685dc64d6500f042b0c31bbb6fd34f9a.ppt
- Количество слайдов: 24
Chapter 16 Synthesis and Reactions of β- Dicarbonyl Compounds (Ester Enolates) 16. 1 β – Dicarbonyl compounds 16. 2 Acidity of β – dicarbonyl compounds 16. 3 The Claisen Condensation 16. 4 Intramolecular Claisen condensation: The Dieckmann Reaction 16. 5 Mixed (crossed) Claisen Condensation 16. 6 Acylation of ketones 16. 7 Ketone synthesis via β- keto esters 16. 8 The acetoacetic ester synthesis 16. 9 The Malonic ester synthesis 16. 10 α- Deprotonation of carbonyl compounds by Lithium dialkylamides
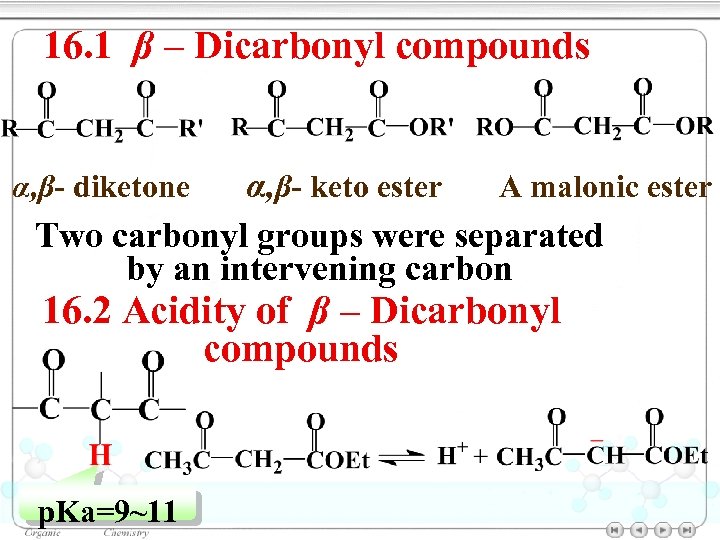
16. 1 β – Dicarbonyl compounds α, β- keto ester A malonic ester Two carbonyl groups were separated by an intervening carbon α, β- diketone 16. 2 Acidity of β – Dicarbonyl compounds p. Ka=9~11
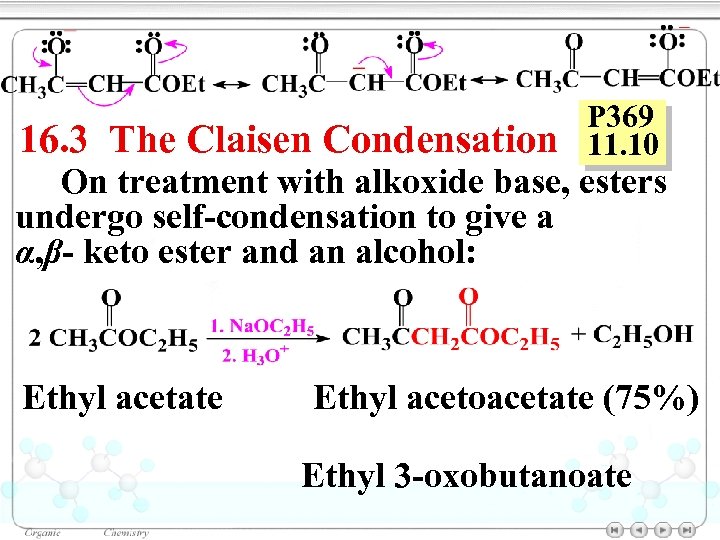
