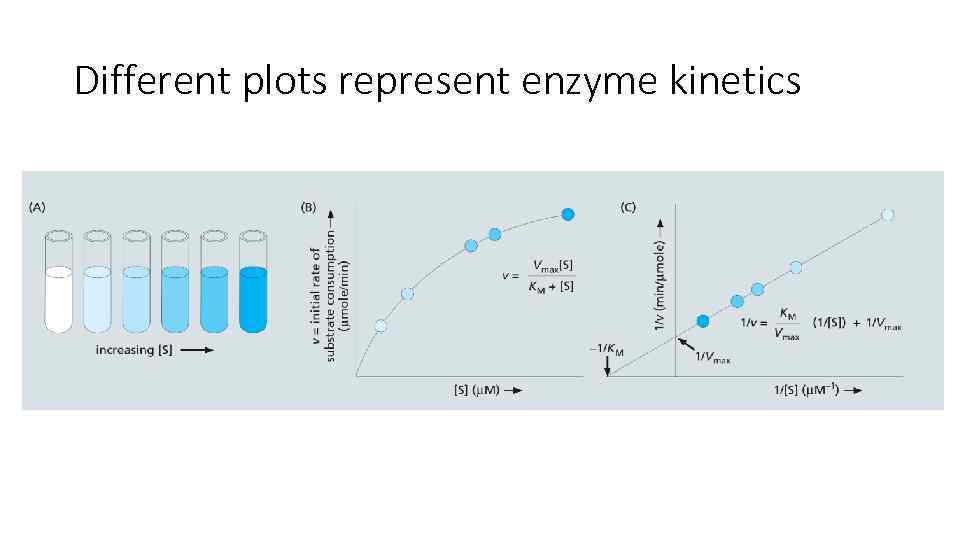
Different plots represent enzyme kinetics
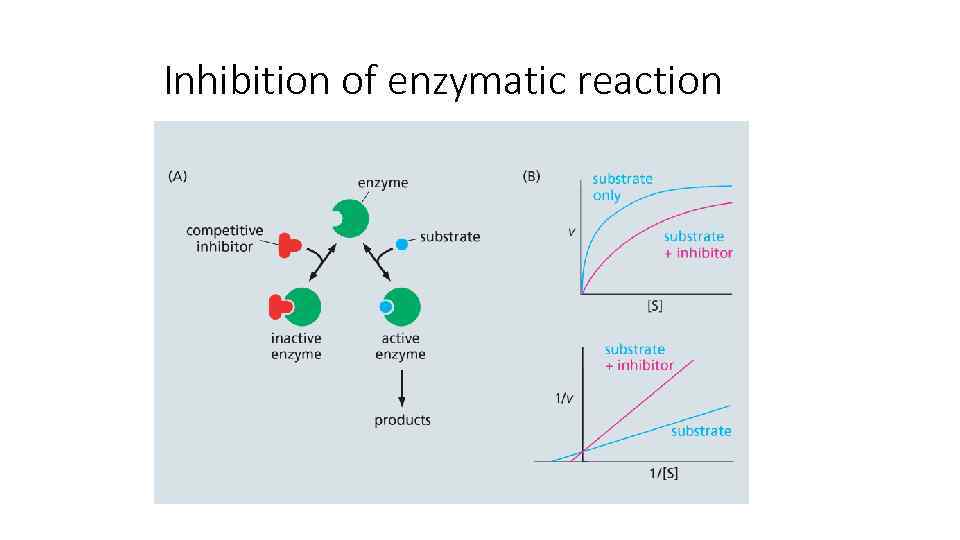
Inhibition of enzymatic reaction
Energy.pptx