1614bd9eeac6a87757870076addb7640.ppt
- Количество слайдов: 72

Chapter 3 Bioenergetics

Chapter 3 Bioenergetics Converting foodstuffs (fats, proteins, carbohydrates) into energy
Metabolism l Total of all chemical reactions that occur in the body – Anabolic reactions • Synthesis of molecules – Catabolic reactions • Breakdown of molecules

Cellular Level l Look back……
Cell Structure l Cell membrane – Protective barrier between interior of cell and extracellular fluid l Nucleus – Contains genes that regulate protein synthesis l Cytoplasm – Fluid portion of cell – Contains organelles (***mitochondria***)
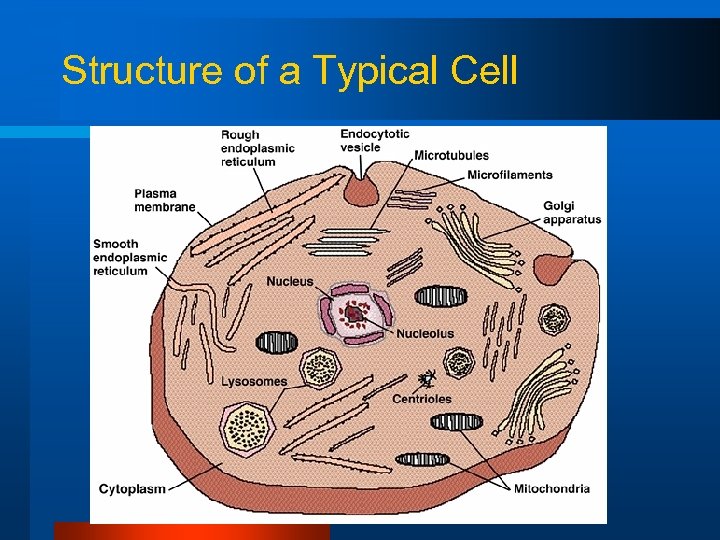
Structure of a Typical Cell

Even the Smallest Cells… …are huge compared to the “bits and pieces” and chemicals in them. If you had to wait for random action, you’d wait forever!
Cellular Chemical Reactions l Endergonic reactions – Require energy to be added l Exergonic reactions – Release energy l Coupled reactions – Liberation of energy in an exergonic reaction drives an endergonic reaction • “Harnessed energy to accomplish a task”
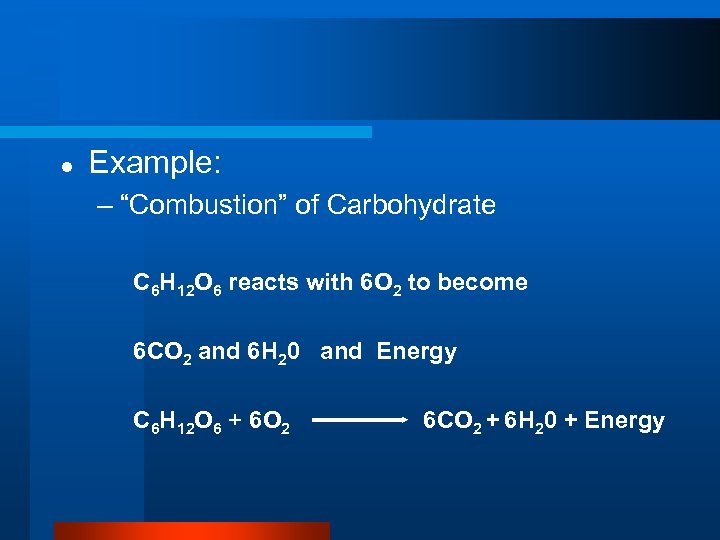
l Example: – “Combustion” of Carbohydrate C 6 H 12 O 6 reacts with 6 O 2 to become 6 CO 2 and 6 H 20 and Energy C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 20 + Energy
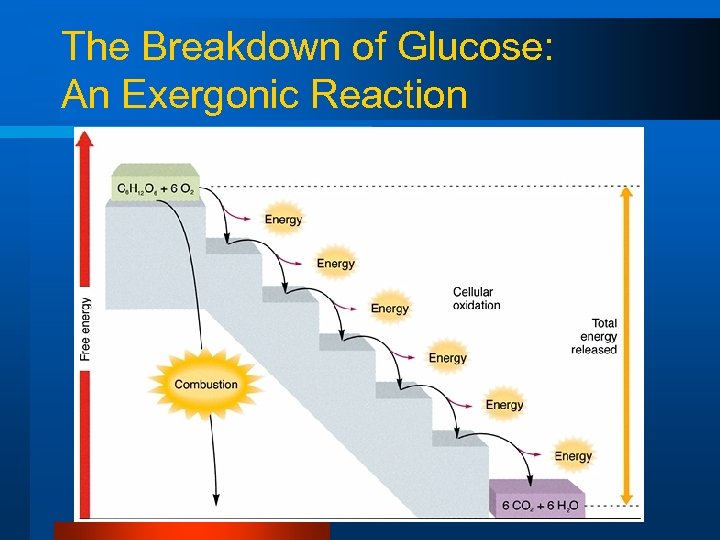
The Breakdown of Glucose: An Exergonic Reaction

Can we regulate reactions? l l l Make sure the reactions occur. Make the reactions occur in a timely manner (speed up the reaction). Prevent the reaction from occurring or slow it down – Rate Limiting