16. 3 The Claisen Condensation P 369 11. 10 On treatment with alkoxide base, esters undergo self-condensation to give a α, β- keto ester and an alcohol: Ethyl acetate Ethyl acetoacetate (75%) Ethyl 3 -oxobutanoate
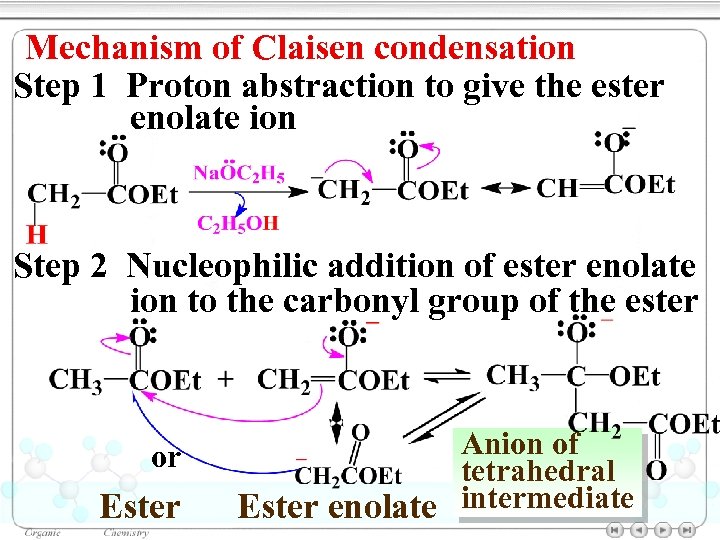
Mechanism of Claisen condensation Step 1 Proton abstraction to give the ester enolate ion Step 2 Nucleophilic addition of ester enolate ion to the carbonyl group of the ester or Ester Anion of tetrahedral Ester enolate intermediate
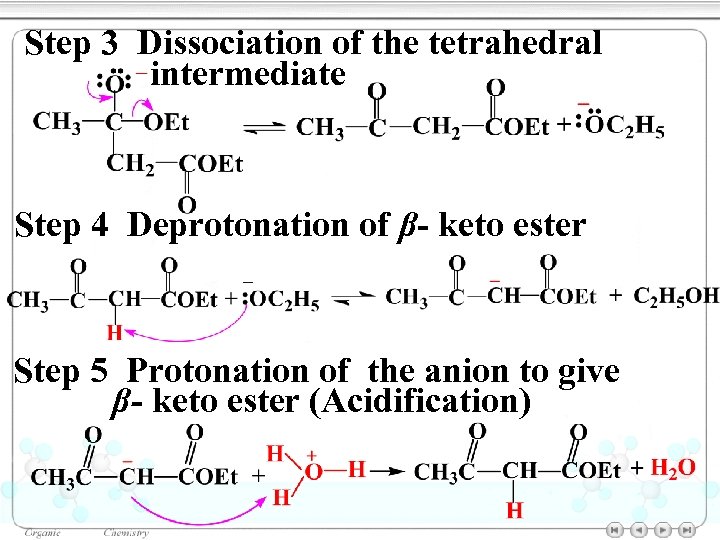
Step 3 Dissociation of the tetrahedral intermediate Step 4 Deprotonation of β- keto ester Step 5 Protonation of the anion to give β- keto ester (Acidification)
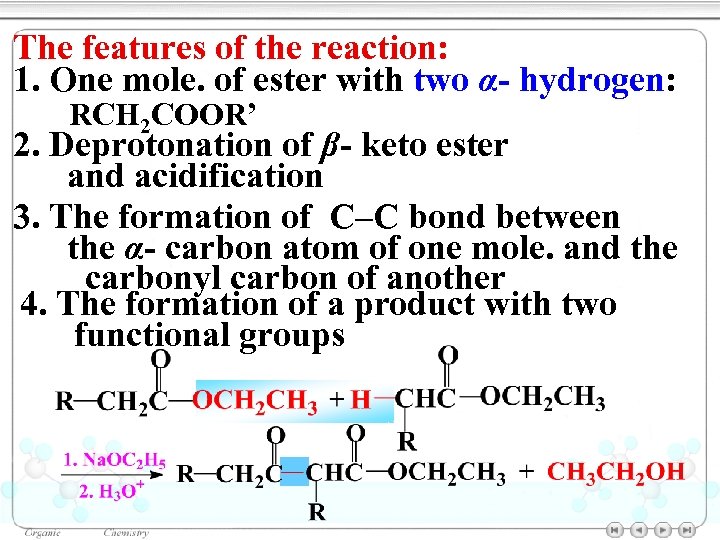
The features of the reaction: 1. One mole. of ester with two α- hydrogen: RCH 2 COOR’ 2. Deprotonation of β- keto ester and acidification 3. The formation of C–C bond between the α- carbon atom of one mole. and the carbonyl carbon of another 4. The formation of a product with two functional groups
