516af8faa9344df02ea7d7e422bdef2c.ppt
- Количество слайдов: 86
 CHAPTER 11 Intermolecular Forces, Liquids and Solids 1
CHAPTER 11 Intermolecular Forces, Liquids and Solids 1
 Description of Liquids & Solids • Solids & liquids are condensed states • Liquids & gases are fluids • • • atoms, ions, molecules are close to one another highly incompressible Solid molecules are packed closely together. The molecules are so rigidly packed that they cannot easily slide past each other. easily flow Liquid molecules are held closer together than gas molecules, but not so rigidly that the molecules cannot slide past each other. 2
Description of Liquids & Solids • Solids & liquids are condensed states • Liquids & gases are fluids • • • atoms, ions, molecules are close to one another highly incompressible Solid molecules are packed closely together. The molecules are so rigidly packed that they cannot easily slide past each other. easily flow Liquid molecules are held closer together than gas molecules, but not so rigidly that the molecules cannot slide past each other. 2
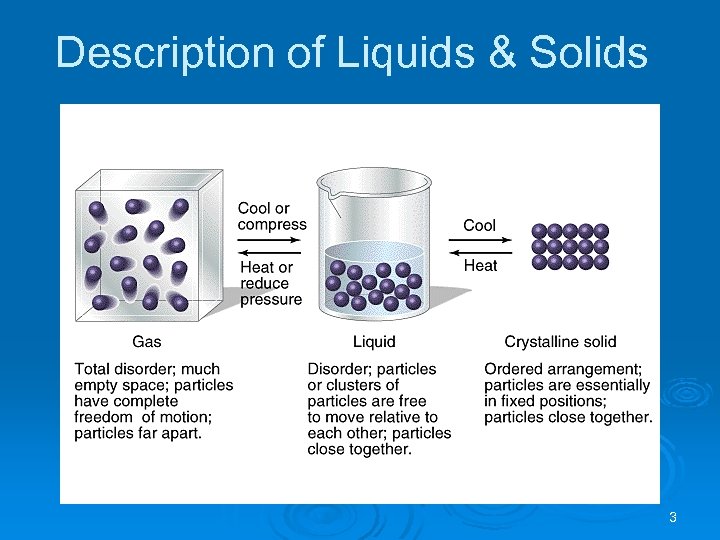 Description of Liquids & Solids 3
Description of Liquids & Solids 3
 Description of Liquids & Solids Ø Converting a gas into a liquid or solid requires the molecules to get closer to each other: l Ø Converting a solid into a liquid or gas requires the molecules to move further apart: l Ø cool or compress. heat or reduce pressure. The forces holding solids and liquids together are called intermolecular forces. 4
Description of Liquids & Solids Ø Converting a gas into a liquid or solid requires the molecules to get closer to each other: l Ø Converting a solid into a liquid or gas requires the molecules to move further apart: l Ø cool or compress. heat or reduce pressure. The forces holding solids and liquids together are called intermolecular forces. 4
 Kinetic-Molecular Description of Liquids & Solids strengths of interactions among particles & degree of ordering of particles Gases< Liquids < Solids 5
Kinetic-Molecular Description of Liquids & Solids strengths of interactions among particles & degree of ordering of particles Gases< Liquids < Solids 5
 Intermolecular Attractions The covalent bond holding a molecule together is an intramolecular forces. Ø The attraction between molecules is an intermolecular force. Ø Intermolecular forces are much weaker than intramolecular forces (e. g. 16 k. J/mol vs. 431 k. J/mol for HCl). Ø When a substance melts or boils the intermolecular forces are broken (not the covalent bonds). Ø When a substance condenses intermolecular forces are formed. Ø 6
Intermolecular Attractions The covalent bond holding a molecule together is an intramolecular forces. Ø The attraction between molecules is an intermolecular force. Ø Intermolecular forces are much weaker than intramolecular forces (e. g. 16 k. J/mol vs. 431 k. J/mol for HCl). Ø When a substance melts or boils the intermolecular forces are broken (not the covalent bonds). Ø When a substance condenses intermolecular forces are formed. Ø 6
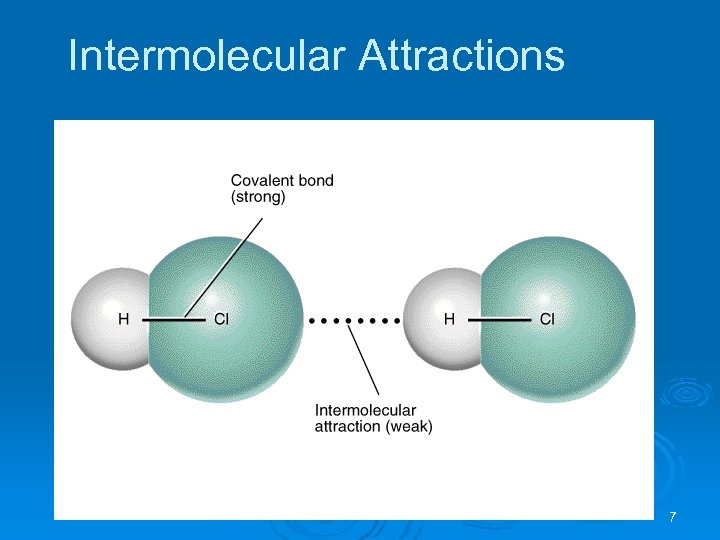 Intermolecular Attractions 7
Intermolecular Attractions 7
 Intermolecular Attractions Ion-Dipole Forces Ø Ion-dipole forces exist between an ion and a the partial charge on the end of a polar molecule. Ø Positive ions are attracted to the negative end of the dipole (and vice versa) Ø Magnitude of the attraction increases with increasing ionic charge or dipole moment. Ø Important when ionic compound are dissolved in water. 8
Intermolecular Attractions Ion-Dipole Forces Ø Ion-dipole forces exist between an ion and a the partial charge on the end of a polar molecule. Ø Positive ions are attracted to the negative end of the dipole (and vice versa) Ø Magnitude of the attraction increases with increasing ionic charge or dipole moment. Ø Important when ionic compound are dissolved in water. 8
 Intermolecular Attractions Dipole-Dipole Forces Ø Dipole-dipole forces exist between neutral polar molecules. Ø Polar molecules need to be close together. Ø Weaker than ion-dipole forces: l Q 1 and Q 2 are partial charges. 9
Intermolecular Attractions Dipole-Dipole Forces Ø Dipole-dipole forces exist between neutral polar molecules. Ø Polar molecules need to be close together. Ø Weaker than ion-dipole forces: l Q 1 and Q 2 are partial charges. 9
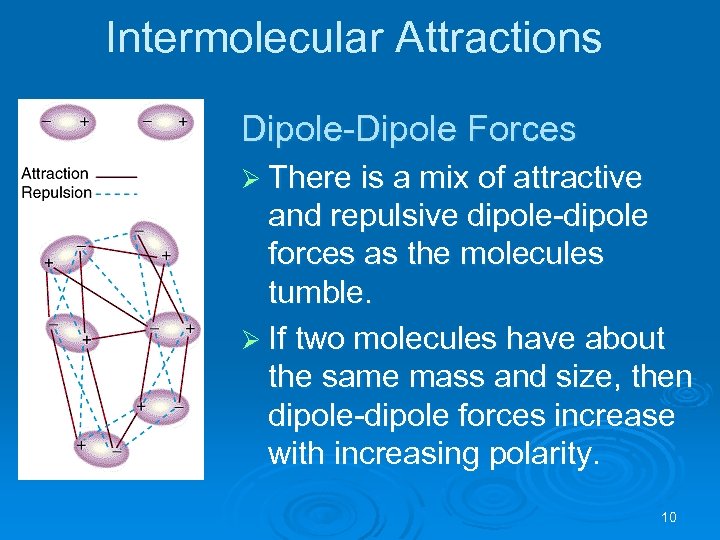 Intermolecular Attractions Dipole-Dipole Forces Ø There is a mix of attractive and repulsive dipole-dipole forces as the molecules tumble. Ø If two molecules have about the same mass and size, then dipole-dipole forces increase with increasing polarity. 10
