3bae44441922acc4e262d46b06ea5a10.ppt
- Количество слайдов: 68
 Chapter 2: Chemicals, Apparatus, and Unit Operations of Analytical Chemistry
Chapter 2: Chemicals, Apparatus, and Unit Operations of Analytical Chemistry
 Introduction At the heart of analytical chemistry is a core set of operations and equipment. The technology of analytical chemistry has improved with the advent of electronic analytical balances, automated titrators, and other computercontrolled instruments. The speed, convenience, accuracy, and precision of analytical methods have improved as well.
Introduction At the heart of analytical chemistry is a core set of operations and equipment. The technology of analytical chemistry has improved with the advent of electronic analytical balances, automated titrators, and other computercontrolled instruments. The speed, convenience, accuracy, and precision of analytical methods have improved as well.
 2 A Selecting and handling reagents and other chemicals The purity of reagents influences the accuracy of analysis. Classifying Chemicals ØRegent grade: conform to the minimum standards set forth by the Reagent Chemical Committee of the American Chemical Society (ACS). ØPrimary-standard: carefully analyzed by the supplier. The National Institute of Standards and Technology (NIST) is an excellent source. ØSpecial-purpose reagent chemicals: are prepared for a specific application such as solvents for spectrophotometry and high-performance liquid chromatography.
2 A Selecting and handling reagents and other chemicals The purity of reagents influences the accuracy of analysis. Classifying Chemicals ØRegent grade: conform to the minimum standards set forth by the Reagent Chemical Committee of the American Chemical Society (ACS). ØPrimary-standard: carefully analyzed by the supplier. The National Institute of Standards and Technology (NIST) is an excellent source. ØSpecial-purpose reagent chemicals: are prepared for a specific application such as solvents for spectrophotometry and high-performance liquid chromatography.
 Rules for Handling Reagents and Solutions 1. Select the best grade of chemical available. Pick the smallest bottle that is sufficient to do the job. 2. Replace the top of every container immediately after removing reagent. 3. Hold the stoppers of reagent bottles between your fingers. Never set a stopper on a desk top. 3. Unless specifically directed otherwise, never return any excess reagent to a bottle.
Rules for Handling Reagents and Solutions 1. Select the best grade of chemical available. Pick the smallest bottle that is sufficient to do the job. 2. Replace the top of every container immediately after removing reagent. 3. Hold the stoppers of reagent bottles between your fingers. Never set a stopper on a desk top. 3. Unless specifically directed otherwise, never return any excess reagent to a bottle.
 Rules for Handling Reagents and Solutions (contd) 5. Never insert spatulas, spoons, or knives into a bottle that contains a solid chemical. Instead, shake the capped bottle vigorously or tap it gently against a wooden table to break up an encrustation. Then pour out the desired quantity. 6. Keep the reagent shelf and the laboratory balance clean and neat. Clean up any spills immediately. 7. Follow local regulations concerning the disposal of surplus reagents and solutions.
Rules for Handling Reagents and Solutions (contd) 5. Never insert spatulas, spoons, or knives into a bottle that contains a solid chemical. Instead, shake the capped bottle vigorously or tap it gently against a wooden table to break up an encrustation. Then pour out the desired quantity. 6. Keep the reagent shelf and the laboratory balance clean and neat. Clean up any spills immediately. 7. Follow local regulations concerning the disposal of surplus reagents and solutions.
 2 B Cleaning and marking of laboratory ware ØEach vessel that holds a sample must be marked. Special marking inks are available for porcelain surfaces. A saturated solution of iron(III) chloride can also be used for marking. ØEvery apparatus must be thoroughly washed with a hot detergent solution and then rinsed, initially with large amounts of tap water and finally with several small portions of deionized water. ØProperly cleaned glassware will be coated with a uniform and unbroken film of water. Do not dry the interior surfaces of glassware. ØAn organic solvent, such as methyl ketone or acetone, may be effective in removing grease films.
2 B Cleaning and marking of laboratory ware ØEach vessel that holds a sample must be marked. Special marking inks are available for porcelain surfaces. A saturated solution of iron(III) chloride can also be used for marking. ØEvery apparatus must be thoroughly washed with a hot detergent solution and then rinsed, initially with large amounts of tap water and finally with several small portions of deionized water. ØProperly cleaned glassware will be coated with a uniform and unbroken film of water. Do not dry the interior surfaces of glassware. ØAn organic solvent, such as methyl ketone or acetone, may be effective in removing grease films.
 2 C Evaporating liquids Evaporation is difficult to control because of the tendency of some solutions to overheat locally. Bumping can cause partial loss of the solution. Careful and gentle heating or use of glass beads will minimize such loss. Some unwanted substances can be eliminated during evaporation. Wet ashing is the oxidation of the organic constituents of a sample with oxidizing reagents such as nitric acid, sulfuric acid, hydrogen peroxide, aqueous bromine, or a combination of these reagents.
2 C Evaporating liquids Evaporation is difficult to control because of the tendency of some solutions to overheat locally. Bumping can cause partial loss of the solution. Careful and gentle heating or use of glass beads will minimize such loss. Some unwanted substances can be eliminated during evaporation. Wet ashing is the oxidation of the organic constituents of a sample with oxidizing reagents such as nitric acid, sulfuric acid, hydrogen peroxide, aqueous bromine, or a combination of these reagents.
 2 D Measuring mass An analytical balance must be used to measure masses with high accuracy. An analytical balance is used for determining mass with a maximum capacity that ranges from 1 g to a few kgs with a precision of at least 1 part in 105 A macrobalance has a maximum load of 160 -200 g and a precision of 0. 1 mg. A semimicroanalytical balance has a maximum load of 10 -30 g and a precision of 0. 01 mg. A microanalytical balance has a maximum load of 1 -3 g and a precision of 0. 001 mg, or 1 µg. 1. The traditional analytical balance had two pans and is considered an equal-arm balance. 2. The single-pan analytical balance was far superior and replaced the traditional balance. 3. The electronic analytical balance is the current balance that is widely used.
2 D Measuring mass An analytical balance must be used to measure masses with high accuracy. An analytical balance is used for determining mass with a maximum capacity that ranges from 1 g to a few kgs with a precision of at least 1 part in 105 A macrobalance has a maximum load of 160 -200 g and a precision of 0. 1 mg. A semimicroanalytical balance has a maximum load of 10 -30 g and a precision of 0. 01 mg. A microanalytical balance has a maximum load of 1 -3 g and a precision of 0. 001 mg, or 1 µg. 1. The traditional analytical balance had two pans and is considered an equal-arm balance. 2. The single-pan analytical balance was far superior and replaced the traditional balance. 3. The electronic analytical balance is the current balance that is widely used.
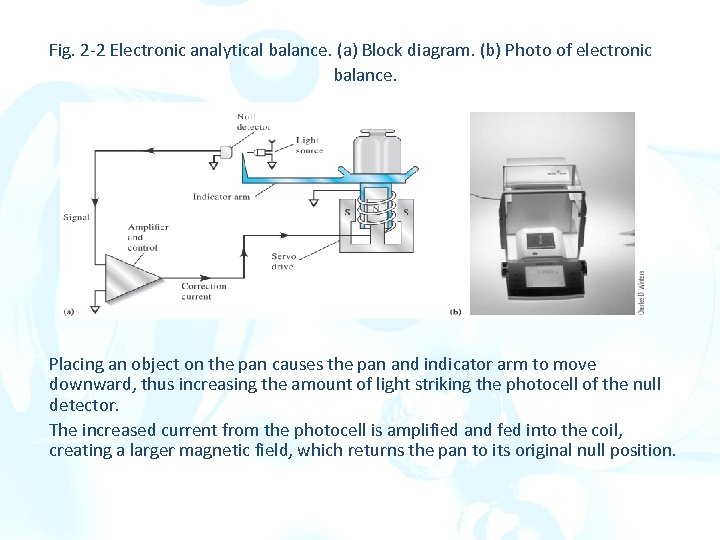 Fig. 2 -2 Electronic analytical balance. (a) Block diagram. (b) Photo of electronic balance. Placing an object on the pan causes the pan and indicator arm to move downward, thus increasing the amount of light striking the photocell of the null detector. The increased current from the photocell is amplified and fed into the coil, creating a larger magnetic field, which returns the pan to its original null position.
Fig. 2 -2 Electronic analytical balance. (a) Block diagram. (b) Photo of electronic balance. Placing an object on the pan causes the pan and indicator arm to move downward, thus increasing the amount of light striking the photocell of the null detector. The increased current from the photocell is amplified and fed into the coil, creating a larger magnetic field, which returns the pan to its original null position.
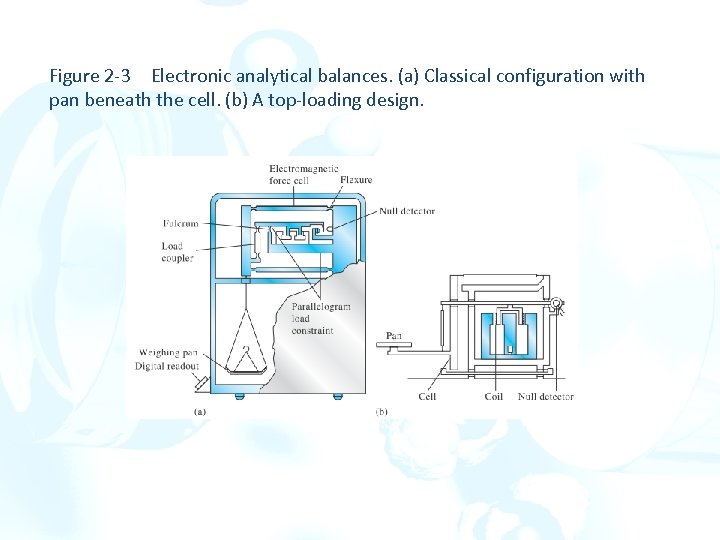 Figure 2 -3 Electronic analytical balances. (a) Classical configuration with pan beneath the cell. (b) A top-loading design.