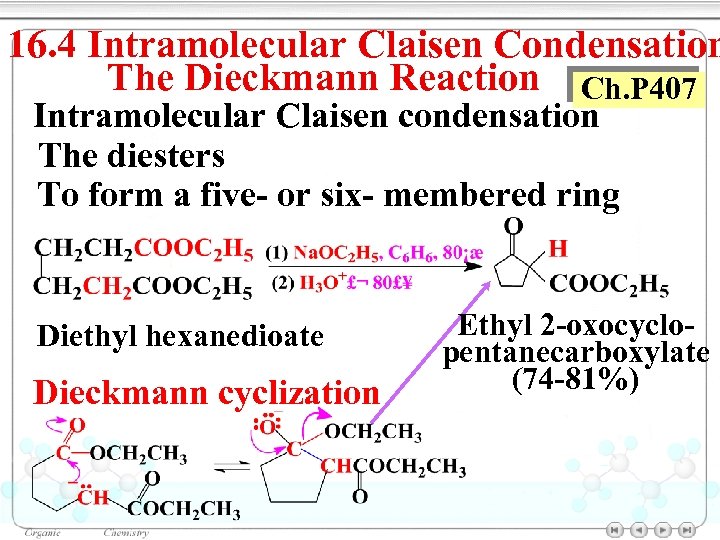
16. 4 Intramolecular Claisen Condensation The Dieckmann Reaction Ch. P 407 Intramolecular Claisen condensation The diesters To form a five- or six- membered ring Diethyl hexanedioate Dieckmann cyclization Ethyl 2 -oxocyclopentanecarboxylate (74 -81%)

C. R. Hauser and B. E. Hudson, Organic Reactions, 1, 274 (1942) D. E. Wolf and K. Folkers, Organic Reactions, 6, 449 (1951) B. S. Thyagarajan, Chem. Revs. , 54, 1029(1954) N. J. Leonard and C. W. Schimelpfenig, J. Org. Chem. , 23, 1708 (1958) H. H. House, Modern Synthetic Reactions, p 261 (New York, 1965) Walter Dieckman (1869 -1925) was born in Harmburg, Germany, and received his Ph. D. at the University of Munich as professor of chemistry
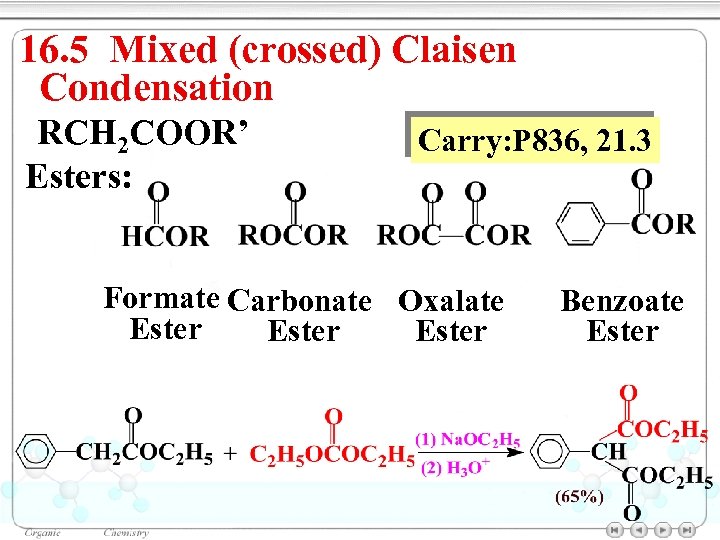
16. 5 Mixed (crossed) Claisen Condensation RCH 2 COOR’ Esters: Carry: P 836, 21. 3 Formate Carbonate Oxalate Ester Benzoate Ester
