7fc9e8218cd5a7303f7c89b57a9f20f3.ppt
- Количество слайдов: 81
 Chapter 11 a Modern Atomic Theory
Chapter 11 a Modern Atomic Theory
 Chapter 11 Table of Contents 11. 1 11. 2 11. 3 11. 4 11. 5 Rutherford’s Atom Electromagnetic Radiation Emission of Energy by Atoms The Energy Levels of Hydrogen The Bohr Model of the Atom 2
Chapter 11 Table of Contents 11. 1 11. 2 11. 3 11. 4 11. 5 Rutherford’s Atom Electromagnetic Radiation Emission of Energy by Atoms The Energy Levels of Hydrogen The Bohr Model of the Atom 2
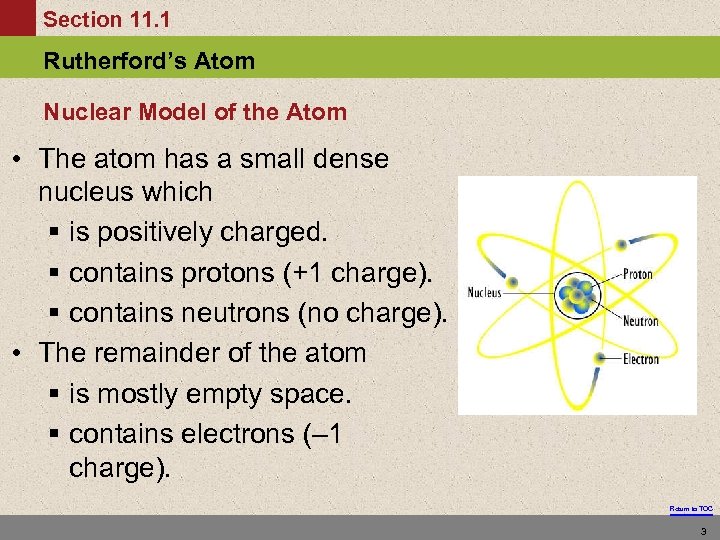 Section 11. 1 Rutherford’s Atom Nuclear Model of the Atom • The atom has a small dense nucleus which § is positively charged. § contains protons (+1 charge). § contains neutrons (no charge). • The remainder of the atom § is mostly empty space. § contains electrons (– 1 charge). Return to TOC 3
Section 11. 1 Rutherford’s Atom Nuclear Model of the Atom • The atom has a small dense nucleus which § is positively charged. § contains protons (+1 charge). § contains neutrons (no charge). • The remainder of the atom § is mostly empty space. § contains electrons (– 1 charge). Return to TOC 3
 Section 11. 1 Rutherford’s Atom • The nuclear charge (n+) is balanced by the presence of n electrons moving in some way around the nucleus. • What are the electrons doing? • How are the electrons arranged and how do they move? Return to TOC Copyright © Cengage Learning. All rights reserved 4
Section 11. 1 Rutherford’s Atom • The nuclear charge (n+) is balanced by the presence of n electrons moving in some way around the nucleus. • What are the electrons doing? • How are the electrons arranged and how do they move? Return to TOC Copyright © Cengage Learning. All rights reserved 4
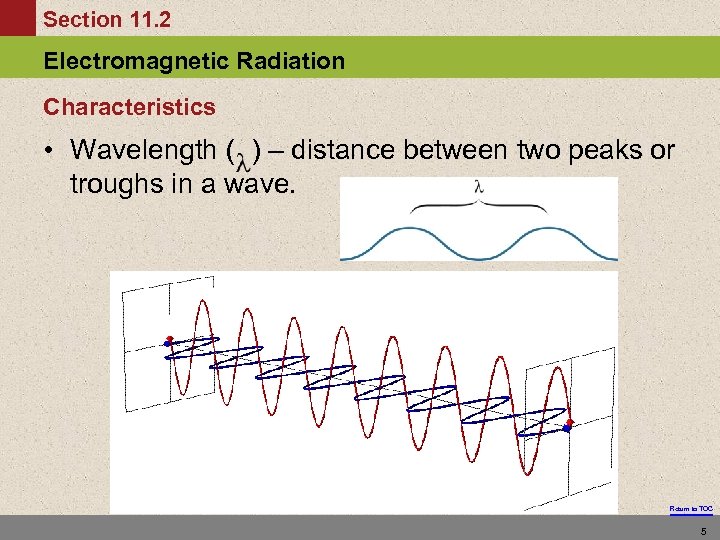 Section 11. 2 Electromagnetic Radiation Characteristics • Wavelength ( ) – distance between two peaks or troughs in a wave. Return to TOC 5
Section 11. 2 Electromagnetic Radiation Characteristics • Wavelength ( ) – distance between two peaks or troughs in a wave. Return to TOC 5
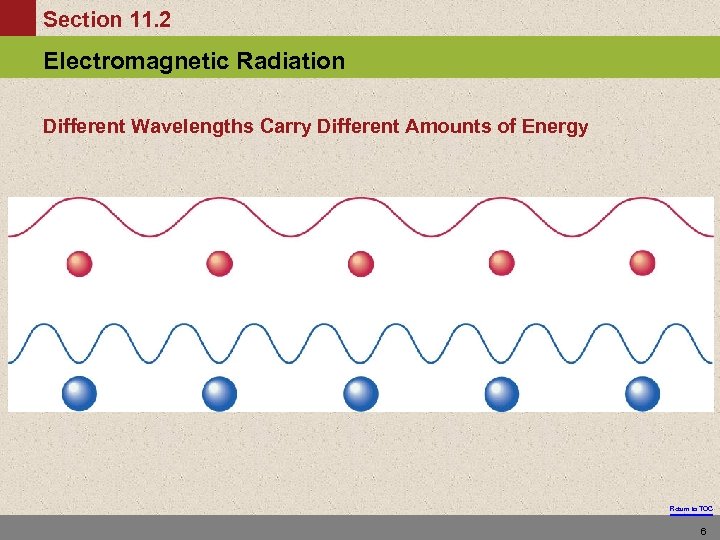 Section 11. 2 Electromagnetic Radiation Different Wavelengths Carry Different Amounts of Energy Return to TOC 6
Section 11. 2 Electromagnetic Radiation Different Wavelengths Carry Different Amounts of Energy Return to TOC 6
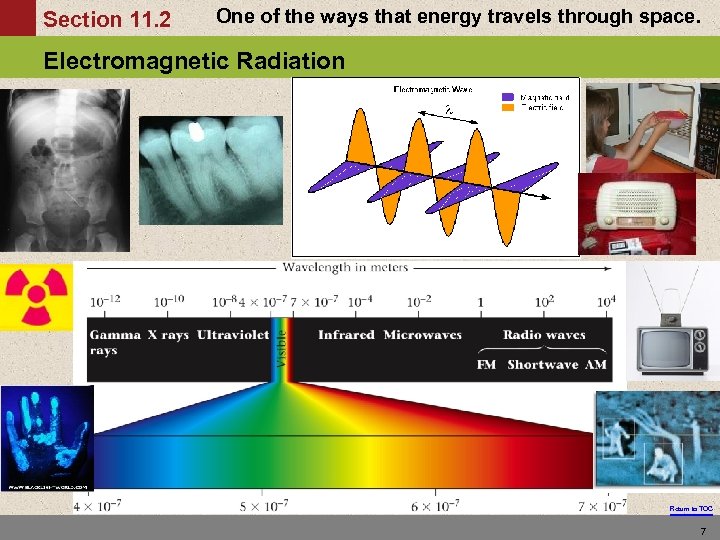 Section 11. 2 One of the ways that energy travels through space. Electromagnetic Radiation Return to TOC 7
Section 11. 2 One of the ways that energy travels through space. Electromagnetic Radiation Return to TOC 7
 Section 11. 2 Electromagnetic Radiation Characteristics • Frequency ( ) – number of waves (cycles) per second that pass a given point in space • Speed (c) – speed of light (2. 9979× 108 m/s) 186, 000 miles/s Return to TOC 8
Section 11. 2 Electromagnetic Radiation Characteristics • Frequency ( ) – number of waves (cycles) per second that pass a given point in space • Speed (c) – speed of light (2. 9979× 108 m/s) 186, 000 miles/s Return to TOC 8
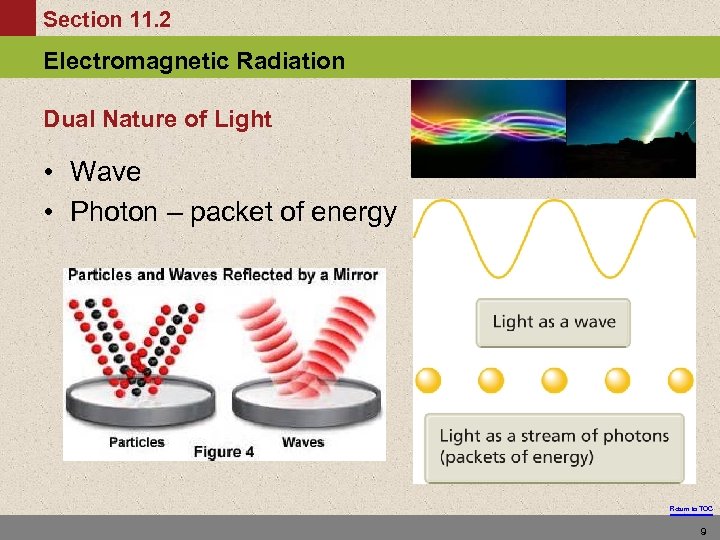 Section 11. 2 Electromagnetic Radiation Dual Nature of Light • Wave • Photon – packet of energy Return to TOC 9
Section 11. 2 Electromagnetic Radiation Dual Nature of Light • Wave • Photon – packet of energy Return to TOC 9
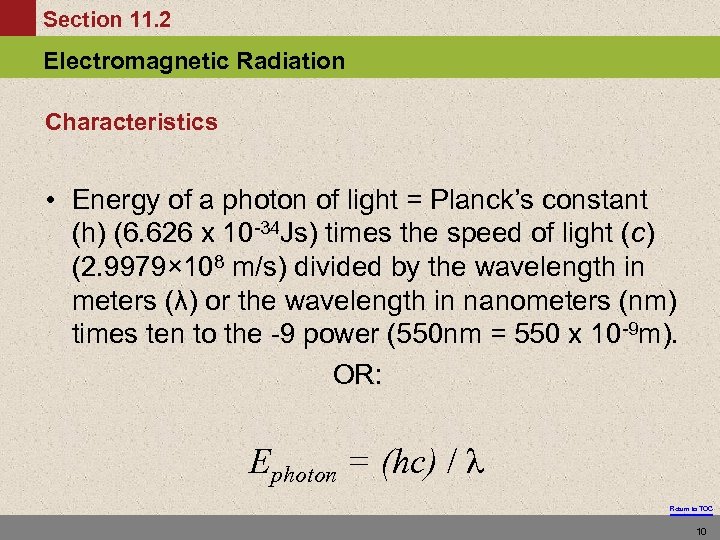 Section 11. 2 Electromagnetic Radiation Characteristics • Energy of a photon of light = Planck’s constant (h) (6. 626 x 10 -34 Js) times the speed of light (c) (2. 9979× 108 m/s) divided by the wavelength in meters (λ) or the wavelength in nanometers (nm) times ten to the -9 power (550 nm = 550 x 10 -9 m). OR: Ephoton = (hc) / λ Return to TOC 10
Section 11. 2 Electromagnetic Radiation Characteristics • Energy of a photon of light = Planck’s constant (h) (6. 626 x 10 -34 Js) times the speed of light (c) (2. 9979× 108 m/s) divided by the wavelength in meters (λ) or the wavelength in nanometers (nm) times ten to the -9 power (550 nm = 550 x 10 -9 m). OR: Ephoton = (hc) / λ Return to TOC 10
 Section 11. 2 Electromagnetic Radiation Let’s Practice! What is the energy of light with a wavelength of 535 nm? E=hc/λ =6. 626 x 10 -34 Js(3. 00 x 108 m/s)/(535 x 10 -9 m) =3. 70 x 10 -19 J Return to TOC 11
Section 11. 2 Electromagnetic Radiation Let’s Practice! What is the energy of light with a wavelength of 535 nm? E=hc/λ =6. 626 x 10 -34 Js(3. 00 x 108 m/s)/(535 x 10 -9 m) =3. 70 x 10 -19 J Return to TOC 11
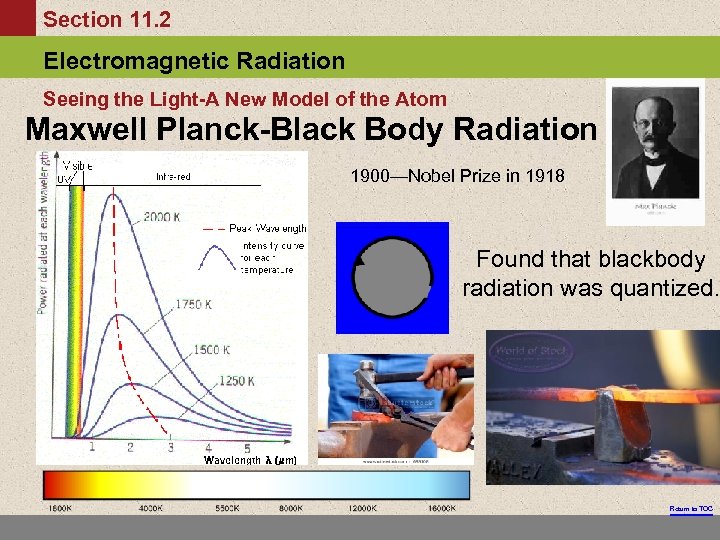 Section 11. 2 Electromagnetic Radiation Seeing the Light-A New Model of the Atom Maxwell Planck-Black Body Radiation 1900—Nobel Prize in 1918 Found that blackbody radiation was quantized. Return to TOC
Section 11. 2 Electromagnetic Radiation Seeing the Light-A New Model of the Atom Maxwell Planck-Black Body Radiation 1900—Nobel Prize in 1918 Found that blackbody radiation was quantized. Return to TOC
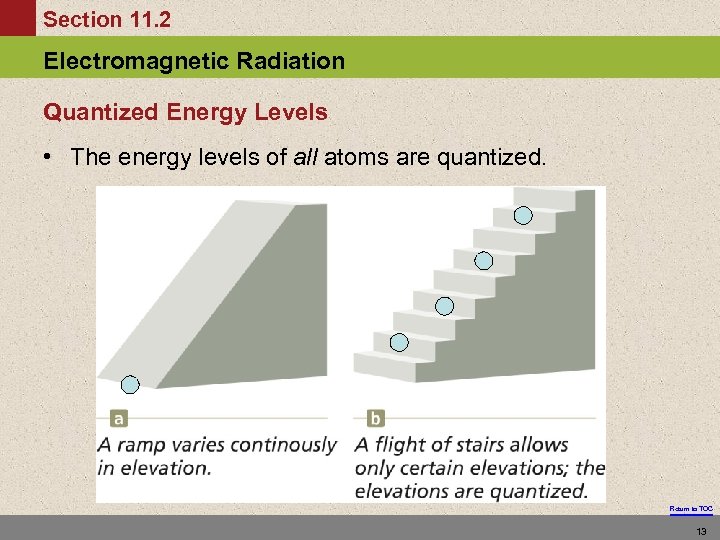 Section 11. 2 Electromagnetic Radiation Quantized Energy Levels • The energy levels of all atoms are quantized. Return to TOC 13
Section 11. 2 Electromagnetic Radiation Quantized Energy Levels • The energy levels of all atoms are quantized. Return to TOC 13
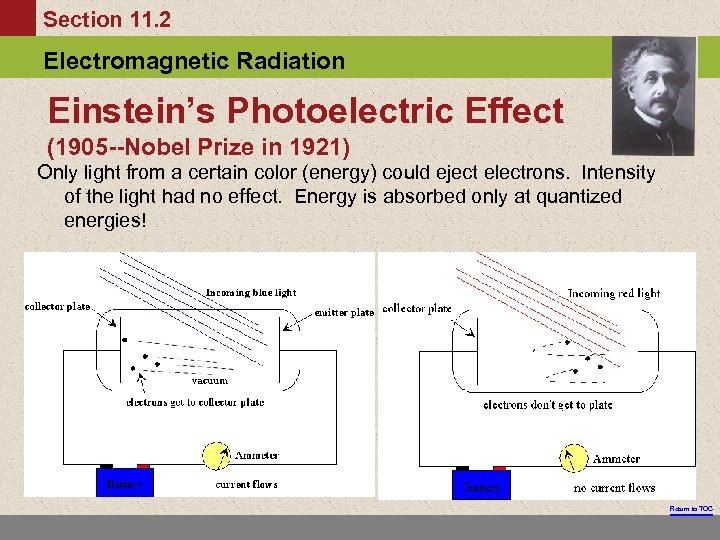 Section 11. 2 Electromagnetic Radiation Einstein’s Photoelectric Effect (1905 --Nobel Prize in 1921) Only light from a certain color (energy) could eject electrons. Intensity of the light had no effect. Energy is absorbed only at quantized energies! Return to TOC
Section 11. 2 Electromagnetic Radiation Einstein’s Photoelectric Effect (1905 --Nobel Prize in 1921) Only light from a certain color (energy) could eject electrons. Intensity of the light had no effect. Energy is absorbed only at quantized energies! Return to TOC
