cc1ecce50517dd1184542d820413a8ae.ppt
- Количество слайдов: 81
 Chapter 17 Properties of Atoms and the Periodic Table Section 3 Page 516 -
Chapter 17 Properties of Atoms and the Periodic Table Section 3 Page 516 -
 Atomic Models • • • Ancient Greek- “cannot be divided” John Dalton’s Theory- simple sphere Thompson’s Model- plum pudding Rutherford- Electron/Proton Model Bohr- Orbital Model/ Neutron Modern – electron Cloud Model – Protons +1 charge, 1 amu – Neutrons 0 charge, 1 amu – Electrons -1 charge, negligible mass
Atomic Models • • • Ancient Greek- “cannot be divided” John Dalton’s Theory- simple sphere Thompson’s Model- plum pudding Rutherford- Electron/Proton Model Bohr- Orbital Model/ Neutron Modern – electron Cloud Model – Protons +1 charge, 1 amu – Neutrons 0 charge, 1 amu – Electrons -1 charge, negligible mass
 Review- Section 1 • Elements are abbreviated in scientific shorthand- first letter or 2 letters of element name. • Atom- smallest piece of matter that still has the properties of the element. • Protons have electrical charge of 1+ • Neutrons do not have an electrical charge • Electrons have electrical charge 1 • Protons and neutrons are made up of smaller particles called quarks. Page 507 -508 • Six quarks are known to exist; the sixth is called the top quark. • Scientists use scaled-up models to represent atoms. • Early models of atoms used a solid sphere • Current models show electrons traveling in specific energy levels around a nucleus of protons and neutrons.
Review- Section 1 • Elements are abbreviated in scientific shorthand- first letter or 2 letters of element name. • Atom- smallest piece of matter that still has the properties of the element. • Protons have electrical charge of 1+ • Neutrons do not have an electrical charge • Electrons have electrical charge 1 • Protons and neutrons are made up of smaller particles called quarks. Page 507 -508 • Six quarks are known to exist; the sixth is called the top quark. • Scientists use scaled-up models to represent atoms. • Early models of atoms used a solid sphere • Current models show electrons traveling in specific energy levels around a nucleus of protons and neutrons.
 Review Section 2 • Mass of the atom composed mostly of the protons and neutrons in the nucleus. • Unit of measurement of mass for atomic particles is atomic mass unit (amu) which is one twelfth the mass of a carbon atom containing 6 protons and 6 neutrons. • Atomic number (Z)the number of protons in an atom; number of protons identifies the element. • The sum of the number of protons and neutrons in the nucleus of an atom is the mass number (atomic mass) (A) • Isotopes- atoms of the same element with different numbers of neutrons. • Different isotopes have different nuclear properties. • Number of neutrons is equal to the mass number minus atomic number.
Review Section 2 • Mass of the atom composed mostly of the protons and neutrons in the nucleus. • Unit of measurement of mass for atomic particles is atomic mass unit (amu) which is one twelfth the mass of a carbon atom containing 6 protons and 6 neutrons. • Atomic number (Z)the number of protons in an atom; number of protons identifies the element. • The sum of the number of protons and neutrons in the nucleus of an atom is the mass number (atomic mass) (A) • Isotopes- atoms of the same element with different numbers of neutrons. • Different isotopes have different nuclear properties. • Number of neutrons is equal to the mass number minus atomic number.
 Identifying Numbers (P 512) • The atoms of each elements contain a unique numbers of protons. • The atomic number of an element is the number of protons in the nucleus of an atom of that element. (Z) • Atoms of an element are identified by the number of protons because this number never changes without changing the identify of the element. The number of electrons equals the number of protons so charge is neutral
Identifying Numbers (P 512) • The atoms of each elements contain a unique numbers of protons. • The atomic number of an element is the number of protons in the nucleus of an atom of that element. (Z) • Atoms of an element are identified by the number of protons because this number never changes without changing the identify of the element. The number of electrons equals the number of protons so charge is neutral
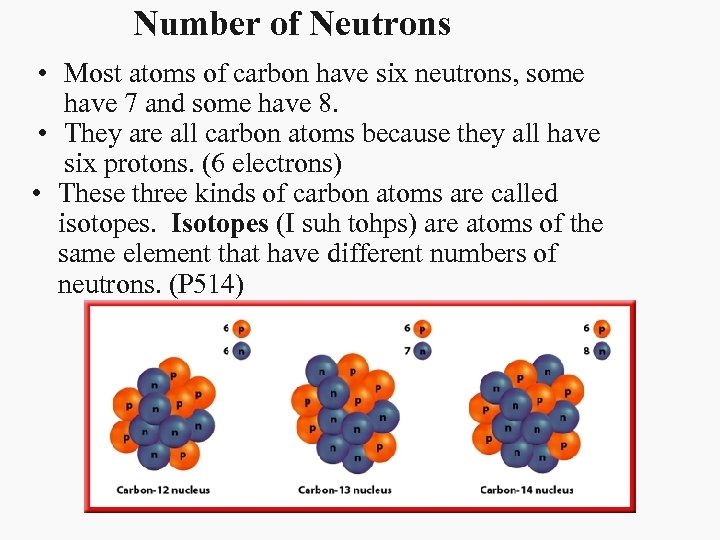 Number of Neutrons • Most atoms of carbon have six neutrons, some have 7 and some have 8. • They are all carbon atoms because they all have six protons. (6 electrons) • These three kinds of carbon atoms are called isotopes. Isotopes (I suh tohps) are atoms of the same element that have different numbers of neutrons. (P 514)
Number of Neutrons • Most atoms of carbon have six neutrons, some have 7 and some have 8. • They are all carbon atoms because they all have six protons. (6 electrons) • These three kinds of carbon atoms are called isotopes. Isotopes (I suh tohps) are atoms of the same element that have different numbers of neutrons. (P 514)
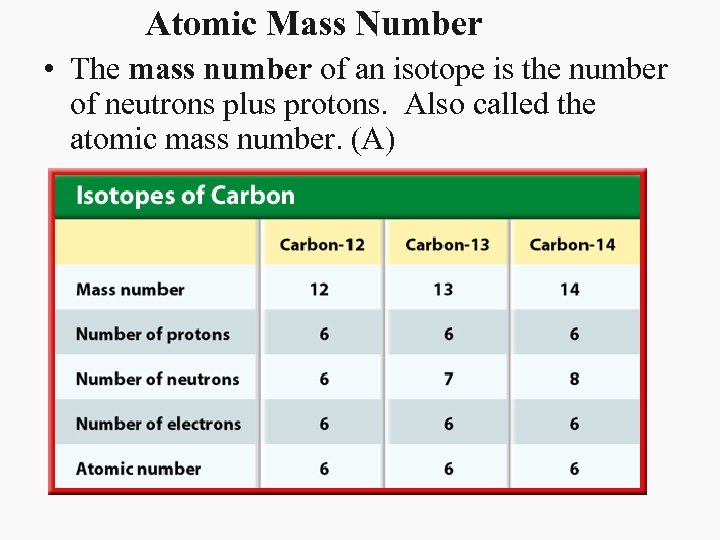 Atomic Mass Number • The mass number of an isotope is the number of neutrons plus protons. Also called the atomic mass number. (A)
Atomic Mass Number • The mass number of an isotope is the number of neutrons plus protons. Also called the atomic mass number. (A)
 Atomic Number, Atomic Mass, and # of Neutrons • mass number = #protons + # neutrons • atomic number = # protons • Number of neutrons = mass number – atomic number • Number of neutrons = A – Z
Atomic Number, Atomic Mass, and # of Neutrons • mass number = #protons + # neutrons • atomic number = # protons • Number of neutrons = mass number – atomic number • Number of neutrons = A – Z
 Symbols for the Elements • The symbols for the elements are either oneor two- letter abbreviations, often based on the element name. • For example, V is the symbol for vanadium, and Sc is the symbol for scandium. • Sometimes the symbols don’t match the names. • In those cases, the symbol might come from Greek or Latin or German names for the elements.
Symbols for the Elements • The symbols for the elements are either oneor two- letter abbreviations, often based on the element name. • For example, V is the symbol for vanadium, and Sc is the symbol for scandium. • Sometimes the symbols don’t match the names. • In those cases, the symbol might come from Greek or Latin or German names for the elements.
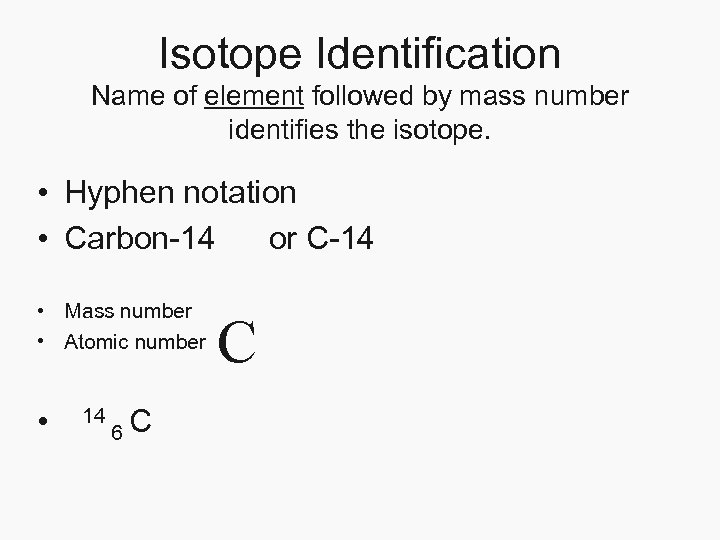 Isotope Identification Name of element followed by mass number identifies the isotope. • Hyphen notation • Carbon-14 or C-14 • Mass number • Atomic number • 14 6 C C
Isotope Identification Name of element followed by mass number identifies the isotope. • Hyphen notation • Carbon-14 or C-14 • Mass number • Atomic number • 14 6 C C
 Average Atomic Mass • Weighted average of the atomic masses of all the isotopes. • Weighted on the relative abundance of the isotopes. • Periodic table gives average atomic mass • Average atomic mass is closest to the element’s most abundant isotope.
