afd3efbd63d5f9da954036a1059cd61f.ppt
- Количество слайдов: 52

CHAPTER 14: SOLUTIONS AND THEIR BEHAVIOR
14. 1 UNITS OF CONCENTRATION • A. Solution terminology – 1. solution • “homogeneous mixture of a solute in a solvent” • Held together by IM Forces – 2. solute • “substance being dissolved” – 3. solvent • “substance doing the dissolving” • *Usually present in the larger amount
14. 1 UNITS OF CONCENTRATION • 4. concentrated vs dilute – Concentrated: large amount of solute in comparison to the solvent – Dilute: small amount of solute compared to solvent
14. 1 UNITS OF CONCENTRATION • 5. gaseous solutions – Mixture of gases = partial pressures! – Ex. – mole fraction from Gas chapter

14. 1 UNITS OF CONCENTRATION • 6. liquid solutions (aqueous and tinctures) – Aqueous water is the solvent – Tincture alcohol is the solvent – H 2 O is most common solvent (Universal Solvent)
14. 1 UNITS OF CONCENTRATION • 7. solid solutions (alloys) – Homogeneous mixture of metals – Change % of metals = change alloy
14. 1 UNITS OF CONCENTRATION • 8. saturated solution – No more solute will dissolve in the solvent • Will start to see undissolved solute on bottom! • Ex. Adding 200 g of sugar to 100 m. L of coffee – Saturation occurs for a specific solute can actually add more of a different solute

