08450a9d2c3fa050a5014a9d2bb57a79.ppt
- Количество слайдов: 57

Chapter 10 Lecture General, Organic, and Biological Chemistry: An Integrated Approach Chapter 10 Laura Frost, Todd Deal and Karen Timberlake Enzymes—Nature’s Chemists by Richard Triplett © 2011 Pearson Education, Inc.
Chapter Outline 10. 1 Enzymes and Their Substrates 10. 2 Thermodynamics of Chemical Reactions 10. 3 Enzymes and Catalysis 10. 4 Factors That Affect Enzyme Activity © 2011 Pearson Education, Inc.
Introduction • Enzymes are biologically active proteins that accelerate the breakdown of food that is eaten. • Enzymes are biological catalysts. They accelerate reactions, but are not consumed or changed in reactions. • Discussions on the production or consumption of energy, specifically heat, during chemical reactions is called thermodynamics. © 2011 Pearson Education, Inc.
10. 1 Enzymes and Their Substrates • Enzymes are large proteins with complex, threedimensional structures. • Enzymes work in an aqueous environment in our body so that the protein chain folds such that the polar amino acids are on the surface. • Consider hexokinase, an enzyme whose job is to transfer a phosphate group from the high energy molecule, adenosine triphosphate, ATP, to D-glucose. © 2011 Pearson Education, Inc.
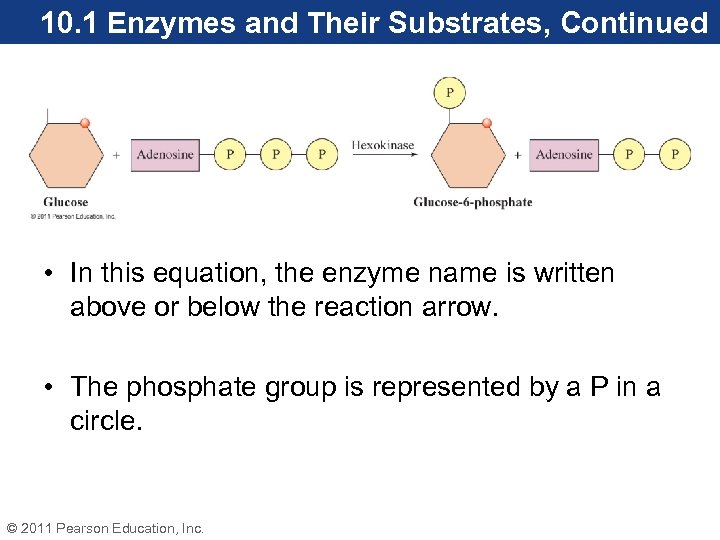
10. 1 Enzymes and Their Substrates, Continued • In this equation, the enzyme name is written above or below the reaction arrow. • The phosphate group is represented by a P in a circle. © 2011 Pearson Education, Inc.
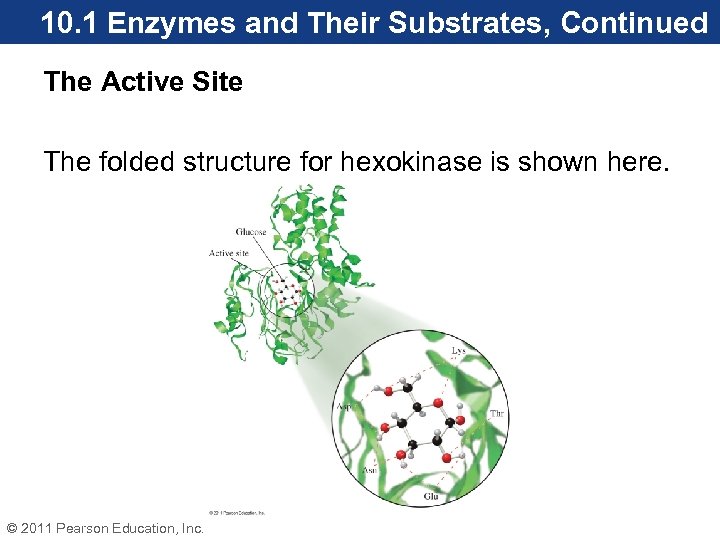
10. 1 Enzymes and Their Substrates, Continued The Active Site The folded structure for hexokinase is shown here. © 2011 Pearson Education, Inc.