Average Atomic Mass • Weighted average of the atomic masses of all the isotopes. • Weighted on the relative abundance of the isotopes. • Periodic table gives average atomic mass • Average atomic mass is closest to the element’s most abundant isotope.
 Find the number • How many neutrons in Iodine-131? • What is the atomic mass number of a copper isotope with 34 neutrons? • Worksheet
Find the number • How many neutrons in Iodine-131? • What is the atomic mass number of a copper isotope with 34 neutrons? • Worksheet
 Development of the Periodic Table– Mendeleev’s Table of Elements • A Russian chemist, Dmitri Mendeleev (men duh LAY uhf), published his first version of the periodic table 1869. • When Mendeleev arranged the elements in order of increasing atomic mass, he saw a pattern. • He noticed repeating patterns of properties.
Development of the Periodic Table– Mendeleev’s Table of Elements • A Russian chemist, Dmitri Mendeleev (men duh LAY uhf), published his first version of the periodic table 1869. • When Mendeleev arranged the elements in order of increasing atomic mass, he saw a pattern. • He noticed repeating patterns of properties.
 Development of the Periodic Table– Mendeleev’s Table of Elements • To make his table work, Mendeleev had to leave three gaps for missing elements. • Based on the groupings in his table, he predicted the properties for the missing elements. • Within 15 years, all three elements— gallium, scandium, and germanium— were discovered. • The most important characteristic of a good scientific theory is the ability to predict future discoveries.
Development of the Periodic Table– Mendeleev’s Table of Elements • To make his table work, Mendeleev had to leave three gaps for missing elements. • Based on the groupings in his table, he predicted the properties for the missing elements. • Within 15 years, all three elements— gallium, scandium, and germanium— were discovered. • The most important characteristic of a good scientific theory is the ability to predict future discoveries.
 Moseley’s Contribution • Although Mendeleev’s table correctly organized most of the elements, a few elements (cobalt and nickel, tellurium and iodine) seemed out of place. • In the early twentieth century, the English physicist Henry Moseley realized that Mendeleev’s table could be improved by arranging the elements according to atomic number rather than atomic mass.
Moseley’s Contribution • Although Mendeleev’s table correctly organized most of the elements, a few elements (cobalt and nickel, tellurium and iodine) seemed out of place. • In the early twentieth century, the English physicist Henry Moseley realized that Mendeleev’s table could be improved by arranging the elements according to atomic number rather than atomic mass.
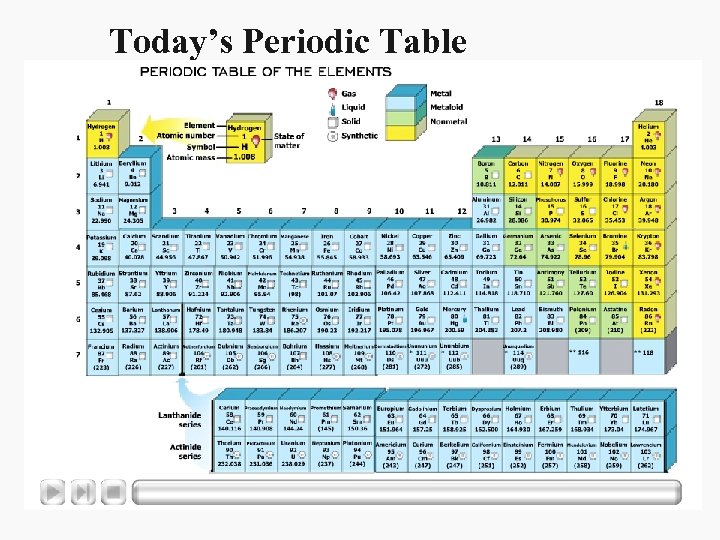 Today’s Periodic Table
Today’s Periodic Table
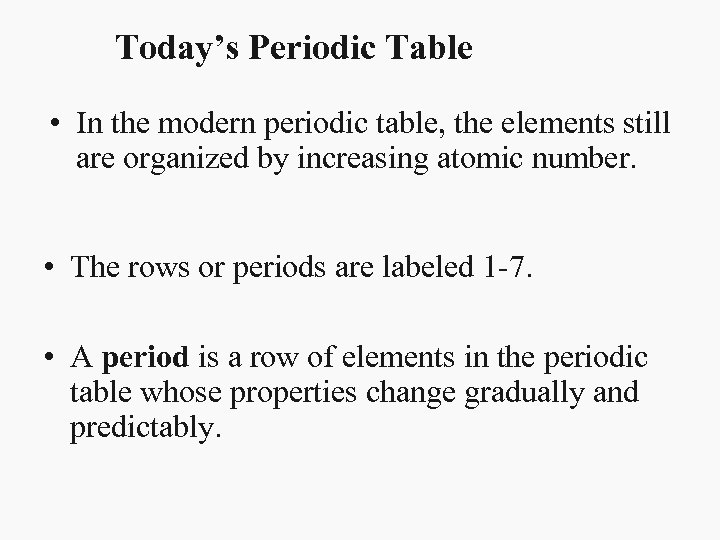 Today’s Periodic Table • In the modern periodic table, the elements still are organized by increasing atomic number. • The rows or periods are labeled 1 -7. • A period is a row of elements in the periodic table whose properties change gradually and predictably.
Today’s Periodic Table • In the modern periodic table, the elements still are organized by increasing atomic number. • The rows or periods are labeled 1 -7. • A period is a row of elements in the periodic table whose properties change gradually and predictably.
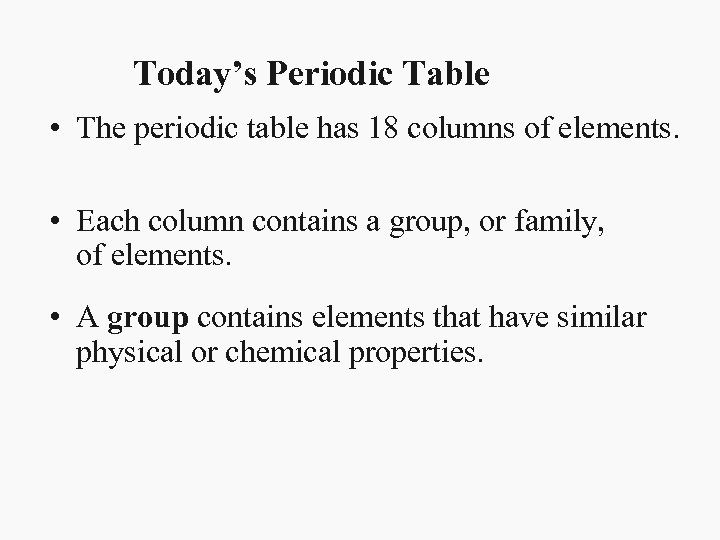 Today’s Periodic Table • The periodic table has 18 columns of elements. • Each column contains a group, or family, of elements. • A group contains elements that have similar physical or chemical properties.
Today’s Periodic Table • The periodic table has 18 columns of elements. • Each column contains a group, or family, of elements. • A group contains elements that have similar physical or chemical properties.