Enzymes l Catalysts that regulate the speed of reactions – Lower the energy of activation – Create specific conditions for reactions
Enzymes l Factors that regulate enzyme activity – Temperature – p. H • work in a narrow range – large gain

Enzymes l Interact with specific substrates – Lock and key model
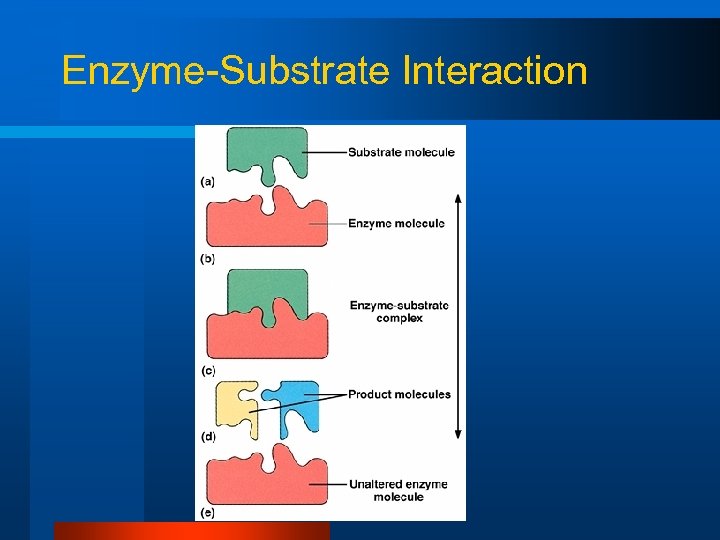
Enzyme-Substrate Interaction
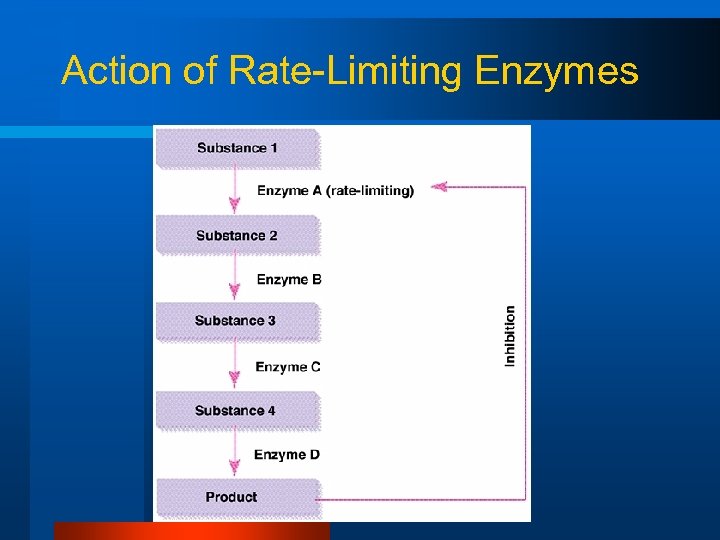
Action of Rate-Limiting Enzymes

How is energy converted?

Energy Systems Overview

Currency System l Money – Gold, $100’s, $50’s, $20’s, $10’s, $5’s, $2’s, $1’s • Dollar coin, fifty cent, quarters, dimes, nickels, pennies. – Exchanged but not destroyed – Recycled?

U. S. Currency System l A dollar has potential to do work for you. l Spend some, but still has potential left l Recycle it and it’s ready to go again l Only so much in circulation
Human Energy Currency System l Adenosine Triphosphate – ATP
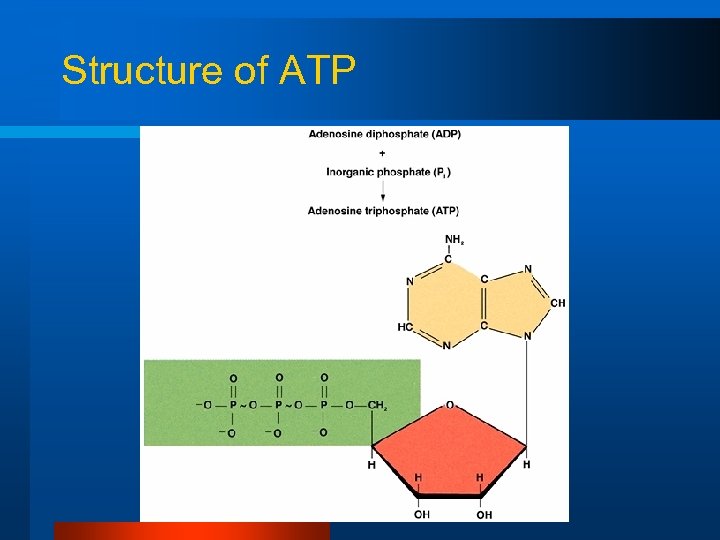
Structure of ATP
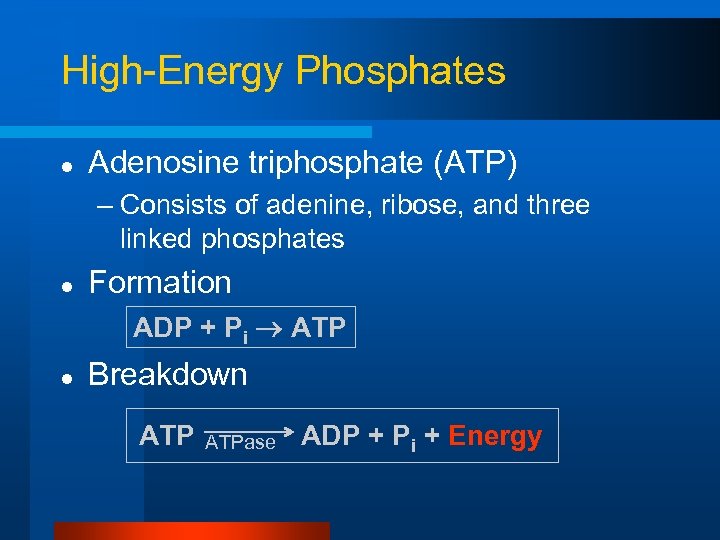
High-Energy Phosphates l Adenosine triphosphate (ATP) – Consists of adenine, ribose, and three linked phosphates l Formation ADP + Pi ATP l Breakdown ATPase ADP + Pi + Energy