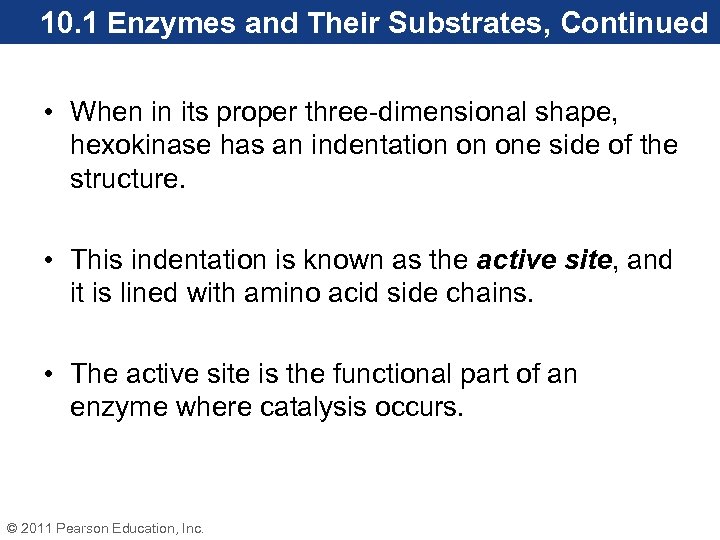
10. 1 Enzymes and Their Substrates, Continued • When in its proper three-dimensional shape, hexokinase has an indentation on one side of the structure. • This indentation is known as the active site, and it is lined with amino acid side chains. • The active site is the functional part of an enzyme where catalysis occurs. © 2011 Pearson Education, Inc.

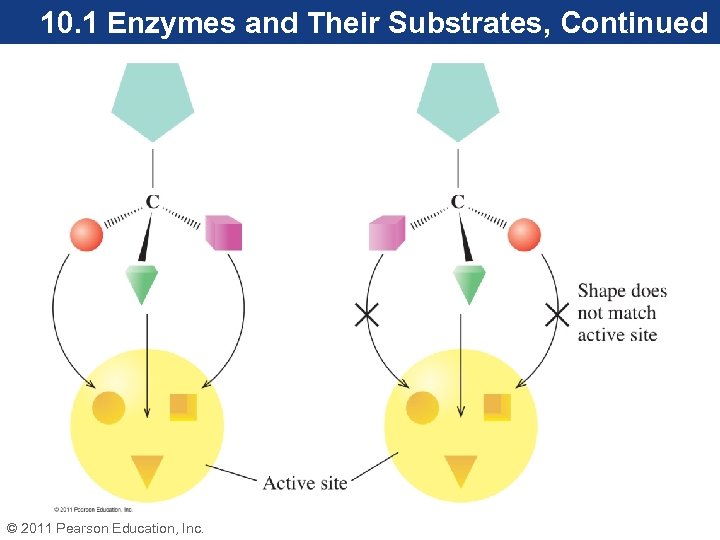
10. 1 Enzymes and Their Substrates, Continued • Glucose, the reactant for hexokinase, fits snugly in the active site. In an enzyme reaction, the reactant is called the substrate. • Enzymes have specific substrates, a property known as substrate specificity. For example, the active site of hexokinase reacts with D-glucose, but will not react with L-glucose. • Enzymes are specific for one enantiomer of the substrate. © 2011 Pearson Education, Inc.
10. 1 Enzymes and Their Substrates, Continued © 2011 Pearson Education, Inc.
10. 1 Enzymes and Their Substrates, Continued Some enzymes, like hexokinase, have nonprotein helpers. Two categories of helpers are as follows: 1. Cofactors are inorganic substances like magnesium ions. 2. Coenzymes are small organic molecules derived from vitamins. Riboflavin found in the coenzyme flavin adenine dinucleotide (FAD) is a coenzyme. © 2011 Pearson Education, Inc.
10. 1 Enzymes and Their Substrates, Continued Enzyme–Substrate Models • A substrate is drawn into the active site by intermolecular attractions like hydrogen bonding. • Hydrogen bonding orients the substrate properly within the active site. • The initial interaction of the enzyme with the substrate is called the enzyme–substrate complex (ES). This complex forms prior to catalysis. © 2011 Pearson Education, Inc.
10. 1 Enzymes and Their Substrates, Continued There are two enzyme–substrate models: 1. In the Lock-and-key model, the active site is thought to be a rigid, inflexible shape that is an exact complement to the shape of the substrate. The substrate fits in the active site much like a key fits in a lock. 2. In the induced-fit model, the active site is flexible, has a shape roughly complementary to the shape of its substrate, and undergoes a conformational change, adjusting to the shape of the substrate when the substrate interacts with the enzyme. © 2011 Pearson Education, Inc.
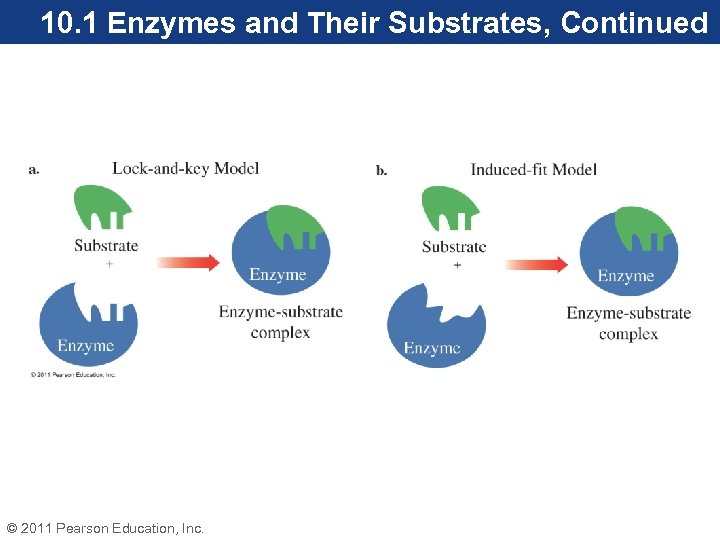
10. 1 Enzymes and Their Substrates, Continued © 2011 Pearson Education, Inc.