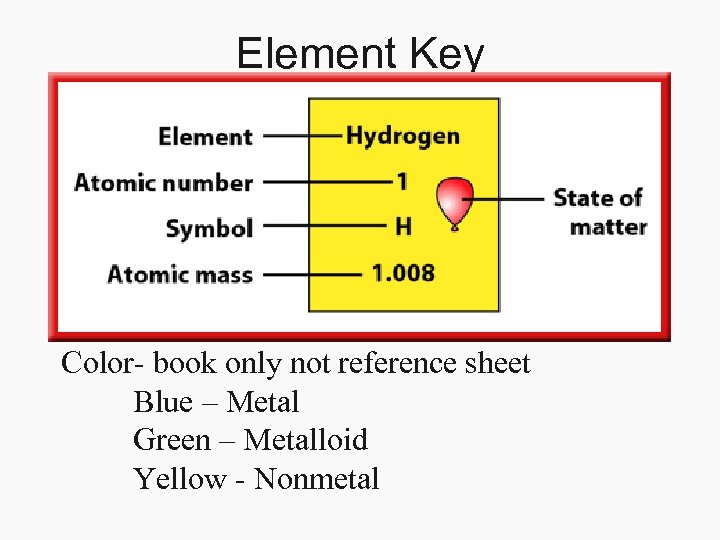 Element Key Color- book only not reference sheet Blue – Metal Green – Metalloid Yellow - Nonmetal
Element Key Color- book only not reference sheet Blue – Metal Green – Metalloid Yellow - Nonmetal
 Common elements - Uncommon Names • • • Sodium Potassium Iron Copper Silver Tin Antimony Tungsten. W Gold Mercury Lead Na K Fe Cu Ag Sn Sb Au Hg Pb Natrium Kalium Ferrum Cuprum Argentum Stannum Stibium Wolfram Aurum Hydragyrum Plumbum
Common elements - Uncommon Names • • • Sodium Potassium Iron Copper Silver Tin Antimony Tungsten. W Gold Mercury Lead Na K Fe Cu Ag Sn Sb Au Hg Pb Natrium Kalium Ferrum Cuprum Argentum Stannum Stibium Wolfram Aurum Hydragyrum Plumbum
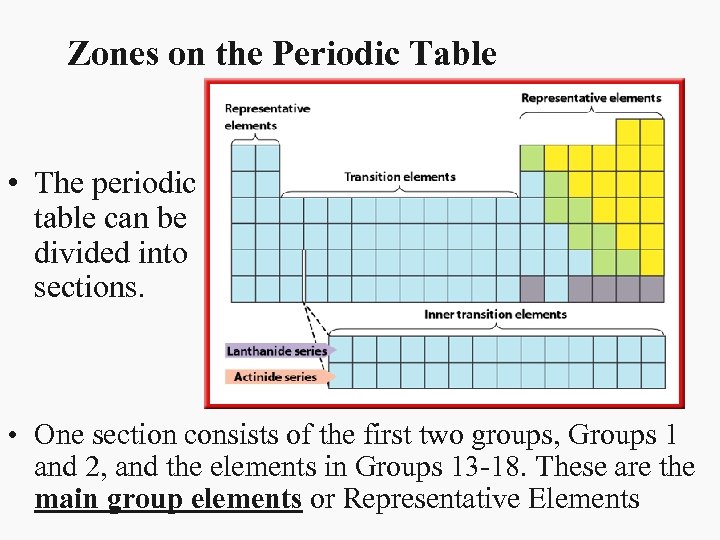 Zones on the Periodic Table • The periodic table can be divided into sections. • One section consists of the first two groups, Groups 1 and 2, and the elements in Groups 13 -18. These are the main group elements or Representative Elements
Zones on the Periodic Table • The periodic table can be divided into sections. • One section consists of the first two groups, Groups 1 and 2, and the elements in Groups 13 -18. These are the main group elements or Representative Elements
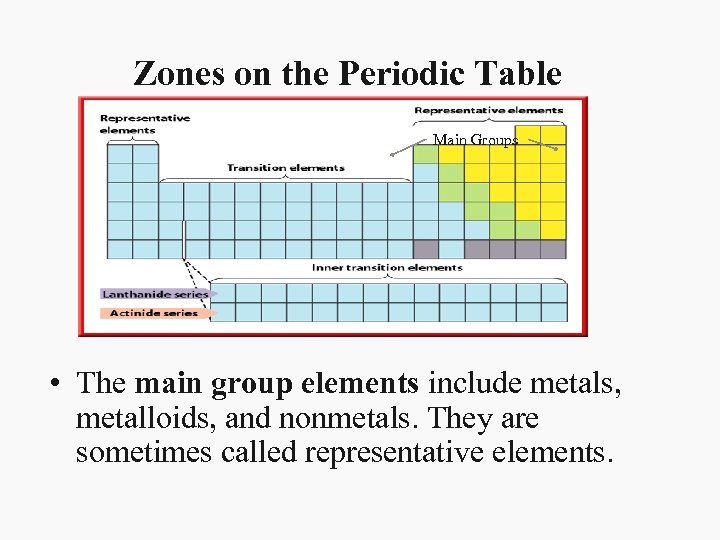 Zones on the Periodic Table Main Groups • The main group elements include metals, metalloids, and nonmetals. They are sometimes called representative elements.
Zones on the Periodic Table Main Groups • The main group elements include metals, metalloids, and nonmetals. They are sometimes called representative elements.
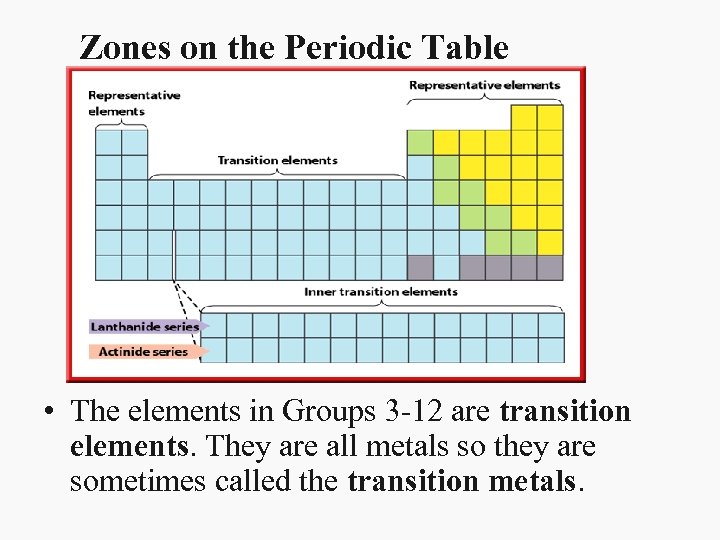 Zones on the Periodic Table • The elements in Groups 3 -12 are transition elements. They are all metals so they are sometimes called the transition metals.
Zones on the Periodic Table • The elements in Groups 3 -12 are transition elements. They are all metals so they are sometimes called the transition metals.
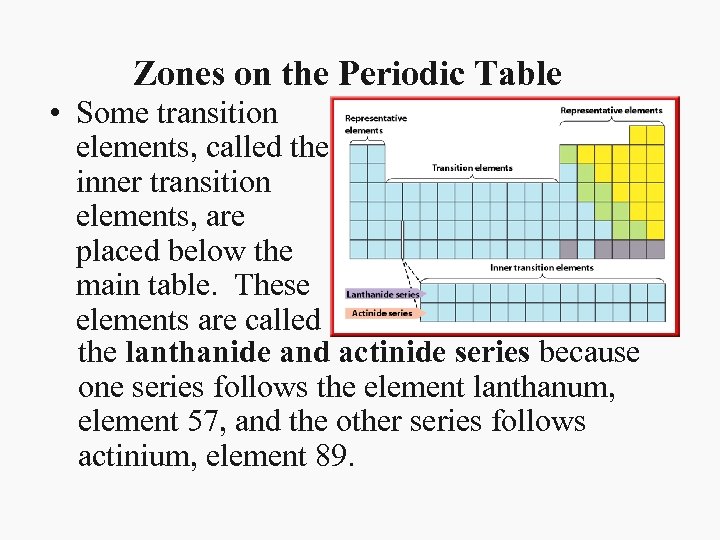 Zones on the Periodic Table • Some transition elements, called the inner transition elements, are placed below the main table. These elements are called the lanthanide and actinide series because one series follows the element lanthanum, element 57, and the other series follows actinium, element 89.
Zones on the Periodic Table • Some transition elements, called the inner transition elements, are placed below the main table. These elements are called the lanthanide and actinide series because one series follows the element lanthanum, element 57, and the other series follows actinium, element 89.
