f567bdbdabd6b48559cac0c7b225d55f.ppt
- Количество слайдов: 55
 Chapter 2 STRUCTURE AND PROPERTIES OF ATOMS
Chapter 2 STRUCTURE AND PROPERTIES OF ATOMS
 The hydrogen atom A set of elementary particles, protons, neutrons and electrons can build an atom. Our model in this chapter is the isolated atom, the atom that does not treat into interactions with its environment. A low pressure gas is its good realisation. The hydrogen atom is the simplest atom therefore this study begins with it.
The hydrogen atom A set of elementary particles, protons, neutrons and electrons can build an atom. Our model in this chapter is the isolated atom, the atom that does not treat into interactions with its environment. A low pressure gas is its good realisation. The hydrogen atom is the simplest atom therefore this study begins with it.
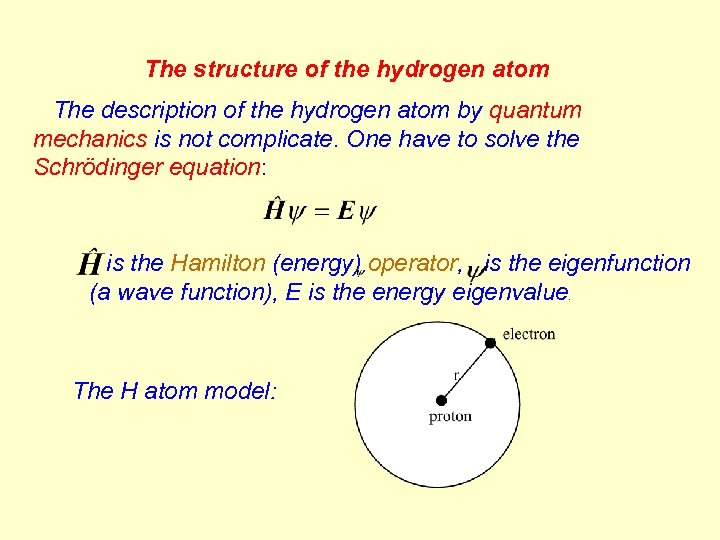 The structure of the hydrogen atom The description of the hydrogen atom by quantum mechanics is not complicate. One have to solve the Schrödinger equation: is the Hamilton (energy) operator, is the eigenfunction (a wave function), E is the energy eigenvalue. The H atom model:
The structure of the hydrogen atom The description of the hydrogen atom by quantum mechanics is not complicate. One have to solve the Schrödinger equation: is the Hamilton (energy) operator, is the eigenfunction (a wave function), E is the energy eigenvalue. The H atom model:
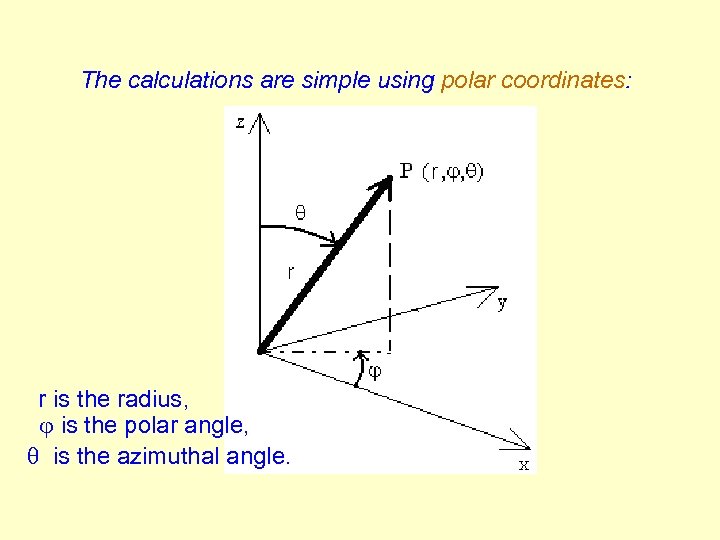 The calculations are simple using polar coordinates: r is the radius, is the polar angle, q is the azimuthal angle.
The calculations are simple using polar coordinates: r is the radius, is the polar angle, q is the azimuthal angle.
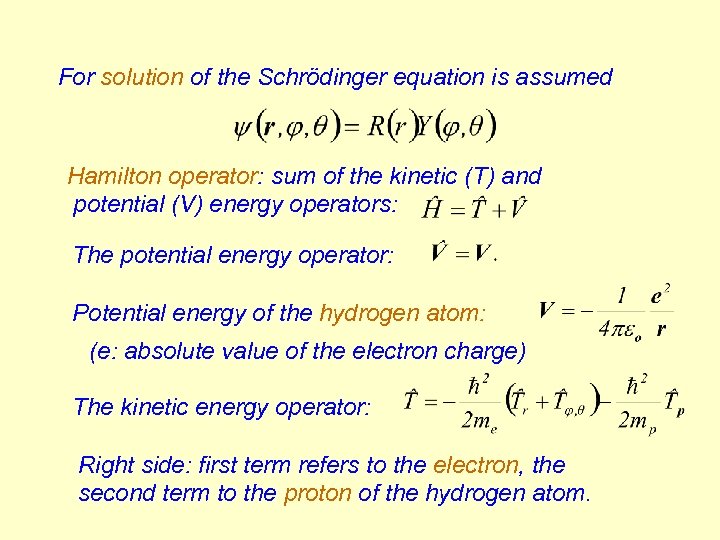 For solution of the Schrödinger equation is assumed Hamilton operator: sum of the kinetic (T) and potential (V) energy operators: The potential energy operator: Potential energy of the hydrogen atom: (e: absolute value of the electron charge) The kinetic energy operator: Right side: first term refers to the electron, the second term to the proton of the hydrogen atom.
For solution of the Schrödinger equation is assumed Hamilton operator: sum of the kinetic (T) and potential (V) energy operators: The potential energy operator: Potential energy of the hydrogen atom: (e: absolute value of the electron charge) The kinetic energy operator: Right side: first term refers to the electron, the second term to the proton of the hydrogen atom.
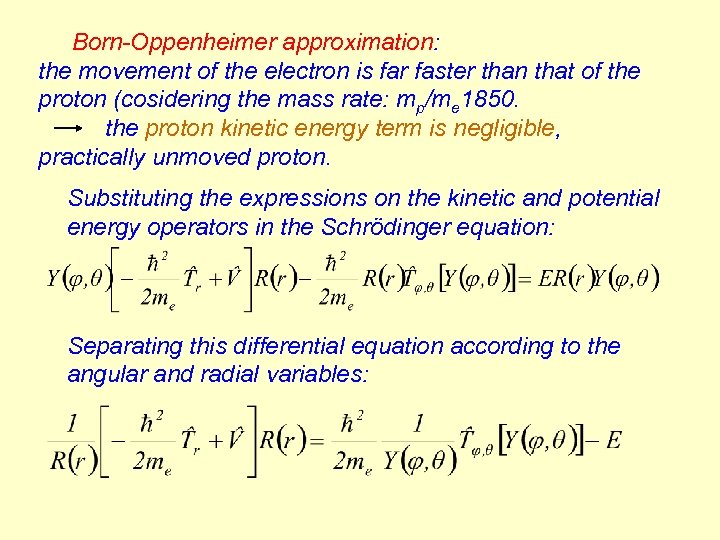 Born-Oppenheimer approximation: the movement of the electron is far faster than that of the proton (cosidering the mass rate: mp/me 1850. the proton kinetic energy term is negligible, practically unmoved proton. Substituting the expressions on the kinetic and potential energy operators in the Schrödinger equation: Separating this differential equation according to the angular and radial variables:
Born-Oppenheimer approximation: the movement of the electron is far faster than that of the proton (cosidering the mass rate: mp/me 1850. the proton kinetic energy term is negligible, practically unmoved proton. Substituting the expressions on the kinetic and potential energy operators in the Schrödinger equation: Separating this differential equation according to the angular and radial variables:
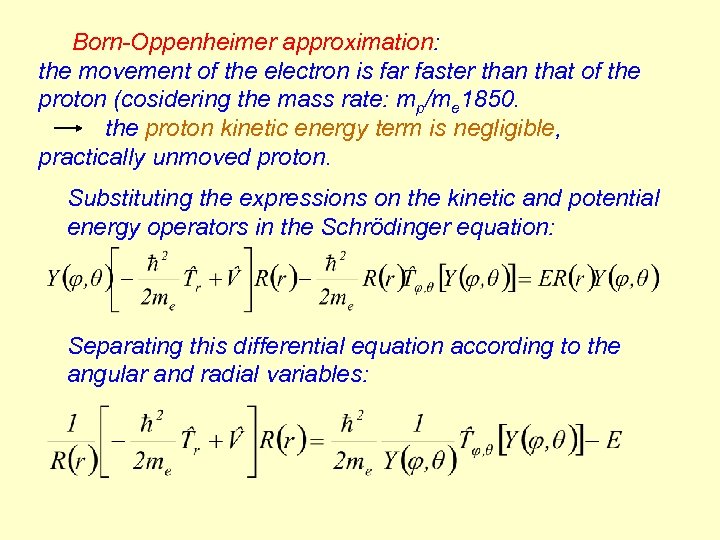 Born-Oppenheimer approximation: the movement of the electron is far faster than that of the proton (cosidering the mass rate: mp/me 1850. the proton kinetic energy term is negligible, practically unmoved proton. Substituting the expressions on the kinetic and potential energy operators in the Schrödinger equation: Separating this differential equation according to the angular and radial variables:
Born-Oppenheimer approximation: the movement of the electron is far faster than that of the proton (cosidering the mass rate: mp/me 1850. the proton kinetic energy term is negligible, practically unmoved proton. Substituting the expressions on the kinetic and potential energy operators in the Schrödinger equation: Separating this differential equation according to the angular and radial variables:
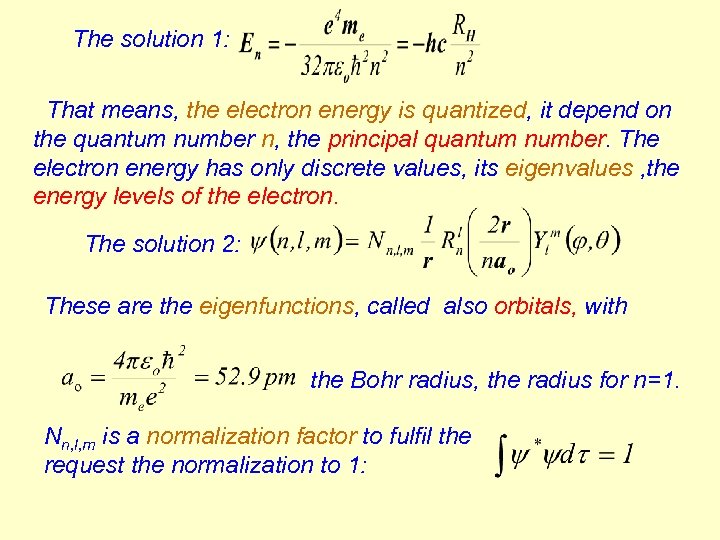 The solution 1: That means, the electron energy is quantized, it depend on the quantum number n, the principal quantum number. The electron energy has only discrete values, its eigenvalues , the energy levels of the electron. The solution 2: These are the eigenfunctions, called also orbitals, with the Bohr radius, the radius for n=1. Nn, l, m is a normalization factor to fulfil the request the normalization to 1:
The solution 1: That means, the electron energy is quantized, it depend on the quantum number n, the principal quantum number. The electron energy has only discrete values, its eigenvalues , the energy levels of the electron. The solution 2: These are the eigenfunctions, called also orbitals, with the Bohr radius, the radius for n=1. Nn, l, m is a normalization factor to fulfil the request the normalization to 1:
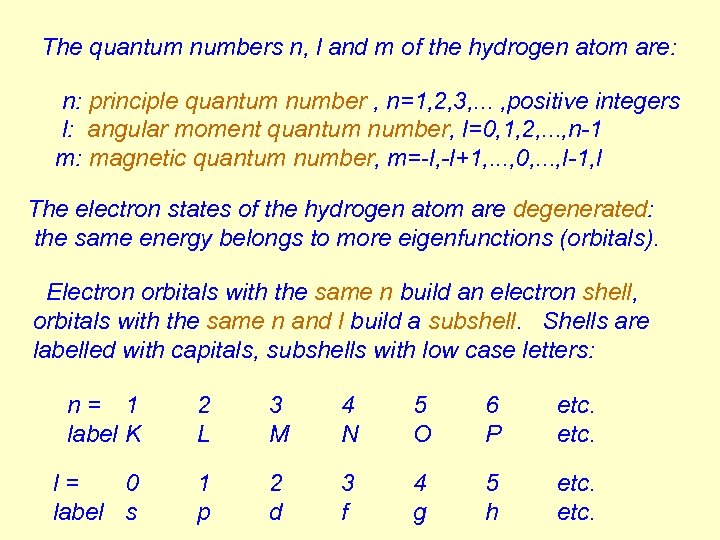 The quantum numbers n, l and m of the hydrogen atom are: n: principle quantum number , n=1, 2, 3, . . . , positive integers l: angular moment quantum number, l=0, 1, 2, . . . , n-1 m: magnetic quantum number, m=-l, -l+1, . . . , 0, . . . , l-1, l The electron states of the hydrogen atom are degenerated: the same energy belongs to more eigenfunctions (orbitals). Electron orbitals with the same n build an electron shell, orbitals with the same n and l build a subshell. Shells are labelled with capitals, subshells with low case letters: n= 1 label K 2 L 3 M 4 N 5 O 6 P etc. l= 0 label s 1 p 2 d 3 f 4 g 5 h etc.