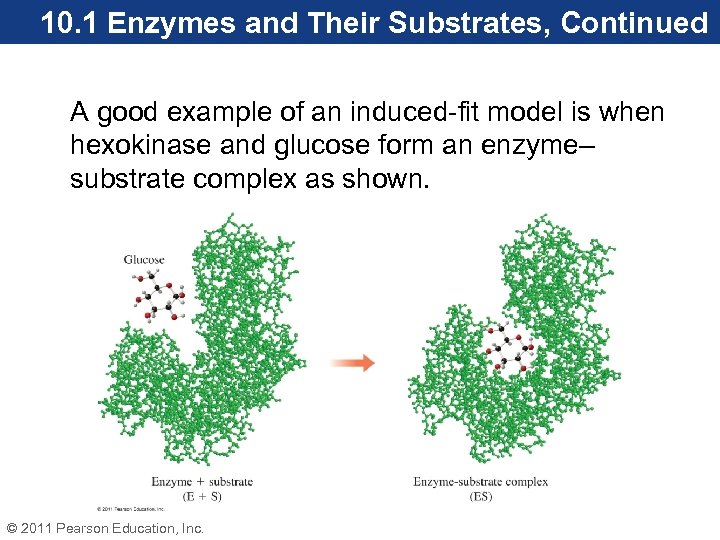
10. 1 Enzymes and Their Substrates, Continued A good example of an induced-fit model is when hexokinase and glucose form an enzyme– substrate complex as shown. © 2011 Pearson Education, Inc.
10. 2 Thermodynamics of Chemical Reactions • As chemical reactions occur, some bonds are formed and some are broken, and in the process, the amount of energy changes. • Some reactions release energy as heat (exothermic reactions), and some absorb energy as heat (endothermic reactions). • A collision of reactant molecules must occur for a chemical reaction to occur. © 2011 Pearson Education, Inc.
10. 2 Thermodynamics of Chemical Reactions, Continued • Energy is required to cause reactant molecules to collide. • Reactant molecules must be aligned properly in order for a reaction to occur. • Activation energy is required to properly align reactant molecules and to cause them to collide to produce products. © 2011 Pearson Education, Inc.
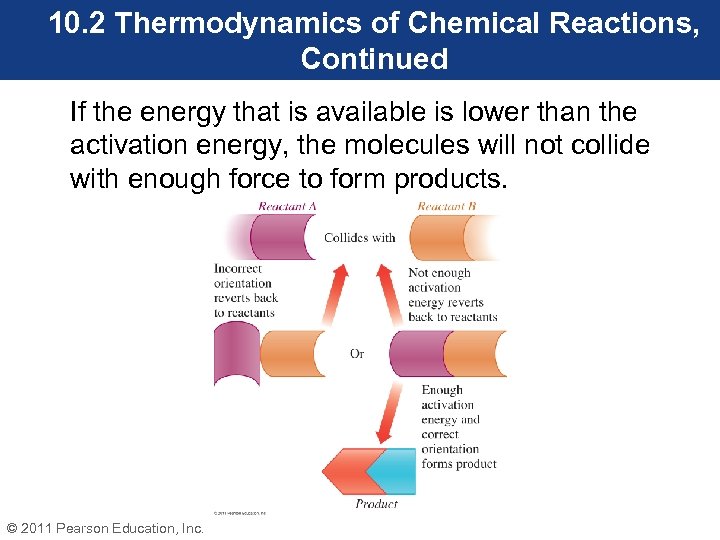
10. 2 Thermodynamics of Chemical Reactions, Continued If the energy that is available is lower than the activation energy, the molecules will not collide with enough force to form products. © 2011 Pearson Education, Inc.
