ef7b5fe582d911f01777ea7f1e62dd40.ppt
- Количество слайдов: 45
 Chapter 1 Chemistry: The Science of Matter Section 1. 1: The Puzzle of Matter Section 1. 2: Properties and Changes of Matter
Chapter 1 Chemistry: The Science of Matter Section 1. 1: The Puzzle of Matter Section 1. 2: Properties and Changes of Matter
 Section 1. 1 Objectives Classify matter according to its composition. n Distinguish among elements, compounds, homogeneous mixtures, and heterogeneous mixtures. n Relate the properties of matter to its structure. n
Section 1. 1 Objectives Classify matter according to its composition. n Distinguish among elements, compounds, homogeneous mixtures, and heterogeneous mixtures. n Relate the properties of matter to its structure. n
 Composition, Structure and Behavior Chemistry- the science that investigates and explains the structure and properties of matter. n Matter- anything that takes up space and has mass n Mass- the measure of the amount of matter that an object contains n
Composition, Structure and Behavior Chemistry- the science that investigates and explains the structure and properties of matter. n Matter- anything that takes up space and has mass n Mass- the measure of the amount of matter that an object contains n
 Matter n Matter is all around you. ¨ ¨ ¨ n Phone Your neighbor The desk The metal of your chair The air you are breathing What isn’t matter ¨ ¨ ¨ Heat Light Thoughts Ideas Radio waves Magnetic fields
Matter n Matter is all around you. ¨ ¨ ¨ n Phone Your neighbor The desk The metal of your chair The air you are breathing What isn’t matter ¨ ¨ ¨ Heat Light Thoughts Ideas Radio waves Magnetic fields
 Mass n On earth we equate mass with weight. ¨A bowling ball has a larger mass than a tennis ball. ¨ I have more mass than the text book.
Mass n On earth we equate mass with weight. ¨A bowling ball has a larger mass than a tennis ball. ¨ I have more mass than the text book.
 Matter The structure of matter refers to its composition-what is it made of and how is it organized n The properties of matter describe the characteristics and behavior of matter. n ¨ Changes matter undergoes
Matter The structure of matter refers to its composition-what is it made of and how is it organized n The properties of matter describe the characteristics and behavior of matter. n ¨ Changes matter undergoes
 Comparing Composition and Behavior (Figure 1. 2 page 5) n Salt and Water ¨ Salt is Na and Cl ¨ Water is H and O ¨ You can wash you hair in water, but not in salt. And you sprinkle water over popcorn. n Aspirin and Sucrose ¨ Both are composed of C, H, and O ¨ You wouldn’t use aspirin to sweeten cereal or use sucrose for a headache
Comparing Composition and Behavior (Figure 1. 2 page 5) n Salt and Water ¨ Salt is Na and Cl ¨ Water is H and O ¨ You can wash you hair in water, but not in salt. And you sprinkle water over popcorn. n Aspirin and Sucrose ¨ Both are composed of C, H, and O ¨ You wouldn’t use aspirin to sweeten cereal or use sucrose for a headache
 Properties n You can determine some of the properties of a particular chunk of matter by examination and manipulation. ¨ What is its color? ¨ Is it a solid, liquid or gas? ¨ Is it soft or hard? ¨ Does it dissolve in water?
Properties n You can determine some of the properties of a particular chunk of matter by examination and manipulation. ¨ What is its color? ¨ Is it a solid, liquid or gas? ¨ Is it soft or hard? ¨ Does it dissolve in water?
 Properties of Iron Strong, but can be flattened and stretched n Does not dissolve in water n Turns to a liquid at high temperatures n Is a gray, shiny solid n Is attracted to a magnet n Conduct electricity n
Properties of Iron Strong, but can be flattened and stretched n Does not dissolve in water n Turns to a liquid at high temperatures n Is a gray, shiny solid n Is attracted to a magnet n Conduct electricity n
 Properties and Composition You may be able to determine many properties of a piece of matter by examining it and doing some simple tests. n However, you can not determine what it is composed of just by looking at it. n
Properties and Composition You may be able to determine many properties of a piece of matter by examining it and doing some simple tests. n However, you can not determine what it is composed of just by looking at it. n
 Examining Matter: The Macroscopic View of Matter n The macroscopic view of matter is one in which you touch, smell, taste and see.
Examining Matter: The Macroscopic View of Matter n The macroscopic view of matter is one in which you touch, smell, taste and see.