 Electron Configuration • Electrons within the electron cloud have different energy levels. • Each electron is assigned 4 different “quantum numbers”. No two electrons within an atom can have the same quantum numbers. – Energy Level quantum number, there are 7 levels (1 -7) – Shape quantum number -suborbits for electrons s, p, d, f – Orientation quantum number – Spin quantum number- right handed or left handed
Electron Configuration • Electrons within the electron cloud have different energy levels. • Each electron is assigned 4 different “quantum numbers”. No two electrons within an atom can have the same quantum numbers. – Energy Level quantum number, there are 7 levels (1 -7) – Shape quantum number -suborbits for electrons s, p, d, f – Orientation quantum number – Spin quantum number- right handed or left handed
 Electron Energy Levels • Energy Levels 1 -7 – Lowest energy near the nucleus – Highest energy far from the nucleus – Energy Levels Overlap • Shapes- s, p, d, f – s- 2 electrons – p- 6 electrons – d- 10 electrons – f- 14 electrons • Spin - right or left
Electron Energy Levels • Energy Levels 1 -7 – Lowest energy near the nucleus – Highest energy far from the nucleus – Energy Levels Overlap • Shapes- s, p, d, f – s- 2 electrons – p- 6 electrons – d- 10 electrons – f- 14 electrons • Spin - right or left
 Review Periodic Table • Mendeleev- arranged in order of increasing atomic mass. • Moseley – in order of increasing atomic number • Atomic mass = #protons + # neutrons, atomic number = #protons • Period- row of elements, properties change gradually • Group – column of elements, similar properties • Element key – name, symbol, atomic number, average atomic mass • Zones- main group elements, transition metals • Electron configuration – all the elements in a group have the same configuration of electrons in their outer shell
Review Periodic Table • Mendeleev- arranged in order of increasing atomic mass. • Moseley – in order of increasing atomic number • Atomic mass = #protons + # neutrons, atomic number = #protons • Period- row of elements, properties change gradually • Group – column of elements, similar properties • Element key – name, symbol, atomic number, average atomic mass • Zones- main group elements, transition metals • Electron configuration – all the elements in a group have the same configuration of electrons in their outer shell
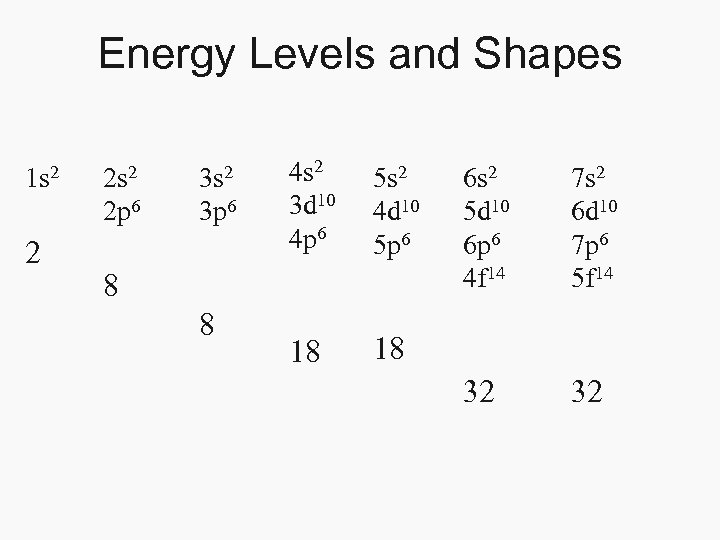 Energy Levels and Shapes 1 s 2 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 18 18 8 8 6 s 2 5 d 10 6 p 6 4 f 14 7 s 2 6 d 10 7 p 6 5 f 14 32 32
Energy Levels and Shapes 1 s 2 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 18 18 8 8 6 s 2 5 d 10 6 p 6 4 f 14 7 s 2 6 d 10 7 p 6 5 f 14 32 32
 Electrons and Periods • Each period of the periodic table represents a different energy level and a different number of total electrons. • • Period 1 - Energy Level 1 – up to 2 electrons 2 elements Period 2 – Energy Level 2 - up to 8 electrons 8 elements Period 3 - energy Level 3 – up to 8 electrons 8 elements Period 4 - energy Level 4 - up to 18 electrons including 10 from level 3 18 elements • Period 5 - Energy Level 5 up to 18 electrons including 10 from level 4 18 elements • Period 6 - Energy Level 6 up to 32 electrons including 14 from level 5 32 Elements • Period 7 - Energy Level 7 up to 32 electrons including 14 from level 6 32 elements
Electrons and Periods • Each period of the periodic table represents a different energy level and a different number of total electrons. • • Period 1 - Energy Level 1 – up to 2 electrons 2 elements Period 2 – Energy Level 2 - up to 8 electrons 8 elements Period 3 - energy Level 3 – up to 8 electrons 8 elements Period 4 - energy Level 4 - up to 18 electrons including 10 from level 3 18 elements • Period 5 - Energy Level 5 up to 18 electrons including 10 from level 4 18 elements • Period 6 - Energy Level 6 up to 32 electrons including 14 from level 5 32 Elements • Period 7 - Energy Level 7 up to 32 electrons including 14 from level 6 32 elements
 Electrons in groups • Group 1 – 1 electron in outer shell s 1 • Group 2 – 2 electrons in outer shell s 2 • Group 3 -12 many electrons in outer shell • • • Group 13 - 3 electrons in outer shell Group 14 – 4 electrons in outer shell Group 15 – 5 electrons in outer shell Group 16 - 6 electrons in outer shell Group 17 – 7 electrons in outer shell Group 18 - 8 electrons in outer shell
Electrons in groups • Group 1 – 1 electron in outer shell s 1 • Group 2 – 2 electrons in outer shell s 2 • Group 3 -12 many electrons in outer shell • • • Group 13 - 3 electrons in outer shell Group 14 – 4 electrons in outer shell Group 15 – 5 electrons in outer shell Group 16 - 6 electrons in outer shell Group 17 – 7 electrons in outer shell Group 18 - 8 electrons in outer shell
 Electron Dot Diagrams Page 522 H Li Be Na Mg K Ca Rb Sr Cs Ba Fr Ra B Al C Si N P O S F Cl He Ne Ar Kr Xe Rn
Electron Dot Diagrams Page 522 H Li Be Na Mg K Ca Rb Sr Cs Ba Fr Ra B Al C Si N P O S F Cl He Ne Ar Kr Xe Rn
 Review Electron Configuration • The properties of an element are determined by the arrangement of electrons in the outer energy level. • The number of electrons in the outer energy level of main group elements can be determined by the group number of the element. • Group 1 and 2: number of electrons = group number. • Group 13 -18: number of electrons = group number - 10
Review Electron Configuration • The properties of an element are determined by the arrangement of electrons in the outer energy level. • The number of electrons in the outer energy level of main group elements can be determined by the group number of the element. • Group 1 and 2: number of electrons = group number. • Group 13 -18: number of electrons = group number - 10
 Liquids and Synthetics • Two elements on the periodic table are liquids at room temperature. • Their logo is a drop. What are they? • Elements that do not occur naturally on Earth are marked with a bull’s-eye logo. • These are synthetic elements.
Liquids and Synthetics • Two elements on the periodic table are liquids at room temperature. • Their logo is a drop. What are they? • Elements that do not occur naturally on Earth are marked with a bull’s-eye logo. • These are synthetic elements.
 Gases • All the gases except hydrogen are on the right side of the table. • They are marked with a balloon logo. • Most of the other elements are solids at room temperature and are marked with a cube.
Gases • All the gases except hydrogen are on the right side of the table. • They are marked with a balloon logo. • Most of the other elements are solids at room temperature and are marked with a cube.
 Diatomic Gases • • Br 2 - bromine I 2 – iodine N 2 – nitrogen Cl 2 - chlorine H 2 – hydrogen O 2 – oxygen F 2 – fluorine Mr. Br. INCl. HOF
Diatomic Gases • • Br 2 - bromine I 2 – iodine N 2 – nitrogen Cl 2 - chlorine H 2 – hydrogen O 2 – oxygen F 2 – fluorine Mr. Br. INCl. HOF
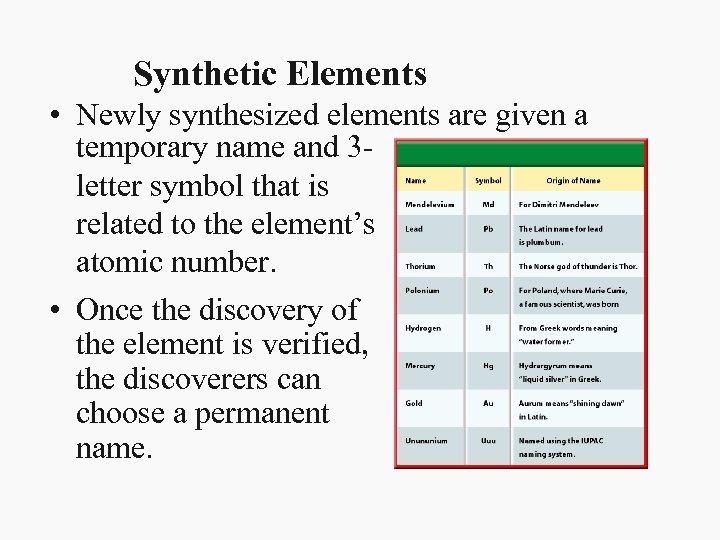 Synthetic Elements • Newly synthesized elements are given a temporary name and 3 letter symbol that is related to the element’s atomic number. • Once the discovery of the element is verified, the discoverers can choose a permanent name.
Synthetic Elements • Newly synthesized elements are given a temporary name and 3 letter symbol that is related to the element’s atomic number. • Once the discovery of the element is verified, the discoverers can choose a permanent name.
 In the beginning • When God started building the atoms of elements he began with a design. We may have discovered that design. • He began adding electrons, protons and neutrons according to the design. • The first element had 1 electron and 1 proton and either 0, 1 or 2 neutrons. • The second element had 2 electrons and 2 protons and either 1, 2 or 3 neutrons.
In the beginning • When God started building the atoms of elements he began with a design. We may have discovered that design. • He began adding electrons, protons and neutrons according to the design. • The first element had 1 electron and 1 proton and either 0, 1 or 2 neutrons. • The second element had 2 electrons and 2 protons and either 1, 2 or 3 neutrons.
 Elements and Their Properties Chapter 19, page 570 • • Metals Group 1 Group 2 Group 13 -17 – – – Boron Carbon Nitrogen Oxygen Fluorine • Group 18 – Noble Gases
Elements and Their Properties Chapter 19, page 570 • • Metals Group 1 Group 2 Group 13 -17 – – – Boron Carbon Nitrogen Oxygen Fluorine • Group 18 – Noble Gases
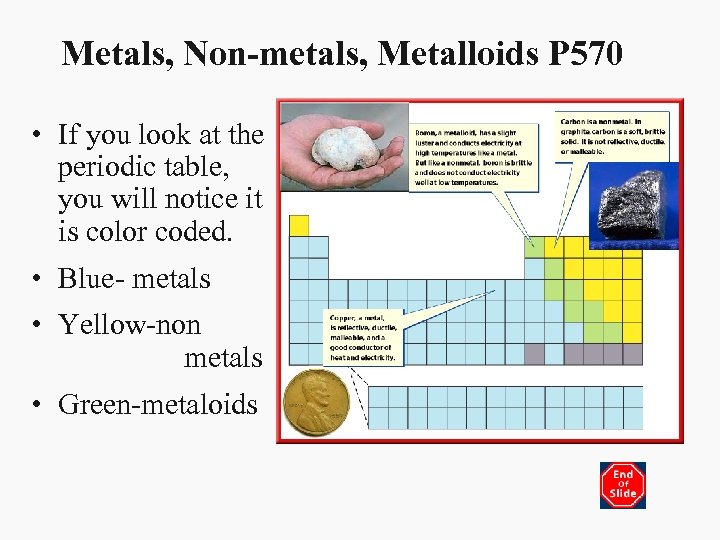 Metals, Non-metals, Metalloids P 570 • If you look at the periodic table, you will notice it is color coded. • Blue- metals • Yellow-non metals • Green-metaloids
Metals, Non-metals, Metalloids P 570 • If you look at the periodic table, you will notice it is color coded. • Blue- metals • Yellow-non metals • Green-metaloids
 Metals P 570 • With the exception of mercury, all the metals are solids, most with high melting points. • A metal is an element that has luster, is a good conductor of heat and electricity, is malleable, and is ductile. • The ability to reflect light is a property of metals called luster.