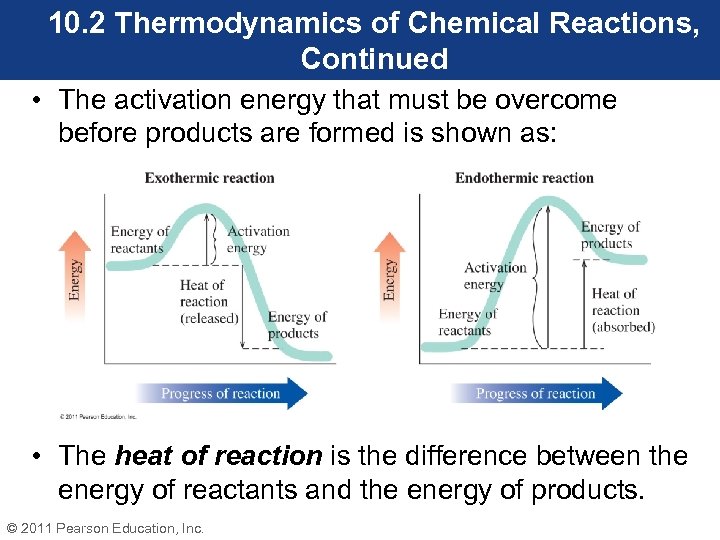
10. 2 Thermodynamics of Chemical Reactions, Continued • The activation energy that must be overcome before products are formed is shown as: • The heat of reaction is the difference between the energy of reactants and the energy of products. © 2011 Pearson Education, Inc.
10. 2 Thermodynamics of Chemical Reactions, Continued • In an exothermic reaction, the energy of reactants is higher than the energy of products, so heat is released. • In an endothermic reaction, the energy of products is higher than the energy of reactants, so heat is absorbed. • The height of the activation energy peak gives an indication of how fast the reaction proceeds. © 2011 Pearson Education, Inc.
10. 2 Thermodynamics of Chemical Reactions, Continued • Reactions with a low activation energy will proceed at a faster rate than reactions with a high activation energy. • Activation energy can be lowered with a catalyst, which will cause the reaction to proceed at a faster rate. • A catalyst will not affect the energy of products or reactants. © 2011 Pearson Education, Inc.
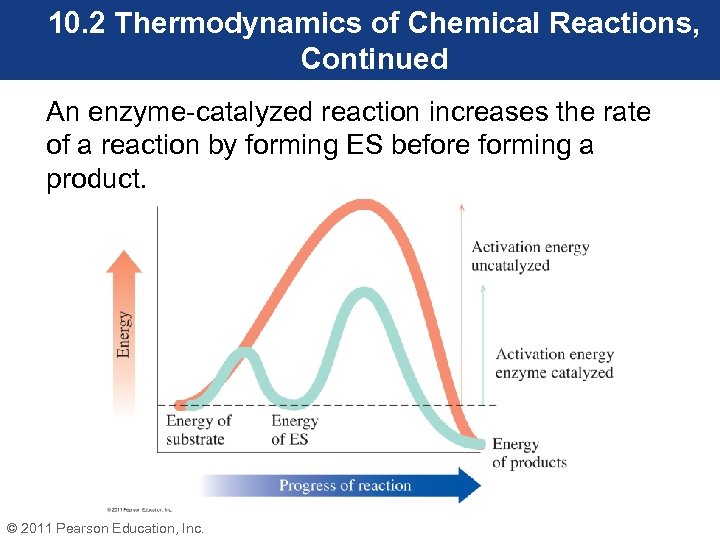
10. 2 Thermodynamics of Chemical Reactions, Continued An enzyme-catalyzed reaction increases the rate of a reaction by forming ES before forming a product. © 2011 Pearson Education, Inc.
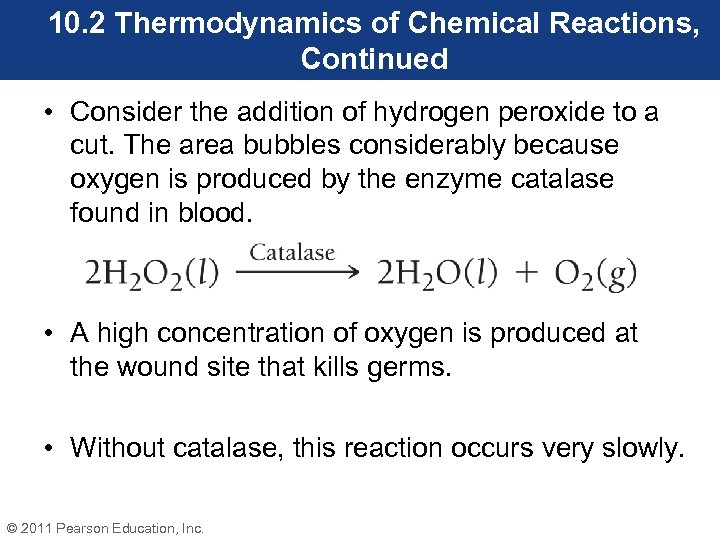
10. 2 Thermodynamics of Chemical Reactions, Continued • Consider the addition of hydrogen peroxide to a cut. The area bubbles considerably because oxygen is produced by the enzyme catalase found in blood. • A high concentration of oxygen is produced at the wound site that kills germs. • Without catalase, this reaction occurs very slowly. © 2011 Pearson Education, Inc.
10. 3 Enzymes and Catalysis • Enzymes lower the activation energy by forming ES complex. • ES is formed through the interactions between the enzyme and substrate. Each interaction releases a small amount of energy to stabilize the complex. • These interactions combine to lower the activation energy of the reaction. • Some interactions that help lower the activation energy are discussed in the next several slides. © 2011 Pearson Education, Inc.