Figure 2 -3 Electronic analytical balances. (a) Classical configuration with pan beneath the cell. (b) A top-loading design.
 In each electronic balance, the pan is tethered to a system of constraints known collectively as a cell. The cell has several flexures that permit limited movement of the pan. At null, the beam is parallel to the gravitational horizon. Electronic balances feature an automatic taring control that causes the display to read zero with a container (such as a boat or weighing bottle) on the pan. Some electronic balances have dual capacities and dual precisions. These features permit the capacity to be decreased from that of a macrobalance to that of a semimicrobalance (30 g) with a corresponding gain in precision to 0. 01 mg.
In each electronic balance, the pan is tethered to a system of constraints known collectively as a cell. The cell has several flexures that permit limited movement of the pan. At null, the beam is parallel to the gravitational horizon. Electronic balances feature an automatic taring control that causes the display to read zero with a container (such as a boat or weighing bottle) on the pan. Some electronic balances have dual capacities and dual precisions. These features permit the capacity to be decreased from that of a macrobalance to that of a semimicrobalance (30 g) with a corresponding gain in precision to 0. 01 mg.
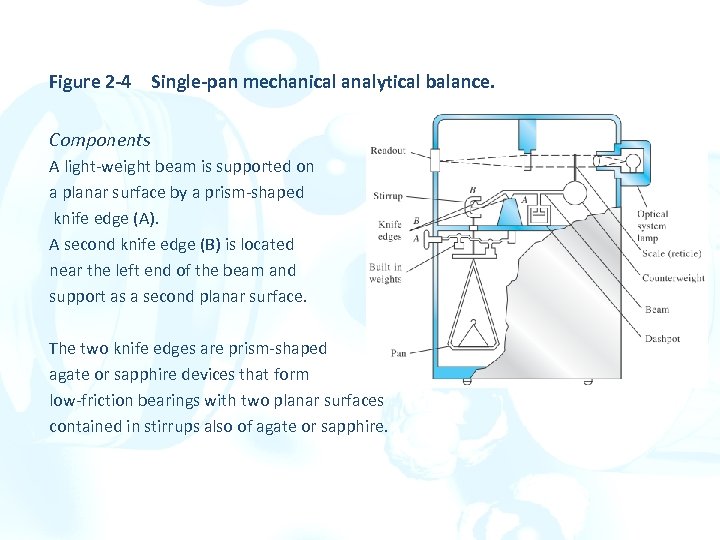 Figure 2 -4 Single-pan mechanical analytical balance. Components A light-weight beam is supported on a planar surface by a prism-shaped knife edge (A). A second knife edge (B) is located near the left end of the beam and support as a second planar surface. The two knife edges are prism-shaped agate or sapphire devices that form low-friction bearings with two planar surfaces contained in stirrups also of agate or sapphire.
Figure 2 -4 Single-pan mechanical analytical balance. Components A light-weight beam is supported on a planar surface by a prism-shaped knife edge (A). A second knife edge (B) is located near the left end of the beam and support as a second planar surface. The two knife edges are prism-shaped agate or sapphire devices that form low-friction bearings with two planar surfaces contained in stirrups also of agate or sapphire.
 Weighing with a Single-Pan Balance The beam of an adjusted balance is in horizontal position. Placing an object on the pan causes the left end of the beam to move downward. Masses are then removed systematically until the imbalance is less than 100 mg. The angle of deflection of the beam is directly proportional to the additional mass that must be removed to restore the beam to its horizontal position. The optical system measures this angle of deflection. A reticle is scribed with a scale that reads 0 to 100 mg. A vernier makes it possible to read this scale to the nearest 0. 1 mg.
Weighing with a Single-Pan Balance The beam of an adjusted balance is in horizontal position. Placing an object on the pan causes the left end of the beam to move downward. Masses are then removed systematically until the imbalance is less than 100 mg. The angle of deflection of the beam is directly proportional to the additional mass that must be removed to restore the beam to its horizontal position. The optical system measures this angle of deflection. A reticle is scribed with a scale that reads 0 to 100 mg. A vernier makes it possible to read this scale to the nearest 0. 1 mg.
 Precautions in using an analytical balance 1. Center the load on the pan as well as possible. 2. Protect the balance from corrosion. 3. Observe special precautions for the weighing of liquids. 4. Consult your instructor if the balance appears to need adjustment. 5. Keep the balance and its case scrupulously clean. A camel’s hair brush is useful for removing spilled material or dust. 6. Always allow an object that has been heated to return to room temperature before weighing it. 7. Use tongs, finger pads, or a glassine paper strip to handle dried objects to prevent transferring moisture to them.
Precautions in using an analytical balance 1. Center the load on the pan as well as possible. 2. Protect the balance from corrosion. 3. Observe special precautions for the weighing of liquids. 4. Consult your instructor if the balance appears to need adjustment. 5. Keep the balance and its case scrupulously clean. A camel’s hair brush is useful for removing spilled material or dust. 6. Always allow an object that has been heated to return to room temperature before weighing it. 7. Use tongs, finger pads, or a glassine paper strip to handle dried objects to prevent transferring moisture to them.
 Sources of error in weighing Correction for Buoyancy A buoyancy error is an error that develops when the object being weighed has a significantly different density than the masses. Buoyancy corrections may be accomplished with the equation: W 1 is the corrected mass of the object, W 2 is the mass of the standard masses, dobj is the density of the object, dwts is the density of the masses, and dair is the density of the air displaced by masses and object. The value of dair is 0. 0012 g/cm 3.
Sources of error in weighing Correction for Buoyancy A buoyancy error is an error that develops when the object being weighed has a significantly different density than the masses. Buoyancy corrections may be accomplished with the equation: W 1 is the corrected mass of the object, W 2 is the mass of the standard masses, dobj is the density of the object, dwts is the density of the masses, and dair is the density of the air displaced by masses and object. The value of dair is 0. 0012 g/cm 3.
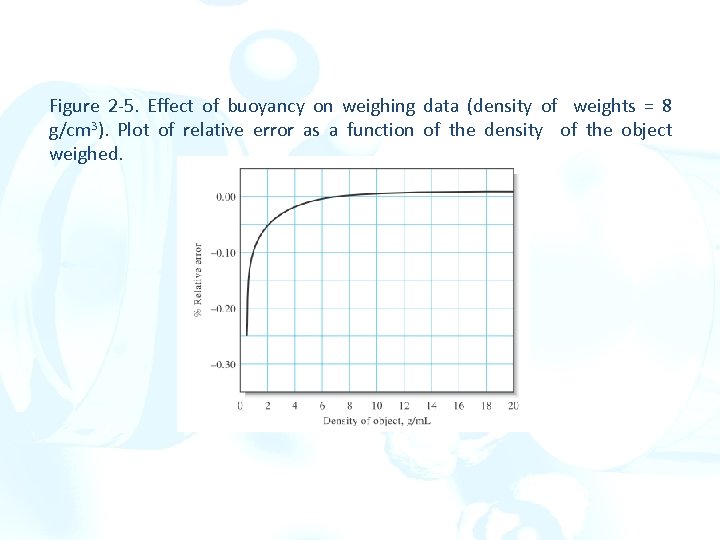 Figure 2 -5. Effect of buoyancy on weighing data (density of weights = 8 g/cm 3). Plot of relative error as a function of the density of the object weighed.
Figure 2 -5. Effect of buoyancy on weighing data (density of weights = 8 g/cm 3). Plot of relative error as a function of the density of the object weighed.

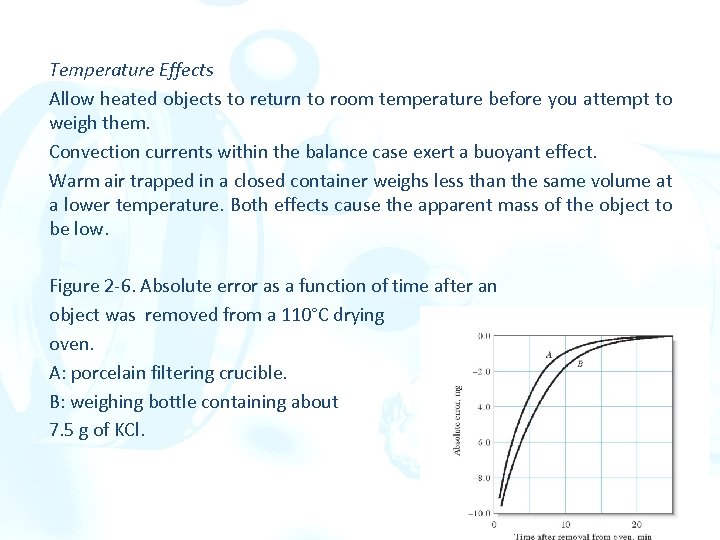 Temperature Effects Allow heated objects to return to room temperature before you attempt to weigh them. Convection currents within the balance case exert a buoyant effect. Warm air trapped in a closed container weighs less than the same volume at a lower temperature. Both effects cause the apparent mass of the object to be low. Figure 2 -6. Absolute error as a function of time after an object was removed from a 110°C drying oven. A: porcelain filtering crucible. B: weighing bottle containing about 7. 5 g of KCl.
Temperature Effects Allow heated objects to return to room temperature before you attempt to weigh them. Convection currents within the balance case exert a buoyant effect. Warm air trapped in a closed container weighs less than the same volume at a lower temperature. Both effects cause the apparent mass of the object to be low. Figure 2 -6. Absolute error as a function of time after an object was removed from a 110°C drying oven. A: porcelain filtering crucible. B: weighing bottle containing about 7. 5 g of KCl.