Where Does ATP Come From? l Is it in our food? l Can you buy some at the store? – GNC? l Does grandma have some in the attic?

Where Does ATP Come From? l Stored in cells (muscle**) l Store limited amounts (heavy molecule) – Use 50 – 100 x body weight in ATP / 24 hrs. l Make it from ADP – Recycle “used” ATP

Bioenergetics How to make (recycle) ATP “Phosphorylation” - adding a phosphate

Bioenergetics l Anaerobic pathways (non-aerobic) – Do not require or involve O 2 – Called direct phosphorylation
Bioenergetics l Aerobic pathways – Require O 2 molecules – Called oxidative phosphorylation
Anaerobic ATP Production l Stored ATP – Immediate (1 st) source of ATP – Amount depends on muscle size – Runs low on substrate almost immediately ~ 1 sec. – Used during initiation of movement
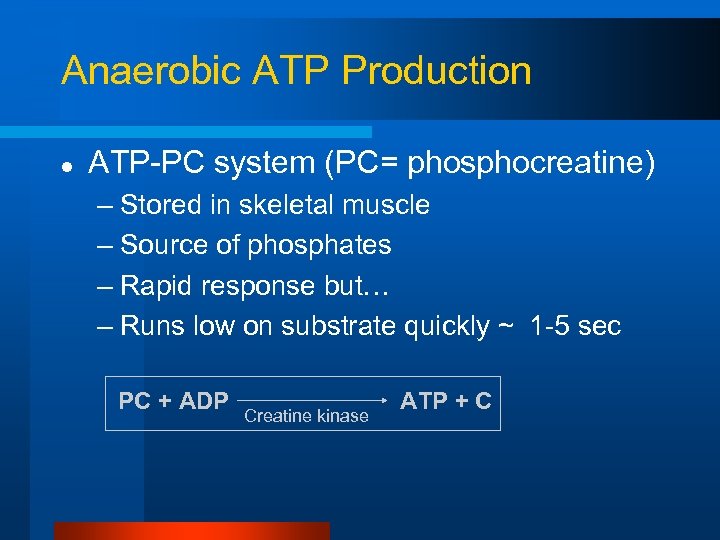
Anaerobic ATP Production l ATP-PC system (PC= phosphocreatine) – Stored in skeletal muscle – Source of phosphates – Rapid response but… – Runs low on substrate quickly ~ 1 -5 sec PC + ADP Creatine kinase ATP + C