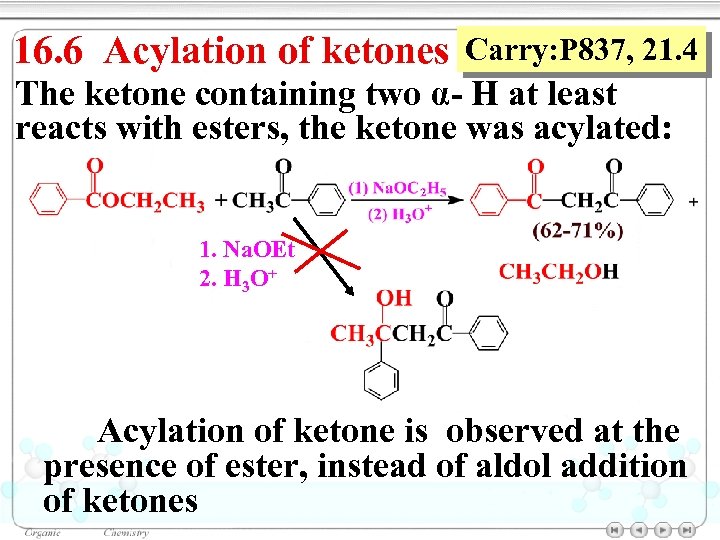
16. 6 Acylation of ketones Carry: P 837, 21. 4 The ketone containing two α- H at least reacts with esters, the ketone was acylated: 1. Na. OEt 2. H 3 O+ Acylation of ketone is observed at the presence of ester, instead of aldol addition of ketones
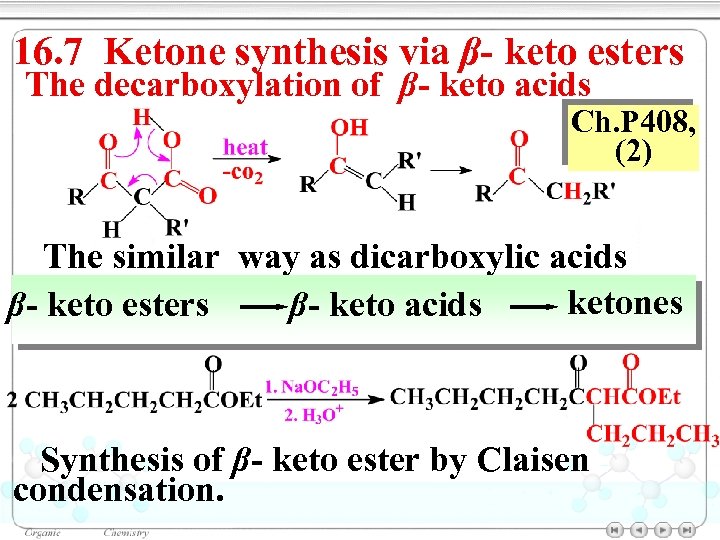
16. 7 Ketone synthesis via β- keto esters The decarboxylation of β- keto acids Ch. P 408, (2) The similar way as dicarboxylic acids ketones β- keto esters β- keto acids Synthesis of β- keto ester by Claisen condensation.
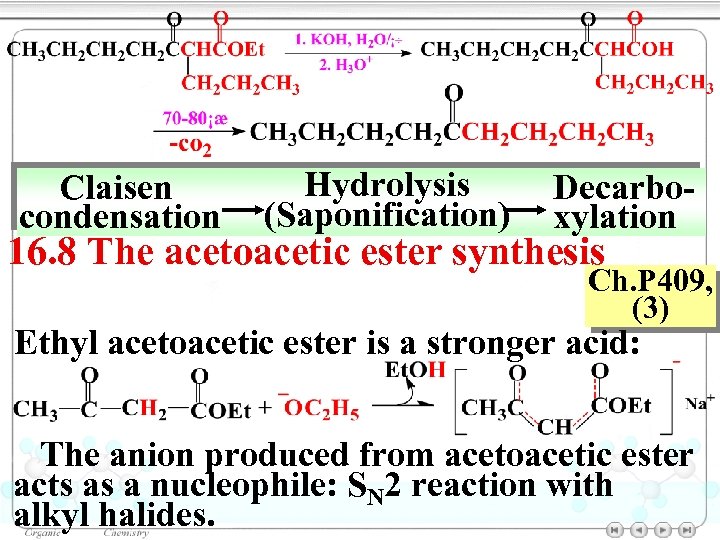
Claisen condensation Hydrolysis (Saponification) Decarboxylation 16. 8 The acetoacetic ester synthesis Ch. P 409, (3) Ethyl acetoacetic ester is a stronger acid: The anion produced from acetoacetic ester acts as a nucleophile: SN 2 reaction with alkyl halides.