Metals P 570 • With the exception of mercury, all the metals are solids, most with high melting points. • A metal is an element that has luster, is a good conductor of heat and electricity, is malleable, and is ductile. • The ability to reflect light is a property of metals called luster.
 Nonmetals • Nonmetals are usually gases or brittle solids at room temperature and poor conductors of heat and electricity. • There are only 17 nonmetals, but they include many elements that are essential for life —carbon, sulfur, nitrogen, oxygen, phosphorus, and iodine.
Nonmetals • Nonmetals are usually gases or brittle solids at room temperature and poor conductors of heat and electricity. • There are only 17 nonmetals, but they include many elements that are essential for life —carbon, sulfur, nitrogen, oxygen, phosphorus, and iodine.
 Metalloids • The elements between metals and nonmetals on the periodic table are called metalloids. • A metalloid is an element that shares some properties with metals and some with nonmetals. • Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium, Astatine
Metalloids • The elements between metals and nonmetals on the periodic table are called metalloids. • A metalloid is an element that shares some properties with metals and some with nonmetals. • Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium, Astatine
 Groups 1 and 2 • Groups 1 and 2 are always found in nature combined with other elements. • They’re called active metals because of their readiness to form new substances with other elements. • They are all metals except hydrogen, the first element in Group 1. • Although hydrogen is placed in Group 1, it shares properties with the elements in Group 1 and Group 17. Group 1 atoms have 1 electron in the outer most suborbits. s 1 Group 2 atoms have 2 electrons in the outer most suborbits. s 2
Groups 1 and 2 • Groups 1 and 2 are always found in nature combined with other elements. • They’re called active metals because of their readiness to form new substances with other elements. • They are all metals except hydrogen, the first element in Group 1. • Although hydrogen is placed in Group 1, it shares properties with the elements in Group 1 and Group 17. Group 1 atoms have 1 electron in the outer most suborbits. s 1 Group 2 atoms have 2 electrons in the outer most suborbits. s 2
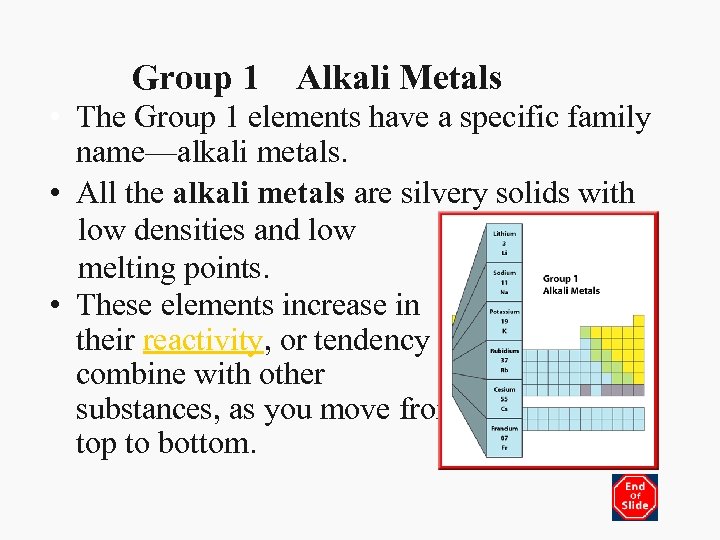 Group 1 Alkali Metals • The Group 1 elements have a specific family name—alkali metals. • All the alkali metals are silvery solids with low densities and low melting points. • These elements increase in their reactivity, or tendency to combine with other substances, as you move from top to bottom.
Group 1 Alkali Metals • The Group 1 elements have a specific family name—alkali metals. • All the alkali metals are silvery solids with low densities and low melting points. • These elements increase in their reactivity, or tendency to combine with other substances, as you move from top to bottom.
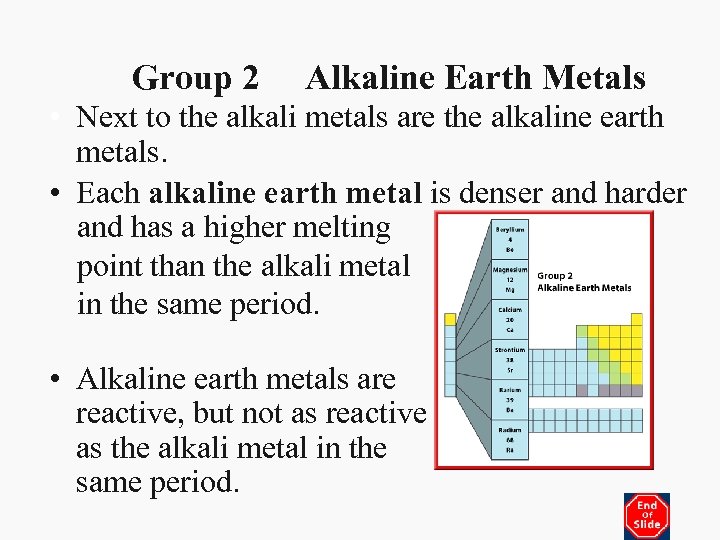 Group 2 Alkaline Earth Metals • Next to the alkali metals are the alkaline earth metals. • Each alkaline earth metal is denser and harder and has a higher melting point than the alkali metal in the same period. • Alkaline earth metals are reactive, but not as reactive as the alkali metal in the same period.
Group 2 Alkaline Earth Metals • Next to the alkali metals are the alkaline earth metals. • Each alkaline earth metal is denser and harder and has a higher melting point than the alkali metal in the same period. • Alkaline earth metals are reactive, but not as reactive as the alkali metal in the same period.
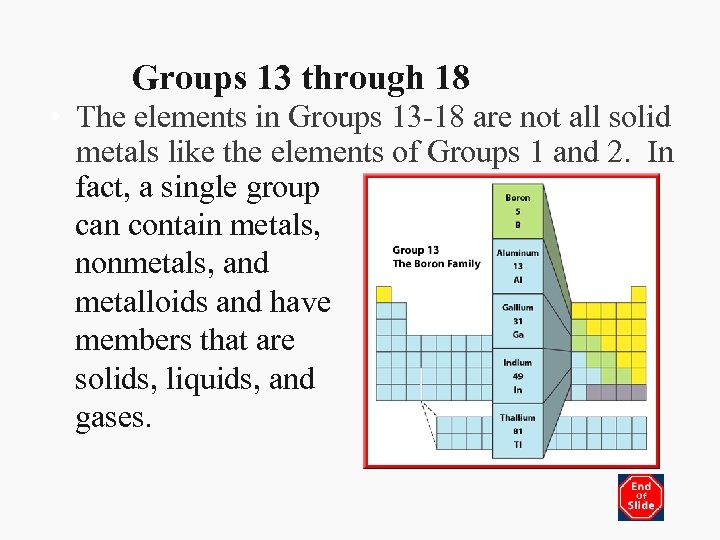 Groups 13 through 18 • The elements in Groups 13 -18 are not all solid metals like the elements of Groups 1 and 2. In fact, a single group can contain metals, nonmetals, and metalloids and have members that are solids, liquids, and gases.
Groups 13 through 18 • The elements in Groups 13 -18 are not all solid metals like the elements of Groups 1 and 2. In fact, a single group can contain metals, nonmetals, and metalloids and have members that are solids, liquids, and gases.
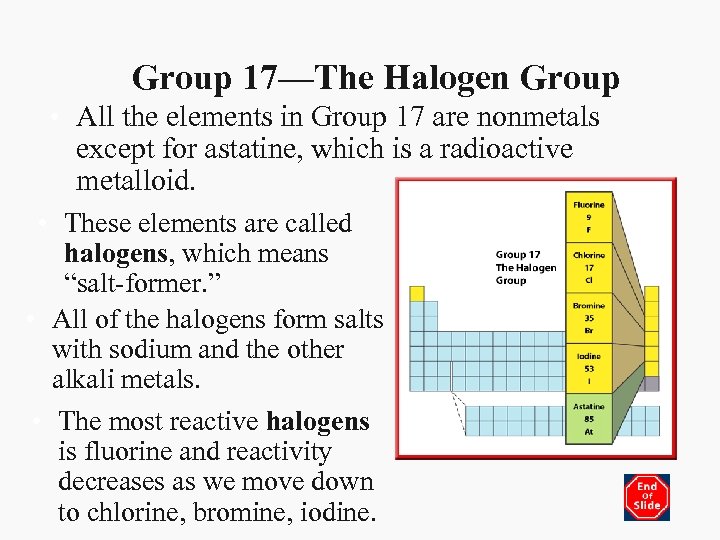 Group 17—The Halogen Group • All the elements in Group 17 are nonmetals except for astatine, which is a radioactive metalloid. • These elements are called halogens, which means “salt-former. ” • All of the halogens form salts with sodium and the other alkali metals. • The most reactive halogens is fluorine and reactivity decreases as we move down to chlorine, bromine, iodine.