Anaerobic ATP Production l Glycolysis – Occurs in cytoplasm – Fairly rapid response (small lag) ~ 3+ sec – High energy response – High energy because of it’s speed of action – Expensive in terms of energy required – Lasts as long as substrates available ~ 30+ sec – Makes product that can limit its action
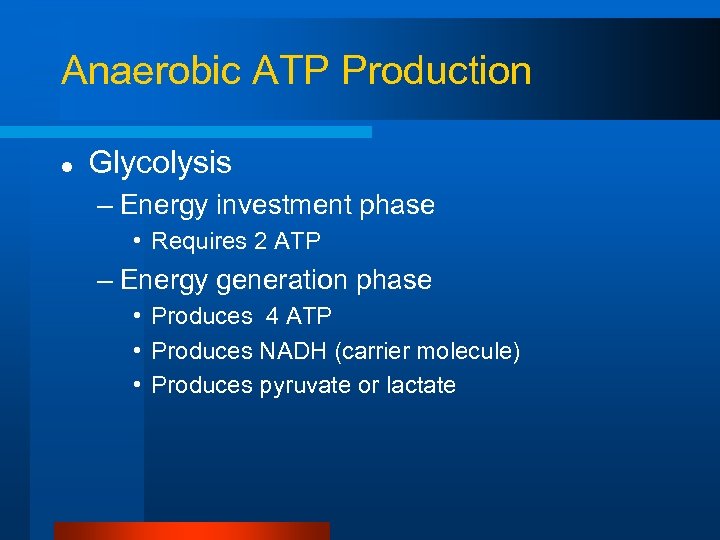
Anaerobic ATP Production l Glycolysis – Energy investment phase • Requires 2 ATP – Energy generation phase • Produces 4 ATP • Produces NADH (carrier molecule) • Produces pyruvate or lactate
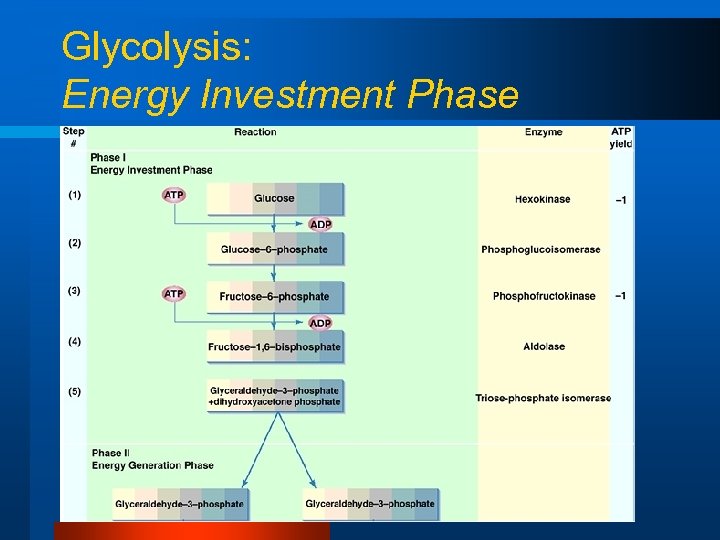
Glycolysis: Energy Investment Phase
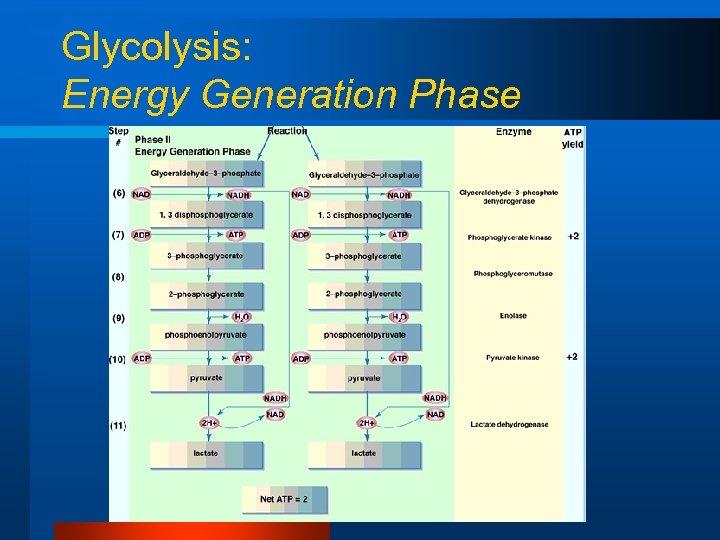
Glycolysis: Energy Generation Phase
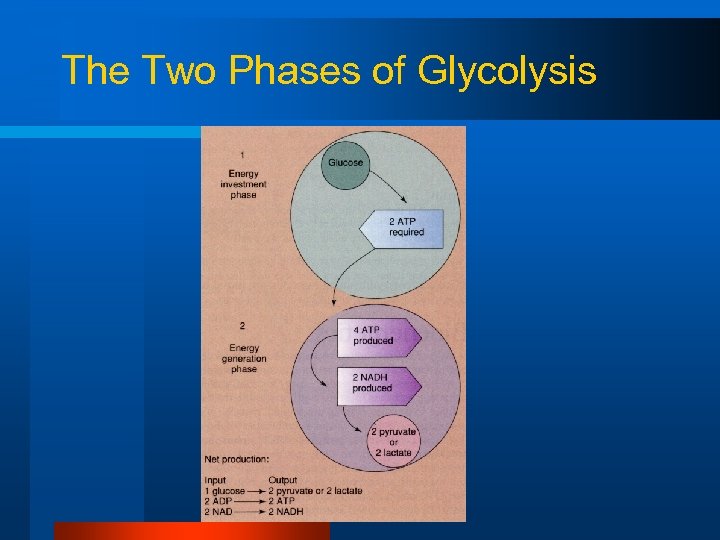
The Two Phases of Glycolysis
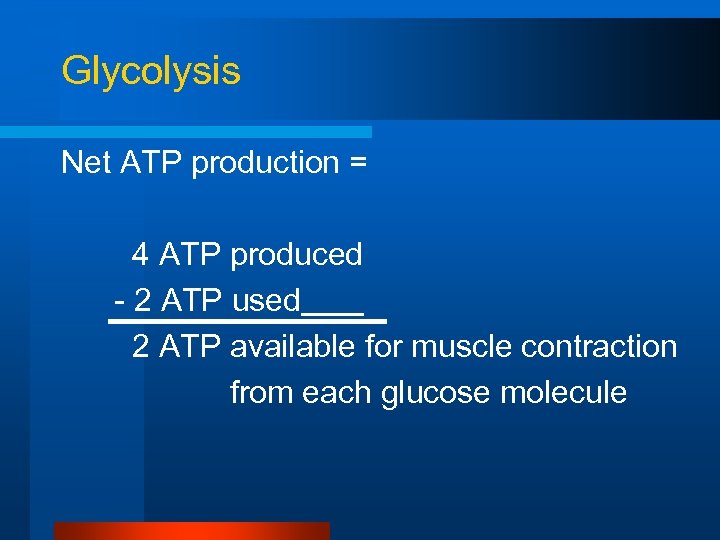
Glycolysis Net ATP production = 4 ATP produced - 2 ATP used 2 ATP available for muscle contraction from each glucose molecule

