506bc8d1feaf7b8bc0994161291640c5.ppt
- Количество слайдов: 28
 Chapter 13: Properties of Solutions Sam White Pd. 2
Chapter 13: Properties of Solutions Sam White Pd. 2
 Introduction A solution is any homogenous mixture, which means the components are uniformly intermingled on a molecular level The Solvent is the most abundant component. It does the dissolving. The Solute are any of the other components. They are the ones being dissolved
Introduction A solution is any homogenous mixture, which means the components are uniformly intermingled on a molecular level The Solvent is the most abundant component. It does the dissolving. The Solute are any of the other components. They are the ones being dissolved
 Formation of Solutions With the exception of gas solutions, solutions form when the attractive forces between solute and solvent are comparable or greater than the intermolecular forces in either component
Formation of Solutions With the exception of gas solutions, solutions form when the attractive forces between solute and solvent are comparable or greater than the intermolecular forces in either component
 Formation of Solutions Example: Salt Water- Attractive forces between Na+ or Cl- and the polar water molecules overcome the lattice energy of solid Na. Cl Once separated, the Na+ and Cl- are surrounded by water. This interaction is known in all solutions as solvation When the solvent is water, this interaction is known as hydration
Formation of Solutions Example: Salt Water- Attractive forces between Na+ or Cl- and the polar water molecules overcome the lattice energy of solid Na. Cl Once separated, the Na+ and Cl- are surrounded by water. This interaction is known in all solutions as solvation When the solvent is water, this interaction is known as hydration
 Energy Change in Solution Formation In order to form a solution, the solvent must form space to house the solute and the solute must be dissolved, both of which take energy
Energy Change in Solution Formation In order to form a solution, the solvent must form space to house the solute and the solute must be dissolved, both of which take energy
 Enthalpy of Solution Overall Enthalpy Change: l DHsolution=DH 1+DH 2+DH 3 Example with Salt Water: DH 1 accounts for the separation of Na. Cl to Na+ and Cll DH 2 accounts for the separation of solvent molecules to accommodate the solute l DH 3 accounts for the attractive interactions between solute and solvent l