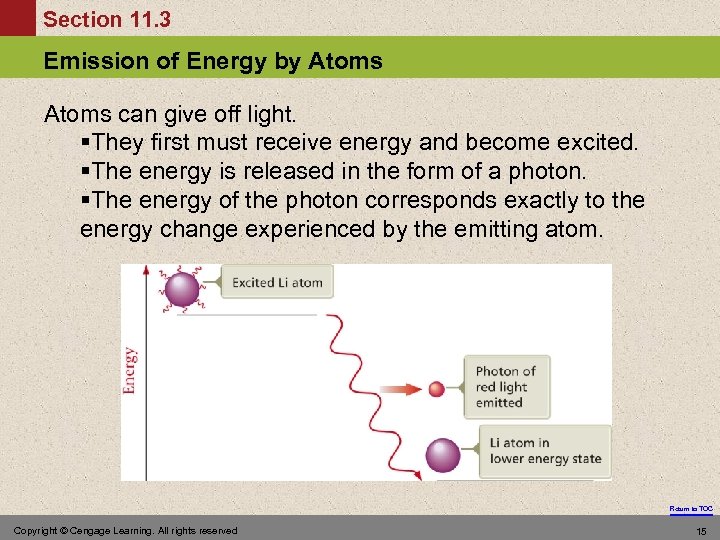 Section 11. 3 Emission of Energy by Atoms can give off light. §They first must receive energy and become excited. §The energy is released in the form of a photon. §The energy of the photon corresponds exactly to the energy change experienced by the emitting atom. Return to TOC Copyright © Cengage Learning. All rights reserved 15
Section 11. 3 Emission of Energy by Atoms can give off light. §They first must receive energy and become excited. §The energy is released in the form of a photon. §The energy of the photon corresponds exactly to the energy change experienced by the emitting atom. Return to TOC Copyright © Cengage Learning. All rights reserved 15
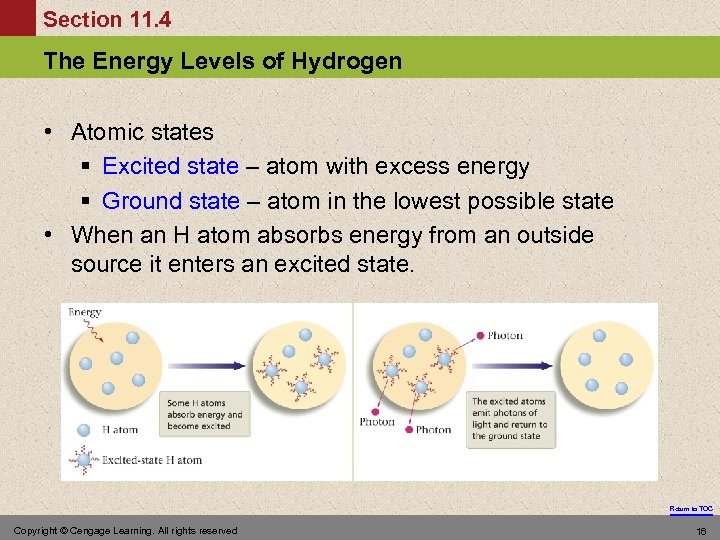 Section 11. 4 The Energy Levels of Hydrogen • Atomic states § Excited state – atom with excess energy § Ground state – atom in the lowest possible state • When an H atom absorbs energy from an outside source it enters an excited state. Return to TOC Copyright © Cengage Learning. All rights reserved 16
Section 11. 4 The Energy Levels of Hydrogen • Atomic states § Excited state – atom with excess energy § Ground state – atom in the lowest possible state • When an H atom absorbs energy from an outside source it enters an excited state. Return to TOC Copyright © Cengage Learning. All rights reserved 16
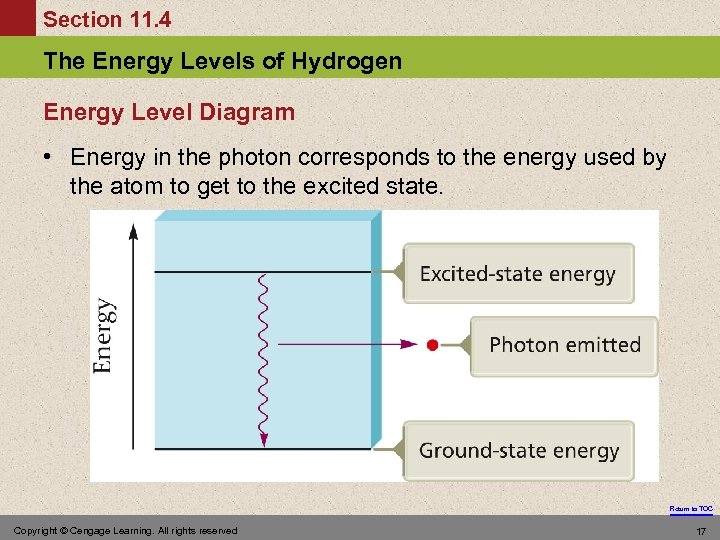 Section 11. 4 The Energy Levels of Hydrogen Energy Level Diagram • Energy in the photon corresponds to the energy used by the atom to get to the excited state. Return to TOC Copyright © Cengage Learning. All rights reserved 17
Section 11. 4 The Energy Levels of Hydrogen Energy Level Diagram • Energy in the photon corresponds to the energy used by the atom to get to the excited state. Return to TOC Copyright © Cengage Learning. All rights reserved 17
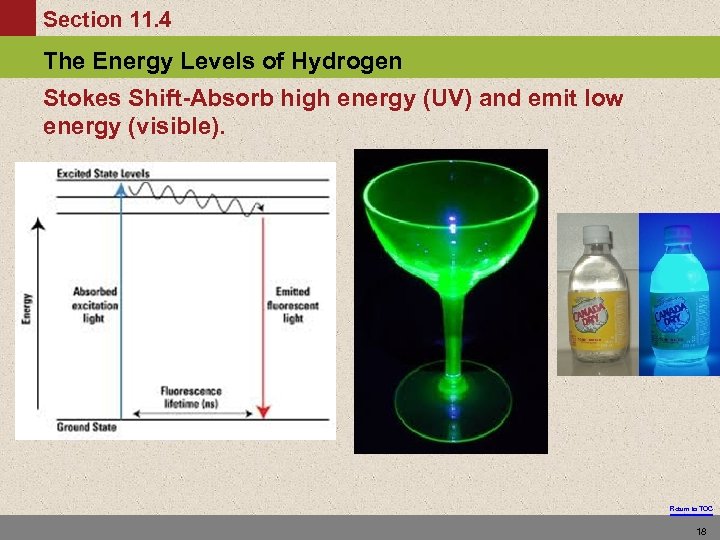 Section 11. 4 The Energy Levels of Hydrogen Stokes Shift-Absorb high energy (UV) and emit low energy (visible). Return to TOC 18
Section 11. 4 The Energy Levels of Hydrogen Stokes Shift-Absorb high energy (UV) and emit low energy (visible). Return to TOC 18
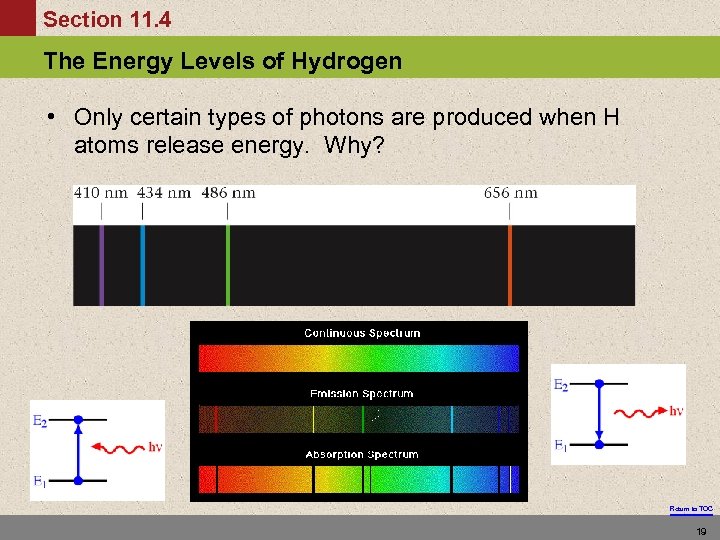 Section 11. 4 The Energy Levels of Hydrogen • Only certain types of photons are produced when H atoms release energy. Why? Return to TOC 19
Section 11. 4 The Energy Levels of Hydrogen • Only certain types of photons are produced when H atoms release energy. Why? Return to TOC 19
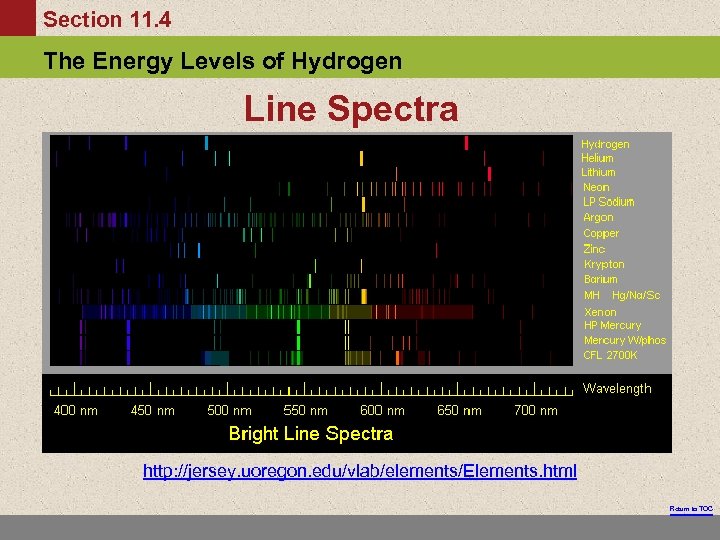 Section 11. 4 The Energy Levels of Hydrogen Line Spectra http: //jersey. uoregon. edu/vlab/elements/Elements. html Return to TOC
Section 11. 4 The Energy Levels of Hydrogen Line Spectra http: //jersey. uoregon. edu/vlab/elements/Elements. html Return to TOC
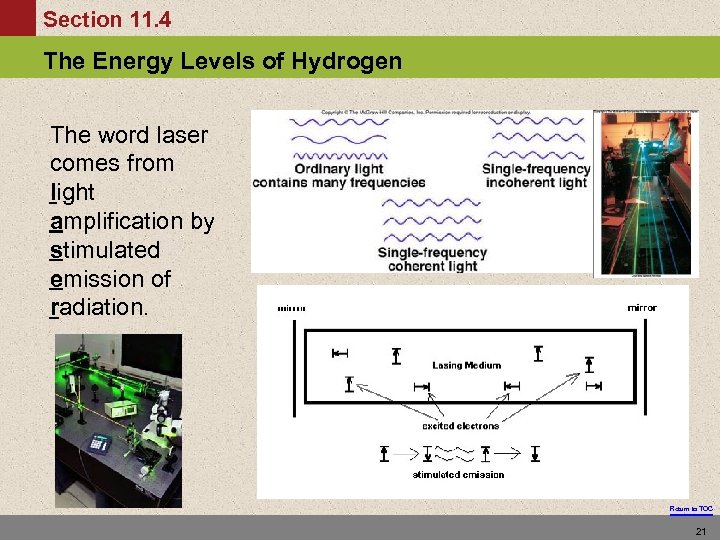 Section 11. 4 The Energy Levels of Hydrogen The word laser comes from light amplification by stimulated emission of radiation. Return to TOC 21
Section 11. 4 The Energy Levels of Hydrogen The word laser comes from light amplification by stimulated emission of radiation. Return to TOC 21
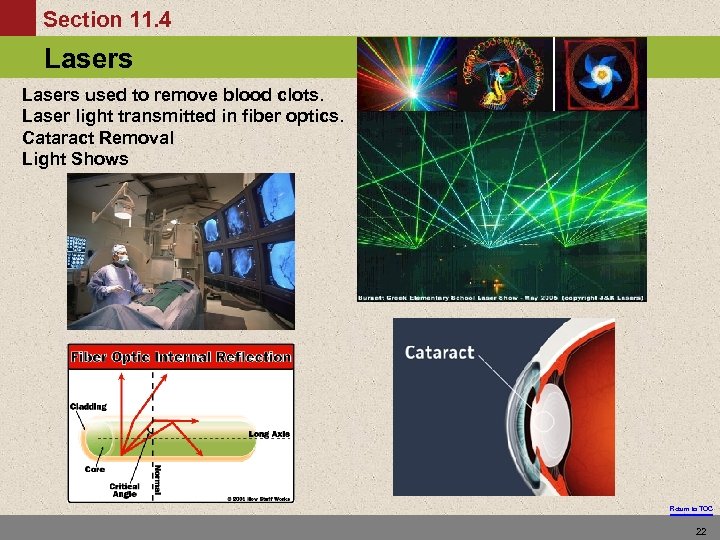 Section 11. 4 Lasers The Energy Levels of Hydrogen Lasers used to remove blood clots. Laser light transmitted in fiber optics. Cataract Removal Light Shows Return to TOC 22
Section 11. 4 Lasers The Energy Levels of Hydrogen Lasers used to remove blood clots. Laser light transmitted in fiber optics. Cataract Removal Light Shows Return to TOC 22
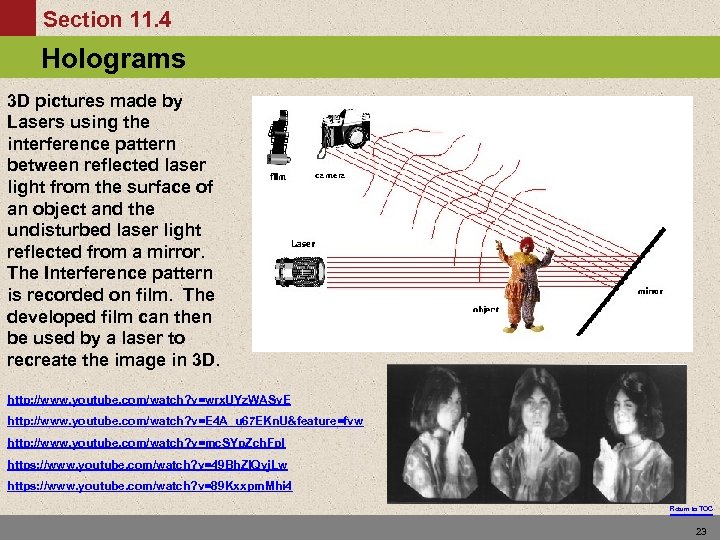 Section 11. 4 Holograms The Energy Levels of Hydrogen 3 D pictures made by Lasers using the interference pattern between reflected laser light from the surface of an object and the undisturbed laser light reflected from a mirror. The Interference pattern is recorded on film. The developed film can then be used by a laser to recreate the image in 3 D. http: //www. youtube. com/watch? v=wrx. UYz. WASv. E http: //www. youtube. com/watch? v=E 4 A_u 67 EKn. U&feature=fvw http: //www. youtube. com/watch? v=mc. SYp. Zch. Fp. I https: //www. youtube. com/watch? v=49 Bh. Zl. Qvj. Lw https: //www. youtube. com/watch? v=89 Kxxpm. Mhi 4 Return to TOC 23
Section 11. 4 Holograms The Energy Levels of Hydrogen 3 D pictures made by Lasers using the interference pattern between reflected laser light from the surface of an object and the undisturbed laser light reflected from a mirror. The Interference pattern is recorded on film. The developed film can then be used by a laser to recreate the image in 3 D. http: //www. youtube. com/watch? v=wrx. UYz. WASv. E http: //www. youtube. com/watch? v=E 4 A_u 67 EKn. U&feature=fvw http: //www. youtube. com/watch? v=mc. SYp. Zch. Fp. I https: //www. youtube. com/watch? v=49 Bh. Zl. Qvj. Lw https: //www. youtube. com/watch? v=89 Kxxpm. Mhi 4 Return to TOC 23
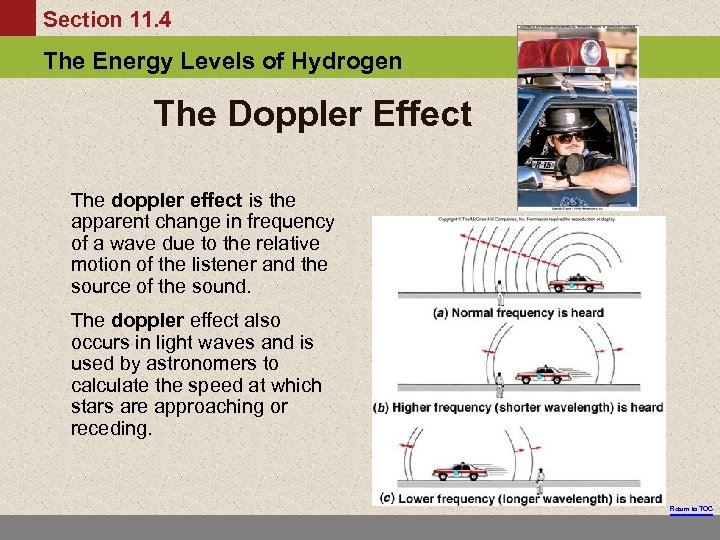 Section 11. 4 The Energy Levels of Hydrogen The Doppler Effect The doppler effect is the apparent change in frequency of a wave due to the relative motion of the listener and the source of the sound. The doppler effect also occurs in light waves and is used by astronomers to calculate the speed at which stars are approaching or receding. Return to TOC
Section 11. 4 The Energy Levels of Hydrogen The Doppler Effect The doppler effect is the apparent change in frequency of a wave due to the relative motion of the listener and the source of the sound. The doppler effect also occurs in light waves and is used by astronomers to calculate the speed at which stars are approaching or receding. Return to TOC
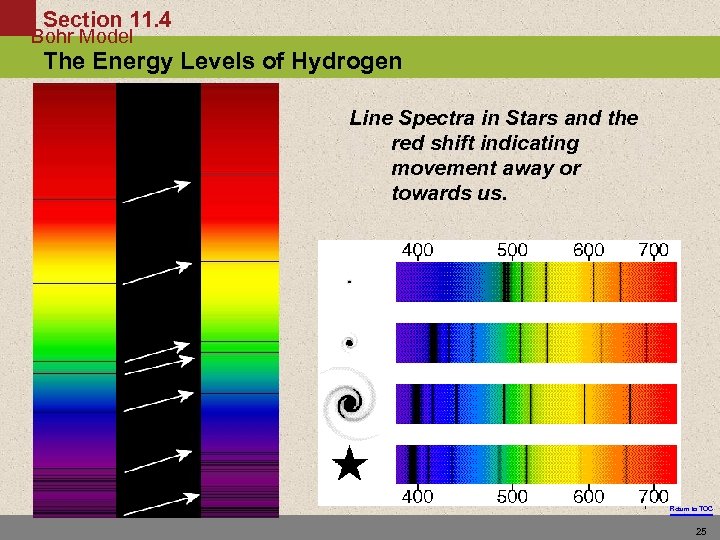 Section 11. 4 Bohr Model The Energy Levels of Hydrogen Line Spectra in Stars and the red shift indicating movement away or towards us. 7 - Return to TOC 25