10. 3 Enzymes and Catalysis, Continued Proximity • The active site of enzymes has a small volume. • When ES forms, the active site is filled with substrate. • The reacting molecules are in close proximity to each other, and the closer they are the more likely a reaction will occur. • Amino acid side chains in the active site are used to facilitate the reaction. © 2011 Pearson Education, Inc.
10. 3 Enzymes and Catalysis, Continued Orientation • In the active site, substrate molecules are held at the appropriate distance and in correct alignment to each other for the reaction to occur. • Amino acid side chains in the active site create interactions that orient the substrates. • This lowers the activation energy needed for the reaction to occur. © 2011 Pearson Education, Inc.
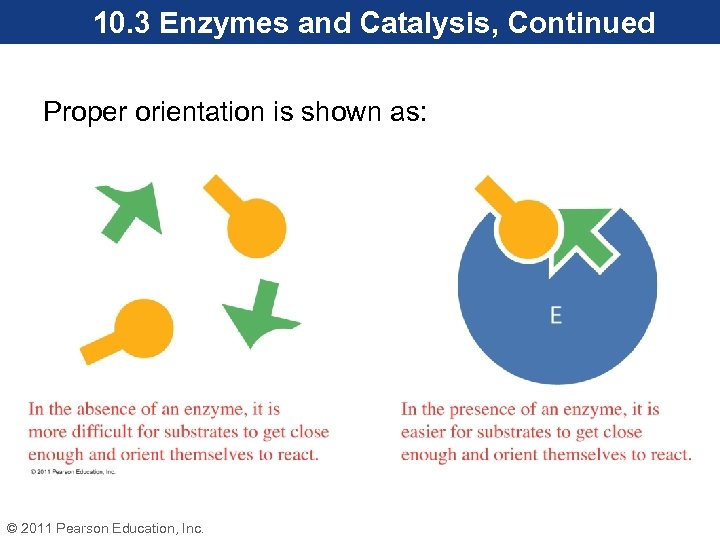
10. 3 Enzymes and Catalysis, Continued Proper orientation is shown as: © 2011 Pearson Education, Inc.
10. 3 Enzymes and Catalysis, Continued Bond Energy • When an enzyme interacts with substrate to form ES, the bonds of the substrate molecule are weakened (strained). • Strained bonds in the substrate means that the reaction will proceed more rapidly because the activation energy is lowered by this effect. © 2011 Pearson Education, Inc.
10. 3 Enzymes and Catalysis, Continued • Consider the hexokinase reaction that catalyzes glucose to glucose-6 -phosphate. • Mg 2+ (a coenzyme) holds ATP in one area of the active site and glucose interacts with another area. • Amino acid side chains in the active site form multiple hydrogen bonds with glucose, which stabilizes ES and lowers the activation energy. © 2011 Pearson Education, Inc.
10. 3 Enzymes and Catalysis, Continued • A conformational change in the enzyme occurs when glucose enters the active site. • ATP is in close proximity to the glucose and is in proper orientation for the reaction. • Glucose-6 -phosphate is formed along with ADP. • The enzyme is less attracted to the products, so the lobes of the enzyme move apart and the products are released. © 2011 Pearson Education, Inc.
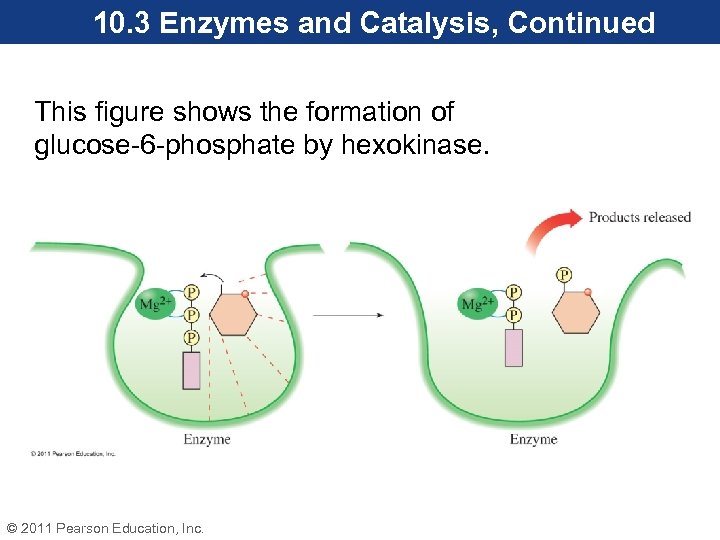
10. 3 Enzymes and Catalysis, Continued This figure shows the formation of glucose-6 -phosphate by hexokinase. © 2011 Pearson Education, Inc.