Enthalpy of Solution Overall Enthalpy Change: l DHsolution=DH 1+DH 2+DH 3 Example with Salt Water: DH 1 accounts for the separation of Na. Cl to Na+ and Cll DH 2 accounts for the separation of solvent molecules to accommodate the solute l DH 3 accounts for the attractive interactions between solute and solvent l
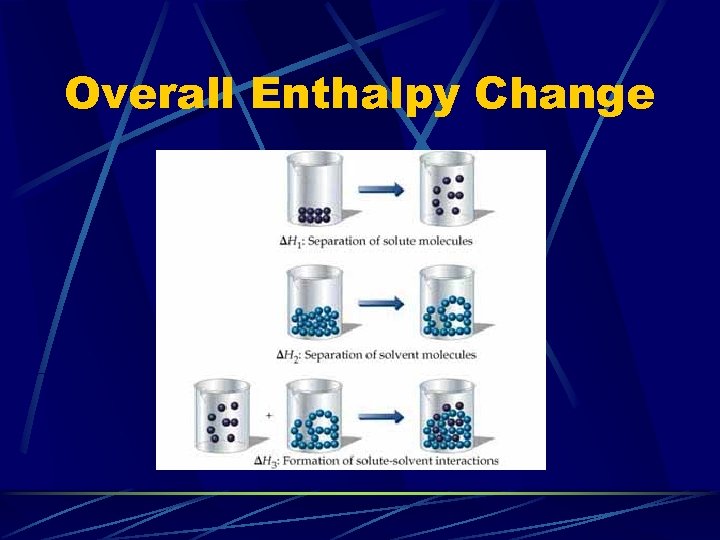 Overall Enthalpy Change
Overall Enthalpy Change
 Saturated Solutions As concentration of a solid solute increases, so does it’s chance of of colliding with the surface of the solid and becoming reattached to the solid This is called crystallization Solute + Solvent Solution
Saturated Solutions As concentration of a solid solute increases, so does it’s chance of of colliding with the surface of the solid and becoming reattached to the solid This is called crystallization Solute + Solvent Solution
 Saturated Solutions When the rates of crystallization and dissolving become equal, no increase of solute in solution will occur When a solution will not dissolve any more solute, it is a saturated solution When a solution that can still dissolve solute into it is an unsaturated solution
Saturated Solutions When the rates of crystallization and dissolving become equal, no increase of solute in solution will occur When a solution will not dissolve any more solute, it is a saturated solution When a solution that can still dissolve solute into it is an unsaturated solution
 Supersaturation Under suitable conditions, it is sometimes possible to form a solution with more solute than that needed for a saturated solution These solutions are supersaturated
Supersaturation Under suitable conditions, it is sometimes possible to form a solution with more solute than that needed for a saturated solution These solutions are supersaturated
 Supersaturation usually occurs because many solutes are more soluble at one temperature than another Example: Sodium acetate, Na. C 2 H 3 O 2, will dissolve in water more readily at higher temperatures. When a saturated solution is made at higher temperatures then slowly cooled, all of the solute may remain dissolved even though the solubility decreases
Supersaturation usually occurs because many solutes are more soluble at one temperature than another Example: Sodium acetate, Na. C 2 H 3 O 2, will dissolve in water more readily at higher temperatures. When a saturated solution is made at higher temperatures then slowly cooled, all of the solute may remain dissolved even though the solubility decreases
