decdce639e1468dccf45a8828b2d9ddd.ppt
- Количество слайдов: 35
 Chapter 15 Air Pollution and Stratospheric Ozone Depletion
Chapter 15 Air Pollution and Stratospheric Ozone Depletion
 Air Pollution © Air pollution- the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough to harm plants, animals, and materials such as buildings, or to alter ecosystems. © Refers to pollution in the troposphere – first 16 km (10 miles) of atmosphere.
Air Pollution © Air pollution- the introduction of chemicals, particulate matter, or microorganisms into the atmosphere at concentrations high enough to harm plants, animals, and materials such as buildings, or to alter ecosystems. © Refers to pollution in the troposphere – first 16 km (10 miles) of atmosphere.
 Major Air Pollutants 1. 2. 3. Sulfur Dioxide (SO 2) – corrosive gas from combustion of coal and oil. © Respiratory irritant; adversely affects plant tissue © Also released in large quantities from volcanic eruptions Nitrogen Oxides (Nox) © NO – colorless, odorless gas © NO 2 – pungent, reddish-brown gas © Motor vehicles and stationary fossil fuel combustion © Natural sources – forest fires, lightening, microbial action in soil © Play a role in forming tropospheric ozone and smog Carbon Oxides © Carbon monoxide (CO) – colorless, odorless gas that is formed during incomplete combustion – common emission in vehicle exhaust. In developing countries – poor ventilation when cooking with manure, charcoal, or kerosene. © Carbon dioxide (CO 2) – colorless, odorless gas formed during complete combustion of fossil fuels and biomass. © Absorbed by plants during photosynthesis © Burning fossil fuels has contributed additional CO 2 to atmosphere and is becoming a major pollutant.
Major Air Pollutants 1. 2. 3. Sulfur Dioxide (SO 2) – corrosive gas from combustion of coal and oil. © Respiratory irritant; adversely affects plant tissue © Also released in large quantities from volcanic eruptions Nitrogen Oxides (Nox) © NO – colorless, odorless gas © NO 2 – pungent, reddish-brown gas © Motor vehicles and stationary fossil fuel combustion © Natural sources – forest fires, lightening, microbial action in soil © Play a role in forming tropospheric ozone and smog Carbon Oxides © Carbon monoxide (CO) – colorless, odorless gas that is formed during incomplete combustion – common emission in vehicle exhaust. In developing countries – poor ventilation when cooking with manure, charcoal, or kerosene. © Carbon dioxide (CO 2) – colorless, odorless gas formed during complete combustion of fossil fuels and biomass. © Absorbed by plants during photosynthesis © Burning fossil fuels has contributed additional CO 2 to atmosphere and is becoming a major pollutant.
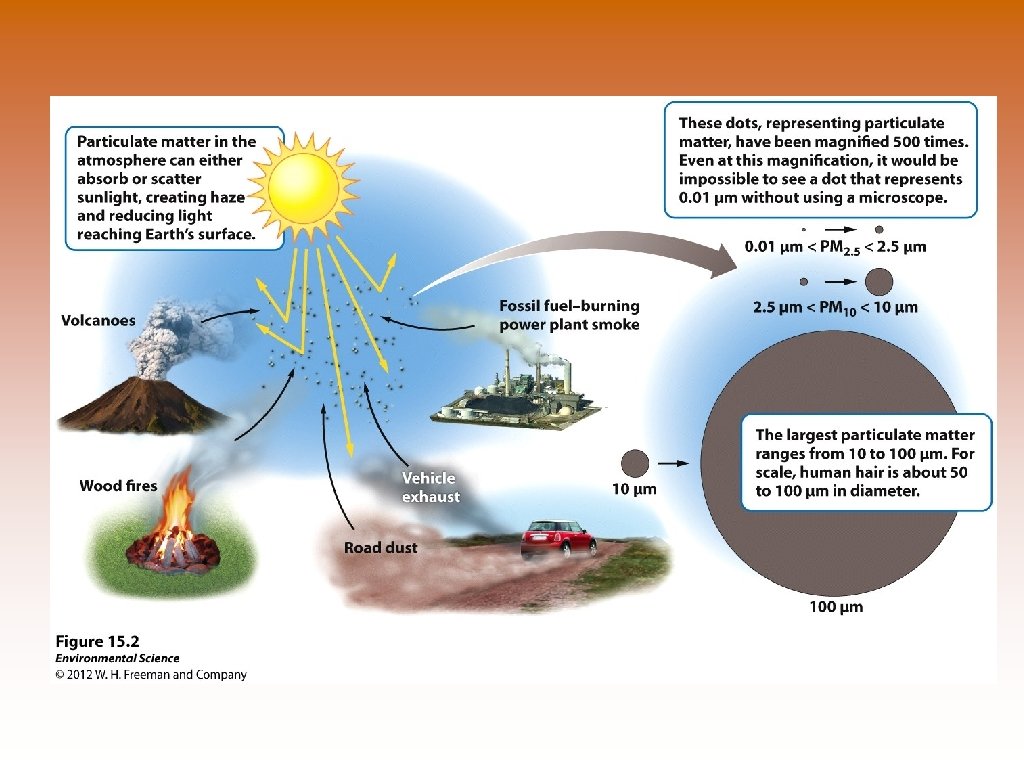
 Major Air Pollutants 4. Particulate Matter – solid or liquid particles suspended in air © Combustion of wood, animal manure biofuels, coal, oil, and gasoline © Diesel vehicles give off more in the form of black smoke © Also comes from road and rock-crushing operations © Volcanoes, forest fires, dust storms – natural sources © Size ranges from 0. 01 micrometers to 100 micrometers © Human hair has a diameter = 50 -100 micrometers © PM 10 – smaller than 10µm – concern to air pollution scientists because they are not filtered out by the nose and throat and can be deposited deep within the respiratory tract. © PM 2. 5 – greater health concern – deposited deep in respiratory tract and are composed of more toxic substances © Scatters and absorbs sunlight © Can cause reduces visibility known as haze – caused when particulate matter from air pollutions scatters light.
Major Air Pollutants 4. Particulate Matter – solid or liquid particles suspended in air © Combustion of wood, animal manure biofuels, coal, oil, and gasoline © Diesel vehicles give off more in the form of black smoke © Also comes from road and rock-crushing operations © Volcanoes, forest fires, dust storms – natural sources © Size ranges from 0. 01 micrometers to 100 micrometers © Human hair has a diameter = 50 -100 micrometers © PM 10 – smaller than 10µm – concern to air pollution scientists because they are not filtered out by the nose and throat and can be deposited deep within the respiratory tract. © PM 2. 5 – greater health concern – deposited deep in respiratory tract and are composed of more toxic substances © Scatters and absorbs sunlight © Can cause reduces visibility known as haze – caused when particulate matter from air pollutions scatters light.