 Other Sources of Error A porcelain or glass object will occasionally acquire a static charge causing a balance to perform erratically, especially when the relative humidity is low. Spontaneous discharge frequently occurs after a short period. A low level source of radioactivity in the balance case will ionize enough ions to neutralize the charge. The optical scale of a single-pan mechanical balance should be checked regularly for accuracy, particularly under loading conditions that require the full-scale range. A standard 100 -mg mass is used for this check.
Other Sources of Error A porcelain or glass object will occasionally acquire a static charge causing a balance to perform erratically, especially when the relative humidity is low. Spontaneous discharge frequently occurs after a short period. A low level source of radioactivity in the balance case will ionize enough ions to neutralize the charge. The optical scale of a single-pan mechanical balance should be checked regularly for accuracy, particularly under loading conditions that require the full-scale range. A standard 100 -mg mass is used for this check.
 Auxiliary Balances 1. Less precise than analytical balances. 2. Offer the advantages of speed, ruggedness, large capacity, and convenience. 3. A sensitive top-loading balance will accommodate 150 -200 g with a precision of about 1 mg. 4. Most are equipped with a taring device. 5. Some are fully automatic, require no manual dialing or mass handling, and provide a digital readout of the mass. 6. Modern top-loading balances are electronic. A triple-beam balance that is less sensitive than a typical top-loading auxiliary balance is also useful. This is a single-pan balance with three decades of masses that slide along individual calibrated scales. The precision may be one or two orders of magnitude less.
Auxiliary Balances 1. Less precise than analytical balances. 2. Offer the advantages of speed, ruggedness, large capacity, and convenience. 3. A sensitive top-loading balance will accommodate 150 -200 g with a precision of about 1 mg. 4. Most are equipped with a taring device. 5. Some are fully automatic, require no manual dialing or mass handling, and provide a digital readout of the mass. 6. Modern top-loading balances are electronic. A triple-beam balance that is less sensitive than a typical top-loading auxiliary balance is also useful. This is a single-pan balance with three decades of masses that slide along individual calibrated scales. The precision may be one or two orders of magnitude less.
 2 E Equipment and manipulations associated with weighing Drying or ignition to constant mass is a process in which a solid is cycled through heating, cooling, and weighing steps until its mass becomes constant to within 0. 2 to 0. 3 mg. Constant mass ensures that the chemical or physical processes that occur during the heating (or ignition) are complete. Figure 2 -7. Weighing Bottles are convenient for drying and storing solids. Plastic bottles are rugged but abrade easily and not easily cleaned as compared to glass.
2 E Equipment and manipulations associated with weighing Drying or ignition to constant mass is a process in which a solid is cycled through heating, cooling, and weighing steps until its mass becomes constant to within 0. 2 to 0. 3 mg. Constant mass ensures that the chemical or physical processes that occur during the heating (or ignition) are complete. Figure 2 -7. Weighing Bottles are convenient for drying and storing solids. Plastic bottles are rugged but abrade easily and not easily cleaned as compared to glass.
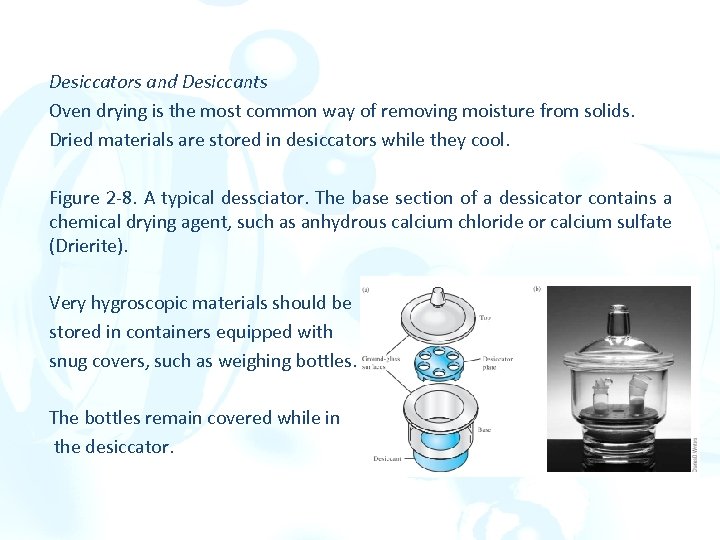 Desiccators and Desiccants Oven drying is the most common way of removing moisture from solids. Dried materials are stored in desiccators while they cool. Figure 2 -8. A typical dessciator. The base section of a dessicator contains a chemical drying agent, such as anhydrous calcium chloride or calcium sulfate (Drierite). Very hygroscopic materials should be stored in containers equipped with snug covers, such as weighing bottles. The bottles remain covered while in the desiccator.
Desiccators and Desiccants Oven drying is the most common way of removing moisture from solids. Dried materials are stored in desiccators while they cool. Figure 2 -8. A typical dessciator. The base section of a dessicator contains a chemical drying agent, such as anhydrous calcium chloride or calcium sulfate (Drierite). Very hygroscopic materials should be stored in containers equipped with snug covers, such as weighing bottles. The bottles remain covered while in the desiccator.
 Manipulating Weighing Bottles Heating at 105°C to 110°C for 1 hour is sufficient to remove the moisture. Figure 2 -9. The recommended way to dry a sample. Figure 2 -10. Avoid touching dried objects with your fingers. Instead, use tongs, chamois finger cots, clean cotton gloves, or strips of paper to handle dried objects for weighing.
Manipulating Weighing Bottles Heating at 105°C to 110°C for 1 hour is sufficient to remove the moisture. Figure 2 -9. The recommended way to dry a sample. Figure 2 -10. Avoid touching dried objects with your fingers. Instead, use tongs, chamois finger cots, clean cotton gloves, or strips of paper to handle dried objects for weighing.
 Weighing by Difference: 1. The bottle and its contents are weighed. 2. One sample is then transferred from the bottle to a container. 3. The bottle and its residual contents are then weighed. 4. The mass of the sample is the difference between the two masses. Weighing Hygroscopic Solids: 1. Place the approximate amount of sample needed in the individual bottle and heat for an appropriate time. 2. Then, quickly cap the bottles and cool in a desiccator. 3. Weigh one of the bottles after opening it momentarily to relieve any vacuum. 4. Quickly empty the contents of the bottle into its receiving vessel, cap immediately, and weigh the bottle again along with any solid that did not get transferred. 5. Repeat for each sample and determine the sample masses by difference.
Weighing by Difference: 1. The bottle and its contents are weighed. 2. One sample is then transferred from the bottle to a container. 3. The bottle and its residual contents are then weighed. 4. The mass of the sample is the difference between the two masses. Weighing Hygroscopic Solids: 1. Place the approximate amount of sample needed in the individual bottle and heat for an appropriate time. 2. Then, quickly cap the bottles and cool in a desiccator. 3. Weigh one of the bottles after opening it momentarily to relieve any vacuum. 4. Quickly empty the contents of the bottle into its receiving vessel, cap immediately, and weigh the bottle again along with any solid that did not get transferred. 5. Repeat for each sample and determine the sample masses by difference.
 Weighing Liquids ØThe mass of a liquid is always obtained by difference. ØLiquids that are noncorrosive and relatively nonvolatile can be transferred to previously weighed containers. ØThe mass of the container is subtracted from the total mass. ØA volatile or corrosive liquid should be sealed in a weighed glass ampoule. The ampoule is heated, and the neck is then immersed in the sample. ØAs cooling occurs, the liquid is drawn into the bulb. ØThe ampoule is then inverted and the neck sealed off with a small flame. The ampoule and its contents, along with any glass removed during sealing, are cooled to room temperature and weighed. ØThe ampoule is then transferred to an appropriate container and broken. ØA volume correction for the glass of the ampoule may be needed if the receiving vessel is a volumetric flask.
Weighing Liquids ØThe mass of a liquid is always obtained by difference. ØLiquids that are noncorrosive and relatively nonvolatile can be transferred to previously weighed containers. ØThe mass of the container is subtracted from the total mass. ØA volatile or corrosive liquid should be sealed in a weighed glass ampoule. The ampoule is heated, and the neck is then immersed in the sample. ØAs cooling occurs, the liquid is drawn into the bulb. ØThe ampoule is then inverted and the neck sealed off with a small flame. The ampoule and its contents, along with any glass removed during sealing, are cooled to room temperature and weighed. ØThe ampoule is then transferred to an appropriate container and broken. ØA volume correction for the glass of the ampoule may be needed if the receiving vessel is a volumetric flask.
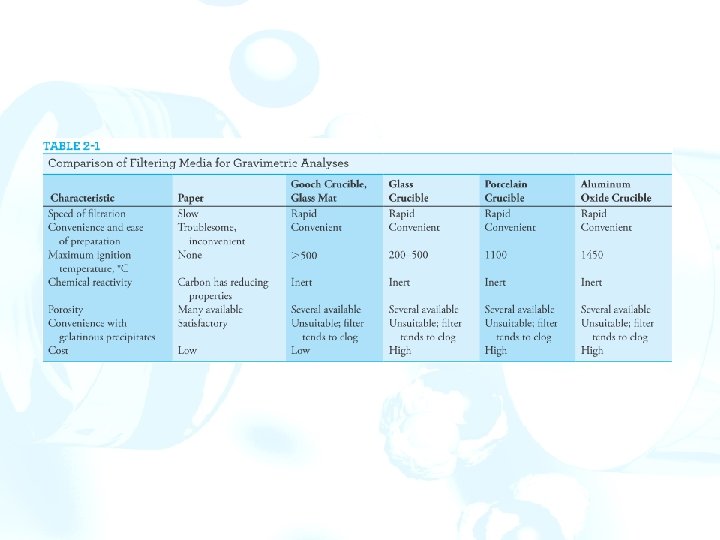
 2 F Filtration and ignition of solids Apparatus Simple crucibles serve only as containers. Porcelain, aluminum oxide, silica, and platinum crucibles maintain constant mass. The solid is first collected on a filter paper. The filter and contents are then transferred to a weighed crucible, and the paper is ignited. Filtering crucibles serve not only as containers but also as filters. A vacuum is used to hasten the filtration. Figure 2 -11 Adaptors for filtering crucibles.