Section 11. 4 Bohr Model The Energy Levels of Hydrogen Line Spectra in Stars and the red shift indicating movement away or towards us. 7 - Return to TOC 25
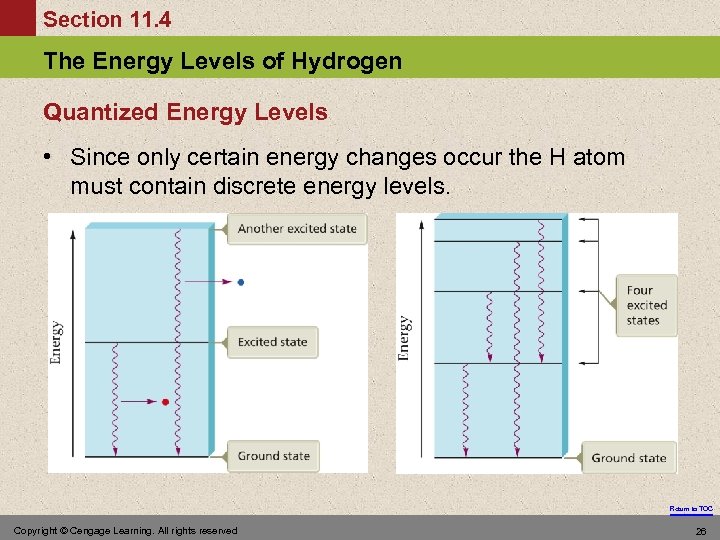 Section 11. 4 The Energy Levels of Hydrogen Quantized Energy Levels • Since only certain energy changes occur the H atom must contain discrete energy levels. Return to TOC Copyright © Cengage Learning. All rights reserved 26
Section 11. 4 The Energy Levels of Hydrogen Quantized Energy Levels • Since only certain energy changes occur the H atom must contain discrete energy levels. Return to TOC Copyright © Cengage Learning. All rights reserved 26
 Section 11. 4 The Energy Levels of Hydrogen Concept Check Why is it significant that the color emitted from the hydrogen emission spectrum is not white? How does the emission spectrum support the idea of quantized energy levels? Return to TOC 27
Section 11. 4 The Energy Levels of Hydrogen Concept Check Why is it significant that the color emitted from the hydrogen emission spectrum is not white? How does the emission spectrum support the idea of quantized energy levels? Return to TOC 27
 Section 11. 4 The Energy Levels of Hydrogen Concept Check When an electron is excited in an atom or ion a) only specific quantities of energy are released in order for the electron to return to its ground state. b) white light is never observed when the electron returns to its ground state. c) the electron is only excited to certain energy levels. d) All of the above statements are true when an electron is excited. Return to TOC Copyright © Cengage Learning. All rights reserved 28
Section 11. 4 The Energy Levels of Hydrogen Concept Check When an electron is excited in an atom or ion a) only specific quantities of energy are released in order for the electron to return to its ground state. b) white light is never observed when the electron returns to its ground state. c) the electron is only excited to certain energy levels. d) All of the above statements are true when an electron is excited. Return to TOC Copyright © Cengage Learning. All rights reserved 28
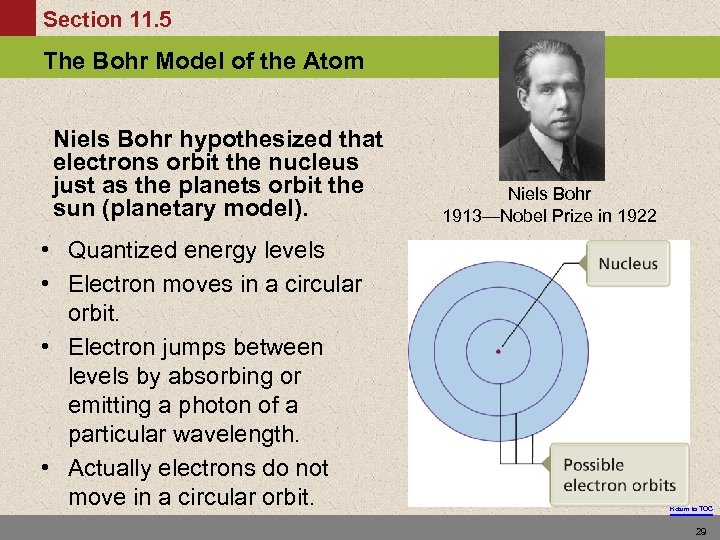 Section 11. 5 The Bohr Model of the Atom Niels Bohr hypothesized that electrons orbit the nucleus just as the planets orbit the sun (planetary model). • Quantized energy levels • Electron moves in a circular orbit. • Electron jumps between levels by absorbing or emitting a photon of a particular wavelength. • Actually electrons do not move in a circular orbit. Niels Bohr 1913—Nobel Prize in 1922 Return to TOC 29
Section 11. 5 The Bohr Model of the Atom Niels Bohr hypothesized that electrons orbit the nucleus just as the planets orbit the sun (planetary model). • Quantized energy levels • Electron moves in a circular orbit. • Electron jumps between levels by absorbing or emitting a photon of a particular wavelength. • Actually electrons do not move in a circular orbit. Niels Bohr 1913—Nobel Prize in 1922 Return to TOC 29
 D Section 11. 5 The Bohr Model of the Atom Chapter 11 b Modern Atomic Theory Return to TOC 30
D Section 11. 5 The Bohr Model of the Atom Chapter 11 b Modern Atomic Theory Return to TOC 30
 Chapter 11. 5 Section 11 Modern Model of of the Atom The Bohr Model the Atom 11. 6 11. 7 11. 8 11. 9 The Wave Mechanical Model of the Atom The Hydrogen Orbitals The Wave Mechanical Model: Further Development Electron Arrangements in the First Eighteen Atoms on the Periodic Table 11. 10 Electron Configurations and the Periodic Table 11. 11 Atomic Properties and the Periodic Table Return to TOC 31
Chapter 11. 5 Section 11 Modern Model of of the Atom The Bohr Model the Atom 11. 6 11. 7 11. 8 11. 9 The Wave Mechanical Model of the Atom The Hydrogen Orbitals The Wave Mechanical Model: Further Development Electron Arrangements in the First Eighteen Atoms on the Periodic Table 11. 10 Electron Configurations and the Periodic Table 11. 11 Atomic Properties and the Periodic Table Return to TOC 31
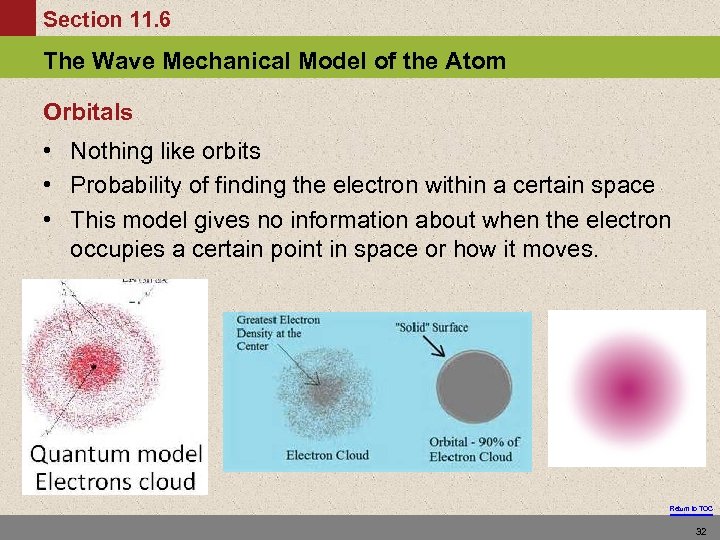 Section 11. 6 The Wave Mechanical Model of the Atom Orbitals • Nothing like orbits • Probability of finding the electron within a certain space • This model gives no information about when the electron occupies a certain point in space or how it moves. Return to TOC 32
Section 11. 6 The Wave Mechanical Model of the Atom Orbitals • Nothing like orbits • Probability of finding the electron within a certain space • This model gives no information about when the electron occupies a certain point in space or how it moves. Return to TOC 32
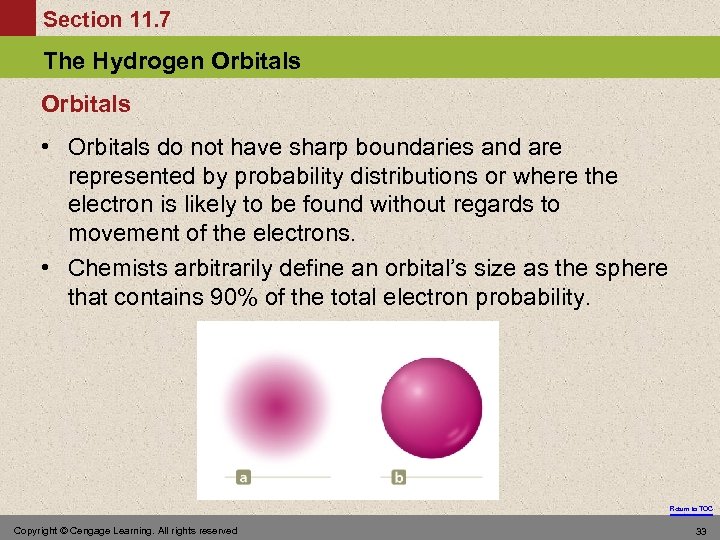 Section 11. 7 The Hydrogen Orbitals • Orbitals do not have sharp boundaries and are represented by probability distributions or where the electron is likely to be found without regards to movement of the electrons. • Chemists arbitrarily define an orbital’s size as the sphere that contains 90% of the total electron probability. Return to TOC Copyright © Cengage Learning. All rights reserved 33
Section 11. 7 The Hydrogen Orbitals • Orbitals do not have sharp boundaries and are represented by probability distributions or where the electron is likely to be found without regards to movement of the electrons. • Chemists arbitrarily define an orbital’s size as the sphere that contains 90% of the total electron probability. Return to TOC Copyright © Cengage Learning. All rights reserved 33
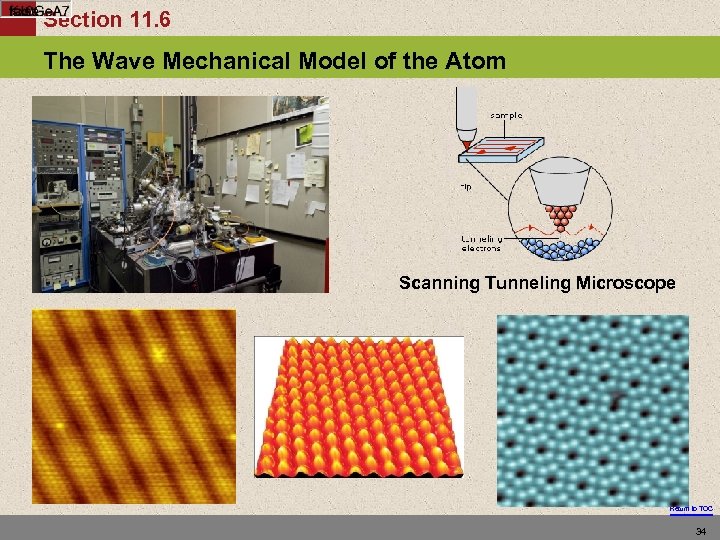 Section 11. 6 The Wave Mechanical Model of the Atom Scanning Tunneling Microscope Return to TOC 34
Section 11. 6 The Wave Mechanical Model of the Atom Scanning Tunneling Microscope Return to TOC 34