10. 4 Factors That Affect Enzyme Activity • If allowed to sit untouched, the flesh of sliced apples will turn brown by a process known as oxidation, caused by an enzyme. • If lemon juice is sprinkled on the sliced apple, the vitamin C in the lemon juice will inhibit the formation of this brown color by changing the p. H of the environment of the enzyme. • Enzyme reactions are affected by reaction conditions such as substrate concentration, p. H, temperature, and the presence of inhibitors. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued Substrate Concentration • Recall that the first step in an enzyme-catalyzed reaction is the formation of ES. • At a constant concentration of enzyme, an increase in substrate concentration will cause an increase in the enzyme activity up to the point where the enzyme becomes saturated with substrate. © 2011 Pearson Education, Inc.
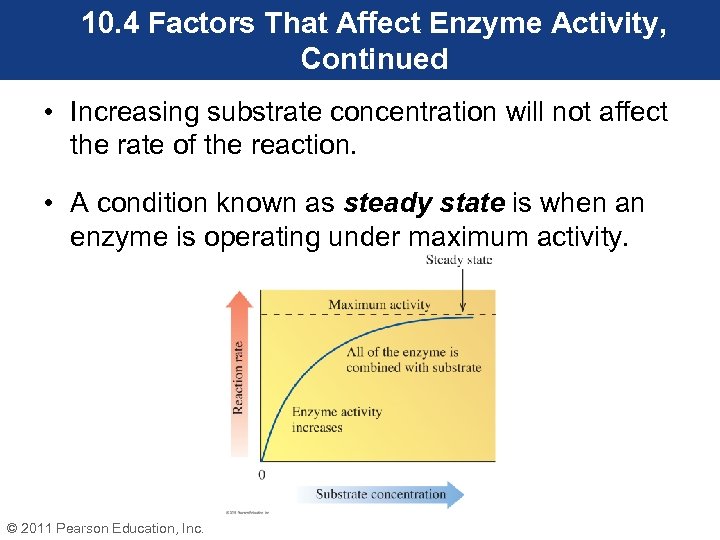
10. 4 Factors That Affect Enzyme Activity, Continued • Increasing substrate concentration will not affect the rate of the reaction. • A condition known as steady state is when an enzyme is operating under maximum activity. © 2011 Pearson Education, Inc.
10. 4 Factors That Affect Enzyme Activity, Continued p. H • When the enzyme environment is changed by p. H, its tertiary structure is disrupted, altering the active site and causing the enzyme’s activity to decrease. • Enzymes are most active at a p. H known as their optimum p. H. • At optimum p. H, the enzyme maintains its tertiary structure and its active site. © 2011 Pearson Education, Inc.
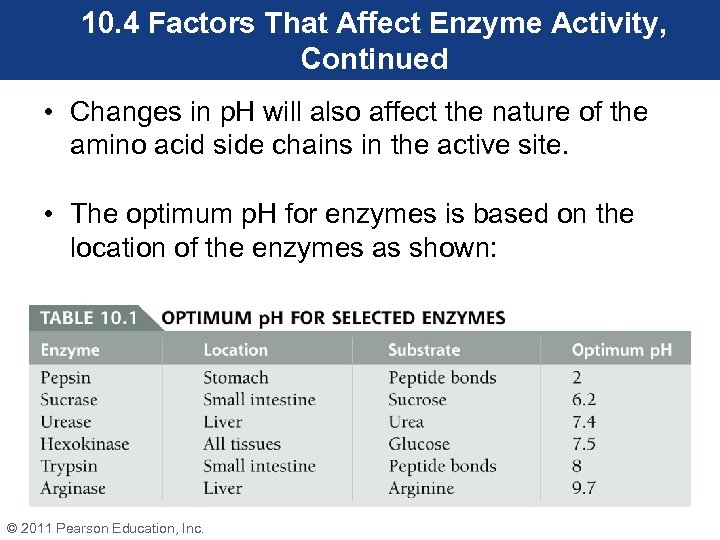
10. 4 Factors That Affect Enzyme Activity, Continued • Changes in p. H will also affect the nature of the amino acid side chains in the active site. • The optimum p. H for enzymes is based on the location of the enzymes as shown: © 2011 Pearson Education, Inc.
10. 4 Factors That Affect Enzyme Activity, Continued Temperature • Enzymes have an optimum temperature at which they are most active. • The optimum temperature for most human enzymes is normal body temperature, 37 o. C. • Above optimum temperature, enzymes lose activity due to disruption of intermolecular forces stabilizing the tertiary structure. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued • At high temperatures, enzymes denature, which modifies the active site. • At low temperatures, enzyme activity is low due to a lack of energy for the reaction to occur. • Food is stored in a refrigerator or freezer to slow spoilage brought on by enzymes. • Boiling contaminated water will destroy enzymes in bacteria that are present in the water. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued Inhibitors • Inhibitors are types of molecules that will cause enzymes to lose activity. • Enzyme inhibitors prevent the active site from interacting with substrate to form ES. • Some inhibitors cause temporary loss of activity, while others cause permanent loss of activity. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued • Reversible inhibition occurs when the inhibitor causes a temporary loss of activity. However, activity is regained if the inhibitor is removed. • Reversible inhibitors can be competitive or noncompetitive. • Competitive inhibitors are molecules that compete with a substrate for the active site, and have a structure similar to the substrate. © 2011 Pearson Education, Inc.