2 F Filtration and ignition of solids Apparatus Simple crucibles serve only as containers. Porcelain, aluminum oxide, silica, and platinum crucibles maintain constant mass. The solid is first collected on a filter paper. The filter and contents are then transferred to a weighed crucible, and the paper is ignited. Filtering crucibles serve not only as containers but also as filters. A vacuum is used to hasten the filtration. Figure 2 -11 Adaptors for filtering crucibles.
 Sintered-glass crucibles are manufactured in fine, medium, and coarse porosities. The upper temperature limit is usually ~200°C. Made of quartz and can tolerate substantially higher temperatures without damage. A Gooch crucible has a perforated bottom that supports a fibrous mat. Small circles of glass matting are used in pairs to protect against breaking during the filtration. Glass mats can tolerate temperatures in excess of 500°C and are substantially less hygroscopic.
Sintered-glass crucibles are manufactured in fine, medium, and coarse porosities. The upper temperature limit is usually ~200°C. Made of quartz and can tolerate substantially higher temperatures without damage. A Gooch crucible has a perforated bottom that supports a fibrous mat. Small circles of glass matting are used in pairs to protect against breaking during the filtration. Glass mats can tolerate temperatures in excess of 500°C and are substantially less hygroscopic.
 Filter paper Paper is an important filtering medium. ØAshless paper is manufactured from cellulose fibers that have been treated with hydrochloric and hydrofluoric acids. Ø 9 - or 11 -cm circles of ashless paper leave a residue that weighs less than 0. 1 mg, which is negligible under most circumstances. ØAshless paper can be obtained in several porosities. ØA coarse-porosity ashless paper is most effective for filtering solids, but even with such paper, clogging occurs. This problem can be minimized by mixing a dispersion of ashless filter paper with the precipitate prior to filtration.
Filter paper Paper is an important filtering medium. ØAshless paper is manufactured from cellulose fibers that have been treated with hydrochloric and hydrofluoric acids. Ø 9 - or 11 -cm circles of ashless paper leave a residue that weighs less than 0. 1 mg, which is negligible under most circumstances. ØAshless paper can be obtained in several porosities. ØA coarse-porosity ashless paper is most effective for filtering solids, but even with such paper, clogging occurs. This problem can be minimized by mixing a dispersion of ashless filter paper with the precipitate prior to filtration.
 Heating Equipment 1. Many precipitates can be weighed directly after being brought to constant mass in a low-temperature drying oven. 2. The maximum attainable temperature ranges from 140°C to 260°C. 3. Microwave laboratory ovens greatly shorten drying cycles. 4. An ordinary heat lamp can be used to dry a precipitate that has been collected on ashless paper and to char the paper as well. 5. Burners are convenient sources of intense heat. The maximum attainable temperature depends on the design of the burner and the fuel combustion properties. 6. The Meker burner provides the highest temperatures, followed by the Tirrill and Bunsen types. 7. A heavy-duty electric furnace (muffle furnace) is capable of maintaining controlled temperatures of 1100°C or higher.
Heating Equipment 1. Many precipitates can be weighed directly after being brought to constant mass in a low-temperature drying oven. 2. The maximum attainable temperature ranges from 140°C to 260°C. 3. Microwave laboratory ovens greatly shorten drying cycles. 4. An ordinary heat lamp can be used to dry a precipitate that has been collected on ashless paper and to char the paper as well. 5. Burners are convenient sources of intense heat. The maximum attainable temperature depends on the design of the burner and the fuel combustion properties. 6. The Meker burner provides the highest temperatures, followed by the Tirrill and Bunsen types. 7. A heavy-duty electric furnace (muffle furnace) is capable of maintaining controlled temperatures of 1100°C or higher.
 Filtering and Igniting Precipitates Preparation of Crucibles A crucible used to convert a precipitate to a form suitable for weighing must maintain a constant mass throughout drying or ignition. Backwashing a filtering crucible is done by turning the crucible upside down in the adaptor and sucking water through the inverted crucible. Figure 2 -12 (a) Washing by decantation. (b) Transferring the precipitate. The steps in filtering an analytical precipitate are decantation, washing, and transfer.
Filtering and Igniting Precipitates Preparation of Crucibles A crucible used to convert a precipitate to a form suitable for weighing must maintain a constant mass throughout drying or ignition. Backwashing a filtering crucible is done by turning the crucible upside down in the adaptor and sucking water through the inverted crucible. Figure 2 -12 (a) Washing by decantation. (b) Transferring the precipitate. The steps in filtering an analytical precipitate are decantation, washing, and transfer.
 The last traces of precipitate on the inside of the beaker are dislodged with a rubber policeman. Many precipitates possess the property of creeping, or spreading over a wetted surface against the force of gravity. Filters are never filled to more than three quarters of capacity to prevent the possible loss of precipitate through creeping. The addition of a small amount of nonionic detergent, such as Triton X 100, to the supernatant liquid or wash liquid can help minimize creeping. A gelatinous precipitate must be completely washed before it is allowed to dry.
The last traces of precipitate on the inside of the beaker are dislodged with a rubber policeman. Many precipitates possess the property of creeping, or spreading over a wetted surface against the force of gravity. Filters are never filled to more than three quarters of capacity to prevent the possible loss of precipitate through creeping. The addition of a small amount of nonionic detergent, such as Triton X 100, to the supernatant liquid or wash liquid can help minimize creeping. A gelatinous precipitate must be completely washed before it is allowed to dry.
 Figure 2 -13. Preparation of a Filter Paper
Figure 2 -13. Preparation of a Filter Paper
 Figure 2 -14 Transferring Paper and Precipitate to a Crucible
Figure 2 -14 Transferring Paper and Precipitate to a Crucible
 Ashing Filter Papers If a heat lamp is used, the crucible is placed on a clean, nonreactive surface, such as a wire screen covered with aluminum foil. The lamp is positioned above the rim of the crucible. Charring is accelerated if the paper is moistened with a drop of concentrated ammonium nitrate solution. A burner produces much higher temperatures than a heat lamp. Partial reduction of some precipitates can occur through reaction with the hot carbon of the charring paper. Figure 2 -15 Ignition of a precipitate.
Ashing Filter Papers If a heat lamp is used, the crucible is placed on a clean, nonreactive surface, such as a wire screen covered with aluminum foil. The lamp is positioned above the rim of the crucible. Charring is accelerated if the paper is moistened with a drop of concentrated ammonium nitrate solution. A burner produces much higher temperatures than a heat lamp. Partial reduction of some precipitates can occur through reaction with the hot carbon of the charring paper. Figure 2 -15 Ignition of a precipitate.
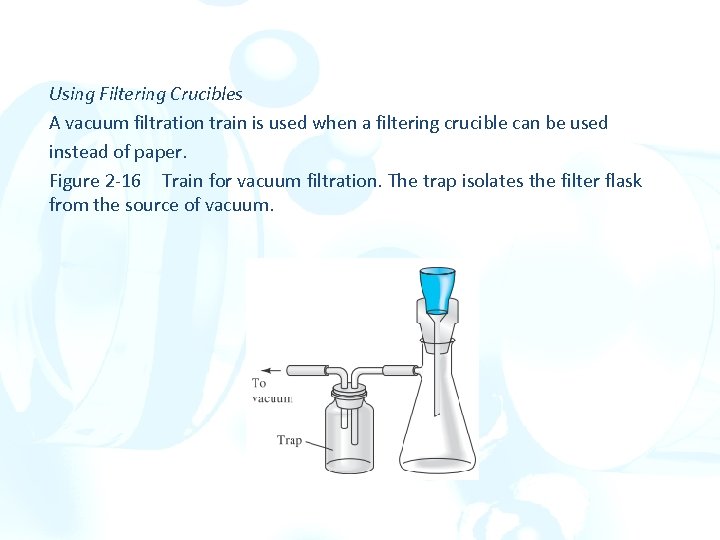 Using Filtering Crucibles A vacuum filtration train is used when a filtering crucible can be used instead of paper. Figure 2 -16 Train for vacuum filtration. The trap isolates the filter flask from the source of vacuum.
Using Filtering Crucibles A vacuum filtration train is used when a filtering crucible can be used instead of paper. Figure 2 -16 Train for vacuum filtration. The trap isolates the filter flask from the source of vacuum.
 Rules for Manipulating Heated Objects 1. Practice unfamiliar manipulations before putting them to use. 2. Never place a heated object on the benchtop. Instead, place it on a wire gauze or a heat-resistant ceramic plate. 3. Allow a crucible that has been subjected to the full flame of a burner or to a muffle furnace to cool momentarily. 4. Keep the tongs and forceps used to handle heated objects scrupulously clean. In particular, do not allow the tips to touch the benchtop.
Rules for Manipulating Heated Objects 1. Practice unfamiliar manipulations before putting them to use. 2. Never place a heated object on the benchtop. Instead, place it on a wire gauze or a heat-resistant ceramic plate. 3. Allow a crucible that has been subjected to the full flame of a burner or to a muffle furnace to cool momentarily. 4. Keep the tongs and forceps used to handle heated objects scrupulously clean. In particular, do not allow the tips to touch the benchtop.
 2 G Measuring volume The precise measurement of volume is as important to many analytical methods as the precise measurement of mass. Units of Volume The unit of volume is the liter (L), defined as one cubic decimeter. The milliliter(m. L) is one-thousandth of a liter (0. 001 L) and the microliter (µL) is 1026 L or 10 -3 m. L. The Effect of Temperature on Volume Measurements Most volumetric measuring devices are made of glass, which has a small coefficient of expansion.