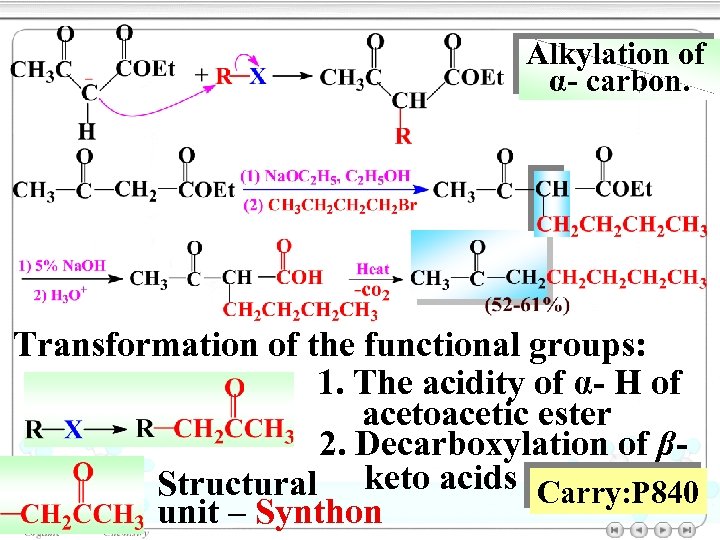
Alkylation of α- carbon. Transformation of the functional groups: 1. The acidity of α- H of acetoacetic ester 2. Decarboxylation of βStructural keto acids Carry: P 840 unit – Synthon
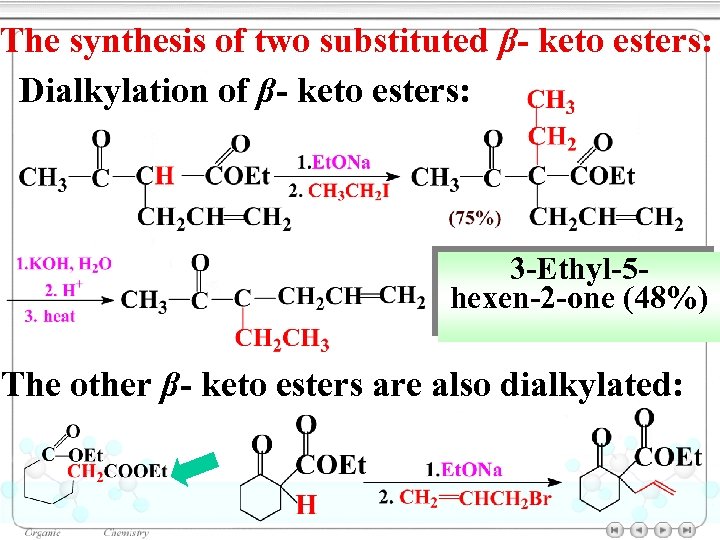
The synthesis of two substituted β- keto esters: Dialkylation of β- keto esters: 3 -Ethyl-5 hexen-2 -one (48%) The other β- keto esters are also dialkylated:
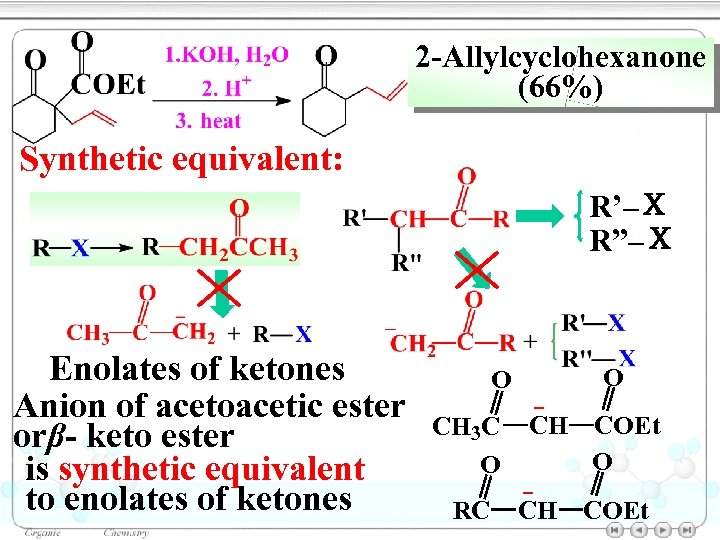
2 -Allylcyclohexanone (66%) Synthetic equivalent: R’–X R”–X Enolates of ketones Anion of acetoacetic ester orβ- keto ester is synthetic equivalent to enolates of ketones O CH 3 C O RC O CH COEt
