2bc49b713c4b4aefc02af2d79e5933c7.ppt
- Количество слайдов: 24
 Chapter 10 - Gases What are the characteristics of gases? Variable shape Variable volume The atmosphere is composed of gases. The two major components are nitrogen(78%) and oxygen(21%).
Chapter 10 - Gases What are the characteristics of gases? Variable shape Variable volume The atmosphere is composed of gases. The two major components are nitrogen(78%) and oxygen(21%).
 Kinetic Molecular Theory A theory that explains the behavior of gases at the molecular level Based on the Ideal Gas a gas for which the product of the pressure and volume is proportional to the absolute temperature If a gas is moving it has energy Kinetic Energy KE=1/2 mv 2
Kinetic Molecular Theory A theory that explains the behavior of gases at the molecular level Based on the Ideal Gas a gas for which the product of the pressure and volume is proportional to the absolute temperature If a gas is moving it has energy Kinetic Energy KE=1/2 mv 2
 Four Variables describe a Gas 1. Temperature Based on absolute temperature Kelvin How do we convert to Kelvin? K=°C + 273
Four Variables describe a Gas 1. Temperature Based on absolute temperature Kelvin How do we convert to Kelvin? K=°C + 273
 Cont. 2. Volume V= l • w • h Common units are L, ml, or cm 3 3. Pressure - force exerted over an area P=Force/area Force is measured in Newtons(N) Area is measured m 2 or other similar units
Cont. 2. Volume V= l • w • h Common units are L, ml, or cm 3 3. Pressure - force exerted over an area P=Force/area Force is measured in Newtons(N) Area is measured m 2 or other similar units
 Pressure cont. How is pressure measured? Barometer- device used to measure atmospheric pressure What are the units of Pressure? A. Millimeters of mercury(mm Hg) 1 mm of Hg= 1 torr Atmospheric pressure at sea level at 0° C is 760 mm
Pressure cont. How is pressure measured? Barometer- device used to measure atmospheric pressure What are the units of Pressure? A. Millimeters of mercury(mm Hg) 1 mm of Hg= 1 torr Atmospheric pressure at sea level at 0° C is 760 mm
 Pressure cont. B. Atmosphere of pressure(atm) 1 atm= 760 mm Hg C. Pascal(Pa) – the pressure exerted by a force of one newton acting on an area of one square meter. 1 atm= 1. 01325 x 105 Pa or 101. 325 k. Pa
Pressure cont. B. Atmosphere of pressure(atm) 1 atm= 760 mm Hg C. Pascal(Pa) – the pressure exerted by a force of one newton acting on an area of one square meter. 1 atm= 1. 01325 x 105 Pa or 101. 325 k. Pa
 Standard Temperature and Pressure To compare volumes of gases, it is necessary to know the temperature and pressure at which the volumes are measured. The standard conditions are called Standard Temperature and Pressure(STP) STP= 0°C and 1. 0000 atm
Standard Temperature and Pressure To compare volumes of gases, it is necessary to know the temperature and pressure at which the volumes are measured. The standard conditions are called Standard Temperature and Pressure(STP) STP= 0°C and 1. 0000 atm
 4. Number of moles(n) The volume of one mole of gas at STP is called Molar Volume.
4. Number of moles(n) The volume of one mole of gas at STP is called Molar Volume.
 The Gas Laws 1. Charles’s Law -the volume of a gas at constant pressure is directly proportional to the temperature. V 1/T 1=V 2/T 2
The Gas Laws 1. Charles’s Law -the volume of a gas at constant pressure is directly proportional to the temperature. V 1/T 1=V 2/T 2
 Example of Charles’s Law A sample of gas occupies 24 m 3 at 100. K. What volume would the gas occupy at 400. K? What do you know? V 1=24 m 3 T 1=100. K T 2= 400. K V 2= ?
Example of Charles’s Law A sample of gas occupies 24 m 3 at 100. K. What volume would the gas occupy at 400. K? What do you know? V 1=24 m 3 T 1=100. K T 2= 400. K V 2= ?
 Boyle’s Law The volume of a gas at constant temperature is inversely proportional to the pressure. P 1 V 1=P 2 V 2
Boyle’s Law The volume of a gas at constant temperature is inversely proportional to the pressure. P 1 V 1=P 2 V 2
 Example of Boyle’s Law The gas in a balloon has a volume of 4 L at 100 k. Pa. The balloon is released into the atmosphere, and the gas in it expands to a volume of 8 L. What is the pressure on the balloon at the new volume? What do you know? V 1= 4 L P 1= 100 k. Pa V 2= 8 L P 2 = ?