Group 17—The Halogen Group • All the elements in Group 17 are nonmetals except for astatine, which is a radioactive metalloid. • These elements are called halogens, which means “salt-former. ” • All of the halogens form salts with sodium and the other alkali metals. • The most reactive halogens is fluorine and reactivity decreases as we move down to chlorine, bromine, iodine.
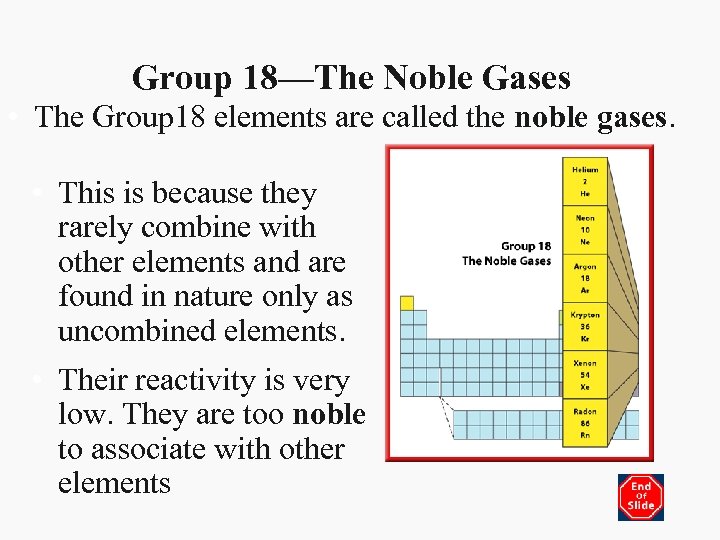 Group 18—The Noble Gases • The Group 18 elements are called the noble gases. • This is because they rarely combine with other elements and are found in nature only as uncombined elements. • Their reactivity is very low. They are too noble to associate with other elements
Group 18—The Noble Gases • The Group 18 elements are called the noble gases. • This is because they rarely combine with other elements and are found in nature only as uncombined elements. • Their reactivity is very low. They are too noble to associate with other elements
 Group 18—The Noble Gases • Helium is less dense than air, so it’s great for all kinds of balloons. • Even though hydrogen is lighter than helium, helium is preferred because it will not burn (because it is noble). • Helium balloons lift instruments into the upper atmosphere to measure atmospheric conditions.
Group 18—The Noble Gases • Helium is less dense than air, so it’s great for all kinds of balloons. • Even though hydrogen is lighter than helium, helium is preferred because it will not burn (because it is noble). • Helium balloons lift instruments into the upper atmosphere to measure atmospheric conditions.
 Uses for the Noble Gases • The “neon” lights you see in advertising signs can contain any of the noble gases, not just neon. • Electricity passing through the glass tubes make the gas glow. Each noble gas glows a different color. • Helium glows yellow, neon glows red-orange, and argon produces a bluish-violet color.
Uses for the Noble Gases • The “neon” lights you see in advertising signs can contain any of the noble gases, not just neon. • Electricity passing through the glass tubes make the gas glow. Each noble gas glows a different color. • Helium glows yellow, neon glows red-orange, and argon produces a bluish-violet color.
 Uses for the Noble Gases • At the bottom of the group is radon, a radioactive gas produced naturally as uranium decays in rocks and soil. • If radon seeps into a home, the gas can be harmful because it continues to emit radiation. • When people breathe gas over a period of time, it can cause lung cancer. • To sell your house you must check for radon gas.
Uses for the Noble Gases • At the bottom of the group is radon, a radioactive gas produced naturally as uranium decays in rocks and soil. • If radon seeps into a home, the gas can be harmful because it continues to emit radiation. • When people breathe gas over a period of time, it can cause lung cancer. • To sell your house you must check for radon gas.
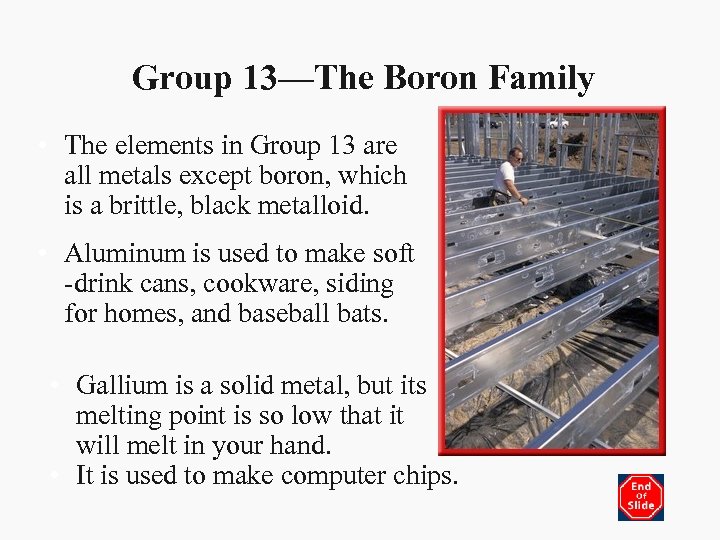 Group 13—The Boron Family • The elements in Group 13 are all metals except boron, which is a brittle, black metalloid. • Aluminum is used to make soft -drink cans, cookware, siding for homes, and baseball bats. • Gallium is a solid metal, but its melting point is so low that it will melt in your hand. • It is used to make computer chips.
Group 13—The Boron Family • The elements in Group 13 are all metals except boron, which is a brittle, black metalloid. • Aluminum is used to make soft -drink cans, cookware, siding for homes, and baseball bats. • Gallium is a solid metal, but its melting point is so low that it will melt in your hand. • It is used to make computer chips.
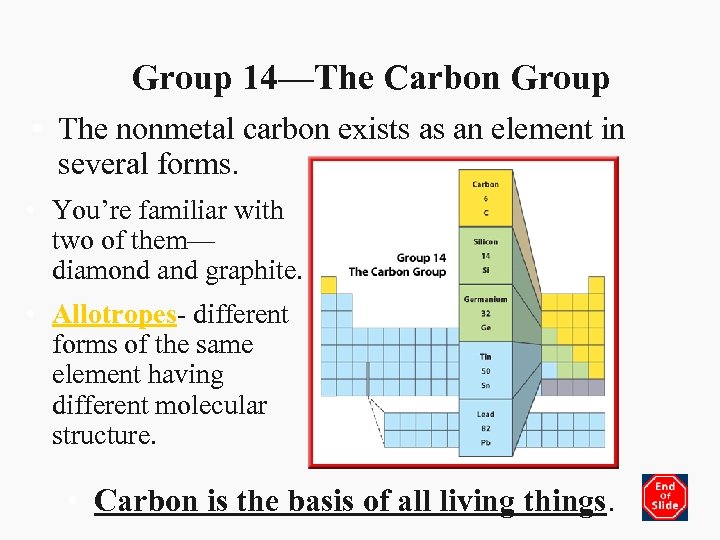 Group 14—The Carbon Group • The nonmetal carbon exists as an element in several forms. • You’re familiar with two of them— diamond and graphite. • Allotropes- different forms of the same element having different molecular structure. • Carbon is the basis of all living things.
Group 14—The Carbon Group • The nonmetal carbon exists as an element in several forms. • You’re familiar with two of them— diamond and graphite. • Allotropes- different forms of the same element having different molecular structure. • Carbon is the basis of all living things.
 Group 14—The Carbon Group Silicon • Carbon is followed by the metalloid silicon, an abundant element contained in sand. • Sand contains ground up particles of minerals such as quartz, which is composed of silicon and oxygen. • Glass is an important product made from sand.
Group 14—The Carbon Group Silicon • Carbon is followed by the metalloid silicon, an abundant element contained in sand. • Sand contains ground up particles of minerals such as quartz, which is composed of silicon and oxygen. • Glass is an important product made from sand.
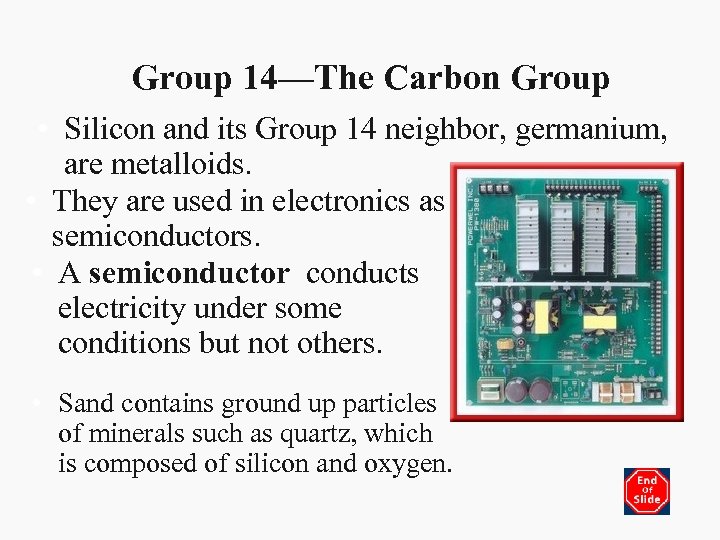 Group 14—The Carbon Group • Silicon and its Group 14 neighbor, germanium, are metalloids. • They are used in electronics as semiconductors. • A semiconductor conducts electricity under some conditions but not others. • Sand contains ground up particles of minerals such as quartz, which is composed of silicon and oxygen.