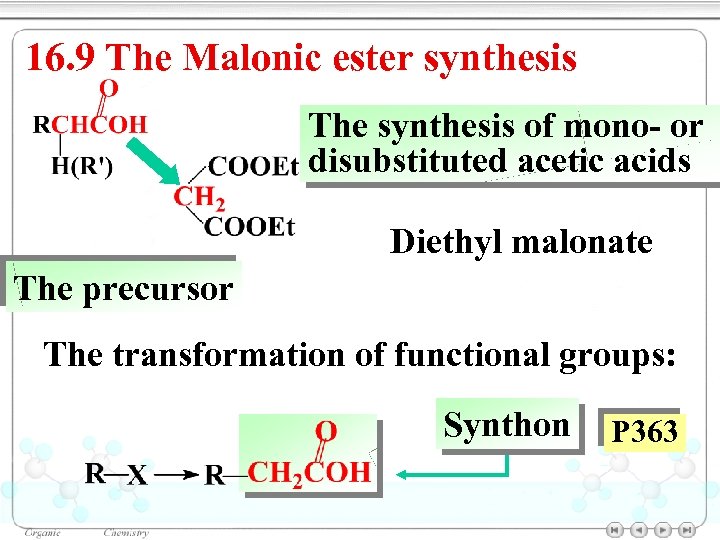
16. 9 The Malonic ester synthesis The synthesis of mono- or disubstituted acetic acids Diethyl malonate The precursor The transformation of functional groups: Synthon P 363
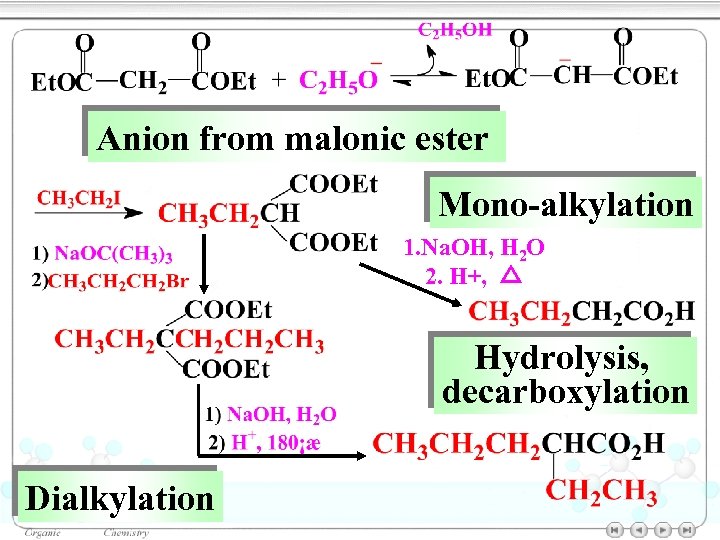
Anion from malonic ester Mono-alkylation 1. Na. OH, H 2 O 2. H+, △ Hydrolysis, decarboxylation Dialkylation
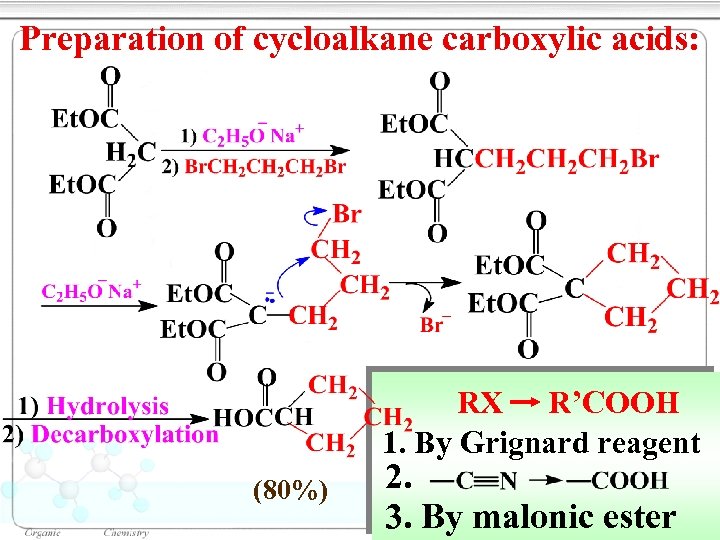
Preparation of cycloalkane carboxylic acids: RX R’COOH 1. By Grignard reagent (80%) 2. 3. By malonic ester
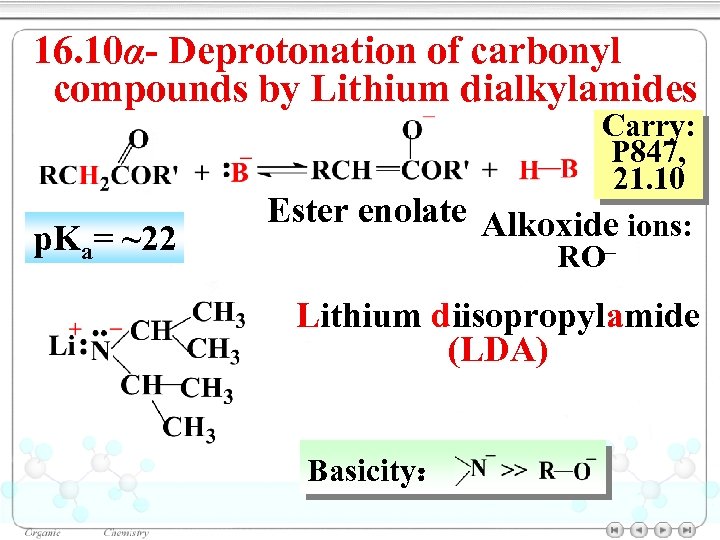