Example of Boyle’s Law The gas in a balloon has a volume of 4 L at 100 k. Pa. The balloon is released into the atmosphere, and the gas in it expands to a volume of 8 L. What is the pressure on the balloon at the new volume? What do you know? V 1= 4 L P 1= 100 k. Pa V 2= 8 L P 2 = ?
 Gay-Lussac’s Law The pressure of a fixed mass of gas at constant volume varies directly with the Kelvin temperature. P 1/T 1=P 2/T 2 The gas in a container is at a pressure of 3. 00 atm at 25°C. Directions on the container warn the user not to keep it in a place where the temp. exceeds 52°C. What would the gas pressure in the container be at 52°C?
Gay-Lussac’s Law The pressure of a fixed mass of gas at constant volume varies directly with the Kelvin temperature. P 1/T 1=P 2/T 2 The gas in a container is at a pressure of 3. 00 atm at 25°C. Directions on the container warn the user not to keep it in a place where the temp. exceeds 52°C. What would the gas pressure in the container be at 52°C?
 Dalton’s Law of Partial Pressures For the if it a mixture of gases in a container total pressure exerted is the sum of pressures that each gas would exert were alone. PTotal = P 1 + P 2 + P 3 + …
Dalton’s Law of Partial Pressures For the if it a mixture of gases in a container total pressure exerted is the sum of pressures that each gas would exert were alone. PTotal = P 1 + P 2 + P 3 + …
 Example Hydrogen gas is collected over water at a total pressure of 95. 0 k. Pa. The volume of hydrogen collected is 28 ml at 25° C. If the pressure of water vapor at 25° C is 3. 17 k. Pa, what is the partial pressure of hydrogen gas?
Example Hydrogen gas is collected over water at a total pressure of 95. 0 k. Pa. The volume of hydrogen collected is 28 ml at 25° C. If the pressure of water vapor at 25° C is 3. 17 k. Pa, what is the partial pressure of hydrogen gas?
 Graham’s Law Diffusion- movement of particles from regions of high density to regions of low density Example: smelling food throughout the house Effusion- passage of a gas under pressure through a tiny opening Example: a helium balloon is buoyant at first but later hangs toward the ground because helium has escaped through holes in the balloon
Graham’s Law Diffusion- movement of particles from regions of high density to regions of low density Example: smelling food throughout the house Effusion- passage of a gas under pressure through a tiny opening Example: a helium balloon is buoyant at first but later hangs toward the ground because helium has escaped through holes in the balloon
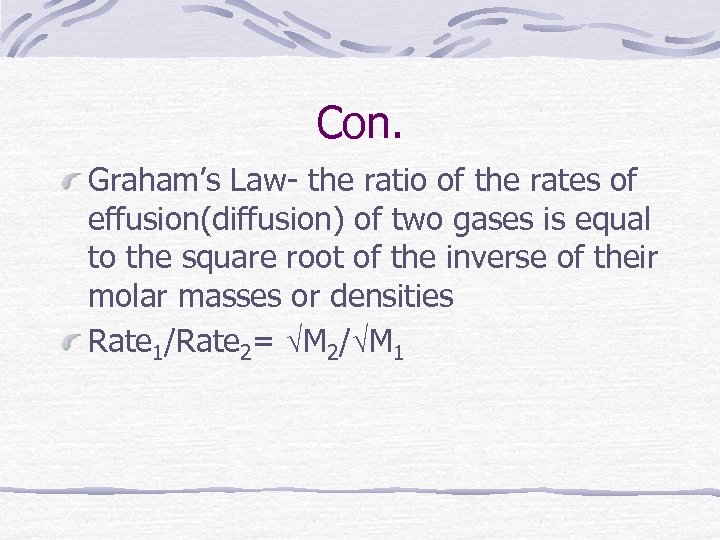 Con. Graham’s Law- the ratio of the rates of effusion(diffusion) of two gases is equal to the square root of the inverse of their molar masses or densities Rate 1/Rate 2= M 2/ M 1
Con. Graham’s Law- the ratio of the rates of effusion(diffusion) of two gases is equal to the square root of the inverse of their molar masses or densities Rate 1/Rate 2= M 2/ M 1
 Example An oxygen molecule travels at about 480 m/s at room temperature. How fast would a molecule of sulfur trioxide travel at the same temperature?