 The Submicroscopic View of Matter n n n Gives you a glimpse into the world of atoms You cannot see this world even with the most powerful microscopic. Matter is made up of atoms Atoms are so small that a period at the end of a sentence is made up of 100, 000, 000 (100 quintillion) carbon atoms. If you could count all 100 quintillion atoms at a rate of three per second it would take you a trillion years to finish counting.
The Submicroscopic View of Matter n n n Gives you a glimpse into the world of atoms You cannot see this world even with the most powerful microscopic. Matter is made up of atoms Atoms are so small that a period at the end of a sentence is made up of 100, 000, 000 (100 quintillion) carbon atoms. If you could count all 100 quintillion atoms at a rate of three per second it would take you a trillion years to finish counting.
 Macro, micro, submicro n Macroscopic – I can see with my “naked” eye n Microscopic – I need a microscope to see n Submicroscopic – I can’t see even with the most powerful microscope
Macro, micro, submicro n Macroscopic – I can see with my “naked” eye n Microscopic – I need a microscope to see n Submicroscopic – I can’t see even with the most powerful microscope
 Scanning Tunneling Microscope (STM) Although you cannot see atoms the STM can produce images on a computer screen that show the location of individual atoms. n Platinum n
Scanning Tunneling Microscope (STM) Although you cannot see atoms the STM can produce images on a computer screen that show the location of individual atoms. n Platinum n
 Using Models in Chemistry In chemistry you will use macroscopic and sub-microscopic models to understand certain concepts. n Scientific model- a thinking device, built on experimentation, that helps us to understand explain macroscopic observations. n
Using Models in Chemistry In chemistry you will use macroscopic and sub-microscopic models to understand certain concepts. n Scientific model- a thinking device, built on experimentation, that helps us to understand explain macroscopic observations. n
 Models A model for the atom was discussed in Greece about 2, 500 years ago. However, this was not a scientific model. n The scientific model of the atom was not proposed until the 1800’s and it has with stood much experimentation with little changes. n
Models A model for the atom was discussed in Greece about 2, 500 years ago. However, this was not a scientific model. n The scientific model of the atom was not proposed until the 1800’s and it has with stood much experimentation with little changes. n
 Classifying Matter can be classified by its composition n There are two main types of classifications n ¨ Qualitative- an observation made without measurement. ¨ Quantitative- an observation made with measurement
Classifying Matter can be classified by its composition n There are two main types of classifications n ¨ Qualitative- an observation made without measurement. ¨ Quantitative- an observation made with measurement
 Qualitative vs. Quantitative n Qualitative ¨ There are students in this room ¨ Sucrose contains carbon, oxygen and hydrogen n Quantitative ¨ There are 24 students in this room ¨ Sucrose contains 42. 1 g of carbon, 51. 4 g of oxygen and 6. 5 g of hydrogen.
Qualitative vs. Quantitative n Qualitative ¨ There are students in this room ¨ Sucrose contains carbon, oxygen and hydrogen n Quantitative ¨ There are 24 students in this room ¨ Sucrose contains 42. 1 g of carbon, 51. 4 g of oxygen and 6. 5 g of hydrogen.
 Pure vs. Mixture n n Matter can be classified by its purity. Is the matter pure or is it a mixture? Pure in chemistry means it contains only the same substance. Substance- matter with the same fixed composition and properties. ¨ Can be an element or a compound ¨ Any sample of pure matter is a substance
Pure vs. Mixture n n Matter can be classified by its purity. Is the matter pure or is it a mixture? Pure in chemistry means it contains only the same substance. Substance- matter with the same fixed composition and properties. ¨ Can be an element or a compound ¨ Any sample of pure matter is a substance
 Substances n The bag of sugar you buy at the store is pure sucrose. It all has the same properties and a fixed composition. Therefore, it is a substance.
Substances n The bag of sugar you buy at the store is pure sucrose. It all has the same properties and a fixed composition. Therefore, it is a substance.
 Mixed Matter n n Mixed matter is referred to as a mixture. Mixture- combination of two or more substances in which the basic identity of the substances are not changed. Mixtures do not have a specific composition. Mixtures can be separated into its components by physical means.
Mixed Matter n n Mixed matter is referred to as a mixture. Mixture- combination of two or more substances in which the basic identity of the substances are not changed. Mixtures do not have a specific composition. Mixtures can be separated into its components by physical means.