2 G Measuring volume The precise measurement of volume is as important to many analytical methods as the precise measurement of mass. Units of Volume The unit of volume is the liter (L), defined as one cubic decimeter. The milliliter(m. L) is one-thousandth of a liter (0. 001 L) and the microliter (µL) is 1026 L or 10 -3 m. L. The Effect of Temperature on Volume Measurements Most volumetric measuring devices are made of glass, which has a small coefficient of expansion.
 The coefficient of expansion for dilute aqueous solutions (approximately 0. 025%/°C) is such that a 5°C change has a measurable effect on the reliability of ordinary volumetric measurements.
The coefficient of expansion for dilute aqueous solutions (approximately 0. 025%/°C) is such that a 5°C change has a measurable effect on the reliability of ordinary volumetric measurements.
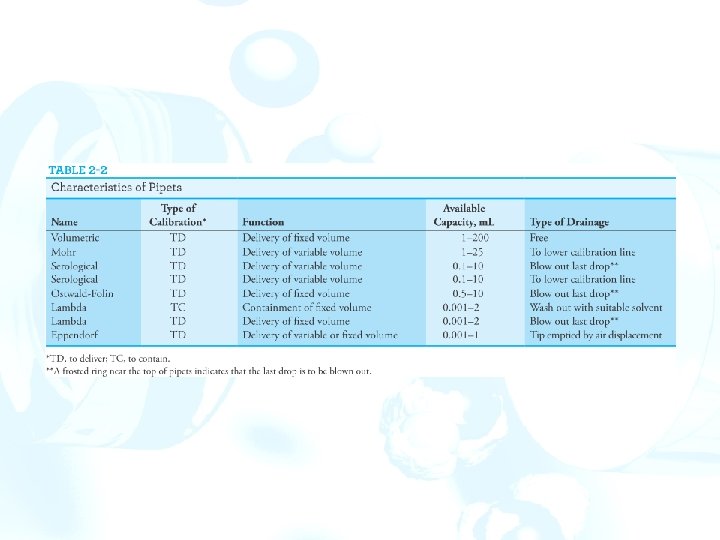
 Apparatus for Precisely Measuring Volume 1. Volume may be measured with a pipet, a buret, or a volumetric flask. 2. Volumetric equipment is marked TD for “to deliver” or TC for “to contain” and also marked for the temperature at which the calibration strictly applies. 3. Pipets and burets are usually calibrated to deliver specified volumes. Volumetric flasks are calibrated to contain a specific volume. Pipets permit the transfer of accurately known volumes. A volumetric pipet delivers a single fixed volume between 0. 5 and 200 m. L. Measuring pipets are calibrated in convenient units to permit delivery of any volume up to a maximum capacity ranging from 0. 1 to 25 m. L.
Apparatus for Precisely Measuring Volume 1. Volume may be measured with a pipet, a buret, or a volumetric flask. 2. Volumetric equipment is marked TD for “to deliver” or TC for “to contain” and also marked for the temperature at which the calibration strictly applies. 3. Pipets and burets are usually calibrated to deliver specified volumes. Volumetric flasks are calibrated to contain a specific volume. Pipets permit the transfer of accurately known volumes. A volumetric pipet delivers a single fixed volume between 0. 5 and 200 m. L. Measuring pipets are calibrated in convenient units to permit delivery of any volume up to a maximum capacity ranging from 0. 1 to 25 m. L.
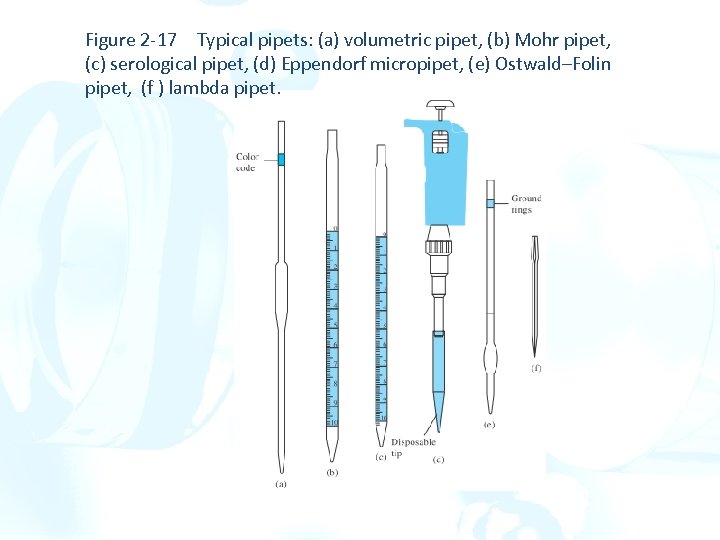 Figure 2 -17 Typical pipets: (a) volumetric pipet, (b) Mohr pipet, (c) serological pipet, (d) Eppendorf micropipet, (e) Ostwald–Folin pipet, (f ) lambda pipet.
Figure 2 -17 Typical pipets: (a) volumetric pipet, (b) Mohr pipet, (c) serological pipet, (d) Eppendorf micropipet, (e) Ostwald–Folin pipet, (f ) lambda pipet.
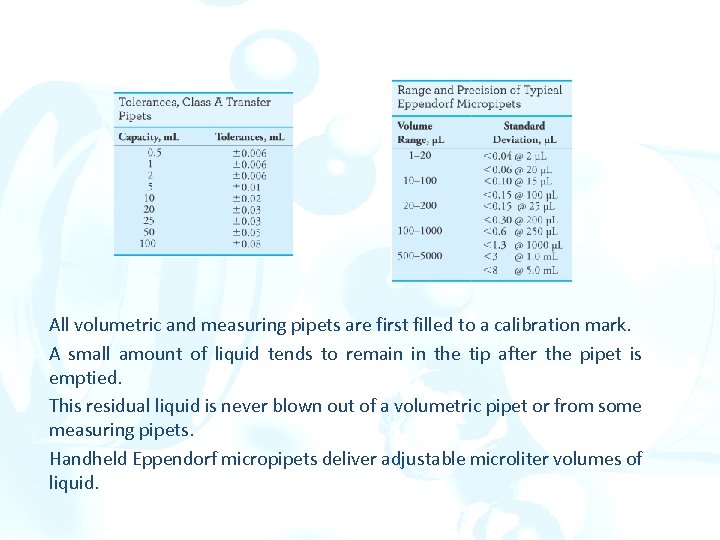 All volumetric and measuring pipets are first filled to a calibration mark. A small amount of liquid tends to remain in the tip after the pipet is emptied. This residual liquid is never blown out of a volumetric pipet or from some measuring pipets. Handheld Eppendorf micropipets deliver adjustable microliter volumes of liquid.
All volumetric and measuring pipets are first filled to a calibration mark. A small amount of liquid tends to remain in the tip after the pipet is emptied. This residual liquid is never blown out of a volumetric pipet or from some measuring pipets. Handheld Eppendorf micropipets deliver adjustable microliter volumes of liquid.
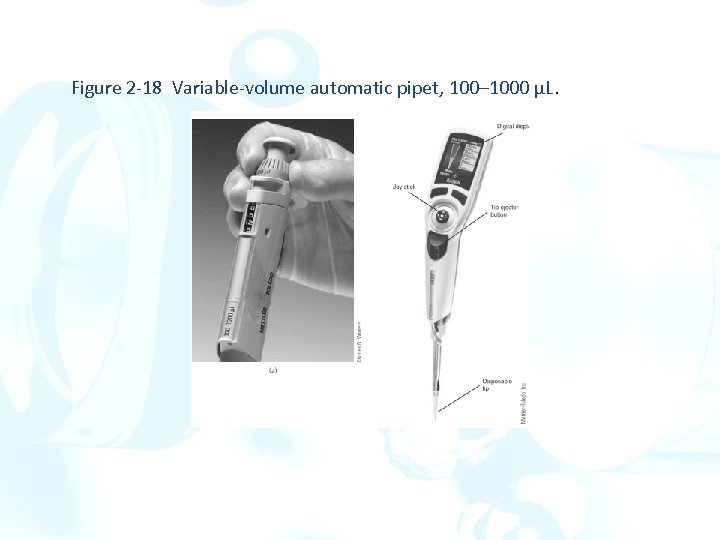 Figure 2 -18 Variable-volume automatic pipet, 100– 1000 µL.
Figure 2 -18 Variable-volume automatic pipet, 100– 1000 µL.
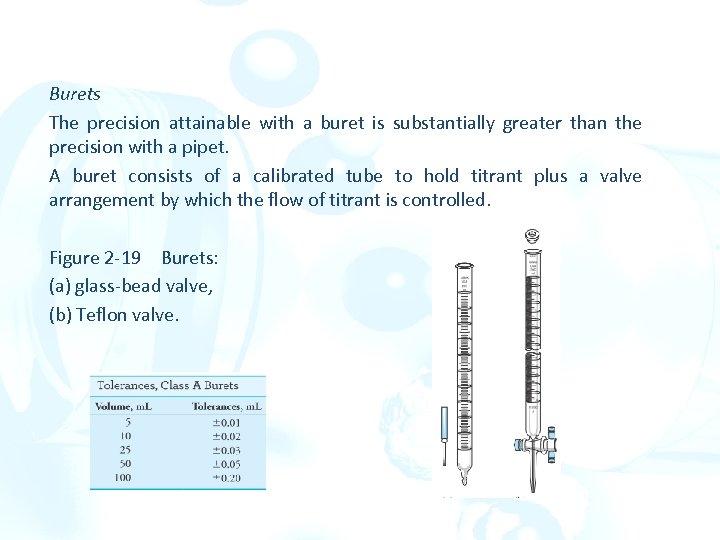 Burets The precision attainable with a buret is substantially greater than the precision with a pipet. A buret consists of a calibrated tube to hold titrant plus a valve arrangement by which the flow of titrant is controlled. Figure 2 -19 Burets: (a) glass-bead valve, (b) Teflon valve.