 Major Air Pollutants 5. 6. Photochemical Oxidants – formed as a result of sunlight acting on compounds such as nitrogen oxides and sulfur dioxide. q Harmful to plant tissue, human respiratory tissue, and construction materials q Smog – mixture of oxidants and particulate matter 1. Photochemical smog (Los Angeles-type smog, brown smog) – dominated by oxidants such as ozone 2. Sulfurous smog (London-type smog, gray smog) – dominated by sulfur dioxide and sulfate compounds. Lead and Other Metals q Lead – trace metal occurs naturally in soils q Toxic to central nervous system (CNS) q Added to gas to improve vehicle performance – phased out between 1975 and 1996 – since then air concentration dropped considerably q Lead in paint in older buildings – chips off – can affect learning and intelligence especially in young children who may ingest due to sweet taste q Mercury – trace metal found in coal and oil – toxic to CNS q EPA regulates mercury through its hazardous air pollutants program – decreased air and water concentrations dramatically q Bioaccumulations – mercury concentrations in some fish have increased
Major Air Pollutants 5. 6. Photochemical Oxidants – formed as a result of sunlight acting on compounds such as nitrogen oxides and sulfur dioxide. q Harmful to plant tissue, human respiratory tissue, and construction materials q Smog – mixture of oxidants and particulate matter 1. Photochemical smog (Los Angeles-type smog, brown smog) – dominated by oxidants such as ozone 2. Sulfurous smog (London-type smog, gray smog) – dominated by sulfur dioxide and sulfate compounds. Lead and Other Metals q Lead – trace metal occurs naturally in soils q Toxic to central nervous system (CNS) q Added to gas to improve vehicle performance – phased out between 1975 and 1996 – since then air concentration dropped considerably q Lead in paint in older buildings – chips off – can affect learning and intelligence especially in young children who may ingest due to sweet taste q Mercury – trace metal found in coal and oil – toxic to CNS q EPA regulates mercury through its hazardous air pollutants program – decreased air and water concentrations dramatically q Bioaccumulations – mercury concentrations in some fish have increased
 Major Air Pollutants 7. Volatile Organic Compounds (VOCs) – organic compounds that become compounds at typical atmospheric temperatures q Many are hydrocarbons – compounds that contain carbonhydrogen bonds, such as gas, lighter fluid, dry-cleaning fluid, oil-based paints, and perfumes q Compounds that give off a strong aroma – often VOCs – chemicals easily released into the air q Lead to formation of photochemical smog
Major Air Pollutants 7. Volatile Organic Compounds (VOCs) – organic compounds that become compounds at typical atmospheric temperatures q Many are hydrocarbons – compounds that contain carbonhydrogen bonds, such as gas, lighter fluid, dry-cleaning fluid, oil-based paints, and perfumes q Compounds that give off a strong aroma – often VOCs – chemicals easily released into the air q Lead to formation of photochemical smog
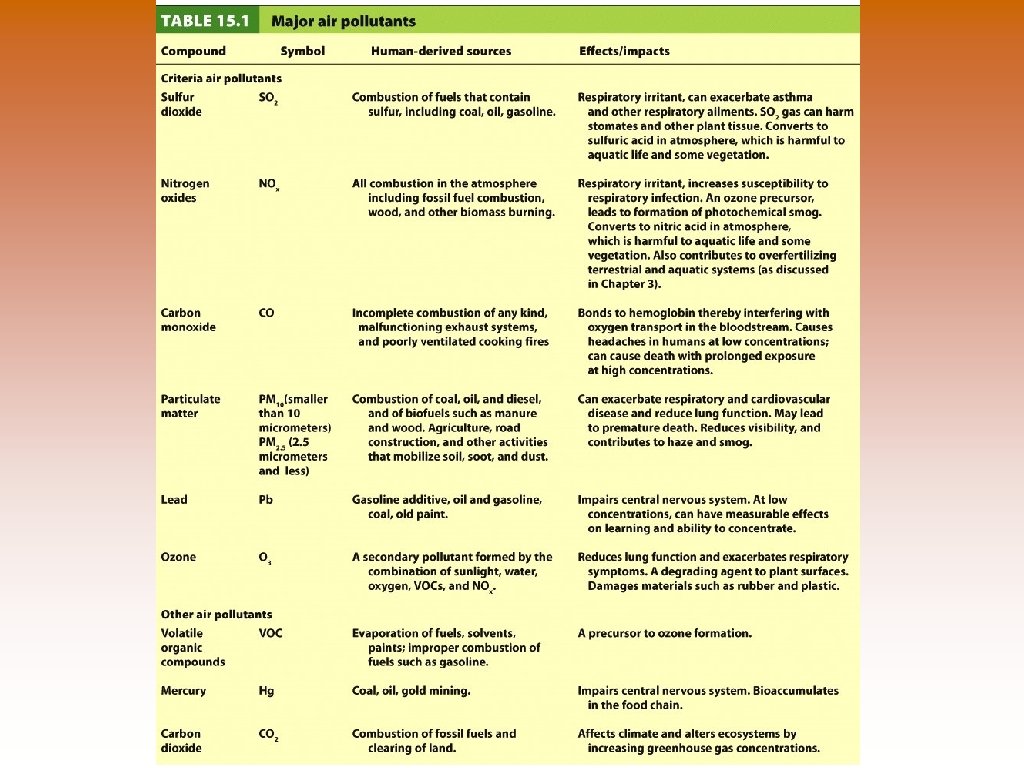
 Pollutants © © Primary pollutants- polluting compounds that come directly out of the smoke-stack, exhaust pipe, or natural emission source. © Examples: CO, CO 2, SO 2, NOx, and most suspended particulate matter. Secondary pollutants- pollutants that have undergone transformation in the presence of sunlight, water, oxygen, or other compounds. © Examples: ozone, sulfate and nitrate
Pollutants © © Primary pollutants- polluting compounds that come directly out of the smoke-stack, exhaust pipe, or natural emission source. © Examples: CO, CO 2, SO 2, NOx, and most suspended particulate matter. Secondary pollutants- pollutants that have undergone transformation in the presence of sunlight, water, oxygen, or other compounds. © Examples: ozone, sulfate and nitrate
 Natural Sources of Air Pollution © Volcanoes © Lightning © Forest fires © Plants
Natural Sources of Air Pollution © Volcanoes © Lightning © Forest fires © Plants
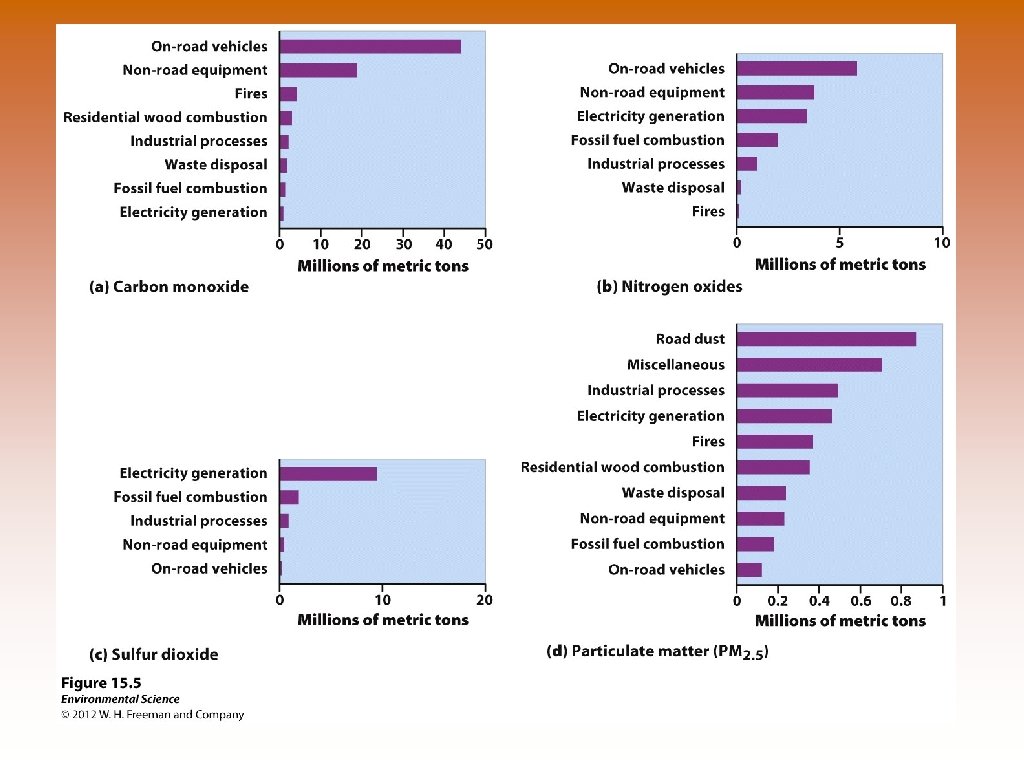
 Anthropogenic Sources of Air Pollution © © © On-road vehicles Power plants Industrial processes Waste disposal The Clean Air Act and its various amendments require that the EPA establish standards to control pollutants that are harmful to “human health and welfare. ” Through the National Ambient Air Quality Standards (NAAQS) the EPA periodically specifies concentration limits for each air pollutant. © For each pollutant the NAAQS note a concentration that should not be exceeded over a specified time period. © If a locality violates the air quality standard and does not make an attempt to make improvements, it is subject to penalties.