 Factors Affecting Solubility The stronger the intermolecular attractive forces between solute and solvent, the greater the solubility As a result of favorable dipole-dipole attractions, polar liquids tend to dissolve more readily in polar solvents Water is not only polar, but has hydrogen bonds, making solutes that have hydrogen bonds able to dissolve in water as well
Factors Affecting Solubility The stronger the intermolecular attractive forces between solute and solvent, the greater the solubility As a result of favorable dipole-dipole attractions, polar liquids tend to dissolve more readily in polar solvents Water is not only polar, but has hydrogen bonds, making solutes that have hydrogen bonds able to dissolve in water as well
 Factors Affecting Solubility Pairs of liquids that mix in all proportions are miscible Liquids that do not dissolve significantly in one another are immiscible
Factors Affecting Solubility Pairs of liquids that mix in all proportions are miscible Liquids that do not dissolve significantly in one another are immiscible
 Hydrocarbons vs. Alcohols Many hydrocarbons are immiscible in water because they are nonpolar molecules Alcohols have an OH group, which are both polar and have hydrogen bonds, making them more readily soluble in water As the carbon chain become larger, the effect of the OH group becomes smaller, meaning that larger alcohol chains begin to become less soluble
Hydrocarbons vs. Alcohols Many hydrocarbons are immiscible in water because they are nonpolar molecules Alcohols have an OH group, which are both polar and have hydrogen bonds, making them more readily soluble in water As the carbon chain become larger, the effect of the OH group becomes smaller, meaning that larger alcohol chains begin to become less soluble
 Pressure Effects Pressure only affects the solubility of gas in a solvent As pressure increases, solubility of the gas increases
Pressure Effects Pressure only affects the solubility of gas in a solvent As pressure increases, solubility of the gas increases
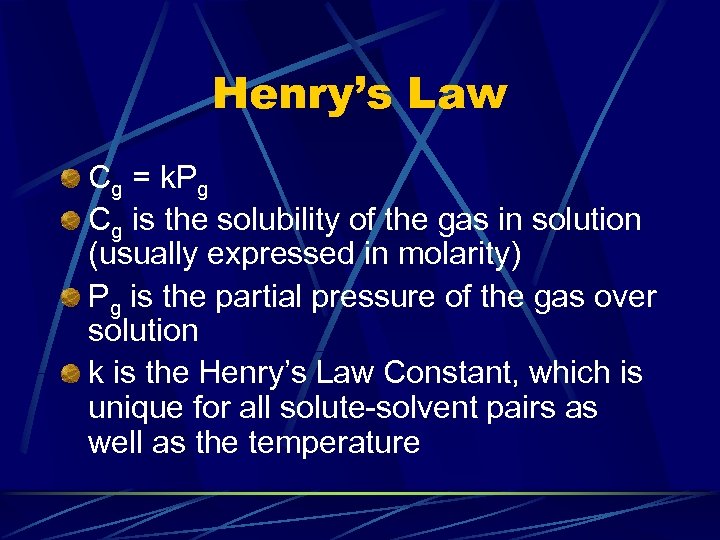 Henry’s Law Cg = k. Pg Cg is the solubility of the gas in solution (usually expressed in molarity) Pg is the partial pressure of the gas over solution k is the Henry’s Law Constant, which is unique for all solute-solvent pairs as well as the temperature