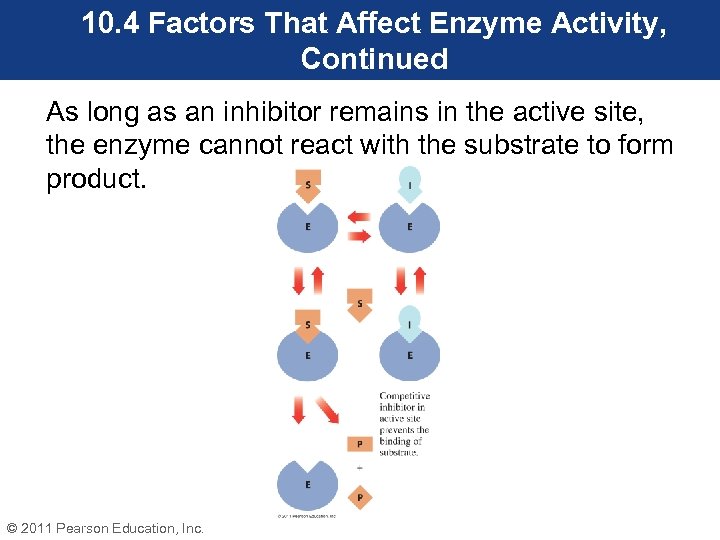
10. 4 Factors That Affect Enzyme Activity, Continued As long as an inhibitor remains in the active site, the enzyme cannot react with the substrate to form product. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued • An example of a medical therapy that involves a competitive inhibitor involves liver alcohol dehydrogenase (LAD). This enzyme oxidizes ethanol, the alcohol found in alcoholic beverages. • This enzyme will also react with ethylene glycol and methanol, which are found in antifreeze, and will compete with ethanol for the active site. • If a pet is poisoned by drinking antifreeze, a slow intravenous infusion of ethanol is administrated. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued • Administration of ethanol slows the production of the toxic metabolites of ethylene glycol and methanol, giving the kidneys time to eliminate these two substrates. • Noncompetitive inhibitors do not resemble the substrate. They do not compete for the enzyme’s active site. • Noncompetitive inhibitors bind at a site on the enzyme that is usually remote to the active site. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued • When a noncompetitive inhibitor binds to an enzyme, it causes a conformational change in the enzyme. This change in shape causes the active site to no longer interact with the substrate. • As long as this type of inhibitor is bound to the enzyme, it will no longer function effectively. © 2011 Pearson Education, Inc.
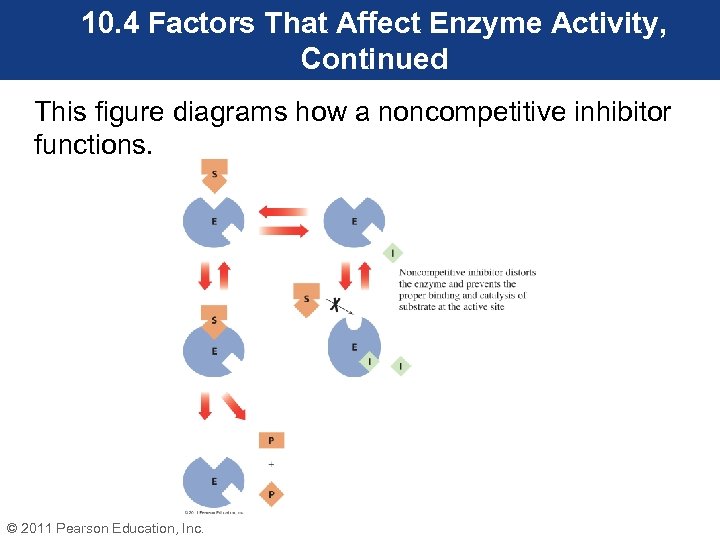
10. 4 Factors That Affect Enzyme Activity, Continued This figure diagrams how a noncompetitive inhibitor functions. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued • Inhibitions caused by competitive and noncompetitive inhibitors can be reversed. • Inhibition by competitive inhibitors can be reversed by adding more substrate. The higher the concentration of substrate, the more likely it will overcome the competition for the active site. • Adding more substrate with noncompetitive inhibitors has no effect on overcoming inhibition. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued • Reversing a noncompetitive inhibitor requires a special chemical reagent to remove the inhibitor and restore catalytic activity. • An irreversible inhibitor forms a covalent bond with an amino acid side chain in the enzyme’s active site. • Irreversible inhibition causes the substrate to be excluded from the active site. • Irreversible inhibition is a permanent inhibition. © 2011 Pearson Education, Inc.