Anthropogenic Sources of Air Pollution © © © On-road vehicles Power plants Industrial processes Waste disposal The Clean Air Act and its various amendments require that the EPA establish standards to control pollutants that are harmful to “human health and welfare. ” Through the National Ambient Air Quality Standards (NAAQS) the EPA periodically specifies concentration limits for each air pollutant. © For each pollutant the NAAQS note a concentration that should not be exceeded over a specified time period. © If a locality violates the air quality standard and does not make an attempt to make improvements, it is subject to penalties.
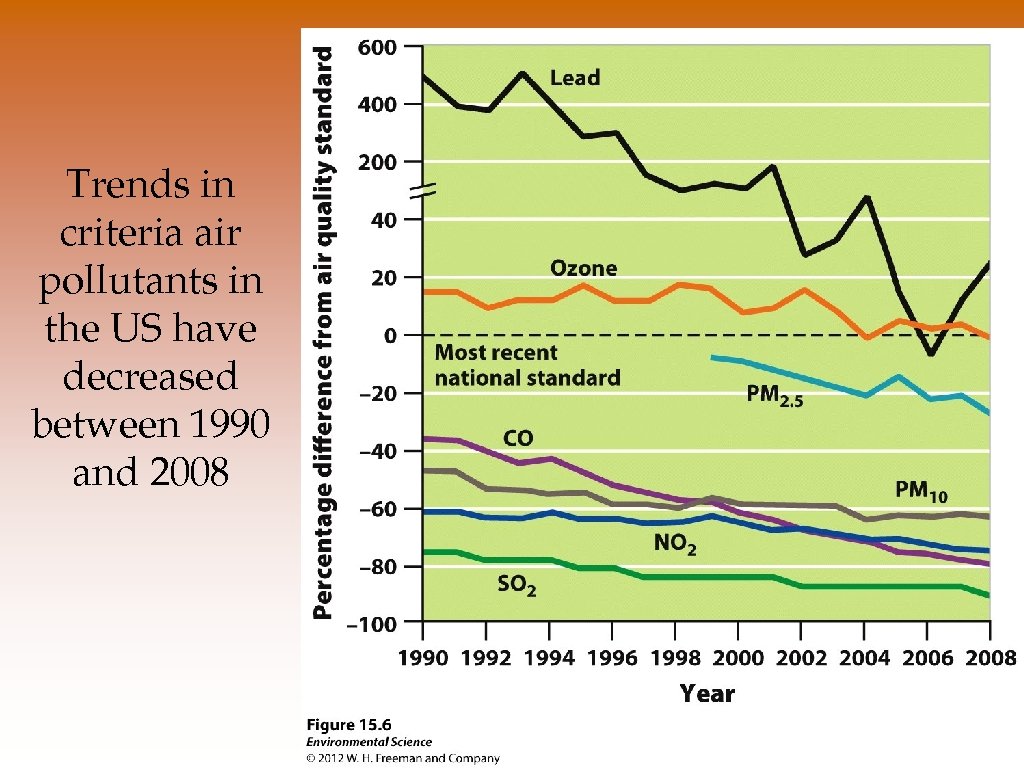 Trends in criteria air pollutants in the US have decreased between 1990 and 2008
Trends in criteria air pollutants in the US have decreased between 1990 and 2008
 The Chemistry of Smog Formation • During the day, in the presence of sunlight, if there is an abundance of nitrogen oxides in the atmosphere, but very few VOCs, ozone (O 3) forms. When sunlight decreases, nitrogen oxide is still present in the atmosphere and the ozone recombines with the NO and reforms into O 2 + NO 2. When petrochemicals or VOCs are absent or limited, the cycle of ozone destruction and formation generally takes place with relatively small amounts of smog formation. When VOCs are present, they combine with nitrogen oxide. Nitrogen oxide is not available to break down ozone by recombining with it and a larger amount of ozone accumulates Daytime accumulation of ozone in urban areas with an abundance of VOCs and Nox. Trees and shrubs, and forest fires can contribute to photochemical smog in rural areas • • •
The Chemistry of Smog Formation • During the day, in the presence of sunlight, if there is an abundance of nitrogen oxides in the atmosphere, but very few VOCs, ozone (O 3) forms. When sunlight decreases, nitrogen oxide is still present in the atmosphere and the ozone recombines with the NO and reforms into O 2 + NO 2. When petrochemicals or VOCs are absent or limited, the cycle of ozone destruction and formation generally takes place with relatively small amounts of smog formation. When VOCs are present, they combine with nitrogen oxide. Nitrogen oxide is not available to break down ozone by recombining with it and a larger amount of ozone accumulates Daytime accumulation of ozone in urban areas with an abundance of VOCs and Nox. Trees and shrubs, and forest fires can contribute to photochemical smog in rural areas • • •