The quantum numbers n, l and m of the hydrogen atom are: n: principle quantum number , n=1, 2, 3, . . . , positive integers l: angular moment quantum number, l=0, 1, 2, . . . , n-1 m: magnetic quantum number, m=-l, -l+1, . . . , 0, . . . , l-1, l The electron states of the hydrogen atom are degenerated: the same energy belongs to more eigenfunctions (orbitals). Electron orbitals with the same n build an electron shell, orbitals with the same n and l build a subshell. Shells are labelled with capitals, subshells with low case letters: n= 1 label K 2 L 3 M 4 N 5 O 6 P etc. l= 0 label s 1 p 2 d 3 f 4 g 5 h etc.
 There exist a special angular moment of the electron from its nature, the spin. The eigenvalue equation for the spin component in the direction of the external field (z): The quantum number ms is the magnetic spin quantum number, and has only two possible values: +1/2 and -1/2, labelled and , respectively. is the so-called "spin coordinate". The spin quantum number is labelled with s and has similarly the same values. Considering the restrictions for the l, m and s, if the principal quantum number is n, the possible maximal number of electrons with n is 2 n 2.
There exist a special angular moment of the electron from its nature, the spin. The eigenvalue equation for the spin component in the direction of the external field (z): The quantum number ms is the magnetic spin quantum number, and has only two possible values: +1/2 and -1/2, labelled and , respectively. is the so-called "spin coordinate". The spin quantum number is labelled with s and has similarly the same values. Considering the restrictions for the l, m and s, if the principal quantum number is n, the possible maximal number of electrons with n is 2 n 2.
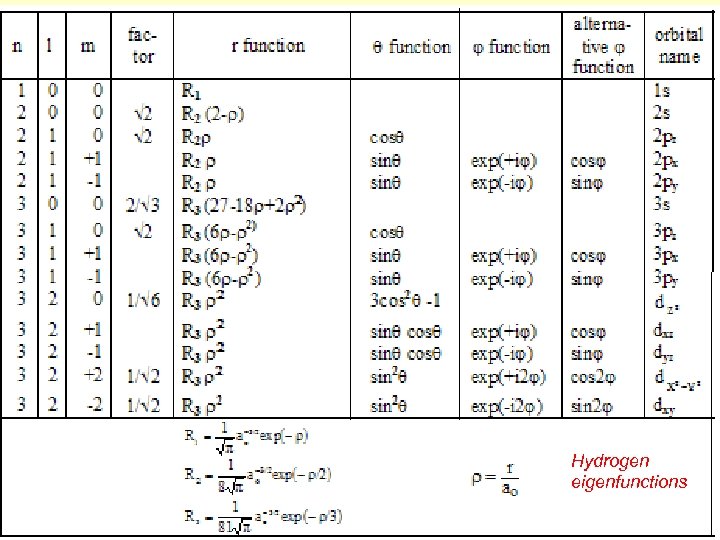 Hydrogen eigenfunctions
Hydrogen eigenfunctions
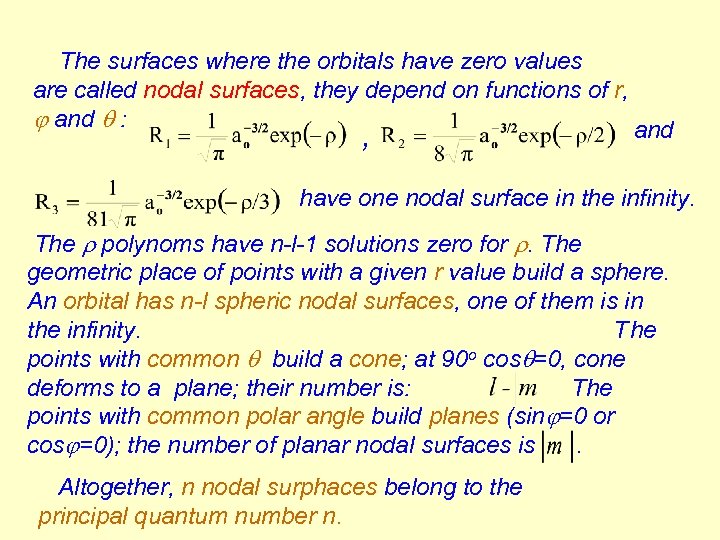 The surfaces where the orbitals have zero values are called nodal surfaces, they depend on functions of r, and : and , have one nodal surface in the infinity. The r polynoms have n-l-1 solutions zero for r. The geometric place of points with a given r value build a sphere. An orbital has n-l spheric nodal surfaces, one of them is in the infinity. The points with common build a cone; at 90 o cos =0, cone deforms to a plane; their number is: The points with common polar angle build planes (sin =0 or cos =0); the number of planar nodal surfaces is. Altogether, n nodal surphaces belong to the principal quantum number n.
The surfaces where the orbitals have zero values are called nodal surfaces, they depend on functions of r, and : and , have one nodal surface in the infinity. The r polynoms have n-l-1 solutions zero for r. The geometric place of points with a given r value build a sphere. An orbital has n-l spheric nodal surfaces, one of them is in the infinity. The points with common build a cone; at 90 o cos =0, cone deforms to a plane; their number is: The points with common polar angle build planes (sin =0 or cos =0); the number of planar nodal surfaces is. Altogether, n nodal surphaces belong to the principal quantum number n.
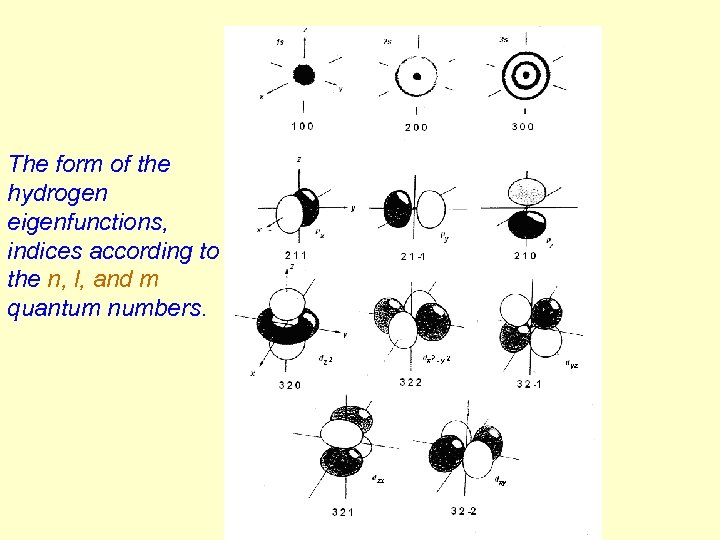 The form of the hydrogen eigenfunctions, indices according to the n, l, and m quantum numbers.
The form of the hydrogen eigenfunctions, indices according to the n, l, and m quantum numbers.
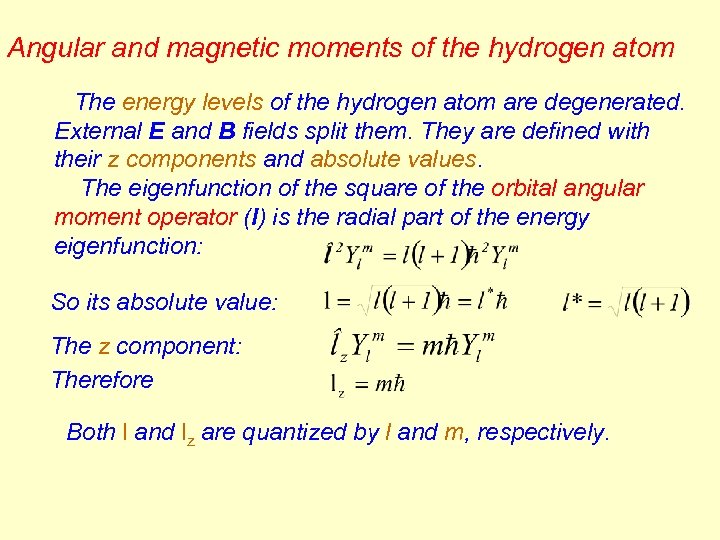 Angular and magnetic moments of the hydrogen atom The energy levels of the hydrogen atom are degenerated. External E and B fields split them. They are defined with their z components and absolute values. The eigenfunction of the square of the orbital angular moment operator (l) is the radial part of the energy eigenfunction: So its absolute value: The z component: Therefore Both l and lz are quantized by l and m, respectively.