16. 10α- Deprotonation of carbonyl compounds by Lithium dialkylamides p. Ka= ~22 Carry: P 847, 21. 10 Ester enolate Alkoxide ions: RO– Lithium diisopropylamide (LDA) Basicity:
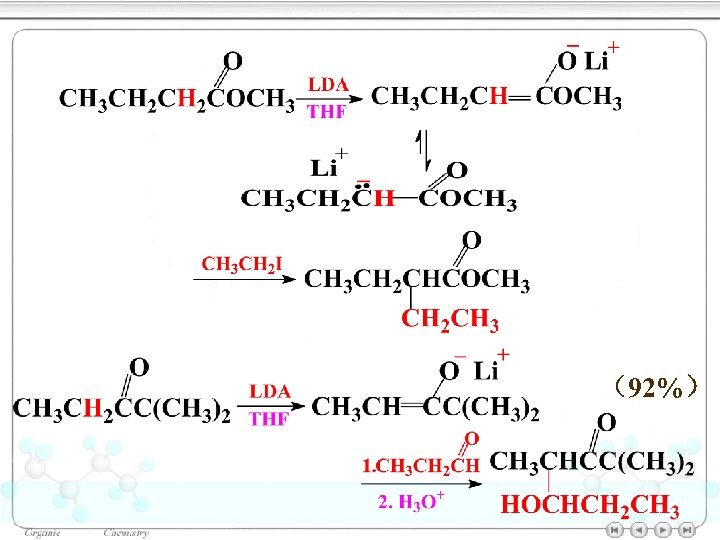
(92%)
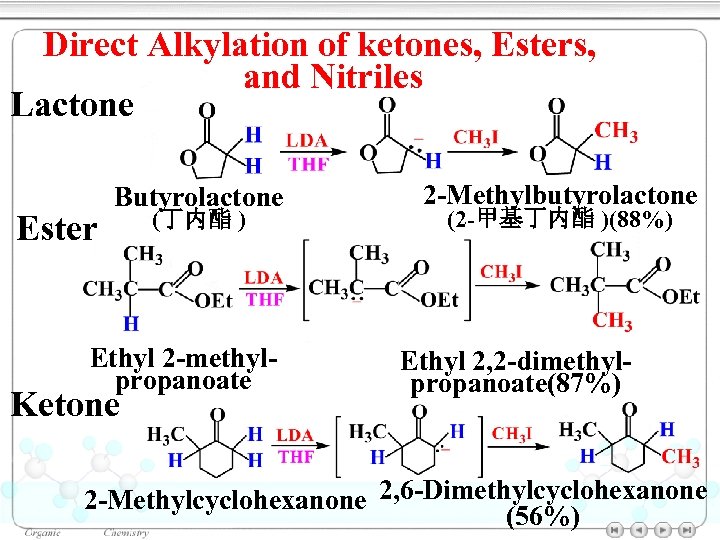
Direct Alkylation of ketones, Esters, and Nitriles Lactone Ester Butyrolactone (丁内酯 ) Ethyl 2 -methylpropanoate Ketone 2 -Methylbutyrolactone (2 -甲基丁内酯 )(88%) Ethyl 2, 2 -dimethylpropanoate(87%) 2 -Methylcyclohexanone 2, 6 -Dimethylcyclohexanone (56%)
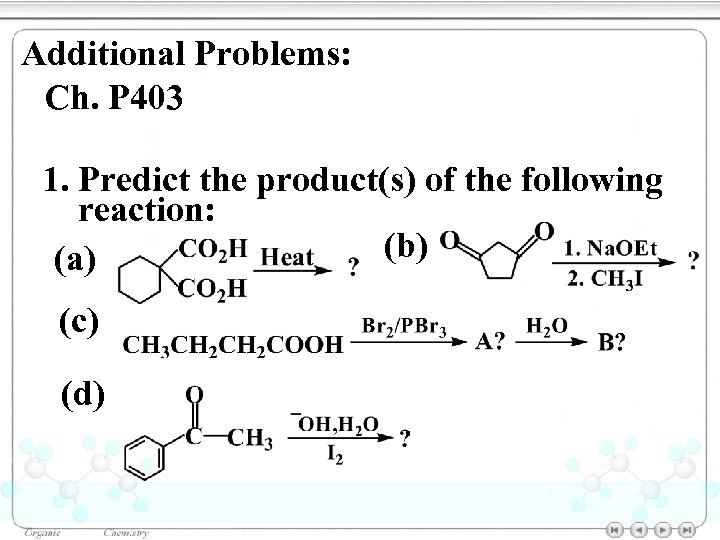
Additional Problems: Ch. P 403 1. Predict the product(s) of the following reaction: (b) (a) (c) (d)
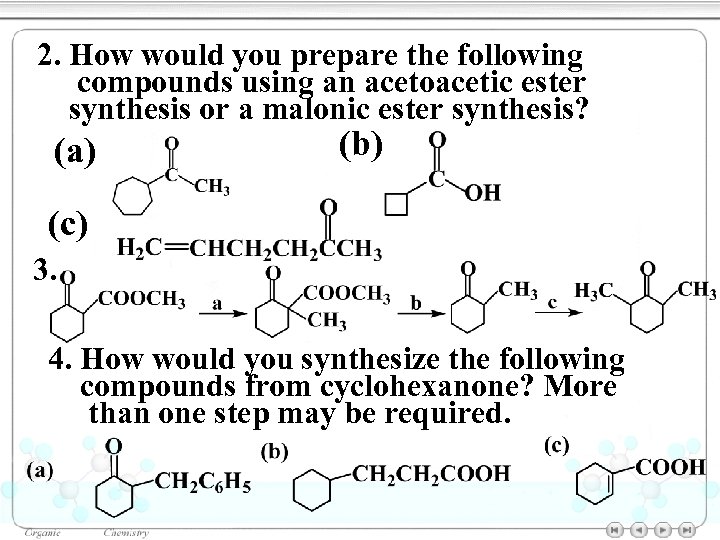