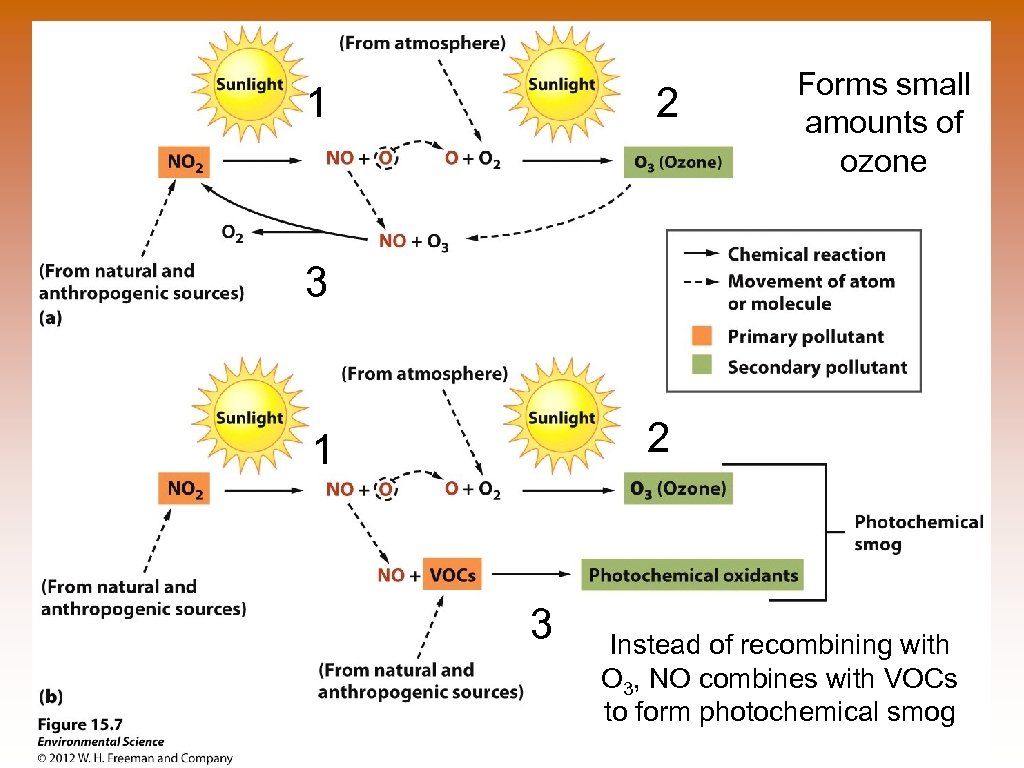 1 2 Forms small amounts of ozone 3 2 1 3 Instead of recombining with O 3, NO combines with VOCs to form photochemical smog
1 2 Forms small amounts of ozone 3 2 1 3 Instead of recombining with O 3, NO combines with VOCs to form photochemical smog
 • • Virtual sunlight: The LED screen shows the rising sun in Tiananmen Square which is shrouded with heavy smog on January 16, 2014 in Beijing, China. http: //www. dailymail. co. uk
• • Virtual sunlight: The LED screen shows the rising sun in Tiananmen Square which is shrouded with heavy smog on January 16, 2014 in Beijing, China. http: //www. dailymail. co. uk
 • • Tourists in masks use mobile phone cameras to snap shots of themselves during a heavily polluted day on Tiananmen Square in Beijing, China http: //www. dailymail. co. uk/
• • Tourists in masks use mobile phone cameras to snap shots of themselves during a heavily polluted day on Tiananmen Square in Beijing, China http: //www. dailymail. co. uk/
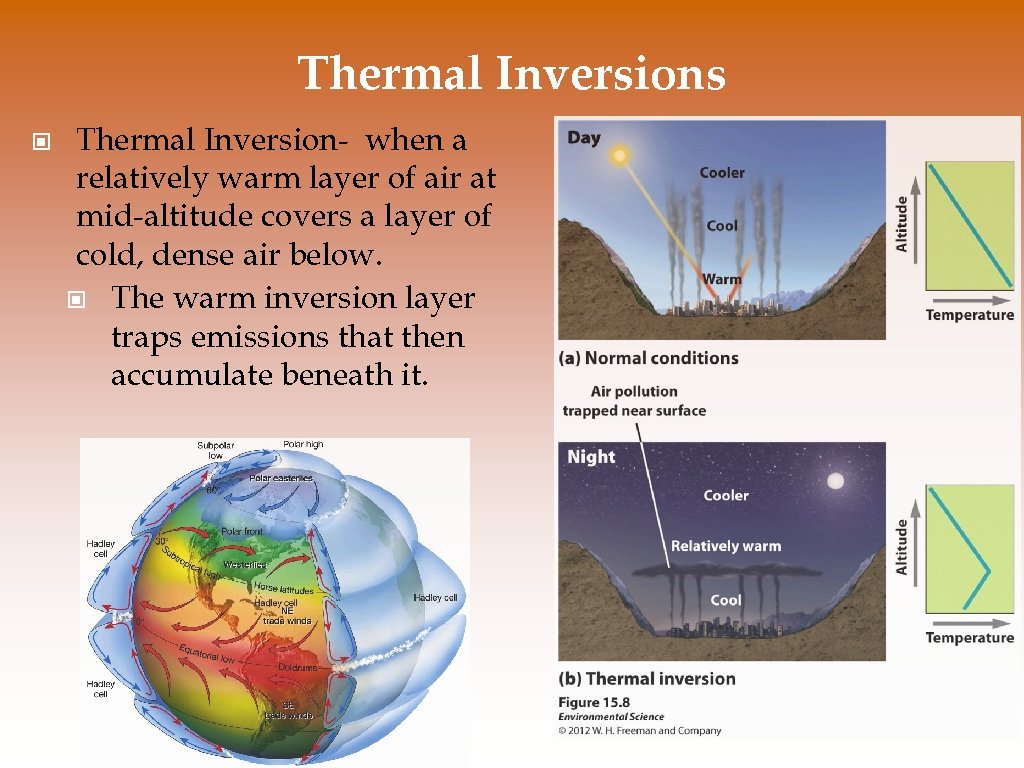 Thermal Inversions © Thermal Inversion- when a relatively warm layer of air at mid-altitude covers a layer of cold, dense air below. © The warm inversion layer traps emissions that then accumulate beneath it.
Thermal Inversions © Thermal Inversion- when a relatively warm layer of air at mid-altitude covers a layer of cold, dense air below. © The warm inversion layer traps emissions that then accumulate beneath it.
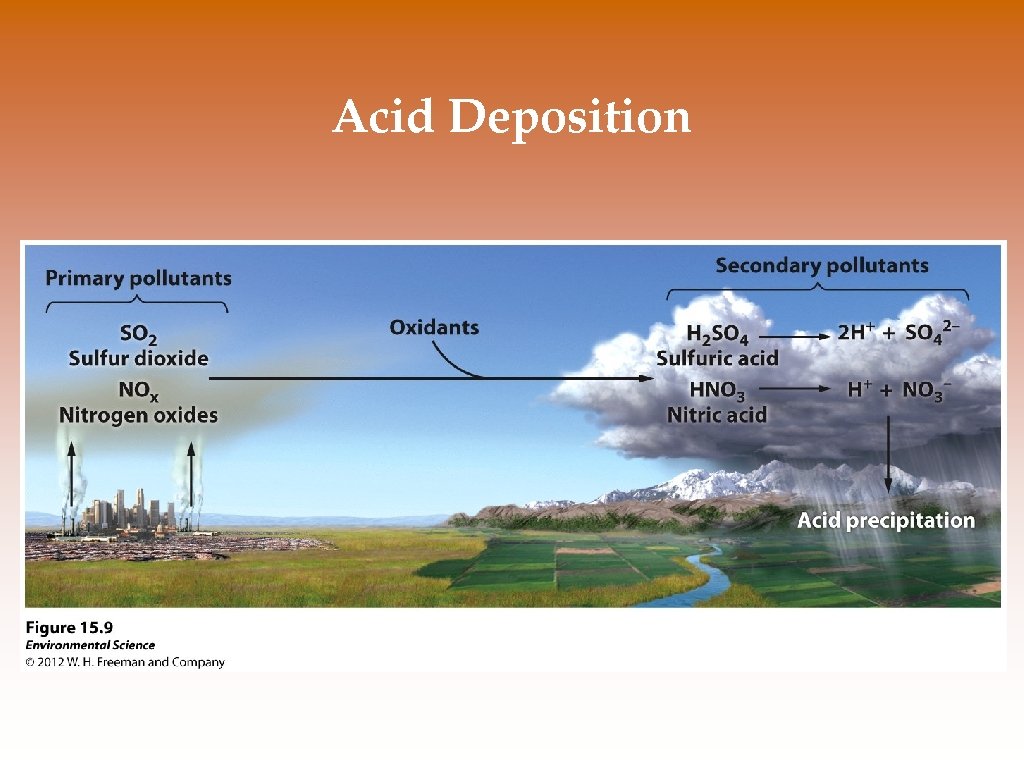
 Acid Deposition © Acid deposition- occurs when nitrogen oxides and sulfur oxides are released into the atmosphere and combine with atmospheric oxygen and water. These form the secondary pollutants nitric acid and sulfuric acid. © These secondary pollutants further break down into nitrate and sulfate and hydrogen ions (H+) which cause the acidity in acid deposition. © Transformations occur over a number of days and pollutants may travels a thousand kilometers or more. © Acid deposition has been reduced in the US as a result of the Clean Air Act.
Acid Deposition © Acid deposition- occurs when nitrogen oxides and sulfur oxides are released into the atmosphere and combine with atmospheric oxygen and water. These form the secondary pollutants nitric acid and sulfuric acid. © These secondary pollutants further break down into nitrate and sulfate and hydrogen ions (H+) which cause the acidity in acid deposition. © Transformations occur over a number of days and pollutants may travels a thousand kilometers or more. © Acid deposition has been reduced in the US as a result of the Clean Air Act.