14. 1 UNITS OF CONCENTRATION • 9. unsaturated solution – Can dissolve more solute in the solvent
14. 1 UNITS OF CONCENTRATION • 10. supersaturated solution – Solute added beyond saturation point, but everything is still dissolved – “trick” the solution into doing this by heating and slowly cooling – Ex. Fudge
14. 1 UNITS OF CONCENTRATION • 11. test for saturation – Add a single crystal of solute what happens? • Dissolve = unsaturated • Sit on bottom = saturated • Crystallize into a solid rock = supersaturated
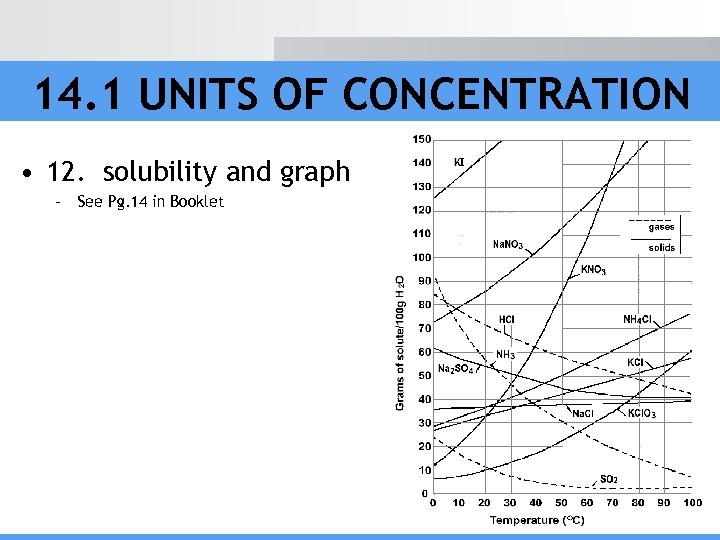
14. 1 UNITS OF CONCENTRATION • 12. solubility and graph – See Pg. 14 in Booklet
14. 1 UNITS OF CONCENTRATION • B. Types of Solutions – 1. Nonelectrolytes • Substances stay in molecular form when in soln. • Ex. Sucrose C 12 H 22 O 11 – 2. Electrolytes, strong and weak • Compounds break into ions (to varying degrees) and conduct e • Ex. Na. Cl
14. 1 UNITS OF CONCENTRATION • 3. Dissociation equations for strong electrolytes – Solute breaks completely into ions – “ ”
14. 1 UNITS OF CONCENTRATION 4. Ex 14. 1 Write the dissociation equations for the strong electrolytes: KCl, (NH 4)2 CO 3 and Al 2(SO 4)3. State the concentration of each ion if the original solutions are all 0. 23 M. NOTE: [KCl] means:
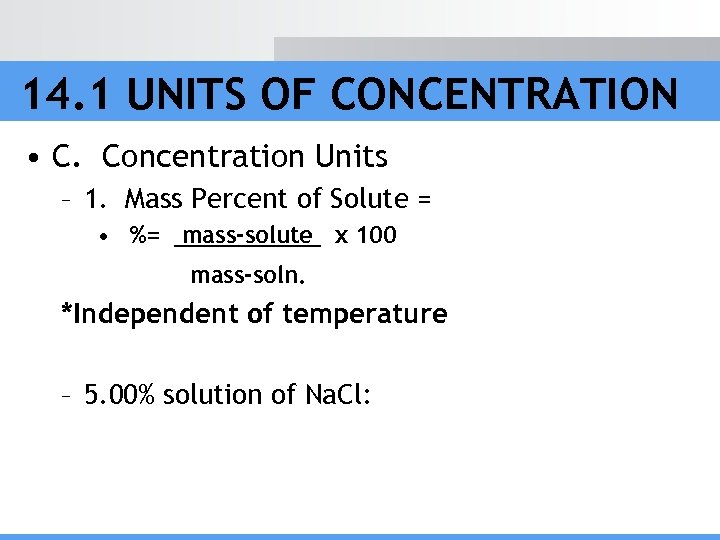
14. 1 UNITS OF CONCENTRATION • C. Concentration Units – 1. Mass Percent of Solute = • %= mass-solute x 100 mass-soln. *Independent of temperature – 5. 00% solution of Na. Cl:
14. 1 UNITS OF CONCENTRATION • 2. Ex 14. 2 What is the mass percent of a solution prepared by dissolving 13. 5 g of Na. Cl in 150 g of water?
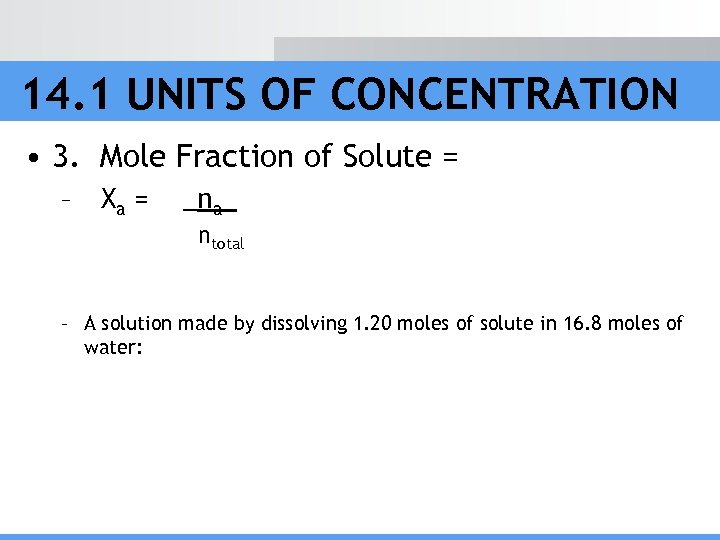
14. 1 UNITS OF CONCENTRATION • 3. Mole Fraction of Solute = – Xa = _na_ ntotal – A solution made by dissolving 1. 20 moles of solute in 16. 8 moles of water:
14. 1 UNITS OF CONCENTRATION – 4. Ex 14. 3 What are the mole fractions of solute and solvent in a solution made by dissolving 3. 45 g of ethyl alcohol (C 2 H 5 OH) in 21. 2 g of water?