Group 14—The Carbon Group • Silicon and its Group 14 neighbor, germanium, are metalloids. • They are used in electronics as semiconductors. • A semiconductor conducts electricity under some conditions but not others. • Sand contains ground up particles of minerals such as quartz, which is composed of silicon and oxygen.
 Group 14—The Carbon Group • Tin and lead are the two heaviest elements in Group 14. • Lead is used to protect you during dental X rays, protective shielding around nuclear reactors, and containers used for storing and transporting radioactive materials. • Lead was once used in paints but because it is toxic, lead is no longer widely used. • Lead also is used in car batteries- caution • Tin is used in pewter, toothpaste, and the coating on steel cans used for food.
Group 14—The Carbon Group • Tin and lead are the two heaviest elements in Group 14. • Lead is used to protect you during dental X rays, protective shielding around nuclear reactors, and containers used for storing and transporting radioactive materials. • Lead was once used in paints but because it is toxic, lead is no longer widely used. • Lead also is used in car batteries- caution • Tin is used in pewter, toothpaste, and the coating on steel cans used for food.
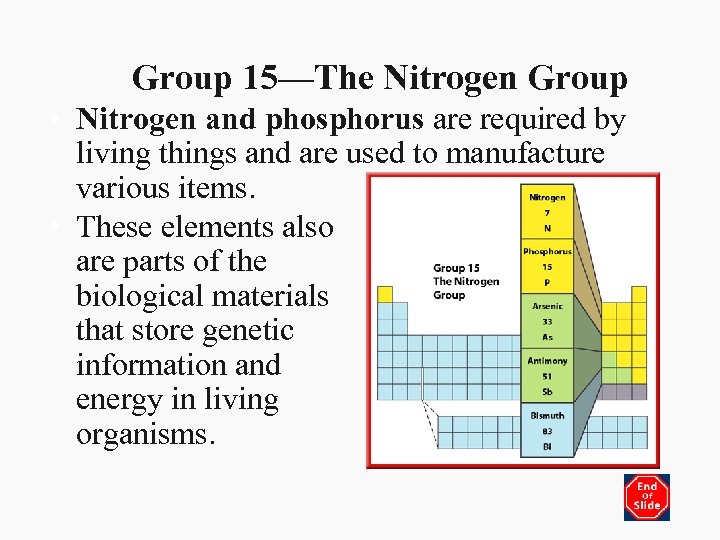 Group 15—The Nitrogen Group • Nitrogen and phosphorus are required by living things and are used to manufacture various items. • These elements also are parts of the biological materials that store genetic information and energy in living organisms.
Group 15—The Nitrogen Group • Nitrogen and phosphorus are required by living things and are used to manufacture various items. • These elements also are parts of the biological materials that store genetic information and energy in living organisms.
 Group 15—The Nitrogen Group • Although almost 80 percent of the air you breathe is nitrogen, plants and animals can’t get the nitrogen needed by breathing nitrogen gas. • Bacteria in the soil must first change nitrogen gas into substances that can be absorbed through the roots of plants. • Then, by eating some plants, nitrogen becomes available to your body.
Group 15—The Nitrogen Group • Although almost 80 percent of the air you breathe is nitrogen, plants and animals can’t get the nitrogen needed by breathing nitrogen gas. • Bacteria in the soil must first change nitrogen gas into substances that can be absorbed through the roots of plants. • Then, by eating some plants, nitrogen becomes available to your body.
 Group 15—The Nitrogen Group • The element phosphorus comes in two forms—white and red. • White phosphorus is so active it can’t be exposed to oxygen in the air or it will burst into flames. • The heads of matches contain the less active red phosphorus, which ignites from the heat produced by friction when the match is struck.
Group 15—The Nitrogen Group • The element phosphorus comes in two forms—white and red. • White phosphorus is so active it can’t be exposed to oxygen in the air or it will burst into flames. • The heads of matches contain the less active red phosphorus, which ignites from the heat produced by friction when the match is struck.
 Group 15—The Nitrogen Group • Phosphorous compounds are essential ingredients for healthy teeth and bones. • Plants also need phosphorus, so it is one of the nutrients in most fertilizers.
Group 15—The Nitrogen Group • Phosphorous compounds are essential ingredients for healthy teeth and bones. • Plants also need phosphorus, so it is one of the nutrients in most fertilizers.
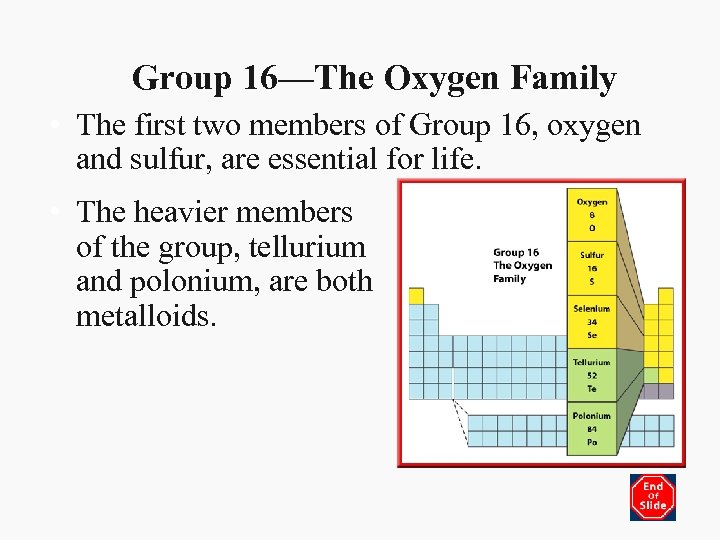 Group 16—The Oxygen Family • The first two members of Group 16, oxygen and sulfur, are essential for life. • The heavier members of the group, tellurium and polonium, are both metalloids.
Group 16—The Oxygen Family • The first two members of Group 16, oxygen and sulfur, are essential for life. • The heavier members of the group, tellurium and polonium, are both metalloids.
 Group 16—The Oxygen Family • About 20 percent of Earth’s atmosphere is the oxygen you breathe. • Oxygen is abundant in Earth’s rocks and minerals because it readily combines with other elements. • Oxygen also is required for combustion to occur.
Group 16—The Oxygen Family • About 20 percent of Earth’s atmosphere is the oxygen you breathe. • Oxygen is abundant in Earth’s rocks and minerals because it readily combines with other elements. • Oxygen also is required for combustion to occur.
 Group 16—The Oxygen Family sulfur • Sulfur is a solid, yellow nonmetal. • Large amounts of sulfur are used to manufacture sulfuric acid, one of the most commonly used chemicals in the world. • Sulfuric acid is a combination of sulfur, hydrogen, and oxygen. • It is used in the manufacture of paints, fertilizers, detergents, synthetic fibers, and rubber.
Group 16—The Oxygen Family sulfur • Sulfur is a solid, yellow nonmetal. • Large amounts of sulfur are used to manufacture sulfuric acid, one of the most commonly used chemicals in the world. • Sulfuric acid is a combination of sulfur, hydrogen, and oxygen. • It is used in the manufacture of paints, fertilizers, detergents, synthetic fibers, and rubber.
 Group 16—The Oxygen Family • Selenium conducts electricity when exposed to light, so it is used in solar cells, light meters and photographic materials. Its most important use is as the lightsensitive component in photocopy machines. • Traces of selenium are also necessary for good health.
Group 16—The Oxygen Family • Selenium conducts electricity when exposed to light, so it is used in solar cells, light meters and photographic materials. Its most important use is as the lightsensitive component in photocopy machines. • Traces of selenium are also necessary for good health.
 Why do elements behave as they do? • To explain why, scientist developed a theory. • Elements behave as they do (react or combine with other elements) because of the position of the electrons. • The electrons in the outer orbits (highest energy level) determine the chemical properties of an element. • 8 electrons in the outer orbits is a “magic” number (full outer orbit)
Why do elements behave as they do? • To explain why, scientist developed a theory. • Elements behave as they do (react or combine with other elements) because of the position of the electrons. • The electrons in the outer orbits (highest energy level) determine the chemical properties of an element. • 8 electrons in the outer orbits is a “magic” number (full outer orbit)
 The “magic” 8 • For elements with atomic number >2, the magic number is 8 electrons in the outer orbits. – s 2 p 6 • The Noble gases have 8 electrons • Elements with fewer electrons combine with other elements in ways that produce 8 electrons in the outer orbits. • Elements with few electrons lose electrons to “make 8”. • Elements with many electrons gain electrons to “make 8”.
The “magic” 8 • For elements with atomic number >2, the magic number is 8 electrons in the outer orbits. – s 2 p 6 • The Noble gases have 8 electrons • Elements with fewer electrons combine with other elements in ways that produce 8 electrons in the outer orbits. • Elements with few electrons lose electrons to “make 8”. • Elements with many electrons gain electrons to “make 8”.