 Acid Deposition
Acid Deposition
 Effects of Acid Deposition © © © Lowering the p. H of lake water © Decreased species diversity – a change p. H change of 6. 5 to 6. 0 acidifies the lake and may cause developmental or reproductive problems in amphibians. © Below 5. 0, most salamander species will die. Mobilizing metals © Lower p. H causes metals bound in organic or inorganic compounds in soils and sediments to be released into surface water. © Aluminum and mercury can impair physiological functioning of aquatic organisms – can lead to species loss. Damaging statues, monuments, and buildings © When the hydrogen ions in acid deposition interacts with limestone or marble, the calcium carbonate reacts with H+ and give off Ca+. © More acidic the precipitation, the more H+ to interact with calcium carbonate. © Acropolis - Athens, Greece
Effects of Acid Deposition © © © Lowering the p. H of lake water © Decreased species diversity – a change p. H change of 6. 5 to 6. 0 acidifies the lake and may cause developmental or reproductive problems in amphibians. © Below 5. 0, most salamander species will die. Mobilizing metals © Lower p. H causes metals bound in organic or inorganic compounds in soils and sediments to be released into surface water. © Aluminum and mercury can impair physiological functioning of aquatic organisms – can lead to species loss. Damaging statues, monuments, and buildings © When the hydrogen ions in acid deposition interacts with limestone or marble, the calcium carbonate reacts with H+ and give off Ca+. © More acidic the precipitation, the more H+ to interact with calcium carbonate. © Acropolis - Athens, Greece
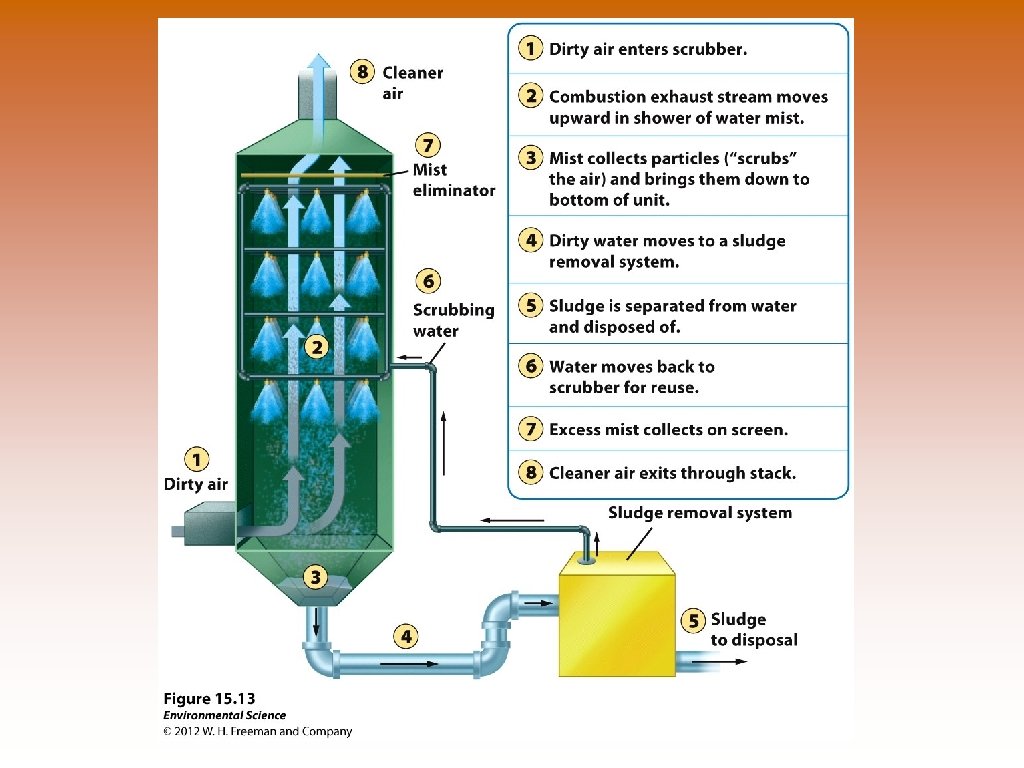
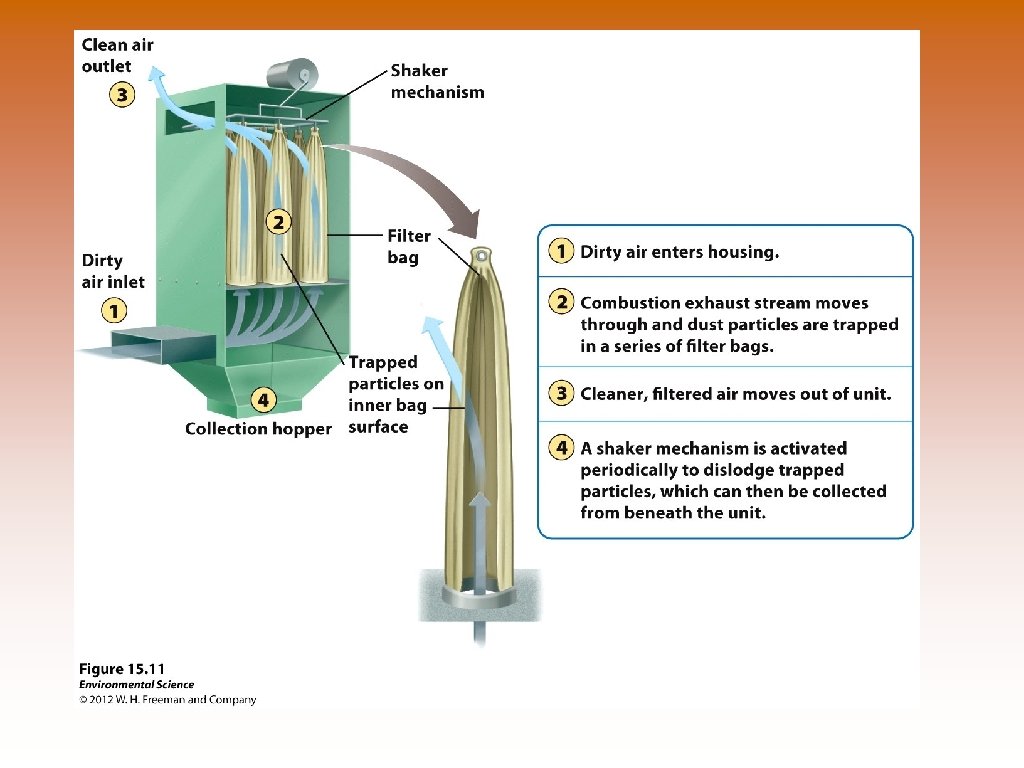
 Ways to Prevent Air Pollution © © © Removing sulfur dioxide from coal by fluidized bed combustion – granulated coal burned in close proximity to calcium carbonate – reduces sulfur dioxide emissions. © Heated calcium carbonate absorbs sulfur dioxide and produces calcium sulfate – used in production of gypsum wallboard (sheetrock), for houses. Catalytic converters on cars – reduce nitrogen oxide and carbon monoxide emissions. Scrubbers on smoke stacks – uses a combination of water and air that actually separates and removes particles. Particles removed in liquid or sludge form and clean gas exits. © Reduce emissions of SO 2. Baghouse filters – fabric filters that allow gases to pass through them but remove particulate matter. Electrostatic precipitators – use an electrical charge to make particles coalesce so they could be removed. © Polluted air enters and the electrically charged particles within are attracted to negative or positive charges on the sides of the precipitator.
Ways to Prevent Air Pollution © © © Removing sulfur dioxide from coal by fluidized bed combustion – granulated coal burned in close proximity to calcium carbonate – reduces sulfur dioxide emissions. © Heated calcium carbonate absorbs sulfur dioxide and produces calcium sulfate – used in production of gypsum wallboard (sheetrock), for houses. Catalytic converters on cars – reduce nitrogen oxide and carbon monoxide emissions. Scrubbers on smoke stacks – uses a combination of water and air that actually separates and removes particles. Particles removed in liquid or sludge form and clean gas exits. © Reduce emissions of SO 2. Baghouse filters – fabric filters that allow gases to pass through them but remove particulate matter. Electrostatic precipitators – use an electrical charge to make particles coalesce so they could be removed. © Polluted air enters and the electrically charged particles within are attracted to negative or positive charges on the sides of the precipitator.