Is That All That Happens? l No
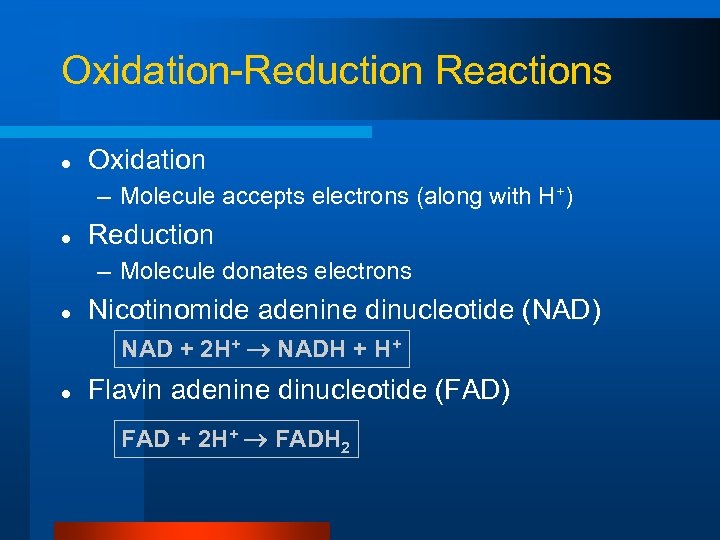
Oxidation-Reduction Reactions l Oxidation – Molecule accepts electrons (along with H+) l Reduction – Molecule donates electrons l Nicotinomide adenine dinucleotide (NAD) NAD + 2 H+ NADH + H+ l Flavin adenine dinucleotide (FAD) FAD + 2 H+ FADH 2

Conversion of Pyruvic Acid to Lactic Acid l l l Normally, O 2 is available in the mitochondria to accept H+ (and electrons) from NADH produced in glycolysis In anaerobic pathways, O 2 is not available H+ and electrons from NADH are accepted by pyruvic acid to form lactic acid
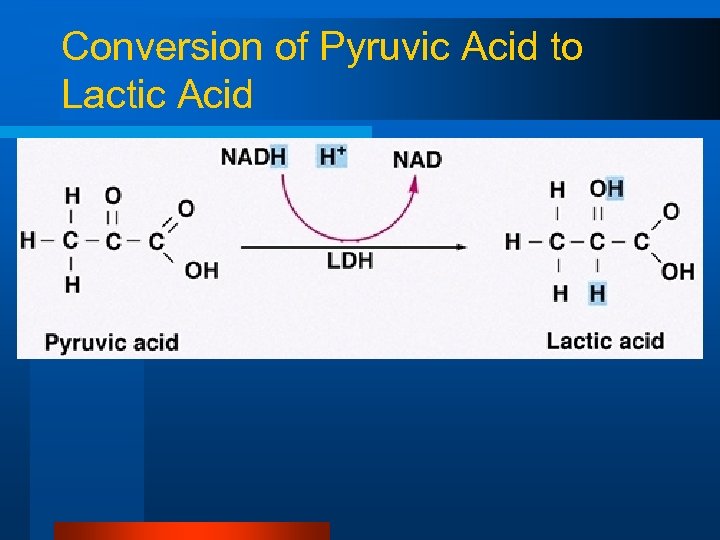
Conversion of Pyruvic Acid to Lactic Acid

So glycolysis is…. . l 1 x 6 carbon molecule split into 2 x 3 carbon molecules (costs 2 ATP) 2 x 3 C molecule rearranged to make 2 x 3 C pyruvic acids (makes 2 ATP each) and 4 H+ are shuttled by 2 NAD to 2 x 3 C pyruvic acids to make 2 x 3 C lactic acids (if H+ s removed from lactic acid = pyruvic acid)