 Section 11. 6 The Wave Mechanical Model of the Atom Louis De. Broglie 1924 – Nobel Prize in 1929 • He found that matter (electrons) moved in waves. Just as light behaved like particles and waves, so did matter. • An 18 -wheeler moving down Hwy 99 at 60 mph has a wavelength smaller than an atom. • However, an electron (very light) moves much faster and its wavelength is much larger than its size. Return to TOC
Section 11. 6 The Wave Mechanical Model of the Atom Louis De. Broglie 1924 – Nobel Prize in 1929 • He found that matter (electrons) moved in waves. Just as light behaved like particles and waves, so did matter. • An 18 -wheeler moving down Hwy 99 at 60 mph has a wavelength smaller than an atom. • However, an electron (very light) moves much faster and its wavelength is much larger than its size. Return to TOC
 Section 11. 6 The Wave Mechanical Model of the Atom Probability of Finding Electrons Like Fireflies Return to TOC
Section 11. 6 The Wave Mechanical Model of the Atom Probability of Finding Electrons Like Fireflies Return to TOC
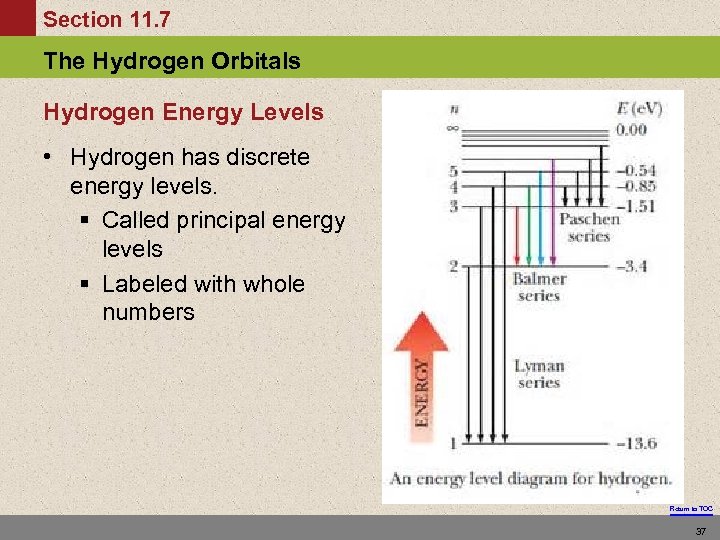 Section 11. 7 The Hydrogen Orbitals Hydrogen Energy Levels • Hydrogen has discrete energy levels. § Called principal energy levels § Labeled with whole numbers Return to TOC 37
Section 11. 7 The Hydrogen Orbitals Hydrogen Energy Levels • Hydrogen has discrete energy levels. § Called principal energy levels § Labeled with whole numbers Return to TOC 37
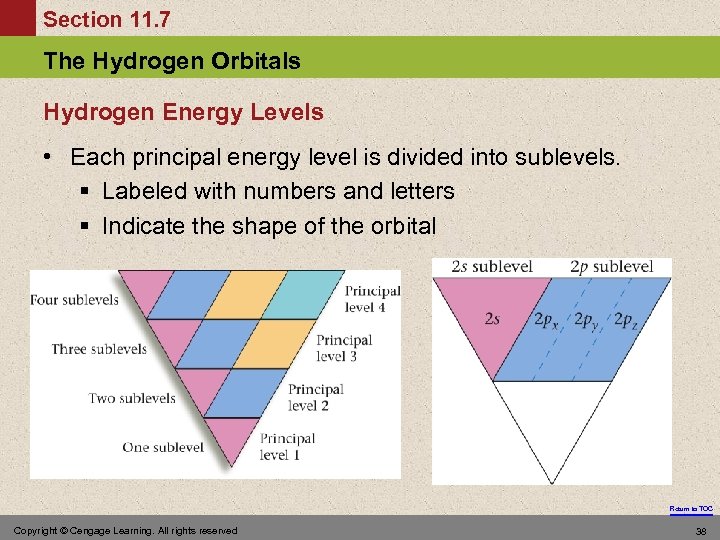 Section 11. 7 The Hydrogen Orbitals Hydrogen Energy Levels • Each principal energy level is divided into sublevels. § Labeled with numbers and letters § Indicate the shape of the orbital Return to TOC Copyright © Cengage Learning. All rights reserved 38
Section 11. 7 The Hydrogen Orbitals Hydrogen Energy Levels • Each principal energy level is divided into sublevels. § Labeled with numbers and letters § Indicate the shape of the orbital Return to TOC Copyright © Cengage Learning. All rights reserved 38
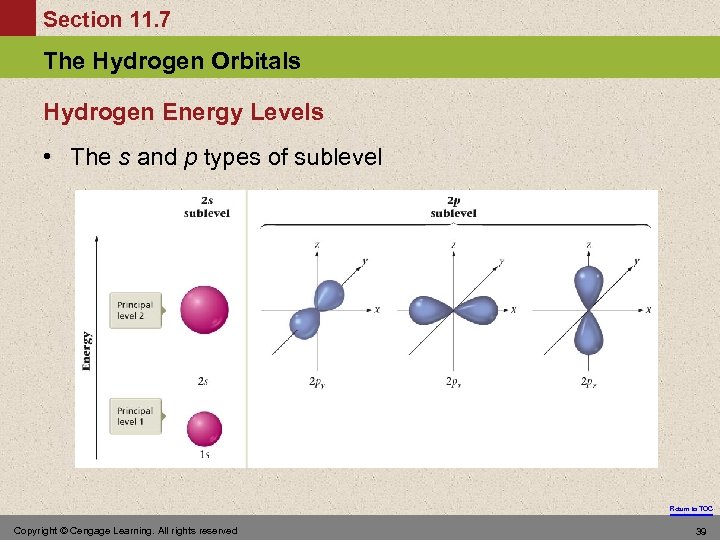 Section 11. 7 The Hydrogen Orbitals Hydrogen Energy Levels • The s and p types of sublevel Return to TOC Copyright © Cengage Learning. All rights reserved 39
Section 11. 7 The Hydrogen Orbitals Hydrogen Energy Levels • The s and p types of sublevel Return to TOC Copyright © Cengage Learning. All rights reserved 39
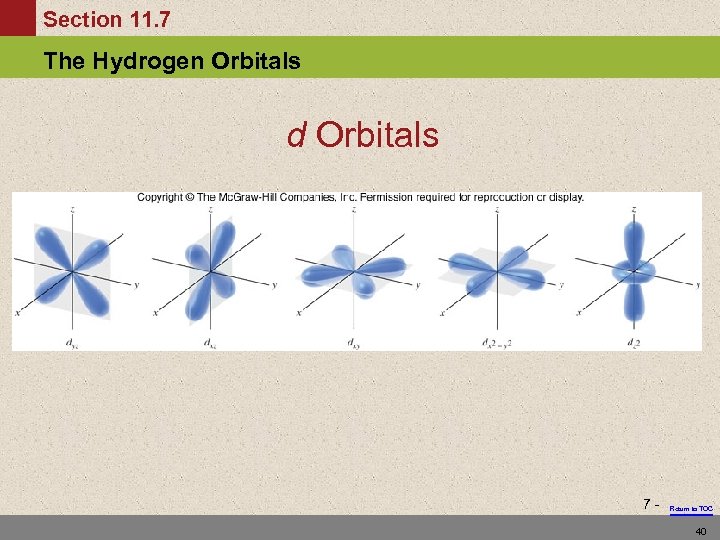 Section 11. 7 The Hydrogen Orbitals d Orbitals 7 - Return to TOC 40
Section 11. 7 The Hydrogen Orbitals d Orbitals 7 - Return to TOC 40
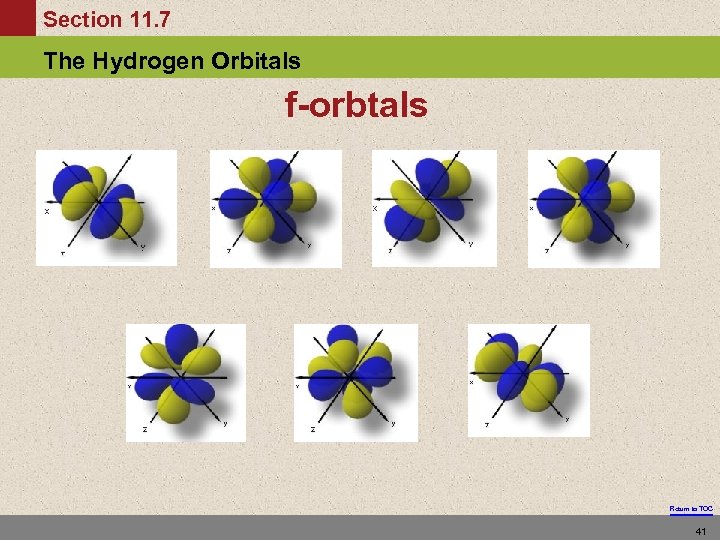 Section 11. 7 The Hydrogen Orbitals f-orbtals Return to TOC 41
Section 11. 7 The Hydrogen Orbitals f-orbtals Return to TOC 41
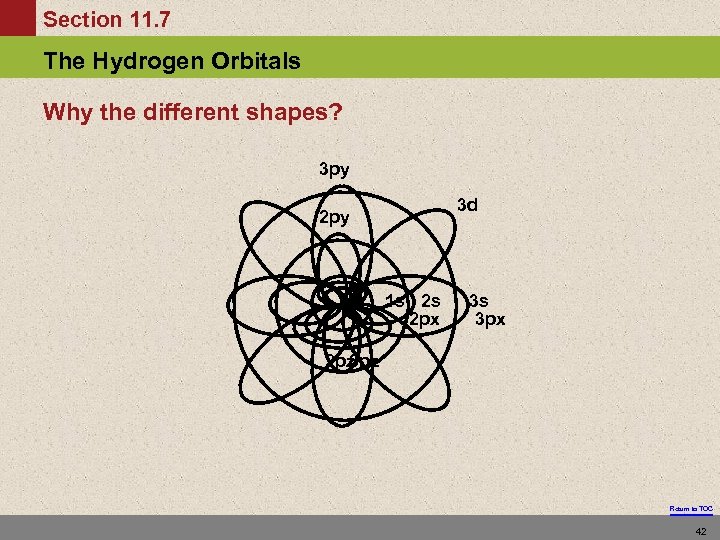 Section 11. 7 The Hydrogen Orbitals Why the different shapes? 3 py 3 d 2 py 1 s 2 s 2 px 3 s 3 px 2 pz 3 pz Return to TOC 42
Section 11. 7 The Hydrogen Orbitals Why the different shapes? 3 py 3 d 2 py 1 s 2 s 2 px 3 s 3 px 2 pz 3 pz Return to TOC 42
 Section 11. 7 The Hydrogen Orbitals Orbital Labels 1. The number tells the principal energy level. 2. The letter tells the shape. § The letter s means a spherical orbital or shape of the probability distribution of the electron. § The letter p means the orientation. The x, y, or z subscript on a p orbital label tells along which of the coordinate axes the two lobes lie. Return to TOC 43
Section 11. 7 The Hydrogen Orbitals Orbital Labels 1. The number tells the principal energy level. 2. The letter tells the shape. § The letter s means a spherical orbital or shape of the probability distribution of the electron. § The letter p means the orientation. The x, y, or z subscript on a p orbital label tells along which of the coordinate axes the two lobes lie. Return to TOC 43
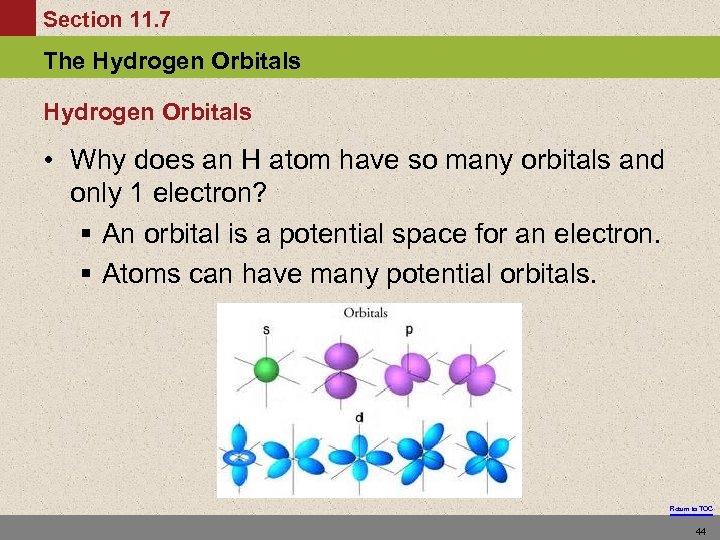 Section 11. 7 The Hydrogen Orbitals • Why does an H atom have so many orbitals and only 1 electron? § An orbital is a potential space for an electron. § Atoms can have many potential orbitals. Return to TOC 44
Section 11. 7 The Hydrogen Orbitals • Why does an H atom have so many orbitals and only 1 electron? § An orbital is a potential space for an electron. § Atoms can have many potential orbitals. Return to TOC 44
 Section 11. 8 The Wave Mechanical Model: Further Development Atoms Beyond Hydrogen • The Bohr model was discarded because it does not apply to all atoms. It did not consider the different energy sublevels or suborbitals within each orbital. • Atoms beyond hydrogen have multiple electrons that distorts the energy levels due to electron-electron interactions. • Need one more property to determine how the electrons are arranged: Spin – electrons spin like a top causing a magnetic field. Opposite magnetic fields can attract allowing electrons to occur in pairs if their spin or magnetic field is opposite. Return to TOC 45
Section 11. 8 The Wave Mechanical Model: Further Development Atoms Beyond Hydrogen • The Bohr model was discarded because it does not apply to all atoms. It did not consider the different energy sublevels or suborbitals within each orbital. • Atoms beyond hydrogen have multiple electrons that distorts the energy levels due to electron-electron interactions. • Need one more property to determine how the electrons are arranged: Spin – electrons spin like a top causing a magnetic field. Opposite magnetic fields can attract allowing electrons to occur in pairs if their spin or magnetic field is opposite. Return to TOC 45
 Section 11. 8 The Wave Mechanical Model: Further Development Atoms Beyond Hydrogen • Pauli Exclusion Principle – an atomic orbital can hold a maximum of 2 electrons and those 2 electrons must have opposite spins. Return to TOC 46