Burets The precision attainable with a buret is substantially greater than the precision with a pipet. A buret consists of a calibrated tube to hold titrant plus a valve arrangement by which the flow of titrant is controlled. Figure 2 -19 Burets: (a) glass-bead valve, (b) Teflon valve.
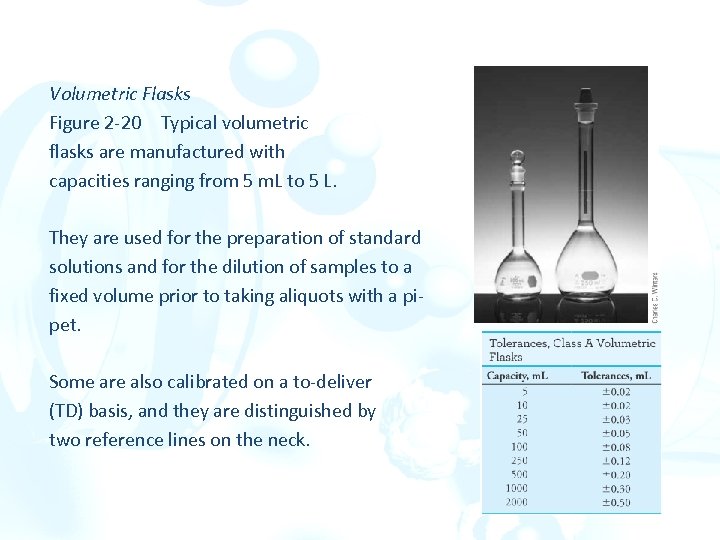 Volumetric Flasks Figure 2 -20 Typical volumetric flasks are manufactured with capacities ranging from 5 m. L to 5 L. They are used for the preparation of standard solutions and for the dilution of samples to a fixed volume prior to taking aliquots with a pipet. Some are also calibrated on a to-deliver (TD) basis, and they are distinguished by two reference lines on the neck.
Volumetric Flasks Figure 2 -20 Typical volumetric flasks are manufactured with capacities ranging from 5 m. L to 5 L. They are used for the preparation of standard solutions and for the dilution of samples to a fixed volume prior to taking aliquots with a pipet. Some are also calibrated on a to-deliver (TD) basis, and they are distinguished by two reference lines on the neck.
 Using Volumetric Equipment Volume markings are blazed on clean volumetric equipment by the manufacturer. Only clean glass surfaces support a uniform film of liquid. Dirt or oil causes breaks in this film. Cleaning 1. Brief soaking in a warm detergent solution is usually sufficient. 2. The apparatus must be thoroughly rinsed with tap water and then with three or four portions of distilled water. 3. It is seldom necessary to dry volumetric ware. 4. Prolonged soaking will cause the formation of a ring at a detergent/air interface. 5. This ring cannot be removed and causes a film break.
Using Volumetric Equipment Volume markings are blazed on clean volumetric equipment by the manufacturer. Only clean glass surfaces support a uniform film of liquid. Dirt or oil causes breaks in this film. Cleaning 1. Brief soaking in a warm detergent solution is usually sufficient. 2. The apparatus must be thoroughly rinsed with tap water and then with three or four portions of distilled water. 3. It is seldom necessary to dry volumetric ware. 4. Prolonged soaking will cause the formation of a ring at a detergent/air interface. 5. This ring cannot be removed and causes a film break.
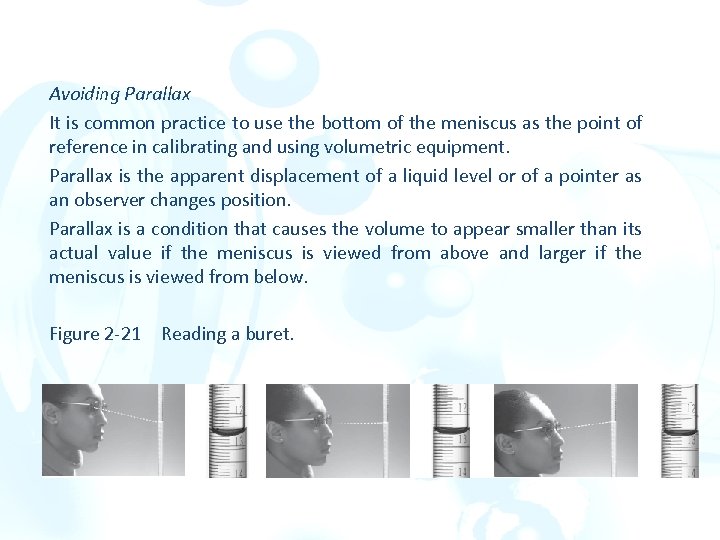 Avoiding Parallax It is common practice to use the bottom of the meniscus as the point of reference in calibrating and using volumetric equipment. Parallax is the apparent displacement of a liquid level or of a pointer as an observer changes position. Parallax is a condition that causes the volume to appear smaller than its actual value if the meniscus is viewed from above and larger if the meniscus is viewed from below. Figure 2 -21 Reading a buret.
Avoiding Parallax It is common practice to use the bottom of the meniscus as the point of reference in calibrating and using volumetric equipment. Parallax is the apparent displacement of a liquid level or of a pointer as an observer changes position. Parallax is a condition that causes the volume to appear smaller than its actual value if the meniscus is viewed from above and larger if the meniscus is viewed from below. Figure 2 -21 Reading a buret.
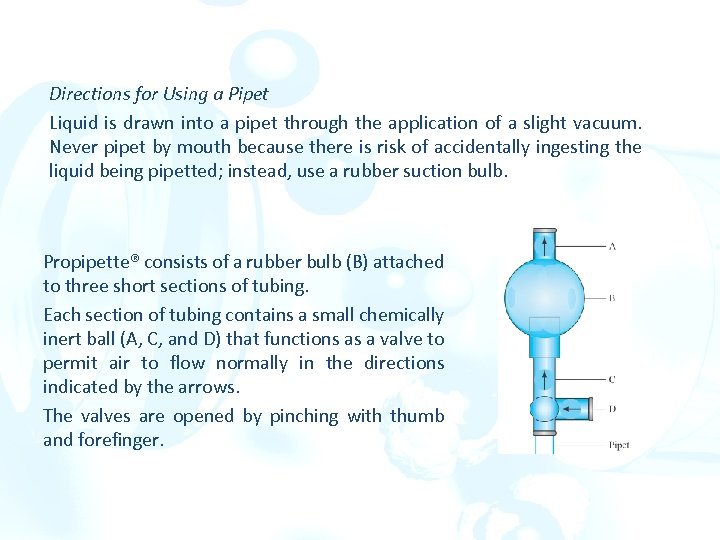 Directions for Using a Pipet Liquid is drawn into a pipet through the application of a slight vacuum. Never pipet by mouth because there is risk of accidentally ingesting the liquid being pipetted; instead, use a rubber suction bulb. Propipette® consists of a rubber bulb (B) attached to three short sections of tubing. Each section of tubing contains a small chemically inert ball (A, C, and D) that functions as a valve to permit air to flow normally in the directions indicated by the arrows. The valves are opened by pinching with thumb and forefinger.
Directions for Using a Pipet Liquid is drawn into a pipet through the application of a slight vacuum. Never pipet by mouth because there is risk of accidentally ingesting the liquid being pipetted; instead, use a rubber suction bulb. Propipette® consists of a rubber bulb (B) attached to three short sections of tubing. Each section of tubing contains a small chemically inert ball (A, C, and D) that functions as a valve to permit air to flow normally in the directions indicated by the arrows. The valves are opened by pinching with thumb and forefinger.
 Cleaning 1. Draw detergent solution to a level 2 to 3 cm above the calibration mark of the pipet. 2. Drain this solution and then rinse the pipet with several portions of tap water. 3. Inspect for film breaks, and repeat this portion of the cleaning cycle if necessary. 4. Finally, fill the pipet with distilled water to perhaps one third of its capacity and carefully rotate it so that the entire interior surface is wetted. 5. Repeat this rinsing step at least twice.
Cleaning 1. Draw detergent solution to a level 2 to 3 cm above the calibration mark of the pipet. 2. Drain this solution and then rinse the pipet with several portions of tap water. 3. Inspect for film breaks, and repeat this portion of the cleaning cycle if necessary. 4. Finally, fill the pipet with distilled water to perhaps one third of its capacity and carefully rotate it so that the entire interior surface is wetted. 5. Repeat this rinsing step at least twice.
 Measuring an Aliquot: 1. Draw a small volume of the sample liquid into the pipet and thoroughly wet the entire interior surface. Repeat twice. 2. Carefully fill the pipet to a level above the graduation mark. Be sure that there are no bubbles. 3. Touch the pipet tip to the wall of a glass vessel and slowly allow the liquid level to drop. 4. When the bottom of the meniscus coincides exactly with the graduation mark, stop the flow. 5. Remove the pipet from the volumetric flask, tilt it until liquid is drawn slightly up into the pipet, and wipe the tip with a lintless tissue. 6. Place the pipet tip well within the receiving vessel, and allow the liquid to drain. 7. Finally, withdraw the pipet with a rotating motion to remove any liquid adhering to the tip. 8. Rinse the pipet thoroughly after use.
Measuring an Aliquot: 1. Draw a small volume of the sample liquid into the pipet and thoroughly wet the entire interior surface. Repeat twice. 2. Carefully fill the pipet to a level above the graduation mark. Be sure that there are no bubbles. 3. Touch the pipet tip to the wall of a glass vessel and slowly allow the liquid level to drop. 4. When the bottom of the meniscus coincides exactly with the graduation mark, stop the flow. 5. Remove the pipet from the volumetric flask, tilt it until liquid is drawn slightly up into the pipet, and wipe the tip with a lintless tissue. 6. Place the pipet tip well within the receiving vessel, and allow the liquid to drain. 7. Finally, withdraw the pipet with a rotating motion to remove any liquid adhering to the tip. 8. Rinse the pipet thoroughly after use.