So glycolysis is…. . l l l A fast method of providing energy for movement without the need for O 2 A self-limiting method *Also a potential provider of H+ for aerobic energy production
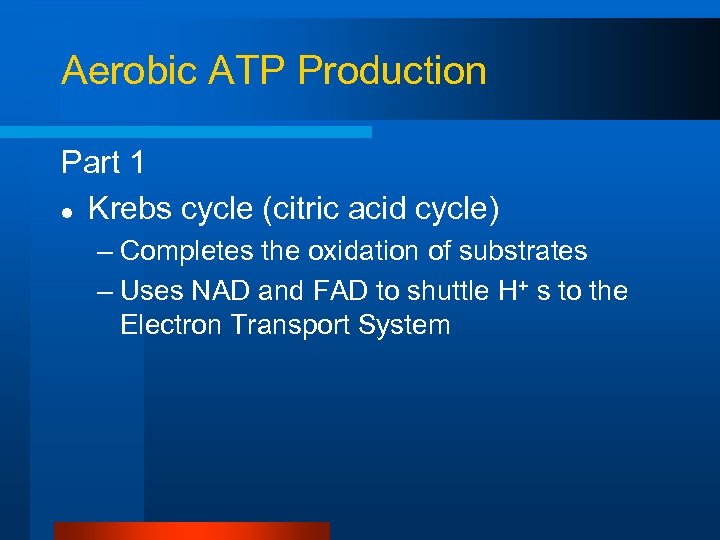
Aerobic ATP Production Part 1 l Krebs cycle (citric acid cycle) – Completes the oxidation of substrates – Uses NAD and FAD to shuttle H+ s to the Electron Transport System
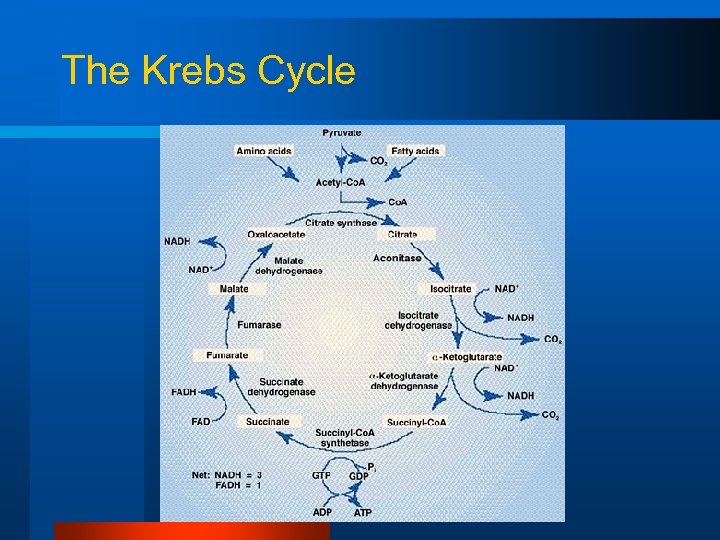
The Krebs Cycle
So the Krebs Cycle is…. l A 2 C molecule added to a 4 C molecule (6 C) – – – rearranged to a 5 C and then 4 C (version) 2 x CO 2 are made 3 x NADH are made 1 x FADH is made 1 x GTP (ATP) is made
Aerobic ATP Production Part 2 l Electron Transport System “ETS” – Oxidative Phosphorylation
Aerobic ATP Production l ETS – Electrons removed from NADH and FADH are passed along a series of carriers to drive phosphorylation of ADP to ATP – H+ from NADH and FADH are accepted by O 2 to form water – H 2 O (neutral)
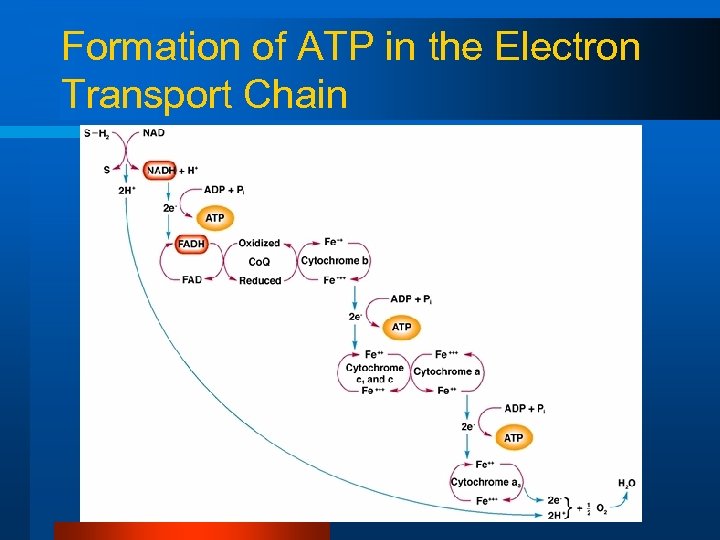
Formation of ATP in the Electron Transport Chain

So for each…… l 1 NADH through the ETS = 3 ATP made l 1 FADH through the ETS = 2 ATP made
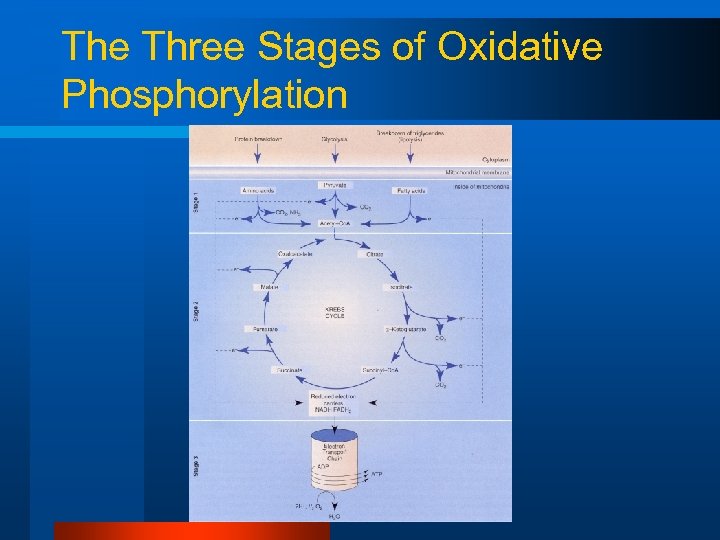
The Three Stages of Oxidative Phosphorylation
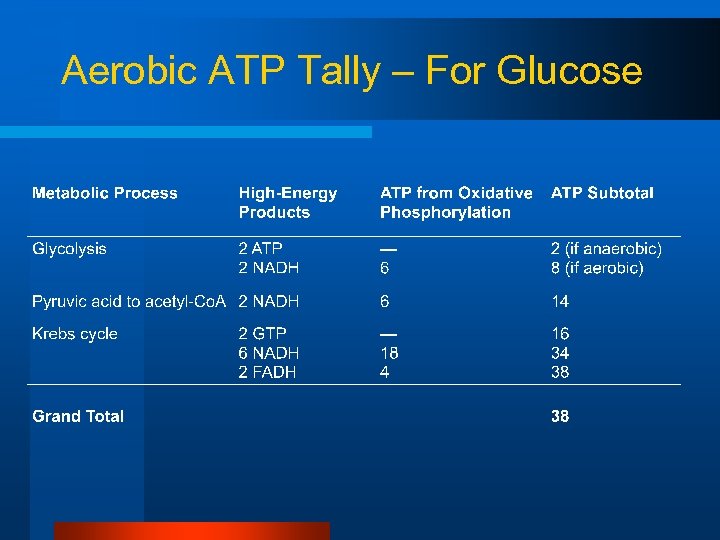
Aerobic ATP Tally – For Glucose
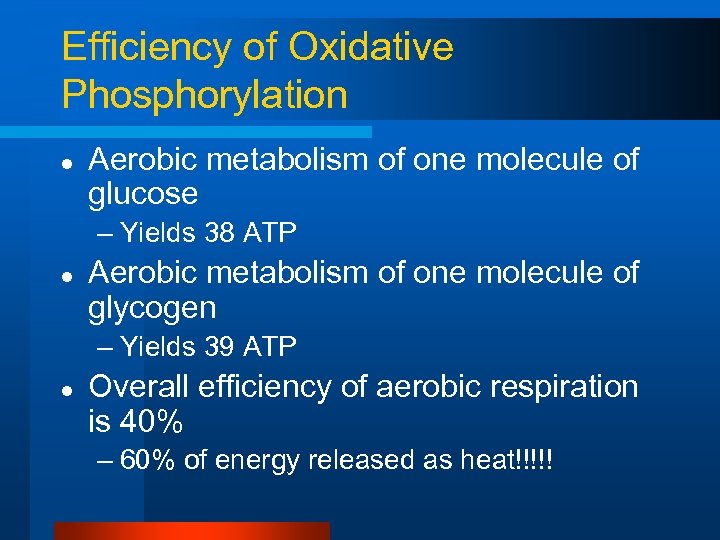
Efficiency of Oxidative Phosphorylation l Aerobic metabolism of one molecule of glucose – Yields 38 ATP l Aerobic metabolism of one molecule of glycogen – Yields 39 ATP l Overall efficiency of aerobic respiration is 40% – 60% of energy released as heat!!!!!