 Separating Mixtures n n One way is by physical changes. Physical change- a change in matter that does not involve a change in the identity of individual substances. ¨ Boiling ¨ Freezing ¨ Melting ¨ Evaporation ¨ Dissolving ¨ Crystallization
Separating Mixtures n n One way is by physical changes. Physical change- a change in matter that does not involve a change in the identity of individual substances. ¨ Boiling ¨ Freezing ¨ Melting ¨ Evaporation ¨ Dissolving ¨ Crystallization
 Physical Properties n n Separation by physical changes takes advantage of the physical properties of the mixture. Physical properties- characteristics that a sample of matter exhibits without any changes in its identity ¨ ¨ ¨ Solubility Melting and boiling point Color Density Electrical conductivity Physical state (solid, liquid or gas)
Physical Properties n n Separation by physical changes takes advantage of the physical properties of the mixture. Physical properties- characteristics that a sample of matter exhibits without any changes in its identity ¨ ¨ ¨ Solubility Melting and boiling point Color Density Electrical conductivity Physical state (solid, liquid or gas)
 Types of Mixture n There are two types of mixtures: ¨ Heterogeneous n Hetero means “different” ¨ Homogeneous n Homo means “the same”
Types of Mixture n There are two types of mixtures: ¨ Heterogeneous n Hetero means “different” ¨ Homogeneous n Homo means “the same”
 Heterogeneous Mixture n Heterogeneous Mixture- a mixture that does not have a uniform composition. ¨ You can see the different composition. ¨ Examples: Granite n Chef Salad n Lucky Charms Cereal n Orange Juice with pulp n
Heterogeneous Mixture n Heterogeneous Mixture- a mixture that does not have a uniform composition. ¨ You can see the different composition. ¨ Examples: Granite n Chef Salad n Lucky Charms Cereal n Orange Juice with pulp n
 Homogeneous Mixture n Homogeneous Mixture- a mixture with a uniform composition. ¨ You cannot tell that it is composed of more than one substance ¨ Another name is a solution ¨ Examples: n n n Salt water Tea Sugar water
Homogeneous Mixture n Homogeneous Mixture- a mixture with a uniform composition. ¨ You cannot tell that it is composed of more than one substance ¨ Another name is a solution ¨ Examples: n n n Salt water Tea Sugar water
 Solutions Homogeneous mixture n Examples: n ¨ Salt water ¨ Gasoline ¨ Air ¨ Steel
Solutions Homogeneous mixture n Examples: n ¨ Salt water ¨ Gasoline ¨ Air ¨ Steel
 Alloys- solid solutions that contain different metals and sometimes nonmetallic substance n Table 1. 1 page 23 n