Intermolecular Attractions Dipole-Dipole Forces Ø There is a mix of attractive and repulsive dipole-dipole forces as the molecules tumble. Ø If two molecules have about the same mass and size, then dipole-dipole forces increase with increasing polarity. 10
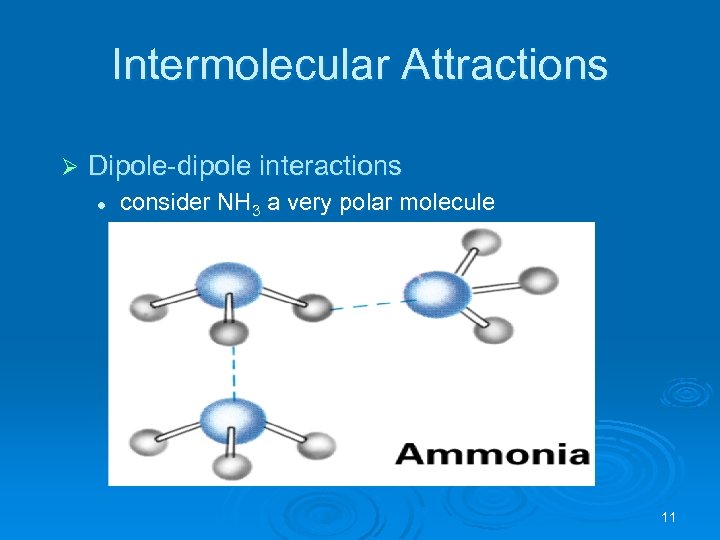 Intermolecular Attractions Ø Dipole-dipole interactions l consider NH 3 a very polar molecule 11
Intermolecular Attractions Ø Dipole-dipole interactions l consider NH 3 a very polar molecule 11
 Intermolecular Attractions London Dispersion Forces Weakest of all intermolecular forces. Ø It is possible for two adjacent neutral molecules to affect each other. Ø The nucleus of one molecule (or atom) attracts the electrons of the adjacent molecule (or atom). Ø For an instant, the electron clouds become distorted. Ø In that instant a dipole is formed (called an instantaneous dipole). Ø 12
Intermolecular Attractions London Dispersion Forces Weakest of all intermolecular forces. Ø It is possible for two adjacent neutral molecules to affect each other. Ø The nucleus of one molecule (or atom) attracts the electrons of the adjacent molecule (or atom). Ø For an instant, the electron clouds become distorted. Ø In that instant a dipole is formed (called an instantaneous dipole). Ø 12
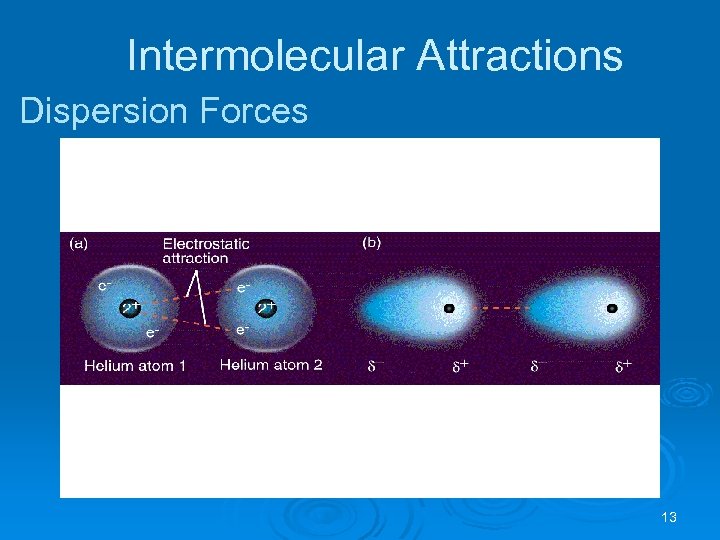 Intermolecular Attractions Dispersion Forces 13
Intermolecular Attractions Dispersion Forces 13
 Intermolecular Attractions Ø Polarizability is the ease with which an electron cloud can be deformed. Ø The larger the molecule (the greater the number of electrons) the more polarizable. 14
Intermolecular Attractions Ø Polarizability is the ease with which an electron cloud can be deformed. Ø The larger the molecule (the greater the number of electrons) the more polarizable. 14
 Intermolecular Attractions 15
Intermolecular Attractions 15
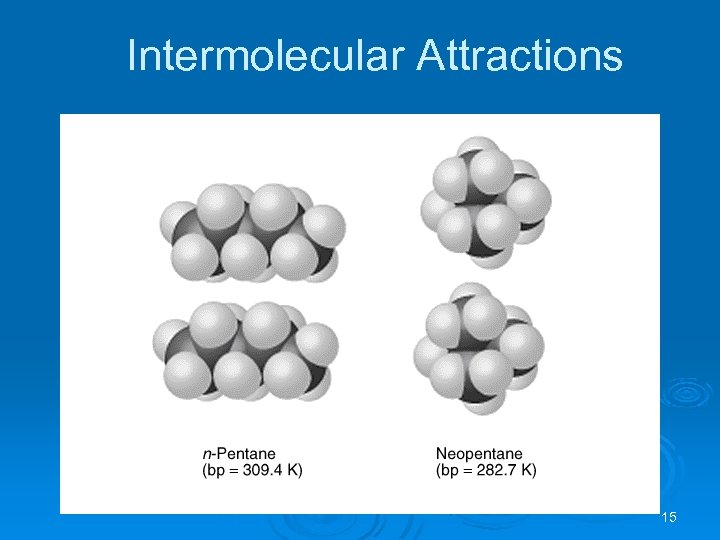
 Intermolecular Attractions Dispersion Forces Ø London dispersion forces depend on the shape of the molecule. Ø The greater the surface area available for contact, the greater the dispersion forces. Ø London dispersion forces between spherical molecules are smaller than between sausage-like molecules. 16
Intermolecular Attractions Dispersion Forces Ø London dispersion forces depend on the shape of the molecule. Ø The greater the surface area available for contact, the greater the dispersion forces. Ø London dispersion forces between spherical molecules are smaller than between sausage-like molecules. 16
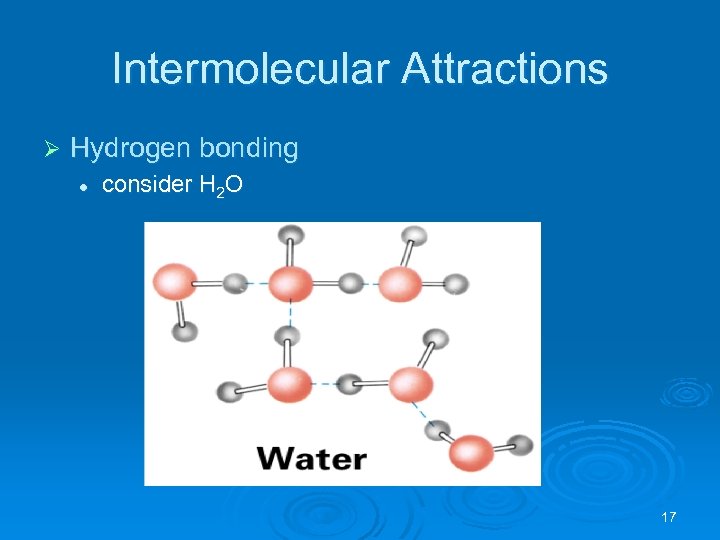 Intermolecular Attractions Ø Hydrogen bonding l consider H 2 O 17
Intermolecular Attractions Ø Hydrogen bonding l consider H 2 O 17
 Intermolecular Attractions Hydrogen Bonding Ø Special case of dipole-dipole forces. Ø By experiments: boiling points of compounds with H-F, H-O, and H-N bonds are abnormally high. Ø Intermolecular forces are abnormally strong. 18
Intermolecular Attractions Hydrogen Bonding Ø Special case of dipole-dipole forces. Ø By experiments: boiling points of compounds with H-F, H-O, and H-N bonds are abnormally high. Ø Intermolecular forces are abnormally strong. 18
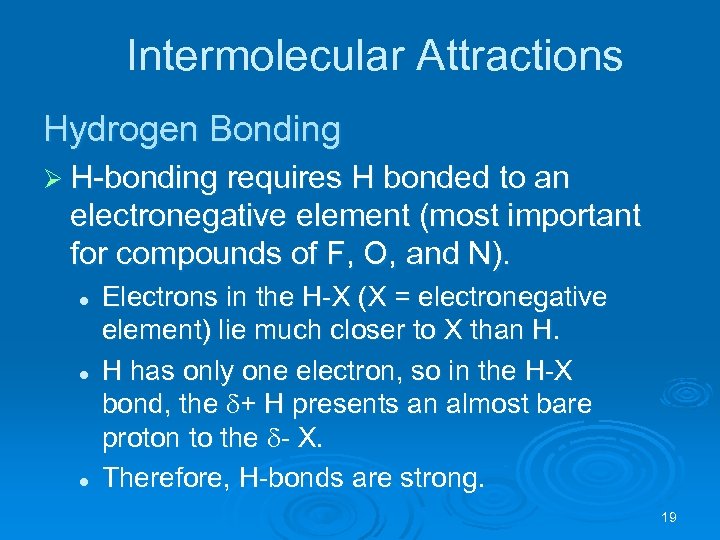 Intermolecular Attractions Hydrogen Bonding Ø H-bonding requires H bonded to an electronegative element (most important for compounds of F, O, and N). l l l Electrons in the H-X (X = electronegative element) lie much closer to X than H. H has only one electron, so in the H-X bond, the + H presents an almost bare proton to the - X. Therefore, H-bonds are strong. 19
Intermolecular Attractions Hydrogen Bonding Ø H-bonding requires H bonded to an electronegative element (most important for compounds of F, O, and N). l l l Electrons in the H-X (X = electronegative element) lie much closer to X than H. H has only one electron, so in the H-X bond, the + H presents an almost bare proton to the - X. Therefore, H-bonds are strong. 19
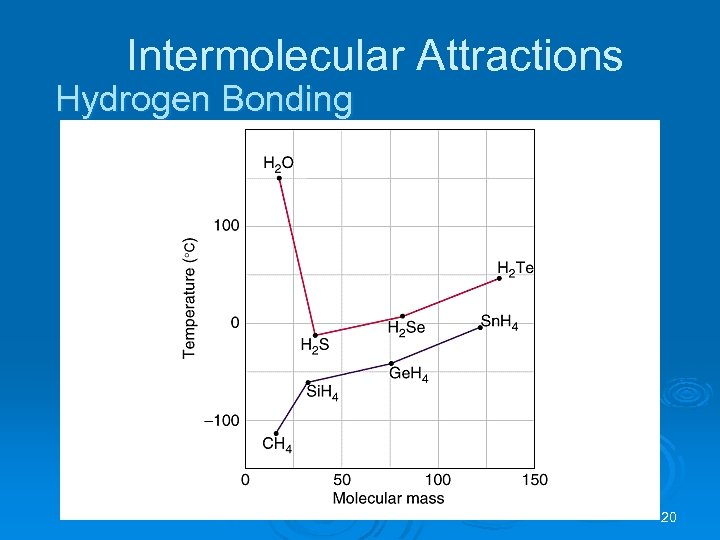 Intermolecular Attractions Hydrogen Bonding 20
Intermolecular Attractions Hydrogen Bonding 20
 Intermolecular Attractions Hydrogen Bonding l Ice Floating • • • Solids are usually more closely packed than liquids; therefore, solids are more dense than liquids. Ice is ordered with an open structure to optimize H-bonding. Therefore, ice is less dense than water. In water the H-O bond length is 1. 0 Å. The O…H hydrogen bond length is 1. 8 Å. Ice has waters arranged in an open, regular hexagon. Each + H points towards a lone pair on O. Ice floats, so it forms an insulating layer on top of lakes, rivers, etc. Therefore, aquatic life can survive in winter. 21