Angular and magnetic moments of the hydrogen atom The energy levels of the hydrogen atom are degenerated. External E and B fields split them. They are defined with their z components and absolute values. The eigenfunction of the square of the orbital angular moment operator (l) is the radial part of the energy eigenfunction: So its absolute value: The z component: Therefore Both l and lz are quantized by l and m, respectively.
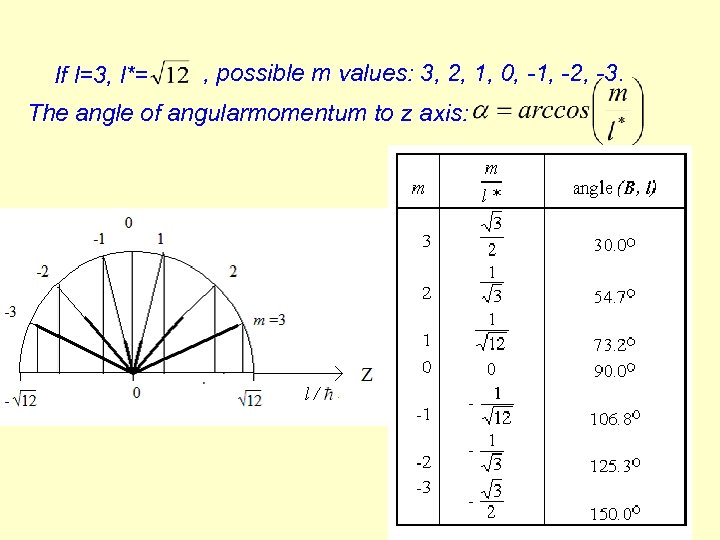 If l=3, l*= , possible m values: 3, 2, 1, 0, -1, -2, -3. The angle of angularmomentum to z axis:
If l=3, l*= , possible m values: 3, 2, 1, 0, -1, -2, -3. The angle of angularmomentum to z axis:
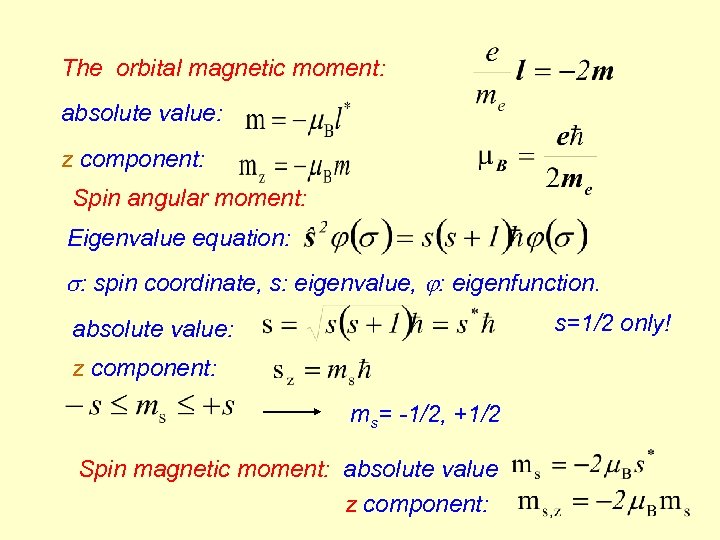 The orbital magnetic moment: absolute value: z component: Spin angular moment: Eigenvalue equation: : spin coordinate, s: eigenvalue, : eigenfunction. s=1/2 only! absolute value: z component: ms= -1/2, +1/2 Spin magnetic moment: absolute value z component:
The orbital magnetic moment: absolute value: z component: Spin angular moment: Eigenvalue equation: : spin coordinate, s: eigenvalue, : eigenfunction. s=1/2 only! absolute value: z component: ms= -1/2, +1/2 Spin magnetic moment: absolute value z component:
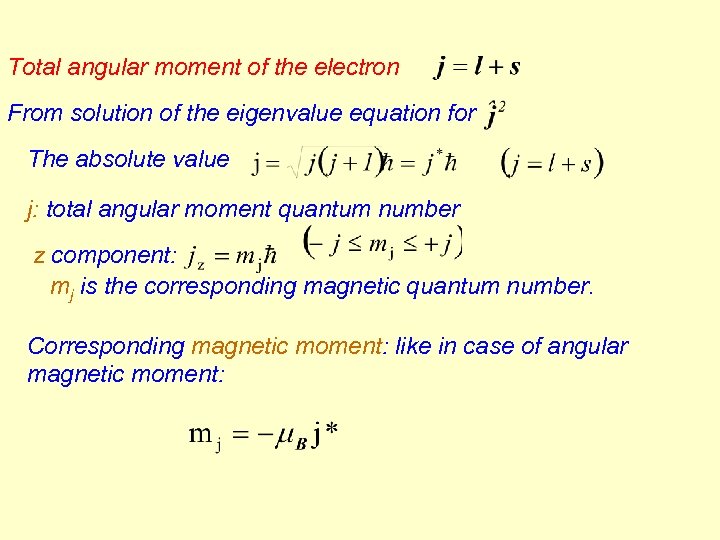 Total angular moment of the electron From solution of the eigenvalue equation for The absolute value j: total angular moment quantum number z component: mj is the corresponding magnetic quantum number. Corresponding magnetic moment: like in case of angular magnetic moment:
Total angular moment of the electron From solution of the eigenvalue equation for The absolute value j: total angular moment quantum number z component: mj is the corresponding magnetic quantum number. Corresponding magnetic moment: like in case of angular magnetic moment:
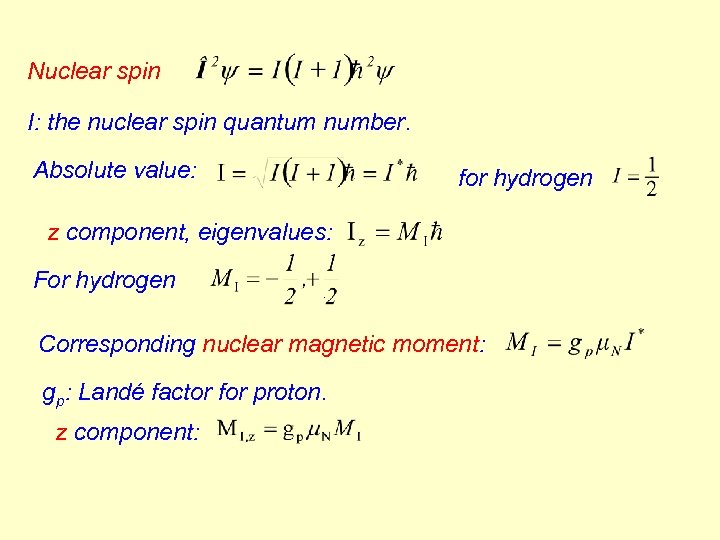 Nuclear spin I: the nuclear spin quantum number. Absolute value: for hydrogen z component, eigenvalues: For hydrogen . Corresponding nuclear magnetic moment: gp: Landé factor for proton. z component:
Nuclear spin I: the nuclear spin quantum number. Absolute value: for hydrogen z component, eigenvalues: For hydrogen . Corresponding nuclear magnetic moment: gp: Landé factor for proton. z component:
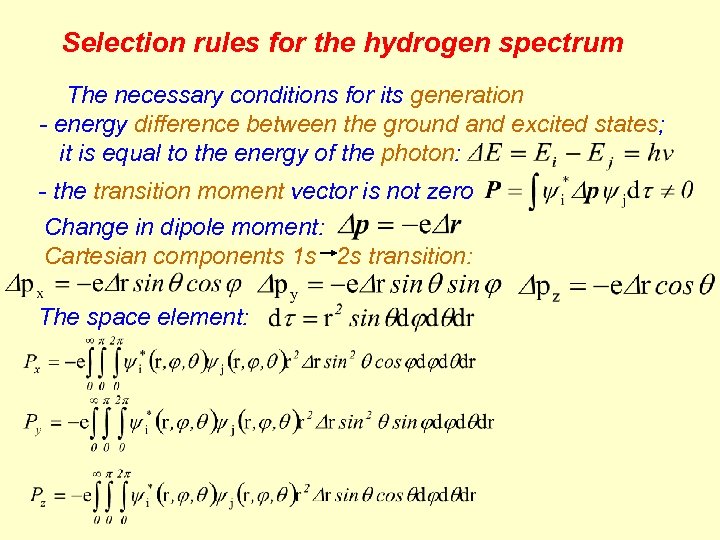 Selection rules for the hydrogen spectrum The necessary conditions for its generation - energy difference between the ground and excited states; it is equal to the energy of the photon: - the transition moment vector is not zero Change in dipole moment: Cartesian components 1 s 2 s transition: The space element:
Selection rules for the hydrogen spectrum The necessary conditions for its generation - energy difference between the ground and excited states; it is equal to the energy of the photon: - the transition moment vector is not zero Change in dipole moment: Cartesian components 1 s 2 s transition: The space element:
 Integrating with respect to from 0 to all integer powers of sin : the result is non-zero, even powers of cos : the result is non-zero, odd powers of cos : the result is zero. Integrating with respect to from 0 to 2 even powers of sin or cos : the result is non-zero, odd powers of sin and cos : the result is zero. Angle functions for excitation from 1 s (100) state to 2 s (200) state the integrals are zero (no transition), since: 1 s 2 s transition x component contains sin 2 cos is not possible. 2 sin y component contains sin z component contains sin cos
Integrating with respect to from 0 to all integer powers of sin : the result is non-zero, even powers of cos : the result is non-zero, odd powers of cos : the result is zero. Integrating with respect to from 0 to 2 even powers of sin or cos : the result is non-zero, odd powers of sin and cos : the result is zero. Angle functions for excitation from 1 s (100) state to 2 s (200) state the integrals are zero (no transition), since: 1 s 2 s transition x component contains sin 2 cos is not possible. 2 sin y component contains sin z component contains sin cos
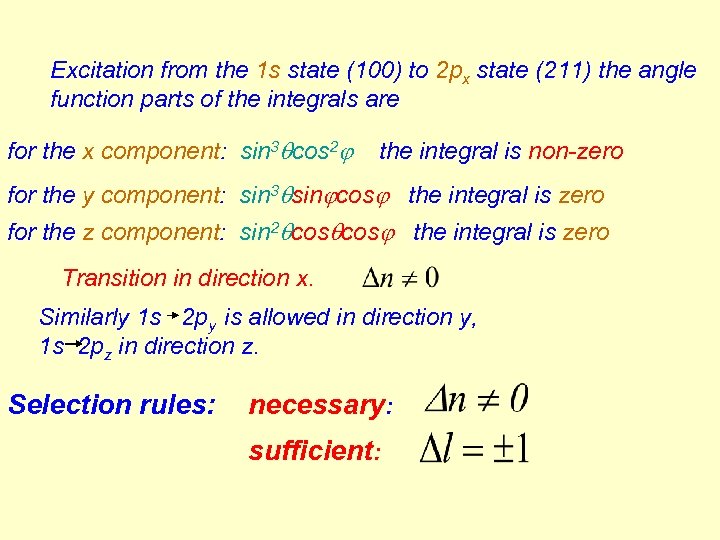 Excitation from the 1 s state (100) to 2 px state (211) the angle function parts of the integrals are for the x component: sin 3 cos 2 the integral is non-zero for the y component: sin 3 sin cos the integral is zero for the z component: sin 2 cos the integral is zero Transition in direction x. Similarly 1 s 2 py is allowed in direction y, 1 s 2 pz in direction z. Selection rules: necessary: sufficient:
Excitation from the 1 s state (100) to 2 px state (211) the angle function parts of the integrals are for the x component: sin 3 cos 2 the integral is non-zero for the y component: sin 3 sin cos the integral is zero for the z component: sin 2 cos the integral is zero Transition in direction x. Similarly 1 s 2 py is allowed in direction y, 1 s 2 pz in direction z. Selection rules: necessary: sufficient:
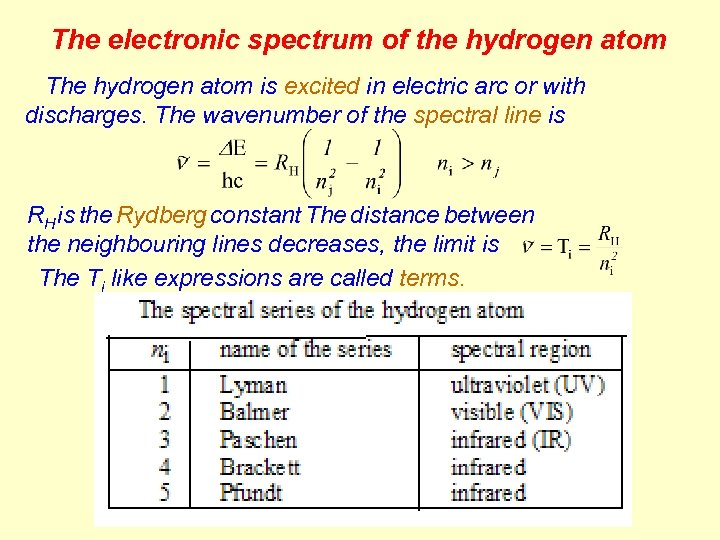 The electronic spectrum of the hydrogen atom The hydrogen atom is excited in electric arc or with discharges. The wavenumber of the spectral line is RH is the Rydberg constant The distance between the neighbouring lines decreases, the limit is The Ti like expressions are called terms.
The electronic spectrum of the hydrogen atom The hydrogen atom is excited in electric arc or with discharges. The wavenumber of the spectral line is RH is the Rydberg constant The distance between the neighbouring lines decreases, the limit is The Ti like expressions are called terms.
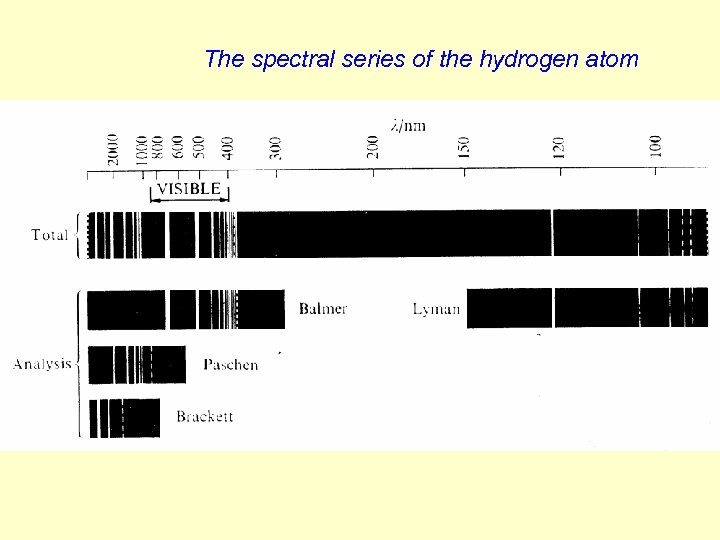 The spectral series of the hydrogen atom
The spectral series of the hydrogen atom
 Many-electron atoms More than one electrons move around the nucleus. Electric and magnetic interactions between the electrons of the electron cloud. Hydrogenic atoms (or ions) have only one electron on their outside (valence) shell. This is a good approximation for these type atoms. All other atoms are far from this model, their description is very complicate. Hydrogenic atoms where Rz is the Rydberg constant for the atom with atomic number z. Comparing this equation with the spectra
Many-electron atoms More than one electrons move around the nucleus. Electric and magnetic interactions between the electrons of the electron cloud. Hydrogenic atoms (or ions) have only one electron on their outside (valence) shell. This is a good approximation for these type atoms. All other atoms are far from this model, their description is very complicate. Hydrogenic atoms where Rz is the Rydberg constant for the atom with atomic number z. Comparing this equation with the spectra
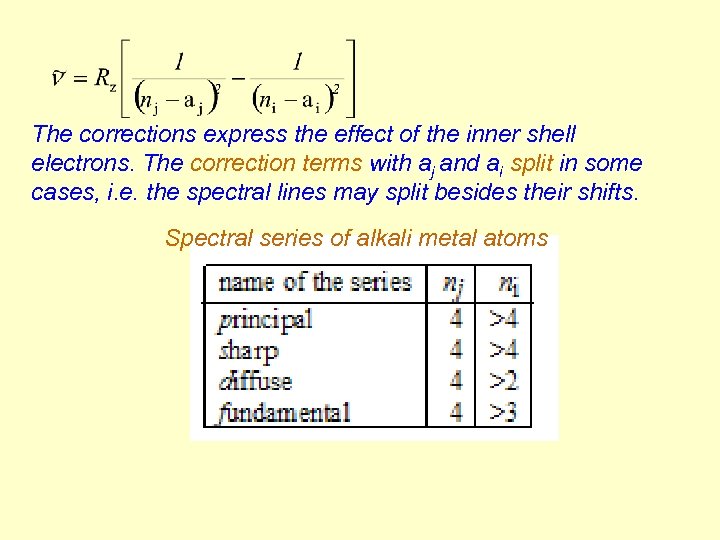 The corrections express the effect of the inner shell electrons. The correction terms with aj and ai split in some cases, i. e. the spectral lines may split besides their shifts. Spectral series of alkali metal atoms
The corrections express the effect of the inner shell electrons. The correction terms with aj and ai split in some cases, i. e. the spectral lines may split besides their shifts. Spectral series of alkali metal atoms
 Other many-electron atoms Strong electron interactions exist in open (not absolutely closed) electron shells and clouds. The Hamilton operator: Sums are extended to all electrons; ri: electron-nucleus distance; rij is the electron-electron one. First term: kinetic energy operator, second term: electron-nucleus, third term: electron-electron potential energy. The atomic energy depend at these atoms also on the angular moment quantum number l. Quantum mechanical calculations: energy level series for the subshells.
Other many-electron atoms Strong electron interactions exist in open (not absolutely closed) electron shells and clouds. The Hamilton operator: Sums are extended to all electrons; ri: electron-nucleus distance; rij is the electron-electron one. First term: kinetic energy operator, second term: electron-nucleus, third term: electron-electron potential energy. The atomic energy depend at these atoms also on the angular moment quantum number l. Quantum mechanical calculations: energy level series for the subshells.
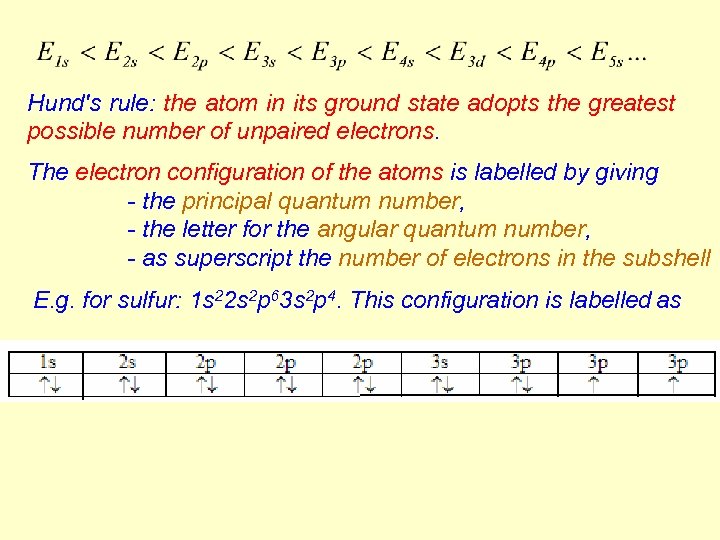 Hund's rule: the atom in its ground state adopts the greatest possible number of unpaired electrons. The electron configuration of the atoms is labelled by giving - the principal quantum number, - the letter for the angular quantum number, - as superscript the number of electrons in the subshell E. g. for sulfur: 1 s 22 s 2 p 63 s 2 p 4. This configuration is labelled as
Hund's rule: the atom in its ground state adopts the greatest possible number of unpaired electrons. The electron configuration of the atoms is labelled by giving - the principal quantum number, - the letter for the angular quantum number, - as superscript the number of electrons in the subshell E. g. for sulfur: 1 s 22 s 2 p 63 s 2 p 4. This configuration is labelled as
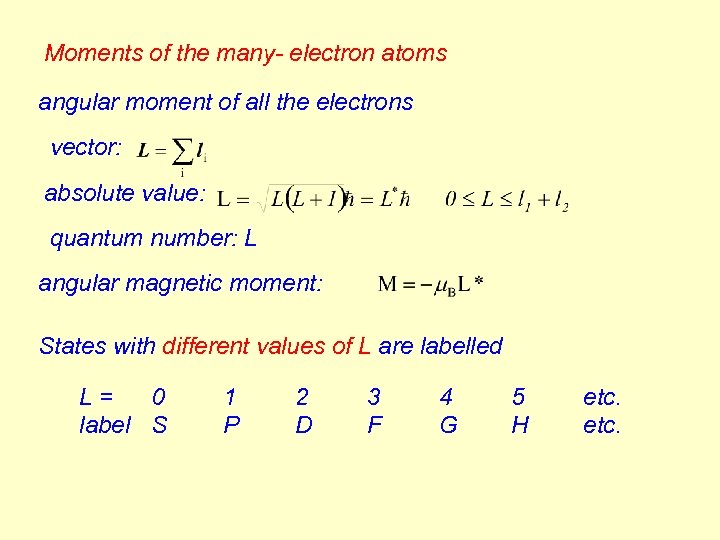 Moments of the many- electron atoms angular moment of all the electrons vector: absolute value: quantum number: L angular magnetic moment: States with different values of L are labelled L= 0 label S 1 P 2 D 3 F 4 G 5 H etc.