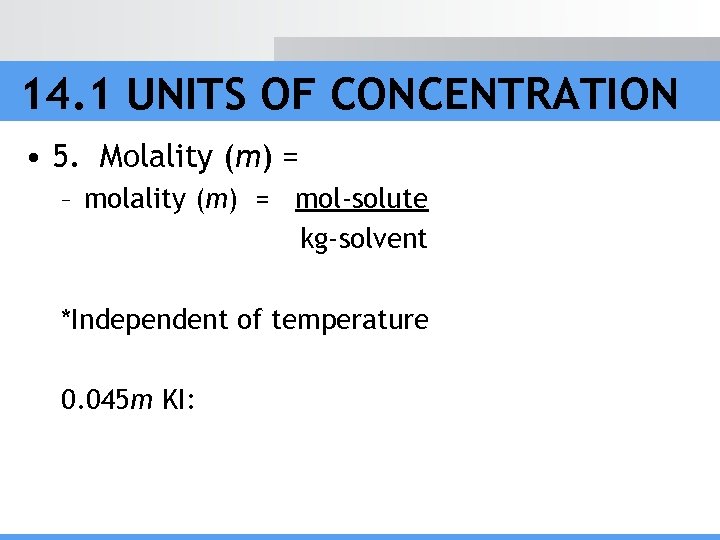
14. 1 UNITS OF CONCENTRATION • 5. Molality (m) = – molality (m) = mol-solute kg-solvent *Independent of temperature 0. 045 m KI:
14. 1 UNITS OF CONCENTRATION – 6. Ex 14. 4 Calculate the molality of an aqueous solution of urea, (NH 2)2 CO, containing 15. 5 g of solute and 74. 3 g of solvent.
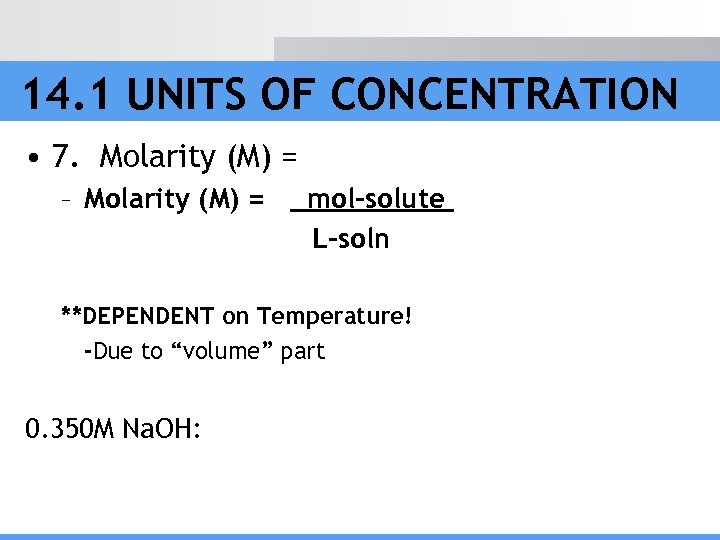
14. 1 UNITS OF CONCENTRATION • 7. Molarity (M) = – Molarity (M) = mol-solute L-soln **DEPENDENT on Temperature! -Due to “volume” part 0. 350 M Na. OH:
14. 1 UNITS OF CONCENTRATION • 8. Ex 14. 5 Explain how you would prepare 250. m. L of 0. 300 M Na 2 CO 3 starting with solid solute and water.
14. 1 UNITS OF CONCENTRATION – 9. Ex 14. 6 What mass of sodium carbonate would be crystallized from 5. 00 m. L of the above solution?
14. 1 UNITS OF CONCENTRATION • 10. Dilution Problems – – Add additional solvent to a concentrated solutions M 1 V 1 = M 2 V 2
14. 1 UNITS OF CONCENTRATION – 11. Ex. 14. 7 How would you prepare 0. 250 L of 0. 300 M sodium carbonate starting with a 1. 33 M solution?
14. 1 UNITS OF CONCENTRATION • D. Conversions between concentration units – 1. Ex 14. 8 The mass percent of hydrochloric acid (HCl) in concentrated acid from a supply house is 36. 0 and the density of this solution is 1. 18 g/cm 3. Determine the mole fraction of solute, the molality, and the molarity of this concentrated acid.
14. 1 UNITS OF CONCENTRATION – 2. Ex 14. 9 A certain solution of sulfuric acid (H 2 SO 4) has a molarity of 2. 87 and a density of 1. 17 g/cm 3. Calculate the molality, mole fraction of solute, and mass percent of this solution.
14. 1 UNITS OF CONCENTRATION – 3. Ex 14. 10 A 6. 00 M HCl solution has a density of 1. 10 g/cm 3. How many grams of water are there in 1. 00 liter of this solution? What is the molality of this solution?