Intermolecular Attractions Hydrogen Bonding l Ice Floating • • • Solids are usually more closely packed than liquids; therefore, solids are more dense than liquids. Ice is ordered with an open structure to optimize H-bonding. Therefore, ice is less dense than water. In water the H-O bond length is 1. 0 Å. The O…H hydrogen bond length is 1. 8 Å. Ice has waters arranged in an open, regular hexagon. Each + H points towards a lone pair on O. Ice floats, so it forms an insulating layer on top of lakes, rivers, etc. Therefore, aquatic life can survive in winter. 21
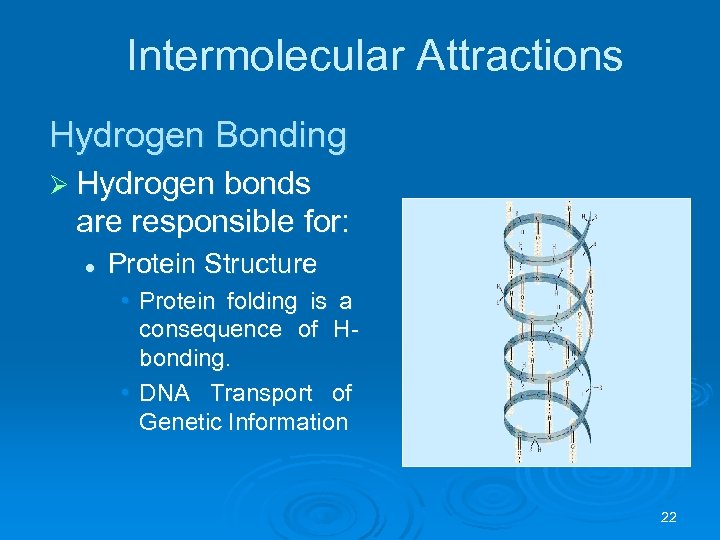 Intermolecular Attractions Hydrogen Bonding Ø Hydrogen bonds are responsible for: l Protein Structure • Protein folding is a consequence of Hbonding. • DNA Transport of Genetic Information 22
Intermolecular Attractions Hydrogen Bonding Ø Hydrogen bonds are responsible for: l Protein Structure • Protein folding is a consequence of Hbonding. • DNA Transport of Genetic Information 22
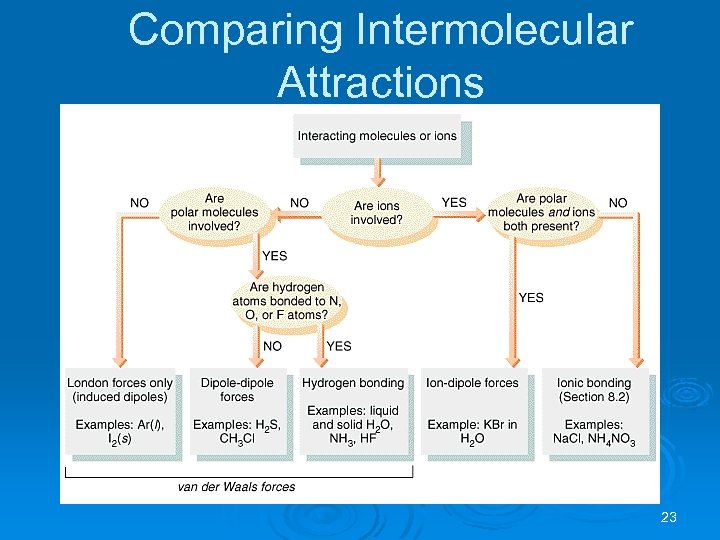 Comparing Intermolecular Attractions 23
Comparing Intermolecular Attractions 23
 Intermolecular Attractions Ø Intermolecular interactions determine: l l melting & boiling points of ionic compounds the solubility of ionic compounds Ø Arrange the following ionic compounds in the expected order of increasing melting and boiling points. Na. F, Ca. O, Ca. F 2 24
Intermolecular Attractions Ø Intermolecular interactions determine: l l melting & boiling points of ionic compounds the solubility of ionic compounds Ø Arrange the following ionic compounds in the expected order of increasing melting and boiling points. Na. F, Ca. O, Ca. F 2 24
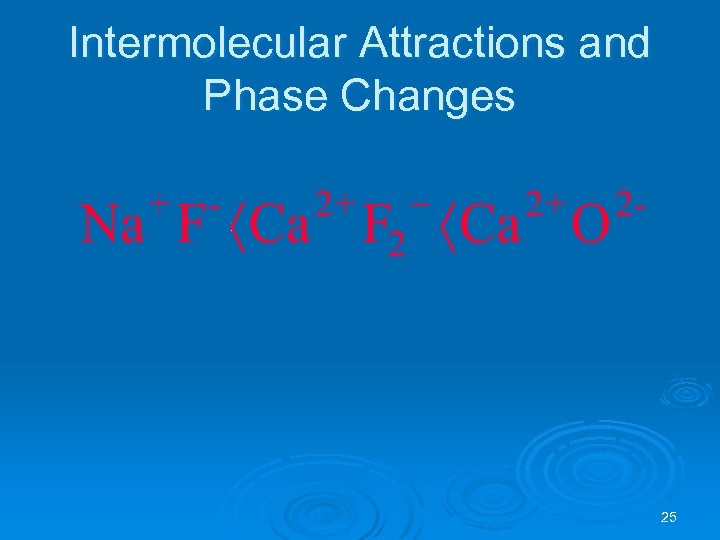 Intermolecular Attractions and Phase Changes 25
Intermolecular Attractions and Phase Changes 25
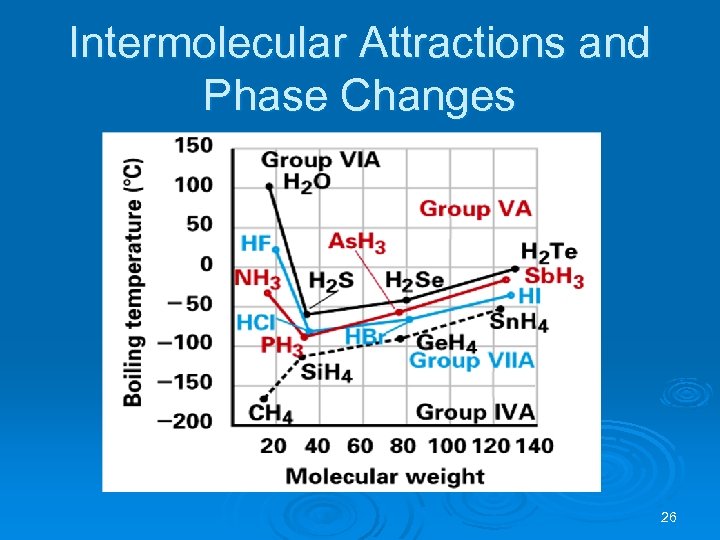 Intermolecular Attractions and Phase Changes 26
Intermolecular Attractions and Phase Changes 26
 Vapor Pressure pressure exerted by a liquid’s vapor on its surface at equilibrium Ø Vap. Press. (torr) for 3 Liquids 0 o. C Ø diethyl ether Ø ethanol Ø water Norm. B. P. 20 o. C 30 o. C 185 442 647 12 44 74 5 18 32 36 o. C 78 o. C 100 o. C 27
Vapor Pressure pressure exerted by a liquid’s vapor on its surface at equilibrium Ø Vap. Press. (torr) for 3 Liquids 0 o. C Ø diethyl ether Ø ethanol Ø water Norm. B. P. 20 o. C 30 o. C 185 442 647 12 44 74 5 18 32 36 o. C 78 o. C 100 o. C 27
 Vapor Pressure Some of the molecules on the surface of a liquid have enough energy to escape the attraction of the bulk liquid. Ø These molecules move into the gas phase. Ø As the number of molecules in the gas phase increases, some of the gas phase molecules strike the surface and return to the liquid. Ø After some time the pressure of the gas will be constant at the vapor pressure. Ø 28
Vapor Pressure Some of the molecules on the surface of a liquid have enough energy to escape the attraction of the bulk liquid. Ø These molecules move into the gas phase. Ø As the number of molecules in the gas phase increases, some of the gas phase molecules strike the surface and return to the liquid. Ø After some time the pressure of the gas will be constant at the vapor pressure. Ø 28
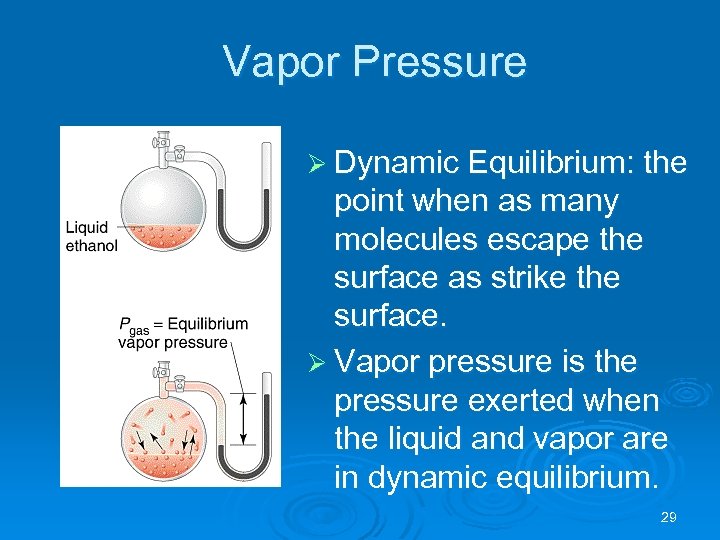 Vapor Pressure Ø Dynamic Equilibrium: the point when as many molecules escape the surface as strike the surface. Ø Vapor pressure is the pressure exerted when the liquid and vapor are in dynamic equilibrium. 29
Vapor Pressure Ø Dynamic Equilibrium: the point when as many molecules escape the surface as strike the surface. Ø Vapor pressure is the pressure exerted when the liquid and vapor are in dynamic equilibrium. 29
 Vapor Pressure Ø If equilibrium is never established then the liquid evaporates. Ø Volatile substances evaporate rapidly. Ø The higher the temperature, the higher the average kinetic energy, the faster the liquid evaporates. 30
Vapor Pressure Ø If equilibrium is never established then the liquid evaporates. Ø Volatile substances evaporate rapidly. Ø The higher the temperature, the higher the average kinetic energy, the faster the liquid evaporates. 30
 Vapor Pressure Liquids boil when the external pressure equals the vapor pressure. Ø Temperature of boiling point increases as pressure increases. Ø Two ways to get a liquid to boil: increase temperature or decrease pressure. Ø l Pressure cookers operate at high pressure. At high pressure the boiling point of water is higher than at 1 atm. Therefore, there is a higher temperature at which the food is cooked, reducing the cooking time required. 31
Vapor Pressure Liquids boil when the external pressure equals the vapor pressure. Ø Temperature of boiling point increases as pressure increases. Ø Two ways to get a liquid to boil: increase temperature or decrease pressure. Ø l Pressure cookers operate at high pressure. At high pressure the boiling point of water is higher than at 1 atm. Therefore, there is a higher temperature at which the food is cooked, reducing the cooking time required. 31