Moments of the many- electron atoms angular moment of all the electrons vector: absolute value: quantum number: L angular magnetic moment: States with different values of L are labelled L= 0 label S 1 P 2 D 3 F 4 G 5 H etc.
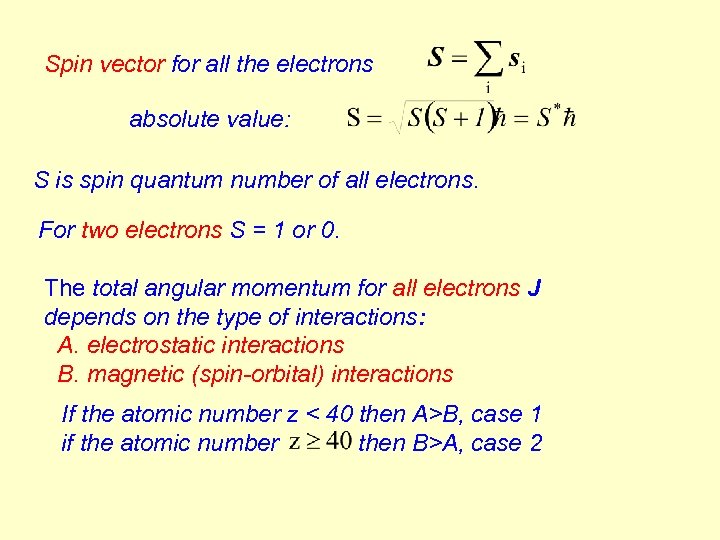 Spin vector for all the electrons absolute value: S is spin quantum number of all electrons. For two electrons S = 1 or 0. The total angular momentum for all electrons J depends on the type of interactions: A. electrostatic interactions B. magnetic (spin-orbital) interactions If the atomic number z < 40 then A>B, case 1 if the atomic number then B>A, case 2
Spin vector for all the electrons absolute value: S is spin quantum number of all electrons. For two electrons S = 1 or 0. The total angular momentum for all electrons J depends on the type of interactions: A. electrostatic interactions B. magnetic (spin-orbital) interactions If the atomic number z < 40 then A>B, case 1 if the atomic number then B>A, case 2
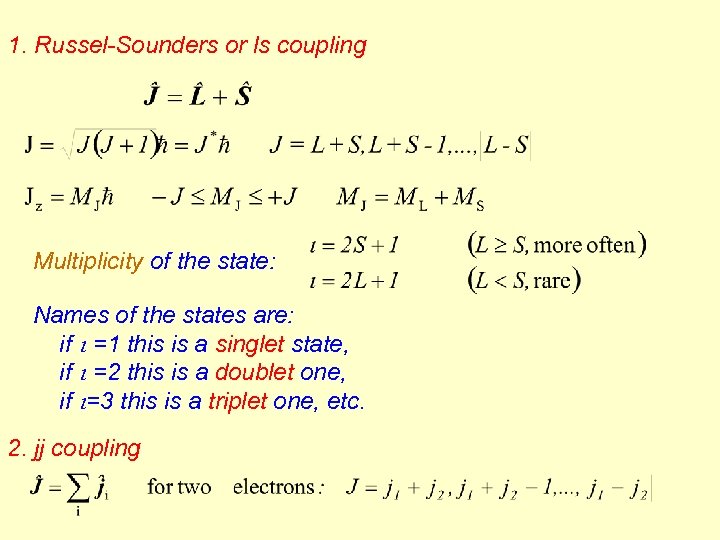 1. Russel-Sounders or ls coupling Multiplicity of the state: Names of the states are: if i =1 this is a singlet state, if i =2 this is a doublet one, if i=3 this is a triplet one, etc. 2. jj coupling
1. Russel-Sounders or ls coupling Multiplicity of the state: Names of the states are: if i =1 this is a singlet state, if i =2 this is a doublet one, if i=3 this is a triplet one, etc. 2. jj coupling
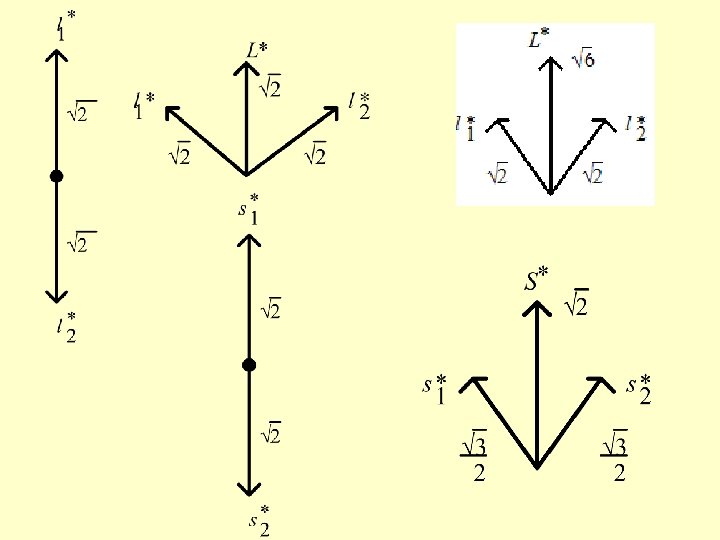
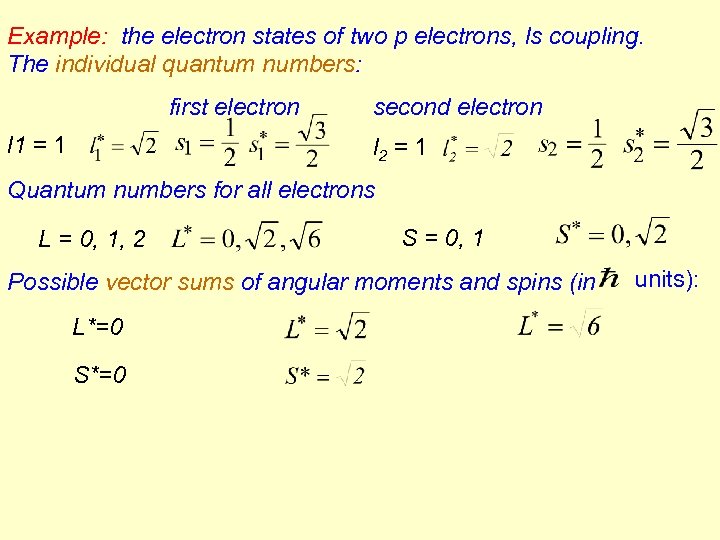 Example: the electron states of two p electrons, ls coupling. The individual quantum numbers: first electron l 1 = 1 second electron l 2 = 1 Quantum numbers for all electrons L = 0, 1, 2 S = 0, 1 Possible vector sums of angular moments and spins (in L*=0 S*=0 units):
Example: the electron states of two p electrons, ls coupling. The individual quantum numbers: first electron l 1 = 1 second electron l 2 = 1 Quantum numbers for all electrons L = 0, 1, 2 S = 0, 1 Possible vector sums of angular moments and spins (in L*=0 S*=0 units):
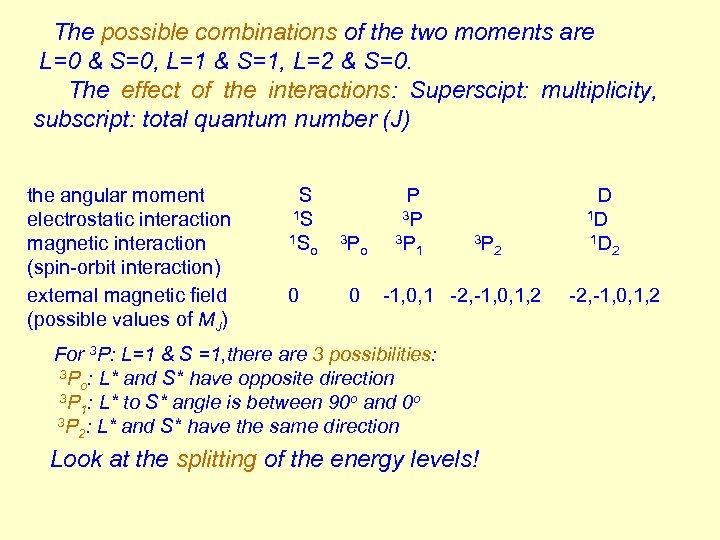 The possible combinations of the two moments are L=0 & S=0, L=1 & S=1, L=2 & S=0. The effect of the interactions: Superscipt: multiplicity, subscript: total quantum number (J) the angular moment electrostatic interaction magnetic interaction (spin-orbit interaction) external magnetic field (possible values of MJ) S 1 S 1 S o 0 3 P o 0 P 3 P 3 P 1 3 P 2 -1, 0, 1 -2, -1, 0, 1, 2 For 3 P: L=1 & S =1, there are 3 possibilities: 3 P : L* and S* have opposite direction o 3 P : L* to S* angle is between 90 o and 0 o 1 3 P : L* and S* have the same direction 2 Look at the splitting of the energy levels! D 1 D 1 D 2 -2, -1, 0, 1, 2
The possible combinations of the two moments are L=0 & S=0, L=1 & S=1, L=2 & S=0. The effect of the interactions: Superscipt: multiplicity, subscript: total quantum number (J) the angular moment electrostatic interaction magnetic interaction (spin-orbit interaction) external magnetic field (possible values of MJ) S 1 S 1 S o 0 3 P o 0 P 3 P 3 P 1 3 P 2 -1, 0, 1 -2, -1, 0, 1, 2 For 3 P: L=1 & S =1, there are 3 possibilities: 3 P : L* and S* have opposite direction o 3 P : L* to S* angle is between 90 o and 0 o 1 3 P : L* and S* have the same direction 2 Look at the splitting of the energy levels! D 1 D 1 D 2 -2, -1, 0, 1, 2
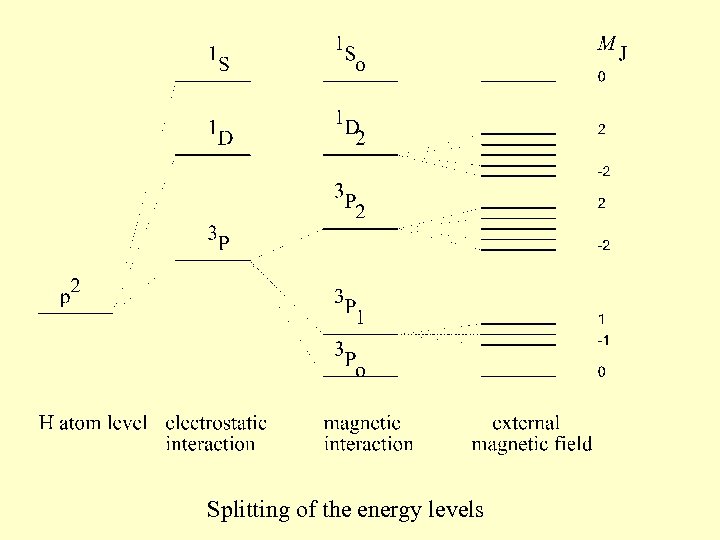 Splitting of the energy levels
Splitting of the energy levels