14. 2 SOLUTION PROCESS • 1. "like dissolves like“ – Substances with similar IM Forces dissolve in one another • Ex. Ethanol (C 2 H 5 OH) dissolves in water – Why?

14. 2 SOLUTION PROCESS • 2. nonpolar substances are miscible in one another – – “like dissolves like” Mostly dispersion forces at work Ex. Nail Polish and Acetone Ex 2. Paint and Gasoline

14. 2 SOLUTION PROCESS • 3. nonpolar solutes and polar solvents are immiscible – IM Forces do not match = no mixing! – Ex. Oil and Water (salad dressing)

14. 2 SOLUTION PROCESS • 4. molecular substances that are soluble in water – What characteristics should our substance have if we want it to dissolve?

14. 2 SOLUTION PROCESS • 5. ionic substances in water - solution process – Ionic compounds are usually soluble in water due to H-Bonding

14. 2 SOLUTION PROCESS • All this being said, how does soap work? – What do the structures of all parties involved look like? – What forces are at work?
14. 3 FACTORS AFFECTING SOLUBILITY: PRESSURE AND TEMPERATURE • 1. Solubility-temperature relationship and endothermic and exothermic processes – As temp. increases, so does the space between molecules – Solids more soluble in liquids at higher Temp. – Gases less soluble in liquids at higher Temp.
14. 3 FACTORS AFFECTING SOLUBILITY: PRESSURE AND TEMPERATURE • 2. Effect of pressure on solubility (gasliquid systems) – As pressure increases, solubility of gases in liquids increases – “The Bends”!!!
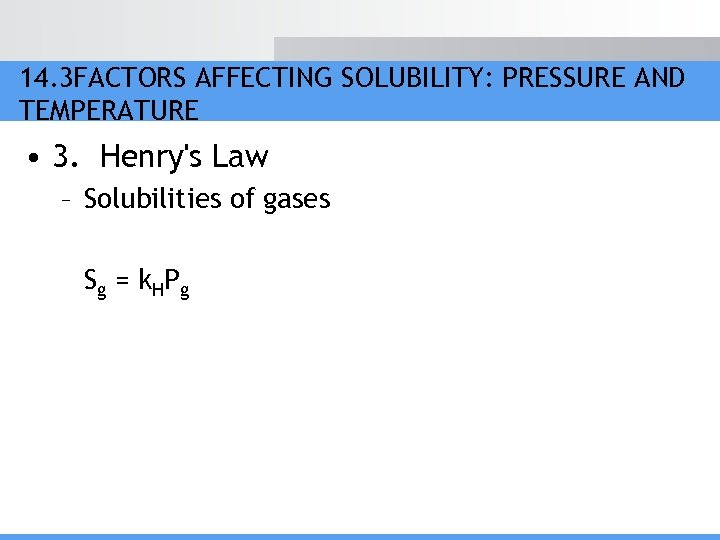
14. 3 FACTORS AFFECTING SOLUBILITY: PRESSURE AND TEMPERATURE • 3. Henry's Law – Solubilities of gases S g = k HP g

14. 3 FACTORS AFFECTING SOLUBILITY: PRESSURE AND TEMPERATURE • 4. Ex 14. 11 The solubility of pure oxygen gas in water at 20. 0 o. C and 1. 00 atm is 1. 38 x 10 -3 mol/L. Calculate the concentration of oxygen at 20. 0 o. C and a pressure of 0. 21 atm.