 Boiling Points Ø Boiling point is the temperature at which the liquid’s vapor pressure is equal to the applied pressure l normal boiling point is boiling point @ 1 atm 32
Boiling Points Ø Boiling point is the temperature at which the liquid’s vapor pressure is equal to the applied pressure l normal boiling point is boiling point @ 1 atm 32
 Distillation Ø Process in which a mixture or solution is separated into its components on the basis of the differences in boiling points of the components l Distillation is another vapor pressure phenomenon. 33
Distillation Ø Process in which a mixture or solution is separated into its components on the basis of the differences in boiling points of the components l Distillation is another vapor pressure phenomenon. 33
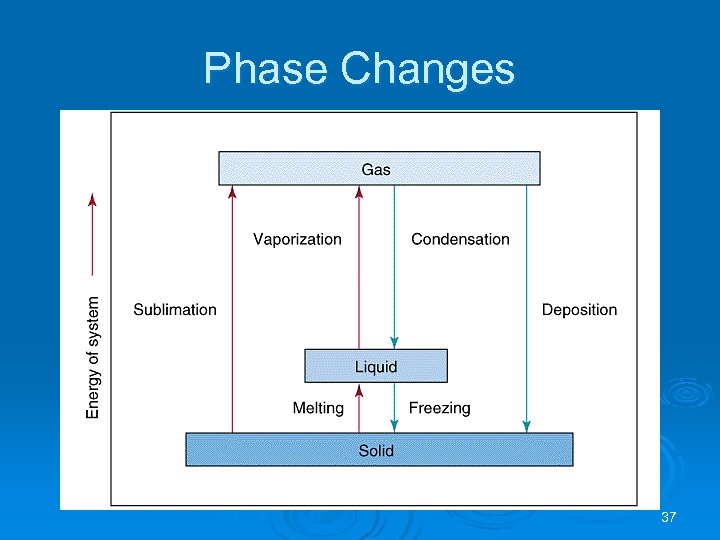
 Phase Changes Ø Surface molecules are only attracted inwards towards the bulk molecules. Ø Sublimation: solid gas. Ø Vaporization: liquid gas. Ø Melting or fusion: solid liquid. Ø Deposition: gas solid. Ø Condensation: gas liquid. Ø Freezing: liquid solid. 34
Phase Changes Ø Surface molecules are only attracted inwards towards the bulk molecules. Ø Sublimation: solid gas. Ø Vaporization: liquid gas. Ø Melting or fusion: solid liquid. Ø Deposition: gas solid. Ø Condensation: gas liquid. Ø Freezing: liquid solid. 34
 Phase Changes l l l Sublimation: Hsub > 0 (endothermic). Vaporization: Hvap > 0 (endothermic). Melting or Fusion: Hfus > 0 (endothermic). Deposition: Hdep < 0 (exothermic). Condensation: Hcon < 0 (exothermic). Freezing: Hfre < 0 (exothermic). Ø Generally heat of fusion (enthalpy of fusion) is less than heat of vaporization: l it takes more energy to completely separate molecules, than partially separate them. 35
Phase Changes l l l Sublimation: Hsub > 0 (endothermic). Vaporization: Hvap > 0 (endothermic). Melting or Fusion: Hfus > 0 (endothermic). Deposition: Hdep < 0 (exothermic). Condensation: Hcon < 0 (exothermic). Freezing: Hfre < 0 (exothermic). Ø Generally heat of fusion (enthalpy of fusion) is less than heat of vaporization: l it takes more energy to completely separate molecules, than partially separate them. 35
 Phase Changes All phase changes are possible under the right conditions (e. g. water sublimes when snow disappears without forming puddles). Ø The sequence heat solid melt heat liquid boil heat gas is endothermic. Ø The sequence cool gas condense cool liquid freeze cool solid is exothermic. 36
Phase Changes All phase changes are possible under the right conditions (e. g. water sublimes when snow disappears without forming puddles). Ø The sequence heat solid melt heat liquid boil heat gas is endothermic. Ø The sequence cool gas condense cool liquid freeze cool solid is exothermic. 36
 Phase Changes 37
Phase Changes 37
 Phase Changes Ø Plot of temperature change versus heat added is a heating curve. Ø During a phase change, adding heat causes no temperature change. l These points are used to calculate Hfus and Hvap. Ø Supercooling: When a liquid is cooled below its melting point and it still remains a liquid. Ø Achieved by keeping the temperature low and increasing kinetic energy to break intermolecular forces. 38
Phase Changes Ø Plot of temperature change versus heat added is a heating curve. Ø During a phase change, adding heat causes no temperature change. l These points are used to calculate Hfus and Hvap. Ø Supercooling: When a liquid is cooled below its melting point and it still remains a liquid. Ø Achieved by keeping the temperature low and increasing kinetic energy to break intermolecular forces. 38
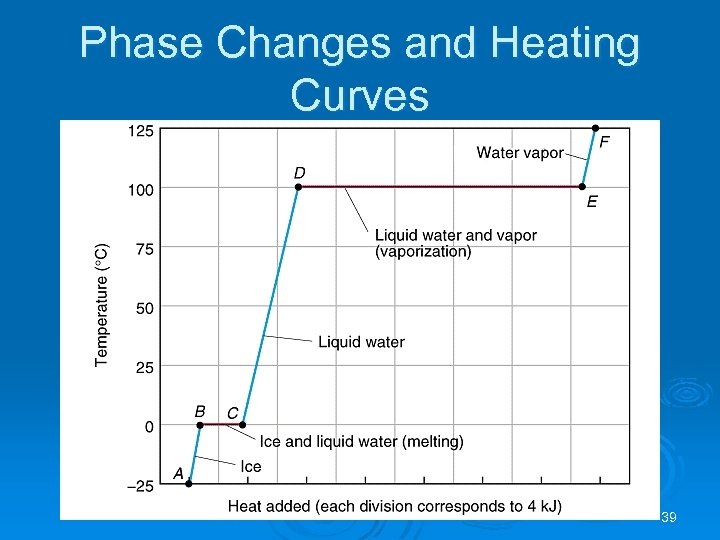 Phase Changes and Heating Curves 39
Phase Changes and Heating Curves 39
 Critical Temperature and Pressure Gases liquefied by increasing pressure at some temperature. Ø Critical temperature: the maximum temperature a liquid can exist. Ø Critical pressure: pressure required for liquefaction at the critical temperature. 40
Critical Temperature and Pressure Gases liquefied by increasing pressure at some temperature. Ø Critical temperature: the maximum temperature a liquid can exist. Ø Critical pressure: pressure required for liquefaction at the critical temperature. 40
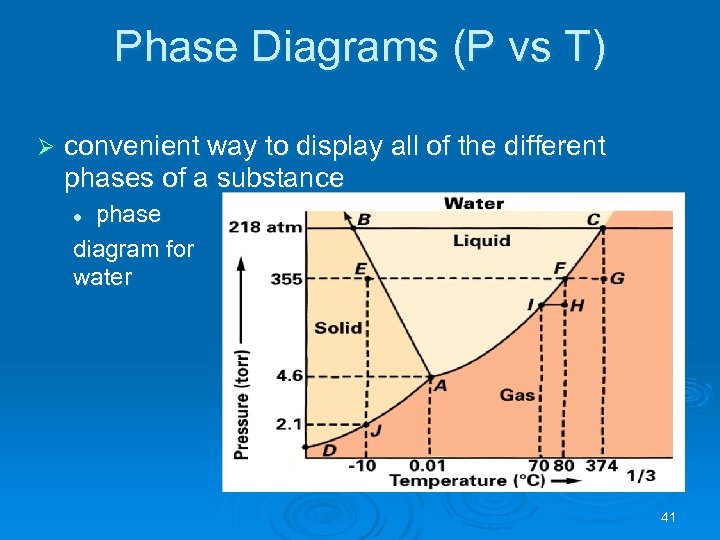 Phase Diagrams (P vs T) Ø convenient way to display all of the different phases of a substance phase diagram for water l 41
Phase Diagrams (P vs T) Ø convenient way to display all of the different phases of a substance phase diagram for water l 41
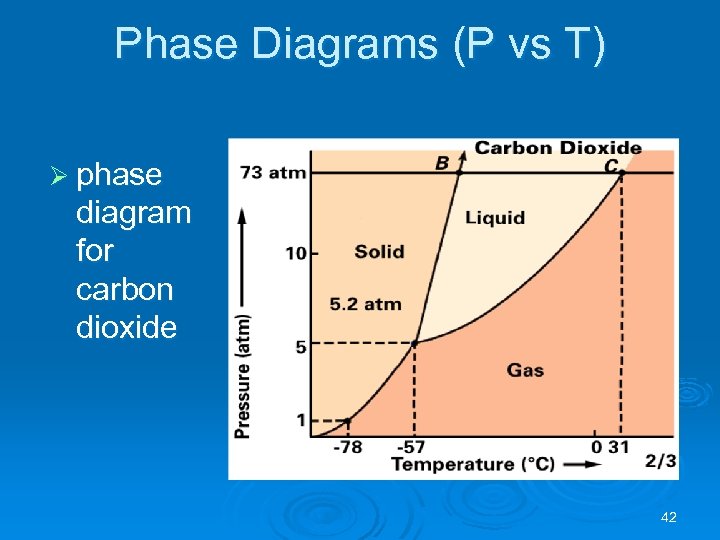 Phase Diagrams (P vs T) Ø phase diagram for carbon dioxide 42