Is Glucose the Only Fuel Source? l No
Fuels for Phosphorylation l Carbohydrates – Glucose (6 carbon molecule) – Glycogen (branched carbon molecules) • Glucose stored in muscle cells and liver • Glycogenolysis - makes carbons available for metabolism – Occurring in muscle cells - glucose stays in muscle cells – Occurring in liver – glucose put into circulation
Fuels for Phosphorylation l Fats (18+ carbon molecule) – Primarily fatty acids (FA) • Stored as triglycerides • Stored in muscle cells and in adipose cells • Beta oxidation makes FAs available for Krebs Cycle – Release of FAs from adipose to blood stream – Hormone initiated – All working muscles have access to FAs via cellular storage or via blood stream
Fuels for Phosphorylation l Proteins – Amino Acids (AA) – Storage is structural (no extra depot) – Carbons are available – Not a primary energy source during exercise (too expensive) – But usable – long duration exercise (up to 15%)
Fuels for Phosphorylation What is common between all these fuel sources? - A 2 carbon molecule called…. . Acetyl Co-A
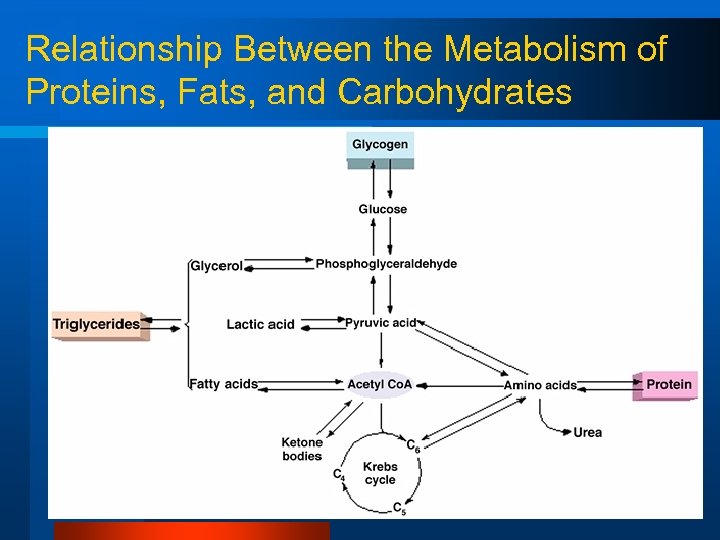
Relationship Between the Metabolism of Proteins, Fats, and Carbohydrates

There’s one other potential fuel source.
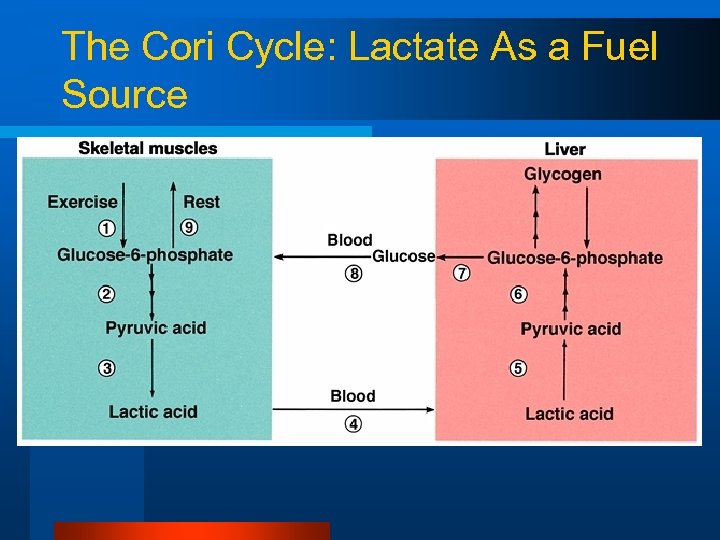
The Cori Cycle: Lactate As a Fuel Source

Can You See the “Big Picture” Regarding Aerobic ATP Production? What’s it all about? Does anybody know? Here’s how I found out.