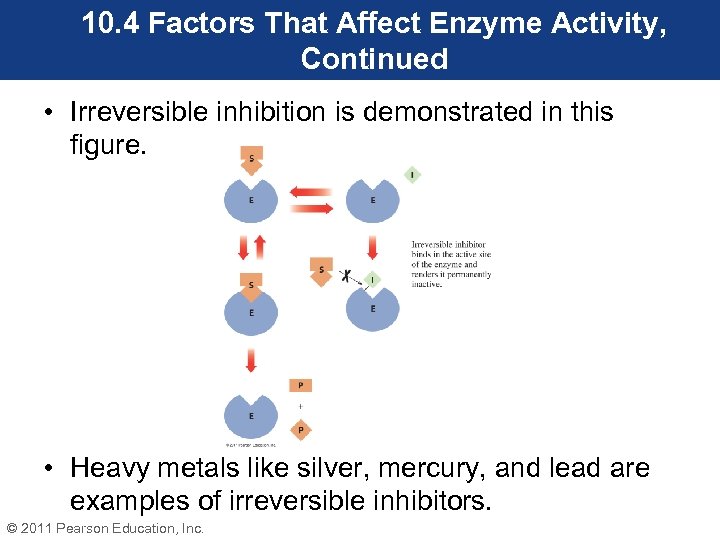
10. 4 Factors That Affect Enzyme Activity, Continued • Irreversible inhibition is demonstrated in this figure. • Heavy metals like silver, mercury, and lead are examples of irreversible inhibitors. © 2011 Pearson Education, Inc.

10. 4 Factors That Affect Enzyme Activity, Continued Antibiotics Inhibit Bacterial Enzymes • Enzyme inhibitors are used to fight bacterial infections. • Penicillin is an example of an irreversible inhibitor. It binds to the enzyme that bacteria use to synthesize cell walls, and slows the growth of cell walls. • Without a cell wall, bacteria cannot survive and the infection stops. © 2011 Pearson Education, Inc.
Chapter Summary 10. 1 Enzymes and Their Substrates • Enzymes are large, globular proteins that serve as biological catalysts. • The functional part of an enzyme is the active site. • Substrates are the reactants for an enzyme reaction, and they bind to the active site to form ES. © 2011 Pearson Education, Inc.
Chapter Summary, Continued 10. 1 Enzymes and Their Substrates, Continued • Enzymes are specific for one substrate that will bind to the active site and react. • Two theories, lock-and-key and induced-fit, explain how an enzyme interacts with its substrate to form ES. © 2011 Pearson Education, Inc.
Chapter Summary, Continued 10. 2 Thermodynamics of Chemical Reactions • Thermodynamics is a study of the energy changes that occur during a chemical reaction. • Activation energy is the energy required to start a reaction, and plays a role in the rate of reaction. • The lower the activation energy, the faster the rate of reaction. • Heat of reaction is a measure of the production or consumption of energy in a reaction. © 2011 Pearson Education, Inc.
Chapter Summary, Continued 10. 2 Thermodynamics of Chemical Reactions, Continued • An exothermic reaction releases heat to its environment. • An endothermic reaction absorbs heat from its environment. • Catalysts lower the activation energy causing an increase in the rate of reaction. • Enzymes form ES before catalysis, which causes a lowering of the activation energy. © 2011 Pearson Education, Inc.
Chapter Summary, Continued 10. 3 Enzymes and Catalysis • Formation of the ES complex lowers the activation energy for a catalyzed reaction. • In the active site, atoms are brought close together and aligned with amino acid side chains. © 2011 Pearson Education, Inc.
Chapter Summary, Continued 10. 3 Enzymes and Catalysis, Continued • The active site also aligns the reactants with the optimal orientation for a reaction to occur. • The interaction of substrate with the active site weakens bonds between atoms in the substrate so the reaction can form products easier. © 2011 Pearson Education, Inc.
Chapter Summary, Continued 10. 4 Factors That Affect Enzyme Activity • Factors such as p. H, temperature, substrate concentration, and the presence of inhibitors can affect the activity of an enzyme. • An increase in substrate concentration increases the rate of an enzyme-catalyzed reaction. When substrate concentration is increased, the active site becomes saturated with substrate. © 2011 Pearson Education, Inc.
Chapter Summary, Continued 10. 4 Factors That Affect Enzyme Activity, Continued • When the active site is saturated, the enzyme is operating at steady state. • Enzymes have an optimum p. H and temperature at which they function best. • Inhibitors decrease or eliminate an enzyme’s catalytic abilities. © 2011 Pearson Education, Inc.

Chapter Summary, Continued 10. 4 Factors That Affect Enzyme Activity, Continued • The effect of inhibitors can be reversible or irreversible. • Reversible inhibitors can be competitive inhibitors, which compete with the substrate for the active site. • Reversible inhibitors can also be noncompetitive, which bind to a site on the enzyme other than the active site, and causes a conformational change in the enzyme. © 2011 Pearson Education, Inc.
08450a9d2c3fa050a5014a9d2bb57a79.ppt