Phase Diagrams (P vs T) Ø phase diagram for carbon dioxide 42
 Structures of Solids have maximum intermolecular forces. Ø Molecular crystals are formed by close packing of the molecules (model by packing spheres). Ø We rationalize maximum intermolecular force in a crystal by the close packing of spheres. Ø When spheres are packed as closely as possible, there are small spaces between adjacent spheres. Ø The spaces are called interstitial holes. Ø 43
Structures of Solids have maximum intermolecular forces. Ø Molecular crystals are formed by close packing of the molecules (model by packing spheres). Ø We rationalize maximum intermolecular force in a crystal by the close packing of spheres. Ø When spheres are packed as closely as possible, there are small spaces between adjacent spheres. Ø The spaces are called interstitial holes. Ø 43
 Structures of Solids 44
Structures of Solids 44
 Structures of Solids A crystal is built up by placing close packed layers of spheres on top of each other. Ø There is only one place for the second layer of spheres. Ø There are two choices for the third layer of spheres: Ø l l Third layer eclipses the first (ABAB arrangement). This is called hexagonal close packing (hcp); Third layer is in a different position relative to the first (ABCABC arrangement). This is called cubic close packing (ccp). 45
Structures of Solids A crystal is built up by placing close packed layers of spheres on top of each other. Ø There is only one place for the second layer of spheres. Ø There are two choices for the third layer of spheres: Ø l l Third layer eclipses the first (ABAB arrangement). This is called hexagonal close packing (hcp); Third layer is in a different position relative to the first (ABCABC arrangement). This is called cubic close packing (ccp). 45
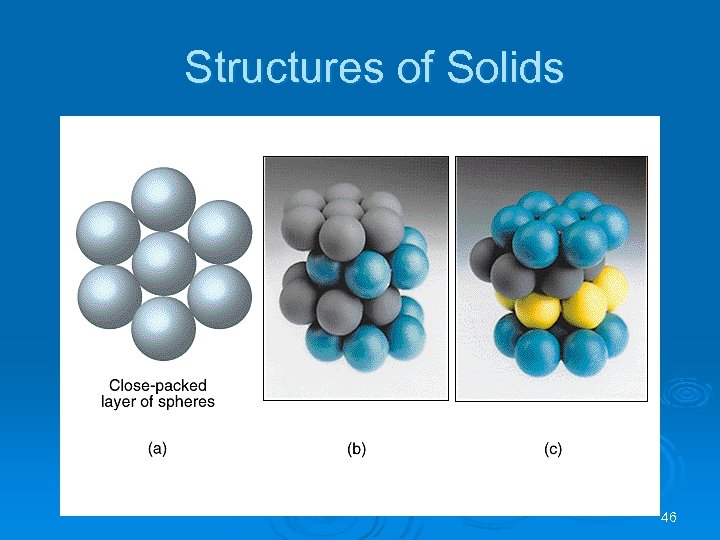 Structures of Solids 46
Structures of Solids 46
 Structures of Solids Close Packing of Spheres Each sphere is surrounded by 12 other spheres (6 in one plane, 3 above and 3 below). Ø Coordination number: the number of spheres directly surrounding a central sphere. Ø Hexagonal and cubic close packing are different from the cubic unit cells. Ø If unequally sized spheres are used, the smaller spheres are placed in the interstitial holes. Ø 47
Structures of Solids Close Packing of Spheres Each sphere is surrounded by 12 other spheres (6 in one plane, 3 above and 3 below). Ø Coordination number: the number of spheres directly surrounding a central sphere. Ø Hexagonal and cubic close packing are different from the cubic unit cells. Ø If unequally sized spheres are used, the smaller spheres are placed in the interstitial holes. Ø 47
 Structures of Solids X-Ray Diffraction When waves are passed through a narrow slit they are diffracted. Ø When waves are passed through a diffraction grating (many narrow slits in parallel) they interact to form a diffraction pattern (areas of light and dark bands). Ø Efficient diffraction occurs when the wavelength of light is close to the size of the slits. Ø The spacing between layers in a crystal is 2 - 20 Å, which is the wavelength range for X-rays. Ø 48
Structures of Solids X-Ray Diffraction When waves are passed through a narrow slit they are diffracted. Ø When waves are passed through a diffraction grating (many narrow slits in parallel) they interact to form a diffraction pattern (areas of light and dark bands). Ø Efficient diffraction occurs when the wavelength of light is close to the size of the slits. Ø The spacing between layers in a crystal is 2 - 20 Å, which is the wavelength range for X-rays. Ø 48
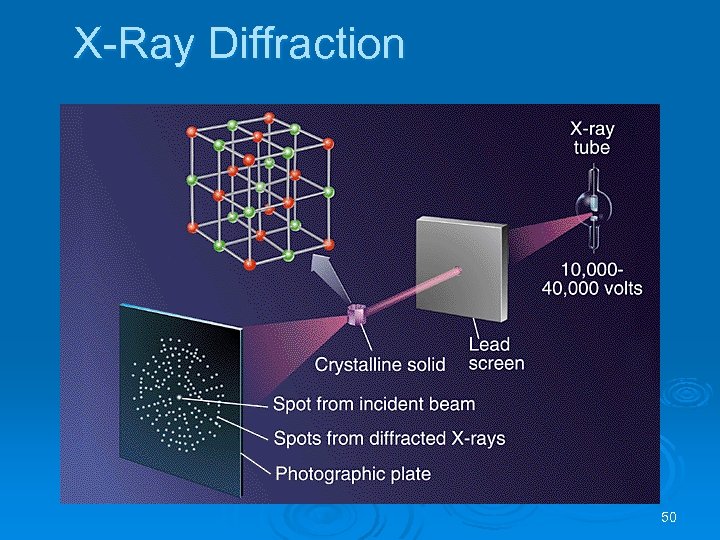
 Structures of Solids X-ray diffraction (X-ray crystallography): l l l X-rays are passed through the crystal and are detected on a photographic plate. The photographic plate has one bright spot at the center (incident beam) as well as a diffraction pattern. Each close packing arrangement produces a different diffraction pattern. Knowing the diffraction pattern, we can calculate the positions of the atoms required to produce that pattern. We calculate the crystal structure based on a knowledge of the diffraction pattern. 49
Structures of Solids X-ray diffraction (X-ray crystallography): l l l X-rays are passed through the crystal and are detected on a photographic plate. The photographic plate has one bright spot at the center (incident beam) as well as a diffraction pattern. Each close packing arrangement produces a different diffraction pattern. Knowing the diffraction pattern, we can calculate the positions of the atoms required to produce that pattern. We calculate the crystal structure based on a knowledge of the diffraction pattern. 49
 X-Ray Diffraction 50
X-Ray Diffraction 50