14. 4 COLLIGATIVE PROPERTIES • A. Definition and examples – “Physical properties of solutions that depend on the number of solute particles” – Examples: • Boiling Point Elevation • Freezing Point Depression • Vapor Pressure Lowering • Osmotic Pressure
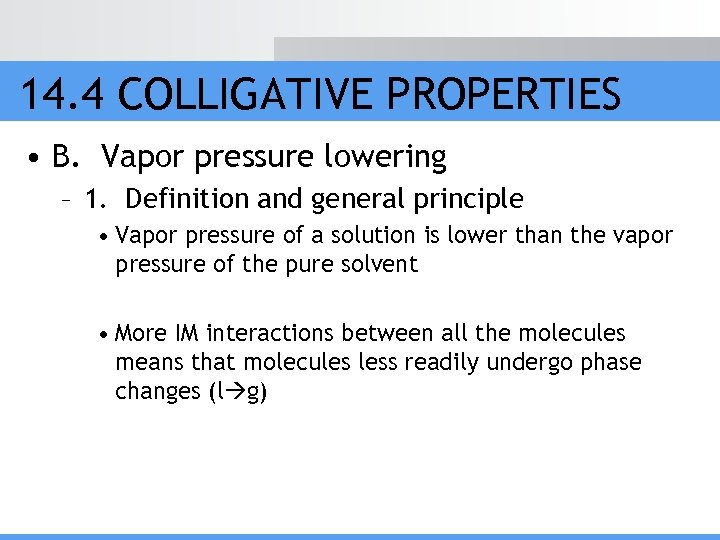
14. 4 COLLIGATIVE PROPERTIES • B. Vapor pressure lowering – 1. Definition and general principle • Vapor pressure of a solution is lower than the vapor pressure of the pure solvent • More IM interactions between all the molecules means that molecules less readily undergo phase changes (l g)
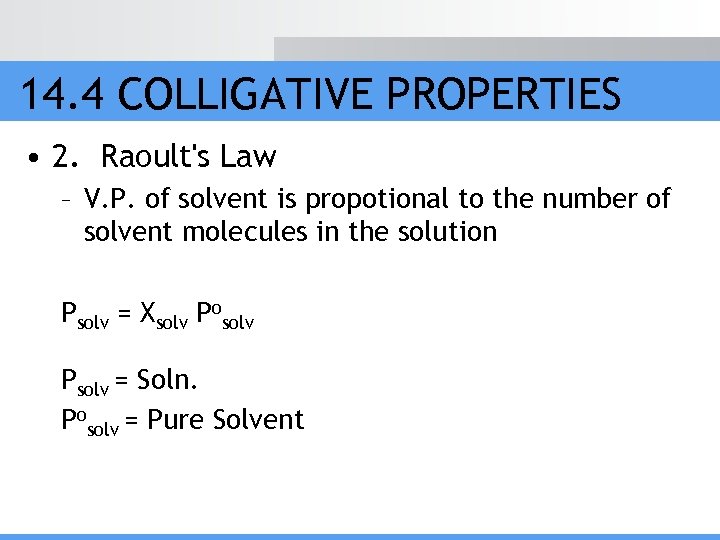
14. 4 COLLIGATIVE PROPERTIES • 2. Raoult's Law – V. P. of solvent is propotional to the number of solvent molecules in the solution Psolv = Xsolv Posolv Psolv = Soln. Posolv = Pure Solvent

14. 4 COLLIGATIVE PROPERTIES • 3. Ex 14. 12 A solution contains 25. 0 g of urea, (NH 2)2 CO, dissolved in 435 g of water. Calculate the vapor pressure of this solution at 25. 0 o. C.
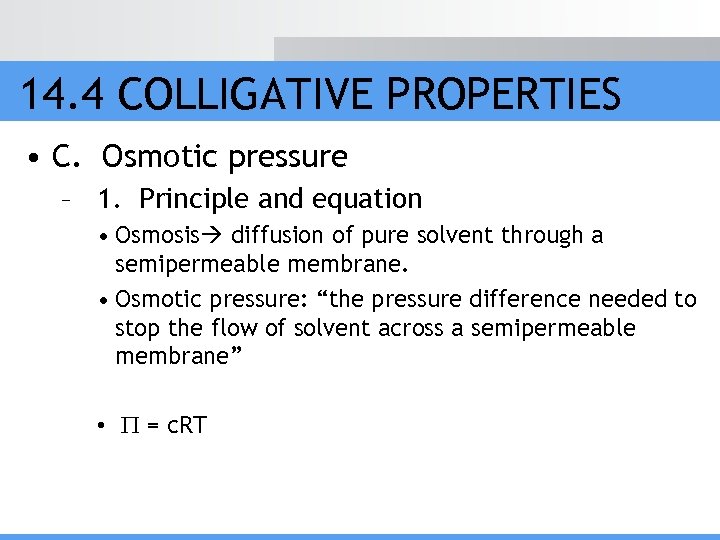
14. 4 COLLIGATIVE PROPERTIES • C. Osmotic pressure – 1. Principle and equation • Osmosis diffusion of pure solvent through a semipermeable membrane. • Osmotic pressure: “the pressure difference needed to stop the flow of solvent across a semipermeable membrane” • = c. RT