Alloys- solid solutions that contain different metals and sometimes nonmetallic substance n Table 1. 1 page 23 n
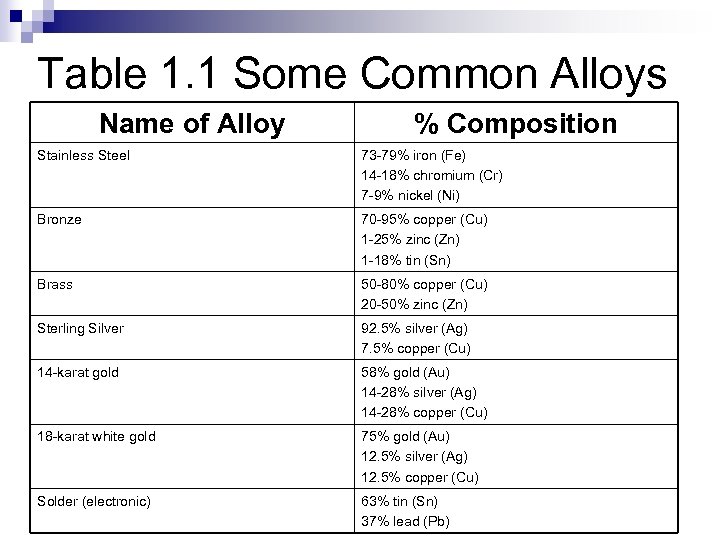 Table 1. 1 Some Common Alloys Name of Alloy % Composition Stainless Steel 73 -79% iron (Fe) 14 -18% chromium (Cr) 7 -9% nickel (Ni) Bronze 70 -95% copper (Cu) 1 -25% zinc (Zn) 1 -18% tin (Sn) Brass 50 -80% copper (Cu) 20 -50% zinc (Zn) Sterling Silver 92. 5% silver (Ag) 7. 5% copper (Cu) 14 -karat gold 58% gold (Au) 14 -28% silver (Ag) 14 -28% copper (Cu) 18 -karat white gold 75% gold (Au) 12. 5% silver (Ag) 12. 5% copper (Cu) Solder (electronic) 63% tin (Sn) 37% lead (Pb)
Table 1. 1 Some Common Alloys Name of Alloy % Composition Stainless Steel 73 -79% iron (Fe) 14 -18% chromium (Cr) 7 -9% nickel (Ni) Bronze 70 -95% copper (Cu) 1 -25% zinc (Zn) 1 -18% tin (Sn) Brass 50 -80% copper (Cu) 20 -50% zinc (Zn) Sterling Silver 92. 5% silver (Ag) 7. 5% copper (Cu) 14 -karat gold 58% gold (Au) 14 -28% silver (Ag) 14 -28% copper (Cu) 18 -karat white gold 75% gold (Au) 12. 5% silver (Ag) 12. 5% copper (Cu) Solder (electronic) 63% tin (Sn) 37% lead (Pb)
 Solutions n When you are dissolving a substance into another substance there are two important terms: ¨ Solute – the substance being dissolved ¨ Solvent – the dissolving agent n Salt Water ¨ Solute = Na. Cl ¨ Solvent = H 2 O
Solutions n When you are dissolving a substance into another substance there are two important terms: ¨ Solute – the substance being dissolved ¨ Solvent – the dissolving agent n Salt Water ¨ Solute = Na. Cl ¨ Solvent = H 2 O
 Aqueous Solution n n Aqueous Solution- a solution in which the solvent is water. Examples: ¨ Soda ¨ Tea ¨ Contact-lens cleaner ¨ Clear cleaning liquids n Most processes of life take place in aqueous solutions.
Aqueous Solution n n Aqueous Solution- a solution in which the solvent is water. Examples: ¨ Soda ¨ Tea ¨ Contact-lens cleaner ¨ Clear cleaning liquids n Most processes of life take place in aqueous solutions.
 Substance: Pure Matter n You and everything around you is made up of chemicals.
Substance: Pure Matter n You and everything around you is made up of chemicals.
 Elements: The Building Blocks If you classify an unknown piece of matter as pure; it means that it is made up of one substance n There are two types of substances n ¨ Compounds ¨ Elements
Elements: The Building Blocks If you classify an unknown piece of matter as pure; it means that it is made up of one substance n There are two types of substances n ¨ Compounds ¨ Elements