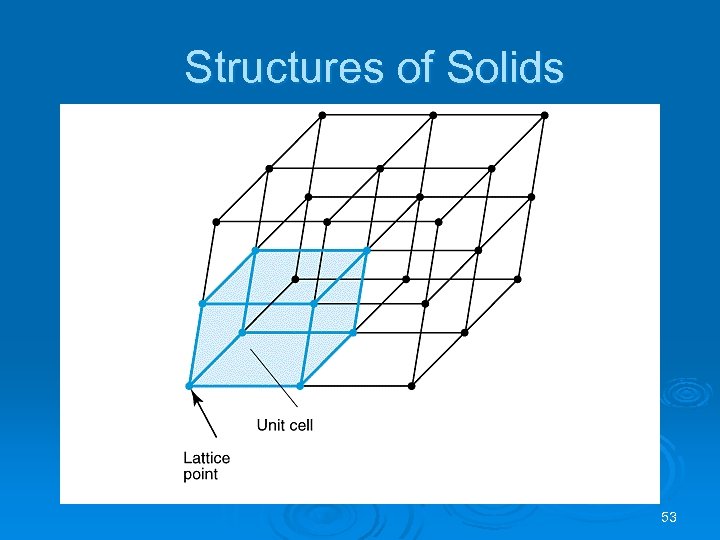
 Structures of Solids Unit Cells Crystalline solid: well-ordered, definite arrangements of molecules, atoms or ions. Ø Crystals have an ordered, repeated structure. Ø The smallest repeating unit in a crystal is a unit cell. Ø Unit cell is the smallest unit with all the symmetry of the entire crystal. Ø Three-dimensional stacking of unit cells is the crystal lattice. Ø 51
Structures of Solids Unit Cells Crystalline solid: well-ordered, definite arrangements of molecules, atoms or ions. Ø Crystals have an ordered, repeated structure. Ø The smallest repeating unit in a crystal is a unit cell. Ø Unit cell is the smallest unit with all the symmetry of the entire crystal. Ø Three-dimensional stacking of unit cells is the crystal lattice. Ø 51
 Structures of Solids Unit Cells 52
Structures of Solids Unit Cells 52
 Structures of Solids 53
Structures of Solids 53
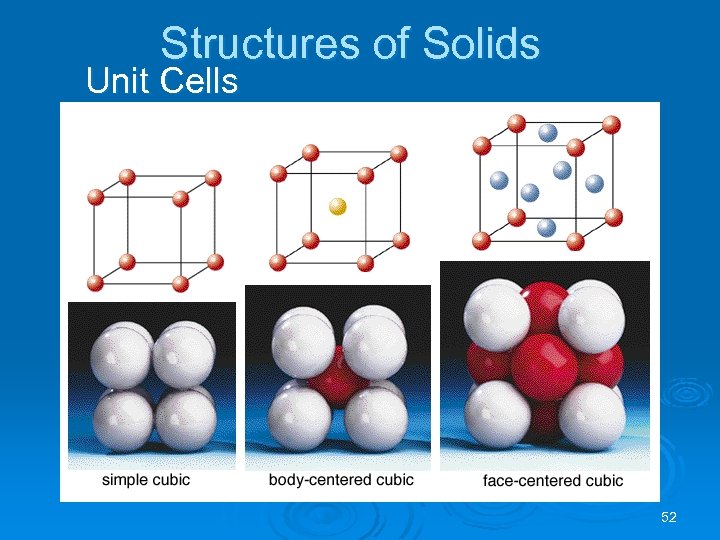
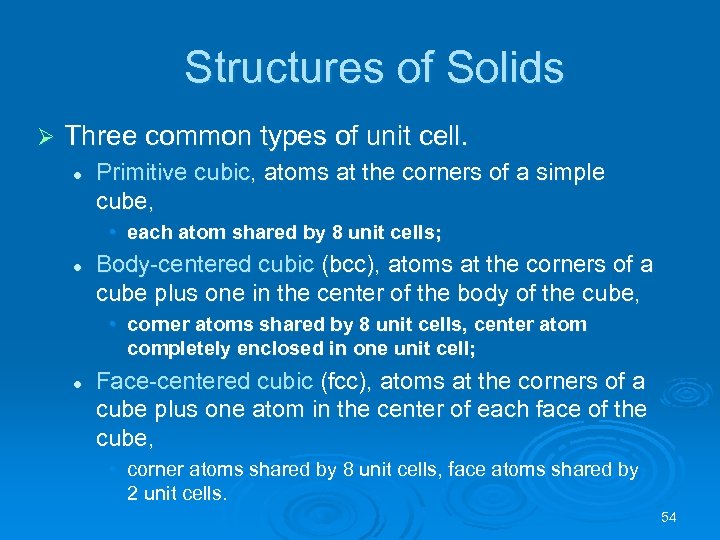 Structures of Solids Ø Three common types of unit cell. l Primitive cubic, atoms at the corners of a simple cube, • each atom shared by 8 unit cells; l Body-centered cubic (bcc), atoms at the corners of a cube plus one in the center of the body of the cube, • corner atoms shared by 8 unit cells, center atom completely enclosed in one unit cell; l Face-centered cubic (fcc), atoms at the corners of a cube plus one atom in the center of each face of the cube, • corner atoms shared by 8 unit cells, face atoms shared by 2 unit cells. 54
Structures of Solids Ø Three common types of unit cell. l Primitive cubic, atoms at the corners of a simple cube, • each atom shared by 8 unit cells; l Body-centered cubic (bcc), atoms at the corners of a cube plus one in the center of the body of the cube, • corner atoms shared by 8 unit cells, center atom completely enclosed in one unit cell; l Face-centered cubic (fcc), atoms at the corners of a cube plus one atom in the center of each face of the cube, • corner atoms shared by 8 unit cells, face atoms shared by 2 unit cells. 54
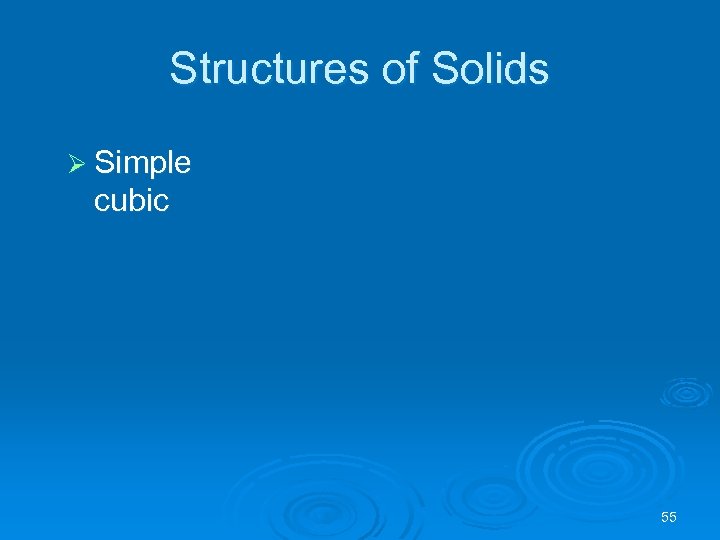 Structures of Solids Ø Simple cubic 55
Structures of Solids Ø Simple cubic 55
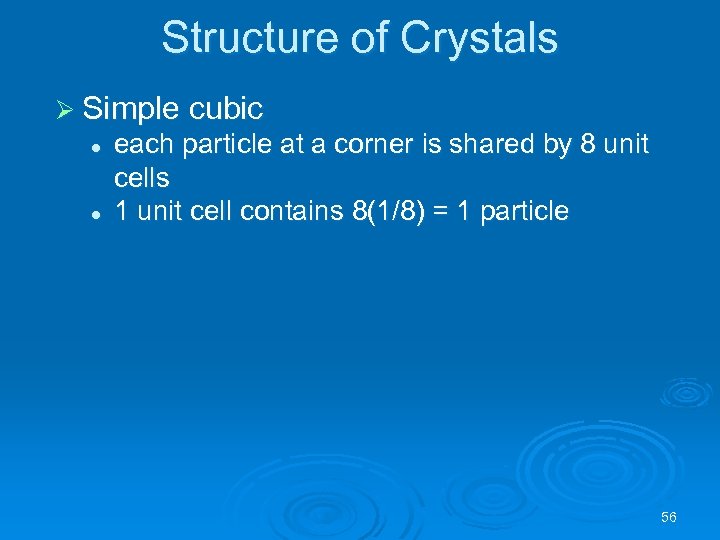 Structure of Crystals Ø Simple cubic l l each particle at a corner is shared by 8 unit cells 1 unit cell contains 8(1/8) = 1 particle 56
Structure of Crystals Ø Simple cubic l l each particle at a corner is shared by 8 unit cells 1 unit cell contains 8(1/8) = 1 particle 56
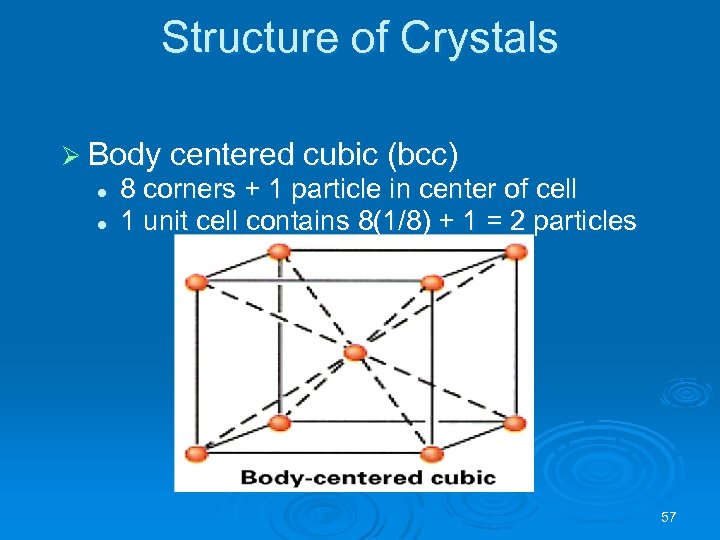 Structure of Crystals Ø Body centered cubic (bcc) l l 8 corners + 1 particle in center of cell 1 unit cell contains 8(1/8) + 1 = 2 particles 57
Structure of Crystals Ø Body centered cubic (bcc) l l 8 corners + 1 particle in center of cell 1 unit cell contains 8(1/8) + 1 = 2 particles 57
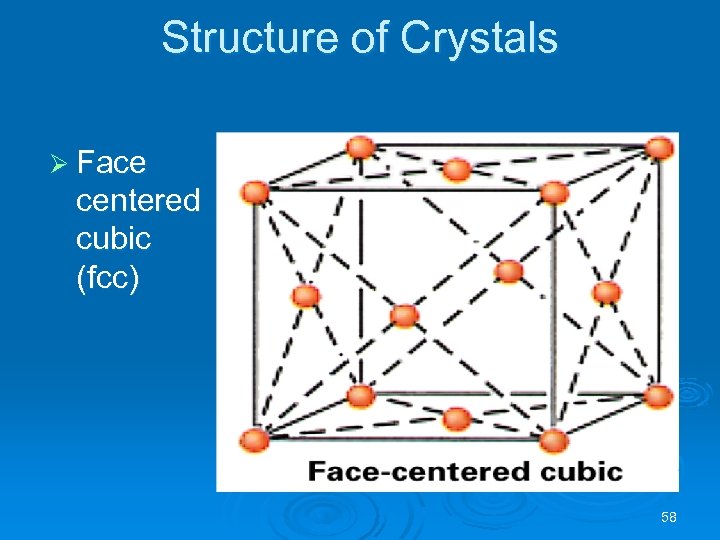 Structure of Crystals Ø Face centered cubic (fcc) 58
Structure of Crystals Ø Face centered cubic (fcc) 58
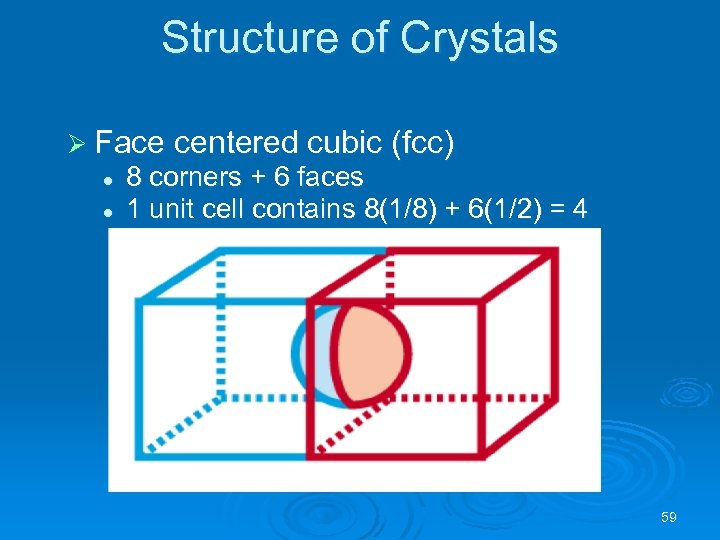 Structure of Crystals Ø Face centered cubic (fcc) l l 8 corners + 6 faces 1 unit cell contains 8(1/8) + 6(1/2) = 4 particles 59
Structure of Crystals Ø Face centered cubic (fcc) l l 8 corners + 6 faces 1 unit cell contains 8(1/8) + 6(1/2) = 4 particles 59
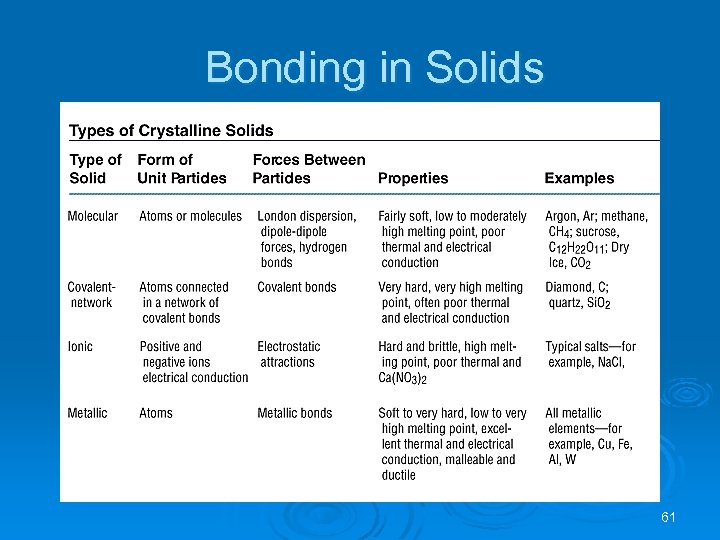
 Bonding in Solids Molecular (formed from molecules) - usually soft with low melting points and poor conductivity. Covalent network (formed from atoms) - very hard with very high melting points and poor conductivity. Ions (formed form ions) - hard, brittle, high melting points and poor conductivity. Metallic (formed from metal atoms) - soft or hard, high melting points, good conductivity, malleable and ductile. 60
Bonding in Solids Molecular (formed from molecules) - usually soft with low melting points and poor conductivity. Covalent network (formed from atoms) - very hard with very high melting points and poor conductivity. Ions (formed form ions) - hard, brittle, high melting points and poor conductivity. Metallic (formed from metal atoms) - soft or hard, high melting points, good conductivity, malleable and ductile. 60
 Bonding in Solids 61
Bonding in Solids 61
 Molecular Solids Ø Intermolecular forces: dipole-dipole, London dispersion and H-bonds. Ø Weak intermolecular forces give rise to low melting points. Ø Room temperature gases and liquids usually form molecular solids and low temperature. Ø Efficient packing of molecules is important (since they are not regular spheres). 62
Molecular Solids Ø Intermolecular forces: dipole-dipole, London dispersion and H-bonds. Ø Weak intermolecular forces give rise to low melting points. Ø Room temperature gases and liquids usually form molecular solids and low temperature. Ø Efficient packing of molecules is important (since they are not regular spheres). 62