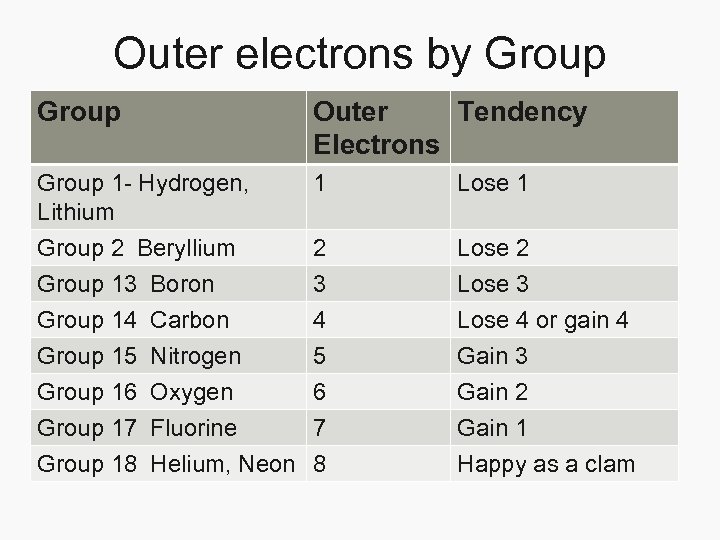 Outer electrons by Group Outer Tendency Electrons Group 1 - Hydrogen, Lithium 1 Lose 1 Group 2 Beryllium Group 13 Boron Group 14 Carbon Group 15 Nitrogen 2 3 4 5 Lose 2 Lose 3 Lose 4 or gain 4 Gain 3 Group 16 Oxygen 6 Group 17 Fluorine 7 Group 18 Helium, Neon 8 Gain 2 Gain 1 Happy as a clam
Outer electrons by Group Outer Tendency Electrons Group 1 - Hydrogen, Lithium 1 Lose 1 Group 2 Beryllium Group 13 Boron Group 14 Carbon Group 15 Nitrogen 2 3 4 5 Lose 2 Lose 3 Lose 4 or gain 4 Gain 3 Group 16 Oxygen 6 Group 17 Fluorine 7 Group 18 Helium, Neon 8 Gain 2 Gain 1 Happy as a clam
 The Metals in the Middle • Groups 3 -12 are called the transition elements. • All of them are metals, so they are sometimes called transition metals. • Across any period from Group 3 through 12, the properties of the elements change less noticeably than they do across a period of representative elements. • Most transition elements are found combined with other elements in ores.
The Metals in the Middle • Groups 3 -12 are called the transition elements. • All of them are metals, so they are sometimes called transition metals. • Across any period from Group 3 through 12, the properties of the elements change less noticeably than they do across a period of representative elements. • Most transition elements are found combined with other elements in ores.
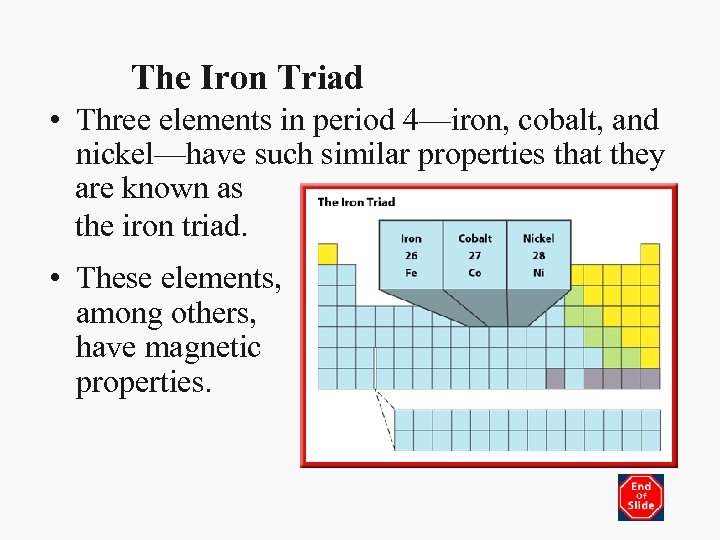 The Iron Triad • Three elements in period 4—iron, cobalt, and nickel—have such similar properties that they are known as the iron triad. • These elements, among others, have magnetic properties.
The Iron Triad • Three elements in period 4—iron, cobalt, and nickel—have such similar properties that they are known as the iron triad. • These elements, among others, have magnetic properties.
 The Iron Triad • Industrial magnets are made from an alloy of nickel, cobalt, and aluminum. • Nickel is used in batteries along with cadmium. • Iron is a necessary part of hemoglobin, the substance that transports oxygen in the blood. • Iron also is mixed with other metals and with carbon to create a variety of steels with different properties.
The Iron Triad • Industrial magnets are made from an alloy of nickel, cobalt, and aluminum. • Nickel is used in batteries along with cadmium. • Iron is a necessary part of hemoglobin, the substance that transports oxygen in the blood. • Iron also is mixed with other metals and with carbon to create a variety of steels with different properties.
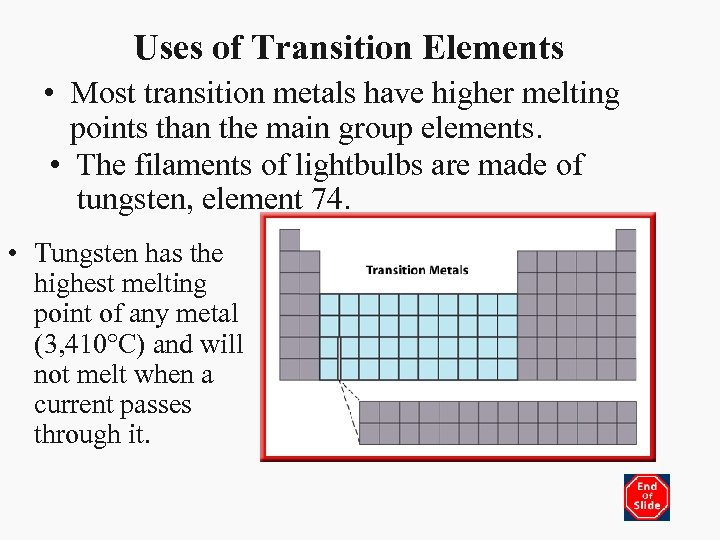 Uses of Transition Elements • Most transition metals have higher melting points than the main group elements. • The filaments of lightbulbs are made of tungsten, element 74. • Tungsten has the highest melting point of any metal (3, 410°C) and will not melt when a current passes through it.
Uses of Transition Elements • Most transition metals have higher melting points than the main group elements. • The filaments of lightbulbs are made of tungsten, element 74. • Tungsten has the highest melting point of any metal (3, 410°C) and will not melt when a current passes through it.
 Chapter 17 Assignments • • Note Taking Worksheet Atomic Mass worksheet Periodic Table worksheet Chapter Review Page 530: 1 -18, 27 Monday- Complete Periodic Table Tuesday – Writing Test Wednesday – Chapter Review Thursday- Friday – Chapter 19 Elements and their properties • Monday – Chapter 19 Note Taking Worksheet • Tuesday - Test
Chapter 17 Assignments • • Note Taking Worksheet Atomic Mass worksheet Periodic Table worksheet Chapter Review Page 530: 1 -18, 27 Monday- Complete Periodic Table Tuesday – Writing Test Wednesday – Chapter Review Thursday- Friday – Chapter 19 Elements and their properties • Monday – Chapter 19 Note Taking Worksheet • Tuesday - Test
 Uses of Transition Elements • Mercury, which has the lowest melting point of any metal (– 39°C), was used in thermometers and in barometers. • Mercury is the only metal that is a liquid at room temperatures. • Like many of the heavy metals, mercury is poisonous to living beings.
Uses of Transition Elements • Mercury, which has the lowest melting point of any metal (– 39°C), was used in thermometers and in barometers. • Mercury is the only metal that is a liquid at room temperatures. • Like many of the heavy metals, mercury is poisonous to living beings.
 Catalytic Converter • The catalytic converter on a car uses platinum, palladium, and rhodium as catalysts to speed up a chemical reaction that removes toxic fumes from the exhaust of the car. • The catalytic converter contains about $200 worth of these metals which can be sold on the black market.
Catalytic Converter • The catalytic converter on a car uses platinum, palladium, and rhodium as catalysts to speed up a chemical reaction that removes toxic fumes from the exhaust of the car. • The catalytic converter contains about $200 worth of these metals which can be sold on the black market.
 Precious (coinage) Metals • Copper, Silver, Gold, Platinum • Metal Properties – Malleable and Ductile – Can be shaped into beautiful objects • Rare – Valuable • Often used for coins
Precious (coinage) Metals • Copper, Silver, Gold, Platinum • Metal Properties – Malleable and Ductile – Can be shaped into beautiful objects • Rare – Valuable • Often used for coins
 Element Practice • • • Element: Iron Symbol _____ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
Element Practice • • • Element: Iron Symbol _____ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
 Element Practice • • • Element: Oxygen Symbol ____ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
Element Practice • • • Element: Oxygen Symbol ____ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
 Element Practice • • • Element: Sodium Symbol _______ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
Element Practice • • • Element: Sodium Symbol _______ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
 Element Practice • • • Element: Neon Symbol _______ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
Element Practice • • • Element: Neon Symbol _______ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
 Element Practice • • • Element: Tungsten Symbol _______ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
Element Practice • • • Element: Tungsten Symbol _______ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
 Element Practice • • • Element: Iodine Symbol _______ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____
Element Practice • • • Element: Iodine Symbol _______ Period ____ Group ____ Atomic Number _____ Average Atomic Mass _____