Section 11. 8 The Wave Mechanical Model: Further Development Atoms Beyond Hydrogen • Pauli Exclusion Principle – an atomic orbital can hold a maximum of 2 electrons and those 2 electrons must have opposite spins. Return to TOC 46
 Section 11. 8 The Wave Mechanical Model: Further Development Principal Components of the Wave Mechanical Model of the Atom 1. Atoms have a series of energy levels called principal energy levels (n = 1, 2, 3, etc. ). 2. The energy of the level increases as the value of n increases. 3. Each principal energy level contains one or more types of orbitals, called sublevels. 4. The number of sublevels present in a given principal energy level equals n. Return to TOC Copyright © Cengage Learning. All rights reserved 47
Section 11. 8 The Wave Mechanical Model: Further Development Principal Components of the Wave Mechanical Model of the Atom 1. Atoms have a series of energy levels called principal energy levels (n = 1, 2, 3, etc. ). 2. The energy of the level increases as the value of n increases. 3. Each principal energy level contains one or more types of orbitals, called sublevels. 4. The number of sublevels present in a given principal energy level equals n. Return to TOC Copyright © Cengage Learning. All rights reserved 47
 Section 11. 8 The Wave Mechanical Model: Further Development Principal Components of the Wave Mechanical Model of the Atom 5. The n value is always used to label the orbitals of a given principal level and is followed by a letter that indicates the type (shape) of the orbital (1 s, 3 p, etc. ). 6. An orbital can be empty or it can contain one or two electrons, but never more than two. If two electrons occupy the same orbital, they must have opposite spins. Return to TOC Copyright © Cengage Learning. All rights reserved 48
Section 11. 8 The Wave Mechanical Model: Further Development Principal Components of the Wave Mechanical Model of the Atom 5. The n value is always used to label the orbitals of a given principal level and is followed by a letter that indicates the type (shape) of the orbital (1 s, 3 p, etc. ). 6. An orbital can be empty or it can contain one or two electrons, but never more than two. If two electrons occupy the same orbital, they must have opposite spins. Return to TOC Copyright © Cengage Learning. All rights reserved 48
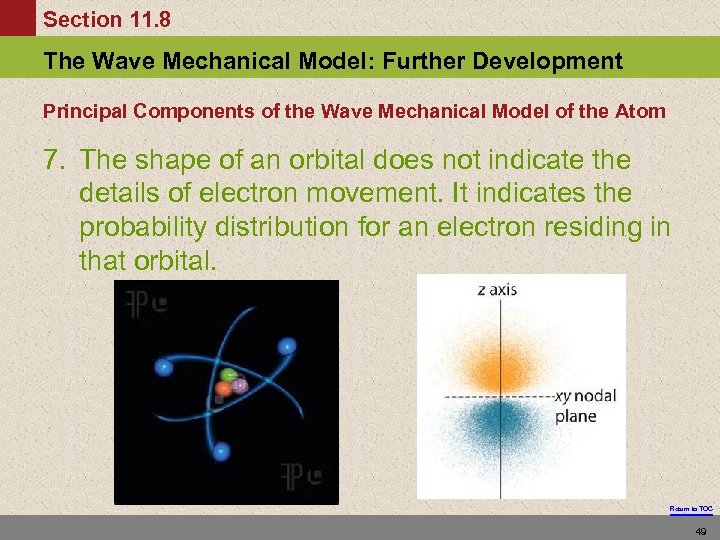 Section 11. 8 The Wave Mechanical Model: Further Development Principal Components of the Wave Mechanical Model of the Atom 7. The shape of an orbital does not indicate the details of electron movement. It indicates the probability distribution for an electron residing in that orbital. Return to TOC 49
Section 11. 8 The Wave Mechanical Model: Further Development Principal Components of the Wave Mechanical Model of the Atom 7. The shape of an orbital does not indicate the details of electron movement. It indicates the probability distribution for an electron residing in that orbital. Return to TOC 49
 Section 11. 8 The Wave Mechanical Model: Further Development Concept Check Which of the following statements best describes the movement of electrons in a p orbital? a) The electron movement cannot be exactly determined. b) The electrons move within the two lobes of the p orbital, but never beyond the outside surface of the orbital. c) The electrons are concentrated at the center (node) of the two lobes. d) The electrons move along the outer surface of the p orbital, similar to a “figure 8” type of movement. Return to TOC Copyright © Cengage Learning. All rights reserved 50
Section 11. 8 The Wave Mechanical Model: Further Development Concept Check Which of the following statements best describes the movement of electrons in a p orbital? a) The electron movement cannot be exactly determined. b) The electrons move within the two lobes of the p orbital, but never beyond the outside surface of the orbital. c) The electrons are concentrated at the center (node) of the two lobes. d) The electrons move along the outer surface of the p orbital, similar to a “figure 8” type of movement. Return to TOC Copyright © Cengage Learning. All rights reserved 50
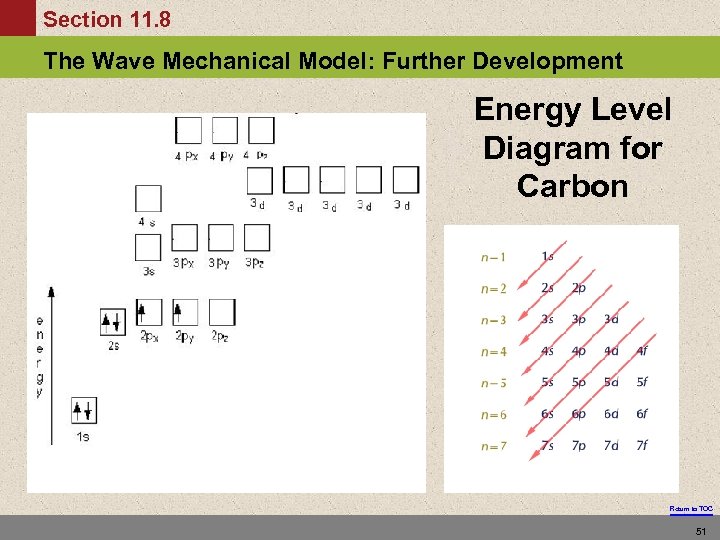 Section 11. 8 The Wave Mechanical Model: Further Development Energy Level Diagram for Carbon Return to TOC 51
Section 11. 8 The Wave Mechanical Model: Further Development Energy Level Diagram for Carbon Return to TOC 51
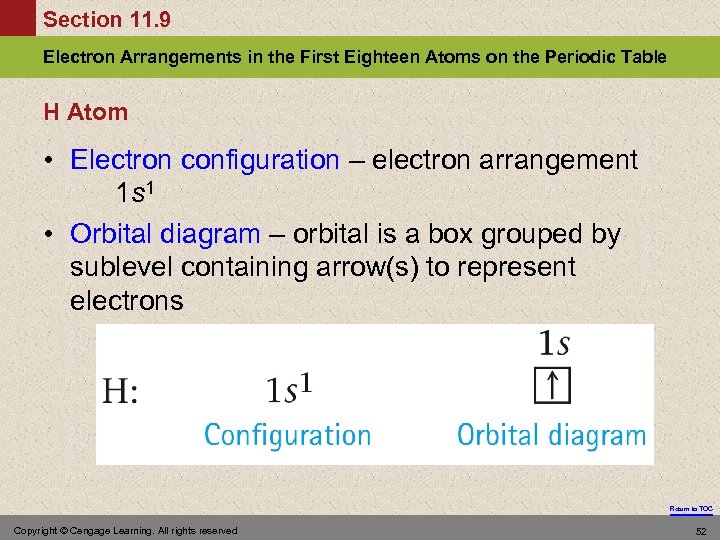 Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table H Atom • Electron configuration – electron arrangement 1 s 1 • Orbital diagram – orbital is a box grouped by sublevel containing arrow(s) to represent electrons Return to TOC Copyright © Cengage Learning. All rights reserved 52
Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table H Atom • Electron configuration – electron arrangement 1 s 1 • Orbital diagram – orbital is a box grouped by sublevel containing arrow(s) to represent electrons Return to TOC Copyright © Cengage Learning. All rights reserved 52
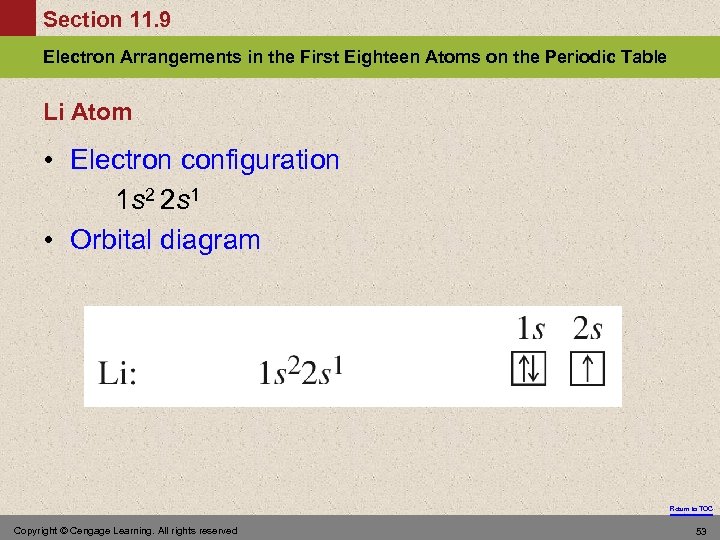 Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Li Atom • Electron configuration 1 s 2 2 s 1 • Orbital diagram Return to TOC Copyright © Cengage Learning. All rights reserved 53
Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Li Atom • Electron configuration 1 s 2 2 s 1 • Orbital diagram Return to TOC Copyright © Cengage Learning. All rights reserved 53
 Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table O Atom • The lowest energy configuration for an atom is the one having the maximum number of unpaired electrons in a particular set of degenerate (same energy) orbitals. Oxygen: 1 s 2 p Return to TOC Copyright © Cengage Learning. All rights reserved 54
Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table O Atom • The lowest energy configuration for an atom is the one having the maximum number of unpaired electrons in a particular set of degenerate (same energy) orbitals. Oxygen: 1 s 2 p Return to TOC Copyright © Cengage Learning. All rights reserved 54
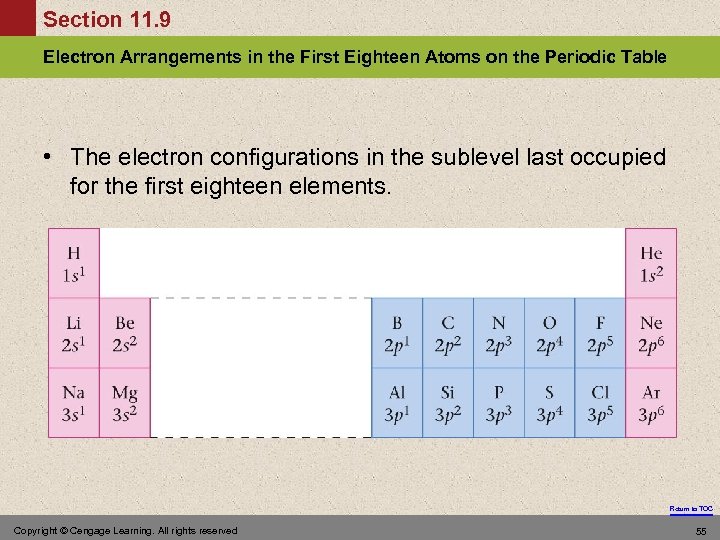 Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table • The electron configurations in the sublevel last occupied for the first eighteen elements. Return to TOC Copyright © Cengage Learning. All rights reserved 55
Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table • The electron configurations in the sublevel last occupied for the first eighteen elements. Return to TOC Copyright © Cengage Learning. All rights reserved 55
 Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Classifying Electrons • Core electrons – inner electrons • Valence electrons – electrons in the outermost (highest) principal energy level of an atom § 1 s 22 p 6 (valence electrons = 8) § The elements in the same group on the periodic table have the same valence electron configuration. § Elements with the same valence electron arrangement show very similar chemical behavior. Return to TOC 56
Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Classifying Electrons • Core electrons – inner electrons • Valence electrons – electrons in the outermost (highest) principal energy level of an atom § 1 s 22 p 6 (valence electrons = 8) § The elements in the same group on the periodic table have the same valence electron configuration. § Elements with the same valence electron arrangement show very similar chemical behavior. Return to TOC 56
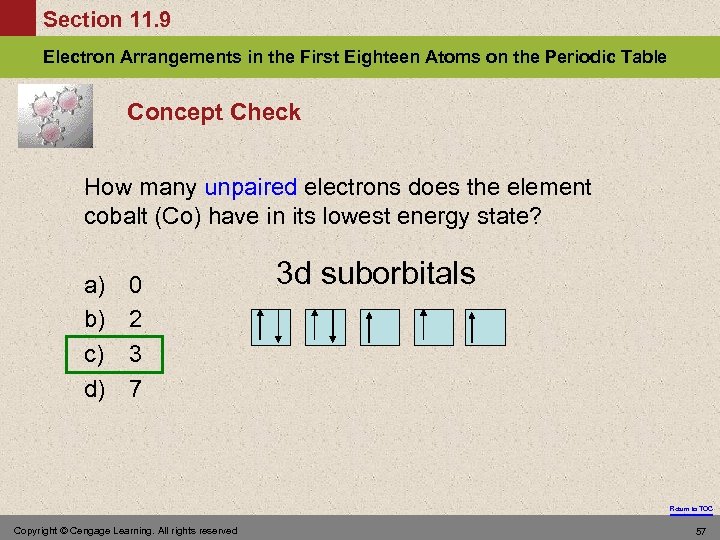 Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Concept Check How many unpaired electrons does the element cobalt (Co) have in its lowest energy state? a) b) c) d) 0 2 3 7 3 d suborbitals Return to TOC Copyright © Cengage Learning. All rights reserved 57
Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Concept Check How many unpaired electrons does the element cobalt (Co) have in its lowest energy state? a) b) c) d) 0 2 3 7 3 d suborbitals Return to TOC Copyright © Cengage Learning. All rights reserved 57
 Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Concept Check Can an electron in a phosphorus atom ever be in a 3 d orbital? Choose the best answer. a) Yes. An electron can be excited into a 3 d orbital. b) Yes. A ground-state electron in phosphorus is located in a 3 d orbital. c) No. Only transition metal atoms can have electrons located in the d orbitals. d) No. This would not correspond to phosphorus’ electron arrangement in its ground state. Return to TOC Copyright © Cengage Learning. All rights reserved 58
Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Concept Check Can an electron in a phosphorus atom ever be in a 3 d orbital? Choose the best answer. a) Yes. An electron can be excited into a 3 d orbital. b) Yes. A ground-state electron in phosphorus is located in a 3 d orbital. c) No. Only transition metal atoms can have electrons located in the d orbitals. d) No. This would not correspond to phosphorus’ electron arrangement in its ground state. Return to TOC Copyright © Cengage Learning. All rights reserved 58
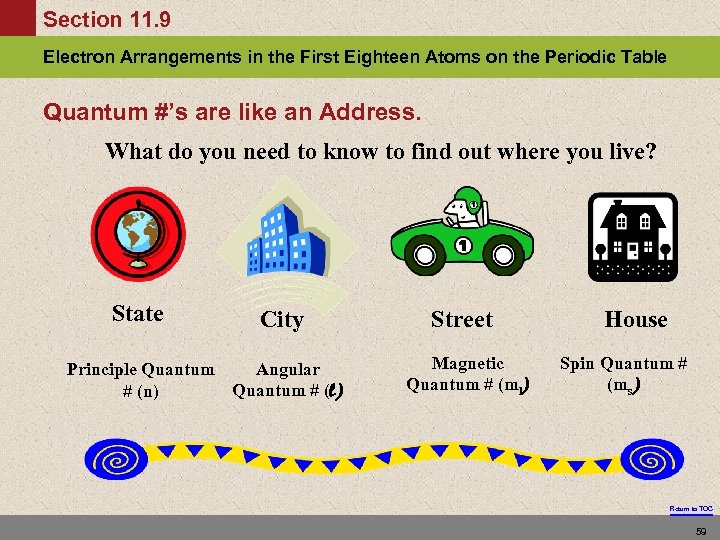 Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Quantum #’s are like an Address. What do you need to know to find out where you live? State City Principle Quantum Angular Quantum # (l) # (n) Street Magnetic Quantum # (ml) House Spin Quantum # (ms) Return to TOC 59
Section 11. 9 Electron Arrangements in the First Eighteen Atoms on the Periodic Table Quantum #’s are like an Address. What do you need to know to find out where you live? State City Principle Quantum Angular Quantum # (l) # (n) Street Magnetic Quantum # (ml) House Spin Quantum # (ms) Return to TOC 59
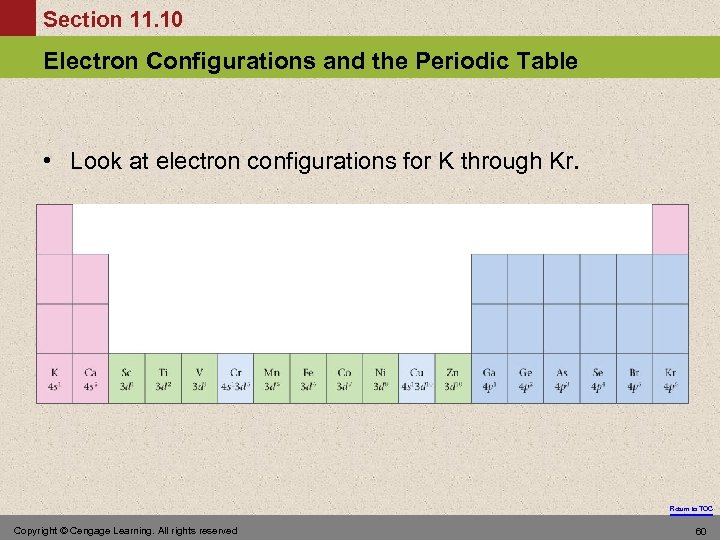 Section 11. 10 Electron Configurations and the Periodic Table • Look at electron configurations for K through Kr. Return to TOC Copyright © Cengage Learning. All rights reserved 60
Section 11. 10 Electron Configurations and the Periodic Table • Look at electron configurations for K through Kr. Return to TOC Copyright © Cengage Learning. All rights reserved 60
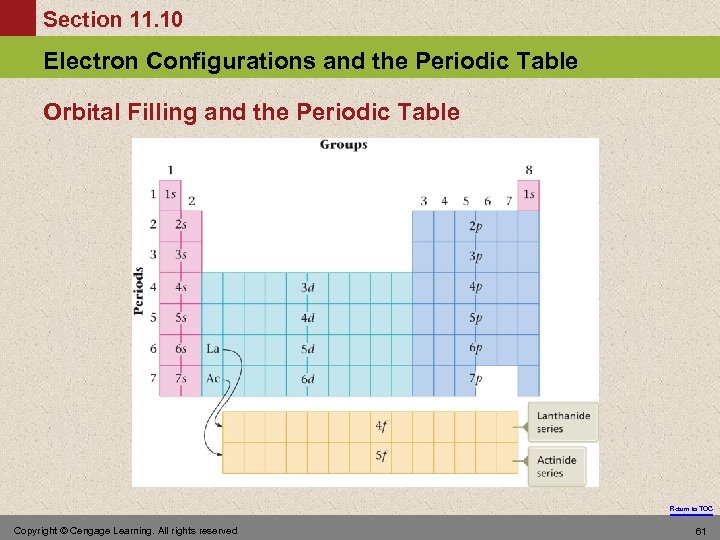 Section 11. 10 Electron Configurations and the Periodic Table Orbital Filling and the Periodic Table Return to TOC Copyright © Cengage Learning. All rights reserved 61
Section 11. 10 Electron Configurations and the Periodic Table Orbital Filling and the Periodic Table Return to TOC Copyright © Cengage Learning. All rights reserved 61
 Section 11. 10 Electron Configurations and the Periodic Table Orbital Filling 1. In a principal energy level that has d orbitals, the s orbital from the next level fills before the d orbitals in the current level. 2. After lanthanum, which has the electron configuration [Xe]6 s 25 d 1, a group of fourteen elements called the lanthanide series, or the lanthanides, occurs. This series of elements corresponds to the filling of the seven 4 f orbitals. Return to TOC Copyright © Cengage Learning. All rights reserved 62
Section 11. 10 Electron Configurations and the Periodic Table Orbital Filling 1. In a principal energy level that has d orbitals, the s orbital from the next level fills before the d orbitals in the current level. 2. After lanthanum, which has the electron configuration [Xe]6 s 25 d 1, a group of fourteen elements called the lanthanide series, or the lanthanides, occurs. This series of elements corresponds to the filling of the seven 4 f orbitals. Return to TOC Copyright © Cengage Learning. All rights reserved 62