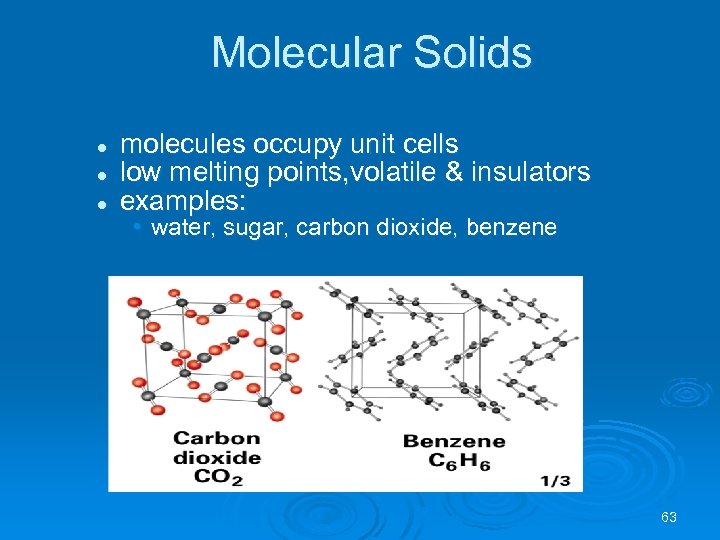 Molecular Solids l l l molecules occupy unit cells low melting points, volatile & insulators examples: • water, sugar, carbon dioxide, benzene 63
Molecular Solids l l l molecules occupy unit cells low melting points, volatile & insulators examples: • water, sugar, carbon dioxide, benzene 63
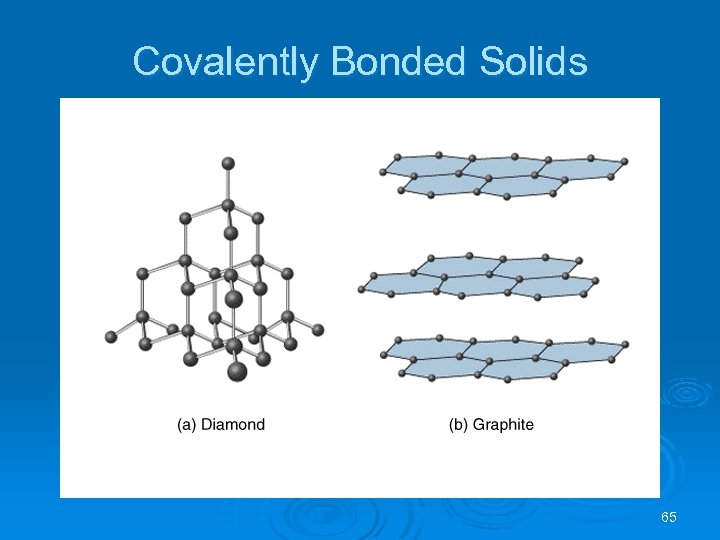
 Covalently Bonded Solids Intermolecular forces: dipole-dipole, London dispersion and H-bonds. Ø Atoms held together in large networks. Ø Examples: diamond, graphite, quartz (Si. O 2), silicon carbide (Si. C), and boron nitride (BN). Ø Ø In diamond: l l each C atom has a coordination number of 4; each C atom is tetrahedral; there is a three-dimensional array of atoms. Diamond is hard, and has a high melting point (3550 C). 64
Covalently Bonded Solids Intermolecular forces: dipole-dipole, London dispersion and H-bonds. Ø Atoms held together in large networks. Ø Examples: diamond, graphite, quartz (Si. O 2), silicon carbide (Si. C), and boron nitride (BN). Ø Ø In diamond: l l each C atom has a coordination number of 4; each C atom is tetrahedral; there is a three-dimensional array of atoms. Diamond is hard, and has a high melting point (3550 C). 64
 Covalently Bonded Solids 65
Covalently Bonded Solids 65
 Covalently Bonded Solids In graphite l l l each C atom is arranged in a planar hexagonal ring; layers of interconnected rings are placed on top of each other; the distance between C atoms is close to benzene (1. 42 Å vs. 1. 395 Å in benzene); the distance between layers is large (3. 41 Å); electrons move in delocalized orbitals (good conductor). 66
Covalently Bonded Solids In graphite l l l each C atom is arranged in a planar hexagonal ring; layers of interconnected rings are placed on top of each other; the distance between C atoms is close to benzene (1. 42 Å vs. 1. 395 Å in benzene); the distance between layers is large (3. 41 Å); electrons move in delocalized orbitals (good conductor). 66
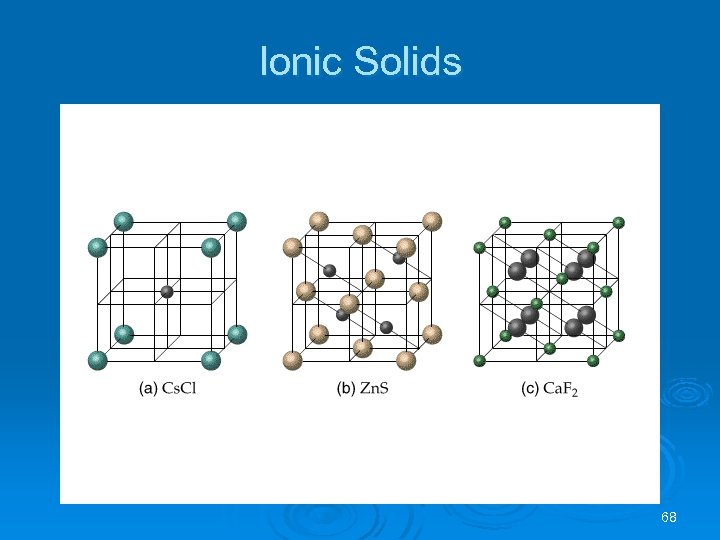
 Ionic Solids Ø Ions (spherical) held together by electrostatic forces of attraction: l Ø The higher the charge (Q) and smaller the distance (d) between ions, the stronger the ionic bond. There are some simple classifications for ionic lattice types: 67
Ionic Solids Ø Ions (spherical) held together by electrostatic forces of attraction: l Ø The higher the charge (Q) and smaller the distance (d) between ions, the stronger the ionic bond. There are some simple classifications for ionic lattice types: 67
 Ionic Solids 68
Ionic Solids 68
 Ionic Solids Na. Cl Structure • • Each ion has a coordination number of 6. Face-centered cubic lattice. Cation to anion ratio is 1: 1. Examples: Li. F, KCl, Ag. Cl and Ca. O. Cs. Cl Structure • Cs+ has a coordination number of 8. • Different from the Na. Cl structure (Cs+ is larger than Na+). • Cation to anion ratio is 1: 1. 69
Ionic Solids Na. Cl Structure • • Each ion has a coordination number of 6. Face-centered cubic lattice. Cation to anion ratio is 1: 1. Examples: Li. F, KCl, Ag. Cl and Ca. O. Cs. Cl Structure • Cs+ has a coordination number of 8. • Different from the Na. Cl structure (Cs+ is larger than Na+). • Cation to anion ratio is 1: 1. 69
 Ionic Solids Crystal Structure of Sodium Chloride Face-centered cubic lattice. Ø Two equivalent ways of defining unit cell: Ø l l Cl- (larger) ions at the corners of the cell, or Na+ (smaller) ions at the corners of the cell. The cation to anion ratio in a unit cell is the same for the crystal. In Na. Cl each unit cell contains same number of Na+ and Cl- ions. Ø Note the unit cell for Ca. Cl 2 needs twice as many Cl- ions as Ca 2+ ions. Ø 70
Ionic Solids Crystal Structure of Sodium Chloride Face-centered cubic lattice. Ø Two equivalent ways of defining unit cell: Ø l l Cl- (larger) ions at the corners of the cell, or Na+ (smaller) ions at the corners of the cell. The cation to anion ratio in a unit cell is the same for the crystal. In Na. Cl each unit cell contains same number of Na+ and Cl- ions. Ø Note the unit cell for Ca. Cl 2 needs twice as many Cl- ions as Ca 2+ ions. Ø 70
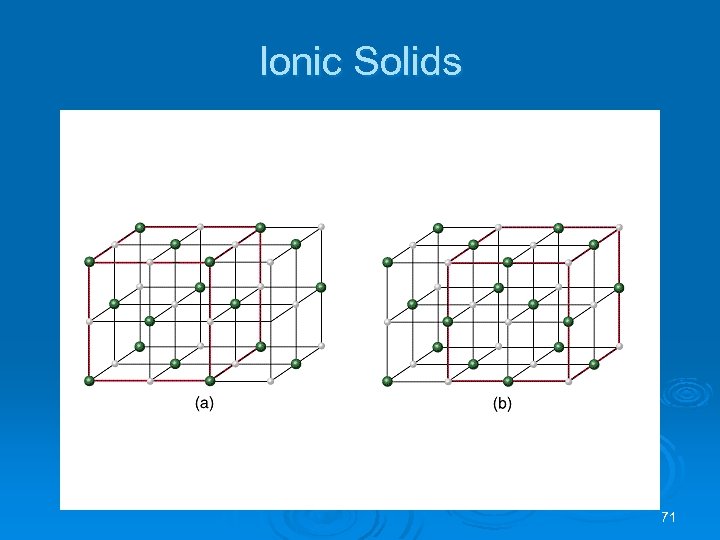 Ionic Solids 71
Ionic Solids 71
 Metallic Solids Ø Metallic solids have metal atoms in hcp, fcc or bcc arrangements. Ø Coordination number for each atom is either 8 or 12. Ø Problem: the bonding is too strong for London dispersion and there are not enough electrons for covalent bonds. 72
Metallic Solids Ø Metallic solids have metal atoms in hcp, fcc or bcc arrangements. Ø Coordination number for each atom is either 8 or 12. Ø Problem: the bonding is too strong for London dispersion and there are not enough electrons for covalent bonds. 72