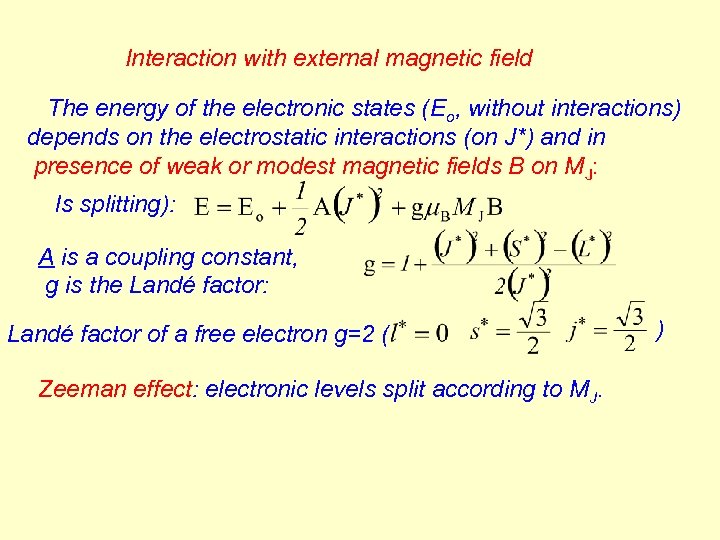 Interaction with external magnetic field The energy of the electronic states (Eo, without interactions) depends on the electrostatic interactions (on J*) and in presence of weak or modest magnetic fields B on MJ: ls splitting): A is a coupling constant, g is the Landé factor: Landé factor of a free electron g=2 ( Zeeman effect: electronic levels split according to MJ. )
Interaction with external magnetic field The energy of the electronic states (Eo, without interactions) depends on the electrostatic interactions (on J*) and in presence of weak or modest magnetic fields B on MJ: ls splitting): A is a coupling constant, g is the Landé factor: Landé factor of a free electron g=2 ( Zeeman effect: electronic levels split according to MJ. )
 Paschen-Back effect: very strong splitting, strong magnetic field the magnetic interactions dominate (jj coupling) The energy levels of the nucleus splits also in magnetic field: The splitting of the energy levels in external magnetic field is important in the methods of nuclear magnetic resonance and electron spin (paramagnetic) resonance.
Paschen-Back effect: very strong splitting, strong magnetic field the magnetic interactions dominate (jj coupling) The energy levels of the nucleus splits also in magnetic field: The splitting of the energy levels in external magnetic field is important in the methods of nuclear magnetic resonance and electron spin (paramagnetic) resonance.
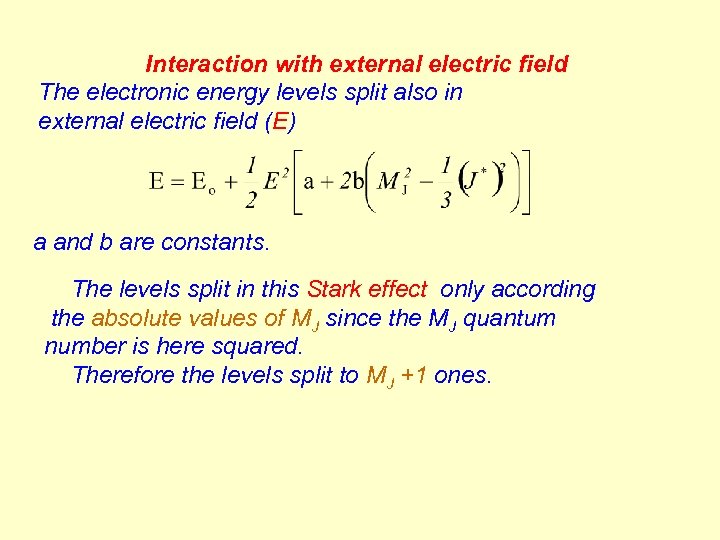 Interaction with external electric field The electronic energy levels split also in external electric field (E) a and b are constants. The levels split in this Stark effect only according the absolute values of MJ since the MJ quantum number is here squared. Therefore the levels split to MJ +1 ones.
Interaction with external electric field The electronic energy levels split also in external electric field (E) a and b are constants. The levels split in this Stark effect only according the absolute values of MJ since the MJ quantum number is here squared. Therefore the levels split to MJ +1 ones.
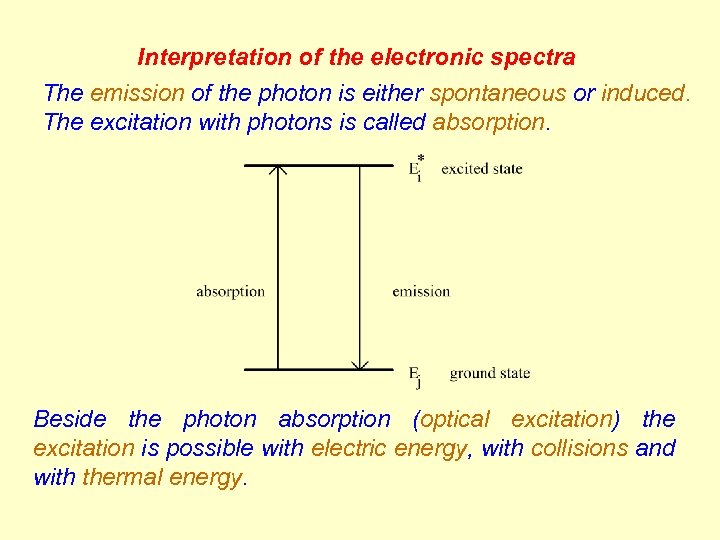 Interpretation of the electronic spectra The emission of the photon is either spontaneous or induced. The excitation with photons is called absorption. Beside the photon absorption (optical excitation) the excitation is possible with electric energy, with collisions and with thermal energy.
Interpretation of the electronic spectra The emission of the photon is either spontaneous or induced. The excitation with photons is called absorption. Beside the photon absorption (optical excitation) the excitation is possible with electric energy, with collisions and with thermal energy.
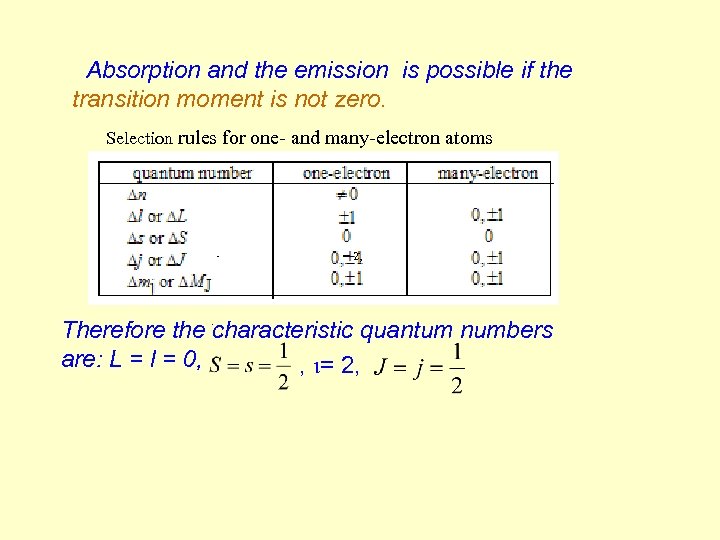 Absorption and the emission is possible if the transition moment is not zero. Selection rules for one- and many-electron atoms = 2, . Therefore the characteristic quantum numbers are: L = l = 0, , i= 2,
Absorption and the emission is possible if the transition moment is not zero. Selection rules for one- and many-electron atoms = 2, . Therefore the characteristic quantum numbers are: L = l = 0, , i= 2,
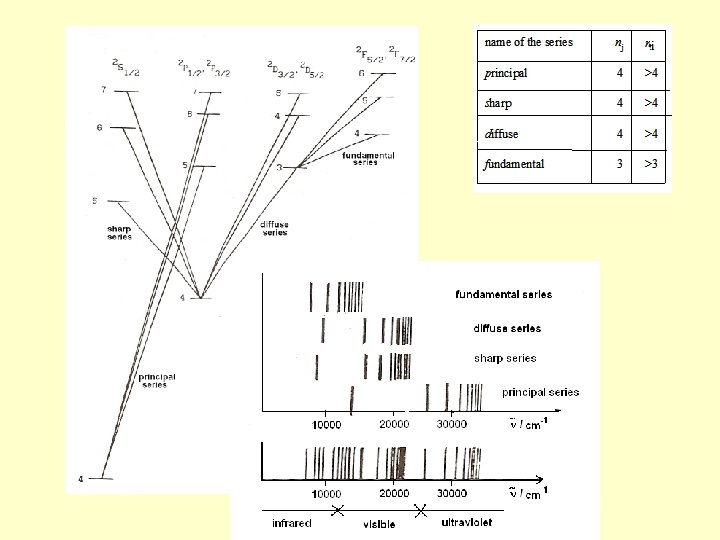
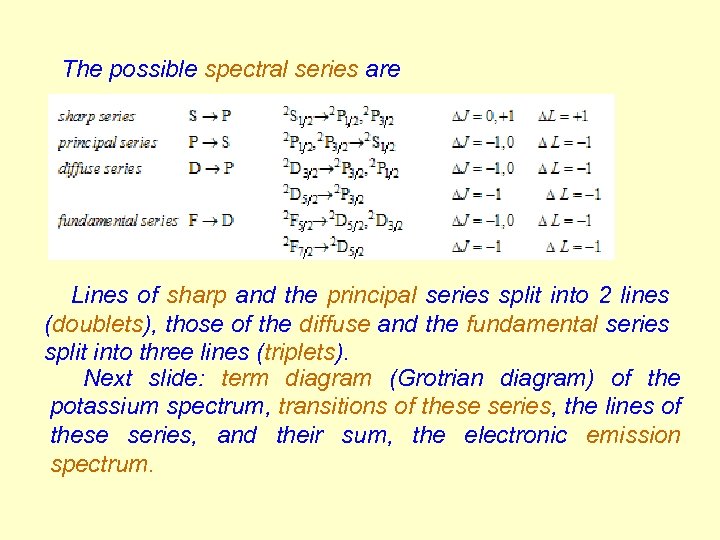 The possible spectral series are Lines of sharp and the principal series split into 2 lines (doublets), those of the diffuse and the fundamental series split into three lines (triplets). Next slide: term diagram (Grotrian diagram) of the potassium spectrum, transitions of these series, the lines of these series, and their sum, the electronic emission spectrum.