 Section 11. 10 Electron Configurations and the Periodic Table Orbital Filling 3. After actinum, which has the configuration [Rn]7 s 26 d 1, a group of fourteen elements called the actinide series, or actinides, occurs. This series corresponds to the filling of the seven 5 f orbitals. Return to TOC 63
Section 11. 10 Electron Configurations and the Periodic Table Orbital Filling 3. After actinum, which has the configuration [Rn]7 s 26 d 1, a group of fourteen elements called the actinide series, or actinides, occurs. This series corresponds to the filling of the seven 5 f orbitals. Return to TOC 63
 Section 11. 10 Electron Configurations and the Periodic Table Orbital Filling 4. Except for helium, the group numbers indicate the sum of electrons in the ns and np orbitals in the highest principal energy level that contains electrons (where n is the number that indicates a particular principal energy level). These electrons are the valence electrons. Return to TOC 64
Section 11. 10 Electron Configurations and the Periodic Table Orbital Filling 4. Except for helium, the group numbers indicate the sum of electrons in the ns and np orbitals in the highest principal energy level that contains electrons (where n is the number that indicates a particular principal energy level). These electrons are the valence electrons. Return to TOC 64
 Section 11. 10 Electron Configurations and the Periodic Table Exercise Determine the expected electron configurations for each of the following. a) S 1 s 22 p 63 s 23 p 4 or [Ne]3 s 23 p 4 b) Ba [Xe]6 s 2 c) Eu [Xe]6 s 24 f 6 Return to TOC Copyright © Cengage Learning. All rights reserved 65
Section 11. 10 Electron Configurations and the Periodic Table Exercise Determine the expected electron configurations for each of the following. a) S 1 s 22 p 63 s 23 p 4 or [Ne]3 s 23 p 4 b) Ba [Xe]6 s 2 c) Eu [Xe]6 s 24 f 6 Return to TOC Copyright © Cengage Learning. All rights reserved 65
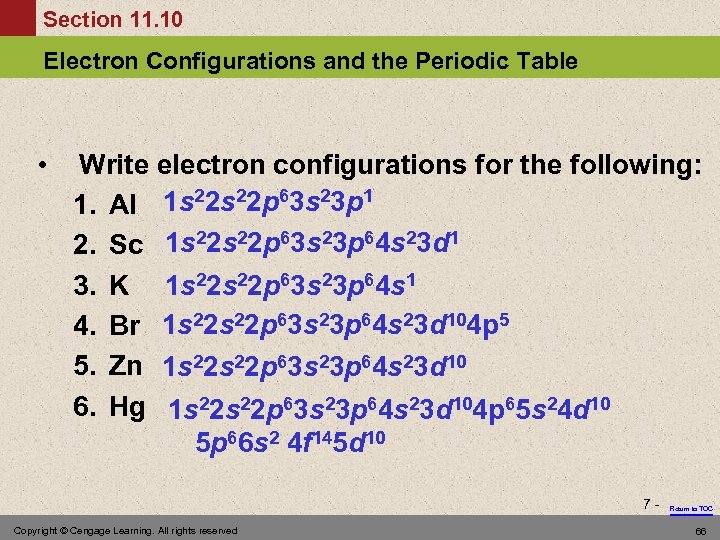 Section 11. 10 Electron Configurations and the Periodic Table • Write electron configurations for the following: 1. Al 1 s 22 p 63 s 23 p 1 2. Sc 1 s 22 p 63 s 23 p 64 s 23 d 1 3. 4. 5. 6. K Br Zn Hg 1 s 22 p 63 s 23 p 64 s 1 1 s 22 p 63 s 23 p 64 s 23 d 104 p 5 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10 5 p 66 s 2 4 f 145 d 10 7 - Copyright © Cengage Learning. All rights reserved Return to TOC 66
Section 11. 10 Electron Configurations and the Periodic Table • Write electron configurations for the following: 1. Al 1 s 22 p 63 s 23 p 1 2. Sc 1 s 22 p 63 s 23 p 64 s 23 d 1 3. 4. 5. 6. K Br Zn Hg 1 s 22 p 63 s 23 p 64 s 1 1 s 22 p 63 s 23 p 64 s 23 d 104 p 5 1 s 22 s 22 p 63 s 23 p 64 s 23 d 104 p 65 s 24 d 10 5 p 66 s 2 4 f 145 d 10 7 - Copyright © Cengage Learning. All rights reserved Return to TOC 66
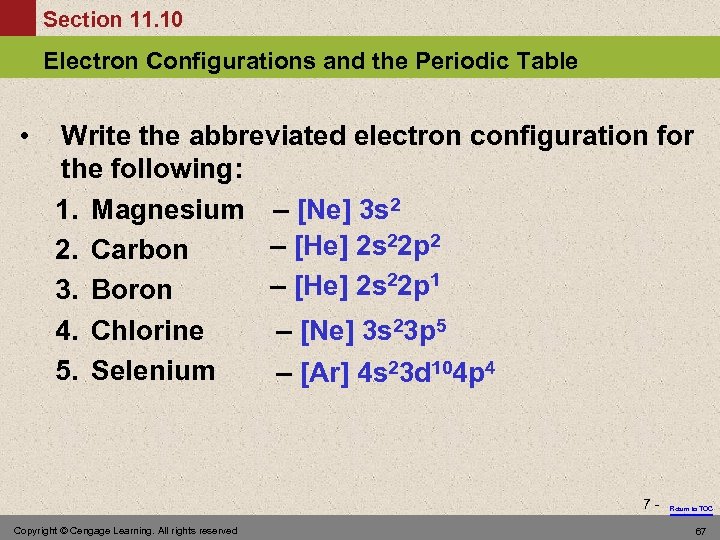 Section 11. 10 Electron Configurations and the Periodic Table • Write the abbreviated electron configuration for the following: 1. Magnesium – [Ne] 3 s 2 – [He] 2 s 22 p 2 2. Carbon – [He] 2 s 22 p 1 3. Boron 4. Chlorine – [Ne] 3 s 23 p 5 5. Selenium – [Ar] 4 s 23 d 104 p 4 7 - Copyright © Cengage Learning. All rights reserved Return to TOC 67
Section 11. 10 Electron Configurations and the Periodic Table • Write the abbreviated electron configuration for the following: 1. Magnesium – [Ne] 3 s 2 – [He] 2 s 22 p 2 2. Carbon – [He] 2 s 22 p 1 3. Boron 4. Chlorine – [Ne] 3 s 23 p 5 5. Selenium – [Ar] 4 s 23 d 104 p 4 7 - Copyright © Cengage Learning. All rights reserved Return to TOC 67
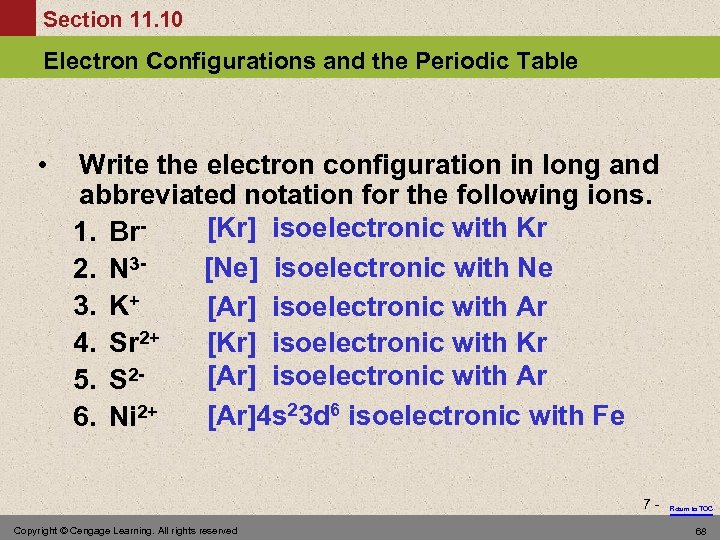 Section 11. 10 Electron Configurations and the Periodic Table • Write the electron configuration in long and abbreviated notation for the following ions. [Kr] isoelectronic with Kr 1. Br[Ne] isoelectronic with Ne 2. N 33. K+ [Ar] isoelectronic with Ar 4. Sr 2+ [Kr] isoelectronic with Kr [Ar] isoelectronic with Ar 5. S 2[Ar]4 s 23 d 6 isoelectronic with Fe 6. Ni 2+ 7 - Copyright © Cengage Learning. All rights reserved Return to TOC 68
Section 11. 10 Electron Configurations and the Periodic Table • Write the electron configuration in long and abbreviated notation for the following ions. [Kr] isoelectronic with Kr 1. Br[Ne] isoelectronic with Ne 2. N 33. K+ [Ar] isoelectronic with Ar 4. Sr 2+ [Kr] isoelectronic with Kr [Ar] isoelectronic with Ar 5. S 2[Ar]4 s 23 d 6 isoelectronic with Fe 6. Ni 2+ 7 - Copyright © Cengage Learning. All rights reserved Return to TOC 68
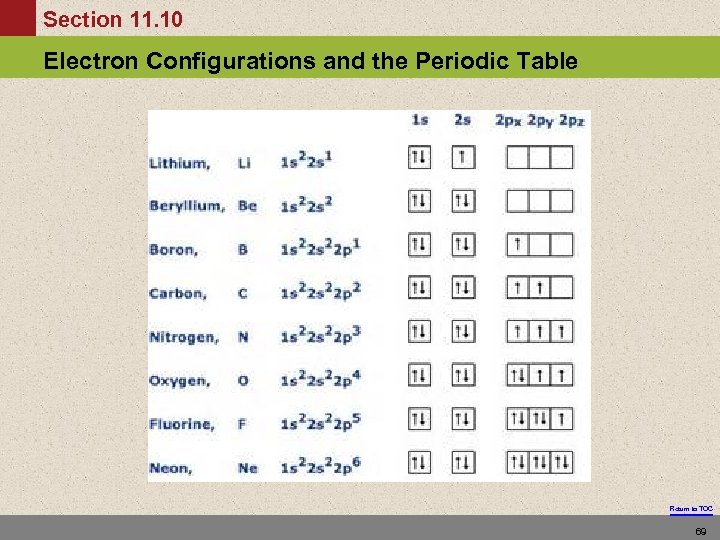 Section 11. 10 Electron Configurations and the Periodic Table Return to TOC 69
Section 11. 10 Electron Configurations and the Periodic Table Return to TOC 69
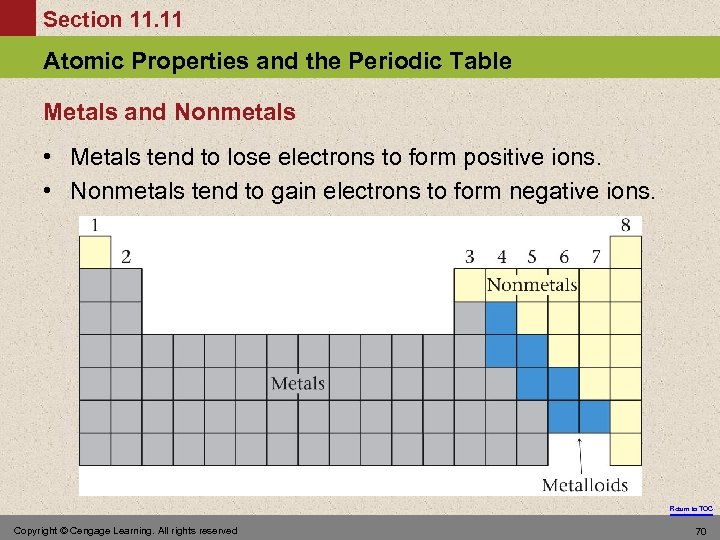 Section 11. 11 Atomic Properties and the Periodic Table Metals and Nonmetals • Metals tend to lose electrons to form positive ions. • Nonmetals tend to gain electrons to form negative ions. Return to TOC Copyright © Cengage Learning. All rights reserved 70
Section 11. 11 Atomic Properties and the Periodic Table Metals and Nonmetals • Metals tend to lose electrons to form positive ions. • Nonmetals tend to gain electrons to form negative ions. Return to TOC Copyright © Cengage Learning. All rights reserved 70
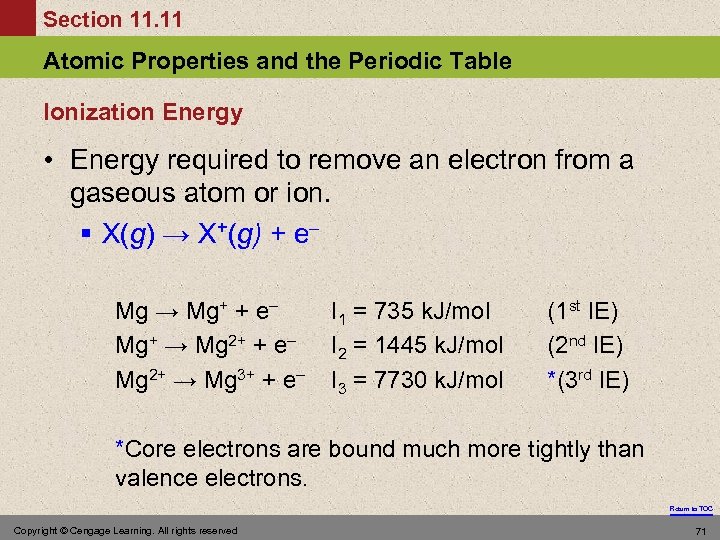 Section 11. 11 Atomic Properties and the Periodic Table Ionization Energy • Energy required to remove an electron from a gaseous atom or ion. § X(g) → X+(g) + e– Mg → Mg+ + e– Mg+ → Mg 2+ + e– Mg 2+ → Mg 3+ + e– I 1 = 735 k. J/mol I 2 = 1445 k. J/mol I 3 = 7730 k. J/mol (1 st IE) (2 nd IE) *(3 rd IE) *Core electrons are bound much more tightly than valence electrons. Return to TOC Copyright © Cengage Learning. All rights reserved 71