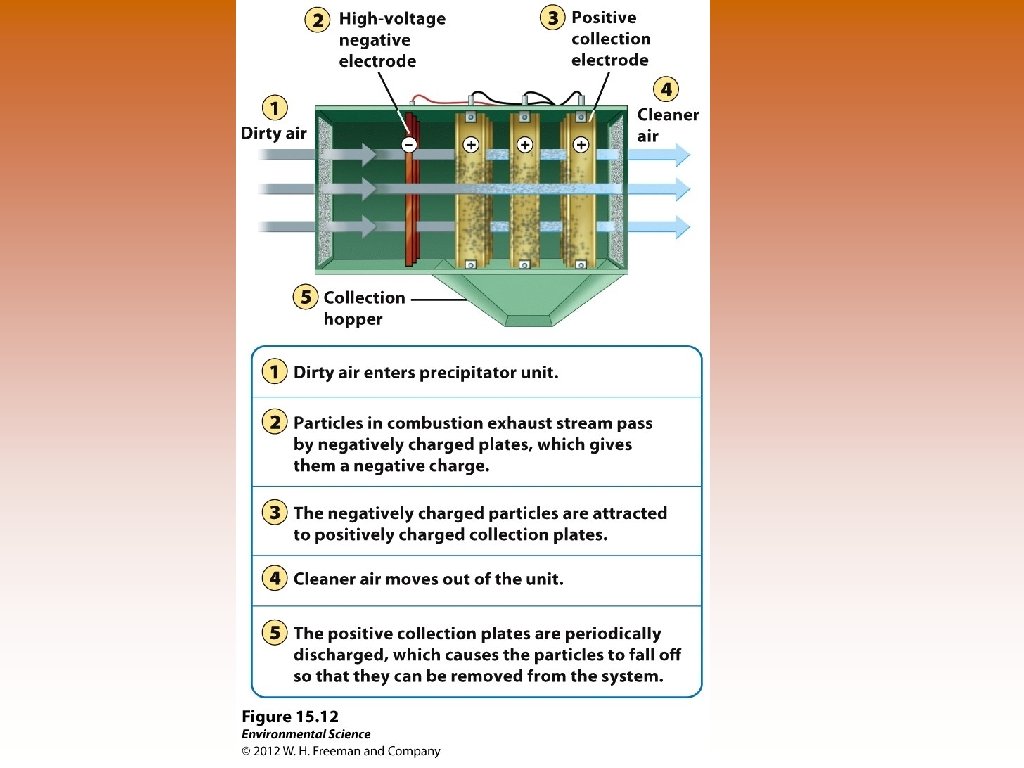
 Innovative Pollution Control © © Mexico City – permits cars to be driven every other day – license plates ending in odd numbers may be used one day with even-numbered on alternate days China – during 2008 Beijing Olympics – government successfully expanded public transportation networks, imposed motor vehicle restrictions, and temporarily shut down a number of industries to reduce photochemical smog. London – charging individual user fees to use roads at certain times of the day or within certain parts of the city. Clean Air Act – buying and selling allowances that authorizes a power plant or industrial source to emit one ton of SO 2 per year. © Facilities that emit above their allowances must pay a penalty © Sulfur allowances can be bought and sold on the open market © If emitters wanted to exceed their allowance they would need to purchase more allowances. © SO 2 emissions in US has declined from 23. 5 million metric tons in 1982 to 10. 3 million metric tons in 2008
Innovative Pollution Control © © Mexico City – permits cars to be driven every other day – license plates ending in odd numbers may be used one day with even-numbered on alternate days China – during 2008 Beijing Olympics – government successfully expanded public transportation networks, imposed motor vehicle restrictions, and temporarily shut down a number of industries to reduce photochemical smog. London – charging individual user fees to use roads at certain times of the day or within certain parts of the city. Clean Air Act – buying and selling allowances that authorizes a power plant or industrial source to emit one ton of SO 2 per year. © Facilities that emit above their allowances must pay a penalty © Sulfur allowances can be bought and sold on the open market © If emitters wanted to exceed their allowance they would need to purchase more allowances. © SO 2 emissions in US has declined from 23. 5 million metric tons in 1982 to 10. 3 million metric tons in 2008
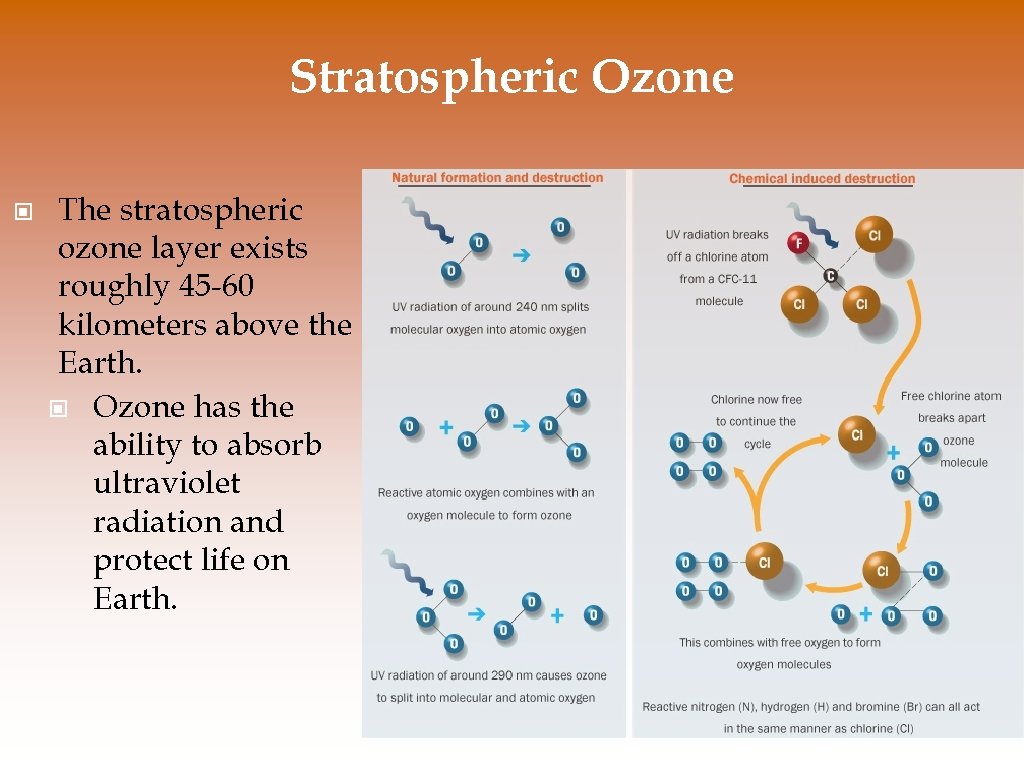 Stratospheric Ozone © The stratospheric ozone layer exists roughly 45 -60 kilometers above the Earth. © Ozone has the ability to absorb ultraviolet radiation and protect life on Earth.
Stratospheric Ozone © The stratospheric ozone layer exists roughly 45 -60 kilometers above the Earth. © Ozone has the ability to absorb ultraviolet radiation and protect life on Earth.