Henry’s Law Cg = k. Pg Cg is the solubility of the gas in solution (usually expressed in molarity) Pg is the partial pressure of the gas over solution k is the Henry’s Law Constant, which is unique for all solute-solvent pairs as well as the temperature
 Temperature Effects As temperature increases, the solubility of solid solutes (such as salts) normally increases In contrast, as temperature increases, the solubility of gaseous solutes normally decreases
Temperature Effects As temperature increases, the solubility of solid solutes (such as salts) normally increases In contrast, as temperature increases, the solubility of gaseous solutes normally decreases
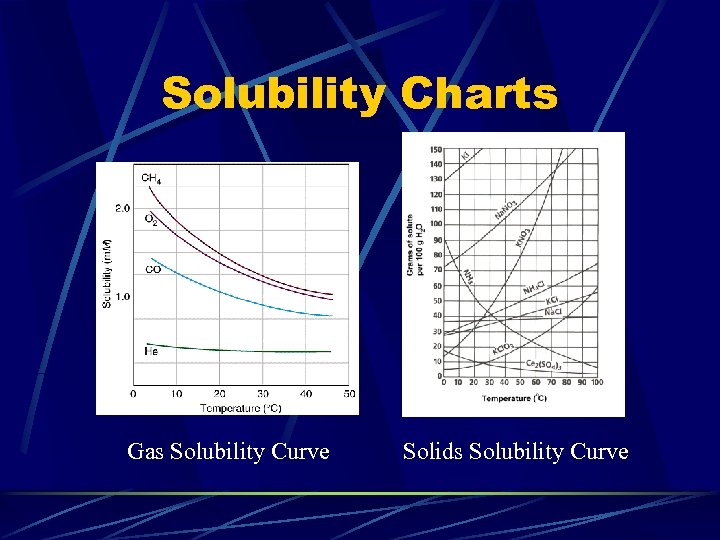 Solubility Charts Gas Solubility Curve Solids Solubility Curve
Solubility Charts Gas Solubility Curve Solids Solubility Curve
 Ways of Expressing Concentration Mass percentage, ppm Mole Fraction Molarity Molality
Ways of Expressing Concentration Mass percentage, ppm Mole Fraction Molarity Molality
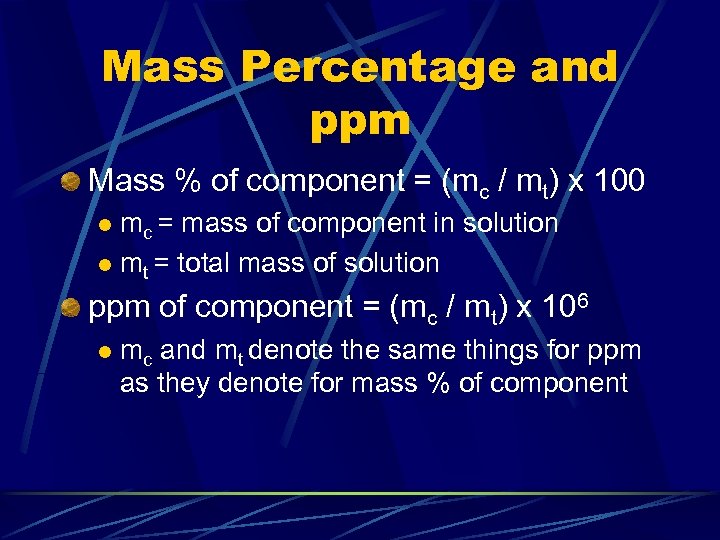 Mass Percentage and ppm Mass % of component = (mc / mt) x 100 mc = mass of component in solution l mt = total mass of solution l ppm of component = (mc / mt) x 106 l mc and mt denote the same things for ppm as they denote for mass % of component
Mass Percentage and ppm Mass % of component = (mc / mt) x 100 mc = mass of component in solution l mt = total mass of solution l ppm of component = (mc / mt) x 106 l mc and mt denote the same things for ppm as they denote for mass % of component
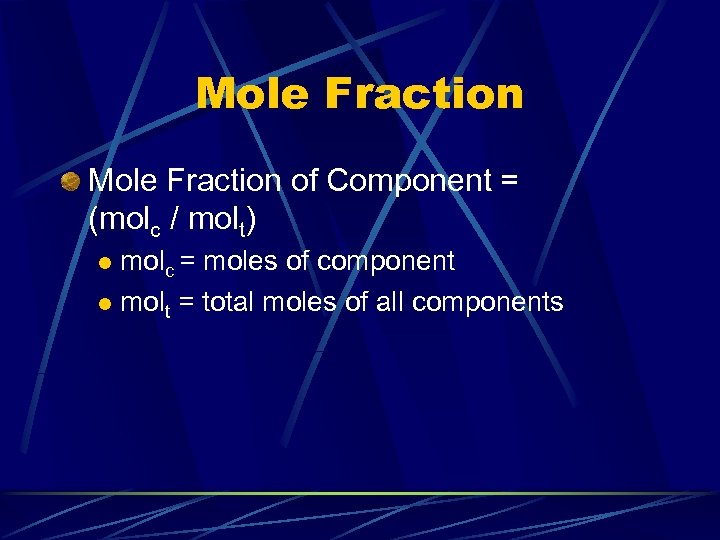 Mole Fraction of Component = (molc / molt) molc = moles of component l molt = total moles of all components l
Mole Fraction of Component = (molc / molt) molc = moles of component l molt = total moles of all components l
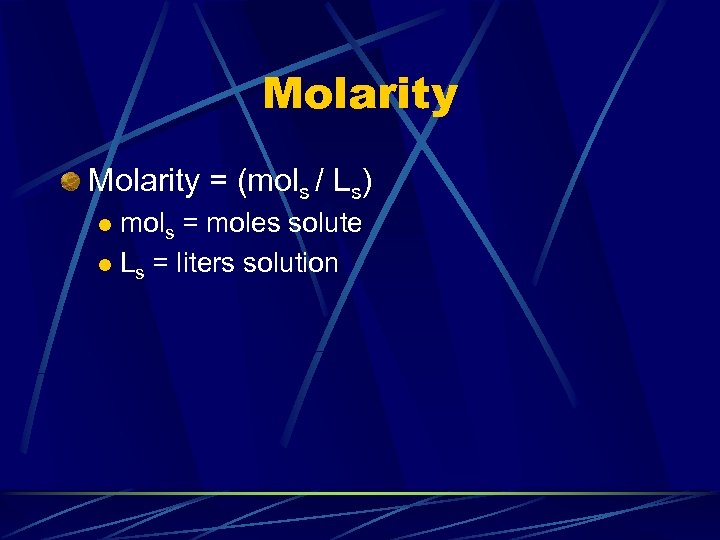 Molarity = (mols / Ls) mols = moles solute l Ls = liters solution l
Molarity = (mols / Ls) mols = moles solute l Ls = liters solution l
 Molality = (mols / kgs) mols = moles solute l kgs = kilograms solvent l
Molality = (mols / kgs) mols = moles solute l kgs = kilograms solvent l
 Colligative Properties Colligative properties depend on the quanity of solute, not the type of solute The colligative properties are: Vapor-Pressure Reduction l Boiling-Point Elevation l Freezing-Point Depression l Osmotic Pressure l
Colligative Properties Colligative properties depend on the quanity of solute, not the type of solute The colligative properties are: Vapor-Pressure Reduction l Boiling-Point Elevation l Freezing-Point Depression l Osmotic Pressure l