How is control maintained?
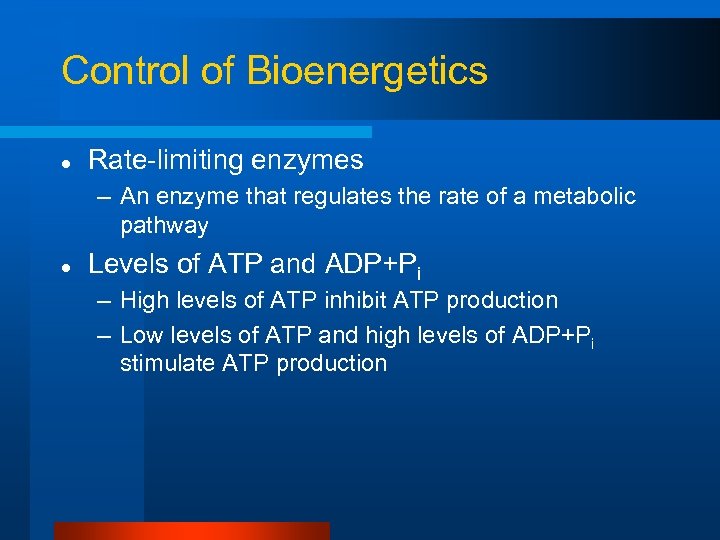
Control of Bioenergetics l Rate-limiting enzymes – An enzyme that regulates the rate of a metabolic pathway l Levels of ATP and ADP+Pi – High levels of ATP inhibit ATP production – Low levels of ATP and high levels of ADP+Pi stimulate ATP production
Control of Bioenergetics l Rate-limiting enzymes – An enzyme that regulates the rate of a metabolic pathway l Levels of ATP and ADP+Pi – High levels of ATP inhibit ATP production – Low levels of ATP and high levels of ADP+Pi stimulate ATP production l Calcium may stimulate aerobic ATP production
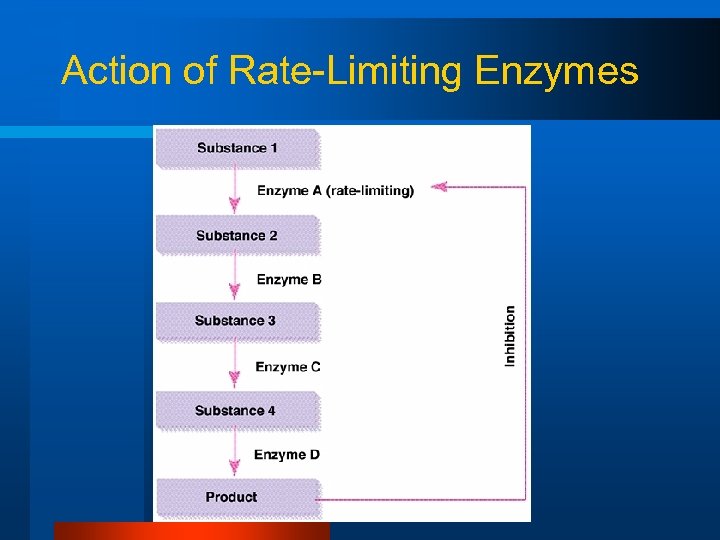
Action of Rate-Limiting Enzymes
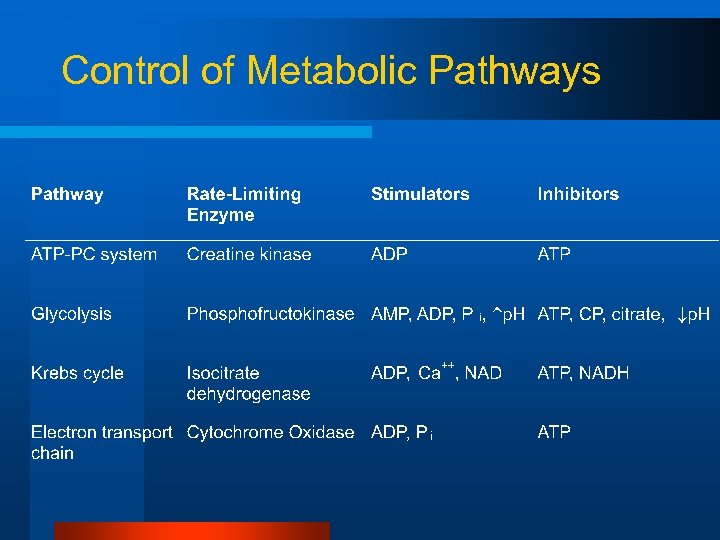
Control of Metabolic Pathways

Remember…. . 1 cell = absolute Whole body = not absolute Where is there interaction between energy systems?

Interaction Between Aerobic and Anaerobic ATP Production l Energy to perform exercise comes from an interaction between aerobic and anaerobic pathways
Interaction Between Aerobic and Anaerobic ATP Production l Effect of duration and intensity – Immediate movement • Stored ATP – Short-term, high-intensity activities • Greater contribution of anaerobic energy systems – ATP-PC and Glycolysis – Long-term, low to moderate-intensity exercise • Majority of ATP produced from aerobic sources – Aerobic glycolysis, beta oxidation, protein

Questions?

End
1614bd9eeac6a87757870076addb7640.ppt