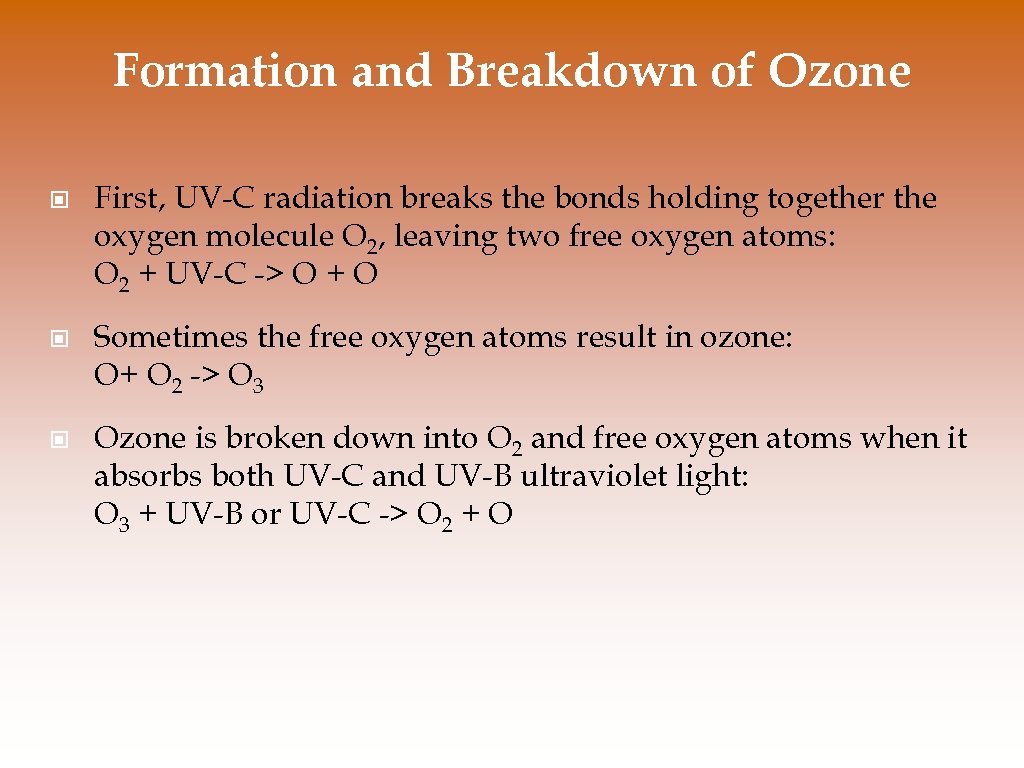 Formation and Breakdown of Ozone © First, UV-C radiation breaks the bonds holding together the oxygen molecule O 2, leaving two free oxygen atoms: O 2 + UV-C -> O + O © Sometimes the free oxygen atoms result in ozone: O+ O 2 -> O 3 © Ozone is broken down into O 2 and free oxygen atoms when it absorbs both UV-C and UV-B ultraviolet light: O 3 + UV-B or UV-C -> O 2 + O
Formation and Breakdown of Ozone © First, UV-C radiation breaks the bonds holding together the oxygen molecule O 2, leaving two free oxygen atoms: O 2 + UV-C -> O + O © Sometimes the free oxygen atoms result in ozone: O+ O 2 -> O 3 © Ozone is broken down into O 2 and free oxygen atoms when it absorbs both UV-C and UV-B ultraviolet light: O 3 + UV-B or UV-C -> O 2 + O
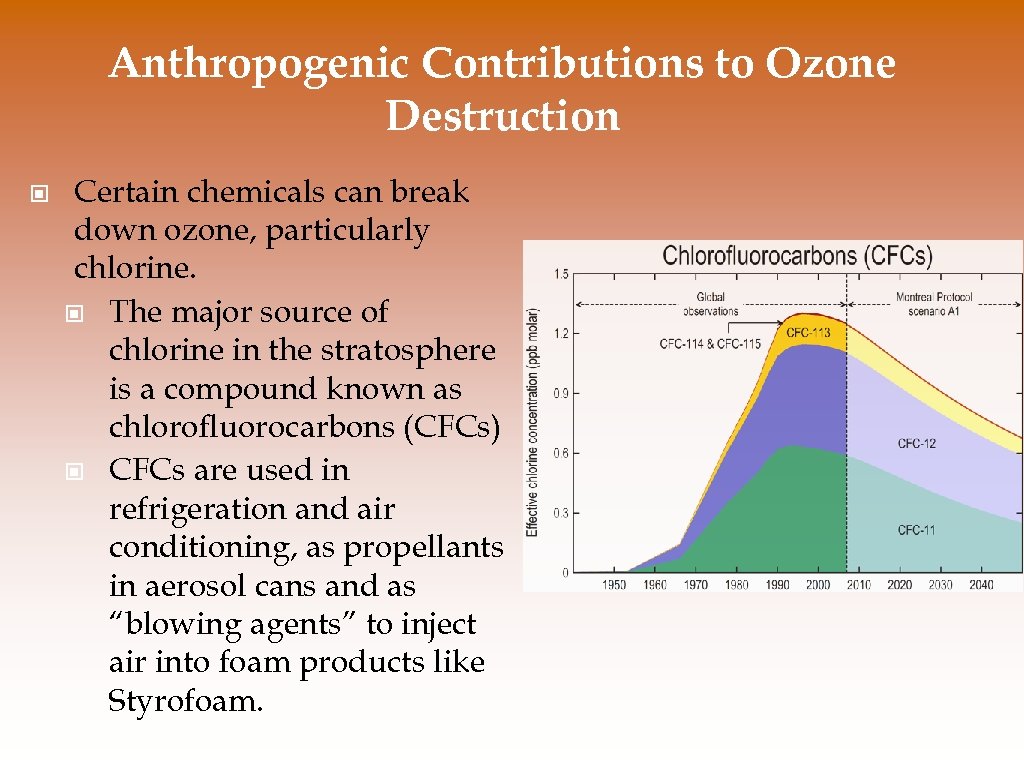 Anthropogenic Contributions to Ozone Destruction © Certain chemicals can break down ozone, particularly chlorine. © The major source of chlorine in the stratosphere is a compound known as chlorofluorocarbons (CFCs) © CFCs are used in refrigeration and air conditioning, as propellants in aerosol cans and as “blowing agents” to inject air into foam products like Styrofoam.
Anthropogenic Contributions to Ozone Destruction © Certain chemicals can break down ozone, particularly chlorine. © The major source of chlorine in the stratosphere is a compound known as chlorofluorocarbons (CFCs) © CFCs are used in refrigeration and air conditioning, as propellants in aerosol cans and as “blowing agents” to inject air into foam products like Styrofoam.
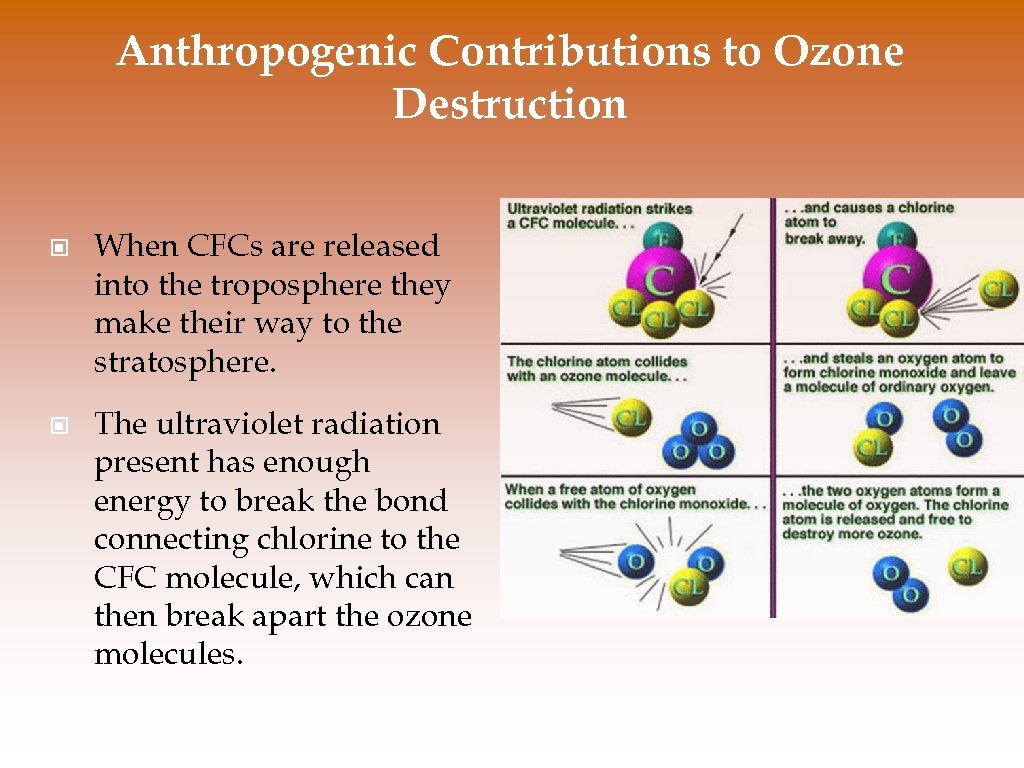 Anthropogenic Contributions to Ozone Destruction © When CFCs are released into the troposphere they make their way to the stratosphere. © The ultraviolet radiation present has enough energy to break the bond connecting chlorine to the CFC molecule, which can then break apart the ozone molecules.
Anthropogenic Contributions to Ozone Destruction © When CFCs are released into the troposphere they make their way to the stratosphere. © The ultraviolet radiation present has enough energy to break the bond connecting chlorine to the CFC molecule, which can then break apart the ozone molecules.