Example An oxygen molecule travels at about 480 m/s at room temperature. How fast would a molecule of sulfur trioxide travel at the same temperature?
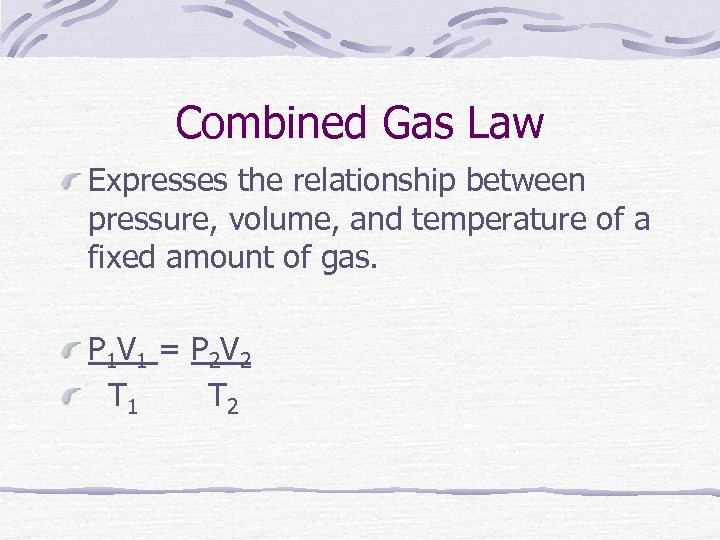 Combined Gas Law Expresses the relationship between pressure, volume, and temperature of a fixed amount of gas. P 1 V 1 = P 2 V 2 T 1 T 2
Combined Gas Law Expresses the relationship between pressure, volume, and temperature of a fixed amount of gas. P 1 V 1 = P 2 V 2 T 1 T 2
 Example of Combined Gas Law A helium balloon with a volume of 410. m. L is cooled from 27° C to -27° C. The pressure on the gas is reduced from 110. k. Pa to 25 k. Pa. What is the volume of the gas at the lower temperature and pressure? What do you know? V 1= 410. m. L V 2= ? P 1= 110. k. Pa P 2= 25. k. Pa T 1= 27°C= 300 K T 2= -27°= 246 K
Example of Combined Gas Law A helium balloon with a volume of 410. m. L is cooled from 27° C to -27° C. The pressure on the gas is reduced from 110. k. Pa to 25 k. Pa. What is the volume of the gas at the lower temperature and pressure? What do you know? V 1= 410. m. L V 2= ? P 1= 110. k. Pa P 2= 25. k. Pa T 1= 27°C= 300 K T 2= -27°= 246 K
 Ideal Gas Law Is the mathematical relationship among pressure, volume, temperature, and the number of moles of a gas. PV= n. RT P = pressure V=Volume n= number of moles T= temperature R=8. 314 L • k. Pa
Ideal Gas Law Is the mathematical relationship among pressure, volume, temperature, and the number of moles of a gas. PV= n. RT P = pressure V=Volume n= number of moles T= temperature R=8. 314 L • k. Pa
 Example of Ideal Gas Law A sample of carbon dioxide with a mass of. 250 g was placed in a 350 ml container at 400. K. What is the pressure exerted by the gas? What do you know? V= 350 ml T= 400. K M=. 250 g of carbon dioxide P= ?
Example of Ideal Gas Law A sample of carbon dioxide with a mass of. 250 g was placed in a 350 ml container at 400. K. What is the pressure exerted by the gas? What do you know? V= 350 ml T= 400. K M=. 250 g of carbon dioxide P= ?
 Ideal gas and Stoichiometry How many liters of hydrogen gas will be produced at 280. K and 96. 0 k. Pa if 40. 0 g of sodium react with excess hydrochloric acid according to the following equation? 2 Na(s) + 2 HCl(aq) 2 Na. Cl(aq) + H 2(g)
Ideal gas and Stoichiometry How many liters of hydrogen gas will be produced at 280. K and 96. 0 k. Pa if 40. 0 g of sodium react with excess hydrochloric acid according to the following equation? 2 Na(s) + 2 HCl(aq) 2 Na. Cl(aq) + H 2(g)
 What do we know? P= 96. 0 k. Pa R= 8. 314 L • k. Pa/mol • K T= 280. K N= calculate with molar mass and mole ratio V=?
What do we know? P= 96. 0 k. Pa R= 8. 314 L • k. Pa/mol • K T= 280. K N= calculate with molar mass and mole ratio V=?