The possible spectral series are Lines of sharp and the principal series split into 2 lines (doublets), those of the diffuse and the fundamental series split into three lines (triplets). Next slide: term diagram (Grotrian diagram) of the potassium spectrum, transitions of these series, the lines of these series, and their sum, the electronic emission spectrum.
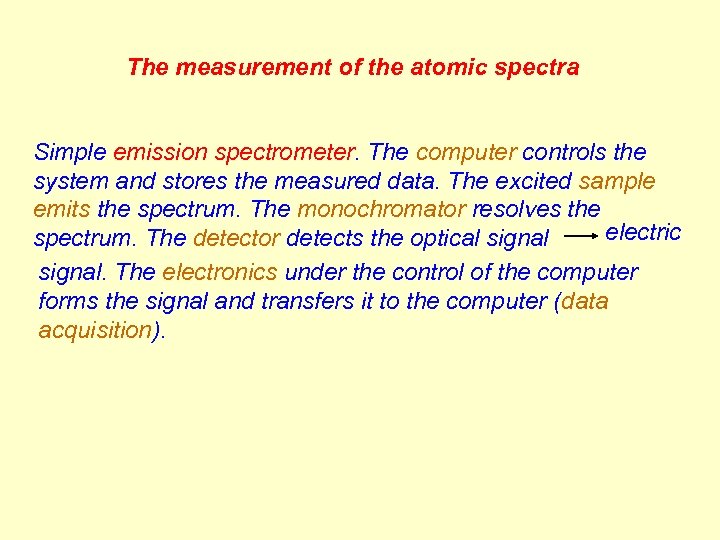 The measurement of the atomic spectra Simple emission spectrometer. The computer controls the system and stores the measured data. The excited sample emits the spectrum. The monochromator resolves the electric spectrum. The detector detects the optical signal. The electronics under the control of the computer forms the signal and transfers it to the computer (data acquisition).
The measurement of the atomic spectra Simple emission spectrometer. The computer controls the system and stores the measured data. The excited sample emits the spectrum. The monochromator resolves the electric spectrum. The detector detects the optical signal. The electronics under the control of the computer forms the signal and transfers it to the computer (data acquisition).
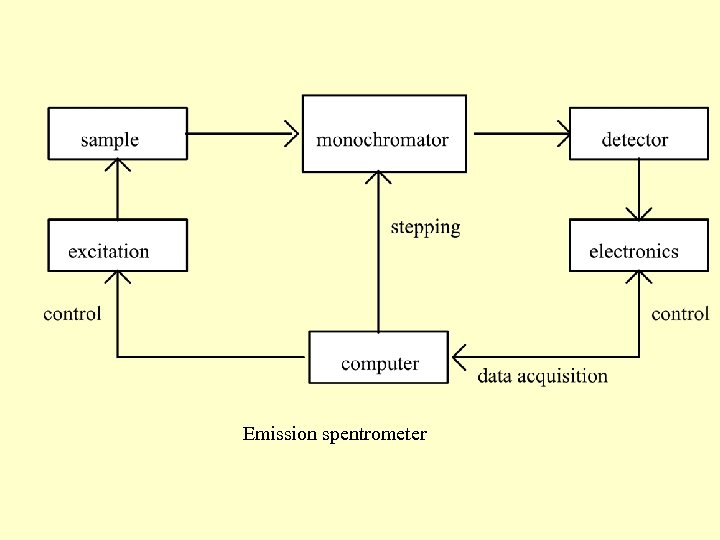 Emission spentrometer
Emission spentrometer
 Absorption spectrometer. The sample is here passive, a light source is necessary for the working. A part of the light will be absorbed by the sample. The monochromator resolve the light, the detector transforms the optical signal to electric one. The speciality of the absorption spectrometer is the necessity of a reference. The reference will be measured either by dividing the source light into a sample and a reference beam; the measurement of them will detected in short time spans during the same mesurement. Otherwise, the sample and the reference will be measured in different measurements. The resulted spectrum will be produced by the computer.
Absorption spectrometer. The sample is here passive, a light source is necessary for the working. A part of the light will be absorbed by the sample. The monochromator resolve the light, the detector transforms the optical signal to electric one. The speciality of the absorption spectrometer is the necessity of a reference. The reference will be measured either by dividing the source light into a sample and a reference beam; the measurement of them will detected in short time spans during the same mesurement. Otherwise, the sample and the reference will be measured in different measurements. The resulted spectrum will be produced by the computer.
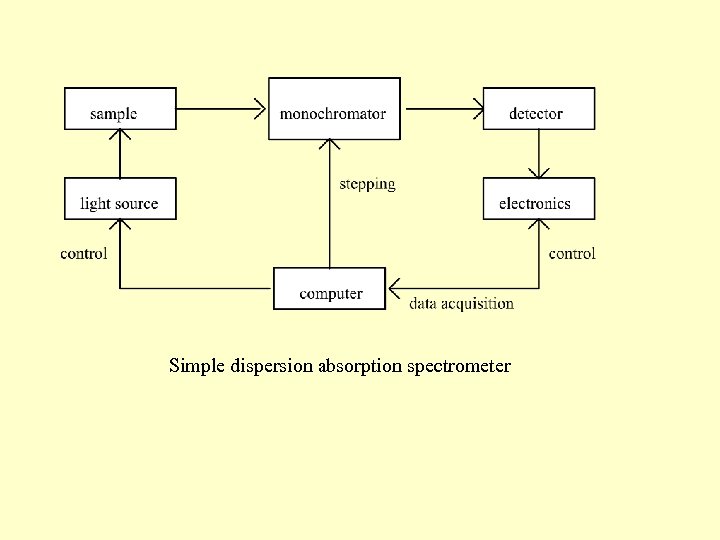 Simple dispersion absorption spectrometer
Simple dispersion absorption spectrometer

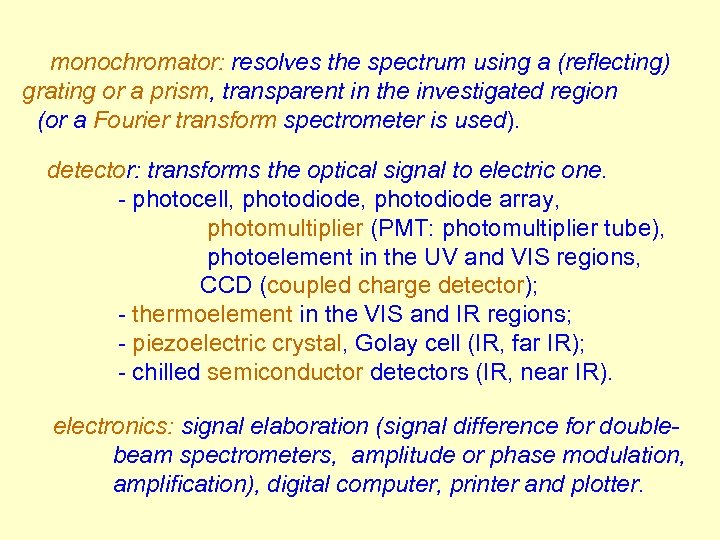 monochromator: resolves the spectrum using a (reflecting) grating or a prism, transparent in the investigated region (or a Fourier transform spectrometer is used). detector: transforms the optical signal to electric one. - photocell, photodiode array, photomultiplier (PMT: photomultiplier tube), photoelement in the UV and VIS regions, CCD (coupled charge detector); - thermoelement in the VIS and IR regions; - piezoelectric crystal, Golay cell (IR, far IR); - chilled semiconductor detectors (IR, near IR). electronics: signal elaboration (signal difference for doublebeam spectrometers, amplitude or phase modulation, amplification), digital computer, printer and plotter.
monochromator: resolves the spectrum using a (reflecting) grating or a prism, transparent in the investigated region (or a Fourier transform spectrometer is used). detector: transforms the optical signal to electric one. - photocell, photodiode array, photomultiplier (PMT: photomultiplier tube), photoelement in the UV and VIS regions, CCD (coupled charge detector); - thermoelement in the VIS and IR regions; - piezoelectric crystal, Golay cell (IR, far IR); - chilled semiconductor detectors (IR, near IR). electronics: signal elaboration (signal difference for doublebeam spectrometers, amplitude or phase modulation, amplification), digital computer, printer and plotter.
 The spectral information: - frequency, i. e. position of the line; - intensity, i. e. area of the line; - profile or shape of the line. Applications of the atomic spectra: mostly quantitative and qualitative chemical analysis, rarely physics (theory of atomic structure).
The spectral information: - frequency, i. e. position of the line; - intensity, i. e. area of the line; - profile or shape of the line. Applications of the atomic spectra: mostly quantitative and qualitative chemical analysis, rarely physics (theory of atomic structure).