 Anthropogenic Contributions to Ozone Destruction © First, chlorine breaks ozone’s bonds and pulls off one atom of oxygen, forming a chlorine monoxide molecule and O 2: O 3 + Cl -> Cl. O + O 2 © Next, a free oxygen atoms pulls the oxygen atom from Cl. O, liberating the chlorine and creating one oxygen molecule: Cl. O + O -> Cl + O 2 © One chlorine atom can catalyze the breakdown of as many as 100, 000 ozone molecules before it leaves the stratosphere.
Anthropogenic Contributions to Ozone Destruction © First, chlorine breaks ozone’s bonds and pulls off one atom of oxygen, forming a chlorine monoxide molecule and O 2: O 3 + Cl -> Cl. O + O 2 © Next, a free oxygen atoms pulls the oxygen atom from Cl. O, liberating the chlorine and creating one oxygen molecule: Cl. O + O -> Cl + O 2 © One chlorine atom can catalyze the breakdown of as many as 100, 000 ozone molecules before it leaves the stratosphere.
 Depletion of the Ozone Layer © © © Since the late 1970 s, global ozone concentrations had decreased by more than 10%. Depletion was greatest at the poles © Antarctic – ozone depletion was seasonal – occurring from August through November © Extremely cold weather conditions during the polar winter cause a buildup of ice crystals mixed with nitrogen oxide. © This provides a surface for the formation of the stable molecule Cl 2, which accumulates as atmospheric chlorine interacts with ice crystals. © When sun reappears in spring, UV radiation breaks down this molecule into Cl again, which in turn catalyzes the destruction of ozone © Because almost no ozone had been formed in the dark polar winter, a large ozone “hole” occurs. Decreased stratospheric ozone has increased the amount of UV-B radiation that reaches the surface of Earth. © UV-B – harmful to cells, reduces photosynthesis, increased skin cancer, cataracts, suppresses immune systems © Significant increases in skin cancer have already been recorded, especially in countries near the ozone hole, such as Chile and Australia
Depletion of the Ozone Layer © © © Since the late 1970 s, global ozone concentrations had decreased by more than 10%. Depletion was greatest at the poles © Antarctic – ozone depletion was seasonal – occurring from August through November © Extremely cold weather conditions during the polar winter cause a buildup of ice crystals mixed with nitrogen oxide. © This provides a surface for the formation of the stable molecule Cl 2, which accumulates as atmospheric chlorine interacts with ice crystals. © When sun reappears in spring, UV radiation breaks down this molecule into Cl again, which in turn catalyzes the destruction of ozone © Because almost no ozone had been formed in the dark polar winter, a large ozone “hole” occurs. Decreased stratospheric ozone has increased the amount of UV-B radiation that reaches the surface of Earth. © UV-B – harmful to cells, reduces photosynthesis, increased skin cancer, cataracts, suppresses immune systems © Significant increases in skin cancer have already been recorded, especially in countries near the ozone hole, such as Chile and Australia
 Efforts to Reduce Ozone Depletion © Montreal Protocol on Substances that Deplete the Ozone Layer – signed in 1987 by 24 nations – committed to concrete steps toward a solution and resolving to reduce CFC production 50% by the year 2000. © A series of increasingly stringent amendments was eventually signed by more than 180 countries, requiring the elimination of CFC production and use in the developed world by 1996. © Because of these efforts, the chlorine concentration in the stratosphere has stabilized at about 5 ppb and should fall to 1 ppb by 2100. © http: //www. youtube. com/watch? v=XLY 8 m-d. XOxo – NASA research
Efforts to Reduce Ozone Depletion © Montreal Protocol on Substances that Deplete the Ozone Layer – signed in 1987 by 24 nations – committed to concrete steps toward a solution and resolving to reduce CFC production 50% by the year 2000. © A series of increasingly stringent amendments was eventually signed by more than 180 countries, requiring the elimination of CFC production and use in the developed world by 1996. © Because of these efforts, the chlorine concentration in the stratosphere has stabilized at about 5 ppb and should fall to 1 ppb by 2100. © http: //www. youtube. com/watch? v=XLY 8 m-d. XOxo – NASA research
 Indoor Air Pollutants © © © Wood, animal manure or coal used for cooking and heating in developing countries. Asbestos – long, thin, fibrous silicate mineral with insulating properties. © Was used as an insulator on steam and hot-water pipes and in shingles for building siding. © Greatest health risk – respiratory diseases such as asbestosis and lung cancer © When insulating materials become old or damaged fine fibers become airborne and can enter the respiratory tract. Carbon Monoxide – result of malfunctioning exhaust systems – natural gas heaters © Colorless and odorless – occupants of house do notice © CO binds with hemoglobin more efficiently than oxygen interfering with oxygen transport in the blood. Leads to oxygen deprivation and death. Radon – Radon-222 – radioactive gas that occurs naturally from decay of uranium. Exists in igneous rock, granite, all around the world © Seeps into a home through cracks in foundation or soil, or from drinking water from underlying rock, soil, or groundwater © Decays within 4 days to radioactive Polonium-210 © Either can attach to dust in the air and then be inhaled. © EPA estimate 21, 000 people die each year from radon-induced lung cancer © Radon detector, increase ventilation, seal cracks in basement VOCs in home products – building materials, furniture, glues, paints © Formaldehyde – used to manufacture particle board and carpeting glue © Pungent smell from new carpeting © Found to cause cancer in lab rats and is a suspected human carcinogen
Indoor Air Pollutants © © © Wood, animal manure or coal used for cooking and heating in developing countries. Asbestos – long, thin, fibrous silicate mineral with insulating properties. © Was used as an insulator on steam and hot-water pipes and in shingles for building siding. © Greatest health risk – respiratory diseases such as asbestosis and lung cancer © When insulating materials become old or damaged fine fibers become airborne and can enter the respiratory tract. Carbon Monoxide – result of malfunctioning exhaust systems – natural gas heaters © Colorless and odorless – occupants of house do notice © CO binds with hemoglobin more efficiently than oxygen interfering with oxygen transport in the blood. Leads to oxygen deprivation and death. Radon – Radon-222 – radioactive gas that occurs naturally from decay of uranium. Exists in igneous rock, granite, all around the world © Seeps into a home through cracks in foundation or soil, or from drinking water from underlying rock, soil, or groundwater © Decays within 4 days to radioactive Polonium-210 © Either can attach to dust in the air and then be inhaled. © EPA estimate 21, 000 people die each year from radon-induced lung cancer © Radon detector, increase ventilation, seal cracks in basement VOCs in home products – building materials, furniture, glues, paints © Formaldehyde – used to manufacture particle board and carpeting glue © Pungent smell from new carpeting © Found to cause cancer in lab rats and is a suspected human carcinogen
 Sick Building Syndrome © Insulation and prevention of air leaks in order to reduce the amount of heating or cooling necessary for a comfortable existence © Reduces energy use – side effect of allowing buildup of toxic compounds and pollutants in an airtight space. © Causes Sick Building Syndrome © New buildings contain many products made with synthetic materials and glues that may not have fully dried out – outgassing occurs © Office buildings – large numbers of workers have headaches, nausea, throat or eye irritations, and fatigue © EPA identified 4 reasons: 1. Inadequate or faulty ventilation 2. Chemical contamination – glues, carpeting, furniture, cleaning agents, copy machines 3. Chemical contamination from outdoor sources – vehicle exhaust transferred in through air intakes 4. Biological contaminants – mold or pollen
Sick Building Syndrome © Insulation and prevention of air leaks in order to reduce the amount of heating or cooling necessary for a comfortable existence © Reduces energy use – side effect of allowing buildup of toxic compounds and pollutants in an airtight space. © Causes Sick Building Syndrome © New buildings contain many products made with synthetic materials and glues that may not have fully dried out – outgassing occurs © Office buildings – large numbers of workers have headaches, nausea, throat or eye irritations, and fatigue © EPA identified 4 reasons: 1. Inadequate or faulty ventilation 2. Chemical contamination – glues, carpeting, furniture, cleaning agents, copy machines 3. Chemical contamination from outdoor sources – vehicle exhaust transferred in through air intakes 4. Biological contaminants – mold or pollen