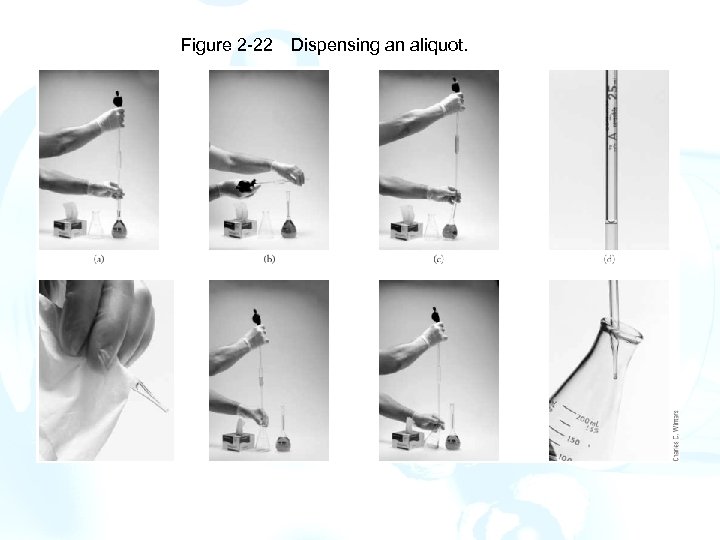 Figure 2 -22 Dispensing an aliquot.
Figure 2 -22 Dispensing an aliquot.
 Directions for Using a Buret Cleaning Clean the tube with detergent and a long brush. Rinse thoroughly with tap water and then with distilled water. Inspect for water breaks. Repeat if necessary. Lubricating a Glass Stopcock Carefully remove all old grease from a glass stopcock and its barrel with a paper towel and dry both parts completely. Lightly grease the stopcock. Insert the stopcock into the barrel and rotate it vigorously with slight inward pressure. A proper amount of lubricant has been used when: (1) the area of contact between stopcock and barrel appears nearly transparent, (2) the seal is liquid-tight, and (3) no grease has worked its way into the tip. Buret readings should be estimated to the nearest 0. 01 m. L.
Directions for Using a Buret Cleaning Clean the tube with detergent and a long brush. Rinse thoroughly with tap water and then with distilled water. Inspect for water breaks. Repeat if necessary. Lubricating a Glass Stopcock Carefully remove all old grease from a glass stopcock and its barrel with a paper towel and dry both parts completely. Lightly grease the stopcock. Insert the stopcock into the barrel and rotate it vigorously with slight inward pressure. A proper amount of lubricant has been used when: (1) the area of contact between stopcock and barrel appears nearly transparent, (2) the seal is liquid-tight, and (3) no grease has worked its way into the tip. Buret readings should be estimated to the nearest 0. 01 m. L.
 Filling 1. Ensure that the stopcock is closed. 2. Add 5 to 10 m. L of the titrant, and carefully rotate the buret to wet the interior completely. 3. Allow the liquid to drain through the tip. Repeat twice. 4. Then fill the buret well above the zero mark. 5. Free the tip of air bubbles by rapidly rotating the stopcock and permitting small quantities of the titrant to pass. 6. Lower the level of the liquid just to or somewhat below the zero mark. 7. Allow for drainage (<1 min), and then record the initial volume reading, estimating to the nearest 0. 01 m. L.
Filling 1. Ensure that the stopcock is closed. 2. Add 5 to 10 m. L of the titrant, and carefully rotate the buret to wet the interior completely. 3. Allow the liquid to drain through the tip. Repeat twice. 4. Then fill the buret well above the zero mark. 5. Free the tip of air bubbles by rapidly rotating the stopcock and permitting small quantities of the titrant to pass. 6. Lower the level of the liquid just to or somewhat below the zero mark. 7. Allow for drainage (<1 min), and then record the initial volume reading, estimating to the nearest 0. 01 m. L.
 Titration Figure 2 -23 Recommended method for manipulating a buret stopcock. Be sure the tip of the buret is well within the titration flask, and introduce the titrant in increments of about 1 m. L. Swirl (or stir) constantly. Decrease the volume of the increments progresseively toward the end point. When only a few more drops are needed to reach the end point, rinse the walls of the container. Allow the titrant to drain from the inner wall of the buret (at least 30 seconds) at the completion of the titration. Then, record the final volume, again to the nearest 0. 01 m. L.
Titration Figure 2 -23 Recommended method for manipulating a buret stopcock. Be sure the tip of the buret is well within the titration flask, and introduce the titrant in increments of about 1 m. L. Swirl (or stir) constantly. Decrease the volume of the increments progresseively toward the end point. When only a few more drops are needed to reach the end point, rinse the walls of the container. Allow the titrant to drain from the inner wall of the buret (at least 30 seconds) at the completion of the titration. Then, record the final volume, again to the nearest 0. 01 m. L.
 Directions for Using a Volumetric Flask Volumetric flasks should be washed with detergent and thoroughly rinsed. Only rarely do they need to be dried. Drying is best accomplished by clamping the flask in an inverted position. Direct Weighing into a Volumetric Flask 1. The direct preparation of a standard solution requires the introduction of a known mass of solute to a volumetric flask. 2. Use of a powder funnel minimizes the possibility of losing solid during the transfer. 3. Rinse the funnel thoroughly, and collect the washings in the flask.
Directions for Using a Volumetric Flask Volumetric flasks should be washed with detergent and thoroughly rinsed. Only rarely do they need to be dried. Drying is best accomplished by clamping the flask in an inverted position. Direct Weighing into a Volumetric Flask 1. The direct preparation of a standard solution requires the introduction of a known mass of solute to a volumetric flask. 2. Use of a powder funnel minimizes the possibility of losing solid during the transfer. 3. Rinse the funnel thoroughly, and collect the washings in the flask.
 Quantitative Transfer of Liquid to a Volumetric Flask 1. The solute should be completely dissolved before diluting to the mark. 2. Insert a funnel into the neck of the volumetric flask, and use a stirring rod to direct the flow of liquid from the beaker into the funnel. 3. With the stirring rod, tip off the last drop of liquid on the spout of the beaker. 4. Rinse both the stirring rod and the interior of the beaker with distilled water and transfer the washings to the volumetric flask as before. Repeat the rinsing process.
Quantitative Transfer of Liquid to a Volumetric Flask 1. The solute should be completely dissolved before diluting to the mark. 2. Insert a funnel into the neck of the volumetric flask, and use a stirring rod to direct the flow of liquid from the beaker into the funnel. 3. With the stirring rod, tip off the last drop of liquid on the spout of the beaker. 4. Rinse both the stirring rod and the interior of the beaker with distilled water and transfer the washings to the volumetric flask as before. Repeat the rinsing process.
 Diluting to the Mark 1. After the solute has been transferred, fill the flask about half full and swirl the contents to hasten solution. 2. Add more solvent and again mix well. 3. Bring the liquid level almost to the mark, and allow time for drainage (1 min). Then, use a medicine dropper to make any necessary final additions of solvent. 4. Firmly stopper the flask, and invert it repeatedly to ensure thorough mixing. 5. Transfer the contents to a storage bottle that either is dry or has been thoroughly rinsed with several small portions of the solution from the flask.
Diluting to the Mark 1. After the solute has been transferred, fill the flask about half full and swirl the contents to hasten solution. 2. Add more solvent and again mix well. 3. Bring the liquid level almost to the mark, and allow time for drainage (1 min). Then, use a medicine dropper to make any necessary final additions of solvent. 4. Firmly stopper the flask, and invert it repeatedly to ensure thorough mixing. 5. Transfer the contents to a storage bottle that either is dry or has been thoroughly rinsed with several small portions of the solution from the flask.
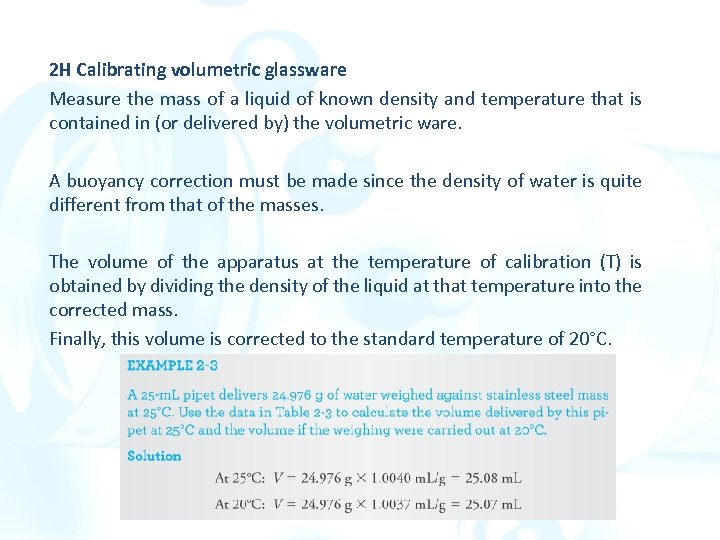 2 H Calibrating volumetric glassware Measure the mass of a liquid of known density and temperature that is contained in (or delivered by) the volumetric ware. A buoyancy correction must be made since the density of water is quite different from that of the masses. The volume of the apparatus at the temperature of calibration (T) is obtained by dividing the density of the liquid at that temperature into the corrected mass. Finally, this volume is corrected to the standard temperature of 20°C.