2. How would you prepare the following compounds using an acetoacetic ester synthesis or a malonic ester synthesis? (a) (b) (c) 3. 4. How would you synthesize the following compounds from cyclohexanone? More than one step may be required.
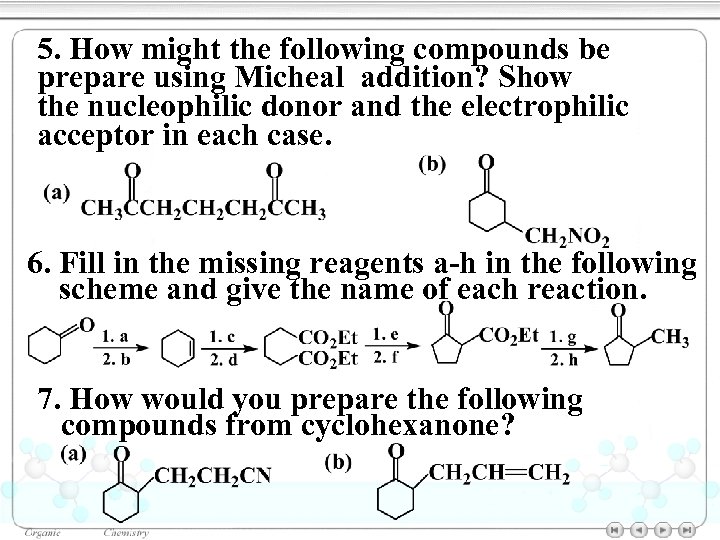
5. How might the following compounds be prepare using Micheal addition? Show the nucleophilic donor and the electrophilic acceptor in each case. 6. Fill in the missing reagents a-h in the following scheme and give the name of each reaction. 7. How would you prepare the following compounds from cyclohexanone?
685dc64d6500f042b0c31bbb6fd34f9a.ppt