 Ions Atomic ions and molecular ions Ionization is generation of positive ions. Generation of a positive ion from a neutral atom: The external (outside) ionization is the ablation of an electron from the outmost (valence) shell of the atom. The internal (inner) ionization is called the ablation of an electron from the core (inner electron shells) of the atom. The electron gain is the building of a negative (or more negative) ion by receiving an electron:
Ions Atomic ions and molecular ions Ionization is generation of positive ions. Generation of a positive ion from a neutral atom: The external (outside) ionization is the ablation of an electron from the outmost (valence) shell of the atom. The internal (inner) ionization is called the ablation of an electron from the core (inner electron shells) of the atom. The electron gain is the building of a negative (or more negative) ion by receiving an electron:
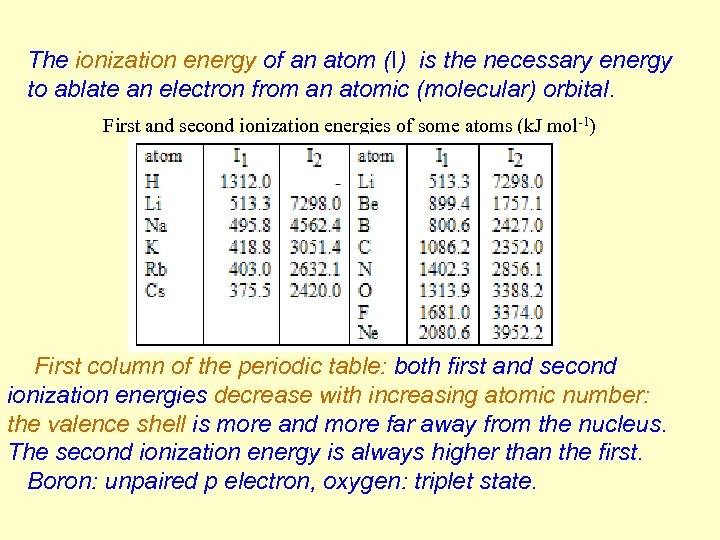 The ionization energy of an atom (I) is the necessary energy to ablate an electron from an atomic (molecular) orbital. First and second ionization energies of some atoms (k. J mol-1) First column of the periodic table: both first and second ionization energies decrease with increasing atomic number: the valence shell is more and more far away from the nucleus. The second ionization energy is always higher than the first. Boron: unpaired p electron, oxygen: triplet state.
The ionization energy of an atom (I) is the necessary energy to ablate an electron from an atomic (molecular) orbital. First and second ionization energies of some atoms (k. J mol-1) First column of the periodic table: both first and second ionization energies decrease with increasing atomic number: the valence shell is more and more far away from the nucleus. The second ionization energy is always higher than the first. Boron: unpaired p electron, oxygen: triplet state.
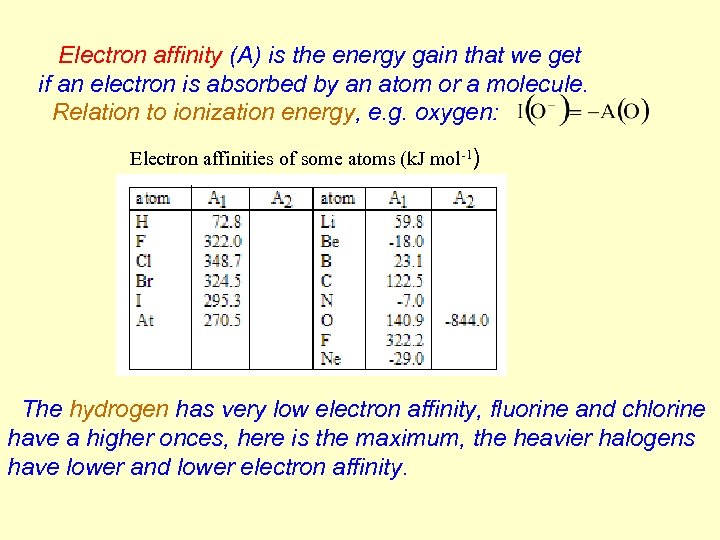 Electron affinity (A) is the energy gain that we get if an electron is absorbed by an atom or a molecule. Relation to ionization energy, e. g. oxygen: Electron affinities of some atoms (k. J mol-1) The hydrogen has very low electron affinity, fluorine and chlorine have a higher onces, here is the maximum, the heavier halogens have lower and lower electron affinity.
Electron affinity (A) is the energy gain that we get if an electron is absorbed by an atom or a molecule. Relation to ionization energy, e. g. oxygen: Electron affinities of some atoms (k. J mol-1) The hydrogen has very low electron affinity, fluorine and chlorine have a higher onces, here is the maximum, the heavier halogens have lower and lower electron affinity.
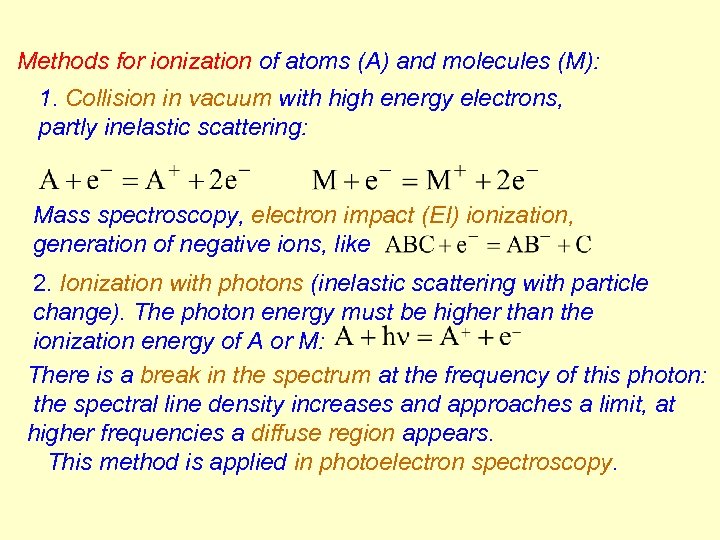 Methods for ionization of atoms (A) and molecules (M): 1. Collision in vacuum with high energy electrons, partly inelastic scattering: Mass spectroscopy, electron impact (EI) ionization, generation of negative ions, like 2. Ionization with photons (inelastic scattering with particle change). The photon energy must be higher than the ionization energy of A or M: There is a break in the spectrum at the frequency of this photon: the spectral line density increases and approaches a limit, at higher frequencies a diffuse region appears. This method is applied in photoelectron spectroscopy.
Methods for ionization of atoms (A) and molecules (M): 1. Collision in vacuum with high energy electrons, partly inelastic scattering: Mass spectroscopy, electron impact (EI) ionization, generation of negative ions, like 2. Ionization with photons (inelastic scattering with particle change). The photon energy must be higher than the ionization energy of A or M: There is a break in the spectrum at the frequency of this photon: the spectral line density increases and approaches a limit, at higher frequencies a diffuse region appears. This method is applied in photoelectron spectroscopy.
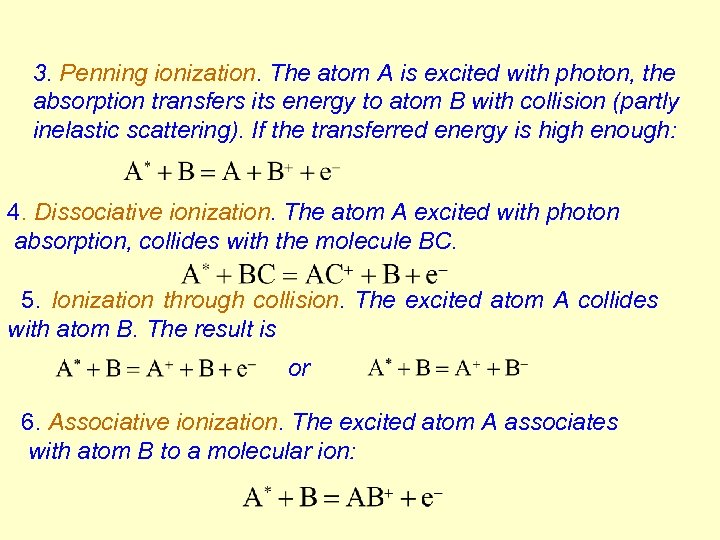 3. Penning ionization. The atom A is excited with photon, the absorption transfers its energy to atom B with collision (partly inelastic scattering). If the transferred energy is high enough: 4. Dissociative ionization. The atom A excited with photon absorption, collides with the molecule BC. 5. Ionization through collision. The excited atom A collides with atom B. The result is or 6. Associative ionization. The excited atom A associates with atom B to a molecular ion:
3. Penning ionization. The atom A is excited with photon, the absorption transfers its energy to atom B with collision (partly inelastic scattering). If the transferred energy is high enough: 4. Dissociative ionization. The atom A excited with photon absorption, collides with the molecule BC. 5. Ionization through collision. The excited atom A collides with atom B. The result is or 6. Associative ionization. The excited atom A associates with atom B to a molecular ion:
 7. Auger effect. After an inner ionization an electron gap appears on an inner electron shell. This gap will be filled by an electron jumps from a higher level. The energy difference between the two states can be assign to the emission of an X-ray photon or to a second ionization: to the emission of a second photon. The latter is the Auger effect. 8. Chemical ionization (CI). This is a special method for the ionization of molecules. A high velocity molecular ion (let us denote it with X+) collides with M (mass spectroscopy): or If X contains hydrogen atoms (XH): 9. Fast atomic bombardment (FAB). The molecules are bombarded with high velocity atom or ion radiation (Ar, Xe, Cs+), applied in mass spectroscopy and secondary ion emission spectroscopy.
7. Auger effect. After an inner ionization an electron gap appears on an inner electron shell. This gap will be filled by an electron jumps from a higher level. The energy difference between the two states can be assign to the emission of an X-ray photon or to a second ionization: to the emission of a second photon. The latter is the Auger effect. 8. Chemical ionization (CI). This is a special method for the ionization of molecules. A high velocity molecular ion (let us denote it with X+) collides with M (mass spectroscopy): or If X contains hydrogen atoms (XH): 9. Fast atomic bombardment (FAB). The molecules are bombarded with high velocity atom or ion radiation (Ar, Xe, Cs+), applied in mass spectroscopy and secondary ion emission spectroscopy.
 Interactions of ions The interaction of ions with electromagnetic waves is similar to those of the neutral atoms: spectral line series appear in the emission and absorption spectra. Electric and magnetic fields act on ions having Q charge and v velocity: Lorentz force: Coulomb force: Applying these equations can be direct detected. Constant B, ions can be detected by velocities. Electrons of different velocities are detectable with the same (in the space fixed) detector. Changing B, particles with different velocity can be directed to the same in space fixed detector. These methods are applied in mass spectroscopy and in different electron spectroscopic methods.
Interactions of ions The interaction of ions with electromagnetic waves is similar to those of the neutral atoms: spectral line series appear in the emission and absorption spectra. Electric and magnetic fields act on ions having Q charge and v velocity: Lorentz force: Coulomb force: Applying these equations can be direct detected. Constant B, ions can be detected by velocities. Electrons of different velocities are detectable with the same (in the space fixed) detector. Changing B, particles with different velocity can be directed to the same in space fixed detector. These methods are applied in mass spectroscopy and in different electron spectroscopic methods.