2 H Calibrating volumetric glassware Measure the mass of a liquid of known density and temperature that is contained in (or delivered by) the volumetric ware. A buoyancy correction must be made since the density of water is quite different from that of the masses. The volume of the apparatus at the temperature of calibration (T) is obtained by dividing the density of the liquid at that temperature into the corrected mass. Finally, this volume is corrected to the standard temperature of 20°C.
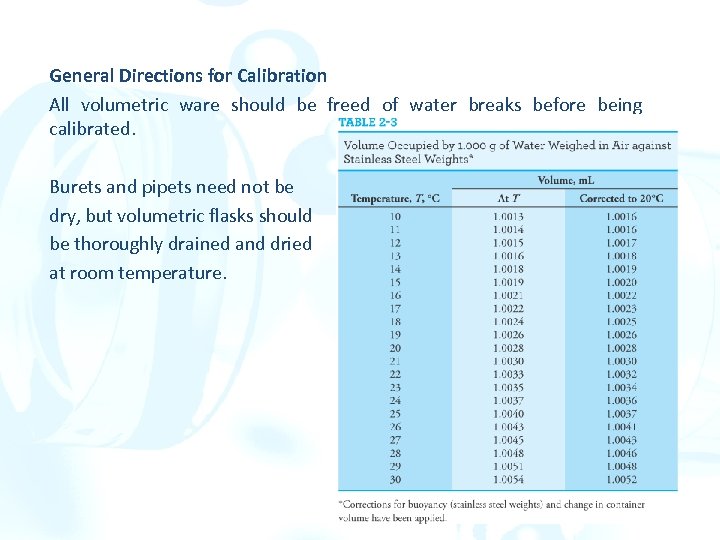 General Directions for Calibration All volumetric ware should be freed of water breaks before being calibrated. Burets and pipets need not be dry, but volumetric flasks should be thoroughly drained and dried at room temperature.
General Directions for Calibration All volumetric ware should be freed of water breaks before being calibrated. Burets and pipets need not be dry, but volumetric flasks should be thoroughly drained and dried at room temperature.
 Calibrating a Volumetric Pipet 1. Determine the empty mass of the stoppered receiver to the nearest milligram. 2. Transfer a portion of temperature-equilibrated water to the receiver with the pipet, weigh the receiver and its contents (to the nearest milligram), and calculate the mass of water delivered from the difference in these masses. 3. Calculate the volume delivered. 4. Repeat the calibration several times, and calculate the mean volume delivered and its standard deviation.
Calibrating a Volumetric Pipet 1. Determine the empty mass of the stoppered receiver to the nearest milligram. 2. Transfer a portion of temperature-equilibrated water to the receiver with the pipet, weigh the receiver and its contents (to the nearest milligram), and calculate the mass of water delivered from the difference in these masses. 3. Calculate the volume delivered. 4. Repeat the calibration several times, and calculate the mean volume delivered and its standard deviation.
 Calibrating a Buret ØFill the buret with temperature-equilibrated water. ØLower the liquid level to bring the bottom of the meniscus to the 0. 00 m. L mark. ØTouch the tip to the wall of a beaker to remove any adhering drop. Wait 10 minutes and recheck the volume. ØWeigh (to the nearest milligram) a 125 -m. L conical flask fitted with a rubber stopper. ØSlowly transfer (at about 10 m. L/min) approximately 10 m. L of water to the flask. Touch the tip to the wall of the flask. ØWait 1 minute, record the volume that was apparently delivered, and refill the buret.
Calibrating a Buret ØFill the buret with temperature-equilibrated water. ØLower the liquid level to bring the bottom of the meniscus to the 0. 00 m. L mark. ØTouch the tip to the wall of a beaker to remove any adhering drop. Wait 10 minutes and recheck the volume. ØWeigh (to the nearest milligram) a 125 -m. L conical flask fitted with a rubber stopper. ØSlowly transfer (at about 10 m. L/min) approximately 10 m. L of water to the flask. Touch the tip to the wall of the flask. ØWait 1 minute, record the volume that was apparently delivered, and refill the buret.
 ØWeigh the flask and its contents to the nearest milligram. The difference between this mass and the initial value is the mass of water delivered. Convert this mass to the true volume. Subtract the apparent volume from the true volume. ØThis difference is the correction that should be applied to the apparent volume to give the true volume. ØRepeat the calibration until agreement within 60. 02 m. L is achieved. Calibrating a Volumetric Flask ØWeigh the clean, dry flask to the nearest milligram. ØThen fill to the mark with equilibrated water and reweigh. ØWith the aid of the table, calculate the volume contained.
ØWeigh the flask and its contents to the nearest milligram. The difference between this mass and the initial value is the mass of water delivered. Convert this mass to the true volume. Subtract the apparent volume from the true volume. ØThis difference is the correction that should be applied to the apparent volume to give the true volume. ØRepeat the calibration until agreement within 60. 02 m. L is achieved. Calibrating a Volumetric Flask ØWeigh the clean, dry flask to the nearest milligram. ØThen fill to the mark with equilibrated water and reweigh. ØWith the aid of the table, calculate the volume contained.
 2 I The laboratory notebook It is needed to record measurements and observations concerning an analysis. It should be permanently bound with consecutively numbered pages. Maintaining a Laboratory Notebook 1. Record all data and observations directly into the notebook in ink. 2. Supply each entry or series of entries with a heading or label. 3. Date each page of the notebook as it is used. 4. Never attempt to erase or obliterate an incorrect entry. 5. Never remove a page from the notebook. Draw diagonal lines across any page that is to be disregarded. Provide a brief rationale for disregarding the page.
2 I The laboratory notebook It is needed to record measurements and observations concerning an analysis. It should be permanently bound with consecutively numbered pages. Maintaining a Laboratory Notebook 1. Record all data and observations directly into the notebook in ink. 2. Supply each entry or series of entries with a heading or label. 3. Date each page of the notebook as it is used. 4. Never attempt to erase or obliterate an incorrect entry. 5. Never remove a page from the notebook. Draw diagonal lines across any page that is to be disregarded. Provide a brief rationale for disregarding the page.
 Notebook Format In one convention, data and observations are recorded on consecutive pages as they occur. The completed analysis is then summarized on the next available page spread. The first of these two facing pages should contain the following entries: 1. The title of the experiment. 2. A brief statement of the principles on which the analysis is based. 3. A complete summary of the weighing, volumetric, and/or instrument response data needed to calculate the results. 4. A report of the best value for the set and a statement of its precision. The second page should contain the following items: 1. Equations for the principal reactions in the analysis. 2. An equation showing how the results were calculated. 3. A summary of observations that appear to bear on the validity.
Notebook Format In one convention, data and observations are recorded on consecutive pages as they occur. The completed analysis is then summarized on the next available page spread. The first of these two facing pages should contain the following entries: 1. The title of the experiment. 2. A brief statement of the principles on which the analysis is based. 3. A complete summary of the weighing, volumetric, and/or instrument response data needed to calculate the results. 4. A report of the best value for the set and a statement of its precision. The second page should contain the following items: 1. Equations for the principal reactions in the analysis. 2. An equation showing how the results were calculated. 3. A summary of observations that appear to bear on the validity.
 Figure 2 -24 Laboratory notebook data page.
Figure 2 -24 Laboratory notebook data page.
 2 J Safety in the laboratory 1. Learn the location of the nearest eye fountain, fire blanket, shower, and fire extinguisher. Do not hesitate to use this equipment if the need arises. 2. Wear eye protection at all times. 3. Most of the chemicals in a laboratory are toxic. Avoid contact with these liquids. In the event of such contact, immediately flood the affected area with large quantities of water. 4. NEVER perform an unauthorized experiment. Unauthorized experiments are grounds for disqualification at many institutions. 5. Never work alone in the laboratory. Always be certain that someone is within earshot. 6. Never bring food or beverages into the laboratory. NEVER drink from laboratory glassware. NEVER smoke in the laboratory.
2 J Safety in the laboratory 1. Learn the location of the nearest eye fountain, fire blanket, shower, and fire extinguisher. Do not hesitate to use this equipment if the need arises. 2. Wear eye protection at all times. 3. Most of the chemicals in a laboratory are toxic. Avoid contact with these liquids. In the event of such contact, immediately flood the affected area with large quantities of water. 4. NEVER perform an unauthorized experiment. Unauthorized experiments are grounds for disqualification at many institutions. 5. Never work alone in the laboratory. Always be certain that someone is within earshot. 6. Never bring food or beverages into the laboratory. NEVER drink from laboratory glassware. NEVER smoke in the laboratory.
 7. Always use a bulb or other device to draw liquids into a pipet. NEVER pipet by mouth. 8. Wear adequate foot covering (no sandals). Confine long hair with a net. A laboratory coat or apron will provide some protection and may be required. 9. Be extremely tentative in touching objects that have been heated because hot glass looks exactly like cold glass. 10. Always fire-polish the ends of freshly cut glass tubing. NEVER attempt to force glass tubing through the hole of a stopper. 11. Use fume hoods whenever toxic or noxious gases are likely to be evolved. Be cautious in testing for odors. 12. Notify your instructor immediately in the event of an injury. 13. Dispose off solutions and chemicals as instructed. It is illegal to flush solutions containing heavy metal ions or organic liquids down the drain in most localities.
7. Always use a bulb or other device to draw liquids into a pipet. NEVER pipet by mouth. 8. Wear adequate foot covering (no sandals). Confine long hair with a net. A laboratory coat or apron will provide some protection and may be required. 9. Be extremely tentative in touching objects that have been heated because hot glass looks exactly like cold glass. 10. Always fire-polish the ends of freshly cut glass tubing. NEVER attempt to force glass tubing through the hole of a stopper. 11. Use fume hoods whenever toxic or noxious gases are likely to be evolved. Be cautious in testing for odors. 12. Notify your instructor immediately in the event of an injury. 13. Dispose off solutions and chemicals as instructed. It is illegal to flush solutions containing heavy metal ions or organic liquids down the drain in most localities.