 Metallic Solids Resolution: the metal nuclei float in a sea of electrons. Ø Metals conduct because the electrons are delocalized and are mobile. Ø Ø positively charged nuclei surrounded by a sea of electrons l positive ions occupy lattice positions l examples: • Na, Li, Au, Ag, ……. . 73
Metallic Solids Resolution: the metal nuclei float in a sea of electrons. Ø Metals conduct because the electrons are delocalized and are mobile. Ø Ø positively charged nuclei surrounded by a sea of electrons l positive ions occupy lattice positions l examples: • Na, Li, Au, Ag, ……. . 73
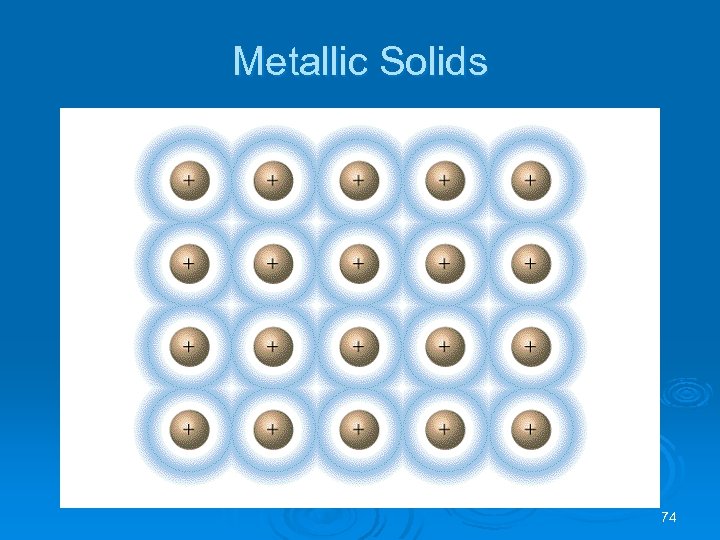 Metallic Solids 74
Metallic Solids 74
 Bonding in Solids Ø Variations in Melting Points Ø Molecular Solids Compound (o C ) ice ammonia benzene, C 6 H 6 napthalene, C 10 H 8 benzoic acid, C 6 H 5 CO 2 H Melting Point 0 -77. 7 5. 5 80. 6 122. 4 75
Bonding in Solids Ø Variations in Melting Points Ø Molecular Solids Compound (o C ) ice ammonia benzene, C 6 H 6 napthalene, C 10 H 8 benzoic acid, C 6 H 5 CO 2 H Melting Point 0 -77. 7 5. 5 80. 6 122. 4 75
 Bonding in Solids Covalent Solids Substance sand, Si. O 2 carborundum, Si. C diamond graphite Ø Melting Point (o. C) 1713 ~2700 >3550 3652 -3697 76
Bonding in Solids Covalent Solids Substance sand, Si. O 2 carborundum, Si. C diamond graphite Ø Melting Point (o. C) 1713 ~2700 >3550 3652 -3697 76
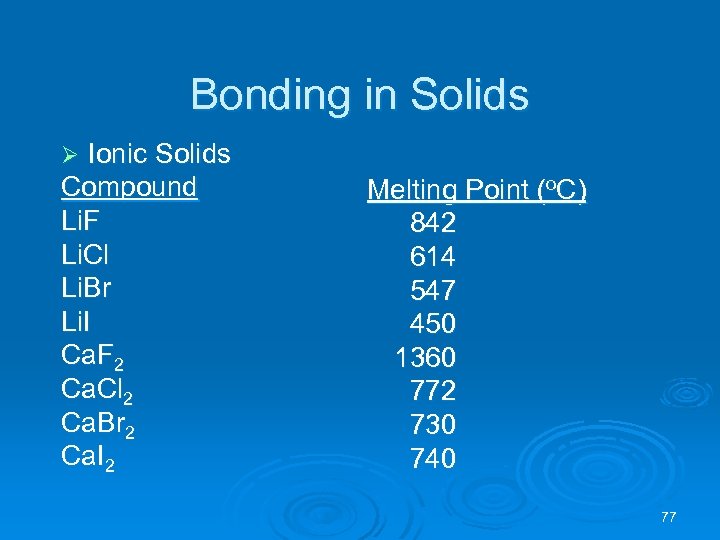 Bonding in Solids Ionic Solids Compound Li. F Li. Cl Li. Br Li. I Ca. F 2 Ca. Cl 2 Ca. Br 2 Ca. I 2 Ø Melting Point (o. C) 842 614 547 450 1360 772 730 740 77
Bonding in Solids Ionic Solids Compound Li. F Li. Cl Li. Br Li. I Ca. F 2 Ca. Cl 2 Ca. Br 2 Ca. I 2 Ø Melting Point (o. C) 842 614 547 450 1360 772 730 740 77
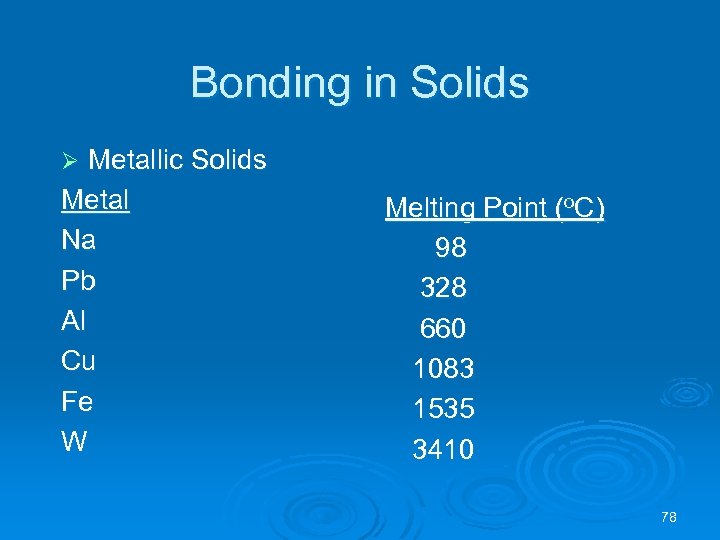 Bonding in Solids Metallic Solids Metal Na Pb Al Cu Fe W Ø Melting Point (o. C) 98 328 660 1083 1535 3410 78
Bonding in Solids Metallic Solids Metal Na Pb Al Cu Fe W Ø Melting Point (o. C) 98 328 660 1083 1535 3410 78
 Band Theory of Metals Ø Na’s 3 s orbitals can interact to produce overlapping orbitals 79
Band Theory of Metals Ø Na’s 3 s orbitals can interact to produce overlapping orbitals 79
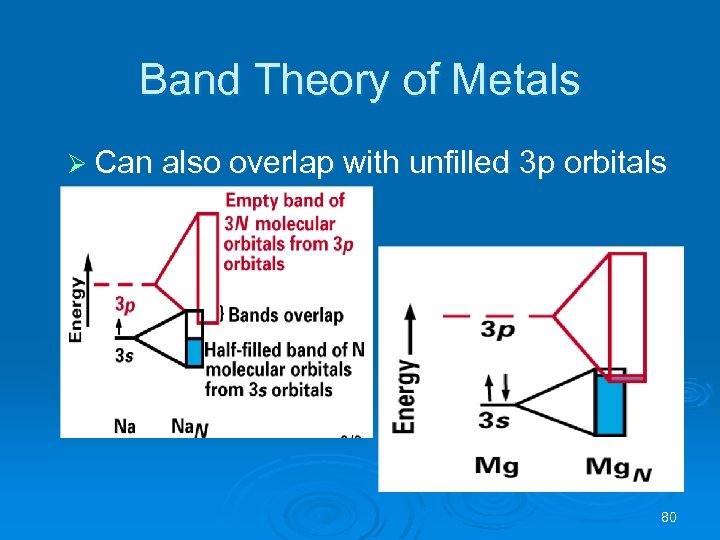 Band Theory of Metals Ø Can also overlap with unfilled 3 p orbitals 80
Band Theory of Metals Ø Can also overlap with unfilled 3 p orbitals 80
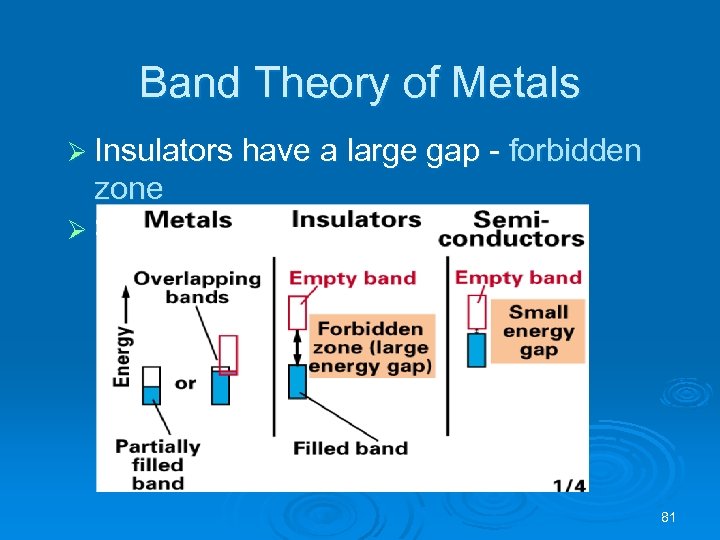 Band Theory of Metals Ø Insulators have a large gap - forbidden zone Ø Semiconductors have a small gap 81
Band Theory of Metals Ø Insulators have a large gap - forbidden zone Ø Semiconductors have a small gap 81
 Synthesis Question Ø Maxwell House Coffee Company decaffeinates its coffee beans using an extractor that is 7. 0 feet in diameter and 70. 0 feet long. Supercritical carbon dioxide at a pressure of 300. 0 atm and temperature of 100. 00 C is passed through the stainless steel extractor. The extraction vessel contains 100, 000 pounds of coffee beans soaked in water until they have a water content of 50%. 82
Synthesis Question Ø Maxwell House Coffee Company decaffeinates its coffee beans using an extractor that is 7. 0 feet in diameter and 70. 0 feet long. Supercritical carbon dioxide at a pressure of 300. 0 atm and temperature of 100. 00 C is passed through the stainless steel extractor. The extraction vessel contains 100, 000 pounds of coffee beans soaked in water until they have a water content of 50%. 82
 Synthesis Question Ø This process removes 90% of the caffeine in a single pass of the beans through the extractor. Carbon dioxide that has passed over the coffee is then directed into a water column that washes the caffeine from the supercritical CO 2. How many moles of carbon dioxide are present in the extractor? 83
Synthesis Question Ø This process removes 90% of the caffeine in a single pass of the beans through the extractor. Carbon dioxide that has passed over the coffee is then directed into a water column that washes the caffeine from the supercritical CO 2. How many moles of carbon dioxide are present in the extractor? 83
 Synthesis Question 84
Synthesis Question 84
 Synthesis Question 85
Synthesis Question 85
 Group Question Ø How many CO 2 molecules are there in 1. 0 cm 3 of the Maxwell House Coffee Company extractor? How many more CO 2 molecules are there in a cm 3 of the supercritical fluid in the Maxwell House extractor than in a mole of CO 2 at STP? 86
Group Question Ø How many CO 2 molecules are there in 1. 0 cm 3 of the Maxwell House Coffee Company extractor? How many more CO 2 molecules are there in a cm 3 of the supercritical fluid in the Maxwell House extractor than in a mole of CO 2 at STP? 86