 Elements n n Elements- a substance that cannot be broken down into simpler substances. Simplest form of matter Building blocks for other types of matter All substance in the universe are: ¨ Elements ¨ Compounds formed from elements ¨ Or mixtures of elements and compound
Elements n n Elements- a substance that cannot be broken down into simpler substances. Simplest form of matter Building blocks for other types of matter All substance in the universe are: ¨ Elements ¨ Compounds formed from elements ¨ Or mixtures of elements and compound
 Elements n n 118 elements Examples: ¨ Gold ¨ Carbon ¨ Lead n n Elements combine to form millions of compounds. Chemical elements are often referred to as the building blocks of matter
Elements n n 118 elements Examples: ¨ Gold ¨ Carbon ¨ Lead n n Elements combine to form millions of compounds. Chemical elements are often referred to as the building blocks of matter
 Elements 118 elements n 90 occur naturally n ¨ Less n than half of these are abundant The remainder are synthesized
Elements 118 elements n 90 occur naturally n ¨ Less n than half of these are abundant The remainder are synthesized
 Organizing the Elements are organized in the Periodic Table n The periodic table tells you: n ¨ Name ¨ Symbol ¨ Atomic mass
Organizing the Elements are organized in the Periodic Table n The periodic table tells you: n ¨ Name ¨ Symbol ¨ Atomic mass
 Symbols n n The symbols of the elements are extremely important to know. You will only have to know the most common ones. The symbols are a one to two letter representation of the elements. Not all the symbols are the first or second letter of the elements name
Symbols n n The symbols of the elements are extremely important to know. You will only have to know the most common ones. The symbols are a one to two letter representation of the elements. Not all the symbols are the first or second letter of the elements name
 Symbols n Oxygen ¨O n Hydrogen ¨H n Bromine ¨ Br n Chlorine ¨ Cl
Symbols n Oxygen ¨O n Hydrogen ¨H n Bromine ¨ Br n Chlorine ¨ Cl
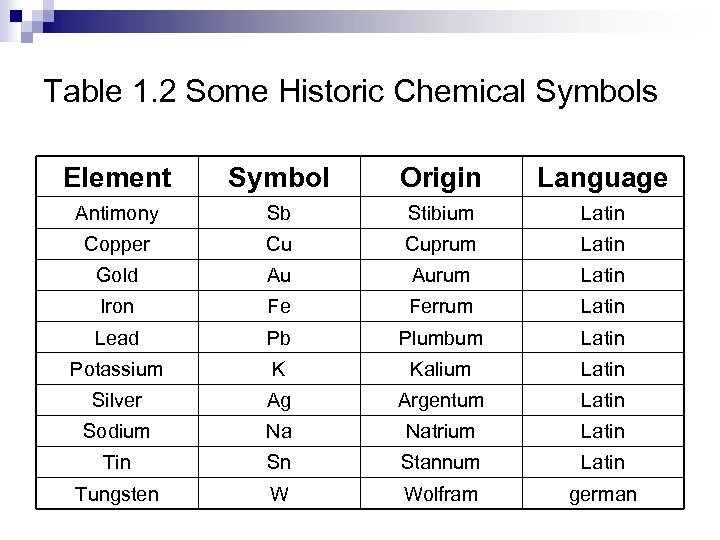 Table 1. 2 Some Historic Chemical Symbols Element Symbol Origin Language Antimony Sb Stibium Latin Copper Cu Cuprum Latin Gold Au Aurum Latin Iron Fe Ferrum Latin Lead Pb Plumbum Latin Potassium K Kalium Latin Silver Ag Argentum Latin Sodium Na Natrium Latin Tin Sn Stannum Latin Tungsten W Wolfram german
Table 1. 2 Some Historic Chemical Symbols Element Symbol Origin Language Antimony Sb Stibium Latin Copper Cu Cuprum Latin Gold Au Aurum Latin Iron Fe Ferrum Latin Lead Pb Plumbum Latin Potassium K Kalium Latin Silver Ag Argentum Latin Sodium Na Natrium Latin Tin Sn Stannum Latin Tungsten W Wolfram german