Section 11. 11 Atomic Properties and the Periodic Table Ionization Energy • Energy required to remove an electron from a gaseous atom or ion. § X(g) → X+(g) + e– Mg → Mg+ + e– Mg+ → Mg 2+ + e– Mg 2+ → Mg 3+ + e– I 1 = 735 k. J/mol I 2 = 1445 k. J/mol I 3 = 7730 k. J/mol (1 st IE) (2 nd IE) *(3 rd IE) *Core electrons are bound much more tightly than valence electrons. Return to TOC Copyright © Cengage Learning. All rights reserved 71
 Section 11. 11 Atomic Properties and the Periodic Table Ionization Energy • In general, as we go across a period from left to right, the first ionization energy increases. • Why? § Electrons added in the same principal quantum level do not completely shield the increasing nuclear charge caused by the added protons. § Electrons in the same principal quantum level are generally more strongly bound from left to right on the periodic table. Return to TOC Copyright © Cengage Learning. All rights reserved 72
Section 11. 11 Atomic Properties and the Periodic Table Ionization Energy • In general, as we go across a period from left to right, the first ionization energy increases. • Why? § Electrons added in the same principal quantum level do not completely shield the increasing nuclear charge caused by the added protons. § Electrons in the same principal quantum level are generally more strongly bound from left to right on the periodic table. Return to TOC Copyright © Cengage Learning. All rights reserved 72
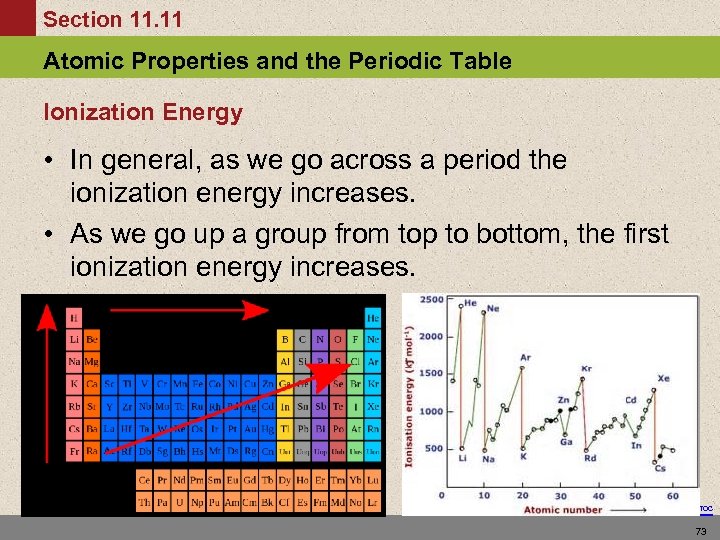 Section 11. 11 Atomic Properties and the Periodic Table Ionization Energy • In general, as we go across a period the ionization energy increases. • As we go up a group from top to bottom, the first ionization energy increases. Return to TOC 73
Section 11. 11 Atomic Properties and the Periodic Table Ionization Energy • In general, as we go across a period the ionization energy increases. • As we go up a group from top to bottom, the first ionization energy increases. Return to TOC 73
 Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which atom would require more energy to remove an electron? Why? Na Cl Return to TOC Copyright © Cengage Learning. All rights reserved 74
Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which atom would require more energy to remove an electron? Why? Na Cl Return to TOC Copyright © Cengage Learning. All rights reserved 74
 Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which atom would require more energy to remove an electron? Why? Li Cs Return to TOC Copyright © Cengage Learning. All rights reserved 75
Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which atom would require more energy to remove an electron? Why? Li Cs Return to TOC Copyright © Cengage Learning. All rights reserved 75
 Section 11. 11 Atomic Properties and the Periodic Table Atomic Size • In general as we go across a period from left to right, the atomic radius decreases. § Effective nuclear charge increases, therefore the valence electrons are drawn closer to the nucleus, decreasing the size of the atom. • In general atomic radius increases in going down a group. § Orbital sizes increase in successive principal quantum levels. Return to TOC Copyright © Cengage Learning. All rights reserved 76
Section 11. 11 Atomic Properties and the Periodic Table Atomic Size • In general as we go across a period from left to right, the atomic radius decreases. § Effective nuclear charge increases, therefore the valence electrons are drawn closer to the nucleus, decreasing the size of the atom. • In general atomic radius increases in going down a group. § Orbital sizes increase in successive principal quantum levels. Return to TOC Copyright © Cengage Learning. All rights reserved 76
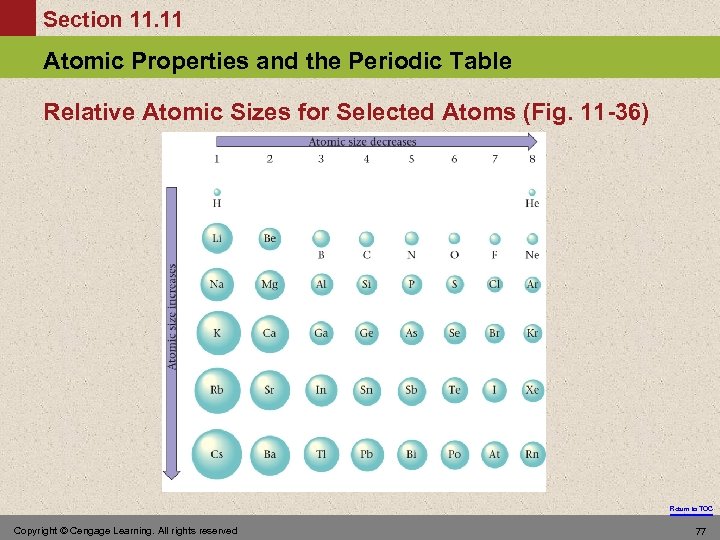 Section 11. 11 Atomic Properties and the Periodic Table Relative Atomic Sizes for Selected Atoms (Fig. 11 -36) Return to TOC Copyright © Cengage Learning. All rights reserved 77
Section 11. 11 Atomic Properties and the Periodic Table Relative Atomic Sizes for Selected Atoms (Fig. 11 -36) Return to TOC Copyright © Cengage Learning. All rights reserved 77
 Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which should be the larger atom? Why? Na Cl Return to TOC 78
Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which should be the larger atom? Why? Na Cl Return to TOC 78
 Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which should be the larger atom? Why? Li Cs Return to TOC Copyright © Cengage Learning. All rights reserved 79
Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which should be the larger atom? Why? Li Cs Return to TOC Copyright © Cengage Learning. All rights reserved 79
 Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which is larger? • The hydrogen 1 s orbital • The lithium 2 s orbital Which is lower in energy? • The hydrogen 1 s orbital • The lithium 2 s orbital Return to TOC Copyright © Cengage Learning. All rights reserved 80
Section 11. 11 Atomic Properties and the Periodic Table Concept Check Which is larger? • The hydrogen 1 s orbital • The lithium 2 s orbital Which is lower in energy? • The hydrogen 1 s orbital • The lithium 2 s orbital Return to TOC Copyright © Cengage Learning. All rights reserved 80
 Section 11. 11 Atomic Properties and the Periodic Table Exercise Arrange the elements oxygen, fluorine, and sulfur according to increasing: § Ionization energy S, O, F § Atomic size F, O, S Return to TOC Copyright © Cengage Learning. All rights reserved 81
Section 11. 11 Atomic Properties and the Periodic Table Exercise Arrange the elements oxygen, fluorine, and sulfur according to increasing: § Ionization energy S, O, F § Atomic size F, O, S Return to TOC Copyright © Cengage Learning. All rights reserved 81