 Vapor-Pressure Reduction As the amount of solute increases, the vapor pressure of solution decreases This relationship can be expessed through Raoult’s Law: P A = X A P o. A l PA = Partial pressure exerted by solvent l XA = Mole fraction of solvent l Po. A = Vapor pressure of pure solvent l
Vapor-Pressure Reduction As the amount of solute increases, the vapor pressure of solution decreases This relationship can be expessed through Raoult’s Law: P A = X A P o. A l PA = Partial pressure exerted by solvent l XA = Mole fraction of solvent l Po. A = Vapor pressure of pure solvent l
 Boiling-Point Elevation As amount of solute increase, boiling point increases This relationship can be expressed as: DTb = d. Kbm l l DTb = total boiling point elevation d = dissociation factor of the solute Kb = molal boiling point elevation constant solvent m = molality of solution of the
Boiling-Point Elevation As amount of solute increase, boiling point increases This relationship can be expressed as: DTb = d. Kbm l l DTb = total boiling point elevation d = dissociation factor of the solute Kb = molal boiling point elevation constant solvent m = molality of solution of the
 Freezing-Point Depression As amount of solute increases, freezing point decreases This relationship can be expressed as: DTf = d. Kfm l l DTf = total freezing point depression d = dissociation factor of the solute Kf = molal freezing point depression constant of the solvent m = molality of solution
Freezing-Point Depression As amount of solute increases, freezing point decreases This relationship can be expressed as: DTf = d. Kfm l l DTf = total freezing point depression d = dissociation factor of the solute Kf = molal freezing point depression constant of the solvent m = molality of solution
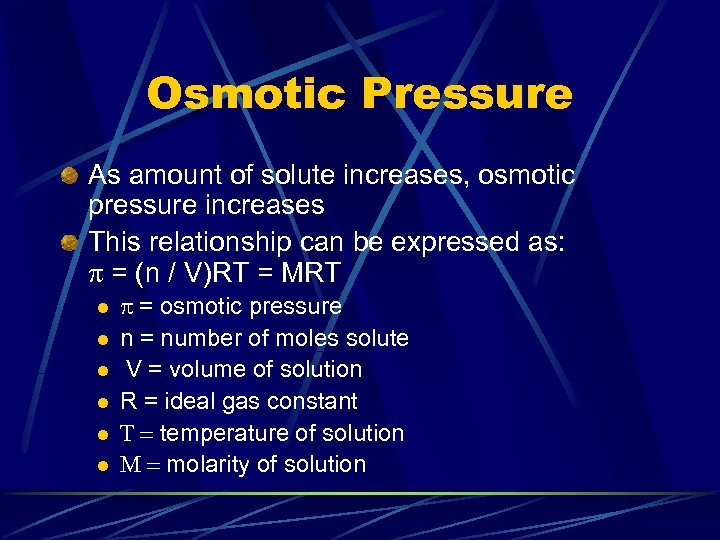 Osmotic Pressure As amount of solute increases, osmotic pressure increases This relationship can be expressed as: p = (n / V)RT = MRT l l l p = osmotic pressure n = number of moles solute V = volume of solution R = ideal gas constant T = temperature of solution M = molarity of solution
Osmotic Pressure As amount of solute increases, osmotic pressure increases This relationship can be expressed as: p = (n / V)RT = MRT l l l p = osmotic pressure n = number of moles solute V = volume of solution R = ideal gas constant T = temperature of solution M = molarity of solution