 Compounds Are More Than One Element n n Compound – a chemical combination of two or more different elements joined together in a fixed proportion. Every compound has its own fixed composition Therefore, every compound has unique chemical and physical properties. The properties of a compound are different from the properties of the elements that make them up.
Compounds Are More Than One Element n n Compound – a chemical combination of two or more different elements joined together in a fixed proportion. Every compound has its own fixed composition Therefore, every compound has unique chemical and physical properties. The properties of a compound are different from the properties of the elements that make them up.
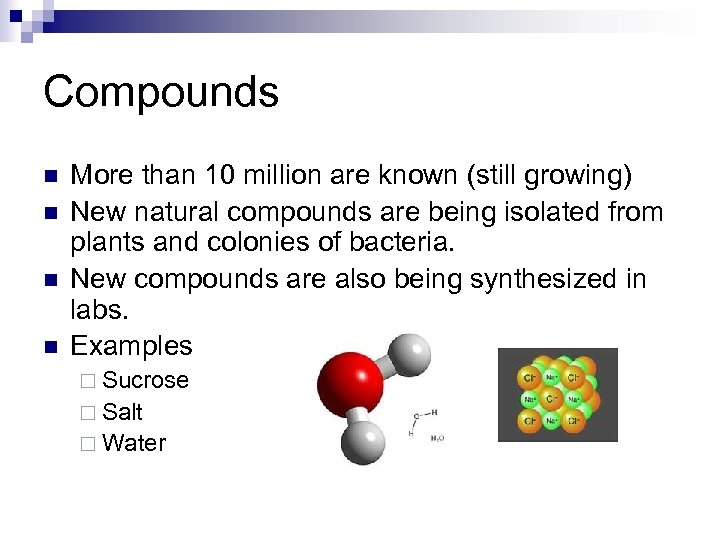 Compounds n n More than 10 million are known (still growing) New natural compounds are being isolated from plants and colonies of bacteria. New compounds are also being synthesized in labs. Examples ¨ Sucrose ¨ Salt ¨ Water
Compounds n n More than 10 million are known (still growing) New natural compounds are being isolated from plants and colonies of bacteria. New compounds are also being synthesized in labs. Examples ¨ Sucrose ¨ Salt ¨ Water
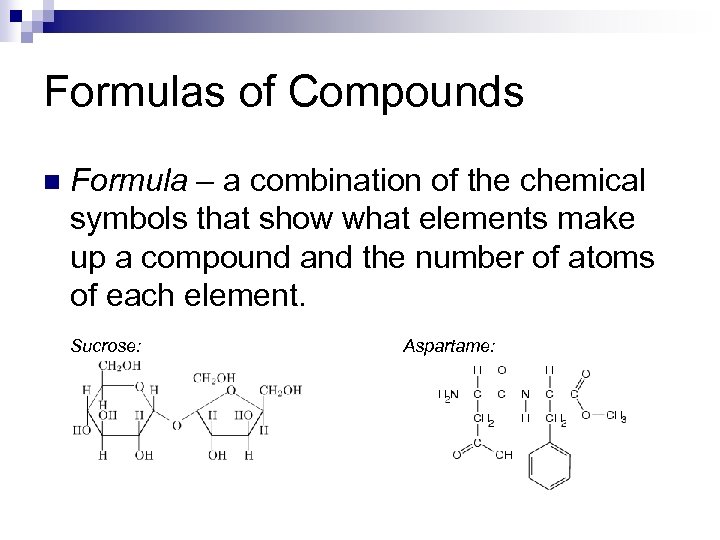 Formulas of Compounds n Formula – a combination of the chemical symbols that show what elements make up a compound and the number of atoms of each element. Sucrose: Aspartame:
Formulas of Compounds n Formula – a combination of the chemical symbols that show what elements make up a compound and the number of atoms of each element. Sucrose: Aspartame:
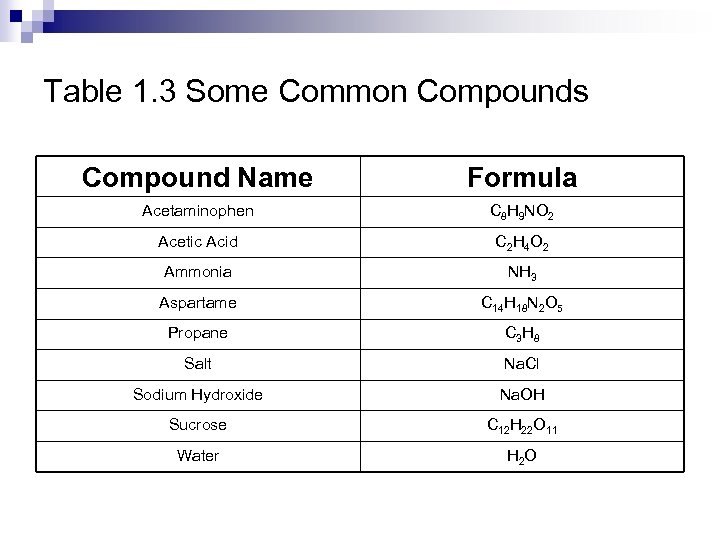 Table 1. 3 Some Common Compounds Compound Name Formula Acetaminophen C 8 H 9 NO 2 Acetic Acid C 2 H 4 O 2 Ammonia NH 3 Aspartame C 14 H 18 N 2 O 5 Propane C 3 H 8 Salt Na. Cl Sodium Hydroxide Na. OH Sucrose C 12 H 22 O 11 Water H 2 O
Table 1. 3 Some Common Compounds Compound Name Formula Acetaminophen C 8 H 9 NO 2 Acetic Acid C 2 H 4 O 2 Ammonia NH 3 Aspartame C 14 H 18 N 2 O 5 Propane C 3 H 8 Salt Na. Cl Sodium Hydroxide Na. OH Sucrose C 12 H 22 O 11 Water H 2 O
 Review What is the difference between an element and a compound? n What is the difference between a homogeneous mixture and a heterogeneous mixture? n What is the difference between a mixture and a compound? n
Review What is the difference between an element and a compound? n What is the difference between a homogeneous mixture and a heterogeneous mixture? n What is the difference between a mixture and a compound? n