14. 4 COLLIGATIVE PROPERTIES • 2. Ex 14. 13 A solution contains 4. 00 g of ribonuclease (an enzyme that digests RNA) in 1. 00 L of water. The osmotic pressure at 25. 0 o. C is 5. 35 mm. Hg. Calculate the molarity of the ribonuclease solution and the molar mass of the enzyme.

14. 4 COLLIGATIVE PROPERTIES • D. Boiling Point Elevation and Freezing Point Depression, Nonelectrolyte – 1. Definition • In a soln. , the greater attractions between solute and solvent increase the boiling point and decrease the freezing point of a solution compared to the pure solvent • Ex. Antifreeze (Ethylene Glycol), Lakes freezing
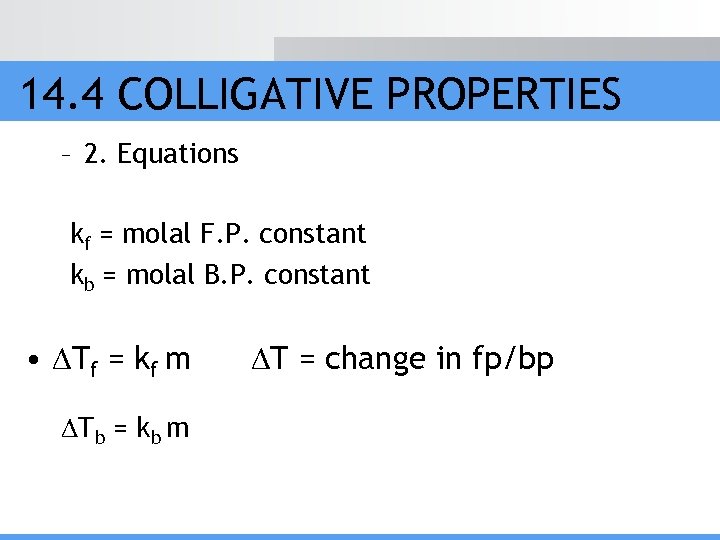
14. 4 COLLIGATIVE PROPERTIES – 2. Equations kf = molal F. P. constant kb = molal B. P. constant • Tf = kf m Tb = kb m T = change in fp/bp

14. 4 COLLIGATIVE PROPERTIES – 3. Ex 14. 14 Calculate the boiling point and freezing point of an aqueous solution of 50. 0 g of glucose (180 g/mol) in 400. g of solute.

14. 4 COLLIGATIVE PROPERTIES – 4. Ex 14. 15 Calculate the boiling and freezing points of an antifreeze solution that is 50% water and 50% ethylene glycol (C 2 H 4 O 2)

14. 4 COLLIGATIVE PROPERTIES – 5. Ex 14. 16 A student dissolves 1. 00 g of a new compound in 25. 0 g of cyclohexane. Pure cyclohexane has a freezing point of 6. 50 o. C and the solution freezes at 2. 00 o. C. Determine the molar mass of the compound if the kfp for cyclohexane is 20. 2 o. C/m.
14. 4 COLLIGATIVE PROPERTIES • E. Boiling point and freezing point effects for electrolytes – 1. Equations • Modified equation - Van’t Hoff factor (Correction factor for solns. of weak and strong electrolytes) • Tf = kf m i Tb = kb m i • i = Van’t Hoff factor for # particles(ions)
14. 4 COLLIGATIVE PROPERTIES – 2. Ex 14. 17 Estimate the freezing and boiling points of aqueous solutions of 0. 20 m Mg. SO 4 and 0. 20 m Cr(NO 3)3.

End of Chapter 14! Test Time! How did Mr. Wood do on this test in High School? Pgs. 14 -33
afd3efbd63d5f9da954036a1059cd61f.ppt